Published online Nov 14, 2022. doi: 10.3748/wjg.v28.i42.6045
Peer-review started: July 1, 2022
First decision: August 1, 2022
Revised: August 13, 2022
Accepted: October 14, 2022
Article in press: October 14, 2022
Published online: November 14, 2022
Processing time: 132 Days and 5.2 Hours
Assessment of liver reserve function (LRF) is essential for predicting the prognosis of patients with chronic liver disease (CLD) and determines the extent of liver resection in patients with hepatocellular carcinoma.
To establish noninvasive models for LRF assessment based on liver stiffness measurement (LSM) and to evaluate their clinical performance.
A total of 360 patients with compensated CLD were retrospectively analyzed as the training cohort. The new predictive models were established through logistic regression analysis and were validated internally in a prospective cohort (132 patients).
Our study defined indocyanine green retention rate at 15 min (ICGR15) ≥ 10% as mildly impaired LRF and ICGR15 ≥ 20% as severely impaired LRF. We constru
The new models had a good predictive performance for LRF and could replace the indocyanine green (ICG) clearance test, especially in patients who are unable to undergo ICG testing.
Core Tip: This study aimed to establish predictive models of liver stiffness measurement (LSM) in patients with compensated chronic liver disease based on LSM and evaluate their clinical value. The results showed that the new models had a good predictive performance for liver reserve function (LRF). The area under the curve of the models was higher than that of the model for end-stage liver disease, albumin-bilirubin grade and prothrombin time international normalized ratio to albumin ratio. Moreover, the predictive performance of the new models was validated in a prospective cohort. We believe that these models could replace the indocyanine green (ICG) clearance test to assess LRF, especially in patients who are unable to undergo ICG testing.
- Citation: Lai RM, Wang MM, Lin XY, Zheng Q, Chen J. Clinical value of predictive models based on liver stiffness measurement in predicting liver reserve function of compensated chronic liver disease. World J Gastroenterol 2022; 28(42): 6045-6055
- URL: https://www.wjgnet.com/1007-9327/full/v28/i42/6045.htm
- DOI: https://dx.doi.org/10.3748/wjg.v28.i42.6045
The high prevalence of chronic liver disease (CLD) in China has become a severe public health problem. Cirrhosis, hepatocellular carcinoma (HCC), hepatic encephalopathy and other decompensated complications are the leading causes of mortality in CLD patients without treatment. Liver reserve function (LRF) is defined as the compensated ability of the liver to maintain normal physiological functions in the presence of injury, which mainly depends on the quality and quantity of hepatocytes in the remnant liver[1,2]. There are no obvious clinical symptoms in CLD patients in the early stage, but their LRF may be impaired. Early evaluation of LRF is of great help in identifying disease progression, timely implementation of interventions and appropriate treatment strategies in CLD patients. Several scoring systems, including the Child-Turcotte-Pugh (CTP), model for end-stage liver disease (MELD), albumin-bilirubin (ALBI) and APRI, can be used to evaluate LRF[1,3-5]. Although the CTP score is widely used to assess LRF, it includes subjective criteria, such as ascites and hepatic encephalopathy. The MELD score is initially used as a standard model to assess the prognosis of patients with decompensated cirrhosis, but its creatinine (Cr) value can be significantly affected by age and gender.
The indocyanine green (ICG) clearance test is commonly used for LRF assessment, which is considered the most valuable method for evaluating LRF. ICGR15 had become a standard dynamic preoperative instrument to evaluate the hepatic functional reserve before liver resection and predict post-hepatectomy liver failure[6,7]. However, the ICG clearance test process is tedious and requires a technical operator; thus, most of these tests can only be carried out in major hospitals. In addition, some patients are allergic to ICG, which can lead to failure of the test. Due to impossible implementation of the ICG clearance test in CLD patients, a new method to accurately assess LRF is needed.
Liver stiffness measurement (LSM) is commonly used to evaluate the degree of liver fibrosis, and due to its non-invasiveness, cost-efficiency and safety, it has been widely applied in clinical treatment. Previous studies have shown that LSM can predict the occurrence of liver failure after HCC resection[8-10]. Therefore, LSM has potential value in evaluating hepatic functional reserve.
The purpose of this study was to analyze the association between LSM and ICGR15 in evaluating LRF. We constructed the predictive models based on LSM and examined their clinical application value in evaluating LRF in compensated CLD patients.
All patients with CLD (≥ 18 years old) consecutively observed in the inpatient department of the Hepatology Research Institute of the First Affiliated Hospital, Fujian Medical University, China, from March 2016 to June 2019 were retrospectively analyzed as the training cohort. From September 2019 to August 2020, patients with CLD were prospectively evaluated to validate the new models. Information regarding the patients’ demographics, ICG clearance test, laboratory data and Fibro-scan examination was abstracted from the electronic medical record system of the First Affiliated Hospital of Fujian Medical University. Patients with the following conditions were excluded: (1) Decompensated cirrhosis with CTP grade B and C; (2) insufficient data; and (3) complicated with other tumors, or gestation. After exclusion, 492 patients were identified for study inclusion, comprising 389 chronic hepatitis B patients, 35 fatty liver disease patients, 21 autoimmune liver disease patients, 8 hepatitis C virus patients and 39 patients with other etiologies. All enrolled patients were divided into the training cohort (360 patients) and validation cohort (132 patients), including 105 HCC patients who met the diagnostic criteria in the guidelines for diagnosis and treatment of primary liver cancer in China (2019 edition)[11].
The demographic data collected included age and gender. The clinical laboratory information included prothrombin time (PT), international normalized ratio (INR), total bilirubin (TBIL), aspartate aminotransferase, alanine aminotransferase, albumin (ALB), glomerular filtration rate, alkaline phosphatase, gamma-glutamyltransferase, cholinesterase, platelet count, and hemoglobin. The parameters were detected using an Olympus AU2700 automatic biochemical analyzer. The calculation of CTP score included five items, namely ALB, TBIL, PT, hepatic encephalopathy and ascites[12]. The CTP classifications were defined as grade A (5-6 points), grade B (7-9 points), and grade C (10-15 points). The MELD score was calculated by the formula 3.78 × ln[TBIL (mg/dL)] + 11.2 × ln (INR) + 9.57 × ln[Cr (mg/dL)] + 6.43 × etiology (0 for cholestasis and alcohol, and 1 for others)[13]. The prothrombin time international normalized ratio to albumin ratio (PTAR) score was calculated by the formula INR/ALB (g/dL)[14]. The ALBI score was calculated by the formula ln[TBIL (mol/L)] × 0.66 + ln[ALB (g/L)]-0.0852[15].
All patients received the ICG clearance test after overnight fasting, a dose of 0.5 mg/kg of ICG was rapidly injected into patients via a peripheral vein in the forearm. An optical probe attached to the patient’s nose was used to monitor plasma ICG concentrations, and the value of ICGR15 was calculated by a Pulse Dye Densito-Graph Analyzer (DDG-3300K, Nihon Kohden, Tokyo, Japan)[16]. The LRF was defined as normal if ICGR15 < 10%, mild impairment if ICGR15 ≥ 10%, and severe impairment if ICGR15 ≥ 20%.
The Fibro-Scan 502 Touch (Echosens, Paris, France) was performed by the same trained operator according to the manufacturer’s instructions. LSM was performed on the right lobe of the liver through the intercostal spaces. Ten successful acquisitions were performed for each patient. The success rate (≥ 60%) was calculated as the number of successful measurements divided by the total number of measurements recorded[17]. LSM was expressed as the median and IQR [in kilopascals (kPa)] of all valid measurements obtained. A LSM was considered reliable if 10 valid acquisitions were obtained. Patients with poorly reliable measurements (IQR/median ratio > 0.30 with a median LSM > 7.1 kPa) were excluded[18]. This retrospective study was approved by the ethics committee of the First Affiliated Hospital of Fujian Medical University, China.
Statistical analyses were performed using SPSS 23.0. The normally distributed continuous variables are presented as mean ± SD, which were further evaluated by Student’s t-test in the different groups. Whereas, variables showing skewed distributions were evaluated by the Mann-Whitney U test, and are presented as median (interquartile range). Categorical variables are described using frequencies and proportions, and the Pearson’s chi-squared test was used to compare categorical variables.
Multivariable analyses were conducted on variables that reached P < 0.1 at univariate analysis. Multivariate analysis was performed using the logistic regression analysis, and we established regression prediction models to predict the hepatic functional reserve. The continuous variables (cut-off value of LSM was 12.4 and PTAR was 0.280) were transformed into dichotomous variables. In order to avoid collinearity of some clinical indicators, stepwise forward regression was used in multivariate analysis. The optimal cut-off level of the model was determined by a receiver operator characteristic curve analysis. The areas under the curve (AUCs) were measured and compared to evaluate the discrimination ability of different models. The final predictive model was fitted on an internal validation dataset and on the entire prospective population. A two sided P value less than 0.05 was considered significant.
Overall, 492 patients were included in the study, including 103 patients with HCC (Table 1). 350 (71.14%) of 492 patients were male, the predominant etiology of liver disease was related to HBV (n = 389, 79.07%). Patients in the validation cohort were older than those in the training cohort (mean age, 54.84 ± 27.70 vs 48.71 ± 13.34, P < 0.001), and there was a statistically significant difference in ALB and TBIL levels. However, the two cohorts had a similar level of LSM and MELD (P = 0.066, P = 0.241, respectively).
| Validation cohort, n = 132 | Training cohort, n = 360 | P value | |
| Gender (male/female, n) | 90/42 | 260/100 | 0.381 |
| Age (yr) | 54.84 ± 27.70 | 48.71 ± 13.34 | 0.001 |
| Etiology | 0.097 | ||
| HBV | 111 | 278 | |
| Others | 21 | 82 | |
| HCC (n) | 42 | 63 | 0.001 |
| HB (g/dL) | 14.28 ± 10.86 | 13.71 ± 2.16 | 0.346 |
| PLT (× 109/L) | 174.96 ± 83.93 | 170.95 ± 74.69 | 0.610 |
| PT (s) | 13.21 ± 1.66 | 12.84 ± 1.67 | 0.030 |
| APTT (s) | 33.28 ± 7.73 | 32.54 ± 6.14 | 0.273 |
| INR | 1.16 ± 0.16 | 1.09 ± 0.16 | 0.000 |
| AST (U/L) | 72.14 ± 78.19 | 86.16 ± 135.81 | 0.264 |
| ALT (U/L) | 106.35 ± 166.60 | 136.73 ± 247.37 | 0.192 |
| ALB (g/L) | 38.03 ± 5.22 | 40.03 ± 5.08 | 0.000 |
| TBIL (μmol/L) | 28.29 ± 31.05 | 19.94 ± 16.99 | 0.000 |
| CHE (U/L) | 6163.11 ± 2647.19 | 6748.44 ± 2127.44 | 0.015 |
| ALP (U/L) | 118.60 ± 95.93 | 179.64 ± 721.94 | 0.334 |
| GGT (U/L) | 128.91 ± 201.79 | 116.89 ± 188.75 | 0.539 |
| GLO (g/L) | 28.70 ± 4.81 | 29.02 ± 5.19 | 0.534 |
| Cr (μmol/L) | 65.36 ± 23.23 | 65.68 ± 15.38 | 0.860 |
| MELD | 8.14 ± 3.46 | 7.80 ± 2.56 | 0.241 |
| ALBI | -2.36 ± 0.54 | -2.60 ± 0.48 | 0.000 |
| PTAR | 0.31 ± 0.07 | 0.28 ± 0.07 | 0.000 |
| ICGR15 (%) | 11.55 ± 11.82 | 8.16 ± 8.56 | 0.000 |
| LSM (kPa) | 19.54 ± 18.28 | 16.34 ± 16.62 | 0.066 |
With ICGR15 ≥ 10% and ICGR15 ≥ 20% as the predictive points, the new models of mildly impaired LRF (mLPaM) and severely impaired LRF (sLPaM) were constructed based on LSM. In the training cohort, 360 patient variables were included in the multivariate logistic stepwise regression analysis. LSM (OR = 4.357, 95%CI: 2.248-8.445), PTAR (OR = 3.544, 95%CI: 1.838-6.835), age (OR = 1.048, 95%CI: 1.024-1.073) and MELD score (OR = 1.340, 95%CI: 1.150-1.562) were independent influencing factors of ICGR15 ≥ 10% (Table 2). LSM (OR = 3.120, 95%CI: 1.125-8.656), PTAR (OR = 3.524, 95%CI: 1.267-9.801), age (OR = 1.059, 95%CI: 1.024-1.096) and MELD score (OR = 1.377, 95%CI: 1.146-1.655) were independent influencing factors of ICGR15 ≥ 20% (Table 3). The predictive models using the above 4 variables were constructed as follows: mLPaM = 1.472 LSM (LSM ≥ 12.4 = 2, LSM < 12.4 = 1) + 1.265 PTAR (PTAR ≥ 0.280 = 2, PTAR < 0.280 = 1) + 0.047 age (years) + 0.291 MELD-7.600 and sLPaM = 1.138 LSM (LSM ≥ 12.4 = 2, LSM < 12.4 = 1) + 1.260 PTAR (PTAR ≥ 0.280 = 2, PTAR < 0.280 = 1) + 0.058 age (years) + 0.320 MELD-9.750.
| Variable | B | SE | Wald | P value | OR (95%CI) |
| LSM (kPa) | 1.472 | 0.338 | 19.008 | < 0.001 | 4.357 (2.248-8.445) |
| PTAR | 1.265 | 0.335 | 14.260 | < 0.001 | 3.544 (1.838-6.835) |
| Age (yr) | 0.047 | 0.012 | 15.329 | < 0.001 | 1.048 (1.024-1.073) |
| MELD | 0.291 | 0.078 | 13.844 | < 0.001 | 1.337 (1.147-1.558) |
| Constant | -7.600 | 1.022 | 55.302 | < 0.001 | 0.007 |
| Variable | B | SE | Wald | P value | OR (95%CI) |
| LSM (kPa) | 1.138 | 0.520 | 4.778 | 0.029 | 3.120 (1.125-8.656) |
| PTAR | 1.260 | 0.521 | 5.825 | 0.016 | 3.524 (1.267-9.801) |
| Age (yr) | 0.058 | 0.017 | 11.226 | 0.001 | 1.059 (1.024-1.096) |
| MELD | 0.320 | 0.094 | 11.652 | 0.001 | 1.377 (1.146-1.655) |
| Constant | -9.750 | -1.454 | 44.963 | < 0.001 | 0.001 |
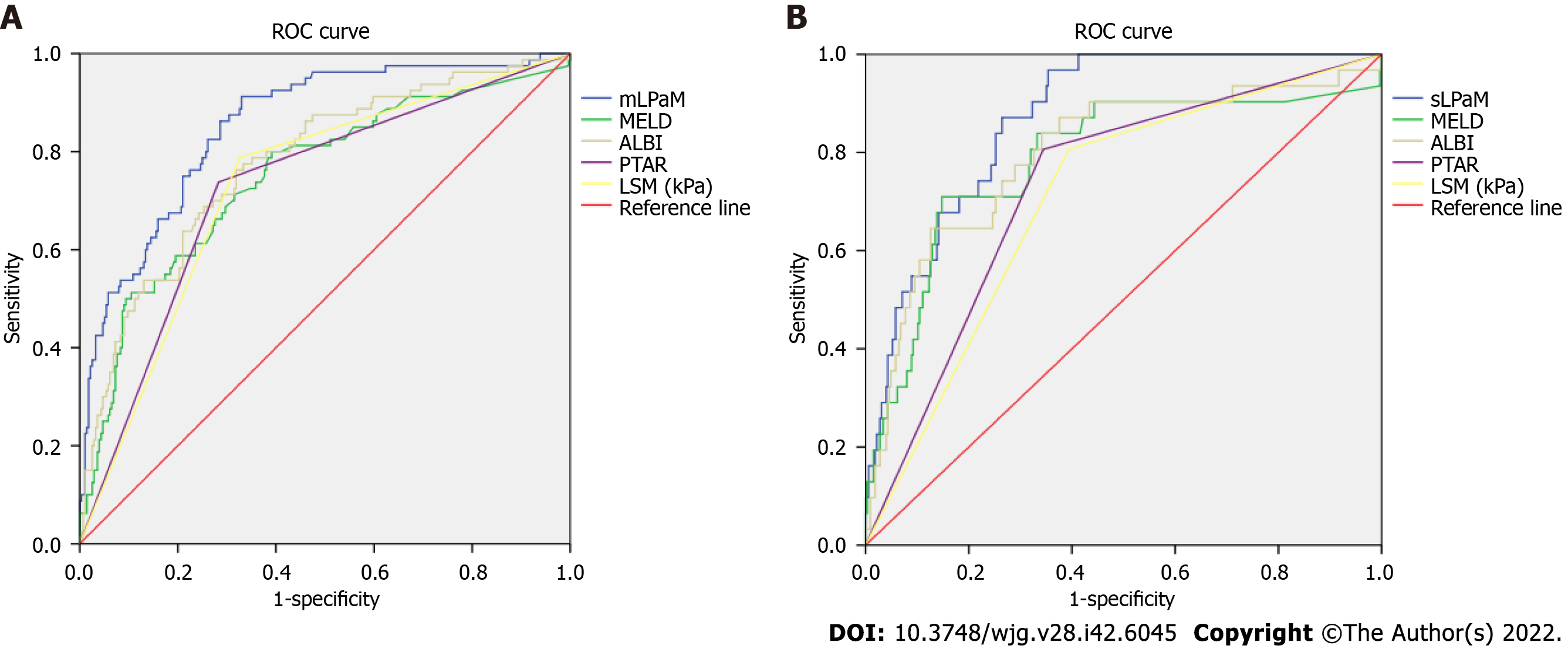
The AUC values of the mLPaM model (0.855) and sLPaM model (0.872) were greater than that of MELD score, PTAR and ALBI evaluation tools, and their sensitivity and negative predictive values were better than these evaluation methods (Table 4 and Figure 1).
| AUC (95%CI) | Optimal cut-off | Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) | Accuracy (%) | |
| mLPaM | 0.855 (0.809-0.901) | 0.135 | 91.3 | 66.4 | 36.09 | 97.35 | 70.68 |
| MELD | 0.752 (0.688-0.817) | 7.662 | 80.0 | 61.4 | 31.25 | 93.33 | 54.75 |
| ALBI | 0.776 (0.717-0.835) | -2.557 | 76.3 | 67.9 | 37.67 | 91.85 | 69.90 |
| PTAR | 0.728 (0.664-0.791) | 0.150 | 73.8 | 71.8 | 42.11 | 90.79 | 72.24 |
| LSM (kPa) | 0.733 (0.672-0.794) | 1.50 | 78.8 | 67.9 | 37.67 | 92.86 | 70.05 |
| sLPaM | 0.872 (0.823-0.921) | 0.046 | 96.8 | 64.6 | 14.83 | 99.69 | 66.53 |
| MELD | 0.786 (0.687-0.886) | 9.380 | 71.0 | 85.2 | 35.45 | 96.25 | 83.74 |
| ALBI | 0.798 (0.706-0.890) | -2.220 | 64.5 | 87.4 | 39.81 | 95.01 | 84.78 |
| PTAR | 0.731 (0.644-0.818) | 0.150 | 80.6 | 65.5 | 15.33 | 97.76 | 66.59 |
| LSM (kPa) | 0.706 (0.618-0.795) | 1.50 | 80.6 | 60.6 | 12.79 | 97.76 | 61.94 |
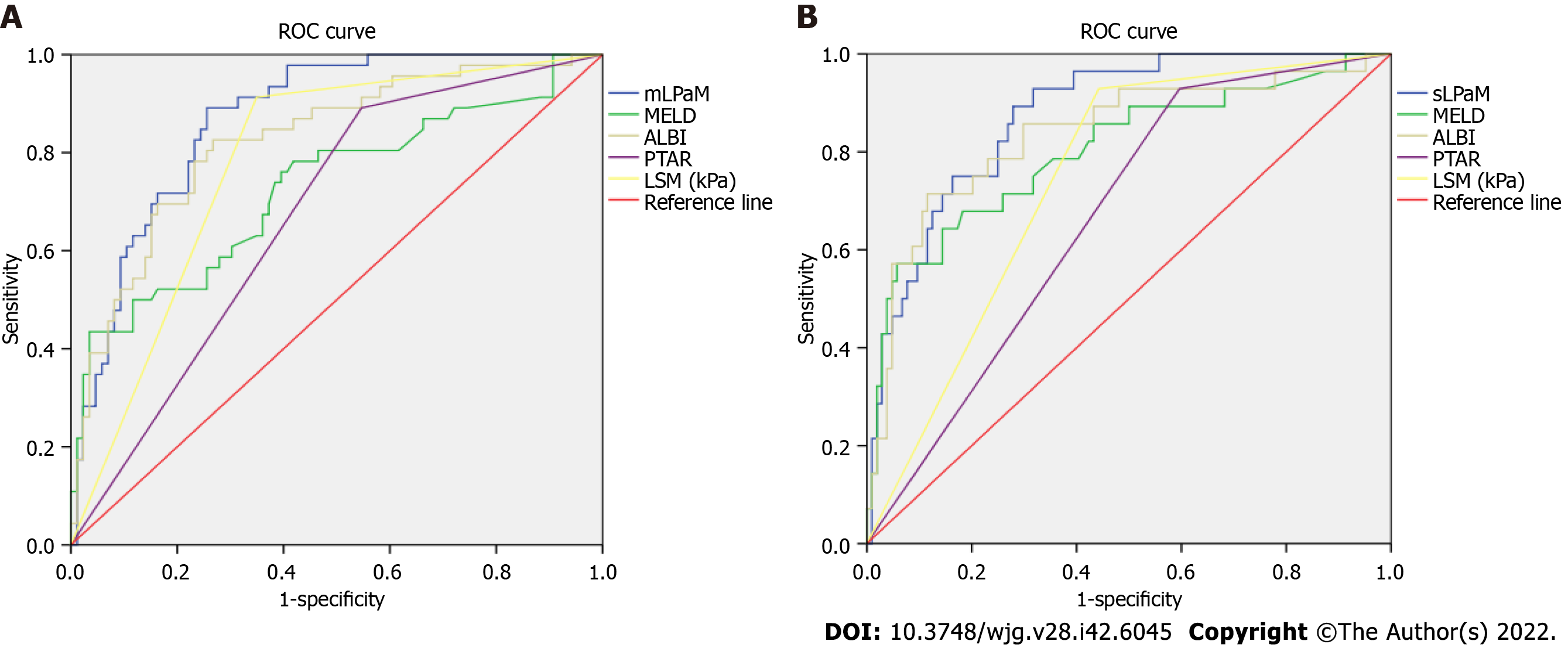
132 CLD patients were prospectively considered for enrollment in the internal validation cohort. The performance of the various methods at predicting LRF is reported in Table 5. The AUC values of the mLPaM model (0.869) and sLPaM model (0.876) were greater than other LRF predictive methods. The mLPaM model showed good sensitivity (89.1%) and optimal accuracy (78.94%) for the diagnosis of mild LRF impairment, and the sLPaM model showed optimal sensitivity (92.9%) for the diagnosis of severe LRF impairment (Table 5 and Figure 2).
| AUC (95%CI) | Optimal cut-off | Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) | Accuracy (%) | |
| mLPaM | 0.869 (0.810-0.929) | 0.240 | 89.1 | 74.4 | 60.85 | 93.86 | 78.94 |
| MELD | 0.729 (0.633-0.824) | 9.743 | 43.5 | 96.5 | 93.65 | 59.01 | 67.74 |
| ALBI | 0.824 (0.749-0.900) | -2.315 | 78.3 | 76.7 | 63.78 | 87.09 | 77.25 |
| PTAR | 0.672 (0.580-0.765) | 1.500 | 89.1 | 45.3 | 30.70 | 93.86 | 54.66 |
| LSM (kPa) | 0.782 (0.702-0.862) | 1.500 | 91.3 | 65.1 | 49.93 | 95.15 | 72.33 |
| sLPaM | 0.876 (0.812-0.940) | 0.073 | 92.9 | 68.3 | 49.94 | 95.15 | 72.33 |
| MELD | 0.803 (0.701-0.904) | 9.187 | 64.3 | 85.6 | 61.54 | 87.00 | 79.98 |
| ALBI | 0.836 (0.743-0.929) | -1.897 | 71.4 | 88.5 | 67.44 | 90.27 | 84.22 |
| PTAR | 0.666 (0.566-0.767) | 1.500 | 92.9 | 59.6 | 28.43 | 97.98 | 64.50 |
| LSM (kPa) | 0.743 (0.653-0.833) | 1.500 | 92.9 | 55.8 | 25.37 | 97.98 | 60.96 |
To date, accurate evaluation of LRF has been a hot topic in national and international research. As classic scoring systems, the CTP score and MELD score have been widely used in clinical practice. The CTP has introduced an element of bias into the scoring system due to the subjective nature of how clinical encephalopathy and ascites variables may be graded[19]. The MELD score is a continuous variable, and each indicator is given a corresponding weight through statistical analysis, which has further accuracy in evaluating LRF. In recent years, the ALBI and PTAR models have been gradually applied in clinical practice, which better evaluated LRF[20,21]. However, the ICG clearance test is currently considered the most valuable test in assessing LRF.
Although the ICG clearance test is a simple and helpful tool to assess individual LRF, it is an invasive and complex procedure, and the result is influenced by many factors (such as biliary excretion disorder and low proteinemia). In particular, the ICG clearance test is not applicable in pregnant women, patients with a history of iodine allergy or hyperthyroidism[22]. Transient elastography (TE) is a non-invasive and reproducible technique for assessing liver fibrosis, and is even a replacement for liver biopsy[23,24]. The Baveno VII Consensus showed that TE was an accurate tool for the prediction of CSPH[25]. In a previous study, it was found that LSM could predict postoperative liver failure in patients with HCC[26]. Therefore, LSM is considered to have a strong relationship with liver function.
As liver function impairment is the primary determinant of the development of post-hepatectomy liver failure, the vast majority of candidates for liver resection had CTP grade A[27]. According to the CTP classification, the majority of patients with HCC were classified as grade A, but their liver function may vary significantly[15]. A previous study revealed that ICGR15 was more accurate than the CTP score and MELD score in predicting hepatic functional reserve before hepatectomy[3]. The study showed that ICGR15 > 15% was an accurate method of predicting postoperative hepatic decompen
Our research constructed new models for clinical prediction of LRF impairment based on LSM, and the models were superior to other existing methods for predicting LRF (Table 4 and Figure 1). Moreover, compared to the other four methods, the models also showed better performance for predicting LRF in the prospective validation cohort (Table 5 and Figure 2). Therefore, based on the analysis of the above research results, these models could be an alternative tool for LRF assessment, especially in evaluating a population almost entirely stratified as CTP grade A.
Despite the significant findings in this study, our research also had a few limitations. First, the study was limited by its single-center prospective cohort nature. The patients were recruited from the same medical facility, and not all patients with complete clinical data were obtained from a treatment database. Second, most of the patients in this study were Asians with B viral hepatitis. Therefore, the performance of the model in patients of other ethnicities (e.g., Caucasians, Africans, etc.) still needs further investigation. Third, the models were mainly used to evaluate LRF in patients with compensated CLD, and their predictive value in patients with decompensated stage needs further evaluation. Finally, although the formula for the models was relatively complex, a mobile app or web-based calculator could calculate the score easily and rapidly in the current high-tech era. Despite these limitations, this study provided the first accurate models for evaluating LRF based on LSM in China.
The first predicted models based on LSM could facilitate accurate, reliable and simple-to-use prediction of LRF irrespective of etiology. They are entirely objective based on routine clinical and laboratory parameters. These models would be a useful tool for realizing individualized LRF evaluation to improve the popularity of testing and avoid possible risks during the ICG clearance test, ultimately achieving a clinically feasible and safe LRF test.
There are no obvious clinical symptoms in chronic liver disease (CLD) patients at the early stage, but their liver reserve function (LRF) may be impaired. Early evaluation of LRF is of great help in identifying disease progression. Assessment of LRF is essential for predicting the prognosis of patients with CLD and determines the extent of liver resection in patients with hepatocellular carcinoma (HCC).
Liver function impairment is the primary determinant in the development of post-hepatectomy liver failure. There are no obvious clinical symptoms in CLD patients at Child-Turcotte-Pugh A stage, but their LRF may be impaired. Due to impossible implementation of the indocyanine green (ICG) clearance test in some CLD patients, a new method to accurately assess LRF is needed.
This study aimed to establish noninvasive models of LRF assessment based on LSM. The new predictive models were established through logistic regression analysis and were validated internally in a prospective cohort. The new models had a good predictive performance on LRF and could replace the ICG clearance test, especially in the patients who are unable to undergo ICG testing.
Clinical data from 360 patients with compensated CLD were retrospectively collected and analyzed in the training cohort. The new predictive models were established through logistic regression analysis and were validated internally in a prospective cohort (132 patients). The areas under the ROC curve (AUCs) were measured and compared to evaluate the discrimination ability of different models.
Our study defined the indocyanine green retention rate at 15 min (ICGR15) ≥ 10% as mildly impaired LRF and ICGR15 ≥ 20% as severely impaired LRF. We constructed predictive models of LRF, named the mLPaM and sLPaM, which involved only LSM, prothrombin time international normalized ratio to albumin ratio, age and model for end-stage liver disease. The AUC of the mLPaM model (0.855, 0.872, respectively) and sLPaM model (0.869, 0.876, respectively) were higher than that of other methods in the two cohorts. In addition, the new models showed good sensitivity and accuracy for the diagnosis of LRF impairment in the validation cohort.
Our study found that the new models had a good predictive performance for LRF and could replace the ICG clearance test, especially in patients who are unable to undergo ICG testing.
This was not a multicenter study and most of the CLD patients in this study were Asians. Therefore, a multi-center prospective cohort study could further evaluate the performance of the predictive models, and the models in patients of other ethnicities need further investigation. The predictive value of the models in patients with a decompensated stage need further evaluation.
We would like to thank the patients who participated in this study.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Infectious diseases
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Enomoto H, Japan; Rodrigues AT, Brazil S-Editor: Gong ZM L-Editor: Webster JR P-Editor: Gong ZM
| 1. | Mai RY, Zeng J, Lu HZ, Liang R, Lin Y, Piao XM, Gao X, Wu GB, Wu FX, Ma L, Xiang BD, Li LQ, Ye JZ. Combining Aspartate Aminotransferase-to-Platelet Ratio Index with Future Liver Remnant to Assess Preoperative Hepatic Functional Reserve in Patients with Hepatocellular Carcinoma. J Gastrointest Surg. 2021;25:688-697. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 2. | Cieslak KP, Runge JH, Heger M, Stoker J, Bennink RJ, van Gulik TM. New perspectives in the assessment of future remnant liver. Dig Surg. 2014;31:255-268. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 64] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 3. | Wang YY, Zhao XH, Ma L, Ye JZ, Wu FX, Tang J, You XM, Xiang BD, Li LQ. Comparison of the ability of Child-Pugh score, MELD score, and ICG-R15 to assess preoperative hepatic functional reserve in patients with hepatocellular carcinoma. J Surg Oncol. 2018;118:440-445. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 71] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 4. | Lin ZH, Xin YN, Dong QJ, Wang Q, Jiang XJ, Zhan SH, Sun Y, Xuan SY. Performance of the aspartate aminotransferase-to-platelet ratio index for the staging of hepatitis C-related fibrosis: an updated meta-analysis. Hepatology. 2011;53:726-736. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 821] [Cited by in RCA: 791] [Article Influence: 56.5] [Reference Citation Analysis (0)] |
| 5. | Demirtas CO, D'Alessio A, Rimassa L, Sharma R, Pinato DJ. ALBI grade: Evidence for an improved model for liver functional estimation in patients with hepatocellular carcinoma. JHEP Rep. 2021;3:100347. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 57] [Cited by in RCA: 122] [Article Influence: 30.5] [Reference Citation Analysis (0)] |
| 6. | De Gasperi A, Mazza E, Prosperi M. Indocyanine green kinetics to assess liver function: Ready for a clinical dynamic assessment in major liver surgery? World J Hepatol. 2016;8:355-367. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 101] [Cited by in RCA: 130] [Article Influence: 14.4] [Reference Citation Analysis (4)] |
| 7. | Sato N, Kenjo A, Suzushino S, Kimura T, Okada R, Ishigame T, Kofunato Y, Marubashi S. Predicting Post-Hepatectomy Liver Failure Using Intra-Operative Measurement of Indocyanine Green Clearance in Anatomical Hepatectomy. World J Surg. 2021;45:3660-3667. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 8. | Lei Q, Zhang Y, Ke C, Yan C, Huang P, Shen H, Lei H, Chen Y, Luo J, Meng Z. Value of the albumin-bilirubin score in the evaluation of hepatitis B virus-related acute-on-chronic liver failure, liver cirrhosis, and hepatocellular carcinoma. Exp Ther Med. 2018;15:3074-3079. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 20] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 9. | Huang Z, Huang J, Zhou T, Cao H, Tan B. Prognostic value of liver stiffness measurement for the liver-related surgical outcomes of patients under hepatic resection: A meta-analysis. PLoS One. 2018;13:e0190512. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 25] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 10. | Serenari M, Han KH, Ravaioli F, Kim SU, Cucchetti A, Han DH, Odaldi F, Ravaioli M, Festi D, Pinna AD, Cescon M. A nomogram based on liver stiffness predicts postoperative complications in patients with hepatocellular carcinoma. J Hepatol. 2020;73:855-862. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 76] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 11. | Department of Medical Administration, National Health and Health Commission of the People's Republic of China. [Guidelines for diagnosis and treatment of primary liver cancer in China (2019 edition)]. Zhonghua Gan Zang Bing Za Zhi. 2020;28:112-128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 65] [Reference Citation Analysis (0)] |
| 12. | Durand F, Valla D. Assessment of the prognosis of cirrhosis: Child-Pugh versus MELD. J Hepatol. 2005;42 Suppl:S100-S107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 376] [Cited by in RCA: 426] [Article Influence: 21.3] [Reference Citation Analysis (0)] |
| 13. | Perdigoto DN, Figueiredo P, Tomé L. The Role of the CLIF-C OF and the 2016 MELD in Prognosis of Cirrhosis with and without Acute-on-Chronic Liver Failure. Ann Hepatol. 2019;18:48-57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 14. | Haruki K, Shiba H, Saito N, Horiuchi T, Shirai Y, Fujiwara Y, Furukawa K, Sakamoto T, Yanaga K. Risk stratification using a novel liver functional reserve score of combination prothrombin time-international normalized ratio to albumin ratio and albumin in patients with hepatocellular carcinoma. Surgery. 2018;164:404-410. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 25] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 15. | Johnson PJ, Berhane S, Kagebayashi C, Satomura S, Teng M, Reeves HL, O'Beirne J, Fox R, Skowronska A, Palmer D, Yeo W, Mo F, Lai P, Iñarrairaegui M, Chan SL, Sangro B, Miksad R, Tada T, Kumada T, Toyoda H. Assessment of liver function in patients with hepatocellular carcinoma: a new evidence-based approach-the ALBI grade. J Clin Oncol. 2015;33:550-558. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1962] [Cited by in RCA: 2012] [Article Influence: 201.2] [Reference Citation Analysis (0)] |
| 16. | Wang L, Xie L, Zhang N, Zhu W, Zhou J, Pan Q, Mao A, Lin Z, Wang L, Zhao Y. Predictive Value of Intraoperative Indocyanine Green Clearance Measurement on Postoperative Liver Function After Anatomic Major Liver Resection. J Gastrointest Surg. 2020;24:1342-1351. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 16] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 17. | Naveau S, Voican CS, Lebrun A, Gaillard M, Lamouri K, Njiké-Nakseu M, Courie R, Tranchart H, Balian A, Prévot S, Dagher I, Perlemuter G. Controlled attenuation parameter for diagnosing steatosis in bariatric surgery candidates with suspected nonalcoholic fatty liver disease. Eur J Gastroenterol Hepatol. 2017;29:1022-1030. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 23] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 18. | Boursier J, Zarski JP, de Ledinghen V, Rousselet MC, Sturm N, Lebail B, Fouchard-Hubert I, Gallois Y, Oberti F, Bertrais S, Calès P; Multicentric Group from ANRS/HC/EP23 FIBROSTAR Studies. Determination of reliability criteria for liver stiffness evaluation by transient elastography. Hepatology. 2013;57:1182-1191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 392] [Cited by in RCA: 496] [Article Influence: 41.3] [Reference Citation Analysis (0)] |
| 19. | Kok B, Abraldes JG. Child-Pugh Classification: Time to Abandon? Semin Liver Dis. 2019;39:96-103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 55] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 20. | Feng D, Wang M, Hu J, Li S, Zhao S, Li H, Liu L. Prognostic value of the albumin-bilirubin grade in patients with hepatocellular carcinoma and other liver diseases. Ann Transl Med. 2020;8:553. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 42] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 21. | Levesque E, Martin E, Dudau D, Lim C, Dhonneur G, Azoulay D. Current use and perspective of indocyanine green clearance in liver diseases. Anaesth Crit Care Pain Med. 2016;35:49-57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 64] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 22. | Gao F, Cai MX, Lin MT, Xie W, Zhang LZ, Ruan QZ, Huang ZM. Prognostic value of international normalized ratio to albumin ratio among critically ill patients with cirrhosis. Eur J Gastroenterol Hepatol. 2019;31:824-831. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 23. | Singh S, Muir AJ, Dieterich DT, Falck-Ytter YT. American Gastroenterological Association Institute Technical Review on the Role of Elastography in Chronic Liver Diseases. Gastroenterology. 2017;152:1544-1577. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 171] [Cited by in RCA: 210] [Article Influence: 26.3] [Reference Citation Analysis (0)] |
| 24. | Park CC, Nguyen P, Hernandez C, Bettencourt R, Ramirez K, Fortney L, Hooker J, Sy E, Savides MT, Alquiraish MH, Valasek MA, Rizo E, Richards L, Brenner D, Sirlin CB, Loomba R. Magnetic Resonance Elastography vs Transient Elastography in Detection of Fibrosis and Noninvasive Measurement of Steatosis in Patients With Biopsy-Proven Nonalcoholic Fatty Liver Disease. Gastroenterology. 2017;152:598-607.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 479] [Cited by in RCA: 543] [Article Influence: 67.9] [Reference Citation Analysis (0)] |
| 25. | de Franchis R, Bosch J, Garcia-Tsao G, Reiberger T, Ripoll C; Baveno VII Faculty. Baveno VII - Renewing consensus in portal hypertension. J Hepatol. 2022;76:959-974. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1537] [Cited by in RCA: 1496] [Article Influence: 498.7] [Reference Citation Analysis (2)] |
| 26. | Shen Y, Zhou C, Zhu G, Shi G, Zhu X, Huang C, Zhou J, Fan J, Ding H, Ren N, Sun HC. Liver Stiffness Assessed by Shear Wave Elastography Predicts Postoperative Liver Failure in Patients with Hepatocellular Carcinoma. J Gastrointest Surg. 2017;21:1471-1479. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 27] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 27. | Maluccio M, Covey A. Recent progress in understanding, diagnosing, and treating hepatocellular carcinoma. CA Cancer J Clin. 2012;62:394-399. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 577] [Cited by in RCA: 698] [Article Influence: 53.7] [Reference Citation Analysis (0)] |
| 28. | Le Roy B, Grégoire E, Cossé C, Serji B, Golse N, Adam R, Cherqui D, Mabrut JY, Le Treut YP, Vibert E. Indocyanine Green Retention Rates at 15 min Predicted Hepatic Decompensation in a Western Population. World J Surg. 2018;42:2570-2578. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 14] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 29. | Yamamoto Y, Ikoma H, Morimura R, Konishi H, Murayama Y, Komatsu S, Shiozaki A, Kuriu Y, Kubota T, Nakanishi M, Ichikawa D, Fujiwara H, Okamoto K, Sakakura C, Ochiai T, Otsuji E. Clinical analysis of anatomical resection for the treatment of hepatocellular carcinoma based on the stratification of liver function. World J Surg. 2014;38:1154-1163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 20] [Article Influence: 1.8] [Reference Citation Analysis (0)] |