Published online Apr 21, 2023. doi: 10.3748/wjg.v29.i15.2336
Peer-review started: December 9, 2022
First decision: January 3, 2023
Revised: January 15, 2023
Accepted: March 23, 2023
Article in press: March 23, 2023
Published online: April 21, 2023
Processing time: 126 Days and 11.3 Hours
Transjugular intrahepatic portosystemic shunt (TIPS) is placed important role in the therapy of complications of portal hypertension, there is still no suitable criterion for a reduction in portosystemic gradient (PSG), which can both reduce PSG and maximize clinical results and minimize hepatic encephalopathy (HE).
To compare the clinical outcomes and incidence of HE after one-third PSG reduction during TIPS in patients with variceal bleeding and refractory ascites.
A total of 1280 patients with portal-hypertension-related complications of refractory ascites or variceal bleeding who underwent TIPS from January 2016 to January 2019 were analyzed retrospectively. Patients were divided into group A (variceal hemorrhage and PSG reduced by one third, n = 479); group B (variceal hemorrhage and PSG reduced to < 12 mmHg, n = 412); group C (refractory ascites and PSG reduced by one third, n = 217); and group D (refractory ascites and PSG reduced to < 12 mmHg of PSG, plus medication, n = 172). The clinical outcomes were analyzed.
By the endpoint of follow-up, recurrent bleeding was no different between groups A and B (χ2 = 7.062, P = 0.374), but recurrent ascites did differ significantly between groups C and D (χ2 = 14.493, P = 0.006). The probability of total hepatic impairment within 3 years was significantly different between groups A and B (χ2 = 11.352, P = 0.005) and groups C and D (χ2 = 13.758, P = 0.002). The total incidence of HE differed significantly between groups A and B (χ2 = 7.932, P = 0.016), groups C and D (χ2 = 13.637, P = 0.007). There were no differences of survival rate between groups A and B (χ2 = 3.376, P = 0.369, log-rank test), but did differ significantly between groups C and D (χ2 = 13.582, P = 0.014, log-rank test).
The PSG reduction by one third may reduce the risk of HE, hepatic function damage and achieve good clinical results.
Core Tip: Patients with cirrhosis who underwent transjugular intrahepatic portosystemic shunt for recurrent variceal bleeding and refractory ascites were evaluated. Reduction in portosystemic gradient (PSG) should be based on the original basal pressure and reduction by one third may reduce the risk of hepatic encephalopathy, hepatic function damage and achieve similar clinical results as for the refractory ascites patients. Appropriate reduction of PSG directly influences the patient prognosis.
- Citation: Luo SH, Zhou MM, Cai MJ, Han SL, Zhang XQ, Chu JG. Reduction of portosystemic gradient during transjugular intrahepatic portosystemic shunt achieves good outcome and reduces complications. World J Gastroenterol 2023; 29(15): 2336-2348
- URL: https://www.wjgnet.com/1007-9327/full/v29/i15/2336.htm
- DOI: https://dx.doi.org/10.3748/wjg.v29.i15.2336
Transjugular intrahepatic portosystemic shunt (TIPS) is placed important role in the therapy of complications of portal hypertension[1], It has been progressively recognized as an effective therapeutic option in a growing number of clinical situations[2,3]. Measurement of portosystemic pressure gradient (PSG) is important during the TIPS procedure. Reduction of PSG can achieve good clinical results, but when PSG is too low, TIPS can have many complications, of which, hepatic encephalopathy (HE) and liver function damage are the most frequent[4]. Post-TIPS HE could depend mainly on portocaval pressure gradient and volume of blood shunted through the liver[5].
Several guidelines[6,7] recommend that the PSG should be reduced to 12 mmHg after TIPS creation to achieve a better clinical outcome. In that situation, however, the incidence of HE is higher than in clinical practice. This has prompted many centers to anecdotally adopt the technique of dilation of stent grafts using balloons with a nominal diameter of ≤ 8 mm at TIPS positioning. Recently, a new controlled expansion stent has been introduced in clinical practice (Viatorr Controlled Expansion Endoprosthesis; Gore and Associates, Flagstaff, AZ, United States), which allows lasting diameter control within a range of 8–10 mm during implantation to reach a targeted portal pressure gradient[8].
However, there is still no suitable criterion for a reduction in PSG, which can both reduce PSG and maximize clinical results and minimize HE, and few data are available to calculate an appropriate PSG value[9]. Here, we report our multicenter retrospective study to compare the occurrence of HE and clinical results of one-third reduction of PSG with PSG reduced to < 12 mmHg in patients who required TIPS placement.
This was a multicenter retrospective study. The Ethics Committee approved the study protocol and all procedures were conducted according to the guidelines approved by the Committee. Between January 2016 and January 2019, 1280 patients were referred on an intention-to-treat basis and underwent a TIPS procedure. Indications for stent graft shunt were variceal hemorrhage or refractory ascites. The outcomes of HE, recurrent variceal bleeding and ascites, and mortality were compared between the groups. The patients’ medical records and images were reviewed to gather information regarding underlying etiology, clinical presentation, age, sex, and severity of cirrhosis (Table 1).
| Characteristics | Group A | Group B | Pvalue | Group C | Group D | Pvalue |
| Gender, M/F | 256/223 | 237/175 | 0.369 | 126/91 | 93/79 | 0.319 |
| Age (mean ± SD) (yr) | 54.616 ± 17.27 | 56.39 ± 12.19 | 0.319 | 56.24 ± 13.67 | 58.27 ± 13.25 | 0.246 |
| Child–Pugh A/B/C | 39/342/98 | 32/318/62 | 0.187 | 0/60/157 | 0/41/131 | 0.215 |
| MELD score (mean ± SD) | 8.42 ± 1.37 | 9.29 ± 2.16 | 0.576 | 13.26 ± 4.56 | 14.39 ± 5.38 | 0.472 |
| Viral hepatitis | 324 | 276 | 0.528 | 136 | 107 | 0.632 |
| Chronic ethanol consumption | 102 | 87 | 0.317 | 55 | 38 | 0.258 |
| Cryptogenic hepatitis | 53 | 49 | 0.492 | 26 | 27 | 0.146 |
| Variceal hemorrhage | 479 | 412 | 0.721 | 0 | 0 | 0 |
| Refractory ascites | 0 | 0 | 0 | 217 | 172 | 0.562 |
| Laboratory tests | ||||||
| Alanine transaminase (U/L) | 48.36 ± 4.21 | 53.19 ± 3.27 | 0.462 | 62.13 ± 6.48 | 57.49 ± 7.29 | 0.368 |
| Aspartate Transaminase (U/L) | 54.17 ± 9.25 | 58.27 ± 12.37 | 0.361 | 67.43 ± 15.7 | 64.28 ± 17.24 | 0.357 |
| Alkaline phosphatase (U/L) | 145.36 ± 23.45 | 167.18 ± 27.36 | 0.382 | 89.67 ± 13.24 | 92.36 ± 16.58 | 0.413 |
| γ-Glutamyl transpeptidase (U/L) | 278.54 ± 37.47 | 259.74 ± 46.37 | 0.463 | 364.27 ± 58.74 | 382.17 ± 47.26 | 0.482 |
| Total bilirubin (mmol/L) | 29.45 ± 3.17 | 32.46 ± 4.28 | 0.147 | 37.18 ± 7.69 | 35.24 ± 8.54 | 0.367 |
| Albumin (g/L) | 31.28 ± 1.47 | 32.07 ± 1.25 | 0.106 | 28.07 ± 1.29 | 29.36 ± 1.48 | 0.294 |
| Prothrombin time (s) | 14.02 ± 1.35 | 15.04 ± 1.19 | 0.236 | 18.12 ± 2.39 | 19.23 ± 2.41 | 0.241 |
| Clinical presentations | ||||||
| Abdominal distention | 89 | 93 | 0.261 | 217 | 172 | 0.562 |
| Abdominal pain | 48 | 52 | 0.273 | 134 | 97 | 0.183 |
| Weakness | 364 | 314 | 0.148 | 189 | 154 | 0.136 |
| Poor appetite | 373 | 362 | 0.302 | 196 | 153 | 0.324 |
| Jaundice | 28 | 24 | 0.532 | 21 | 16 | 0.214 |
| Splenomegaly | 264 | 249 | 0.357 | 205 | 168 | 0.436 |
| Lower limbs edema | 47 | 62 | 0.159 | 189 | 157 | 0.327 |
| Ascites paracentesis | 0 | 0 | 0 | 217 | 172 | 0.562 |
| Endoscopic therapy | 453 | 407 | 0.372 | 0 | 0 | 0 |
This was a multicenter retrospective study that compared the rate of HE and clinical outcomes after TIPS with PSG reduced by one third with PSG reduced to < 12 mmHg in patients who required TIPS placement for portal-hypertension-related complications of ascites or variceal bleeding. The patients were divided into four groups[10]: Group A (variceal hemorrhage and PSG reduced by one third, n = 479); group B (variceal hemorrhage and PSG reduced to < 12 mmHg, n = 412); group C (refractory ascites and PSG reduced by one third, n = 217); and group D (refractory ascites and PSG reduced to < 12 mmHg, plus medication, n = 172). The clinical outcomes were analyzed.
The inclusion criteria were: Recurrent variceal bleeding after a session of variceal sclerotherapy, and refractory ascites that required TIPS placement with portal-hypertension-related complications. Only de novo TIPS procedures using Viatorr stent grafts (Gore & Associates) were included. We excluded: TIPS procedures performed with bare stents, TIPS with bare stents followed by revision with Viatorr stent grafts, and TIPS performed with other types of stent grafts; variceal bleeding as an emergency indication; portal vein thrombosis; history of HE; severe right-sided heart failure; severe liver failure (bilirubin > 4 mg/dL), polycystic liver disease, and dilated biliary ducts; age > 75 years; Child–Pugh score > 11; Model of End-Stage Liver Disease (MELD) score > 18; hepatic carcinoma; sepsis spontaneous bacterial peritonitis; and liver transplantation.
TIPS was performed under standard local anesthesia as described previously[11]. The entire length of the intrahepatic tract was covered by the stent graft. Hepatic venous pressure gradient and portal venous pressure were measured during the procedure, and the shunts were dilated to an appropriate diameter to reach a target PSG of < 12 mmHg or reduced by one third. To reduce PSG by one third of basal value, the stent was not fully expanded, the diameter was retained, and the pressure was measured several times until it was reduced by one third. Obvious gastroesophageal collateral vessels observed during the TIPS procedure were embolized with coils (Cook Inc., Bloomington, IL, United States; or Interlock Coil, Boston Scientific Corporation, Natikeshi, MA, United States). Subsequent direct portography was performed to evaluate whether the portal venous system was completely patent.
After the TIPS procedure, intravenous Dalteparin Sodium Injection (5000 U/d; VetterPharma-Fertigung, Germany) was administered for 3 d. No patients had portal vein thrombosis, and oral warfarin was not given.
After TIPS deployment, baseline duplex sonography was performed. Shunt velocities were compared with this baseline result during follow-up. Patients were placed into a routine follow-up protocol identical for each group. They were seen as outpatients 1 mo after the procedure and then at 3 and 6 mo and 1 and 3 years, or whenever needed. Each consultation included a clinical examination, blood chemistry, upper abdominal ultrasonography, and assessment of HE.
TIPS angiography was performed in patients with recurrent symptoms or suspected shunt dysfunction. TIPS revision was performed when hemodynamically significant shunt stenosis (> 50%) was present with recurrent variceal bleeding, or recurrent or gradually worsening ascites. HE was defined according to the practice guidelines of the European Association for the Study of the Liver (EASL) and American Association for the Study of Liver Diseases (AASLD)[12,13]. Patients lost to follow-up were censored at the time of the last known imaging of the shunt (duplex ultrasonography or shunt venography).
Data measurements results of the four groups were normally distributed, and expressed as mean ±SD, and their differences were determined using t test. Categorical variables were expressed as frequencies and compared using χ2 test, and their differences among the four groups were determined by one-way ANOVA. A P value of less than 0.05 was considered significant. The statistical analyses were performed with SPSS version 22.0 (SPSS, Armonk, NY, United States).
All TIPS procedures showed similar efficacy in all four groups by reducing the PSG before and after TIPS. PSG was reduced after TIPS placement from 24.58 ± 2.41 to 15.72 ± 1.04 mmHg in group A (P = 0.012), 25.37 ± 2.54 to 11.27 ± 2.04 mmHg in group B (P = 0.004), 25.12 ± 3.16 to 16.15 ± 1.37 mmHg in group C (P = 0.016), and 24.48 ± 3.24 to 10.28 ± 1.18 mmHg in group D (P = 0.003) (Table 2). It showed that there were significant differences between groups A and B (P = 0.017) and groups C and D (P = 0.026).
| Groups | PSG (mmHg) | tvalue | Pvalue | |
| Before | After | |||
| Group A | 24.58 ± 2.41 | 15.72 ± 1.04 | 11.48 | 0.012 |
| Group B | 25.37 ± 2.54 | 11.27 ± 2.04 | 14.25 | 0.004 |
| (t value) 0.649 | 6.382 | |||
| (P value) 0.483 | 0.026 | |||
| Group C | 25.12 ± 3.16 | 16.15 ± 1.37 | 12.43 | 0.016 |
| Group D | 24.48 ± 3.24 | 10.28 ± 1.18 | 15.47 | 0.003 |
| (t value) 0.367 | 5.734 | |||
| (P value) 0.534 | 0.017 | |||
No patient died within 30 d after TIPS, with an early survival of 100%. None of the patients in groups A and B had recurrent bleeding within the first week. The symptoms of ascites in 198 (91.24%) patients in group C and 161 (93.60%) in group D disappeared or were relieved without paracentesis, with no significant differences (P = 0.327).
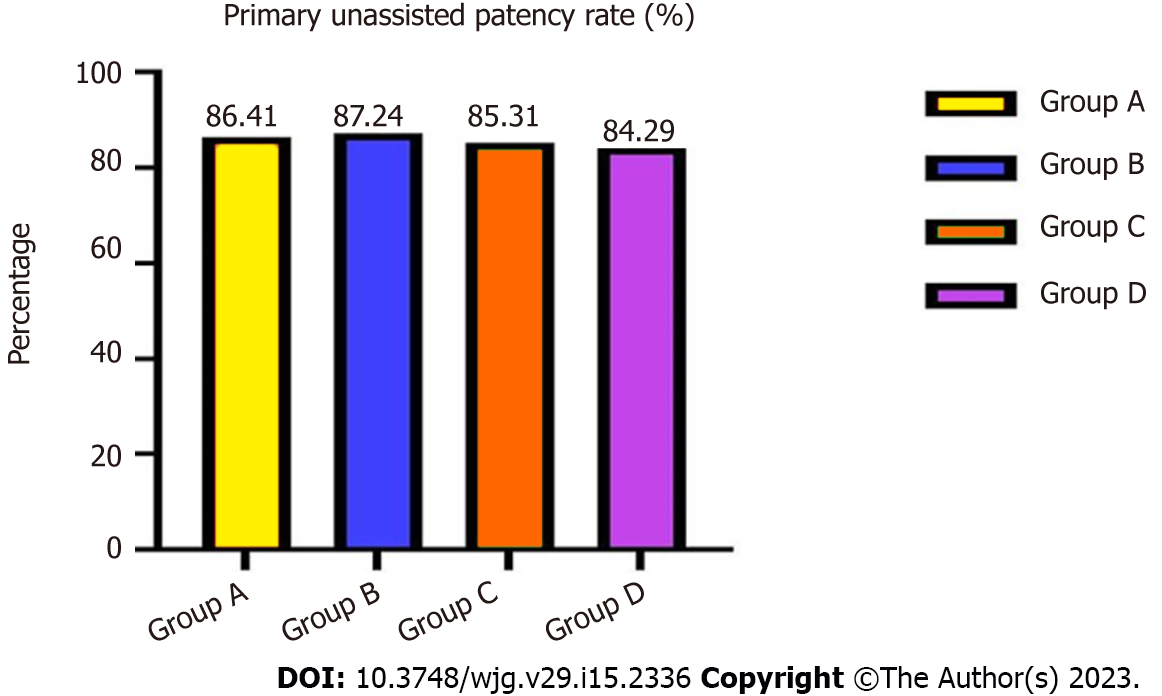
During 3-years’ follow-up, the total primary unassisted patency rates in groups A and B were 86.41% vs 87.24% (χ2 = 4.486, P = 0.257), and in groups C and D were 85.31% vs 84.29% (χ2 = 4.529, P = 0.248), with no significant differences (Figure 1).
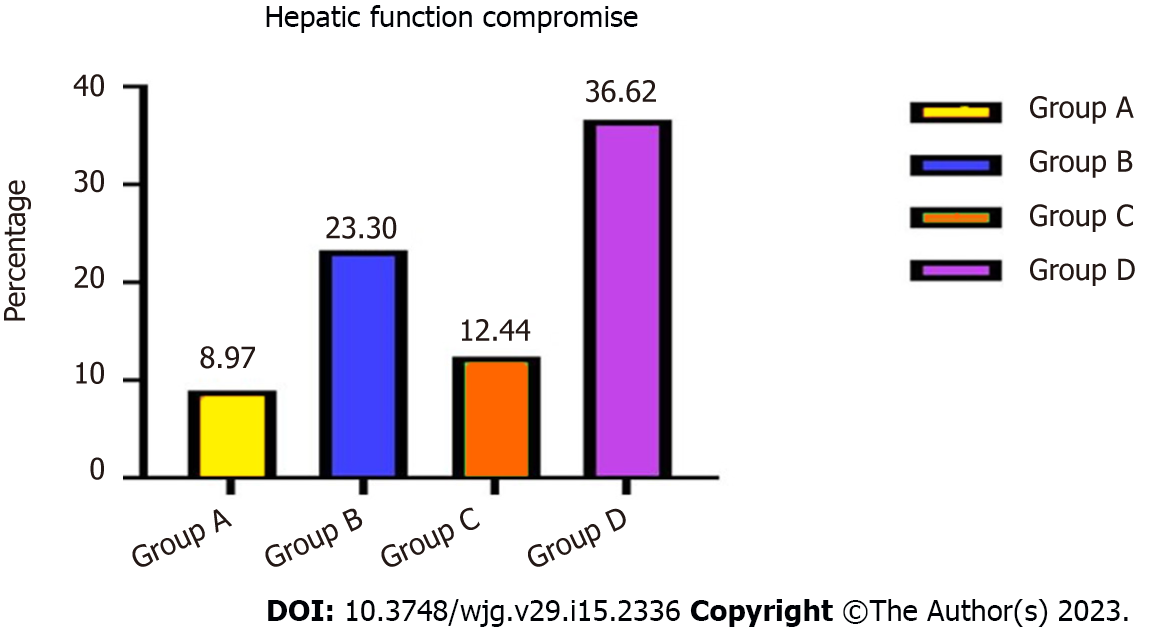
Forty-three (8.97%) patients in group A, 96 (23.30%) in group B, 27 (12.44%) in group C and 63 (36.62%) in group D developed hepatic function compromise after TIPS placement. The probability of total hepatic impairment within 3 years differed significantly between groups A and B (χ2 = 11.352, P = 0.005) and groups C and D (χ2 = 13.758, P = 0.002) (Figure 2). Mean aspartate transaminase, alanine transaminase, and total bilirubin concentrations were elevated, albumin levels decreased, and prothrombin time was prolonged compared with pre-TIPS.
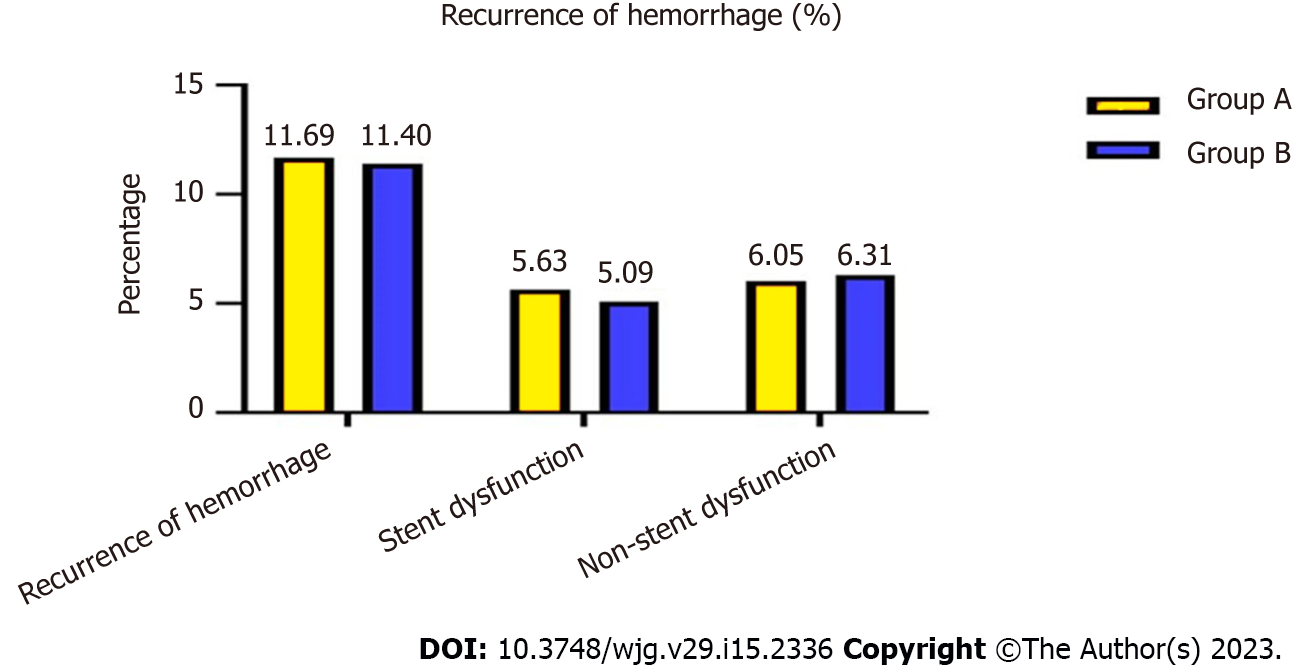
At the end of follow-up, 56 (11.69%) patients in group A and 47 (11.40%) in group B had recurrent variceal bleeding, which was not a significant difference (χ2 = 7.062, P = 0.374) (Figure 3).
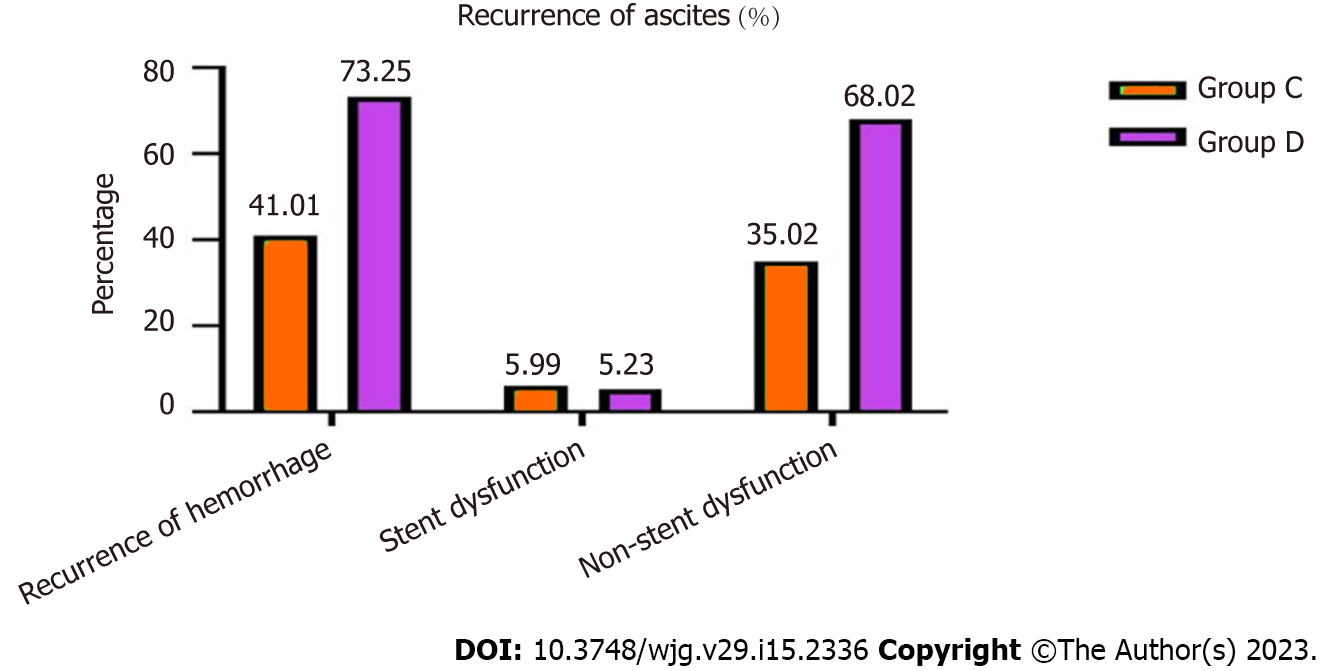
Eighty-Nine (41.01%) patients in group C, 126 (73.25%) in group D with recurrent ascites, which was a significant difference (χ2 = 14.493, P = 0.006) (Figure 4).
Of these, 27 (5.63%) patients in group A, 21 (5.09%) in group B, 13 (5.99%) in group C and nine (5.23%) in group D were caused by stent dysfunction, and after stent revision, the symptoms disappeared, and there was no significant difference between the groups (χ2 = 834, P = 0.358; χ2 = 4.574, P = 0.375).
The bleeding in patients in groups A and B that was not caused by stent dysfunction was relieved after medical treatment. However, 76 (35.02%) patients in group C and 117 (68.02%) in group D was not caused by stent graft dysfunction but rather hepatic dysfunction and hypoalbuminemia, which differed significantly between the two groups (χ2 = 13.356, P = 0.006). After medication and albumin supplementation, the symptoms recurred many times (Table 3).
| Symptoms | Group A | Group B | χ2 | Pvalue | Group C | Group D | χ2 | Pvalue |
| Ascites within 1 wk | / | / | / | 198 (198/217, 91.24%) | 161 (161/172, 93.60%) | 0.327 | ||
| Hemorrhage within 1 wk | 0 | 0 | 0 | / | / | / | ||
| Primary unassisted patency rate | 0.8641 | 0.8724 | 4.486 | 0.257 | 0.8531 | 0.8429 | 4.529 | 0.248 |
| Hepatic function compromise | 43 (43/479, 8.97%) | 96 (96/412, 23.30%) | 11.352 | 0.005 | 27 (27/217, 12.44%) | 63 (63/172, 36.62%) | 13.758 | 0.002 |
| Recurrence of hemorrhage | 56 (56/479, 11.69%) | 47 (47/412, 11.40%) | 7.062 | 0.374 | / | / | / | / |
| Stent dysfunction | 27 (27/479, 5.63%) | 21 (21/412, 5.09%) | 6.834 | 0.358 | / | / | / | / |
| Non-stent dysfunction | 29 (29/479, 6.05%) | 26 (26/412, 6.31%) | 6.486 | 0.362 | / | / | / | / |
| Recurrence of ascites | / | / | / | / | 89 (89/217, 41.01%) | 126 (126/172,73.25%) | 14.493 | 0.006 |
| Stent dysfunction | / | / | / | / | 13 (13/217, 5.99%) | 9 (9/172, 5.23%) | 4.574 | 0.375 |
| Non-stent dysfunction | / | / | / | / | 76 (76/217, 35.02%) | 117 (117/172, 68.02%) | 13.356 | 0.006 |
Symptoms of variceal bleeding in groups A and B disappeared within 1 wk, and symptoms of ascites in groups C and D disappeared or were relieved within 1 wk without paracentesis, and total primary unassisted patency rates were not significantly different. The probability of total hepatic impairment and recurrent symptoms was significantly different between the groups.
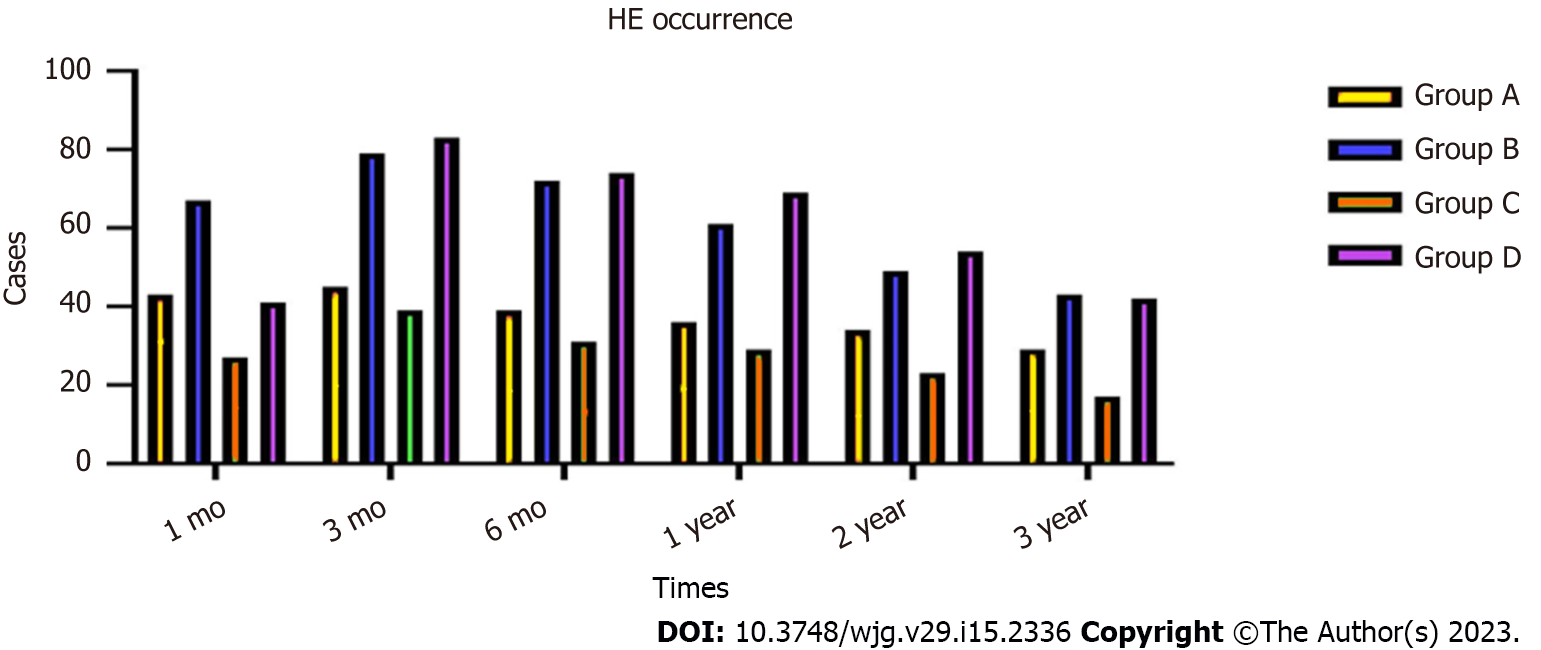
During the 3-year follow-up period, 46 (9.60%) patients in group A, 67 (16.26%) in group B, 47 (21.65%) in group C and 69 (40.11%) in group D developed HE, and the incidence of HE in group A compared with group B, and group C compared with group D differed significantly (χ2 = 7.932, P = 0.016; χ2 = 13.637, P = 0.007, respectively). There were significant differences in the occurrence of HE between groups A and B at 1 mo (χ2 = 6.463, P = 0.027), 3 mo (χ2 = 5.0368, P = 0.023), 6 mo (χ2 = 6.473, P = 0.017), 1 year (χ2 = 4.538, P = 0.027), 2 years (χ2 = 5.452, P = 0.026) and 3 years (χ2 = 5.467, P = 0.028). There were also significant differences in HE occurrence between groups C and D at 1 mo (χ2 = 14.673, P = 0.014), 3 mo (χ2 = 17.478, P = 0.009), 6 mo (χ2 = 13.957, P = 0.011), 1 year (χ2 = 14.576, P = 0.014), 2 years (χ2 = 11.476, P = 0.013) and 3 years (χ2 = 8.473, P = 0.017) (Figure 5).
The incidence of HE in the four groups showed a downward trend. After drug treatment, the symptoms disappeared in patients with covert and grade II HE. In patients with grade III or IV HE, the symptoms disappeared after shunt reduction, but five patients who underwent shunt reduction still had hepatic myelopathy (Table 4).
| Time | Group | HE occurrence | Occurrence rate (%) | χ2 | Pvalue | |
| Yes | No | |||||
| 1 mo | A | 43 | 436 | 8.97 | 6.463 | 0.027 |
| B | 67 | 345 | 16.26 | |||
| C | 27 | 190 | 12.44 | 14.673 | 0.014 | |
| D | 41 | 131 | 23.83 | |||
| 3 mo | A | 45 | 434 | 9.39 | 5.368 | 0.023 |
| B | 79 | 333 | 19.17 | |||
| C | 39 | 178 | 17.97 | 17.478 | 0.009 | |
| D | 83 | 89 | 48.25 | |||
| 6 mo | A | 39 | 440 | 8.14 | 6.473 | 0.017 |
| B | 72 | 340 | 17.47 | |||
| C | 31 | 186 | 14.28 | 13.957 | 0.011 | |
| D | 74 | 98 | 43.02 | |||
| 1 year | A | 36 | 443 | 7.51 | 4.538 | 0.027 |
| B | 61 | 351 | 14.8 | |||
| C | 29 | 188 | 13.36 | 14.576 | 0.014 | |
| D | 69 | 103 | 40.11 | |||
| 2 year | A | 34 | 445 | 7.09 | 5.452 | 0.026 |
| B | 49 | 363 | 11.89 | |||
| C | 23 | 194 | 10.59 | 11.476 | 0.013 | |
| D | 54 | 118 | 31.39 | |||
| 3 year | A | 29 | 443 | 6.14 | 5.467 | 0.028 |
| B | 43 | 369 | 10.43 | |||
| C | 17 | 155 | 9.88 | 8.473 | 0.017 | |
| D | 42 | 130 | 24.41 | |||
| Total HE rate | A | 46 | 433 | 9.6 | 7.932 | 0.016 |
| B | 67 | 158 | 16.26 | |||
| C | 47 | 170 | 21.65 | 13.637 | 0.007 | |
| D | 69 | 103 | 40.11 | |||
During 3 years’ follow-up, 262 patients in group A, 234 in group B, 189 in group C and 160 in group D were lost to follow-up. Total survival rates were no different compared groups A with B (χ2 = 3.376, P = 0.369, log-rank test), but there were significant differences between groups C and D (χ2 =13.582, P = 0.014, log-rank test) (Figure 6).
The 3-mo, 6-mo, and 1-, 2-, and 3-year survival rates were different between groups A and B (χ2 = 5.368, P = 0.425; χ2 = 4.557, P = 0.436; χ2 = 4.562, P = 0.427, χ2 = 5.487, P = 0.382, and χ2 = 4.582, P = 0.375, respectively); and significantly different between groups C and D (χ2 = 13.364, P = 0.012; χ2 = 12.463, P = 0.013; χ2 = 12.568, P = 0.016; χ2 = 11.467, P = 0.017, and χ2 = 10.367, P = 0.027, respectively). Four hundred and forty-nine patients died of multiorgan failure, 127 of hepatic tumor, and 298 of other causes (Table 5).
| Time | Group | Survival | Survival rate (%) | χ2 | Pvalue | |
| Yes | No | |||||
| 3 mo | A | 470 | 9 | 98.12 | 5.368 | 0.425 |
| B | 401 | 11 | 97.33 | |||
| C | 184 | 33 | 84.79 | 13.364 | 0.012 | |
| D | 127 | 45 | 73.83 | |||
| 6 mo | A | 442 | 37 | 92.27 | 4.557 | 0.436 |
| B | 381 | 31 | 92.47 | |||
| C | 162 | 55 | 74.65 | 12.463 | 0.013 | |
| D | 107 | 65 | 62.2 | |||
| 1 year | A | 397 | 82 | 82.88 | 4.562 | 0.427 |
| B | 335 | 77 | 81.31 | |||
| C | 117 | 100 | 53.91 | 12.568 | 0.016 | |
| D | 65 | 107 | 37.79 | |||
| 2 year | A | 293 | 186 | 61.16 | 5.487 | 0.382 |
| B | 245 | 167 | 59.46 | |||
| C | 59 | 158 | 27.18 | 11.467 | 0.017 | |
| D | 26 | 146 | 15.11 | |||
| 3 year | A | 229 | 250 | 47.8 | 4.582 | 0.375 |
| B | 193 | 219 | 46.84 | |||
| C | 32 | 185 | 14.74 | 10.367 | 0.027 | |
| D | 16 | 156 | 9.3 | |||
| Total survival rate | A | 217 | 262 | 45.3 | 3.376 | 0.369 |
| B | 178 | 234 | 43.2 | |||
| C | 28 | 189 | 12.9 | 13.582 | 0.014 | |
| D | 12 | 160 | 6.97 | |||
During the TIPS procedure, measuring PSG is an important step because reducing PSG can achieve good clinical results, in which a conduit is constructed within the liver between the systemic venous and portal systems, with the aim of decreasing portal systemic pressure[14]. However, too low portal pressure can lead to some complications, and to avoid the recurrence of bleeding and uncontrolled ascites induced by excess reduction of portal vein pressure, appropriate PSG levels are required[15].
Most guidelines recommend[16] that the upper threshold of the post-TIPS PSG for a patient with variceal bleeding is < 12 mmHg or 50% of baseline, and the AASLD practice guidelines suggest a gradient of ≤ 8 mmHg[17]. Most centers presently use the thresholds for TIPS procedures.
The complications of TIPS are classified as related to the TIPS procedure itself, the stent, portosystemic shunting, etc.[18]. Among them, HE and deterioration of liver function, as complications related to portosystemic shunting, are associated with reduced PSG. Some of the patients with low PSG after TIPS have complications such as worsening of HE, which causes multiple admissions to hospital and increased liver enzymes and bilirubin, even though they are ultimately medically controlled[19].
This creates a paradoxical dilemma in which low PSG results in complications such as severe HE or liver function failure, and inappropriate reduction of PSG also leads to recurrence of symptoms of portal hypertension, such as variceal bleeding and ascites. The current concept of small balloon expansion is intended to reduce PSG appropriately to reduce portal hypertension without associated serious complications[20].
Self-expandable stents may continue to dilate until achieving their nominal diameter[21]. This means that if PSG is 11 mmHg after dilating a 10-mm stent to only 8 mm, the stent may continue to self-dilate until reaching approximately 10 mm in diameter, leading to a further decrease in PSG and an increased risk of HE. How frequently this spontaneous expansion is clinically relevant is a matter of debate, but certainly represents a limitation. This led to a further technical improvement, the controlled-expansion stents, that cannot spontaneously dilate over preset limits. More crucially, the exact reduction of PSG is unknown.
However, we do not have an answer for simple questions such as how large the shunt should be, or what PSG reduction should be targeted to prevent recurrent bleeding or ascites during TIPS. PSG should be decreased to ≤ 12 mmHg, or by > 50% of baseline (which in most cases means < 12 mmHg) to prevent the complications of portal hypertension[22]. This comes from observations that recurrent bleeding and ascites occurred almost exclusively when patients had a PSG of at least 12 mmHg after TIPS[23].
A study has suggested that despite a traditional PSG reduction to below 12 mmHg or > 50% of baseline, a PSG decrease to 14 mmHg or > 30% of baseline would be appropriate when uncovered stents are used[24]. This goal is achieved in a large proportion of patients with small diameter TIPS such as 7 or 6 mm dilated shunts that are likely to result in less worsening of portosystemic shunting and hence a lower likelihood of severe HE and post-TIPS liver failure[25]. Of note, in high-risk situations, such as refractory ascites, the recent EASL guidelines recommended small diameter TIPS, although not as small as 6 mm[15].
It has been shown that complementing a small diameter TIPS with drugs can be converted into a satisfactory one by adding propranolol, even at low doses[26]. The synergistic effect of combining two different mechanisms decreases PSG by bypassing liver resistance to portal flow, and propranolol decreases PSG by reducing splanchnic blood flow. This approach achieves good clinical results and lower incidence of HE. Post-TIPS HE is a complex condition that is determined by TIPS diameter and many nonhemodynamic factors. Age, degree of liver and kidney failure, chronic inflammation, urease-producing intestinal bacteria, bacterial translocation and malnutrition/sarcopenia, are other important factors that can modulate the therapeutic effect[27]. Several of them are associated with post-TIPS HE and survival[28]. Therefore, appropriate reduction of PSG can reduce the occurrence of TIPS-related complications, such as HE. Combined with drugs, if good clinical results are achieved, it is not necessary to reduce PSG too low to produce higher TIPS-related complications.
Based on the above, we hypothesize that for patients with gastrointestinal bleeding and refractory ascites requiring TIPS, one-third reduction of PSG of the baseline is appropriate, which was supplemented by drug-lowering portal pressure therapy. We should reduce portal hypertension as much as possible, achieve good therapeutic results, and minimize complications, especially the incidence of HE and compromise of liver function.
In this study, we divided the patients with gastrointestinal bleeding and refractory ascites who required TIPS into four groups, to make a detailed evaluation of the clinical effects. The results showed that, as for patients with variceal bleeding who required TIPS placement, PSG reduced by one third compared with < 12 mmHg baseline, the two groups had a similar effect on variceal bleeding, but the incidence of HE and compromise of liver function differed. During the TIPS procedure, to achieve the goal of reducing PSG by one third, a small balloon was required for gradual dilatation, slowly from 6 mm to 8 mm, which would also be useful for the controlled expansion stent that has been introduced in clinical practice. During the dilatation process, the operating procedure will be slower, PSG measurement will take approximately 30 min longer, and two more balloons will be used, resulting in increased cost. However, the cost should be worthwhile in comparison to the cost of complications.
For patients with refractory ascites who required TIPS, the incidence of HE and compromise of liver function were obviously different. In the short term, the symptoms of ascites disappear or subside, but in the medium and long term, PSG drops less, ascites still recurs in some cases, and drug therapy is necessary. Post-TIPS HE is a complex condition that is determined by TIPS and many nonhemodynamic factors. The liver function reserve of patients with refractory ascites and survival rate are poor, and the patients are prone to hypoproteinemia and electrolyte disturbances, which are likely to cause recurrence of ascites and require drug treatment[29]. In this circumstance, to reduce TIPS-related complications and liver function damage, it is not necessary to reduce PSG drastically, one-third PSG reduction plus drug therapy would be appropriate.
As a retrospective analysis, our study may manifestaed some limitations. For the one, it maybe need randomized controlled trials to validate the results. Next, to achieve main goal of reducing the PSG by one third, it requires gradual balloon dilatation from 6 mm to 8 mm, the operating procedure will be slower, PSG measurement will take approximately 30 min longer, and two more balloons will be used, resulting in increased cost. Finally, our hypothesis needs to be validated by a comparative study on the results of small balloon dilatation.
This multicenter retrospective study showed that patients who underwent TIPS creation with PSG reduced to one third of baseline or to < 12 mmHg or 50% of baseline had similar successful clinical outcomes. However, PSG reduced to < 12 mmHg had a lower rate of HE and liver compromise. Given that the PSG will become more controllable in the future with the advent of controllable stents, we believe that our concept is worthy of clinical application.
Transjugular intrahepatic portosystemic shunt (TIPS) is placed important role in the therapy of complications of liver cirrhosis. Measuring portosystemic pressure gradient (PSG) is important during the TIPS procedure. Reducing PSG can achieve good clinical results, but when PSG is too low, TIPS leads to many complications. Factors associated with post-TIPS complications depend mainly on portocaval pressure gradient and the volume of blood shunted through the liver. Several guidelines recommend that PSG reduced to 12 mmHg after TIPS creation achieves better clinical outcomes. However, in that situation, the incidence of hepatic encephalopathy (HE) was higher in clinical practice. There is still no suitable criterion for a reduction in PSG, which can both reduce PSG and maximize clinical results and minimize HE, and few data are available to calculate an appropriate PSG value.
We report our multicenter retrospective study to compare the rate of HE and clinical results of reducing PSG by one third of baseline with PSG reduction to < 12 mmHg in patients with portal hypertension who required TIPS placement wtih of variceal bleeding and ascites.
The main objective was to establish that patients who underwent TIPS PSG reduced by one third of baseline compared with PSG reduced to < 12 mmHg of baseline were associated with similar successful clinical outcomes.
We hypothesized that reducing PSG by one third of baseline compared with < 12 mmHg of baseline would result in a lower rate of HE and liver compromise. The patients were divided into four groups: Group A (variceal hemorrhage and PSG reduced by one third, n = 479); group B (variceal hemorrhage and PSG reduced to < 12 mmHg, n = 412); group C (refractory ascites and PSG reduced by one third, n = 217); and group D (refractory ascites and PSG reduced to < 12 mmHg, plus medication, n = 172). The clinical outcomes were compared and evaluated. Data measurements results of the four groups were normally distributed, and expressed as mean ± standard deviation, and their differences were determined using t-test. The statistical analyses were performed with SPSS version 22.0.
This study showed that during TIPS placement, when PSG was reduced by one third compared with < 12 mmHg of baseline, recurrent bleeding showed no significant difference, but recurrent ascites did differ significantly. The probability of total hepatic impairment within 3 years was significantly different. During follow-up, the total incidence of HE differed significantly. The total survival rates were no different for the variceal bleeding patients but were significantly different for the patients with refractory ascites.
We found that patients who underwent TIPS PSG reduced by one third of baseline compared with reduced to < 12 mmHg of baseline were associated with similar successful clinical outcomes, but PSG reduced by one third resulted in a lower rate of HE and liver compromise.
Measuring PSG is important during the TIPS procedure. Reducing PSG can achieve good clinical results, but when PSG is too low, TIPS leads to many complications. Reduction of PSG by one third of baseline is recommended to decrease the probability of liver function impairment after TIPS, decrease the incidence of HE, and increase survival in patients with refractory ascites.
We thank all the patients participated in this study, and department of gastroenterology and department of interventional radiology of all the hospital for their contributions to the data collection. We also acknowledge the cooperation of all participating units for the collection and processing of case data.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Garbuzenko DV, Russia; Mahmoud MZ, Saudi Arabia S-Editor: Li L L-Editor: A P-Editor: Li L
| 1. | Bureau C, Thabut D, Oberti F, Dharancy S, Carbonell N, Bouvier A, Mathurin P, Otal P, Cabarrou P, Péron JM, Vinel JP. Transjugular Intrahepatic Portosystemic Shunts With Covered Stents Increase Transplant-Free Survival of Patients With Cirrhosis and Recurrent Ascites. Gastroenterology. 2017;152:157-163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 233] [Cited by in RCA: 305] [Article Influence: 38.1] [Reference Citation Analysis (0)] |
| 2. | Philips CA, Ahamed R, Rajesh S, George T, Mohanan M, Augustine P. Beyond the scope and the glue: update on evaluation and management of gastric varices. BMC Gastroenterol. 2020;20:361. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 34] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 3. | Saab S, Kim NG, Lee EW. Practical Tips on TIPS: When and When Not to Request It. Am J Gastroenterol. 2020;115:797-800. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 4. | Cui J, Smolinski SE, Liu F, Xu D, Dulaimy K, Irani Z. Incrementally Expandable Transjugular Intrahepatic Portosystemic Shunts: Single-Center Experience. AJR Am J Roentgenol. 2018;210:438-446. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 14] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 5. | Schindler P, Heinzow H, Trebicka J, Wildgruber M. Shunt-Induced Hepatic Encephalopathy in TIPS: Current Approaches and Clinical Challenges. J Clin Med. 2020;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 39] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 6. | Nicoară-Farcău O, Han G, Rudler M, Angrisani D, Monescillo A, Torres F, Casanovas G, Bosch J, Lv Y, Thabut D, Fan D, Hernández-Gea V, García-Pagán JC; Preemptive TIPS Individual Data Metanalysis, International Variceal Bleeding Study and Baveno Cooperation Study groups. Effects of Early Placement of Transjugular Portosystemic Shunts in Patients With High-Risk Acute Variceal Bleeding: a Meta-analysis of Individual Patient Data. Gastroenterology. 2021;160:193-205.e10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 112] [Article Influence: 28.0] [Reference Citation Analysis (0)] |
| 7. | Morrison JD, Mendoza-Elias N, Lipnik AJ, Lokken RP, Bui JT, Ray CE Jr, Gaba RC. Gastric Varices Bleed at Lower Portosystemic Pressure Gradients than Esophageal Varices. J Vasc Interv Radiol. 2018;29:636-641. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 30] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 8. | Mollaiyan A, Bettinger D, Rössle M. The underdilation of nitinol stents at TIPS implantation: Solution or illusion? Eur J Radiol. 2017;89:123-128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 28] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 9. | Kloster ML, Ren A, Shah KY, Alqadi MM, Bui JT, Lipnik AJ, Niemeyer MM, Ray CE, Gaba RC. High Incidence of Hepatic Encephalopathy After Viatorr Controlled Expansion Transjugular Intrahepatic Portosystemic Shunt Creation. Dig Dis Sci. 2021;66:4058-4062. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 15] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 10. | de Franchis R, Bosch J, Garcia-Tsao G, Reiberger T, Ripoll C; Baveno VII Faculty. Baveno VII - Renewing consensus in portal hypertension. J Hepatol. 2022;76:959-974. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1537] [Cited by in RCA: 1496] [Article Influence: 498.7] [Reference Citation Analysis (2)] |
| 11. | Luo SH, Chu JG, Huang H, Yao KC. Effect of initial stent position on patency of transjugular intrahepatic portosystemic shunt. World J Gastroenterol. 2017;23:4779-4787. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 14] [Cited by in RCA: 14] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 12. | Vilstrup H, Amodio P, Bajaj J, Cordoba J, Ferenci P, Mullen KD, Weissenborn K, Wong P. Hepatic encephalopathy in chronic liver disease: 2014 Practice Guideline by the American Association for the Study of Liver Diseases and the European Association for the Study of the Liver. Hepatology. 2014;60:715-735. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1583] [Cited by in RCA: 1408] [Article Influence: 128.0] [Reference Citation Analysis (1)] |
| 13. | Chalasani N, Younossi Z, Lavine JE, Charlton M, Cusi K, Rinella M, Harrison SA, Brunt EM, Sanyal AJ. The diagnosis and management of nonalcoholic fatty liver disease: Practice guidance from the American Association for the Study of Liver Diseases. Hepatology. 2018;67:328-357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3544] [Cited by in RCA: 4949] [Article Influence: 707.0] [Reference Citation Analysis (9)] |
| 14. | Pieper CC, Jansen C, Meyer C, Nadal J, Lehmann J, Schild HH, Trebicka J, Thomas D. Prospective Evaluation of Passive Expansion of Partially Dilated Transjugular Intrahepatic Portosystemic Shunt Stent Grafts-A Three-Dimensional Sonography Study. J Vasc Interv Radiol. 2017;28:117-125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 43] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 15. | Bosch J. Small diameter shunts should lead to safe expansion of the use of TIPS. J Hepatol. 2021;74:230-234. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 41] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 16. | Tripathi D, Stanley AJ, Hayes PC, Travis S, Armstrong MJ, Tsochatzis EA, Rowe IA, Roslund N, Ireland H, Lomax M, Leithead JA, Mehrzad H, Aspinall RJ, McDonagh J, Patch D. Transjugular intrahepatic portosystemic stent-shunt in the management of portal hypertension. Gut. 2020;69:1173-1192. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 235] [Cited by in RCA: 219] [Article Influence: 43.8] [Reference Citation Analysis (0)] |
| 17. | Northup PG, Garcia-Pagan JC, Garcia-Tsao G, Intagliata NM, Superina RA, Roberts LN, Lisman T, Valla DC. Vascular Liver Disorders, Portal Vein Thrombosis, and Procedural Bleeding in Patients With Liver Disease: 2020 Practice Guidance by the American Association for the Study of Liver Diseases. Hepatology. 2021;73:366-413. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 171] [Cited by in RCA: 376] [Article Influence: 94.0] [Reference Citation Analysis (1)] |
| 18. | Khan A, Maheshwari S, Gupta K, Naseem K, Chowdry M, Singh S. Rate, reasons, predictors, and burden of readmissions after transjugular intrahepatic portosystemic shunt placement. J Gastroenterol Hepatol. 2021;36:775-781. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 13] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 19. | Li W, Duan Y, Liu Z, Lu X, She J, Qing J, Zhang C. Clinical value of hemodynamic changes in diagnosis of hepatic encephalopathy after transjugular intrahepatic portosystemic shunt. Scand J Gastroenterol. 2022;1-6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Reference Citation Analysis (0)] |
| 20. | Trebicka J, Bastgen D, Byrtus J, Praktiknjo M, Terstiegen S, Meyer C, Thomas D, Fimmers R, Treitl M, Euringer W, Sauerbruch T, Rössle M. Smaller-Diameter Covered Transjugular Intrahepatic Portosystemic Shunt Stents Are Associated With Increased Survival. Clin Gastroenterol Hepatol. 2019;17:2793-2799.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 83] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 21. | Miraglia R, Maruzzelli L, Di Piazza A, Mamone G, Caruso S, Gentile G, Tuzzolino F, Floridia G, Petridis I, Volpes R, Luca A. Transjugular Intrahepatic Portosystemic Shunt Using the New Gore Viatorr Controlled Expansion Endoprosthesis: Prospective, Single-Center, Preliminary Experience. Cardiovasc Intervent Radiol. 2019;42:78-86. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 56] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 22. | Slowik V, Monroe EJ, Friedman SD, Hsu EK, Horslen S. Pressure gradients, laboratory changes, and outcomes with transjugular intrahepatic portosystemic shunts in pediatric portal hypertension. Pediatr Transplant. 2019;23:e13387. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 11] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 23. | Zhao D, Zhang G, Wang M, Zhang C, Li J. Portal pressure gradient and serum albumin: A simple combined parameter associated with the appearance of ascites in decompensated cirrhosis treated with transjugular intrahepatic portosystemic shunt. Clin Mol Hepatol. 2019;25:210-217. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 24. | Perello MP, Mur JP, Vives MS, Riutort JMM, Artigues AP, Garcia CN, Vidal MLB, Gelabert AE, Garau MV. Long-term follow-up of transjugular intrahepatic portosystemic shunt (TIPS) with stent-graft. Diagn Interv Radiol. 2019;25:346-352. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 19] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 25. | Huang Z, Yao Q, Zhu J, He Y, Chen Y, Wu F, Hua T. Efficacy and safety of transjugular intrahepatic portosystemic shunt (TIPS) created using covered stents of different diameters: A systematic review and meta-analysis. Diagn Interv Imaging. 2021;102:279-285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 21] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 26. | Bellis L, Moitinho E, Abraldes JG, Graupera M, García-Pagán JC, Rodés J, Bosch J. Acute propranolol administration effectively decreases portal pressure in patients with TIPS dysfunction. Transjugular intrahepatic portosystemic shunt. Gut. 2003;52:130-133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 22] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 27. | García-Pagán JC, Saffo S, Mandorfer M, Garcia-Tsao G. Where does TIPS fit in the management of patients with cirrhosis? JHEP Rep. 2020;2:100122. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 61] [Cited by in RCA: 80] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 28. | Horhat A, Bureau C, Thabut D, Rudler M. Transjugular intrahepatic portosystemic shunt in patients with cirrhosis: Indications and posttransjugular intrahepatic portosystemic shunt complications in 2020. United European Gastroenterol J. 2021;9:203-208. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 26] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 29. | Adebayo D, Neong SF, Wong F. Refractory Ascites in Liver Cirrhosis. Am J Gastroenterol. 2019;114:40-47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 49] [Article Influence: 8.2] [Reference Citation Analysis (0)] |