Published online Oct 21, 2022. doi: 10.3748/wjg.v28.i39.5679
Peer-review started: June 27, 2022
First decision: August 1, 2022
Revised: August 5, 2022
Accepted: September 9, 2022
Article in press: September 9, 2022
Published online: October 21, 2022
Processing time: 112 Days and 10.4 Hours
Compelling evidence derived from clinical and experimental research has de
Core tip: A growing body of data has demonstrated a positive association between the risk of gallbladder cancer and high dietary intake of advanced glycation end-products (AGEs). These noxious compounds are important contributors to the onset of a chronic inflammatory response, through the activation of the receptor of AGEs (RAGE). We herein discuss how RAGE activation is crucial in the development of gallbladder cancer and the relevance of new incoming data supporting the role of dietary interventions to reduce the risk of gallbladder cancer.
- Citation: Rojas A, Lindner C, Schneider I, Gonzàlez I, Morales MA. Receptor of advanced glycation end-products axis and gallbladder cancer: A forgotten connection that we should reconsider. World J Gastroenterol 2022; 28(39): 5679-5690
- URL: https://www.wjgnet.com/1007-9327/full/v28/i39/5679.htm
- DOI: https://dx.doi.org/10.3748/wjg.v28.i39.5679
Gallbladder cancer development is linked to both genetic and environmental factors, and where the onset of chronic inflammation is a crucial contributor to gallbladder carcinogenesis. This chronic inflammatory condition can be triggered by several factors including not only chronic infection by Salmonella spp., or Helicobacter pylori[1-4] but also some dietary habits or metabolic conditions[5-9], which are associated with an overactivation of the receptor of advanced glycation end-products (RAGE).
At present, the onset of many of both age- and diet-related noncommunicable diseases, including different cancer types, is widely associated with the chronicity of low-grade inflammation[10,11]. At this point, the diet is widely recognized as an important modulator of this chronic and systemic inflammation[12,13], particularly the western-type dietary patterns[14].
One common and important element in this unhealthy diet is the advanced glycation end-products (AGEs), which are a large and heterogeneous group of compounds that were initially recognized in the Maillard reaction, but they can also form by other reactions, including the oxidation of sugars, lipids, and amino acids[15,16].
Food-derived AGEs, also known as dietary AGEs, substantially contribute to the systemic pool of AGEs. Their intake has been linked in humans and mice to an increased level of oxidative stress and inflammation, thus playing an important role in the onset and development of several health disorders[17,18].
The pathogenic mechanisms of dietary AGEs are the same as those endogenously produced, either by activation of the RAGE or by covalent crosslinking of proteins, thus altering protein structure and function. The receptor-dependent and receptor-independent mechanisms are recognized as important contributors to tumor growth and development[19,20].
In the present review, we aim to highlight the burden of RAGE axis activation on gallbladder cancer, its therapeutic potential, as well as the significance of lowering dietary consumption of AGEs in subjects at risk.
There is growing evidence supporting the key role of dietary AGEs as major contributors to the systemic pool of AGEs[21], which notably increase oxidative stress and chronic/acute inflammation, contributing to the pathophysiology of many human inflammatory and malignant diseases[18,22,23].
Since the multicenter prospective European Prospective Investigation into Cancer and Nutrition (EPIC) cohort study, which investigated the relationship of dietary and environmental factors with the incidence of cancer and other chronic diseases[24-27], a growing body of evidence has revealed strong findings to support that a proinflammatory diet with high levels of dietary AGEs intake increases the risk of several types of cancer[28], such as breast, skin and those originating from the digestive tract[29-31].
Recently, Mayén et al[32] conducted a multinational cohort study using the EPIC database to characterize the daily dietary intake (mg/d) of three AGEs including Nε-[carboxymethyl] lysine (CML), Nε-[1-carboxyethyl] lysine (CEL), and Nδ-[5-hydro-5-methyl-4-imidazolon-2-yl]-ornithine (MG-H1) for each study participant, to assess AGE consumption with hepatobiliary cancer risk. In this study, the authors found a positive association between the risk of gallbladder cancer and high dietary intake of CML [hazard ratio (HR) = 1.30, 95% confidence interval (CI): 1.07–1.57] and MG-H1 (HR = 1.26, 95%CI: 1.06–1.50), and thus suggesting that higher intakes of dietary AGEs may increase the risk of gallbladder cancer.
Although the study of Mayen et al[32] has some limitations, particularly in estimating dietary AGEs exposure, other epidemiological studies have revealed an increased tumor progression and mortality of gallbladder cancer patients, with inflammatory comorbidities related to overactivation of the RAGE axis, such as high-fat diet consumption[33], metabolic syndrome[34], and diabetes mellitus [35-38], due to the increased endogenous formation of AGEs reported in these entities.
Some studies have shown increased expression of RAGE in gallbladder cancer cells, which were directly in concordance with the invasive ability of the neoplastic cell lines[39]. Additionally, compelling evidence has been reported of a strong increase in AGEs formation under hyperglycemic conditions[40,41]. Noteworthy, the gallbladder accumulation of AGEs is significantly higher in the gallbladder of diabetic mice when compared to control animals. These findings support the role of the RAGE/AGEs axis activation in gallbladder carcinogenesis[42].
Furthermore, other in vivo analyses of adenocarcinoma cells treated under a hyperglycemic milieu, a condition favoring the increased accumulation of AGEs, have been revealed to promote tumor cell proliferation and migration[43].
Emerging in vitro and in vivo analyses have revealed overexpression of several RAGE ligands such as high mobility group B1 (HMGB1) and members of the S100P protein family in malignant gallbladder epithelial cells compared to benign tissue[44,45].
This increased expression of those RAGE ligands in gallbladder cancer cells has been closely correlated with malignant progression and therefore may then be considered an independent risk factor for poor prognosis and proliferation in gallbladder cancer[45-47].
A key consequence of RAGE binding with its ligands is the activation of multiple and crucial signaling pathways[48], that are involved in gallbladder carcinogenesis, such as reactive oxygen species (ROS)[49], Erk1/2 (p44/42) mitogen-activated protein kinases (MAPKs)[50], C-Jun n-terminal kinase and p38 MAPK[51], and phosphatidylinositol 3-kinase pathways[52].
These signals trigger important downstream inflammatory and procarcinogenic consequences such as activation of signal transducer and activator of transcription 3[53,54], activator protein-1[55], and nuclear factor (NF)-κB pathways[49,54,56-58], favoring the secretion of several proinflammatory cytokines also involved in the promotion of gallbladder cancer invasion and migration such as tumor necrosis factor-α[59,60]. Hence, this proinflammatory milieu continuously fuels chronic inflammation in gallbladder carcinogenesis in a RAGE-dependent manner[19,61].
RAGE is recognized as a pattern recognition receptor, and its activation plays a pivotal role in the propagation of immune responses and inflammatory reactions[62]. It is expressed at low levels in most differentiated adult cells in a regulated manner. However, upregulation of RAGE expression is associated with many inflammation-related pathological entities, including cancer[63].
RAGE engagement subsequently converts transient cellular stimulation into a sustained cellular dysfunctional state driven by long-term activation of NF-κB[64]. There is compelling evidence that RAGE activation promotes many crucial steps during tumorigenesis, from DNA damage and genetic instability to supporting many phenotypic changes in tumor cells favoring their growth and dissemination[65].
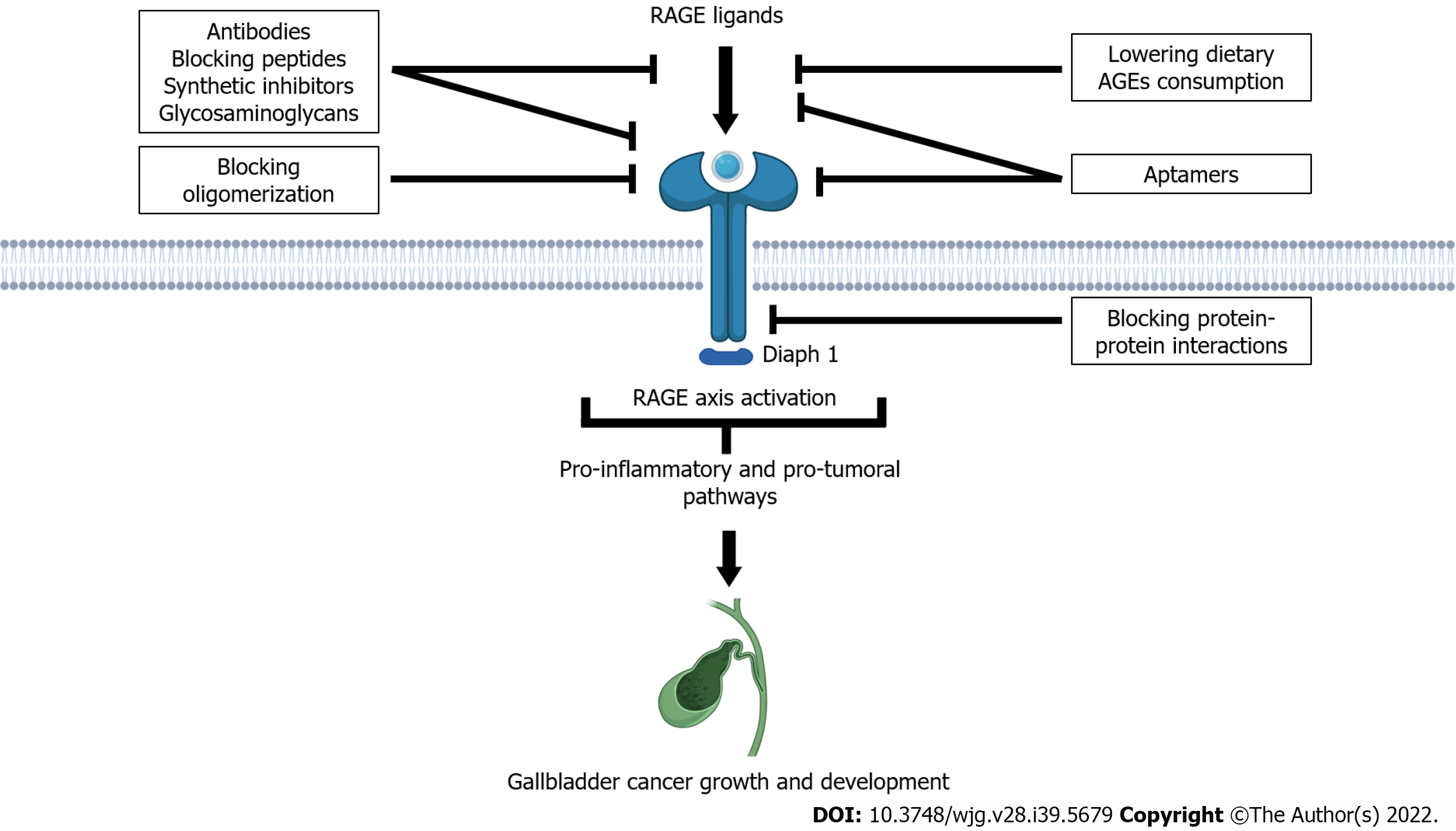
Since the work by Taguchi et al[66], which experimentally reported that in vivo blockade of the RAGE–amphotericin axis suppresses tumor growth and dissemination, intense research efforts have been focused towards the development of new therapeutic approaches to modulate both deleterious proinflammatory and procarcinogenesis effects of RAGE axis activation[67,68].
The use of novel RAGE-targeting antibodies and blocking peptides derived from RAGE ligands such as S100P and HMGB1 has demonstrated to block the ability of ligands to stimulate RAGE activation in cancer cells both in vitro and in vivo models, thus inhibiting tumor growth, metastasis, and inflammation[69], as well as significant reductions in tumor growth with acceptable toxicity levels in several in vivo mouse adenocarcinoma models[70,71]. Furthermore, the treatment of cancer cell lines with anti-RAGE antibodies demonstrates that RAGE blocking may even enhance the chemotherapeutic effects of antineoplastic drugs[72,73].
Recent evidence has also revealed that the antibody targeting of RAGE ligands such as HMGB1 and AGEs may effectively decrease tumor progression in solid malignancies[74]. This approach can even enhance the antitumoral response of cancer immunotherapies by remobilizing the antitumor immune response[75].
Another emerging therapeutic approach is based on the high binding affinity to RAGE of some members of the family of glycosaminoglycans such as chondroitin sulfate, heparan sulfate (HS), and low molecular weight and semisynthetic glycosaminoglycan[76]. These molecules have been reported to be involved in effectively inhibiting RAGE signaling pathways in both in vitro and in vivo models[71,77].
Strikingly, new evidence has revealed that HS acts as a crucial element for RAGE signaling, leading to the formation of stable RAGE–HS complexes, which drive the RAGE oligomerization and subsequent downstream functional signaling[78,79].
These observations have revealed a new strategy for treating RAGE-associated diseases by hindering RAGE oligomerization.
The use of synthetic compounds with both anticarcinogenic and anti-inflammatory activities based on their capacities to interfere with the HMGB1–RAGE axis seems to be a promising strategy for several cancer types, including gallbladder cancer[80,81].
A novel molecule, recently discovered by Tanuma et al[82], 7-methoxy-3-hydroxy-styrylchromone (c6), is not only an effective suppressor of cell cycle/proliferation but also an initiator of apoptosis in cancer cells, and a promising potentiator of the anticancer effects of DNA-damaging antineoplastic agents.
RAGE gene silencing has been demonstrated to significantly downregulate AGE-induced inflammation and RAGE-dependent release of proinflammatory cytokines in normal human cells[83], while in malignant cells, RAGE gene silencing can decrease the colony-forming ability, proliferation, migration, and the invasive potential of cancer cells, through inhibiting RAGE-dependent mechanisms that sustain cancer cell progression and invasion[84].
The requirement of the cytoplasmic tail of RAGE to interact with its molecular effector DIAPH1 to mediate downstream signal transduction has been highlighted as a promising approach to inhibit RAGE signaling[85-87].
This novel screening strategy of searching for molecules able to block protein–protein interactions has been demonstrated to be successful to inhibit the RAGE-mediated expression of inflammatory genes in diabetes complications[88,89] and atherosclerosis[90].
A growing body of experimental data using the DNA-aptamer technology against RAGE has demonstrated that this novel approach can inhibit the inflammatory reactions triggered by activation of the RAGE axis in different in vivo models[91-93].
Experimental research has reported interesting results in different cancer types, as revealed in tumor-bearing mice treated with RAGE-aptamers, where marked inhibition of tumor growth was achieved[94]. The use of this technology on tumor-bearing mice is also able to inhibit macrophage infiltration and neoangiogenesis through the inhibition of RAGE/NF-κB/VEGF-A-dependent signaling pathways[94-96] (Figure 1).
In many clinical entities where the activation of the RAGE/AGEs axis is crucial in the underlying pathogenic mechanisms, restriction of dietary AGEs has been extensively studied in clinical trials[97-101]. Under the same rationale, and based on the active role of RAGE-mediated mechanisms in tumor biology, different interventional clinical studies already published[102-107] (Table 1), or in progress, have supported the use of restriction of AGEs intake in human cancers, as documented on the website ClinicalTrials.gov (ClinicalTrials.gov identifier: NCT03712371, NCT04716764, NCT02946996, NCT03092635, NCT01820299, NCT01363141, NCT03147339). However, it must be emphasized that therapeutic interventions, including dietary interventional actions on the RAGE axis, have been focused on achieving clinical improvements in disease course, including dietary interventional actions, and therefore the potential of modulating RAGE activation in terms of cancer prevention is still controversial.
| Ref. | Year | Condition | Outcome |
| Jiao et al[102] | 2015 | Pancreatic cancer | Increased risk of pancreatic cancer |
| Peterson et al[103] | 2020 | Breast cancer | Increased breast cancer risk in postmenopausal women |
| Omofuma et al[104] | 2020 | Breast cancer | Increased risk of breast cancer |
| Aglago et al[105] | 2021 | Colorectal cancer | Increased risk of CRC |
| Mao et al[106] | 2021 | Colorectal cancer | Increased CRC mortality in non-T2D patients |
| Omofuma et al[107] | 2021 | Breast cancer | Increased breast cancer mortality |
| Mayén et al[32] | 2021 | Hepatobiliary cancers | Increased risk of gallbladder cancer |
International consensus estimates that almost 40% of cancer cases are preventable through a healthy lifestyle[108]. Compelling evidence derived from epidemiological studies of different cancer types suggests that lifestyle changes, including dietary habits, may play a crucial role in determining the risk of various cancers[109-113].
Currently, the western diet is considered a major driver of chronic, low-grade, metabolic inflammation, which is a crosswise element in the pathogenesis of many human diseases, including cancer[114]. Data derived from preclinical investigations, and observational and interventional studies, has provided conclusive evidence that the western diet is associated with an increased incidence of many malignancies, such as colorectal, pancreatic, prostate and breast cancers[115-118].
In modern society, dietary AGE consumption – as a component of modern westernized diets – is markedly increased. Therefore, dietary AGE restriction is now recognized as a useful intervention, as demonstrated in several pathologies[119-123].
Western diet generally contains large amounts of fructose, thus promoting AGE formation[124]. This diet is also an important source of AGE precursors, such as methylglyoxal and glyoxal[125]. In light of these findings, dietary AGEs have gained particular importance due to their capacity to support the onset of many human diseases, including cancer, mainly due to their proinflammatory and pro-oxidant properties[17,18].
The role of RAGE/AGEs axis activation has emerged as a crucial element in the tumor microenvironment to promote cancer cell migration, invasion, survival, and even resistance to chemotherapy[19]. Additionally, the accumulation of AGEs in tissues can promote protein structural damage and modification of the mechanical and physiological functions of the extracellular matrix, thus contributing to carcinogenesis and inflammation[20].
Therefore, the report recently published by Mayén et al[32] showed a positive association between dietary AGEs and the risk of gallbladder cancer in the EPIC cohort, which deserves special attention. We believe that actions such as dietary recommendations for the reduction of dietary AGEs intake to individuals at risk of gallbladder cancer will be beneficial. In this regard, it is important to highlight that some pre-existing clinical conditions such as diabetes mellitus and metabolic syndrome are risk factors for the development of gallbladder cancer[34,35-38]. Additionally, the demonstrated links between genetic ancestry and gallbladder cancer development may represent another risk factor for some populations[126,127]. Other recommendations that focus on reducing the RAGE/AGEs axis activation are attractive, particularly the consumption of polyphenol-rich foods due to the inhibitory activities of polyphenols on the RAGE/AGEs axis at different levels, such as by inhibition of ROS formation during glycation reactions, chelation of transition metal ions, trapping dicarbonyls, and activation of AGE detoxification pathways[128].
Gallbladder cancer is an aggressive and rare neoplasm with an unusual geographic distribution. Most patients are diagnosed in the advanced stages of the disease, and therefore the life expectancy is low. Compelling evidence supports the role of several risk factors, which are linked to the onset, and chronicity of an inflammatory reaction. The report of Taguchi et al[66] represented a critical point in understanding the role of the RAGE axis in tumor biology, and highlighting the potential of therapeutic interventions on a hyperactive cellular signaling pathway that causes disease, as the RAGE axis is[129].
The role of RAGE axis activation in gallbladder cancer is supported by its active contribution to the pathogenic framework of the main risk factors associated with this neoplasm, such as infectious agents[130,131], some metabolic conditions[132,133], and dietary habits[32].
Although much research is needed, lowering dietary AGEs intake as well as increasing the consumption of foods rich in polyphenols in subjects at risk of gallbladder cancer, either by pre-existing metabolic conditions or genetic ancestry, seems to be a plausible recommendation, to avoid the hyperactivation of the RAGE/AGEs axis.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Chile
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Shalaby MN, Egypt; Yasukawa K, Japan S-Editor: Gong ZM L-Editor: Kerr C P-Editor: Gong ZM
| 1. | Dutta U, Garg PK, Kumar R, Tandon RK. Typhoid carriers among patients with gallstones are at increased risk for carcinoma of the gallbladder. Am J Gastroenterol. 2000;95:784-787. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 160] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 2. | Leong RW, Sung JJ. Review article: Helicobacter species and hepatobiliary diseases. Aliment Pharmacol Ther. 2002;16:1037-1045. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 67] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 3. | Nath G, Gulati AK, Shukla VK. Role of bacteria in carcinogenesis, with special reference to carcinoma of the gallbladder. World J Gastroenterol. 2010;16:5395-5404. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 61] [Cited by in RCA: 58] [Article Influence: 3.9] [Reference Citation Analysis (2)] |
| 4. | Scanu T, Spaapen RM, Bakker JM, Pratap CB, Wu LE, Hofland I, Broeks A, Shukla VK, Kumar M, Janssen H, Song JY, Neefjes-Borst EA, te Riele H, Holden DW, Nath G, Neefjes J. Salmonella Manipulation of Host Signaling Pathways Provokes Cellular Transformation Associated with Gallbladder Carcinoma. Cell Host Microbe. 2015;17:763-774. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 184] [Article Influence: 18.4] [Reference Citation Analysis (0)] |
| 5. | Strom BL, Soloway RD, Rios-Dalenz JL, Rodriguez-Martinez HA, West SL, Kinman JL, Polansky M, Berlin JA. Risk factors for gallbladder cancer. An international collaborative case-control study. Cancer. 1995;76:1747-1756. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 6. | Zatonski WA, Lowenfels AB, Boyle P, Maisonneuve P, Bueno de Mesquita HB, Ghadirian P, Jain M, Przewozniak K, Baghurst P, Moerman CJ, Simard A, Howe GR, McMichael AJ, Hsieh CC, Walker AM. Epidemiologic aspects of gallbladder cancer: a case-control study of the SEARCH Program of the International Agency for Research on Cancer. J Natl Cancer Inst. 1997;89:1132-1138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 188] [Cited by in RCA: 179] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 7. | Kato K, Akai S, Tominaga S, Kato I. A case-control study of biliary tract cancer in Niigata Prefecture, Japan. Jpn J Cancer Res. 1989;80:932-938. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 60] [Cited by in RCA: 59] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 8. | Larsson SC, Wolk A. Obesity and the risk of gallbladder cancer: a meta-analysis. Br J Cancer. 2007;96:1457-1461. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 117] [Cited by in RCA: 118] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 9. | Park JH, Hong JY, Park YS, Kang G, Han K, Park JO. Association of prediabetes, diabetes, and diabetes duration with biliary tract cancer risk: A nationwide cohort study. Metabolism. 2021;123:154848. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 23] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 10. | Duncan BB, Schmidt MI, Pankow JS, Ballantyne CM, Couper D, Vigo A, Hoogeveen R, Folsom AR, Heiss G; Atherosclerosis Risk in Communities Study. Low-grade systemic inflammation and the development of type 2 diabetes: the atherosclerosis risk in communities study. Diabetes. 2003;52:1799-1805. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 796] [Cited by in RCA: 775] [Article Influence: 35.2] [Reference Citation Analysis (0)] |
| 11. | Furman D, Campisi J, Verdin E, Carrera-Bastos P, Targ S, Franceschi C, Ferrucci L, Gilroy DW, Fasano A, Miller GW, Miller AH, Mantovani A, Weyand CM, Barzilai N, Goronzy JJ, Rando TA, Effros RB, Lucia A, Kleinstreuer N, Slavich GM. Chronic inflammation in the etiology of disease across the life span. Nat Med. 2019;25:1822-1832. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1084] [Cited by in RCA: 2766] [Article Influence: 461.0] [Reference Citation Analysis (0)] |
| 12. | Minihane AM, Vinoy S, Russell WR, Baka A, Roche HM, Tuohy KM, Teeling JL, Blaak EE, Fenech M, Vauzour D, McArdle HJ, Kremer BH, Sterkman L, Vafeiadou K, Benedetti MM, Williams CM, Calder PC. Low-grade inflammation, diet composition and health: current research evidence and its translation. Br J Nutr. 2015;114:999-1012. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 442] [Cited by in RCA: 621] [Article Influence: 62.1] [Reference Citation Analysis (0)] |
| 13. | Ricordi C, Garcia-Contreras M, Farnetti S. Diet and Inflammation: Possible Effects on Immunity, Chronic Diseases, and Life Span. J Am Coll Nutr. 2015;34 Suppl 1:10-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 57] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 14. | Malesza IJ, Malesza M, Walkowiak J, Mussin N, Walkowiak D, Aringazina R, Bartkowiak-Wieczorek J, Mądry E. High-Fat, Western-Style Diet, Systemic Inflammation, and Gut Microbiota: A Narrative Review. Cells. 2021;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 408] [Article Influence: 102.0] [Reference Citation Analysis (0)] |
| 15. | Twarda-Clapa A, Olczak A, Białkowska AM, Koziołkiewicz M. Advanced Glycation End-Products (AGEs): Formation, Chemistry, Classification, Receptors, and Diseases Related to AGEs. Cells. 2022;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 325] [Article Influence: 108.3] [Reference Citation Analysis (0)] |
| 16. | Ruiz HH, Ramasamy R, Schmidt AM. Advanced Glycation End Products: Building on the Concept of the "Common Soil" in Metabolic Disease. Endocrinology. 2020;161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 128] [Article Influence: 25.6] [Reference Citation Analysis (0)] |
| 17. | Garay-Sevilla ME, Rojas A, Portero-Otin M, Uribarri J. Dietary AGEs as Exogenous Boosters of Inflammation. Nutrients. 2021;13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 61] [Cited by in RCA: 61] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 18. | Uribarri J, del Castillo MD, de la Maza MP, Filip R, Gugliucci A, Luevano-Contreras C, Macías-Cervantes MH, Markowicz Bastos DH, Medrano A, Menini T, Portero-Otin M, Rojas A, Sampaio GR, Wrobel K, Garay-Sevilla ME. Dietary advanced glycation end products and their role in health and disease. Adv Nutr. 2015;6:461-473. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 283] [Cited by in RCA: 274] [Article Influence: 27.4] [Reference Citation Analysis (0)] |
| 19. | Rojas A, Figueroa H, Morales E. Fueling inflammation at tumor microenvironment: the role of multiligand/RAGE axis. Carcinogenesis. 2010;31:334-341. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 123] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 20. | Rojas A, Añazco C, González I, Araya P. Extracellular matrix glycation and receptor for advanced glycation end-products activation: a missing piece in the puzzle of the association between diabetes and cancer. Carcinogenesis. 2018;39:515-521. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 62] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 21. | Davis KE, Prasad C, Vijayagopal P, Juma S, Adams-Huet B, Imrhan V. Contribution of dietary advanced glycation end products (AGE) to circulating AGE: role of dietary fat. Br J Nutr. 2015;114:1797-1806. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 34] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 22. | Gill V, Kumar V, Singh K, Kumar A, Kim JJ. Advanced Glycation End Products (AGEs) May Be a Striking Link Between Modern Diet and Health. Biomolecules. 2019;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 66] [Cited by in RCA: 130] [Article Influence: 21.7] [Reference Citation Analysis (0)] |
| 23. | Ahmad S, Khan H, Siddiqui Z, Khan MY, Rehman S, Shahab U, Godovikova T, Silnikov V, Moinuddin. AGEs, RAGEs and s-RAGE; friend or foe for cancer. Semin Cancer Biol. 2018;49:44-55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 140] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 24. | Riboli E, Hunt KJ, Slimani N, Ferrari P, Norat T, Fahey M, Charrondière UR, Hémon B, Casagrande C, Vignat J, Overvad K, Tjønneland A, Clavel-Chapelon F, Thiébaut A, Wahrendorf J, Boeing H, Trichopoulos D, Trichopoulou A, Vineis P, Palli D, Bueno-De-Mesquita HB, Peeters PH, Lund E, Engeset D, González CA, Barricarte A, Berglund G, Hallmans G, Day NE, Key TJ, Kaaks R, Saracci R. European Prospective Investigation into Cancer and Nutrition (EPIC): study populations and data collection. Public Health Nutr. 2002;5:1113-1124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1350] [Cited by in RCA: 1482] [Article Influence: 64.4] [Reference Citation Analysis (0)] |
| 25. | Bingham S, Riboli E. Diet and cancer--the European Prospective Investigation into Cancer and Nutrition. Nat Rev Cancer. 2004;4:206-215. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 276] [Cited by in RCA: 265] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 26. | Riboli E, Kaaks R. The EPIC Project: rationale and study design. European Prospective Investigation into Cancer and Nutrition. Int J Epidemiol. 1997;26 Suppl 1:S6-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 686] [Cited by in RCA: 744] [Article Influence: 26.6] [Reference Citation Analysis (0)] |
| 27. | Hainaut P, Vozar B, Rinaldi S, Riboli E, Caboux E. The European Prospective Investigation into Cancer and Nutrition biobank. Methods Mol Biol. 2011;675:179-191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 28. | Ubago-Guisado E, Rodríguez-Barranco M, Ching-López A, Petrova D, Molina-Montes E, Amiano P, Barricarte-Gurrea A, Chirlaque MD, Agudo A, Sánchez MJ. Evidence Update on the Relationship between Diet and the Most Common Cancers from the European Prospective Investigation into Cancer and Nutrition (EPIC) Study: A Systematic Review. Nutrients. 2021;13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 86] [Article Influence: 21.5] [Reference Citation Analysis (0)] |
| 29. | Agudo A, Cayssials V, Bonet C, Tjønneland A, Overvad K, Boutron-Ruault MC, Affret A, Fagherazzi G, Katzke V, Schübel R, Trichopoulou A, Karakatsani A, La Vecchia C, Palli D, Grioni S, Tumino R, Ricceri F, Panico S, Bueno-de-Mesquita B, Peeters PH, Weiderpass E, Skeie G, Nøst TH, Lasheras C, Rodríguez-Barranco M, Amiano P, Chirlaque MD, Ardanaz E, Ohlsson B, Dias JA, Nilsson LM, Myte R, Khaw KT, Perez-Cornago A, Gunter M, Huybrechts I, Cross AJ, Tsilidis K, Riboli E, Jakszyn P. Inflammatory potential of the diet and risk of gastric cancer in the European Prospective Investigation into Cancer and Nutrition (EPIC) study. Am J Clin Nutr. 2018;107:607-616. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 58] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 30. | Aglago EK, Schalkwijk CG, Freisling H, Fedirko V, Hughes DJ, Jiao L, Dahm CC, Olsen A, Tjønneland A, Katzke V, Johnson T, Schulze MB, Aleksandrova K, Masala G, Sieri S, Simeon V, Tumino R, Macciotta A, Bueno-de-Mesquita B, Skeie G, Gram IT, Sandanger T, Jakszyn P, Sánchez MJ, Amiano P, Colorado-Yohar SM, Gurrea AB, Perez-Cornago A, Mayén AL, Weiderpass E, Gunter MJ, Heath AK, Jenab M. Plasma concentrations of advanced glycation end-products and colorectal cancer risk in the EPIC study. Carcinogenesis. 2021;42:705-713. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 31. | González CA, Jakszyn P, Pera G, Agudo A, Bingham S, Palli D, Ferrari P, Boeing H, del Giudice G, Plebani M, Carneiro F, Nesi G, Berrino F, Sacerdote C, Tumino R, Panico S, Berglund G, Simán H, Nyrén O, Hallmans G, Martinez C, Dorronsoro M, Barricarte A, Navarro C, Quirós JR, Allen N, Key TJ, Day NE, Linseisen J, Nagel G, Bergmann MM, Overvad K, Jensen MK, Tjonneland A, Olsen A, Bueno-de-Mesquita HB, Ocke M, Peeters PH, Numans ME, Clavel-Chapelon F, Boutron-Ruault MC, Trichopoulou A, Psaltopoulou T, Roukos D, Lund E, Hemon B, Kaaks R, Norat T, Riboli E. Meat intake and risk of stomach and esophageal adenocarcinoma within the European Prospective Investigation Into Cancer and Nutrition (EPIC). J Natl Cancer Inst. 2006;98:345-354. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 215] [Cited by in RCA: 228] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 32. | Mayén AL, Aglago EK, Knaze V, Cordova R, Schalkwijk CG, Wagner KH, Aleksandrova K, Fedirko V, Keski-Rahkonen P, Leitzmann MF, Katzke V, Srour B, Schulze MB, Masala G, Krogh V, Panico S, Tumino R, Bueno-de-Mesquita B, Brustad M, Agudo A, Chirlaque López MD, Amiano P, Ohlsson B, Ramne S, Aune D, Weiderpass E, Jenab M, Freisling H. Dietary intake of advanced glycation endproducts and risk of hepatobiliary cancers: A multinational cohort study. Int J Cancer. 2021;. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 15] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 33. | Pérez-Moreno P, Riquelme I, García P, Brebi P, Roa JC. Environmental and Lifestyle Risk Factors in the Carcinogenesis of Gallbladder Cancer. J Pers Med. 2022;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 21] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 34. | Borena W, Edlinger M, Bjørge T, Häggström C, Lindkvist B, Nagel G, Engeland A, Stocks T, Strohmaier S, Manjer J, Selmer R, Tretli S, Concin H, Hallmans G, Jonsson H, Stattin P, Ulmer H. A prospective study on metabolic risk factors and gallbladder cancer in the metabolic syndrome and cancer (Me-Can) collaborative study. PLoS One. 2014;9:e89368. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 38] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 35. | Jing C, Wang Z, Fu X. Effect of diabetes mellitus on survival in patients with gallbladder Cancer: a systematic review and meta-analysis. BMC Cancer. 2020;20:689. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 16] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 36. | Tsilidis KK, Kasimis JC, Lopez DS, Ntzani EE, Ioannidis JP. Type 2 diabetes and cancer: umbrella review of meta-analyses of observational studies. BMJ. 2015;350:g7607. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 549] [Cited by in RCA: 564] [Article Influence: 56.4] [Reference Citation Analysis (0)] |
| 37. | Pearson-Stuttard J, Papadimitriou N, Markozannes G, Cividini S, Kakourou A, Gill D, Rizos EC, Monori G, Ward HA, Kyrgiou M, Gunter MJ, Tsilidis KK. Type 2 Diabetes and Cancer: An Umbrella Review of Observational and Mendelian Randomization Studies. Cancer Epidemiol Biomarkers Prev. 2021;30:1218-1228. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 126] [Cited by in RCA: 158] [Article Influence: 39.5] [Reference Citation Analysis (0)] |
| 38. | Gu J, Yan S, Wang B, Shen F, Cao H, Fan J, Wang Y. Type 2 diabetes mellitus and risk of gallbladder cancer: a systematic review and meta-analysis of observational studies. Diabetes Metab Res Rev. 2016;32:63-72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 30] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 39. | Hirata K, Takada M, Suzuki Y, Kuroda Y. Expression of receptor for advanced glycation end products (RAGE) in human biliary cancer cells. Hepatogastroenterology. 2003;50:1205-1207. [PubMed] |
| 40. | Berg TJ, Snorgaard O, Faber J, Torjesen PA, Hildebrandt P, Mehlsen J, Hanssen KF. Serum levels of advanced glycation end products are associated with left ventricular diastolic function in patients with type 1 diabetes. Diabetes Care. 1999;22:1186-1190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 155] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 41. | Bellier J, Nokin MJ, Lardé E, Karoyan P, Peulen O, Castronovo V, Bellahcène A. Methylglyoxal, a potent inducer of AGEs, connects between diabetes and cancer. Diabetes Res Clin Pract. 2019;148:200-211. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 97] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 42. | Swartz-Basile DA, Lu D, Tran KQ, Graewin S, Nakeeb A, Pitt HA. Advanced glycation end products accumulate in the diabetic gallbladder. J Surg Res. 2006;130:302. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 43. | Liao YF, Yin S, Chen ZQ, Li F, Zhao B. High glucose promotes tumor cell proliferation and migration in lung adenocarcinoma via the RAGENOXs pathway. Mol Med Rep. 2018;17:8536-8541. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 8] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 44. | Wang W, Ai KX, Yuan Z, Huang XY, Zhang HZ. Different expression of S100A8 in malignant and benign gallbladder diseases. Dig Dis Sci. 2013;58:150-162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 45. | Shi Z, Huang Q, Chen J, Yu P, Wang X, Qiu H, Chen Y, Dong Y. Correlation of HMGB1 expression to progression and poor prognosis of adenocarcinoma and squamous cell/adenosquamous carcinoma of gallbladder. Am J Transl Res. 2015;7:2015-2025. [PubMed] |
| 46. | Li Z, Chen Y, Wang X, Zhang H, Zhang Y, Gao Y, Weng M, Wang L, Liang H, Li M, Zhang F, Zhao S, Liu S, Cao Y, Shu Y, Bao R, Zhou J, Liu X, Yan Y, Zhen L, Dong Q, Liu Y. LASP-1 induces proliferation, metastasis and cell cycle arrest at the G2/M phase in gallbladder cancer by down-regulating S100P via the PI3K/AKT pathway. Cancer Lett. 2016;372:239-250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 38] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 47. | Liu BX, Tang CT, Dai XJ, Zeng L, Cheng F, Chen Y, Zeng C. Prognostic Value of S100P Expression in Patients With Digestive System Cancers: A Meta-Analysis. Front Oncol. 2021;11:593728. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 48. | Rojas A, Delgado-López F, González I, Pérez-Castro R, Romero J, Rojas I. The receptor for advanced glycation end-products: a complex signaling scenario for a promiscuous receptor. Cell Signal. 2013;25:609-614. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 77] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 49. | Yu J, Shi L, Lin W, Lu B, Zhao Y. UCP2 promotes proliferation and chemoresistance through regulating the NF-κB/β-catenin axis and mitochondrial ROS in gallbladder cancer. Biochem Pharmacol. 2020;172:113745. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 38] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 50. | Tang H, Shi X, Zhu P, Guo W, Li J, Yan B, Zhang S. Melatonin inhibits gallbladder cancer cell migration and invasion via ERK-mediated induction of epithelial-to-mesenchymal transition. Oncol Lett. 2021;22:609. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 51. | Liu S, Chu B, Cai C, Wu X, Yao W, Wu Z, Yang Z, Li F, Liu Y, Dong P, Gong W. DGCR5 Promotes Gallbladder Cancer by Sponging MiR-3619-5p via MEK/ERK1/2 and JNK/p38 MAPK Pathways. J Cancer. 2020;11:5466-5477. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 23] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 52. | Tong C, Wang Y, Li J, Cen W, Zhang W, Zhu Z, Yu J, Lu B. Pterostilbene inhibits gallbladder cancer progression by suppressing the PI3K/Akt pathway. Sci Rep. 2021;11:4391. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 53. | Pan MS, Wang H, Ansari KH, Li XP, Sun W, Fan YZ. Gallbladder cancer-associated fibroblasts promote vasculogenic mimicry formation and tumor growth in gallbladder cancer via upregulating the expression of NOX4, a poor prognosis factor, through IL-6-JAK-STAT3 signal pathway. J Exp Clin Cancer Res. 2020;39:234. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 50] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 54. | Nakada S, Kuboki S, Nojima H, Yoshitomi H, Furukawa K, Takayashiki T, Takano S, Miyazaki M, Ohtsuka M. Roles of Pin1 as a Key Molecule for EMT Induction by Activation of STAT3 and NF-κB in Human Gallbladder Cancer. Ann Surg Oncol. 2019;26:907-917. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 28] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 55. | Hong H, He C, Zhu S, Zhang Y, Wang X, She F, Chen Y. CCR7 mediates the TNF-α-induced lymphatic metastasis of gallbladder cancer through the "ERK1/2 - AP-1" and "JNK - AP-1" pathways. J Exp Clin Cancer Res. 2016;35:51. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 23] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 56. | Zhang DC, Liu JL, Ding YB, Xia JG, Chen GY. Icariin potentiates the antitumor activity of gemcitabine in gallbladder cancer by suppressing NF-κB. Acta Pharmacol Sin. 2013;34:301-308. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 66] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 57. | Bao D, Yuan RX, Zhang Y. Effects of lncRNA MEG3 on proliferation and apoptosis of gallbladder cancer cells through regulating NF-κB signaling pathway. Eur Rev Med Pharmacol Sci. 2020;24:6632-6638. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 58. | Ouyang B, Pan N, Zhang H, Xing C, Ji W. miR146b5p inhibits tumorigenesis and metastasis of gallbladder cancer by targeting Tolllike receptor 4 via the nuclear factorκB pathway. Oncol Rep. 2021;45. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 59. | Zhu G, Du Q, Wang X, Tang N, She F, Chen Y. TNF-α promotes gallbladder cancer cell growth and invasion through autocrine mechanisms. Int J Mol Med. 2014;33:1431-1440. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 38] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 60. | Hong H, Jiang L, Lin Y, He C, Zhu G, Du Q, Wang X, She F, Chen Y. TNF-alpha promotes lymphangiogenesis and lymphatic metastasis of gallbladder cancer through the ERK1/2/AP-1/VEGF-D pathway. BMC Cancer. 2016;16:240. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 45] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 61. | Li Y, Zhang J, Ma H. Chronic inflammation and gallbladder cancer. Cancer Lett. 2014;345:242-248. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 44] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 62. | González I, Romero J, Rodríguez BL, Pérez-Castro R, Rojas A. The immunobiology of the receptor of advanced glycation end-products: trends and challenges. Immunobiology. 2013;218:790-797. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 48] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 63. | Palanissami G, Paul SFD. RAGE and Its Ligands: Molecular Interplay Between Glycation, Inflammation, and Hallmarks of Cancer-a Review. Horm Cancer. 2018;9:295-325. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 135] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 64. | Bierhaus A, Humpert PM, Morcos M, Wendt T, Chavakis T, Arnold B, Stern DM, Nawroth PP. Understanding RAGE, the receptor for advanced glycation end products. J Mol Med (Berl). 2005;83:876-886. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 863] [Cited by in RCA: 956] [Article Influence: 47.8] [Reference Citation Analysis (0)] |
| 65. | Rojas A, Schneider I, Lindner C, Gonzalez I, Morales MA. The RAGE/multiligand axis: a new actor in tumor biology. Biosci Rep. 2022;42. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 66. | Taguchi A, Blood DC, del Toro G, Canet A, Lee DC, Qu W, Tanji N, Lu Y, Lalla E, Fu C, Hofmann MA, Kislinger T, Ingram M, Lu A, Tanaka H, Hori O, Ogawa S, Stern DM, Schmidt AM. Blockade of RAGE-amphoterin signalling suppresses tumour growth and metastases. Nature. 2000;405:354-360. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 917] [Cited by in RCA: 968] [Article Influence: 38.7] [Reference Citation Analysis (0)] |
| 67. | Rojas A, Morales M, Gonzalez I, Araya P. Inhibition of RAGE Axis Signaling: A Pharmacological Challenge. Curr Drug Targets. 2019;20:340-346. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 25] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 68. | Shen CY, Lu CH, Wu CH, Li KJ, Kuo YM, Hsieh SC, Yu CL. The Development of Maillard Reaction, and Advanced Glycation End Product (AGE)-Receptor for AGE (RAGE) Signaling Inhibitors as Novel Therapeutic Strategies for Patients with AGE-Related Diseases. Molecules. 2020;25. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 162] [Article Influence: 32.4] [Reference Citation Analysis (0)] |
| 69. | Arumugam T, Ramachandran V, Gomez SB, Schmidt AM, Logsdon CD. S100P-derived RAGE antagonistic peptide reduces tumor growth and metastasis. Clin Cancer Res. 2012;18:4356-4364. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 126] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 70. | Healey GD, Pan-Castillo B, Garcia-Parra J, Davies J, Roberts S, Jones E, Dhar K, Nandanan S, Tofazzal N, Piggott L, Clarkson R, Seaton G, Frostell A, Fagge T, McKee C, Margarit L, Conlan RS, Gonzalez D. Antibody drug conjugates against the receptor for advanced glycation end products (RAGE), a novel therapeutic target in endometrial cancer. J Immunother Cancer. 2019;7:280. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 21] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 71. | Mizumoto S, Takahashi J, Sugahara K. Receptor for advanced glycation end products (RAGE) functions as receptor for specific sulfated glycosaminoglycans, and anti-RAGE antibody or sulfated glycosaminoglycans delivered in vivo inhibit pulmonary metastasis of tumor cells. J Biol Chem. 2012;287:18985-18994. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 86] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 72. | Zhao Z, Wang H, Zhang L, Mei X, Hu J, Huang K. Receptor for advanced glycation end product blockade enhances the chemotherapeutic effect of cisplatin in tongue squamous cell carcinoma by reducing autophagy and modulating the Wnt pathway. Anticancer Drugs. 2017;28:187-196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 10] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 73. | Swami P, O'Connell KA, Thiyagarajan S, Crawford A, Patil P, Radhakrishnan P, Shin S, Caffrey TC, Grunkemeyer J, Neville T, Vetter SW, Hollingsworth MA, Leclerc E. Inhibition of the Receptor for Advanced Glycation End Products Enhances the Cytotoxic Effect of Gemcitabine in Murine Pancreatic Tumors. Biomolecules. 2021;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 9] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 74. | Wendel U, Persson N, Risinger C, Bengtsson E, Nodin B, Danielsson L, Welinder C, Nordin Fredrikson G, Jansson B, Blixt O. A novel monoclonal antibody targeting carboxymethyllysine, an advanced glycation end product in atherosclerosis and pancreatic cancer. PLoS One. 2018;13:e0191872. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 75. | Hubert P, Roncarati P, Demoulin S, Pilard C, Ancion M, Reynders C, Lerho T, Bruyere D, Lebeau A, Radermecker C, Meunier M, Nokin MJ, Hendrick E, Peulen O, Delvenne P, Herfs M. Extracellular HMGB1 blockade inhibits tumor growth through profoundly remodeling immune microenvironment and enhances checkpoint inhibitor-based immunotherapy. J Immunother Cancer. 2021;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 91] [Cited by in RCA: 116] [Article Influence: 29.0] [Reference Citation Analysis (0)] |
| 76. | Mizumoto S, Sugahara K. Glycosaminoglycans are functional ligands for receptor for advanced glycation end-products in tumors. FEBS J. 2013;280:2462-2470. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 52] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 77. | Takeuchi A, Yamamoto Y, Munesue S, Harashima A, Watanabe T, Yonekura H, Yamamoto H, Tsuchiya H. Low molecular weight heparin suppresses receptor for advanced glycation end products-mediated expression of malignant phenotype in human fibrosarcoma cells. Cancer Sci. 2013;104:740-749. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 38] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 78. | Xu D, Young JH, Krahn JM, Song D, Corbett KD, Chazin WJ, Pedersen LC, Esko JD. Stable RAGE-heparan sulfate complexes are essential for signal transduction. ACS Chem Biol. 2013;8:1611-1620. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 71] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 79. | Li M, Ong CY, Langouët-Astrié CJ, Tan L, Verma A, Yang Y, Zhang X, Shah DK, Schmidt EP, Xu D. Heparan sulfate-dependent RAGE oligomerization is indispensable for pathophysiological functions of RAGE. Elife. 2022;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 80. | Li ML, Wang XF, Tan ZJ, Dong P, Gu J, Lu JH, Wu XS, Zhang L, Ding QC, Wu WG, Rao LH, Mu JS, Yang JH, Weng H, Ding Q, Zhang WJ, Chen L, Liu YB. Ethyl pyruvate administration suppresses growth and invasion of gallbladder cancer cells via downregulation of HMGB1-RAGE axis. Int J Immunopathol Pharmacol. 2012;25:955-965. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 26] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 81. | Yang Y, Zhao LH, Huang B, Wang RY, Yuan SX, Tao QF, Xu Y, Sun HY, Lin C, Zhou WP. Pioglitazone, a PPARγ agonist, inhibits growth and invasion of human hepatocellular carcinoma via blockade of the rage signaling. Mol Carcinog. 2015;54:1584-1595. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 50] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 82. | Tanuma SI, Oyama T, Okazawa M, Yamazaki H, Takao K, Sugita Y, Amano S, Abe T, Sakagami H. A Dual Anti-Inflammatory and Anti-Proliferative 3-Styrylchromone Derivative Synergistically Enhances the Anti-Cancer Effects of DNA-Damaging Agents on Colon Cancer Cells by Targeting HMGB1-RAGE-ERK1/2 Signaling. Int J Mol Sci. 2022;23. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 8] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 83. | Ramya R, Coral K, Bharathidevi SR. RAGE silencing deters CML-AGE induced inflammation and TLR4 expression in endothelial cells. Exp Eye Res. 2021;206:108519. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 21] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 84. | Yu YX, Pan WC, Cheng YF. Silencing of advanced glycosylation and glycosylation and product-specific receptor (RAGE) inhibits the metastasis and growth of non-small cell lung cancer. Am J Transl Res. 2017;9:2760-2774. [PubMed] |
| 85. | Hudson BI, Kalea AZ, Del Mar Arriero M, Harja E, Boulanger E, D'Agati V, Schmidt AM. Interaction of the RAGE cytoplasmic domain with diaphanous-1 is required for ligand-stimulated cellular migration through activation of Rac1 and Cdc42. J Biol Chem. 2008;283:34457-34468. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 234] [Cited by in RCA: 288] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 86. | Hofmann MA, Drury S, Fu C, Qu W, Taguchi A, Lu Y, Avila C, Kambham N, Bierhaus A, Nawroth P, Neurath MF, Slattery T, Beach D, McClary J, Nagashima M, Morser J, Stern D, Schmidt AM. RAGE mediates a novel proinflammatory axis: a central cell surface receptor for S100/calgranulin polypeptides. Cell. 1999;97:889-901. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1392] [Cited by in RCA: 1484] [Article Influence: 57.1] [Reference Citation Analysis (0)] |
| 87. | Touré F, Fritz G, Li Q, Rai V, Daffu G, Zou YS, Rosario R, Ramasamy R, Alberts AS, Yan SF, Schmidt AM. Formin mDia1 mediates vascular remodeling via integration of oxidative and signal transduction pathways. Circ Res. 2012;110:1279-1293. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 82] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 88. | Manigrasso MB, Pan J, Rai V, Zhang J, Reverdatto S, Quadri N, DeVita RJ, Ramasamy R, Shekhtman A, Schmidt AM. Small Molecule Inhibition of Ligand-Stimulated RAGE-DIAPH1 Signal Transduction. Sci Rep. 2016;6:22450. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 63] [Cited by in RCA: 89] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 89. | Manigrasso MB, Rabbani P, Egaña-Gorroño L, Quadri N, Frye L, Zhou B, Reverdatto S, Ramirez LS, Dansereau S, Pan J, Li H, D'Agati VD, Ramasamy R, DeVita RJ, Shekhtman A, Schmidt AM. Small-molecule antagonism of the interaction of the RAGE cytoplasmic domain with DIAPH1 reduces diabetic complications in mice. Sci Transl Med. 2021;13:eabf7084. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 42] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 90. | Leerach N, Munesue S, Harashima A, Kimura K, Oshima Y, Kawano S, Tanaka M, Niimura A, Sakulsak N, Yamamoto H, Hori O, Yamamoto Y. RAGE signaling antagonist suppresses mouse macrophage foam cell formation. Biochem Biophys Res Commun. 2021;555:74-80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 91. | Koga Y, Sotokawauchi A, Higashimoto Y, Nishino Y, Hashizume N, Kakuma T, Akiba J, Tanaka Y, Matsui T, Yagi M, Yamagishi SI. DNA-Aptamer Raised against Receptor for Advanced Glycation End Products Improves Survival Rate in Septic Mice. Oxid Med Cell Longev. 2021;2021:9932311. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 92. | Sotokawauchi A, Matsui T, Higashimoto Y, Nishino Y, Koga Y, Yagi M, Yamagishi SI. DNA aptamer raised against receptor for advanced glycation end products suppresses renal tubular damage and improves insulin resistance in diabetic mice. Diab Vasc Dis Res. 2021;18:1479164121990533. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 9] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 93. | Kaida Y, Fukami K, Matsui T, Higashimoto Y, Nishino Y, Obara N, Nakayama Y, Ando R, Toyonaga M, Ueda S, Takeuchi M, Inoue H, Okuda S, Yamagishi S. DNA aptamer raised against AGEs blocks the progression of experimental diabetic nephropathy. Diabetes. 2013;62:3241-3250. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 59] [Cited by in RCA: 67] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 94. | Ojima A, Matsui T, Maeda S, Takeuchi M, Inoue H, Higashimoto Y, Yamagishi S. DNA aptamer raised against advanced glycation end products inhibits melanoma growth in nude mice. Lab Invest. 2014;94:422-429. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 33] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 95. | Zheng J, Zhu W, He F, Li Z, Cai N, Wang HH. An Aptamer-Based Antagonist against the Receptor for Advanced Glycation End-Products (RAGE) Blocks Development of Colorectal Cancer. Mediators Inflamm. 2021;2021:9958051. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 96. | Nakamura N, Matsui T, Ishibashi Y, Sotokawauchi A, Fukami K, Higashimoto Y, Yamagishi SI. RAGE-aptamer Attenuates the Growth and Liver Metastasis of Malignant Melanoma in Nude Mice. Mol Med. 2017;23:295-306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 27] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 97. | Lotan R, Ganmore I, Shelly S, Zacharia M, Uribarri J, Beisswenger P, Cai W, Troen AM, Schnaider Beeri M. Long Term Dietary Restriction of Advanced Glycation End-Products (AGEs) in Older Adults with Type 2 Diabetes Is Feasible and Efficacious-Results from a Pilot RCT. Nutrients. 2020;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 98. | Yacoub R, Nugent M, Cai W, Nadkarni GN, Chaves LD, Abyad S, Honan AM, Thomas SA, Zheng W, Valiyaparambil SA, Bryniarski MA, Sun Y, Buck M, Genco RJ, Quigg RJ, He JC, Uribarri J. Advanced glycation end products dietary restriction effects on bacterial gut microbiota in peritoneal dialysis patients; a randomized open label controlled trial. PLoS One. 2017;12:e0184789. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 78] [Cited by in RCA: 112] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 99. | Berdún R, Jové M, Sol J, Cai W, He JC, Rodriguez-Mortera R, Martin-Garí M, Pamplona R, Uribarri J, Portero-Otin M. Restriction of Dietary Advanced Glycation End Products Induces a Differential Plasma Metabolome and Lipidome Profile. Mol Nutr Food Res. 2021;65:e2000499. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 100. | Goudarzi R, Sedaghat M, Hedayati M, Hekmatdoost A, Sohrab G. Low advanced Glycation end product diet improves the central obesity, insulin resistance and inflammatory profiles in Iranian patients with metabolic syndrome: a randomized clinical trial. J Diabetes Metab Disord. 2020;19:1129-1138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 12] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 101. | Gutierrez-Mariscal FM, Cardelo MP, de la Cruz S, Alcala-Diaz JF, Roncero-Ramos I, Guler I, Vals-Delgado C, López-Moreno A, Luque RM, Delgado-Lista J, Perez-Martinez P, Yubero-Serrano EM, Lopez-Miranda J. Reduction in Circulating Advanced Glycation End Products by Mediterranean Diet Is Associated with Increased Likelihood of Type 2 Diabetes Remission in Patients with Coronary Heart Disease: From the Cordioprev Study. Mol Nutr Food Res. 2021;65:e1901290. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 41] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 102. | Jiao L, Stolzenberg-Solomon R, Zimmerman TP, Duan Z, Chen L, Kahle L, Risch A, Subar AF, Cross AJ, Hollenbeck A, Vlassara H, Striker G, Sinha R. Dietary consumption of advanced glycation end products and pancreatic cancer in the prospective NIH-AARP Diet and Health Study. Am J Clin Nutr. 2015;101:126-134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 71] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 103. | Peterson LL, Park S, Park Y, Colditz GA, Anbardar N, Turner DP. Dietary advanced glycation end products and the risk of postmenopausal breast cancer in the National Institutes of Health-AARP Diet and Health Study. Cancer. 2020;126:2648-2657. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 27] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 104. | Omofuma OO, Turner DP, Peterson LL, Merchant AT, Zhang J, Steck SE. Dietary Advanced Glycation End-products (AGE) and Risk of Breast Cancer in the Prostate, Lung, Colorectal and Ovarian Cancer Screening Trial (PLCO). Cancer Prev Res (Phila). 2020;13:601-610. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 34] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 105. | Aglago EK, Mayén AL, Knaze V, Freisling H, Fedirko V, Hughes DJ, Jiao L, Eriksen AK, Tjønneland A, Boutron-Ruault MC, Rothwell JA, Severi G, Kaaks R, Katzke V, Schulze MB, Birukov A, Palli D, Sieri S, Santucci de Magistris M, Tumino R, Ricceri F, Bueno-de-Mesquita B, Derksen JWG, Skeie G, Gram IT, Sandanger T, Quirós JR, Luján-Barroso L, Sánchez MJ, Amiano P, Chirlaque MD, Gurrea AB, Johansson I, Manjer J, Perez-Cornago A, Weiderpass E, Gunter MJ, Heath AK, Schalkwijk CG, Jenab M. Dietary Advanced Glycation End-Products and Colorectal Cancer Risk in the European Prospective Investigation into Cancer and Nutrition (EPIC) Study. Nutrients. 2021;13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 106. | Mao Z, Aglago EK, Zhao Z, Schalkwijk C, Jiao L, Freisling H, Weiderpass E, Hughes DJ, Eriksen AK, Tjønneland A, Severi G, Rothwell J, Boutron-Ruault MC, Katzke V, Kaaks R, Schulze MB, Birukov A, Krogh V, Panico S, Tumino R, Ricceri F, Bueno-de-Mesquita HB, Vermeulen RCH, Gram IT, Skeie G, Sandanger TM, Quirós JR, Crous-Bou M, Sánchez MJ, Amiano P, Chirlaque MD, Barricarte Gurrea A, Manjer J, Johansson I, Perez-Cornago A, Jenab M, Fedirko V. Dietary Intake of Advanced Glycation End Products (AGEs) and Mortality among Individuals with Colorectal Cancer. Nutrients. 2021;13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 107. | Omofuma OO, Peterson LL, Turner DP, Merchant AT, Zhang J, Thomson CA, Neuhouser ML, Snetselaar LG, Caan BJ, Shadyab AH, Saquib N, Banack HR, Uribarri J, Steck SE. Dietary Advanced Glycation End-Products and Mortality after Breast Cancer in the Women's Health Initiative. Cancer Epidemiol Biomarkers Prev. 2021;30:2217-2226. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 20] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 108. | Islami F, Goding Sauer A, Miller KD, Siegel RL, Fedewa SA, Jacobs EJ, McCullough ML, Patel AV, Ma J, Soerjomataram I, Flanders WD, Brawley OW, Gapstur SM, Jemal A. Proportion and number of cancer cases and deaths attributable to potentially modifiable risk factors in the United States. CA Cancer J Clin. 2018;68:31-54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 902] [Cited by in RCA: 1003] [Article Influence: 143.3] [Reference Citation Analysis (2)] |
| 109. | Du H, Cao T, Lu X, Zhang T, Luo B, Li Z. Mediterranean Diet Patterns in Relation to Lung Cancer Risk: A Meta-Analysis. Front Nutr. 2022;9:844382. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 14] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 110. | Long T, Liu K, Long J, Li J, Cheng L. Dietary glycemic index, glycemic load and cancer risk: a meta-analysis of prospective cohort studies. Eur J Nutr. 2022;61:2115-2127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 17] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 111. | Papadimitriou N, Markozannes G, Kanellopoulou A, Critselis E, Alhardan S, Karafousia V, Kasimis JC, Katsaraki C, Papadopoulou A, Zografou M, Lopez DS, Chan DSM, Kyrgiou M, Ntzani E, Cross AJ, Marrone MT, Platz EA, Gunter MJ, Tsilidis KK. An umbrella review of the evidence associating diet and cancer risk at 11 anatomical sites. Nat Commun. 2021;12:4579. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 95] [Cited by in RCA: 139] [Article Influence: 34.8] [Reference Citation Analysis (0)] |
| 112. | Kazemi A, Barati-Boldaji R, Soltani S, Mohammadipoor N, Esmaeilinezhad Z, Clark CCT, Babajafari S, Akbarzadeh M. Intake of Various Food Groups and Risk of Breast Cancer: A Systematic Review and Dose-Response Meta-Analysis of Prospective Studies. Adv Nutr. 2021;12:809-849. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 59] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 113. | Key TJ, Allen NE, Spencer EA, Travis RC. The effect of diet on risk of cancer. Lancet. 2002;360:861-868. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 325] [Cited by in RCA: 301] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 114. | Kopp W. How Western Diet And Lifestyle Drive The Pandemic Of Obesity And Civilization Diseases. Diabetes Metab Syndr Obes. 2019;12:2221-2236. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 266] [Cited by in RCA: 431] [Article Influence: 71.8] [Reference Citation Analysis (0)] |
| 115. | Benninghoff AD, Hintze KJ, Monsanto SP, Rodriguez DM, Hunter AH, Phatak S, Pestka JJ, Wettere AJV, Ward RE. Consumption of the Total Western Diet Promotes Colitis and Inflammation-Associated Colorectal Cancer in Mice. Nutrients. 2020;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 36] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 116. | Zheng J, Guinter MA, Merchant AT, Wirth MD, Zhang J, Stolzenberg-Solomon RZ, Steck SE. Dietary patterns and risk of pancreatic cancer: a systematic review. Nutr Rev. 2017;75:883-908. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 73] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 117. | Fabiani R, Minelli L, Bertarelli G, Bacci S. A Western Dietary Pattern Increases Prostate Cancer Risk: A Systematic Review and Meta-Analysis. Nutrients. 2016;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 51] [Cited by in RCA: 59] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 118. | Xiao Y, Xia J, Li L, Ke Y, Cheng J, Xie Y, Chu W, Cheung P, Kim JH, Colditz GA, Tamimi RM, Su X. Associations between dietary patterns and the risk of breast cancer: a systematic review and meta-analysis of observational studies. Breast Cancer Res. 2019;21:16. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 81] [Cited by in RCA: 108] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 119. | Uribarri J, Peppa M, Cai W, Goldberg T, Lu M, He C, Vlassara H. Restriction of dietary glycotoxins reduces excessive advanced glycation end products in renal failure patients. J Am Soc Nephrol. 2003;14:728-731. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 244] [Cited by in RCA: 240] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 120. | Vlassara H, Cai W, Crandall J, Goldberg T, Oberstein R, Dardaine V, Peppa M, Rayfield EJ. Inflammatory mediators are induced by dietary glycotoxins, a major risk factor for diabetic angiopathy. Proc Natl Acad Sci U S A. 2002;99:15596-15601. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 519] [Cited by in RCA: 485] [Article Influence: 21.1] [Reference Citation Analysis (0)] |
| 121. | Vlassara H, Cai W, Goodman S, Pyzik R, Yong A, Chen X, Zhu L, Neade T, Beeri M, Silverman JM, Ferrucci L, Tansman L, Striker GE, Uribarri J. Protection against loss of innate defenses in adulthood by low advanced glycation end products (AGE) intake: role of the antiinflammatory AGE receptor-1. J Clin Endocrinol Metab. 2009;94:4483-4491. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 167] [Cited by in RCA: 177] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 122. | Luévano-Contreras C, Garay-Sevilla ME, Wrobel K, Malacara JM. Dietary advanced glycation end products restriction diminishes inflammation markers and oxidative stress in patients with type 2 diabetes mellitus. J Clin Biochem Nutr. 2013;52:22-26. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 53] [Cited by in RCA: 68] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 123. | de Courten B, de Courten MP, Soldatos G, Dougherty SL, Straznicky N, Schlaich M, Sourris KC, Chand V, Scheijen JL, Kingwell BA, Cooper ME, Schalkwijk CG, Walker KZ, Forbes JM. Diet low in advanced glycation end products increases insulin sensitivity in healthy overweight individuals: a double-blind, randomized, crossover trial. Am J Clin Nutr. 2016;103:1426-1433. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 99] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 124. | Gugliucci A. Formation of Fructose-Mediated Advanced Glycation End Products and Their Roles in Metabolic and Inflammatory Diseases. Adv Nutr. 2017;8:54-62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 158] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 125. | Aragno M, Mastrocola R. Dietary Sugars and Endogenous Formation of Advanced Glycation Endproducts: Emerging Mechanisms of Disease. Nutrients. 2017;9:385. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 117] [Cited by in RCA: 156] [Article Influence: 19.5] [Reference Citation Analysis (0)] |
| 126. | Lorenzo Bermejo J, Boekstegers F, González Silos R, Marcelain K, Baez Benavides P, Barahona Ponce C, Müller B, Ferreccio C, Koshiol J, Fischer C, Peil B, Sinsheimer J, Fuentes Guajardo M, Barajas O, Gonzalez-Jose R, Bedoya G, Cátira Bortolini M, Canizales-Quinteros S, Gallo C, Ruiz Linares A, Rothhammer F. Subtypes of Native American ancestry and leading causes of death: Mapuche ancestry-specific associations with gallbladder cancer risk in Chile. PLoS Genet. 2017;13:e1006756. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 47] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 127. | Boekstegers F, Marcelain K, Barahona Ponce C, Baez Benavides PF, Müller B, de Toro G, Retamales J, Barajas O, Ahumada M, Morales E, Rojas A, Sanhueza V, Loader D, Rivera MT, Gutiérrez L, Bernal G, Ortega A, Montalvo D, Portiño S, Bertrán ME, Gabler F, Spencer L, Olloquequi J, González Silos R, Fischer C, Scherer D, Jenab M, Aleksandrova K, Katzke V, Weiderpass E, Moradi T, Fischer K, Bossers W, Brenner H, Hveem K, Eklund N, Völker U, Waldenberger M, Fuentes Guajardo M, Gonzalez-Jose R, Bedoya G, Bortolini MC, Canizales S, Gallo C, Ruiz Linares A, Rothhammer F, Lorenzo Bermejo J. ABCB1/4 gallbladder cancer risk variants identified in India also show strong effects in Chileans. Cancer Epidemiol. 2020;65:101643. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 128. | González I, Morales MA, Rojas A. Polyphenols and AGEs/RAGE axis. Trends and challenges. Food Res Int. 2020;129:108843. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 64] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 129. | Liotta LA, Clair T. Cancer. Checkpoint for invasion. Nature. 2000;405:287-288. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 21] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 130. | Rojas A, González I, Rodríguez B, Romero J, Figueroa H, Llanos J, Morales E, Pérez-Castro R. Evidence of involvement of the receptor for advanced glycation end-products (RAGE) in the adhesion of Helicobacter pylori to gastric epithelial cells. Microbes Infect. 2011;13:818-823. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 131. | Yamamoto Y, Harashima A, Saito H, Tsuneyama K, Munesue S, Motoyoshi S, Han D, Watanabe T, Asano M, Takasawa S, Okamoto H, Shimura S, Karasawa T, Yonekura H, Yamamoto H. Septic shock is associated with receptor for advanced glycation end products ligation of LPS. J Immunol. 2011;186:3248-3257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 147] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 132. | Garay-Sevilla ME, Gomez-Ojeda A, González I, Luévano-Contreras C, Rojas A. Contribution of RAGE axis activation to the association between metabolic syndrome and cancer. Mol Cell Biochem. 2021;476:1555-1573. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 16] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 133. | Rojas A, González I, Morales E, Pérez-Castro R, Romero J, Figueroa H. Diabetes and cancer: Looking at the multiligand/RAGE axis. World J Diabetes. 2011;2:108-113. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 36] [Cited by in RCA: 42] [Article Influence: 3.0] [Reference Citation Analysis (0)] |