Published online May 21, 2023. doi: 10.3748/wjg.v29.i19.3003
Peer-review started: February 17, 2023
First decision: March 24, 2023
Revised: March 28, 2023
Accepted: April 24, 2023
Article in press: April 24, 2023
Published online: May 21, 2023
Processing time: 87 Days and 18.5 Hours
The interruption of mother-to-child transmission (MTCT) is considered important to decrease the individual and population morbidity of hepatitis B virus (HBV) infection as well as the global burden of hepatitis B. Serum vitamin D (VD) is associated with hepatitis B.
To assess whether baseline VD levels and single nucleotide polymorphisms of the VD receptor gene (VDR SNPs) are associated with the efficacy of tenofovir disoproxil fumarate (TDF) in the prevention of MTCT in pregnant women with high HBV viral loads.
Thirty-eight pregnant women who were at high risk for MTCT of HBV (those with an HBV DNA level ≥ 2 × 105 IU/mL during 12-24 wk of gestation) receiving antiviral therapy of TDF between June 1, 2019 and June 30, 2021 in Mianyang were included in this retrospective study. The women received 300 mg TDF once daily from gestational weeks 24-28 until 3 mo after delivery. To further characterize the clinical relevance of maternal serum HBV DNA levels, we stratified patients according to HBV DNA level as follows: Those with levels < 2 × 105 (full responder group) vs those levels ≥ 2 × 105 IU/mL (partial responder group) at delivery. Serum levels of 25-hydroxyvitamin D [25(OH)D], liver function markers, virological parameters, VDR SNPs and other clinical parameters were collected to analyze their association with the efficacy of TDF. The Mann-Whitney U test or t test was used to analyze the serum levels of 25(OH)D in different groups. Multiple linear regressions were utilized to analyze the determinants of the maternal HBV DNA level at delivery. Univariate and multi
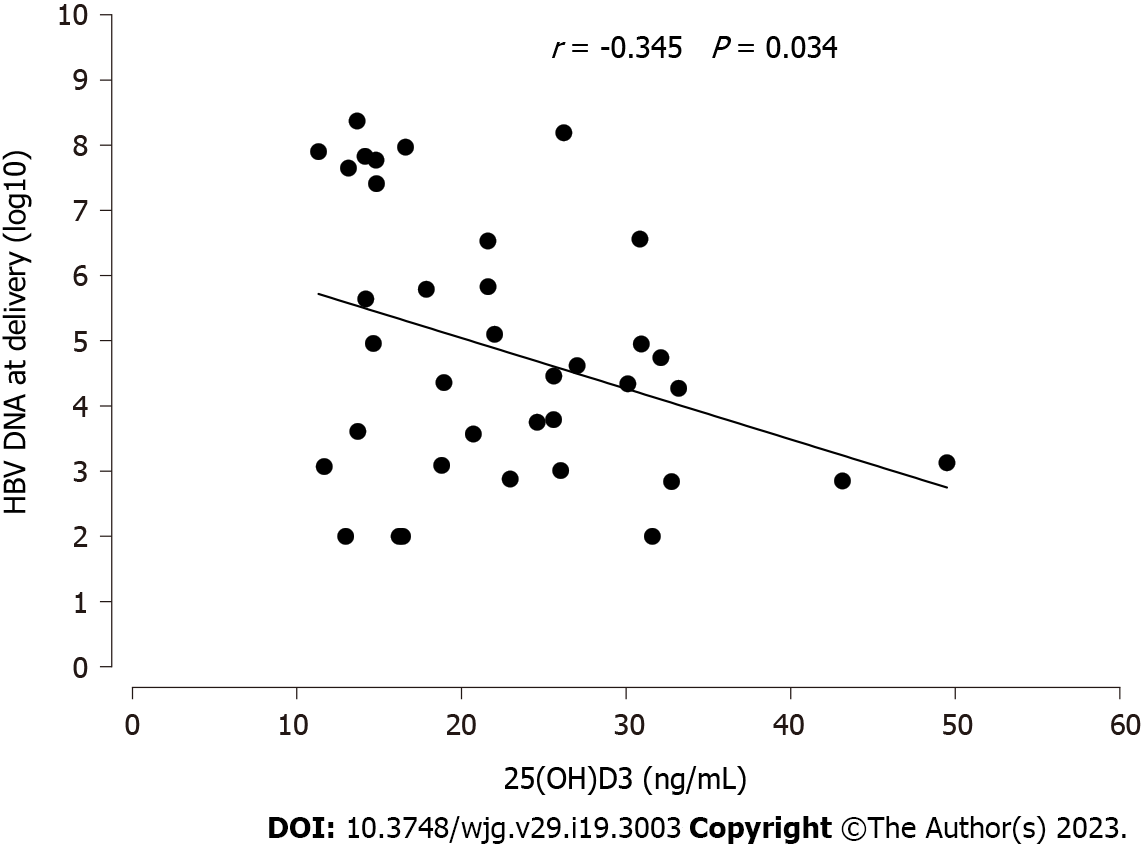
A total of 38 pregnant women in Mianyang City at high risk for MTCT of HBV were enrolled in the study. The MTCT rate was 0%. No mother achieved hepatitis B e antigen or hepatitis B surface antigen (HBsAg) clearance at delivery. Twenty-three (60.5%) participants were full responders, and 15 (39.5%) participants were partial responders according to antiviral efficacy. The present study showed that a high percentage (76.3%) of pregnant women with high HBV viral loads had deficient (< 20 ng/mL) or insufficient (≥ 20 but < 31 ng/mL) VD levels. Serum 25(OH)D levels in partial responders appeared to be significantly lower than those in full responders both at baseline (25.44 ± 9.42 vs 17.66 ± 5.34 ng/mL, P = 0.006) and delivery (26.76 ± 8.59 vs 21.24 ± 6.88 ng/mL, P = 0.044). Serum 25(OH)D levels were negatively correlated with maternal HBV DNA levels [log(10) IU/mL] at delivery after TDF therapy (r = -0.345, P = 0.034). In a multiple linear regression analysis, maternal HBV DNA levels were associated with baseline maternal serum 25(OH)D levels (P < 0.0001, β = -0.446), BMI (P = 0.03, β = -0.245), baseline maternal log10 HBsAg levels (P = 0.05, β = 0.285) and cholesterol levels at delivery (P = 0.015, β = 0.341). Multivariate logistic regression analysis showed that baseline serum 25(OH)D levels (OR = 1.23, 95%CI: 1.04-1.44), maternal VDR Cdx2 TT (OR = 0.09, 95%CI: 0.01-0.88) and cholesterol levels at delivery (OR = 0.39, 95%CI: 0.17-0.87) were associated with targeted antiviral effects (maternal HBV DNA levels < 2 × 105 at delivery).
Maternal VD levels and VDR SNPs may be associated with the efficacy of antiviral therapy in pregnant women with high HBV viral loads. Future studies to evaluate the therapeutic value of VD and its analogs in reducing the MTCT of HBV may be justified.
Core Tip: This retrospective study investigated the influence of vitamin D (VD) levels and single nucleotide polymorphisms of the VD receptor gene (VDR SNPs) on the efficacy of tenofovir disoproxil fumarate in preventing mother-to-child transmission in 38 pregnant women with high hepatitis B viral loads. We demonstrate a significant association between low serum levels of 25-hydroxyvitamin D and high levels of hepatitis B virus replication in pregnant women with high hepatitis B viral loads, and maternal VD levels as well as VDR SNPs may be associated with the efficacy of antiviral therapy.
- Citation: Wang R, Zhu X, Zhang X, Liu H, Ji YL, Chen YH. Association of vitamin D and polymorphisms of its receptor with antiviral therapy in pregnant women with hepatitis B. World J Gastroenterol 2023; 29(19): 3003-3012
- URL: https://www.wjgnet.com/1007-9327/full/v29/i19/3003.htm
- DOI: https://dx.doi.org/10.3748/wjg.v29.i19.3003
Hepatitis B virus (HBV) infection is a serious public health problem that causes a very large medical and economic burden worldwide. Mother-to-child transmission (MTCT) is the main route of HBV trans
Vitamin D (VD) is a fat-soluble steroid hormone that is widely found in systemic organs and tissues, including the brain, bones, cardiac system and immune system, and has multiple effects on human health and many diseases, including infectious diseases. In fact, VD deficiency has been detected in a variety of chronic liver diseases, including chronic viral hepatitis[5-8]. Moreover, VD deficiency is common among patients with chronic hepatitis B infection and is associated with adverse clinical outcomes[9]. Similarly, VD levels are lower in pregnant women with chronic HBV infection than in healthy pregnant women[10]. Clinical and epidemiological studies support the role of VD in inhibiting HBV infection, and this antiviral effect is widely attributed to the VD receptor (VDR)[11].
Different studies have focused on single nucleotide polymorphisms of the VDR gene (VDR SNPs) and HBV. VDR ApaI has been associated with the presence of hepatitis B surface antigen (HBsAg) and viral loads at different times[12]. In addition to its effect on viruses, VD plays an important role for the mother and fetus during pregnancy and has been associated with influencing adverse perinatal events.
However, few data are available with regard to the association of VD levels and VDR SNPs with clinical parameters and treatment outcomes in pregnant women with high HBV viral loads. The aims of our present retrospective study were to study whether baseline VD levels and VDR SNPs were associated with the efficacy of tenofovir disoproxil fumarate (TDF) in the prevention of MTCT in pregnant women with high HBV viral loads.
This was a retrospective study. As part of “The National Science & Technology Pillar Program during the 13th Five-year Plan Period”, HBsAg-positive pregnant women receiving TDF were included from Mianyang between June 1, 2019 and June 30, 2021. The present study was approved by the Institutional Review Board of the West China Hospital, Sichuan University (No. 2019-151), and informed consent was obtained from all patients before recruitment.
Pregnant women screened for HBV DNA at high risk for MTCT (HBV DNA thresholds ≥ 2 × 105 IU/mL) according to WHO recommendations during 12-24 wk of gestation and completed antiviral therapy as required were included in the study. Women received 300 mg TDF once daily from gestational weeks 24-28 until 3 mo after delivery, in addition to HBV immune globulin and three doses of HBV vaccination, including a birth dose given to the neonate. The main exclusion criteria were as follows: (1) Coinfection of syphilis, Toxoplasma gondii, human immunodeficiency viruses, or types of viral hepatitis other than HBV; (2) major systemic disease, including heart disease, malignant neoplasm, or renal insufficiency; (3) evidence of liver cirrhosis, hepatic decompensation and other liver diseases such as drug-induced hepatitis, autoimmune liver disease, or alcoholic liver disease; (4) evidence of congenital anomalies of the fetus; (5) antiviral treatment within a short period of time prior to treatment with TDF or failure to complete TDF antiviral therapy as required; and (6) incomplete data, including basic information, VD levels before and after treatment, VDR SNPs, virological indicators, etc.
The basic information of the pregnant woman including age, height, weight, season of blood sample collection and other basic information was collected. Meanwhile, maternal virological indicators, including HBsAg, hepatitis B e antigen (HBeAg) and HBV DNA, were recorded before antiviral treatment and at delivery after antiviral treatment. Moreover, common clinical parameters before antiviral treatment and at delivery after antiviral treatment including peripheral blood count, liver function, kidney function and other parameters were also collected. Laboratory tests were performed according to our previous description[13,14].
Previously collected blood samples were used to assess maternal VD levels and VDR SNPs. In particular, VD was assessed at baseline and at the time of delivery by measuring serum 25-hydroxyvitamin D [25(OH)D] levels. Serum 25(OH)D was analyzed by LCMS/MS (Agilent Technologies Inc., LCMS/MS1260-6470, CA, United States) after hexane extraction with deuterated 25(OH)D as a control as previously described[15]. Levels of VD were categorized as follows: < 20 ng/mL = deficient; ≥ 20 but < 31 ng/mL = insufficient; and ≥ 31 ng/mL = normal. Genomic DNA was isolated from blood samples (MagNA Pure Compact, Roche). VDR SNPs were assessed through a real-time PCR allelic discrimination system (LightCycler 96, Roche). We investigated the following gene SNPs: VDR: rs7975232 (ApaI)C>A, rs11568820 (Cdx2)T>C, rs2228570 (FokI)A>G, rs1544410 (BsmI)C>T, rs731236 (TaqI)A>G.
The primary outcomes were the changes in the maternal viral load (HBV DNA level) at baseline and the time of delivery. A sustained virological response was defined as an HBV DNA level lower than 2 × 105 IU/mL at delivery. We aimed to determine whether the levels of VD and VD SNPs were associated with the antiviral effects of TDF in interrupting MTCT during the peripartum period.
All statistical analyses were carried out using SPSS Version 26. Categorical variables are represented as frequencies and percentages, and continuous variables are represented as medians (interquartile ranges) or mean ± SD. The outcomes were compared between the two groups using χ2 tests or Fisher’s exact test for categorical variables and the Wilcoxon signed-rank test or Student’s t test for continuous variables. Associations between VD and each of the baseline demographic and lab values were assessed in univariate analyses using general linear models. Univariate and multivariate logistic regression analyses were employed to explore the association of targeted antiviral effects (HBV DNA levels < 2 × 105 at delivery) with various characteristics at baseline and delivery. Factors with a P value < 0.1 in univariate analysis were considered in multivariate analysis. The statistical test was 2-sided, and a P value < 0.05 was considered statistically significant.
The baseline characteristics, laboratory data, and VDR SNPs of the patients are presented in Table 1. No mother achieved HBeAg or HBsAg clearance at delivery. The decrease in HBV DNA levels from baseline to delivery was significant (P < 0.001). A total of 100% of the infants had negative HBsAg and undetectable HBV DNA levels at delivery; thus, the MTCT rate was 0%. To further characterize the clinical relevance of maternal serum HBV DNA levels, we stratified patients according to serum HBV DNA levels as follows: those with levels < 2 × 105 (full responder group) vs those with levels ≥ 2 × 105 IU/mL (partial responder group) at delivery. Mothers with serum HBV DNA viral loads below this threshold are generally considered low risk for MTCT. Twenty-three (60.5%) participants were full responders, and 15 (39.5%) participants were partial responders according to antiviral efficacy. Full and partial responders were similar in age (29.09 vs 28.73 years) and body mass index (BMI) (23.15 vs 21.42 kg/m2).
| Before treatment initiation | P value | At delivery after treatment | P value | |||
| Full responders | Partial responders | Full responders | Partial responders | |||
| Age, yr, mean ± SD | 29.09 ± 3.55 | 28.73 ± 3.01 | 0.75 | NA | NA | |
| BMI, kg/m2, mean ± SD | 23.15 ± 3.33 | 21.42 ± 3.61 | 0.14 | NA | NA | |
| Season of blood draw | 0.552 | 0.311 | ||||
| Winter or spring | 10 (43.5%) | 8 (53.3%) | 16 (69.6%) | 8 (53.3%) | ||
| Summer or autumn | 13 (56.5%) | 7 (46.7%) | 7 (30.4%) | 7 (46.7%) | ||
| HBsAg log10 IU/mL mean (IQR) | 4.33 (3.73, 4.52) | 4.36 (4.1, 4.72) | 0.663 | 4.11 (2.56, 5.44) | 4.33 (3.95, 4.56) | 0.256 |
| HBeAg log10 IU/mL mean (IQR) | 3.16 (2.66, 3.2) | 3.16 (3.12, 3.19) | 0.928 | 3.18 (2.85, 3.19) | 6.75 (6.21, 8.49) | 0.510 |
| HBV DNA, log10 mean (IQR) | 8.06 (7.61, 8.44) | 8.09 (7.53, 8.16) | 0.56 | 3.61 (2.88, 4.46) | 7.41 (5.79, 7.9) | < 0.0001 |
| Vitamin D, ng/mL, mean ± SD | 25.44 ± 9.42 | 17.66 ± 5.34 | 0.006 | 26.76 ± 8.59 | 21.24 ± 6.88 | 0.044 |
| ≥ 30 | 8 (34.8%) | 1 (6.7%) | 0.021 | 10 (43.5%) | 2 (13.3%) | 0.127 |
| 20-30 | 8 (34.8%) | 3 (20%) | 6 (26.1%) | 6 (40%) | ||
| < 20 | 7 (30.4%) | 11 (73.3%) | 7 (30.4%) | 7 (46.7%) | ||
| Hemoglobin, g/dL, mean ± SD | 113.78 ± 9.29 | 115.47 ± 8.98 | 0.583 | 114.3 ± 12.78 | 118 ± 10.17 | 0.353 |
| WBC count, × 106/µL, mean ± SD | 7.83 ± 1.54 | 8.61 ± 2.26 | 0.282 | 7.63 ± 1.77 | 7.7 ± 2.17 | 0.918 |
| Platelet, × 103/µL, mean ± SD | 149.52 ± 56.73 | 155.6 ± 53.01 | 0.742 | 137.17 ± 53.92 | 149.4 ± 56.21 | 0.506 |
| Neutrophil, mean ± SD | 5.78 ± 1.38 | 6.51 ± 2.0 | 0.289 | 5.33 ± 1.62 | 5.53 ± 1.96 | 0.731 |
| Lymphocyte, mean ± SD | 1.46 ± 0.4 | 1.49 ± 0.49 | 0.823 | 1.63 ± 1.14 | 1.61 ± 0.49 | 0.964 |
| Total bilirubin, mg/dL, mean ± SD | 14.73 ± 3.24 | 13.61 ± 3.09 | 0.299 | 14.97 ± 4.15 | 17.13 ± 4.23 | 0.128 |
| Albumin, g/dL, mean ± SD | 39.21 ± 2.36 | 39.93 ± 2.28 | 0.355 | 26.42 ± 3.19 | 37.33 ± 3.68 | 0.698 |
| ALT, U/L, mean (IQR) | 22 (19, 28) | 21 (15, 32) | 0.891 | 22 (18, 30) | 21 (17, 36) | 0.893 |
| Triglyceride, mg/dL, mean (IQR) | 1.74 (1.59, 2.38) | 1.58 (1.34, 1.79) | 0.189 | 3.06 (2.41, 3.67) | 2.52 (2.03, 3.21) | 0.131 |
| Cholesterol, mg/dL, mean ± SD | 5.33 ± 0.85 | 5.7 ± 1.97 | 0.347 | 4.87 ± 0.79 | 5.56 ± 0.96 | 0.001 |
| Creatinine, mg/dL, mean (IQR) | 43 (38, 49) | 43 (39, 45) | 0.951 | 44 (37, 51) | 47.5 (39.8, 55) | 0.397 |
| Alkaline phosphatase, U/L, mean (IQR) | 7 (5, 10) | 10 (4, 23) | 0.234 | 48 (19, 72) | 52 (47, 111) | 0.199 |
| GGT, U/L, mean ± SD | 12.91 ± 8.1 | 13.87 ± 8.4 | 0.675 | 14.48 ± 7.57 | 22 ± 15.19 | 0.115 |
| VDR SNPs | ||||||
| Cdx2 TT | 9 (39.1%) | 2 (13.3%) | 0.076 | NA | ||
| TC/CC | 14 (30.9%) | 13 (86.7%) | ||||
| Bsm1 CC | 20 (87%) | 13 (86.7%) | 0.979 | NA | ||
| CT/TT | 3 (13%) | 2 (13.3%) | ||||
| Fokl AA/AG | 18 (78.3%) | 10 (66.7%) | 0.431 | NA | ||
| GG | 5 (21.7%) | 5 (33.3%) | ||||
| Taq1 AA | 15 (65.2%) | 9 (60%) | 0.744 | NA | ||
| AG/GG | 8 (34.8%) | 6 (40%) | ||||
| Apa1 CC | 10 (43.5%) | 7 (46.7%) | 0.847 | NA | ||
| CA/AA | 13 (56.5%) | 8 (53.3%) | ||||
In the virological indicators related to HBV, there was no significant difference in the HBsAg log10 and HBeAg log10 values between the two groups both at baseline and delivery. The serum HBV DNA concentration was not significantly different between the two groups at baseline, but the serum HBV DNA concentration of full responders was significantly lower than that of partial responders at delivery after antiviral treatment [log10, 3.61 (2.88, 4.46) vs 7.41 (5.79, 7.9), P < 0.0001].
For the laboratory test results, there were no significant differences between full and partial responders in most of the main laboratory indices before and after antiviral therapy, including hemoglobin, white blood cell count, platelets, neutrophils, lymphocytes, total bilirubin, albumin, alanine aminotransferase, triglycerides, creatinine, alkaline phosphatase, and gamma-glutamyl transferase. However, cholesterol levels were lower in complete responders than in partial responders at delivery after treatment (4.87 ± 0.79 vs 5.56 ± 0.96 mg/dL, P = 0.001), but there was no significant difference at baseline.
There was no VD or multivitamin supplementation between baseline and delivery. The mean baseline serum 25(OH)D level of the entire cohort was similar to the serum 25(OH)D level at delivery, with no significant difference (22.37 ± 8.85 vs 24.58 ± 8.32 ng/mL, P = 0.139). Of the 38 patients in the entire cohort, 18 (47.4%), 11 (28.9%), and 9 (23.7%) had severe VD deficiency, VD insufficiency or normal serum VD levels, respectively.
In the absence of significant seasonal differences for the collected blood samples, VD deficiency and insufficiency were highly prevalent in partial responders (73.3%, 20% vs 30.4%, 34.8%, P = 0.021). Overall, the serum 25-hydroxyvitamin D3 [25(OH)D3] level in partial responders appeared to be significantly lower than that in full responders both at baseline (25.44 ± 9.42 vs 17.66 ± 5.34 ng/mL, P = 0.006) and delivery (26.76 ± 8.59 vs 21.24 ± 6.88 ng/mL, P = 0.044). In addition, the VDR SNP assay showed no significant difference between full and partial responders based on VDR SNPs, including VDR Cdx2, Bsm1, Fokl, Taq1 and Apa1.
Maternal HBsAg serum levels were not associated with serum 25(OH)D levels (data not shown). Interestingly, maternal Log10 HBV DNA levels at delivery and baseline serum 25(OH)D levels showed a significant, inverse correlation (P = 0.034, Figure 1). Therefore, we performed multiple linear regression analysis of the determinants of maternal HBV DNA levels at delivery. In both univariate and multivariate analyses, baseline maternal serum 25(OH)D levels were the strongest determinant of low maternal HBV DNA levels (P = 0.034 and < 0.0001, respectively; Table 2), together with BMI, baseline maternal log10 HBsAg levels and cholesterol levels at delivery.
| Variable | P value, univariate | P value, multivariate | Standard beta, multivariate |
| Age (yr) | 0.377 | ||
| BMI (kg/m2) | 0.049 | 0.03 | -0.245 |
| 25(OH)D3 (ng/mL) | 0.034 | < 0.0001 | -0.446 |
| HBsAg (log10 IU/mL) | 0.007 | ||
| HBV DNA log10 | 0.046 | ||
| Alkaline phosphatase | 0.056 | ||
| HBeAg log10 | 0.025 | ||
| WBC count | 0.064 | ||
| Maternal HBsAg at delivery, log10 | 0.003 | 0.05 | 0.285 |
| Maternal alkaline phosphatase at delivery | 0.025 | ||
| Maternal cholesterol at delivery | 0.001 | 0.015 | 0.341 |
We further performed univariate and multivariate regression analyses to characterize the relationship between the serum level of 25(OH)D and targeted antiviral effects. The baseline serum 25(OH)D level was independently associated with targeted antiviral effects (maternal HBV DNA levels < 2 × 105 at delivery) in a multivariate regression model [OR 1.23 (1.04-1.44), P = 0.026], together with maternal VDR Cdx2 TT and cholesterol levels at delivery (Table 3).
| Variable | Univariate | P value | Multivariate | P value |
| Age (yr, continuous) | 1.034 (0.85-1.26) | 0.745 | 0.727 | |
| BMI (kg/m2, continuous) | 1.18 (0.95-1.47) | 0.146 | 0.071 | |
| 25(OH) D3 (ng/mL, continuous) | 1.16 (1.03-1.31) | 0.014 | 1.23 (1.04-1.44) | 0.026 |
| VDR Cdx2 TT | 0.2 (0.036-1.097) | 0.064 | 0.09 (0.01-0.88) | 0.039 |
| Maternal GGT at delivery | 0.94 (0.86-1.006) | 0.075 | 0.05 | |
| Maternal VD at delivery (ng/mL, continuous) | 1.1 (0.999-1.21) | 0.053 | 0.385 | |
| Maternal alkaline phosphatase at delivery | 0.98 (0.96-1.002) | 0.081 | 0.64 | |
| Maternal cholesterol at delivery | 0.47 (0.26-0.86) | 0.015 | 0.39 (0.17-0.87) | 0.021 |
The present study showed that a high percentage (76.3%) of pregnant women with high HBV viral loads had deficient (< 20 ng/mL) or insufficient (≥ 20 but < 31 ng/mL) VD levels. There was a profound association between low serum 25(OH)D levels and higher levels of maternal HBV replication at delivery after TDF therapy. In a multiple linear regression analysis, maternal HBV DNA levels were associated with baseline maternal serum 25(OH)D levels, BMI, baseline maternal log10 HBsAg levels and cholesterol levels at delivery. Finally, we observed that baseline serum 25(OH)D levels, maternal VDR Cdx2 TT and cholesterol levels at delivery were associated with targeted antiviral effects (maternal HBV DNA levels < 2 × 105 at delivery) in a multivariate regression model.
In the human body, VD and its receptors are widely involved in a variety of life processes, regulating the nervous, immune and endocrine systems through related signaling pathways. Many studies have been conducted to reveal the association effect of VD and its receptors on HBV infection and its development. VD deficiency or declines can be detected in a variety of chronic liver diseases[5,7,16] and is associated with adverse clinical outcomes[9,16]. Abnormally low VD levels are highly prevalent among untreated patients with active chronic hepatitis B infection[6].
For a special group of people, such as pregnant females, VD is a vital nutrient that is important for both the mother and fetus in the perinatal period, and prenatal VD supplementation may reduce the risk of many adverse events and yield potential benefits. A previous study showed that pregnant women with HBV in China had lower VD levels than healthy pregnant women[10]. Our present study also showed that a high percentage (76.3%) of pregnant women with high HBV viral loads had deficient (18/38) or insufficient (11/38) VD levels, while only approximately 25.00% (9/38) had adequate VD levels. Our results suggested that abnormally low VD levels may be a common phenomenon in untreated pregnant women with high HBV viral loads in China.
Based on the evidence provided by a companion systematic review that addressed HBV DNA thresholds for identifying pregnant women at risk of MTCT, the WHO recommends administering TDF to pregnant women infected with HBV with high viral loads (≥ HBV DNA thresholds ≥ 2 × 105 IU/mL) from week 28 of pregnancy until at least childbirth to prevent MTCT, in addition to three doses of hepatitis B vaccination, including a birth dose given to the neonate. A recent meta-analysis showed that peripartum antiviral prophylaxis is highly effective at reducing the risk of the MTCT of HBV[17], which supports the 2020 WHO recommendation of administering antivirals during pregnancy, specifically TDF, for the prevention of the MTCT of HBV.
There is growing evidence that VD is associated with infectious diseases and immunity against infection and that VD supplementation has therapeutic potential in the treatment of infectious diseases[18,19]. Low maternal VD levels (< 32 ng/mL) were associated with a higher risk of the MTCT of HIV, and children born to women with low VD levels had a higher risk of death during follow-up[20]. Clinical and epidemiological studies support the role of VD in inhibiting HBV infection, and this antiviral effect is widely attributed to the VDR[11]. Hepatic VDR protein expression was significantly lower in patients with chronic HBV infection, and hepatic VDR expression was inversely correlated with hepatic inflammation and fibrosis[21], which could partly explain the more pronounced decrease in viral DNA in patients with higher VD levels after receiving antiviral therapy in our study.
The present study revealed a profound association between low serum 25(OH)D levels and higher levels of maternal HBV replication at delivery after TDF therapy. Consistent with our previous study, serum 25(OH)D levels were highly negatively correlated with HBV DNA levels[14]. Therefore, for HBV-infected patients, especially pregnant women, monitoring of VD levels is advocated, and increasing VD levels to a normal range in appropriate ways may be beneficial in maintaining low levels of HBV DNA. It is expected that more in-depth studies will be performed to elucidate the mechanism of the effect of VD on HBV infection and its development, treatment and prognosis, which may offer attractive therapeutic opportunities for the treatment of chronic hepatitis B infection.
A number of studies have recently focused on the association between VDR SNPs and the disease characteristics of HBV infection. Some genotypes in VDR FokI increased the risk of HBV infection in a meta-analysis[22]. In addition, the VDR ApaI SNP was associated with viral load and the presence of HBsAg at different times, and pharmacogenetic data could help physicians identify HBV patients with a higher probability of achieving a good response[12]. In addition, VDR SNPs are correlated with HBV viral load and the severity of liver disease[23] and may be associated with occult hepatitis B infection[9]. Our results revealed that VDR Cdx2 TT was a hindering factor in achieving targeted antiviral therapeutic effects (HBV DNA levels < 2 × 105 at delivery) after TDF therapy. In pregnant women, increasing VD levels to within the normal range may help to achieve targeted antiviral treatment effects, especially in those with VDR Cdx2 TT. More basic and clinical studies are warranted for VD supplementation combined with antiviral therapy and immunoprophylaxis to block MTCT.
VD and cholesterol metabolism overlap significantly in the pathways that promote their biosynthesis and have a complex bidirectional relationship[24]. In our study, there was no significant difference in cholesterol levels between the full and partial responders before antiviral therapy, but cholesterol levels were lower in full responders after treatment. Similarly, in another study, for treatment-naive patients with chronic hepatitis B infection, total cholesterol levels showed a decreasing trend during 42 mo of TDF treatment[25]. Moreover, higher total cholesterol concentrations were associated with lower 25(OH)D concentrations[26], and VD supplementation appeared to have a beneficial effect on reducing total serum cholesterol levels[27]. In addition, VDR SNPs were associated with dyslipidemia in Chinese populations, and some variants may increase susceptibility to dyslipidemia[28]. Together, the difference in cholesterol levels after antiviral therapy may be due to differences in VD levels, VDR SNPs, and reactions to antiviral therapy.
Some limitations of the present study should be acknowledged. Most importantly, due to the type of study, the clinical correlations cannot be interpreted as causal relationships. Therefore, a suggestive functional link between VD metabolism and HBV replication remains elusive. Furthermore, the sample size included in this study was limited. Third, there are still some possible confounding factors that have not been considered. Although factors such as the season of blood collection were taken into account, other factors such as dietary habits, the duration of sunlight exposure and the ultraviolet intensity of pregnant women’s living environments may also affect maternal VD levels.
In summary, we demonstrate a significant association between low serum levels of 25(OH)D and high levels of HBV replication in pregnant women with high HBV viral loads, and maternal VD levels as well as VDR SNPs may be associated with the efficacy of antiviral therapy. Future studies to evaluate the therapeutic value of VD and its analogs in reducing the MTCT of HBV may be justified.
Mother-to-child transmission (MTCT) is the main route of hepatitis B virus (HBV) transmission, and HBV infection is associated with human vitamin D (VD) levels.
The role of VD and single nucleotide polymorphisms of the VD receptor gene (VDR SNPs) in blocking MTCT in pregnant women with high HBV viral load receiving antiviral therapy is unclear.
This study aimed to assess whether baseline VD levels and VDR SNPs are associated with the efficacy of tenofovir disoproxil fumarate (TDF) in the prevention of MTCT in pregnant women with high HBV viral loads.
This retrospective study investigated VD levels, common clinical indicators, and virological parameters before and after antiviral therapy in 38 pregnant women with high HBV viral load, and further analyzed the effect of VD levels and VDR SNPs on the efficacy of TDF for the prevention of MTCT.
The present study showed that a high percentage (76.3%) of pregnant women with high HBV viral loads had deficient (< 20 ng/mL) or insufficient (≥ 20 but < 31 ng/mL) VD levels. There was a profound association between low serum 25-hydroxyvitamin D [25(OH)D] levels and higher levels of maternal HBV replication at delivery after TDF therapy. Multivariate logistic regression analysis showed that baseline serum 25(OH)D levels (OR = 1.23, 95%CI: 1.04-1.44), maternal VDR Cdx2 TT (OR = 0.09, 95%CI: 0.01-0.88) and cholesterol levels at delivery (OR = 0.39, 95%CI: 0.17-0.87) were associated with targeted antiviral effects (maternal HBV DNA levels < 2 × 105 at delivery).
We demonstrate a significant association between low serum levels of 25(OH)D and high levels of HBV replication in pregnant women with high HBV viral loads, and maternal VD levels as well as VDR SNPs may be associated with the efficacy of antiviral therapy.
Future studies to evaluate the therapeutic value of VD and its analogs in reducing the MTCT of HBV may be justified.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Dabla PK, India; Shahid M, Pakistan S-Editor: Yan JP L-Editor: A P-Editor: Yuan YY
| 1. | Xu Y, Liu H, Wang Y, Hao R, Li Z, Song H. The next step in controlling HBV in China. BMJ. 2013;347:f4503. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 30] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 2. | Waheed Y, Siddiq M, Jamil Z, Najmi MH. Hepatitis elimination by 2030: Progress and challenges. World J Gastroenterol. 2018;24:4959-4961. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 80] [Cited by in RCA: 76] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 3. | Liu Z, Chen Z, Cui F, Ding Y, Gao Y, Han G, Jia J, Li J, Li Z, Liu Y, Mao Q, Wang A, Wang W, Wei L, Xia J, Xie Q, Yang X, Yin X, Zhang H, Zhang L, Zhang W, Zhuang H, Dou X, Hou J. Management Algorithm for Prevention of Mother-to-child Transmission of Hepatitis B Virus (2022). J Clin Transl Hepatol. 2022;10:1004-1010. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 10] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 4. | Hou J, Cui F, Ding Y, Dou X, Duan Z, Han G, Jia J, Mao Q, Li J, Li Z, Liu Z, Wei L, Xie Q, Yang X, Zhang H, Zhuang H. Management Algorithm for Interrupting Mother-to-Child Transmission of Hepatitis B Virus. Clin Gastroenterol Hepatol. 2019;17:1929-1936.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 56] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 5. | Pop TL, Sîrbe C, Benţa G, Mititelu A, Grama A. The Role of Vitamin D and Vitamin D Binding Protein in Chronic Liver Diseases. Int J Mol Sci. 2022;23. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 46] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 6. | Chan HL, Elkhashab M, Trinh H, Tak WY, Ma X, Chuang WL, Kim YJ, Martins EB, Lin L, Dinh P, Charuworn P, Foster GR, Marcellin P. Association of baseline vitamin D levels with clinical parameters and treatment outcomes in chronic hepatitis B. J Hepatol. 2015;63:1086-1092. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 48] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 7. | Farnik H, Bojunga J, Berger A, Allwinn R, Waidmann O, Kronenberger B, Keppler OT, Zeuzem S, Sarrazin C, Lange CM. Low vitamin D serum concentration is associated with high levels of hepatitis B virus replication in chronically infected patients. Hepatology. 2013;58:1270-1276. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 111] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 8. | Hoan NX, Tong HV, Song LH, Meyer CG, Velavan TP. Vitamin D deficiency and hepatitis viruses-associated liver diseases: A literature review. World J Gastroenterol. 2018;24:445-460. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 37] [Cited by in RCA: 38] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 9. | Wong GL, Chan HL, Chan HY, Tse CH, Chim AM, Lo AO, Wong VW. Adverse effects of vitamin D deficiency on outcomes of patients with chronic hepatitis B. Clin Gastroenterol Hepatol. 2015;13:783-90.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 51] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 10. | Gao XR, Wang CM, Wang WJ, Han GR, Zhang JQ. Serum 25-hydroxyvitamin D status in pregnant women with chronic hepatitis B virus infection. J Infect Dev Ctries. 2016;10:851-856. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 11. | Ahluwalia S, Choudhary D, Tyagi P, Kumar V, Vivekanandan P. Vitamin D signaling inhibits HBV activity by directly targeting the HBV core promoter. J Biol Chem. 2021;297:101233. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 20] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 12. | Cusato J, Boglione L, De Nicolò A, Imbornone R, Cardellino CS, Ghisetti V, Carcieri C, Cariti G, Di Perri G, D'Avolio A. Association of vitamin D pathway SNPs and clinical response to interferon in a cohort of HBeAg-negative patients. Pharmacogenomics. 2017;18:651-661. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 8] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 13. | Zhu X, Wang J, Wang M, Du LY, Ji YL, Zhang X, Tang H. The positive rates of hepatitis B surface antibody in youth after booster vaccination: a 4-year follow-up study with large sample. Biosci Rep. 2021;41. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 14. | Chen EQ, Bai L, Zhou TY, Fe M, Zhang DM, Tang H. Sustained suppression of viral replication in improving vitamin D serum concentrations in patients with chronic hepatitis B. Sci Rep. 2015;5:15441. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 25] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 15. | Lankes U, Elder PA, Lewis JG, George P. Differential extraction of endogenous and exogenous 25-OH-vitamin D from serum makes the accurate quantification in liquid chromatography-tandem mass spectrometry assays challenging. Ann Clin Biochem. 2015;52:151-160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 16. | Huang CZ, Zhang J, Zhang L, Yu CH, Mo Y, Mo LY. Serum vitamin D and vitamin-D-binding protein levels in children with chronic hepatitis B. World J Gastroenterol. 2021;27:255-266. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 3] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 17. | Funk AL, Lu Y, Yoshida K, Zhao T, Boucheron P, van Holten J, Chou R, Bulterys M, Shimakawa Y. Efficacy and safety of antiviral prophylaxis during pregnancy to prevent mother-to-child transmission of hepatitis B virus: a systematic review and meta-analysis. Lancet Infect Dis. 2021;21:70-84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 129] [Article Influence: 25.8] [Reference Citation Analysis (0)] |
| 18. | Ismailova A, White JH. Vitamin D, infections and immunity. Rev Endocr Metab Disord. 2022;23:265-277. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 169] [Article Influence: 56.3] [Reference Citation Analysis (0)] |
| 19. | White JH. Emerging Roles of Vitamin D-Induced Antimicrobial Peptides in Antiviral Innate Immunity. Nutrients. 2022;14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 67] [Article Influence: 22.3] [Reference Citation Analysis (0)] |
| 20. | Mehta S, Hunter DJ, Mugusi FM, Spiegelman D, Manji KP, Giovannucci EL, Hertzmark E, Msamanga GI, Fawzi WW. Perinatal outcomes, including mother-to-child transmission of HIV, and child mortality and their association with maternal vitamin D status in Tanzania. J Infect Dis. 2009;200:1022-1030. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 133] [Cited by in RCA: 124] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 21. | Yang K, Pan Y, Zhang H, Jin L, Wang X. Hepatic vitamin D receptor expression is negatively associated with liver inflammation and fibrosis in patients with chronic HBV infection. Clin Exp Med. 2022;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 6] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 22. | He Q, Huang Y, Zhang L, Yan Y, Liu J, Song X, Chen W. Association between vitamin D receptor polymorphisms and hepatitis B virus infection susceptibility: A meta-analysis study. Gene. 2018;645:105-112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 25] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 23. | Suneetha PV, Sarin SK, Goyal A, Kumar GT, Shukla DK, Hissar S. Association between vitamin D receptor, CCR5, TNF-alpha and TNF-beta gene polymorphisms and HBV infection and severity of liver disease. J Hepatol. 2006;44:856-863. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 93] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 24. | Warren T, McAllister R, Morgan A, Rai TS, McGilligan V, Ennis M, Page C, Kelly C, Peace A, Corfe BM, Mc Auley M, Watterson S. The Interdependency and Co-Regulation of the Vitamin D and Cholesterol Metabolism. Cells. 2021;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 35] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 25. | Zhang Q, Liang J, Yin J, Jiang Y, Yu N, Liao X, Zhao S, Wu L, Fan R. Real-life impact of tenofovir disoproxil fumarate and entecavir therapy on lipid profile, glucose, and uric acid in chronic hepatitis B patients. J Med Virol. 2022;94:5465-5474. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 26. | Vitezova A, Voortman T, Zillikens MC, Jansen PW, Hofman A, Uitterlinden AG, Franco OH, Kiefte-de Jong JC. Bidirectional associations between circulating vitamin D and cholesterol levels: The Rotterdam Study. Maturitas. 2015;82:411-417. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 22] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 27. | Dibaba DT. Effect of vitamin D supplementation on serum lipid profiles: a systematic review and meta-analysis. Nutr Rev. 2019;77:890-902. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 120] [Article Influence: 24.0] [Reference Citation Analysis (0)] |
| 28. | Jia J, Tang Y, Shen C, Zhang N, Ding H, Zhan Y. Vitamin D receptor polymorphism rs2228570 is significantly associated with risk of dyslipidemia and serum LDL levels in Chinese Han population. Lipids Health Dis. 2018;17:193. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 23] [Article Influence: 3.3] [Reference Citation Analysis (0)] |