Published online Mar 21, 2023. doi: 10.3748/wjg.v29.i11.1757
Peer-review started: January 16, 2023
First decision: January 30, 2023
Revised: February 6, 2023
Accepted: February 27, 2023
Article in press: February 27, 2023
Published online: March 21, 2023
Processing time: 59 Days and 19.5 Hours
Eosinophilic gastrointestinal disease (EGID) is a disorder characterized by infiltration of eosinophils causing mucosal damage and dysfunction of the gas
We report the case of a 10-year-old boy who had suffered abdominal pain and fatigue for the preceding 6 mo. He was referred to our institute for investigation of suspected gastrointestinal bleeding because of severe anemia with hypoproteinemia and positive fecal human hemoglobin. The upper and lower gastrointestinal endoscopic findings were normal; however, double-balloon small bowel endoscopy showed multiple oblique and circular ulcers with discrete margins and mild constriction of the intestinal lumen in the ileum. The findings were highly consistent with CEAS, but urine prostaglandin metabolites were within normal limits, and no previously reported mutations in the SLCO2A1 gene were identified. Histological evaluation demonstrated moderate to severe eosinophilic infiltration localized to the small intestine suggesting a diagnosis of EoN. Clinical remission was maintained with montelukast and a partial elemental diet, but emergent surgery for bowel obstruction due to small intestinal stenosis was performed two years after the initial treatment.
EoN should be considered in the differential diagnosis of CEAS-like small intestinal ulcerative lesions and normal urinary prostaglandin metabolite levels.
Core Tip: Eosinophilic enteritis (EoN), a form of eosinophilic gastrointestinal disease localized to the small intestine, is extremely rare in children. The present pediatric case of EoN displayed multiple ulcerative lesions mimicking chronic enteropathy associated with SLCO2A1 and bowel obstruction due to small intestinal stenosis. The diagnosis was confirmed by small intestinal biopsy using double-balloon enteroscopy and analysis of urine prostaglandin metabolites.
- Citation: Kimura K, Jimbo K, Arai N, Sato M, Suzuki M, Kudo T, Yano T, Shimizu T. Eosinophilic enteritis requiring differentiation from chronic enteropathy associated with SLCO2A1 gene: A case report. World J Gastroenterol 2023; 29(11): 1757-1764
- URL: https://www.wjgnet.com/1007-9327/full/v29/i11/1757.htm
- DOI: https://dx.doi.org/10.3748/wjg.v29.i11.1757
In eosinophilic gastrointestinal disease (EGID), tissue and functional disorders of the gastrointestinal tract are caused by inflammation due to abnormal infiltration of eosinophils within the gastrointestinal wall[1]. EGID can occur in any location between the esophagus and the colon, but localization to the small intestine is extremely rare[2]. The disease was re-classified from eosinophilic gastroenteritis (EGE) to eosinophilic enteritis (EoN) in 2022[3]. In addition, the nonspecific gastrointestinal endoscopic findings of EGID (edema, erythema, erosions, and ulcers) lead to difficulty in differentiating EGID from other digestive disorders[4].
In contrast, chronic enteropathy associated with SLCO2A1 (CEAS) is a chronic persistent small bowel disease characterized by multiple oblique and circular ulcers with discrete margins in the ileum endoscopically. It is complicated by small intestinal obstruction due to ulcerative scarring and stenosis in the natural course[5].
Herein, we report a pediatric case of EoN involving multiple ulcerative lesions mimicking CEAS with diagnostic and therapeutic difficulties.
A 10-year-old Japanese boy presented to his family pediatrician with the complaints of easy fatigue and abdominal pain for 6 mo.
The patient presented to the family pediatrician with facial pallor and severe anemia (Hb: 2.9 g/dL) and was referred to his previous physician for admission. He then received red blood cell transfusion and iron supplementation. Further analysis also showed positive fecal human hemoglobin, indicating anemia due to gastrointestinal bleeding, and the patient was transferred to our institution for further evaluation.
The patient had no previous medical history.
Prior to the patient's birth, the father had been treated with antibiotics for iron deficiency anemia caused by Helicobacter pylori infection.
On physical examination, vital signs were as follows: Temperature, 36.8 °C; blood pressure, 99/60 mmHg; heart rate, 80 beats per min; respiratory rate, 18 breaths per min. His height was 122.7 cm (-1.63 standard deviation), and his weight was 24.1 kg (-0.92 standard deviation), with no significant growth disturbance on the growth curve and no other abnormal physical findings other than pale eyelid conjunctiva.
Blood analysis demonstrated low levels of hemoglobin (9.7 g/dL) and albumin (2.9 g/dL), and fecal analysis showed elevated levels of human hemoglobin (2018 ng/mL) and calprotectin (510 μg/g). No elevation of inflammatory markers and no eosinophilia were observed (Table 1).
| Laboratory data | Reference range | |
| White blood cell count (/μL) | 5300 | 4000-8000 |
| Differential (percent) | ||
| Neutrophils | 2597 (49.0) | 1800-4800 |
| Lymphocytes | 2067 (39.0) | 1000-3600 |
| Eosinophils | 53 (1.0) | 40-400 |
| Hemoglobin (g/dL) | 9.7 | 14-18 |
| Hematocrit (%) | 35.0 | 40-48 |
| MCV (fL) | 74.9 | 84-99 |
| MCHC (g/dL) | 27.7 | 32-36 |
| Ferrum (μg/dL) | 39 | 50-190 |
| Ferritin (ng/mL) | 23 | 30-400 |
| Total protein (g/dL) | 6.1 | 6.7-8.3 |
| Albumin (g/dL) | 2.9 | 3.9-4.9 |
| Creatinine (mg/dL) | 0.34 | 0.61-1.04 |
| C-reactive protein (mg/dL) | 0.1 | < 0.3 |
| Erythrocyte sedimentation rate (mm/h) | 4 | 0-15 |
| IgG (mg/dL) | 665 | 870-1700 |
| IgE (IU/mL) | 515 | 0-173 |
| Fecal human hemoglobin (ng/mL) | 2018 | < 50 |
| Fecal calprotectin (μg/g) | 510 | < 50 |
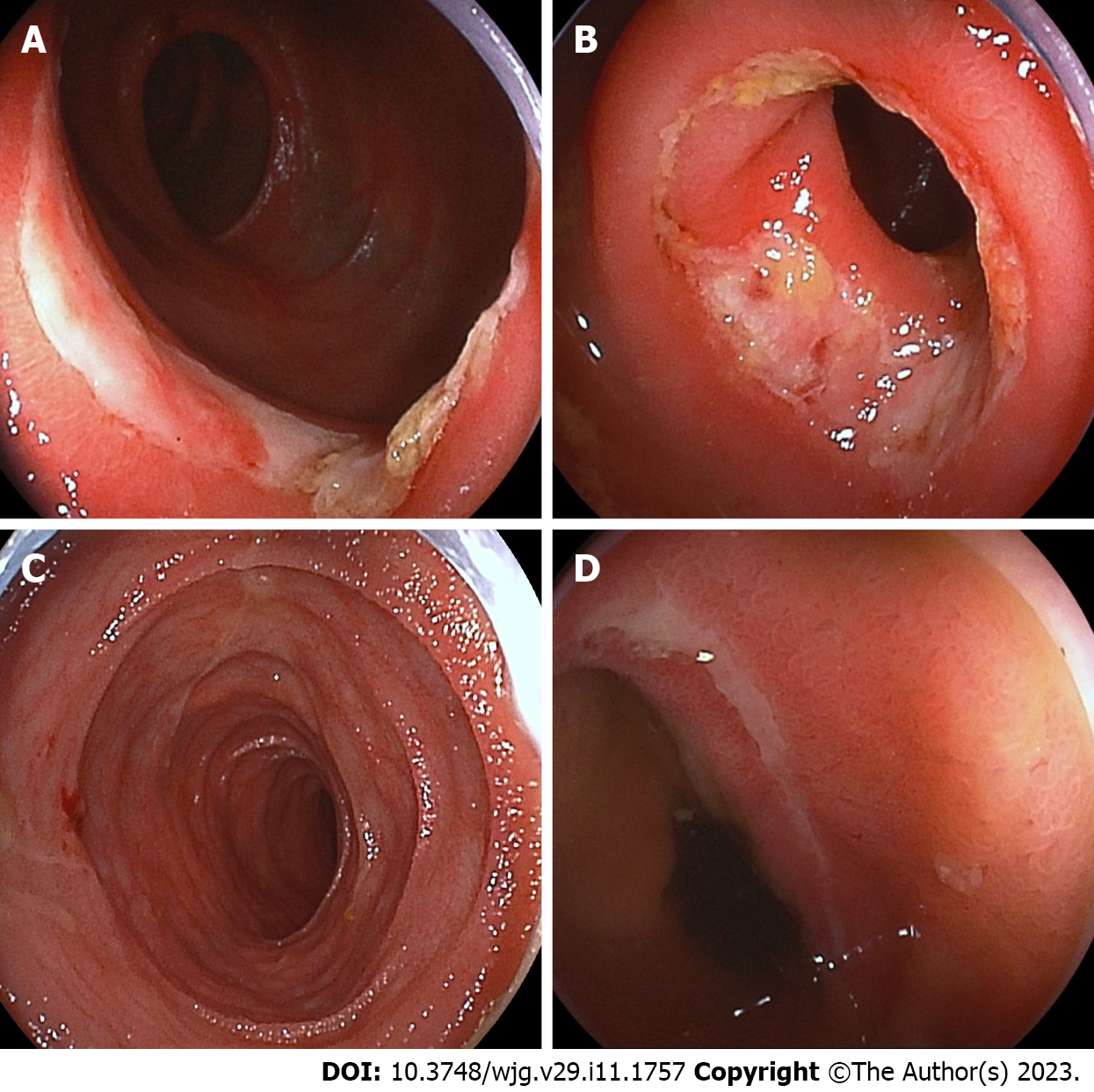
Upper and lower gastrointestinal endoscopy showed normal mucosal findings. Small intestinal capsule endoscopy was not performed because of the patency capsule retention in the stomach, and transanal double-balloon enteroscopy (DBE) was performed. DBE showed multiple oblique and circular ulcers with discrete margins at 70-100 cm proximal from the ileocecal valve with slight constriction of the small intestinal lumen (Figure 1).
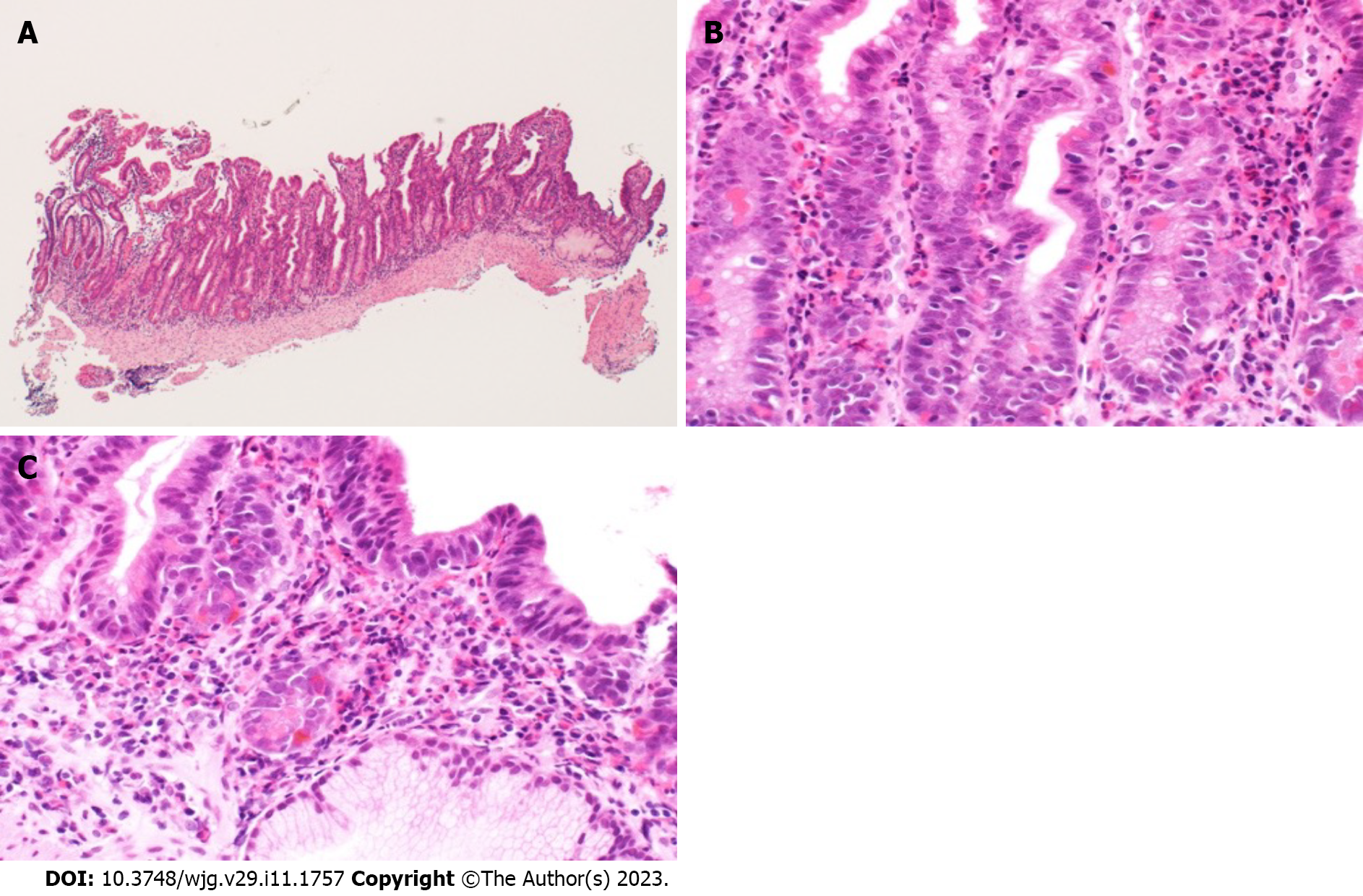
No histological abnormalities were identified on biopsies conducted by upper and lower gastrointestinal endoscopy. The ileal biopsy with DBE showed moderate to severe histological eosinophilic infiltration [maximum 80 eosinophils/high-power field (HPF)] and cryptitis within the mucosa (Figure 2). Of the urine prostaglandin metabolites that are elevated in CEAS, the levels in the present patient were as follows: Prostaglandin F2α metabolite, 3.2 (normal range: 3.0-4.0) ng/mg Cre; prostaglandin E2 metabolite, 2.09 (normal range: 2.0-3.0) ng/mg Cre; and prostaglandin D2 metabolite, 8.5 (normal range: 9.0-10) ng/mg Cre, all of which were within the normal ranges[6,7]. No previously reported mutations in the SLCO2A1 gene or in the targeted gene panels for very early-onset inflammatory bowel disease were identified[8].
The diagnostic findings and medical history indicated a final diagnosis of EoN.
The patient was treated with montelukast (10 mg/d for a total of 26 mo), which reduced the frequency of abdominal pain. Partial elemental diet therapy (600 kcal/day for a total of 24 mo) was also implemented due to insufficient response of hypoalbuminemia and anemia[9]. Corticosteroids were not administered because the patient's family preferred that steroids be avoided.
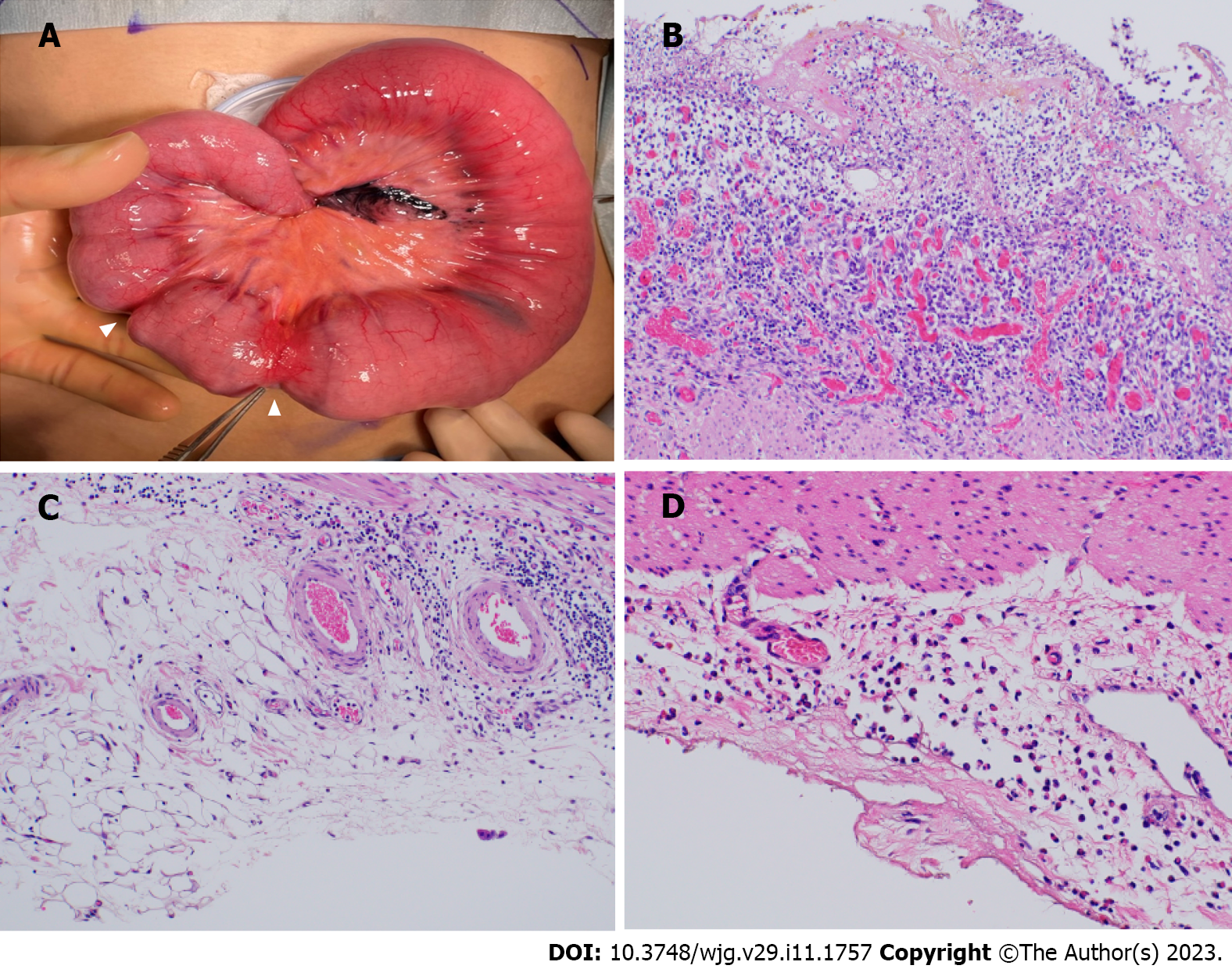
The abdominal pain resolved completely 2 mo after the administration of montelukast and the partial elemental diet, and improvement of hemoglobin (11.2 g/dL) and hypoalbuminemia (3.5 g/dL) and normalization of fecal human hemoglobin (56 ng/mL) were observed after 4 mo. At follow-up of the small intestine by DBE performed 1 year later, mucosal healing was achieved, except for the oblique ulcer and scars at 70 cm proximal to the ileal valve, and no intestinal stenosis caused by the healing ulcer was observed (Figure 1). Eosinophilic infiltration had also disappeared on biopsy, suggesting histological remission. The patient was in clinical remission thereafter, but 2 years and 2 mo after the first visit, sudden bowel obstruction was induced by small intestinal stenosis, and emergent surgery was performed. The ileal macroscopic findings showed strictures at 40 cm and 44 cm proximal to the ileocecal valve, leading to ileal resection of the strictures and ileostomy (Figure 3). The histological findings of the resected specimen were of ulcer formation and peri-ulcer mucosal damage, suggesting intestinal stenosis in the process of ulcerative scarring. No significant granuloma or eosinophilic infiltration was observed (Figure 3). The patient’s postoperative course was uneventful. Ileostomy closure was performed 2 mo later, and the patient is currently being followed on an outpatient basis. An ileal resection specimen obtained at ileostomy closure showed marked eosinophilic infiltration (> 50/HPF) in the subserosa (Figure 3).
The incidence of EGE in the United States is 2.5-30 cases per 100000 people, whereas the incidence in Japan is estimated to be 5.5 times higher[10]. EGE is usually difficult to diagnose because of the variety of gastrointestinal symptoms, as well as the extremely nonspecific findings of gastrointestinal endoscopy[11]. A case series that reported the small intestinal capsule endoscopic findings in 10 EGE cases found small intestinal lesions such as multiple erythematous lesions in 6 cases, erosions and ulcers in 5 cases, flattened or missing villi in 4 cases, and intestinal stenosis in 7 cases[9]. In that study, EGE was defined as EGID with extensive lesions extending from the stomach to the large intestine; however, only one pediatric and 6 adult cases of EoN localized to the small intestine have been reported (Table 2)[12-17]. These reports described various forms of ulcerative lesions and strictures in the small intestine, but all patients were diagnosed with EGE because of the difficulty of distinguishing EGE from CEAS based on the endoscopic images, as in the present case, and the patients had histologically significant eosinophilic infiltrates[12-17]. In contrast, the present case showed hypoproteinemia and iron deficiency anemia combined with multiple oblique and circular ileal ulcers, consistent with the diagnostic criteria for CEAS[18].
| Case | Age/sex | Location | Endoscopic findings | Laboratory findings | ||||
| Multiple erythema | Erosions | Ulcer | Stricture | Anemia | Hypoproteinemia | |||
| 1 | 62/F | Throughout | - | + | + | - | NR | NR |
| 2 | 66/F | Throughout | - | + | + | - | NR | NR |
| 3 | 48/M | Upper jejunum/ileum | - | - | + | - | - | - |
| 4 | 2/M | Jejunum/proximal ileum | + | - | - | - | - | NR |
| 5 | 70/F | Ileum | - | - | - | - | - | + |
| 6 | 54/M | Ileum | - | - | - | + | - | - |
| 7 | 68/M | Distal jejunum/proximal ileum | - | - | - | + | - | - |
CEAS was first reported in Japan in 1968 as "nonspecific multiple ulcers of the small intestine"[5] and is caused by SLCO2A1 germline variants encoding a prostaglandin transporter. The identification of hot spots of SLCO2A1 variants is thus valuable for diagnosis but not currently included in the definitive diagnostic guidelines[5,7,18]. In addition, the clinical manifestations of CEAS are chronic and intractable nonspecific gastrointestinal symptoms comparable to EGID, and no effective treatment for these disorders has been established[18]. Ulcers of CEAS have been described as shallow oblique, circular, or longitudinal with discrete margins in case series of the endoscopic findings of CEAS[13,19]. CEAS-like diseases without SLCO2A1 mutations have also been reported, including inherited eicosanoid metabolic disorders, inherited human cPLA2α deficiency, and cryptogenic multifocal ulcerous stenosing enteritis; histologically, however, these diseases show nonspecific inflammatory cell infiltration predominantly by neutrophils, rather than eosinophils[20,21]. The present case thus meets the diagnostic criteria for CEAS, but the diagnosis of EoN was reasonable based on the histological findings and the therapeutic course and responsiveness.
The patient developed bowel obstruction induced by small bowel stricture during the clinical course. Most cases of CEAS manifest with small bowel stricture associated with the healing of ulcers and require long-term endoscopic follow-up and treatment, whereas few cases of small bowel stricture have been reported in EGID[16,17,22,23]. In particular, two EoN cases with stricture showed a transmural eosinophilic infiltration at the resected intestinal tract[16,17], suggesting that the eosinophilic infiltration in the muscle layer and serosa is a risk factor for intestinal stricture. However, identification of these by endoscopic mucosal biopsy may be extremely difficult. In the present case, the mucosal eosinophilic infiltration at the time of the small bowel resection was insignificant compared to that at the ileostomy closure, in which insufficient therapeutic agents were regularly administered, suggesting the treatment for EoN was unlikely to be inadequate. This fact indicates that periodic endoscopic follow-up with consideration of the possibility of small bowel stricture would be required in EoN localized to the small bowel with CEAS-like ulcerative lesions.
EoN should be included in the differential diagnosis of patients who exhibit CEAS-like ulcerative lesions localized to the small intestine and have normal urinary prostaglandin metabolites. In addition, EoN with CEAS-like ulcerative lesions may require periodic endoscopic follow-up taking into account the potential complication of small bowel stricture.
The authors would like to thank the patient’s family for permission to publish this case report.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Japan
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Anand A, Nepal; Bernabe-Ortiz JC, Peru; Hakimi T, Afghanistan; Paparoupa M, Germany S-Editor: Yan JP L-Editor: A P-Editor: Yan JP
| 1. | Rothenberg ME. Eosinophilic gastrointestinal disorders (EGID). J Allergy Clin Immunol. 2004;113:11-28; quiz 29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 627] [Cited by in RCA: 584] [Article Influence: 27.8] [Reference Citation Analysis (0)] |
| 2. | Ingle SB, Hinge Ingle CR. Eosinophilic gastroenteritis: an unusual type of gastroenteritis. World J Gastroenterol. 2013;19:5061-5066. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 69] [Cited by in RCA: 84] [Article Influence: 7.0] [Reference Citation Analysis (1)] |
| 3. | Dellon ES, Gonsalves N, Abonia JP, Alexander JA, Arva NC, Atkins D, Attwood SE, Auth MKH, Bailey DD, Biederman L, Blanchard C, Bonis PA, Bose P, Bredenoord AJ, Chang JW, Chehade M, Collins MH, Di Lorenzo C, Dias JA, Dohil R, Dupont C, Falk GW, Ferreira CT, Fox AT, Genta RM, Greuter T, Gupta SK, Hirano I, Hiremath GS, Horsley-Silva JL, Ishihara S, Ishimura N, Jensen ET, Gutiérrez-Junquera C, Katzka DA, Khoury P, Kinoshita Y, Kliewer KL, Koletzko S, Leung J, Liacouras CA, Lucendo AJ, Martin LJ, McGowan EC, Menard-Katcher C, Metz DC, Miller TL, Moawad FJ, Muir AB, Mukkada VA, Murch S, Nhu QM, Nomura I, Nurko S, Ohtsuka Y, Oliva S, Orel R, Papadopoulou A, Patel DA, Pesek RD, Peterson KA, Philpott H, Putnam PE, Richter JE, Rosen R, Ruffner MA, Safroneeva E, Schreiner P, Schoepfer A, Schroeder SR, Shah N, Souza RF, Spechler SJ, Spergel JM, Straumann A, Talley NJ, Thapar N, Vandenplas Y, Venkatesh RD, Vieira MC, von Arnim U, Walker MM, Wechsler JB, Wershil BK, Wright BL, Yamada Y, Yang GY, Zevit N, Rothenberg ME, Furuta GT, Aceves SS. International Consensus Recommendations for Eosinophilic Gastrointestinal Disease Nomenclature. Clin Gastroenterol Hepatol. 2022;20:2474-2484.e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 91] [Article Influence: 30.3] [Reference Citation Analysis (0)] |
| 4. | Chen MJ, Chu CH, Lin SC, Shih SC, Wang TE. Eosinophilic gastroenteritis: clinical experience with 15 patients. World J Gastroenterol. 2003;9:2813-2816. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 154] [Cited by in RCA: 146] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 5. | Umeno J, Hisamatsu T, Esaki M, Hirano A, Kubokura N, Asano K, Kochi S, Yanai S, Fuyuno Y, Shimamura K, Hosoe N, Ogata H, Watanabe T, Aoyagi K, Ooi H, Watanabe K, Yasukawa S, Hirai F, Matsui T, Iida M, Yao T, Hibi T, Kosaki K, Kanai T, Kitazono T, Matsumoto T. A Hereditary Enteropathy Caused by Mutations in the SLCO2A1 Gene, Encoding a Prostaglandin Transporter. PLoS Genet. 2015;11:e1005581. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 112] [Cited by in RCA: 103] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 6. | Matsuno Y, Umeno J, Esaki M, Hirakawa Y, Fuyuno Y, Okamoto Y, Hirano A, Yasukawa S, Hirai F, Matsui T, Hosomi S, Watanabe K, Hosoe N, Ogata H, Hisamatsu T, Yanai S, Kochi S, Kurahara K, Yao T, Torisu T, Kitazono T, Matsumoto T. Measurement of prostaglandin metabolites is useful in diagnosis of small bowel ulcerations. World J Gastroenterol. 2019;25:1753-1763. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 18] [Cited by in RCA: 18] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 7. | Jimbo K, Okuno T, Ohgaki R, Nishikubo K, Kitamura Y, Sakurai Y, Quan L, Shoji H, Kanai Y, Shimizu T, Yokomizo T. A novel mutation in the SLCO2A1 gene, encoding a prostaglandin transporter, induces chronic enteropathy. PLoS One. 2020;15:e0241869. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 8. | Arai K. Very Early-Onset Inflammatory Bowel Disease: A Challenging Field for Pediatric Gastroenterologists. Pediatr Gastroenterol Hepatol Nutr. 2020;23:411-422. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 16] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 9. | Higuchi T, Tokunaga M, Murai T, Takeuchi K, Nakayama Y. Elemental diet therapy for eosinophilic gastroenteritis and dietary habits. Pediatr Int. 2022;64:e14894. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 10. | Zhang M, Li Y. Eosinophilic gastroenteritis: A state-of-the-art review. J Gastroenterol Hepatol. 2017;32:64-72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 97] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 11. | Kinoshita Y, Furuta K, Ishimaura N, Ishihara S, Sato S, Maruyama R, Ohara S, Matsumoto T, Sakamoto C, Matsui T, Ishikawa S, Chiba T. Clinical characteristics of Japanese patients with eosinophilic esophagitis and eosinophilic gastroenteritis. J Gastroenterol. 2013;48:333-339. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 114] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 12. | Mizumoto N, Sasaki Y, Abe Y, Yagi M, Kon T, Onozato Y, Sakai T, Ito M, Umehara M, Ueno Y. Small-bowel Capsule Endoscopic Features in Patients with Eosinophilic Gastroenteritis: Three Case Reports. Intern Med. 2021;60:2961-2965. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 13. | Okuda K, Daimon Y, Iwase T, Mitsufuji S. Novel findings of capsule endoscopy and double-balloon enteroscopy in a case of eosinophilic gastroenteritis. Clin J Gastroenterol. 2013;6:16-19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 14. | Nguyen N, Kramer RE, Friedlander JA. Videocapsule Endoscopy Identifies Small Bowel Lesions in Patients With Eosinophilic Enteritis. Clin Gastroenterol Hepatol. 2018;16:e64-e65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 11] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 15. | Yamaga Y, Mizuno M, Okae S, Nio-Tamaoki M, Masuo K, Mashimo-Matsuo Y, Tanaka J, Nabeshima M. Eosinophilic enteritis accompanied by cytomegalovirus disease: a case report. BMC Gastroenterol. 2022;22:209. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 16. | Jagtap SV, Nikumbh DB, Kshirsagar AY, Ahuja N. Unusual presentation of eosinophilic enteritis as multiple strictures of small intestine. Clin Pract. 2012;2:e24. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 17. | Shivathirthan N, Maheshwari G, Kamath D, Haldar P. Enterolithiasis complicating eosinophilic enteritis: A case report and review of literature. World J Gastrointest Surg. 2009;1:68-70. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 6] [Cited by in RCA: 7] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 18. | Hosoe N, Ohmiya N, Hirai F, Umeno J, Esaki M, Yamagami H, Onodera K, Bamba S, Imaeda H, Yanai S, Hisamatsu T, Ogata H, Matsumoto T; CEAS Atlas Group. Chronic Enteropathy Associated With SLCO2A1 Gene [CEAS]-Characterisation of an Enteric Disorder to be Considered in the Differential Diagnosis of Crohn's Disease. J Crohns Colitis. 2017;11:1277-1281. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 36] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 19. | Umeno J, Esaki M, Hirano A, Fuyuno Y, Ohmiya N, Yasukawa S, Hirai F, Kochi S, Kurahara K, Yanai S, Uchida K, Hosomi S, Watanabe K, Hosoe N, Ogata H, Hisamatsu T, Nagayama M, Yamamoto H, Abukawa D, Kakuta F, Onodera K, Matsui T, Hibi T, Yao T, Kitazono T, Matsumoto T; CEAS study group. Clinical features of chronic enteropathy associated with SLCO2A1 gene: a new entity clinically distinct from Crohn's disease. J Gastroenterol. 2018;53:907-915. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 47] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 20. | Chang C, Jiang C, Miao Y, Fang B, Zhang L. A case report of intestinal obstruction caused by cryptogenic multifocal ulcerous stenosing enteritis. BMC Gastroenterol. 2020;20:300. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 21. | Adler DH, Cogan JD, Phillips JA 3rd, Schnetz-Boutaud N, Milne GL, Iverson T, Stein JA, Brenner DA, Morrow JD, Boutaud O, Oates JA. Inherited human cPLA(2alpha) deficiency is associated with impaired eicosanoid biosynthesis, small intestinal ulceration, and platelet dysfunction. J Clin Invest. 2008;118:2121-2131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 56] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 22. | Tan HL, Sithasanan N, Foley P, Davidson GP. The successful medical management of severe duodenal strictures secondary to eosinophilic gastroenteritis in an infant. Pediatr Surg Int. 2003;19:562-563. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 23. | Attar A, Cazals-Hatem D, Ponsot P. Videocapsule endoscopy identifies stenoses missed by other imaging techniques in a patient with eosinophilic gastroenteritis. Clin Gastroenterol Hepatol. 2011;9:A28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |