Published online Aug 21, 2022. doi: 10.3748/wjg.v28.i31.4249
Peer-review started: January 16, 2022
First decision: May 10, 2022
Revised: May 21, 2022
Accepted: July 24, 2022
Article in press: July 24, 2022
Published online: August 21, 2022
Processing time: 212 Days and 9.7 Hours
After more than four decades of hepatitis B virus (HBV) vaccine implementation, its safety and efficacy in preventing HBV infection have been proven and several milestones have been achieved. Most countries have included HBV immunization schedules in their health policies and progress has been made regarding universalization of the first HBV vaccine dose at birth. All of these actions have significantly contributed to reducing both the incidence of HBV infection and its related complications. However, there are still many drawbacks to overcome. The main concerns are the deficient coverage rate of the dose at birth and the large adult population that has not been reached timely by universal immunization. Additionally, the current most widely used second-generation vaccines do not induce protective immunity in 5% to 10% of the population, particularly in people over 40-years-old, obese (body mass index > 25 kg/m2), heavy smokers, and patients undergoing dialysis or infection with human immunodeficiency virus. Recently developed and approved novel vaccine formulations using more potent adjuvants or multiple antigens have shown better performance, particularly in difficult settings. These advances re-launch the expectations of achieving the World Health Organization’s objective of completing hepatitis control by 2030.
Core Tip: Second-generation vaccines induce the production of anti-hepatitis B surface antibodies (anti-HBs). Anti-HBs levels ≥ 10 mIU/mL prevent against infection. More than 90% of immunized persons achieve protective anti-HBs levels 1 mo after completing the three-dose vaccination schedule. Although antibody titers significantly drop during the 1st years after vaccination, memory immunity is sufficient to prevent infection regardless of the antibody levels. In some specific settings showing lower immune response rates, schemes with larger or additional doses and novel vaccine formulations are recommended.
- Citation: Di Lello FA, Martínez AP, Flichman DM. Insights into induction of the immune response by the hepatitis B vaccine. World J Gastroenterol 2022; 28(31): 4249-4262
- URL: https://www.wjgnet.com/1007-9327/full/v28/i31/4249.htm
- DOI: https://dx.doi.org/10.3748/wjg.v28.i31.4249
Hepatitis B infection has been a major public health concern for a long time. Identification of the hepatitis B virus (HBV) in the 1960s and the subsequent development of a safe and effective vaccine were the kickoff to begin to retrace the path and achieve the desired control of this health problem.
First-generation vaccines based on heat-treated plasma derived from hepatitis B surface antigen (HBsAg)-positive donors raised concerns about its safety and availability to meet the vaccine manufacturer’s needs. Shortly afterwards, second-generation DNA vaccines prepared in yeast transfected with recombinant plasmids encoding small HBV surface proteins (SHBs) were developed and approved in 1986[1]. Lastly, third-generation vaccines have been produced in mammalian cells that express and secrete SHBs and middle pre-S2 proteins (MHBs) or the three HBV envelope proteins (SHBs, MHBs, and large HBs).
It is worth noting that several studies have compared the immune response to plasma-derived first-generation vaccines to the recombinant second-generation ones. Most of the studies showed that the lowering rate of anti-HBs was higher in people receiving the recombinant HBV vaccine. However, plasma-derived vaccines were replaced by the recombinant ones due to safety concerns about human blood-derived products[2-5]. In addition, it has been shown that third-generation vaccines containing the pre-S2 and pre-S1 antigens would induce a higher anti-HBs response than second-generation ones, particularly in people ≥ 45-years-old[6-8]. Moreover, compared to plasma-derived vaccines, it has been observed that the HBsAg seropositive rate drops by about 71% and that the anti-HBc seropositive rate decreases by approximately 65% when recombinant HBV vaccines are used, supporting their higher effectiveness[4].
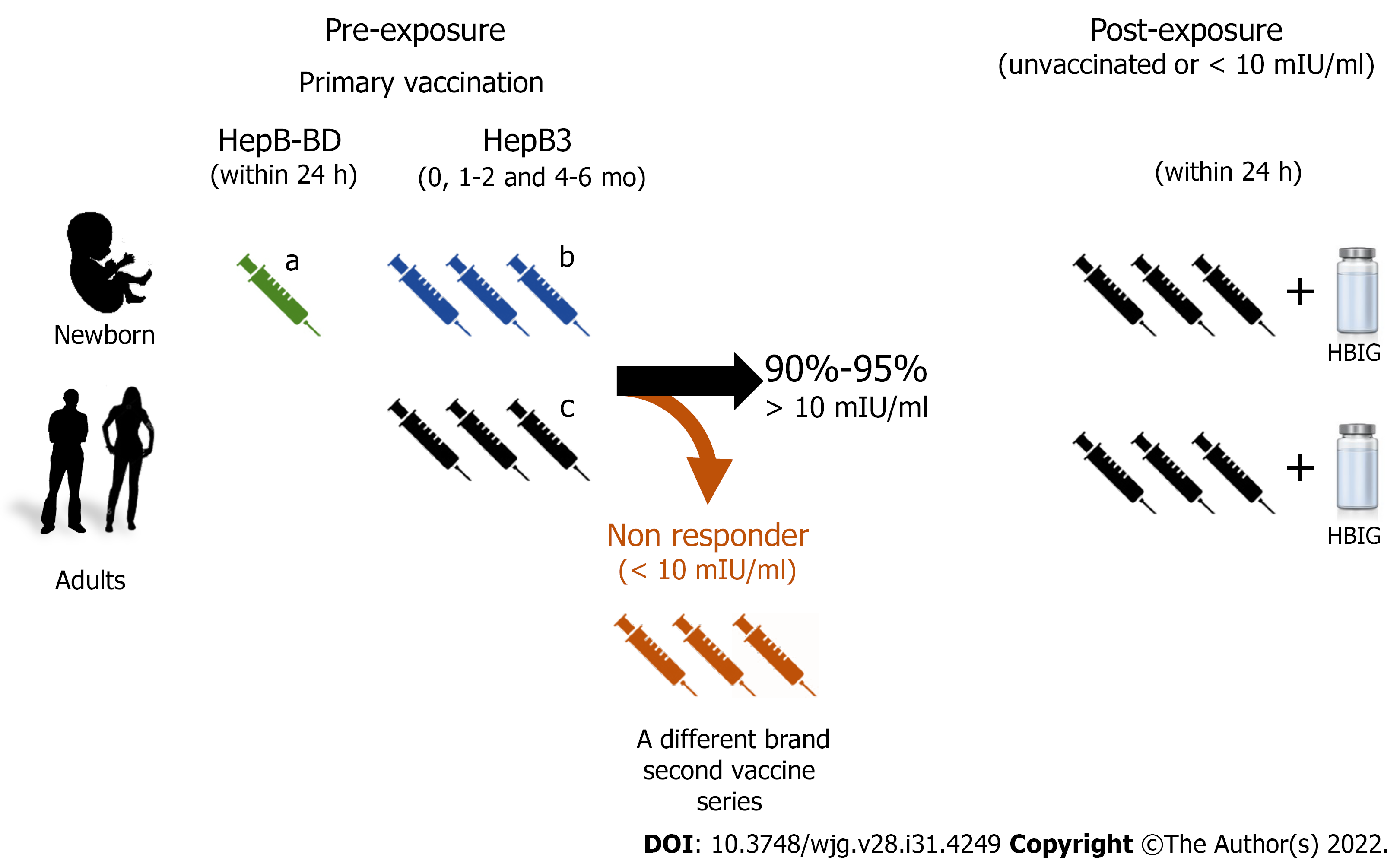
The HBV vaccine has been introduced progressively in the national vaccination calendars. Currently, second-generation HBV vaccines have been widely implemented for newborns in most countries. The pentavalent vaccine formulation protecting against diphtheria, pertussis, tetanus, hepatitis B, and Haemophilus influenzae type B is administered in three doses, 4 wk apart (recommended dosing at 6, 10, and 14 postnatal wk), and aims to lessen horizontal transmission. In addition, a monovalent single dose of the HBV vaccine (HepB-BD) administered within 24 h after delivery is also recommended to reduce mother-to-child transmission (Figure 1). The advised immunization schedule for the adult population includes three vaccine doses (HepB3) according to the individuals’ age. Immunocompromised adults or patients on dialysis treatment require higher or additional doses of HBV vaccines. Recently, a novel vaccine (Heplisav-B) with a different adjuvant was approved for adult immunization with a recommended schedule of two doses 1 mo apart.
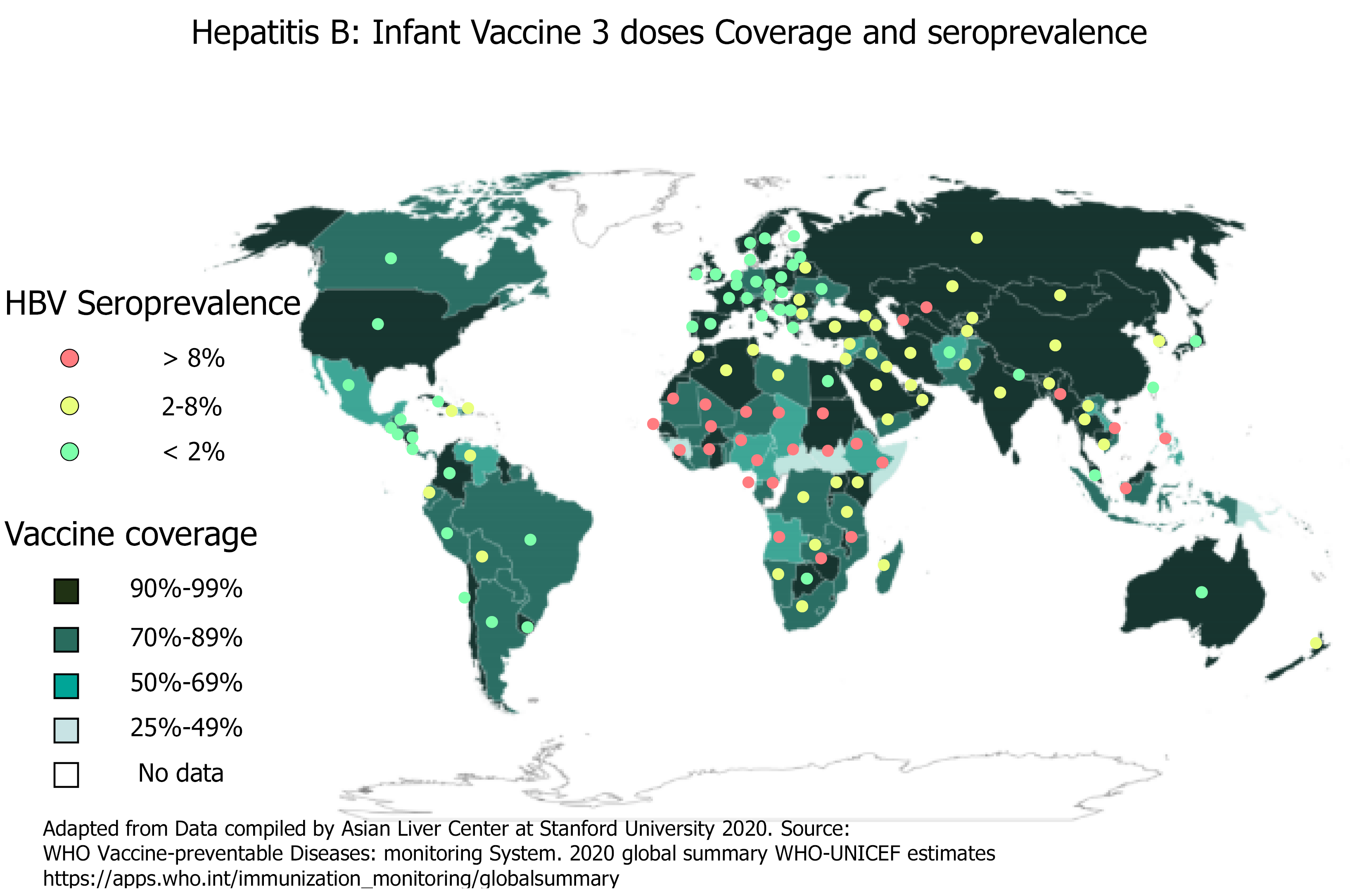
Since 1990, the proportion of children receiving all three doses of the HBV vaccine has increased globally from 1% to 85%. By 2020, the HBV vaccine was introduced to 190 countries with 83% of three-dose coverage rate. Few countries with very low endemicity that consider HBV infection as a limited public health problem provide the HBV vaccine to only well-defined risk groups. Likewise, a dose of HepB-BD has been introduced in the national calendar of 113 countries. Figure 2 shows the HBV worldwide three-dose infant vaccine coverage and the HBsAg seroprevalence[2,9]. However, the at birth dose coverage rate is poor (estimated at 43%) with remarkable disparities according to region and development level[10-12].
Universal vaccination is the most effective strategy to prevent and control HBV infection. In 2016, the World Health Organization (WHO) set the goal of controlling HBV by 2030. The proposed targets include the 90% global coverage of three-dose infant vaccination by 2020, birth-dose vaccination of 50% of infants by 2020, and of 90% of them by 2030[13].
One of the goals of the strategy to achieve HBV control by 2030 is to reduce HBsAg prevalence to 0.1% in 5-year-old children and many countries are already on track to that milestone[14].
The global implementation of the HBV vaccine as part of the national health policies has contributed to directly reducing the global burden of infection, and indirectly, the HBV-related mortality. After the inclusion of HBV vaccination schedules, several surveillance studies have shown an overtime global HBsAg prevalence decrease in most countries[15], either in hyperendemic ones or in those with low or medium HBV infection prevalence[16-20].
Taiwan was the first country to implement a mass vaccination program against HBV in 1984 and it is the paradigm of its impact on the control of hepatitis. After 30 years of sustained immunization programs, the prevalence of HBsAg has decreased from 9.8% in the pre-vaccination period to 0.5% in the cohort reached by HBV vaccination protocols[21]. The main reason for Taiwan’s success was its high three-dose hepatitis infant vaccine coverage rate, which increased from 88.9% in 1985[22,23] to 98.1% in 2018[21].
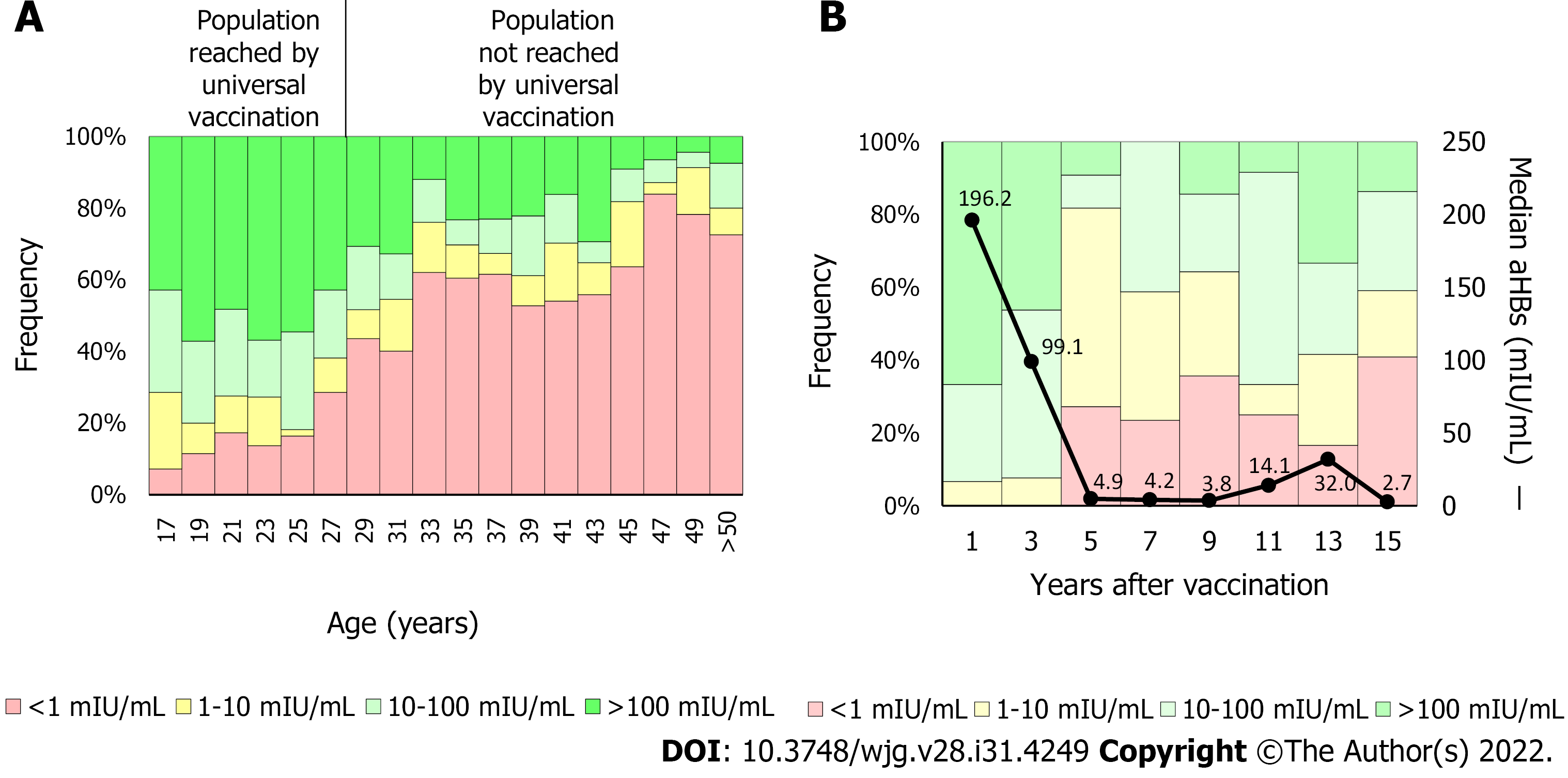
In the United States, since first HBV vaccine recommendations, the infection incidence has decreased by approximately 90%, from 9.6/100000 cases in 1982 to 1.0/100000 cases in 2018[24]. Similarly, in China, where the coverage of the three-dose vaccine schedule has increased from 30.0% to 93.4% and the at birth dose increased from 22.2% to 82.6%, the HBsAg prevalence decreased from 5.5% to 0.9% between 1992 and 2005[25]. In Argentina, a country with low HBV endemicity, the HBV vaccine was included in newborns’ schedules in 2000 and, later in 2003, the catch-up strategy was implemented in 11-year-old adolescents. Currently, the coverage rate of protective antibodies is significantly higher in persons born after 1992 than in those born previously (Figure 3A)[26,27]. In fact, new infections generally occur in the population over 20-years-old not reached by vaccination[28]. These results emphasize the need to raise awareness among people not reached by universal HBV immunization programs and to focus vaccination campaigns on this group.
However, the most outstanding HBV preventive action impact has been detected in regions that were hyperendemic before introduction of the vaccine. In a Southern Italian area, where the vaccine was introduced in 1991, the HBsAg prevalence dropped from 13.4% in 1978 to 0.91% in 2006[29]. Likewise, in Alaska, where one of the highest HBV infection incidences has been reported, universal childhood vaccination was implemented for newborns in 1993. The HBsAg prevalence dropped from 13% detected before the HBV vaccination program, to 0% HBsAg-positive children less than 10-years-old[30].
Although slowly and delayed, a significant reduction in the hepatocellular carcinoma annual average incidence has been observed concomitantly with the HBV infection incidence decline, particularly in those countries where early vaccine protocols have been introduced[31-33].
Several studies have shown that HBV vaccines induced both humoral and cellular immunity providing long-term protection[34,35]. On the one hand, neutralizing antibodies are elicited and two types of them have been identified. The first type targets the “a” determinant and neutralizes cell viral penetration by blocking the interaction with heparan sulfate[36], required by the virus at an early stage of hepatocyte entrance[37]. The second type targets the high-affinity receptor-binding site of the HBV pre-S1 domain and blocks the binding to the Na+-taurocholate cotransporting polypeptide receptor preventing the infection of hepatocytes[38,39]. On the other hand, immune memory cells are generated, which upon contact with the HBV can be activated to expand rapidly. This response has been well demonstrated in studies that administered a booster dose to previously vaccinated persons whose antibody titers had fallen below protective titers[16,40-42].
Pre-exposure: Efficacy and effectiveness studies carried out in animal models first and then in human beings have shown that the HBV vaccine induces the production of neutralizing antibodies against HBV surface antigen (anti-HBs)[43]. Two main questions raised suddenly once the HBV vaccine was developed: What are the levels of antibodies that protect against infection and how long does immunity last? Soon after the release of the vaccine, several studies have shown that anti-HBs levels ≥ 10 mIU/mL, determined 1 to 3 mo after the complete three-dose vaccination scheme administration, were a surrogate marker for vaccine-induced protective immunity[44-46].
The overall response rate to the HBV vaccine, defined as individuals achieving anti-HBs levels > 10 mIU/mL, is 90% to 95% of immunized persons. Different factors such as host genetics, age, body weight, smoking, and concomitant disease have been shown to affect the response rate to the vaccine[47]. These variations probably rely on the strength of the cellular immune response. Velu et al[48] have characterized the cellular immune response and the cytokine profile of vaccine responders and non-responders to investigate the immunization outcome underlying mechanisms. The authors reported that HBsAg-specific interferon gamma, interleukin 10, and tumor necrosis factor alpha secretion correlated with the HBV vaccine-induced humoral immune response. Likewise, non-responders had lower levels of T helper type 1 (Th1) and Th2 cytokines. In addition, Körber et al[49] observed a higher frequency of regulatory B cells in HBV vaccine non-responders. Regulatory B cells suppress immunopathology by skewing T-cell differentiation. Overall, these results suggest that impaired lymphocyte activation is associated with a weak or no response to HBV vaccination.
Notably, although the HBV vaccine induces protective immunity against infection it would not be sterilizing. Consequently, vaccinated people can become infected although episodes are usually asymptomatic and self-limited[50-52]. These benign infection results are possibly due to long-lasting HBV cellular immunity induced by the vaccine despite antibody loss against HBV surface antigens[53].
Post-exposure: Immunization with HBV vaccines combined with different injection sites of HBV immunoglobulin administration, within 12 h after birth, showed a greater than 85% efficacy in preventing infection in infants born to HBsAg-positive mothers[54]. In adult persons, post-exposure prophylaxis is also recommended depending on the individual’s vaccination and anti-HBs status. In unvaccinated subjects or with non-protective levels of anti-HBs, the HBV vaccine has shown high efficacy in preventing infection when administered within 24 h after percutaneous or mucosal exposure to HBV-positive blood. Additional post-exposure immunoprophylaxis is not suggested in individuals who achieved anti-HBs protective levels after vaccination.
Although, as previously mentioned, the massive implementation of the HBV vaccine substantially reduced the incidence and prevalence of the infection, few works have addressed the effectiveness of the vaccine and most of them have been carried out in high endemic countries. In general, a 70% to 94% effectiveness range has been reported, depending mainly on the follow-up time, the exposure risk rate (HBsAg prevalence of the population), and the studied cohort age[18,23,55]. A recent study showed an approximately 58% effectiveness in a birth cohort (mean age, 12 years) and 85% in participants at least 20-years-old[56]. The lower efficacy observed in the birth cohort could be a consequence of a lower level of exposure.
Protective antibodies levels tend to decrease over time, especially during the 1st years after vaccination[57]. In a study carried out by our group, including 132 children born after infants’ vaccine implementation, we observed that anti-HBs titers were significantly higher 1 year post-vaccination compared to the 2 years and 3 years earliest vaccinated population (Figure 3B). In addition, approximately 20% of the 5-year post-vaccination cohort showed anti-HBs levels lower than 10 mIU/mL[27]. These results are in line with a study conducted in Germany, where anti-HBs levels were determined in 106 teenagers, mean age 13.7-years-old, after primary vaccination. Forty percent of cases had anti-HBs levels < 10 mIU/mL. However, almost all (97%) teenagers who received a booster vaccine achieved anti-HBs levels ≥ 100 mIU/mL regardless of their pre-booster levels[58]. Besides time elapsed since primary vaccination, a systematic meta-analysis including 46 studies analyzed the anti-HBs levels from 5 years to 20 years after the primary vaccination and identified the vaccination dose and the less than 6-mo interval between the last dose and the previous one as the main factors associated with anti-HBs titer loss[59]. On the other hand, the duration of antibody levels is directly correlated with the titers reached when completing the vaccination scheme[60].
Another interesting issue regarding antibodies duration refers to the subjects’ age at the time of vaccination. Numerous studies have shown that vaccination in adolescence generates higher and long-lasting titers compared to children vaccinated at birth[61-64], being the age at the time of vaccination an independent variable associated with an anti-HBs titer < 10 mIU/mL. However, childhood HBV vaccination, together with other vaccines, guarantees a higher coverage rate.
Overall, it is widely accepted that a large proportion of vaccinated individuals, particularly those immunized during childhood, rapidly lose their anti-HBs titers below protective levels[40]. However, it has also been extensively described that individuals who achieved anti-HBs protective titers at the time of vaccination, show a rapid anamnestic response when boosted[41,42], suggesting that memory immunity plays a decisive role in the protection against clinical disease and the development of a carrier state regardless of anti-HBs antibody levels[35]. In this regard, it has been observed that even in the lack of anti-HBs, a significant amount of HBsAg-specific memory T and B cells are detected in vaccine responders. For this reason, although it remains a controversial issue, vaccine booster doses are not recommended currently for children and adults with normal immune status, despite the overtime drop of anti-HBs antibody titers[35,65]. Nonetheless, the anti-HBs titer decline could represent a problem for high-risk groups.
Adult persons with increased risk factors for infection are one of the WHO identified obstacles to HBV elimination as a public health problem[66,67]. These groups mainly include health care providers, illicit injected drug users, sexually active individuals (more than 1 partner in the past 6 mo), persons with diabetes, dialysis patients, and people living with human immunodeficiency virus (HIV). The last two groups, in addition to showing a higher risk of HBV infection compared to the general population[68-70] due to the frequent use of percutaneous materials and the common route of HBV and HIV trans
It has been reported that patients living with HIV present a poor initial HBV immunization response, lower seroconversion rates, and difficulty in maintaining immunity over time, mainly due to B-cell dysfunction[67,71,78,79]. For this group, the efficacy of the standard vaccine scheme in the era of the highly active antiretroviral therapy ranges from 17.5% to 71%[80-83].
Consequently, for patients on dialysis and/or living with HIV, other approaches are recommended to enhance the HBV vaccine immune response. For patients undergoing dialysis therapy, alternative strategies include the use of adjuvants, additional vaccination cycles, different vaccine formulations, greater number and concentration of doses, greater frequency of doses, dual vaccination, alternative administration routes, and/or use of booster vaccines[67,72,84-86]. On the other hand, for HIV-infected individuals with negative or < 10 mIU/mL anti-HBs levels after a primary vaccine series, a second HBV vaccine series using larger or additional doses is recommended[71,87]. Furthermore, revaccination should be attempted after HIV viral load suppression and CD4 cell count improvement[83].
As mentioned above, the average response rate to the HBV vaccine is greater than 90% with 5% to 10% vaccinated persons failing to mount a protective immunity level once the vaccination schedule is completed. The response to the vaccine ultimately relies on the individual immune system; however, different factors impairing the response rate have been identified. Response rates can drop drastically when more than one of these factors are present.
Several studies have addressed, through different experimental approaches, the role of genetic polymorphisms in the response to the HBV vaccine. Single nucleotide polymorphisms in HLA loci[88-92], ILs (with a key role in the cellular and humoral response interplay)[93,94], or even other genes have been associated with the response rate to HBV vaccination[90,95,96]. However, these findings have not been widely validated in different cohorts and should be considered with caution.
One of the most recognized consequences of aging is the declination of the immune function and the concomitant vaccination response reduction. The HBV vaccine response rate decreases in people 40-years-old and even more in people older than 60 years[97,98]. This highlights the need to vaccinate the population not covered by health policies before they reach 40-years-old, which will result in important cost-benefit profits.
Overweight and obesity are a growing public health problem worldwide that affects all age groups[99]. They are caused by the deposition of lipids into the adipose tissue and are defined as a body mass index ≥ 25 kg/m.
Shortly after the HBV vaccine was developed and implemented, obesity was found to be a factor impairing the strength of the immune response[100]. This finding has been widely validated in sub
As described for obesity, the link between tobacco smoking and impaired vaccine response has been proposed to be mediated by inflammation[109]. However, data from different studies are less robust. Some studies have reported lower responses to vaccination while others showed no association[110]. This controversy could be based on the level of daily cigarette intake. A recent study reported that subjects in the non-responder group were almost exclusively ‘heavy smokers’ defined as consumers of ≥ 10 cigarettes per day[111]. The development of new vaccine formulations including either additional antigens or more potent adjuvants could represent a solution to improve the response rate of individuals affected by these factors as well as for dialyzed or immunosuppressed patients.
As previously mentioned, one of the main drawbacks of the second-generation vaccines is the poor induction of immune response in 5% to 10% of the general population and individuals presenting detrimental factors that impair vaccine response. Therefore, efforts have been made to find more effective formulations to overcome this limitation. Two advances have been reported in recent years. One of them is the development and evaluation of new and more powerful adjuvants to enhance immunogenicity[112,113].
Heplisav-B (HepB-CpG), a single-antigen vaccine with a novel immunostimulatory adjuvant, has been approved for its use in people at least 18-years-old. This vaccine is administered in two doses, 1 mo apart[114]. The new adjuvant is a small synthetic cytidine-phosphate-guanosine oligodeoxynucleotide containing non-methylated CpG patterns, similar to those present in microbial DNA. This structure acts as an agonist of the toll-like receptor 9 that enhances the immune response. Several studies have shown higher response rates to other second-generation vaccines both in general population[115-117] and in persons with detrimental factors for vaccination response[118-120]. In addition, the two doses-1 mo apart-simplified schedule could help increase patient compliance and raise the coverage rate.
Alternatively, third-generation vaccines derived from mammalian cells, containing the medium and large HBV envelope proteins have been developed. The advantage of this approach is that antigens display the same in vivo post-translational modifications and protein folding.
In 2021, Sci-B-Vac was licensed, and phase III trials showed faster seroprotection and higher response rates than the second-generation vaccines[8,121]. These data making turn Sci-B-Vac of particular interest for its use in people with poor or no response. Particularly, Sci-B-Vac has shown greater efficacy in HIV-infected individuals’ immunization and in the prevention of vertical infection transmission[122,123]. Furthermore, the multiple antigen display of the third-generation vaccines would protect against HBV vaccine breakthrough infections caused by the HBV S gene mutants widely described[124-126]. The results obtained through novel vaccine formulation approaches suppose a contribution to the prophylaxis of HBV infection and represent a promising future.
Beyond significant advances in the prevention of HBV infection, several pitfalls have been identified that need to be overcome in order to eliminate HBV as a health problem[127]. Particularly, in deve
Regarding the at birth dose, out-of-hospital deliveries, shortage of monovalent vaccine formulation in some regions, insufficient training of health care providers, weak monitoring and reporting systems, and low government commitment impair its implementation. On the other hand, in the adult popu
The worldwide application of HBV vaccines has led to a significant decrease in HBV infection incidence and its related death rates. As a general strategy, surveys are necessary to identify local constraints (in regions or countries) in order to achieve the implementation of WHO guidelines, both at the proph
To Silvina Heisecke, from CEMIC-CONICET, for the copyediting of the manuscript.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Argentina
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: JAN CFJ, Taiwan; Kumar R, India; Maslennikov R, Russia S-Editor: Chang KL L-Editor: Filipodia P-Editor: Chang KL
| 1. | Beasley RP. Development of hepatitis B vaccine. JAMA. 2009;302:322-324. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 2. | Kao JT, Wang JH, Hung CH, Yen YH, Hung SF, Hu TH, Lee CM, Lu SN. Long-term efficacy of plasma-derived and recombinant hepatitis B vaccines in a rural township of Central Taiwan. Vaccine. 2009;27:1858-1862. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 42] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 3. | Kim YJ, Li P, Hong JM, Ryu KH, Nam E, Chang MS. A Single Center Analysis of the Positivity of Hepatitis B Antibody after Neonatal Vaccination Program in Korea. J Korean Med Sci. 2017;32:810-816. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 4. | Hsu SH, Chih AH, Lee YC, Huang KC, Jan CF. Higher disappearance rate of anti-HBs in Taiwanese freshers neonatally vaccinated with recombinant yeast hepatitis B vaccine. Liver Int. 2017;37:1780-1787. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 5. | Hu YC, Yeh CC, Chen RY, Su CT, Wang WC, Bai CH, Chan CF, Su FH. Seroprevalence of hepatitis B virus in Taiwan 30 years after the commencement of the national vaccination program. PeerJ. 2018;6:e4297. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 51] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 6. | Shouval D, Roggendorf H, Roggendorf M. Enhanced immune response to hepatitis B vaccination through immunization with a Pre-S1/Pre-S2/S vaccine. Med Microbiol Immunol. 2015;204:57-68. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 92] [Cited by in RCA: 113] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 7. | Krawczyk A, Ludwig C, Jochum C, Fiedler M, Heinemann FM, Shouval D, Roggendorf M, Roggendorf H, Lindemann M. Induction of a robust T- and B-cell immune response in non- and low-responders to conventional vaccination against hepatitis B by using a third generation PreS/S vaccine. Vaccine. 2014;32:5077-5082. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 48] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 8. | Vesikari T, Langley JM, Segall N, Ward BJ, Cooper C, Poliquin G, Smith B, Gantt S, McElhaney JE, Dionne M, van Damme P, Leroux-Roels I, Leroux-Roels G, Machluf N, Spaans JN, Yassin-Rajkumar B, Anderson DE, Popovic V, Diaz-Mitoma F; PROTECT Study Group. Immunogenicity and safety of a tri-antigenic vs a mono-antigenic hepatitis B vaccine in adults (PROTECT): a randomised, double-blind, phase 3 trial. Lancet Infect Dis. 2021;21:1271-1281. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 52] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 9. | Polaris Observatory Collaborators. Global prevalence, treatment, and prevention of hepatitis B virus infection in 2016: a modelling study. Lancet Gastroenterol Hepatol. 2018;3:383-403. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1260] [Cited by in RCA: 1215] [Article Influence: 173.6] [Reference Citation Analysis (2)] |
| 10. | World Health Organization. Hepatitis B Control Through Immunization: A Reference Guide. [cited 5 February 2021]. In: World Health Organization [Internet]. Available from: http://iris.wpro.who.int/bitstream/handle/10665.1/10820/9789290616696_eng.pdf;jsessionid=FEDDD4672274909D7E6998B86BED45C8?sequence=3%0Ahttp://www.who.int/immunization/sage/meetings/2015/october/8_WPRO_Hepatitis_B_Prevention_Through_Immunization_Regional_R. |
| 11. | de Villiers MJ, Nayagam S, Hallett TB. The impact of the timely birth dose vaccine on the global elimination of hepatitis B. Nat Commun. 2021;12:6223. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 59] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 12. | World Health Organization. Progress Towards Global Immunization Goals – 2019. Summary presentations of key indicators, Updated July 2020. [cited 5 February 2021]. In: World Health Organization [Internet]. Available from: https://cdn.who.int/media/docs/default-source/immunization/global_monitoring/slidesglobalimmunization.pdf?sfvrsn=25385c3b_7. |
| 13. | World Health Organization. Combating hepatitis B and C to reach elimination by 2030: advocacy brief (World Health Organization, Geneva, 2016). [cited 5 February 2021]. In: World Health Organization [Internet]. Available from: https://www.who.int/publications/i/item/combating-hepatitis-b-and-c-to-reach-elimination-by-2030. |
| 14. | World Health Organization. Global health sector strategy on viral hepatitis 2016–2021. Towards ending viral hepatitis. [cited 5 February 2021]. In: World Health Organization [Internet]. Available from: https://www.who.int/publications/i/item/WHO-HIV-2016.06. |
| 15. | Zanetti AR, Van Damme P, Shouval D. The global impact of vaccination against hepatitis B: a historical overview. Vaccine. 2008;26:6266-6273. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 273] [Cited by in RCA: 287] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 16. | McMahon BJ, Dentinger CM, Bruden D, Zanis C, Peters H, Hurlburt D, Bulkow L, Fiore AE, Bell BP, Hennessy TW. Antibody levels and protection after hepatitis B vaccine: results of a 22-year follow-up study and response to a booster dose. J Infect Dis. 2009;200:1390-1396. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 176] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 17. | Mohaghegh Shelmani H, Karayiannis P, Ashtari S, Mahmanzar MA, Khanabadi B, Modami N, Gholipour F, Zare F, Zali MR. Demographic changes of hepatitis B virus infection in Iran for the last two decades. Gastroenterol Hepatol Bed Bench. 2017;10:S38-S43. [PubMed] |
| 18. | Garcia D, Porras A, Rico Mendoza A, Alvis N, Navas MC, De La Hoz F, De Neira M, Osorio E, Valderrama JF. Hepatitis B infection control in Colombian Amazon after 15 years of hepatitis B vaccination. Effectiveness of birth dose and current prevalence. Vaccine. 2018;36:2721-2726. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 16] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 19. | Stroffolini T, Guadagnino V, Rapicetta M, Menniti Ippolito F, Caroleo B, De Sarro G, Focà A, Liberto MC, Giancotti A, Barreca GS, Marascio N, Lombardo F, Staltari O; Sersale's Study Collaborating Group. The impact of a vaccination campaign against hepatitis B on the further decrease of hepatitis B virus infection in a southern Italian town over 14 years. Eur J Intern Med. 2012;23:e190-e192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 20. | Huynh C, Minuk GY, Uhanova J, Baikie M, Wong T, Osiowy C. Serological and molecular epidemiological outcomes after two decades of universal infant hepatitis B virus (HBV) vaccination in Nunavut, Canada. Vaccine. 2017;35:4515-4522. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 21. | Lu FT, Ni YH. Elimination of Mother-to-Infant Transmission of Hepatitis B Virus: 35 Years of Experience. Pediatr Gastroenterol Hepatol Nutr. 2020;23:311-318. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 22. | Gust ID. Immunisation against hepatitis B in Taiwan. Gut. 1996;38 Suppl 2:S67-S68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 23. | Chien YC, Jan CF, Kuo HS, Chen CJ. Nationwide hepatitis B vaccination program in Taiwan: effectiveness in the 20 years after it was launched. Epidemiol Rev. 2006;28:126-135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 166] [Cited by in RCA: 168] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 24. | Centers for Diseases Control and Prevention. Epidemiology and Prevention of Vaccine-Preventable Diseases, Chapter 10: Hepatitis B. [cited 5 February 2021]. Available From: https://www.cdc.gov/vaccines/pubs/pinkbook/hepb.html. |
| 25. | Liang X, Bi S, Yang W, Wang L, Cui G, Cui F, Zhang Y, Liu J, Gong X, Chen Y, Wang F, Zheng H, Guo J, Jia Z, Ma J, Wang H, Luo H, Li L, Jin S, Hadler SC, Wang Y. Evaluation of the impact of hepatitis B vaccination among children born during 1992-2005 in China. J Infect Dis. 2009;200:39-47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 203] [Cited by in RCA: 231] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 26. | Stecher D, Katz N, Vizzotti C. Hepatitis B en Argentina. Situación actual y estrategia de vacunación universal para su control y eliminación. Actualizaciones en Sida e Infectología. 2014;22:18-21. |
| 27. | Di Lello FA, Blejer J, Alter A, Bartoli S, Vargas F, Ruiz R, Galli C, Blanco S, Gallego S, Fernández R, Martínez AP, Flichman DM. Hepatitis B surface antibodies seroprevalence among people born before and after implementation of universal HBV vaccination. Vaccine. 2020;38:2678-2682. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 28. | Ministerio de Salud y desarrollo Social. Boletín sobre las Hepatitis Virales en Argentina, N 1, AÑO 1, Octubre 2019. [cited 5 February 2021]. Available from: https://bancos.salud.gob.ar/sites/default/files/2020-01/0000001592cnt-2019-10_boletin-hepatitis.pdf. |
| 29. | Da Villa G, Romanò L, Sepe A, Iorio R, Paribello N, Zappa A, Zanetti AR. Impact of hepatitis B vaccination in a highly endemic area of south Italy and long-term duration of anti-HBs antibody in two cohorts of vaccinated individuals. Vaccine. 2007;25:3133-3136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 55] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 30. | Harpaz R, McMahon BJ, Margolis HS, Shapiro CN, Havron D, Carpenter G, Bulkow LR, Wainwright RB. Elimination of new chronic hepatitis B virus infections: results of the Alaska immunization program. J Infect Dis. 2000;181:413-418. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 92] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 31. | Lin CL, Kao JH. Hepatitis B: Immunization and Impact on Natural History and Cancer Incidence. Gastroenterol Clin North Am. 2020;49:201-214. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 16] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 32. | Chang MH, Chen CJ, Lai MS, Hsu HM, Wu TC, Kong MS, Liang DC, Shau WY, Chen DS. Universal hepatitis B vaccination in Taiwan and the incidence of hepatocellular carcinoma in children. Taiwan Childhood Hepatoma Study Group. N Engl J Med. 1997;336:1855-1859. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1328] [Cited by in RCA: 1196] [Article Influence: 42.7] [Reference Citation Analysis (0)] |
| 33. | Chang MH, You SL, Chen CJ, Liu CJ, Lee CM, Lin SM, Chu HC, Wu TC, Yang SS, Kuo HS, Chen DS; Taiwan Hepatoma Study Group. Decreased incidence of hepatocellular carcinoma in hepatitis B vaccinees: a 20-year follow-up study. J Natl Cancer Inst. 2009;101:1348-1355. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 433] [Cited by in RCA: 427] [Article Influence: 26.7] [Reference Citation Analysis (0)] |
| 34. | Said ZN, Abdelwahab KS. Induced immunity against hepatitis B virus. World J Hepatol. 2015;7:1660-1670. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 23] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 35. | Van Damme P, Dionne M, Leroux-Roels G, Van Der Meeren O, Di Paolo E, Salaun B, Surya Kiran P, Folschweiller N. Persistence of HBsAg-specific antibodies and immune memory two to three decades after hepatitis B vaccination in adults. J Viral Hepat. 2019;26:1066-1075. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 32] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 36. | Sureau C, Salisse J. A conformational heparan sulfate binding site essential to infectivity overlaps with the conserved hepatitis B virus a-determinant. Hepatology. 2013;57:985-994. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 156] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 37. | Schulze A, Gripon P, Urban S. Hepatitis B virus infection initiates with a large surface protein-dependent binding to heparan sulfate proteoglycans. Hepatology. 2007;46:1759-1768. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 303] [Cited by in RCA: 355] [Article Influence: 19.7] [Reference Citation Analysis (0)] |
| 38. | Urban S, Bartenschlager R, Kubitz R, Zoulim F. Strategies to inhibit entry of HBV and HDV into hepatocytes. Gastroenterology. 2014;147:48-64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 242] [Cited by in RCA: 259] [Article Influence: 23.5] [Reference Citation Analysis (0)] |
| 39. | Yan H, Zhong G, Xu G, He W, Jing Z, Gao Z, Huang Y, Qi Y, Peng B, Wang H, Fu L, Song M, Chen P, Gao W, Ren B, Sun Y, Cai T, Feng X, Sui J, Li W. Sodium taurocholate cotransporting polypeptide is a functional receptor for human hepatitis B and D virus. Elife. 2012;1:e00049. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1280] [Cited by in RCA: 1600] [Article Influence: 123.1] [Reference Citation Analysis (1)] |
| 40. | Lu CY, Ni YH, Chiang BL, Chen PJ, Chang MH, Chang LY, Su IJ, Kuo HS, Huang LM, Chen DS, Lee CY. Humoral and cellular immune responses to a hepatitis B vaccine booster 15-18 years after neonatal immunization. J Infect Dis. 2008;197:1419-1426. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 153] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 41. | Bruce MG, Bruden D, Hurlburt D, Zanis C, Thompson G, Rea L, Toomey M, Townshend-Bulson L, Rudolph K, Bulkow L, Spradling PR, Baum R, Hennessy T, McMahon BJ. Antibody Levels and Protection After Hepatitis B Vaccine: Results of a 30-Year Follow-up Study and Response to a Booster Dose. J Infect Dis. 2016;214:16-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 210] [Cited by in RCA: 208] [Article Influence: 23.1] [Reference Citation Analysis (0)] |
| 42. | Wang ZZ, Gao YH, Lu W, Jin CD, Zeng Y, Yan L, Ding F, Li T, Liu XE, Zhuang H. Long-term persistence in protection and response to a hepatitis B vaccine booster among adolescents immunized in infancy in the western region of China. Hum Vaccin Immunother. 2017;13:909-915. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 24] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 43. | Bitter GA, Egan KM, Burnette WN, Samal B, Fieschko JC, Peterson DL, Downing MR, Wypych J, Langley KE. Hepatitis B vaccine produced in yeast. J Med Virol. 1988;25:123-140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 17] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 44. | Jack AD, Hall AJ, Maine N, Mendy M, Whittle HC. What level of hepatitis B antibody is protective? J Infect Dis. 1999;179:489-492. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 227] [Cited by in RCA: 244] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 45. | Hall AJ. Hepatitis B vaccination: protection for how long and against what? BMJ. 1993;307:276-277. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 35] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 47. | Yang S, Tian G, Cui Y, Ding C, Deng M, Yu C, Xu K, Ren J, Yao J, Li Y, Cao Q, Chen P, Xie T, Wang C, Wang B, Mao C, Ruan B, Jiang T, Li L. Factors influencing immunologic response to hepatitis B vaccine in adults. Sci Rep. 2016;6:27251. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 73] [Cited by in RCA: 116] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 48. | Velu V, Saravanan S, Nandakumar S, Shankar EM, Vengatesan A, Jadhav SS, Kulkarni PS, Thyagarajan SP. Relationship between T-lymphocyte cytokine levels and sero-response to hepatitis B vaccines. World J Gastroenterol. 2008;14:3534-3540. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 26] [Cited by in RCA: 28] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 49. | Körber N, Pohl L, Weinberger B, Grubeck-Loebenstein B, Wawer A, Knolle PA, Roggendorf H, Protzer U, Bauer T. Hepatitis B Vaccine Non-Responders Show Higher Frequencies of CD24highCD38high Regulatory B Cells and Lower Levels of IL-10 Expression Compared to Responders. Front Immunol. 2021;12:713351. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 19] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 50. | Werner JM, Abdalla A, Gara N, Ghany MG, Rehermann B. The hepatitis B vaccine protects re-exposed health care workers, but does not provide sterilizing immunity. Gastroenterology. 2013;145:1026-1034. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 45] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 51. | Su TH, Chen PJ. Emerging hepatitis B virus infection in vaccinated populations: a rising concern? Emerg Microbes Infect. 2012;1:e27. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 52. | Poovorawan Y, Chongsrisawat V, Theamboonlers A, Leroux-Roels G, Kuriyakose S, Leyssen M, Jacquet JM. Evidence of protection against clinical and chronic hepatitis B infection 20 years after infant vaccination in a high endemicity region. J Viral Hepat. 2011;18:369-375. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 78] [Cited by in RCA: 81] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 53. | Simons BC, Spradling PR, Bruden DJ, Zanis C, Case S, Choromanski TL, Apodaca M, Brogdon HD, Dwyer G, Snowball M, Negus S, Bruce MG, Morishima C, Knall C, McMahon BJ. A Longitudinal Hepatitis B Vaccine Cohort Demonstrates Long-lasting Hepatitis B Virus (HBV) Cellular Immunity Despite Loss of Antibody Against HBV Surface Antigen. J Infect Dis. 2016;214:273-280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 64] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 54. | Yu AS, Cheung RC, Keeffe EB. Hepatitis B vaccines. Clin Liver Dis. 2004;8:283-300. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 31] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 55. | Peto TJ, Mendy ME, Lowe Y, Webb EL, Whittle HC, Hall AJ. Efficacy and effectiveness of infant vaccination against chronic hepatitis B in the Gambia Hepatitis Intervention Study (1986-90) and in the nationwide immunisation program. BMC Infect Dis. 2014;14:7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 90] [Cited by in RCA: 85] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 56. | He WQ, Guo GN, Li C. The impact of hepatitis B vaccination in the United States, 1999-2018. Hepatology. 2022;75:1566-1578. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 16] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 57. | Jilg W, Schmidt M, Deinhardt F. Four-year experience with a recombinant hepatitis B vaccine. Infection. 1989;17:70-76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 47] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 58. | Anderson CL, Remschmidt C, Drobnitzky FP, Falkenhorst G, Zimmermann R, Wichmann O, Harder T. Hepatitis B immune status in adolescents vaccinated during infancy: A retrospective cohort study from a pediatric practice in Germany. Hum Vaccin Immunother. 2016;12:779-784. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 15] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 59. | Schönberger K, Riedel C, Rückinger S, Mansmann U, Jilg W, Kries RV. Determinants of Long-term protection after hepatitis B vaccination in infancy: a meta-analysis. Pediatr Infect Dis J. 2013;32:307-313. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 58] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 60. | Mendy M, Peterson I, Hossin S, Peto T, Jobarteh ML, Jeng-Barry A, Sidibeh M, Jatta A, Moore SE, Hall AJ, Whittle H. Observational study of vaccine efficacy 24 years after the start of hepatitis B vaccination in two Gambian villages: no need for a booster dose. PLoS One. 2013;8:e58029. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 78] [Cited by in RCA: 93] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 61. | Coppola N, Corvino AR, De Pascalis S, Signoriello G, Di Fiore E, Nienhaus A, Sagnelli E, Lamberti M. The long-term immunogenicity of recombinant hepatitis B virus (HBV) vaccine: contribution of universal HBV vaccination in Italy. BMC Infect Dis. 2015;15:149. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 38] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 62. | Trevisan A, Mason P, Nicolli A, Maso S, Fonzo M, Scarpa B, Bertoncello C. Future Healthcare Workers and Hepatitis B Vaccination: A New Generation. Int J Environ Res Public Health. 2021;18. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 63. | Pileggi C, Papadopoli R, Bianco A, Pavia M. Hepatitis B vaccine and the need for a booster dose after primary vaccination. Vaccine. 2017;35:6302-6307. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 33] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 64. | Stefanati A, Bolognesi N, Sandri F, Dini G, Massa E, Montecucco A, Lupi S, Gabutti G. Long-term persistency of hepatitis B immunity: an observational cross-sectional study on medical students and resident doctors. J Prev Med Hyg. 2019;60:E184-E190. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 65. | Bauer T, Jilg W. Hepatitis B surface antigen-specific T and B cell memory in individuals who had lost protective antibodies after hepatitis B vaccination. Vaccine. 2006;24:572-577. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 165] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 66. | World Health Organization. Hepatitis B vaccines: WHO position paper – July 2017. [cited 5 February 2021]. In: World Health Organization [Internet]. Available from: https://www.who.int/immunization/policy/position_papers/hepatitis_b/en/. |
| 67. | Schillie S, Harris A, Link-Gelles R, Romero J, Ward J, Nelson N. Recommendations of the Advisory Committee on Immunization Practices for Use of a Hepatitis B Vaccine with a Novel Adjuvant. MMWR Morb Mortal Wkly Rep. 2018;67:455-458. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 114] [Cited by in RCA: 145] [Article Influence: 20.7] [Reference Citation Analysis (0)] |
| 68. | Burdick RA, Bragg-Gresham JL, Woods JD, Hedderwick SA, Kurokawa K, Combe C, Saito A, LaBrecque J, Port FK, Young EW. Patterns of hepatitis B prevalence and seroconversion in hemodialysis units from three continents: the DOPPS. Kidney Int. 2003;63:2222-2229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 141] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 69. | Thio CL. Hepatitis B and human immunodeficiency virus coinfection. Hepatology. 2009;49:S138-S145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 205] [Cited by in RCA: 233] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 70. | Pereson MJ, Martínez AP, Isaac K, Laham G, Ridruejo E, Garcia GH, Flichman DM, Di Lello FA. Seroprevalence of hepatitis B, hepatitis C and HIV infection among patients undergoing haemodialysis in Buenos Aires, Argentina. J Med Microbiol. 2021;70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 71. | Whitaker JA, Rouphael NG, Edupuganti S, Lai L, Mulligan MJ. Strategies to increase responsiveness to hepatitis B vaccination in adults with HIV-1. Lancet Infect Dis. 2012;12:966-976. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 60] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 72. | Fabrizi F, Cerutti R, Alfieri CM, Ridruejo E. An Update on Hepatocellular Carcinoma in Chronic Kidney Disease. Cancers (Basel). 2021;13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 73. | Fabrizi F, Martin P. Hepatitis B virus infection in dialysis patients. Am J Nephrol. 2000;20:1-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 25] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 74. | Cohen G, Hörl WH. Immune dysfunction in uremia—an update. Toxins (Basel). 2012;4:962-990. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 99] [Cited by in RCA: 114] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 75. | Sampani E, Vagiotas L, Daikidou DV, Nikolaidou V, Xochelli A, Kasimatis E, Lioulios G, Dimitriadis C, Fylaktou A, Papagianni A, Stangou M. End stage renal disease has an early and continuous detrimental effect on regulatory T cells. Nephrology (Carlton). 2022;27:281-287. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 9] [Article Influence: 2.3] [Reference Citation Analysis (1)] |
| 76. | Sampani E, Stangou M, Daikidou DV, Nikolaidou V, Asouchidou D, Dimitriadis C, Lioulios G, Xochelli A, Fylaktou A, Papagianni A. Influence of end stage renal disease on CD28 expression and T-cell immunity. Nephrology (Carlton). 2021;26:185-196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 77. | Fabrizi F, Lunghi G, Martin P. Treatment of HBV-related liver disease in the dialysis population: reality and promises. Int J Artif Organs. 2001;24:598-605. [PubMed] |
| 78. | Kernéis S, Launay O, Turbelin C, Batteux F, Hanslik T, Boëlle PY. Long-term immune responses to vaccination in HIV-infected patients: a systematic review and meta-analysis. Clin Infect Dis. 2014;58:1130-1139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 130] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 79. | Moretto F, Catherine FX, Esteve C, Blot M, Piroth L. Isolated Anti-HBc: Significance and Management. J Clin Med. 2020;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 22] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 80. | Rey D, Krantz V, Partisani M, Schmitt MP, Meyer P, Libbrecht E, Wendling MJ, Vetter D, Nicolle M, Kempf-Durepaire G, Lang JM. Increasing the number of hepatitis B vaccine injections augments anti-HBs response rate in HIV-infected patients. Effects on HIV-1 viral load. Vaccine. 2000;18:1161-1165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 16] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 81. | Overton ET, Sungkanuparph S, Powderly WG, Seyfried W, Groger RK, Aberg JA. Undetectable plasma HIV RNA load predicts success after hepatitis B vaccination in HIV-infected persons. Clin Infect Dis. 2005;41:1045-1048. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 120] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 82. | Paitoonpong L, Suankratay C. Immunological response to hepatitis B vaccination in patients with AIDS and virological response to highly active antiretroviral therapy. Scand J Infect Dis. 2008;40:54-58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 35] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 83. | Landrum ML, Huppler Hullsiek K, Ganesan A, Weintrob AC, Crum-Cianflone NF, Barthel RV, Peel S, Agan BK. Hepatitis B vaccine responses in a large U.S. military cohort of HIV-infected individuals: another benefit of HAART in those with preserved CD4 count. Vaccine. 2009;27:4731-4738. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 65] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 84. | Janus N, Vacher LV, Karie S, Ledneva E, Deray G. Vaccination and chronic kidney disease. Nephrol Dial Transplant. 2008;23:800-807. [PubMed] |
| 85. | Zhang W, Du X, Zhao G, Jin H, Kang Y, Xiao C, Liu M, Wang B. Levamisole is a potential facilitator for the activation of Th1 responses of the subunit HBV vaccination. Vaccine. 2009;27:4938-4946. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 24] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 86. | Haddiya I. Current Knowledge of Vaccinations in Chronic Kidney Disease Patients. Int J Nephrol Renovasc Dis. 2020;13:179-185. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 22] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 87. | Vargas JI, Jensen D, Martínez F, Sarmiento V, Peirano F, Acuña P, Provoste F, Bustos V, Cornejo F, Fuster A, Acuña M, Fuster F, Soto S, Estay D, Jensen W, Ahumada R, Arab JP, Soza A. Comparative Efficacy of a High-Dose vs Standard-Dose Hepatitis B Revaccination Schedule Among Patients With HIV: A Randomized Clinical Trial. JAMA Netw Open. 2021;4:e2120929. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 17] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 88. | Wang C, Tang J, Song W, Lobashevsky E, Wilson CM, Kaslow RA. HLA and cytokine gene polymorphisms are independently associated with responses to hepatitis B vaccination. Hepatology. 2004;39:978-988. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 152] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 89. | Davila S, Froeling FE, Tan A, Bonnard C, Boland GJ, Snippe H, Hibberd ML, Seielstad M. New genetic associations detected in a host response study to hepatitis B vaccine. Genes Immun. 2010;11:232-238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 68] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 90. | Pan LP, Zhang W, Zhang L, Wu XP, Zhu XL, Yan BY, Li JY, Xu AQ, Liu Y, Li H. CD3Z genetic polymorphism in immune response to hepatitis B vaccination in two independent Chinese populations. PLoS One. 2012;7:e35303. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 91. | Pan L, Zhang L, Zhang W, Wu X, Li Y, Yan B, Zhu X, Liu X, Yang C, Xu J, Zhou G, Xu A, Li H, Liu Y. A genome-wide association study identifies polymorphisms in the HLA-DR region associated with non-response to hepatitis B vaccination in Chinese Han populations. Hum Mol Genet. 2014;23:2210-2219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 64] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 92. | Ou G, Liu X, Jiang Y. HLA-DPB1 alleles in hepatitis B vaccine response: A meta-analysis. Medicine (Baltimore). 2021;100:e24904. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 93. | Cui W, Sun CM, Deng BC, Liu P. Association of polymorphisms in the interleukin-4 gene with response to hepatitis B vaccine and susceptibility to hepatitis B virus infection: a meta-analysis. Gene. 2013;525:35-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 20] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 94. | Höhler T, Reuss E, Freitag CM, Schneider PM. A functional polymorphism in the IL-10 promoter influences the response after vaccination with HBsAg and hepatitis A. Hepatology. 2005;42:72-76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 62] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 95. | Duan Z, Chen X, Liang Z, Zeng Y, Zhu F, Long L, McCrae MA, Zhuang H, Shen T, Lu F. Genetic polymorphisms of CXCR5 and CXCL13 are associated with non-responsiveness to the hepatitis B vaccine. Vaccine. 2014;32:5316-5322. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 35] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 96. | Höhler T, Reuss E, Evers N, Dietrich E, Rittner C, Freitag CM, Vollmar J, Schneider PM, Fimmers R. Differential genetic determination of immune responsiveness to hepatitis B surface antigen and to hepatitis A virus: a vaccination study in twins. Lancet. 2002;360:991-995. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 150] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 97. | Van Der Meeren O, Crasta P, Cheuvart B, De Ridder M. Characterization of an age-response relationship to GSK's recombinant hepatitis B vaccine in healthy adults: An integrated analysis. Hum Vaccin Immunother. 2015;11:1726-1729. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 24] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 98. | Weinberger B, Haks MC, de Paus RA, Ottenhoff THM, Bauer T, Grubeck-Loebenstein B. Impaired Immune Response to Primary but Not to Booster Vaccination Against Hepatitis B in Older Adults. Front Immunol. 2018;9:1035. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 32] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 99. | Ng M, Fleming T, Robinson M, Thomson B, Graetz N, Margono C, Mullany EC, Biryukov S, Abbafati C, Abera SF, Abraham JP, Abu-Rmeileh NM, Achoki T, AlBuhairan FS, Alemu ZA, Alfonso R, Ali MK, Ali R, Guzman NA, Ammar W, Anwari P, Banerjee A, Barquera S, Basu S, Bennett DA, Bhutta Z, Blore J, Cabral N, Nonato IC, Chang JC, Chowdhury R, Courville KJ, Criqui MH, Cundiff DK, Dabhadkar KC, Dandona L, Davis A, Dayama A, Dharmaratne SD, Ding EL, Durrani AM, Esteghamati A, Farzadfar F, Fay DF, Feigin VL, Flaxman A, Forouzanfar MH, Goto A, Green MA, Gupta R, Hafezi-Nejad N, Hankey GJ, Harewood HC, Havmoeller R, Hay S, Hernandez L, Husseini A, Idrisov BT, Ikeda N, Islami F, Jahangir E, Jassal SK, Jee SH, Jeffreys M, Jonas JB, Kabagambe EK, Khalifa SE, Kengne AP, Khader YS, Khang YH, Kim D, Kimokoti RW, Kinge JM, Kokubo Y, Kosen S, Kwan G, Lai T, Leinsalu M, Li Y, Liang X, Liu S, Logroscino G, Lotufo PA, Lu Y, Ma J, Mainoo NK, Mensah GA, Merriman TR, Mokdad AH, Moschandreas J, Naghavi M, Naheed A, Nand D, Narayan KM, Nelson EL, Neuhouser ML, Nisar MI, Ohkubo T, Oti SO, Pedroza A, Prabhakaran D, Roy N, Sampson U, Seo H, Sepanlou SG, Shibuya K, Shiri R, Shiue I, Singh GM, Singh JA, Skirbekk V, Stapelberg NJ, Sturua L, Sykes BL, Tobias M, Tran BX, Trasande L, Toyoshima H, van de Vijver S, Vasankari TJ, Veerman JL, Velasquez-Melendez G, Vlassov VV, Vollset SE, Vos T, Wang C, Wang X, Weiderpass E, Werdecker A, Wright JL, Yang YC, Yatsuya H, Yoon J, Yoon SJ, Zhao Y, Zhou M, Zhu S, Lopez AD, Murray CJ, Gakidou E. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980-2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2014;384:766-781. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7951] [Cited by in RCA: 8004] [Article Influence: 727.6] [Reference Citation Analysis (0)] |
| 100. | Weber DJ, Rutala WA, Samsa GP, Santimaw JE, Lemon SM. Obesity as a predictor of poor antibody response to hepatitis B plasma vaccine. JAMA. 1985;254:3187-3189. [PubMed] |
| 101. | Young KM, Gray CM, Bekker LG. Is obesity a risk factor for vaccine non-responsiveness? PLoS One. 2013;8:e82779. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 52] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 102. | Liu F, Guo Z, Dong C. Influences of obesity on the immunogenicity of Hepatitis B vaccine. Hum Vaccin Immunother. 2017;13:1014-1017. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 49] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 103. | Painter SD, Ovsyannikova IG, Poland GA. The weight of obesity on the human immune response to vaccination. Vaccine. 2015;33:4422-4429. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 150] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 104. | Tagliabue C, Principi N, Giavoli C, Esposito S. Obesity: impact of infections and response to vaccines. Eur J Clin Microbiol Infect Dis. 2016;35:325-331. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 51] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 105. | Ellulu MS, Patimah I, Khaza'ai H, Rahmat A, Abed Y. Obesity and inflammation: the linking mechanism and the complications. Arch Med Sci. 2017;13:851-863. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 793] [Cited by in RCA: 1214] [Article Influence: 151.8] [Reference Citation Analysis (0)] |
| 106. | de Heredia FP, Gómez-Martínez S, Marcos A. Obesity, inflammation and the immune system. Proc Nutr Soc. 2012;71:332-338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 410] [Cited by in RCA: 556] [Article Influence: 42.8] [Reference Citation Analysis (0)] |
| 107. | Thomas AL, Alarcon PC, Divanovic S, Chougne CA, Hildeman DA, Moreno-Fernandez ME. Implications of Inflammatory States on Dysfunctional Immune Responses in Aging and Obesity. Front Aging. 2021;2:732414. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 19] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 108. | Frasca D, Ferracci F, Diaz A, Romero M, Lechner S, Blomberg BB. Obesity decreases B cell responses in young and elderly individuals. Obesity (Silver Spring). 2016;24:615-625. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 174] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 109. | Younas M, Carrat F, Desaint C, Launay O, Corbeau P; ANRS HB03 VIHVAC-B Trial Group. Immune activation, smoking, and vaccine response. AIDS. 2017;31:171-173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 110. | Zimmermann P, Curtis N. Factors That Influence the Immune Response to Vaccination. Clin Microbiol Rev. 2019;32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 300] [Cited by in RCA: 611] [Article Influence: 101.8] [Reference Citation Analysis (0)] |
| 111. | Meier MA, Berger CT. A simple clinical score to identify likely hepatitis B vaccination non-responders - data from a retrospective single center study. BMC Infect Dis. 2020;20:891. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 112. | Leroux-Roels G. Old and new adjuvants for hepatitis B vaccines. Med Microbiol Immunol. 2015;204:69-78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 18] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 113. | Lee S, Nguyen MT. Recent advances of vaccine adjuvants for infectious diseases. Immune Netw. 2015;15:51-57. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 232] [Cited by in RCA: 290] [Article Influence: 29.0] [Reference Citation Analysis (0)] |
| 114. | Food and Drug Administration. Product approval information: package insert. Heplisav-B. Silver Spring, MD: US Department of Health and Human Services, Food and Drug Administration; 2018. [cited 5 February 2021]. Available from: https://www.fda.gov/BiologicsBloodVaccines/Vaccines/ApprovedProducts/ucm584752.htm. |
| 115. | Jackson S, Lentino J, Kopp J, Murray L, Ellison W, Rhee M, Shockey G, Akella L, Erby K, Heyward WL, Janssen RS; HBV-23 Study Group. Immunogenicity of a two-dose investigational hepatitis B vaccine, HBsAg-1018, using a toll-like receptor 9 agonist adjuvant compared with a licensed hepatitis B vaccine in adults. Vaccine. 2018;36:668-674. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 150] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 116. | Splawn LM, Bailey CA, Medina JP, Cho JC. Heplisav-B vaccination for the prevention of hepatitis B virus infection in adults in the United States. Drugs Today (Barc). 2018;54:399-405. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 117. | Halperin SA, Ward B, Cooper C, Predy G, Diaz-Mitoma F, Dionne M, Embree J, McGeer A, Zickler P, Moltz KH, Martz R, Meyer I, McNeil S, Langley JM, Martins E, Heyward WL, Martin JT. Comparison of safety and immunogenicity of two doses of investigational hepatitis B virus surface antigen co-administered with an immunostimulatory phosphorothioate oligodeoxyribonucleotide and three doses of a licensed hepatitis B vaccine in healthy adults 18-55 years of age. Vaccine. 2012;30:2556-2563. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 104] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 118. | Heyward WL, Kyle M, Blumenau J, Davis M, Reisinger K, Kabongo ML, Bennett S, Janssen RS, Namini H, Martin JT. Immunogenicity and safety of an investigational hepatitis B vaccine with a Toll-like receptor 9 agonist adjuvant (HBsAg-1018) compared to a licensed hepatitis B vaccine in healthy adults 40-70 years of age. Vaccine. 2013;31:5300-5305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 98] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 119. | Halperin SA, Ward BJ, Dionne M, Langley JM, McNeil SA, Smith B, Mackinnon-Cameron D, Heyward WL, Martin JT. Immunogenicity of an investigational hepatitis B vaccine (hepatitis B surface antigen co-administered with an immunostimulatory phosphorothioate oligodeoxyribonucleotide) in nonresponders to licensed hepatitis B vaccine. Hum Vaccin Immunother. 2013;9:1438-1444. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 20] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 120. | Champion CR. Heplisav-B: A Hepatitis B Vaccine With a Novel Adjuvant. Ann Pharmacother. 2021;55:783-791. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 37] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 121. | Vesikari T, Finn A, van Damme P, Leroux-Roels I, Leroux-Roels G, Segall N, Toma A, Vallieres G, Aronson R, Reich D, Arora S, Ruane PJ, Cone CL, Manns M, Cosgrove C, Faust SN, Ramasamy MN, Machluf N, Spaans JN, Yassin-Rajkumar B, Anderson D, Popovic V, Diaz-Mitoma F; CONSTANT Study Group. Immunogenicity and Safety of a 3-Antigen Hepatitis B Vaccine vs a Single-Antigen Hepatitis B Vaccine: A Phase 3 Randomized Clinical Trial. JAMA Netw Open. 2021;4:e2128652. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 44] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 122. | Alon D, Stein GY, Hadas-Golan V, Tau L, Brosh T, Turner D. Immunogenicity of Sci-B-Vac (a Third-Generation Hepatitis B Vaccine) in HIV-Positive Adults. Isr Med Assoc J. 2017;19:143-146. [PubMed] |
| 123. | Safadi R, Khoury T, Saed N, Hakim M, Jamalia J, Nijim Y, Farah N, Nuser T, Natur N, Mahamid M, Amer J, Roppert PL, Gerlich WH, Glebe D. Efficacy of Birth Dose Vaccination in Preventing Mother-to-Child Transmission of Hepatitis B: A Randomized Controlled Trial Comparing Engerix-B and Sci-B-Vac. Vaccines (Basel). 2021;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 124. | Di Lello FA, Ridruejo E, Martínez AP, Pérez PS, Campos RH, Flichman DM. Molecular epidemiology of hepatitis B virus mutants associated with vaccine escape, drug resistance and diagnosis failure. J Viral Hepat. 2019;26:552-560. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 125. | Campos-Valdez M, Monroy-Ramírez HC, Armendáriz-Borunda J, Sánchez-Orozco LV. Molecular Mechanisms during Hepatitis B Infection and the Effects of the Virus Variability. Viruses. 2021;13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 16] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 126. | Qin Y, Liao P. Hepatitis B virus vaccine breakthrough infection: surveillance of S gene mutants of HBV. Acta Virol. 2018;62:115-121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 27] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 127. | Ropero Alvarez AM, Vilajeliu A, Magariños M, Jauregui B, Guzmán L, Whittembury A, Cain E, Garcia O, Montesanos R, Ruiz Matus C; PAHO MNI working group. Enablers and barriers of maternal and neonatal immunization programs in Latin America. Vaccine. 2021;39 Suppl 2:B34-B43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |