Published online Jan 7, 2023. doi: 10.3748/wjg.v29.i1.144
Peer-review started: September 26, 2022
First decision: November 5, 2022
Revised: November 23, 2022
Accepted: December 13, 2022
Article in press: December 13, 2022
Published online: January 7, 2023
Processing time: 99 Days and 15.1 Hours
Minimal hepatic encephalopathy (MHE) is a frequent neurological and psychiatric complication of liver cirrhosis. The precise pathogenesis of MHE is complicated and has yet to be fully elucidated. Studies in cirrhotic patients and experimental animals with MHE have indicated that gut microbiota dysbiosis induces systemic inflammation, hyperammonemia, and endotoxemia, subsequently leading to neuroinflammation in the brain via the gut-liver-brain axis. Related mechanisms initiated by gut microbiota dysbiosis have significant roles in MHE pathogenesis. The currently available therapeutic strategies for MHE in clinical practice, including lactulose, rifaximin, probiotics, synbiotics, and fecal microbiota transplantation, exert their effects mainly by modulating gut microbiota dysbiosis. Microbiome therapies for MHE have shown promised efficacy and safety; how
Core Tip: Minimal hepatic encephalopathy (MHE) is a common neuropsychiatric complication of liver cirrhosis. Gut microbiota dysbiosis has an essential role in the pathogenesis of MHE via the gut-liver-brain axis. Current therapeutic strategies for MHE are based on the modulation of gut microbiota dysbiosis. This review presents the recent evidence on the roles of gut microbiota dysbiosis in the pathogenesis and treatment of MHE via the gut-liver-brain axis.
- Citation: Luo M, Xin RJ, Hu FR, Yao L, Hu SJ, Bai FH. Role of gut microbiota in the pathogenesis and therapeutics of minimal hepatic encephalopathy via the gut-liver-brain axis. World J Gastroenterol 2023; 29(1): 144-156
- URL: https://www.wjgnet.com/1007-9327/full/v29/i1/144.htm
- DOI: https://dx.doi.org/10.3748/wjg.v29.i1.144
Hepatic encephalopathy (HE) is a central nervous system complication of chronic liver disease or portal systemic shunting that is characterized by a broad range of neuropsychiatric symptoms[1]. Depending on the severity of clinical manifestations, HE can be classified as overt or covert, such as minimal HE (MHE) and West Haven grade I HE[1]. Overt HE (OHE) exhibits obvious neurological and psychiatric manifestations, such as flapping tremors, drowsiness, and sometimes coma[2]. In contrast, MHE presents with slight cognitive deficits in the executive function, including psychomotor speed, response inhibition, and working memory, with no clinical evidence of OHE[3]. MHE is diagnosed using neurophysiological and psychometric tests, and its prevalence range from approximately 30% to 70% in different populations with liver cirrhosis[1,3,4]. MHE compromises daily functions, affects the health-related quality of life, and increases the risk of progression to OHE[5,6].
The exact pathogenesis of MHE is complex and not completely understood. Furthermore, the pathophysiological basis of MHE is multifactorial, with ammonia, inflammation, and endotoxins considered causative factors[7]. Recently, gut microbiota dysbiosis has been demonstrated to be associated with hyperammonemia, systemic inflammation, neuroinflammation, and endotoxemia in cirrhotic patients and experimental animals with MHE[8-11]. With decompensated liver cirrhosis and hepatic dysfunction, dysbiotic gut microbiota and its metabolites cross the impaired intestinal barrier and induce hyperammonemia, systemic inflammation, and endotoxemia, which influence the permeability of the blood-brain barrier (BBB), resulting in neuroinflammation and low-grade edema in the cerebrum and contributing to central nervous system dysfunction[7]. It has been increasingly recognized that gut microbiota dysbiosis is the predominant factor accounting for MHE pathogenesis via the gut-liver-brain axis[12,13]. Furthermore, an increasing number of clinical studies have shown that currently available therapies for MHE patients, including lactulose, probiotics, synbiotics, rifaximin, and fecal microbiota transplantation (FMT), improve cognitive dysfunction through the modulation of gut microbiota dysbiosis[14-17].
Although several published reviews have elucidated the involvement of gut microbiota dysbiosis in the pathogenesis and treatment of HE, no specific review has focused on this involvement in MHE[13,18-20]. Therefore, this review aimed to comprehensively elucidate the roles of gut microbiota in MHE pathogenesis via the gut-liver-brain axis and systematically analyze the underlying mechanisms linked with microbiome therapies to modulate gut microbiota dysbiosis in cirrhotic patients with MHE.
Small intestinal bacterial overgrowth (SIBO) is a pathological dysregulation of gut microbiota, characterized by excessive bacteria and/or abnormal bacterial composition in the small intestine. Approximately 48% to 73% of cirrhotic patients have SIBO[21]. Intestinal immune dysfunction, intestinal dysmotility, and decreased bile acid synthesis are implicated in the pathogenesis of SIBO[22]. SIBO is closely associated with the severity of advanced liver cirrhosis, and it has been validated as a significant risk factor for MHE[23,24]. In MHE patients, the gut microbiota dysbiosis resulting from the SIBO has been characterized by lower bacterial diversity, decreased autochthonous beneficial bacteria, and increased pathogenic Gram-negative bacteria[8,14,25]. The gut microbiota dysbiosis in cirrhotic patients with MHE is summarized in Table 1. Notably, Bajaj et al[26] found that MHE patients have higher abundances of Enterococcus and Veillonella and a lower abundance of Roseburia in the gut mucosal microbiota; these signatures are significantly different from those of the fecal microbiota. It is hypothesized that the adherence and overgrowth of pathogenic bacteria in the gut mucosal microbiota, rather than the fecal microbiota, might be implicated in the pathogenesis of bacterial translocation.
| Ref. | Nationality | Number of patients | Etiology of cirrhosis | MHE diagnosis | Sample | Method | Microbiota alteration |
| Zhang et al[8] | China | 51 | AIH, HBV, PBC, alcohol | NCT-A DST | Stool | 16S rRNA pyrosequencing | Enriched Streptococcus salivarius |
| Wang et al[14] | China | 98 | HBV, HCV, others | NCT-A DST | Stool | 16S rRNA sequencing | Enriched Proteobacteria, especially Pasteurellaceae Haemophilus and Alcaligenaceae Parasutterella |
| Luo et al[29] | China | 143 | HBV | PHES | Stool | 16S rRNA sequencing | Enriched Streptococcus salivarius and Veillonella |
| Liu et al[76] | China | 55 | HBV, HCV, alcohol, others | NCT-A BAEP | Stool | Stool bacterial culture | Overgrowth of E. coli and Staphylococcus spp. |
| Bajaj et al[27] | United States | 97 | HCV, alcohol, NASH, others | ICT PHES | Stool | Shotgun metagenomic sequencing | Alistipes ihumii, Prevotella copri, and Eubacterium spp. were higher, while Enterococcus spp. were uniquely lower in MHE diagnosed by ICT |
| Bajaj et al[28] | United States | 247 | HCV, alcohol, NASH, others | PHES ICT Stroop | Stool | Multi-tagged sequencing | Enriched Lactobacillaceae |
Because of the lack of uniform criteria for diagnosing MHE, the results of the currently available diagnostic methods for MHE are inconsistent in clinical practice. Specific signatures of fecal microbiota correspond to unique cognitive impairments determined by different diagnostic methods for MHE, including the psychometric hepatic encephalopathy score, inhibitory control test, and EncephalApp Stroop test (Table 1). For example, the abundances of Enterococcus and Streptococcus were higher in cirrhotic patients with MHE diagnosed by the psychometric hepatic encephalopathy score only; however, the abundances of Prevotella copri, Eggerthela, and Alistipes spp. were higher in those with MHE diagnosed by the inhibitory control test only[27]. Of note, the Lactobacillaceae abundance was also higher in fecal samples of MHE patients, regardless of MHE testing; therefore, this might be able to be used as a substitution for MHE testing[28].
Gut microbiota signatures of MHE patients vary depending on the etiology of liver cirrhosis. In a Chinese cohort with cirrhosis, the abundances of Streptococcaceae and Veillonellaceae were overrepresented in cirrhotic patients, and MHE patients had a higher abundance of Streptococcus salivarius[8]. Moreover, Streptococcus salivarius was also enriched in the gut microbiome of patients with MHE due to hepatitis B-associated liver cirrhosis, especially in those with sleep disturbances[29]. In contrast, in a cohort with cirrhosis in the United States, the fecal Lactobacillaceae abundance was higher in MHE patients; however, the abundance of fecal Lachnospiraceae genera, such as Clostridium XIVb and Ruminococcus, was correlated with better cognitive function independent of clinical variables[28]. Additionally, another study in the United States revealed that a higher Veillonellaceae abundance was found in the fecal microbiota of MHE patients, and that Porphyromonadaceae and Alcaligeneceae were positively associated with cognitive dysfunction in MHE[30]. The altered gut microbiota in Chinese MHE patients differs from that of MHE patients in the United States because the primary etiology of liver cirrhosis in the Chinese population is hepatitis B; however, in the United States, hepatitis C and excessive alcohol consumption are the predominant etiologies[31,32].
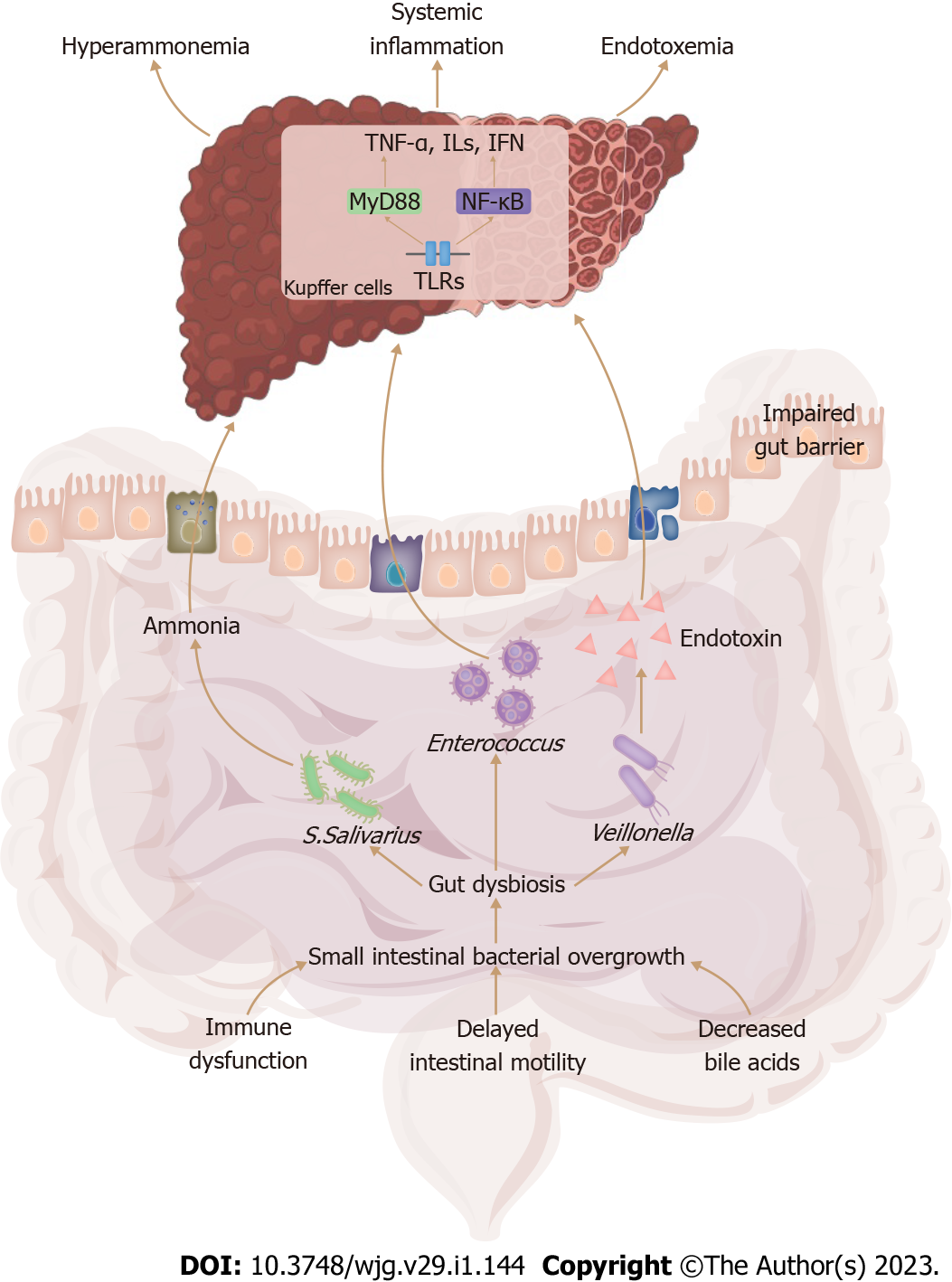
In healthy individuals, the characteristic structure and immune system of the intestinal mucosa can prevent bacteria and their byproducts from entering the systemic circulation. In patients with liver cirrhosis, the SIBO decreases the synthesis of secondary bile acids by inhibiting the activation of Farnesoid X receptor and Takeda G protein-coupled receptor, which reduces intestinal immunoglobulin A levels and further compromises the immune function of the intestinal mucosa[33,34]. Moreover, the SIBO induces decreased synthesis of antimicrobial peptide and activates mucosal immune responses, resulting in intestinal inflammation and impaired intestinal epithelium integrity[35]. Furthermore, the SIBO increases the permeability of epithelial intercellular junctions via the down-regulation of tight junction protein expressions[36]. These potential mechanisms induce a “leaky gut” which facilitates the transfer of pathogenic bacteria and their metabolites from the intestinal tract to the circulatory system, resulting in systemic inflammation (Figure 1).
Translocated bacteria and their products, such as pathogen-associated molecular patterns, are transported to the liver through the portal vein. At the molecular level, pathogen-associated molecular patterns are recognized by Toll-like receptors and cytoplasmic nucleotide-binding oligomerization domain-like receptors, and primarily stimulate hepatic Kupffer cells through the activation of MyD88-dependent and NF-κB signaling pathways[37,38]. Innate immune responses in the liver are triggered, resulting in liver damage, with the release of damage-associated molecular patterns and the production of proinflammatory cytokines and chemokines (Figure 1)[37,38]. MHE patients present the systemic proinflammatory environment reflected by increased circulatory levels of proinflammatory cytokines such as tumor necrosis factor-alpha (TNF-α), interleukins (ILs), and interferon, and chemokines such as CCL20, CXCL13 and CX3CL1[39].
In experimental mice with MHE, higher abundances of Staphylococcaceae, Enterobacteriaceae, and Lactobacillaceae in the large intestine and of Staphylococcaceae, Streptococcaceae, and Enterobacteriaceae in the small intestine were associated with systemic inflammation along with higher circulating concentrations of TNF-α and IL-1β[10]. Similarly, the abundances of Enterobacteriaceae, Fusobacteriaceae, and Veillonellaceae were positively associated with higher serum concentrations of IL-2, IL-13, and IL-23 in cirrhotic patients with MHE, and these increased cytokines were significantly correlated with MHE severity[30]. Furthermore, the association between MHE severity and increased proinflammatory cytokines has been demonstrated to be independent of the severity of liver cirrhosis and ammonia levels, suggesting that systemic inflammation with its proinflammatory cytokines is potentially implicated in the development of MHE[40].
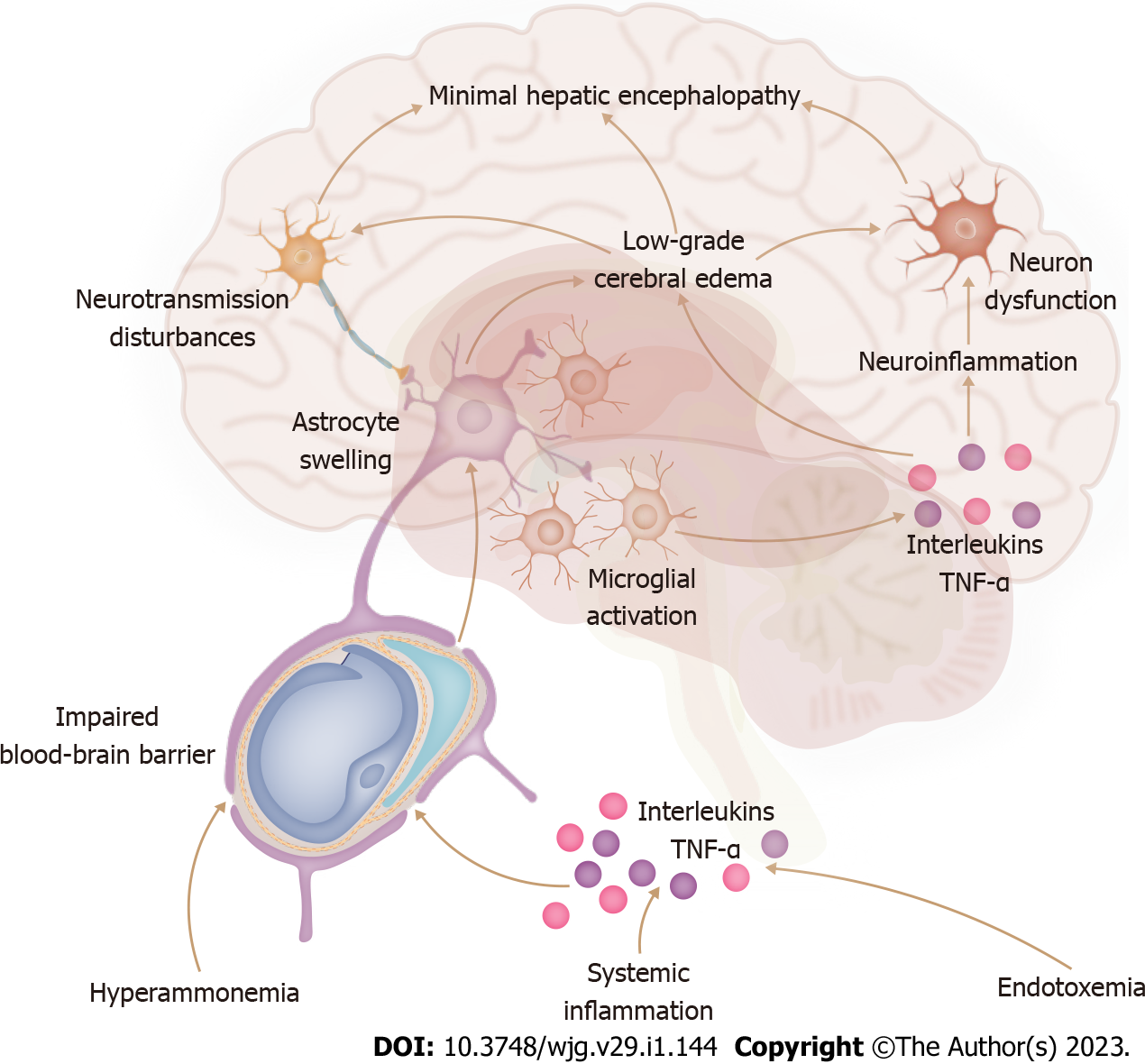
The blood-brain barrier is composed of capillary endothelial cells surrounded by capillary basement membrane and astrocytic perivascular endfeet. The BBB separates the systemic circulation and brain, prevents the entry of potentially harmful substances into the brain, and maintains the homeostasis of the brain microenvironment. Circulating proinflammatory cytokines cannot directly cross the BBB and exert their effects on the brain. However, these cytokines, including TNF-α, ILs, and interferon, down-regulate the expression of endothelial tight junction proteins, compromise cerebrovascular endothelial cells, activate astrocytes to an inflammatory reactive state, and alter BBB receptor expression and transport pathways, which consequently impair BBB integrity and further increase BBB permeability (Figure 2)[41,42]. Through the aforementioned mechanisms, the proinflammatory signaling, which is initiated by systemic inflammation, crosses the damaged BBB and underlies the neuroinflammatory response that develops in the cerebrum.
Neuroinflammation refers to a series of inflammatory response processes characterized by microglial activation and proinflammatory cytokine production in the cerebrum[43]. The proinflammatory signaling, originating from systemic inflammation and crossing the BBB, induces microglial activation, stimulates Toll-like receptors, and activates NF-κB and myeloid protein-dependent pathways to produce proinflammatory mediators in the cerebrum[41]. Neuroinflammation interferes with neurotransmission, affects neuronal function, and induces low-grade cerebral edema in concert with hyperammonemia (Figure 2)[43]. Balzano et al[44] found that MHE rats experienced not only increased serum levels of prostaglandin E2, IL-6, and IL-17 but also microglial activation with increased mRNA expression of TNF-α and IL-1β in the hippocampus, which indicated the existence of both systemic inflammation and neuroinflammation in MHE. Additionally, Enterobacteriaceae in the cecum and Staphylococcaceae in the small intestine are linked to serum proinflammatory cytokines and neuroinflammation in cirrhotic mice[10]. Moreover, germ-free mice colonized with feces from MHE patients containing high abundances of Enterobacteriaceae, Staphylococcaceae, and Streptococcaceae had remarkable microglial activation and neuroinflammation[9]. Therefore, neuroinflammation is closely associated with gut microbiota dysbiosis in experimental animal models of MHE.
In contrast, the neuroinflammation in MHE patients has not been extensively studied. Postmortem examination of cerebral specimens from MHE patients showed that mRNA expressions of TNF-α, IL-1β, and IL-6 remained unchanged in the cerebral cortex, although genes related to microglial activation were upregulated[45,46]. Current evidence of the involvement of gut microbiota dysbiosis in the pathogenesis of neuroinflammation has been derived from experimental animal models of MHE; however, related studies of MHE patients are lacking. Magnetic resonance imaging (MRI) has been successfully used to quantify the manganese deposition in the brain and noradrenaline in MHE rats[47,48]. It is presumed that cerebral MRI examinations of MHE patients could facilitate further research concerning the involvement of gut microbiota dysbiosis in the pathogenesis of neuroinflammation.
Ammonia, an important causative agent of MHE, is predominantly derived from the degradation of amino acids and urea by the gut bacteria. Urea hydrolysis is catalyzed by urease, an enzyme mainly produced by Gram-negative Enterobacteriaceae[49]. In MHE patients, an increased abundance of Streptococcus salivarius is associated with hyperammonemia because Streptococcus salivarius has a considerable number of urea catabolite genes that activate urease activity, facilitating ammonia production and accumulation, leading to further hyperammonemia[8,29,50]. Streptococcus salivarius might be a potential therapeutic target for ammonia-lowering strategies in MHE patients.
Hyperammonemia induces a leaky BBB, promotes glutamine accumulation in astrocytes, and leads to astrocyte swelling and subsequent low-grade cerebral edema that influences neurotransmission (Figure 2)[51,52]. Similar to systemic inflammation, chronic hyperammonemia induces microglial activation with the increased production of TNF-α, IL-1β, and IL-6 and impaired glutamatergic and GABAergic neurotransmission, resulting in cognitive deficits in MHE rats[53,54]. Moreover, treating MHE rats with anti-TNF-α, which does not cross the BBB, attenuated systemic inflammation, alleviated hyperammonemia-induced neuroinflammation, and ameliorated neurotransmission and cognitive function[44]. Experimental animal evidence indicated that hyperammonemia might be exerted in concert with systemic inflammation to drive the development of neuroinflammation.
Endotoxins, also known as lipopolysaccharides, are components of the outer membrane of Gram-negative bacteria. In patients with liver cirrhosis, serum endotoxin levels are increased and correlated with MHE severity, and functional modules associated with endotoxin production are abundant in the gut microbiome of MHE patients[11,29]. Several studies reported that increased endotoxin production was related to a higher Veillonella abundance in MHE patients[55,56]. Due to the impaired intestinal barrier and portal-systemic shunting, endotoxins enter the systemic circulation and cause endotoxemia with increased production of pro-inflammatory cytokines (Figure 1)[57]. Similar to pro-inflammatory cytokines, endotoxin is also unable to cross the BBB. Nevertheless, endotoxin stimulates microglia to release TNF-α, IL-1β, and reactive oxygen species, which increases the permeability of BBB tight junctions[58]. Peripheral lipopolysaccharide injection induces microglial hyperactivation, increases mRNA expressions of TNF-α, IL-1β, and IL-10 in the cerebral cortex, and impairs glutamate transmission, resulting in memory and learning deficits in mice[59,60]. Based on the synergistic effect of hyperammonemia, peripheral lipopolysaccharide injection induced cytotoxic brain swelling and a subsequent pre-coma status in cirrhotic rats[61]. However, the exact mechanism of the interaction between hyperammonemia and endotoxemia in the pathogenesis of MHE remains unclear and requires further research.
The majority of current therapeutic strategies for MHE in clinical practice exert their effects through modulation of gut microbiota dysbiosis. These microbiome therapies, including lactulose, rifaximin, probiotics, synbiotics, and FMT, alter the composition and function of the gut microbiota, inhibit pathogenic bacterial overgrowth, increase the abundance of beneficial bacteria, and reduce the production and absorption of ammonia (Table 2).
| Ref. | Design | Patients | Duration | Sample | Method | Microbiota alteration | Therapeutic effect |
| Lactulose | |||||||
| Wang et al[14] | Multi-centre, open-label, randomized controlled trial | lactulose (n = 67), control (n = 31) | 60 d | Stool | 16S rRNA sequencing | Higher abundances of Bacteroidetes, Firmicutes, Actinobacteria, and Proteobacteria were in non-responders for lactulose | Significantly ameliorated MHE |
| Rifaximin | |||||||
| Bajaj et al[56] | Controlled clinical trial | MHE patients before/after rifaximin (n = 20) | 8 wk | Stool | Multi-tagged pyrosequencing, GC/LC-MS | Modest decrease in Veillonellaceae and increase in Eubacteriaceae, with significant changes in metabolite correlations | Significant improvement in endotoxemia and cognition |
| Probiotics | |||||||
| Lactobacillus GG | |||||||
| Bajaj et al[74] | Randomized phase I, placebo-controlled trial | Probiotic (n = 14), placebo (n = 16) | 8 wk | Stool | Multi-tagged pyrosequencing, GC/LC-MS | Decreased Enterobacteriaceae and increased Lachnospiraceae and Clostridiales Incertae Sedis XIV, with significant alterations in metabolite correlations with amino acid and secondary bile acid metabolism | Attenuated endotoxemia and decreased TNF-α without change in cognition |
| Probiotics | |||||||
| Clostridium butyricum combined with Bifidobacterium infantis | |||||||
| Xia et al[73] | Randomized controlled trial | Probiotic (n = 30), placebo (n = 37) | 3 mo | Stool | 16S rRNA sequencing | Increased Clostridium cluster I and Bifidobacterium, decreased Enterobacteriaceae and Enterococcus | Reduced ammonia and improved cognition |
| Escherichia coli Nissle 1917 strain | |||||||
| Manzhalii et al[75] | Single-centre, open-label, randomized trial | Probiotic (n = 15), lactulose (n = 15), rifaximin (n = 15) | 1 mo | Stool | 16S rRNA sequencing | Normalized Bifidobacterium and Lactobacilli abundance | Reduced ammonia and pro-inflammatory cytokines and improved cognition |
| Rifaximin plus probiotic | |||||||
| Zuo et al[84] | Controlled clinical trial | Rifaximin (n = 7), rifaximin plus probiotic (n = 7) | 4 wk | Stool | 16S rRNA sequencing | Both treatments alone reduced the overall microbiome diversity, with decreased Streptococcus and Faecalibacterium, Clostridium and increased Lactobacillus | Rifaximin plus probiotics showed a more apparent effect |
| Rifaximin plus lactulose | |||||||
| Schulz et al[72] | Randomized controlled trial | Rifaximin (n = 1), rifaximin plus lactulose (n = 4) | 3 mo | Stool | 16S rRNA sequencing | Rifaximin with or without lactulose did not affect microbiota composition | MHE improvement with rifaximin lasted after the end of treatment |
| Synbiotics | |||||||
| Probiotics plus fermentable fiber | |||||||
| Liu et al[76] | Controlled clinical trial | Synbiotic (n = 20), fermentable fiber (n = 20), placebo (n = 15) | 30 d | Stool | Stool quantitative bacteriological culture | Significant increase in non-urease-producing Lactobacillus species | Reduced ammonia and endotoxemia levels, reversal in 50% of MHE patients |
Lactulose, the standard therapy for MHE, is considered a prebiotic. A multicenter, randomized, controlled trial in China suggested that lactulose reduces ammonia production and absorption by inhibiting the growth of ammonia-producing bacteria, such as Streptococcus salivarius, and facilitates the growth of beneficial saccharolytic bacteria, such as Bifidobacterium and Lactobacillus[14]. Moreover, several studies have revealed that lactulose reduces the serum concentrations of TNF-α, ILs, and endotoxins by inhibiting SIBO and bacterial translocation, thus improving cognitive dysfunction of MHE patients[11,62,63].
Despite lactulose treatment for MHE patients, there was still an increased gut microbiota dysbiosis with a lower cirrhosis dysbiosis ratio and enriched Gram-negative bacteria such as Enterobacteriaceae and Bacteroidaceae[64]. Similarly, Sarangi et al[65] indicated that lactulose did not significantly influence bacterial diversity, species richness, or taxa abundance in the gut microbiome of cirrhotic patients with MHE. Moreover, lactulose withdrawal only decreased the Faecalibacterium abundance and did not remarkably alter the gut microbiota composition[24]. These studies suggest that alterations in the gut microbiota function, rather than changes in the gut microbiota composition, may be associated with the therapeutic effects of lactulose in MHE patients.
Rifaximin is an oral semisynthetic and nonsystemic antibiotic that inhibits transcription and RNA synthesis by binding to the β-subunit of bacterial RNA polymerase, with lower gastrointestinal absorption and better antimicrobial activity[66]. As an antibiotic, rifaximin also reduced pro-inflammatory cytokines and attenuated systemic and intestinal inflammation in a mouse model of MHE[10]. Similarly, rifaximin-α inhibited serum neutrophil TLR-4 expression, decreased TNF-α and IL levels, and ameliorated MHE in cirrhotic patients[67].
A systematic review and meta-analysis showed that rifaximin is an effective and safe therapy for SIBO with a higher overall eradication rate[68]. In a mouse model of MHE, rifaximin therapy decreased intestinal ammonia production and serum IL-1β and IL-6 Levels by altering the gut microbiota function with increased secondary bile acids and decreased deconjugation without altering the gut microbiota composition[69]. Similarly, several clinical studies revealed that rifaximin attenuated hyperammonemia and endotoxemia in patients with MHE and resulted in significant changes in gut metabolites with modest alterations in gut microbiota composition, such as decreased Streptococcus and Veillonella abundance[56,70,71]. Furthermore, long-term treatment with rifaximin with or without lactulose did not affect the gut microbiota composition over a period of 3 mo in cirrhotic patients with MHE[72]. The results of these studies further support the theory that rifaximin treats MHE by modulating the metabolic function of the gut microbiota rather than gut microbiota composition, similar to the mechanism of lactulose treatment for MHE.
Probiotics, which are added to yogurt or consumed as food supplements, are live bacteria with various health benefits. Treatment with probiotics containing B. infantis and C. butyricum increased Bifidobacterium and Clostridium cluster I abundances and decreased Enterococcus and Enterobacteriaceae abundances, thereby significantly lowering serum ammonia levels of patients with hepatitis B-associated liver cirrhosis[73]. Additionally, the probiotic Lactobacillus GG increased Clostridiales XIV and Lachnospiraceae abundances, decreased the Enterobacteriaceae abundance, and decreased serum endotoxemia and TNF-α levels, resulting in alterations in metabolites associated with amino acid and secondary bile acid metabolism[74]. Moreover, the probiotic Escherichia coli Nissle strain reduced the levels of ammonia and pro-inflammatory cytokines, normalized Lactobacilli and Bifidobacterium abundances, and improved the cognitive function of MHE patients[75]. A systematic review of 19 trials showed that probiotics increased beneficial bacteria such as Lactobacillus and Bifidobacterium, decreased SIBO and endotoxemia, and reversed MHE without affecting systemic inflammation[15]. Compared with other modalities, including lactulose, rifaximin, and L-ornithine-aspartate, probiotics have similar therapeutic effects on MHE reversal and OHE prevention, and no significant differences were observed in the gut microbiota composition when probiotics and lactulose were compared[15,73]. Probiotics are regarded as alternative therapies for MHE. In contrast to lactulose and rifaximin, probiotics have therapeutic effects on MHE by altering the gut microbiota composition. Because of the complicated interconnections within the gut microbiome, a single change in the gut microbiota composition may have an unexpected effect or no effect at all. The interactions among supplemental probiotics, autochthonous beneficial bacteria, and pathogenic bacteria in the intestinal tract remain to be determined.
Synbiotics are a combination of probiotics and prebiotics. It is hypothesized that synbiotics improve the effectiveness of probiotics in the human intestine. A synbiotic containing probiotics and fermentable fibers significantly increased the nonurease-producing Lactobacillus abundance, decreased serum ammonia levels, and reversed MHE in cirrhotic patients[76]. Moreover, a combination of Bifidobacterium longum and fructo-oligosaccharide, which is another symbiotic, decreased serum ammonia levels and improved the cognitive function of MHE patients[77]. A systematic review revealed that synbiotic supplementation decreased SIBO, increased beneficial commensal bacteria such as Lactobacillus and Bifidobacterium, reduced blood ammonia and endotoxin levels, and decreased the risk of MHE recurrence[15]. Both a synbiotic and a prebiotic alone reduced ammonia and endotoxin levels, decreased the fecal Escherichia coli abundance, and reversed MHE; however, the synbiotic did not show better efficacy than the prebiotic alone[76]. Compared with prebiotics and probiotics used alone, the clinical benefits of synbiotics have yet to be demonstrated.
FMT refers to the process of transferring fecal bacteria from healthy donors to patients with gut microbiota dysbiosis[78]. FMT is an effective therapy for Clostridioides difficile infection and inflammatory bowel diseases[78,79]. In germ-free mice colonized with feces from MHE patients, FMT modulated gut microbiota dysbiosis and ameliorated microglial activation and neuroinflammation independent of active liver inflammation[9]. In cirrhotic patients with MHE, oral FMT capsules increased Ruminococcaceae and Bifidobacteriaceae abundances, decreased Streptococcaceae and Veillonellaceae abundances, and reduced serum IL-6 and lipopolysaccharide-binding protein[17]. Furthermore, long-term treatment with FMT increased Burkholderiaceae abundance and decreased Acidaminoccocaceae abundance, which prevented HE recurrence and improved cognitive function during the follow-up[80]. The role of FMT in preventing OHE recurrence by modulating gut microbiota dysbiosis has been demonstrated; however, no clinical trial regarding the FMT for MHE treatment has been reported so far. It could be presumed that FMT is a potential and effective microbiome therapy for MHE; this would require rigorous clinical trials for verification.
Clinical studies of FMT for MHE have used different routes, doses, and dosing times. Owing to these differences, uniform criteria for selecting ideal FMT donors are lacking, and the optimal FMT dosing regimen remains unclear. Moreover, the identification of pathogens in FMT donors is difficult. FMT is associated with Shigatoxin-producing Escherichia coli, extended-spectrum-β-lactamase-producing Escherichia coli, and enteropathogenic Escherichia coli infections due to the lack of donor screening[81,82]. Patients with liver cirrhosis are vulnerable to infection because of their weakened immune systems; therefore, rigorous screening and selection of FMT donors would improve FMT safety for patients with MHE.
One major challenge of microbiome therapies for MHE is that host factors, dietary habits, and long-term medications may influence the gut microbiome of MHE. Ruminococcus gnavus and Streptococcus salivarius were the predictors of response to rifaximin treatment, and a higher abundance of Bacteroidetes, Firmicutes, Actinobacteria, and Proteobacteria could predict poor response to lactulose treatment[14,83]. Moreover, patients with MHE caused by non-alcoholic cirrhosis responded better to the treatment with rifaximin plus probiotics because they presented a significant decrease in ammonia-producing bacteria genera, such as Clostridium and Streptococcus[84]. Before the microbiome therapies, the baseline signatures of gut microbiota are identified to match the appropriate microbiome therapies, and gut microbiota biomarkers are explored to predict the therapeutic effects. Based on the different baseline compositions of gut microbiota, targeted and personalized microbiome therapies might be potentially effective strategies for MHE treatment.
Furthermore, cirrhosis is a chronic liver disease, and gut microbiota dysbiosis caused by liver cirrhosis may persist for a long time and require maintenance treatment. However, most microbiome therapeutics are currently single and short-term therapies. After probiotic yogurt supplementation for more than 2 mo, patients with liver cirrhosis had significant MHE reversal rates and excellent compliance; moreover, the potential for long-term compliance existed[85]. Additionally, MHE amelioration with rifaximin treatment for more than 3 mo lasted after the end of treatment, thus indicating a long-term effect on the metabolic function of the gut microbiota[72]. The efficacy and safety of long-term microbiome therapies for MHE require multicenter studies with large populations.
Another related challenge is that rifaximin, a validated antibiotic for MHE, potentially increases antibiotic resistance in liver cirrhosis. A large European cohort study of patients with liver cirrhosis revealed a significant increase in the prevalence of multidrug-resistant bacteria from 29% to 38% during the past decade[86]. Moreover, Chang et al[87] reported that rifampin-resistant staphylococcal isolates appeared after rifaximin treatment and disappeared during the short term in cirrhotic patients. Prophylactic use of rifaximin did not alter the diversity and composition of gut microbiota or the overall resistance over 12 wk[88]. Rifaximin has not induced significant bacterial resistance and has shown active antimicrobial activity against most bacteria. Multi-drug resistant bacteria should be monitored when using rifaximin in MHE patients, especially in cirrhotic patients previously treated with antibiotics.
Gut microbiota dysbiosis initiates the pathophysiological mechanisms of hyperammonemia, systemic inflammation, and endotoxemia, which contribute to neuroinflammation via the gut-liver-brain axis in MHE. Currently available strategies for MHE treatment mainly involve the modulation of gut microbiota dysbiosis. In the future, based on the specific microbial signatures identified, personalized and targeted microbiome therapies with optimal regimens and doses may improve the efficacy and safety of MHE treatments.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Corresponding Author's Membership in Professional Societies: Society of Gastroenterology, Chinese Medical Association; Gastroenterologist Branch, Chinese Physicians Association.
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Gazouli M, Greece; Kumar S, United States; Oura S, Japan; Pitton Rissardo J, Brazil S-Editor: Zhang H L-Editor: A P-Editor: Zhang H
| 1. | Vilstrup H, Amodio P, Bajaj J, Cordoba J, Ferenci P, Mullen KD, Weissenborn K, Wong P. Hepatic encephalopathy in chronic liver disease: 2014 Practice Guideline by the American Association for the Study of Liver Diseases and the European Association for the Study of the Liver. Hepatology. 2014;60:715-735. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1583] [Cited by in RCA: 1408] [Article Influence: 128.0] [Reference Citation Analysis (1)] |
| 2. | Patidar KR, Bajaj JS. Covert and Overt Hepatic Encephalopathy: Diagnosis and Management. Clin Gastroenterol Hepatol. 2015;13:2048-2061. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 151] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 3. | Karanfilian BV, Park T, Senatore F, Rustgi VK. Minimal Hepatic Encephalopathy. Clin Liver Dis. 2020;24:209-218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 24] [Article Influence: 4.8] [Reference Citation Analysis (1)] |
| 4. | Elsaid MI, Rustgi VK. Epidemiology of Hepatic Encephalopathy. Clin Liver Dis. 2020;24:157-174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 54] [Article Influence: 10.8] [Reference Citation Analysis (1)] |
| 5. | Labenz C, Baron JS, Toenges G, Schattenberg JM, Nagel M, Sprinzl MF, Nguyen-Tat M, Zimmermann T, Huber Y, Marquardt JU, Galle PR, Wörns MA. Prospective evaluation of the impact of covert hepatic encephalopathy on quality of life and sleep in cirrhotic patients. Aliment Pharmacol Ther. 2018;48:313-321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 71] [Article Influence: 10.1] [Reference Citation Analysis (1)] |
| 6. | Hanai T, Shiraki M, Nishimura K, Miwa T, Maeda T, Ogiso Y, Imai K, Suetsugu A, Takai K, Shimizu M. Usefulness of the Stroop Test in Diagnosing Minimal Hepatic Encephalopathy and Predicting Overt Hepatic Encephalopathy. Hepatol Commun. 2021;5:1518-1526. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 15] [Article Influence: 3.8] [Reference Citation Analysis (2)] |
| 7. | Butterworth RF. Hepatic Encephalopathy in Cirrhosis: Pathology and Pathophysiology. Drugs. 2019;79:17-21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52] [Cited by in RCA: 88] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 8. | Zhang Z, Zhai H, Geng J, Yu R, Ren H, Fan H, Shi P. Large-scale survey of gut microbiota associated with MHE Via 16S rRNA-based pyrosequencing. Am J Gastroenterol. 2013;108:1601-1611. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 146] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 9. | Liu R, Kang JD, Sartor RB, Sikaroodi M, Fagan A, Gavis EA, Zhou H, Hylemon PB, Herzog JW, Li X, Lippman RH, Gonzalez-Maeso J, Wade JB, Ghosh S, Gurley E, Gillevet PM, Bajaj JS. Neuroinflammation in Murine Cirrhosis Is Dependent on the Gut Microbiome and Is Attenuated by Fecal Transplant. Hepatology. 2020;71:611-626. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 76] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 10. | Kang DJ, Betrapally NS, Ghosh SA, Sartor RB, Hylemon PB, Gillevet PM, Sanyal AJ, Heuman DM, Carl D, Zhou H, Liu R, Wang X, Yang J, Jiao C, Herzog J, Lippman HR, Sikaroodi M, Brown RR, Bajaj JS. Gut microbiota drive the development of neuroinflammatory response in cirrhosis in mice. Hepatology. 2016;64:1232-1248. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 84] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 11. | Jain L, Sharma BC, Srivastava S, Puri SK, Sharma P, Sarin S. Serum endotoxin, inflammatory mediators, and magnetic resonance spectroscopy before and after treatment in patients with minimal hepatic encephalopathy. J Gastroenterol Hepatol. 2013;28:1187-1193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 56] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 12. | Ahluwalia V, Betrapally NS, Hylemon PB, White MB, Gillevet PM, Unser AB, Fagan A, Daita K, Heuman DM, Zhou H, Sikaroodi M, Bajaj JS. Impaired Gut-Liver-Brain Axis in Patients with Cirrhosis. Sci Rep. 2016;6:26800. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 123] [Cited by in RCA: 175] [Article Influence: 19.4] [Reference Citation Analysis (0)] |
| 13. | Ding JH, Jin Z, Yang XX, Lou J, Shan WX, Hu YX, Du Q, Liao QS, Xie R, Xu JY. Role of gut microbiota via the gut-liver-brain axis in digestive diseases. World J Gastroenterol. 2020;26:6141-6162. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 64] [Cited by in RCA: 102] [Article Influence: 20.4] [Reference Citation Analysis (6)] |
| 14. | Wang JY, Bajaj JS, Wang JB, Shang J, Zhou XM, Guo XL, Zhu X, Meng LN, Jiang HX, Mi YQ, Xu JM, Yang JH, Wang BS, Zhang NP. Lactulose improves cognition, quality of life, and gut microbiota in minimal hepatic encephalopathy: A multicenter, randomized controlled trial. J Dig Dis. 2019;20:547-556. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 56] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 15. | Viramontes Hörner D, Avery A, Stow R. The Effects of Probiotics and Symbiotics on Risk Factors for Hepatic Encephalopathy: A Systematic Review. J Clin Gastroenterol. 2017;51:312-323. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 27] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 16. | Schulz C, Schütte K, Kropf S, Schmitt FC, Vasapolli R, Kliegis LM, Riegger A, Malfertheiner P. RiMINI - the influence of rifaximin on minimal hepatic encephalopathy (MHE) and on the intestinal microbiome in patients with liver cirrhosis: study protocol for a randomized controlled trial. Trials. 2016;17:111. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 17. | Bajaj JS, Salzman NH, Acharya C, Sterling RK, White MB, Gavis EA, Fagan A, Hayward M, Holtz ML, Matherly S, Lee H, Osman M, Siddiqui MS, Fuchs M, Puri P, Sikaroodi M, Gillevet PM. Fecal Microbial Transplant Capsules Are Safe in Hepatic Encephalopathy: A Phase 1, Randomized, Placebo-Controlled Trial. Hepatology. 2019;70:1690-1703. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 220] [Article Influence: 36.7] [Reference Citation Analysis (0)] |
| 18. | Bloom PP, Tapper EB, Young VB, Lok AS. Microbiome therapeutics for hepatic encephalopathy. J Hepatol. 2021;75:1452-1464. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 44] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 19. | Liu J, Xu Y, Jiang B. Novel Insights Into Pathogenesis and Therapeutic Strategies of Hepatic Encephalopathy, From the Gut Microbiota Perspective. Front Cell Infect Microbiol. 2021;11:586427. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 20. | Fallahzadeh MA, Rahimi RS. Hepatic Encephalopathy: Current and Emerging Treatment Modalities. Clin Gastroenterol Hepatol. 2022;20:S9-S19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 23] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 21. | Shah A, Shanahan E, Macdonald GA, Fletcher L, Ghasemi P, Morrison M, Jones M, Holtmann G. Systematic Review and Meta-Analysis: Prevalence of Small Intestinal Bacterial Overgrowth in Chronic Liver Disease. Semin Liver Dis. 2017;37:388-400. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 51] [Article Influence: 6.4] [Reference Citation Analysis (1)] |
| 22. | Ghosh G, Jesudian AB. Small Intestinal Bacterial Overgrowth in Patients With Cirrhosis. J Clin Exp Hepatol. 2019;9:257-267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 42] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 23. | Qin N, Yang F, Li A, Prifti E, Chen Y, Shao L, Guo J, Le Chatelier E, Yao J, Wu L, Zhou J, Ni S, Liu L, Pons N, Batto JM, Kennedy SP, Leonard P, Yuan C, Ding W, Hu X, Zheng B, Qian G, Xu W, Ehrlich SD, Zheng S, Li L. Alterations of the human gut microbiome in liver cirrhosis. Nature. 2014;513:59-64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1230] [Cited by in RCA: 1537] [Article Influence: 139.7] [Reference Citation Analysis (40)] |
| 24. | Gupta A, Dhiman RK, Kumari S, Rana S, Agarwal R, Duseja A, Chawla Y. Role of small intestinal bacterial overgrowth and delayed gastrointestinal transit time in cirrhotic patients with minimal hepatic encephalopathy. J Hepatol. 2010;53:849-855. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 152] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 25. | Bajaj JS, Torre A, Rojas ML, Fagan A, Nandez IE, Gavis EA, De Leon Osorio O, White MB, Fuchs M, Sikaroodi M, Gillevet PM. Cognition and hospitalizations are linked with salivary and faecal microbiota in cirrhosis cohorts from the USA and Mexico. Liver Int. 2020;40:1395-1407. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 19] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 26. | Bajaj JS, Hylemon PB, Ridlon JM, Heuman DM, Daita K, White MB, Monteith P, Noble NA, Sikaroodi M, Gillevet PM. Colonic mucosal microbiome differs from stool microbiome in cirrhosis and hepatic encephalopathy and is linked to cognition and inflammation. Am J Physiol Gastrointest Liver Physiol. 2012;303:G675-G685. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 354] [Cited by in RCA: 429] [Article Influence: 33.0] [Reference Citation Analysis (0)] |
| 27. | Bajaj JS, Shamsaddini A, Fagan A, McGeorge S, Gavis E, Sikaroodi M, Brenner LA, Wade JB, Gillevet PM. Distinct gut microbial compositional and functional changes associated with impaired inhibitory control in patients with cirrhosis. Gut Microbes. 2021;13:1953247. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 20] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 28. | Bajaj JS, Fagan A, White MB, Wade JB, Hylemon PB, Heuman DM, Fuchs M, John BV, Acharya C, Sikaroodi M, Gillevet PM. Specific Gut and Salivary Microbiota Patterns Are Linked With Different Cognitive Testing Strategies in Minimal Hepatic Encephalopathy. Am J Gastroenterol. 2019;114:1080-1090. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 54] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 29. | Luo M, Hu FR, Xin RJ, Yao L, Hu SJ, Bai FH. Altered gut microbiota is associated with sleep disturbances in patients with minimal hepatic encephalopathy caused by hepatitis B-related liver cirrhosis. Expert Rev Gastroenterol Hepatol. 2022;16:797-807. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 30. | Bajaj JS, Ridlon JM, Hylemon PB, Thacker LR, Heuman DM, Smith S, Sikaroodi M, Gillevet PM. Linkage of gut microbiome with cognition in hepatic encephalopathy. Am J Physiol Gastrointest Liver Physiol. 2012;302:G168-G175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 347] [Cited by in RCA: 423] [Article Influence: 32.5] [Reference Citation Analysis (0)] |
| 31. | Scaglione S, Kliethermes S, Cao G, Shoham D, Durazo R, Luke A, Volk ML. The Epidemiology of Cirrhosis in the United States: A Population-based Study. J Clin Gastroenterol. 2015;49:690-696. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 416] [Cited by in RCA: 504] [Article Influence: 50.4] [Reference Citation Analysis (0)] |
| 32. | Xu XY, Ding HG, Li WG, Xu JH, Han Y, Jia JD, Wei L, Duan ZP, Ling-Hu EQ, Zhuang H. Chinese guidelines on the management of liver cirrhosis (abbreviated version). World J Gastroenterol. 2020;26:7088-7103. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 69] [Cited by in RCA: 68] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 33. | Donaldson GP, Ladinsky MS, Yu KB, Sanders JG, Yoo BB, Chou WC, Conner ME, Earl AM, Knight R, Bjorkman PJ, Mazmanian SK. Gut microbiota utilize immunoglobulin A for mucosal colonization. Science. 2018;360:795-800. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 330] [Cited by in RCA: 440] [Article Influence: 62.9] [Reference Citation Analysis (0)] |
| 34. | Wahlström A, Sayin SI, Marschall HU, Bäckhed F. Intestinal Crosstalk between Bile Acids and Microbiota and Its Impact on Host Metabolism. Cell Metab. 2016;24:41-50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1317] [Cited by in RCA: 1850] [Article Influence: 205.6] [Reference Citation Analysis (0)] |
| 35. | Zong X, Fu J, Xu B, Wang Y, Jin M. Interplay between gut microbiota and antimicrobial peptides. Anim Nutr. 2020;6:389-396. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 77] [Cited by in RCA: 109] [Article Influence: 21.8] [Reference Citation Analysis (0)] |
| 36. | Alaish SM, Smith AD, Timmons J, Greenspon J, Eyvazzadeh D, Murphy E, Shea-Donahue T, Cirimotich S, Mongodin E, Zhao A, Fasano A, Nataro JP, Cross A. Gut microbiota, tight junction protein expression, intestinal resistance, bacterial translocation and mortality following cholestasis depend on the genetic background of the host. Gut Microbes. 2013;4:292-305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 45] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 37. | Nakamoto N, Kanai T. Role of toll-like receptors in immune activation and tolerance in the liver. Front Immunol. 2014;5:221. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 85] [Cited by in RCA: 125] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 38. | Trivedi PJ, Adams DH. Gut-liver immunity. J Hepatol. 2016;64:1187-1189. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 71] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 39. | Mangas-Losada A, García-García R, Urios A, Escudero-García D, Tosca J, Giner-Durán R, Serra MA, Montoliu C, Felipo V. Minimal hepatic encephalopathy is associated with expansion and activation of CD(4+)CD28(-), Th22 and Tfh and B lymphocytes. Sci Rep. 2017;7:6683. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 36] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 40. | Shawcross DL, Wright G, Olde Damink SW, Jalan R. Role of ammonia and inflammation in minimal hepatic encephalopathy. Metab Brain Dis. 2007;22:125-138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 186] [Cited by in RCA: 200] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 41. | Lukiw WJ. Gastrointestinal (GI) Tract Microbiome-Derived Neurotoxins-Potent Neuro-Inflammatory Signals From the GI Tract via the Systemic Circulation Into the Brain. Front Cell Infect Microbiol. 2020;10:22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 52] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 42. | Galea I. The blood-brain barrier in systemic infection and inflammation. Cell Mol Immunol. 2021;18:2489-2501. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 53] [Cited by in RCA: 355] [Article Influence: 88.8] [Reference Citation Analysis (0)] |
| 43. | Butterworth RF. The liver-brain axis in liver failure: neuroinflammation and encephalopathy. Nat Rev Gastroenterol Hepatol. 2013;10:522-528. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 160] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 44. | Balzano T, Dadsetan S, Forteza J, Cabrera-Pastor A, Taoro-Gonzalez L, Malaguarnera M, Gil-Perotin S, Cubas-Nuñez L, Casanova B, Castro-Quintas A, Ponce-Mora A, Arenas YM, Leone P, Erceg S, Llansola M, Felipo V. Chronic hyperammonemia induces peripheral inflammation that leads to cognitive impairment in rats: Reversed by anti-TNF-α treatment. J Hepatol. 2020;73:582-592. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 96] [Article Influence: 19.2] [Reference Citation Analysis (0)] |
| 45. | Zemtsova I, Görg B, Keitel V, Bidmon HJ, Schrör K, Häussinger D. Microglia activation in hepatic encephalopathy in rats and humans. Hepatology. 2011;54:204-215. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 136] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 46. | Görg B, Bidmon HJ, Häussinger D. Gene expression profiling in the cerebral cortex of patients with cirrhosis with and without hepatic encephalopathy. Hepatology. 2013;57:2436-2447. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 50] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 47. | Zhang C, Li Y, Lu J, Yang X, Wang J, Qiang J. MR T1 mapping for quantifying brain manganese deposition in type C hepatic encephalopathy rats. Biometals. 2021;34:841-854. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 48. | Yang X, Liu W, Dang P, Wang Y, Ge X, Huang X, Wang M, Zheng J, Ding X, Wang X. Decreased brain noradrenaline in minimal hepatic encephalopathy is associated with cognitive impairment in rats. Brain Res. 2022;1793:148041. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 49. | Collins CM, D'Orazio SE. Bacterial ureases: structure, regulation of expression and role in pathogenesis. Mol Microbiol. 1993;9:907-913. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 155] [Cited by in RCA: 160] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 50. | Chen YY, Weaver CA, Burne RA. Dual functions of Streptococcus salivarius urease. J Bacteriol. 2000;182:4667-4669. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 59] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 51. | Liotta EM, Kimberly WT. Cerebral edema and liver disease: Classic perspectives and contemporary hypotheses on mechanism. Neurosci Lett. 2020;721:134818. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 52. | Jayakumar AR, Rao KV, Murthy ChR, Norenberg MD. Glutamine in the mechanism of ammonia-induced astrocyte swelling. Neurochem Int. 2006;48:623-628. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 101] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 53. | Rodrigo R, Cauli O, Gomez-Pinedo U, Agusti A, Hernandez-Rabaza V, Garcia-Verdugo JM, Felipo V. Hyperammonemia induces neuroinflammation that contributes to cognitive impairment in rats with hepatic encephalopathy. Gastroenterology. 2010;139:675-684. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 231] [Cited by in RCA: 265] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 54. | Hernández-Rabaza V, Cabrera-Pastor A, Taoro-González L, Malaguarnera M, Agustí A, Llansola M, Felipo V. Hyperammonemia induces glial activation, neuroinflammation and alters neurotransmitter receptors in hippocampus, impairing spatial learning: reversal by sulforaphane. J Neuroinflammation. 2016;13:41. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 69] [Cited by in RCA: 111] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 55. | Deng YD, Peng XB, Zhao RR, Ma CQ, Li JN, Yao LQ. The intestinal microbial community dissimilarity in hepatitis B virus-related liver cirrhosis patients with and without at alcohol consumption. Gut Pathog. 2019;11:58. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 28] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 56. | Bajaj JS, Heuman DM, Sanyal AJ, Hylemon PB, Sterling RK, Stravitz RT, Fuchs M, Ridlon JM, Daita K, Monteith P, Noble NA, White MB, Fisher A, Sikaroodi M, Rangwala H, Gillevet PM. Modulation of the metabiome by rifaximin in patients with cirrhosis and minimal hepatic encephalopathy. PLoS One. 2013;8:e60042. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 254] [Cited by in RCA: 339] [Article Influence: 28.3] [Reference Citation Analysis (0)] |
| 57. | Bellot P, Francés R, Such J. Pathological bacterial translocation in cirrhosis: pathophysiology, diagnosis and clinical implications. Liver Int. 2013;33:31-39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 157] [Cited by in RCA: 183] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 58. | Chastre A, Bélanger M, Nguyen BN, Butterworth RF. Lipopolysaccharide precipitates hepatic encephalopathy and increases blood-brain barrier permeability in mice with acute liver failure. Liver Int. 2014;34:353-361. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 46] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 59. | Henry CJ, Huang Y, Wynne AM, Godbout JP. Peripheral lipopolysaccharide (LPS) challenge promotes microglial hyperactivity in aged mice that is associated with exaggerated induction of both pro-inflammatory IL-1beta and anti-inflammatory IL-10 cytokines. Brain Behav Immun. 2009;23:309-317. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 460] [Cited by in RCA: 449] [Article Influence: 28.1] [Reference Citation Analysis (0)] |
| 60. | Tanaka S, Ide M, Shibutani T, Ohtaki H, Numazawa S, Shioda S, Yoshida T. Lipopolysaccharide-induced microglial activation induces learning and memory deficits without neuronal cell death in rats. J Neurosci Res. 2006;83:557-566. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 46] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 61. | Wright G, Davies NA, Shawcross DL, Hodges SJ, Zwingmann C, Brooks HF, Mani AR, Harry D, Stadlbauer V, Zou Z, Williams R, Davies C, Moore KP, Jalan R. Endotoxemia produces coma and brain swelling in bile duct ligated rats. Hepatology. 2007;45:1517-1526. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 168] [Cited by in RCA: 148] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 62. | Moratalla A, Ampuero J, Bellot P, Gallego-Durán R, Zapater P, Roger M, Figueruela B, Martínez-Moreno B, González-Navajas JM, Such J, Romero-Gómez M, Francés R. Lactulose reduces bacterial DNA translocation, which worsens neurocognitive shape in cirrhotic patients with minimal hepatic encephalopathy. Liver Int. 2017;37:212-223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 30] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 63. | Yuan Q, Xin L, Han S, Su Y, Wu R, Liu X, Wuri J, Li R, Yan T. Lactulose Improves Neurological Outcomes by Repressing Harmful Bacteria and Regulating Inflammatory Reactions in Mice After Stroke. Front Cell Infect Microbiol. 2021;11:644448. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 23] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 64. | Bajaj JS, Heuman DM, Hylemon PB, Sanyal AJ, White MB, Monteith P, Noble NA, Unser AB, Daita K, Fisher AR, Sikaroodi M, Gillevet PM. Altered profile of human gut microbiome is associated with cirrhosis and its complications. J Hepatol. 2014;60:940-947. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 659] [Cited by in RCA: 837] [Article Influence: 76.1] [Reference Citation Analysis (0)] |
| 65. | Sarangi AN, Goel A, Singh A, Sasi A, Aggarwal R. Faecal bacterial microbiota in patients with cirrhosis and the effect of lactulose administration. BMC Gastroenterol. 2017;17:125. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 43] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 66. | Scarpignato C, Pelosini I. Rifaximin, a poorly absorbed antibiotic: pharmacology and clinical potential. Chemotherapy. 2005;51 Suppl 1:36-66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 174] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 67. | Patel VC, Lee S, McPhail MJW, Da Silva K, Guilly S, Zamalloa A, Witherden E, Støy S, Manakkat Vijay GK, Pons N, Galleron N, Huang X, Gencer S, Coen M, Tranah TH, Wendon JA, Bruce KD, Le Chatelier E, Ehrlich SD, Edwards LA, Shoaie S, Shawcross DL. Rifaximin-α reduces gut-derived inflammation and mucin degradation in cirrhosis and encephalopathy: RIFSYS randomised controlled trial. J Hepatol. 2022;76:332-342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 131] [Article Influence: 43.7] [Reference Citation Analysis (0)] |
| 68. | Gatta L, Scarpignato C. Systematic review with meta-analysis: rifaximin is effective and safe for the treatment of small intestine bacterial overgrowth. Aliment Pharmacol Ther. 2017;45:604-616. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 109] [Cited by in RCA: 166] [Article Influence: 20.8] [Reference Citation Analysis (0)] |
| 69. | Kang DJ, Kakiyama G, Betrapally NS, Herzog J, Nittono H, Hylemon PB, Zhou H, Carroll I, Yang J, Gillevet PM, Jiao C, Takei H, Pandak WM, Iida T, Heuman DM, Fan S, Fiehn O, Kurosawa T, Sikaroodi M, Sartor RB, Bajaj JS. Rifaximin Exerts Beneficial Effects Independent of its Ability to Alter Microbiota Composition. Clin Transl Gastroenterol. 2016;7:e187. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 64] [Cited by in RCA: 75] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 70. | Kaji K, Takaya H, Saikawa S, Furukawa M, Sato S, Kawaratani H, Kitade M, Moriya K, Namisaki T, Akahane T, Mitoro A, Yoshiji H. Rifaximin ameliorates hepatic encephalopathy and endotoxemia without affecting the gut microbiome diversity. World J Gastroenterol. 2017;23:8355-8366. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 82] [Cited by in RCA: 79] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 71. | Kaji K, Saikawa S, Takaya H, Fujinaga Y, Furukawa M, Kitagawa K, Ozutsumi T, Kaya D, Tsuji Y, Sawada Y, Kawaratani H, Moriya K, Namisaki T, Akahane T, Mitoro A, Yoshiji H. Rifaximin Alleviates Endotoxemia with Decreased Serum Levels of Soluble CD163 and Mannose Receptor and Partial Modification of Gut Microbiota in Cirrhotic Patients. Antibiotics (Basel). 2020;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 31] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 72. | Schulz C, Schütte K, Vilchez-Vargas R, Vasapolli R, Malfertheiner P. Long-Term Effect of Rifaximin with and without Lactulose on the Active Bacterial Assemblages in the Proximal Small Bowel and Faeces in Patients with Minimal Hepatic Encephalopathy. Dig Dis. 2019;37:161-169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 73. | Xia X, Chen J, Xia J, Wang B, Liu H, Yang L, Wang Y, Ling Z. Role of probiotics in the treatment of minimal hepatic encephalopathy in patients with HBV-induced liver cirrhosis. J Int Med Res. 2018;46:3596-3604. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 74] [Cited by in RCA: 69] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 74. | Bajaj JS, Heuman DM, Hylemon PB, Sanyal AJ, Puri P, Sterling RK, Luketic V, Stravitz RT, Siddiqui MS, Fuchs M, Thacker LR, Wade JB, Daita K, Sistrun S, White MB, Noble NA, Thorpe C, Kakiyama G, Pandak WM, Sikaroodi M, Gillevet PM. Randomised clinical trial: Lactobacillus GG modulates gut microbiome, metabolome and endotoxemia in patients with cirrhosis. Aliment Pharmacol Ther. 2014;39:1113-1125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 214] [Cited by in RCA: 222] [Article Influence: 20.2] [Reference Citation Analysis (1)] |
| 75. | Manzhalii E, Moyseyenko V, Kondratiuk V, Molochek N, Falalyeyeva T, Kobyliak N. Effect of a specific Escherichia coli Nissle 1917 strain on minimal/mild hepatic encephalopathy treatment. World J Hepatol. 2022;14:634-646. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 21] [Article Influence: 7.0] [Reference Citation Analysis (3)] |
| 76. | Liu Q, Duan ZP, Ha DK, Bengmark S, Kurtovic J, Riordan SM. Synbiotic modulation of gut flora: effect on minimal hepatic encephalopathy in patients with cirrhosis. Hepatology. 2004;39:1441-1449. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 444] [Cited by in RCA: 454] [Article Influence: 21.6] [Reference Citation Analysis (0)] |
| 77. | Malaguarnera M, Greco F, Barone G, Gargante MP, Malaguarnera M, Toscano MA. Bifidobacterium longum with fructo-oligosaccharide (FOS) treatment in minimal hepatic encephalopathy: a randomized, double-blind, placebo-controlled study. Dig Dis Sci. 2007;52:3259-3265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 138] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 78. | Weingarden AR, Vaughn BP. Intestinal microbiota, fecal microbiota transplantation, and inflammatory bowel disease. Gut Microbes. 2017;8:238-252. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 186] [Cited by in RCA: 335] [Article Influence: 41.9] [Reference Citation Analysis (0)] |
| 79. | Adelman MW, Woodworth MH, Shaffer VO, Martin GS, Kraft CS. Critical Care Management of the Patient with Clostridioides difficile. Crit Care Med. 2021;49:127-139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 80. | Bajaj JS, Fagan A, Gavis EA, Kassam Z, Sikaroodi M, Gillevet PM. Long-term Outcomes of Fecal Microbiota Transplantation in Patients With Cirrhosis. Gastroenterology. 2019;156:1921-1923.e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 131] [Article Influence: 21.8] [Reference Citation Analysis (0)] |
| 81. | Zellmer C, Sater MRA, Huntley MH, Osman M, Olesen SW, Ramakrishna B. Shiga Toxin-Producing Escherichia coli Transmission via Fecal Microbiota Transplant. Clin Infect Dis. 2021;72:e876-e880. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 28] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 82. | DeFilipp Z, Bloom PP, Torres Soto M, Mansour MK, Sater MRA, Huntley MH, Turbett S, Chung RT, Chen YB, Hohmann EL. Drug-Resistant E. coli Bacteremia Transmitted by Fecal Microbiota Transplant. N Engl J Med. 2019;381:2043-2050. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 575] [Cited by in RCA: 831] [Article Influence: 138.5] [Reference Citation Analysis (0)] |
| 83. | Yukawa-Muto Y, Kamiya T, Fujii H, Mori H, Toyoda A, Sato I, Konishi Y, Hirayama A, Hara E, Fukuda S, Kawada N, Ohtani N. Distinct responsiveness to rifaximin in patients with hepatic encephalopathy depends on functional gut microbial species. Hepatol Commun. 2022;6:2090-2104. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 19] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 84. | Zuo Z, Fan H, Tang XD, Chen YM, Xun LT, Li Y, Song ZJ, Zhai HQ. Effect of different treatments and alcohol addiction on gut microbiota in minimal hepatic encephalopathy patients. Exp Ther Med. 2017;14:4887-4895. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 13] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 85. | Bajaj JS, Saeian K, Christensen KM, Hafeezullah M, Varma RR, Franco J, Pleuss JA, Krakower G, Hoffmann RG, Binion DG. Probiotic yogurt for the treatment of minimal hepatic encephalopathy. Am J Gastroenterol. 2008;103:1707-1715. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 191] [Cited by in RCA: 200] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 86. | Fernández J, Prado V, Trebicka J, Amoros A, Gustot T, Wiest R, Deulofeu C, Garcia E, Acevedo J, Fuhrmann V, Durand F, Sánchez C, Papp M, Caraceni P, Vargas V, Bañares R, Piano S, Janicko M, Albillos A, Alessandria C, Soriano G, Welzel TM, Laleman W, Gerbes A, De Gottardi A, Merli M, Coenraad M, Saliba F, Pavesi M, Jalan R, Ginès P, Angeli P, Arroyo V; European Foundation for the Study of Chronic Liver Failure (EF-Clif). Multidrug-resistant bacterial infections in patients with decompensated cirrhosis and with acute-on-chronic liver failure in Europe. J Hepatol. 2019;70:398-411. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 264] [Cited by in RCA: 248] [Article Influence: 41.3] [Reference Citation Analysis (0)] |
| 87. | Chang JY, Kim SE, Kim TH, Woo SY, Ryu MS, Joo YH, Lee KE, Lee J, Lee KH, Moon CM, Jung HK, Shim KN, Jung SA. Emergence of rifampin-resistant staphylococci after rifaximin administration in cirrhotic patients. PLoS One. 2017;12:e0186120. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 20] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 88. | Yu X, Jin Y, Zhou W, Xiao T, Wu Z, Su J, Gao H, Shen P, Zheng B, Luo Q, Li L, Xiao Y. Rifaximin Modulates the Gut Microbiota to Prevent Hepatic Encephalopathy in Liver Cirrhosis Without Impacting the Resistome. Front Cell Infect Microbiol. 2021;11:761192. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 23] [Article Influence: 7.7] [Reference Citation Analysis (0)] |