Published online Mar 14, 2023. doi: 10.3748/wjg.v29.i10.1602
Peer-review started: August 29, 2022
First decision: December 12, 2022
Revised: December 23, 2022
Accepted: January 16, 2023
Article in press: January 16, 2023
Published online: March 14, 2023
Processing time: 192 Days and 20.7 Hours
The level of Ki-67 expression has served as a prognostic factor in gastric cancer. The quantitative parameters based on the novel dual-layer spectral detector computed tomography (DLSDCT) in discriminating the Ki-67 expression status are unclear.
To investigate the diagnostic ability of DLSDCT-derived parameters for Ki-67 expression status in gastric carcinoma (GC).
Dual-phase enhanced abdominal DLSDCT was performed preoperatively in 108 patients with gastric adenocarcinoma. Primary tumor monoenergetic CT attenuation value at 40-100 kilo electron volt (kev), the slope of the spectral curve (λHU), iodine concentration (IC), normalized IC (nIC), effective atomic number (Zeff) and normalized Zeff (nZeff) in the arterial phase (AP) and venous phase (VP) were retrospectively compared between patients with low and high Ki-67 expression in gastric adenocarcinoma. Spearman’s correlation coefficient was used to analyze the association between the above parameters and Ki-67 expression status. Receiver operating characteristic (ROC) curve analysis was performed to compare the diagnostic efficacy of the statistically significant parameters between two groups.
Thirty-seven and 71 patients were classified as having low and high Ki-67 expression, respectively. CT40 kev-VP, CT70 kev-VP, CT100 kev-VP, and Zeff-related parameters were significantly higher, but IC-related parameters were lower in the group with low Ki-67 expression status than the group with high Ki-67 expression status, and other analyzed parameters showed no statistical difference between the two groups. Spearman’s correlation analysis showed that CT40 kev-VP, CT70 kev-VP, CT100 kev-VP, Zeff, and nZeff exhibited a negative correlation with Ki-67 status, whereas IC and nIC had positive correlation with Ki-67 status. The ROC analysis demonstrated that the multi-variable model of spectral parameters performed well in identifying the Ki-67 status [area under the curve (AUC) = 0.967; sensitivity 95.77%; specificity 91.89%)]. Nevertheless, the differentiating capabilities of single-variable model were moderate (AUC value 0.630 - 0.835). In addition, the nZeffVP and nICVP (AUC 0.835 and 0.805) showed better performance than CT40 kev-VP, CT70 kev-VP and CT100 kev-VP (AUC 0.630, 0.631 and 0.662) in discriminating the Ki-67 status.
Quantitative spectral parameters are feasible to distinguish low and high Ki-67 expression in gastric adenocarcinoma. Zeff and IC may be useful parameters for evaluating the Ki-67 expression.
Core Tip: This is a retrospective study to preoperatively distinguish the expression of Ki-67 index based on the parameters of spectral computer tomography (CT) in patients with gastric adenocarcinoma. The CT attenuation of virtual monoenergetic images in venous phase and effective atomic number were negatively related to the expression of Ki-67, while iodine concentration exhibited positive associations with it. Multi-variable model of spectral parameters exhibited a better diagnostic efficiency than other single-variable model of spectral parameter in discriminating low and high Ki-67 expression in gastric adenocarcinoma.
- Citation: Mao LT, Chen WC, Lu JY, Zhang HL, Ye YS, Zhang Y, Liu B, Deng WW, Liu X. Quantitative parameters in novel spectral computed tomography: Assessment of Ki-67 expression in patients with gastric adenocarcinoma. World J Gastroenterol 2023; 29(10): 1602-1613
- URL: https://www.wjgnet.com/1007-9327/full/v29/i10/1602.htm
- DOI: https://dx.doi.org/10.3748/wjg.v29.i10.1602
Gastric carcinoma (GC) is the fifth commonest malignancy and the fourth most predominant cause of cancer-related mortality worldwide according to the Global Cancer Statistics 2020[1]. Although the global age-standardized rates of incidence and mortality presented a slight decrease from 1990 to 2019, China had a high incidence-mortality ratio (0.845) and five-year prevalence (27.6/100 000)[2]. A large number of GC cases are found at the advanced stage and have a relatively poor prognosis, with an overall survival rate of 25% worldwide[3]. The high proportion of tumor metastasis, intratumor heterogeneity and chemotherapeutic resistance leads to unfavorable survival outcomes in patients with GC. Conventionally, the tumor-node-metastasis (TNM) stage, histologic classification and differentiation are the major prognostic indicators for GC[4]. In addition, some oncogenic protein markers, such as antigen Ki-67, have been associated with the prognosis of GC patients[5].
Cell proliferation is a distinguishing feature of cancer. The Ki-67 protein, a nucleus-associated antigen, which is a convenient and reproducible biomarker in this process, is expressed during the cell proliferation cycle including G1, S, G2, and mitosis phases[6]. Some studies have demonstrated that Ki-67 proliferation index could be a potential indicator to predict the prognosis and identify high-risk GC cases, and was relevant to TNM stage, tumor differentiation grade, invasion depth and distant metastasis[5,7,8]. Additionally, Ki-67 index was associated with chemotherapy efficacy in the advanced GC, because cytotoxic chemotherapeutic drugs are effective against tumor cells that have entered the cell division cycle. Thus, identifying the Ki-67 status noninvasively would be beneficial for predicting the prognosis and chemotherapeutic response in the patients with GC.
Recently, a novel dual-layer spectral detector CT (DLSDCT), which utilizes a detector-based dual-energy separation technology to acquire low- and high-energy data synchronously with two layers of detectors, makes for beam-hardening rectification, material decomposition, and image de-noising[9,10]. This system applies projection-space decomposition and generates various spectral basis images (SBIs) except for the conventional polyenergetic images, including material-specific images [iodine concentration (IC) images, virtual non-contrast images, effective atomic number (Zeff) images] and energy-specific images [virtual monoenergetic images (VMIs)]. These spectral images are widely used for enhancing image contrast, improving lesion detection, characterizing materials, reducing artifact, and lowering radiation dosage[9-12]. Some studies have investigated the gastric lesions based on the IC derived from dual-energy spectral CT, such as distinguishing between malignant and benign gastric mucosal lesions[13], diagnosing GC and its histological type[14], and detecting serosal invasion of GC[15]. The latest study demonstrated that virtual monochromatic CT values were related to proliferative activity of tumor cells[16]. The Zeff was correlated with Ki-67 expression in laryngeal squamous cell carcinoma[17], and could predict the vascular density of affected lesions[18]. However, only a few studies have applied the quantitative parameters [IC and normalized IC (nIC)] from spectral CT to evaluate the Ki-67 expression status in patients with gastric adenocarcinoma, but not including the CT attenuation value and Zeff derived from the novel DLSDCT. Thus, the aim of this study is to explore the clinical usefulness of the quantitative parameters of novel DLSDCT in assessing the Ki-67 expression status in patients with gastric adenocarcinoma.
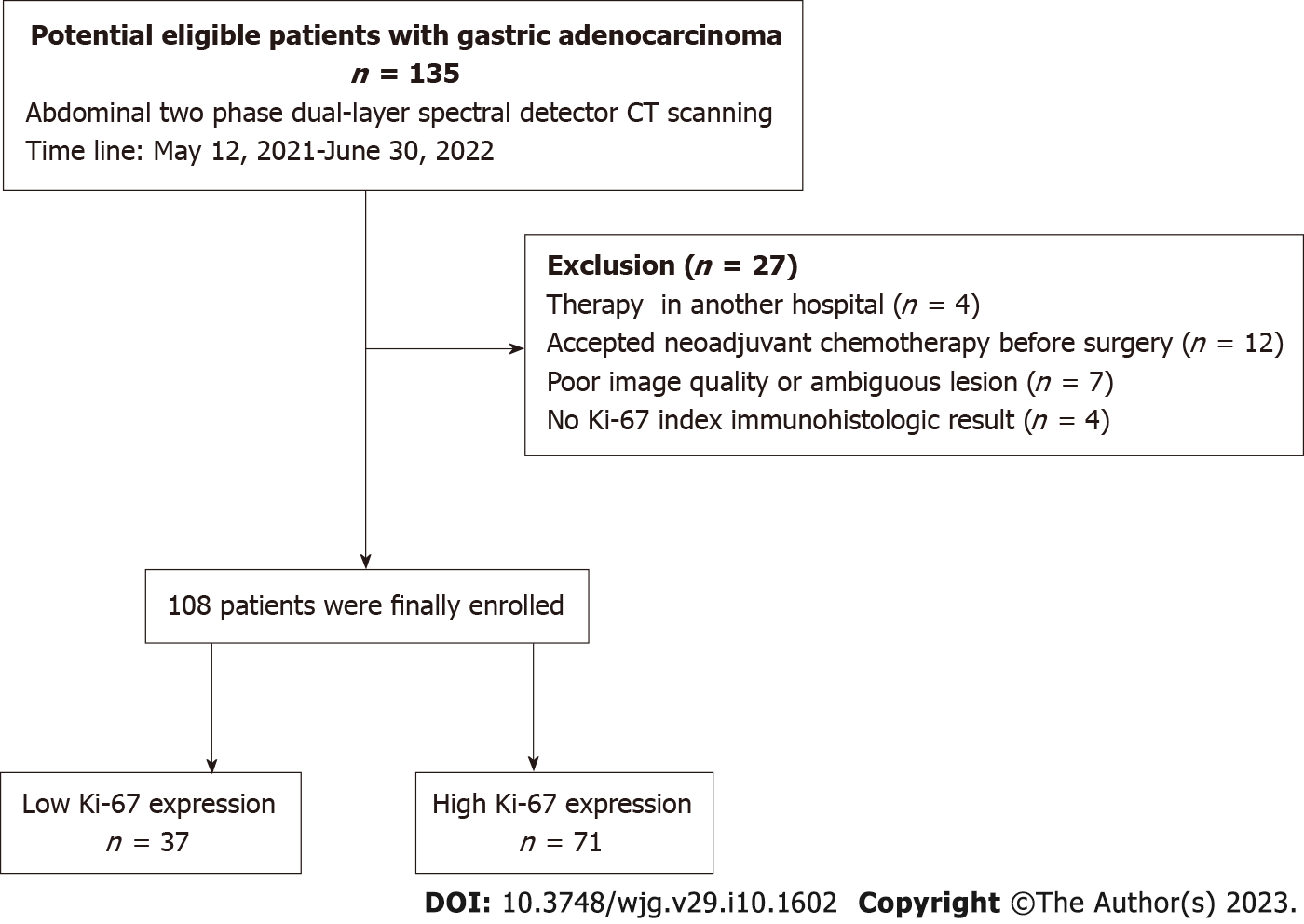
This retrospective study was approved by Institutional Review Board of our hospital, with a waiver for written informed consent. From May 2021 to June 2022, 135 patients diagnosed with gastric adenocarcinoma through endoscopic biopsy underwent non-contrast and contrast-enhanced CT scans on a DLSDCT scanner (IQon Spectral CT, Philips Healthcare, Best, The Netherlands). Twenty-eight cases were excluded for the following factors: (1) Transferred to another hospital for further treatment (n = 4); (2) Accepted or required neo-adjuvant chemotherapy prior to surgery (n = 12); (3) Poor image quality or the lesion is ambiguous on the image (n = 7); or (4) Lack of the Ki-67 index immunohistologic result (n = 4). Finally, 108 patients were analyzed in this study. The detailed procedure of patient selection is shown in Figure 1.
The interval between the DLSDCT examination and surgery was less than one week. Before CT examination, patients were required to be fast for 6-8 h and drank 800-1000 mL of water. The scan ranged from the diaphragm to the symphysis pubis in a supine position and cranio-caudal direction. Nonionic contrast agent (Ultravist, Byer HealthCare) (370 mg/mL, 80 mL) was injected intravenously at a flow rate of 2.5 mL/s, with an automated injector (Stellant, Medrad, Byer HealthCare), following 30 mL of normal saline flushing at the same flow rate. Using bolus-tracking technique, the arterial phase (AP) scan was triggered at a threshold of 200 hounsfield unit (HU) and an additional delay of 6 s. The venous phase (VP) images were respectively collected at 35 s after injecting the contrast agent.
CT scan parameters were as follows: Tube voltage 120 kVp, automatic tube current 37-84 mAs, detector collimation 64 mm × 0.625 mm, reconstruction matrix 512 × 512. Conventional and SBI were reconstructed using the iDose 4 algorithm (Philips Healthcare). All CT images were reconstructed with a slice thickness of 1 mm and an increment of 1 mm, using a standard kernel. All image data were transferred to a workstation (IntelliSpace Portal, version 10.1, Philips Healthcare) for post-processing and analysis.
The image analysis was conducted by two gastrointestinal radiologists (reader 1 and reader 2, with 10 and 26 years of experience, respectively), who were blind to pathological results. The two-dimensional region-of-interest (ROI) was drawn on lesion manually, according to the following principles: (1) Polygon ROIs covered the enhanced areas of the lesions as much as possible; (2) Be careful to avoid the areas of necrosis, calcification, and vessels; (3) All lesions were measured on three consecutive axial layers by the same evaluator, and average values were calculated; and (4) The size, form, and position of the ROIs were maintained consistent between two phases images, by applying the copy-and-paste function of the workstation. A circular ROI was placed in the abdominal aorta parallel with lesion. The intraclass correlation coefficient (ICC) between the two radiologists was calculated. The final results of all ROIs were measured by the radiologist with 10 years’ experience.
The following quantitative spectral parameters were automatically calculated using the post-processing software: The CT attenuation values of monochromatic images [40 kilo electron volt (kev), 70 kev and 100 kev], IC, and the Zeff. Additionally, three related parameters were measured: (1) The nIC was computed as Nic = IClesion/ICaorta, where IClesion and ICaorta are the ICs of the lesions and abdominal aorta, which help to minimize individual variation; (2) The normalized Zeff (nZeff) was counted similarly to nIC, nZeff = Zefflesion/Zeffaorta; and (3) The slope of the spectral curve (λHU) was calculated as the CT attenuation values, λHU = (CT40 kev - CT70 kev)/30, where CT40 kev and CT70 kev are the attenuation of the tumors at 40 kev and 70 kev monochromatic images, respectively[19]. Two phases (AP and VP) of CT attenuation value, IC, nIC, Zeff, nZeff, and λHU were measured.
A pathologist with 22 years of experience (Yu Zhang) conducted the pathologic analysis. The TNM staging was determined on the basis of the American Joint Committee on Cancer (AJCC) 8th manual of gastric cancer[20]. The Ki-67 proliferation index was evaluated according to the normal immunohistochemistry process and evaluated by the pathologist blindly. The Ki-67 polyclonal antibody used (Roche #) was produced by Shanghai Rebiosci Biotech Co., Ltd. The specimens were analyzed in a high power field (× 400). The pathologist selected five fields of view randomly and each region was observed. Then 100 cells were selected in each field and the number and intensity of positively stained cells were counted. Finally, an average number of five fields were recorded. The Ki-67 indexes were categorized as low expression (< 50% positive cells) or high expression (≥ 50% positive cells), according to relevant reports[7,21].
Statistical analysis was performed using MedCalc Statistical Software version 19.4.1 (MedCalc Software, Ostend, Belgium; https://www.medcalc.org; 2020). Continuous variables were presented as the mean ± SD, and categorical variables as proportions. ICC analyses were performed with the data of 20 patients to evaluate the reliability of spectral parameters measurement. An ICC > 0.75 was considered good.
The Shapiro-Wilk test was used to test the normality of data distributions. The Student’s t-test was employed to analyze the differences in clinical demographics and imaging parameters between low and high Ki-67 expressing status. Spearman’s correlation coefficient was used to assess the correlation between the quantitative imaging parameters and the Ki-67 expression status.
Receiver operating characteristic (ROC) analysis was performed to evaluate the diagnostic efficacy in classifying Ki-67 statuses. Area under the curve (AUC) and their 95% confidence intervals (CIs) were determined using unstratified bootstrap replicates 10 000 times. The optimal cutoff value was determined according to the Youden index. The difference between ROC curves were evaluated by pairwise comparison test. A two-sided P value of less than 0.05 was regarded statistically significant.
A total of 108 patients (mean age 61.9 years, range 34-85 years) were analyzed, consisting of 46 females and 62 males. The gastric tumors were located in the antrum in 48 cases (44.4%), in the corpus in 24 cases (22.2%), in the fundus in 11 cases (10.1%), in the antrum and corpus in 9 cases (8.3%), in the gastric angle in 9 cases (8.3%), and in the cardia in 7 case (6.4%).
Referring to the pathologic TNM staging of GC (AJCC 8th edition), 27 patients had gastric cancer of less than stage pT2, 32 patients had stage pT3 gastric cancer, and 49 patients had stage T4 gastric cancer. Twenty-six patients had no regional lymph node metastasis, whereas 24 patients had less than three lymph nodes invaded (pN1), 30 patients had 3-6 regional lymph nodes invaded (pN2), and 28 patients had seven or more regional lymph nodes invaded (pN3). The immunohistochemical staining results revealed that 37 cases were categorized as having low expression of the Ki-67, while 71 cases were categorized as having high expression. According to the World Health Organization grading criteria, 29 (26.8%) and 79 (73.1%) tumors were respectively classified as moderately and poorly differentiated adenocarcinoma.
The ICC values for Zefflesion, Zeffaorta, IClesion, ICaorta, CT40 kev, CT70 kev, CT100 kev in AP and VP were all more than 0.85, and the specific values are shown in Table 1.
| Parameters | ICC (95%CI) | |
| AP | VP | |
| Zefflesion | 0.88 (0.71, 0.95) | 0.86 (0.69, 0.94) |
| Zeffaorta | 0.94 (0.85, 0.97) | 0.95 (0.88, 0.98) |
| IClesion (mg/mL) | 0.86 (0.56, 0.95) | 0.87 (0.73, 0.95) |
| ICaorta (mg/mL) | 0.98 (0.94, 0.99) | 0.95 (0.86, 0.98) |
| CT40 kev (HU) | 0.95 (0.88, 0.98) | 0.96 (0.79, 0.99) |
| CT70 kev (HU) | 0.92 (0.78, 0.97) | 0.91 (0.50, 0.98) |
| CT100 kev (HU) | 0.86 (0.69, 0.94) | 0.89 (0.70, 0.96) |
Zeff and nZeff in AP and VP presented a moderate negative correlation with Ki-67 status (P < 0.001), whereas the IC and nIC in AP and VP were moderately positive-correlated with Ki-67 status (P < 0.001). The CT40 kev-VP, CT70 kev-VP and CT100 kev-VP were weakly negative-correlated with Ki-67 status (all P < 0.05). However, the CT40 kev-AP, CT70 kev-AP, CT100 kev-AP and λHU-AP and λHU-VP were not correlated with Ki-67 expression (all P > 0.05). The correlation coefficients and 95%CIs are shown in Table 2.
| Parameters | Ki-67 status | ||
| r (95%CI) | P value | ||
| AP | CT40 kev | -0.162 (-0.362, 0.043) | 0.093 |
| CT70 kev | -0.097 (-0.290, 0.102) | 0.316 | |
| CT100 kev | -0.074 (-0.277, 0.117) | 0.449 | |
| λHU | -0.137 (-0.328, 0.056) | 0.156 | |
| Zeff | -0.427 (-0.606, -0.208) | < 0.001 | |
| nZeff | -0.487 (-0.649, -0.318) | < 0.001 | |
| IC | 0.409 (0.228, 0.567) | < 0.001 | |
| nIC | 0.449 (0.288, 0.598) | < 0.001 | |
| VP | CT40 kev | -0.213 (-0.397, -0.005) | 0.027 |
| CT70 kev | -0.216 (-0.407, -0.004) | 0.025 | |
| CT100 kev | -0.266 (-0.438, -0.096) | 0.005 | |
| λHU | -0.090 (-0.278, 0.093) | 0.354 | |
| Zeff | -0.455 (-0.647, -0.215) | < 0.001 | |
| nZeff | -0.555 (-0.692, -0.403) | < 0.001 | |
| IC | 0.405 (0.223, 0.571) | < 0.001 | |
| nIC | 0.502 (0.355, 0.651) | < 0.001 | |
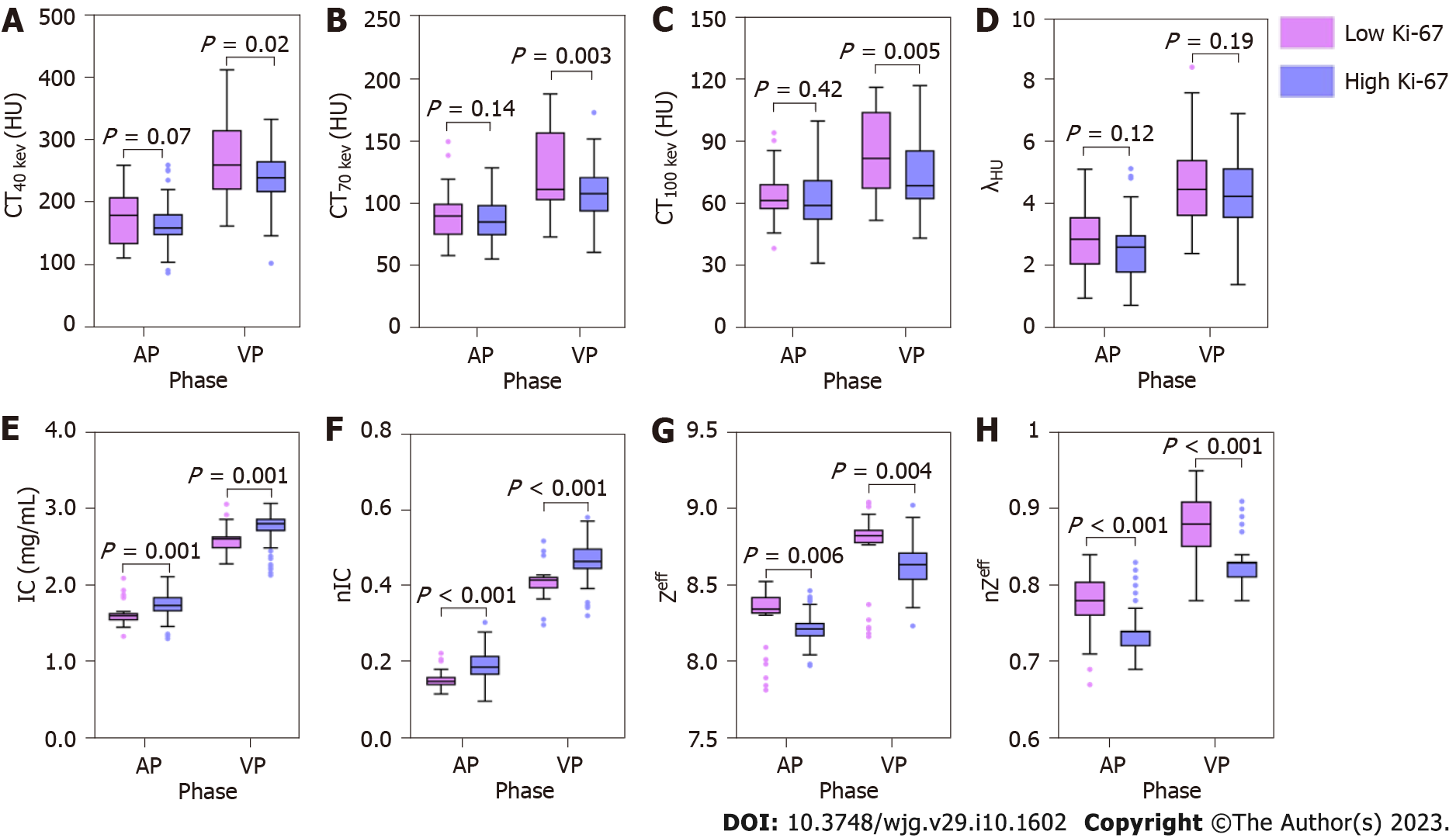
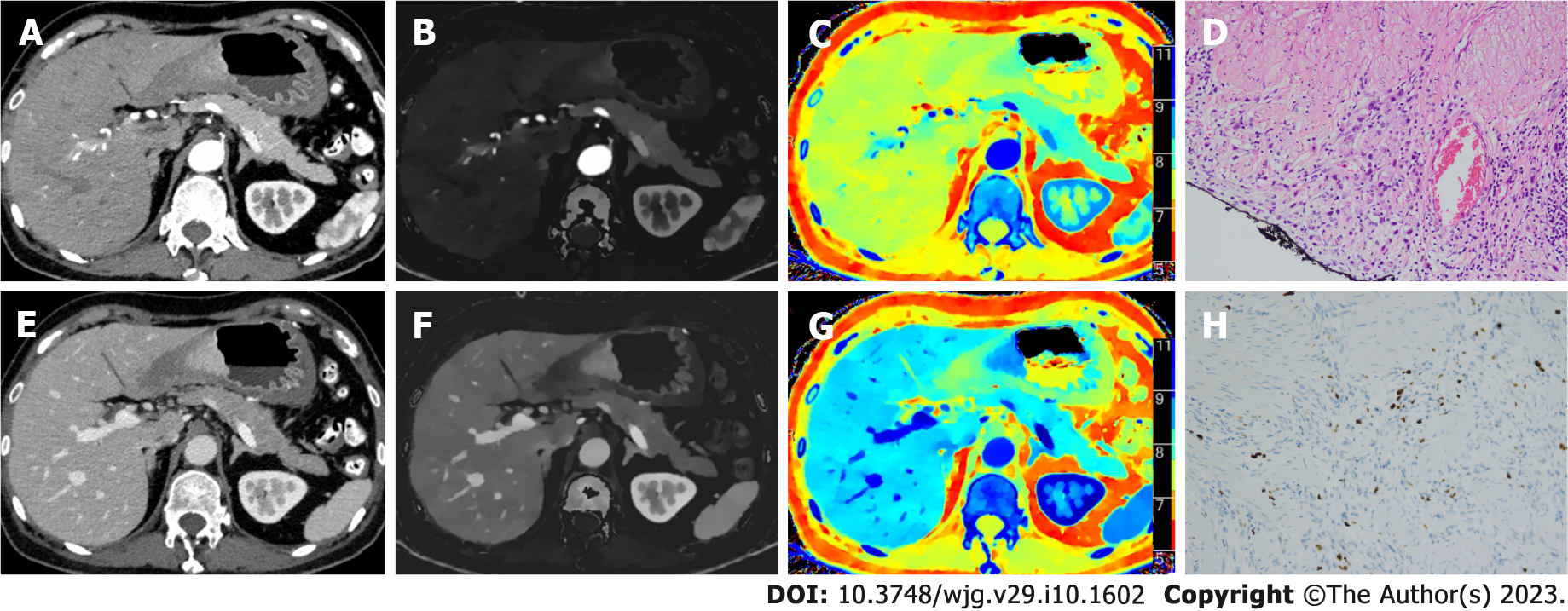
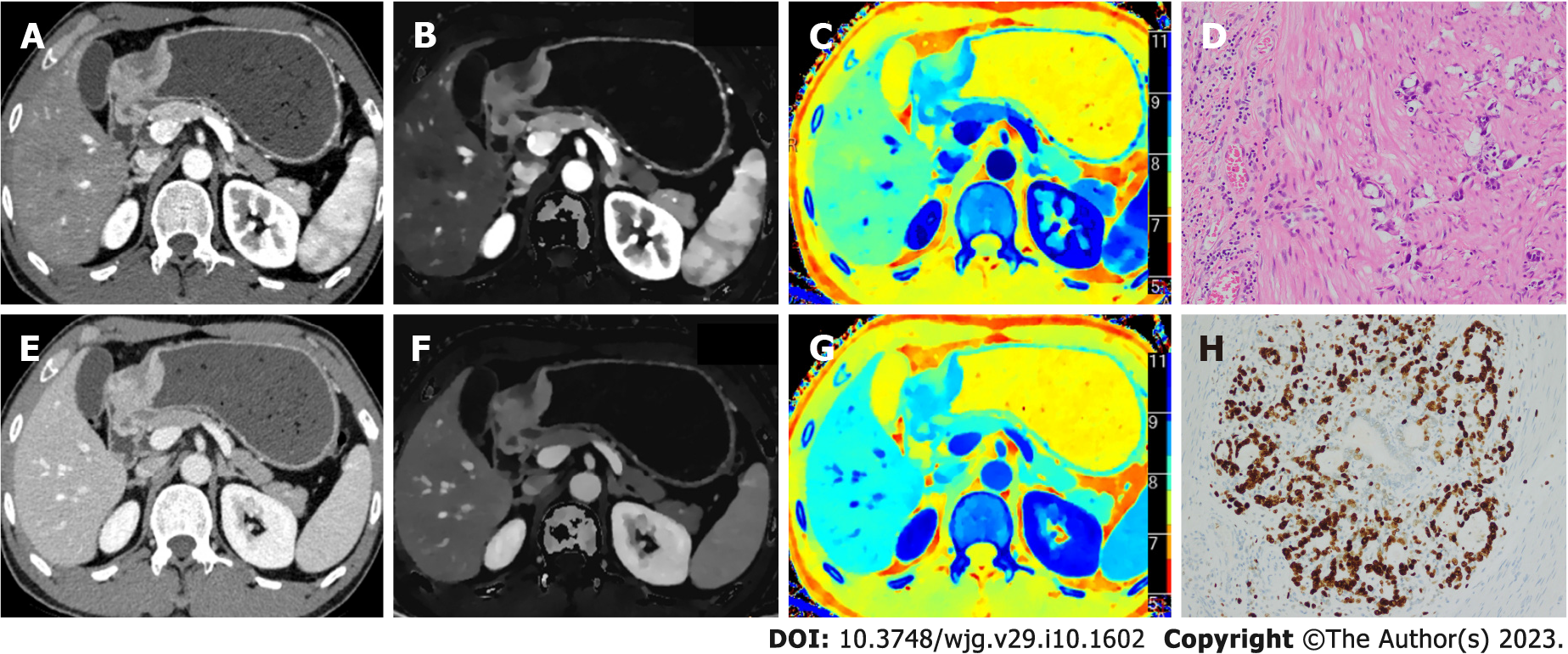
Table 3 and Figure 2 show the results of quantitative analysis. Compared with high Ki-67 status, the low Ki-67 status had higher ZeffAP, ZeffVP, nZeffAP, nZeffVP, CT40 kev-VP, CT70 kev-VP and CT100 kev-VP, and had lower ICAP, ICVP, nICAP and nICVP. Although the CT40 kev-AP, CT70 kev-AP and CT100 kev-AP of low Ki-67 status were slightly higher than that of high Ki-67 status, it was not statistically significant. Similarly, no significant differences were found in the λHU of AP and VP between the two groups. Figures 3 and 4 show the spectral parameter images of two cases with high and low Ki-67 expression, respectively.
| Parameters | Low Ki-67 status | High Ki-67 status | t value | P value | |
| AP | CT40 kev | 177.3 ± 43.9 | 162.3 ± 37.8 | 1.85 | 0.068 |
| CT70 kev | 90.9 ± 20.1 | 85.5 ± 16.6 | 1.48 | 0.141 | |
| CT100 kev | 63.8 ± 12.7 | 61.7 ± 12.6 | 0.81 | 0.422 | |
| λHU | 2.88 ± 1.04 | 2.56 ± 0.98 | 1.58 | 0.118 | |
| Zeff | 8.30 ± 0.19 | 8.21 ± 0.10 | 2.87 | 0.006 | |
| nZeff | 0.78 ± 0.04 | 0.74 ± 0.03 | 5.33 | < 0.001 | |
| IC (mg/mL) | 1.61 ± 0.15 | 1.73 ± 0.19 | -3.53 | 0.001 | |
| nIC | 0.15 ± 0.02 | 0.19 ± 0.04 | -5.88 | < 0.001 | |
| VP | CT40 kev | 265.9 ± 61.4 | 237.3 ± 45.8 | 2.50 | 0.016 |
| CT70 kev | 126.5 ± 33.4 | 108.0 ± 20.9 | 3.07 | 0.003 | |
| CT100 kev | 84.3 ± 18.7 | 73.9 ± 17.2 | 3.71 | 0.005 | |
| λHU | 4.65 ± 1.43 | 4.31 ± 1.15 | 1.33 | 0.185 | |
| Zeff | 8.75 ± 0.24 | 8.62 ± 0.15 | 3.04 | 0.004 | |
| nZeff | 0.87 ± 0.04 | 0.83 ± 0.03 | 6.59 | < 0.001 | |
| IC (mg/mL) | 2.62 ± 0.17 | 2.75 ± 0.21 | -3.40 | 0.001 | |
| nIC | 0.41 ± 0.04 | 0.46 ± 0.06 | -5.87 | < 0.001 |
Table 4 summarizes the results of ROC analysis for evaluating the diagnostic performance of 12 significant spectral parameters in discriminating the Ki-67 status. For diagnosing the high Ki-67 labeling index, the Zeff, nZeff, IC and nIC in AP and VP performed moderate efficiency (AUC value, ranged from 0.747 to 0.835), and there were no significant differences among the AUC values of these parameters. Nevertheless, the CT40 kev-VP, CT70 kev-VP, and CT100 kev-VP showed general differentiating capabilities (AUC value 0.630, 0.631, 0.662, respectively) in differentiating low from high Ki-67 expression in GC, and the AUC values of these parameters had no statistical differences. Comparing the AUC values of CT attenuation with that of the Zeff- and IC-related parameters, the AUC value of nZeffAP was higher than CT40 kev-VP (0.796 vs 0.630, P = 0.047), and the AUC values of nZeffVP and nICVP were higher than CT40 kev-VP, CT70 kev-VP and CT100 kev-VP (P = 0.02, 0.01, 0.009 and 0.03, 0.01, 0.02, respectively). In addition, the multi-variable model (CT70 kev-VP, nZeffAP, nZeffVP, nICAP, nICVP) was selected for most powerful parameters by multivariate logistic regression, and the model demonstrated excellent efficiency (AUC = 0.967; sensitivity 95.77%; specificity 91.89%) in discriminating high expression of Ki-67 in GC.
| AUC (95%CI) | TV | YI | Sen (%) | Spe (%) | PPV (%) | NPV (%) | Acc (%) | ||
| AP | Zeff | 0.759 (0.668, 0.836) | ≤ 8.27 | 0.67 | 85.92 | 81.08 | 92.96 | 37.84 | 71.30 |
| nZeff | 0.796 (0.707, 0.867) | ≤ 0.74 | 0.67 | 80.28 | 86.49 | 85.92 | 54.05 | 75.00 | |
| IC | 0.752 (0.660, 0.830) | > 1.63 | 0.60 | 78.87 | 81.08 | 88.73 | 21.62 | 65.74 | |
| nIC | 0.773 (0.683, 0.848) | > 0.16 | 0.61 | 74.65 | 86.49 | 81.69 | 48.65 | 70.37 | |
| VP | CT40 kev | 0.630 (0.531, 0.721) | ≤ 271.2 | 0.26 | 80.28 | 45.95 | 95.77 | 24.32 | 71.30 |
| CT70 kev | 0.631 (0.533, 0.722) | ≤ 138.8 | 0.31 | 92.96 | 37.84 | 94.37 | 32.43 | 73.15 | |
| CT100 kev | 0.662 (0.565, 0.750) | ≤ 89.3 | 0.28 | 81.69 | 45.95 | 87.32 | 32.43 | 68.52 | |
| Zeff | 0.777 (0.687, 0.851) | ≤ 8.75 | 0.73 | 88.73 | 83.78 | 92.96 | 29.73 | 74.07 | |
| nZeff | 0.835 (0.751, 0.899) | ≤ 0.83 | 0.68 | 78.87 | 89.19 | 85.92 | 51.35 | 74.07 | |
| IC | 0.747 (0.654, 0.826) | > 2.65 | 0.63 | 84.51 | 78.38 | 85.92 | 27.03 | 65.74 | |
| nIC | 0.805 (0.718, 0.875) | > 0.43 | 0.71 | 78.87 | 91.89 | 85.92 | 37.84 | 69.44 | |
| Multi-parameters | 0.967 (0.913, 0.992) | - | 0.88 | 95.77 | 91.89 | 97.18 | 86.49 | 93.52 | |
The recently developed DLSDCT could quantitatively map the IC and Zeff of the tissue in enhanced images, and offer CT attenuation values on a wide range of VMIs. In this study, we explored the association between quantitative parameters derived from DLSDCT and the Ki-67 labeling index of gastric adenocarcinoma. Our results revealed that the CT40 kev-VP, CT70 kev-VP, CT100 kev-VP, and Zeff-related parameters were significantly higher, but IC-related parameters were lower in the group with low Ki-67 status. Additionally, the CT40 kev-VP, CT70 kev-VP, CT100 kev-VP, and Zeff-related parameters exhibited negative correlations with Ki-67 status, whereas IC-related parameters positively correlated with it. These results were partially in agreement with previous reports that used different spectral CT systems, which demonstrated that IC and nIC were positively associated with Ki-67 status, and the values of nIC were higher in poorly differentiated gastric adenocarcinomas significantly[22,23].
We found a negative correlation between Zeff and Ki-67 labeling index, which is seemingly at variance with a previous study that Zeff was positively correlated with Ki-67 expression in the laryngeal squamous cell carcinoma[17] and invasive breast cancer[24]. Zeff reflects the total atomic numbers of complex or mixture of materials, and has a close relationship with fundamental properties of the elements[25]. Previous research indicated that the evaluation of Zeff could be able to distinguish the different tissues showing similar attenuative properties at given energy[26]. On account of the concentrations of elements (Cl, K, Ca, Ti, Mn, Fe, Co, Cu, and Zn) are lower in the stomach cancerous tissue than normal tissue[25], gastric cancer tissue exhibits a lower Zeff than its healthy counterpart[27]. It is known that deficiency or excess of certain essential trace metals is relevant to carcinogenesis of the specific organs[27,28]. The abnormal levels of these elements lead to the discrepancy of toxicity and proliferation activity of cancer cells. We hypothesize that Zeff difference between high and low Ki-67 expression status is more significant due to abnormal metal concentration than tumor heterogeneity and angiogenesis, especially in case of using nZeff, which eliminate individual differences in hemodynamics.
The blood vessels of tumors are supplied by tumor angiogenesis and invasion of vessels around the tumor. The degree of tumor angiogenesis is strongly linked to tumor growth, progression, and metastasis[29,30]. Wang et al[31] found that the degree of CT enhancement is correlated with tumor angiogenesis and the malignancy of the tumor. Compared to CT contrast enhancement, IC can quantitatively indicate the degree of tumor neovascularization and reflect the deposition of iodine in the tissue objectively[32]. In this study, we detected significantly higher IC and nIC values in the AP and VP of the high-expression Ki-67 group, indicating a richer blood supply in these tumors. Compared to IC, the normalized parameter nIC minimized hemodynamic variations between individuals, which could be more comparable among different groups. These findings are consistent with the fact that the high proliferative activity is accompanied by abundant angiogenesis.
In our study, we found no statistical difference in the CT attenuation values at 40-100 kev (at 30 kev interval) in AP between low- and high-expression Ki-67 groups. Likewise, the λHU of AP and VP between the two groups showed no significant differences, which was different from the result reported by Cheng et al[22], who found that the λHU values were significantly different among the low, medium and high level Ki-67 groups in both VP and delayed phase, and had positive correlation with Ki-67 level. We deemed that the discrepancy might be attributable to the grouping method of Ki-67 index and the constituent ratio of differentiation degree of the analyzed cases was distinct from our study. It is necessary to further explore the usefulness of λHU values.
When evaluating the diagnostic performance of spectral parameters in discriminating the Ki-67 status, the results from ROC analysis demonstrated that the multi-variable model of spectral parameters performed excellent capacity, with high sensitivity and specificity. In contrast, the single-variable model of Zeff- and IC-related parameters demonstrated moderate efficiency, and 40-100 kev (at 30 kev interval) in VP showed general efficiency. Therefore, the parameters derived from DLSDCT were feasible for the prediction of Ki-67 expression in the gastric adenocarcinoma, which is of great significance in predicting prognosis and guiding treatment for the patients with GC.
There were several limitations in our study. First, this is a retrospective study in which case grouping is not random, hence, an unconscious selection bias may exist. Second, a relatively small number of patients might overstate the consequence of association. Third, the number of cases with different degrees of differentiation was disproportionate, resulting in significance hard to achieve for partially analyzed variables. Fourth, our uniform standard of enhanced scanning protocol with DLSDCT may be different from other centers, thus, the results acquired from other vendors or different scanning parameters may not be directly concluded from our results. It demands further studies to confirm and outspread our preliminary results.
Quantitative spectral parameters are feasible to distinguish low and high Ki-67 expression in gastric adenocarcinoma. Multi-variable model of spectral parameters exhibited a better diagnostic efficiency than single-variable model of spectral parameter in discriminating low and high Ki-67 expression in gastric adenocarcinoma. Zeff and IC derived from DLSDCT may be useful parameters for evaluating the Ki-67 proliferation index for gastric adenocarcinoma.
The level of Ki-67 expression is a valuable prognostic factor in gastric cancer. However, the quantitative parameters based on the novel dual-layer spectral detector computed tomography (DLSDCT) in discriminating the Ki-67 expression status are unclear.
The relationship between the Ki-67 expression in gastric carcinoma (GC) and part spectral parameters (including the effective atomic number (Zeff) and the monoenergetic CT attenuation) is unclear.
This study aimed to investigate the diagnostic ability of DLSDCT-derived parameters for Ki-67 expression status in GC.
Dual-phase enhanced abdominal CT was performed preoperatively in 108 patients with GC. The monoenergetic CT attenuation value at 40-100 kilo electron volt (kev), the slope of the spectral curve (λHU), iodine concentration (IC), normalized IC (nIC), Zeff and normalized Zeff (nZeff) in the arterial phase (AP) and venous phase (VP) were retrospectively compared between the groups of low and high Ki-67 expression status. The relationship between the spectral parameters and Ki-67 expression status were analyzed, and the diagnostic efficacy of the statistically significant parameters between the two groups was compared.
The low and high Ki-67 expression groups consisted of 37 and 71 patients respectively. CT40 kev-VP, CT70 kev-VP, CT100 kev-VP, and Zeff-related parameters were significantly higher, but IC-related parameters were lower in the low Ki-67 expression group than in the high Ki-67 expression group. CT40 kev-VP, CT70 kev-VP, CT100 kev-VP, Zeff, and nZeff exhibited negative correlations with Ki-67 status, whereas IC and nIC positively correlated with it. The results of receiver operating characteristic analysis showed that the multi-variable model of spectral parameters performed well in identifying the Ki-67 status [area under the curve (AUC) = 0.967; sensitivity 95.77%; specificity 91.89%]. Nevertheless, the differentiating capabilities of single-variable model were moderate (AUC value from 0.630 to 0.835).
Zeff and IC may be useful parameters for evaluating the Ki-67 expression in GC.
The spectral CT images are prospective to provide the pathological information of Ki-67 expression of GC in the future.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Shariati MBH, Iran; Vorobjova T, Estonia S-Editor: Wang JJ L-Editor: Ma JY-MedE P-Editor: Wang JJ
| 1. | Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75126] [Cited by in RCA: 64681] [Article Influence: 16170.3] [Reference Citation Analysis (177)] |
| 2. | He Y, Wang Y, Luan F, Yu Z, Feng H, Chen B, Chen W. Chinese and global burdens of gastric cancer from 1990 to 2019. Cancer Med. 2021;10:3461-3473. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 70] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 3. | He X, Wu W, Lin Z, Ding Y, Si J, Sun LM. Validation of the American Joint Committee on Cancer (AJCC) 8th edition stage system for gastric cancer patients: a population-based analysis. Gastric Cancer. 2018;21:391-400. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 48] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 4. | Zhang H, Pan Z, Du L, Yan C, Ding B, Song Q, Ling H, Chen K. Advanced gastric cancer and perfusion imaging using a multidetector row computed tomography: correlation with prognostic determinants. Korean J Radiol. 2008;9:119-127. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 27] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 5. | Xiong DD, Zeng CM, Jiang L, Luo DZ, Chen G. Ki-67/MKI67 as a Predictive Biomarker for Clinical Outcome in Gastric Cancer Patients: an Updated Meta-analysis and Systematic Review involving 53 Studies and 7078 Patients. J Cancer. 2019;10:5339-5354. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 40] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 6. | Scholzen T, Gerdes J. The Ki-67 protein: from the known and the unknown. J Cell Physiol. 2000;182:311-322. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 67] [Reference Citation Analysis (0)] |
| 7. | Wei Z, Huang L, Zhang X, Xu A. Expression and significance of Her2 and Ki-67 in gastric adenocarcinoma without distant metastasis: a cohort study. BMC Gastroenterol. 2020;20:343. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 8. | Böger C, Behrens HM, Röcken C. Ki67--An unsuitable marker of gastric cancer prognosis unmasks intratumoral heterogeneity. J Surg Oncol. 2016;113:46-54. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 38] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 9. | Rassouli N, Etesami M, Dhanantwari A, Rajiah P. Detector-based spectral CT with a novel dual-layer technology: principles and applications. Insights Imaging. 2017;8:589-598. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 96] [Cited by in RCA: 188] [Article Influence: 23.5] [Reference Citation Analysis (0)] |
| 10. | van Hamersvelt RW, Willemink MJ, de Jong PA, Milles J, Vlassenbroek A, Schilham AMR, Leiner T. Feasibility and accuracy of dual-layer spectral detector computed tomography for quantification of gadolinium: a phantom study. Eur Radiol. 2017;27:3677-3686. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 22] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 11. | Hickethier T, Baeßler B, Kroeger JR, Doerner J, Pahn G, Maintz D, Michels G, Bunck AC. Monoenergetic reconstructions for imaging of coronary artery stents using spectral detector CT: In-vitro experience and comparison to conventional images. J Cardiovasc Comput Tomogr. 2017;11:33-39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 66] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 12. | Hickethier T, Byrtus J, Hauger M, Iuga AI, Pahn G, Maintz D, Haneder S, Doerner J. Utilization of virtual mono-energetic images (MonoE) derived from a dual-layer spectral detector CT (SDCT) for the assessment of abdominal arteries in venous contrast phase scans. Eur J Radiol. 2018;99:28-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 28] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 13. | Meng X, Ni C, Shen Y, Hu X, Chen X, Li Z, Hu D. Differentiating malignant from benign gastric mucosal lesions with quantitative analysis in dual energy spectral computed tomography: Initial experience. Medicine (Baltimore). 2017;96:e5878. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 12] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 14. | Li R, Li J, Wang X, Liang P, Gao J. Detection of gastric cancer and its histological type based on iodine concentration in spectral CT. Cancer Imaging. 2018;18:42. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 42] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 15. | Küpeli A, Bulut E, Cansu A, Güner A, Soytürk M, Danışan G. Contribution of DECT in detecting serosal invasion of gastric cancer. Turk J Med Sci. 2019;49:782-788. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 16. | Chen M, Li X, Wei Y, Qi L, Sun YS. Spectral CT imaging parameters and Ki-67 labeling index in lung adenocarcinoma. Chin J Cancer Res. 2020;32:96-104. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 17. | Wang P, Tang Z, Xiao Z, Wu L, Hong R, Duan F, Wang Y, Zhan Y. Dual-energy CT in predicting Ki-67 expression in laryngeal squamous cell carcinoma. Eur J Radiol. 2021;140:109774. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 14] [Reference Citation Analysis (0)] |
| 18. | Onishi S, Fujioka C, Kaichi Y, Amatya VJ, Ishifuro M, Takeshima Y, Awai K, Sugiyama K, Kurisu K, Yamasaki F. Utility of dual-energy CT for predicting the vascularity of meningiomas. Eur J Radiol. 2020;123:108790. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 19. | Zhang X, Bai L, Wang D, Huang X, Wei J, Zhang W, Zhang Z, Zhou J. Gastrointestinal stromal tumor risk classification: spectral CT quantitative parameters. Abdom Radiol (NY). 2019;44:2329-2336. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 24] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 20. | Amin MB, Edge SB, Greene FL, Byrd DR, Brookland RK, Washington MK, Gershenwald JE, Compton CC, Hess KR, Sullivan DC, Milburn Jessup J, Brierley JD, Gaspar LE, Schilsky RL, Balch CM, Winchester DP, Asare EA, Madera M, Gress DM, Meyer LR. AJCC cancer staging manual. Switzerland: Springer, 2017. |
| 21. | Li N, Deng W, Ma J, Wei B, Guo K, Shen W, Zhang Y, Luo S. Prognostic evaluation of Nanog, Oct4, Sox2, PCNA, Ki67 and E-cadherin expression in gastric cancer. Med Oncol. 2015;32:433. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 83] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 22. | Cheng SM, Ling W, Zhu J, Xu JR, Wu LM, Gong HX. Dual Energy Spectral CT Imaging in the assessment of Gastric Cancer and cell proliferation: A Preliminary Study. Sci Rep. 2018;8:17619. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 26] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 23. | Liang P, Ren XC, Gao JB, Chen KS, Xu X. Iodine Concentration in Spectral CT: Assessment of Prognostic Determinants in Patients With Gastric Adenocarcinoma. AJR Am J Roentgenol. 2017;209:1033-1038. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 37] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 24. | Wang X, Liu D, Zeng X, Jiang S, Li L, Yu T, Zhang J. Dual-energy CT quantitative parameters for evaluating Immunohistochemical biomarkers of invasive breast cancer. Cancer Imaging. 2021;21:4. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 31] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 25. | Taylor ML. Quantification of differences in the effective atomic numbers of healthy and cancerous tissues: a discussion in the context of diagnostics and dosimetry. Med Phys. 2012;39:5437-5445. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 26. | Gorshkov V, Rozhkova N, Prokopenko S. Dual-energy dividing mammography. Berlin, Heidelberg: Springer, 2010. |
| 27. | Reddy SB, John Charles M, Naga Raju GJ, Vijayan V, Seetharami Reddy B, Ravi Kumar M, Sundareswar B. Trace elemental analysis of carcinoma kidney and stomach by PIXE method. Nucl Instrum Methods Phys Res B. 2003;207:345-355. [DOI] [Full Text] |
| 28. | Yaman M. Comprehensive comparison of trace metal concentrations in cancerous and non-cancerous human tissues. Curr Med Chem. 2006;13:2513-2525. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 64] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 29. | Hu S, Huang W, Chen Y, Song Q, Lin X, Wang Z, Chen K. Spectral CT evaluation of interstitial brachytherapy in pancreatic carcinoma xenografts: preliminary animal experience. Eur Radiol. 2014;24:2167-2173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 22] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 30. | Thaiss WM, Haberland U, Kaufmann S, Spira D, Thomas C, Nikolaou K, Horger M, Sauter AW. Iodine concentration as a perfusion surrogate marker in oncology: Further elucidation of the underlying mechanisms using Volume Perfusion CT with 80 kVp. Eur Radiol. 2016;26:2929-2936. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 42] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 31. | Wang SH, Sun YF, Liu Y, Zhou Y. CT contrast enhancement correlates with pathological grade and microvessel density of pancreatic cancer tissues. Int J Clin Exp Pathol. 2015;8:5443-5449. [PubMed] |
| 32. | Wu J, Lv Y, Wang N, Zhao Y, Zhang P, Liu Y, Chen A, Li J, Li X, Guo Y, Wu T, Liu A. The value of single-source dual-energy CT imaging for discriminating microsatellite instability from microsatellite stability human colorectal cancer. Eur Radiol. 2019;29:3782-3790. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 54] [Article Influence: 9.0] [Reference Citation Analysis (0)] |