Published online Oct 7, 2022. doi: 10.3748/wjg.v28.i37.5457
Peer-review started: June 22, 2022
First decision: September 2, 2022
Revised: September 14, 2022
Accepted: September 20, 2022
Article in press: September 20, 2022
Published online: October 7, 2022
Processing time: 98 Days and 23.1 Hours
Gastrointestinal stromal tumors (GISTs) with a diameter of < 2 cm are called small GISTs. Currently, endoscopic ultrasound (EUS) is widely used as a regular follow-up method for GISTs, which can also provide a preliminary basis for judging the malignancy potential of lesions. However, there are no studies on the accuracy of EUS to assess the malignant potential of small GISTs.
To evaluate the efficacy of EUS in the diagnosis and risk assessment of small GISTs.
We collected data from patients with small GISTs who were admitted to Shengjing Hospital of China Medical University between October 2014 and July 2019. The accurate diagnosis and risk classifications of patients were based on the pathological assessment according to the modified National Institute of Health criteria after endoscopic resection or laparoscopic surgery. Preoperative EUS features (marginal irregularity, cystic changes, homogeneity, ulceration, and strong echogenic foci) were retrospectively analyzed. The assessment results based on EUS features were compared with the pathological features.
A total of 256 patients (69 men and 187 women) were enrolled. Pathological results included 232, 16, 7, and 1 very low-, low-, intermediate-, and high-risk cases, respectively. The most frequent tumor location was the gastric fundus (78.1%), and mitoses were calculated as > 5/50 high power field in 8 (3.1%) patients. Marginal irregularity, ulceration, strong echo foci, and heterogeneity were detected in 1 (0.4%), 2 (0.8%), 22 (8.6%), and 67 (65.1%) patients, respectively. However, cystic changes were not detected. Tumor size was positively correlated with the mitotic index (P < 0.001). Receiver operating curve analysis identified 1.48 cm as the best cut-off value to predict malignant potential (95% confidence interval: 0.824–0.956). EUS heterogeneity with tumor diameters > 1.48 cm was associated with higher risk classification (P < 0.05).
Small GISTs (diameters > 1.48 cm) with positive EUS features should receive intensive surveillance or undergo endoscopic surgery. EUS and dissection are efficient diagnostic and therapeutic approaches for small GISTs.
Core Tip: Endoscopic ultrasound (EUS) has been the recommended follow-up method for small gastrointestinal stromal tumors (GISTs); however, it is not clear whether positive EUS features can predict the malignant potential of small GISTs. Besides, undergoing close follow-up is an economic and mental burden on patients with small GISTs. This study illustrates an optimal cut-off value for the tumor size (1.48 cm) of small GISTs and uses heterogeneity to evaluate risk prediction. Overall, small GISTs with diameters > 1.48 cm with positive EUS features should receive more intensive follow-up or undergo endoscopic surgery.
- Citation: Ge QC, Wu YF, Liu ZM, Wang Z, Wang S, Liu X, Ge N, Guo JT, Sun SY. Efficacy of endoscopic ultrasound in the evaluation of small gastrointestinal stromal tumors. World J Gastroenterol 2022; 28(37): 5457-5468
- URL: https://www.wjgnet.com/1007-9327/full/v28/i37/5457.htm
- DOI: https://dx.doi.org/10.3748/wjg.v28.i37.5457
Gastrointestinal stromal tumors (GISTs) are the most common mesenchy-derived tumors of the gastrointestinal tract[1,2]. They occur most frequently in the stomach, accounting for approximately 60% of all cases[3,4]. Most GISTs contain mutations that activate the c-kit or platelet-derived growth factor receptor α gene[5,6]. The golden standard for the diagnosis of GISTs relies on pathological features assessed by methods, such as hematoxylin and eosin (H&E) and immunohistochemical staining. Currently, the risk classification of GISTs is mainly based on the National Institute of Health (NIH) 2008 (modified) or Fletcher criteria[7,8], which include tumor size (2, 5, and 10 cm), mitotic index, primary tumor sites, and tumor rupture.
GISTs with a diameter of < 2 cm are called small GISTs[9]. Currently, the management of small GISTs is controversial. There is no consensus on the treatment of GIST with a diameter of < 2 cm in the latest guidelines of the National Comprehensive Cancer Network (NCCN) (United States), European Society for Medical Oncology (ESMO), and Chinese Society of Clinical Oncology (CSCO)[10-12]. Most small GISTs are discovered incidentally during regular endoscopic scanning, and they are now easier to be detected than before using endoscopic ultrasound (EUS). EUS has been the widely recommended surveillance method for small GISTs. In addition, EUS elastography, contrast-enhanced EUS, and EUS-guided fine needle aspiration and biopsy (EUS-FNA/FNB) have further improved their diagnostic accuracy[13-16]. Furthermore, EUS features have been used in some studies to predict the risk degree of GISTs[17-19]. However, the management of small GISTs remains controversial, and endoscopic resection via endoscopic submucosal dissection (ESD) or endoscopic full-thickness resection (EFTR) is not recommended as a routine treatment. Conversely, no study has focused on the positive EUS features for determining the degree of risk for small GISTs. Therefore, in this study, we aimed to identify the efficiency of EUS in the risk assessment and safety of endoscopic dissection for small GISTs.
Data on consecutive patients with pathologically confirmed GISTs who were admitted to Shengjing Hospital of China Medical University between September 2014 and July 2019 were collected. The study included patients who underwent endoscopic resection and were pathologically or genotypically diagnosed with GISTs with a diameter of ≤ 2 cm. All the patients underwent preoperative EUS examination. Patients with other sarcomatous malignancies and/or with incomplete data were excluded. This study was approved by the Institutional Review Board and the Ethics Committee of China Medical University (No. 2022PS009K), and all the patients provided informed consent to participate in the study.
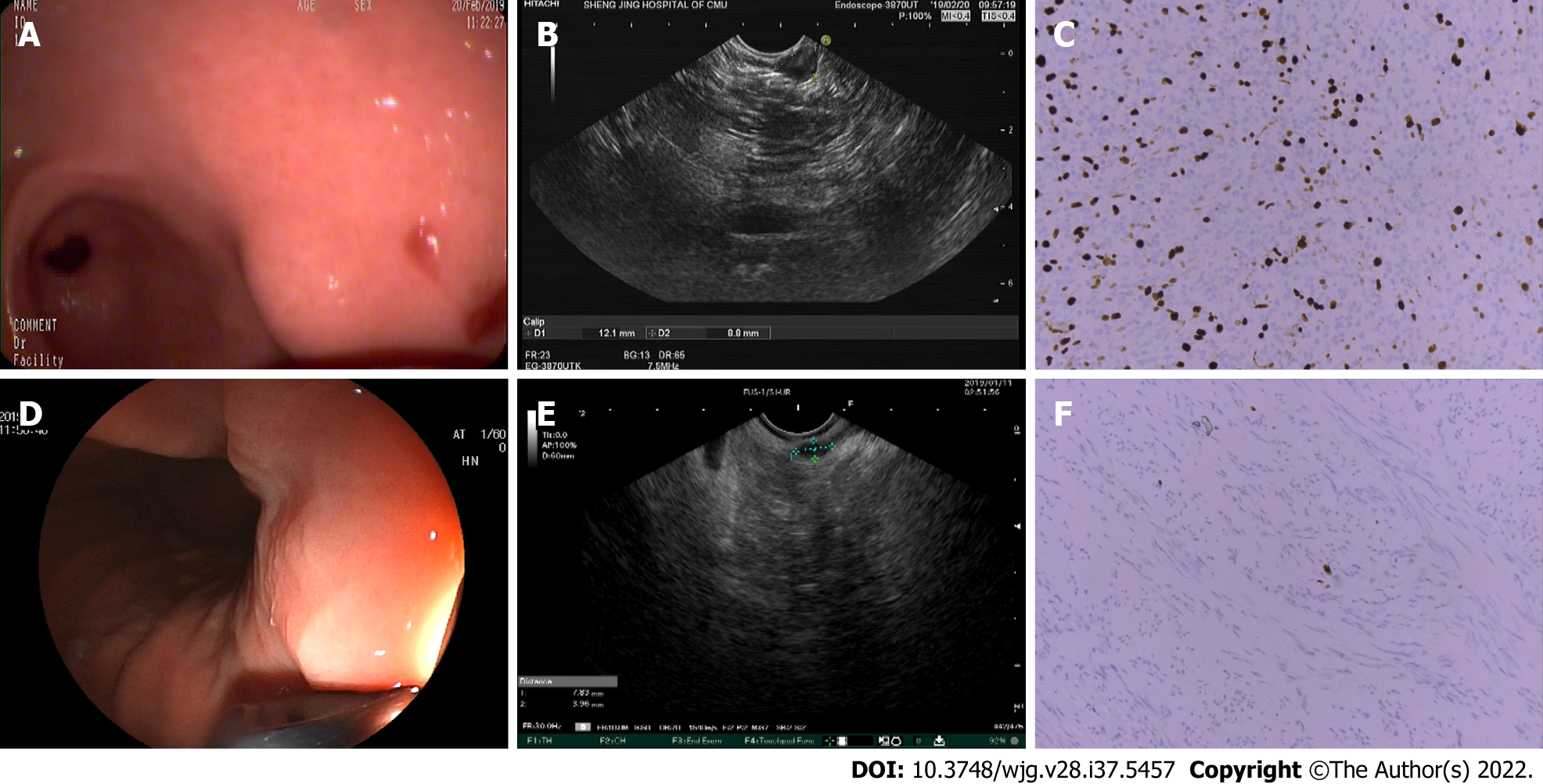
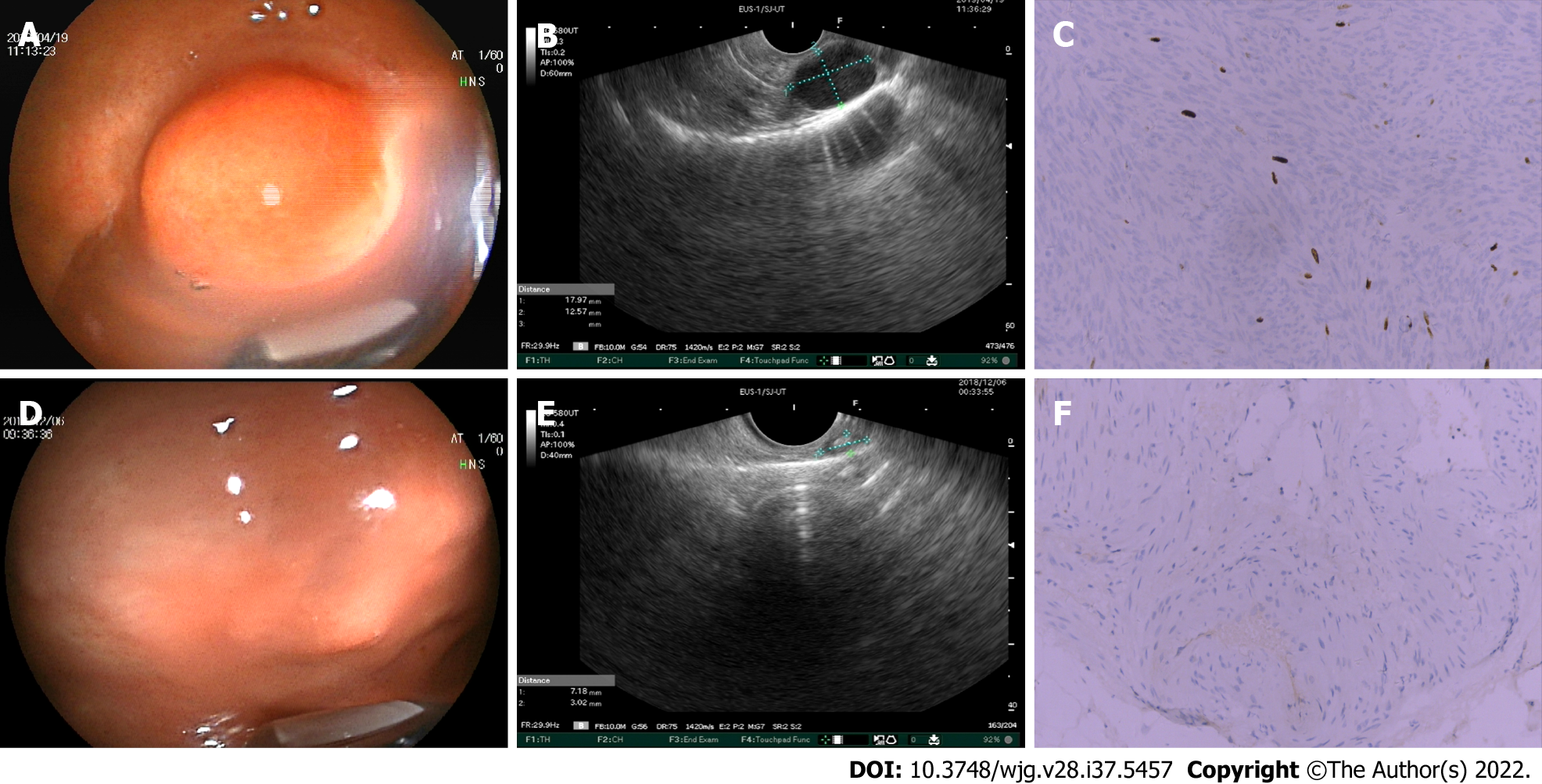
Linear EUS (EG3870UT; Pentax, Tokyo, Japan) was used for the examination. Two experienced endoscopists, who were blinded to the pathological results of these lesions, retrospectively evaluated the characteristics of the EUS images to determine whether there were positive features, including irregular borders, cystic changes, ulcerations, strong echogenic foci, heterogeneity, and the presence of an exogenous type of development (Figures 1 and 2). According to the NCCN guidelines, one or more positive EUS features indicate the malignant potential of small GISTs.
The equipment and accessories used during the endoscopic procedure included a standard single-channel gastroscope (EG29-i10, Pentax, Tokyo, Japan) and a transparent cap. Triangle- (TT knife, Olympus Corporation, Tokyo, Japan) and insulation- tipped knifes (IT knife, Olympus Corporation, Tokyo, Japan) were used for the dissection and resection of tumors. During the procedure, hot biopsy forceps (FD-410LR, Olympus Corporation, Tokyo, Japan) were used for hemostasis. Metal clips (Boston Resolution™, Boston, United States) and an over-the-scope clips (OTSC) (Ovesco Endoscopy GmbH, Tuebingen, Germany) were used for the closure of defects.
All endoscopic resections were performed by skilled gastroenterologists, following the standard endoscopic treatment regimen. All procedures were performed using propofol sedation under continuous cardiorespiratory monitoring. Ligation-assisted endoscopic resection, ESD, and EFTR were performed to resect gastric GISTs.
All resected GIST specimens were routinely processed using a standard surgical pathology protocol after overnight fixation in 10% neutral buffered formalin solution. The details of the tumor site, size, shape, surface color, and consistency were documented by a surgical pathologist. Conventional immunochemistry with a panel of antibodies, such as CD117 (c-kit), CD34, Dog-1, S-100, and smooth muscle actin, was used to support the histopathological diagnosis of GIST. Based on the modified NIH criteria, patients with GISTs were divided into very low-, low-, intermediate-, and high-risk groups (Table 1).
| Risk classification | Tumor diameter (d/cm) | Mitotic count (number/50 HPF) |
| Very-low | D ≤ 2 | Mitotic ≤ 5 |
| Intermediate | D ≤ 2 | 5 < mitotic ≤ 10 |
| High | D ≤ 2 | Mitotic > 10 |
All the patients were routinely followed-up with conventional endoscopic surveillance at 1, 3, 6, and 12 mo after resection. This was followed by annual abdominal computed tomography (CT) for 3 years to rule out recurrence and metastasis. If no residual tumor or tumor recurrence was observed, endoscopic examinations were performed once every 2 years. The follow-up period was up to October 2020. If residual tumor or tumor recurrence was detected, endoscopic resection or surgery could be performed.
Data processing was performed using the SPSS statistical software (SPSS 23.0, Chicago, IL, United States). Continuous data are expressed as the mean ± SD, and categorical data are displayed as number (n) or percentage (%). The accuracy of the diagnostic test was evaluated by sensitivity, specificity, false negative and positive rates, positive and negative likelihood ratios, and Youden’s index. The Chi-square test was applied for intergroup comparisons. A receiver operating characteristic (ROC) curve was performed to determine the optimal tumor diameter for predicting malignant potential. Lesions with positive EUS features or mitoses more than 5/50 high power field (HPF) were defined as true positives. P value < 0.05 was considered statistically significant.
A total of 256 patients, including 69 men (27%) and 187 women (73%), were enrolled in the study (Table 2), with an average age of 57.4 ± 8.6 years. The most frequent locations of the tumors were the gastric fundus (78.1%) and body (17.2%), and the average diameter of all lesions was 1.05 cm (range, 0.30–2.00 cm). These patients were eventually diagnosed with GISTs mainly based on pathological standards, such as H&E and immunohistochemistry staining. According to the modified NIH criterion, mitoses were calculated as > 5/50 HPF and ≤ 5/50 HPF in 8 (3.1%) and 248 (96.9%) patients, respectively. The patients’ features in the EUS images are shown in Table 2, illustrating that 67 (65.1%) and 22 (8.6%) cases were identified with heterogenous and hyperechoic foci, respectively. However, a lobular border and ulceration were detected in only 3 cases (1.2%).
| Characteristics | Cases (n = 256) |
| Age (year) | 57.4 ± 8.6 |
| Sex, n (%) | |
| Male | 69 (27.0) |
| Female | 187 (73.0) |
| Tumor location, n (%) | |
| Cardia | 6 (2.3) |
| Fundus | 200 (78.1) |
| Body | 44 (17.2) |
| Antrum | 6 (2.4) |
| Endoscopic resection, n (%) | |
| ESD | 136 (53.1) |
| EFTR | 120 (46.9) |
| Tumor size (cm), n (%) | |
| ≤ 1.48 | 208 (81.3) |
| >1.48 | 48 (18.7) |
| Mitotic index (/50HPF), n (%) | |
| ≤ 5 | 248 (96.9) |
| > 5 | 8 (3.1) |
| Modified NIH classification, n (%) | |
| Very-low | 232 (90.6) |
| Low | 16 (6.3) |
| Intermediate | 7 (2.7) |
| High | 1 (0.4) |
| Positive EUS features, n (%) | |
| Heterogenous | 67 (65.1) |
| Hyperechoic foci | 22 (8.6) |
| Lobular border | 1 (0.4) |
| Ulceration | 2 (0.8) |
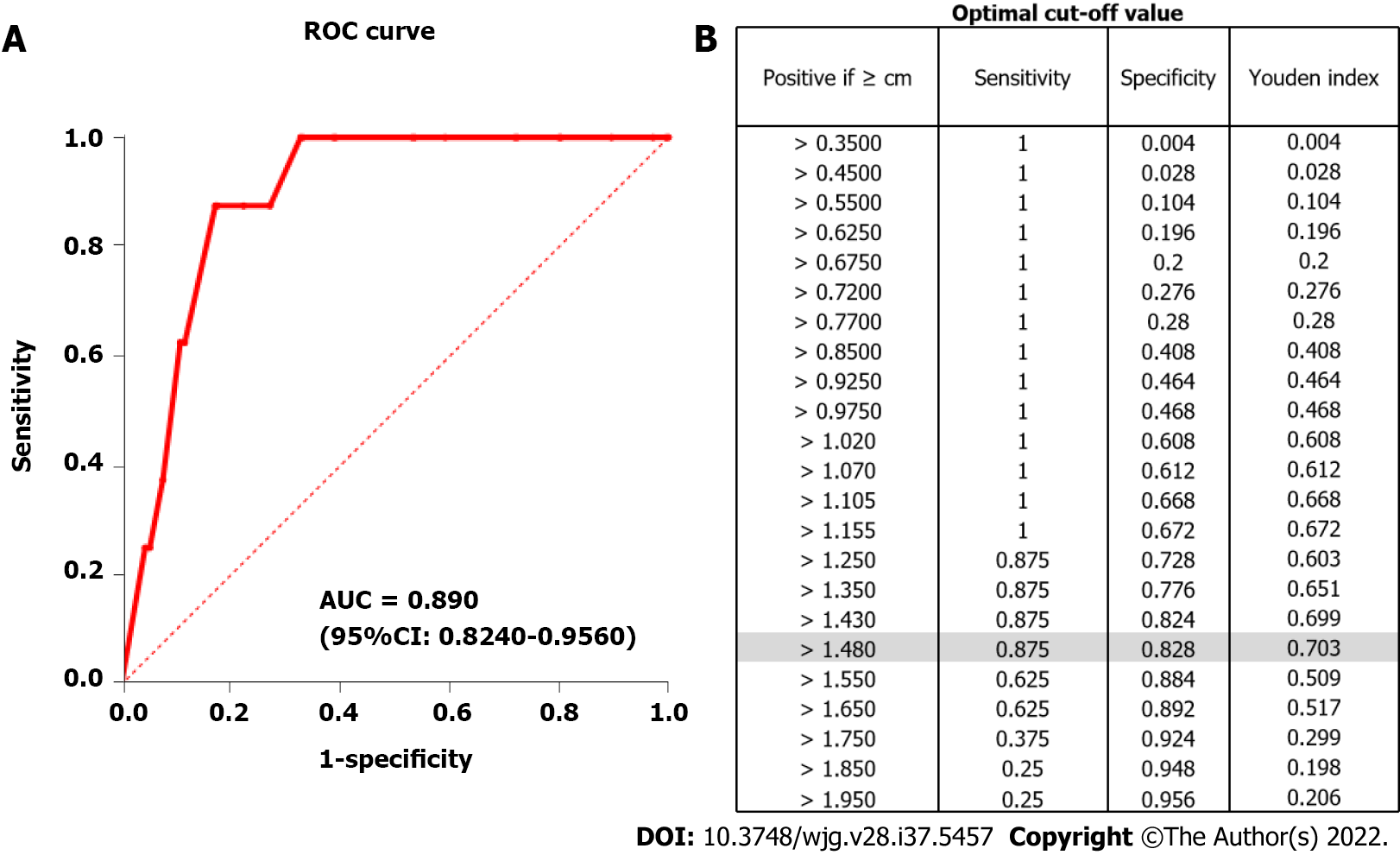
To determine a suitable cut-off value for predicting the higher risk potential of small GISTs, aggressive biological behaviors were expanded to mitoses more than 5/50 HPF. Lesions with mitoses more than 5/50 HPF were defined as true positives. A tumor diameter of 1.48 cm was finally identified as the optimal cut-off value, with the area under the curve (AUC) being 0.89 (95% confidence interval: 0.824–0.956; P < 0.001) (Figure 3A), a sensitivity of 0.875, and a specificity of 0.828 (Figure 3B). Subsequently, 208 and 48 patients were classified into groups with tumor size ≤ 1.48 cm and > 1.48 cm, respectively. The comparison of the clinicopathological characteristics between the two groups is presented in Table 3. In the > 1.48 cm group, a higher percentage of patients had more than 5 mitotic indexes/50 HPFs compared to those in the ≤ 1.48 cm group (14.58% vs 0.48%, P < 0.001). Moreover, patients with larger tumor diameter tended to display positive EUS features (56.25% vs 31.25%, P = 0.002).
| Characteristic | Tumor size | ||
| ≤ 1.48 cm group | > 1.48 cm group | P value | |
| Age (year) | 57.3 ± 8.6 | 58.2 ± 8.4 | 0.49 |
| Sex | 0.368 | ||
| Men | 59 | 10 | |
| Women | 149 | 38 | |
| Tumor location | 0.01 | ||
| Fundus | 168 | 32 | |
| Body | 34 | 10 | |
| Antrum | 4 | 2 | |
| Cardia | 2 | 4 | |
| Mitotic index (/50 HPFs) | < 0.001 | ||
| ≤ 5 | 207 | 41 | |
| >5 | 1 | 7 | |
| Positive EUS features1 | 0.002 | ||
| Yes | 65 | 25 | |
| No | 143 | 23 | |
We compared the consistency of positive EUS features and pathological risk classification of small GISTs, and found that intermediate- and very low-risk cases presented with heterogeneous (Figure 1A-C) and hypoechoic (Figure 1D-F) EUS findings, respectively. This suggests that EUS imaging features were consistent with the postoperative pathological results. However, some cases showed that EUS imaging features were inconsistent with the postoperative pathological results [intermediate-risk case with hypoechoic mass (Figure 2A-C) and very-low-risk case with strong echogenic foci (Figure 2D-F)]. To further identify the efficiency of positive EUS features in the risk assessment of small GISTs, we divided these patients into non-low-risk and low-risk groups (Table 4). Five of eight patients with non-low-risk GISTs (62.5%) and 62 of 248 patients with low-risk GISTs (25%) had heterogeneous features. There was a significant difference (P = 0.031) in the heterogeneous features between low- and high-grade risk GISTs. However, there was no statistical difference in hyperechoic foci and ulceration between low- and high-grade risk GISTs. Hyperechoic foci were found in 0 of 8 (0%) and 22 of 248 (8.87%) non-low- and low-risk groups, respectively. The ulcerative appearance was found in 0 of 8 (0%) and 2 of 248 (0.8%) non-low- and low-risk groups, respectively. Furthermore, other positive EUS features, such as marginal irregularity and cystic changes, were not detected in small GISTs.
| Positive EUS features | Risk classification | ||
| Intermediate/high | Very low/low | P value | |
| Heterogeneous | 0.031 | ||
| Yes | 5 | 62 | |
| No | 3 | 186 | |
| Hyperechoic foci | NS | ||
| Yes | 0 | 22 | |
| No | 8 | 226 | |
| Ulcers | NS | ||
| Yes | 0 | 2 | |
| No | 8 | 246 | |
The incidence of GIST ranges from 6 to 22 cases per 106 individuals per year[20,21], while that of small GISTs might be higher as more cases of asymptomatic small GISTs have been detected clinically due to the growing popularity of endoscopy in the recent years. Currently, there is no consensus on whether small GISTs (≤ 2 cm) require resection or yearly surveillance in the latest guidelines, including NCCN, ESMO, and CSCO[10-12,22]. However, the ESMO recommends that resection should be the standard treatment for histologically confirmed small GISTs[21]. Small GISTs are generally considered to have a low malignant potential, and few of them may progress to clinically relevant tumors. However, a population-based study reviewed 378 patients with small GISTs found that approximately 11.4% of small GISTs were accompanied by local progression or even distant metastasis when first diagnosed and claimed that small GISTs might progress and become life-threatening, with a mortality of 12% at 5 years[23]. Additionally, some scholars believe that the conservative observational methods for small GISTs can only evaluate whether the tumor diameter has increased, which will cause psychological burden and risk to patients as clinicians would only passively wait for the tumor size to increase before performing surgical resection[24]. Therefore, it is critical to choose optimal approaches for accurately evaluating the clinical features and providing risk suggestions for small GISTs before their progression.
EUS has been recommended as an optimal method for the diagnosis and follow-up of small GISTs, while its efficacy in risk assessment of small GISTs is still not clear. The NCCN guidelines recommend that conservative follow-up should be performed in small GISTs lacking high-risk EUS features (marginal irregularity, cystic changes, ulcers, strong echo foci, and heterogeneity)[10]. Moreover, in recent years, many studies have shown that EUS can provide a preliminary basis for assessing the malignant potential of lesions. Palazzo et al[25] showed that EUS was reliable in predicting the malignant potential of GISTs, including three most predictive EUS features (one or more of the following: Irregular border, cystic changes, and lymph nodes with a malignant pattern) with a sensitivity of 91% and a specificity of 88%[25]. Jeon et al[26] found that the best predictor of high-risk GISTs was the combination of endoscopic and EUS features, including a tumor diameter > 3 cm, irregular border, mucosal ulceration, and a non-oval shape[26]. Concerning small GISTs, Gao et al[18] studied 69 patients suspected to have GISTs as indicated by EUS and concluded that the tumor size of > 9.5 mm was significantly correlated with tumor progression, which was of great value in predicting the malignant potential of the GISTs[18]. Wang et al[27] examined 648 cases of small GISTs and concluded that GISTs with a diameter of < 1.45 cm had an overall good prognosis; however, those with a diameter > 1.45 cm required intensive monitoring or had to undergo endoscopic surgery[27,28].
Our study enrolled a total of 256 patients who underwent ESD or EFTR during 2014–2019 in Shengjing Hospital including 248 very low- or low-risk cases and 8 intermediate/high-risk cases based on the modified NIH classification. We generated a ROC curve based on the potential malignancy predictor (mitoses more than 5/50 HPF) to determine the optimal cut-off value of tumor size. A tumor diameter of 1.48 cm was identified as the optimal cutoff value, with a sensitivity of 87.5%, a specificity of 82.8%, and an AUC of 0.89. Of all the 256 patients with small gastric GISTs included in our study, EUS features for malignancy prediction were studied retrospectively. Heterogenous, hyperechoic foci, lobular border, and ulceration were detected in 67 (65.1%), 22 (8.6%), 1 (0.4%) and 2 (0.8%) patients, respectively. According to the optimal cut-off value, patients were subsequently divided into two groups: ≤ 1.48 cm and >1.48 cm. We identified that tumors with a diameter > 1.48 cm were more likely to present positive EUS features (P = 0.002). To further identify whether positive EUS features can predict the malignant potential of small GISTs, we compared EUS features between the non-low- and low-risk groups. The results showed that there was a significant difference between the two groups regarding the heterogeneity, which might suggest that patients with GISTs experiencing this EUS feature should be followed-up more frequently while considering endoscopic surgery when regular follow-up is not feasible or EUS-guided tissue acquisition for pathological assessment. However, there was no statistical difference in hyperechoic foci and ulceration features between the two groups. We believed that limited cases with these two EUS features might have resulted in a partial evaluation. Therefore, a larger scale study is needed to further comprehensively evaluate the efficacy of EUS for the risk prediction of small GISTs.
Other studies have shown that contrast-enhanced harmonic EUS and EUS-elastography can distinguish GISTs from other submucosal tumors, but the risk grade of GISTs cannot be evaluated[14,29]. Moreover, another study suggests that EUS-FNA/FNB can be used to obtain tissue for assessing the malignancy risk of tumors[30]. However, for small GISTs, the diagnostic rate of EUS-FNA is low, and due to the small size of the lesion and certain mobility limitations, puncturing the tumor is difficult and the possibility of obtaining sufficient samples is low[31,32]. A study of 53 patients[33] showed that the successful diagnostic rate of an adequate specimen was related to the lesion size: Only 71% of lesions measuring < 20 mm had complications led by repeated needle puncture[34,35]. In contrast, with the advance of endoscopic techniques, complete treatment has become possible via endoscopic surgery, such as ESD and EFTR. ESD is currently the most widely used endoscopic treatment for resecting GISTs. Studies have shown that ESD is safe and effective for treating GISTs that originate from the muscularis propria layer, with a diameter ≤3 cm and without extra-gastrointestinal invasion or abdominal metastasis[36-38]. The main adverse events of this technique are bleeding and perforation[39,40]. EFTR is mainly suitable for patients whose lesions are located in the deep muscularis propria layer and are closely connected to the serosal layer, in which metal clips, OTSCs, and other methods are used for rapid closure of defects to prevent pneumoperitoneum and peritonitis. In this study, only one patient treated with endoscopy developed delayed bleeding; however, the patient recovered well after endoscopic hemostasis was performed. Furthermore, all patients who underwent endoscopic resection had no recurrence or metastasis during a mean follow-up period of 40 mo (range, 15–72 mo), suggesting that endoscopic surgery is feasible and safe for small GISTs.
This study had a few limitations. First, the results of this retrospective study may be affected by selection bias. Second, the follow-up period was relatively short, which may result in missing cases of recurrence. Third, limited samples may affect the comprehensive evaluation of positive EUS features for risk classification. Therefore, multicenter randomized controlled trials should be conducted in the future to enhance the robustness of these conclusions.
In conclusion, partial EUS features, such as heterogeneity, can be applied to predict higher-risk small GISTs with a diameter > 1.48 cm. These tumors with diameters > 1.48 cm should undergo more intensive surveillance. Endoscopic surgery should be strongly recommended for small GISTs if regular follow-up is infeasible for low recurrence rate and metastatic rate.
Small gastrointestinal stromal tumors (GISTs) have a high incidence, and their prognosis and treatment remain controversial.
Endoscopic ultrasound (EUS) plays a pivotal role in the diagnosis of GISTs, but its ability to assess the prognosis of small GISTs remains to be explored.
To evaluate the efficacy of EUS in the diagnosis and risk assessment of small GISTs.
We collected data from patients with small GISTs, the diagnosis and risk classifications of which were based on the pathological assessment according to the modified National Institute of Health criteria after endoscopic resection or laparoscopic surgery. The assessment results based on EUS features (marginal irregularity, cystic changes, homogeneity, ulceration, and strong echogenic foci) were compared with the pathological features.
A total of 256 patients (69 men and 187 women) were enrolled. Tumor size was positively correlated with the mitotic index (P < 0.001). Receiver operating curve analysis identified 1.48 cm as the best cut-off value to predict malignant potential (95% confidence interval: 0.824–0.956). EUS heterogeneity with tumor diameters > 1.48 cm was associated with higher risk classification (P < 0.05).
Small GISTs (diameters, > 1.48 cm) with positive EUS features should receive intensive surveillance or undergo endoscopic surgery. EUS and dissection are efficient diagnostic and therapeutic approaches for small GISTs.
EUS provides reference evidence for the precise assessment and early risk assessment of small GISTs.
We thank Professor Si-Yu Sun, for his support and guidance during this study. We also thank all other doctors who participated in this study.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): A, A
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Kim HJ, South Korea; Sundaram S, India; Villa E, United States S-Editor: Fan JR L-Editor: A P-Editor: Fan JR
| 1. | Di Vita M, Zanghì A, Cavallaro A, Cardì F, Uhlig M, Ursi P, Lo Menzo E, Panebianco V, Cappellani A. Gastric GIST and prognostic models. Which is the best to predict survival after surgery? Ann Ital Chir. 2019;90:31-40. [PubMed] |
| 2. | Xu C, Han H, Wang J, Zhang B, Shao Y, Zhang L, Wang H, Wu Y, Li X, Li R, Tian Y. Diagnosis value of CD117 and PDGFRA, alone or in combination DOG1, as biomarkers for gastrointestinal stromal tumors. Ann Transl Med. 2015;3:308. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 3. | Søreide K, Sandvik OM, Søreide JA, Giljaca V, Jureckova A, Bulusu VR. Global epidemiology of gastrointestinal stromal tumours (GIST): A systematic review of population-based cohort studies. Cancer Epidemiol. 2016;40:39-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 579] [Cited by in RCA: 520] [Article Influence: 57.8] [Reference Citation Analysis (1)] |
| 4. | Wang M, Xu J, Zhang Y, Tu L, Qiu WQ, Wang CJ, Shen YY, Liu Q, Cao H. Gastrointestinal stromal tumor: 15-years' experience in a single center. BMC Surg. 2014;14:93. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 22] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 5. | Corless CL, Barnett CM, Heinrich MC. Gastrointestinal stromal tumours: origin and molecular oncology. Nat Rev Cancer. 2011;11:865-878. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 635] [Cited by in RCA: 623] [Article Influence: 44.5] [Reference Citation Analysis (0)] |
| 6. | Hirota S, Isozaki K, Moriyama Y, Hashimoto K, Nishida T, Ishiguro S, Kawano K, Hanada M, Kurata A, Takeda M, Muhammad Tunio G, Matsuzawa Y, Kanakura Y, Shinomura Y, Kitamura Y. Gain-of-function mutations of c-kit in human gastrointestinal stromal tumors. Science. 1998;279:577-580. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3215] [Cited by in RCA: 3114] [Article Influence: 115.3] [Reference Citation Analysis (0)] |
| 7. | Murphey MD. World Health Organization classification of bone and soft tissue tumors: modifications and implications for radiologists. Semin Musculoskelet Radiol. 2007;11:201-214. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 61] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 8. | Fletcher CD, Berman JJ, Corless C, Gorstein F, Lasota J, Longley BJ, Miettinen M, O'Leary TJ, Remotti H, Rubin BP, Shmookler B, Sobin LH, Weiss SW. Diagnosis of gastrointestinal stromal tumors: A consensus approach. Hum Pathol. 2002;33:459-465. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2231] [Cited by in RCA: 2149] [Article Influence: 93.4] [Reference Citation Analysis (1)] |
| 9. | Demetri GD, von Mehren M, Antonescu CR, DeMatteo RP, Ganjoo KN, Maki RG, Pisters PW, Raut CP, Riedel RF, Schuetze S, Sundar HM, Trent JC, Wayne JD. NCCN Task Force report: update on the management of patients with gastrointestinal stromal tumors. J Natl Compr Canc Netw. 2010;8 Suppl 2:S1-41; quiz S42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 826] [Cited by in RCA: 822] [Article Influence: 54.8] [Reference Citation Analysis (0)] |
| 10. | von Mehren M, Randall RL, Benjamin RS, Boles S, Bui MM, Ganjoo KN, George S, Gonzalez RJ, Heslin MJ, Kane JM, Keedy V, Kim E, Koon H, Mayerson J, McCarter M, McGarry SV, Meyer C, Morris ZS, O'Donnell RJ, Pappo AS, Paz IB, Petersen IA, Pfeifer JD, Riedel RF, Ruo B, Schuetze S, Tap WD, Wayne JD, Bergman MA, Scavone JL. Soft Tissue Sarcoma, Version 2.2018, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2018;16:536-563. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 326] [Cited by in RCA: 460] [Article Influence: 76.7] [Reference Citation Analysis (1)] |
| 11. | ESMO/European Sarcoma Network Working Group. Gastrointestinal stromal tumours: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2014;25 Suppl 3:iii21-iii26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 228] [Cited by in RCA: 264] [Article Influence: 26.4] [Reference Citation Analysis (0)] |
| 12. | Li J, Ye Y, Wang J, Zhang B, Qin S, Shi Y, He Y, Liang X, Liu X, Zhou Y, Wu X, Zhang X, Wang M, Gao Z, Lin T, Cao H, Shen L; Chinese Society Of Clinical Oncology Csco Expert Committee On Gastrointestinal Stromal Tumor. Chinese consensus guidelines for diagnosis and management of gastrointestinal stromal tumor. Chin J Cancer Res. 2017;29:281-293. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 142] [Cited by in RCA: 139] [Article Influence: 17.4] [Reference Citation Analysis (1)] |
| 13. | Hu J, Ge N, Wang S, Liu X, Guo J, Wang G, Sun S. The Role of Endoscopic Ultrasound and Endoscopic Resection for Gastric Glomus: A Case Series and Literature Review. J Transl Int Med. 2019;7:149-154. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 16] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 14. | Kovacevic B, Vilmann P. EUS tissue acquisition: From A to B. Endosc Ultrasound. 2020;9:225-231. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 36] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 15. | Fabbri C, Fornelli A, Fuccio L, Giovanelli S, Tarantino I, Antonini F, Liotta R, Frazzoni L, Gusella P, La Marca M, Barresi L, Macarri G, Traina M, De Biase D, Fiorino S, Jovine E, Larghi A, Cennamo V. High diagnostic adequacy and accuracy of the new 20G procore needle for EUS-guided tissue acquisition: Results of a large multicentre retrospective study. Endosc Ultrasound. 2019;8:261-268. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 13] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 16. | Ang TL, Li JW, Kwek ABE, Thurairajah PH, Wang LM. The difference in histological yield between 19G EUS-FNA and EUS-fine-needle biopsy needles. Endosc Ultrasound. 2019;8:255-260. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 22] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 17. | Săftoiu A, Vilmann P, Ciurea T. Utility of endoscopic ultrasound for the diagnosis and treatment of submucosal tumors of the upper gastrointestinal tract. Rom J Gastroenterol. 2003;12:215-229. [PubMed] |
| 18. | Gao Z, Wang C, Xue Q, Wang J, Shen Z, Jiang K, Shen K, Liang B, Yang X, Xie Q, Wang S, Ye Y. The cut-off value of tumor size and appropriate timing of follow-up for management of minimal EUS-suspected gastric gastrointestinal stromal tumors. BMC Gastroenterol. 2017;17:8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 29] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 19. | Kim GH, Kim KB, Lee SH, Jeon HK, Park DY, Jeon TY, Kim DH, Song GA. Digital image analysis of endoscopic ultrasonography is helpful in diagnosing gastric mesenchymal tumors. BMC Gastroenterol. 2014;14:7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 25] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 20. | Kim HJ, Lim SJ, Park K, Yuh YJ, Jang SJ, Choi J. Multiple gastrointestinal stromal tumors with a germline c-kit mutation. Pathol Int. 2005;55:655-659. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 38] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 21. | Sanborn RE, Blanke CD. Gastrointestinal stromal tumors and the evolution of targeted therapy. Clin Adv Hematol Oncol. 2005;3:647-657. [PubMed] |
| 22. | Casali PG, Abecassis N, Aro HT, Bauer S, Biagini R, Bielack S, Bonvalot S, Boukovinas I, Bovee JVMG, Brodowicz T, Broto JM, Buonadonna A, De Álava E, Dei Tos AP, Del Muro XG, Dileo P, Eriksson M, Fedenko A, Ferraresi V, Ferrari A, Ferrari S, Frezza AM, Gasperoni S, Gelderblom H, Gil T, Grignani G, Gronchi A, Haas RL, Hassan B, Hohenberger P, Issels R, Joensuu H, Jones RL, Judson I, Jutte P, Kaal S, Kasper B, Kopeckova K, Krákorová DA, Le Cesne A, Lugowska I, Merimsky O, Montemurro M, Pantaleo MA, Piana R, Picci P, Piperno-Neumann S, Pousa AL, Reichardt P, Robinson MH, Rutkowski P, Safwat AA, Schöffski P, Sleijfer S, Stacchiotti S, Sundby Hall K, Unk M, Van Coevorden F, van der Graaf WTA, Whelan J, Wardelmann E, Zaikova O, Blay JY; ESMO Guidelines Committee and EURACAN. Gastrointestinal stromal tumours: ESMO-EURACAN Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2018;29:iv68-iv78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 270] [Cited by in RCA: 289] [Article Influence: 41.3] [Reference Citation Analysis (1)] |
| 23. | Coe TM, Fero KE, Fanta PT, Mallory RJ, Tang CM, Murphy JD, Sicklick JK. Population-Based Epidemiology and Mortality of Small Malignant Gastrointestinal Stromal Tumors in the USA. J Gastrointest Surg. 2016;20:1132-1140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 57] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 24. | Fang YJ, Cheng TY, Sun MS, Yang CS, Chen JH, Liao WC, Wang HP. Suggested cutoff tumor size for management of small EUS-suspected gastric gastrointestinal stromal tumors. J Formos Med Assoc. 2012;111:88-93. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 25] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 25. | Palazzo L, Landi B, Cellier C, Cuillerier E, Roseau G, Barbier JP. Endosonographic features predictive of benign and malignant gastrointestinal stromal cell tumours. Gut. 2000;46:88-92. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 244] [Cited by in RCA: 221] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 26. | Jeon SW, Park YD, Chung YJ, Cho CM, Tak WY, Kweon YO, Kim SK, Choi YH. Gastrointestinal stromal tumors of the stomach: endosonographic differentiation in relation to histological risk. J Gastroenterol Hepatol. 2007;22:2069-2075. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 36] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 27. | Wang M, Xue A, Yuan W, Gao X, Fu M, Fang Y, Wang L, Shu P, Li H, Hou Y, Shen K, Sun Y, Qin X. Clinicopathological Features and Prognosis of Small Gastric Gastrointestinal Stromal Tumors (GISTs). J Gastrointest Surg. 2019;23:2136-2143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 11] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 28. | Tsuji Y, Kusano C, Gotoda T, Itokawa F, Fukuzawa M, Sofuni A, Matsubayashi J, Nagao T, Itoi T, Moriyasu F. Diagnostic potential of endoscopic ultrasonography-elastography for gastric submucosal tumors: A pilot study. Dig Endosc. 2016;28:173-178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 32] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 29. | Pesenti C, Bories E, Caillol F, Ratone JP, Godat S, Monges G, Poizat F, Raoul JL, Ries P, Giovannini M. Characterization of subepithelial lesions of the stomach and esophagus by contrast-enhanced EUS: A retrospective study. Endosc Ultrasound. 2019;8:43-49. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 40] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 30. | Kuwatani M, Sakamoto N. Evolution and a promising role of EUS-FNA in gene and future analyses. Endosc Ultrasound. 2020;9:151-153. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 31. | Okubo K, Yamao K, Nakamura T, Tajika M, Sawaki A, Hara K, Kawai H, Yamamura Y, Mochizuki Y, Koshikawa T, Inada K. Endoscopic ultrasound-guided fine-needle aspiration biopsy for the diagnosis of gastrointestinal stromal tumors in the stomach. J Gastroenterol. 2004;39:747-753. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 74] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 32. | Attila T, Aydın Ö. Lesion size determines diagnostic yield of EUS-FNA with onsite cytopathologic evaluation for upper gastrointestinal subepithelial lesions. Turk J Gastroenterol. 2018;29:436-441. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 22] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 33. | Akahoshi K, Sumida Y, Matsui N, Oya M, Akinaga R, Kubokawa M, Motomura Y, Honda K, Watanabe M, Nagaie T. Preoperative diagnosis of gastrointestinal stromal tumor by endoscopic ultrasound-guided fine needle aspiration. World J Gastroenterol. 2007;13:2077-2082. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 159] [Cited by in RCA: 184] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 34. | Hunt GC, Smith PP, Faigel DO. Yield of tissue sampling for submucosal lesions evaluated by EUS. Gastrointest Endosc. 2003;57:68-72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 81] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 35. | Sepe PS, Moparty B, Pitman MB, Saltzman JR, Brugge WR. EUS-guided FNA for the diagnosis of GI stromal cell tumors: sensitivity and cytologic yield. Gastrointest Endosc. 2009;70:254-261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 136] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 36. | Levy MJ, Jondal ML, Clain J, Wiersema MJ. Preliminary experience with an EUS-guided trucut biopsy needle compared with EUS-guided FNA. Gastrointest Endosc. 2003;57:101-106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 178] [Cited by in RCA: 171] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 37. | Bai J, Wang Y, Guo H, Zhang P, Ling X, Zhao X. Endoscopic resection of small gastrointestinal stromal tumors. Dig Dis Sci. 2010;55:1950-1954. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 28] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 38. | Yu C, Liao G, Fan C, Yu J, Nie X, Yang S, Bai J. Long-term outcomes of endoscopic resection of gastric GISTs. Surg Endosc. 2017;31:4799-4804. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 28] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 39. | Li B, Chen T, Qi ZP, Yao LQ, Xu MD, Shi Q, Cai SL, Sun D, Zhou PH, Zhong YS. Efficacy and safety of endoscopic resection for small submucosal tumors originating from the muscularis propria layer in the gastric fundus. Surg Endosc. 2019;33:2553-2561. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 30] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 40. | An W, Sun PB, Gao J, Jiang F, Liu F, Chen J, Wang D, Li ZS, Shi XG. Endoscopic submucosal dissection for gastric gastrointestinal stromal tumors: a retrospective cohort study. Surg Endosc. 2017;31:4522-4531. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 56] [Article Influence: 7.0] [Reference Citation Analysis (0)] |