Published online Aug 28, 2022. doi: 10.3748/wjg.v28.i32.4527
Peer-review started: January 19, 2022
First decision: May 29, 2022
Revised: June 28, 2022
Accepted: July 27, 2022
Article in press: July 27, 2022
Published online: August 28, 2022
Processing time: 218 Days and 11 Hours
The microbiota impact on human diseases is well-known, and a growing body of literature is providing evidence about the complex interplay between microbiota-immune system-human physiology/pathology, including cancers. Together with the defined risk factors (e.g., smoke habits, diet, diabetes, and obesity), the oral, gut, biliary, and intrapancreatic microbiota contribute to pancreatic cancer development through different pathways including the interaction with the immune system. Unfortunately, a great majority of the pancreatic cancer patients received a diagnosis in advanced stages not amenable to be radically treated and potentially cured. Given the poor pancreatic cancer prognosis, complete knowledge of these complicated relationships could help researchers better understand the disease pathogenesis and thus provide early potential non-invasive biomarkers, new therapeutic targets, and tools for risk stratification that might result in greater therapeutic possibilities and eventually in a better and longer patient survival.
Core Tip: Despite improvements in traditional patient treatment, pancreatic cancer remains a tumor with an increasing incidence and a poor prognosis, often diagnosed in late stages. The oral, gut, biliary, and intrapancreatic microbiota might contribute to pancreatic cancer through different pathways including a complex interplay with the immune system. Comprehending these complicated relationships could help researchers better understand the pathogenesis of pancreatic cancer, thus providing new promising options for early diagnosis, therapeutic targets, and risk stratification hoping that could translate into better and longer patient survival.
- Citation: Bartolini I, Nannini G, Risaliti M, Matarazzo F, Moraldi L, Ringressi MN, Taddei A, Amedei A. Impact of microbiota-immunity axis in pancreatic cancer management. World J Gastroenterol 2022; 28(32): 4527-4539
- URL: https://www.wjgnet.com/1007-9327/full/v28/i32/4527.htm
- DOI: https://dx.doi.org/10.3748/wjg.v28.i32.4527
Pancreatic cancer (PanCa), with its most common subtype ductal adenocarcinoma, is a relatively uncommon but highly lethal neoplasm. Despite years of research, the PanCa patients are often diagnosed in advanced stages having less than a 30% chance of being alive 1 year after diagnosis and less than a 10% 5-year survival rate[1-3].
The human body is colonized by a huge number of microorganisms, which include bacteria, archaea, viruses, and fungi. This articulate community with the environment and the microorganism metabolites is known as microbiota. Usually, the commensal community lives in equilibrium with the host in a condition defined as “eubiosis;” however, if for some reason the equilibrium is broken, a condition called dysbiosis occurs[4]. A growing body of the literature reported the complex interplay of the microbiota with the immune system and the intestinal barrier in a bidirectional influence that plays a key role in health and disease, including cancer[5-7].
Evidence about the potential causal role of microbiota on PanCa development is still scarce, but it is gaining great attention over the last few years[8,9]. The oral, gut, biliary, and intrapancreatic microbiota seem to contribute to PanCa initiation and development. Furthermore, microbiota both act through a direct effect or indirectly through the interaction with the immune system or through the production of circulating toxins. Understanding these complicated relationships could help researchers better define the causes of different pathologies, including PanCa, and thus provide new therapeutic targets and tools for risk stratification[10].
Finally, given the diagnosis frequently made in advanced stages and the poor prognosis of this tumor together with the lack of markers able to detect an early PanCa, the chance to use the gut microbiota as an early non-invasive biomarker might result in greater therapeutic possibilities and, eventually, in a better and longer patient survival[11].
This review aims to provide the current state of the microbiota immunity axis impact in every step of PanCa, trying to highlight potential promising new therapeutic options.
Although often no identifiable risk factors can be found, genetic factors, lifestyle, and chronic pancreatitis are well-recognized[12]. It is notable that most of them cause dysbiosis too[1,12].
Because an estimated 70% of the bacteria in the human body have not been grown yet, studying the human microbiota is a difficult endeavor[13]. A breakthrough in DNA sequencing occurred in the mid-2000s thanks to next-generation sequencing[14]. Through gene sequencing, metagenomics, metatranscriptomics, metaproteomics, and metabolomics (the recently introduced “meta-omics” techniques) analysis of strain composition, production of metabolites, and other bacterial activities can be performed[15,16]. Consequently, the significant microbiota impact on human physiology and pathology has been increasingly acknowledged over the years. Although the evidence is still in its infancy, being a small part of more complex mechanisms, the potential role of the microbiome itself and microbiome-immune system interplay on PanCa onset and development has been recently advocated.
Although whether changes in the balance between microbial species could be a cause or an effect of disease onset and development is difficult to state[17], microbial composition analyses have been performed in different districts including the oral cavity, duodenum, gut, bile, and pancreatic tissue. Conflicting results can be found in analyzing the studies, but the influence of other risk factors (for example, smoking habit) may create bias and at least, partially explain some differences.
Association between periodontal disease or oral dysbiosis and several kinds of cancers (oral, hematological, digestive tract, prostate, uterus, lung, and breast) has been reported with different strengths of association[18]. In analyzing this relationship, PanCa has been one of the most studied cancers[16,18]. Porphyromonas gingivalis, Fusobacterium, and Aggregatibacter actinomycetemcomitans in the oral microbiota were related to a higher risk of developing PanCa[17,19,20]. Similarly, analyzing the saliva, the presence of Streptococcus and Leptotrichia was associated with a higher risk of human PanCa, and Granulicatella adiacens was found to be increased in these patients. On the contrary, the presence of Veillonella and Neisseria was associated with a lower risk of PanCa, and Streptococcus mitis appeared to be reduced in these patients[21-23]. Higher representation of Prevotella and Vaillonella was more frequently found in patients with jaundice[22].
Fusobacterium, Rothia, Actinomyces, Corynebacterium, Atopobium, Peptostreptococcus, Catonella, Oribacterium, Filifactor, Campylobacter, Moraxella, and Tannerella were more abundant in the PanCa patients’ tongue coatings, while Haemophilus, Porphyromonas, and Paraprevotella were more abundant in healthy people. Haemophilus and Porphyromonas or Leptotrichia and Fusobacterium represented unique signatures able to identify healthy people or patients with PanCa[24].
Helicobacter pylori (H. pylori) is a well-known pathogenetic agent in gastric cancer, and its role in colorectal cancer development has also been suggested[25]. Being more represented in the stomach and duodenum of the PanCa patients than in controls, H. pylori could also have a role in the development of this cancer. It elicits chronic mucosal inflammation and alters the gut microbiota causing bacterial translocation[3,26]. However, this relationship is particularly argued due to the frequent associations with several other known risk factors thus precluding definitive conclusions[9].
Association between gut dysbiosis and several kinds of cancers (breast, lung, digestive tract, and above all colorectal) has been reported. Lower gut microbial biodiversity was found in stool samples of PanCa patients[27]. Furthermore, significantly higher levels of mucus-degrader Verrucomicrobia and Bacteroidetes including the Gram-negative bacteria lipopolysaccharide (LPS) producers Prevotella and Hallella together with other LPS producers Veillonella, Klebsiella, Selenomonas, and Enterobacter have been found in the gut microbiota of PanCa patients compared to healthy people. In parallel, lower levels of other Firmicutes, notably the butyrate-producers Coprococcus and Anaerostipes, have been found[2,27,28]. However, the exact clinical correlation is still unknown, and further studies are needed. Finally, higher levels of Bifidobacterium pseudolongum were documented in both gut and tumor specimens[28].
The pancreas is considered a sterile organ because of its alkaline pH and the presence of digestive enzymes that make bacterial proliferation difficult[29]. However, some potential ways through which microbes could reach it have been recently proposed even if definitive conclusions cannot be drawn. They include colonization from the duodenum or the biliary tract following reflux and colonization through the lymphatic and/or portal system[28]. Finally, the potential tissue contamination during sample collection and processing is another way of colonization. The majority of the human PanCa tissues were found to be colonized, mainly by Gammaproteobacteria[30-33], and a greater abundance of bacteria and fungi have been found in PanCa tissue of both human and animal models[28,30,31].
Acinetobacter, Enterobacter, Pseudomonas, Delftia, Enterococcus, Streptococcus, Corynebacterium, Propionibacterium, Sphingomonas, and Staphylococcus with a significant Klebsiella predominance were found in the human PanCa group compared to the healthy control (samples from organ donations)[32]. In addition, higher levels of Malassezia species in cancer tissue were related to PanCa progression, while Candida, Aspergillus, or Saccharomyces species did not cause cancer development after repopulation following mycobiome ablation[34]. In animal models, a relatively higher abundance of Lactobacillus was found in the pancreatic tissue of the control group, while a relatively higher abundance of Fusobacterium species and Proteobacteria were found in the pancreatic tissue of the cancer group[17,33]. Furthermore, significant differences have been discovered in microbiota composition found in different PanCa stages[28].
Finally, bile contamination, mainly related to biliary stenting and endoscopic procedures, was significantly found in patients affected by PanCa. The more represented species in bile samples (collected during endoscopic procedures or surgery) were Escherichia coli (E. coli) and different species of Pseudomonas, Enterobacter, and Enterococcus[34-37].
The main microbiota modifications are summarized in Table 1.
| Anatomical district | Microbiota | Implications |
| Oral | ||
| Higher microbial biodiversity | Confer higher risk of developing PanCa[17,19,20,23] | |
| Porphyromonas gingivalis, Fusobacterium | ||
| Aggregatibacter actinomycetemcomitans | ||
| Streptococcus, Leptotrichia, Neisseria elongata | ||
| Granulicatella adiacens | More abundant in PanCa than control[23] | |
| Fusobacterium, Rothia, Actinomyces | ||
| Corynebacterium, Atopobium | ||
| Peptostreptococcus, Catonella | ||
| Oribacterium, Filifactor | ||
| Campylobacter, Moraxella, Tannerella | ||
| Fungi: | ||
| Candida | ||
| Gut | ||
| Bacteroidetes | More abundant in PanCa than control[27,28] | |
| Prevotella, Hallella | ||
| Proteobacteria | ||
| Klebsiella, Enterobacter | ||
| Verrucomicrobia | ||
| Bifidobacterium pseudolongum | ||
| Veillonella | ||
| Firmicutes | Less abundant in PanCa than control[27] | |
| Coprococcus, Anaerostipes | ||
| Pancreas | ||
| Proteobacteria | More abundant in PanCa than control[17,28,30-33] | |
| Gammaproteobacteria | ||
| Pseudomonas, Acinetobacter, Enterobacter | ||
| Delftia, Klebsiella, Sphingomonas | ||
| Firmicutes | ||
| Enterococcus, Streptococcus, Staphylococcus | ||
| Fusobacteria | ||
| Fusobacterium species | ||
| Acinetobacteria | ||
| Bifidobacterium pseudolongum | ||
| Corynebacterium, Propionibacterium | ||
| Lactobacillus | Less abundant in PanCa than control[17,33] | |
| Fungi | Confer higher risk of PanCa progression[31] | |
| Malassezia |
The pathogenetic relationship between microbiota and PanCa is far from being completely understood, and the proposed potential pathogenetic mechanisms are derived mainly from animal models[17]. The microbiota promotes carcinogenesis in direct or indirect ways. Indirect action could be elicited through the production of circulating toxins or harmful metabolites or through the interaction with the immune system. Both innate and adaptive immune responses are active in fighting human cancers[38]. The anti-cancer immunological mechanisms include the cytotoxic CD 8+ T cells, T helper (Th) cells, mature dendritic cells, macrophages, and natural killer cells. Myeloid-derived suppressor cells (MDSCs) are immature myeloid cells able to suppress both innate and adaptive immunity with different mechanisms, promoting regulatory T cell development and simultaneously inhibiting the effector T cells and natural killer cells[39].
Most of these models have also been reported in other cancer types.
A direct carcinogenic effect of Porphyromonas gingivalis is related to the apoptosis inhibition of the epithelial cells, the activation of the p53 gene, and the induction of KRAS mutation, the latter two through the production of the enzyme peptidyl-arginine deiminase[40]. KRAS mutation can be found in almost all PanCa. Interestingly, germ-free KRAS-mutated mice demonstrated slower PanCa progression suggesting that gene mutation itself is not sufficient to cause cancer development[28,29]. Intestinal microbiota has a direct long-distance effect on PanCa development through up/downregulation of pro/anti-cancer pathways[32]. Pseudomonas aeruginosa is related to higher levels of the anti-apoptotic and pro-proliferative proteins Bel-XL, Mcl-1, survivin, c-Myc, and cyclin DI[41]. The hepatotropic viruses that cause hepatitis B or C can contribute to PanCa development through the modulation of the PI3K/AKT signaling pathway and tissue inflammation[22].
One of the most common bacterial components related to cancer development is LPS, which is a direct cause of KRAS gene mutation and can stimulate nuclear factor-κB that further increases KRAS mutation risk. Furthermore, LPS is an activator of the toll-like receptor (TLR) signaling pathway thus enhancing pancreatic inflammation[21,29]. H. pylori is an LPS producer[29]. Deoxycholic acid, a bacterial metabolite of cholic acid, promotes carcinogenesis through the activation of the epidermal growth factor and STAT3 signaling pathway[2]. Desulfovibrio vulgaris and other sulfate-reducing bacteria produce genotoxic hydrogen sulfide when the diet is enriched in proteins and fats[42]. Other carcinogenic bacterial metabolites from proteolytic fermentation include phenols, ammonia, and other nitrogen-rich products. Candida induces PanCa through the production of the carcinogen nitrosamines or acetaldehyde[10,43].
Finally, many studies highlighted the role of the immune system on PanCa[44-46]. The activation of the immune system by the microbiota of different districts can cause local and/or systemic inflammation that worsen dysbiosis sustaining a vicious circle. For example, H. pylori presence in the stomach is related to reduced production of acid/achlorhydria that might trigger dysbiosis[9]. Gut dysbiosis induces chronic pancreatic inflammation. Pancreatic microbiota can further elicit an inflammatory response interacting with both innate and specific immune systems. The immunosuppressive cells, regulatory T cells and MDSC, are predominant at the expense of cytotoxic T lymphocytes, indicating that the phenotype is immunosuppressive[32,44,47]. This aspect implies that the immune system is faulty, most likely due to cancer itself, which causes local and systemic immunological dysfunction escaping the immune system’s detection[48,49].
Furthermore, the tumor microenvironment contributes to the development of PanCa promoting a Th2 polarization with the production of higher levels of interleukin (IL)-17 and IL-10 and reduced production of interferon-γ, which promotes cancer progression[28,50]. The presence of this microenvironment kind and a dense stroma represents a unique PanCa peculiarity[51]. Interestingly, smoke habits impact microbiota inducing immunosuppression with reduced levels of IgG and the making of biofilms, hence possibly promoting the proliferation of harmful bacteria[30]. In an experimental setting, Pushalkar et al[28] demonstrated that germ-free mice did not show PanCa progression, while fecal microbiota transplantation received from sick mice hampered Th1 differentiation of CD4+ and activation of CD8+ T cells hence causing cancer progression.
In humans, Citrobacter freundii was found to be associated with immunosuppression and the activation of several oncogenic pathways. Furthermore, the enrichment of nine microbes including Acidovorax ebreus and Shigella sonnei was related to the downregulation of tumor-suppressive pathways and immune dysregulation with reduced levels of M2 macrophages, activated memory, and CD8+ T cells[51]. H. pylori infection of human PanCa tissue was reported to be associated with higher levels of IL-8 and vascular endothelial growth factor and with the proliferation of several factors including nuclear factor-κB, activator protein 1, and serum response element[52].
Malassezia can interact with the MAPK pathway causing the production of high levels of proinflammatory cytokines, including IL-6, and can activate mast cells[31]. Similarly, Trichosporon can increase the level of IL-6, tumor necrosis factor-α, and interferon-γ[8]. Candida triggers inflammatory response (Th17-mediated), activating the MDSCs[10,43]. Porphyromonas gingivalis and Aggregatibacter activate, similarly to Bifidobacterium pseudolongum[53], the TLR signaling pathway increasing the risk of PanCa development in both animal models and humans[28,54]. Different studies documented that mice deficient in pattern recognition receptors signaling, including TLR 4, TLR7, TLR9, and mincle, show less PanCa progression[53,55,56].
In this scenario, another critical pattern recognition receptors is the mannose-binding lectin (MBL). The complement cascade is triggered when MBL binds to the glycans of the fungal wall, for example, Malassezia. The MBL-C3 axis might be involved in cancer development given that extratumoral suppression of MBL or C3 was associated with a lower risk of PanCa onset and MBL- or C3-deficient mice showed greater defense against PanCa progression[31].
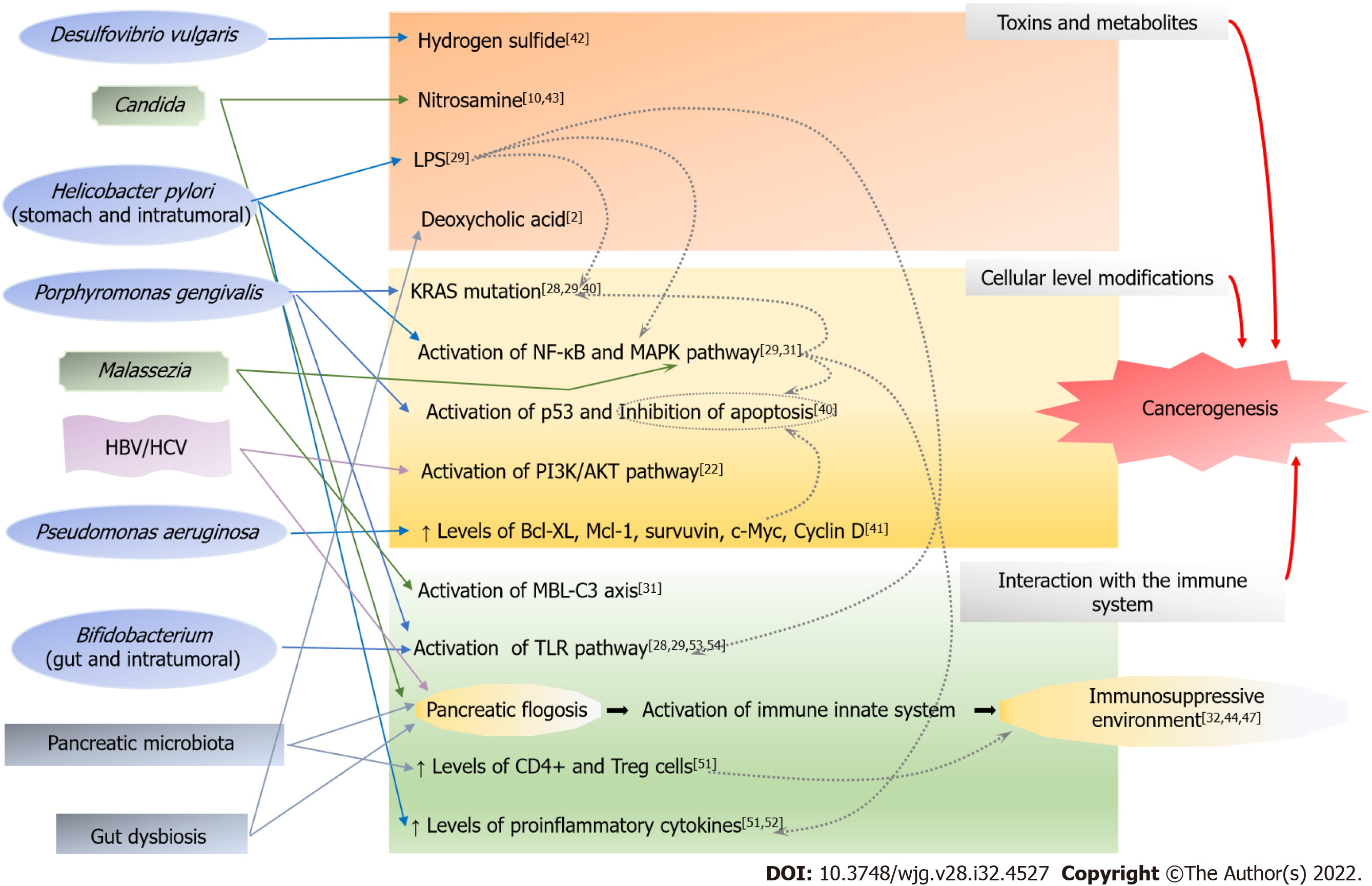
Each component of the microbiota might be implicated in PanCa through different pathogenetic mechanisms and the role of fungal dysbiosis is still far from being completely explored and understood. Figure 1 summarizes this complex relationship.
Whenever technically feasible and oncologically appropriate, surgical treatment is the best chance of cure that could be offered to the patients.
In a small prospective study, both pre- and postoperative gut microbiota was found to be enriched in Akkermansia, Aeromonas, Enterobacteriaceae, Bacteroidales and decreased in Lachnospiraceae, Prevotella, and Faecatitalea. Furthermore, Bacteroides were more frequently associated with higher postoperative morbidity, longer hospital stays, and longer intensive care unit stay[57].
When patients present with severe jaundice, a biliary drain is required before surgery. On the one hand, biliary drain could cause bile contamination; on the other hand, it could restore physiologic bile flow permitting the maintenance of gut eubiosis. The percentage of bile contamination in stented patients was reported to be up to 92% of the samples collected during surgery, while fungal presence was found in 25% of the patients. Fungal presence was directly related to the longer period between biliary drain positioning and surgery[58]. A frequent presence of at least one bacterial species showing antibiotic resistance was also reported, and these patients presented with significantly higher rates of postoperative morbidity[37]. In a large retrospective analysis of 1964 patients undergoing pancreatoduodenectomy and receiving preoperative biliary drain, 30.8% of them presented biliary contamination with the most common contaminants being Enterococcus, Klebsiella, and Enterobacter.
Postoperative pancreatic fistula (POPF) is the main postoperative complication following pancreatic surgery, and it is related to higher morbidity rates. Bile contamination was an independent risk factor for POPF grade B/C with an odds ratio of 1.33. Furthermore, bile contamination was associated with wound and catheter infections[36]. Similar data were reported in successively published papers[59]. Coppola et al[60] found in the bile different microbes related to different complications: E. coli, Klebsiella pneumoniae (K. pneumoniae), and Enterococcus faecalis were prevalent in surgical site infections; E. coli, K. pneumoniae, Enterococcus faecalis, and Enterococcus faecium were more abundant in POPF; and E. coli, Enterococcus faecalis and Enterococcus faecium were found in delayed empty gastric syndrome. Abe et al[61] analyzed the cultures of drainage fluid from patients undergoing pancreatic surgery. They found that the presence of Enterobacter species, Enterococcus species, and Candida species was significantly associated with clinically relevant POPF. Furthermore, the presence of Candida species resulted in being an independent factor for POPF C and POPF-related hemorrhages[61,62]. On the contrary, in other studies (with a restricted number of patients), bactobilia or fungal bile contamination were not reported to be associated with higher POPF rates or different global morbidity and mortality rates[58,63]. Different population samples and geographic bias in defining POPF or in sample collection and analysis could at least partially explain the differences.
In the setting of advanced stages, traditional chemotherapy, mainly based on the FOLFIRINOX regimen (5-fluorouracil, oxaliplatin, and irinotecan) or gemcitabine with or without cisplatin or nab-paclitaxel, has a pivotal role. Chemoresistance is one of the most challenging aspects of systemic treatment.
Microbiota affects gemcitabine response thus influencing patients’ prognoses. Most of the PanCa-associated abundant bacteria, including K. pneumoniae and other species from the Gammaproteobacteria, are cytidine deaminase producers. The production of this enzyme results in higher gemcitabine degradation and consequent chemoresistance[30]. Consistently, quinolone administration in patients infected with K. pneumoniae allowed for a better prognosis[64]. Nevertheless, gemcitabine use can alter the gut microbiota composition being a potential cause of PanCa progression[65], and the use of antibiotics increases side effects[66]. Adding Lactobacillus paracasei improved gemcitabine response in mouse models[67]. Similarly, Lactobacillus spp. reduced diarrhea, which is one of the most common side effects of chemotherapy based on irinotecan[42].
Although evidence is still scarce, the use of oncolytic viruses, mostly adenovirus, in combination with gemcitabine may improve its efficacy through different mechanisms including an antitumor immunity booster activity[68].
Other relations between microbiota and response to chemotherapy have been tested for other kinds of solid cancer, and specific data on PanCa are still needed[42].
Immunotherapy resulted in a game-changer in the treatment of several solid malignancies in the recent decade, and the inhibition of immunological checkpoints has been the most explored strategy for eliciting an antitumor immune response[69,70]. The immune checkpoint inhibitors are monoclonal antibodies targeting molecules, such as the programmed cell death protein 1/programmed death-ligand 1 or cytotoxic T-lymphocyte antigen 4 expressed by tumor or immune cells.
PanCa is one of the cancers for which no immunotherapeutic drugs have been licensed since many clinical trials have failed to show a benefit in terms of response rate and survival[42]. The weak success of the immune checkpoint inhibitor treatment is due to the immunologically “cold” phenotype characterized by the low mutation load and limited expression of neoantigens in PanCa in addition to the immunosuppressive action of the resident stromal cells. Although the fine mechanism of interaction between the microbiota and the immune system in PanCa needs to be examined in-depth, the combination of immune checkpoint inhibitors and chemotherapy could produce promising results. For example, specific polymeric nanoformulations of oxaliplatin and doxorubicin can upregulate the damage-associated molecular patterns expression in PanCa models, activating dendritic cell maturation and adaptive immune response[71]. These two nanoformulations act both by preventing tumor growth and by showing a prophylactic action through inoculation in Pan02 mouse models.
Vaccines are the most effective way to overcome the PanCa hypoimmunogenicity, and nanotechnologies can enhance their immunostimulatory effects[72].
Recent studies have highlighted the importance of stromal macrophages demonstrating the possibility to restore antitumor immunity. The employment of TLR agonists and kinase inhibitors can induce an M1-like polarization. Different PanCa models have been treated with nanoformulations with the ability to reprogram the macrophages phenotype, showing excellent results[73,74].
As previously reported, several modifiable risk factors have been recognized including smoking habits, alcohol abuse, and diet (high meat intake and low fruit consumption). It is expected that about a quarter of PanCa could be avoided by acting on these factors[9,75]. Since most of them are strictly connected with the microbiota, the possibility to modulate its composition or function could be a promising tool against cancer. There is an ongoing phase II randomized clinical trial (NCT04631445) analyzing the effect of a ketogenic diet in metastatic patients receiving chemotherapy, but unluckily the microbiota evaluation is not included.
A growing number of studies are analyzing the potential role of the so-called “next-generation probiotics” or “live biotherapeutics” that includes short-chain fatty acids producers. These microbes are implied in maintaining the intestinal barrier integrity with the reduction of bacterial translocation and in the reduction of fungal overgrowth[2]. But, to date, specific results of the effects on PanCa are lacking.
In animal models, the 4-wk oral administration of the probiotics Lactobacillus paracasei and Lactobacillus reuteri alone or combined with an intraperitoneal injection of gentamicin was related to a lower chance of developing precursors of PanCa. Furthermore, they allowed a lower increase of liver enzymes caused by gemcitabine[76]. Similarly, oral administration of the probiotic Aspergillus oryzae showed an antitumoral activity. The heptelidic acid was the involved bacterial-produced molecule able to pass the intestinal barrier, reach other organs, and induce apoptosis through the p38 MAPK signaling pathway[77].
In experimental studies with xenografts, Sethi et al[50] found that administration of oral antibiotics causing bacterial depletion achieved an antitumor effect through a switch from Th2 immune phenotype toward a Th1 differentiation of CD4+ T cells, with the activation of CD8+ T cells, lower levels of MDSCs, and finally an increase in M1 macrophage differentiation together with lower levels of the protumor IL-17a and IL-10 in the tumor microenvironment. Furthermore, these changes were not seen in T and B cells-deprived animals. Similarly, the administration of oral antibiotics to wild-type mice caused a reduction of the PanCa size by 50% through the reversing of the immunosuppressive tumor microenvironment[28].
Further research about microbial engineering strategies that could allow on-site recruitment of CD8+ T cells via interferon-γ production or interaction with the complement cascade is strongly warranted[2].
Despite few specific studies being available, the fecal microbiota transplant through modifying the entire gut microbiota ecology, could represent a great opportunity in PanCa treatment. Experiments in fecal microbiota transplantation animal models confirmed the responsibility of gut microbiota in the modulation of the intratumoral microbiome[78].
Drug delivery systems are bio-responsive elements able to change and thus release drugs in a particular microenvironment (e.g., low level of pH, hypoxia, or presence of specific enzymes). Consequently, the drug delivery systems have been recently proposed as a promising instrument for microbiota modulation through the delivery of pre/probiotics with the capability of depleting protumoral bacteria and eliminating their toxins[79].
However, specific studies are needed to introduce this promising tool in routine clinical practice.
Since complete PanCa prevention is not expected, an early diagnosis would be of great help in widening the chance to cure the patients.
Within the species found to be increased/decreased in the saliva of the affected patients compared to the control group, Neisseria elongata and Streptococcus mitis resulted in a distinguishing signature of PanCa with a sensitivity of 96.4% and a specificity of 82.1% representing a potential biomarker for early cancer detection[23]. In the saliva of the PanCa patients, the ratio of Leptotrichia and Porphyromonas was reported to be significantly higher compared with the control group[53]. The presence of a high level of serum antibodies against Porphyromonas gingivalis was found to be associated with a two-fold higher risk of developing PanCa within 5 years, while high levels of anti-commensal microbe antibodies seemed related to a reduced risk of PanCa[11].
Kim et al[80] found that altered human microbiota composition evaluated with microbial extracellular vesicles from blood samples could represent a novel biomarker. Six species, Ruminococcaceae UCG-014, Lachnospiraceae NK4A136 group, Akkermansia, Turicibacter, Ruminiclostridium, and Lachnospiraceae UCG-001, were more abundant, while four species, Stenotrophomonas, Sphingomonas, Propionibacterium, and Corynebacterium, were less represented in PanCa patients. With these findings, a prediction model for PanCa was built, and the related area under the receiver operating characteristic curve was 1.000[80].
Furthermore, experiments with spontaneous PanCa in animal models demonstrated that the polyamine metabolism of some gut bacteria including Lactobacillus was significantly dysregulated in the very early stages of PanCa[1].
Unfortunately, none of the presented potential biomarkers is available in clinical practice[21]. So, further studies are strongly warranted to validate new diagnostic tools.
One of the most frequently asked questions by patients affected by cancers is about their prognosis. The finding of further prognostic markers, other than the well-known staging systems, could help doctors answer this patient concern.
Riquelme et al[78] compared the disparity in microbiota composition of the PanCa tissue of the patients with different prognoses using 16S rRNA gene sequencing. Long-term survivors (alive 5 years after surgery) showed the highest alpha-diversity and a particular signature composed of Pseudoxanthomonas, Streptomyces, Saccharopolyspora, and Bacillus clausii with an area under the curve of 97.5%. Furthermore, this group of bacteria was associated with greater activation of CD8+ T cells. Furthermore, gut microbiome from short-term survival could induce PanCa onset in a mouse model through the establishment of an immunosuppressive environment suggesting the role of both intrapancreatic and gut microbiome[28,78]. Tumors showing a lower immunosuppressive environment (more neoantigens MUC16 - CA 125 - and CD8+ T cells) confer longer survival rates[81].
Using data from The Cancer Genome Atlas, Chakladar et al[51] found that only Acidovorax ebreus was linked to a high tumor grade. A significantly higher microbial biodiversity in metastatic and short-survival patients with a predominant percentage of Proteobacteria, particularly Acidovorax ebreus and members of the Gammaproteobacteria, was also reported. Citrobacter freundii and Shigella sonnei were associated with the dysregulation of cancer-associated pathways. Furthermore, they found different microbes associated with survival and metastasis. Analyzing The Cancer Genome Atlas database, a prognostic score was proposed by other authors. The high tumor score was associated with p53 mutation, higher tumor mutational burden, and unfavorable and immunosuppressive tumor microenvironment. This high score was found to be related to increased cell proliferation and a higher chance of achieving a positive margin following surgery[82].
In a study with almost 300 PanCa patients, the presence of Fusobacterium species was found to be an independent factor of higher mortality[83]. Similarly, the presence of Pseudomonas aeruginosa was found to be a negative prognostic biomarker[41].
The complex interplay between microbiota and the immune system at different anatomical districts (mostly gut and pancreas/intratumor) might have a role in both the onset and progression of PanCa. Due to the poor prognosis of this tumor despite global advances in surgery and perioperative management, every effort to find new potential targets is welcome. Modifications in microbial composition related to cancer onset could be both a promising target for microbiota shaping and cancer treatment and a potential biomarker for early cancer detection. With the progressive advances in microbiota knowledge, new possibilities for treatment and patient stratification could be expected in the coming years with an improved prognosis for patients with PanCa.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Italy
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C, C, C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Jiang M, China; Leowattana W, Thailand; Li HL, China; Luo ZW, China; Uhlmann D, Germany; Volynets GV, Russia S-Editor: Gong ZM L-Editor: Filipodia P-Editor: Gong ZM
| 1. | Mendez R, Kesh K, Arora N, Di Martino L, McAllister F, Merchant N, Banerjee S. Microbial dysbiosis and polyamine metabolism as predictive markers for early detection of pancreatic cancer. Carcinogenesis. 2020;41:561-570. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 83] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 2. | Brandi G, Turroni S, McAllister F, Frega G. The Human Microbiomes in Pancreatic Cancer: Towards Evidence-Based Manipulation Strategies? Int J Mol Sci. 2021;22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 3. | Wang Y, Yang G, You L, Yang J, Feng M, Qiu J, Zhao F, Liu Y, Cao Z, Zheng L, Zhang T, Zhao Y. Role of the microbiome in occurrence, development and treatment of pancreatic cancer. Mol Cancer. 2019;18:173. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 76] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 4. | Iebba V, Totino V, Gagliardi A, Santangelo F, Cacciotti F, Trancassini M, Mancini C, Cicerone C, Corazziari E, Pantanella F, Schippa S. Eubiosis and dysbiosis: the two sides of the microbiota. New Microbiol. 2016;39:1-12. [PubMed] |
| 5. | Pagliari D, Piccirillo CA, Larbi A, Cianci R. The Interactions between Innate Immunity and Microbiota in Gastrointestinal Diseases. J Immunol Res. 2015;2015:898297. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 26] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 6. | Russo E, Giudici F, Ricci F, Scaringi S, Nannini G, Ficari F, Luceri C, Niccolai E, Baldi S, D'Ambrosio M, Ramazzotti M, Amedei A. Diving into Inflammation: A Pilot Study Exploring the Dynamics of the Immune-Microbiota Axis in Ileal Tissue Layers of Patients with Crohn's Disease. J Crohns Colitis. 2021;15:1500-1516. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 18] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 7. | Niccolai E, Russo E, Baldi S, Ricci F, Nannini G, Pedone M, Stingo FC, Taddei A, Ringressi MN, Bechi P, Mengoni A, Fani R, Bacci G, Fagorzi C, Chiellini C, Prisco D, Ramazzotti M, Amedei A. Significant and Conflicting Correlation of IL-9 With Prevotella and Bacteroides in Human Colorectal Cancer. Front Immunol. 2020;11:573158. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 43] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 8. | Kaźmierczak-Siedlecka K, Dvořák A, Folwarski M, Daca A, Przewłócka K, Makarewicz W. Fungal Gut Microbiota Dysbiosis and Its Role in Colorectal, Oral, and Pancreatic Carcinogenesis. Cancers (Basel). 2020;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 51] [Cited by in RCA: 61] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 9. | Archibugi L, Signoretti M, Capurso G. The Microbiome and Pancreatic Cancer: An Evidence-based Association? J Clin Gastroenterol. 2018;52 Suppl 1:S82-S85. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 19] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 10. | Kaźmierczak-Siedlecka K, Stachowska E, Folwarski M, Przewłócka K, Makarewicz W, Bryl E. The potential of gut microbiome as a non-invasive predictive biomarker for early detection of pancreatic cancer and hepatocellular carcinoma. Eur Rev Med Pharmacol Sci. 2021;25:7275-7284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 11. | Michaud DS, Izard J, Wilhelm-Benartzi CS, You DH, Grote VA, Tjønneland A, Dahm CC, Overvad K, Jenab M, Fedirko V, Boutron-Ruault MC, Clavel-Chapelon F, Racine A, Kaaks R, Boeing H, Foerster J, Trichopoulou A, Lagiou P, Trichopoulos D, Sacerdote C, Sieri S, Palli D, Tumino R, Panico S, Siersema PD, Peeters PH, Lund E, Barricarte A, Huerta JM, Molina-Montes E, Dorronsoro M, Quirós JR, Duell EJ, Ye W, Sund M, Lindkvist B, Johansen D, Khaw KT, Wareham N, Travis RC, Vineis P, Bueno-de-Mesquita HB, Riboli E. Plasma antibodies to oral bacteria and risk of pancreatic cancer in a large European prospective cohort study. Gut. 2013;62:1764-1770. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 295] [Cited by in RCA: 310] [Article Influence: 25.8] [Reference Citation Analysis (0)] |
| 12. | Yadav D, Lowenfels AB. The epidemiology of pancreatitis and pancreatic cancer. Gastroenterology. 2013;144:1252-1261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1151] [Cited by in RCA: 1347] [Article Influence: 112.3] [Reference Citation Analysis (0)] |
| 13. | Lagier JC, Khelaifia S, Alou MT, Ndongo S, Dione N, Hugon P, Caputo A, Cadoret F, Traore SI, Seck EH, Dubourg G, Durand G, Mourembou G, Guilhot E, Togo A, Bellali S, Bachar D, Cassir N, Bittar F, Delerce J, Mailhe M, Ricaboni D, Bilen M, Dangui Nieko NP, Dia Badiane NM, Valles C, Mouelhi D, Diop K, Million M, Musso D, Abrahão J, Azhar EI, Bibi F, Yasir M, Diallo A, Sokhna C, Djossou F, Vitton V, Robert C, Rolain JM, La Scola B, Fournier PE, Levasseur A, Raoult D. Culture of previously uncultured members of the human gut microbiota by culturomics. Nat Microbiol. 2016;1:16203. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 756] [Cited by in RCA: 665] [Article Influence: 73.9] [Reference Citation Analysis (0)] |
| 14. | Szychowiak P, Villageois-Tran K, Patrier J, Timsit JF, Ruppé É. The role of the microbiota in the management of intensive care patients. Ann Intensive Care. 2022;12:3. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 42] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 15. | Zhang X, Li L, Butcher J, Stintzi A, Figeys D. Advancing functional and translational microbiome research using meta-omics approaches. Microbiome. 2019;7:154. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 144] [Cited by in RCA: 188] [Article Influence: 31.3] [Reference Citation Analysis (0)] |
| 16. | Herremans KM, Riner AN, Cameron ME, McKinley KL, Triplett EW, Hughes SJ, Trevino JG. The oral microbiome, pancreatic cancer and human diversity in the age of precision medicine. Microbiome. 2022;10:93. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 26] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 17. | Akshintala VS, Talukdar R, Singh VK, Goggins M. The Gut Microbiome in Pancreatic Disease. Clin Gastroenterol Hepatol. 2019;17:290-295. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 79] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 18. | Irfan M, Delgado RZR, Frias-Lopez J. The Oral Microbiome and Cancer. Front Immunol. 2020;11:591088. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 184] [Article Influence: 36.8] [Reference Citation Analysis (0)] |
| 19. | Michaud DS. Role of bacterial infections in pancreatic cancer. Carcinogenesis. 2013;34:2193-2197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 116] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 20. | Fan X, Alekseyenko AV, Wu J, Peters BA, Jacobs EJ, Gapstur SM, Purdue MP, Abnet CC, Stolzenberg-Solomon R, Miller G, Ravel J, Hayes RB, Ahn J. Human oral microbiome and prospective risk for pancreatic cancer: a population-based nested case-control study. Gut. 2018;67:120-127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 386] [Cited by in RCA: 552] [Article Influence: 78.9] [Reference Citation Analysis (0)] |
| 21. | Amara S, Yang LV, Tiriveedhi V, Muzaffar M. Complex Role of Microbiome in Pancreatic Tumorigenesis: Potential Therapeutic Implications. Cells. 2022;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 22. | Wei MY, Shi S, Liang C, Meng QC, Hua J, Zhang YY, Liu J, Zhang B, Xu J, Yu XJ. The microbiota and microbiome in pancreatic cancer: more influential than expected. Mol Cancer. 2019;18:97. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 197] [Cited by in RCA: 193] [Article Influence: 32.2] [Reference Citation Analysis (0)] |
| 23. | Farrell JJ, Zhang L, Zhou H, Chia D, Elashoff D, Akin D, Paster BJ, Joshipura K, Wong DT. Variations of oral microbiota are associated with pancreatic diseases including pancreatic cancer. Gut. 2012;61:582-588. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 403] [Cited by in RCA: 487] [Article Influence: 37.5] [Reference Citation Analysis (0)] |
| 24. | Lu H, Ren Z, Li A, Li J, Xu S, Zhang H, Jiang J, Yang J, Luo Q, Zhou K, Zheng S, Li L. Tongue coating microbiome data distinguish patients with pancreatic head cancer from healthy controls. J Oral Microbiol. 2019;11:1563409. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 101] [Cited by in RCA: 96] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 25. | Butt J, Jenab M, Pawlita M, Tjønneland A, Kyrø C, Boutron-Ruault MC, Carbonnel F, Dong C, Kaaks R, Kühn T, Boeing H, Schulze MB, Trichopoulou A, Karakatsani A, La Vecchia C, Palli D, Agnoli C, Tumino R, Sacerdote C, Panico S, Bueno-de-Mesquita B, Vermeulen R, Gram IT, Weiderpass E, Borch KB, Quirós JR, Agudo A, Rodríguez-Barranco M, Santiuste C, Ardanaz E, Van Guelpen B, Harlid S, Imaz L, Perez-Cornago A, Gunter MJ, Zouiouich S, Park JY, Riboli E, Cross AJ, Heath AK, Waterboer T, Hughes DJ. Antibody Responses to Helicobacter pylori and Risk of Developing Colorectal Cancer in a European Cohort. Cancer Epidemiol Biomarkers Prev. 2020;29:1475-1481. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 26. | Mei QX, Huang CL, Luo SZ, Zhang XM, Zeng Y, Lu YY. Characterization of the duodenal bacterial microbiota in patients with pancreatic head cancer vs. healthy controls. Pancreatology. 2018;18:438-445. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 75] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 27. | Ren Z, Li A, Jiang J, Zhou L, Yu Z, Lu H, Xie H, Chen X, Shao L, Zhang R, Xu S, Zhang H, Cui G, Sun R, Wen H, Lerut JP, Kan Q, Li L, Zheng S. Gut microbiome analysis as a tool towards targeted non-invasive biomarkers for early hepatocellular carcinoma. Gut. 2019;68:1014-1023. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 542] [Cited by in RCA: 509] [Article Influence: 84.8] [Reference Citation Analysis (0)] |
| 28. | Pushalkar S, Hundeyin M, Daley D, Zambirinis CP, Kurz E, Mishra A, Mohan N, Aykut B, Usyk M, Torres LE, Werba G, Zhang K, Guo Y, Li Q, Akkad N, Lall S, Wadowski B, Gutierrez J, Kochen Rossi JA, Herzog JW, Diskin B, Torres-Hernandez A, Leinwand J, Wang W, Taunk PS, Savadkar S, Janal M, Saxena A, Li X, Cohen D, Sartor RB, Saxena D, Miller G. The Pancreatic Cancer Microbiome Promotes Oncogenesis by Induction of Innate and Adaptive Immune Suppression. Cancer Discov. 2018;8:403-416. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1016] [Cited by in RCA: 970] [Article Influence: 138.6] [Reference Citation Analysis (0)] |
| 29. | Daniluk J, Daniluk U, Rogalski P, Dabrowski A, Swidnicka-Siergiejko A. Microbiome-Friend or Foe of Pancreatic Cancer? J Clin Med. 2021;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 30. | Geller LT, Barzily-Rokni M, Danino T, Jonas OH, Shental N, Nejman D, Gavert N, Zwang Y, Cooper ZA, Shee K, Thaiss CA, Reuben A, Livny J, Avraham R, Frederick DT, Ligorio M, Chatman K, Johnston SE, Mosher CM, Brandis A, Fuks G, Gurbatri C, Gopalakrishnan V, Kim M, Hurd MW, Katz M, Fleming J, Maitra A, Smith DA, Skalak M, Bu J, Michaud M, Trauger SA, Barshack I, Golan T, Sandbank J, Flaherty KT, Mandinova A, Garrett WS, Thayer SP, Ferrone CR, Huttenhower C, Bhatia SN, Gevers D, Wargo JA, Golub TR, Straussman R. Potential role of intratumor bacteria in mediating tumor resistance to the chemotherapeutic drug gemcitabine. Science. 2017;357:1156-1160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 944] [Cited by in RCA: 1206] [Article Influence: 150.8] [Reference Citation Analysis (0)] |
| 31. | Aykut B, Pushalkar S, Chen R, Li Q, Abengozar R, Kim JI, Shadaloey SA, Wu D, Preiss P, Verma N, Guo Y, Saxena A, Vardhan M, Diskin B, Wang W, Leinwand J, Kurz E, Kochen Rossi JA, Hundeyin M, Zambrinis C, Li X, Saxena D, Miller G. The fungal mycobiome promotes pancreatic oncogenesis via activation of MBL. Nature. 2019;574:264-267. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 265] [Cited by in RCA: 577] [Article Influence: 96.2] [Reference Citation Analysis (0)] |
| 32. | Thomas RM, Gharaibeh RZ, Gauthier J, Beveridge M, Pope JL, Guijarro MV, Yu Q, He Z, Ohland C, Newsome R, Trevino J, Hughes SJ, Reinhard M, Winglee K, Fodor AA, Zajac-Kaye M, Jobin C. Intestinal microbiota enhances pancreatic carcinogenesis in preclinical models. Carcinogenesis. 2018;39:1068-1078. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 131] [Cited by in RCA: 165] [Article Influence: 23.6] [Reference Citation Analysis (0)] |
| 33. | Del Castillo E, Meier R, Chung M, Koestler DC, Chen T, Paster BJ, Charpentier KP, Kelsey KT, Izard J, Michaud DS. The Microbiomes of Pancreatic and Duodenum Tissue Overlap and Are Highly Subject Specific but Differ between Pancreatic Cancer and Noncancer Subjects. Cancer Epidemiol Biomarkers Prev. 2019;28:370-383. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 141] [Article Influence: 23.5] [Reference Citation Analysis (0)] |
| 34. | Shrader HR, Miller AM, Tomanek-Chalkley A, McCarthy A, Coleman KL, Ear PH, Mangalam AK, Salem AK, Chan CHF. Effect of bacterial contamination in bile on pancreatic cancer cell survival. Surgery. 2021;169:617-622. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 15] [Article Influence: 3.0] [Reference Citation Analysis (1)] |
| 35. | Serra N, Di Carlo P, Gulotta G, d' Arpa F, Giammanco A, Colomba C, Melfa G, Fasciana T, Sergi C. Bactibilia in women affected with diseases of the biliary tract and pancreas. A STROBE guidelines-adherent cross-sectional study in Southern Italy. J Med Microbiol. 2018;67:1090-1095. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 24] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 36. | Okano K, Suzuki Y. Influence of bile contamination for patients who undergo pancreaticoduodenectomy after biliary drainage. World J Gastroenterol. 2019;25:6847-6856. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 11] [Cited by in RCA: 14] [Article Influence: 2.3] [Reference Citation Analysis (1)] |
| 37. | Stecca T, Nistri C, Pauletti B, Greco A, Di Giacomo A, Caratozzolo E, Bonariol L, Massani M. Bacteriobilia resistance to antibiotic prophylaxis increases morbidity after pancreaticoduodenectomy: a monocentric retrospective study of 128 patients. Updates Surg. 2020;72:1073-1080. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 38. | Balkwill FR, Mantovani A. Cancer-related inflammation: common themes and therapeutic opportunities. Semin Cancer Biol. 2012;22:33-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 456] [Cited by in RCA: 515] [Article Influence: 39.6] [Reference Citation Analysis (0)] |
| 39. | Ostrand-Rosenberg S. Myeloid-derived suppressor cells: more mechanisms for inhibiting antitumor immunity. Cancer Immunol Immunother. 2010;59:1593-1600. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 396] [Cited by in RCA: 431] [Article Influence: 28.7] [Reference Citation Analysis (0)] |
| 40. | Zhou Y, Luo GH. Porphyromonas gingivalis and digestive system cancers. World J Clin Cases. 2019;7:819-829. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 24] [Cited by in RCA: 23] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 41. | Joukar F, Mavaddati S, Mansour-Ghanaei F, Samadani AA. Gut Microbiota as a Positive Potential Therapeutic Factor in Carcinogenesis: an Overview of Microbiota-Targeted Therapy. J Gastrointest Cancer. 2020;51:363-378. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 42. | Abdul Rahman R, Lamarca A, Hubner RA, Valle JW, McNamara MG. The Microbiome as a Potential Target for Therapeutic Manipulation in Pancreatic Cancer. Cancers (Basel). 2021;13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 20] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 43. | Chung LM, Liang JA, Lin CL, Sun LM, Kao CH. Cancer risk in patients with candidiasis: a nationwide population-based cohort study. Oncotarget. 2017;8:63562-63573. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 61] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 44. | Amedei A, Niccolai E, Benagiano M, Della Bella C, Cianchi F, Bechi P, Taddei A, Bencini L, Farsi M, Cappello P, Prisco D, Novelli F, D'Elios MM. Ex vivo analysis of pancreatic cancer-infiltrating T lymphocytes reveals that ENO-specific Tregs accumulate in tumor tissue and inhibit Th1/Th17 effector cell functions. Cancer Immunol Immunother. 2013;62:1249-1260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 86] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 45. | Niccolai E, Cappello P, Taddei A, Ricci F, D'Elios MM, Benagiano M, Bechi P, Bencini L, Ringressi MN, Coratti A, Cianchi F, Bonello L, Di Celle PF, Prisco D, Novelli F, Amedei A. Peripheral ENO1-specific T cells mirror the intratumoral immune response and their presence is a potential prognostic factor for pancreatic adenocarcinoma. Int J Oncol. 2016;49:393-401. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 19] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 46. | Niccolai E, Taddei A, Ricci F, Rolla S, D'Elios MM, Benagiano M, Bechi P, Bencini L, Ringressi MN, Pini A, Castiglione F, Giordano D, Satolli MA, Coratti A, Cianchi F, Bani D, Prisco D, Novelli F, Amedei A. Intra-tumoral IFN-γ-producing Th22 cells correlate with TNM staging and the worst outcomes in pancreatic cancer. Clin Sci (Lond). 2016;130:247-258. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 27] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 47. | Feig C, Gopinathan A, Neesse A, Chan DS, Cook N, Tuveson DA. The pancreas cancer microenvironment. Clin Cancer Res. 2012;18:4266-4276. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 841] [Cited by in RCA: 1029] [Article Influence: 85.8] [Reference Citation Analysis (0)] |
| 48. | Inman KS, Francis AA, Murray NR. Complex role for the immune system in initiation and progression of pancreatic cancer. World J Gastroenterol. 2014;20:11160-11181. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 93] [Cited by in RCA: 109] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 49. | Zou W. Immunosuppressive networks in the tumour environment and their therapeutic relevance. Nat Rev Cancer. 2005;5:263-274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1527] [Cited by in RCA: 1564] [Article Influence: 78.2] [Reference Citation Analysis (0)] |
| 50. | Sethi V, Kurtom S, Tarique M, Lavania S, Malchiodi Z, Hellmund L, Zhang L, Sharma U, Giri B, Garg B, Ferrantella A, Vickers SM, Banerjee S, Dawra R, Roy S, Ramakrishnan S, Saluja A, Dudeja V. Gut Microbiota Promotes Tumor Growth in Mice by Modulating Immune Response. Gastroenterology. 2018;155:33-37.e6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 194] [Cited by in RCA: 299] [Article Influence: 42.7] [Reference Citation Analysis (0)] |
| 51. | Chakladar J, Kuo SZ, Castaneda G, Li WT, Gnanasekar A, Yu MA, Chang EY, Wang XQ, Ongkeko WM. The Pancreatic Microbiome is Associated with Carcinogenesis and Worse Prognosis in Males and Smokers. Cancers (Basel). 2020;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 56] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 52. | Takayama S, Takahashi H, Matsuo Y, Okada Y, Manabe T. Effects of Helicobacter pylori infection on human pancreatic cancer cell line. Hepatogastroenterology. 2007;54:2387-2391. [PubMed] |
| 53. | Zambirinis CP, Levie E, Nguy S, Avanzi A, Barilla R, Xu Y, Seifert L, Daley D, Greco SH, Deutsch M, Jonnadula S, Torres-Hernandez A, Tippens D, Pushalkar S, Eisenthal A, Saxena D, Ahn J, Hajdu C, Engle DD, Tuveson D, Miller G. TLR9 ligation in pancreatic stellate cells promotes tumorigenesis. J Exp Med. 2015;212:2077-2094. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 110] [Cited by in RCA: 143] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 54. | Olsen I, Lambris JD, Hajishengallis G. Porphyromonas gingivalis disturbs host-commensal homeostasis by changing complement function. J Oral Microbiol. 2017;9:1340085. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 70] [Cited by in RCA: 111] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 55. | Seifert L, Werba G, Tiwari S, Ly NNG, Alothman S, Alqunaibit D, Avanzi A, Barilla R, Daley D, Greco SH, Torres-Hernandez A, Pergamo M, Ochi A, Zambirinis CP, Pansari M, Rendon M, Tippens D, Hundeyin M, Mani VR, Hajdu C, Engle D, Miller G. Author Correction: The necrosome promotes pancreatic oncogenesis via CXCL1 and Mincle-induced immune suppression. Nature. 2021;591:E28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 56. | Ochi A, Nguyen AH, Bedrosian AS, Mushlin HM, Zarbakhsh S, Barilla R, Zambirinis CP, Fallon NC, Rehman A, Pylayeva-Gupta Y, Badar S, Hajdu CH, Frey AB, Bar-Sagi D, Miller G. MyD88 inhibition amplifies dendritic cell capacity to promote pancreatic carcinogenesis via Th2 cells. J Exp Med. 2012;209:1671-1687. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 205] [Cited by in RCA: 242] [Article Influence: 18.6] [Reference Citation Analysis (0)] |
| 57. | Schmitt FCF, Brenner T, Uhle F, Loesch S, Hackert T, Ulrich A, Hofer S, Dalpke AH, Weigand MA, Boutin S. Gut microbiome patterns correlate with higher postoperative complication rates after pancreatic surgery. BMC Microbiol. 2019;19:42. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 46] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 58. | Tortajada P, Sauvanet A, Truant S, Regenet N, Souche R, Benoist S, Muscari F, Regimbeau JM, Gaujoux S, Cunha AS, Schwarz L; French-Achbt Working Group. Does Fungal Biliary Contamination after Preoperative Biliary Drainage Increase Postoperative Complications after Pancreaticoduodenectomy? Cancers (Basel). 2020;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 59. | Parapini ML, Skipworth JRA, Mah A, Desai S, Chung S, Scudamore CH, Segedi M, Vasilyeva E, Li J, Kim PT. The association between bacterobilia and the risk of postoperative complications following pancreaticoduodenectomy. HPB (Oxford). 2022;24:277-285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 60. | Coppola A, La Vaccara V, Farolfi T, Fiore M, Cascone C, Ramella S, Spoto S, Ciccozzi M, Angeletti S, Coppola R, Caputo D. Different Biliary Microbial Flora Influence Type of Complications after Pancreaticoduodenectomy: A Single Center Retrospective Analysis. J Clin Med. 2021;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 61. | Abe K, Kitago M, Shinoda M, Yagi H, Abe Y, Oshima G, Hori S, Yokose T, Endo Y, Kitagawa Y. High risk pathogens and risk factors for postoperative pancreatic fistula after pancreatectomy; a retrospective case-controlled study. Int J Surg. 2020;82:136-142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 62. | Sato A, Masui T, Nakano K, Sankoda N, Anazawa T, Takaori K, Kawaguchi Y, Uemoto S. Abdominal contamination with Candida albicans after pancreaticoduodenectomy is related to hemorrhage associated with pancreatic fistulas. Pancreatology. 2017;17:484-489. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 22] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 63. | Müssle B, Hempel S, Kahlert C, Distler M, Weitz J, Welsch T. Prognostic Impact of Bacterobilia on Morbidity and Postoperative Management After Pancreatoduodenectomy: A Systematic Review and Meta-analysis. World J Surg. 2018;42:2951-2962. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 47] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 64. | Weniger M, Hank T, Qadan M, Ciprani D, Michelakos T, Niess H, Heiliger C, Ilmer M, D'Haese JG, Ferrone CR, Warshaw AL, Lillemoe KD, Werner J, Liss A, Fernández-Del Castillo C. Influence of Klebsiella pneumoniae and quinolone treatment on prognosis in patients with pancreatic cancer. Br J Surg. 2021;108:709-716. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 29] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 65. | Panebianco C, Adamberg K, Jaagura M, Copetti M, Fontana A, Adamberg S, Kolk K, Vilu R, Andriulli A, Pazienza V. Influence of gemcitabine chemotherapy on the microbiota of pancreatic cancer xenografted mice. Cancer Chemother Pharmacol. 2018;81:773-782. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 74] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 66. | Corty RW, Langworthy BW, Fine JP, Buse JB, Sanoff HK, Lund JL. Antibacterial Use Is Associated with an Increased Risk of Hematologic and Gastrointestinal Adverse Events in Patients Treated with Gemcitabine for Stage IV Pancreatic Cancer. Oncologist. 2020;25:579-584. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 27] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 67. | Chen SM, Chieng WW, Huang SW, Hsu LJ, Jan MS. The synergistic tumor growth-inhibitory effect of probiotic Lactobacillus on transgenic mouse model of pancreatic cancer treated with gemcitabine. Sci Rep. 2020;10:20319. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 64] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 68. | Lu SY, Hua J, Xu J, Wei MY, Liang C, Meng QC, Liu J, Zhang B, Wang W, Yu XJ, Shi S. Microorganisms in chemotherapy for pancreatic cancer: An overview of current research and future directions. Int J Biol Sci. 2021;17:2666-2682. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 69. | Ciardiello D, Vitiello PP, Cardone C, Martini G, Troiani T, Martinelli E, Ciardiello F. Immunotherapy of colorectal cancer: Challenges for therapeutic efficacy. Cancer Treat Rev. 2019;76:22-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 220] [Article Influence: 36.7] [Reference Citation Analysis (0)] |
| 70. | Postow MA, Callahan MK, Wolchok JD. Immune Checkpoint Blockade in Cancer Therapy. J Clin Oncol. 2015;33:1974-1982. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1664] [Cited by in RCA: 2094] [Article Influence: 209.4] [Reference Citation Analysis (0)] |
| 71. | Zhao X, Yang K, Zhao R, Ji T, Wang X, Yang X, Zhang Y, Cheng K, Liu S, Hao J, Ren H, Leong KW, Nie G. Inducing enhanced immunogenic cell death with nanocarrier-based drug delivery systems for pancreatic cancer therapy. Biomaterials. 2016;102:187-197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 197] [Article Influence: 21.9] [Reference Citation Analysis (0)] |
| 72. | Liu L, Kshirsagar P, Christiansen J, Gautam SK, Aithal A, Gulati M, Kumar S, Solheim JC, Batra SK, Jain M, Wannemuehler MJ, Narasimhan B. Polyanhydride nanoparticles stabilize pancreatic cancer antigen MUC4β. J Biomed Mater Res A. 2021;109:893-902. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 28] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 73. | Zhou W, Zhou Y, Chen X, Ning T, Chen H, Guo Q, Zhang Y, Liu P, Li C, Chu Y, Sun T, Jiang C. Pancreatic cancer-targeting exosomes for enhancing immunotherapy and reprogramming tumor microenvironment. Biomaterials. 2021;268:120546. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 306] [Article Influence: 61.2] [Reference Citation Analysis (0)] |
| 74. | Zhang F, Parayath NN, Ene CI, Stephan SB, Koehne AL, Coon ME, Holland EC, Stephan MT. Genetic programming of macrophages to perform anti-tumor functions using targeted mRNA nanocarriers. Nat Commun. 2019;10:3974. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 175] [Cited by in RCA: 329] [Article Influence: 54.8] [Reference Citation Analysis (0)] |
| 75. | Capurso G, Lahner E. The interaction between smoking, alcohol and the gut microbiome. Best Pract Res Clin Gastroenterol. 2017;31:579-588. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 129] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 76. | Chen SM, Hsu LJ, Lee HL, Lin CP, Huang SW, Lai CJ, Lin CW, Chen WT, Chen YJ, Lin YC, Yang CC, Jan MS. Lactobacillus Attenuate the Progression of Pancreatic Cancer Promoted by Porphyromonas Gingivalis in K-rasG12DTransgenic Mice. Cancers (Basel). 2020;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 29] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 77. | Konishi H, Isozaki S, Kashima S, Moriichi K, Ichikawa S, Yamamoto K, Yamamura C, Ando K, Ueno N, Akutsu H, Ogawa N, Fujiya M. Probiotic Aspergillus oryzae produces anti-tumor mediator and exerts anti-tumor effects in pancreatic cancer through the p38 MAPK signaling pathway. Sci Rep. 2021;11:11070. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 20] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 78. | Riquelme E, Zhang Y, Zhang L, Montiel M, Zoltan M, Dong W, Quesada P, Sahin I, Chandra V, San Lucas A, Scheet P, Xu H, Hanash SM, Feng L, Burks JK, Do KA, Peterson CB, Nejman D, Tzeng CD, Kim MP, Sears CL, Ajami N, Petrosino J, Wood LD, Maitra A, Straussman R, Katz M, White JR, Jenq R, Wargo J, McAllister F. Tumor Microbiome Diversity and Composition Influence Pancreatic Cancer Outcomes. Cell. 2019;178:795-806.e12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 469] [Cited by in RCA: 1038] [Article Influence: 207.6] [Reference Citation Analysis (0)] |
| 79. | Zhu R, Lang T, Yan W, Zhu X, Huang X, Yin Q, Li Y. Gut Microbiota: Influence on Carcinogenesis and Modulation Strategies by Drug Delivery Systems to Improve Cancer Therapy. Adv Sci (Weinh). 2021;8:2003542. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 34] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 80. | Kim JR, Han K, Han Y, Kang N, Shin TS, Park HJ, Kim H, Kwon W, Lee S, Kim YK, Park T, Jang JY. Microbiome Markers of Pancreatic Cancer Based on Bacteria-Derived Extracellular Vesicles Acquired from Blood Samples: A Retrospective Propensity Score Matching Analysis. Biology (Basel). 2021;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 26] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 81. | Balachandran VP, Łuksza M, Zhao JN, Makarov V, Moral JA, Remark R, Herbst B, Askan G, Bhanot U, Senbabaoglu Y, Wells DK, Cary CIO, Grbovic-Huezo O, Attiyeh M, Medina B, Zhang J, Loo J, Saglimbeni J, Abu-Akeel M, Zappasodi R, Riaz N, Smoragiewicz M, Kelley ZL, Basturk O; Australian Pancreatic Cancer Genome Initiative; Garvan Institute of Medical Research; Prince of Wales Hospital; Royal North Shore Hospital; University of Glasgow; St Vincent’s Hospital; QIMR Berghofer Medical Research Institute; University of Melbourne, Centre for Cancer Research; University of Queensland, Institute for Molecular Bioscience; Bankstown Hospital; Liverpool Hospital; Royal Prince Alfred Hospital, Chris O’Brien Lifehouse; Westmead Hospital; Fremantle Hospital; St John of God Healthcare; Royal Adelaide Hospital; Flinders Medical Centre; Envoi Pathology; Princess Alexandria Hospital; Austin Hospital; Johns Hopkins Medical Institutes; ARC-Net Centre for Applied Research on Cancer, Gönen M, Levine AJ, Allen PJ, Fearon DT, Merad M, Gnjatic S, Iacobuzio-Donahue CA, Wolchok JD, DeMatteo RP, Chan TA, Greenbaum BD, Merghoub T, Leach SD. Identification of unique neoantigen qualities in long-term survivors of pancreatic cancer. Nature. 2017;551:512-516. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 920] [Cited by in RCA: 879] [Article Influence: 109.9] [Reference Citation Analysis (0)] |
| 82. | Katsuta E, Huyser M, Yan L, Takabe K. A prognostic score based on long-term survivor unique transcriptomic signatures predicts patient survival in pancreatic ductal adenocarcinoma. Am J Cancer Res. 2021;11:4294-4307. [PubMed] |
| 83. | Mitsuhashi K, Nosho K, Sukawa Y, Matsunaga Y, Ito M, Kurihara H, Kanno S, Igarashi H, Naito T, Adachi Y, Tachibana M, Tanuma T, Maguchi H, Shinohara T, Hasegawa T, Imamura M, Kimura Y, Hirata K, Maruyama R, Suzuki H, Imai K, Yamamoto H, Shinomura Y. Association of Fusobacterium species in pancreatic cancer tissues with molecular features and prognosis. Oncotarget. 2015;6:7209-7220. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 301] [Cited by in RCA: 288] [Article Influence: 28.8] [Reference Citation Analysis (0)] |