Published online Mar 21, 2023. doi: 10.3748/wjg.v29.i11.1745
Peer-review started: October 24, 2022
First decision: November 2, 2022
Revised: November 7, 2022
Accepted: March 6, 2023
Article in press: March 6, 2023
Published online: March 21, 2023
Processing time: 143 Days and 13.2 Hours
Human immunodeficiency virus (HIV)-positive patients coinfected with hepatitis B virus (HBV) are eligible for liver transplantation (LT) in Africa and Southeast Asia, particularly China. However, the outcome of HIV-HBV coinfected patients referred for ABO-incompatible LT (ABOi-LT) is unknown.
To clarify the outcome of ABOi-LT for HIV-HBV coinfected patients with end-stage liver disease (ESLD).
We report on two Chinese HIV-HBV coinfected patients with ESLD who underwent A to O brain-dead donor LT and reviewed the literature on HIV-HBV coinfected patients treated with ABO-compatible LT. The pretransplantation HIV viral load was undetectable, with no active opportunistic infections. Induction therapy consisted of two sessions of plasmapheresis and a single dose of rituximab in two split doses, followed by an intraoperative regimen of intravenous immunoglobulin, methylprednisolone, and basiliximab. Post-transplant maintenance immunosuppressive agents consisted of tacrolimus and mycophenolate mofetil, and prednisone.
At the intermediate-term follow-up, patients showed undetectable HIV viral load, CD4(+) T cell counts greater than 150 cells/μL, no HBV recurrence, and stable liver function. A liver allograft biopsy showed no evidence of acute cellular rejection. Both patients survived at 36-42 mo of follow-up.
This is the first report of ABOi-LT in HIV-HBV recipients with good intermediate-term outcomes, suggesting that ABOi-LT may be feasible and safe for HIV-HBV coinfected patients with ESLD.
Core Tip: The outcome of human immunodeficiency virus (HIV)-hepatitis B virus (HBV) coinfected patients referred for ABO-incompatible liver transplantation (LT) (ABOi-LT) is unknown. We report on two Chinese HIV-HBV coinfected patients with end-stage liver disease (ESLD) who underwent A to O brain-dead donor LT and reviewed the literature on HIV-HBV coinfected patients treated with ABO-compatible LT. At intermediate-term follow-up, patients showed undetectable HIV viral load, CD4(+) T cell counts greater than 150 cells/μL, no HBV recurrence, and stable liver function. Both patients survived at 36-42 mo of follow-up. This is the first report of ABOi-LT in HIV-HBV recipients with good intermediate-term outcomes, suggesting that ABOi-LT may be feasible and safe for HIV-HBV coinfected patients with ESLD.
- Citation: Tang JX, Zhang KJ, Fang TS, Weng RH, Liang ZM, Yan X, Jin X, Xie LJ, Zeng XC, Zhao D. Outcomes of ABO-incompatible liver transplantation in end-stage liver disease patients co-infected with hepatitis B and human immunodeficiency virus. World J Gastroenterol 2023; 29(11): 1745-1756
- URL: https://www.wjgnet.com/1007-9327/full/v29/i11/1745.htm
- DOI: https://dx.doi.org/10.3748/wjg.v29.i11.1745
The recent introduction of highly active antiretroviral therapy (HAART) has dramatically changed the natural history of human immunodeficiency virus (HIV) infection[1]. Both the mortality rate and the incidence of acquired immune deficiency syndrome (AIDS) due to HIV infection have decreased with effective suppression of viral replication and prophylaxis against opportunistic infections[2]. However, HIV-infected patients are frequently coinfected with hepatitis B virus (HBV) since both viruses share similar modes of transmission, resulting in an increased risk of developing chronic liver disease[3,4]. After the dramatic improvement in the survival of HIV-infected patients with HAART, hepatitis cirrhosis and its complications have replaced opportunistic infections as the leading cause of mortality in the HIV-HBV coinfected patient[5]. In addition, almost all of the antiretroviral agents are metabolized in the liver. Patients with hepatic metabolic impairment cannot use these agents, accounting for the increased mortality associated with AIDS[6]. Accordingly, end-stage liver disease (ESLD) accounts for up to 50% of deaths in HIV-infected patients[7,8].
It has long been thought that HIV is a contraindication to liver transplantation (LT) in the pre-HAART era since immunosuppression can reportedly aggravate HIV infection and complications[9]. Case reports have shown nearly 25% AIDS-related mortality in HIV patients 6 mo after transplantation[10,11]. However, an increasing body of evidence suggests comparable survival rates between HIV-positive and HIV-negative recipients after LT in the HAART era[12-14]. These results suggest that HIV infection should not be a contraindication to LT, provided the underlying HIV disease is under control. Recent studies have shown that common indicators of controlled HIV disease-infected patient pretransplantation include an HIV viral load < 200 copies/mm3, a CD4(+) T cell count greater than 200 cells/μL, and the absence of active opportunistic infections for at least 6 mo[15].
ABO-incompatible LT (ABOi-LT) is considered to be a high-risk procedure, compared to ABO-compatible LT, associated with a higher rate of antibody-mediated rejection, biliary complications, hepatic artery thrombosis, and mortality[16]. Hence, ABO-incompatible liver grafts have been used as a rescue option. Advances in the treatment strategies for ABOi-LT[17] include plasmapheresis, intravenous immunoglobulin (IVIG), splenectomy, rituximab, antilymphocyte antibodies, and immunosuppressant medications have improved the post-LT outcomes. The past decade has witnessed an increase in ABOi-LT procedures with increasing success. No significant difference between rejection and allograft survival at 1, 3, and 5 years after transplantation was found in a United Network of Organ Sharing Database analysis between 1990 and 2010 that compared ABOi liver transplants with ABO-compatible transplants[18].
Notably, the China Liver Transplant Registry does not prohibit HIV patients from receiving organs. With the introduction and effectiveness of HAART therapy, the outcomes of HIV-positive recipients with ESLD are reportedly similar to HIV-negative recipients after LT[19]. However, the incompatibility between the ABO blood group and living organ donation severely limits the transplantation opportunities for this patient population[20]. Besides, LT in HIV patients using ABOi organs brings additional complexity and difficulty to this already intricate patient population. Therefore, assessing the practicability of ABOi-LT in HIV-positive recipients is essential. To our knowledge, ABOi-LT in an HIV-HBV coinfected recipient with ESLD has hitherto not been documented in the literature. Here, we report on two cases and review major clinical and research issues related to HIV-HBV coinfected patients treated with ABO-compatible LT. We present the following article in accordance with the AME Case Series reporting checklist.
From January 2019 to December 2021, 7 patients with HIV infection underwent LT in our LT center, including 2 patients with ABOi-LT. These 2 patients underwent ABOi-LT between April 2019 and December 2019, and their clinical data were extracted from our database. Both patients received HAART and anti-HBV therapy before transplantation and presented undetectable HIV RNA and HBV DNA levels, while the CD4(+) T cell count of one patient was less than 100 cells/μL. The surgical technique of ABOi-LT was a modified piggyback technique with triangulation of the hepatic veins. The vena cava anastomosis was completed with three separate continuous sutures, first completing the right side of the triangle. Subsequently, we released the vena cava blood flow to reduce the cold ischemia time. Next, we successively performed portal vein and hepatic artery vascular anastomosis. Bile duct reconstruction was performed by end-to-end anastomosis (continuous for the posterior wall and interrupted for the anterior wall). The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This retrospective study was a single-center consecutive case series analysis approved by the Ethics Committee of the Third People's Hospital of Shenzhen (No. 2022-038-02). Written informed consent was obtained from all participants.
To the best of our knowledge, no cases of ABO incompatibility involving HIV-HBV coinfection recipients have been reported in the literature. Little information is available on HIV-HBV coinfected patients that undergo LT and methods to improve survival and reduce complications. We systematically searched for all patients diagnosed with HIV who underwent LT from 1995 to 2022. Search terms were (HIV or human immunodeficiency virus) AND (HBV or hepatitis B virus) AND (liver or hepatic) AND (transplantation or transplant) in Embase, MEDLINE, and PubMed. When data were missing, we contacted the study authors for additional information.
Case 1: A 61-year-old HIV-positive Chinese man with grade IV hepatic encephalopathy and hepatorenal syndrome secondary to decompensated HBV cirrhosis was referred for LT. He was considered a good candidate for LT because he had undetectable HIV RNA levels for at least 5 years, the CD4(+) T cell count was 42 cell/μL, and he received lamivudine and efavirenz as part of his Reverse Transcriptase inhibitor-based HAART therapy regimen for approximately 6 years. The model for ESLD (MELD) score was 40. However, given the absence of a suitable ABO-compatible liver donor and the patient's critical condition requiring urgent LT, a man with blood type A+ was used as the donor (O+ recipient, A+ donor). The recipient's latest anti-A titers were 1:128 for immunoglobulin G (IgG) and 1:32 for immunoglobulin M (IgM) before transplantation.
The decision was made to proceed with LT on April 4, 2019. Induction therapy consisted of two sessions of plasmapheresis, and rituximab 375 mg/m2 in split doses was administered intravenously before surgery. IVIG 400 mg/kg, rituximab 375 mg/m2 in split doses, and methylprednisolone 0.5 g were administered intravenously during the operation; and basiliximab 20 mg was administered intravenously prior to the release of circulation in the graft.
Following transplantation, the patient was started on methylprednisolone for 7 d and switched to prednisone 48 mg daily and tapered to 8 mg daily over 4 wk. Maintenance immunosuppressive agents included tacrolimus and mycophenolate mofetil. Initially, 1 mg tacrolimus twice daily was started on postoperative day (POD) 1 and titrated to achieve a trough of 12 to 15 ng/mL during the first 2 postoperative weeks. The tacrolimus dose was adjusted to maintain a concentration of 8 to 12 ng/mL from 2 wk to 1 mo after transplantation, 6 to 8 ng/mL within 1 to 6 mo after transplantation, and then 4 to 6 ng/mL for long-term follow-up in our institution. Mycophenolate mofetil was started on POD4 and adjusted reasonably according to the postoperative liver function, infection index, white blood cell count, and drug concentration. IVIG 400 mg/kg was given daily for 7 d, then every other day for two more sessions. In addition, the second dose of basiliximab 20 mg was given on POD4. HAART therapy consisting of dolutegravir and lamivudine was restarted on POD1. Post-transplant anti-HBV therapy included tenofovir alafenamide and monthly infusions of high-dose hepatitis B immune globulin (HBIG). Given the presence of HIV-associated immunodeficiency and antirejection medication use, infection prophylaxis is critical. The patient was treated with antibiotics, ganciclovir, and antifungal combination therapy.
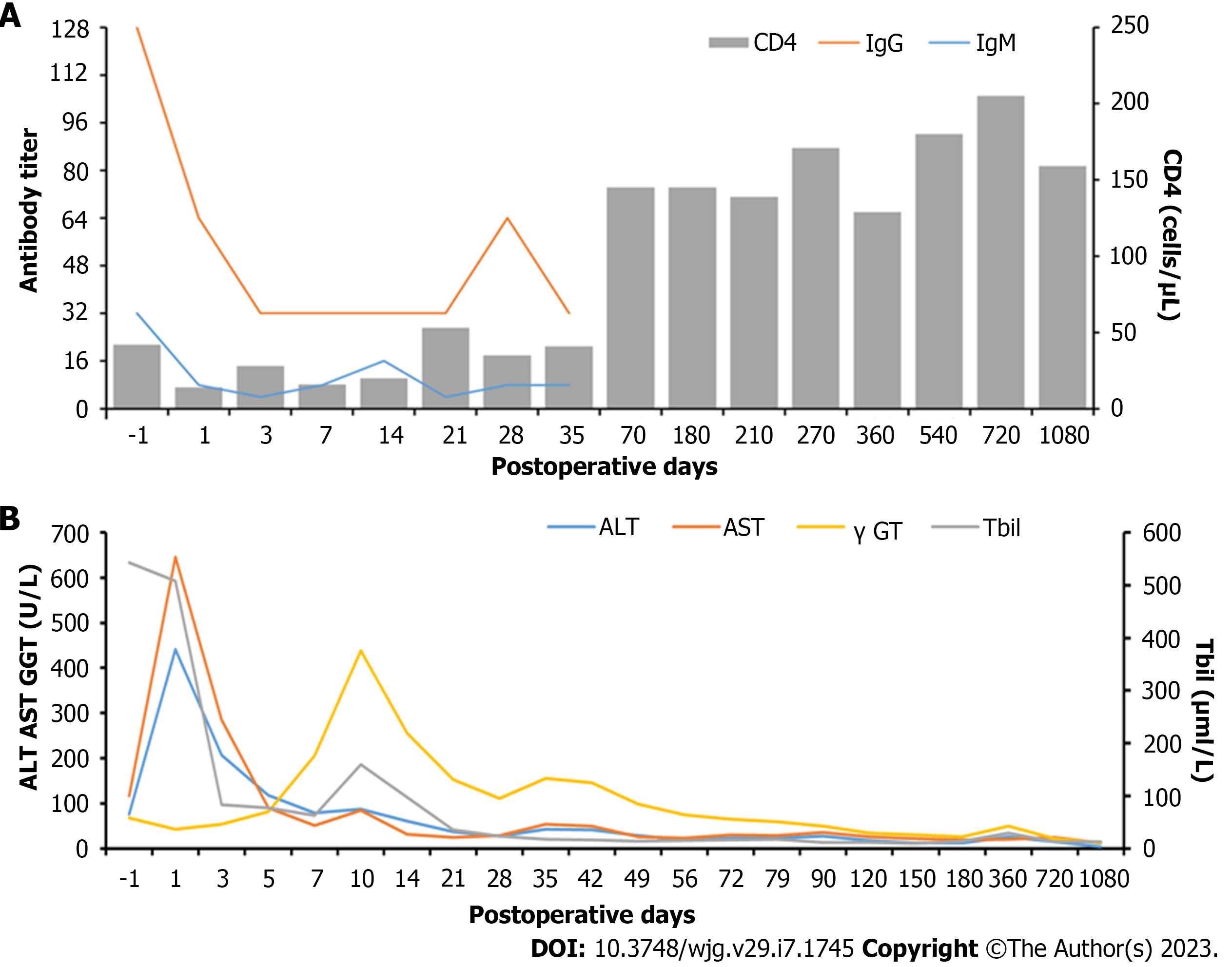
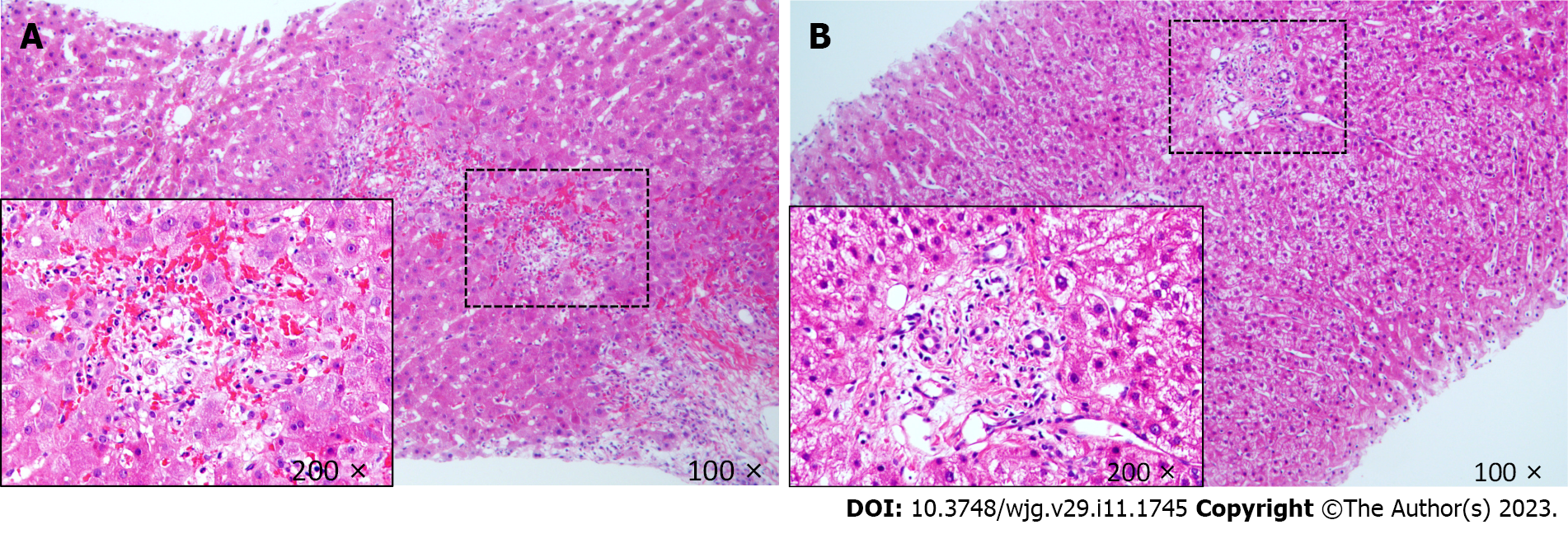
Due to a high preoperative anti-A IgM/IgG titer, the anti-A IgM/IgG titer, CD4(+) T lymphocyte count, postoperative rejection, and the risk of opportunistic infection after LT were dynamically monitored. From POD1, ABO blood group antibody titers and T lymphocyte subsets were detected in the blood collected from the patient every other day. Postoperative monitoring showed that anti-A IgG/IgM decreased from 1:128/1:32 to 1:32/1:16 at 2 wk postoperatively (Figure 1A). There were no problems with postoperative infection and HIV management; the patient received post-transplant HAART and anti-HBV therapy with CD4(+) T cell counts ranging from 60 to 148 cells/μL with undetectable HIV RNA and HBV DNA levels. Allograft ultrasound showed normal blood vessels and biliary tract. Serum transaminase peaked at 458 U/L on POD1, then began to trend down. However, graft function worsened on POD14. Mild elevation of the hepatic enzymes [total bilirubin (Tbil): 97 μmol/L; γ-glutamyltransferase: 255 U/L; aspartate aminotransferase: 39 U/L; alanine aminotransferase (ALT): 61 U/L] was observed (Figure 1B). The IgG and IgM titers remained stable (1:32 and 1:16, respectively). The follow-up liver allograft biopsy showed mild acute cellular rejection, with a Banff rejection activity index score of 4 (Figure 2A). After the patient received intravenous pulse steroid therapy (80 mg of methylprednisolone for 3 d, then tapered), liver enzymes decreased and subsequently remained in the normal range. The patient was discharged on POD37 without any infection and exhibited normal liver function. On subsequent clinical follow-up, normal hepatic enzymes were maintained. His latest CD4(+) T cell count was 159 cells/μL on June 6, 2022. A follow-up liver biopsy 1 year after transplantation revealed no evidence of graft rejection. More than 3 years after LT, the patient and graft function remained stable.
Case 2: A 46-year-old HIV-HBV coinfected Chinese man with acute-on-chronic liver failure was referred for LT. The preoperative Tbil was 320 μmol/L, and the international normalized ratio was 9.85. A multidisciplinary team discussion concluded that he was considered a good candidate for emergency LT with a MELD score of 40 and received a HAART therapy regimen for approximately 3 years with undetectable HIV RNA levels and a CD4(+) T cell count of 120 cells/μL. However, in the absence of suitable ABO-compatible liver donors, a man with blood type A+ was used as the donor (O+ recipient, A+ donor). The recipient's most recent anti-A titers for IgG and IgM were both 1:32 before transplantation. On November 19, 2019, the patient underwent ABOi-LT in our LT center. The induction treatment and maintenance immunosuppression scheme is shown in patient 1. HAART therapy was restarted on POD1 without changes. Given the immunodeficiency status of the patient treated with antirejection medication, the patient was treated with antibiotics, ganciclovir, and an antifungal combination therapy for infection prophylaxis.
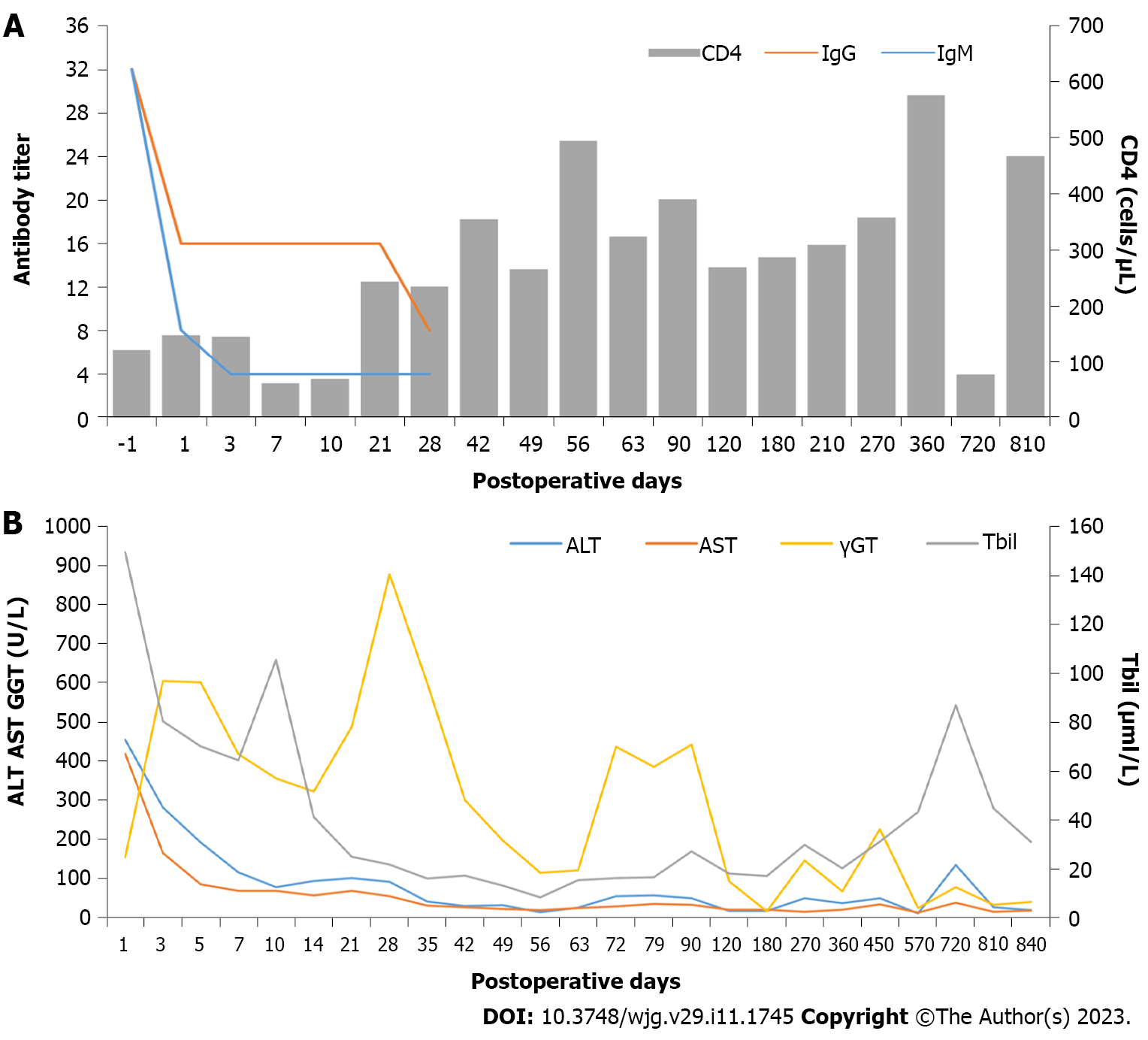
The baseline anti-A IgG/IgM titers were both 1:32. Although the baseline anti-A titer was high and the CD4(+) T cell count was less than 200 cells/μL, an emergency ABOi-LT was successfully performed. The anti-A IgG/IgM titer, CD4(+) T cell count, postoperative rejection, and the risk of opportunistic infection after LT were dynamically monitored. Postoperatively the anti-A IgG/IgM titers decreased from 1:32/1:32 to 1:16/1:4 at 2 wk. Postoperative infection was not observed, and HAART and anti-HBV therapy were continued; the post-transplant CD4(+) T cell counts ranged from 62 to 494 cells/μL with undetectable HIV RNA and HBV DNA levels. The patient was finally discharged on POD63 with normal liver function, IgG and IgM titers of 1:8 and 1:4, respectively, and the absence of any complications (Figure 3A).
Deteriorating graft function was observed three months after LT, with relatively stable liver function (Figure 3B). The blood drug concentration of tacrolimus was 3 ng/mL, and the CD4(+) T-cell count was 390 cells/μL. A follow-up liver biopsy at 6 mo after transplantation revealed no evidence of graft rejection. Twenty-five months after LT, he was hospitalized due to abnormal hepatic enzymes and underwent a liver biopsy. The pathology report suggested chronic cholangitis with bile duct sclerosis and the possibility of early chronic rejection (Figure 2B). After the adjustment of the immunosuppressive drug regimen, the patient's liver function gradually stabilized. During the subsequent follow-up, the hepatic enzymes remained within the normal range with a CD4(+) T cell count of 467 cells/μL on February 7, 2022. Nearly 36 mo after LT, the patient and graft function remained stable.
To date, there have been no reports of successful ABOi-LT in HIV-HBV coinfected patients. To understand the prognosis and risk of HIV complications in patients with simultaneous HBV infection after LT, we reviewed the literature for LT in HBV-HIV coinfected patients[12-15,21-27]. Eleven studies were screened, reporting the characteristics of 69 patients with HIV-HBV coinfection that underwent LT from 1995 to 2022 (Table 1). The etiology of liver disease was HBV-related cirrhosis (n = 62) and fulminant liver failure due to HBV (n = 7).
| Ref. | Patients, n | Study period in yr | Pre-LT | Post-LT | ||||||||
| Liver disease | CD4(+) T cell count as cells/μL | HIV-RNA as copies/mm3 | HBV-DNA as IU/mL | Median follow-up in mo | Latest CD4(+) T cell count as cells/μL | Latest HIV-RNA as copies/mm3 | Latest HBV-DNA as IU/mL | Rejections, n | Survival after LT in mo | |||
| Tateo et al[12] | 13 | 1999-2007 | HBV | 173 (118-615)1 | 100% < 40 | 100% < 12 | 32 ± 5.22 | 281 (10-810)1 | 100% < 40 | 100% < 12 | 2 | 100% 1 yr, 100% 3 yr, 100% 5 yr |
| Schreibman et al[27] | 6 | 1999-2006 | 5 HBV, 1 ALF | 100% > 100 | 100% < 200 | 66.7% < 12 | 60 (2-64)1 | 83.3% > 100 | 100% < 100 | 83.3% ND | 1 | 66.7% 1 yr, 66.7% 3 yr, 66.7% 5 yr |
| Norris et al[25] | 5 | 1995-2003 | 4 HBV, 1 ALF | 187 (124-293)1 | 20% < 50 | 100% < 12 | 15 (6-65)1 | 467 (241-754)1 | 100% < 50 | 100% < 12 | 1 | 100% 1 yr, 100% 3 yr |
| Coffin et al[15] | 22 | 2001-2007 | 21 HBV, 1 ALF | 317 (38–1070)1 | 100% < 40 | 45.4% ND | 42 (0.6-84)1 | 289 (48–744)1 | NA | 68% ND | 5 | 85% 1 yr, 85% 3 yr |
| Vernadakis et al[1] | 2 | 1996-2009 | 1 HBV, 1 ALF | 219, 403 | 50% < 50 | NA | 3, 34 | NA | NA | NA | 0 | 3, 34 |
| Neff et al[22] | 4 | 1997-2001 | 2 HBV, 2 ALF | 75% > 100 | 50% < 50 | ND | 21 (5-36)1 | 100% > 100 | 100% < 50 | ND | 3 | 100% 1 yr, 100% 3 yr |
| Anadol et al[23] | 10 | 1997-2011 | HBV | 100% > 100 | 80% < 50 | ND | NA | NA | ND | ND | 1 | 80% 1 yr, 80% 3 yr, 80% 5 yr |
| Radecke et al[24] | 1 | 1998-2001 | HBV | 196 | 380 | NA | 3 | > 100 | NA | NA | 1 | 3 |
| Schliefer et al[26] | 1 | 1997-1999 | ALF | 477 | < 80 | NA | 27 | > 100 | < 80 | NA | 0 | 27 (Alive) |
| Roland et al[21] | 1 | NA | HBV | 439 | ND | ND | 20 | 305 to 700 | ND | ND | 0 | 20 (Alive) |
| Terrault et al[1] | 4 | 2000-2002 | HBV | 175 (104-439) | 100% < 75 | ND | 18, 25, 42, 48 | 315 (125-505) | ND | ND | 0 | 100% 1 yr, 100% 3 yr |
Fifty-nine patients had HIV infection under control, with undetectable or low HIV viral loads and no previous AIDS events or opportunistic infections upon LT waiting list registration. At the time of transplantation, all but one patient had CD4(+) T cell counts above ≥ 100 cells/μL[22]. Polymerase chain reaction showed that 17.4% of patients (12/69) had detectable HBV DNA prior to transplant (among these, 10 patients received adefovir and/or entecavir therapy, and 2 patients received lamivudine combined with tenofovir therapy).
All patients received HAART following LT and plasma HIV-RNA remained low to undetectable in all patients (one case presented with a viral load of 76 copies/mL) during follow-up. No viral breakthrough was observed. CD4(+) T cell counts were maintained at more than ≥ 100 cells/μL. All patients received dual immunoprophylaxis with hepatitis B immunoglobulin and anti-HBV medications. Eight patients tested positive for HBV DNA (among these, low-level HBV viremia was intermittently detected in 7 patients[15] but not associated with hepatitis B surface antigen detection or ALT elevation; for one patient[27] with a transiently positive HBV DNA, serum HBV DNA results were undetectable after tenofovir was added for antiviral therapy) (Table 1). 20.3% (14/69) of HIV-HBV coinfected patients were treated with high-dose prednisone or adjustment of immunosuppressive therapy after developing acute cellular rejection. However, one patient[23] died of hepatic artery thrombosis and graft failure due to rejection. The cumulative patient survival at one and three years in the HIV-HBV coinfected patients was 85.9% and 77.3%, respectively.
Current evidence suggests that in the era of HAART therapy, morbidity and mortality have declined in HIV-infected patients[28,29]. Mortality in this patient population is mainly attributed to comorbidities such as viral hepatitis infection, which is well-established to be associated with an increased prevalence of HIV-infected patients with ESLD. In recent years, studies have shown that the outcomes of HIV and non-HIV recipients were comparable, which has led to much controversy on transplantation in HIV patients[15]. Multiple centers have established protocols for HIV recipients, including an undetectable HIV RNA viral load and CD4(+) T cell counts greater than 200 cells/μL for sustained HAART therapy without other contraindications to LT. Overwhelming evidence[30-32] suggests promising outcomes for HAART-treated HIV-infected patients with maximally suppressed viral loads and no significant increase in opportunistic infections after LT.
Although LT is more common in selected HIV recipients, no studies have reported ABO incompatibility involving HIV-HBV coinfected recipients in the literature, while only 2 cases involving ABO-incompatible kidney transplantation have been reported in HIV recipients[33,34]. Our study provides the first documented cases of A to O incompatible LT in HIV-HBV coinfected recipients with ESLD. Ample evidence suggests that HIV-positive liver transplant recipients are prone to rejection due to immunosuppression or immune dysregulation from the virus itself[35-37]. Interestingly, the United Network of Organ Sharing Database[38] showed that the overall incidence of acute rejection in non-HIV recipients was 24.7% within 1 year after LT, which is similar to the incidence (20.3%) in our review of HIV-infected recipients. Regardless of the HIV infection status, acute rejection is common in ABOi transplantation, with an acute rejection rate of nearly 22%[18]. In our report, emergency ABOi-LT was successfully performed in HIV-HBV coinfected recipients that received induction therapy and adjunctive immunosuppressive regimens, including plasmapheresis, rituximab, basiliximab, methylprednisolone, and IVIG. Liver biopsy at mid-term follow-up did not show acute cellular rejection.
The two patients described in our study are the first cases of A to O brain-dead donor LT reported in the literature. The A-blood group is unique since it exhibits two phenotypes (A1 and A2) that harbor different immunogenicity. The A2 phenotype is characterized by reduced reactivity with anti-A isoagglutinin since it expresses fewer A epitopes on two of four possible core saccharide chains of the ABO antigen[39]. Besides, an increasing body of evidence[40,41] suggests that transplantation of A2 Liver grafts does not elevate anti-A titers after LT. Kluger et al[18] reported that blood group O recipients with A2 grafts exhibited no significant differences in rejection during the transplant hospitalization and at 12 mo postoperatively, with the same overall and graft survival rates as recipients with O grafts at 1, 5, and 10 years. The patient's preoperative anti-A IgG/IgM titers in case 1 were 1:128 and 1:32, respectively. No definite graft rejection was observed 3 years after LT because the donor's blood group was A2. Our center's protocol for A to O LT is based on baseline (pretransplantation) titer data. If the anti-IgG and the IgM titers are > 1:16, the preoperative management consists of two sessions of plasmapheresis, IVIG 400 mg/kg, and rituximab 375 mg/m2 in split doses. In our article, both patients received this protocol. Postoperative anti-A titer monitoring in patient 1 showed that anti-A IgG/IgM decreased from 1:128/1:32 pre-LT to 1:32/1:16 at 2 wk. During the subsequent three years of clinical follow-up, the patient exhibited normal liver enzyme levels.
Preventing HBV recurrence and HIV progression has become a research hotspot in patients with HIV-HBV coinfection after LT. No consensus has been reached on the optimal anti-HBV therapy in patients with HIV-HBV coinfection. Anti-HIV drugs with anti-HBV activity include lamivudine, tenofovir, and emtricitabine. Drug therapy for HIV infection has important implications for preventing the recurrence of HBV infection. However, treating HBV infection in a coinfected patient with lamivudine or tenofovir alone can result in HIV resistance to these drugs, affecting anti-HIV treatment options in the future[42,43]. Accordingly, clinicians should be aware of the potential impact of nucleos/tide analogue selection on managing HIV-HBV coinfected recipients. Terrault et al[13] suggested that the combination therapy using nucleos(t)ide analogues and HBIG in the HIV-HBV coinfected recipient could effectively prevent post-transplantation HBV recurrence. In this report, the patient had excellent short outcomes after treatment with anti-HBV therapy, including tenofovir alafenamide and HBIG, and the HAART regimen consisting of dolutegravir and lamivudine. Subsequently, the two patients that underwent ABOi-LT with HIV were switched to albuvirtide and dolutegravir, well-recognized for their low hepatorenal toxicity and non-CYP450 enzyme inhibitors[44], reducing the impact of calcineurin inhibitor-type immunosuppressive drugs, which achieved intermediate-term excellent outcomes. Overall, it is essential to continuously monitor HIV RNA and HBV DNA levels and optimize anti-HIV and anti-HBV therapies to reduce postoperative complications and prolong survival in HBV-HIV coinfected recipients. Our HIV patients remained infection-free with good CD4(+) T cell count and stable liver function. Moreover, Albuvirtide and dolutegravir represent good options for HIV patients undergoing ABOi-LT.
Given that the number of HIV-infected patients with ESLD is expected to rise in the coming years, the same organ shortage issues that plague non-HIV patients will become increasingly severe for HIV patients[45]. To our knowledge, these two cases are the first reports of ABOi-LT in HIV-HBV coinfected patients with ESLD in the literature. In this study, the early course of ABOi-LT in HIV recipients was similar to that of ABO-compatible LT, without severe acute rejection. Desensitization regimens for ABOi-LT include rituximab, plasmapheresis, and IVIG. In previous studies[46,47] and our previous clinical practice, it has been observed that multiple doses of rituximab could increase the risk of infection, especially in immunodeficient HIV patients. Therefore, a sufficient dose and course of prophylactic antibiotics are crucial to prevent postoperative infection. Furthermore, the number of targeted B cells was significantly smaller in HIV transplant patients, and multiple doses of rituximab yielded no significant benefit for acute rejection or survival in transplant patients[48], suggesting that a single dose of rituximab may be sufficient. The desensitization protocol is usually initiated 2 to 3 d before transplantation. In these two cases, we administered a single dose of rituximab in two split doses, and two sessions of plasmapheresis were performed to reduce the anti-A titers with satisfactory results.
We successfully performed emergency ABOi-LT in HIV-HBV coinfected patients. Their intermediate-term outcomes are encouraging, with normal graft function. Thus, ABOi-LT may be safe and feasible in HIV-HBV coinfected patients with ESLD. We are cautiously optimistic that ABOi transplantation can be extended to other HIV-positive patients with ESLD.
Human immunodeficiency virus (HIV)-positive patients coinfected with hepatitis B virus (HBV) are eligible for liver transplantation (LT) in Africa and Southeast Asia, particularly China. However, the outcome of HIV-HBV coinfected patients referred for ABO-incompatible LT (ABOi-LT) is unknown.
There have been no reports about the intermediate-term outcome of ABOi-LT in HIV-HBV coinfected recipients.
We sought to clarify the outcome of ABOi-LT for HIV-HBV coinfected patients with end-stage liver disease (ESLD).
We report on two Chinese HIV-HBV coinfected patients with ESLD who underwent A to O brain-dead donor LT and reviewed the literature on HIV-HBV coinfected patients treated with ABO-compatible LT. Data of the pre- and post-transplantation were collected, including HIV viral load, CD4(+) T cell count, induction therapy methods, the immunosuppressive regimen and the clinical materials.
After follow-up for 36-42 mo, both patients survived with undetectable HIV viral load, CD4(+) T cell counts greater than 150 cells/μL, no HBV recurrence, and stable liver function. Liver biopsy showed no evidence of acute cellular rejection.
This is the first study of ABOi-LT in HIV-HBV recipients with good intermediate-term outcomes, which suggests that ABOi-LT may be feasible and safe for HIV-HBV coinfected patients with ESLD.
Due to the relatively small number of cases in the study, follow-up studies with large samples are still required.
We thank all the patients for cooperating with our investigation.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Lo SY, Taiwan; Tavan H, Iran S-Editor: Fan JR L-Editor: Filipodia P-Editor: Fan JR
| 1. | Falade-Nwulia O, Seaberg EC, Snider AE, Rinaldo CR, Phair J, Witt MD, Thio CL. Incident Hepatitis B Virus Infection in HIV-Infected and HIV-Uninfected Men Who Have Sex With Men From Pre-HAART to HAART Periods: A Cohort Study. Ann Intern Med. 2015;163:673-680. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 26] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 2. | Sezgin E, Van Natta ML, Thorne JE, Puhan MA, Jabs DA; Longitudinal Studies of the Ocular Complications of AIDS (SOCA) Research Group. Secular trends in opportunistic infections, cancers and mortality in patients with AIDS during the era of modern combination antiretroviral therapy. HIV Med. 2018;19:411-419. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 3. | Liu L, Wang L, Zhang H, Ou W, Li D, Feng Y, Zhuang H, Shao Y. Changing Epidemiology of Hepatitis B Virus and Hepatitis C Virus Coinfection in a Human Immunodeficiency Virus-Positive Population in China: Results From the Third and Fourth Nationwide Molecular Epidemiologic Surveys. Clin Infect Dis. 2021;73:642-649. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 4. | Su M, Liao L, Xing H, Wang S, Li Y, Lu W, He L, Deng J, Shao Y, Li T, Zhuang H. Characteristics of HBV infection in 705 HIV-infected patients under lamivudine-based antiretroviral treatment from three regions in China. Infect Drug Resist. 2018;11:1635-1644. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 5. | Lacombe K, Rockstroh J. HIV and viral hepatitis coinfections: advances and challenges. Gut. 2012;61 Suppl 1:i47-i58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 105] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 6. | Deeks SG, Tracy R, Douek DC. Systemic effects of inflammation on health during chronic HIV infection. Immunity. 2013;39:633-645. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 496] [Cited by in RCA: 625] [Article Influence: 52.1] [Reference Citation Analysis (0)] |
| 7. | Bica I, McGovern B, Dhar R, Stone D, McGowan K, Scheib R, Snydman DR. Increasing mortality due to end-stage liver disease in patients with human immunodeficiency virus infection. Clin Infect Dis. 2001;32:492-497. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 726] [Cited by in RCA: 710] [Article Influence: 29.6] [Reference Citation Analysis (0)] |
| 8. | Tuma P, Jarrin I, Del Amo J, Vispo E, Medrano J, Martin-Carbonero L, Labarga P, Barreiro P, Soriano V. Survival of HIV-infected patients with compensated liver cirrhosis. AIDS. 2010;24:745-753. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 29] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 9. | Baccarani U, Righi E, Adani GL, Lorenzin D, Pasqualucci A, Bassetti M, Risaliti A. Pros and cons of liver transplantation in human immunodeficiency virus infected recipients. World J Gastroenterol. 2014;20:5353-5362. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 17] [Cited by in RCA: 18] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 10. | Fox AN, Vagefi PA, Stock PG. Liver transplantation in HIV patients. Semin Liver Dis. 2012;32:177-185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 27] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 11. | Bouscarat F, Samuel D, Simon F, Debat P, Bismuth H, Saimot AG. An observational study of 11 French liver transplant recipients infected with human immunodeficiency virus type 1. Clin Infect Dis. 1994;19:854-859. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 57] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 12. | Tateo M, Roque-Afonso AM, Antonini TM, Medja F, Lombes A, Jardel C, Teicher E, Sebagh M, Roche B, Castaing D, Samuel D, Duclos-Vallee JC. Long-term follow-up of liver transplanted HIV/hepatitis B virus coinfected patients: perfect control of hepatitis B virus replication and absence of mitochondrial toxicity. AIDS. 2009;23:1069-1076. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 41] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 13. | Terrault NA, Carter JT, Carlson L, Roland ME, Stock PG. Outcome of patients with hepatitis B virus and human immunodeficiency virus infections referred for liver transplantation. Liver Transpl. 2006;12:801-807. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 52] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 14. | Vernadakis S, Sotiropoulos GC, Brokalaki EI, Esser S, Kaiser GM, Cicinnati VR, Beckebaum S, Paul A, Mathé Z. Long-term outcomes of liver transplant patients with human immunodeficiency virus infection and end-stage-liver-disease: single center experience. Eur J Med Res. 2011;16:342-348. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 15. | Coffin CS, Stock PG, Dove LM, Berg CL, Nissen NN, Curry MP, Ragni M, Regenstein FG, Sherman KE, Roland ME, Terrault NA. Virologic and clinical outcomes of hepatitis B virus infection in HIV-HBV coinfected transplant recipients. Am J Transplant. 2010;10:1268-1275. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 75] [Cited by in RCA: 66] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 16. | Lee EC, Kim SH, Park SJ. Outcomes after liver transplantation in accordance with ABO compatibility: A systematic review and meta-analysis. World J Gastroenterol. 2017;23:6516-6533. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 24] [Cited by in RCA: 25] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 17. | Kim SH, Lee EC, Shim JR, Park SJ. A simplified protocol using rituximab and immunoglobulin for ABO-incompatible low-titre living donor liver transplantation. Liver Int. 2018;38:932-939. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 24] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 18. | Kluger MD, Guarrera JV, Olsen SK, Brown RS Jr, Emond JC, Cherqui D. Safety of blood group A2-to-O liver transplantation: an analysis of the United Network of Organ Sharing database. Transplantation. 2012;94:526-531. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 29] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 19. | Lieveld FI, Smit C, Richter C, van Erpecum KJ, Spanier BWM, Gisolf EH, Vrolijk JM, Siersema PD, Hoepelman AIM, Reiss P, Arends JE. Liver decompensation in HIV/Hepatitis B coinfection in the combination antiretroviral therapy era does not seem increased compared to hepatitis B mono-infection. Liver Int. 2019;39:470-483. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 15] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 20. | Taege AJ. Human Immunodeficiency Virus Organ Transplantation. Infect Dis Clin North Am. 2018;32:615-634. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 21. | Roland ME, Adey D, Carlson LL, Terrault NA. Kidney and liver transplantation in HIV-infected patients: case presentations and review. AIDS Patient Care STDS. 2003;17:501-507. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 14] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 22. | Neff GW, Bonham A, Tzakis AG, Ragni M, Jayaweera D, Schiff ER, Shakil O, Fung JJ. Orthotopic liver transplantation in patients with human immunodeficiency virus and end-stage liver disease. Liver Transpl. 2003;9:239-247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 123] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 23. | Anadol E, Beckebaum S, Radecke K, Paul A, Zoufaly A, Bickel M, Hitzenbichler F, Ganten T, Kittner J, Stoll M, Berg C, Manekeller S, Kalff JC, Sauerbruch T, Rockstroh JK, Spengler U. Orthotopic liver transplantation in human-immunodeficiency-virus-positive patients in Germany. AIDS Res Treat. 2012;2012:197501. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 24. | Radecke K, Frühauf NR, Miller M, Ross B, Köditz R, Malagó M, Broelsch CE, Gerken G, Treichel U. Outcome after orthotopic liver transplantation in five HIV-infected patients with virus hepatitis-induced cirrhosis. Liver Int. 2005;25:101-108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 27] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 25. | Norris S, Taylor C, Muiesan P, Portmann BC, Knisely AS, Bowles M, Rela M, Heaton N, O'Grady JG. Outcomes of liver transplantation in HIV-infected individuals: the impact of HCV and HBV infection. Liver Transpl. 2004;10:1271-1278. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 72] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 26. | Schliefer K, Paar WD, Aydemir G, Wolff M, Rockstroh JK, Spengler U, Sauerbruch T. [Orthotopic liver transplantation in a 33-year-old patient with fulminant hepatitis B and HIV infection]. Dtsch Med Wochenschr. 2000;125:523-526. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 27. | Schreibman I, Gaynor JJ, Jayaweera D, Pyrsopoulos N, Weppler D, Tzakis A, Schiff ER, Regev A. Outcomes after orthotopic liver transplantation in 15 HIV-infected patients. Transplantation. 2007;84:697-705. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 40] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 28. | Bandera A, Gori A, Clerici M, Sironi M. Phylogenies in ART: HIV reservoirs, HIV latency and drug resistance. Curr Opin Pharmacol. 2019;48:24-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 58] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 29. | Frigati L, Archary M, Rabie H, Penazzato M, Ford N. Priorities for Decreasing Morbidity and Mortality in Children With Advanced HIV Disease. Clin Infect Dis. 2018;66:S147-S151. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 23] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 30. | Bonny TS, Kirby C, Martens C, Rose R, Desai N, Seisa M, Petropoulos C, Florman S, Friedman-Moraco RJ, Turgeon NA, Brown D, Segev DL, Durand CM, Tobian AAR, Redd AD. Outcomes of donor-derived superinfection screening in HIV-positive to HIV-positive kidney and liver transplantation: a multicentre, prospective, observational study. Lancet HIV. 2020;7:e611-e619. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 32] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 31. | Miro JM, Agüero F, Duclos-Vallée JC, Mueller NJ, Grossi P, Moreno A; ESCMID Study Group of Infection in Compromised Hosts. Infections in solid organ transplant HIV-infected patients. Clin Microbiol Infect. 2014;20 Suppl 7:119-130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 24] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 32. | Tan-Tam CC, Frassetto LA, Stock PG. Liver and kidney transplantation in HIV-infected patients. AIDS Rev. 2009;11:190-204. [PubMed] |
| 33. | Forbes RC, DeMers A, Concepcion BP, Moore DR, Schaefer HM, Shaffer D. A2 to B Blood Type Incompatible Deceased Donor Kidney Transplantation in a Recipient Infected with the Human Immunodeficiency Virus: A Case Report. Transplant Proc. 2017;49:206-209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 34. | Campara M, West-Thielke P, Thielke J, Ommert T, Oberholzer J, Benedetti E, Kaplan B. ABO incompatible renal transplantation in an HIV-seropositive patient. Transplantation. 2008;86:176-178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 35. | Agüero F, Rimola A, Stock P, Grossi P, Rockstroh JK, Agarwal K, Garzoni C, Barcan LA, Maltez F, Manzardo C, Mari M, Ragni MV, Anadol E, Di Benedetto F, Nishida S, Gastaca M, Miró JM; FIPSE/NIH HIVTR/NEAT023 Investigators. Liver Retransplantation in Patients With HIV-1 Infection: An International Multicenter Cohort Study. Am J Transplant. 2016;16:679-687. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 36. | Terrault N, Reddy KR, Poordad F, Curry M, Schiano T, Johl J, Shaikh O, Dove L, Shetty K, Millis M, Schiff E, Regenstein F, Barnes D, Barin B, Peters M, Roland M, Stock P; HIVTR Investigators. Peginterferon and ribavirin for treatment of recurrent hepatitis C disease in HCV-HIV coinfected liver transplant recipients. Am J Transplant. 2014;14:1129-1135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 37. | Thorsen T, Dahlgren US, Aandahl EM, Grzyb K, Karlsen TH, Boberg KM, Rydberg L, Naper C, Foss A, Bennet W. Liver transplantation with deceased ABO-incompatible donors is life-saving but associated with increased risk of rejection and post-transplant complications. Transpl Int. 2015;28:800-812. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 12] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 38. | Kim WR, Lake JR, Smith JM, Schladt DP, Skeans MA, Noreen SM, Robinson AM, Miller E, Snyder JJ, Israni AK, Kasiske BL. OPTN/SRTR 2017 Annual Data Report: Liver. Am J Transplant. 2019;19 Suppl 2:184-283. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 224] [Cited by in RCA: 245] [Article Influence: 40.8] [Reference Citation Analysis (0)] |
| 39. | Böhmig GA, Farkas AM, Eskandary F, Wekerle T. Strategies to overcome the ABO barrier in kidney transplantation. Nat Rev Nephrol. 2015;11:732-747. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 47] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 40. | Puri Y, Rammohan A, Sachan D, Vij M, Rela M. ABO-Incompatible Living Donor Liver Transplant From a Blood Type A2 Donor to a Type B Recipient: A Note of Caution. Exp Clin Transplant. 2022;20:100-103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 41. | Skogsberg U, Breimer ME, Friman S, Mjörnstedt L, Mölne J, Olausson M, Rydberg L, Svalander CT, Bäckman L. Successful ABO-incompatible liver transplantation using A2 donors. Transplant Proc. 2006;38:2667-2670. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 23] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 42. | Aoudjane S, Chaponda M, González Del Castillo AA, O'Connor J, Noguera M, Beloukas A, Hopkins M, Khoo S, van Oosterhout JJ, Geretti AM. Hepatitis B virus sub-genotype A1 infection is characterized by high replication levels and rapid emergence of drug resistance in HIV-positive adults receiving first-line antiretroviral therapy in Malawi. Clin Infect Dis. 2014;59:1618-1626. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 26] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 43. | Boyd A, Gozlan J, Maylin S, Delaugerre C, Peytavin G, Girard PM, Zoulim F, Lacombe K. Persistent viremia in human immunodeficiency virus/hepatitis B coinfected patients undergoing long-term tenofovir: virological and clinical implications. Hepatology. 2014;60:497-507. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 63] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 44. | Esposito I, Labarga P, Barreiro P, Fernandez-Montero JV, de Mendoza C, Benítez-Gutiérrez L, Peña JM, Soriano V. Dual antiviral therapy for HIV and hepatitis C - drug interactions and side effects. Expert Opin Drug Saf. 2015;14:1421-1434. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 45. | Neuberger J. An update on liver transplantation: A critical review. J Autoimmun. 2016;66:51-59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 55] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 46. | Egawa H, Teramukai S, Haga H, Tanabe M, Mori A, Ikegami T, Kawagishi N, Ohdan H, Kasahara M, Umeshita K. Impact of rituximab desensitization on blood-type-incompatible adult living donor liver transplantation: a Japanese multicenter study. Am J Transplant. 2014;14:102-114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 141] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 47. | Egawa H, Umeshita K, Uemoto S. Optimal dosage regimen for rituximab in ABO-incompatible living donor liver transplantation. J Hepatobiliary Pancreat Sci. 2017;24:89-94. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 29] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 48. | Wang XZ, Wan Z, Xue WJ, Zheng J, Li Y, Ding CG. B-Cell Activating Factor Predicts Acute Rejection Risk in Kidney Transplant Recipients: A 6-Month Follow-Up Study. Front Immunol. 2019;10:1046. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 17] [Article Influence: 2.8] [Reference Citation Analysis (0)] |