Published online Aug 21, 2022. doi: 10.3748/wjg.v28.i31.4417
Peer-review started: March 3, 2022
First decision: April 25, 2022
Revised: May 19, 2022
Accepted: July 25, 2022
Article in press: July 25, 2022
Published online: August 21, 2022
Processing time: 166 Days and 5.7 Hours
Autoimmune liver disease (AILD) has been considered a relatively uncommon disease in China, epidemiological data for AILD in patients with cirrhosis and acute decompensation (AD) is sparse.
To investigate the prevalence, outcome and risk factors for AILD in cirrhotic patients complicated with AD in China.
We collected data from patients with cirrhosis and AD from two prospective, multicenter cohorts in hepatitis B virus endemic areas. Patients were regularly followed up at the end of 28-d, 90-d and 365-d, or until death or liver transplantation (LT). The primary outcome in this study was 90-d LT-free mortality. Acute-on-chronic liver failure (ACLF) was assessed on admission and during 28-d hospitalization, according to the diagnostic criteria of the European Association for the Study of the Liver (EASL). Risk factors for death were analyzed with logistic regression model.
In patients with cirrhosis and AD, the overall prevalence of AILD was 9.3% (242/2597). Prevalence of ACLF was significantly lower in AILD cases (14%) than those with all etiology groups with cirrhosis and AD (22.8%) (P < 0.001). Among 242 enrolled AILD patients, the prevalence rates of primary biliary cirrhosis (PBC), autoimmune hepatitis (AIH) and PBC-AIH overlap syndrome (PBC/AIH) were 50.8%, 28.5% and 12.0%, respectively. In ACLF patients, the proportions of PBC, AIH and PBC/AIH were 41.2%, 29.4% and 20.6%. 28-d and 90-d mortality were 43.8% and 80.0% in AILD-related ACLF. The etiology of AILD had no significant impact on 28-d, 90-d or 365-d LT-free mortality in patients with cirrhosis and AD in both univariate and multivariate analysis. Total bilirubin (TB), hepatic encephalopathy (HE) and blood urea nitrogen (BUN) were independent risk factors for 90-d LT-free mortality in multivariate analysis. The development of ACLF during hospitalization only independently correlated to TB and international normalized ratio.
AILD was not rare in hospitalized patients with cirrhosis and AD in China, among which PBC was the most common etiology. 90-d LT-free mortality were independently associated with TB, HE and BUN.
Core Tip: Autoimmune liver disease (AILD) has been regarded as a relatively rare disease in China. Our study reported that the overall prevalence of AILD was 9.3% hospitalized patients with cirrhosis and acute decompensation, among which primary biliary cirrhosis was the most prevalent type. In AILD patients with cirrhosis and acute decompensation, the etiology types of AILD had no significant effect on short-term mortality, total bilirubin, hepatic encephalopathy (HE) and blood urea nitrogen were independently associated with 90-d mortality in multivariate analysis. Strategies are needed to prevent presence of HE and closely monitor the changes of liver and renal function in clinical practice. These data will be a crucial complement to the public epidemiology research on AILD in Asian-Pacific regions.
- Citation: Shen ZX, Wu DD, Xia J, Wang XB, Zheng X, Huang Y, Li BL, Meng ZJ, Gao YH, Qian ZP, Liu F, Lu XB, Shang J, Yan HD, Zheng YB, Gu WY, Zhang Y, Wei JY, Tan WT, Hou YX, Zhang Q, Xiong Y, Zou CC, Chen J, Huang ZB, Jiang XH, Luo S, Chen YY, Gao N, Liu CY, Yuan W, Mei X, Li J, Li T, Zhou XY, Deng GH, Chen JJ, Ma X, Li H. Prevalence and clinical characteristics of autoimmune liver disease in hospitalized patients with cirrhosis and acute decompensation in China. World J Gastroenterol 2022; 28(31): 4417-4430
- URL: https://www.wjgnet.com/1007-9327/full/v28/i31/4417.htm
- DOI: https://dx.doi.org/10.3748/wjg.v28.i31.4417
Autoimmune liver disease (AILD) includes primary biliary cirrhosis (PBC), autoimmune hepatitis (AIH), primary sclerosing cholangitis (PSC) and immunoglobulin G 4-related diseases (IgG4-RD)[1-3]. Some patients display clinical manifestations of two different entities based on clinical and histological features, which is described as “overlap syndrome”, among which PBC-AIH overlap syndrome (PBC/AIH) is the most common[4]. AILD has been considered to be a relatively rare etiology of chronic liver disease (CLD) in China where viral hepatitis has a high prevalence. However, recent findings indicated that the prevalence of AILD is increasing in the Asia-Pacific region. In Japan, AIH prevalence increased from 8.7 to 23.9 per 100000 population from 2004 to 2016[5]. In a city in northern China, PBC prevalence rose from 0.5 to 8.0 per 100000 population in the past 10 years[6]. Several studies have illustrated the prevalence and clinical characteristics of AILD in general population[7-9]. Few studies have been conducted to investigate the nature history of AILD, especially in the late stage of CLD-cirrhosis and acute decompensation (AD).
The Chinese Acute on Chronic Liver Failure (CATCH-LIFE) study consisted of two prospective, multicenter cohorts that enrolled hospitalized patients with CLD of various etiologies, most of whom had cirrhosis and complicated with AD. It is nationally representative of hospitalized CLD patients in China. In this study, we used the data from the CATCH-LIFE study to investigate the following: (1) The prevalence and short-term outcome of AILD in patients with cirrhosis and AD; (2) The impact of AILD etiology on mortality; and (3) Risk factors for 90-d mortality in AILD patients with cirrhosis and AD.
Data were collected from 2 prospective, observational cohorts of the CATCH-LIFE study which enrolled hospitalized patients with CLD and acute events, from January 2015 to December 2016 and July 2018 to January 2019, respectively. The rationale and design of the cohorts have been described elsewhere[10,11]. The medical ethics boards of the Shanghai Renji Hospital, approved the study. Written informed consent were obtained from every participant or his or her legal surrogates before enrollment.
Cirrhosis was diagnosed according to a computed tomography/magnetic resonance imaging scan, clinical symptoms, laboratory tests and medical history[12]. AD was defined as ascites, variceal hemorrhage, hepatic encephalopathy (HE), infection and jaundice within 1 mo[13]. The diagnosis of AILD was exclusionary. The following patients were excluded: Coinfections with other viruses [hepatitis A virus, hepatitis B virus (HBV), hepatitis C virus and hepatitis E virus], alcoholic liver disease, nonalcoholic fatty liver disease, schistosomiasis, metabolic liver diseases, chronic drug-induced liver disease, and cryptogenic liver disease.
PBC was defined according to the American Association for the Study of Liver Diseases clinical guidance in 2008[14], with at least 2 of the following: (1) Elevated serum alkaline phosphatase (ALP) [> 1.5 folds the upper limit of normal (ULN)] or gamma glutamyl transpeptidase (GGT) (> 3 ULN); (2) Positive test for antimitochondrial antibodies (AMA) (titer > 1:40); and (3) Compatible liver biopsy suggestive of suppurative destructive cholangitis (ductopenia, cholestasis, fibrosis and portal inflammation). AIH was defined based on simplified criteria (score > 6) proposed by the International Autoimmune Hepatitis Group in 2008[15]. PBC/AIH overlap syndrome was strictly diagnosed according to the Paris Criteria proposed by Chazouillères et al[16] in 1998, which associated PBC and AIH either synchronously or consecutively. Presence of at least 2 of the 3 criteria was required. PBC criteria were the following: (1) ALP > 2 ULN or GGT > 5 ULN; (2) A positive serology for AMA; and (3) A liver biopsy specimen indicating florid bile duct lesions. AIH criteria were the following: (1) Serum alanine transaminase (ALT) > 5 ULN; (2) IgG > 2 ULN or a positive serology for anti-smooth muscle antibodies; and (3) A liver biopsy indicating moderate to severe periportal or periseptal lymphocytic piecemeal interface hepatitis.
Some uncommon etiologies of AILD in this study, such as PSC, PBC-PSC overlap syndrome, AIH-PSC overlap syndrome and IgG4-RD were categorized into a “others” group, as the number of cases of “others” was quite small to allow robust statistical analysis. Acute-on-chronic liver failure (ACLF) was diagnosed based on the European Association for the Study of the Liver (EASL) criteria[17]. Just as follows: (1) ACLF grade 1: Single kidney failure or single cerebral failure with renal dysfunction (1.5 mg/dL < creatinine < 1.9 mg/dL) or other single organ failure [serum bilirubin ≥ 12 mg/dL for liver; (international normalized ratio) INR = 2.5 for coagulation; vasopressors to maintain arterial pressure for circulation; PaO2/FiO2 ≤ 200 or SpO2/FiO2 ≤ 214 for respiration] with renal dysfunction and/or mild HE; (2) ACLF grade 2: The presence of 2 organ failures; and (3) ACLF grade 3: The failure no less than 3 organs.
Demographics, medical history, imagological examination and laboratory parameters were obtained from every participant on admission. Participants were regularly followed up for 28 d, 90 d and 365 d until death or liver transplantation (LT). Development of ACLF was assessed on admission and during hospitalization within 28 d, respectively. The primary outcome of this study was 90-d LT-free mortality. The secondary outcome was ACLF development during 28-d hospitalization.
Continuous variables were summarized as mean (SD) or median (interquartile range) according to their distribution, while categorical variables were summarized as frequency (proportion). Comparisons between four groups were performed using Kruskal-Wallis test for continuous variables, Chi-square test or Fisher’s exact test for categorical variables. The impact of the etiology of AILD on short-term mortality were analyzed using a multivariable logistic regression model, adjusting for potential confounders, including demographics (age, sex) and AD events (HE, gastrointestinal bleeding, infection and ascites). Logistic regression was also used to determine the risk factors for 90-d mortality and ACLF development during hospitalization. Variables with P values less than 0.1 were included in the multivariate logistic regression model[18,19]. All continuous variables were log2 transformed before entering the model. Two-tailed P values of 0.05 were considered statistically significant. Statistical analysis was conducted using R 3.6.2 and SPSS 19.0.
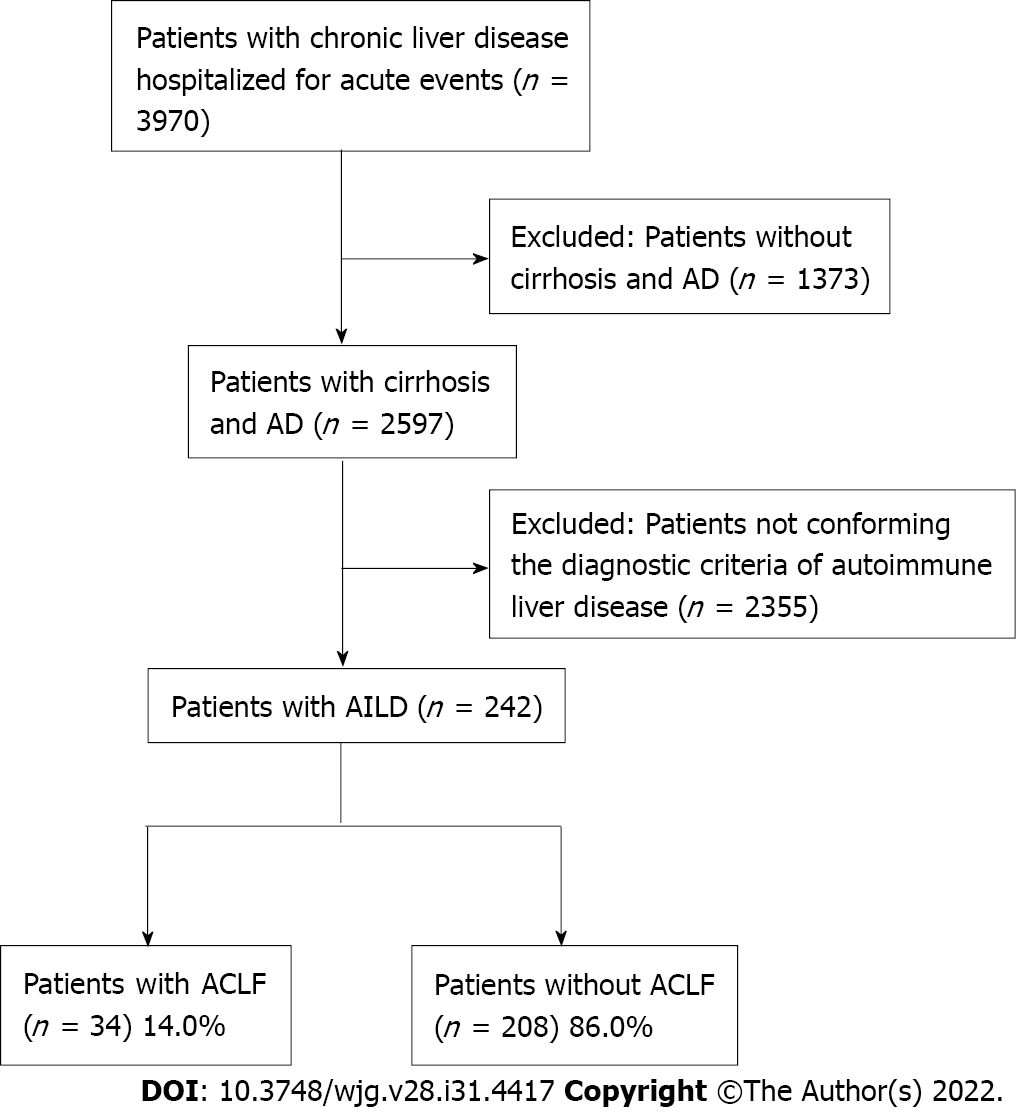
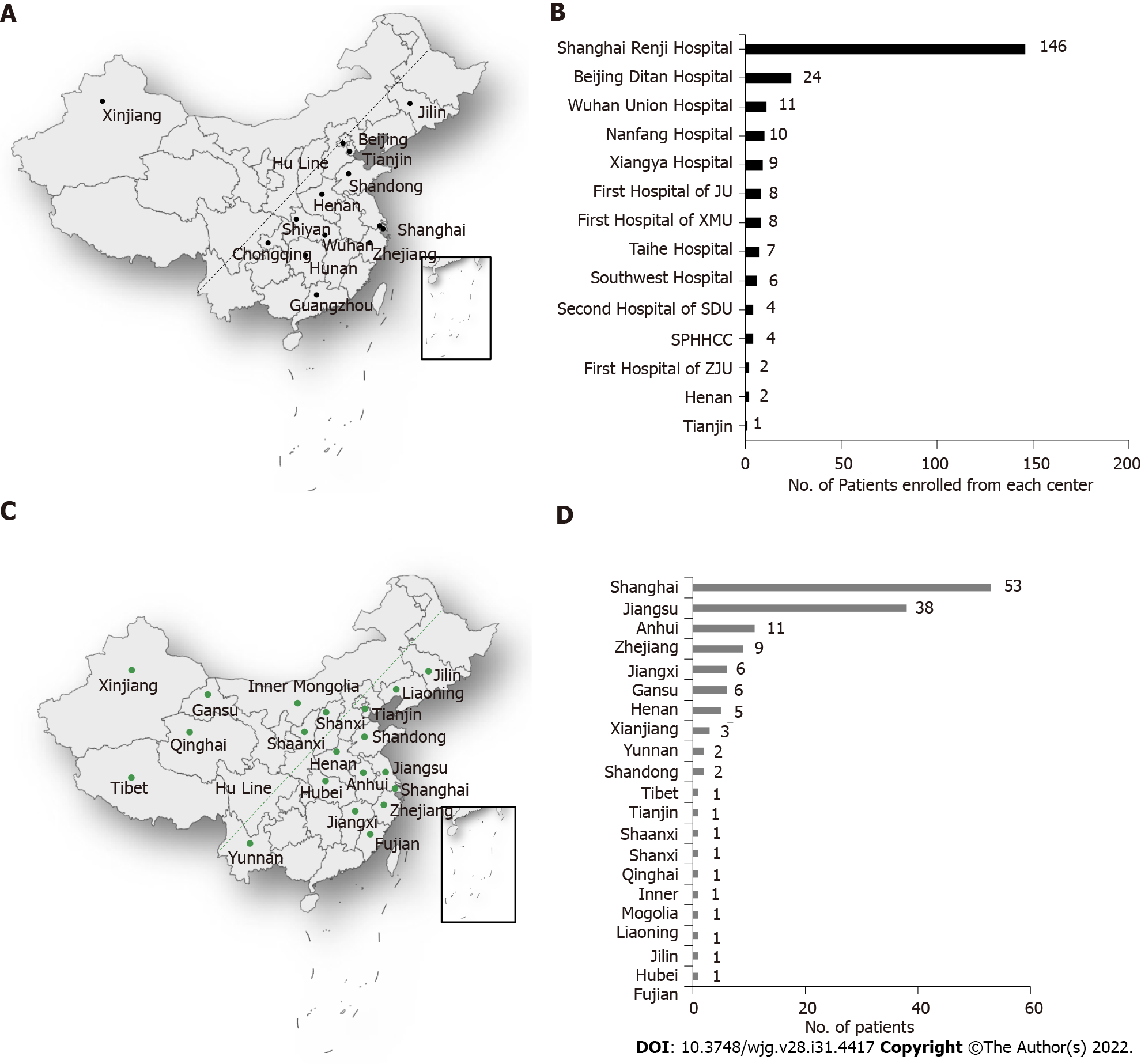
A total of 3970 patients from CATCH-LIFE study were screened, 2597 of whom had cirrhosis and AD. Eventually, 242 patients conforming the diagnostic criteria of AILD were finally enrolled (Figure 1). In patients with cirrhosis and AD, the overall prevalence of AILD was 9.3% (242/2597). Prevalence of ACLF was significantly lower in AILD cases (14%) than those with all etiology groups with cirrhosis and AD (22.8%) (P < 0.001). Distribution of participating centers in this study and number of AILD patients enrolled from each center were displayed in Figures 2A and 2B, respectively. Among the 242 enrolled AILD patients, 234 (96.6%) are from eastern China, where 94% of Chinese population resides; 8 (3.4%) are from western China, where 6% of Chinese population resides. Due to Renji Hospital accounted for 60.3% (146/242) of the total enrolled patients, we further analyzed district distribution of patients enrolled from Renji Hospital. Actually, these patients were from 20 different district all over the country (Figure 2C), the number of patients from each district was shown in Figure 2D. Distribution of patients enrolled from Renji Hospital were also consistent with population density distribution (divided by Hu Line) in China.
Baseline characteristics of 242 AILD patients with cirrhosis and AD categorized by etiology are depicted in Table 1. 123 (50.8%) patients showed PBC, 69 (28.5%) displayed AIH, 29 (12.0%) had AIH-PBC overlap syndrome (AIH/PBC) and 21 (8.7%) patients manifested other uncommon etiologies of AILD. Most patients were female (81.0%) and their mean age was 56.04 years. Patients with PBC/AIH had higher white blood cell (P < 0.001) and neutrophil-lymphocyte (NL) ratio (P < 0.001) levels than patients with PBC, AIH or others. ALT (P < 0.001) and AST (P = 0.04) levels were higher in the AIH/PBC group. In addition, the PBC group tended to have lower hemoglobin (P = 0.003) and higher ALP (P < 0.001) levels than other AILD etiologies.
| PBC | AIH | PBC/AIH | Others | P value | |
| n = 123 | n = 69 | n = 29 | n = 21 | ||
| Demographics | |||||
| Age | 56.00 (48.02, 63.83) | 57.47 (49.33, 65.34) | 52.62 (45.53, 60.85) | 52.94 (41.15, 62.48) | 0.28 |
| Male, no. (%) | 25 (20.3) | 11 (15.9) | 4 (13.8) | 6 (28.6) | 0.51 |
| AD, no. (%) | |||||
| Bacterial infection | 34 (27.6) | 21 (30.4) | 13 (44.8) | 8 (38.1) | 0.30 |
| Ascites | 82 (66.7) | 42 (60.9) | 17 (58.6) | 14 (66.7) | 0.77 |
| Gastrointestinal bleeding | 27 (22.0) | 11 (15.9) | 4 (13.8) | 2 (9.5) | 0.42 |
| Hepatic encephalopathy | 10 (8.1) | 4 (5.8) | 6 (20.7) | 3 (14.3) | 0.11 |
| Jaundice | 61 (49.6) | 35 (50.7) | 19 (65.5) | 9 (42.9) | 0.38 |
| Laboratory results, median (IQR) | |||||
| TB, mg/dL | 4.88 (1.60, 10.82) | 5.15 (1.86, 11.14) | 7.31 (3.04, 23.60) | 4.88 (2.05, 11.72) | 0.17 |
| INR | 1.29 (1.13, 1.52) | 1.39 (1.23, 1.64) | 1.39 (1.16, 1.49) | 1.24 (1.15, 1.44) | 0.044 |
| Creatinine, mg/dL | 0.65 (0.50, 0.88) | 0.64 (0.51, 0.76) | 0.67 (0.53, 0.79) | 0.67 (0.45, 0.73) | 0.51 |
| Blood urea nitrogen, mmol/L | 5.38 (3.70, 8.27) | 4.73 (3.63, 5.95) | 5.37 (3.90, 7.20) | 4.50 (3.37, 6.60) | 0.26 |
| White blood cell, 109/L | 4.39 (2.75, 6.34) | 4.01 (2.81, 5.78) | 6.85 (4.56, 11.76) | 3.82 (3.10, 5.72) | 0.001 |
| Neutrophil-lymphocyte ratio | 2.80 (1.73, 4.29) | 2.00 (1.25, 3.25) | 3.61 (3.01, 5.67) | 2.89 (2.35, 4.86) | < 0.001 |
| Platelet, 109/L | 0.17 (0.11, 0.30) | 0.15 (0.11, 0.25) | 0.32 (0.14, 0.45) | 0.12 (0.08, 0.30) | 0.11 |
| Albumin, g/L | 30.30 (25.70, 33.70) | 28.50 (25.70, 32.55) | 29.90 (26.10, 33.80) | 30.40 (28.45, 34.92) | 0.262 |
| ALT, IU/L | 48.00 (24.50, 80.50) | 59.00 (37.00, 116.00) | 81.00 (61.00, 148.00) | 57.90 (39.00, 85.40) | < 0.001 |
| AST, IU/L | 79.60 (48.40, 124.50) | 87.00 (52.90, 187.00) | 120.70 (79.00, 153.00) | 90.00 (39.00, 148.00) | 0.037 |
| Hemoglobin, g/L | 90.00 (68.00, 110.00) | 99.00 (87.00, 119.00) | 99.00 (85.00, 115.00) | 98.00 (89.00, 112.00) | 0.003 |
| ALP, IU/L | 208.50 (136.25, 313.00) | 143.00 (105.74, 181.70) | 175.50 (128.50, 243.00) | 202.50 (133.50, 298.85) | < 0.001 |
| GGT, IU/L | 105.00 (51.23, 192.50) | 86.63 (37.00, 126.00) | 106.20 (58.70, 235.25) | 122.70 (32.92, 220.22) | 0.19 |
| Pre-albumin, mg/L | 78.30 (46.80, 113.90) | 63.20 (40.93, 83.25) | 77.30 (57.75, 97.80) | 68.65 (54.75, 88.90) | 0.25 |
| Score, median (IQR) | |||||
| Child Turcotte Pugh | 9.00 (8.00, 10.00) | 9.00 (8.00, 10.25) | 10.00 (8.00, 11.00) | 9.00 (8.00, 11.00) | 0.58 |
| MELD | 11.00 (7.00, 17.00) | 15.00 (11.00, 21.00) | 18.00 (14.00, 23.00) | 12.00 (10.00, 16.00) | < 0.001 |
| MELD Na | 14.00 (7.75, 19.00) | 17.00 (12.00, 23.00) | 20.50 (15.75, 25.00) | 13.00 (10.00, 19.00) | < 0.001 |
| CLIF SOFA | 5.00 (3.00, 6.00) | 5.00 (3.00, 6.00) | 6.00 (3.00, 7.00) | 5.00 (4.00, 6.00) | 0.44 |
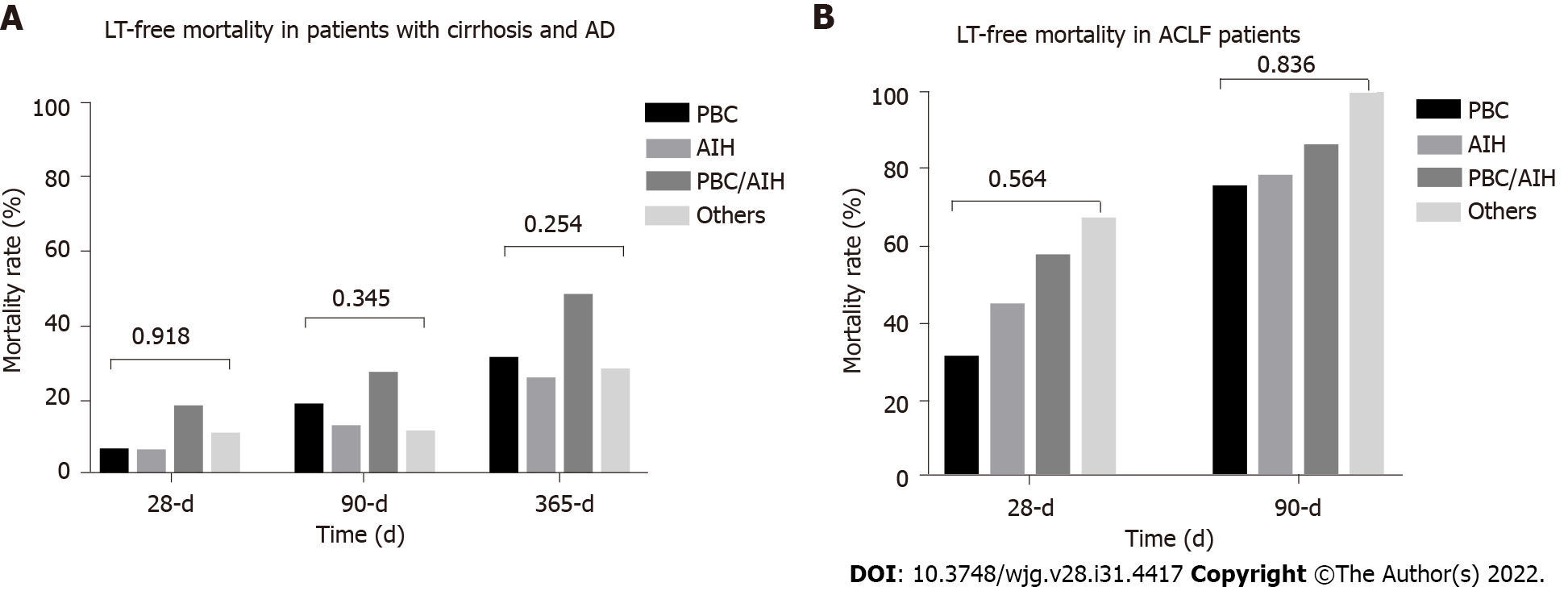
Baseline characteristics of 34 ACLF patients categorized by etiology are shown in Table 2. PBC (41.2%) was still the most common etiology among ACLF patients with AILD, followed by AIH (29.4%), AIH/PBC overlap syndrome (20.6%) and others (8.8%). However, as the number of patients in some subgroups was small, the results may not be robust and require further validation in a larger population. At the end of 28-d and 90-d, no patients were lost to follow up. At the end of 365-d, 3 (1.2%) patients were lost to follow-up. Short-term (28-d, 90-d and 365-d) LT-free mortality of AILD patients with cirrhosis and AD are presented in Figure 3. Generally, among AILD patients with cirrhosis and AD, 90-d LT-free mortality were 17%. AIH/PBC had higher 28-d, 90-d and 365-d mortality, although the results were not statistically significant. Among patients with AILD-related ACLF, 28-d and 90-d mortality were 43.8% and 80.0%, respectively.
| Variable | PBC | AIH | PBC/AIH | Others | P value |
| n = 14 | n = 10 | n = 7 | n = 3 | ||
| Demographics | |||||
| Age, median (IQR) | 55.34 (48.88, 58.96) | 55.94 (50.00, 64.01) | 53.46 (47.97, 63.46) | 50.85 (46.87, 58.00) | 0.88 |
| Male, no. (%) | 6 (42.9) | 1 (10.0) | 0 (0.0) | 2 (66.7) | 0.04 |
| AD, no. (%) | |||||
| Bacterial infection | 7 (50.0) | 3 (30.0) | 6 (85.7) | 2 (66.7) | 0.14 |
| Ascites | 9 (64.3) | 6 (60.0) | 5 (71.4) | 3 (100.0) | 0.61 |
| Gastrointestinal bleeding | 1 (7.1) | 1 (10.0) | 0 (0.0) | 1 (33.3) | 0.39 |
| Hepatic encephalopathy | 4 (28.6) | 3 (30.0) | 4 (57.1) | 2 (66.7) | 0.40 |
| Jaundice | 10 (71.4) | 9 (90.0) | 6 (85.7) | 2 (66.7) | 0.64 |
| Laboratory results, median (IQR) | |||||
| TB, mg/dL | 16.78 (4.79, 27.10) | 12.56 (8.94, 17.64) | 23.60 (14.64, 27.52) | 14.56 (8.94, 19.29) | 0.71 |
| INR | 1.57 (1.34, 1.77) | 2.64 (1.67, 2.80) | 1.49 (1.44, 2.31) | 1.50 (1.47, 1.97) | 0.35 |
| Creatinine, mg/dL | 0.83 (0.79, 2.01) | 0.58 (0.50, 0.74) | 0.79 (0.75, 0.84) | 0.68 (0.36, 1.46) | 0.08 |
| Blood urea nitrogen, mmol/L | 12.05 (6.04, 20.65) | 5.40 (2.74, 8.12) | 7.70 (7.20, 9.25) | 8.73 (6.92, 21.00) | 0.09 |
| White blood cell, 109/L | 5.55 (3.62, 10.19) | 4.57 (3.13, 5.34) | 8.82 (6.55, 16.27) | 6.71 (4.91, 9.37) | 0.08 |
| Neutrophil-lymphocyte ratio | 4.55 (3.78, 5.84) | 1.80 (1.06, 4.40) | 7.64 (5.66, 12.77) | 5.40 (4.86, 6.30) | 0.05 |
| Albumin, g/L | 28.90 (26.75, 33.72) | 27.80 (25.38, 31.48) | 27.20 (25.05, 28.60) | 27.40 (25.00, 31.15) | 0.60 |
| ALT, IU/L | 105.15 (33.30, 186.90) | 59.05 (38.65, 99.47) | 113.90 (102.60, 204.10) | 69.90 (57.55, 146.65) | 0.37 |
| AST, IU/L | 121.50 (62.72, 182.23) | 99.45 (61.10, 172.75) | 153.00 (111.65, 238.05) | 34.20 (30.30, 85.15) | 0.33 |
| Hemoglobin, g/L | 88.00 (40.75, 138.00) | 64.00 (33.00, 116.00) | 65.00 (46.00, 77.50) | 57.00 (55.20, 74.50) | 0.89 |
| ALP, IU/L | 76.00 (69.00, 96.25) | 86.50 (82.00, 90.75) | 108.00 (89.00, 127.50) | 91.40 (65.80, 102.90) | 0.29 |
| GGT, IU/L | 187.00 (111.25, 252.80) | 149.00 (125.65, 195.00) | 153.00 (109.00, 365.00) | NA (NA, NA) | 0.76 |
| Pre-albumin, mg/L | 89.00 (52.00, 119.57) | 106.50 (45.25, 116.50) | 63.00 (58.70, 258.60) | NA (NA, NA) | 0.85 |
| Albumin, g/L | 66.50 (54.60, 115.00) | 33.40 (23.55, 60.40) | 87.65 (60.35, 91.55) | NA (NA, NA) | 0.13 |
| Score, median (IQR) | |||||
| Child Turcotte Pugh | 9.50 (8.25, 10.75) | 12.00 (10.00, 12.75) | 11.00 (10.50, 12.50) | 11.00 (11.00, 12.50) | 0.04 |
| MELD | 20.00 (18.00, 22.75) | 27.50 (18.50, 28.75) | 24.00 (22.00, 28.00) | 21.00 (20.50, 23.00) | 0.17 |
| MELD Na | 21.00 (19.00, 25.00) | 26.50 (23.00, 28.75) | 29.00 (23.00, 32.00) | 41.00 (39.00, 41.50) | 0.12 |
| CLIF SOFA | 7.00 (6.25, 8.00) | 7.00 (7.00, 8.00) | 8.00 (6.50, 9.00) | 9.00 (8.00, 10.00) | 0.32 |
To investigate the effect of etiology on short-term (28-d, 90-d and 365-d) LT-free mortality in AILD patients with cirrhosis and AD, we constructed 3 models to gradually control other potential confounding factors (Table 3). Both univariate (unadjusted) and multivariate (adjusted I and adjusted II) analyses showed that compared to PBC, AIH patients were at lower risk for death at 90-d and 365-d, whereas PBC/AIH patients were at higher risk for death at all time periods. However, none of the associations were statistically significant. Subgroup analysis was conducted according to Child Turcotte Pugh and model for end-stage liver disease (MELD) score, there was no heterogeneity in the impact of etiology types on 90-d LT-free mortality in different AILD subgroups, as was shown in Supplemen
| n | Num of death (percentage) | Unadjusted OR (95%CI), P value | Adjusted I1, OR (95%CI), P value | Adjusted II2, OR (95%CI), P value | |
| 28-d LT-free mortality | |||||
| PBC | 111 | 7 (6.3) | 1.0 | 1.0 | 1.0 |
| AIH | 67 | 4 (6.0) | 0.94 (0.24-3.25), 0.93 | 0.94 (0.24-3.24), 0.92 | 1.26 (0.3-4.85), 0.74 |
| PBC/AIH | 28 | 5 (17.9) | 3.23 (0.89-11.05), 0.06 | 3.43 (0.93-11.93), 0.05 | 2.61 (0.63-10.21), 0.17 |
| Others | 19 | 2 (10.5) | 1.75 (0.25-7.99), 0.51 | 1.75 (0.24-8.13), 0.51 | 1.46 (0.17-8.28), 0.70 |
| 90-d LT-free mortality | |||||
| PBC | 103 | 19 (18.4) | 1.0 | 1.0 | 1.0 |
| AIH | 64 | 8 (12.5) | 0.63 (0.25-1.50), 0.31 | 0.64 (0.25-1.53), 0.33 | 0.74 (0.27-1.88), 0.54 |
| PBC/AIH | 26 | 7 (26.9) | 1.63 (0.57-4.31), 0.34 | 1.70 (0.59-4.56), 0.30 | 1.18 (0.36-3.56), 0.77 |
| Others | 18 | 2 (11.1) | 0.55 (0.08-2.17), 0.45 | 0.51 (0.08-2.02), 0.40 | 0.44 (0.05-2.08), 0.36 |
| 365-d LT-free mortality | |||||
| PBC | 97 | 30 (30.9) | 1.0 | 1.0 | 1.0 |
| AIH | 63 | 16 (25.4) | 0.76 (0.37-1.54), 0.45 | 0.76 (0.36-1.53), 0.44 | 0.81 (0.38-1.68), 0.58 |
| PBC/AIH | 23 | 11 (47.8) | 2.05 (0.9-5.20), 0.13 | 2.06 (0.81-5.24), 0.13 | 1.72 (0.63-4.61), 0.28 |
| Others | 18 | 5 (27.8) | 0.86 (0.26-2.51), 0.79 | 0.89 (0.26-2.62), 0.84 | 0.82 (0.24-2.49), 0.74 |
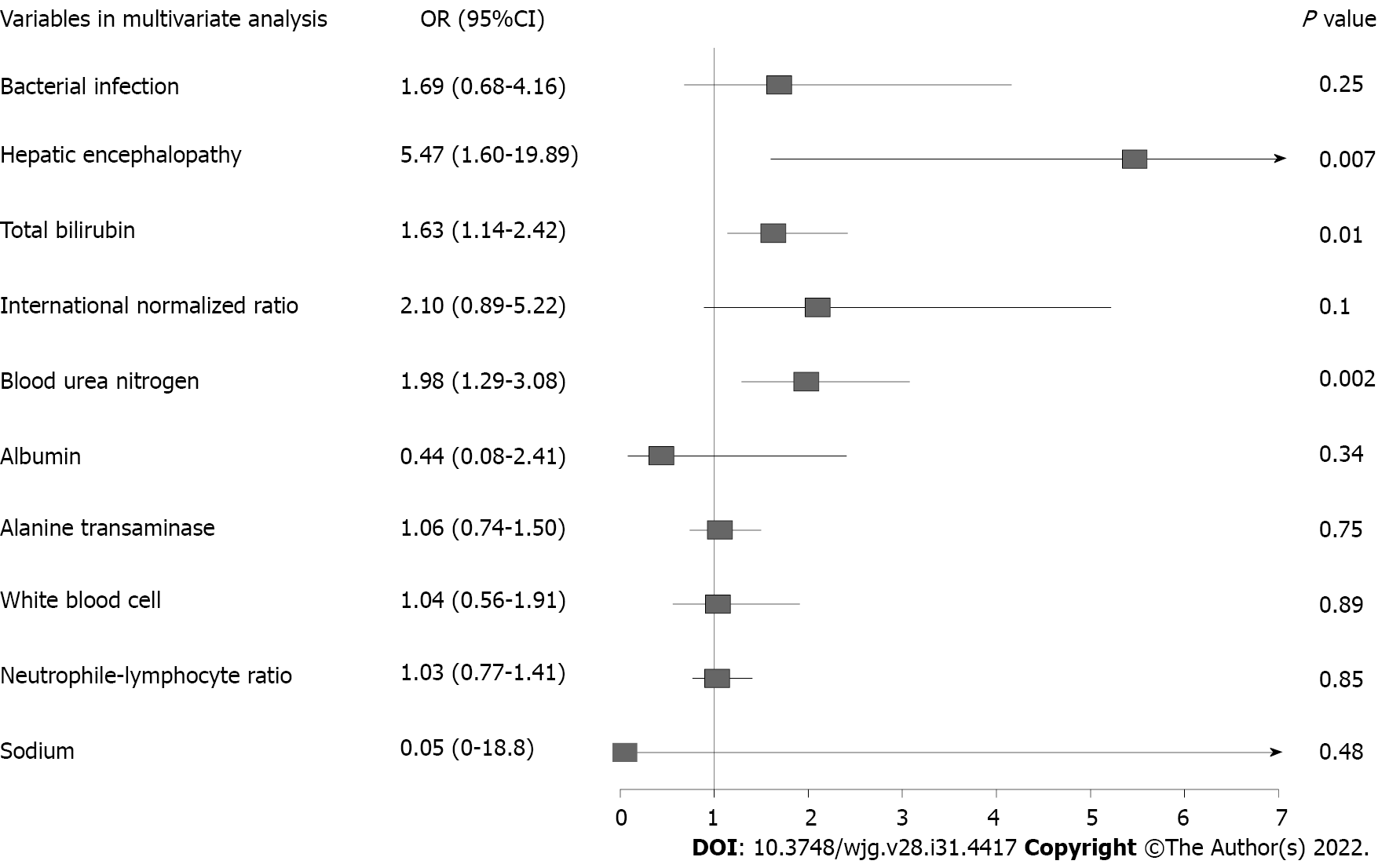
Logistic regression model was conducted to assess the risk factors for 90-d LT-free mortality. Univariable analysis identified 10 variables on admission correlated with 90-d prognosis: Bacterial infection, HE, total bilirubin (TB), INR, blood urea nitrogen (BUN), albumin, ALT, white blood cell, NL-ratio and sodium (Supplementary Table 1). Variables with P < 0.1 were selected into multivariable model for further analysis. Only HE, TB and BUN were found to be independently associated with 90-d mortality in AILD patients with cirrhosis and AD (Figure 4). We also investigated risk factors for ACLF development during hospitalization (Table 4). 21 ACLF patients diagnosed on admission were excluded from analysis. Multivariable analysis revealed that only TB (P = 0.046) and INR (P = 0.048) independently correlated with ACLF development.
| OR | 95%CI | P value | |
| Univariable logistic regression | |||
| Demographic data | |||
| Age | 0.96 | 0.21-6.18 | 0.96 |
| Male | 2.89 | 0.83-9.17 | 0.08 |
| Etiology | 0.90 | 0.43-1.70 | 0.76 |
| Acute decompensation events | |||
| Overt ascites | 0.92 | 0.3-3.14 | 0.89 |
| Gastrointestinal bleeding | 0.74 | 0.11-2.9 | 0.7 |
| Bacterial infection | 2.22 | 0.69-6.95 | 0.17 |
| Hepatic encephalopathy | 3.6 | 0.51-15.95 | 0.13 |
| Laboratory data | |||
| Total bilirubin | 1.68 | 1.14-2.61 | 0.012 |
| International normalized ratio | 6.53 | 1.9-28.55 | 0.006 |
| Creatinine | 0.85 | 0.51-1.87 | 0.6 |
| Blood urea nitrogen | 1.52 | 0.84-2.63 | 0.12 |
| Albumin | 0.44 | 0.07-3.19 | 0.4 |
| ALT | 1.25 | 0.85-1.82 | 0.24 |
| AST | 1.14 | 0.73-1.75 | 0.55 |
| White blood cells | 0.88 | 0.45-1.75 | 0.72 |
| Hemoglobin | 0.39 | 0.11-1.5 | 0.16 |
| K | 1.53 | 0.13-18.28 | 0.74 |
| Sodium | 0 | 0-288.55 | 0.29 |
| Neutrophil-lymphocyte ratio | 1.37 | 0.84-2.23 | 0.21 |
| ALP | 1.15 | 0.59-2.27 | 0.7 |
| GGT | 0.86 | 0.54-1.34 | 0.52 |
| Multivariable logistic regression | |||
| Male | 3.24 | 0.84-11.63 | 0.07 |
| TB | 1.56 | 1.03-2.47 | 0.046 |
| INR | 4.31 | 1.15-21.70 | 0.048 |
The current study is the first nationwide investigation of the prevalence and short-term outcome of end-stage AILD in China, which is a traditional HBV high endemic area. Our study showed that the overall prevalence of AILD was 9.3% in tertiary hospitalized patients with cirrhosis and AD. PBC was responsible for half of AILD cases. The prevalence of ACLF was significantly lower in AILD patients than those with all etiology groups with cirrhosis and AD. Short-term mortality were extremely high in AILD-related ACLF.
EASL-CLIF study reported that the prevalence of ACLF was 30.9% in alcohol-related cirrhosis and AD, whereas COSSH study showed that the prevalence of ACLF reached up to 26.2% in patients with HBV-related cirrhosis and AD[17,20]. However, in this study, among 242 AILD patients with cirrhosis and AD, only 34 (14%) had ACLF, the prevalence was significantly lower than that of patients with other etiology types. One plausible explanation may be that AILD patients have distinct clinical characteristics (higher severity, higher prevalence of infection and lower prevalence of organ failures) that differ distinctly from those of patients with other etiologies, which led to lower prevalence of ACLF but higher short-term mortality.
Despite having higher levels of inflammation and liver injury, PBC/AIH had similar short-term mortality rates compared to PBC individually after adjusting for confounding factors. Neuhauser et al[21] and Yang et al[22] showed that a more aggressive clinical course and worse clinical consequences were observed in patients with PBC/AIH than patients with pure PBC. Based on these results, some experts suggested that PBC/AIH should be identified and aggressively treated. Notably, these studies only evaluate the univariate prognostic value of etiology and did not take confounders into consideration. It is, therefore, likely that they fail to objectively reveal the independent impact of etiology on the prognosis of AILD. PBC, AIH and PBC/AIH are all complex disorders but result in significant morbidity and mortality. Once progressed to liver failure, no effective treatment is clinically available. Herein, our results clearly showed that presence of HE, higher TB and BUN levels, rather than liver disease etiologies, were independently associated with short-term mortality in AILD patients with cirrhosis and AD. Therefore, in clinical management of AILD, physicians are supposed to pay more attention to the presence of HE and closely monitor the changes of liver and renal function.
This study had several strengths. With national samples, our multicenter data provided an overview of epidemiological features of end-stage AILD in China, where it is considered uncommon. Thus, we presented a national view of the disease. Indeed, most of the studies in this field originated from single center or other geographic areas[7,23-25]. We focused on the role of the etiology on short-term mortality. Moreover, we clarified that HE, TB and BUN were significant risk factors of short-term mortality in AILD patients with cirrhosis and AD. These data will be an important complement to the public epidemiological data of AILDs in Asian-Pacific regions.
There were still several limitations in our study. First, since AILD is a relatively rare disease, the number of patients recruited in our study was not quite large, especially ACLF patients. The limited sample size could also slightly reduce the accuracy of the results of risk factor analysis. Second, one of our centers, Renji hospital, is a nationwide center of AILD. Thus, some degree of selection bias could be present in the prevalence rates of AILD due to the participation of this center. However, our further analysis showed that patients recruited from Renji Hospital were from 20 different districts all over the country, the population distribution of these patients were also consistent with that of Chinese population density.
In conclusion, our study showed that AILD was not rare in China. The etiology of AILD had no significant impact on short-term mortality in AILD patients with cirrhosis and AD. HE, elevated levels of TB and BUN were significantly associated with high 90-d mortality in these patients. Strategies are needed to the presence of HE and closely monitor the changes of liver and renal function in clinical practice. These data will be a crucial complement to the public epidemiology of AILD in Asian-Pacific regions.
Epidemiological research on autoimmune liver disease (AILD) in patients with end-stage liver disease is sparse in Asian-pacific areas.
This study aimed to provide a national view of epidemiological features of end-stage AILD in China.
To investigate the disease burden and risk factor of short-term mortality in AILD patients with cirrhosis and acute decompensation (AD), and thus provide clues on clinical management.
Data were collected from two prospective, multicenter cohorts which enrolled patients with chronic liver disease and acute exacerbation from China. Logistic regression model was conducted to analyze risk factors.
The overall prevalence of AILD was 9.3% (242/2597) in patients with cirrhosis and AD, among which primary biliary cirrhosis was the most prevalent type. The etiology of AILD had no significant impact on short-term mortality in patients with cirrhosis and AD in univariate and multivariate analysis. Total bilirubin, hepatic encephalopathy (HE) and blood urea nitrogen independently correlated with 90-d LT-free mortality in multivariate analysis.
AILD was not rare in hospitalized patients with cirrhosis and AD in China. 90-d mortality was independently associated with severity of the disease.
In clinical management of AILD, strategies are needed to prevent presence of HE and closely monitor the changes of liver and renal function.
We acknowledge the following Chinese (Acute on) Chronic Liver Failure Consortium members and participants for their hard work. Department of Gastroenterology, Renji Hospital, School of Medicine, Shanghai Jiao Tong University - Shi-Jin Wang, Wen-Yi Gu, Liang Qiao, Yan Zhang; Clinical Research Institute, Shanghai Jiao Tong University School of Medicine, Shanghai, China, Zhang Wei-Tuo; Centre of Integrative Medicine, Beijing Ditan Hospital, Capital Medical University - Qun Zhang, Yi-Xin Hou, Yu-Xin Li, Yun-Yi Huang; Department of Infectious Diseases, Southwest Hospital, Third Military Medical University (Army Medical University) - Jie Xia; Yi Zhou; Bao-Yan Xu; Shu-Ning Sun, Yun-Jie Dan, Wen-Ting Tan; Department of Infectious Disease, Hunan Key Laboratory of Viral Hepatitis, Xiangya Hospital, Central South University - Jun Chen, Ruo-Chan Chen, Xiao-Xiao Liu; Department of Infectious Diseases, Institute of Infection and Immunology, Union Hospital, Tongji Medical College, Huazhong University of Science and Technology - Jing Liu, Ling Xu, Shue Xiong; Hepatology Unit, Department of Infectious Diseases, Nan-fang Hospital, Southern Medical University - Xiu-Hua Jiang, Bei-Ling Li, Cong-Yan Zhu; Department of Hepatology, First Hospital of Jilin University - Chang Jiang, Xiao-Yu Wen, Na Gao, Chun-Yan Liu; Department of Infectious Disease, Taihe Hospital, Hubei University of Medicine - Yuan-Yuan Chen, Sen Luo, Qing Lei; Department of Liver Intensive Care Unit, Shanghai Public Health Clinical Centre, Fudan University - Xue Mei, Liu-Juan Ji, Jie-Fei Wang; Department of Infectious Diseases and Hepatology, Second Hospital of Shandong University - Tao Li, Xuan-Qiong Fang, Zi-Yu Wang; Liver Disease Centre, First Affiliated Hospital of Xinjiang Medical University - Rong-Jiu Zheng, Nan Li; Department of Infectious Disease, Henan Provincial People’s Hospital - Hui-Ming Jin; Affiliated Hospital of Logistics University of People’s Armed Police Force - Hai Li, Qing Zhang, Xue-Qun Zheng; Department of Infectious Diseases, Affiliated Hospital of Logistics University of People’s Armed Police Force - Shao-Yang Wang. We thank all the patients participated in the study.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Haile D, Ethiopia; Hayat U, United States S-Editor: Wang JJ L-Editor: A P-Editor: Wang JJ
| 1. | Carbone M, Neuberger JM. Autoimmune liver disease, autoimmunity and liver transplantation. J Hepatol. 2014;60:210-223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 193] [Cited by in RCA: 165] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 2. | Floreani A, De Martin S, Secchi MF, Cazzagon N. Extrahepatic autoimmunity in autoimmune liver disease. Eur J Intern Med. 2019;59:1-7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 31] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 3. | Chang C, Tanaka A, Bowlus C, Gershwin ME. The use of biologics in the treatment of autoimmune liver disease. Expert Opin Investig Drugs. 2020;29:385-398. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 4. | Zhang H, Yang J, Zhu R, Zheng Y, Zhou Y, Dai W, Wang F, Chen K, Li J, Wang C, Li S, Liu T, Abudumijiti H, Zhou Z, Wang J, Lu W, Xia Y, Lu J, Guo C. Combination therapy of ursodeoxycholic acid and budesonide for PBC-AIH overlap syndrome: a meta-analysis. Drug Des Devel Ther. 2015;9:567-574. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 13] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 5. | Katsumi T, Ueno Y. Epidemiology and surveillance of autoimmune hepatitis in Asia. Liver Int. 2022;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 24] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 6. | Zhang L, Bai SS. Epidemiological survey of hospitalized patients with primary biliary cirrhosis in urban areas of Hohhot. World Latest Med Information. 2016;16:6-7. |
| 7. | Jeong SH. Current epidemiology and clinical characteristics of autoimmune liver diseases in South Korea. Clin Mol Hepatol. 2018;24:10-19. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 19] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 8. | Abe M, Mashiba T, Zeniya M, Yamamoto K, Onji M, Tsubouchi H; Autoimmune Hepatitis Study Group-Subgroup of the Intractable Hepato-Biliary Disease Study Group in Japan. Present status of autoimmune hepatitis in Japan: a nationwide survey. J Gastroenterol. 2011;46:1136-1141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 83] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 9. | Lv T, Chen S, Li M, Zhang D, Kong Y, Jia J. Regional variation and temporal trend of primary biliary cholangitis epidemiology: A systematic review and meta-analysis. J Gastroenterol Hepatol. 2021;36:1423-1434. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 76] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 10. | Gu WY, Xu BY, Zheng X, Chen J, Wang XB, Huang Y, Gao YH, Meng ZJ, Qian ZP, Liu F, Lu XB, Shang J, Li H, Wang SY, Sun X. Acute-on-Chronic Liver Failure in China: Rationale for Developing a Patient Registry and Baseline Characteristics. Am J Epidemiol. 2018;187:1829-1839. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 49] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 11. | Qiao L, Wang X, Deng G, Huang Y, Chen J, Meng Z, Zheng X, Shi Y, Qian Z, Liu F, Gao Y, Lu X, Liu J, Gu W, Zhang Y, Wang T, Wu D, Dong F, Sun X, Li H. Cohort profile: a multicentre prospective validation cohort of the Chinese Acute-on-Chronic Liver Failure (CATCH-LIFE) study. BMJ Open. 2021;11:e037793. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 24] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 12. | Tsochatzis EA, Bosch J, Burroughs AK. Liver cirrhosis. Lancet. 2014;383:1749-1761. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1139] [Cited by in RCA: 1311] [Article Influence: 119.2] [Reference Citation Analysis (0)] |
| 13. | Zhang Y, Xu BY, Wang XB, Zheng X, Huang Y, Chen J, Meng ZJ, Gao YH, Qian ZP, Liu F, Lu XB, Shi Y, Shang J, Li H, Wang SY, Yin S, Sun SN, Hou YX, Xiong Y, Li BL, Lei Q, Gao N, Ji LJ, Li J, Jie FR, Zhao RH, Liu JP, Lin TF, Chen LY, Tan WT, Zhang Q, Zou CC, Huang ZB, Jiang XH, Luo S, Liu CY, Zhang YY, Li T, Ren HT, Wang SJ, Deng GH, Xiong SE, Liu XX, Wang C, Yuan W, Gu WY, Qiao L, Wang TY, Wu DD, Dong FC, Hua J. Prevalence and Clinical Significance of Portal Vein Thrombosis in Patients With Cirrhosis and Acute Decompensation. Clin Gastroenterol Hepatol. 2020;18:2564-2572.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 47] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 14. | Lindor KD, Gershwin ME, Poupon R, Kaplan M, Bergasa NV, Heathcote EJ; American Association for Study of Liver Diseases. Primary biliary cirrhosis. Hepatology. 2009;50:291-308. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 933] [Cited by in RCA: 896] [Article Influence: 56.0] [Reference Citation Analysis (0)] |
| 15. | Hennes EM, Zeniya M, Czaja AJ, Parés A, Dalekos GN, Krawitt EL, Bittencourt PL, Porta G, Boberg KM, Hofer H, Bianchi FB, Shibata M, Schramm C, Eisenmann de Torres B, Galle PR, McFarlane I, Dienes HP, Lohse AW; International Autoimmune Hepatitis Group. Simplified criteria for the diagnosis of autoimmune hepatitis. Hepatology. 2008;48:169-176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1205] [Cited by in RCA: 1252] [Article Influence: 73.6] [Reference Citation Analysis (0)] |
| 16. | Chazouillères O, Wendum D, Serfaty L, Montembault S, Rosmorduc O, Poupon R. Primary biliary cirrhosis-autoimmune hepatitis overlap syndrome: clinical features and response to therapy. Hepatology. 1998;28:296-301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 506] [Cited by in RCA: 479] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 17. | Moreau R, Jalan R, Gines P, Pavesi M, Angeli P, Cordoba J, Durand F, Gustot T, Saliba F, Domenicali M, Gerbes A, Wendon J, Alessandria C, Laleman W, Zeuzem S, Trebicka J, Bernardi M, Arroyo V; CANONIC Study Investigators of the EASL–CLIF Consortium. Acute-on-chronic liver failure is a distinct syndrome that develops in patients with acute decompensation of cirrhosis. Gastroenterology. 2013;144:1426-1437, 1437.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1720] [Cited by in RCA: 2167] [Article Influence: 180.6] [Reference Citation Analysis (5)] |
| 18. | Jalan R, Stadlbauer V, Sen S, Cheshire L, Chang YM, Mookerjee RP. Role of predisposition, injury, response and organ failure in the prognosis of patients with acute-on-chronic liver failure: a prospective cohort study. Crit Care. 2012;16:R227. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 99] [Cited by in RCA: 98] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 19. | Montalvo M, Mistry E, Chang AD, Yakhkind A, Dakay K, Azher I, Kaushal A, Mistry A, Chitale R, Cutting S, Burton T, Mac Grory B, Reznik M, Mahta A, Thompson BB, Ishida K, Frontera J, Riina HA, Gordon D, Parella D, Scher E, Farkas J, McTaggart R, Khatri P, Furie KL, Jayaraman M, Yaghi S. Predicting symptomatic intracranial haemorrhage after mechanical thrombectomy: the TAG score. J Neurol Neurosurg Psychiatry. 2019;90:1370-1374. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 30] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 20. | Wu T, Li J, Shao L, Xin J, Jiang L, Zhou Q, Shi D, Jiang J, Sun S, Jin L, Ye P, Yang L, Lu Y, Li T, Huang J, Xu X, Chen J, Hao S, Chen Y, Xin S, Gao Z, Duan Z, Han T, Wang Y, Gan J, Feng T, Pan C, Li H, Huang Y, Xie Q, Lin S, Li L; Chinese Group on the Study of Severe Hepatitis B (COSSH). Development of diagnostic criteria and a prognostic score for hepatitis B virus-related acute-on-chronic liver failure. Gut. 2018;67:2181-2191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 167] [Cited by in RCA: 297] [Article Influence: 42.4] [Reference Citation Analysis (2)] |
| 21. | Neuhauser M, Bjornsson E, Treeprasertsuk S, Enders F, Silveira M, Talwalkar J, Lindor K. Autoimmune hepatitis-PBC overlap syndrome: a simplified scoring system may assist in the diagnosis. Am J Gastroenterol. 2010;105:345-353. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 74] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 22. | Yang F, Wang Q, Wang Z, Miao Q, Xiao X, Tang R, Chen X, Bian Z, Zhang H, Yang Y, Sheng L, Fang J, Qiu D, Krawitt EL, Gershwin ME, Ma X. The Natural History and Prognosis of Primary Biliary Cirrhosis with Clinical Features of Autoimmune Hepatitis. Clin Rev Allergy Immunol. 2016;50:114-123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 47] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 23. | Zhang X, Chen P, Gao H, Hao S, Yang M, Zhao H, Hu J, Ma W, Li L. Bacterial Infection and Predictors of Mortality in Patients with Autoimmune Liver Disease-Associated Acute-On-Chronic Liver Failure. Can J Gastroenterol Hepatol. 2018;2018:5108781. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 19] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 24. | Grønbæk L, Vilstrup H, Jepsen P. Autoimmune hepatitis in Denmark: incidence, prevalence, prognosis, and causes of death. A nationwide registry-based cohort study. J Hepatol. 2014;60:612-617. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 204] [Cited by in RCA: 246] [Article Influence: 22.4] [Reference Citation Analysis (0)] |
| 25. | Choudhuri G, Somani SK, Baba CS, Alexander G. Autoimmune hepatitis in India: profile of an uncommon disease. BMC Gastroenterol. 2005;5:27. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 49] [Article Influence: 2.5] [Reference Citation Analysis (0)] |