Published online Oct 14, 2022. doi: 10.3748/wjg.v28.i38.5557
Peer-review started: May 11, 2022
First decision: June 19, 2022
Revised: June 21, 2022
Accepted: September 8, 2022
Article in press: September 8, 2022
Published online: October 14, 2022
Processing time: 153 Days and 18.4 Hours
The thyroid-gut axis has a great influence on the maintenance of human health; however, we know very little about the effects of low-dose ionizing radiation (LDR) on thyroid hormone levels and gut microbiota composition.
To investigate the potential effects of low-dose X-ray radiation to male C57BL/6J mice.
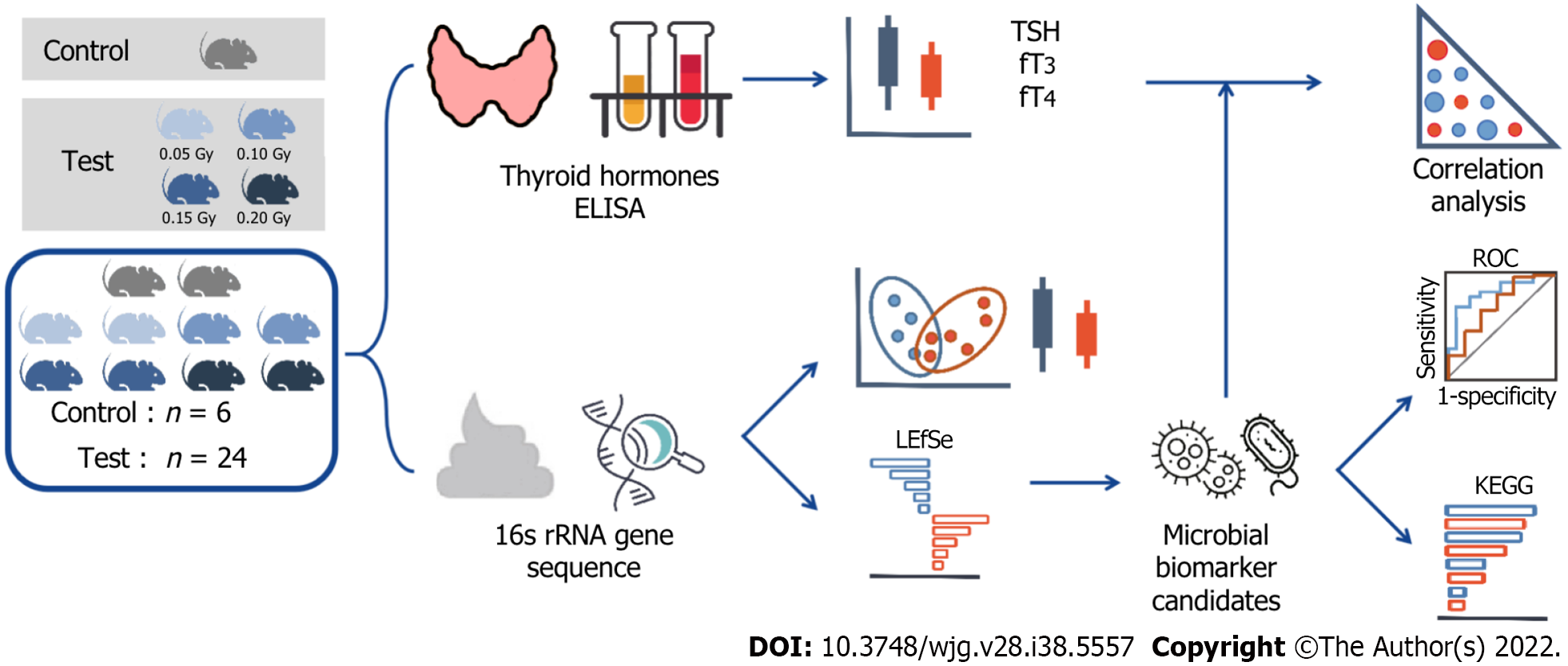
Peripheral blood was collected for enzyme-linked immunosorbent assay (ELISA), and stool samples were taken for 16S ribosomal RNA (rRNA) gene sequencing after irradiation.
We found that LDR caused changes in thyroid stimulating hormone (TSH) levels in the irradiated mice, suggesting a dose-dependent response in thyroid function to ionizing radiation. No changes in the diversity and richness of the gut microbiota were observed in the LDR-exposed group in comparison to the controls. The abundance of Moraxellaceae and Enterobacteriaceae decreased in the LDR-exposed groups compared with the controls, and the Lachnospiraceae abundance increased in a dose-dependent manner in the radiated groups. And the abundances of uncultured_bacterium_g_Acinetobacter, uncultured_bacterium_ o_Mollicutes_RF39, uncultured_bacterium_g_Citrobacter, and uncultured_ bacterium_g_Lactococcus decreased in the radiated groups at the genus level, which showed a correlation with radiation exposure and diagnostic efficacy. Analysis of functional metabolic pathways revealed that biological metabolism was predicted to have an effect on functional activities, such as nucleotide metabolism, carbohydrate metabolism, and glycan biosynthesis and metabolism. Furthermore, Kyoto Encyclopedia of Genes and Genomes pathway annotation also suggested that changes in the gut microbiota were related to processing functions, including translation, replication and repair.
LDR can change thyroid function and the gut microbiota, and changes in the abundances of bacteria are correlated with the radiation dose.
Core Tip: In this study, we administered low-dose X-ray radiation to male C57BL/6J mice to investigate the potential effects. Peripheral blood was collected for enzyme-linked immunosorbent assay, and stool samples were taken for 16S ribosomal RNA gene sequencing after irradiation. We found that LDR caused changes in TSH levels in the irradiated mice and the gut microbiota, and changes in the abundances of bacteria are correlated with the radiation dose. Our research aims to explore the influence of LDR on homeostasis from the “thyroid-gut axis” perspective for the first time.
- Citation: Tong JY, Jiang W, Yu XQ, Wang R, Lu GH, Gao DW, Lv ZW, Li D. Effect of low-dose radiation on thyroid function and the gut microbiota. World J Gastroenterol 2022; 28(38): 5557-5572
- URL: https://www.wjgnet.com/1007-9327/full/v28/i38/5557.htm
- DOI: https://dx.doi.org/10.3748/wjg.v28.i38.5557
According to microecological theory, the ecological balance of the nervous system, endocrine system, metabolic system, microbial system and immune system is the essence of life[1]. The thyroid is an important endocrine gland that regulates multiple metabolic pathways by producing thyroid hormones and has a great impact on the regulation of the internal environment[2]. Several investigations have indicated that the gut microbiota may indirectly participate in disease development and might become a promising biomarker for immune-related adverse events[3]. Studies have found that intestinal flora are related to various thyroid diseases, and the “thyroid-gut axis” theory has been proposed accordingly[4,5]. The mechanism by which the thyroid-gut axis maintains homeostasis under external stimulation deserves further study.
As one of the environmental stressors affecting people worldwide, low-dose ionizing radiation (LDR) exposure is usually from medical radiotherapy, radiation-contaminated areas and human space exploration[6,7]. The studies previously reported have indicated that the biological responses of LDR exposure on epidemiology, animals, and cells are paradoxical due to discrepancies in the dose rate[8]. The role of LDR in inducing apoptosis, stimulating antioxidant capacity and repairing DNA damage has been well established[9]. Conversely, LDR exposure from radiotherapy for the treatment of autoimmune diseases or carcinoma is associated with an increasing risk of cardiovascular disease or secondary carcinoma[10]. For the thyroid, the levels of thyroid stimulating hormone (TSH), free triiodothyronine (fT3) and free thyroxine (fT4) have been reported to be affected by LDR exposure[11]. In addition, changes between external irradiation and the gut microbiota have been preliminarily confirmed[12]. Thus, we want to know how the thyroid-gut axis responds to radiation exposure and what roles the thyroid and gut microbiota play in an individual’s response to radiation injury.
The objective of our research was to investigate changes in thyroid hormone levels and gut microbiota composition in LDR-exposed mice by enzyme-linked immunosorbent assay (ELISA) and 16S rRNA gene sequencing, respectively. Our research revealed the abundances of microbial species in the gut ecosystem following dose-modulated LDR exposure. These investigations predicted possible LDR-induced gut microbiota disorders based on variations in microbial species abundance. Our research aims to explore the influence of LDR on homeostasis from the “thyroid-gut axis” perspective for the first time and may provide a concrete conceptual and analytical basis for the additional study into the long-term impact of LDR on human health.
Thirty 6-wk-old female wild-type C57BL/6J mice were divided equally into five groups after one week of adaptive feeding at the Science and Innovation Center of Shanghai Tenth People’s Hospital (ID Number: SHDSYY-2022-4381). The low-dose irradiation conditions were as follows: Dose rate, 0.05 Gy/min; 1 min/time; and once every other day. Irradiation was completed by the Irradiation Center of the Institute of Radiation Medicine, Tongji University (160 keV, 1 mA). The two groups were the test group and control group. And the test group was further divided into four groups according to the radiation dose, namely, the 0.05 Gy group, 0.10 Gy group, 0.15 Gy group, and 0.20 Gy group. The general condition of the mice was observed during the experiment.
Peripheral blood was collected from the mouse tail vein to measure thyroid hormone levels when the mice completed the planned dose of radiation exposure within 24 h. Serum levels of TSH, fT4, and fT3 were assayed by an ELISA kit (Rayto RT-6100). Histological sections of mouse thyroid tissue were prepared by paraffin embedding and stained with hematoxylin and eosin (HE).
Stool samples were stored in a sterile container and snap-frozen with liquid nitrogen following collection at -80 °C (Sarstedt, 80.734.311, Germany). Using the E.Z.N.A. Soil Kit for DNA extraction (Omega, Bio-Tek, Norcross, GA, United States) and using a Nanodrop 2000 to measure the concentration of bacterial DNA (Thermo Scientific, Wilmington, United States). All the fecal samples were stored at -20 °C until ready for sequencing. Then, the V3-V4 region of the bacterial 16S rRNA was amplified by polymerase chain reaction with barcode index primers using TransStart FastPfu DNA Polymerase (TransGen BioTech, AP221-02, Beijing, China) on an ABI GeneAmp 9700 polymerase chain reaction system (United States). Amplicons were then purified by gel extraction (AxyPrep DNA Gel Extraction Kit, Axygen Biosciences, United States) and quantified using QuantiFluor-ST (Promega, United States). The adapter sequences were added to the purified amplicons using a TruSeqTM DNA Sample Prep Kit (Illumina, San Diego, United States), and paired-end sequencing was performed using an Illumina MiSeq instrument (Illumina, San Diego, United States) (Figure 1).
The paired-end readings obtained by MiSeq were first merged by overlapping sequences. Filtering and quality control allow the sequences to be optimized. Operational taxonomic units (OTUs) were clustered using a 97% similarity cutoff with UPARSE (version 10.0). Cohort was randomly generated for a cross-sectional study that included 24 mice exposed to radiation and 6 controls. The alpha diversity was analyzed in terms of the species richness, including CHAO, ACE, SHANNON, and SIMPSON indices. Beta diversity was used to calculate structural variation by principal coordinates analysis (PCoA) and nonmetric multidimensional scaling (NMDS) and was mapped from principal coordinate analysis. Functional pathway analysis was conducted in accordance with the Kyoto Encyclopedia of Genes and Genomes (KEGG) database based on our microbial information.
Data that fit the normal distribution are expressed as the mean ± SD, and nonnormally distributed data are expressed as the median (interquartile). The t test or rank-sum test was used for comparing the thyroid hormone levels between the control group (n = 6) and test group (n = 24). Significant differences in the diversity index and microbial abundance between groups was determined by the Mann-Whitney U test. The microbial taxa with differences between the two groups were identified by linear discriminant analysis (LDA) effect size (LEfSe), with an LDA threshold value of > 2.0. The correlation between microbiota abundance and thyroid function was analyzed by Spearman correlation coefficient. The area under the curve (AUC) reflected the diagnostic accuracy of the bacterial indicators based on the receiver operating characteristic (ROC) curve. Unless otherwise stated, all analyses were performed using SPSS Statistics (Version 20.0). Statistical significance was set at P < 0.05 for analyses.
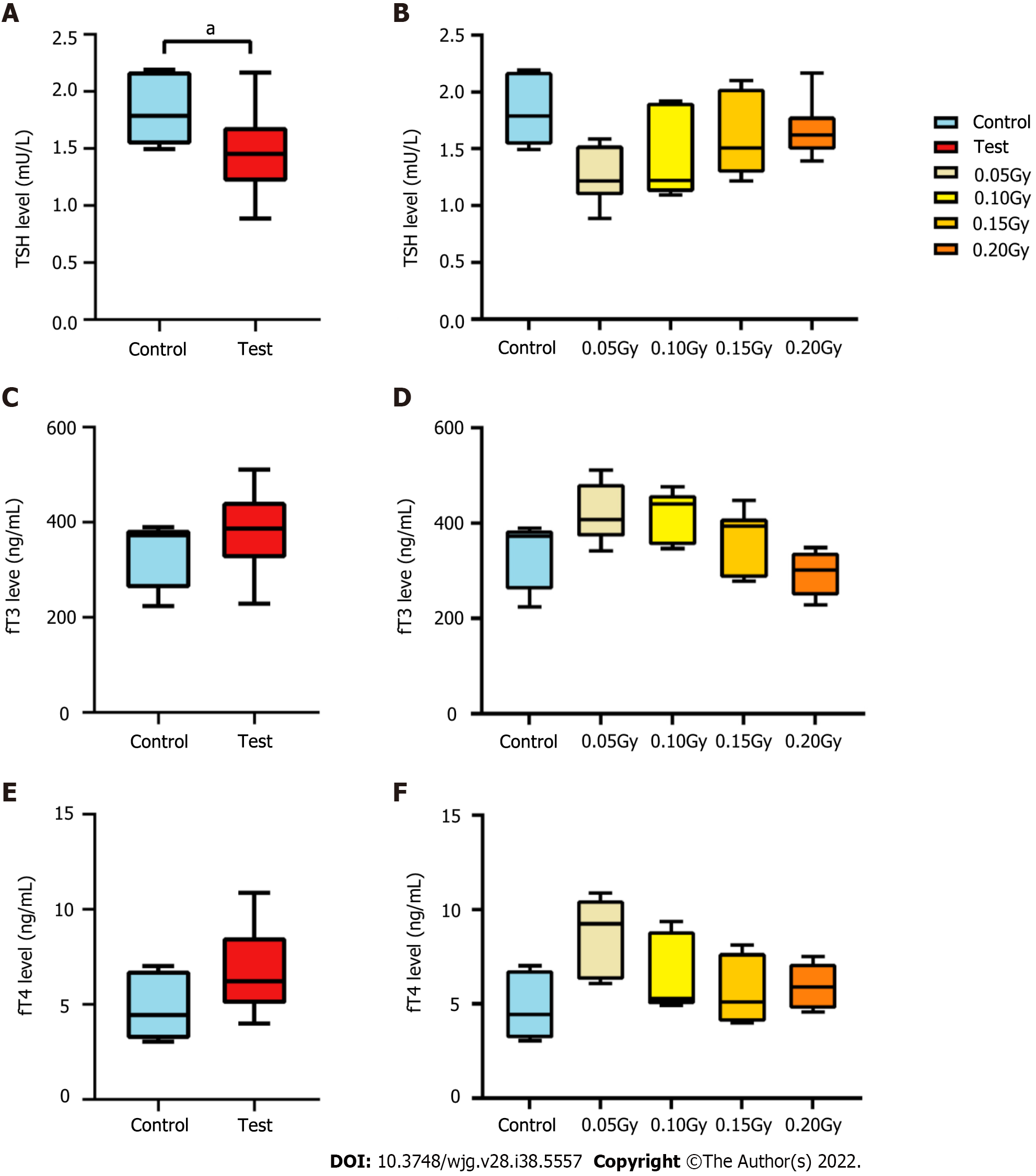
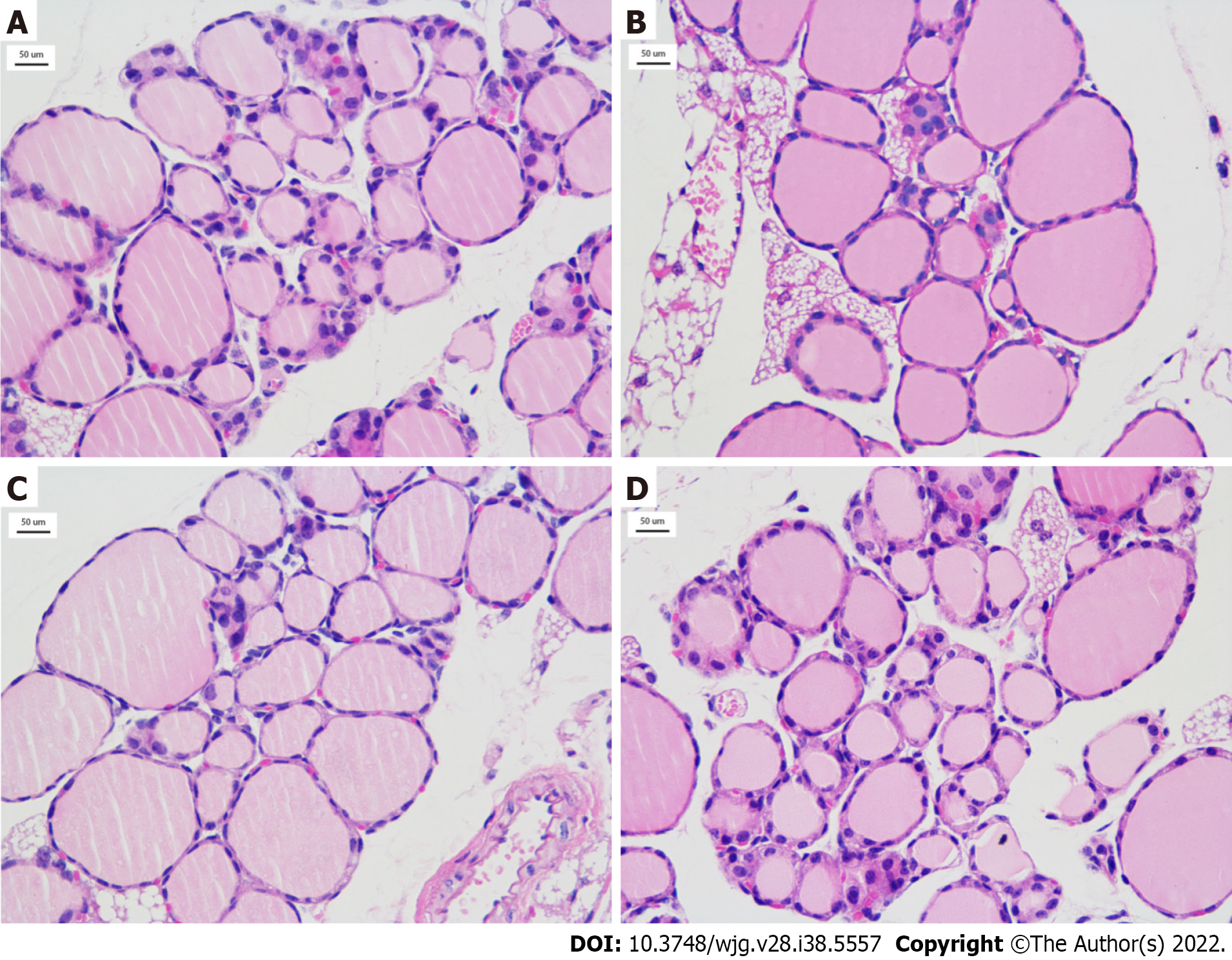
To understand changes in thyroid function in the irradiated mice, the levels of thyroid hormones in peripheral blood were determined by ELISA, including TSH, fT3, and fT4. As shown in Figure 2A, the TSH level of mice in the test mice (1.487 ± 0.347 mU/L) was obviously lower than the control mice (1.799 ± 0.289 mU/L), t = 2.188, P = 0.037). The level of TSH was the lowest in the 0.05 Gy group (1.262 ± 0.254 mU/L), which was the lowest among the four test subgroups, and in the 0.20 Gy group, it was the highest (1.664 ± 0.264 mU/L) (Figure 2A and B). The change in thyroid hormone levels increased with increasing radiation dose and was consistent with TSH regulation in the test mice. The average levels of fT3 and fT4 in the test mice [386.560 (323.973, 442.594) ng/mL and 6.211 (5.018, 8.536) ng/mL, respectively] were higher than those in the control groups [372.664 (260.701, 384.492) ng/mL and 4.428 (3.175, 6.781) ng/mL, respectively]; nevertheless, the difference was not statistically confirmed between the two groups (Figure 2C and E). Moreover, the changes in fT3 and fT4 Levels from the different dose groups were consistent with the regulation of TSH (Figure 2D and F). Figure 3 shows thyroid tissue from the different groups of mice, and there were no obvious structural abnormalities.
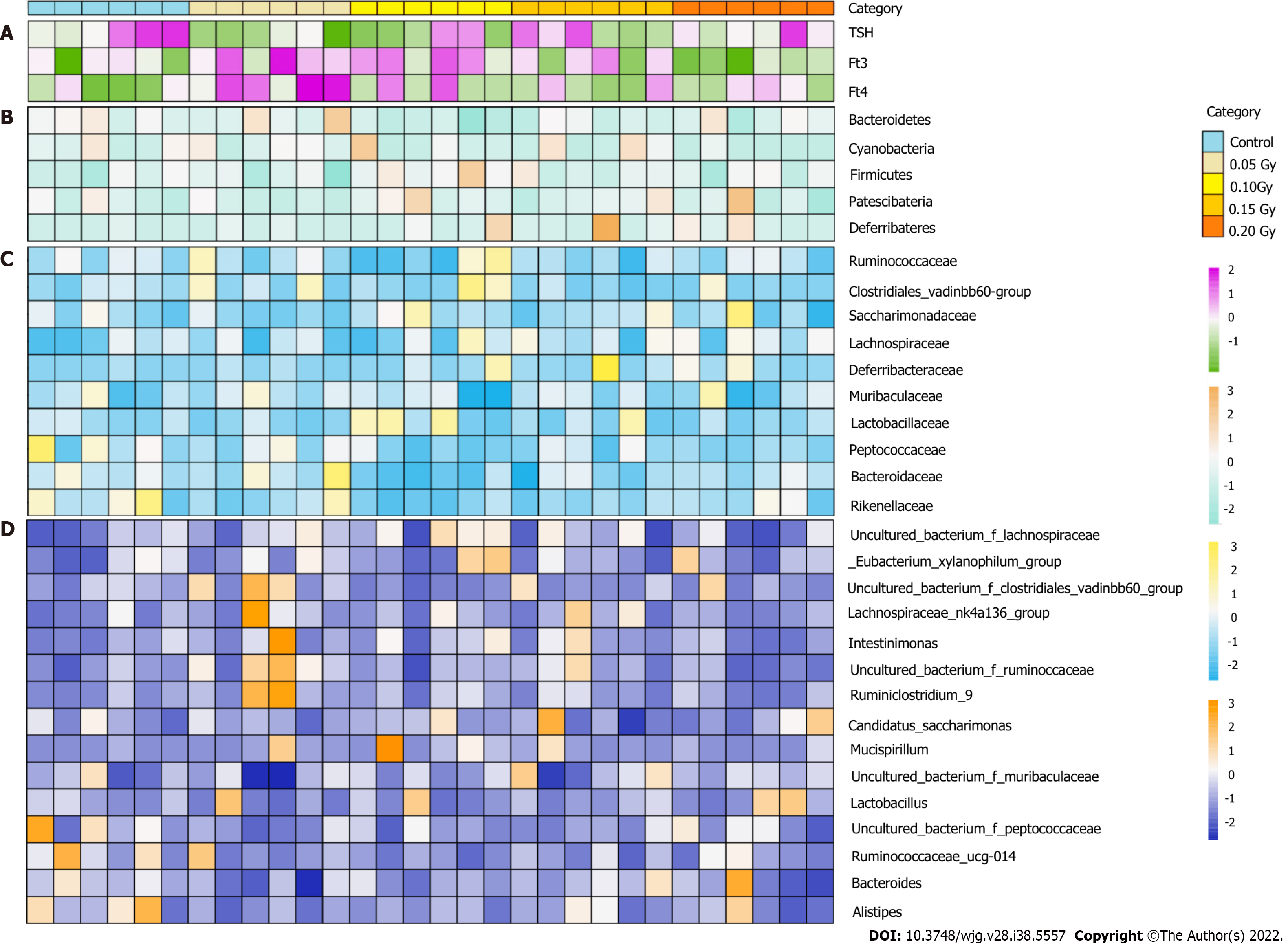
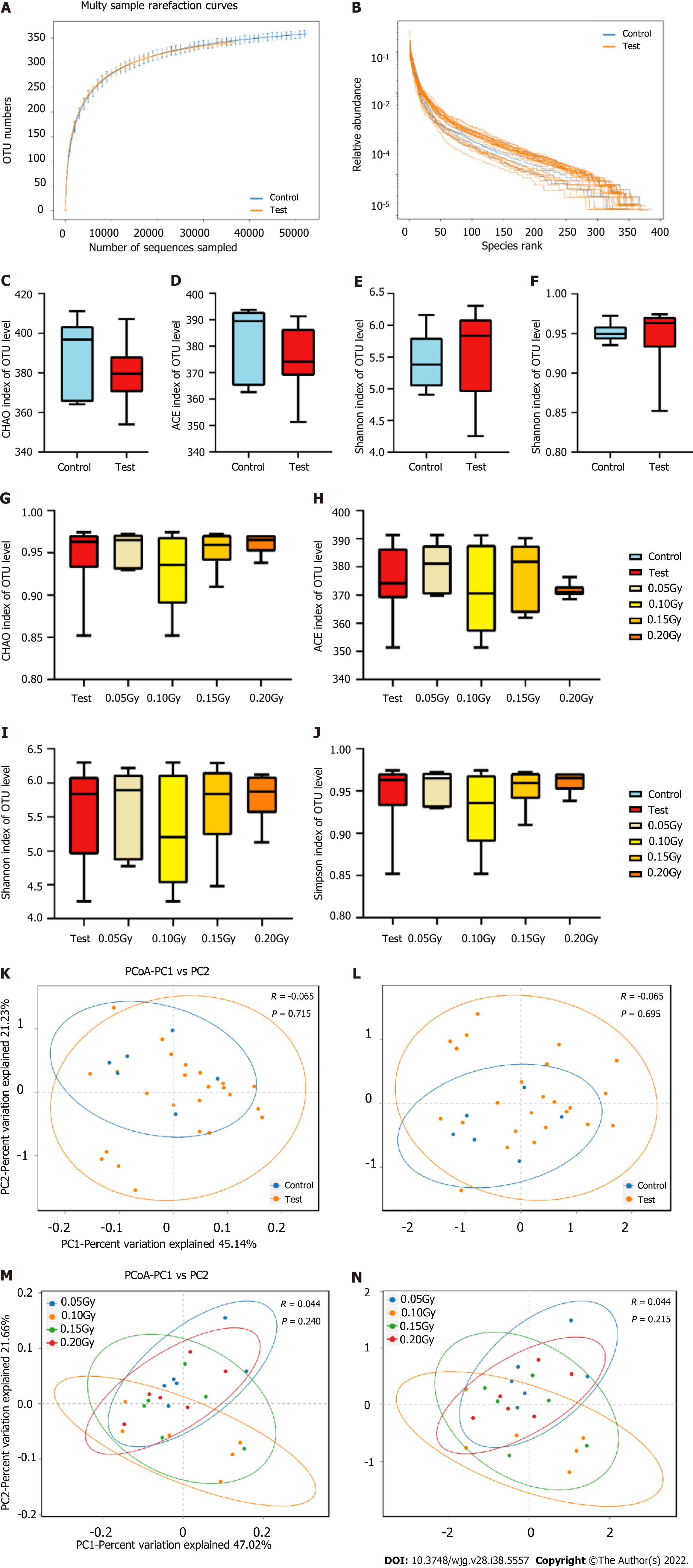
An overview of the thyroid hormone levels and taxonomic data of all mice is visualized in Figure 4. High-throughput sequencing results of the 16S rRNA V3-V4 hypervariable regions obtained from stool samples revealed that the tested taxa were abundant, including 11 phyla, 50 families, 110 genera, and 424 OTUs. Rank-abundance curves according to OTU levels indicated that the species were distributed evenly and richly (Figure 5A). Figure 5B indicates that the curves flattened as increasing the sample size, demonstrating that the sequencing data were adequate and appropriately capturing microbial diversity.
According to the outcome of alpha diversity analysis, the results showed that the test group had no differences in the diversity and abundances of certain microbes compared with the control group. The Chao and Ace indices, reflecting richness, indicated no considerable difference was found between the two groups (P > 0.05) (Figure 5C and D). Moreover, the Shannon and Simpson indices, which represent alpha diversity, indicated no meaningful difference between the two groups (P > 0.05, Figure 5E and F). Figure 5G-J shows the Chao, Ace, Shannon and Simpson indices among the different subgroups from the test group.
PCoA and NMDS analyses of the unweighted UniFrac distance were conducted on evaluation the overall microbiota diversity. PCoA indicated that there were no statistically significant variation in diversity of the microbiota between the test and control mice (R = -0.065, P = 0.715, Figure 5K). The NMDS results demonstrated no differences between the two groups (R = -0.065, P = 0.695, Figure 5L). Figure 5M and N show the results of the PCoA and NMDS analyses in the four test subgroups. These findings suggest that LDR in this study does not lead to altered microbiota diversity.
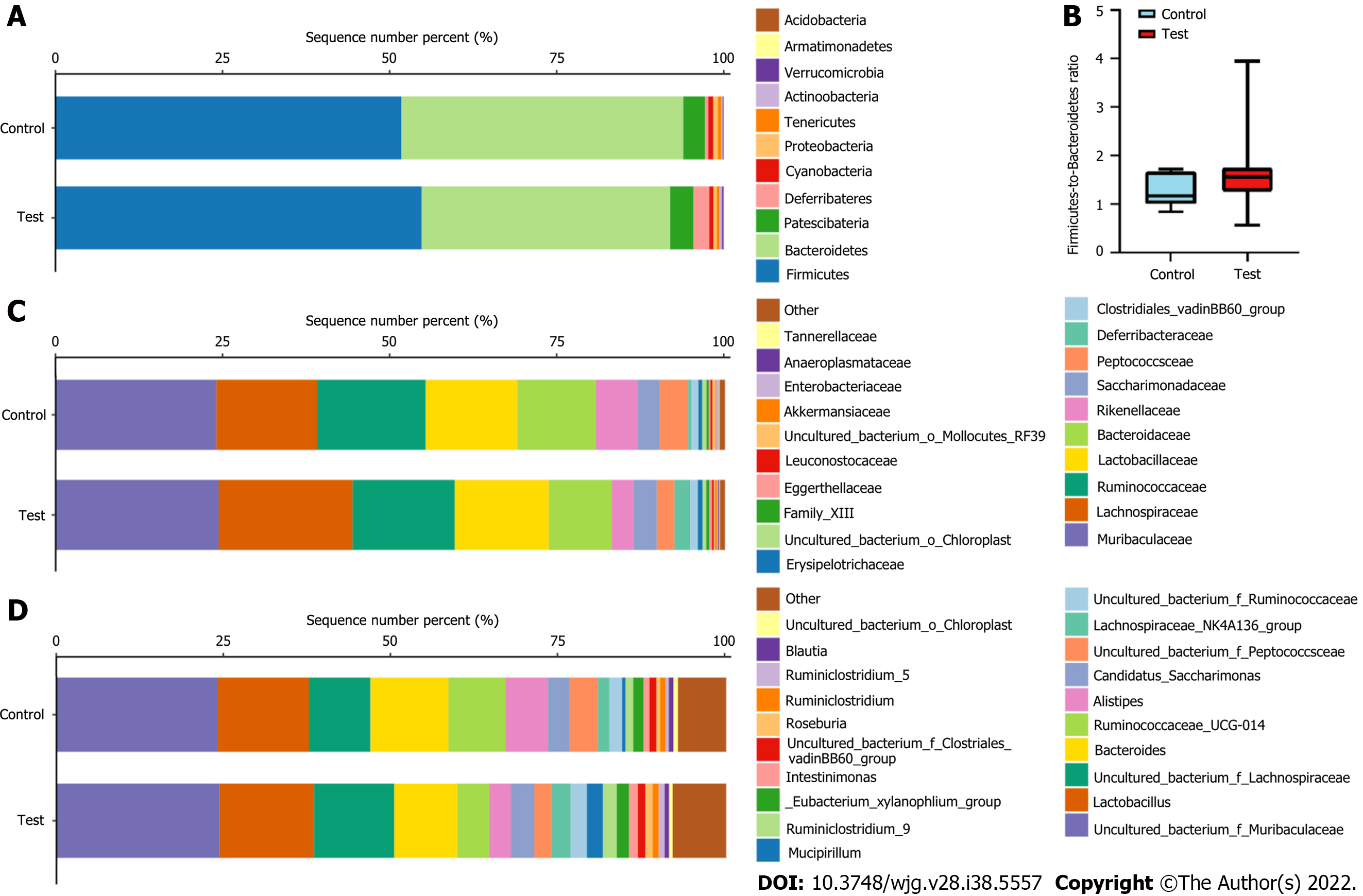
The taxon compositions were further determined in the two groups. Eleven phyla, 50 families, and 110 genera were present in the samples from both groups. Figure 6A shows that Firmicutes, Bacteroidetes, Patescibacteria, and Deferribacteres were the four dominant phyla, with total abundances accounting for 97.83% ± 1.23% and 97.68% ± 0.75% of the microbiota of the test and control groups, respectively. The two dominant phyla were Firmicutes and Bacteroidetes in both the test and control group. At the phylum level, no meaningful variation in the relative abundances of the microorganisms was observed between the two groups (Figure 6B). We found that the Firmicutes-to-Bacteroidetes (F/B) ratio in the test group (median = 1.623 ± 0.679) was higher than controls (median = 1.266 ± 0.345), the differences were not confirmed by statistics. In addition, the microbiota was further analyzed according to the distribution at the family and genus levels.
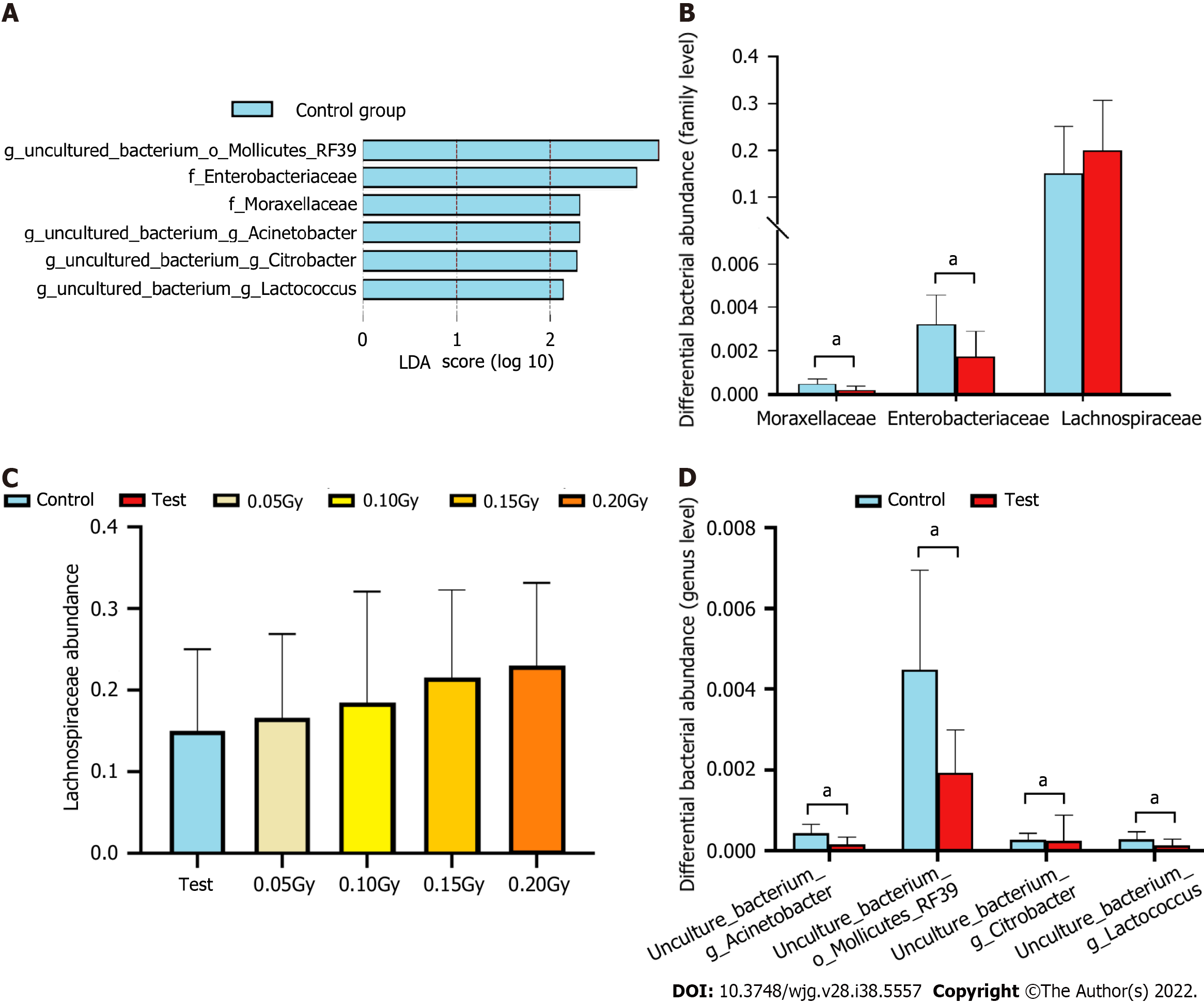
LEfSe analysis results indicated that the control group had relatively high abundance in two families and four genera (LDA > 2, P < 0.05, Figure 7A). Muribaculaceae, Lachnospiraceae, Ruminococcaceae, Lactobacillaceae and Bacteroidaceae were the five dominant families, with total abundances accounting for 83.04% ± 4.59% and 80.68% ± 6.93% of the microbiomes of the test group and control group, respectively (Figure 7A). At the family level, the test group had significantly lower levels of Moraxellaceae and Enterobacteriaceae (all P < 0.05, Figure 7B). We also found that the abundance of Lachnospiraceae in the test group (20.05% ± 10.79%) was significantly higher than that in the controls (15.08% ± 10.02%), which increased in a dose-dependent manner following LDR exposure (Figure 7B and C).
Uncultured_bacterium_f_Muribaculaceae, Lactobacillus, uncultured_bacterium_f_Lachnospiraceae, Bacteroides, and uncultured_bacterium_g_Ruminococcaceae_UCG-014 were the five dominant genera, with total abundances accounting for 59.87% ± 11.92% and 67.05% ± 10.38% of the microbiomes of the test group and control group, respectively (Figure 6C and D). At the genus level, the test group had significantly lower levels of uncultured_bacterium_g_Acinetobacter, uncultured_bacterium_o_ Mollicutes_RF39, uncultured_bacterium_g_Citrobacter, and uncultured_bacterium_g_Lactococcus (all P < 0.05, Figure 7D).
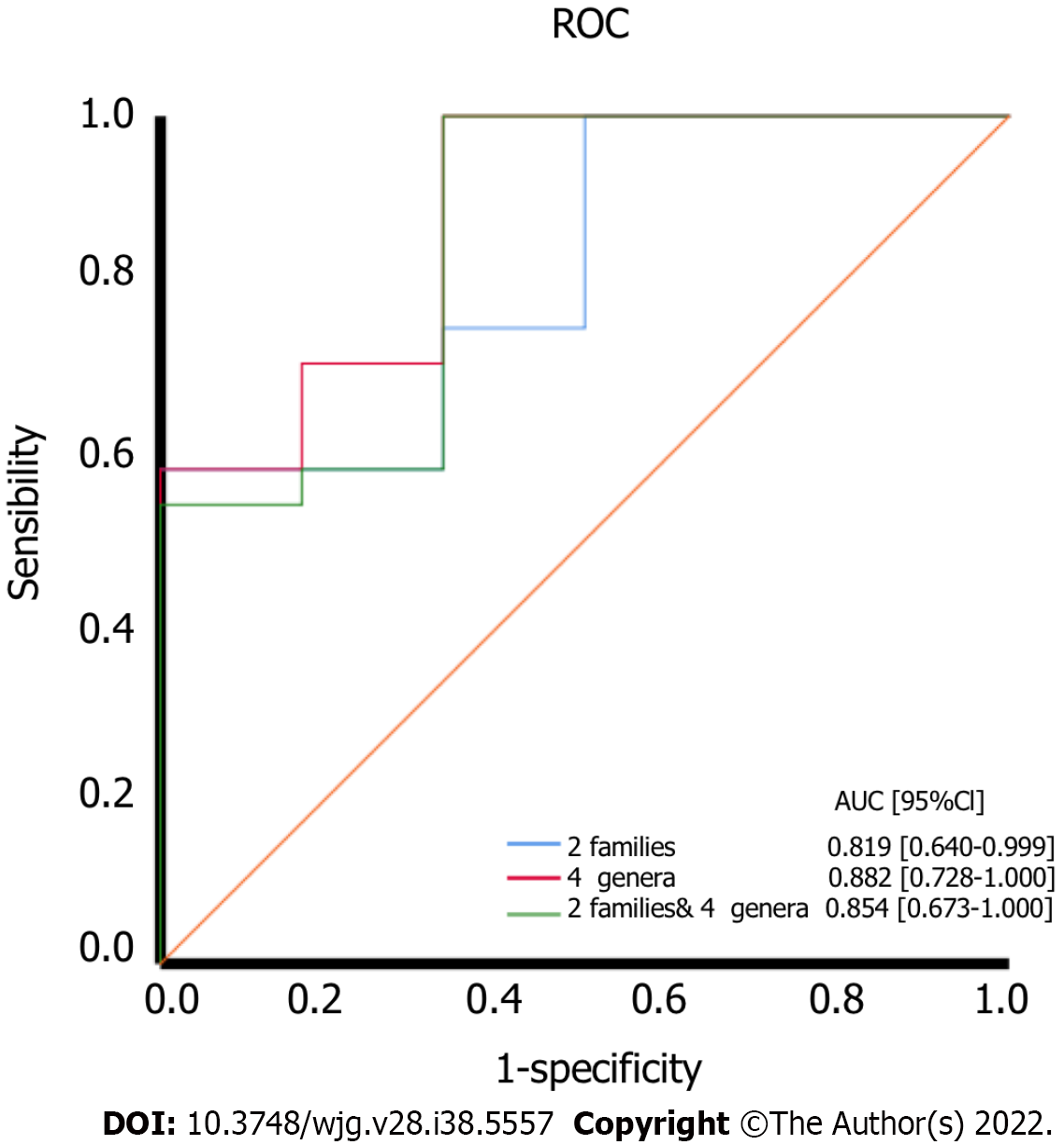
Based on the altered gut microbiota composition between the test and control groups, we found that six kinds of bacteria might serve as diagnostic biomarkers. At the family level, Moraxellaceae and Enterobacteriaceae are the two different bacteria between the two groups, and we selected them to serve as diagnostic biomarkers. ROC curves were generated to evaluate the diagnostic accuracy of these two families [AUC value of 0.819 (95% confidence interval: 0.640-0.999)] (Figure 8A).
At the genus level, we found four different bacteria and selected them to serve as diagnostic biomarkers, including uncultured_bacterium_g_Acinetobacter, uncultured_bacterium_o_Mollicutes_RF39, uncultured_bacterium_g_Citrobacter, and uncultured_bacterium_g_Lactococcus. ROC curves were generated to evaluate the diagnostic accuracy of these four genera [AUC value of 0.882 (confidence interval: 0.728-1.000)]. At the same time, the accuracy of the prognostic variables of two families and four genera was also analyzed by ROC curves [AUC value of 0.854 (confidence interval: 0.673-1.000)] (Figure 8).
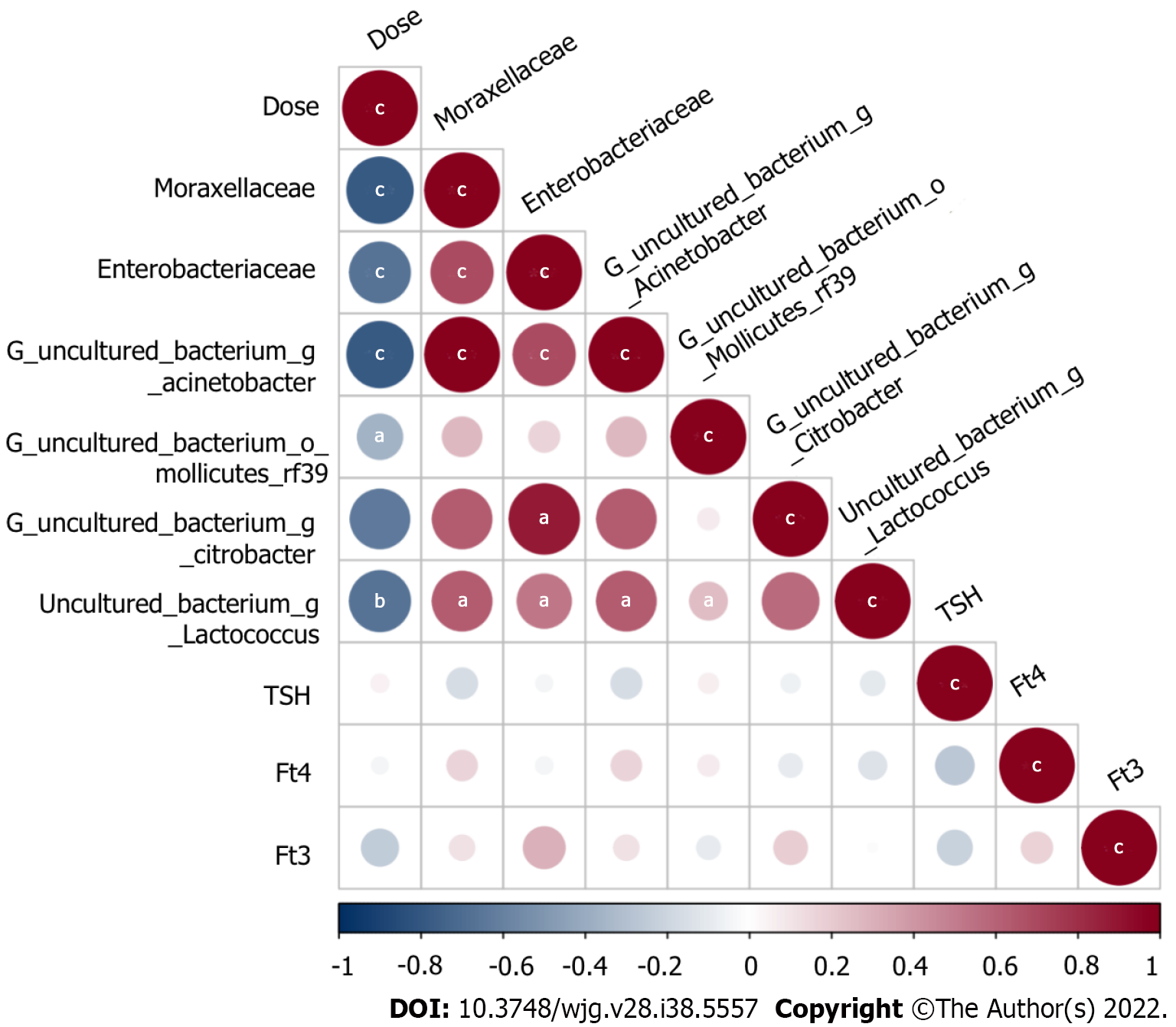
The test groups included the 0.05 Gy group, 0.10 Gy group, 0.15 Gy group, and 0.20 Gy group according to the total radiation dose the mice received. On the basis of these results, we investigated the following relationships among thyroid function tests, absorbed dose, and levels of the gut microorganisms: f_Moraxellaceae, f_Enterobacteriaceae, uncultured_bacterium_g_Acinetobacter, uncultured_ bacterium_o_Mollicutes_RF39, uncultured_bacterium_g_Citrobacter, and uncultured_bacterium_g_Lactococcus. We made an attempt to demarcate the functional influence of these flora according to the KEGG database to determine which biological pathways they may participate. Spearman correlation coefficient testing showed that Moraxellaceae (r = -0.789, P = 7.75E-7), Enterobacteriaceae (r=-0.662, P = 2.92E-4), uncultured_bacterium_g_Acinetobacter (r = -0.789, P = 7.75E-7), uncultured_bacterium_o_Mollicutes_RF39 (r = -0.365, P = 0.0106), and uncultured_bacterium_g_Lactococcus (r = -0.667, P = 4.77E-3) levels were significantly negatively correlated with the absorbed dose (Figure 9).
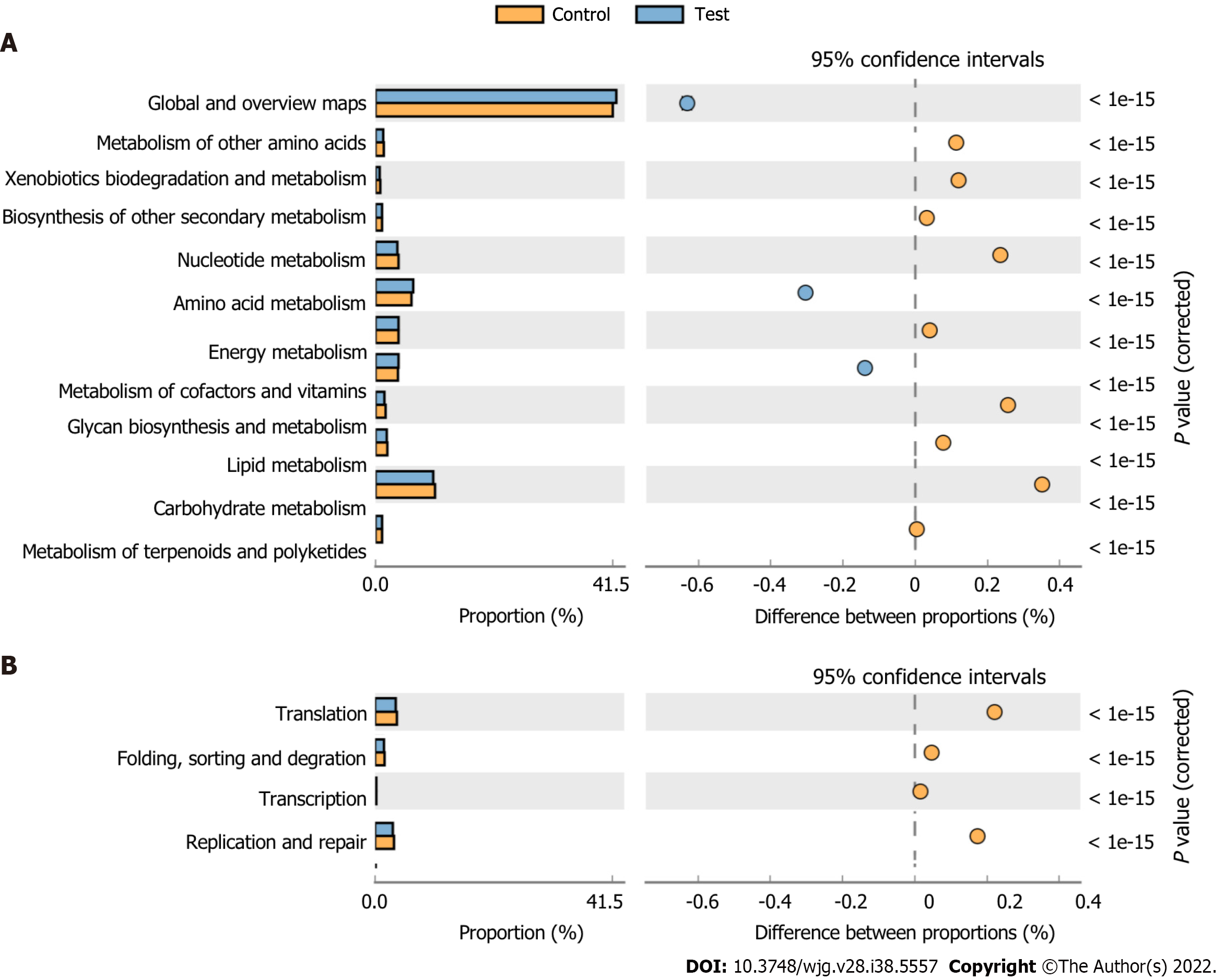
The OTU abundance table was standardized to acquire information about the prediction of metabolic function in the KEGG pathway (Figure 10). According to the effect size, the top five pathways included carbohydrate metabolism, nucleotide metabolism, glycan biosynthesis and metabolism, xenobiotics biodegradation and metabolism, and metabolism of other amino acids. The effect size of the genetic information processing function is second only to metabolic function, which includes translation, replication and repair, folding, sorting and degradation, and transcription.
As an important organ of the endocrine system, the thyroid participates in homeostatic regulation. Recent studies demonstrated that the gut microbiota, a sophisticated and metabolically active community, is closely related to homeostasis in the host[13]. Although LDR exposure has been demonstrated to induce contradictory effects previously, i.e., beneficial or harmful, the impacts of LDR exposure on the “thyroid-gut axis” have yet to be clarified. Therefore, we analyzed the association among LDR exposure, thyroid function and the gut microbiota in mice in this research. Our group indicated that as the LDR radiation dose increased, subclinical thyroid hyperthyroidism appeared in mice, and the observed species and OTU abundances showed no obvious changes in the radiated groups compared with the controls. It was thought that the low doses of radiation applied in this paper have an influence on the thyroid function of mice but are unable to alter the richness of the intestinal flora.
The thyroid is a radiation-sensitive organ, and internal radionuclide contamination external radiation and exposure (such as therapeutic radiation) may lead to thyroid dysfunction[14,15]. Our research found that the TSH level in the test mice was lower than the controls. Double-strand breaks can lead to cytotoxic, mutagenic and carcinogenic effects if unrepaired or misrepaired, although they only constitute a minority of DNA lesions induced by LDR, which will affect cell function[16,17]. Ishizaki et al[18] demonstrated a very small increase in γH2AX-positive foci in a human diploid cell line after exposure to LDR, which indicates the presence of DNA double-strand breaks. Some studies have also suggested that radiation-induced thyroid dysfunction is induced by damage to small thyroid vessels and to the gland capsule, the mechanisms of which include direct thyroid cell injury from radiation damage[19]. Because of the low thyroid dose in patients treated with total body irradiation, the incidence of Graves’ disease accompanying this management was also reported in a total body irradiation series[20]. Strikingly, our results indicate pronounced modulation of the TSH level in mice exposed to 0.05 Gy and 0.10 Gy, while those exposed to a much higher dose (0.20 Gy) consistently clustered with controls in terms of thyroid hormone levels. We speculate that there is an intricate dose-dependent response in thyroid function to ionizing radiation, with enhanced sensitivity for the lowest doses employed here. Therefore, thyroid function and microbial communities might be directly affected by LDR exposure.
Firmicutes and Bacteroidetes were the two dominant microbiota in these two groups. We found that the Firmicutes proportion in the irradiated group was higher than in the controls, while the Bacteroidetes proportion was lower in the irradiated group. One study about environmental radionuclides on the gut microbiota from Lavrinienko came to similar conclusions that the F/B ratio was increased almost 2-fold compared with an unirradiated group[21]. Previous investigation have also revealed that patients with metabolic diseases such as obesity, diabetes, and nonalcoholic fatty liver disease have a reduced F/B ratio compared to healthy individuals[22-24]. Recently, our group demonstrated that thyroid diseases, including Graves’ disease and thyroid carcinoma, can cause changes in the F/B ratio[25,26]. Therefore, we speculate that the composition and function of microbial communities might be affected by LDR exposure directly or indirectly through changes in thyroid function.
Compared with the controls, the abundances of Moraxellaceae and Enterobacteriaceae were decreased in the test group at the family level. Obvious differences in the levels of four species were observed between the two groups at the genus level, and uncultured_bacterium_g_Acinetobacter, uncultured_ bacterium_o_Mollicutes_RF39, uncultured_bacterium_g_Citrobacter and uncultured_bacterium_g_Lactococcus were reduced in the test group. On the one hand, the decreased abundance of the bacterium might be directly caused by radiation damage. On the other hand, changes in microbial abundance inhibit the development of gastrointestinal mucositis and promote intestinal repair mechanisms[27]. We hypothesize that metabolites from bacteria impair intestinal barrier function. By regulating bacterial abundance and reducing the release of proinflammatory factors, the intestinal barrier could repair the damage from LDR exposure. Another significant finding in this research was that the abundance of Lachnospiraceae increased in a dose-dependent manner following LDR exposure, which was in accordance with the performance outcome of the group of mice that have recovered from high-dose radiation to live a normal life span[28].
The synthesis of short-chain fatty acids (SCFAs) by fermenting polysaccharides in food has made members of the Lachnospira family well known[29]. SCFAs are essential substrates for the maintenance of the intestinal epithelium, regulation of the immune system and inflammatory response[30,31]. Broad inhibition of systemic inflammation, through reduction the levels of proinflammatory cytokines or through induction of anti-inflammatory cytokine interleukin-10, mediated by SCFAs may also potentially provide radioprotection. Elevated abundances of Lachnospiraceae have been shown to be connected with the repair of gastrointestinal after exposure to radiation[32].
Additionally, we employed KEGG pathway analysis to characterize changes in the gut microbiota associated with the stress from LDR exposure. KEGG pathway analysis based on OTU abundance indicated that biological metabolism was predicted to influence functional activities, including carbohydrate metabolism, glycan biosynthesis and metabolism, and nucleotide metabolism. The gut microbiota composition and metabolic pathways were influenced by LDR exposure. Moreover, processing functions were second only to metabolic functions according to the effect size, including translation, replication and repair. As the most significant influence of radiation, base modifications are a common type of DNA lesion[33]. KEGG pathway analysis may suggest a relationship between radiation damage and functional repair. Nevertheless, our observations provide circumstantial the proof of host-microbiome interactions; more investigations are required to uncover the crosstalk within LDR-responsive alterations and host-derived targets or signals.
Our research did not consider the following issues, and these limitations will also become the focus of follow-up research. First, the effects of exposure dose and recovery time on the thyroid and gut microbiota in the mice were not considered. Second, this was a descriptive study in nature and lacked evidence regarding the underlying mechanisms of the responses to LDR exposure, which could be used to determine the alterations in metabolites from the gut microbiota. Finally, our experiment was a single-center study that lacked external validation. Despite these limitations, our research is one of the few to indicate the effects of LDR on homeostasis from the “thyroid-gut axis” perspective. In summary, our study constitutes a baseline analysis that can not only be used to further study the effects of LDR on human health but also provide potential targets for preventing or predicting radiation damage.
LDR can change the thyroid function and gut microbiota of mice, and changes in the abundances of bacteria are correlated with the radiation dose.
The thyroid-gut axis has a great influence on the maintenance of human health.
We know very little about the effects of low-dose ionizing radiation (LDR) on thyroid hormone levels and gut microbiota composition.
To investigate the potential effects by administering low-dose X-ray radiation to male C57BL/6J mice.
Peripheral blood was collected for enzyme-linked immunosorbent assay (ELISA), and stool samples were taken for 16S ribosomal RNA (rRNA) gene sequencing after irradiation.
We found that LDR caused changes in thyroid stimulating hormone (TSH) levels in the irradiated mice, suggesting a dose-dependent response in thyroid function to ionizing radiation. No changes in the diversity and richness of the gut microbiota were observed in the LDR-exposed group in comparison to the controls. The abundance of Moraxellaceae and Enterobacteriaceae decreased in the LDR-exposed groups compared with the controls, and the Lachnospiraceae abundance increased in a dose-dependent manner in the radiated groups. And the abundances of uncultured_bacterium_g_Acinetobacter, uncultured_bacterium_o_Mollicutes_RF39, uncultured_bacterium_g_Citrobacter, and uncul-tured_ bacterium_g_Lactococcus decreased in the radiated groups at the genus level, which showed a correlation with radiation exposure and diagnostic efficacy. Analysis of functional metabolic pathways revealed that biological metabolism was predicted to have an effect on functional activities, such as nucleotide metabolism, carbohydrate metabolism, and glycan biosynthesis and metabolism. Furthermore, KEGG pathway annotation also suggested that changes in the gut microbiota were related to processing functions, including translation, replication and repair.
LDR can change thyroid function and the gut microbiota, and changes in the abundances of bacteria are correlated with the radiation dose.
We administered low-dose X-ray radiation to male C57BL/6J mice to investigate the potential effects. Peripheral blood was collected for ELISA, and stool samples were taken for 16S rRNA gene sequencing after irradiation.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Microbiology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Bairwa DBL, India; Eccher A, Italy; Lee KS, South Korea S-Editor: Chen YL L-Editor: A P-Editor: Chen YX
| 1. | Fröhlich E, Wahl R. Microbiota and Thyroid Interaction in Health and Disease. Trends Endocrinol Metab. 2019;30:479-490. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 134] [Article Influence: 22.3] [Reference Citation Analysis (0)] |
| 2. | Baş Y, Hassan HA, Adıgüzel C, Bulur O, Ibrahim İA, Soydan S. The distribution of cancer cases in Somalia. Semin Oncol. 2017;44:178-186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 12] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 3. | von Itzstein MS, Khan S, Gerber DE. Investigational Biomarkers for Checkpoint Inhibitor Immune-Related Adverse Event Prediction and Diagnosis. Clin Chem. 2020;66:779-793. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 82] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 4. | Knezevic J, Starchl C, Tmava Berisha A, Amrein K. Thyroid-Gut-Axis: How Does the Microbiota Influence Thyroid Function? Nutrients. 2020;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 131] [Cited by in RCA: 193] [Article Influence: 38.6] [Reference Citation Analysis (0)] |
| 5. | Samimi H, Haghpanah V. Gut Microbiome and Radioiodine-Refractory Papillary Thyroid Carcinoma Pathophysiology. Trends Endocrinol Metab. 2020;31:627-630. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 23] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 6. | Huang R, Zhou P. Double-edged effects of noncoding RNAs in responses to environmental genotoxic insults: Perspectives with regards to molecule-ecology network. Environ Pollut. 2019;247:64-71. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 27] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 7. | Liu X, Zhou Y, Wang S, Guan H, Hu S, Huang R, Zhou P. Impact of Low-dose Ionising Radiation on the Composition of the Gut Microbiota of Mice. Toxicol Sci. 2019;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 35] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 8. | Ebrahimian TG, Beugnies L, Surette J, Priest N, Gueguen Y, Gloaguen C, Benderitter M, Jourdain JR, Tack K. Chronic Exposure to External Low-Dose Gamma Radiation Induces an Increase in Anti-inflammatory and Anti-oxidative Parameters Resulting in Atherosclerotic Plaque Size Reduction in ApoE-/- Mice. Radiat Res. 2018;189:187-196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 38] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 9. | Farooque A, Mathur R, Verma A, Kaul V, Bhatt AN, Adhikari JS, Afrin F, Singh S, Dwarakanath BS. Low-dose radiation therapy of cancer: role of immune enhancement. Expert Rev Anticancer Ther. 2011;11:791-802. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 52] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 10. | Skov KA. Radioresponsiveness at low doses: hyper-radiosensitivity and increased radioresistance in mammalian cells. Mutat Res. 1999;430:241-253. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 42] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 11. | Cioffi DL, Fontana L, Leso V, Dolce P, Vitale R, Vetrani I, Galdi A, Iavicoli I. Low dose ionizing radiation exposure and risk of thyroid functional alterations in healthcare workers. Eur J Radiol. 2020;132:109279. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 12. | Li Y, Dong J, Xiao H, Zhang S, Wang B, Cui M, Fan S. Gut commensal derived-valeric acid protects against radiation injuries. Gut Microbes. 2020;11:789-806. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 114] [Article Influence: 22.8] [Reference Citation Analysis (0)] |
| 13. | Virili C, Centanni M. "With a little help from my friends" - The role of microbiota in thyroid hormone metabolism and enterohepatic recycling. Mol Cell Endocrinol. 2017;458:39-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 72] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 14. | Sassolas G, Hafdi-Nejjari Z, Casagranda L, Berger C, Bournaud C, Decaussin-Petrucci M, Berger N, Borson-Chazot F. Thyroid cancers in children, adolescents, and young adults with and without a history of childhood exposure to therapeutic radiation for other cancers. Thyroid. 2013;23:805-810. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 23] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 15. | Bang HS, Choi MH, Kim CS, Choi SJ. Gene expression profiling in undifferentiated thyroid carcinoma induced by high-dose radiation. J Radiat Res. 2016;57:238-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 16. | Asaithamby A, Chen DJ. Mechanism of cluster DNA damage repair in response to high-atomic number and energy particles radiation. Mutat Res. 2011;711:87-99. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 131] [Cited by in RCA: 119] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 17. | Bhattacharya S, Asaithamby A. Ionizing radiation and heart risks. Semin Cell Dev Biol. 2016;58:14-25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 50] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 18. | Ishizaki K, Hayashi Y, Nakamura H, Yasui Y, Komatsu K, Tachibana A. No induction of p53 phosphorylation and few focus formation of phosphorylated H2AX suggest efficient repair of DNA damage during chronic low-dose-rate irradiation in human cells. J Radiat Res. 2004;45:521-525. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 41] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 19. | Jereczek-Fossa BA, Alterio D, Jassem J, Gibelli B, Tradati N, Orecchia R. Radiotherapy-induced thyroid disorders. Cancer Treat Rev. 2004;30:369-384. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 182] [Cited by in RCA: 176] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 20. | Thomas O, Mahé M, Campion L, Bourdin S, Milpied N, Brunet G, Lisbona A, Le Mevel A, Moreau P, Harousseau J, Cuillière J. Long-term complications of total body irradiation in adults. Int J Radiat Oncol Biol Phys. 2001;49:125-131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 87] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 21. | Lavrinienko A, Mappes T, Tukalenko E, Mousseau TA, Møller AP, Knight R, Morton JT, Thompson LR, Watts PC. Environmental radiation alters the gut microbiome of the bank vole Myodes glareolus. ISME J. 2018;12:2801-2806. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 42] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 22. | Riva A, Borgo F, Lassandro C, Verduci E, Morace G, Borghi E, Berry D. Pediatric obesity is associated with an altered gut microbiota and discordant shifts in Firmicutes populations. Environ Microbiol. 2017;19:95-105. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 314] [Cited by in RCA: 302] [Article Influence: 37.8] [Reference Citation Analysis (0)] |
| 23. | Loomba R, Seguritan V, Li W, Long T, Klitgord N, Bhatt A, Dulai PS, Caussy C, Bettencourt R, Highlander SK, Jones MB, Sirlin CB, Schnabl B, Brinkac L, Schork N, Chen CH, Brenner DA, Biggs W, Yooseph S, Venter JC, Nelson KE. Gut Microbiome-Based Metagenomic Signature for Non-invasive Detection of Advanced Fibrosis in Human Nonalcoholic Fatty Liver Disease. Cell Metab. 2017;25:1054-1062.e5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 551] [Cited by in RCA: 752] [Article Influence: 94.0] [Reference Citation Analysis (0)] |
| 24. | Brown CT, Davis-Richardson AG, Giongo A, Gano KA, Crabb DB, Mukherjee N, Casella G, Drew JC, Ilonen J, Knip M, Hyöty H, Veijola R, Simell T, Simell O, Neu J, Wasserfall CH, Schatz D, Atkinson MA, Triplett EW. Gut microbiome metagenomics analysis suggests a functional model for the development of autoimmunity for type 1 diabetes. PLoS One. 2011;6:e25792. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 496] [Cited by in RCA: 590] [Article Influence: 42.1] [Reference Citation Analysis (0)] |
| 25. | Jiang W, Yu X, Kosik RO, Song Y, Qiao T, Tong J, Liu S, Fan S, Luo Q, Chai L, Lv Z, Li D. Gut Microbiota May Play a Significant Role in the Pathogenesis of Graves' Disease. Thyroid. 2021;31:810-820. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 56] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 26. | Yu X, Jiang W, Kosik RO, Song Y, Luo Q, Qiao T, Tong J, Liu S, Deng C, Qin S, Lv Z, Li D. Gut microbiota changes and its potential relations with thyroid carcinoma. J Adv Res. 2022;35:61-70. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 68] [Article Influence: 22.7] [Reference Citation Analysis (0)] |
| 27. | Touchefeu Y, Montassier E, Nieman K, Gastinne T, Potel G, Bruley des Varannes S, Le Vacon F, de La Cochetière MF. Systematic review: the role of the gut microbiota in chemotherapy- or radiation-induced gastrointestinal mucositis - current evidence and potential clinical applications. Aliment Pharmacol Ther. 2014;40:409-421. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 356] [Cited by in RCA: 218] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 28. | Guo H, Chou WC, Lai Y, Liang K, Tam JW, Brickey WJ, Chen L, Montgomery ND, Li X, Bohannon LM, Sung AD, Chao NJ, Peled JU, Gomes ALC, van den Brink MRM, French MJ, Macintyre AN, Sempowski GD, Tan X, Sartor RB, Lu K, Ting JPY. Multi-omics analyses of radiation survivors identify radioprotective microbes and metabolites. Science. 2020;370. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 305] [Cited by in RCA: 356] [Article Influence: 71.2] [Reference Citation Analysis (0)] |
| 29. | Reichardt N, Duncan SH, Young P, Belenguer A, McWilliam Leitch C, Scott KP, Flint HJ, Louis P. Phylogenetic distribution of three pathways for propionate production within the human gut microbiota. ISME J. 2014;8:1323-1335. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 598] [Cited by in RCA: 868] [Article Influence: 78.9] [Reference Citation Analysis (0)] |
| 30. | Smith PM, Howitt MR, Panikov N, Michaud M, Gallini CA, Bohlooly-Y M, Glickman JN, Garrett WS. The microbial metabolites, short-chain fatty acids, regulate colonic Treg cell homeostasis. Science. 2013;341:569-573. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2937] [Cited by in RCA: 3958] [Article Influence: 329.8] [Reference Citation Analysis (1)] |
| 31. | Arpaia N, Campbell C, Fan X, Dikiy S, van der Veeken J, deRoos P, Liu H, Cross JR, Pfeffer K, Coffer PJ, Rudensky AY. Metabolites produced by commensal bacteria promote peripheral regulatory T-cell generation. Nature. 2013;504:451-455. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2516] [Cited by in RCA: 3434] [Article Influence: 286.2] [Reference Citation Analysis (0)] |
| 32. | Xiao HW, Cui M, Li Y, Dong JL, Zhang SQ, Zhu CC, Jiang M, Zhu T, Wang B, Wang HC, Fan SJ. Gut microbiota-derived indole 3-propionic acid protects against radiation toxicity via retaining acyl-CoA-binding protein. Microbiome. 2020;8:69. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 112] [Cited by in RCA: 150] [Article Influence: 30.0] [Reference Citation Analysis (0)] |
| 33. | Cadet J, Delatour T, Douki T, Gasparutto D, Pouget JP, Ravanat JL, Sauvaigo S. Hydroxyl radicals and DNA base damage. Mutat Res. 1999;424:9-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 465] [Cited by in RCA: 443] [Article Influence: 17.0] [Reference Citation Analysis (0)] |