Published online Apr 7, 2023. doi: 10.3748/wjg.v29.i13.2034
Peer-review started: December 13, 2022
First decision: January 11, 2023
Revised: January 21, 2023
Accepted: March 20, 2023
Article in press: March 20, 2023
Published online: April 7, 2023
Processing time: 114 Days and 21.2 Hours
Sepsis exacerbates intestinal microecological disorders leading to poor prognosis. Proper modalities of nutritional support can improve nutrition, immunity, and intestinal microecology.
To identify the optimal modality of early nutritional support for patients with sepsis from the perspective of intestinal microecology.
Thirty patients with sepsis admitted to the intensive care unit of the General Hospital of Ningxia Medical University, China, between 2019 and 2021 with indications for nutritional support, were randomly assigned to one of three different modalities of nutritional support for a total of 5 d: Total enteral nutrition (TEN group), total parenteral nutrition (TPN group), and supplemental parenteral nutrition (SPN group). Blood and stool specimens were collected before and after nutritional support, and changes in gut microbiota, short-chain fatty acids (SCFAs), and immune and nutritional indicators were detected and compared among the three groups.
In comparison with before nutritional support, the three groups after nutritional support presented: (1) Differences in the gut bacteria (Enterococcus increased in the TEN group, Campylobacter decreased in the TPN group, and Dialister decreased in the SPN group; all P < 0.05); (2) different trends in SCFAs (the TEN group showed improvement except for Caproic acid, the TPN group showed improvement only for acetic and propionic acid, and the SPN group showed a decreasing trend); (3) significant improvement of the nutritional and immunological indicators in the TEN and SPN groups, while only immunoglobulin G improved in the TPN group (all P < 0.05); and (4) a significant correlation was found between the gut bacteria, SCFAs, and nutritional and immunological indicators (all P < 0.05).
TEN is recommended as the preferred mode of early nutritional support in sepsis based on clinical nutritional and immunological indicators, as well as changes in intestinal microecology.
Core Tip: Nutritional support is an important component of treatment for sepsis, and an appropriate modality of nutritional support can improve patient nutrition, immunity, and intestinal microecology. We applied different nutritional modalities for early and short-duration nutritional support in patients with sepsis and found differences in intestinal bacterial composition, short-chain fatty acids, and nutritional and immune indicators. We concluded that total enteral nutrition is a good modality of early nutritional support for sepsis. These findings provide a new perspective for optimizing nutritional support modalities in sepsis.
- Citation: Yang XJ, Wang XH, Yang MY, Ren HY, Chen H, Zhang XY, Liu QF, Yang G, Yang Y, Yang XJ. Exploring choices of early nutritional support for patients with sepsis based on changes in intestinal microecology. World J Gastroenterol 2023; 29(13): 2034-2049
- URL: https://www.wjgnet.com/1007-9327/full/v29/i13/2034.htm
- DOI: https://dx.doi.org/10.3748/wjg.v29.i13.2034
Sepsis is a life-threatening organ dysfunction resulting from a dysregulated response of the body to infection[1]. Sepsis affects nearly 50 million people each year worldwide and accounts for approximately 11 million deaths, corresponding to approximately 20% of all deaths worldwide[2]. The high morbidity and mortality rates associated with sepsis, as well as the difficulties with early detection and treatment, have become challenging healthcare issues.
Our previous study confirmed the presence of gut microbiota disorders in patients with sepsis[3], and the interaction of disease and gut microbiota disorders has been shown to lead to clinical deterioration and the development of multiple organ dysfunction (MODS)[4]. Therefore, the treatment of sepsis requires consideration of the range of its effects on gut microbiota, metabolism, nutrition, and immunity. Nutritional support is an important component of sepsis treatment; however, its focus is still limited to the amount of energy provided, the choice of energy supply routes, the placement of feeding tubes, and nitrogen balance. The clinical recommendations about the choice of nutritional support modalities are largely based on expert consensus, although the supporting evidence is of low level[5,6]. Nutritional support has a significant impact on intestinal microecology; however, the modalities of nutritional support in sepsis have not been evaluated from the perspective of intestinal microecology.
In this context, we used different modalities of nutritional support: Total enteral nutrition (TEN), total parenteral nutrition (TPN), and supplemental parenteral nutrition (SPN) to group patients with sepsis. Moreover, we explored the choices of nutritional modalities for sepsis from the perspective of systemic immunity and nutritional indicators, the degree of their interrelationships, and the effects of the three nutritional modalities on the intestine microecology [including gut microbiota, short-chain fatty acid (SCFA) metabolism]. In this study, 16S rRNA gene sequencing and GC-MS metabolomics were used to detect gut microbiota and SCFAs. The exploration of early nutritional support in patients with sepsis from the perspective of intestinal microecology not only provides a new fundamental perspective for optimizing nutritional support in sepsis but also has far-reaching implications for improving nutritional support.
A randomized clinical trial involving 30 patients with sepsis admitted to the Department of Critical Care Medicine at the General Hospital of Ningxia Medical University (a Grade 3A hospital) between October 1, 2019, and February 1, 2021, was conducted. The inclusion criteria were as follows: (1) Age between 18 and 75 years; (2) meeting the criteria of the latest definition of sepsis of the American Society of Critical Care Medicine "Sepsis-3.0" from 2016[1]; (3) mechanically ventilated patients; and (4) indications for nutritional support [hemodynamic stability (no dose or small dose of vasoactive drugs to maintain vital signs and lactate ≤ 2 mmol/L) and NUTRIC score (Nutritional Risk Rating Instrument for Critically Ill Patients) ≥ 6]. The following exclusion criteria applied: (1) Patients with chest wall, thoracic, or airway injuries, respiratory instability, ventilator parameters requiring adjustment during measurement, or rapid respiratory rate ≥ 35 breaths/min, or oxygen concentration > 60%, or positive end-expiratory pressure ≥ 10 cmH2O; (2) patients with open injuries to the gastrointestinal tract or abdominal cavity; (3) patients with severe acid-base balance or electrolyte metabolism disturbances; and (4) patients whose family members did not provide informed consent to participate in the trial. Informed consent was obtained from the patients or their immediate family members. This study was approved by the Ethics Committee of the Hospital (approval number: 2017-266).
Patients with sepsis included in this study were divided into three groups using a random number table and a random number remainder grouping method. Nutritional support was administered to the patients via the following different modalities based on the order of randomization: (1) TEN group (n = 10): Total enteral nutritional support via a gastric tube or jejunal tube feeding; (2) TPN group (n = 10): Total parenteral nutrition via the internal jugular vein; and (3) SPN group (n = 10): Trophic enteral nutrition via a gastric tube or jejunal tube feeding + SPN via the internal jugular vein, in which trophic enteral is 10–20 kcal/h, not more than 500 kcal in 24 h, and the remaining energy is supplemented by parenteral nutrition[6]. The amount of nutritional support provided to the patients in the three groups was based on the resting energy expenditure (REE) measured by indirect calorimetry in the ventilation circuit; the energy metabolism test (metabolic cart) used in this study was Vmax Encore 229, Medical Graphics, United States, 2015. The selected SPN enteral preparation for enteral nutrition solution was Enteral Nutritional Suspension (SP, 500 mL/500 kcal, Nutricia Pharmaceutical Co., Ltd.). The selected TEN enteral preparation for enteral nutrition solution, due to energy supply and fluid volume regulation, was Enteral Nutritional Emulsion (TPF, total energy 500 mL/750 kcal, Nutricia Pharmaceutical Co., Ltd.), combined or not with SP. Patients who received nutritional support for less than 5 d during the nutritional support period because of changes in patient condition (discharge from hospital after improvement in condition or inability to continue with nutritional support owing to deterioration in condition) were regarded as invalid observation cases and excluded from the study. Subsequently, the next randomized patient was administered nutritional support using the same modality used for the invalid case. Patient age, sex, underlying disease, NUTRIC score, Acute Physiology and Chronic Health Evaluation (APACHE II) score, sequential organ failure assessment (SOFA) score, and general information such as REE during 24 h, were recorded. Before day 1 and on day 5 of nutritional support, stool specimens were collected for 16S rRNA gene sequencing and the analysis of SCFA metabolism and venous blood specimens were collected for lymphocyte subpopulation analysis, immunoglobulin + complement analysis, and complete set of nutritional tests.
Stool specimens were collected from enrolled patients with sepsis on days 1 and 5 of nutritional support. Specimens were collected from the bottom layers of fresh stools of the patients with a sampling spatula after spontaneous defecation or a warm saline enema. The stool specimens were quickly placed in stool specimen boxes, which were enveloped, labeled, and stored in liquid nitrogen tanks and transferred to a -80 °C refrigerator for freezing.
Each stool sample was spiked with 790 µL of lysis solution [4M guanidine thiocyanate, 250 µL; 10% N-lauroyl sarcosine, 40 µL; 5% N-lauroyl sarcosine-0.1 M phosphate buffer (pH 8.0), 500 µL], followed by a vigorous vortex mixing, and then incubated at 70 °C for 1 h. At the end of the incubation, bead-beating with glass beads (0.1 mm, 500–750 µL) was performed for 10 min (25HZ/S); subsequent extraction was performed according to the instructions of the extraction kit (E.Z.N.A.® Stool DNA Kit). The concentration of extracted DNA was measured using NanoDrop (Thermo Scientific). The V3-V4 region of the 16S rRNA gene was amplified by primers 341F/805R (341F: 5′-CCTACGGGNGGCWGCAG-3′; 805R: 5′-GACTACHVGGGTATCTAATCC-3′), and the PCR products were sequenced using an Illumina Miseq 2 × 300 bp platform.
Standard configuration: Appropriate amounts of pure acetic acid, propionic acid, butyric acid, isobutyric acid, valeric acid, isovaleric acid, and caproic acid were used to prepare 10 mixed concentration gradients (0.02 μg/mL, 0.1 μg/mL, 0.5 μg/mL, 2 μg/mL, 10 μg/mL, 25 μg/mL, 50 μg/mL, 100 μg/mL, 250 μg/mL, and 500 μg/mL). The master mixes and working standard solutions were stored at 0 °C.
Sample pre-treatment: Appropriate amount of sample was added along with 50 μL of 15% phosphoric acid, 100 μL of 125 μg/mL of internal standard (isocaproic acid), and 400 μL of ether. The solution was then homogenized for 1 min, centrifuged at 4 °C for 10 min at 12000 rpm, and the supernatant was collected for testing.
GC-MS detection method: The chromatographic conditions were as follows: Agilent HP-INNOWAX capillary column (30 m × 0.25 mm ID × 0.25 μm); split injection, injection volume 1 μL, split ratio 10 : 1; inlet temperature 250 °C; ion source temperature (eng) 230 °C; transmission line temperature 250 °C, quadrupole temperature 150 °C. The temperature ramp-up time was programmed to start at 90 °C, ramping up to 120 °C at 10 °C/min, to 150 °C at 5 °C/min, and ultimately reaching 250 °C at 25 °C/min for 2 min. The carrier gas was helium at a flow rate of 1.0 mL/min.
Lymphocyte subpopulation analysis: Flow cytometry method, contained T lymphocytes (CD3+), B lymphocytes (CD3- CD19+), and NK lymphocytes (natural killer cells, CD3- CD16+ and/or CD56+); T cells were subdivided into helper T cells (CD3+ CD4+) and suppressor T cells (CD3+ CD8+). The DxFLEX flow cytometry (lymphocyte division) analyzer (Beckman Coulter Ltd., United States) and lymphocyte assay kit (Beckman Coulter Ltd., United States) were used.
Immunoglobulin + complement, nutritional panel (scattering and turbidimetric method): The nutritional panel contained albumin (ALB), prealbumin (PAB), and transferrin; immunoglobulin and the complement tests contained immunoglobulin A (IgA), immunoglobulin G (IgG), immunoglobulin M (IgM), C3, and C4. BN II analyzer (SIEMENS GmbH, Germany). The IgM assay kit as well as the nutritional complete assay kit (SIEMENS Healthcare Diagnostics GmbH, Germany) were used.
Clinical data were processed and analyzed using the Statistical Package for Social Sciences version 19.0 (IBM, Armonk, New York), and the data were first tested for normality and variance. Normally dis
Raw sequencing data were processed using USEARCH (version 11.0.667) and operational taxonomic units (OTUs) were classified based on 97% sequence similarity, and representative OTU sequences were compared with the SILVA database (SSU138, http://www.arb-silva.de) to obtain the taxonomic classification of each 16S rDNA sequence. The α-diversity of each sample was assessed using Ace, Chao 1, Shannon-Wiener diversity index, and Simpson diversity index, and differences between the groups were tested using the non-parametric Mann–Whitney U test or Kruskal–Wallis rank sum test. Principal coordinates analysis and Linear discriminant analysis Effect Size analysis were performed and heat maps were constructed using R statistical software. Spearman correlation analysis was performed on gut microbiota, SCFA metabolism, and clinical nutritional and immunological indicators of the patients with sepsis treated with different modalities of nutritional support for 5 d.
Patients with sepsis were grouped into TEN, TPN, and SPN groups according to different nutritional modalities, and basic patient data were compared (Table 1). There were no significant differences between the groups in terms of sex, age, underlying disease, and body mass index (P > 0.05). The APACHE II and SOFA scores were compared among the three groups, and no significant differences were observed in the degree of severity of sepsis (P > 0.05). Furthermore, the NUTRIC score was ≥ 6 in all three groups, and no significant differences were observed (P > 0.05). The daily REE of patients with sepsis obtained by indirect calorimetry performed by the ventilation circuit was used as the set amount for nutritional support, and no significant difference was observed among the three groups in the 24-h REE (P > 0.05). General information were balanced and comparable between the groups.
| Indicator | TEN (n = 10) | TPN (n = 10) | SPN (n = 10) | H/F/χ2/Z/t value | P value |
| Age (yr, mean ± SD) | 59.20 ± 12.52 | 54.30 ± 15.10 | 59.30 ± 16.33 | 0.46 | 0.63 |
| Sex | |||||
| Male (case, %) | 8 (80) | 8 (80) | 8 (80) | 0.00 | 1.00 |
| Female (case, %) | 2 (20) | 2 (20) | 2 (20) | ||
| Underlying disease | |||||
| Hypertension (case, %) | 4 (40) | 3 (30) | 5 (50) | 0.83 | 0.65 |
| Diabetes (case, %) | 2 (20) | 4 (40) | 2 (20) | 1.36 | 0.50 |
| Malignant tumor (case, %) | 0 (0) | 2 (20) | 2 (20) | 2.30 | 0.31 |
| APACHE II score on day 1 (points, mean ± SD) | 14.10 ± 2.84 | 15.30 ± 4.73 | 19.50 ± 7.38 | 2.12 | 0.14 |
| SOFA score on day 1 (points, mean ± SD) | 5.40 ± 1.51 | 9.60 ± 1.95 | 10.20 ± 4.10 | 5.53 | 0.10 |
| BMI (kg/m2, mean ± SD) | 23.90 ± 2.79 | 26.84 ± 3.89 | 26.05 ± 2.67 | 1.90 | 0.17 |
| NUTRIC score on day 1 | |||||
| ≥ 6 points (case, %) | 3 (30) | 6 (60) | 6 (60) | 2.40 | 0.30 |
| < 6 points (case, %) | 7 (70) | 4 (40) | 4 (40) | ||
| REE on day 1 [kcal, M (P25, P75)] | 1950.00 (1759.28, 2050.72) | 1900 (1726.40, 2073.59) | 2036 (1882.46, 2189.53) | 2.49 | 0.28 |
No significant improvement in α-diversity and β-diversity of gut microbiota was observed in the three groups, before and after nutritional support (Supplementary Figures 1 and 2) (P > 0.05).
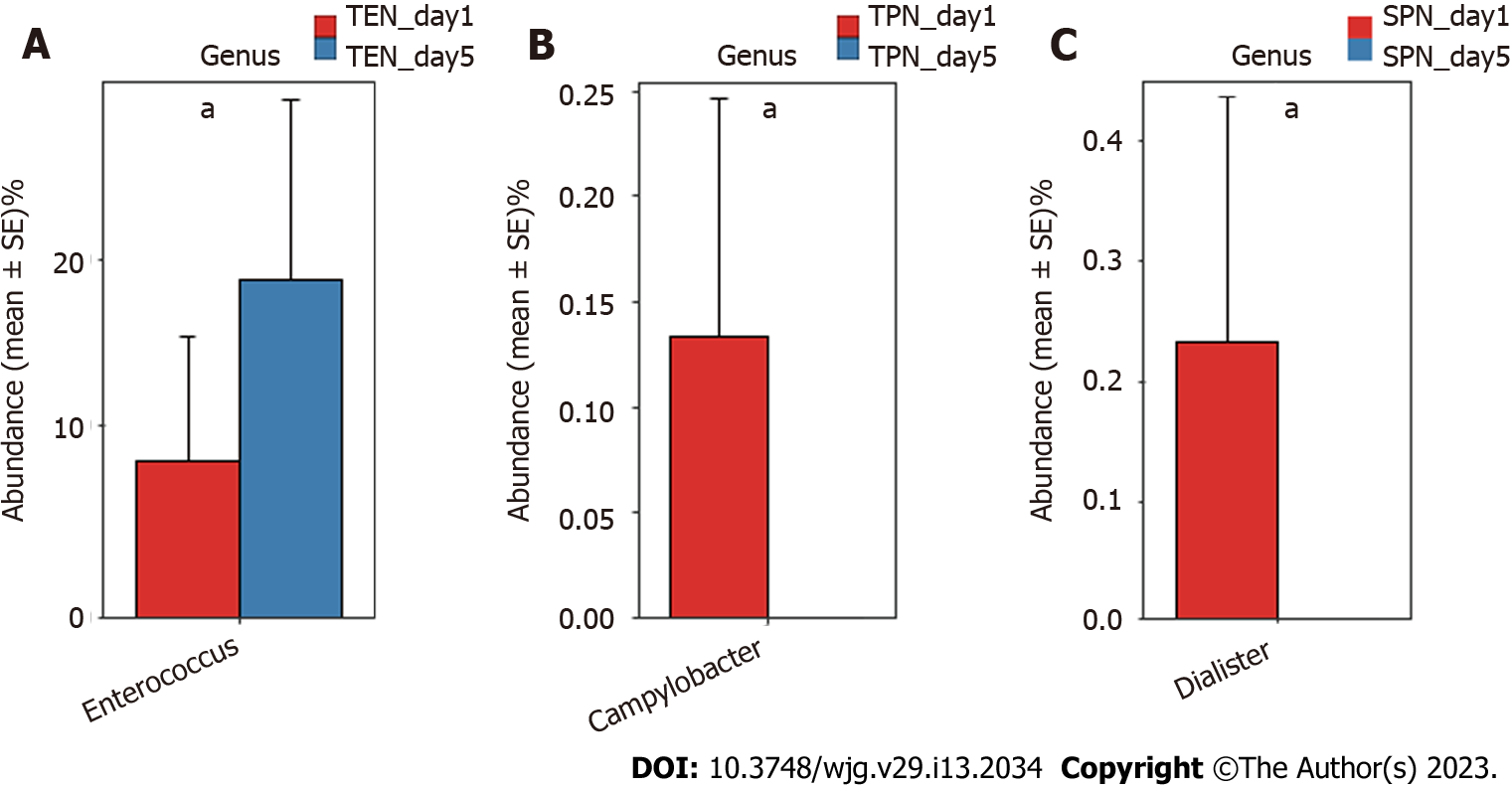
Comparison of gut microbiota composition at the genus and OTU levels: The Mann-Whitney U test was separately performed in each group to compare the genera of the gut microbiota before and after nutritional support. Results indicated that compared with the gut microbiota of patients with sepsis before nutritional support, significant changes occurred in some genera of the gut microbiota after 5 d of nutritional support. Specifically, Enterococcus was significantly higher in the TEN group after nutritional support (P < 0.05) (Figure 1A); Campylobacter was significantly lower in the TPN group after nutritional support (P < 0.05) (Figure 1B); and Dialister was significantly lower in the SPN group after nutritional support (P < 0.05) (Figure 1C).
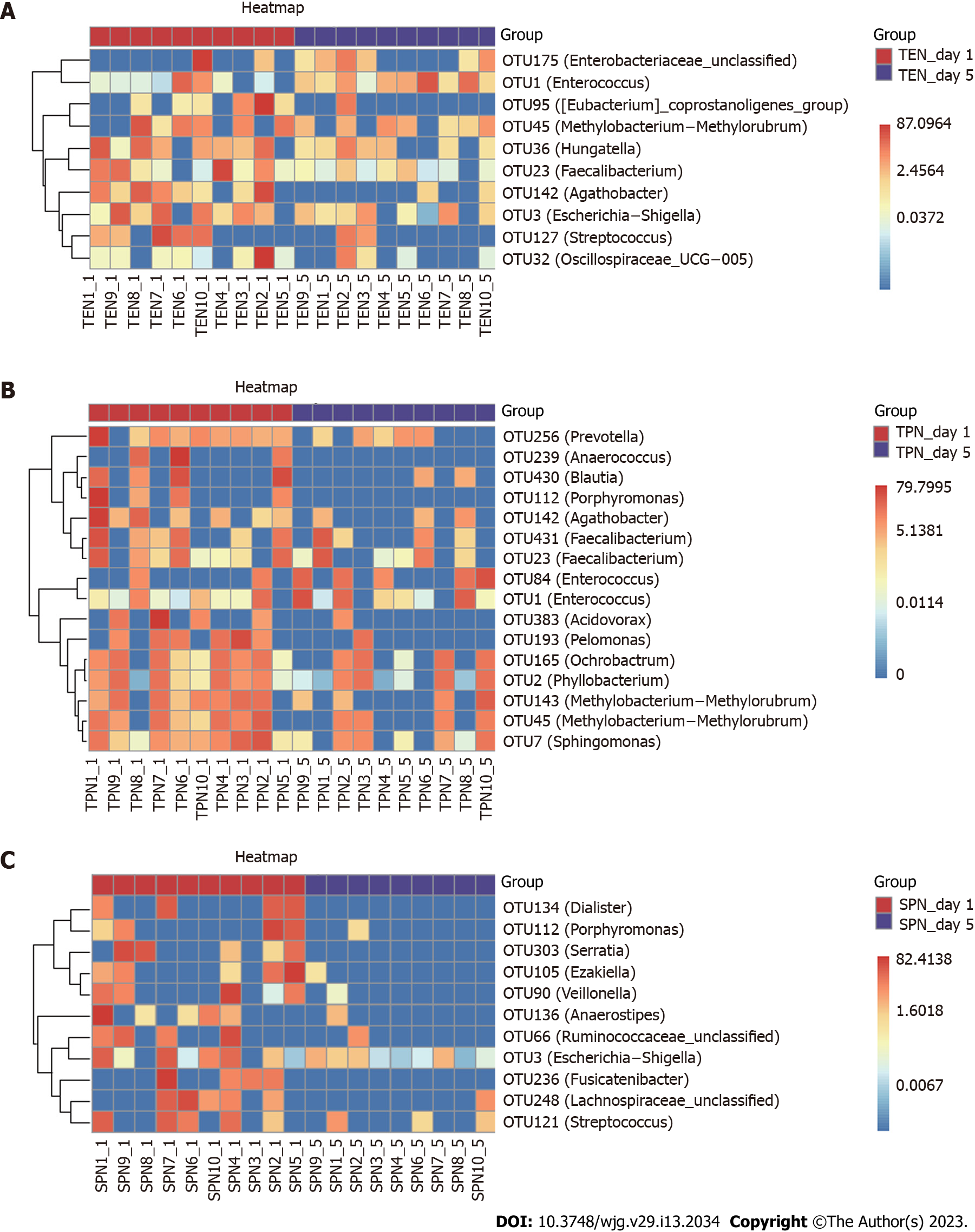
A random forest approach was used to compare the OTU level of the three groups, and changes in key bacterial OTUs before and after nutritional support were identified. After nutritional support in the TEN group, we obtained a significant reduction in the relative abundance of 10 OTUs, including those belonging to the genera Streptococcus, Methylobacterium-Methylorubrum, Oscillospiraceae UCG-005, Faecalibacterium, Escherichia-Shigella, Eubacterium coprostanoligenes group, Agathobacter, and Hungatella. Furthermore, the relative abundance of one OTU belonging to the genus Enterococcus and one unclassified OTU belonging to the Enterobacteriaceae family significantly increased (Figure 2A). After nutritional support in the TPN group, we found a significant reduction in the relative abundance of 16 OTUs, including those belonging to the genera Anaerococcus, Prevotella, Porphyromonas, Methylobacterium-Methylorubrum (2 OTUs), Faecalibacterium (2 OTUs), Ochrobactrum, Sphingo
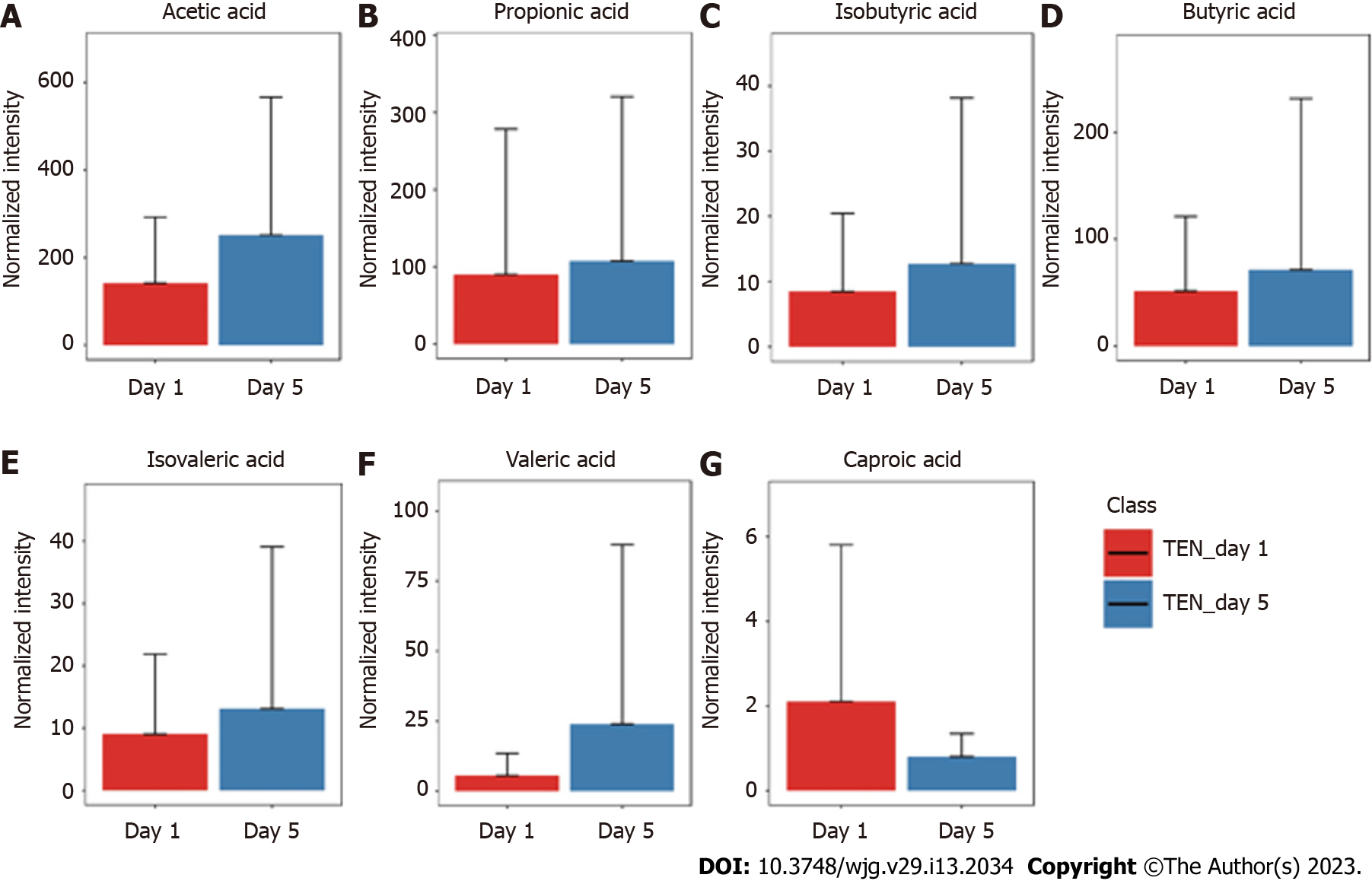
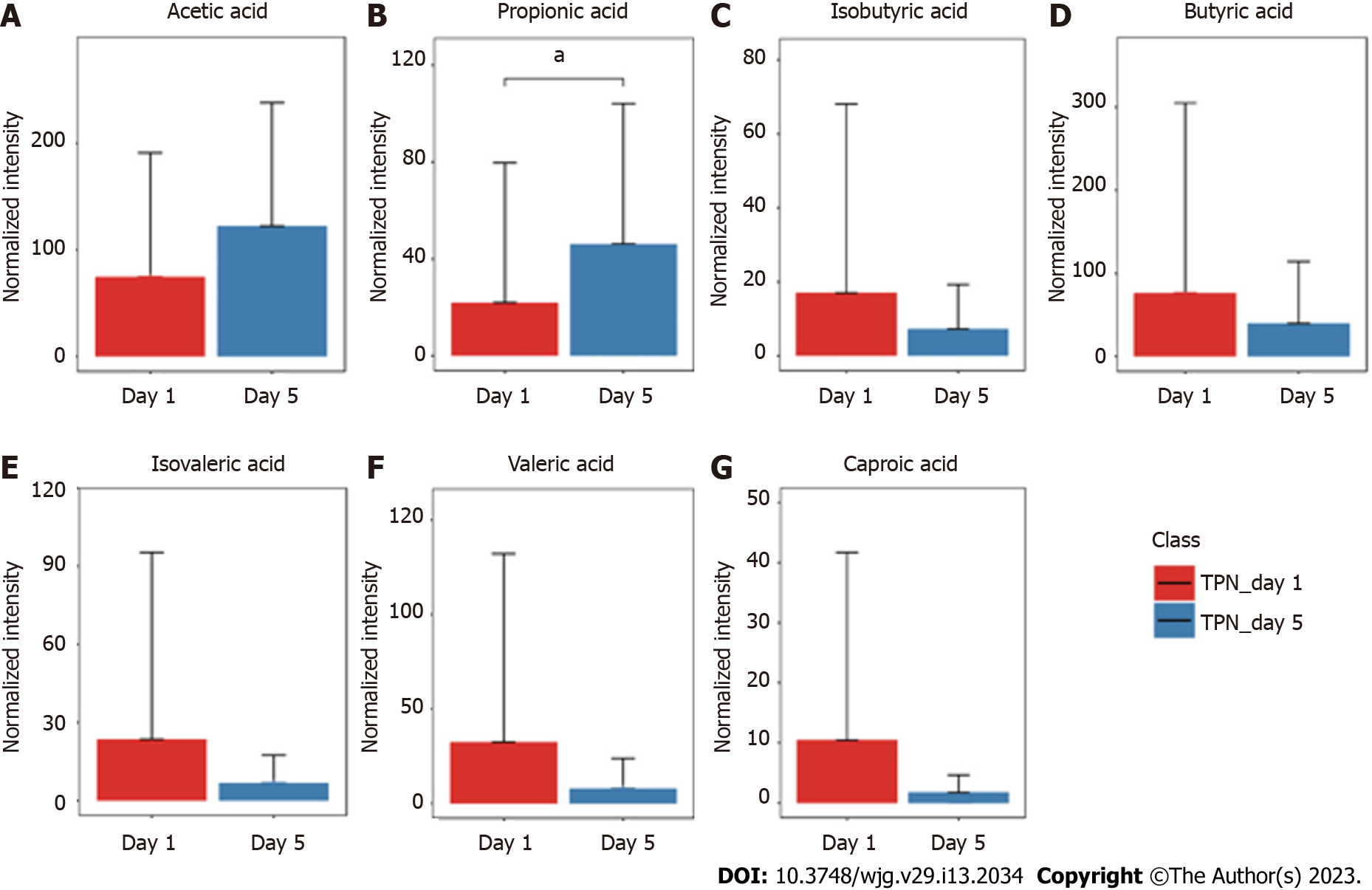
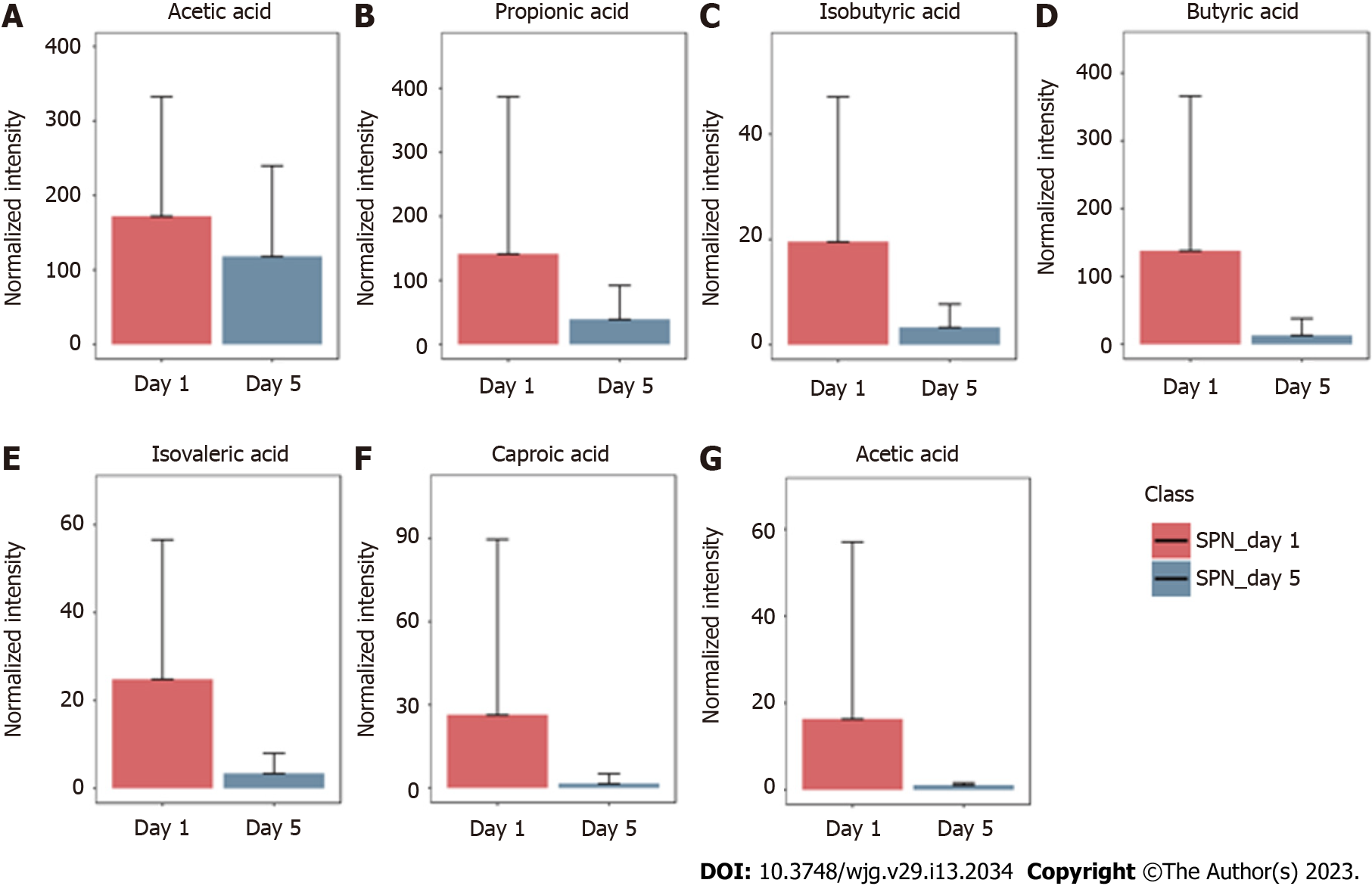
The GC-MS assay was used to detect fecal SCFAs before and after nutritional support in the three groups. The changes in the SCFAs before and after nutrition in the TEN (Figure 3), TPN (Figure 4), and SPN (Figure 5) groups were compared and all SCFAs in the TEN group showed an increasing trend except for caproic acid. Acetic and propionic acid in the TPN group showed an increasing trend, while all SCFAs in the SPN group showed a decreasing trend. However, no significant difference (P > 0.05) was observed in the changes of SCFAs by nutritional support pathways in patients with sepsis, both before and after group nutrition and between groups.
Paired sample t-test and non-parametric test for correlated samples were used to compare the immune and nutritional indicators of the three groups before and after nutrition. The results of these analyses are shown in Table 2. After nutritional support in the TEN group, CD3+ T cells, CD3+/CD4+ T cells, IgG, complement C3, PAB, and ALB levels significantly increased, while CD3- CD19+ B cells significantly decreased (P ≤ 0.05). In the TPN group, only the IgG level significantly increased after nutritional support (P = 0.007), while the remaining immune and nutritional indicators did not change significantly (P > 0.05). In the SPN group, IgA, IgG, complement C3, PAB, ALB, and transferrin levels significantly increased, while CD3- CD19+ B cell levels significantly decreased after nutritional support (P ≤ 0.05).
| Indicator | TEN (n = 20) | SPN (n = 20) | TPN (n = 20) | |||||||||
| Day 1 (n = 10) | Day 5 (n = 10) | t/Z value | P value | Day 1 (n = 10) | Day 5 (n = 10) | t/Z value | P value | Day 1 (n = 10) | Day 5 (n = 10) | t/Z value | P value | |
| CD3+ T cell [%, M (P25, P75)] | 69.85 (63.62, 78.77) | 77.77 (69.40, 80.48) | 2.09 | 0.03 | 71.33 (64.42, 76.50) | 72.25 (64.59, 82.59) | 1.78 | 0.07 | 74.21 (59.82, 83.43) | 74.80 (67.23, 82.74) | 0.76 | 0.44 |
| CD3+ CD4+ T cell [%, M (P25, P75)] | 36.40 (29.47, 43.39) | 40.39 (36.96, 46.50) | 1.98 | 0.04 | 38.21 (32.22, 57.15) | 47.37 (35.61, 58.27) | 1.17 | 0.24 | 38.12 (29.23, 46.40) | 45.67 (28.13, 51.69) | 1.37 | 0.16 |
| CD19+ B cell [%, M (P25, P75)] | 17.04 (11.55, 21.27) | 14.11 (8.99, 18.03) | 1.98 | 0.04 | 16.80 (10.70, 25.14) | 9.75 (6.47, 16.59) | 2.09 | 0.03 | 14.35 (6.62, 22.89) | 12.78 (6.93, 23.61) | 1.02 | 0.30 |
| IgA (g/L mean ± SD) | 2.53 ± 1.18 | 2.93 ± 0.93 | 1.97 | 0.08 | 2.15 ± 0.83 | 2.91 ± 1.19 | 3.87 | 0.00 | 2.10 ± 1.13 | 2.45 ± 0.73 | 1.44 | 0.18 |
| IgG (g/L, mean ± SD) | 11.75 ± 3.46 | 15.19 ± 5.84 | 3.10 | 0.01 | 9.05 ± 3.78 | 13.77 ± 4.52 | 5.48 | 0.00 | 9.32 ± 3.89 | 12.15 ± 3.85 | 3.48 | 0.00 |
| IgM (g/L, mean ± SD) | 0.81 ± 0.36 | 1.11 ± 0.48 | 1.49 | 0.16 | 0.95 ± 0.44 | 1.12 ± 0.75 | 1.11 | 0.29 | 0.77 ± 3.89 | 0.89 ± 0.46 | 1.02 | 0.33 |
| C3 (g/L, mean ± SD) | 0.99 ± 0.30 | 1.21 ± 0.23 | 2.84 | 0.01 | 0.81 ± 0.21 | 1.07 ± 0.23 | 4.73 | 0.00 | 0.77 ± 0.25 | 0.88 ± 0.33 | 0.95 | 0.36 |
| C4 (g/L, mean ± SD) | 0.19 ± 0.04 | 0.22 ± 0.05 | 2.08 | 0.06 | 0.19 ± 0.07 | 0.22 ± 0.07 | 3.64 | 0.00 | 0.17 ± 0.05 | 0.21 ± 0.08 | 1.75 | 0.11 |
| Albumin (g/L, mean ± SD) | 27.14 ± 3.18 | 29.89 ± 3.61 | 3.04 | 0.01 | 27.74 ± 3.45 | 30.97 ± 2.63a | 2.68 | 0.02 | 28.10 ± 3.37 | 27.73 ± 2.64 | 0.24 | 0.81 |
| Pre-albumin (g/L, mean ± SD) | 0.10 ± 0.04 | 0.15 ± 0.07a | 3.88 | 0.00 | 0.08 ± 0.41 | 0.13 ± 0.05 | 3.42 | 0.00 | 0.10 ± 0.03 | 0.11 ± 0.03 | 0.47 | 0.64 |
| Transferrin (g/L, mean ± SD) | 0.01 ± 0.28 | 0.01 ± 0.28 | 1.52 | 0.15 | 1.16 ± 0.36 | 1.36 ± 0.51 | 2.67 | 0.02 | 1.05 ± 0.30 | 1.20 ± 0.37 | 1.11 | 0.29 |
To avoid the influence of immune and nutritional indicators of patients in each group before nutritional support on indicators after nutrition, analysis of covariance was used to compare the three groups. Post-nutritional pre-ALB was significantly higher in the TEN group than in the TPN group (P = 0.05), while ALB was significantly higher in the SPN group than in the TPN group (P = 0.019).
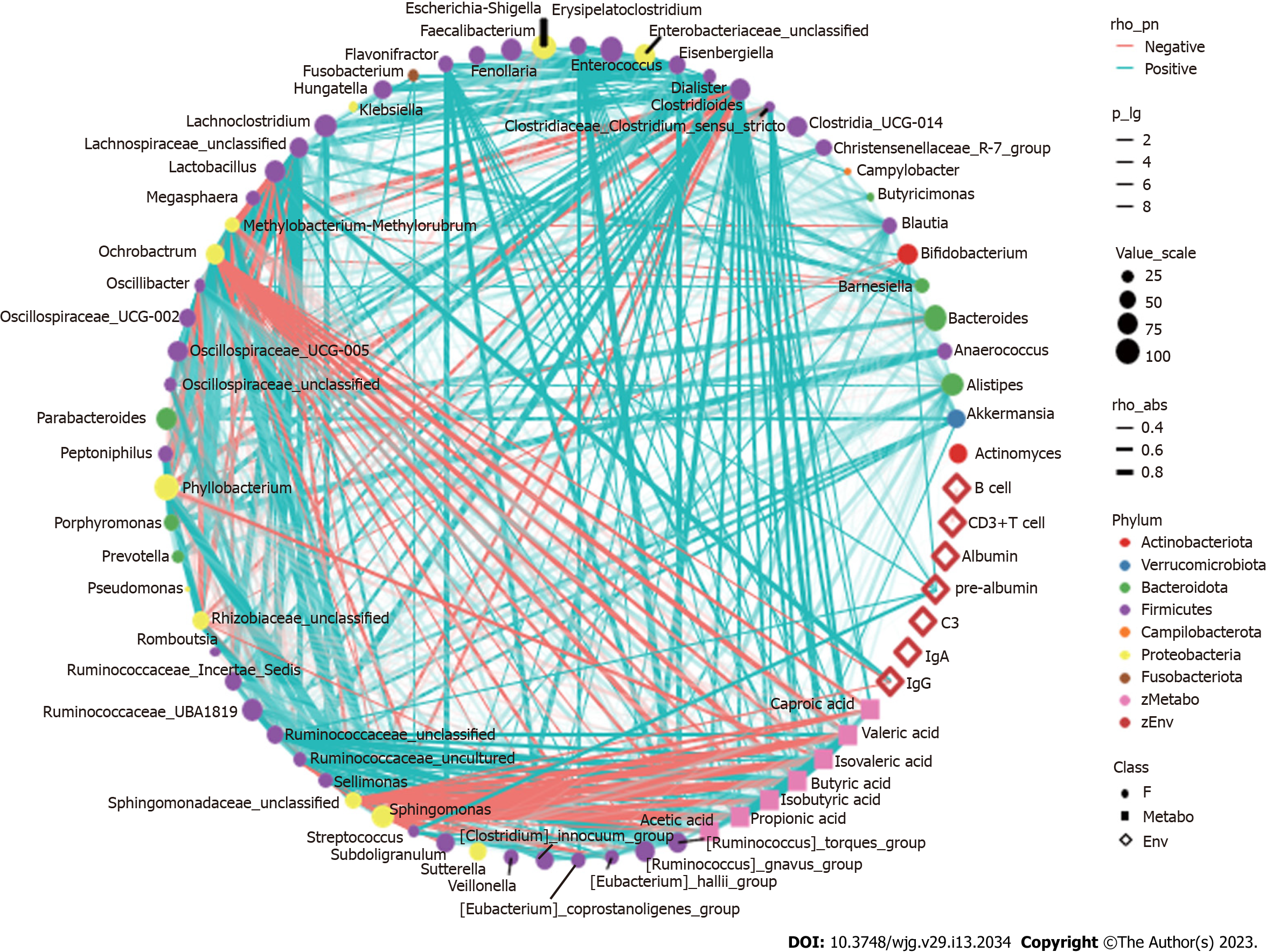
Spearman correlation analysis revealed that after nutritional support there was a correlation between the more dominant species in the gut and SCFAs and clinical immune and nutritional indicators in patients with sepsis (Figure 6). For example, Enterococcus spp., which increased in abundance in the TEN group after the nutritional intervention, showed a significant positive correlation with IgA; Streptococcus and Escherichia-Shigella spp., which decreased in abundance, showed a significant negative correlation with PAB concentrations, as well as a significant positive correlation with intestinal concentrations of multiple SCFAs. Methylobacterium spp. were significantly negatively correlated with IgG and SCFA concentrations (all P < 0.05). Anaerococcus and Porphyromonas spp., which decreased in the TPN group, showed a significant positive correlation with PAB concentrations, and Methylobacterium, Sphingomonas, and Phyllobacterium spp. showed a significant negative correlation with IgG and multiple SCFAs concentrations (all P < 0.05). Streptococcus and Escherichia-Shigella, which showed decreased abundance in the SPN group, were negatively correlated with PAB concentrations and also positively correlated with multiple SCFA concentrations; Porphyromonas and Anaerostipes were positively correlated with PAB concentrations (all P < 0.05). In addition, no significant correlations were observed between clinical nutritional immune indicators such as ALB, CD3+ T cells, CD3- CD19+ B cells, gut bacteria, and SCFAs (all P > 0.05).
By comparing the gut microbiota of septic patients, non-septic patients, and healthy controls, our previous study confirmed that septic patients undergo gut microbiota disorders, which persist for a week or longer[3]. This is consistent with the current knowledge of the characteristics of gut microbiota disorders in patients with sepsis[7,8]. Gut microbiota disorders contribute to the development of sepsis through the proliferation of pathogenic bacteria, dysregulated immune response, and reduced production of microbiota-derived metabolites, such as SCFAs. After the onset of sepsis, alterations in the structure of the normal gut microbiota can exacerbate the condition, leading to a worsening of the prognosis for sepsis, and the development of MODS[4].
Nutritional support is an important part of the treatment of critically ill patients. Diet is by far the most significant among the factors known to affect the gut microbiota[9,10]. Studies have found that diet under normal physiological conditions can affect the gut flora in the short-term, rapidly, and significantly[11]. In this study, we found that nutritional support for 5 d using different modalities of nutritional support did not show significant improvement in the gut microbiota disorders of patients with sepsis. This is in line with the perception of gut microbiota disorders in sepsis. Although the mechanism of intestinal microecological disorders due to sepsis is not clear, multiple factors such as antibiotics[12], sedative and analgesic drugs[13], nutritional support[14], and proton pump inhibitors[15] affect gut flora among patients with sepsis, making it impossible for a single factor to significantly improve the condition in a short period of time.
Although short-term nutritional support could not correct gut microbiota disorders in patients with sepsis, different nutritional support modalities caused some differences in bacterial species: Enterococcus spp. were significantly high in the TEN group, Campylobacter spp. were significantly low in the TPN group, and Dialister spp. were significantly low in the SPN group. In animal studies, it has been demonstrated that Enterococcus can effectively improve animal growth[16,17], enhance the absorption of other nutrients[18], and is a beneficial additive, which is widely used in animal feeds. Current research suggests that drug-resistant strains of Enterococcus may be pathogenic[19]; however, its commensal strains have been identified as probiotics in animal and human intestines[20]. Further studies have shown that Enterococcus can trigger immune signaling pathways and regulate infection[21], autoimmunity[22]. Therefore, we believe that a significant increase of Enterococcus spp. after TEN treatment suggests that early TEN support can improve gut microbiota in sepsis. Campylobacter spp. is the main cause of gastroenteritis in humans, and consumption of contaminated poultry meat is its main transmission route. The significant decrease of this genus after TPN treatment suggests improvements in gut microbiota in sepsis; however, the underlying mechanism is currently unclear and requires further study. Non-oral ingestion during TPN support may have cut-off the transmission route of Campylobacter spp. Other reports suggest that a variety of probiotic bacteria in the intestine reduce the severity of Campylobacter spp. infection by negatively affecting the virulence and survival factors (e.g., adhesion, invasion) of various Campylobacter spp., attenuating intestinal inflammation[23]. Dialister spp. may be positively associated with glucose metabolism disorders, obesity, and insulin resistance[24,25], and our previous study found that Dialister spp. in patients with sepsis was positively associated with diamine oxidase, a highly active intracellular enzyme in the upper villi of the small intestinal mucosa in humans and mammals. Elevated diamine oxidase reflects the degree of damage to the intestinal mechanical barrier[4]. Therefore, the significant decrease in Dialisteria spp. suggests that SPN may improve intestinal barrier function.
Early, short-term nutritional support in sepsis has a beneficial effect on gut bacteria composition, although it did not completely improve gut microbiota disorders.
SCFAs are the main metabolites produced by specific symbiotic bacteria of the gut after fermentation of dietary fiber and indigestible polysaccharides and starches, including acetic, propionic, butyric, isobutyric, pentanoic, isovaleric, and caproic acid. Among them, acetic, propionic, butyric, and isovaleric acid are the most common SCFAs. In addition to their ability to provide direct nutrition to intestinal commensal microorganisms, SCFAs positively influence host immune cell differentiation, immune system, and metabolism as well as modulate host susceptibility[26,27]. Furthermore, they have a protective effect on intestinal barrier function[28,29].
In this study, we found that there was no significant improvement in SCFAs after 5 d of nutritional support in patients with sepsis. This is complemented by the fact that gut microbiota disorders in sepsis are not effectively corrected, and a close correlation exists between SCFAs and gut microbiota, and SCFAs cannot be significantly improved in the absence of effective correction of gut microbiota disorders. In patients with sepsis, there is a significant decrease in SCFA-producing bacteria in the intestine, such as Bifidobacterium and Bacillus. This can cause a dramatic decrease in SCFAs and cause intestinal barrier dysfunction. Concomitantly, decreased levels of SCFAs can further aggravate the gut microbiota disorders in sepsis[30,31].
In this study, we found that all SCFAs in the TEN group showed an increase trend except for caproic acid. In the TPN group, acetic acid showed an improving trend and propionic acid improved significantly, while the remaining SCFAs did not show any improvement. SCFAs in the SPN group showed a continuous decreasing trend. The complete bypassing of the intestine by TPN can serve as both advantage and disadvantage[32]. TPN lacks key intestinal luminal nutrients compared to TEN, resulting in a significant deficiency of glutamine and SCFAs[33,34]. Animal studies have shown that this deficiency cannot be effectively improved by the addition of glutamine and SCFAs to intravenous nutrition[34-36]. Supplemental enteral nutrition during SPN mostly uses ready-made enteral preparations with low fiber or without fiber, which not only fails to promote the production of SCFAs but can also form pathogenic microorganisms with higher virulence[30,37]. The reason for this may be the inability of highly absorbable trophic preparations to adequately feed the intestinal epithelium and intestinal commensal bacteria, leading to disruption of the intestinal barrier, stress signaling, and proliferation of intestinal pathogenic bacteria[38].
Although nutritional support in early sepsis did not significantly improve SCFA metabolism, different nutritional support modalities had different effects on SCFAs. Moreover, TEN had a tendency to improve overall SCFA production in sepsis compared to TPN and SPN and was theoretically more conducive to SCFA production due to dietary fiber supplementation and adequate enteral nutritional supplementation.
In this study, we found that in early and short-term nutritional support for patients with sepsis, TEN can significantly improve cellular immunity, humoral immunity, and nutrition-related indicators; SPN can significantly improve humoral immunity and nutritional indicators; and TPN can only improve IgG. The effects of the disease, vascular leakage, fluid supplementation, diuresis, bed confinement, and other factors, make weight assessment difficult for patients with sepsis. Additionally, muscle mass and other assessment indicators do not truly reflect the nutritional changes of the patient. Therefore, it is more appropriate to select plasma proteins as an indicator of nutrition in patients with sepsis. These proteins also need to have relatively small storage, rapid synthesis, and stable metabolic rate. Pre-ALB has a half-life of only 48 h, a small body storage pool, responds to negative nitrogen balance in the body in a short time, and is a considerably more sensitive indicator of malnutrition than either ALB or transferrin. Combined with the effect on PAB in the three groups in this study, early nutritional support of TEN in sepsis significantly improved the nutritional status of patients compared with TPN. Interestingly, PAB concentration was negatively correlated with the relative abundance of OTUs of Streptococcus and Escherichia-Shigella, while it was positively correlated with the relative abundance of OTUs of Anaerococcus and Porphyromonas. Additionally, the corresponding species were significantly lower in TEN and TPN, respectively. Further studies are needed to clarify whether this may suggest a relationship between improved nutrition and gut microbiota.
The intestine is the largest immune organ in the body. Studies in mice have shown that nutritional pathways and typology can affect gut-associated lymphoid tissue[39]. Relative to TPN, TEN reduces the occurrence of potential gastrointestinal complications, including maintenance of the intestinal epithelial barrier and tight junctions, increases constituent proteins, reduces acute phase reactants and bacterial translocation, and enhances mucosal immunity[40]. A meta-analysis showed that enteral feeding and sepsis are negatively correlated[41]. A more plausible explanation at present is that TPN lacks intraluminal nutrients, such as glutamine and SCFAs, which are essential for local enterocytes and colon cells, and that delivery of these nutrients via extra-gastrointestinal routes does not directly deliver nutrients to the most nutrient-demanding cells of the gastrointestinal tract. In contrast, key intestinal-systemic signaling regulators were significantly downregulated in animals fed via TPN[34]. These findings explain why TEN and SPN in this study could improve nutritional and immune indicators early in patients with sepsis, while TPN did not significantly improve these indicators. Related studies have also shown that, in addition to the nutritional pathway, the composition and adequacy of nutrients in the intestinal lumen determine the gene expression of host intestinal receptors as well as the uptake of luminal metabolites and hormonal signaling[36]. Approximately 20% of the energy supply of SPN passes through the gastrointestinal tract, which allows early improvement of nutrition and humoral immunity in patients with sepsis; however, due to the single formulation of the provided preparation, the frequent absence of dietary fiber, and the insufficient total energy supply through the intestine, SPN does not improve cellular immunity and SCFAs during early nutritional support in sepsis.
IgG and IgA are important immunoglobulins that interact with the gut microbiota to exert corresponding immune effects[42]. In this study, the intestinal Enterococcus spp. and IgA were positively correlated and Methylobacterium spp. and IgG were negatively correlated in patients with sepsis. Accordingly, these two species also showed significant increase and decrease in the TEN group, which suggested interaction between IgG, IgA, and gut microbiota. Meanwhile, the correlation between Enterococcus spp. and IgA may be one of the mechanisms by which Enterococcus spp. exert beneficial effects on the intestinal microecology in sepsis, which remains to be further investigated.
The present study is a preliminary study investigating the modalities of early nutritional support in patients with sepsis from the perspective of intestinal microecology. However, this study is limited by the small sample size and short duration of nutritional support; therefore, significant improvements in intestinal microecology did not have time to materialize. The only differences are observed in the bacterial genera and changes in SCFAs. However, the available preliminary results provide an insight into the effects of different modalities of nutritional support on intestinal microecology, and their link to host nutrition and immunity, suggesting that TEN may be a more appropriate approach for early nutritional support in patients with sepsis. How different nutritional support modalities affect the structural and metabolic changes of gut microbiota composition and the related mechanisms involved need to be studied and explored by designing clinical trials with larger scale and longer intervention time, which is the next direction of our research.
In this study, three groups were classified on the basis of different modalities of nutritional support for sepsis. By comparing the changes in gut microbiota structure, species composition, and SCFAs, early, short-term nutritional support could not completely improve intestinal microecological disorders in sepsis, and accordingly, there was no significant improvement in SCFAs. However, the differences in the species composition of intestinal bacteria suggested a beneficial effect of early nutritional support on gut microbiota disorders. Relative changes in SCFAs showed that TEN support had an improving trend on the indicators. The changes in clinical nutritional and immune indicators before and after nutritional support showed that early TEN support largely improved cellular immunity, humoral immunity, and nutritional indicators. Moreover, TEN support improved clinical nutritional indicators of patients better than TPN. Meanwhile, the correlation between gut microbiota and SCFAs and clinical nutritional and immune indicators suggested some level of interaction. In conclusion, the results of this study suggest that TEN should be the recommended modality for early nutritional support in sepsis from the perspective of intestinal microecology. These findings offer a novel perspective for optimizing the mode of nutritional support in sepsis.
Sepsis is a common disease in intensive care units, with high morbidity and mortality. Our previous study confirmed the presence of gut microbiota disorders in patients with sepsis. The combination of disease and gut microbiota disorders lead to the development of multiple organ dysfunction and clinical deterioration of the patient. Nutritional support is an important part of the treatment of critically ill patients. Proper modalities of nutritional support can improve nutrition, immunity, and intestinal microecology. The exploration of early nutritional support in patients with sepsis from the perspective of intestinal microecology is important to optimize nutritional support and improve prognosis.
The recommendations about the choice of nutritional support modalities are largely based on expert consensus, although the level of evidence is low. Nutritional support has a significant impact on intestinal microecology; to date, the modalities of nutritional support in sepsis have not been evaluated from the perspective of intestinal microecology. This perspective can provide new insights into the optimization of the modalities for nutritional support in sepsis.
The main objective was to determine the optimal modality of early nutritional support for patients with sepsis from the perspective of intestinal microecology. We applied different nutritional modalities for early and short-duration nutritional support in patients with sepsis and found differences in intestinal bacterial composition, short-chain fatty acids (SCFAs), and nutritional and immune indicators. Our results revealed for the first time that total enteral nutrition (TEN) is a good modality for early nutritional support in patients with sepsis. This study offers a new perspective for optimizing nutritional support modalities in sepsis.
Thirty patients with sepsis who were admitted to the intensive care unit of the General Hospital of Ningxia Medical University, China, between 2019 and 2021 with indications for nutritional support, were randomly assigned to one of three different modalities of nutritional support. For 5 d, nutritional support was administered to each patient using one of the following modalities: TEN group, total parenteral nutrition (TPN group), and supplemental parenteral nutrition (SPN group). Blood and stool specimens were collected before and after nutritional support was administered, and changes in gut microbiota, SCFAs, and immune and nutritional indicators were detected and compared among the three groups.
Patients were assessed before and after the administration of nutritional support. The following differences were observed in the three groups after nutritional support: (1) Differences in the gut bacteria (Enterococcus increased in the TEN group, Campylobacter decreased in the TPN group, and Dialister decreased in the SPN group); (2) different trends in SCFA concentrations (increase in SCFAs in the TEN group except for caproic acid, improvement of acetic and propionic acid only in the TPN group, and decreasing trend in SCFA production in the SPN group); and (3) significant improvement in the nutritional and immunological indicators in the TEN and SPN groups, with improvement in immunoglobulin G levels only in the TPN group. Furthermore, and significant correlations were found between the gut bacteria, SCFAs, and nutritional and immunological indicators.
Our results indicate that TEN is the optimal modality for early nutritional support in patients with sepsis from the perspective of intestinal microecology.
Future research should focus on how different nutritional support modalities affect the structural and metabolic changes in gut microbiota composition and the underlying mechanisms. Our research group will explore these questions in a large scale clinical trial with a longer intervention time.
We thank all the patients who participated in this study and the physicians in the Department of Critical Care Medicine, General Hospital, Ningxia Medical University. We also thank Shanghai Mobio Biomedical Technology Co. for technical support.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Dabla PK, India; Ghimire R, Nepal; Tangsuwanaruk T, Thailand S-Editor: Li L L-Editor: A P-Editor: Li L
| 1. | Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, Bellomo R, Bernard GR, Chiche JD, Coopersmith CM, Hotchkiss RS, Levy MM, Marshall JC, Martin GS, Opal SM, Rubenfeld GD, van der Poll T, Vincent JL, Angus DC. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA. 2016;315:801-810. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15803] [Cited by in RCA: 17171] [Article Influence: 1907.9] [Reference Citation Analysis (2)] |
| 2. | van der Poll T, Shankar-Hari M, Wiersinga WJ. The immunology of sepsis. Immunity. 2021;54:2450-2464. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 495] [Article Influence: 123.8] [Reference Citation Analysis (0)] |
| 3. | Yang XJ, Liu D, Ren HY, Zhang XY, Zhang J, Yang XJ. Effects of sepsis and its treatment measures on intestinal flora structure in critical care patients. World J Gastroenterol. 2021;27:2376-2393. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 17] [Cited by in RCA: 31] [Article Influence: 7.8] [Reference Citation Analysis (4)] |
| 4. | Adelman MW, Woodworth MH, Langelier C, Busch LM, Kempker JA, Kraft CS, Martin GS. The gut microbiome's role in the development, maintenance, and outcomes of sepsis. Crit Care. 2020;24:278. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 61] [Cited by in RCA: 206] [Article Influence: 41.2] [Reference Citation Analysis (0)] |
| 5. | McClave SA, Taylor BE, Martindale RG, Warren MM, Johnson DR, Braunschweig C, McCarthy MS, Davanos E, Rice TW, Cresci GA, Gervasio JM, Sacks GS, Roberts PR, Compher C; Society of Critical Care Medicine; American Society for Parenteral and Enteral Nutrition. Guidelines for the Provision and Assessment of Nutrition Support Therapy in the Adult Critically Ill Patient: Society of Critical Care Medicine (SCCM) and American Society for Parenteral and Enteral Nutrition (A.S.P.E.N.). JPEN J Parenter Enteral Nutr. 2016;40:159-211. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1695] [Cited by in RCA: 1866] [Article Influence: 207.3] [Reference Citation Analysis (3)] |
| 6. | Singer P, Blaser AR, Berger MM, Alhazzani W, Calder PC, Casaer MP, Hiesmayr M, Mayer K, Montejo JC, Pichard C, Preiser JC, van Zanten ARH, Oczkowski S, Szczeklik W, Bischoff SC. ESPEN guideline on clinical nutrition in the intensive care unit. Clin Nutr. 2019;38:48-79. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 989] [Cited by in RCA: 1528] [Article Influence: 218.3] [Reference Citation Analysis (0)] |
| 7. | Winter SE, Winter MG, Xavier MN, Thiennimitr P, Poon V, Keestra AM, Laughlin RC, Gomez G, Wu J, Lawhon SD, Popova IE, Parikh SJ, Adams LG, Tsolis RM, Stewart VJ, Bäumler AJ. Host-derived nitrate boosts growth of E. coli in the inflamed gut. Science. 2013;339:708-711. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 602] [Cited by in RCA: 746] [Article Influence: 62.2] [Reference Citation Analysis (0)] |
| 8. | Albenberg L, Esipova TV, Judge CP, Bittinger K, Chen J, Laughlin A, Grunberg S, Baldassano RN, Lewis JD, Li H, Thom SR, Bushman FD, Vinogradov SA, Wu GD. Correlation between intraluminal oxygen gradient and radial partitioning of intestinal microbiota. Gastroenterology. 2014;147:1055-63.e8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 640] [Cited by in RCA: 624] [Article Influence: 56.7] [Reference Citation Analysis (0)] |
| 9. | David LA, Maurice CF, Carmody RN, Gootenberg DB, Button JE, Wolfe BE, Ling AV, Devlin AS, Varma Y, Fischbach MA, Biddinger SB, Dutton RJ, Turnbaugh PJ. Diet rapidly and reproducibly alters the human gut microbiome. Nature. 2014;505:559-563. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5625] [Cited by in RCA: 6818] [Article Influence: 568.2] [Reference Citation Analysis (0)] |
| 10. | Sonnenburg ED, Smits SA, Tikhonov M, Higginbottom SK, Wingreen NS, Sonnenburg JL. Diet-induced extinctions in the gut microbiota compound over generations. Nature. 2016;529:212-215. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 965] [Cited by in RCA: 1167] [Article Influence: 129.7] [Reference Citation Analysis (0)] |
| 11. | Huus KE, Petersen C, Finlay BB. Diversity and dynamism of IgA-microbiota interactions. Nat Rev Immunol. 2021;21:514-525. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 103] [Article Influence: 25.8] [Reference Citation Analysis (0)] |
| 12. | Slimings C, Riley TV. Antibiotics and hospital-acquired Clostridium difficile infection: update of systematic review and meta-analysis. J Antimicrob Chemother. 2014;69:881-891. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 366] [Cited by in RCA: 377] [Article Influence: 31.4] [Reference Citation Analysis (0)] |
| 13. | Zaborin A, Smith D, Garfield K, Quensen J, Shakhsheer B, Kade M, Tirrell M, Tiedje J, Gilbert JA, Zaborina O, Alverdy JC. Membership and behavior of ultra-low-diversity pathogen communities present in the gut of humans during prolonged critical illness. mBio. 2014;5:e01361-e01314. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 247] [Cited by in RCA: 236] [Article Influence: 21.5] [Reference Citation Analysis (0)] |
| 14. | Dahlgren AF, Pan A, Lam V, Gouthro KC, Simpson PM, Salzman NH, Nghiem-Rao TH. Longitudinal changes in the gut microbiome of infants on total parenteral nutrition. Pediatr Res. 2019;86:107-114. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 42] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 15. | Imhann F, Bonder MJ, Vich Vila A, Fu J, Mujagic Z, Vork L, Tigchelaar EF, Jankipersadsing SA, Cenit MC, Harmsen HJ, Dijkstra G, Franke L, Xavier RJ, Jonkers D, Wijmenga C, Weersma RK, Zhernakova A. Proton pump inhibitors affect the gut microbiome. Gut. 2016;65:740-748. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 780] [Cited by in RCA: 933] [Article Influence: 103.7] [Reference Citation Analysis (0)] |
| 16. | Hu C, Xing W, Liu X, Zhang X, Li K, Liu J, Deng B, Deng J, Li Y, Tan C. Effects of dietary supplementation of probiotic Enterococcus faecium on growth performance and gut microbiota in weaned piglets. AMB Express. 2019;9:33. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 17] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 17. | Tanaka M, Ikeji T, Nakanishi R, Hirabayashi T, Ono K, Hirayama Y, Tategaki A, Kondo H, Ishihara A, Fujino H. Protective effects of Enterococcus faecium strain R30 supplementation on decreased muscle endurance under disuse in rats. Exp Physiol. 2021;106:1961-1970. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 18. | Suphoronski SA, de Souza FP, Chideroli RT, Mantovani Favero L, Ferrari NA, Ziemniczak HM, Gonçalves DD, Lopera Barrero NM, Pereira UP. Effect of Enterococcus faecium as a Water and/or Feed Additive on the Gut Microbiota, Hematologic and Immunological Parameters, and Resistance Against Francisellosis and Streptococcosis in Nile Tilapia (Oreochromis niloticus). Front Microbiol. 2021;12:743957. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 19. | Lebreton F, Manson AL, Saavedra JT, Straub TJ, Earl AM, Gilmore MS. Tracing the Enterococci from Paleozoic Origins to the Hospital. Cell. 2017;169:849-861.e13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 186] [Article Influence: 23.3] [Reference Citation Analysis (0)] |
| 20. | Hanchi H, Mottawea W, Sebei K, Hammami R. The Genus Enterococcus: Between Probiotic Potential and Safety Concerns-An Update. Front Microbiol. 2018;9:1791. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 260] [Cited by in RCA: 314] [Article Influence: 44.9] [Reference Citation Analysis (0)] |
| 21. | Rangan KJ, Pedicord VA, Wang YC, Kim B, Lu Y, Shaham S, Mucida D, Hang HC. A secreted bacterial peptidoglycan hydrolase enhances tolerance to enteric pathogens. Science. 2016;353:1434-1437. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 109] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 22. | Manfredo Vieira S, Hiltensperger M, Kumar V, Zegarra-Ruiz D, Dehner C, Khan N, Costa FRC, Tiniakou E, Greiling T, Ruff W, Barbieri A, Kriegel C, Mehta SS, Knight JR, Jain D, Goodman AL, Kriegel MA. Translocation of a gut pathobiont drives autoimmunity in mice and humans. Science. 2018;359:1156-1161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 576] [Cited by in RCA: 596] [Article Influence: 85.1] [Reference Citation Analysis (0)] |
| 23. | Balta I, Butucel E, Stef L, Pet I, Gradisteanu-Pircalabioru G, Chifiriuc C, Gundogdu O, McCleery D, Corcionivoschi N. Anti-Campylobacter Probiotics: Latest Mechanistic Insights. Foodborne Pathog Dis. 2022;19:693-703. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 15] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 24. | Gao J, Guo X, Wei W, Li R, Hu K, Liu X, Jiang W, Liu S, Wang W, Sun H, Wu H, Zhang Y, Gu W, Li Y, Sun C, Han T. The Association of Fried Meat Consumption With the Gut Microbiota and Fecal Metabolites and Its Impact on Glucose Homoeostasis, Intestinal Endotoxin Levels, and Systemic Inflammation: A Randomized Controlled-Feeding Trial. Diabetes Care. 2021;44:1970-1979. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 51] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 25. | Pinart M, Dötsch A, Schlicht K, Laudes M, Bouwman J, Forslund SK, Pischon T, Nimptsch K. Gut Microbiome Composition in Obese and Non-Obese Persons: A Systematic Review and Meta-Analysis. Nutrients. 2021;14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 206] [Article Influence: 51.5] [Reference Citation Analysis (0)] |
| 26. | Hernández-Chirlaque C, Aranda CJ, Ocón B, Capitán-Cañadas F, Ortega-González M, Carrero JJ, Suárez MD, Zarzuelo A, Sánchez de Medina F, Martínez-Augustin O. Germ-free and Antibiotic-treated Mice are Highly Susceptible to Epithelial Injury in DSS Colitis. J Crohns Colitis. 2016;10:1324-1335. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 193] [Article Influence: 21.4] [Reference Citation Analysis (0)] |
| 27. | Wu W, Sun M, Chen F, Cao AT, Liu H, Zhao Y, Huang X, Xiao Y, Yao S, Zhao Q, Liu Z, Cong Y. Microbiota metabolite short-chain fatty acid acetate promotes intestinal IgA response to microbiota which is mediated by GPR43. Mucosal Immunol. 2017;10:946-956. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 245] [Cited by in RCA: 344] [Article Influence: 43.0] [Reference Citation Analysis (0)] |
| 28. | Peng L, He Z, Chen W, Holzman IR, Lin J. Effects of butyrate on intestinal barrier function in a Caco-2 cell monolayer model of intestinal barrier. Pediatr Res. 2007;61:37-41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 388] [Cited by in RCA: 372] [Article Influence: 20.7] [Reference Citation Analysis (0)] |
| 29. | Peng L, Li ZR, Green RS, Holzman IR, Lin J. Butyrate enhances the intestinal barrier by facilitating tight junction assembly via activation of AMP-activated protein kinase in Caco-2 cell monolayers. J Nutr. 2009;139:1619-1625. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1443] [Cited by in RCA: 1339] [Article Influence: 83.7] [Reference Citation Analysis (0)] |
| 30. | Birchenough G, Schroeder BO, Bäckhed F, Hansson GC. Dietary destabilisation of the balance between the microbiota and the colonic mucus barrier. Gut Microbes. 2019;10:246-250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 66] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 31. | Riva A, Kuzyk O, Forsberg E, Siuzdak G, Pfann C, Herbold C, Daims H, Loy A, Warth B, Berry D. A fiber-deprived diet disturbs the fine-scale spatial architecture of the murine colon microbiome. Nat Commun. 2019;10:4366. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 89] [Cited by in RCA: 83] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 32. | Dudrick SJ. History of parenteral nutrition. J Am Coll Nutr. 2009;28:243-251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 42] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 33. | Ziegler TR, Evans ME, Fernández-Estívariz C, Jones DP. Trophic and cytoprotective nutrition for intestinal adaptation, mucosal repair, and barrier function. Annu Rev Nutr. 2003;23:229-261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 147] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 34. | Guzman M, Manithody C, Krebs J, Denton C, Besmer S, Rajalakshmi P, Jain S, Villalona GA, Jain AK. Impaired Gut-Systemic Signaling Drives Total Parenteral Nutrition-Associated Injury. Nutrients. 2020;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 26] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 35. | Bollhalder L, Pfeil AM, Tomonaga Y, Schwenkglenks M. A systematic literature review and meta-analysis of randomized clinical trials of parenteral glutamine supplementation. Clin Nutr. 2013;32:213-223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 99] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 36. | Toni T, Alverdy J, Gershuni V. Re-examining chemically defined liquid diets through the lens of the microbiome. Nat Rev Gastroenterol Hepatol. 2021;18:903-911. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 12] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 37. | Jacobson A, Yang D, Vella M, Chiu IM. The intestinal neuro-immune axis: crosstalk between neurons, immune cells, and microbes. Mucosal Immunol. 2021;14:555-565. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 73] [Cited by in RCA: 172] [Article Influence: 43.0] [Reference Citation Analysis (0)] |
| 38. | Feng Y, Huang Y, Wang Y, Wang P, Wang F. Severe burn injury alters intestinal microbiota composition and impairs intestinal barrier in mice. Burns Trauma. 2019;7:20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 36] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 39. | Pierre JF, Heneghan AF, Tsao FH, Sano Y, Jonker MA, Omata J, Lan J, Kudsk KA. Route and type of nutrition and surgical stress influence secretory phospholipase A2 secretion of the murine small intestine. JPEN J Parenter Enteral Nutr. 2011;35:748-756. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 18] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 40. | Sigalet DL, Mackenzie SL, Hameed SM. Enteral nutrition and mucosal immunity: implications for feeding strategies in surgery and trauma. Can J Surg. 2004;47:109-116. [PubMed] |
| 41. | Lewis SR, Schofield-Robinson OJ, Alderson P, Smith AF. Enteral versus parenteral nutrition and enteral versus a combination of enteral and parenteral nutrition for adults in the intensive care unit. Cochrane Database Syst Rev. 2018;6:CD012276. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 44] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 42. | James KR, Gomes T, Elmentaite R, Kumar N, Gulliver EL, King HW, Stares MD, Bareham BR, Ferdinand JR, Petrova VN, Polański K, Forster SC, Jarvis LB, Suchanek O, Howlett S, James LK, Jones JL, Meyer KB, Clatworthy MR, Saeb-Parsy K, Lawley TD, Teichmann SA. Distinct microbial and immune niches of the human colon. Nat Immunol. 2020;21:343-353. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 170] [Cited by in RCA: 202] [Article Influence: 40.4] [Reference Citation Analysis (0)] |