Published online Oct 7, 2022. doi: 10.3748/wjg.v28.i37.5395
Peer-review started: March 4, 2022
First decision: March 27, 2022
Revised: April 11, 2022
Accepted: August 16, 2022
Article in press: August 16, 2022
Published online: October 7, 2022
Processing time: 208 Days and 13 Hours
The discovery of hepatitis C has been a landmark in public health as it brought the opportunity to save millions of lives through the diagnosis, prevention and cure of the disease. The combined work of three researchers, Alter H, Houghton M and Rice C, which set the basis for the diagnosis, treatment and prevention of hepatitis C apart from laying the ground work for a new approach to study infections in general and developing new antiviral agents. This is a story of a transfusion-associated infection. A series of clinical studies demonstrated the existence of an infectious agent associated with hepatitis. That was followed by the identification of what was later known to be the hepatitis C virus (HCV) and the development of diagnostic tests. It all preceded the full molecular identification and demonstration of a causal effect. Finally it ended up with the development and discovery of a new class of therapeutic drugs, the direct acting antivirals, which are now used not only to cure the disease but most probably, to eliminate the problem. This work started with Dr Alter H who demonstrated that a new virus was responsible for the majority of post-transfusion hepatitis followed by Houghton M who cloned the virus and developed the blood test to identify those cases that carried the virus. Finally, the work of Rice C demonstrated that a cloned HCV produced after applying molecular biology techniques could cause long-standing infection and cause the same disease as the one observed in humans.
Core Tip: The discovery of hepatitis C has been a landmark in public health as it brought the opportunity to save millions of lives through the diagnosis, prevention and cure of a disease that was perhaps noticed 5000 years ago. It was through the combined work of three researchers, Alter H, Houghton M and Rice C, which set the basis for the diagnosis, treatment and prevention of hepatitis C apart from laying the ground work for a new approach to study infections in general and developing new antiviral agents.
- Citation: Campollo O, Amaya G, McCormick PA. Milestones in the discovery of hepatitis C. World J Gastroenterol 2022; 28(37): 5395-5402
- URL: https://www.wjgnet.com/1007-9327/full/v28/i37/5395.htm
- DOI: https://dx.doi.org/10.3748/wjg.v28.i37.5395
“The methodological studies of transfusion-associated hepatitis by Harvey J. Alter demonstrated that an unknown virus was a common cause of chronic hepatitis. Michael Houghton used an untested strategy to isolate the genome of the new virus that was named Hepatitis C virus. Charles M. Rice provided the final evidence showing that Hepatitis C virus alone could cause hepatitis.” Nobel Prize assembly, 2020[1].
The prolonged campaign to discover and treat the various causes of viral hepatitis is a major medical success story as it brought about the opportunity to save thousands of lives through the diagnosis, prevention and cure of the disease, sparing lives that would be lost to chronic hepatitis C and its complications. It was through the combined work of three researchers starting with Dr Alter H working at National Institutes of Health (NIH) who demonstrated that a new virus was responsible for the majority of post-transfusion hepatitis. That was followed by Houghton M who cloned the virus and developed the blood test to identify those cases that carried the virus. And finally, Rice C demonstrated that a cloned hepatitis C virus (HCV), produced after applying molecular biology techniques (a virion), could cause long-standing infection and cause the same disease as the one observed in humans[2]. These scientific breakthroughs enabled the World Health Organization (WHO) to set the, once unthinkable, goal for HCV elimination by 2030[3]. The implications of this work are not limited to hepatitis C as the new diagnostic techniques and methods of drug development may be applicable to other viral pathogens. Furthermore, the complete new approach to treatment based on the study and new knowledge of the HCV genetics and lifecycle together with the molecular biology approach attacking many targets directly (in the HCV lifecycle) i.e., protease inhibitors, polymerase inhibitors and NS5A inhibitors[4-6], the former being the most successful prodrug developed and currently used in the WHO policy for world elimination of hepatitis C aimed at either[6,7], virion processing, RNA replication and virion assembly in the liver cell[6,8].
For those of us who grew up in the hepatology field when non-A non-B hepatitis (what a strange name!) was a common topic of discussion in the clinic and the laboratory, the award of the Nobel prize for the discovery of HCV in 2020 marked a fitting end to a long saga. This is the second Nobel prize awarded to investigators in viral hepatitis recognizing the major advances in this field of clinical and laboratory research [the first Nobel prize was awarded to Blumberg B in 1976 for the discovery of hepatitis B virus (HBV)][9]. Initially viral hepatitis was regarded as an epidemic disease which was a major problem in congregated settings such as residential schools and military establishments. It became a major issue in time of war when epidemics reduced the effectiveness of armies in the field. Much important early research was funded by the military. Only later did it become apparent that chronic viral hepatitis could lead to cirrhosis and hepatocellular carcinoma (HCC). Many millions of people were infected making it one of the leading causes of morbidity and mortality in some parts of the world. In the early 2000’s it was estimated that chronic viral hepatitis was responsible for over one million deaths annually[10]. The development of vaccines for hepatitis B and effective anti-viral therapy for hepatitis B and C have been dramatically effective and offer the prospect of banishing these diseases to the sidelines of human history.
The hepatitis problem was perhaps noticed 5000 years ago when epidemics of jaundice were attributed to a devil called “Ahhazu” by the Sumerians[11] and many years later were described by Hippocrates in the 5th century BC in his book “Epidemics”. They were particular noted in armies during time of war, hence the term “campaign jaundice”. The cause of jaundice was not understood and up till the out-break of the second world war many clinicians accepted Virchow’s theory that a mucous plug at the mouth of the common bile duct caused “catarrhal jaundice”[12]. The large number of hepatitis cases and the impact it had on battle readiness acted as a major stimulus to understanding hepatitis. Most of the subsequent advances in knowledge were described in the British and American literature. Up until D-day in 1944 the British army was mainly engaged in fighting in the Mediterranean theatre. Hepatitis, with malaria and venereal disease were the three most important medical conditions afflicting the troops there[13]. On the other side of the Atlantic ocean, 28585 United States servicemen developed jaundice after receiving the yellow fever vaccine[14]. Clinical, epidemiological and transmission studies established that there were two distinct forms of hepatitis, characterized as infectious or serum hepatitis. Infectious hepatitis was transmitted by the fecal-oral route whereas serum hepatitis was transmitted by injection or blood products[15].
Stokes and Neff of the United States Army Medical Corps had been using concentrated gamma globulin to prevent or attenuate measles infection. Despite several thousand treatments they recorded no cases of hepatitis[16]. They reasoned that there may be neutralizing antibodies in gamma globulin prepared from large pools of adult plasma and suggested that gamma globulin could be useful in the treatment of viral hepatitis. The hypothesis was tested when there was a large epidemic of hepatitis in a summer camp for boys and girls in September 1944. There was sufficient gamma globulin to treat 45 children, who were compared to 246 controls. Hepatitis was subsequently documented in 16% (7/45) treated compared to (70%) 172/246 controls. None of those treated developed clinical jaundice thus demonstrating that gamma globulin could prevent or attenuate infectious hepatitis[17]. Many of the subsequent transmission studies on viral hepatitis of questionable procedures were performed in the Willowbrook State School in New York for children with mental disorders which had a major problem with endemic infectious hepatitis[15]. The investigators clearly demonstrated two types of hepatitis, with different incubation periods and no cross-immunity, subsequently proven to be hepatitis A and hepatitis B. The ethics of that program have since been questioned[18].
The problem of hepatitis continued up until modern times when many cases of un-explained jaundice continued to puzzle clinicians until some 51 years ago when Holland, Schmidt, Purcell, Walsh and Alter began to study what was called “Transfusion-associated hepatitis” in 1969[19]. It would take some 20 years more until the infectious agent, HCV, was discovered[20]. Those of us who grew up in the hepatology field when non-A non-B hepatitis was a common topic for discussion in clinical meetings and on the wards remember the cases of unexplained post-transfusion hepatitis. In addition there were many cases of “nosocomial” hepatitis[21] and cryptogenic hepatitis and a lack of diagnostic tests. The journey to unravel this problem took another 39 years, 14 years from the identification of non-A non-B hepatitis to the discovery of the HCV and 25 years from the discovery of HCV in 1989 to the approval of one of the most prescribed direct acting antiviral agents (DAA) Sofosbuvir in 2013 for the treatment of hepatitis C[22]. In all, those 39 years don’t seem too bad now when we look at the progress made from those early clinical and epidemiologic studies by Alter and others up to the incredibly creative and imaginative pharmacological approach to therapy involving the design of DAA that inhibit HCV infection by blocking viral assembly and replication at the present time[22].
Currently, it is estimated, that there are more than 71 million people around the world infected with HCV. Complications of end-stage liver disease due to hepatitis C make it one of the world’s most important causes of death with 400000 cases a year[23]. It is well-known that HCV can cause chronic hepatitis C, a silent but progressive condition which may progress to cirrhosis and HCC over decades. Hepatitis C is transmitted by the parenteral route. As blood products for transfusion have become increasingly safe due to effective viral testing, the main route of transmission in most countries is through intravenous drug use. Eradication of hepatitis C requires effective harm reduction strategies for intravenous drug use in addition to antiviral therapy[7,20].
Interest in this problem (now known as hepatitis C) was renewed almost 70 years ago when there was a high incidence of chronic hepatitis after blood transfusions or use of blood products. At that time it was impossible to know who of the donors carried the disease. In 1960, Alter[24] started his search for the “source” of post-transfusion hepatitis having previously worked with Blumberg B with whom he had observed a “precipitin line … that stained intensely red” in a reaction between blood from a patient with hemophilia and blood from an Australian aborigine which they called initially the “Australia antigen”. That was later identified as the surface protein of the HBV (HBsAg). Blumberg continued his research on the Australia antigen to establish the link with HBV for which he won the Nobel prize in Medicine in 1976[11]. In his studies, Alter[19] found that even if hepatitis B contaminated blood was excluded from use most post-transfusional hepatitis remained. He tested the blood supplies for the presence of suspected known viruses and followed up patients who developed hepatitis after receiving a blood transfusion and found that an incredible high number of cases could not be explained. Alter[19] and other researchers suspected there was another infectious agent. In 1978, Alter[24] demonstrated that plasma from those patients with post-transfusion hepatitis could infect chimpanzees who developed clinical and laboratory signs of hepatitis suggesting that the cause of that liver inflammation was infectious. Further studies by Alter[24] showed that the causative agent had characteristics of a virus. The next goal was the search for the virus which took lots of effort and time i.e., years! Nevertheless Alter[19] had learnt from Blumberg to work with tenacity and perseverance to carry on with his work. Robert Purcell and Steve Feinstone at the NIH[19] and Alfred Prince in New York noted that most cases of post-transfusion hepatitis were HBsAg negative and hepatitis A virus negative[11]. At that time they started calling it non-A non-B hepatitis (NANBH)[11]. Another important step was the observation that infectivity titers in chimps studied by Purcell were almost identical to the genomic titers in a patient with severe acute NANBH studied by Alter[19]. Alter[19] worked with the assumption that genetic material would be present in pools of DNA sequences isolated from those animals infected with hepatitis, and on the other hand that serum from humans with this form of hepatitis would have specific antibodies against that virus that would bind to some proteins or viral particles and could then be used to identify those samples with the virus. Together with Purcell he attempted every serological approach known at the time to identify the virus without success.
In 1989, Houghton[25] at Chiron Laboratories in California (now part of Novartis) tried a combination of molecular biology and immunology methods. They extracted nucleic acid from plasma and cloned it in an expression vector (GT11) creating a phage expression library. This technique enabled them to identify the first epitope characteristic of the HCV envelope in 1989[11]. They later named it HCV. Houghton rapidly came up with the idea of developing immunoassays to detect antibodies to protein products of those clones establishing a blood test for HCV which he evaluated using Alter’s blood samples’ collection at NIH[26]. They correctly identified all samples which were thought to be infected with the virus as well as all negative controls. This was a major milestone for medicine and public health. It allowed blood banks to screen all blood supplies resulting in an immediate and dramatic drop in the incidence of post-transfusion hepatitis.
At this point there was another crucial question, that is whether this virus could reproduce infection if inoculated into an experimental model, hence probing that the now called HCV was the causative agent of the formerly known NANBH. Rice C a researcher at the University of Washington who had been working with molecular virology of Flavivirus[22] focused on dissecting HCV gene expression using blood from infected chimpanzees to introduce DNA fragments into bacteria to express individual protein fragments. Those products were then screened with the antiviral antibodies until they could isolate one positive clone. The positive clone encoded a sequence that was very similar to sequences of the virus family of flaviviruses. Next, Rice started investigating what was needed for the molecularly cloned HCV to be reproduced in vitro. In 1996, he and his group identified the conserved 3’-terminal region of the HCV genomic RNA which was previously unknown[27], and was crucial for recovery of infectious HCV cDNA clones. However, initially they could not produce infection when injected into the liver of animals speculating that there could be some inactivating random mutations in the genome produced during the replication of the virus[28]. That meant that some individual clones may be defective. He sequenced many clones and compared them with each other and found that some of these clones contained potentially inactivating mutations which he thought could be removed with genetic engineering[29]. He later combined that repaired viral genome with the 3’-end of the genomic RNA hoping he would obtain a functional virus. When he injected this genome into the liver of chimpanzees clinical signs of hepatitis ensued and there was virus present in the blood producing now the evidence that the clone of the HCV could produce the disease associated with hepatitis infection[22,30]. That was a very important advance because the development of HCV replicons provided a live HCV system in the laboratory where viral replication, pathogenesis and evolution in culture could be studied in a viable in vitro replication system[22]. Later on newer constructs were obtained with higher replicative ability in vitro[22]. This was another milestone. Within 1 year of the cloning of HCV the nucleotide sequence of the entire viral genome was determined and the agent was characterized as a single-stranded positive-sense RNA virus of about 9600 nucleotides in length[2,31]. The advent of functional replicons also enabled the assay development for antiviral drug development which made the search for effective anti-viral drugs much easier[22]. Another collaborator of Rice, Ralf Bartenschlager, a molecular biologist who had previously worked with HBV, successfully replicated HCV genomic RNA in a human hepatoma cell line Huh7[32]. Confirmatory reports from various groups worldwide all corroborated that replicons were robust in vitro replication (subgenomic HCV replication system) systems that set the basis for production of infectious virus particles in cell cultures[22,32]. That improved the HCV RNA replicon system model was used in collaboration with Michael Sofia to design the new DAA[22]. One of those developed, DAA PSI-7797 (Sofosbuvir), had a very effective antiviral effect with broader genotype coverage and fewer side effects on a shorter duration treatment[11,22]. This prodrug enters the hepatocyte readily where it is metabolized to produce a triphosphate derivative which is a potent viral replication inhibitor[20,22]. It has just recently been announced that it can cure up to 95% of patients infected with HCV[33]. Different prodrugs were tested in genotype-specific cell lines that have been used in preclinical studies to select and validate novel targets for HCV. Those included NS3-4A protease inhibitors, nucleoside analogue viral polymerase inhibitors, non-nucleoside inhibitors of the viral RNA polymerase, NS5A inhibitors, host targeted agents (HTA), cyclophilin inhibitors and a cellular miRNA antagonist[4]. The discovery of over 30 new DAAs and HTAs revolutionized the treatment of chronic HCV[8]. The swift development of interferon-free protocols using DAA monotherapy or the combined administration of two or three DAAs or HTAs, administered for 8-12 and up to 24 wk, led to sustained virological response (SVR) rates between 90% and 100%[8,33,34].
With the widespread use of DAAs many countries are now reporting a reduction in mortality associated with chronic hepatitis C and it’s complications such as liver cirrhosis and HCC[3]. A strategy of “treatment as prevention” has been proposed with the aim of reducing the population prevalence, interrupting the chain of viral transmission and ultimately leading to elimination of HCV infection[11,20], although patients with HCV infection with cirrhosis or decompensated liver disease present special challenges such as post-SVR complications including HCV reinfection, HCC risk, residual HCC which should be addressed by early detection and treatment, combination and multiple DAA therapy avoiding the use of protease inhibitors and risk reduction counseling[7,34]. As epidemiologists, infectious diseases and liver specialists analyze the natural history, epidemiology and public health figures, world experts have proposed a rationale towards hepatitis C elimination based on infection control and disease elimination and eradication[7,20].
Viral hepatitis is a global health burden affecting 325 million people globally of which 71 million have hepatitis C with 1.5 million infections occurring per year and 542316 global HCV related deaths[3,20]. In 2016, the WHO set the Global health sector strategy for viral hepatitis proposing to eliminate viral hepatitis as a public health problem by 2030[3]. That is a 90% reduction in incidence and a 65% reduction in mortality by 2030 and a new guidance was released in June 2021[35]. These targets are achievable with the tools now at our disposal as demonstrated for hepatitis B in Taiwan[36] and hepatitis C in Egypt[37]. The main global strategies are to increase HCV testing, improve clinical education of providers, utilize simple models for HCV care and provide universal access to antiviral treatment at affordable cost. While there have been technical, geographic and policy limitations such as limited funding, lack of transparency and high in-country process, fragmented procurement, HCV diagnostics inefficiencies[3] and most recently the severe acute respiratory syndrome coronavirus 2 pandemic to mention a few there have been many examples around the globe showing that the goals are feasible[35]. In Egypt the cost of hepatitis C antiviral therapy fell from $1650 to $85 United States dollars between 2015 and 2018. A nationwide screening and treatment program identified 1.15 million infected individuals. By September 2019 over 1.05 million had commenced treatment with sustained virological clearance rates of 98.8%[37]. In spite of those challenges mentioned above, the future of mankind looks promising as millions of people will have the chance of a life free of hepatitis virus.
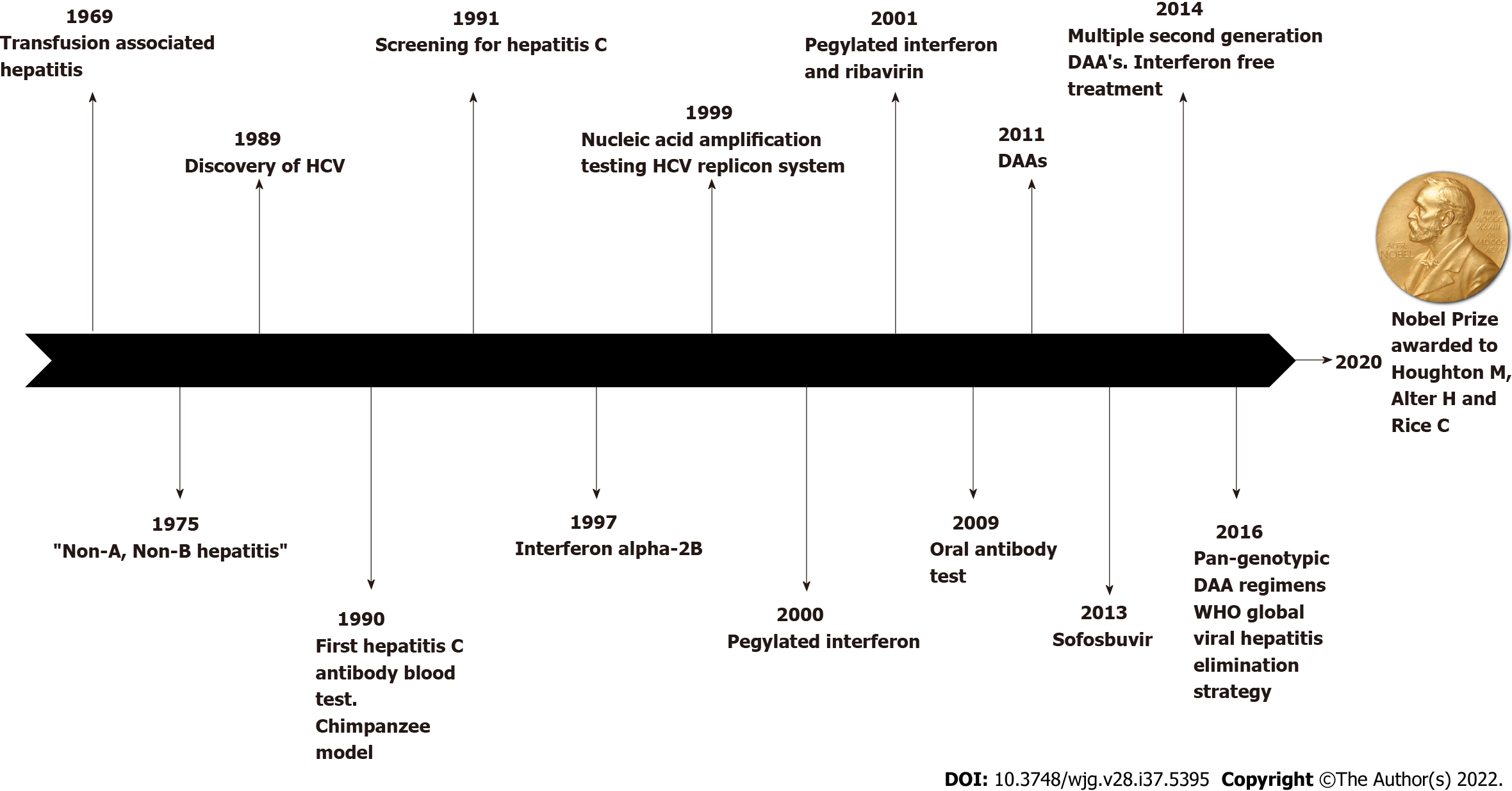
We have summarized the contributions of several groups of medical researchers starting with Dr Alter H who demonstrated that a new virus was responsible for the majority of post-transfusion hepatitis followed by Houghton M who cloned the virus and developed the blood test to identify those cases that carried the virus. That was continued with the work of Rice C that demonstrated that a cloned HCV produced after applying molecular biology techniques could produce long-standing infection and cause the same disease in animals as the one observed in humans[2] (Figure 1). That is a milestone not only in the diagnosis, treatment and prevention of hepatitis C, but in the approach to study infections in general apart from contributing to the understanding of the role genetic and environmental factors play in the development of this infection. In all, that set the basis for the production of new antivirals which are central for hepatitis C control and elimination. Of note, the cloning of HCV in 1989 was a remarkable accomplishment that has not only saved a large number of human lives but also demonstrated the power of molecular biology in unearthing new infectious agents. The discovery of HCV, the first virus ever discovered by molecular cloning technology, with its accompanying development of new methodologies such as new generation sequencing and new generation diagnostic automated systems[38], is also a landmark in public health. The discovery of HCV provides the opportunity to save thousands of lives through prevention and now, cure of the disease, which would otherwise be lost to chronic hepatitis C and its complications.
We thank Octavio Guadalupe Campollo for the art work.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Mexico
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): B, B, B
Grade C (Good): 0
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Ballestín SS, Spain; Lo SY, Taiwan; Villela-Nogueira CA, Brazil; Yu ML, Taiwan S-Editor: Wang JJ L-Editor: Webster JR P-Editor: Wang JJ
| 1. | Masaccio MG, Hedestam GK. The discovery of Hepatitis C virus. The Nobel assembly at Karolinska Institutet. [cited 12 February 2022]. Available from: https://www.nobelprize.org/prizes/medicine/2020/advanced-information/. |
| 2. | Farci P. Choo QL, Kuo G, Weiner AJ, Overby LR, Bradley DW, Houghton M. Isolation of a cDNA clone derived from a blood-borne non-A, non-B viral hepatitis genome [Science 1989; 244: 359-362]. J Hepatol. 2002;36:582-585. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 3. | World Health Organization. Global health sector strategy on viral hepatitis 2016-2021. Towards ending viral hepatitis. [cited 15 February 2022]. Available from: https:// www.who.int/publications/i/item/WHO-HIV-2016.06. |
| 4. | Johnson KA, Dangerfield T. Mechanisms of inhibition of viral RNA replication by nucleotide analogs. Enzymes. 2021;49:39-62. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 16] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 5. | European Association for the Study of the Liver. Clinical Practice Guidelines Panel: Chair:; EASL Governing Board representative:; Panel members:. EASL recommendations on treatment of hepatitis C: Final update of the series☆. J Hepatol. 2020;73:1170-1218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 567] [Cited by in RCA: 779] [Article Influence: 155.8] [Reference Citation Analysis (0)] |
| 6. | Manns MP, Buti M, Gane E, Pawlotsky JM, Razavi H, Terrault N, Younossi Z. Hepatitis C virus infection. Nat Rev Dis Primers. 2017;3:17006. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 280] [Cited by in RCA: 342] [Article Influence: 42.8] [Reference Citation Analysis (1)] |
| 7. | Ghany MG, Morgan TR; AASLD-IDSA Hepatitis C Guidance Panel. Hepatitis C Guidance 2019 Update: American Association for the Study of Liver Diseases-Infectious Diseases Society of America Recommendations for Testing, Managing, and Treating Hepatitis C Virus Infection. Hepatology. 2020;71:686-721. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 348] [Cited by in RCA: 529] [Article Influence: 105.8] [Reference Citation Analysis (0)] |
| 8. | Webster DP, Klenerman P, Dusheiko GM. Hepatitis C. Lancet. 2015;385:1124-1135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 383] [Cited by in RCA: 361] [Article Influence: 36.1] [Reference Citation Analysis (0)] |
| 9. | Gerlich WH. Medical virology of hepatitis B: how it began and where we are now. Virol J. 2013;10:239. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 183] [Cited by in RCA: 221] [Article Influence: 18.4] [Reference Citation Analysis (0)] |
| 10. | Stanaway JD, Flaxman AD, Naghavi M, Fitzmaurice C, Vos T, Abubakar I, Abu-Raddad LJ, Assadi R, Bhala N, Cowie B, Forouzanfour MH, Groeger J, Hanafiah KM, Jacobsen KH, James SL, MacLachlan J, Malekzadeh R, Martin NK, Mokdad AA, Mokdad AH, Murray CJL, Plass D, Rana S, Rein DB, Richardus JH, Sanabria J, Saylan M, Shahraz S, So S, Vlassov VV, Weiderpass E, Wiersma ST, Younis M, Yu C, El Sayed Zaki M, Cooke GS. The global burden of viral hepatitis from 1990 to 2013: findings from the Global Burden of Disease Study 2013. Lancet. 2016;388:1081-1088. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1042] [Cited by in RCA: 989] [Article Influence: 109.9] [Reference Citation Analysis (0)] |
| 11. | Trepo C. A brief history of hepatitis milestones. Liver Int. 2014;34 Suppl 1:29-37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 50] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 12. | MacCallum FO. 1971 International Symposium on Viral Hepatitis. Historical perspectives. Can Med Assoc J. 1972;106 Suppl:423-Suppl:426. [PubMed] |
| 13. | Witts LJ. Some Problems of Infective Hepatitis. Br Med J. 1944;1:739-743. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 14. | Reuben A. The thin red line. Hepatology. 2002;36:770-773. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 15. | Krugman S, Giles JP, Hammond J. Infectious hepatitis. Evidence for two distinctive clinical, epidemiological, and immunological types of infection. JAMA. 1967;200:365-373. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 304] [Cited by in RCA: 296] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 16. | Stokes J, Maris EP, Gellis SS. Chemical, clinical, and immunological studies on the products of human plasma fractionation. xi. the use of concentrated normal human serum gamma globulin (human immune serum globulin) in the prophylaxis and treatment of measles. J Clin Invest. 1944;23:531-540. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 103] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 17. | Stokes JJ, Neefe JR. Prevention and attenuation of infectious hepatitis by gamma globulin: preliminary note. JAMA. 1945;127:144-145. [RCA] [DOI] [Full Text] [Cited by in Crossref: 196] [Cited by in RCA: 194] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 18. | Rothman DJ. Were Tuskegee & Willowbrook 'studies in nature'? Hastings Cent Rep. 1982;12:5-7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 64] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 19. | Alter HJ. The road not taken or how I learned to love the liver: a personal perspective on hepatitis history. Hepatology. 2014;59:4-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 12] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 20. | Ward JW. Hepatitis C virus: the 25-year journey from discovery to cure. Hepatology. 2014;60:1479-1482. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 21. | Campollo O, Valencia-Salinas JJ, Berumen-Arellano A, Pérez-Aranda MA, Panduro-Cerda A, Segura-Ortega J. [Epidemiological characteristics of liver cirrhosis at the Hospital Civil of Guadalajara]. Salud Publica Mex. 1997;39:195-200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 11] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 22. | Zhao Q, Xia N. The 2016 Lasker-DeBakey Clinical Medical Research Award: Innovative hepatitis C virus (HCV) replicons leading to drug development for hepatitis C cure. Sci China Life Sci. 2016;59:1198-1201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 23. | EASL. EASL Policy Statement on Hepatitis C Elimination. [cited 15 February 2022]. Available from: https://easl.eu/wp-content/uploads/2019/04/EASL-Policy-Statement-on-Hepatitis-C-Elimination.pdf. |
| 24. | Alter HJ, Purcell RH, Holland PV, Popper H. Transmissible agent in non-A, non-B hepatitis. Lancet. 1978;1:459-463. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 267] [Cited by in RCA: 246] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 25. | Houghton M. The long and winding road leading to the identification of the hepatitis C virus. J Hepatol. 2009;51:939-948. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 75] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 26. | Kuo G, Choo QL, Alter HJ, Gitnick GL, Redeker AG, Purcell RH, Miyamura T, Dienstag JL, Alter MJ, Stevens CE. An assay for circulating antibodies to a major etiologic virus of human non-A, non-B hepatitis. Science. 1989;244:362-364. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2495] [Cited by in RCA: 2343] [Article Influence: 65.1] [Reference Citation Analysis (0)] |
| 27. | Kolykhalov AA, Feinstone SM, Rice CM. Identification of a highly conserved sequence element at the 3' terminus of hepatitis C virus genome RNA. J Virol. 1996;70:3363-3371. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 342] [Cited by in RCA: 323] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 28. | Harak C, Meyrath M, Romero-Brey I, Schenk C, Gondeau C, Schult P, Esser-Nobis K, Saeed M, Neddermann P, Schnitzler P, Gotthardt D, Perez-Del-Pulgar S, Neumann-Haefelin C, Thimme R, Meuleman P, Vondran FW, De Francesco R, Rice CM, Bartenschlager R, Lohmann V. Tuning a cellular lipid kinase activity adapts hepatitis C virus to replication in cell culture. Nat Microbiol. 2016;2:16247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 49] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 29. | Saeed M, Andreo U, Chung HY, Espiritu C, Branch AD, Silva JM, Rice CM. SEC14L2 enables pan-genotype HCV replication in cell culture. Nature. 2015;524:471-475. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 100] [Cited by in RCA: 100] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 30. | Kolykhalov AA, Agapov EV, Blight KJ, Mihalik K, Feinstone SM, Rice CM. Transmission of hepatitis C by intrahepatic inoculation with transcribed RNA. Science. 1997;277:570-574. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 565] [Cited by in RCA: 549] [Article Influence: 19.6] [Reference Citation Analysis (0)] |
| 31. | Choo QL, Kuo G, Weiner AJ, Overby LR, Bradley DW, Houghton M. Isolation of a cDNA clone derived from a blood-borne non-A, non-B viral hepatitis genome. Science. 1989;244:359-362. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4996] [Cited by in RCA: 4657] [Article Influence: 129.4] [Reference Citation Analysis (0)] |
| 32. | Lohmann V, Körner F, Koch J, Herian U, Theilmann L, Bartenschlager R. Replication of subgenomic hepatitis C virus RNAs in a hepatoma cell line. Science. 1999;285:110-113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2294] [Cited by in RCA: 2251] [Article Influence: 86.6] [Reference Citation Analysis (0)] |
| 33. | Shouval D, Friedman SL. Focusing on the past, present, and future of hepatology. J Hepatol. 2014;61:1196-1198. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 34. | Huang CF, Yu ML. Unmet needs of chronic hepatitis C in the era of direct-acting antiviral therapy. Clin Mol Hepatol. 2020;26:251-260. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 46] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 35. | World Health Organization. Interim guidance for country validation of viral hepatitis elimination. [cited 15 February 2022]. Available from: https://www.who.int/publications/i/item/9789240028395. |
| 36. | Chang MH, You SL, Chen CJ, Liu CJ, Lai MW, Wu TC, Wu SF, Lee CM, Yang SS, Chu HC, Wang TE, Chen BW, Chuang WL, Soon MS, Lin CY, Chiou ST, Kuo HS, Chen DS; Taiwan Hepatoma Study Group. Long-term Effects of Hepatitis B Immunization of Infants in Preventing Liver Cancer. Gastroenterology. 2016;151:472-480.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 189] [Cited by in RCA: 170] [Article Influence: 18.9] [Reference Citation Analysis (0)] |
| 37. | Waked I, Esmat G, Elsharkawy A, El-Serafy M, Abdel-Razek W, Ghalab R, Elshishiney G, Salah A, Abdel Megid S, Kabil K, El-Sayed MH, Dabbous H, El Shazly Y, Abo Sliman M, Abou Hashem K, Abdel Gawad S, El Nahas N, El Sobky A, El Sonbaty S, El Tabakh H, Emad E, Gemeah H, Hashem A, Hassany M, Hefnawy N, Hemida AN, Khadary A, Labib K, Mahmoud F, Mamoun S, Marei T, Mekky S, Meshref A, Othman A, Ragab O, Ramadan E, Rehan A, Saad T, Saeed R, Sharshar M, Shawky H, Shawky M, Shehata W, Soror H, Taha M, Talha M, Tealaab A, Zein M, Hashish A, Cordie A, Omar Y, Kamal E, Ammar I, AbdAlla M, El Akel W, Doss W, Zaid H. Screening and Treatment Program to Eliminate Hepatitis C in Egypt. N Engl J Med. 2020;382:1166-1174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 185] [Cited by in RCA: 180] [Article Influence: 36.0] [Reference Citation Analysis (0)] |
| 38. | Panduro A. Viruses and the Liver 2020: Before COVID-19 and the beginning of a new age in medicine. Ann Hepatol. 2021;20:100293. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |