Published online Feb 7, 2022. doi: 10.3748/wjg.v28.i5.532
Peer-review started: July 21, 2021
First decision: October 3, 2021
Revised: October 16, 2021
Accepted: January 6, 2022
Article in press: January 6, 2022
Published online: February 7, 2022
Processing time: 187 Days and 18.8 Hours
Bacillus subtilis (B. subtilis), Enterococcus faecium (E. faecium), and Enterococcus faecalis (E. faecalis) are probiotics that are widely used in the clinical treatment of irritable bowel syndrome (IBS). Whether the supernatants of these three probiotics can improve gastrointestinal sensation and movement by regulating the serotonin transporter (SERT) expression needs to be clarified.
To investigate whether B. subtilis, E. faecium, and E. faecalis supernatants can upregulate SERT expression in vitro and in vivo.
Caco-2 and HT-29 cells were stimulated with probiotic culture supernatants for 12 and 24 h, respectively. A male Sprague-Dawley rat model of post-infectious irritable bowel syndrome (PI-IBS) was established and the rats were treated with phosphate-buffered saline (group A) and three probiotics culture supernatants (groups B, C, and D) for 4 wk. The levels of SERT were detected by quantitative PCR and western blotting.
The levels of SERT at post-treatment 12 and 24 h were significantly elevated in Caco-2 cells treated with B. subtilis supernatant compared with those in the control group (aP < 0.05). Those levels were markedly upregulated in Caco-2 cells stimulated with E. faecium and E. faecalis supernatants at 24 h (aP < 0.05). In addition, SERT expression in groups B, C, and D was significantly higher than that in group A in the 2nd wk (aP < 0.05). Increased SERT expression was only found in group D in the 3rd wk (aP < 0.05). However, there was no significant difference in SERT expression between the groups in the last week (P > 0.05).
The supernatants of B. subtilis, E. faecium, and E. faecalis can upregulate SERT expression in intestinal epithelial cells and the intestinal tissues in the rat model of PI-IBS.
Core Tip: Irritable bowel syndrome (IBS) is a functional gastrointestinal disorder that can cause chronic symptoms and changes in bowel habits. Serotonin and serotonin transporter (SERT) play significant roles in the development of IBS. Bacillus subtilis (B. subtilis), Enterococcus faecium (E. faecium), and Enterococcus faecalis (E. faecalis) are probiotics that are widely used in the clinical treatment of IBS. Here, we explored the effects of B. subtilis, E. faecium, and E. faecalis supernatants on the mRNA and protein expression of SERT in vitro and in vivo, highlighting the significance of the regulation of SERT expression in the treatment of IBS patients.
- Citation: Chen YM, Li Y, Wang X, Wang ZL, Hou JJ, Su S, Zhong WL, Xu X, Zhang J, Wang BM, Wang YM. Effect of Bacillus subtilis, Enterococcus faecium, and Enterococcus faecalis supernatants on serotonin transporter expression in cells and tissues. World J Gastroenterol 2022; 28(5): 532-546
- URL: https://www.wjgnet.com/1007-9327/full/v28/i5/532.htm
- DOI: https://dx.doi.org/10.3748/wjg.v28.i5.532
Irritable bowel syndrome (IBS) is a functional gastrointestinal disorder that can cause chronic symptoms, such as abdominal pain and changes in bowel habits[1]. According to the Rome IV criteria, IBS is divided into four subtypes: IBS with predominant constipation (IBSC), IBS with predominant diarrhea (IBSD), IBS with mixed bowel habits (IBSM), and unclassified IBS (IBSU)[2]. With a prevalence varying between 5% and 10% worldwide, IBS can reduce the health-related quality of life and lower work productivity. Some symptoms of IBS such as an abnormal psychological state, anxiety, and depression may impose an economic burden on individuals, healthcare systems, and society[3,4]. The etiology of IBS is complex and still elusive. The widely recognized pathogenic mechanisms include gastrointestinal dysmotility, varied visceral sensitivity, brain-gut axis disorder, gut microenvironment, and psychoso
Bacillus subtilis (B. subtilis), Enterococcus faecium (E. faecium), and Enterococcus faecalis (E. faecalis) are probiotics that are widely used in the clinical treatment of IBS[13-16]. These probiotics can alleviate IBS symptoms such as decreasing the intensity of abdominal pain and discomfort and reducing stool frequency. Our previous study revealed that Lactobacillus rhamnosus GG supernatants can upregulate the expression level of SERT in epithelial cells and colon tissues in rats with post-infectious IBS (PI-IBS)[7,17]. We also demonstrated that Lactobacillus acidophilus and Bifidobacterium longum supernatants have similar effects on SERT expression in intestinal epithelial cells[18]. However, whether the supernatants of B. subtilis, E. faecium, and E. faecalis can improve gastrointestinal sensation and movement by regulating SERT expression still needs to be clarified.
The current research explored the effects of B. subtilis, E. faecium, and E. faecalis supernatants on the mRNA and protein expression of SERT in vitro and in vivo.
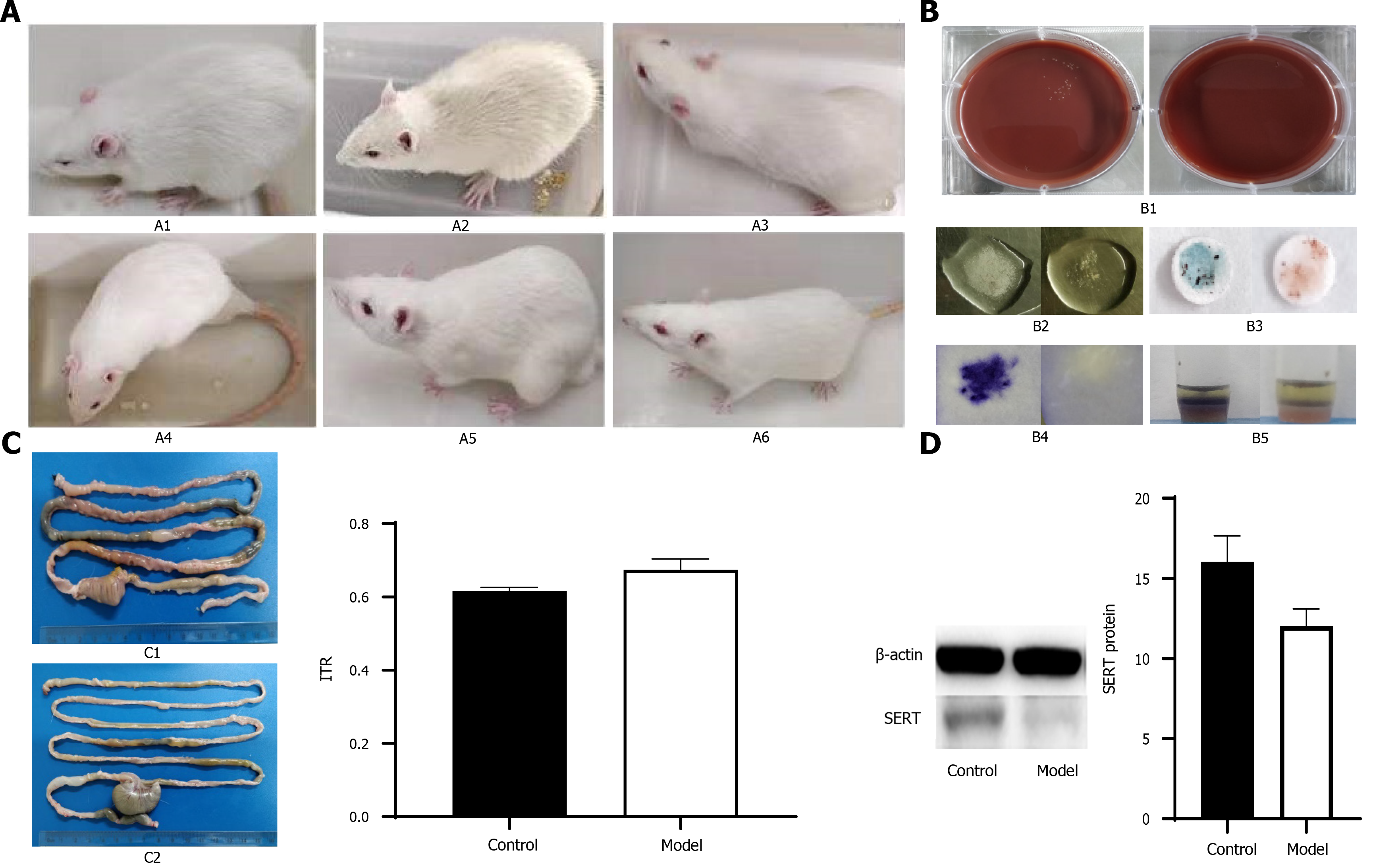
B. subtilis (CGMCC 1.3358), E. faecium (CGMCC 1.2136), and E. faecalis (CGMCC 1.2135) were obtained from China General Microbiological Culture Collection Center (Beijing, China). These bacteria were respectively inoculated into the corresponding liquid medium and cultured at 37 °C for 24 h (B. subtilis: nutrient broth medium, HB0105, Hope Biotechnology Co., Ltd., Qingdao, China; E. faecium: TSB, HB4114, Hope Biotechnology Co., Ltd., Qingdao, China; E. faecalis: TSB, HB4114, Hope Biotechnology Co., Ltd., Qingdao, China). Then they were diluted in the corresponding media, and continued to be cultured to reach the logarithmic stage with an optical density (OD) of 0.5 detected at a wavelength of 600 nm. The supernatants were collected by centrifugation (5000 g, 10 min) and filtered twice through a 0.22 μm filter.
Campylobacter jejuni 81-176 (BAA-2151; ATCC, Manassas, VA, United States) was grown on Skirrow’s selective medium (Columbia Agar Base, Oxoid CM0331) at 42 °C for 24 h. Bacterial colonies were obtained with an inoculating loop and diluted with culture medium until reaching a concentration of 1010 CFU/mL. Determination of bacterial concentration was carried out by the conventional plate-counting method.
Caco-2, a human epithelial colorectal adenocarcinoma cell line, was grown in Eagle's minimal essential medium (MEM; Gibco, New York, NY, United States) supplemented with 20% fetal bovine serum (FBS; Gibco) and 1% nonessential amino acids at 37 °C. The human colonic epithelial carcinoma cell line, HT-29, was grown in Dulbecco's modified Eagle’s medium (DMEM; Gibco, New York, NY, United States) supplemented with 10% FBS and 1% nonessential amino acids at 37 °C. The cells were incubated with the culture supernatants of B. subtilis, E. faecium, and E. faecalis diluted in MEM/DMEM (dilution ratios: 1:100, 1:50, and 1:20, respectively) for 12 and 24 h and classified as the 1:100 group, 1:50 group, and 1:20 group, respectively.
Sixty-six male Sprague-Dawley rats (270-310 g) were maintained in a room at 22 ± 1 °C under a 12-h light:12-h dark cycle in the Institute of Radiation Medicine, Chinese Academy of Medical Sciences (Tianjin, China). Rats in all groups were given the same housing conditions and diet. All animals were euthanized by barbiturate overdose (intravenous injection, 150 mg/kg pentobarbital sodium) for tissue collection. The animal protocol was designed to minimize pain or discomfort to the animals. All procedures involving animals were reviewed and approved by the Institutional Animal Care and Use Committee of the Institute of Radiation Medicine, Chinese Academy of Medical Sciences (IACUC Protocol No. IRM-DWLL-2021142).
Rats were randomly divided into a PI-IBS group (n = 52) and control group (n = 14). Rats in the PI-IBS group were given 1010 CFU/mL C. jejuni for 7 days, while those in the control group were given phosphate-buffered saline (PBS) for 7 days. The rats in both groups were fed separately. C. jejuni stool culture and biochemical tests were carried out to evaluate the infection phase of rats in the PI-IBS group. Four rats were randomly chosen from the PI-IBS and control group to perform the intestinal motility test with carbon solution gavage. The specific experimental process was previously described by Wang et al[19]. After evaluation of the rat model of PI-IBS, the remaining rats were randomly assigned to four different experimental groups (n = 12 for each group): Group A, PBS (i.g); Group B, B. subtilis supernatant (i.g); Group C, E. faecalis supernatant (i.g); Group D, mixed supernatant of B. subtilis and E. faecalis with a dilution of 1:1 (i.g). These rats were treated for 4 wk, and changes in general and fecal states were observed and recorded. Besides, three rats from each group were sacrificed, and protein expression levels of SERT in colonic tissues were detected weekly. The rats were fed 10% activated carbon suspension after fasting for 24 h and were killed 1 h later. The intestinal section from pyloric to terminal rectum was removed immediately. The total length of intestinal tract and the propelling distance of activated carbon were measured. Intestinal transit rate = propelling distance of activated carbon/total length of intestinal tract.
Total RNA was extracted from Caco-2 and HT-29 cells with Trizol reagent (Thermo Fisher Scientific, Waltham, MA, United States). cDNA was synthesized using the iScript cDNA synthesis kit (Bio-Rad Laboratories, Inc., Hercules, CA, United States). The raw materials for PCR synthesis included cDNA, 2x iQSYBR Green Supermix (Thermo Fisher Scientific), primers, and double-distilled water. The primers used for quantitative PCR (qPCR) are listed in Table 1. Relative mRNA expression was calculated using the 2-ΔΔCt method.
| Gene | Sequence (5’-3’) |
| rGAPDH | Forward: 5’-CCATCAACGACCCCTTCATT-3’; Reverse: 5’-GACCAGCTTCCCATTCTCAG-3’ |
| rSERT | Forward: 5’-ACTGTTACCAAGATGCCCTG-3’; Reverse: 5’-ATCTTCATTCCTCATCTCCGC-3’ |
| hGAPDH | Forward: 5’-ACA GCA ACT CCC ATT CTT-3’; Reverse: 5’-TCC AGG GTT TCT TAC TCC-3’ |
| hSERT | Reverse: 5’-AAT GGG TAC TCA GCA GTT CC-3’; Reverse: 5’-CCA CAG CAT AGC CAA TCA C-3’ |
Proteins were extracted from HT-29 cells, Caco-2 cells, and colonic tissues of rats using radio-immunoprecipitation assay lysis buffer (Solarbio Science & Technology Co., Ltd., Beijing, China) according to the manufacturer’s instructions. Protein concentrations were quantified using a bicinchoninic acid protein assay kit (Solarbio Science & Technology Co., Ltd., Beijing, China). A total of 40 g protein samples were isolated by 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis. The isolated protein was transferred to polyvinylidene fluoride (PVDF) membranes. The membranes were blocked in 5% skimmed milk powder at room temperature for 1 h, and incubated with primary rabbit polyclonal antibodies (SERT, Cat. No. ab102048, Abcam, Cambridge, UK; β-actin, Cat No. KM9001, Tianjin Sungene Biotech Co., Ltd., Tianjin, China) at 4 °C overnight. The PVDF membranes were incubated with diluted secondary antibody (LK2001, Tianjin Sungene Biotech Co.) at room temperature for 1 h. Then the membranes were washed with Tris-buffered saline with 0.1% Tween® 20 Detergent and detected using an enhanced chemiluminescence kit (BB-3501; Amersham, Buckinghamshire, UK). The Bio-Rad Image Analysis System (Bio-Rad Laboratories) was utilized to visualize the protein bands. The protein quantitative analysis was undertaken using ImageJ software. The relative expression was calculated using the ratio of the gray value of protein band to that of glyceraldehyde 3-phosphate dehydrogenase.
The Student’s t-test was used to assess differences between two groups. All statistical analyses were performed using SPSS 20.0 software (IBM, Armonk, NY, United States). P < 0.05 was considered statistically significant.
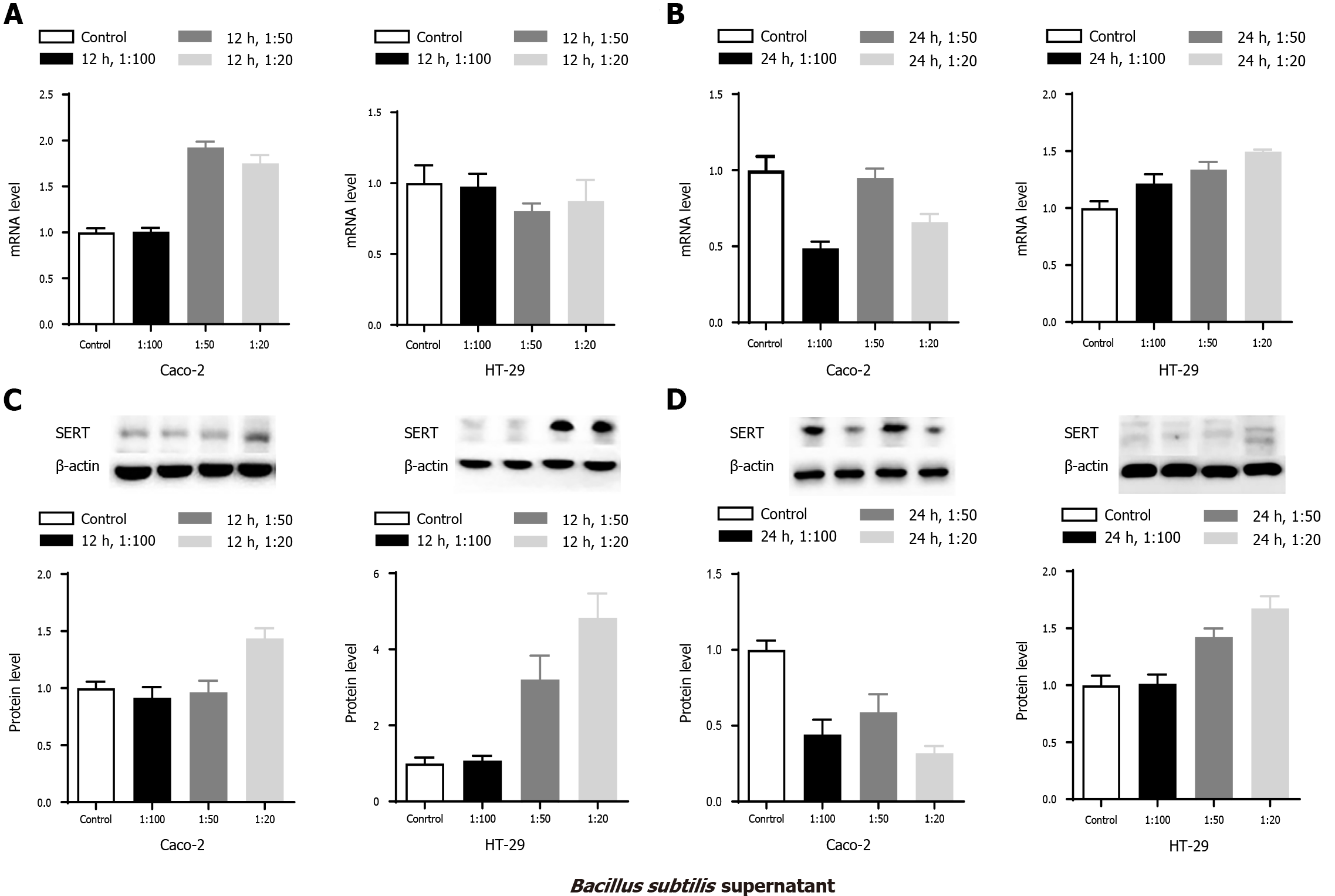
Caco-2 and HT-29 cells were treated with supernatants of B. subtilis from 1:100, 1:50, and 1:20 groups. SERT mRNA and protein expression levels in Caco-2 and HT-29 cells were detected by qPCR and western blotting, respectively. SERT mRNA levels at both 12 and 24 h after treatment were significantly increased in the 1:50 and 1:20 groups in Caco-2 cells (Figure 1 Caco-2 A&B, all aP < 0.05) compared with those in the control group. Increased SERT protein expression was found in the 1:20 group at 12 h after treatment (Figure 1 Caco-2C, aP < 0.05). Both SERT mRNA and protein levels increased at 24 h (Figure 1 Caco-2B&D, aP < 0.05). The mRNA and protein expression levels of SERT in the 1:20 group were significantly higher than those in the 1:100 group at 12 and 24 h (aP < 0.05), which indicated that the effect of B. Subtilis supernatant on the mRNA and protein levels of SERT was concentration-dependent.
SERT protein expression in HT-29 cells was markedly elevated in the 1:50 and 1:20 groups at 12 h compared with that in the control group, and the level of SERT proteins in the 1:20 group was higher than that of the 1:100 and 1:50 group (Figure 1 HT-29C, aP < 0.05). However, there was no significant difference in SERT mRNA level at 12 h among the treatment and control groups (Figure 1 HT-29A, P > 0.05). By contrast, significant reductions in the mRNA and protein levels of SERT were observed among the treatment groups at 24 h (Figure 1 HT-29-B&D, all aP < 0.05).
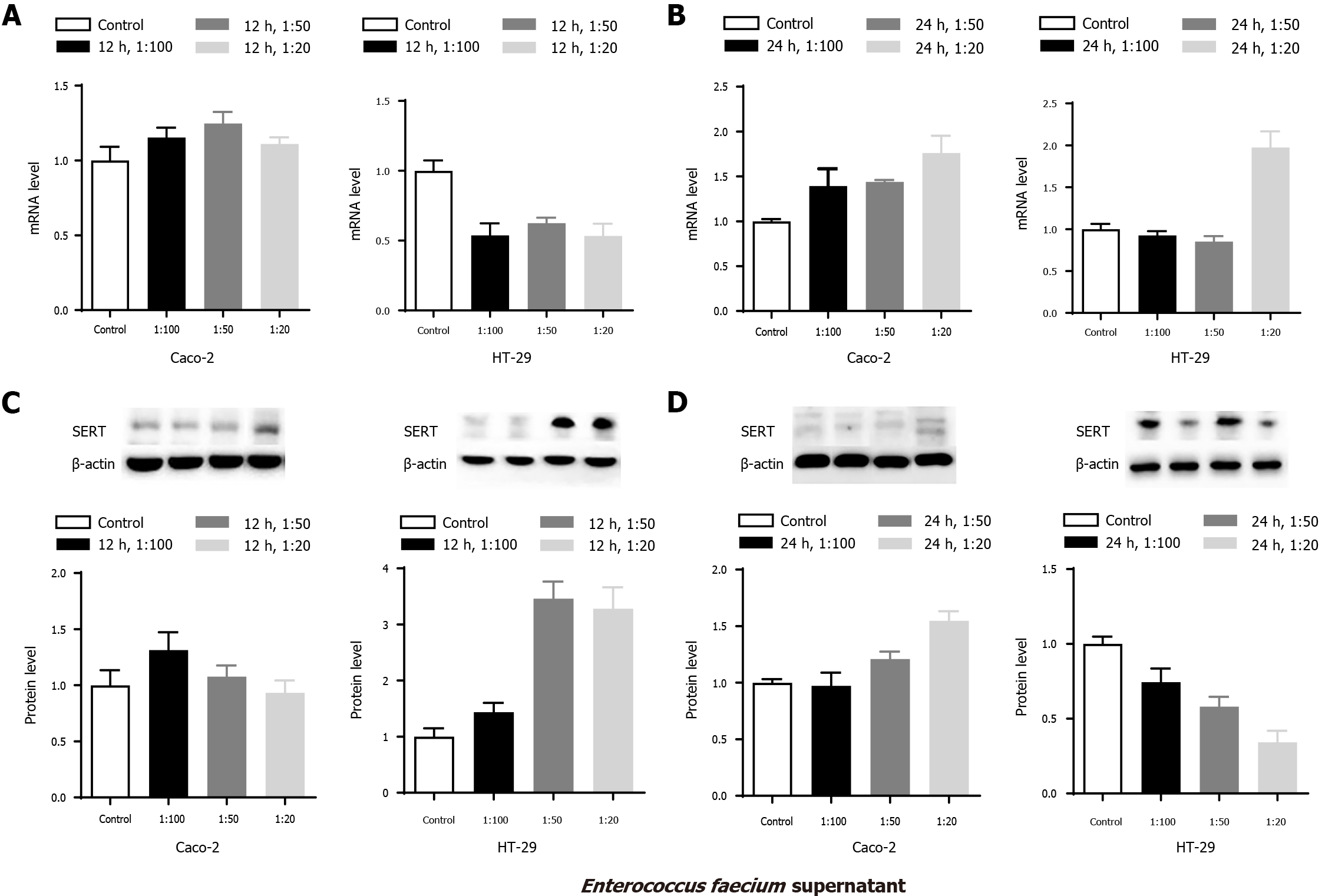
To explore the effects of E. faecium supernatant on the mRNA and protein levels of SERT in Caco-2 and HT-29 cells, similar experiments were performed. The experimental results showed that there were no significant differences in the mRNA and protein levels of SERT at 12 h among the mentioned groups (Figure 2 Caco-2-A&-C, aP > 0.05). SERT mRNA and protein levels were significantly upregulated in the 1:50 and 1:20 groups compared with the control group at 24 h (Figure 2 Caco-2-B&-D, aP < 0.05), indicating that the effects of E. faecium supernatant on SERT mRNA and protein expression were time-dependent. Besides, SERT protein level in the 1:20 group was higher than that in the 1:50 and 1:100 group at 24 h (Figure 2 Caco-2D, aP < 0.05), demonstrating that the effect of E. faecium supernatant was concentration-dependent.
SERT protein levels in the 1:50 and 1:20 groups were markedly higher than those in the control group in HT-29 cells at 12 h (Figure 2 HT-29C, aP < 0.05). However, SERT mRNA levels in the three treatment groups were dramatically reduced at 12 h (Figure 2 HT-29-A, aP < 0.05). Furthermore, compared to the control group, SERT mRNA level was notably elevated in the 1:20 group at 24 h (Figure 2 HT-29B, aP < 0.05), while SERT protein level was significantly reduced in the 1:50 and 1:20 groups at 24 h (Figure 2 HT-29-D, aP < 0.05).
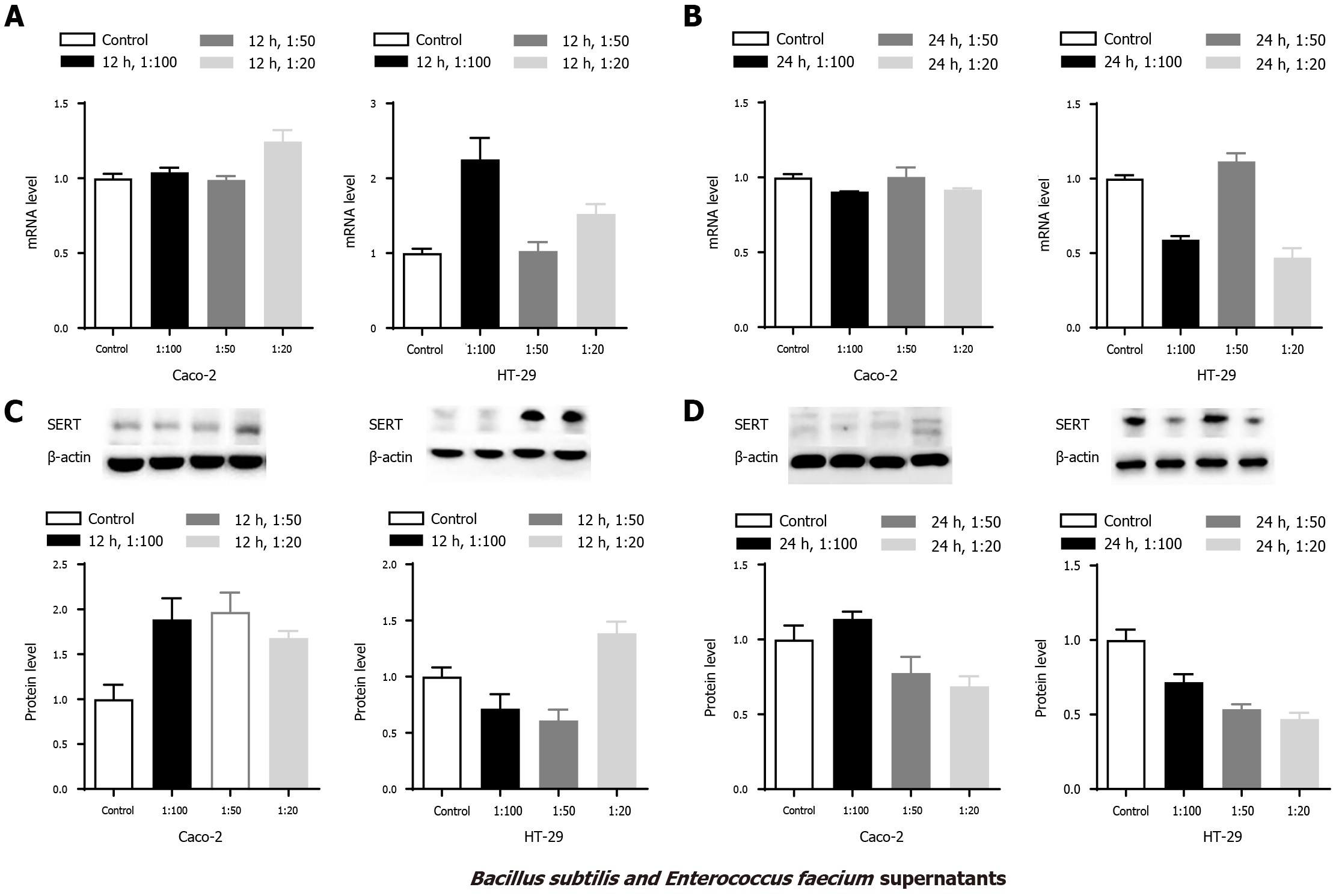
To further investigate the abovementioned results, Caco-2 and HT-29 cells were stimulated with the mixed supernatants of B. subtilis and E. faecium in the 1:1 group. Compared with the control group, SERT mRNA level was upregulated in the 1:20 group in Caco-2 cells at 12 h (Figure 3 Caco-2 A, aP < 0.05), and SERT protein levels were significantly elevated in the three groups at 12 h (Figure 3 Caco-2 C, aP < 0.05). However, there were no significant differences in the mRNA and protein levels of SERT among these groups at 24 h (Figure 3 Caco-2 B&D, P > 0.05).
The expression of SERT mRNA in HT-29 cells was upregulated in the 1:100 and 1:20 groups at 12 h (Figure 3 HT-29A, aP < 0.05) compared to the control group. An increased level of SERT protein was found in the 1:20 group at 12 h, whereas a significantly decreased level was observed in the 1:50 group compared with the control group (Figure 3 HT-29C, aP < 0.05). Furthermore, compared with the control group, SERT mRNA levels were attenuated in the 1:100 and 1:20 groups at 24 h, whereas SERT protein levels were decreased in all the three treatment groups (Figure 3 HT-29 B&D, aP < 0.05).
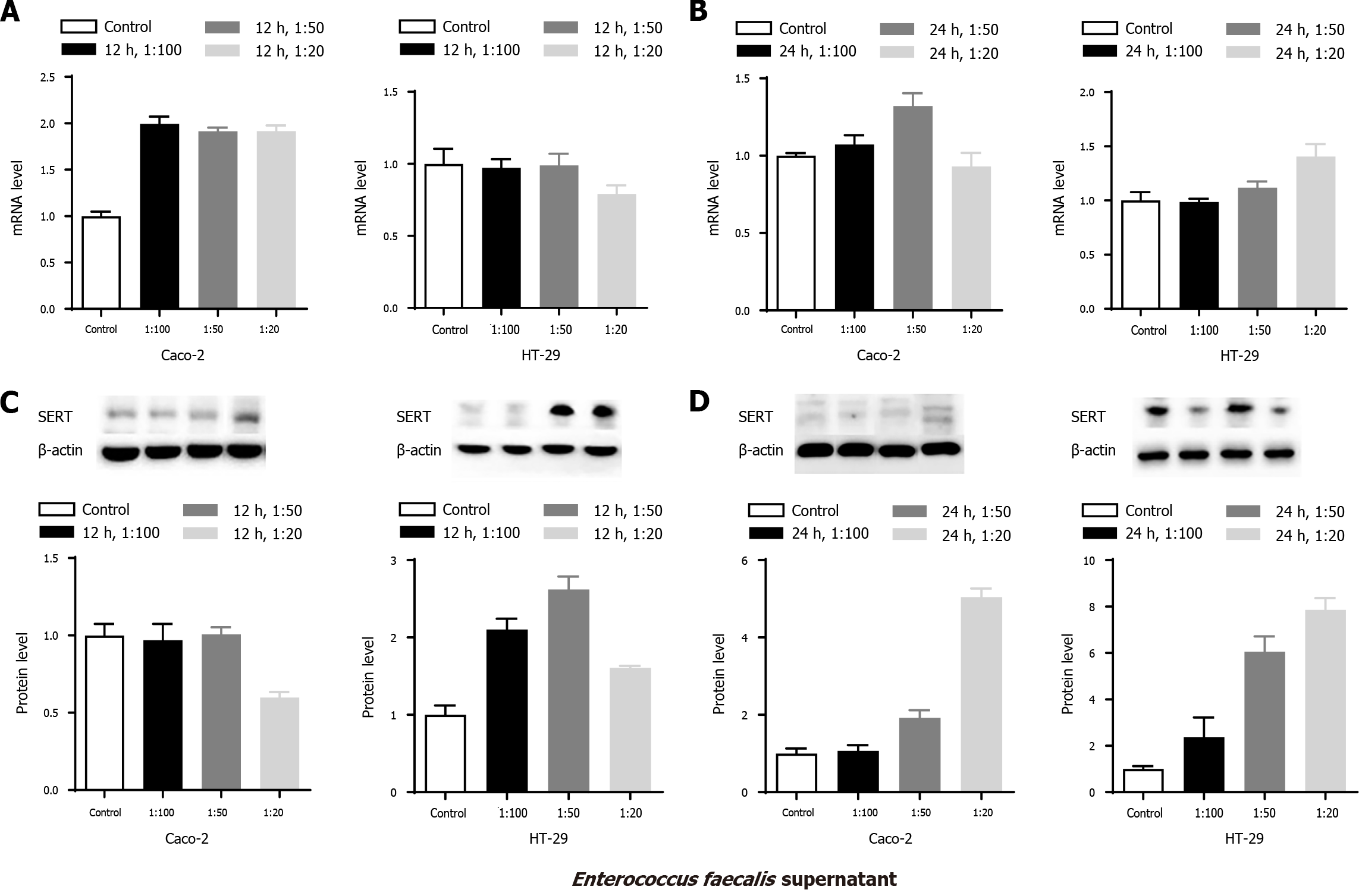
To explore the effects of E. faecalis supernatant on the mRNA and protein levels of SERT in Caco-2 and HT-29 cells, similar experiments were conducted. SERT mRNA levels in Caco-2 cells in the three treatment groups were significantly increased at 12 h (Figure 4 Caco-2 A, aP < 0.05). Western blot analysis showed that there were no significant differences in SERT protein levels among the three treatment groups at 24 h, whereas a decreased level was observed in the 1:20 group at 12 h compared to the other two groups (Figure 4 Caco-2 C, aP < 0.05). In addition, increased SERT mRNA level was only found in the 1:50 group at 24 h (Figure 4 Caco-2-B, aP < 0.05). Similarly, the expression level of SERT protein was markedly upregulated in the 1:50 and 1:20 groups at 24 h (Figure 4 Caco-2 D, aP < 0.05). SERT protein level in 1:20 group was significantly higher than that in the 1:50 and 1:100 groups at 24 h (Figure 4 Caco-2 D,
SERT mRNA level was in steady-state in HT-29 cells at 12 h among the treatment groups (Figure 4 HT-29A, P > 0.05), while western blot analysis showed that the SERT mRNA level was significantly elevated in all three groups (Figure 4 HT-29-C, aP < 0.05) compared to the control group. Additionally, the expression level of SERT protein in the 1:50 group was markedly higher than that in the 1:20 group at 12 h (Figure 4 HT-29C, aP < 0.05). SERT mRNA level was markedly increased in the 1:20 group at 24 h compared to the 1:100 group (Figure 4 HT-29B, aP < 0.05). Similarly, the levels of SERT protein were significantly elevated in the 1:20 and 1:50 groups at 24 h. Meanwhile, the increase in SERT mRNA expression in the 1:20 and 1:50 groups was noticeably higher than that in the 1:100 group at 24 h (Figure 4 HT-29-D, aP < 0.05), confirming that the effect of E. faecalis supernatant on the mRNA and protein levels of SERT was concentration-dependent in HT-29 cells.
To further verify the effect of culture supernatant of probiotics in treating IBS, we constructed a rat model of PI-IBS. Before intervention, all rats were negative for C. jejuni stool culture and biochemical tests. Fifty-two of the rats were used to establish a rat model of PI-IBS as the PI-IBS group. The results of fecal bacteriological culture and biochemical tests were positive on the 7th d after gavage in the PI-IBS group. Twenty-four rats in the control group had lively spirits, quick reactions, bright hair, normal diet, and normal urine and feces. The activities of rats in the PI-IBS group were reduced, lack of luster was observed in the hair of rats, and their appearance was thinner compared with the control group (Figure 5A). However, there were no significant differences between the PI-IBS and control group from 5th wk to 7th wk after gavage. The negative rates of C. jejuni fecal culture and biochemical tests (Figure 5B) in the PI-IBS group were higher than 95% at the 7th wk and 9th wk. After 8 wk of C. jejuni gavage, four rats from each group were randomly selected for model’s evaluation, and the rat model of PI-IBS was confirmed to be successfully constructed. The intestinal transport speed in the PI-IBS group were higher than those in the control group and the expression level of SERT protein in the PI-IBS group were lower than those in the control group (Figure 5C and 5D, aP < 0.05).
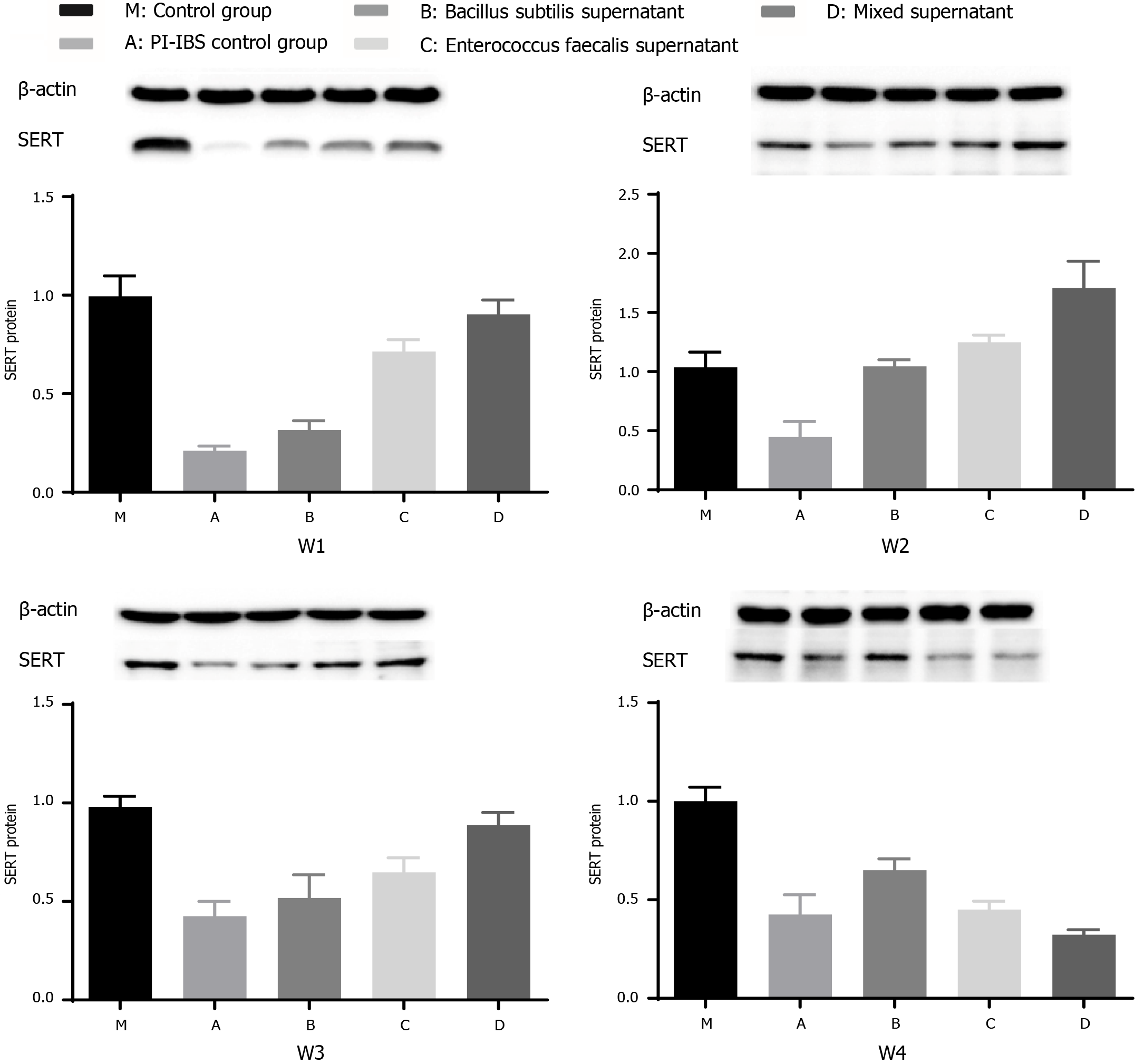
After administration of several probiotic supernatants (group B: B. subtilis supernatant; group C: E. faecalis supernatant; group D: mixed supernatants of B. subtilis and E. faecalis with dilution ratio of 1:1), the expression level of SERT protein was further detected to evaluate the intestinal motility of rats.
SERT protein expression in groups C and D were elevated compared with that in the model group (group A) during the 1st wk, and the efficacy of group C was superior than that of group B (Figure 6 w1, aP < 0.05), demonstrating that the efficacy of E. faecalis supernatant was superior than B. Subtilis supernatant in the 1st wk. The expression levels of SERT protein in groups B, C, and D were markedly higher than that in group A in the 2nd wk (Figure 6 w2, aP < 0.05), and the increase was only observed in the group D in the 3rd wk (Figure 6 w3, aP < 0.05). Besides, the expression level of SERT protein in group D was greater than that in groups B and C in the 2nd wk and 3rd wk (Figure 6 w2, w3, aP < 0.05), reflecting that the efficacy of mixed supernatant was more significant than single supernatant in the 2nd wk and 3rd wk. Meanwhile, the ratio of expression level of SERT protein in group B to that of group A was elevated from the 1st wk to the 2nd wk, indicating that the efficacy of B. Subtilis supernatant was time-dependent. Besides, the ratio of expression level of SERT protein in group C to that of group A was reduced from the 2nd wk to the 3rd wk, representing that the efficacy of E. faecalis supernatant was not significant for long-term. There was no significant increase in the expression levels of SERT protein among the treatment groups at the end of the last week (Figure 6 w4), demonstrating that the long-term efficacy of probiotic therapy was insignificant.
IBS is characterized by recurrent abdominal pain, and is associated with a change in stool frequency[3]. At present, there is no effective medical treatment for relieving the symptoms of IBS. Probiotic therapy is extensively applied in supporting the treatment of a broad range of gastrointestinal diseases, and has shown a modest effect on ameliorating the symptoms of IBS[20-23]. L. rhamnosus GG, L. acidophilus, Bifidobacterium animalis subspecies lactis, and B. longum are commonly used probiotics that can alleviate the symptoms of IBS[24-36]. A review of four clinical studies, which enrolled 214 IBS patients to evaluate the efficacy of a combination of B. subtilis and E. faecium, concluded that the combined therapy had a more significant effect on relieving abdominal pain without adverse events[16].
Previous animal studies have demonstrated that the combination of E. faecium and B. subtilis enhanced intestinal barrier function, increased the expression levels of zonula occludens-1 protein and occludin, and improved gut microbiota with a higher amount of Lactobacilli[37]. The expression level of ileal mucin 2 gene and bacteria population were increased in chickens intervened with B. subtilis in the 1st wk[38]. Besides, heat-killed E. faecalis EF-2001 has shown protective effects on a mouse model of colitis[39]. However, the underlying mechanism of therapeutic effects on IBS remains elusive and requires additional research.
Previous studies have demonstrated that the supernatants of L. rhamnosus GG, L. acidophilus, and B. longum can upregulate the expression of SERT protein in epithelial cells and PI-IBS rats[7,17,18]. SERT, a regulator of the level of 5-HT, plays a significant role in the pathogenesis of IBS and can serve as a novel therapeutic target for IBS. In the present study, we found that the supernatants of B. subtilis, E. faecium, and E. faecalis upregulated the mRNA and protein levels of SERT in intestinal epithelial cells, and the trend of mRNA and protein expression changes were similar. The expression level of SERT protein was upregulated in the intestinal tissues in a rat model of PI-IBS receiving probiotic therapy. Meanwhile, the efficacy of combined supernatants of B. subtilis and E. faecalis was superior to a single supernatant.
The mRNA and protein levels of SERT at 12 and 24 h were significantly increased in Caco-2 cells treated with supernatant of B. subtilis. E. faecium supernatant upregulated the SERT mRNA level in Caco2 cells at 24 h, and the trend of SERT protein level alteration was similar to that of SERT mRNA level. The results were similar in HT-29 cells stimulated with B. subtilis supernatant or E. faecium supernatant. SERT protein level was elevated at 12 h and reduced at 24 h. Thus, we speculated that upregulation of SERT expression in HT-29 cells intervened with B. subtilis or E. faecium supernatant was significant in the short-term. Moreover, we explored the efficacy of combination of B. subtilis and E. faecium supernatants on SERT expression. We found that the mRNA and protein levels of SERT were elevated at 12 h in Caco-2 and HT-29 cells treated with mixed supernatants of B. Subtilis and E. faecium, and the trend of SERT protein expression was similar to that of mRNA expression. Furthermore, our results confirmed the efficacy of mixed supernatants in relieving the symptoms of IBS, which was consistent with the findings of a previous study[14].
Besides, increased mRNA and protein levels of SERT were observed in Caco-2 and HT-29 cells treated with E. faecalis supernatant for 12 and 24 h. The upregulation of SERT expression treated with E. faecalis supernatant could decrease intestinal motility, confirming that ProSymbioflor containing E. faecalis and E. coli was effective on relieving the symptoms of IBS patients[15].
We also assessed the effects of mixed supernatants of B. subtilis and E. faecalis with dilution of 1:1 on the expression level of SERT in intestinal tissues in the rat model of PI-IBS. Western blot analysis revealed that SERT protein expression was upregulated in the three therapeutic groups except for the last week. The efficacy of mixed supernatants was superior than that of single supernatant in the first 3 wk. The abovementioned results indicated that the in vivo regulatory mechanisms might be more complicated. Due to the acidic environment in the stomach, the function of active ingredients in the supernatants might be attenuated, which might justify the weak efficacy of the supernatants in the last week. Thus, establishing a rat model of PI-IBS motivated us to explore a new approach for protecting important organs from possible damages.
The change in trend of mRNA and protein expression was similar, and the difference in SERT expression level between Caco-2 cells and HT-29 cells might be attributed to different types of cells. Occasionally, SERT protein level alterations in the stimulated groups were not identical to that of the mRNA level in the following conditions. First, the difference between reductions or no changes in the SERT mRNA level and significantly increased SERT protein expression could be explained by the fact that the process of mRNA transcription occurred earlier than protein translation, mRNA was easier degraded than protein, and the status of mRNA was less stable than protein. Second, the inconsistent results regarding an increased SERT mRNA level and a reduced SERT protein could be interpreted by unknown factors of supernatants that might be involved in the process of translation from mRNA to protein. Third, the non-change trend of SERT protein expression was in contrast with the increased SERT mRNA level, which was illustrated by the fact that the expression process of SERT protein was slower and more complicated.
The efficacy of the supernatants of B. subtilis, E. faecium, and E. faecalis was found time-dependent, which might help guide physicians to provide continuous treatment for patients with IBS in clinical practice. Moreover, the SERT expression level, which was regulated by the supernatants in a concentration-dependent manner, could be assessed by possible substances, such as bacterial components or small-molecule proteins regulating the SERT expression level. Such a soluble protein, p40, derived from LGG supernatant, could transactivate epidermal growth factor receptor (EGFR) signaling to reduce cytokine-induced apoptosis and epithelial barrier dysfunction in vivo and in vitro[40]. Benmansour et al[41] showed that EGF could upregulate the expression level of SERT in intestinal epithelial cells by activating the activator protien-1 (AP-1), and further interfering with the uptake of 5-HT. Cui et al[42] found that EGF could upregulate the expression level of SERT via EGFR signaling pathway. Other factors influencing the expression level of SERT include SERT gene polymorphisms, immune cells, inflammatory cytokines, microRNA 16 (MiR-16), and MiR-545[12]. B. subtilis has a highly adaptable metabolism and efficient protein expression and secretion system[43], including alkaline phosphatase and thermostable β-galactosidase[43]. A number of studies have demonstrated that B. subtilis and its conditioned media induced cytoprotective heat shock proteins in Caco-2 cells to protect colonic epithelial cells from damages[44-46]. Thus, it deserves to explore which specific factors in the supernatants of B. subtilis, E. faecalis, and E. faecium could regulate the expression level of SERT. The expression level of SERT was upregulated after stimulation with B. subtilis or E. faecalis supernatants for 12 and 24 h. However, the increase on the expression level of SERT was only observed in the cells stimulated with E. faecium supernatant at 24 h, and further research indicated that different potential active ingredients from probiotic supernatants might be effective at different time points or via different signaling pathways.
In conclusion, we found that the supernatants of B. subtilis, E. faecium, and E. faecalis could upregulate the expression level of SERT in intestinal cells, and the effect of combined supernatants of B. subtilis and E. faecalis was superior than that of single supernatant on the expression level of SERT in colonic tissues in the rat model of PI-IBS. Additionally, 5-HT and SERT showed to play important roles in the development of IBS, thus, these proteins were significant to treat IBS patients with lower expression level of SERT or with symptoms of diarrhea. In the next research, we will attempt to explore which effective ingredients in the probiotic supernatants can regulate the SERT expression, and will also find out signaling pathways modulating the SERT expression.
It is worthwhile to explore which effective ingredients in the supernatants of Bacillus subtilis, Enterococcus faecium, and Enterococcus faecalis and which signaling pathways are regulating serotonin transporter (SERT) expression further.
The supernatants of B. subtilis, E. faecium, and E. faecalis can upregulate SERT expression in intestinal epithelial cells and the intestinal tissues in the rat model of PI-IBS. And combined supernatants of B. subtilis and E. faecalis was more efficacious than single supernatant.
The levels of SERT (at post-treatment 12 and 24 h) were significantly elevated in Caco-2 cells treated with B. subtilis supernatant compared with those in the control group (aP < 0.05). Those levels were markedly upregulated in Caco-2 cells stimulated with E. faecium and E. faecalis supernatants at 24 h (aP < 0.05). In addition, the SERT expression in groups B, C and D was significantly higher than that in group A in the 2nd wk (aP < 0.05). Increased SERT expression was found only in group D in the 3rd wk (aP < 0.05). However, there was no significant difference in the SERT expression between the groups in the last week (P > 0.05).
Caco-2 and HT-29 cells were stimulated with probiotic culture supernatants for 12 and 24 h, respectively. A rat (male Sprague-Dawley rat) model of post-infectious irritable bowel syndrome (PI-IBS) was constructed and the rats were treated with PBS (group A) and three probiotics culture supernatants (groups B, C, and D) for 4 wk. The levels of SERT were detected by quantitative PCR and western blotting.
The present study aimed to explore whether B. subtilis, E. faecium, and E. faecalis supernatants could upregulate SERT expression in vitro and in vivo.
5-HT and SERT contribute significantly to the development of IBS. Whether the supernatants of B. subtilis, E. faecium, and E. faecalis can improve gastrointestinal sensation and movement by regulating SERT expression needs to be clarified. The research is significant to the treatment of IBS patients with lower expression level of SERT or with symptoms of diarrhea.
IBS is a functional gastrointestinal disorder, of which the onset and development are associated with serotonin and SERT. Recent studies have shown that B. subtilis, E. faecium, and E. faecalis play important roles in the clinical treatment of IBS. However, the underlying mechanism of therapeutic effects on IBS remains elusive and requires additional research.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Nakaji K, Saraiva MM S-Editor: Wang LL L-Editor: Filipodia P-Editor: Wang LYT
| 1. | Sultan S, Malhotra A. Irritable Bowel Syndrome. Ann Intern Med. 2017;166:ITC81-ITC96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 30] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 2. | Black CJ, Drossman DA, Talley NJ, Ruddy J, Ford AC. Functional gastrointestinal disorders: advances in understanding and management. Lancet. 2020;396:1664-1674. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 287] [Article Influence: 57.4] [Reference Citation Analysis (0)] |
| 3. | Ford AC, Sperber AD, Corsetti M, Camilleri M. Irritable bowel syndrome. Lancet. 2020;396:1675-1688. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 219] [Cited by in RCA: 452] [Article Influence: 90.4] [Reference Citation Analysis (2)] |
| 4. | Lewis ED, Antony JM, Crowley DC, Piano A, Bhardwaj R, Tompkins TA, Evans M. Efficacy of Lactobacillus paracasei HA-196 and Bifidobacterium longum R0175 in Alleviating Symptoms of Irritable Bowel Syndrome (IBS): A Randomized, Placebo-Controlled Study. Nutrients. 2020;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 52] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 5. | Coates MD, Mahoney CR, Linden DR, Sampson JE, Chen J, Blaszyk H, Crowell MD, Sharkey KA, Gershon MD, Mawe GM, Moses PL. Molecular defects in mucosal serotonin content and decreased serotonin reuptake transporter in ulcerative colitis and irritable bowel syndrome. Gastroenterology. 2004;126:1657-1664. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 554] [Cited by in RCA: 570] [Article Influence: 27.1] [Reference Citation Analysis (0)] |
| 6. | Chen MX, Chen Y, Fu R, Liu SY, Yang QQ, Shen TB. Activation of 5-HT and NR2B contributes to visceral hypersensitivity in irritable bowel syndrome in rats. Am J Transl Res. 2016;8:5580-5590. [PubMed] |
| 7. | Cao YN, Feng LJ, Liu YY, Jiang K, Zhang MJ, Gu YX, Wang BM, Gao J, Wang ZL, Wang YM. Effect of Lactobacillus rhamnosus GG supernatant on serotonin transporter expression in rats with post-infectious irritable bowel syndrome. World J Gastroenterol. 2018;24:338-350. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 33] [Cited by in RCA: 35] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 8. | Yuan J, Kang C, Wang M, Wang Q, Li P, Liu H, Hou Y, Su P, Yang F, Wei Y, Yang J. Association study of serotonin transporter SLC6A4 gene with Chinese Han irritable bowel syndrome. PLoS One. 2014;9:e84414. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 9. | Tada Y, Ishihara S, Kawashima K, Fukuba N, Sonoyama H, Kusunoki R, Oka A, Mishima Y, Oshima N, Moriyama I, Yuki T, Ishikawa N, Araki A, Harada Y, Maruyama R, Kinoshita Y. Downregulation of serotonin reuptake transporter gene expression in healing colonic mucosa in presence of remaining low-grade inflammation in ulcerative colitis. J Gastroenterol Hepatol. 2016;31:1443-1452. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 27] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 10. | Kim DY, Camilleri M. Serotonin: a mediator of the brain-gut connection. Am J Gastroenterol. 2000;95:2698-2709. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 268] [Cited by in RCA: 303] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 11. | Mawe GM, Hoffman JM. Serotonin signalling in the gut--functions, dysfunctions and therapeutic targets. Nat Rev Gastroenterol Hepatol. 2013;10:473-486. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 593] [Cited by in RCA: 803] [Article Influence: 66.9] [Reference Citation Analysis (0)] |
| 12. | Jin DC, Cao HL, Xu MQ, Wang SN, Wang YM, Yan F, Wang BM. Regulation of the serotonin transporter in the pathogenesis of irritable bowel syndrome. World J Gastroenterol. 2016;22:8137-8148. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 48] [Cited by in RCA: 56] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 13. | Sohn W, Lee OY, Kwon JG, Park KS, Lim YJ, Kim TH, Jung SW, Kim JI. Tianeptine vs amitriptyline for the treatment of irritable bowel syndrome with diarrhea: a multicenter, open-label, non-inferiority, randomized controlled study. Neurogastroenterol Motil. 2012;24:860-e398. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 14. | Choi CH, Kwon JG, Kim SK, Myung SJ, Park KS, Sohn CI, Rhee PL, Lee KJ, Lee OY, Jung HK, Jee SR, Jeen YT, Choi MG, Choi SC, Huh KC, Park H. Efficacy of combination therapy with probiotics and mosapride in patients with IBS without diarrhea: a randomized, double-blind, placebo-controlled, multicenter, phase II trial. Neurogastroenterol Motil. 2015;27:705-716. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 35] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 15. | Enck P, Zimmermann K, Menke G, Müller-Lissner S, Martens U, Klosterhalfen S. A mixture of Escherichia coli (DSM 17252) and Enterococcus faecalis (DSM 16440) for treatment of the irritable bowel syndrome--a randomized controlled trial with primary care physicians. Neurogastroenterol Motil. 2008;20:1103-1109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 88] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 16. | Tompkins TA, Xu X, Ahmarani J. A comprehensive review of post-market clinical studies performed in adults with an Asian probiotic formulation. Benef Microbes. 2010;1:93-106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 27] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 17. | Wang YM, Ge XZ, Wang WQ, Wang T, Cao HL, Wang BL, Wang BM. Lactobacillus rhamnosus GG supernatant upregulates serotonin transporter expression in intestinal epithelial cells and mice intestinal tissues. Neurogastroenterol Motil. 2015;27:1239-1248. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 26] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 18. | Cao YN, Feng LJ, Wang BM, Jiang K, Li S, Xu X, Wang WQ, Zhao JW, Wang YM. Lactobacillus acidophilus and Bifidobacterium longum supernatants upregulate the serotonin transporter expression in intestinal epithelial cells. Saudi J Gastroenterol. 2018;24:59-66. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 32] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 19. | Wang W, Xin H, Fang X, Dou H, Liu F, Huang D, Han S, Fei G, Zhu L, Zha S, Zhang H, Ke M. Isomalto-oligosaccharides ameliorate visceral hyperalgesia with repair damage of ileal epithelial ultrastructure in rats. PLoS One. 2017;12:e0175276. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 25] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 20. | Tojo González R, Suarez Gonzalez A, Rúas Madiedo P, Mancebo Mata A, Pipa Muñiz M, Barreiro Alonso E, Roman Llorente FJ, Moro Villar MC, Arce González MM, Villegas Diaz MF, Mosquera Sierra E, Ruiz Ruiz M. [Irritable Bowel Syndrome; gut microbiota and probiotic therapy]. Nutr Hosp. 2015;31 Suppl 1:83-88. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 21. | Sarowska J, Choroszy-Król I, Regulska-Ilow B, Frej-Mądrzak M, Jama-Kmiecik A. The therapeutic effect of probiotic bacteria on gastrointestinal diseases. Adv Clin Exp Med. 2013;22:759-766. [PubMed] |
| 22. | Chey WD, Kurlander J, Eswaran S. Irritable bowel syndrome: a clinical review. JAMA. 2015;313:949-958. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 629] [Cited by in RCA: 742] [Article Influence: 74.2] [Reference Citation Analysis (0)] |
| 23. | Moayyedi P, Ford AC, Talley NJ, Cremonini F, Foxx-Orenstein AE, Brandt LJ, Quigley EM. The efficacy of probiotics in the treatment of irritable bowel syndrome: a systematic review. Gut. 2010;59:325-332. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 470] [Cited by in RCA: 452] [Article Influence: 30.1] [Reference Citation Analysis (0)] |
| 24. | Szajewska H, Hojsak I. Health benefits of Lactobacillus rhamnosus GG and Bifidobacterium animalis subspecies lactis BB-12 in children. Postgrad Med. 2020;132:441-451. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 28] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 25. | Martoni CJ, Srivastava S, Leyer GJ. Lactobacillus acidophilus DDS-1 and Bifidobacterium lactis UABla-12 Improve Abdominal Pain Severity and Symptomology in Irritable Bowel Syndrome: Randomized Controlled Trial. Nutrients. 2020;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 60] [Cited by in RCA: 78] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 26. | Bonfrate L, Di Palo DM, Celano G, Albert A, Vitellio P, De Angelis M, Gobbetti M, Portincasa P. Effects of Bifidobacterium longum BB536 and Lactobacillus rhamnosus HN001 in IBS patients. Eur J Clin Invest. 2020;50:e13201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 63] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 27. | Sadrin S, Sennoune S, Gout B, Marque S, Moreau J, Zinoune K, Grillasca JP, Pons O, Maixent JM. A 2-strain mixture of Lactobacillus acidophilus in the treatment of irritable bowel syndrome: A placebo-controlled randomized clinical trial. Dig Liver Dis. 2020;52:534-540. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 23] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 28. | Oh JH, Jang YS, Kang D, Chang DK, Min YW. Efficacy and Safety of New Lactobacilli Probiotics for Unconstipated Irritable Bowel Syndrome: A Randomized, Double-Blind, Placebo-Controlled Trial. Nutrients. 2019;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 32] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 29. | Ding FCL, Karkhaneh M, Zorzela L, Jou H, Vohra S. Probiotics for paediatric functional abdominal pain disorders: A rapid review. Paediatr Child Health. 2019;24:383-394. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 30. | Yoon JY, Cha JM, Oh JK, Tan PL, Kim SH, Kwak MS, Jeon JW, Shin HP. Probiotics Ameliorate Stool Consistency in Patients with Chronic Constipation: A Randomized, Double-Blind, Placebo-Controlled Study. Dig Dis Sci. 2018;63:2754-2764. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 44] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 31. | Bull MJ, Plummer NT. Part 2: Treatments for Chronic Gastrointestinal Disease and Gut Dysbiosis. Integr Med (Encinitas). 2015;14:25-33. [PubMed] |
| 32. | Ducrotté P, Sawant P, Jayanthi V. Clinical trial: Lactobacillus plantarum 299v (DSM 9843) improves symptoms of irritable bowel syndrome. World J Gastroenterol. 2012;18:4012-4018. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 175] [Cited by in RCA: 193] [Article Influence: 14.8] [Reference Citation Analysis (6)] |
| 33. | Hosseini A, Nikfar S, Abdollahi M. Probiotics use to treat irritable bowel syndrome. Expert Opin Biol Ther. 2012;12:1323-1334. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 26] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 34. | Floch MH. Use of diet and probiotic therapy in the irritable bowel syndrome: analysis of the literature. J Clin Gastroenterol. 2005;39:S243-S246. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 33] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 35. | Hasler WL. The irritable bowel syndrome. Med Clin North Am. 2002;86:1525-1551. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 36. | Rousseaux C, Thuru X, Gelot A, Barnich N, Neut C, Dubuquoy L, Dubuquoy C, Merour E, Geboes K, Chamaillard M, Ouwehand A, Leyer G, Carcano D, Colombel JF, Ardid D, Desreumaux P. Lactobacillus acidophilus modulates intestinal pain and induces opioid and cannabinoid receptors. Nat Med. 2007;13:35-37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 525] [Cited by in RCA: 541] [Article Influence: 28.5] [Reference Citation Analysis (0)] |
| 37. | Zhang PW, Yan T, Wang XL, Kuang SC, Xiao YC, Lu WW, Bi DR. Probiotic mixture ameliorates heat stress of laying hens by enhancing intestinal barrier function and improving gut microbiota. Italian Journal of Animal Science. 2017;16:292-300. [RCA] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 48] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 38. | Majidi-Mosleh A, Sadeghi AA, Mousavi SN, Chamani M, Zarei A. Ileal MUC2 gene expression and microbial population, but not growth performance and immune response, are influenced by in ovo injection of probiotics in broiler chickens. Br Poult Sci. 2017;58:40-45. [RCA] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 22] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 39. | Choi EJ, Lee HJ, Kim WJ, Han KI, Iwasa M, Kobayashi K, Debnath T, Tang Y, Kwak YS, Yoon JH, Kim EK. Enterococcus faecalis EF-2001 protects DNBS-induced inflammatory bowel disease in mice model. PLoS One. 2019;14:e0210854. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 23] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 40. | Wang L, Cao H, Liu L, Wang B, Walker WA, Acra SA, Yan F. Activation of epidermal growth factor receptor mediates mucin production stimulated by p40, a Lactobacillus rhamnosus GG-derived protein. J Biol Chem. 2014;289:20234-20244. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 105] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 41. | Benmansour S, Owens WA, Cecchi M, Morilak DA, Frazer A. Serotonin clearance in vivo is altered to a greater extent by antidepressant-induced downregulation of the serotonin transporter than by acute blockade of this transporter. J Neurosci. 2002;22:6766-6772. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 133] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 42. | Cui XF, Zhou WM, Yang Y, Zhou J, Li XL, Lin L, Zhang HJ. Epidermal growth factor upregulates serotonin transporter and its association with visceral hypersensitivity in irritable bowel syndrome. World J Gastroenterol. 2014;20:13521-13529. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 14] [Cited by in RCA: 18] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 43. | Su Y, Liu C, Fang H, Zhang D. Bacillus subtilis: a universal cell factory for industry, agriculture, biomaterials and medicine. Microb Cell Fact. 2020;19:173. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 71] [Cited by in RCA: 218] [Article Influence: 43.6] [Reference Citation Analysis (0)] |
| 44. | Ropeleski MJ, Tang J, Walsh-Reitz MM, Musch MW, Chang EB. Interleukin-11-induced heat shock protein 25 confers intestinal epithelial-specific cytoprotection from oxidant stress. Gastroenterology. 2003;124:1358-1368. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 77] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 45. | Arvans DL, Vavricka SR, Ren H, Musch MW, Kang L, Rocha FG, Lucioni A, Turner JR, Alverdy J, Chang EB. Luminal bacterial flora determines physiological expression of intestinal epithelial cytoprotective heat shock proteins 25 and 72. Am J Physiol Gastrointest Liver Physiol. 2005;288:G696-G704. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 91] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 46. | Fujiya M, Musch MW, Nakagawa Y, Hu S, Alverdy J, Kohgo Y, Schneewind O, Jabri B, Chang EB. The Bacillus subtilis quorum-sensing molecule CSF contributes to intestinal homeostasis via OCTN2, a host cell membrane transporter. Cell Host Microbe. 2007;1:299-308. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 188] [Cited by in RCA: 188] [Article Influence: 10.4] [Reference Citation Analysis (0)] |