Published online Apr 14, 2023. doi: 10.3748/wjg.v29.i14.2188
Peer-review started: December 16, 2022
First decision: January 11, 2023
Revised: January 15, 2023
Accepted: March 23, 2023
Article in press: March 23, 2023
Published online: April 14, 2023
Processing time: 118 Days and 1.1 Hours
Acoustic radiation force impulse (ARFI) is used to measure liver fibrosis and predict outcomes. The performance of elastography in assessment of fibrosis is poorer in hepatitis B virus (HBV) than in other etiologies of chronic liver disease.
To evaluate the performance of ARFI in long-term outcome prediction among different etiologies of chronic liver disease.
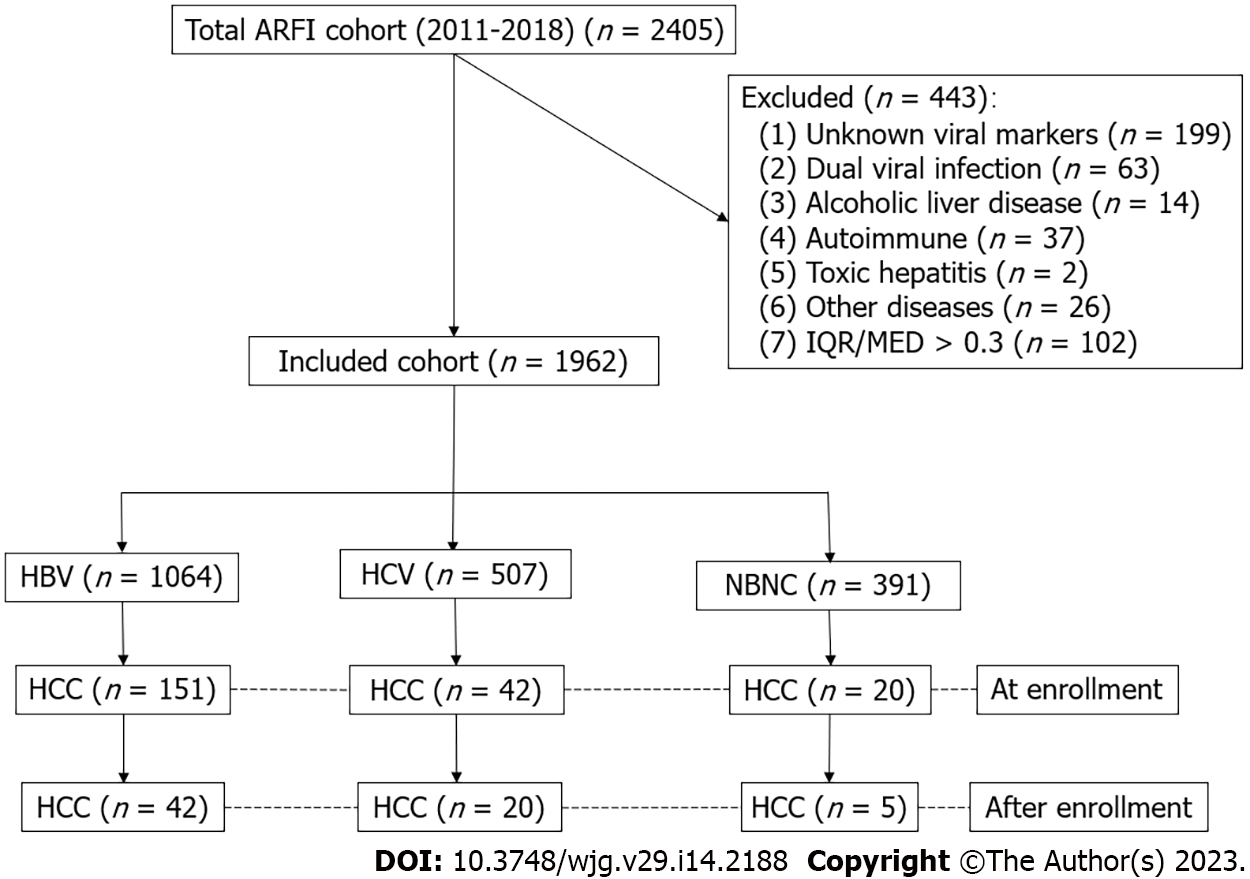
Consecutive patients who received an ARFI study between 2011 and 2018 were enrolled. After excluding dual infection, alcoholism, autoimmune hepatitis, and others with incomplete data, this retrospective cohort were divided into hepatitis B (HBV, n = 1064), hepatitis C (HCV, n = 507), and non-HBV, non-HCV (NBNC, n = 391) groups. The indexed cases were linked to cancer registration (1987-2020) and national mortality databases. The differences in morbidity and mortality among the groups were analyzed.
At the enrollment, the HBV group showed more males (77.5%), a higher prevalence of pre-diagnosed hepatocellular carcinoma (HCC), and a lower prevalence of comorbidities than the other groups (P < 0.001). The HCV group was older and had a lower platelet count and higher ARFI score than the other groups (P < 0.001). The NBNC group showed a higher body mass index and platelet count, a higher prevalence of pre-diagnosed non-HCC cancers (P < 0.001), especially breast cancer, and a lower prevalence of cirrhosis. Male gender, ARFI score, and HBV were independent predictors of HCC. The 5-year risk of HCC was 5.9% and 9.8% for those ARFI-graded with severe fibrosis and cirrhosis. ARFI alone had an area under the receiver operating characteristic curve (AUROC) of 0.742 for prediction of HCC in 5 years. AUROC increased to 0.828 after adding etiology, gender, age, and platelet score. No difference was found in mortality rate among the groups.
The HBV group showed a higher prevalence of HCC but lower comorbidity that made mortality similar among the groups. Those patients with ARFI-graded severe fibrosis or cirrhosis should receive regular surveillance.
Core Tip: Among 1962 patients who received an acoustic radiation force impulse (ARFI) study, the 5-year risk of hepatocellular carcinoma (HCC) was 5.9% and 9.8% for those ARFI-graded with severe fibrosis and cirrhosis, respectively. The prevalence of HCC was highest in the hepatitis B virus (HBV) group. However, the HBV group showed the lowest comorbidities among the groups after adjusting for age, gender, and body mass index. This made the mortality rate similar among the groups. ARFI alone had an area under the receiver operating characteristic curve (AUROC) of 0.742 for prediction of HCC in 5 years. The AUROC increased to 0.828 after adding etiology, gender, age, and platelet score. Those patients with ARFI-estimated severe fibrosis or cirrhosis should receive active surveillance of HCC in all etiologies.
- Citation: Tai J, Harrison AP, Chen HM, Hsu CY, Hsu TH, Chen CJ, Jeng WJ, Chang ML, Lu L, Tai DI. Acoustic radiation force impulse predicts long-term outcomes in a large-scale cohort: High liver cancer, low comorbidity in hepatitis B virus. World J Gastroenterol 2023; 29(14): 2188-2201
- URL: https://www.wjgnet.com/1007-9327/full/v29/i14/2188.htm
- DOI: https://dx.doi.org/10.3748/wjg.v29.i14.2188
Chronic liver diseases are major risk factors for hepatocellular carcinoma (HCC)[1,2]. Regular screening of high-risk groups to detect HCC in the early stage can increase the chance to eradicate HCC and improve survival rates[3,4]. Liver cirrhosis is a major HCC risk factor[1-4]. In the last two decades, Fibroscan has been used to assess the HCC risk for patients with chronic liver diseases[5-9]. An alternative is the acoustic radiation force impulse (ARFI) imaging which uses ultrasound as a push pulse to measure the liver stiffness[10-12]. Both modalities are quite good in patients with chronic hepatitis C virus (HCV) infection and non-alcoholic liver diseases[13]. However, the correlation was relatively poor in patients with chronic hepatitis B virus (HBV) infection. We have been using ARFI to assess liver stiffness since 2011[12,13], which allowed the informatics department in our institute to produce a uniquely available large-scale cohort of patient data for research purposes. This would facilitate us to evaluate the ability of ARFI to assess the HCC risk using a consecutive cohort that is much larger than what has been found in prior work. The short-term correlation between ARFI and liver fibrosis is relatively poor in HBV, but the long-term effect is still not fully investigated. As well, studies into the predictive ability of ARFI for HCC among patients with different etiologies of chronic liver disease are still rare[14]. Finally, the chronic HBV infection is characterized by an initial immune tolerance phase[15]. This could be a survival strategy[16] despite an increased risk of HCC caused by chronic persistent HBV infection[1,2,17,18]. Therefore, we examined the differences in morbidities and mortalities between HBV and other etiologies in this cohort to help shed some light on this question.
This study was approved by the Institutional Review Board (IRB) of the Chang Gung Medical Foundation (CGMH IRB No. 201801283B0 and No. 202200758B0).
ARFI imaging was done with an Acuson S2000 system (Siemens Medical Solutions). Liver stiffness was measured with a standardized protocol at two locations of the right hepatic lobe[12,13]. The mean of the two locations was used as the final measurement. Most of the studies were done by one senior technician (HTW). Because it is not covered by the Taiwanese National Health Insurance Administration, the charge of ARFI (around 50 United States dollars) was mostly paid by the patients. The exceptions were patients undergoing a liver biopsy study or those participating in clinical trials (around 25%), where the cost was paid for by research grants. The first ARFI study was used in this analysis. The ARFI-estimated fibrosis grades was according to the cutoff values in our previous histology proven study[13].
The data of this study were retrieved from the Chang Gung Research Database of the Chang Gung Memorial Hospital, Linkou and Taipei branches. All patients who received ARFI imaging between January 2011 and September 2018 represented as the index patients. A total population of 2405 patients were included. The Chang Gung Research Database includes the original electronic medical records, comprising health care facility information, patient demographics, diagnosis information, drug information, procedure information, and other health digital information. All the personal identifiers were replaced by a code. We organized a team that included researchers from both hepatology and informatics departments of Chang Gung Memorial Hospital, along with outside technical consultants, to deal with this project[19]. We excluded patients with incomplete viral markers and clinical data, dual viral hepatitis, alcoholic liver diseases, autoimmune liver diseases, toxic hepatitis, genetic liver diseases, and those whose ARFI studies were with an interquartile range over a median ratio > 30%, which is a recommended quality assurance criterion (Figure 1)[20]. According to the viral markers, patients were classified into three groups: Hepatitis B, hepatitis C, and non-hepatitis B, non-hepatitis C (NBNC) patients. The NBNC group consisted mostly of patients with non-alcoholic fatty liver disease[19]. All the patients were followed for liver biochemistry, alpha-fetoprotein, and liver ultrasound at 3-12-mo intervals.
The comorbidities under investigation included hypertension [I10-I15], type 2 diabetes mellitus [E8-13], dyslipidemia [E78], myocardial infarction [I21-I23, I1252], atrial fibrillation [I48], heart failure [I50], and ischemic stroke [I63-I66]. The ascertainment of these comorbidities was based on three diagnoses from the outpatient department or one diagnosis from the inpatient department.
Patients could have undergone any of several different antiviral regimens in the study period. Similar interferon regimens had been used in both HBV and HCV groups, and such immune modulatory regimens were not uniform. Therefore, we simply recorded whether interferon therapy was given pre-enrollment or post-enrollment.
Similarly, multiple nucleot(s)ide analogue (NA) regimens for HBV and direct antiviral agent (DAA) regimens for HCV were possibly taken in the study period. Their mechanisms are suppression or elimination of viral replication. We recorded such oral therapies in pre-enrollment and/or post-enrollment periods as one category.
In addition to their medical records, study subjects were linked with the database of Cancer Registration, Chang Gung Memorial Hospital, which records information on all cancers diagnosed in this hospital since 1987. We linked this database up to June 30, 2020.
This study used the national citizen identification numbers of patients to search the mortality data bank established by the Statistics Office, Department of Health, Taiwan. The mortality data bank stored death certificate data, which includes patient demographic data such as the time, place, and cause of death, and the name of the official who issued the document. Causes of death were classified using the International Classifications of Diseases, Injuries and Causes of Death (ICD-10, World Health Organization, 2015). The data was linked up to December 31, 2020.
This mortality data link will collect data for those patients lost to follow-up in this hospital.
Patient characteristics are represented as the number and percentage, or the mean ± SD, as appropriate. Continuous variables of the three independent groups were compared using one-way analysis of variance (ANOVA) with Bonferroni correction. Categorical variables were tested using the chi-square test, or the chi-square test for trend, as applicable. Logistic regression was conducted to identify independent risk factors for HCC for records before the ARFI study. Cox proportional hazards model was conducted to identify independent risk factors of HCC for records after the ARFI study. The Mantel-Cox procedure (Log Rank test) was applied to compare risk factors and the cumulative risk of HCC among different groups after the ARFI study. The area under the receiver operating characteristic curve (AUROC) was calculated using a scoring system for HCC risk prediction. The scoring system was based on the hazard ratio in the multivariate analysis. Briefly, ARFI and FIB4 were according to its values and modified by timing 0.5 or 2. Other factors were calculated as: Gender (G) (male = 2, female = 0); etiology score (E) (HBV = 3, HCV = 2, NBNC = 1); age (year) score (A) (0-35 = 0, 35-40 = 1, 40-45 = 2, 45-50 = 3, 50-55 = 4, 55-60 = 5; > 60 = 6; platelet (109/L) score (A) (0-100 = 3, 100-150 = 2, > 150 = 1). Statistical analyses were performed using the SPSS software (version 22; SPSS Inc., Chicago, IL, United States), and a P value of < 0.05 was judged as statistically significant.
A total of 1962 patients met our inclusion criteria. Among them, 1064, 507, and 391 patients were in the HBV, HCV, and NBNC groups, respectively (Table 1). The HBV group had more men and a lower prevalence of hypertension, diabetes mellitus, heart failure, ischemic stroke, and dyslipidemia. Patients in the HCV group were older and had a lower male ratio, lower platelet counts, higher fibrosis index based on four factors (FIB4), and mean ARFI score. The NBNC group had a higher body mass index (BMI), lower prothrombin time and international normalized ratios (INR), lower FIB4, higher platelet count, lower rate of estimated cirrhosis, and shorter duration of follow-up.
| HBV (n = 1064) | HCV (n = 507) | NBNC (n = 391) | P value | Missing rate | |
| Demographics | |||||
| Age (yr) | 52.05 ± 10.90 | 58.69 ± 10.84 | 51.97 ± 13.10 | < 0.0001 (2 & 3, 1 & 2) | 0.00% |
| Male sex, n (%) | 825 (77.5) | 273 (53.8) | 233 (59.6) | < 0.0001 | 0.00% |
| Weight (kg) | 68.36 ± 12.30 | 64.53 ± 12.17 | 70.94 ± 14.09 | < 0.0001 (2 & 3, 1 & 2, 1 & 3) | 0.97% |
| Height (cm) | 166.34 ± 7.62 | 161.67 ± 8.57 | 164.18 ± 8.75 | < 0.0001 (2 & 3, 1 & 2, 1 & 3) | 2.80% |
| BMI (kg/m2) | 24.66 ± 3.62 | 24.58 ± 3.75 | 26.25 ± 4.23 | < 0.0001 (2 & 3, 1 & 3) | 3.47% |
| Lab data at ARFI study | |||||
| Spleen index (cm2) | 31.97 ± 14.47 | 33.63 ± 16.72 | 34.07 ± 16.05 | 0.0640 (1 & 3) | 0.03% |
| Albumin (mg/dL) | 4.345 ± 0.51 | 4.29 ± 0.51 | 4.43 ± 0.48 | 0.0040 (2 & 3) | 42.15% |
| AST (U/L) | 57.50 ± 102.16 | 62.46 ± 58.08 | 58.40 ± 50.72 | 1.12% | |
| ALT (U/L) | 75.05 ± 162.88 | 71.52 ± 69.61 | 83.46 ± 78.32 | 1.33% | |
| Bilirubin (mg/dL) | 0.93 ± 1.23 | 0.91 ± 1.19 | 0.92 ± 1.21 | 12.79% | |
| Prothrombin time (INR) | 1.10 ± 0.13 | 1.11 ± 0.21 | 1.06 ± 0.13 | 0.0040 (2 & 3, 1 & 3) | 42.15% |
| Platelets (109/L) | 177.86 ± 61.86 | 170.13 ± 60.19 | 218.96 ± 76.08 | < 0.0001 (2 & 3, 1 & 3) | 13.25% |
| FIB4 | 2.454 ± 2.720 | 3.31 ± 3.04 | 2.06 ± 2.13 | < 0.0001 (2 & 3, 1 & 2) | 23.35% |
| Mean ARFI (m/s)1 | 1.40 ± 0.46 | 1.60 ± 0.61 | 1.42 ± 0.62 | < 0.0001 (2 & 3, 1 & 2) | 0.00% |
| Stiffness status1 | < 0.0001 | ||||
| Cirrhosis | 331 (31.1) | 163 (32.1) | 78 (19.9) | ||
| Severe fibrosis | 138 (13.0) | 110 (21.7) | 61 (15.6) | ||
| Moderate fibrosis | 114 (10.7) | 68 (13.4) | 29 (7.4) | ||
| Mild or non-fibrosis | 481(45.2) | 166(32.7) | 223(57.0) | ||
| Comorbidities | |||||
| Hypertension | 181 (17.0) | 147 (29.0) | 115 (29.4) | < 0.0001 | |
| Diabetes | 119 (11.2) | 103 (20.3) | 76 (19.4) | < 0.0001 | |
| Heart failure | 14 (1.3) | 17 (3.4) | 7 (1.8) | 0.023 | |
| Atrial fibrillation | 9 (0.8) | 11 (2.2) | 5 (1.3) | 0.091 | |
| Myocardial infraction | 12 (1.1) | 5 (1.0) | 2 (0.5) | 0.567 | |
| Ischemic stroke | 6 (0.6) | 17 (3.4) | 8 (2.0) | < 0.0001 | |
| Dyslipidemia | 170 (16.0) | 95 (18.7) | 125 (32.0) | < 0.0001 | |
| Follow-up (year) | |||||
| mean ± SD | 4.59 ± 2.22 | 4.34 ± 2.35 | 3.13 ± 2.15 | < 0.0001 (1 & 3, 2 & 3) | |
| Median/IQR | 4.54/3.59 | 3.97/3.88 | 2.61/3.23 | ||
ARFI is strongly influenced by liver inflammation. This especially happens when alanine aminotransferase (ALT) levels are greater than 5 × (180 U/L) the upper limit of normal (0-36). So, we list the ALT level grades in the Supplementary Table 1. In total, 7.3% of patients had ALT level greater than 180 U/L.
There were significant differences in the prevalence of hypertension, diabetes mellitus, heart failure, ischemic stroke, and dyslipidemia between the HBV group and the other two groups (Table 1). After adjusting for age, gender, and BMI, the prevalence of hypertension, diabetes mellitus, ischemic stroke, and dyslipidemia was significantly lower in the HBV group than in the non-HBV groups (Supple
We linked our index cases with cancer registration databases between 1987 and 2020. There were many cancer diagnoses prior to enrollment. These cancers were either well-treated or receiving active therapy. The HBV group had the highest rate of pre-enrollment HCC diagnosis (14.1%, P < 0.001, Table 2, upper panel). On the other hand, the NBNC group had the highest rate of non-HCC cancer (10%, P < 0.001), with breast cancer having the highest rate (4.3% for total or 10.8% for females alone, P < 0.001). Factors associated with a high risk of HCC as identified by logistic regression were male sex, high mean ARFI score, HBV, older age, higher aspartate aminotransferase (AST) level, lower ALT level, and lower platelet count (Table 3). Hypertension and diabetes mellitus were associated with a higher risk of pre-diagnosed HCC while dyslipidemia was associated with a lower risk of pre-diagnosed HCC.
| HBV | HCV | NBNC | P value | |
| Total cases | 1064 | 507 | 391 | |
| Female | 239 (22.5) | 234 (46.2) | 158 (40.4) | < 0.027 |
| Follow years before enrollment | -2.65 ± 3.98 | -2.78 ± 5.56 | -3.30 ± 6.25 | < 0.001 (3 & 1, 3 & 2) |
| Cancers, pre-enrollment | ||||
| HCC | 150 (14.1) | 42 (8.3) | 20 (5.1) | < 0.001 |
| Non-HCC cancer | 36 (3.4) | 30 (5.9) | 39 (10.0) | < 0.001 |
| Colon cancer | 9 (0.8) | 3 (0.6) | 6 (1.5) | NS |
| Breast cancer | 1 (0.1) | 9 (1.8) | 17 (4.3) | < 0.0011 |
| Hematology cancers | 3 (0.3) | 4 (0.8) | 2 (0.5) | NS |
| Follow years before enrollment | -2.76 ± 4.55 | -3.57 ± 5.18 | -3.22 ± 4.40 | NS |
| Cancers, post-enrollment | ||||
| HCC | 43 (4.0) | 19 (3.7) | 5 (1.3) | 0.033 |
| Non-HCC tumor | 21 (2.2) | 14 (2.8) | 12 (3.1) | NS |
| Colon cancer | 2 (0.1) | 3 (0.6) | 1 (0.3) | NS |
| Breast cancer | 2 (0.2) | 0 (0.0) | 1 (0.3) | NS |
| Hematology cancers | 6 (0.6) | 0 (0.0) | 1 (0.5) | NS |
| Prostate cancer | 2 (0.1) | 1 (0.2) | 1 (0.3) | NS |
| Follow years after enrollment | 2.43 ± 1.86 | 2.51 ± 1.86 | 2.25 ± 2.00 | NS |
| Mortality total | 72 (6.8) | 41 (8.1) | 21 (5.4) | NS |
| HCC | 35 (3.3) | 13 (2.6) | 4 (1.0)2 | 0.057 |
| Non-HCC cancers | 16 (1.5) | 8 (1.6) | 7 (1.8)2 | NS |
| Liver disease | 6 (0.6) | 7 (1.4) | 6 (1.5) | NS |
| Non-liver disease | 15 (1.4) | 13 (2.6) | 4 (1.0) | NS |
| Follow years after enrollment | 2.59 ± 1.96 | 2.69 ± 1.99 | 2.00 ± 1.87 | NS |
| Univariate analysis | Multivariate analysis | 95%CI | |||
| P value | P value | Hazard ratio | Lower | Upper | |
| Male | < 0.001 | 0.000 | 3.399 | 2.150 | 5.373 |
| Etiology | < 0.001 | 0.000 | - | - | - |
| HBV | 0.000 | 4.009 | 2.219 | 7.241 | |
| HCV | 0.169 | 1.644 | 0.810 | 3.335 | |
| Age, yr | < 0.001 | 0.000 | 1.047 | 1.029 | 1.066 |
| ALT | 0.243 | 0.001 | 0.993 | 0.989 | 0.997 |
| AST | 0.010 | 0.000 | 1.012 | 1.007 | 1.017 |
| Bilirubin | 0.170 | 0.729 | 0.980 | 0.875 | 1.098 |
| Platelet | 0.138 | 0.035 | 1.003 | 1.000 | 1.005 |
| Spleen index | 0.060 | 0.811 | 1.001 | 0.990 | 1.013 |
| BMI | 0.015 | 0.076 | 0.956 | 0.910 | 1.005 |
| ARFI | < 0.001 | 0.010 | 1.556 | 1.110 | 2.181 |
| Interferon therapy | 0.011 | 0.012 | |||
| Pre-enrollment | 0.618 | 1.204 | 0.581 | 2.496 | |
| Post-enrollment | 0.004 | 0.211 | 0.073 | 0.617 | |
| Oral anti-virus agents | 0.041 | 0.181 | |||
| Pre-enrollment | 0.168 | 1.721 | 0.795 | 3.728 | |
| Post-enrollment | 0.156 | 0.667 | 0.381 | 1.167 | |
| Pre- and post-enrollment | 0.527 | 1.534 | 0.408 | 5.773 | |
| Hypertension | < 0.001 | 0.000 | 2.551 | 1.702 | 3.824 |
| Diabetes mellitus | < 0.001 | 0.031 | 1.618 | 1.044 | 2.508 |
| Dyslipidemia | < 0.001 | 0.000 | 0.358 | 0.207 | 0.620 |
| Ischemic stroke | 0.341 | 0.884 | 1.097 | 0.317 | 3.794 |
After excluding patients with cancer diagnosed before enrollment, the HBV (4.0%) and NBNC (1.3%) groups exhibited the highest and lowest rates of HCC occurrence, respectively (P = 0.033, Table 2, middle panel). Factors associated with HCC diagnosis were male sex (P = 0.013), high ARFI score (< 0.001), and HBV (P = 0.019, Table 4). In Cox’s regression analysis, male gender, HBV, platelet count, and ARFI score were associated with a higher risk of HCC.
| Univariate analysis | Multivariate analysis | 95%CI | |||
| P value | P value | Hazard ratio | Lower | Upper | |
| Male | 0.005 | 0.009 | 20.796 | 1.293 | 6.045 |
| Etiology | 0.019 | 0.057 | |||
| HBV | 0.023 | 40.473 | 1.232 | 16.245 | |
| HCV | 0.150 | 20.670 | 0.701 | 10.173 | |
| Age (yr) | < 0.001 | 0.094 | 10.028 | 0.995 | 1.061 |
| ALT (U/L) | 0.188 | 0.211 | 0.994 | 0.985 | 1.003 |
| AST (U/L) | 0.605 | 0.449 | 10.005 | 0.993 | 1.017 |
| Bilirubin (mg/dL) | 0.962 | 0.326 | 0.772 | 0.460 | 1.295 |
| Platelet (109/L) | < 0.001 | 0.041 | 0.993 | 0.986 | 1.000 |
| Spleen index (cm2) | 0.019 | 0.169 | 10.012 | 0.995 | 1.030 |
| BMI | 0.334 | 0.524 | 0.973 | 0.894 | 1.059 |
| ARFI (m/s) | < 0.001 | 0.000 | 20.775 | 10.624 | 4.742 |
| Interferon therapy | 0.814 | 0.943 | |||
| Pre-enrollment | 0.734 | 0.772 | 0.174 | 3.435 | |
| Post-enrollment | 0.914 | 0.949 | 0.366 | 2.459 | |
| Oral anti-virus therapy | 0.0174 | 0.086 | |||
| Pre-enrollment | 0.977 | 10.031 | 0.134 | 7.948 | |
| Post-enrollment | 0.029 | 20.105 | 10.080 | 4.102 | |
| Pre- and post-enrollment | 0.110 | 30.362 | 0.758 | 14.903 | |
| Hypertension | 0.001 | 0.211 | 10.554 | 0.778 | 3.104 |
| Diabetes mellitus | 0.002 | 0.304 | 10.460 | 0.710 | 3.006 |
| Ischemic stroke | 0.305 | 0.971 | 00.000 | 0.000 | 1.46E+269 |
| Dyslipidemia | 0.233 | 0.065 | 10.920 | 0.960 | 3.840 |
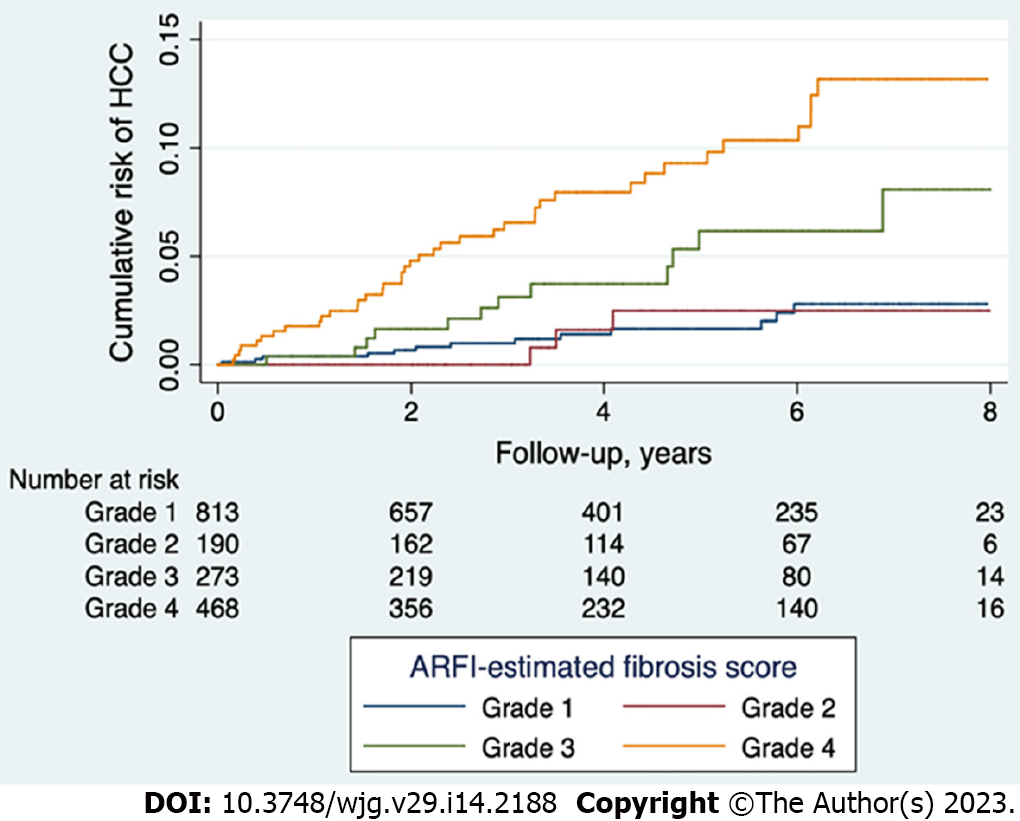
According to the cutoff values of mean ARFI determined in our previous histology proven cases[13], we divided the study population into cirrhosis, severe fibrosis, moderate fibrosis, and mild to non-fibrosis groups. The cumulative risk of HCC development was highest in the cirrhosis group, followed by the severe fibrosis group, and lowest in the none to moderate fibrosis groups (P < 0.001, Figure 2). The 5-year risk of HCC was 5.9% and 9.8% for those patients ARFI-graded with severe fibrosis and cirrhosis, respectively. When those patients with ALT greater than 5 × upper limit normal were removed, the 5-year risk of HCC was 6.1% and 10.4% for those with severe fibrosis and cirrhosis, respectively (Supplementary Figure 1). There was no difference in predictive abilities across different etiologies (Supplementary Figure 2), suggesting that high ARFI score is a good predictor of HCC diagnosis for HBV patients, along with HCV and NBNC patients.
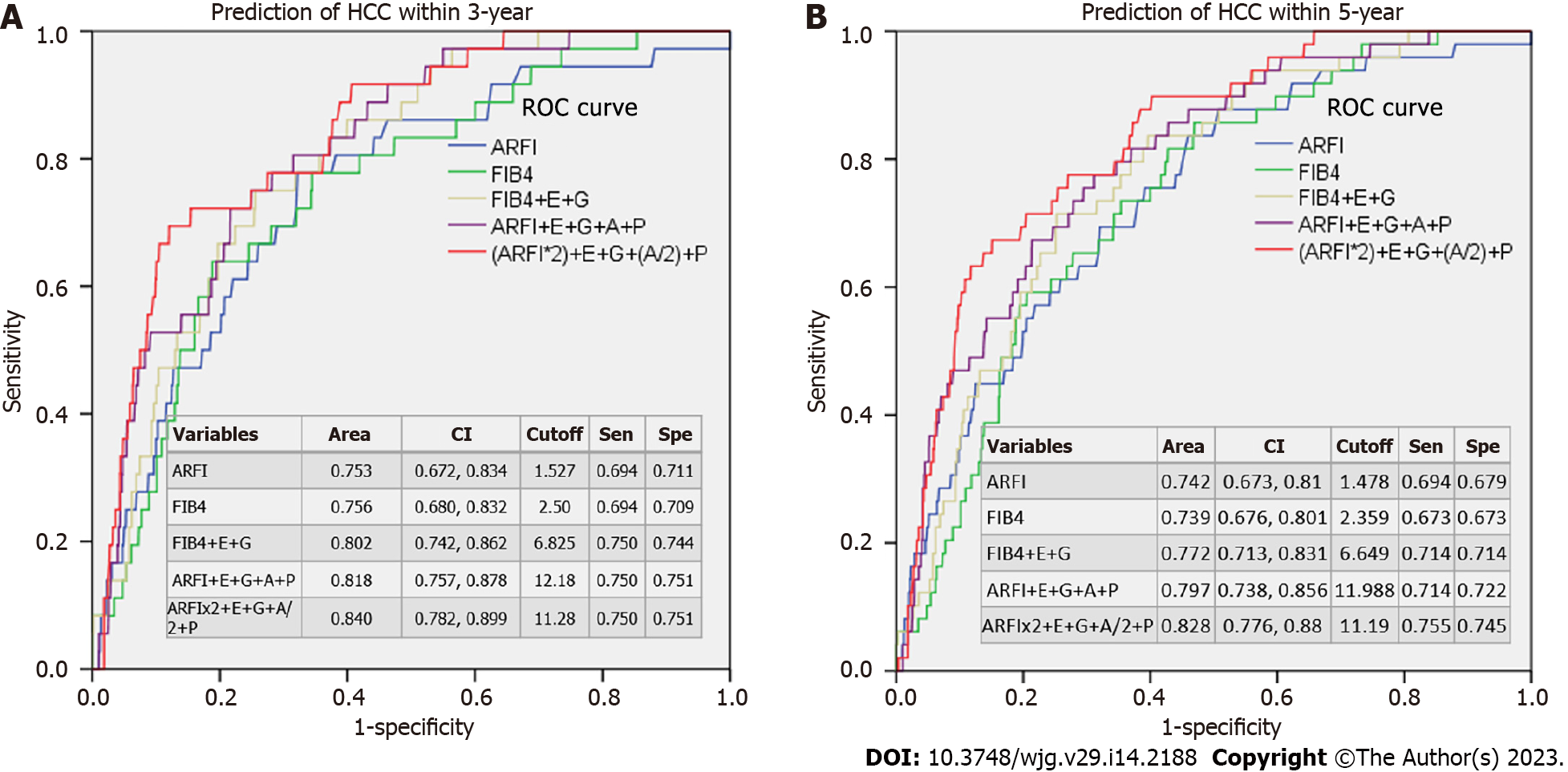
The AUROCs showed that ARFI or FIB4 alone gave similar 3- or 5-year predictions of HCC (AUROCs around 0.739-0.756) (Figure 3). After adding gender, etiology, age, and platelet score, the AUROCs increased to 0.772-0.840.
No significant difference in total mortality was found across the three groups. The HBV group had a marginally higher HCC related mortality (3.3%, P = 0.057) (Table 2, lower panel). However, the non-HCC related mortality was the lowest in the HBV group (P < 0.027). Male sex, age, lower ALT level, higher AST level, lower BMI, higher ARFI score, and ischemic stroke were associated with a higher risk of mortality in Cox’s regression analysis (Supplementary Table 3).
In our patient cohort, 212 patients were diagnosed with HCC prior to enrollment and 67 developed HCC after enrollment (Table 2). High ARFI scores were associated with pre-diagnosed HCC occurrences in a multivariate analysis (P < 0.001, Table 3) and were a good predictor of HCC after enrollment according to Cox’s regression analysis (P < 0.001, Table 4). The ARFI-estimated fibrosis grades showed a severity-dependent increased risk of HCC in the post-enrollment period (Figure 2). The 5-year risk of HCC was 5.9% and 9.8% for those patients who were ARFI-graded with severe fibrosis and cirrhosis, respectively. When age, gender, and platelet count were added, there was only a small rise of AUROC from 0.753 to 0.84 within 3 years and 0.742 to 0.828 within 5 years in the prediction of HCC. These results suggest that liver fibrosis is the main risk factor for HCC (Figure 3). Our finding is consistent with others in a recent study review[21] using non-invasive fibrosis diagnosis models. Patients with grades 3 and 4 fibrosis should receive active surveillance of HCC.
ARFI-graded fibrosis predicts the HCC occurrence well among different etiologies (Supplementary Figure 2). Although the correlation between ARFI and liver histology fibrosis was poorer in the HBV group[13] than in the other two etiologies, HCC risk prediction was as good as that in the other groups. This question was not well-addressed in prior work where such investigations had much smaller clinical datasets or single etiology cohorts. Additional investigations should further pursue this link.
In terms of prior work, ARFI studies on non-HCC patients typically focused on its utility of fibrosis staging[10-12,22]. Exceptions include the work by Sun et al[23], who correlated ARFI with the indocyanine green test and found a positive correlation between the two. ARFI was also correlated to Child-Pugh scores in that study. They suggested that ARFI imaging is a useful tool for assessing liver functional reserve. As another example, a recent meta-analysis reported that ARFI scores may be a good predictor of HCC recurrence-free survival in patients receiving radiofrequency ablation[24]. In a series of 1808 patients who received ARFI, those patients with an ARFI score > 1.33 cm/s showed a higher probability of HCC development than those with an ARFI score ≤ 1.33 cm/s[25]. Therefore, there is good evidence that ARFI can measure liver fibrosis, reflex the liver functional reserve, and predict HCC recurrence. Our study adds to these conclusions, as Cox’s regression analysis confirmed that high ARFI score was a risk factor for HCC and mortality (P < 0.001, Table 3 and Supplementary Table 3).
In aspects of HCC diagnosis, the HBV group had higher occurrences than the NBNC group (Table 2). This aligns with previous investigations, e.g., the study by Chen et al[26] who reported higher occurrences of HCC in their HBV (4.8%) and HCV (4.7%) groups compared to their NBNC group (0.3%). Even so, the prevalence of HCC in our NBNC group was much higher than their value, but this is because Chen et al[26] included healthy subjects in their NBNC group, while our NBNC cohort was a disease group. Even though NBNC patients had a lower incidence of HCC (P < 0.001), this was offset by higher incidences of non-HCC cancers. Consequently, the total mortality rate was similar among different groups (Table 2). This is consistent with a recent meta-analysis by Mantovani et al[27] that concluded that extra-hepatic cancers were increased in non-alcoholic fatty liver disease. It should be noted that such trends were only identified in the pre-enrollment period because it had a longer past history. We can record many cancers in the pre-enrollment period because the diagnosis of all cancer types has been recorded in our cancer registration database since 1987. We found that breast cancer (P < 0.001), colon cancer, and hematologic cancers were the main cancers in the pre-enrollment period. These types of cancers respond to treatment well, allowing us to include such survivors in our cohort.
There were lower rates of comorbidities in the HBV group than in the other groups (Table 1). This seems to be related to the high prevalence of metabolic syndrome in the HCV or NBNC group. However, after adjusting for gender, age, and BMI, such a phenomenon was still present (Supplementary Table 2). As early as 2006, Jan et al[28] had reported a lower prevalence of diabetes, hypertension, obesity, hyperlipidemia, and obesity in HBV than HCV or NBNC in a population-based study in northern Taiwan. Another nationwide study by Kuo et al[29] that examined 1376344 diabetes patients between 2000 and 2012 also confirms these results. Before enrollment, they excluded patients with a history of myocardial infarction (2.28% in HBV group and 4.19% in non-HBV group, P < 0.001) and cerebrovascular diseases (15.6% in HBV group and 24.3% in non-HBV group, P < 0.001). The percentage of excluded patients was lower in their HBV group than the non-HBV groups for both diseases. After enrollment and propensity matching, the risk of all-cause mortalities, myocardial infarction, ischemic stroke, and heart failure was higher in the non-HBV group than in the HBV group (P < 0.001) in a mean follow-up period of 5.3 year[29]. A study by Sung et al[30] from Korea showed similar findings. They pointed out that the difference was more profound in HBV with liver dysfunction than in those without liver dysfunction. They suggested that the HBV-related proinflammatory effect may be the reason for the decreased risk of comorbidity.
Previous studies did not discuss the reason for these low comorbidities in the HBV patients. A recent review suggested that chronic HBV infection may protect infected subjects from the development of metabolic syndrome and hepatic steatosis[31]. The immune system is a double-edged sword. Its efforts against microorganisms may induce host tissue damage[32]. Chronic persistent HBV infection is characterized by an initial immune tolerance phase that allows active HBV replication without immune-mediated inflammation to liver tissue[33]. Liver inflammation occurs only when the immune system is triggered to attack HBV carrying hepatocytes[34]. This contrasts with HCV and NBNC groups with persistently mild inflammation in the liver.
There is a longer HBV-related immune tolerance phase in East Asian than in African, which could be related to genetic polymorphism in human leukocyte antigen (HLA)-DP and -DQ loci[16,35]. Such HBV-related gene variants not only decrease antigen presentation to avoid fatal immune response, but also establish an environment that is suitable for chronic persistent HBV infection[16]. Our recent study indicated that those HBV-related single nucleotide polymorphisms (SNPs) in HLA-DP and -DQ loci were associated with high viral load in the HCC family[36]. Such patients would be more likely to be associated with liver dysfunction as those mentioned in Sung’s series[30]. From the above clues, we suspect that the low comorbidity trend in the HBV group may be partly associated with a low antigen presentation ability.
One of our limitations is that life-long disease consequences are not easy to examine. The mean follow-up duration after enrollment could be considered as relatively short (3.13 to 4.59 years). We may need longer and more specific studies to explore the link between HBV infection and comorbidity. It should be noted that HBV-related SNPs in HLA-DP and -DQ loci in East Asians are quite different from other ethnicities[16]. The low comorbidity in the HBV group may be limited to East Asian. Another limitation is that the contribution of therapy to morbidity and mortality was difficult to evaluate. We noticed that the post-enrollment interferon therapy was associated with a lower prevalence of pretreatment HCC and post-enrollment oral antivirus therapy was associated with a lower risk of post-enrollment HCC. However, these findings may be due to a relatively better condition of chronic liver disease, which could have made the pre-enrollment therapy unnecessary. Similar situations concerning about therapeutic response in HCC-related mortality could be presented. With the success of checkpoint inhibitors in HCC therapy[37], future predictive biomarker study will be needed to clarify the difference in mortality among groups[38].
We conclude that even ARFI-based fibrosis prediction in the HBV group is poorer than that in other groups, its performance or clinical significance in predicting HCC or mortality is as good as that in other etiologies. The HBV group had the highest risk of HCC and the NBNC group had the highest risk of non-HCC tumors, especially breast cancer. Low prevalence of comorbidities in the HBV group was found, which may be a consequence of a low prevalence of metabolic syndrome and low antigen presentation ability.
Acoustic radiation force impulse is used to measure liver fibrosis and predict outcomes. The performance of elastography in assessment of fibrosis is poorer in hepatitis B virus (HBV) than in other etiologies of chronic liver disease.
Whether there are differences in performance of acoustic radiation force impulse (ARFI) in long term outcome prediction among different etiologiesof chronic liver disease remains to be studied.
We collected a cohort of patients who received ARFI studies. After excluding unsuitable cases, 1962 patients were included as the indexed cases. They were classified into HBV, HCV, and non-HBV, non-HCV (NBNC) groups. We examined the differences in demographics, comorbidity, carcinogenesis, and mortality among these groups at and after enrollment.
These indexed cases were linked to the hospital’s cancer registration and national mortality databases to obtain complete outcome data. The data at enrollment were analyzed for differences among three groups and logistic regression was performed to search for predictors associated with cancers. Cox regression analysis and area under the receiver operating characteristic curve (AUROC) were used to assess the performance of ARFI in predicting hepatocellular carcinoma (HCC) and mortality.
At enrollment, the HBV group showed more males (77.5%), a higher prevalence of pre-diagnosed HCC, and a lower prevalence of comorbidities than the other groups (P < 0.001). The HCV group was older and had a lower platelet count and higher ARFI score than the other groups (P < 0.001). The NBNC group showed a higher body mass index, platelet count, and prevalence of pre-diagnosed non-HCC cancers (P < 0.001), especially breast cancer, and a lower prevalence of cirrhosis. After enrollment, male gender, ARFI score, and HBV were independent predictors of HCC. The 5-year risk of HCC was 5.9% and 9.8% for those ARFI-graded with severe fibrosis and cirrhosis, respectively. ARFI alone had an AUROC of 0.742 for prediction of HCC in 5 years. AUROC increased to 0.828 after adding etiology, gender, age, and platelet score. No difference in mortality rate was noted among the groups.
The HBV group showed a higher prevalence of HCC but a lower prevalence of comorbidity that made mortality similar among the groups. Those ARFI-graded as severe fibrosis or cirrhosis should receive regular surveillance.
The immune tolerance is a hallmark of HBV which could be related to poor antigen presentation of human leukocyte antigen-DP and -DQ molecules in HBV surface antigen carriers. Whether such behavior is associated with a low prevalence of comorbidity requires future study.
The authors acknowledge the support and assistance in statistical analysis, study design, and data analysis and interpretation from the Maintenance Project of the Center for Big Data Analytics and Statistics at the Chang Gung Memorial Hospital. This study was based in part on data from the Chang Gung Research Database provided by Chang Gung Memorial Hospital. The interpretation and conclusions contained herein do not represent the position of Chang Gung Memorial Hospital.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Corresponding Author’s Membership in Professional Societies: AASLD, No. 139352; APASL, No. MN-1509; TASL, No. 98.
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Taiwan
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Brandi G, Italy; Li S, China; Tavan H, Iran S-Editor: Chen YL L-Editor: Wang TQ P-Editor: Yu HG
| 1. | El-Serag HB, Rudolph KL. Hepatocellular carcinoma: epidemiology and molecular carcinogenesis. Gastroenterology. 2007;132:2557-2576. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3846] [Cited by in RCA: 4267] [Article Influence: 237.1] [Reference Citation Analysis (2)] |
| 2. | Chen CJ, Yu MW, Liaw YF. Epidemiological characteristics and risk factors of hepatocellular carcinoma. J Gastroenterol Hepatol. 1997;12:S294-S308. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 341] [Cited by in RCA: 338] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 3. | Chen CJ, Tai J, Tai DI. Hepatocellular carcinoma occurred in a Hepatitis B carrier clinic cohort during a mean follow up of 10 years. Hepatoma Res. 2019;5:25. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 4. | Kansagara D, Papak J, Pasha AS, O'Neil M, Freeman M, Relevo R, Quiñones A, Motu'apuaka M, Jou JH. Screening for hepatocellular carcinoma in chronic liver disease: a systematic review. Ann Intern Med. 2014;161:261-269. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 188] [Cited by in RCA: 169] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 5. | Masuzaki R, Tateishi R, Yoshida H, Goto E, Sato T, Ohki T, Imamura J, Goto T, Kanai F, Kato N, Ikeda H, Shiina S, Kawabe T, Omata M. Prospective risk assessment for hepatocellular carcinoma development in patients with chronic hepatitis C by transient elastography. Hepatology. 2009;49:1954-1961. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 294] [Cited by in RCA: 315] [Article Influence: 19.7] [Reference Citation Analysis (0)] |
| 6. | Wong GL, Chan HL, Wong CK, Leung C, Chan CY, Ho PP, Chung VC, Chan ZC, Tse YK, Chim AM, Lau TK, Wong VW. Liver stiffness-based optimization of hepatocellular carcinoma risk score in patients with chronic hepatitis B. J Hepatol. 2014;60:339-345. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 187] [Cited by in RCA: 208] [Article Influence: 18.9] [Reference Citation Analysis (0)] |
| 7. | Jung KS, Kim SU, Song K, Park JY, Kim DY, Ahn SH, Kim BK, Han KH. Validation of hepatitis B virus-related hepatocellular carcinoma prediction models in the era of antiviral therapy. Hepatology. 2015;62:1757-1766. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 93] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 8. | Jeon MY, Lee HW, Kim SU, Heo JY, Han S, Kim BK, Park JY, Kim DY, Ahn SH, Han KH. Subcirrhotic liver stiffness by FibroScan correlates with lower risk of hepatocellular carcinoma in patients with HBV-related cirrhosis. Hepatol Int. 2017;11:268-276. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 18] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 9. | Chon HY, Lee HA, Suh SJ, Lee JI, Kim BS, Kim IH, Lee CH, Jang BK, Lee HW, Hwang JS, Lee JW, Yu JH, Seo YS, Yim HJ, Kim SU; Korean Transient Elastography Study Group. Addition of liver stiffness enhances the predictive accuracy of the PAGE-B model for hepatitis B-related hepatocellular carcinoma. Aliment Pharmacol Ther. 2021;53:919-927. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 10. | Nierhoff J, Chávez Ortiz AA, Herrmann E, Zeuzem S, Friedrich-Rust M. The efficiency of acoustic radiation force impulse imaging for the staging of liver fibrosis: a meta-analysis. Eur Radiol. 2013;23:3040-3053. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 141] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 11. | Gerber L, Kasper D, Fitting D, Knop V, Vermehren A, Sprinzl K, Hansmann ML, Herrmann E, Bojunga J, Albert J, Sarrazin C, Zeuzem S, Friedrich-Rust M. Assessment of liver fibrosis with 2-D shear wave elastography in comparison to transient elastography and acoustic radiation force impulse imaging in patients with chronic liver disease. Ultrasound Med Biol. 2015;41:2350-2359. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 78] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 12. | Tai DI, Tsay PK, Jeng WJ, Weng CC, Huang SF, Huang CH, Lin SM, Chiu CT, Chen WT, Wan YL. Differences in liver fibrosis between patients with chronic hepatitis B and C: evaluation by acoustic radiation force impulse measurements at 2 locations. J Ultrasound Med. 2015;34:813-821. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 21] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 13. | Hsu TH, Tsui PH, Yu WT, Huang SF, Tai J, Wan YL, Tai DI. Cutoff Values of Acoustic Radiation Force Impulse Two-Location Measurements in Different Etiologies of Liver Fibrosis. J Med Ultrasound. 2019;27:130-134. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 14. | Vermehren J, Polta A, Zimmermann O, Herrmann E, Poynard T, Hofmann WP, Bojunga J, Sarrazin C, Zeuzem S, Friedrich-Rust M. Comparison of acoustic radiation force impulse imaging with transient elastography for the detection of complications in patients with cirrhosis. Liver Int. 2012;32:852-858. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 64] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 15. | Chu CM, Liaw YF. Natural history of chronic hepatitis B virus infection: an immunopathological study. J Gastroenterol Hepatol. 1997;12:S218-S222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 64] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 16. | Tai DI, Jeng WJ, Lin CY. A global perspective on hepatitis B-related single nucleotide polymorphisms and evolution during human migration. Hepatol Commun. 2017;1:1005-1013. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 17. | Tai DI, Chen CH, Chang TT, Chen SC, Liao LY, Kuo CH, Chen YY, Chen GH, Yang SS, Tang HS, Lin HH, Lin DY, Lo SK, Du JM, Lin KC, Changchien CS, Chang WY, Sheu JC, Liaw YF, Chen DS, Sung JL. Eight-year nationwide survival analysis in relatives of patients with hepatocellular carcinoma: role of viral infection. J Gastroenterol Hepatol. 2002;17:682-689. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 19] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 18. | Yang HI, Yuen MF, Chan HL, Han KH, Chen PJ, Kim DY, Ahn SH, Chen CJ, Wong VW, Seto WK; REACH-B Working Group. Risk estimation for hepatocellular carcinoma in chronic hepatitis B (REACH-B): development and validation of a predictive score. Lancet Oncol. 2011;12:568-574. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 431] [Cited by in RCA: 521] [Article Influence: 37.2] [Reference Citation Analysis (0)] |
| 19. | Li B, Tai DI, Yan K, Chen YC, Chen CJ, Huang SF, Hsu TH, Yu WT, Xiao J, Le L, Harrison AP. Accurate and generalizable quantitative scoring of liver steatosis from ultrasound images via scalable deep learning. World J Gastroenterol. 2022;28:2494-2508. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 21] [Cited by in RCA: 23] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 20. | Dietrich CF, Bamber J, Berzigotti A, Bota S, Cantisani V, Castera L, Cosgrove D, Ferraioli G, Friedrich-Rust M, Gilja OH, Goertz RS, Karlas T, de Knegt R, de Ledinghen V, Piscaglia F, Procopet B, Saftoiu A, Sidhu PS, Sporea I, Thiele M. EFSUMB Guidelines and Recommendations on the Clinical Use of Liver Ultrasound Elastography, Update 2017 (Long Version). Ultraschall Med. 2017;38:e16-e47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 601] [Cited by in RCA: 584] [Article Influence: 73.0] [Reference Citation Analysis (0)] |
| 21. | Marasco G, Colecchia A, Silva G, Rossini B, Eusebi LH, Ravaioli F, Dajti E, Alemanni LV, Colecchia L, Renzulli M, Golfieri R, Festi D. Non-invasive tests for the prediction of primary hepatocellular carcinoma. World J Gastroenterol. 2020;26:3326-3343. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 31] [Cited by in RCA: 28] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 22. | Chou CT, Chen RC, Wu WP, Lin PY, Chen YL. Prospective Comparison of the Diagnostic Performance of Magnetic Resonance Elastography with Acoustic Radiation Force Impulse Elastography for Pre-operative Staging of Hepatic Fibrosis in Patients with Hepatocellular Carcinoma. Ultrasound Med Biol. 2017;43:2783-2790. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 13] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 23. | Sun XL, Yao H, Men Q, Hou KZ, Chen Z, Xu CQ, Liang LW. Combination of acoustic radiation force impulse imaging, serological indexes and contrast-enhanced ultrasound for diagnosis of liver lesions. World J Gastroenterol. 2017;23:5602-5609. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 6] [Cited by in RCA: 7] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 24. | Zhang Y, Chen H, Chen S, Li W. Prognostic value of liver stiffness measurement in patients with hepatocellular carcinoma (HCC) treated by radiofrequency ablation: a meta-analysis. Int J Hyperthermia. 2021;38:1052-1059. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 25. | Aoki T, Iijima H, Tada T, Kumada T, Nishimura T, Nakano C, Kishino K, Shimono Y, Yoh K, Takata R, Ishii A, Takashima T, Sakai Y, Aizawa N, Nishikawa H, Ikeda N, Iwata Y, Enomoto H, Hirota S, Fujimoto J, Nishiguchi S. Prediction of development of hepatocellular carcinoma using a new scoring system involving virtual touch quantification in patients with chronic liver diseases. J Gastroenterol. 2017;52:104-112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 23] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 26. | Chen CL, Yang HI, Yang WS, Liu CJ, Chen PJ, You SL, Wang LY, Sun CA, Lu SN, Chen DS, Chen CJ. Metabolic factors and risk of hepatocellular carcinoma by chronic hepatitis B/C infection: a follow-up study in Taiwan. Gastroenterology. 2008;135:111-121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 386] [Cited by in RCA: 423] [Article Influence: 24.9] [Reference Citation Analysis (0)] |
| 27. | Mantovani A, Petracca G, Beatrice G, Csermely A, Tilg H, Byrne CD, Targher G. Non-alcoholic fatty liver disease and increased risk of incident extrahepatic cancers: a meta-analysis of observational cohort studies. Gut. 2022;71:778-788. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 208] [Article Influence: 69.3] [Reference Citation Analysis (2)] |
| 28. | Jan CF, Chen CJ, Chiu YH, Chen LS, Wu HM, Huang CC, Yen MF, Chen TH. A population-based study investigating the association between metabolic syndrome and hepatitis B/C infection (Keelung Community-based Integrated Screening study No. 10). Int J Obes (Lond). 2006;30:794-799. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 106] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 29. | Kuo CS, Chen YT, Hsu CY, Chang CC, Chou RH, Li SY, Kuo SC, Huang PH, Chen JW, Lin SJ. The impact of chronic hepatitis B infection on major adverse cardiovascular events and all-cause mortality in patients with diabetes: a nationwide population-based study from Taiwan. BMJ Open. 2017;7:e016179. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 14] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 30. | Sung J, Song YM, Choi YH, Ebrahim S, Davey Smith G. Hepatitis B virus seropositivity and the risk of stroke and myocardial infarction. Stroke. 2007;38:1436-1441. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 48] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 31. | Wang CC, Cheng PN, Kao JH. Systematic review: chronic viral hepatitis and metabolic derangement. Aliment Pharmacol Ther. 2020;51:216-230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 81] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 32. | Luan J, Ju D. Inflammasome: A Double-Edged Sword in Liver Diseases. Front Immunol. 2018;9:2201. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 75] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 33. | Chu CM, Karayiannis P, Fowler MJ, Monjardino J, Liaw YF, Thomas HC. Natural history of chronic hepatitis B virus infection in Taiwan: studies of hepatitis B virus DNA in serum. Hepatology. 1985;5:431-434. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 276] [Cited by in RCA: 260] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 34. | Chang ML, Liaw YF. Hepatitis B flares in chronic hepatitis B: pathogenesis, natural course, and management. J Hepatol. 2014;61:1407-1417. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 201] [Cited by in RCA: 232] [Article Influence: 21.1] [Reference Citation Analysis (0)] |
| 35. | Chang SW, Fann CS, Su WH, Wang YC, Weng CC, Yu CJ, Hsu CL, Hsieh AR, Chien RN, Chu CM, Tai DI. A genome-wide association study on chronic HBV infection and its clinical progression in male Han-Taiwanese. PLoS One. 2014;9:e99724. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 51] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 36. | Hsieh AR, Fann CSJ, Lin HC, Tai J, Hsieh SY, Tai DI. Hepatitis B virus persistent infection-related single nucleotide polymorphisms in HLA regions are associated with viral load in hepatoma families. World J Gastroenterol. 2021;27:6262-6276. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 2] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 37. | Cabibbo G, Reig M, Celsa C, Torres F, Battaglia S, Enea M, Rizzo GEM, Petta S, Calvaruso V, Di Marco V, Craxì A, Singal AG, Bruix J, Cammà C. First-Line Immune Checkpoint Inhibitor-Based Sequential Therapies for Advanced Hepatocellular Carcinoma: Rationale for Future Trials. Liver Cancer. 2022;11:75-84. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 37] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 38. | Rizzo A, Ricci AD, Di Federico A, Frega G, Palloni A, Tavolari S, Brandi G. Predictive Biomarkers for Checkpoint Inhibitor-Based Immunotherapy in Hepatocellular Carcinoma: Where Do We Stand? Front Oncol. 2021;11:803133. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 88] [Article Influence: 29.3] [Reference Citation Analysis (0)] |