Published online Jan 21, 2022. doi: 10.3748/wjg.v28.i3.275
Peer-review started: November 15, 2021
First decision: November 22, 2021
Revised: December 2, 2021
Accepted: January 11, 2022
Article in press: January 11, 2022
Published online: January 21, 2022
Processing time: 58 Days and 14.9 Hours
The coronavirus disease 2019 (COVID-19) infected so far over 250 million people and caused the death of over 5 million worldwide. Aging, diabetes, and cardiovascular diseases, conditions with preexisting impaired endothelial functions predispose to COVID-19. While respiratory epithelium is the main route of virus entry, the endothelial cells (ECs) lining pulmonary blood vessels are also an integral part of lung injury in COVID-19 patients. COVID-19 not only affects the lungs and respiratory system but also gastrointestinal (GI) tract, liver, pancreas, kidneys, heart, brain, and skin. Blood vessels are likely conduits for the virus dissemination to these distant organs. Importantly, ECs are also critical for vascular regeneration during injury/lesions healing and restoration of vascular network. The World Journal of Gastroenterology has published in last two years over 67 outstanding papers on COVID-19 infection with a focus on the GI tract, liver, pancreas, etc., however, the role of the endothelial and vascular components as major targets for COVID-19-induced tissue injury, spreading to various organs, and injury healing have not been sufficiently emphasized. In the present article, we focus on these subjects and on current treatments including the most recent oral drugs molnupiravir and paxlovid that show a dramatic, significant efficacy in controlling severe COVID-19 infection.
Core Tip: The coronavirus disease 2019 (COVID-19) pandemic has enormous health care and economic impact on the entire world - infecting more than 250 million people in 213 countries and territories, causing death of more than 5 million (as of November 1, 2021). We comment here on some outstanding papers on COVID-19 published in World Journal of Gastroenterology and reviewed the important role of endothelium and blood vessels in COVID-19 infection. Endothelial cells and blood vessels are both the targets and a conduit for the spread of severe acute respiratory syndrome coronavirus 2 and play a critical role in COVID-19-induced tissue injury and dissemination to various organs. Pre-existing endothelial impaired function could make endothelial cells more sensitive to COVID-19 or at least COVID-19-induced impairment might be synergistic with pre-existing impairment. That could be one contributing factor explaining why older or diabetic patients have more severe responses to infection, since these conditions are already impacted impaired endothelial function.
- Citation: Tarnawski AS, Ahluwalia A. Endothelial cells and blood vessels are major targets for COVID-19-induced tissue injury and spreading to various organs. World J Gastroenterol 2022; 28(3): 275-289
- URL: https://www.wjgnet.com/1007-9327/full/v28/i3/275.htm
- DOI: https://dx.doi.org/10.3748/wjg.v28.i3.275
Andrzej S Tarnawski, MD, PhD, DSc (Med), AGAF, FACG: Received MD degree, PhD (pathology) and DSc (gastroenterology) from the University Medical School, Krakow, Poland, and became Associate Professor & V-Chair, Dept of Gastroenterology at that University. After completing GI fellowship at the University of Missouri, Columbia, MO, United States he joined the University of California, Irvine, USA as Associate Professor (1982-1986) and full Professor (1986-present). He served as: V-chair and Associate Chair, American Gastroenterological Association (AGA)/EGD 1997-1999 and 2008-2010; Scientific Director, Shimoda Symposia on Mucosal Defense in Japan (8 times); Chair, Research Fora DDW/AGA annual meetings (1996-2011); Chair, Pasteur Institute Euroconference and Chair/Co-chair of 68 other International Symposia. Publications, presentations & grants: 373 full, peer reviewed publications (Lancet, Nature Med, JCI, Gastroenterology, Hepatology, Gut, PNAS, FASEB J, Am J Pathol, Cellular Mol Gastro Hepatol, Am J Physiol, Am J Gastroenterol, Endoscopy, Cell Signal, Cells, and others); 20 book chapters; 533 presentations at international & U.S. meetings; 20 peer reviewed funded grants (NIH, VA Merit Review 1984-present), 4 US patents. Clinical and Research interest: Injury and protection of GI mucosa; cellular and molecular mechanisms of gastric, duodenal and esophageal healing-role of growth factors, signaling pathways, angiogenesis, NSAIDs, prostaglandins and Helicobacter pylori toxins; aging gastric mucosa; confocal endomicroscopy and molecular imaging; gene therapy. Awarded prestigious academic honors (e.g., Glaxo Intl. Res. Award, Athalie-Clarke Award, Merentibus Medal Award, Peregrinator of Science Awards, Andre Robert’s Distinguished Award, Notable Biomedical Research Investigator Award). Memberships: AGA (Fellow), Am. College of Gastroenterology (Fellow), Brit. Soc. of Gastroenterology, Japanese Soc. of Gastroenterology (Honorary), Hungarian Soc. of Gastroenterology (Honorary), Am. Soc. for Investigative Pathology, Association of Am. Physicians (by election) and others. Editorial Boards - 6 scientific journals. Sixteen of his former trainees hold academic positions in US Medical Schools (4 being Chairs of Departments). Twenty of his former international trainees and/or associates hold academic positions abroad (France, Germany, Hungary, Japan, Poland, Sweden, Switzerland) (Figure 1A).
Amrita Ahluwalia, PhD: Research Scientist and coronavirus disease 2019 (COVID-19) Investigator, Veterans Affairs Long Beach Healthcare System, Long Beach, United States. Awarded PhD (Physiology) by the Medical Sciences Program, Indiana University Bloomington, IN, United States. Publications/presentations/grants: 60 peer-reviewed research publications (PNAS, Am J Physiol , Gene Therapy, Endocrinology, Molecular Endocrinology etc.); 95 presentations at International and US research meetings; 6 peer-reviewed funded grants. Research Interests: Endothelial dysfunction, wound and tissue injury healing; gastroprotection; role of growth factors, angiogenesis, molecular imaging & gene therapy. Awards: Quest Diagnostics Young Investigator Award, AACR-AstraZeneca Scholar in Training Award, Robert W. Bullard Award - Outstanding Medical Science Student Award, Indiana University. Memberships: AGA, Am. Assoc. Physiology, Am. Heart Assoc.; Editorial Boards – 2 peer-reviewed scientific journals (Figure 1B).
The coronavirus disease 2019 (COVID-19) pandemic has had enormous health care and economic impact on the entire world - infecting more than 250 million people in 213 countries and territories, causing more than 5 million deaths (as of November 1, 2021). Its enormous magnitude is also reflected by an unprecedented number of publications related to COVID-19 so far approximate 210294 recorded in PubMed; 254358 recorded on PMC, and 3215 clinical trials just in 24 mo. These are staggering numbers compared to 47305 publications recorded on PubMed on Helicobacter pylori (H. pylori)– the world’s most prevalent GI infection - published in about last 40 years.
COVID-19 is caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and is highly infectious and transmitted by aerosol droplets. Therefore, it is not surprising that the respiratory tract including the lungs is the main affected organ by COVID-19 infection that leads to respiratory failure, hypoxia, multiorgan system failure and death. Numerous studies showed that COVID-19 not only affects the lungs and respiratory system but also the gastrointestinal tract (GI), liver, pancreas, kidneys, heart, brain, and skin[1-5]. SARS-CoV-2 RNA was detected in stool or rectal swabs in 34%-59% of infected patients[6]. The viral loads from stool samples peaked 2-3 wk after symptom onset and in some patients were detectable even after viral loads in the respiratory and/or sputum samples were not detectable[6]. The presence and persistence of viral RNA in the stool suggest the potential for enteric infection of SARS-CoV-2. This contention is supported by a study demonstrating that the GI tract is an alternative route for COVID-19 infection in the rhesus monkey model[7]. In that study, the authors showed that intranasal or gastric inoculation with SARS-CoV-2 induced infections and pathologic changes not only in respiratory tissues but also in digestive tissues[7]. In a recent letter to the World Journal of Gastroenterology (WJG) editor[8], Sica et al[8] contended that GI and hepatic involvement are the most common presenting symptoms of COVID-19 and multisystem inflammatory syndrome recently described in children and adolescents. This syndrome can lead to shock and multiple organ failure requiring intensive care[9].
Risk factors for COVID-19 severity include aging and comorbidities such as coronary artery disease, chronic kidney disease, hypertension, obesity, and diabetes[10-12], all of which exhibit preexisting endothelial dysfunction. However, the potential role of endothelial/vascular components as critical target sites for COVID-19-induced tissue injury and spreading to various organs, and the role of preexisting endothelial function impairment, e.g., in aging or diabetes – conditions that facilitate COVID-19 infection have not been sufficiently elaborated on. In the present article, we focus on these topics anticipating that providing a detailed information on endothelial cells (ECs) and vasculature in COVID-19 as critical targets may afford a better insight into the pathomechanism of this disease and add additional new therapies.
The SARS-CoV-2 virus spreads from its primary infection site (respiratory tract) to more distant organs indicating the involvement of ECs and blood vessels for disseminating infection. This contention is supported by some studies demonstrating the presence of SARS-CoV-2-like particles in ECs in several tissues e.g., lung, kidneys, brain, and skin and observation that the clinical course of COVID-19 may include vascular complications such as thrombosis of blood vessels and thromboembolism[3, 5, 13-16].
The WJG has published in the last two years over 67 outstanding papers related to COVID-19 infection with a focus on GI tract and liver. These papers - original papers, retrospective studies and review articles on the pathophysiology, mechanisms, and clinical aspects and manifestations of COVID-19 related diseases of the digestive system including GI tubular system, liver, pancreas provided important information for the gastroenterologists, hepatologists, surgeons, researchers, pharmacologists, and clinicians. These papers provide information on the mechanisms of COVID-19 related tissue damage; the effects of immunosuppression in patients with inflammatory bowel disease and chronic liver disease; and the impact of COVID-19 on GI emergencies, endoscopy, diagnosis and treatments. These WJG articles were frequently viewed on the WJG website and cited in high-impact journals. We wish to point out one important paper by P. Samantha and AR Ghosh: “Environmental perspectives of COVID-19 outbreaks: A review” published in World J Gastroenterol. 2021 Sep 21;27(35):5822-585”[17]. In this paper the authors provided extensive information from an environmental perspective on the origin and current status of COVID-19[17] and summarized the geographical distribution of COVID-19 around the world including specific countries. They also elaborated on the details of coronavirus genus, species and receptors, virus susceptibility and incubation period, and summarized SARS-CoV-2 pathogenesis, the role of angiotensin-converting enzyme 2 (ACE2), the longevity of SARS-CoV-2 virus in the environment, meteorological influences, air quality and social impact. They emphasized that aging, cardiovascular diseases and diabetes predispose to COVID-19. The authors stressed that while drugs such as remdesivir, tocilizumab, lopinavir-ritonavir, azithromycin, etc., are used in COVID-19 patients these drugs do not induce full recovery. The statement that there is no truly effective drug aimed at the causative agent, SARS-CoV-2 is no longer valid. On November 4 and 5, 2021 the released results of most recent clinical trials for COVID-19 treatments demonstrated that oral drugs inhibiting viral replication – Molnupiravir (Merck), and Paxlovid (Pfizer) showed very impressive efficacy in controlling severe COVID-19 infection. The interim analysis of the latter drug showed a dramatic approximate 90% reduction in risk of - hospitalization or death from COVID-19 compared to placebo in patients treated within three - five days of symptom onset. Most likely the vascular component of the disease was important part of this dramatic reduction.
Regarding COVID-19 pathomechanism, the potential role of endothelial and vascular components as critical target sites for COVID-19-induced tissue injury and spreading to various organs and the role of preexisting endothelial function impairment, e.g., aging gastropathy has not been sufficiently emphasized. In this editorial article, we focus on the role vascular endothelium and blood vessels in COVID-19 infection (Table 1).
| COVID-19 and endothelium/blood vessels |
| Endothelium and blood vessels are integral parts of COVID-19-induced tissue injury. Their injury is likely due to either direct viral infection and/or cytokine storm triggered by infection of adjacent epithelial cells and inflammatory response[18]. |
| Blood vessels are critical for virus dissemination to distant organs. |
| Preexisting-impaired endothelial function, e.g., in aging or diabetes are likely predisposing factors COVID-19. Our studies demonstrated that aging gastric mucosa has increased susceptibility to injury and prominent EC abnormalities (decreased VEGF, NGF and impaired mitochondrial function)[19-21]. |
| ECs are critical for vascular regeneration (through angiogenesis and vasculogenesis) during injury/lesions healing and therefore are essential for the delivery of oxygen and nutrients to the healing site[22, 23]. |
| Several growth factors e.g., NGF, IGF-1, HGF and BMD-stem cells may facilitate tissue regeneration in the healing phase[20,24,25]. |
| Long-term effects of COVID-19, its vaccines and treatment on endothelium and vasculature remain to be determined. |
| Recently, new oral drugs inhibiting viral replication–Molnupiravir (Merck) and Paxlovid (Pfizer) showed significant efficacy in controlling severe COVID-19 infection by inhibiting viral replication. The interim analysis of the latter drug showed an 89% reduction in risk of COVID-19-related hospitalization or death from any cause compared to placebo in patients treated within three-five days of symptom onset[26]. |
Increasing evidence suggests the essential role of endothelium and vasculature, in addition to the epithelial cells, in COVID-19 infection as a critical targets for SARS-CoV-2 and the resulting cytokine storm, and as the main effector for the pro-inflammatory and pro-coagulant state in COVID-19 patients[18,27-30]. Focus on ECs and vasculature in COVID-19 may also add additional insight into COVID-19 injury, its healing and tissue regeneration, and new therapies that impact endothelium and the blood vessels.
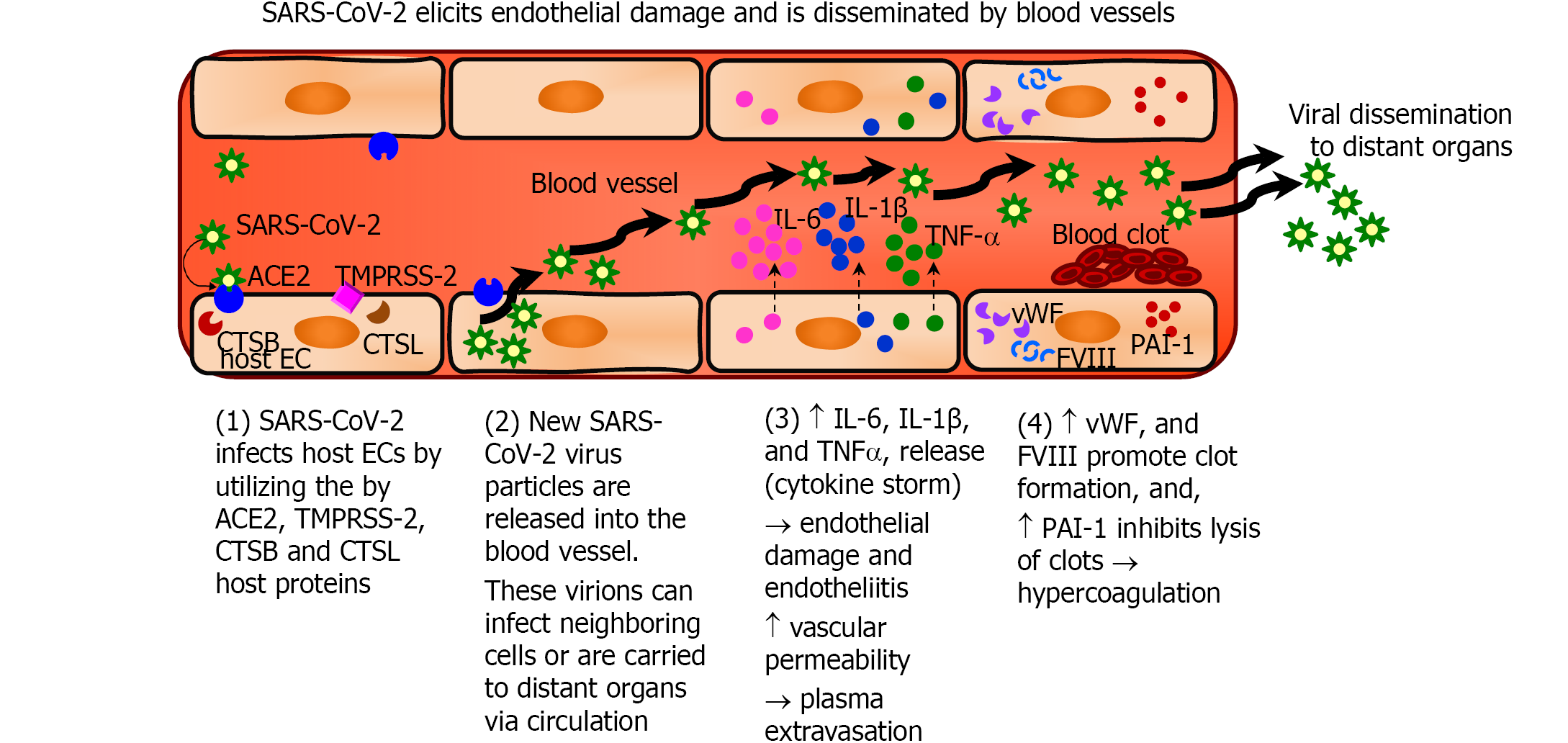
Although SARS-CoV-2 primarily targets the respiratory and alveolar epithelium, the high incidence of vascular complications in COVID-19 patients suggests that impaired function of ECs, which line the blood vessels and microvessels, may be critical factor in COVID-19 progression. SARS-CoV-2 causes endothelial dysfunction and thrombosis by two potential mechanisms: by directly infecting the endothelium, and disrupting its anti-thrombogenic and barrier properties, or indirectly by unleashing a local cytokine storm and systemic inflammatory response that results in endothelial injury (Table 2). Most likely, both these scenarios are in play in COVID-19.
| Scenario A: SARS-CoV-2 infection | Scenario B: Cytokine storm |
| SARS-CoV-2 infects and replicates within vascular ECs and new virus particles are released into the blood vessel. These virions can infect neighboring cells or are carried to distant organs via circulation | ↑ IL-6, IL-1β, and TNFα release (cytokine storm) → endothelial damage |
| ↑ vascular permeability → plasma extravasation | |
| ↑ vWF & FVIII (promote clot formation) and ↑ PAI-1 (inhibits clots lysis) → hypercoagulation |
The endothelium is a key player in vascular homeostasis[29,31-33]. ECs are critical for supplying oxygen and other nutrients to all cells and tissues, and are involved in coagulation and the generation of vasoactive substances, prostanoids, hormones and growth factors[33-38]. The unstimulated vascular endothelium is normally impermeable and acts as a selective barrier regulating exchange of fluids, nutrient delivery and waste removal while preventing entry of pathogens and harmful substances into the tissues. Microvessels consist of a single layer of thin (approximate 0.5-1 μm) ECs and occasional adherent cells such as pericytes[34-38]. The endothelial "barrier between neighboring ECs formed by prominent tight junctions prevents diffusion between cells. ECs act as a barrier between blood and the interstitial tissue, and regulate various physiological processes such as angiogenesis, inflammation, and immune response[31,35,36]. The endothelium contains special vesicles - Weibel-Palade bodies, which store various factors that regulate blood coagulation and leukocyte recruitment and extravasation such as von Willebrand factor (vWF), P-selectin, chemokines, interleukin-8, and eotaxin-3; endothelin-1, angiopoietin-2 and osteoprotegerin[39-42].
In response to local stimuli, ECs secrete endothelin and leukotriene C4 (potent vasoconstrictors), nitric oxide (NO) and prostacyclin (PGI2) (vasodilators) and empty the contents of the Weibel-Palade vesicles that affect the tone of vascular smooth muscle and result in neutrophil adhesion and/or other autocrine and/or paracrine actions. NO, prostacyclin, prostaglandin E2 (PGE2), carbon monoxide (CO), tissue plasminogen activator, vascular endothelial growth factor (VEGF) and bFGF are endothelial mediators that reduce platelet and leukocyte activation, prevent thrombi formation, promote thrombolysis, maintain tissue perfusion, and protect the microvascular wall against acute damage[33,36-38,43-46]. For example, our previous study demonstrated that 16,16 dimethyl PGE2 protects human gastric mucosa against injury by 40% ethanol by protecting and preserving integrity of endothelial cells of gastric microvessels[47]. In response to wounding, infections or injurious stimuli, attachment between ECs is lost, resulting in increased endothelial permeability and edema[48].
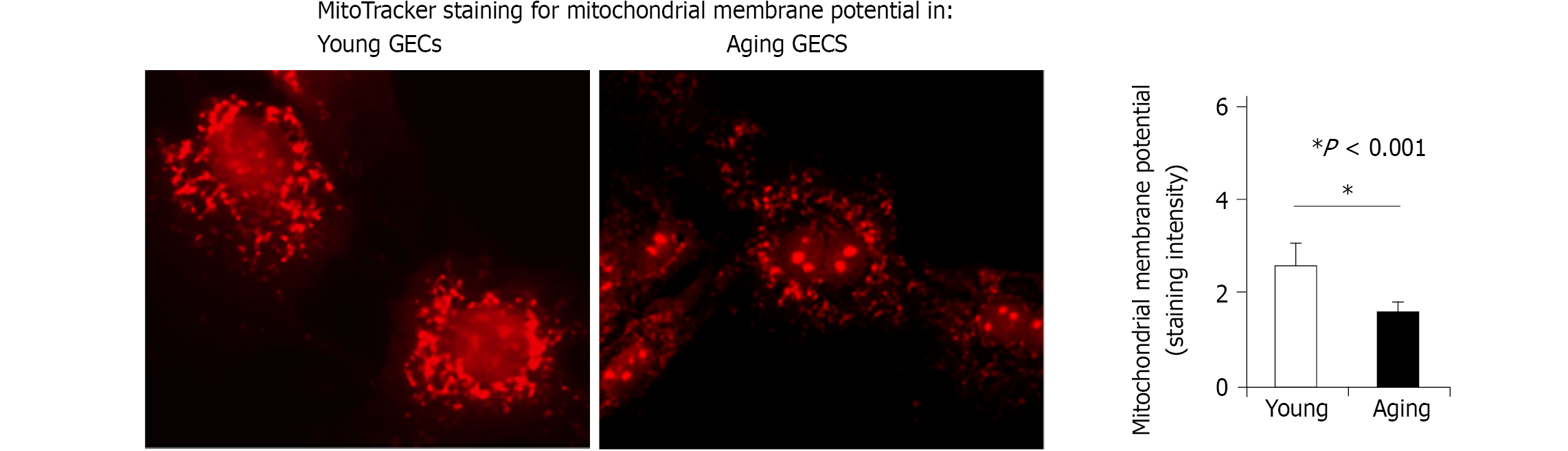
The endothelium and blood vessels are integral parts of any tissue injury including COVID-19. Our previous studies demonstrated that ECs are critical targets of gastric mucosal injury by NSAIDs and ethanol, they initiate angiogenesis, and that age-related endothelial dysfunction of human and rat gastric endothelial cells results in impaired angiogenesis and delayed healing[19,20,24]. Our studies on aging gastropathy showed aging-related defects in ECs functions - angiogenesis, cell migration, proliferation, and healing of injury[19-21,49]. In a recent study, we also showed the critical role of mitochondria in aging gastric ECs; aging ECs have fewer mitochondria, and reduced mitochondrial membrane potential[50] that result in reduced ATP generation (Figure 2). We also demonstrated that treatment with VEGF and nerve growth factor (NGF) restores angiogenesis in cultured aging gastric ECs[20], accelerates healing of gastric ulcers and improves the quality of mucosal regeneration in vivo in aging rats[20, 24].
SARS-CoV-2 is a single, positive-stranded RNA virus that uses a spike-protein (S-protein) expressed on its envelope to bind to the host cell’s human protein receptor ACE2[51-53]. The human ACE2 protein was initially identified as ACE-related carboxypeptidase membrane-associated and secreted enzyme expressed predominantly on the endothelium of the human heart, kidney, and testis [54]. However, it is widely expressed in various cells and tissues[55]. SARS-CoV-2 employs the ACE2 receptor, transmembrane serine protease 2 (TMPRSS-2), and cathepsin B and L (CTSB, and CTSL) for infection[51-53,56,57]. SARS-CoV-2 was detected in the respiratory tract, kidneys, liver, heart, and brain (all of which are highly vascularized tissues) of infected individuals[55]. ECs, which line the blood vessels of all organs and maintain microvascular integrity, express the ACE2 receptor and the cellular proteases TMPRSS-2, CTSB, and CTSL[57]. ECs are, therefore, a target for SARS-CoV-2 and blood vessels likely route of this virus dissemination to various organs. Electron microscopy (EM) and histologic studies detected SARS-CoV-2 virus-like particles and proteins in ECs of the kidney, small bowel, lung, myocardium, skin, and brain[3,5,13-16]. Ackerman et al[15] showed abnormalities within the pulmonary microvasculature with congestion and micro-thrombi in lungs of COVID-19 patients, and visualized endothelial injury and lumen filled with cell fragments and degenerated organelles by electron microscopy. That study also showed increased ACE2-positive ECs and significant changes in endothelial morphology in lung autopsies of COVID-19 patients[15]. Varga et al[5] using EM evaluation reported evidence of viral particles in renal ECs of COVID-19 patients presenting with endotheliitis, which is an immune and inflammatory response within the endothelium of blood vessels.
Other studies visualized SARS-CoV-2 proteins in dermal and renal endothelium[13,58]. While some studies were not able to corroborate presence of SARS-CoV-2 in ECs of some tissues, there is strong evidence to support that SARS-CoV-2 infects ECs. Monteil et al[59] demonstrated that SARS-CoV-2 infects blood vessel organoids. SARS-CoV-2 virus particles range from approximate 70 to 120 nm[60-63]; therefore, in the absence of preexisting tissue injury, the virus would need to pass through the ECs to infect other tissues.
The term endothelial dysfunction was originally used to identify the shift from a normal quiescent endothelium to an impaired endothelium with the inability to generate nitric oxide and other vasodilators. In a broader definition, endothelial dysfunction includes impairment of endothelial function (that we used for aging endothelium in our previous papers) - reduced angiogenesis, pro-inflammatory, pro-vasoconstriction, proliferative, and pro-coagulant phenotype[18,64-66]. In certain pathological conditions characterized by preexisting endothelial dysfunction, the ACE/Ang II axis is upregulated resulting in vasoconstriction, thrombosis, fibrosis, coagulopathy, and thrombophilia.
Emerging evidence indicates that preexisting endothelial dysfunction predisposes to COVID-19 infection and that COVID-19 induced endotheliitis further impairs endothelial integrity and function[27-30,32,34,67-76]. This is evidenced by the critical role of vascular endothelium in inflammation that results in dysregulation of cytokines in acute respiratory distress syndrome as well as multiple cardiovascular pathologies[18,27,30,32,64,71,73]. The ubiquitous expression of ACE-2 on ECs in all tissues suggests that SARS-CoV-2 can spread via circulation throughout the body and affect multiple organs[55].
The sequential steps of SARS-CoV-2 infection of ECs that result in endothelial pathology and a procoagulant, hypofibrinolytic state of the endothelium are summarized in Figure 3. SARS-CoV-2 utilizes the ACE2 receptors and cellular proteases (TMPRSS-2, CTSB and CTSL) infect the host cells including ECs[51-53,56,57]. The virus then replicates within the cells and is released into the blood vessels, which then disseminate the virus to distant organs. Severe COVID-19 results in increased production of pro-inflammatory cytokines such as interleukin-6 (IL-6), interleukin-1β (IL-1β) and tumor necrosis factor-α (TNF-α) which is referred to as cytokine storm[29,77,78]. The binding of IL-6 to its receptors on ECs increases vascular permeability, induces capillary leakage, and unleashes a cytokine storm by further increasing the secretion of IL-6, IL-8, and MCP-1 by ECs[29,70,78]. The cytokine storm in COVID-19 patients exposes the endothelium to pro-inflammatory cytokines resulting in leukocyte recruitment and inflammation and can lead to EC death that contributes to increased vascular permeability and end-organ damage[18,29]. In addition, activated ECs produce increased amounts of vWF and factor VIII, which participate in clot formation thereby inducing a pro-coagulant state. Furthermore, ECs produce increased amounts of PAI-1 that inhibits the degradation of clots and induces a hypofibrinolytic state[29,70,78].
The initial SARS-CoV-2 infection and vascular damage in pulmonary tissues can result in the release of ECs into the circulation. Increased numbers of circulating ECs (CECs) have been demonstrated in conditions associated with vascular damage[79-82]. Increased CECs may potentiate the spread to distant extrapulmonary tissues. Numerous extrapulmonary manifestations of SARS-CoV-2 infection such as acute kidney injury, thrombotic complications, myocardial dysfunction and arrhythmia, heart failure, venous thromboembolism, GI symptoms, hepatocellular injury, neurologic illnesses, ocular symptoms, and dermatologic complications have been documented[1]. Endothelial injury may be the underlying mechanism for both pulmonary and extrapulmonary manifestations of COVID-19.
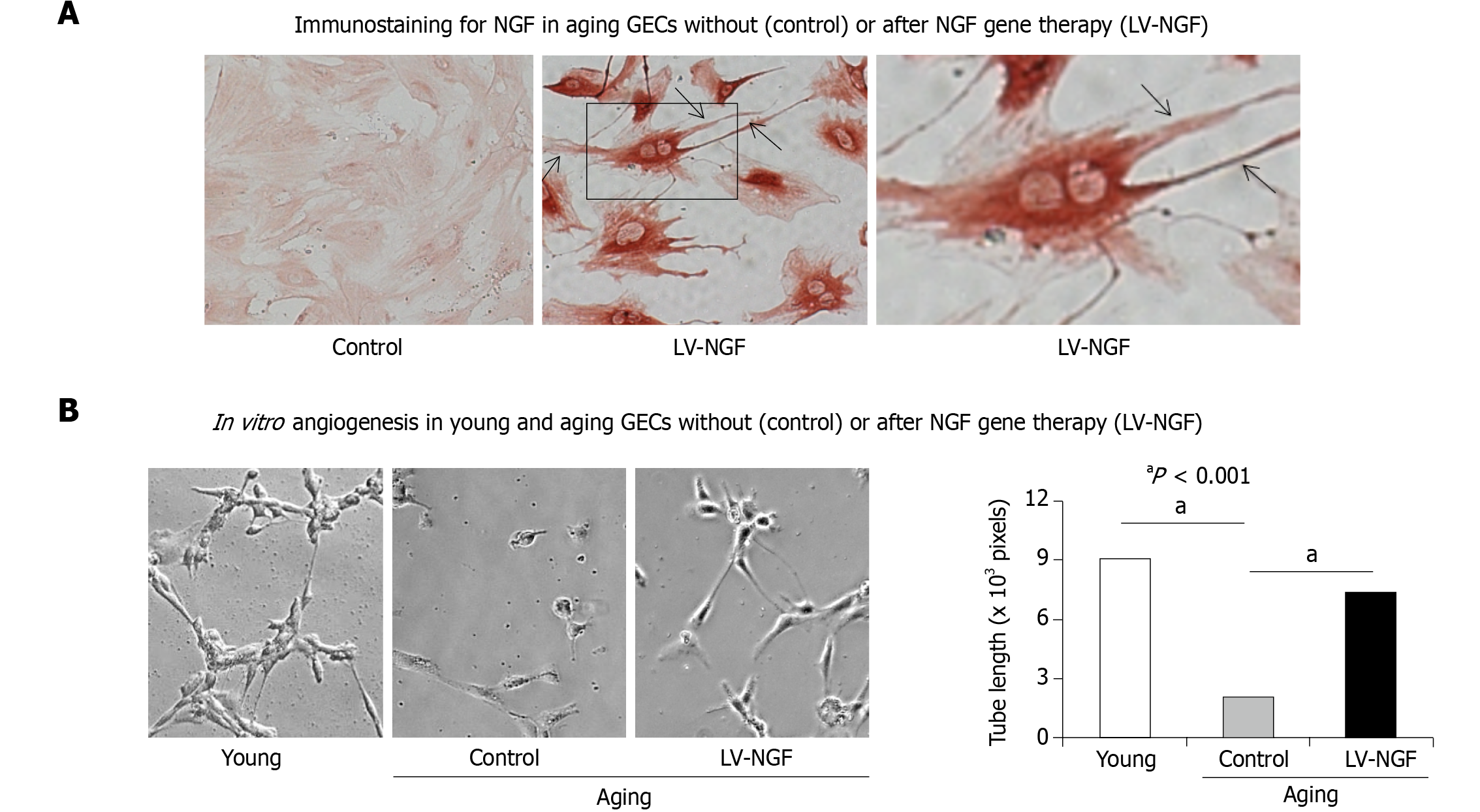
The process of tissue injury healing involves tissue and vascular regeneration[32,34,75,83,84]. The latter is mediated by the sprouting of ECs from pre-existing vessels from areas bordering injury (angiogenesis), or the formation of new blood vessels from bone marrow-derived angiogenic precursor cells (vasculogenesis)[22,23,85]. Blood vessel reconstruction is regulated by angiogenic growth factors and involves the activation of genes such as basic fibroblast growth factor (bFGF or FGF-2) and its receptors; VEGF and its receptor; angiopoietins -Ang 1 and Ang 2, and their receptor, COX-2, serum response factor, NGF, stromal-derived factor 1[25]. Our previous studies demonstrated the aging-related decrease in the expression of VEGF and NGF in ECs and that treatment with VEGF and NGF restore angiogenesis in aging gastric ECs (Figure 4)[20, 21]. Furthermore, we showed that local NGF therapy of gastric ulcers increased angiogenesis, promoted revascularization, and accelerated gastric ulcer healing in aging rats[20].
SARS-CoV-2 infection was first reported in 2019 and rapid, breakthrough research resulted in the development of several effective COVID-19 vaccines. Although these vaccines have proven effective in reducing the infection and severity of COVID-19, the long-term effects of the disease and the vaccines on ECs and blood vasculature are still to be determined.
Two recent outstanding studies published by the Baishideng Publishing Group in the World Journal of Virology outlined the current therapies that have been utilized in COVID-19 treatment[86,87]. We wish to add to this list additional investigational treatments in ongoing clinical trials (Table 3) and describe two additional oral drugs that were announced in early November 2021 as potential COVID-19 treatments Molnupiravir (Merck) and Paxlovid (PF-07321332).
| Intervention/ Treatment | Mode of action | Dose | Route | ClinicalTrials.gov Identifier |
| Ronapreve/REGN-COV2 (REGN10933 and REGN10987) | Monoclonal antibodies against spike proteins | 8 g once, or 4 g twice | IV | NCT04425629 |
| Lopinavir/Ritonavir | Inhibitor of the HIV protease and cytochrome P-450 CYP3A | 200/ 50 mg; (4 tablets twice a day on day 1 followed by 2 tablets twice a day for 9 d) | Oral | NCT04403100 |
| Remdesivir (RDV, GS-5734, Veklury) | Inhibitor of RNA-dependent RNA polymerase | 200 mg on day 1 followed by 100 mg for 4-9 d | IV | NCT04292899 |
| Hyperimmune Plasma (COV19-PLASMA) | Immunotherapy | 250-300 mL up to 3 times over 5 d | IV | NCT04321421 |
| Tocilizumab (TCZ, ROACTEMRA) | Humanized anti-IL6 receptor monoclonal antibody | 8 mg/kg single infusion, up to 800 mg | IV | NCT04320615 |
| Sarilumab (Kevzara, REGN88, SAR153191) | Monoclonal antibody against IL-6 receptor alpha | 200 mg or 400 mg; single dose and multiple doses | IV | NCT04315298 |
| Anakinra (KINERET) | Monoclonal antibody against the IL-1 receptor | 100 mg daily up to 28 d | SC | NCT04330638 |
| Siltuximab (SYLVANT) | Chimeric anti-IL-6 antibody | 11 mg/kg single infusion | IV | NCT04330638 |
| Eculizumab | Monoclonal antibody against complement protein C5 | 900 mg every 7 d | IV | NCT04288713 |
| Methyl-prednisolone (MP) | Immunosuppression against cytokine storm | 80 mg/kg IV bolus, followed by infusion of 80 mg/d for at least 8 d and then oral MP 16 mg or 20 mg IV twice daily | Oral-IV | NCT04323592 |
| Heparin | Antithrombotic agents | 10 units/kg/h | IV | NCT04367831 |
| Enoxaparin (Lovenox) | Antithrombotic agents | 1 mg/kg | SC | NCT04367831 |
| Dexamethasone | Immunosuppression against cytokine storm | 20 mg/d (5 d) then 10 mg/d (5 d) | IV | NCT04325061 |
| Vitamin C | Antioxidant | 12 g infusion twice a day for 7 d | IV | NCT04264533 |
| Melatonin | Antioxidant | 3 or 30 mg three times a day for 14 d | Oral | NCT04784754 |
| CoQ10 | Antioxidant | 500 mg/day for 6 wk | Oral | NCT04960215 |
During recent press releases two newest oral drugs inhibiting SARS-CoV-2 replication were recently presented. Are they game changers? On November 4 and 5, 2021 two oral drugs were announced as novel COVID-19 treatments - Molnupiravir (Merck) and Paxlovid (PF-07321332). Both these drugs showed dramatic efficacy in controlling severe COVID-19 infection. The oral drug Molnupiravir (EIDD-2801) was developed by US-based Merck & Co Inc and Ridgeback Biotherapeutics[88] and investigated in a clinical trial (NCT04405570) to eliminate SARS-CoV-2 virus load in infected patients, has since been approved in the UK to treat patients with mild to moderate COVID-19 and at least one risk factor such as older age, diabetes, obesity, and heart disease that predisposes them for developing severe illness. Molnupiravir is the prodrug of the ribonucleoside analog β-D-N4-hydroxycytidine and is rapidly converted by host kinases in plasma to the active 5′-triphosphate form. The latter is a competitive substrate for SARS-CoV-2 RNA-dependent RNA polymerase and causes mutations in the viral genome during replication that makes the virus non-viable. The specific action of this drug on SARS-CoV-2 infection of ECs is not known.
The second drug, Paxlovid (PF-07321332; ritonavir) is a SARS-CoV-2 protease inhibitor antiviral therapy[26]. PF-07321332 is an inhibitor of the SARS-CoV-2 3- chymotrypsin-like cysteine protease that is essential for SARS-CoV-2 replication[26,89]. Ritonavir is a protease inhibitor that slows down the metabolism/breakdown and therefore, increasing the bioavailability of other protease inhibitors including PF-07321332 in the body[90]. Studies published on November 2, 2021, in Science reported the discovery and characterization of PF-07321332 (Paxlovid)[26]. These studies demonstrated that Paxlovid inhibits SARS-CoV-2 replication in vitro in human adenocarcinoma-derived alveolar basal epithelial and differentiated normal human bronchial epithelial cells[26]. This drug showed in vitro coronavirus antiviral activity against all coronaviruses infecting humans and excellent off-target selectivity and in vivo safety profiles.
That study also showed the efficacy of orally administered 300 or 1000 mg/kg PF-07321332 against SARS-CoV-2 infection in vivo in a mouse model challenged intranasally with SARS-CoV-2 MA10 (CCID50). PF-07321332 Limited cellular infiltration by SARS-CoV-2 and protected lung tissue from damage compared to placebo treatment in that study[26]. Most importantly, the interim analysis of the Paxlovid human clinical trial demonstrated a dramatic approximate 90% reduction in COVID-19-related hospitalization or death in high-risk patients treated within 3 to 5 d of symptom onset compared to placebo. Since this drug inhibits virus replication the chance of endothelial infection and dissemination of virus via blood vessel is reduced. We postulate that ECs and blood vessels are likely an important part of this drug's clinical efficacy. Naturally, this contention requires further careful analysis and confirmation, and in-depth insight, since the biological effects of these drugs are largely unknown[91,92]. This sentiment and discussion regarding these oral drugs are summarized in the November 10, 2021 Nature article titled COVID antiviral pills: what scientists still want to know[91]. On December 22, 2021, the US Food and Drug Administration issued an emergency use authorization of Paxlovid to treat mild and moderate COVID-19 (https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-authorizes-first-oral-antiviral-treatment-covid-19).
Other drugs that may be repurposed for COVID-19 treatment include melatonin, coenzyme Q 10 (CoQ10). Melatonin with its anti-inflammatory and anti-oxidative effects can protect against bacterial and viral infections[93-95] and an ongoing clinical study is investigating the efficacy of melatonin in COVID-19 (NCT: 04784754). A clinical trial is investigating the effect of high-dose CoQ10 in long-term COVID-19 patients (NCT: 04960215). The use of growth factors - VEGF, NGF, EGF and KGF, and treatment with adipose-derived stem cells (ADSCs) may be useful for COVID-19 therapy in both the initial and especially the regenerative, healing phase of the disease. A recent study demonstrated that ADSCs release exosomes that secrete various growth factors such as NGF, IGF1, HGF, etc.) that may alleviate the cytokine storm in COVID-19 patients[96].
While respiratory epithelium is the main route of virus entry, the ECs lining blood vessels are an integral part of COVID-19 disease progression and multi-organ spread. COVID-19 not only affects the lungs and respiratory system but also gastrointestinal tract, liver, pancreas, kidneys, heart, brain, and skin. Blood vessels serve as conduits for the virus dissemination to these distant organs. Importantly, ECs are also critical for vascular regeneration during injury/lesions healing and restoration of vascular network. In the present article, we reviewed the role of the endothelial and vascular components as major targets for COVID-19-induced tissue injury, spreading to various organs, and injury healing, and the current treatments for COVID-19 including the most recent oral drugs Molnupiravir and Paxlovid.
I dedicate this article to my awesome wife Hella Gergely Tarnawski, who encouraged me to write this article and has been my inspiration. Tarnawski A.S.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Watanabe A S-Editor: Wu YXJ L-Editor: A P-Editor: Wu YXJ
| 1. | Gupta A, Madhavan MV, Sehgal K, Nair N, Mahajan S, Sehrawat TS, Bikdeli B, Ahluwalia N, Ausiello JC, Wan EY, Freedberg DE, Kirtane AJ, Parikh SA, Maurer MS, Nordvig AS, Accili D, Bathon JM, Mohan S, Bauer KA, Leon MB, Krumholz HM, Uriel N, Mehra MR, Elkind MSV, Stone GW, Schwartz A, Ho DD, Bilezikian JP, Landry DW. Extrapulmonary manifestations of COVID-19. Nat Med. 2020;26:1017-1032. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2419] [Cited by in RCA: 2045] [Article Influence: 409.0] [Reference Citation Analysis (2)] |
| 2. | Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, Zhang L, Fan G, Xu J, Gu X, Cheng Z, Yu T, Xia J, Wei Y, Wu W, Xie X, Yin W, Li H, Liu M, Xiao Y, Gao H, Guo L, Xie J, Wang G, Jiang R, Gao Z, Jin Q, Wang J, Cao B. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497-506. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35178] [Cited by in RCA: 30084] [Article Influence: 6016.8] [Reference Citation Analysis (3)] |
| 3. | Paniz-Mondolfi A, Bryce C, Grimes Z, Gordon RE, Reidy J, Lednicky J, Sordillo EM, Fowkes M. Central nervous system involvement by severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2). J Med Virol. 2020;92:699-702. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 733] [Cited by in RCA: 689] [Article Influence: 137.8] [Reference Citation Analysis (0)] |
| 4. | South AM, Diz DI, Chappell MC. COVID-19, ACE2, and the cardiovascular consequences. Am J Physiol Heart Circ Physiol. 2020;318:H1084-H1090. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 508] [Cited by in RCA: 515] [Article Influence: 103.0] [Reference Citation Analysis (0)] |
| 5. | Varga Z, Flammer AJ, Steiger P, Haberecker M, Andermatt R, Zinkernagel AS, Mehra MR, Schuepbach RA, Ruschitzka F, Moch H. Endothelial cell infection and endotheliitis in COVID-19. Lancet. 2020;395:1417-1418. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4227] [Cited by in RCA: 4572] [Article Influence: 914.4] [Reference Citation Analysis (0)] |
| 6. | Guo M, Tao W, Flavell RA, Zhu S. Potential intestinal infection and faecal-oral transmission of SARS-CoV-2. Nat Rev Gastroenterol Hepatol. 2021;18:269-283. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 198] [Cited by in RCA: 212] [Article Influence: 53.0] [Reference Citation Analysis (0)] |
| 7. | Jiao L, Li H, Xu J, Yang M, Ma C, Li J, Zhao S, Wang H, Yang Y, Yu W, Wang J, Yang J, Long H, Gao J, Ding K, Wu D, Kuang D, Zhao Y, Liu J, Lu S, Liu H, Peng X. The Gastrointestinal Tract Is an Alternative Route for SARS-CoV-2 Infection in a Nonhuman Primate Model. Gastroenterology. 2021;160:1647-1661. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 77] [Cited by in RCA: 96] [Article Influence: 24.0] [Reference Citation Analysis (0)] |
| 8. | Sica R, Pennoni S, Penta L, Riccioni S, Di Cara G, Verrotti A. Gastrointestinal and hepatic involvement during COVID-19 pandemic: A focus on pediatric population and possible future implications. World J Gastroenterol. 2021;27:7000-7004. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 2] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 9. | Mohamed DZ, Ghoneim ME, Abu-Risha SE, Abdelsalam RA, Farag MA. Gastrointestinal and hepatic diseases during the COVID-19 pandemic: Manifestations, mechanism and management. World J Gastroenterol. 2021;27:4504-4535. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 14] [Cited by in RCA: 15] [Article Influence: 3.8] [Reference Citation Analysis (3)] |
| 10. | Wolff D, Nee S, Hickey NS, Marschollek M. Risk factors for Covid-19 severity and fatality: a structured literature review. Infection. 2021;49:15-28. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 320] [Cited by in RCA: 321] [Article Influence: 80.3] [Reference Citation Analysis (0)] |
| 11. | Zheng Z, Peng F, Xu B, Zhao J, Liu H, Peng J, Li Q, Jiang C, Zhou Y, Liu S, Ye C, Zhang P, Xing Y, Guo H, Tang W. Risk factors of critical & mortal COVID-19 cases: A systematic literature review and meta-analysis. J Infect. 2020;81:e16-e25. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1186] [Cited by in RCA: 1462] [Article Influence: 292.4] [Reference Citation Analysis (0)] |
| 12. | Wu C, Chen X, Cai Y, Xia J, Zhou X, Xu S, Huang H, Zhang L, Du C, Zhang Y, Song J, Wang S, Chao Y, Yang Z, Xu J, Chen D, Xiong W, Xu L, Zhou F, Jiang J, Bai C, Zheng J, Song Y. Risk Factors Associated With Acute Respiratory Distress Syndrome and Death in Patients With Coronavirus Disease 2019 Pneumonia in Wuhan, China. JAMA Intern Med. 2020;180:934-943. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4960] [Cited by in RCA: 5514] [Article Influence: 1102.8] [Reference Citation Analysis (1)] |
| 13. | Colmenero I, Santonja C, Alonso-Riaño M, Noguera-Morel L, Hernández-Martín A, Andina D, Wiesner T, Rodríguez-Peralto JL, Requena L, Torrelo A. SARS-CoV-2 endothelial infection causes COVID-19 chilblains: histopathological, immunohistochemical and ultrastructural study of seven paediatric cases. Br J Dermatol. 2020;183:729-737. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 287] [Cited by in RCA: 321] [Article Influence: 64.2] [Reference Citation Analysis (0)] |
| 14. | Garrido Ruiz MC, Santos-Briz Á, Sánchez A, Alonso-Riaño M, Burgos J, Medina-Miguelañez M, Puebla L, Román-Curto C, Roncero-Riesco M, Garcia R, Ortiz PL, Rodriguez-Peralto JL. Spectrum of Clinicopathologic Findings in COVID-19-induced Skin Lesions: Demonstration of Direct Viral Infection of the Endothelial Cells. Am J Surg Pathol. 2021;45:293-303. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 16] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 15. | Ackermann M, Verleden SE, Kuehnel M, Haverich A, Welte T, Laenger F, Vanstapel A, Werlein C, Stark H, Tzankov A, Li WW, Li VW, Mentzer SJ, Jonigk D. Pulmonary Vascular Endothelialitis, Thrombosis, and Angiogenesis in Covid-19. N Engl J Med. 2020;383:120-128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4313] [Cited by in RCA: 4052] [Article Influence: 810.4] [Reference Citation Analysis (0)] |
| 16. | Bradley BT, Maioli H, Johnston R, Chaudhry I, Fink SL, Xu H, Najafian B, Deutsch G, Lacy JM, Williams T, Yarid N, Marshall DA. Histopathology and ultrastructural findings of fatal COVID-19 infections in Washington State: a case series. Lancet. 2020;396:320-332. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 549] [Cited by in RCA: 622] [Article Influence: 124.4] [Reference Citation Analysis (0)] |
| 17. | Samanta P, Ghosh AR. Environmental perspectives of COVID-19 outbreaks: A review. World J Gastroenterol. 2021;27:5822-5850. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 4] [Cited by in RCA: 4] [Article Influence: 1.0] [Reference Citation Analysis (3)] |
| 18. | Teuwen LA, Geldhof V, Pasut A, Carmeliet P. COVID-19: the vasculature unleashed. Nat Rev Immunol. 2020;20:389-391. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 583] [Cited by in RCA: 799] [Article Influence: 159.8] [Reference Citation Analysis (0)] |
| 19. | Ahluwalia A, Jones MK, Hoa N, Tarnawski AS. NGF protects endothelial cells from indomethacin-induced injury through activation of mitochondria and upregulation of IGF-1. Cell Signal. 2017;40:22-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 15] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 20. | Ahluwalia A, Jones MK, Hoa N, Zhu E, Brzozowski T, Tarnawski AS. Reduced NGF in Gastric Endothelial Cells Is One of the Main Causes of Impaired Angiogenesis in Aging Gastric Mucosa. Cell Mol Gastroenterol Hepatol. 2018;6:199-213. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 21] [Article Influence: 3.0] [Reference Citation Analysis (2)] |
| 21. | Ahluwalia A, Jones MK, Szabo S, Tarnawski AS. Aging impairs transcriptional regulation of vascular endothelial growth factor in human microvascular endothelial cells: implications for angiogenesis and cell survival. J Physiol Pharmacol. 2014;65:209-215. [PubMed] |
| 22. | Risau W. Mechanisms of angiogenesis. Nature. 1997;386:671-674. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4077] [Cited by in RCA: 3938] [Article Influence: 140.6] [Reference Citation Analysis (0)] |
| 23. | Risau W, Flamme I. Vasculogenesis. Annu Rev Cell Dev Biol. 1995;11:73-91. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1190] [Cited by in RCA: 1111] [Article Influence: 37.0] [Reference Citation Analysis (0)] |
| 24. | Jones MK, Kawanaka H, Baatar D, Szabó IL, Tsugawa K, Pai R, Koh GY, Kim I, Sarfeh IJ, Tarnawski AS. Gene therapy for gastric ulcers with single local injection of naked DNA encoding VEGF and angiopoietin-1. Gastroenterology. 2001;121:1040-1047. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 77] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 25. | Tarnawski AS, Ahluwalia A. The Critical Role of Growth Factors in Gastric Ulcer Healing: The Cellular and Molecular Mechanisms and Potential Clinical Implications. Cells. 2021;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 85] [Cited by in RCA: 78] [Article Influence: 19.5] [Reference Citation Analysis (1)] |
| 26. | Owen DR, Allerton CMN, Anderson AS, Aschenbrenner L, Avery M, Berritt S, Boras B, Cardin RD, Carlo A, Coffman KJ, Dantonio A, Di L, Eng H, Ferre R, Gajiwala KS, Gibson SA, Greasley SE, Hurst BL, Kadar EP, Kalgutkar AS, Lee JC, Lee J, Liu W, Mason SW, Noell S, Novak JJ, Obach RS, Ogilvie K, Patel NC, Pettersson M, Rai DK, Reese MR, Sammons MF, Sathish JG, Singh RSP, Steppan CM, Stewart AE, Tuttle JB, Updyke L, Verhoest PR, Wei L, Yang Q, Zhu Y. An oral SARS-CoV-2 Mpro inhibitor clinical candidate for the treatment of COVID-19. Science. 2021;eabl4784. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 362] [Cited by in RCA: 1257] [Article Influence: 314.3] [Reference Citation Analysis (0)] |
| 27. | Nagashima S, Mendes MC, Camargo Martins AP, Borges NH, Godoy TM, Miggiolaro AFRDS, da Silva Dezidério F, Machado-Souza C, de Noronha L. Endothelial Dysfunction and Thrombosis in Patients With COVID-19-Brief Report. Arterioscler Thromb Vasc Biol. 2020;40:2404-2407. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 91] [Cited by in RCA: 130] [Article Influence: 26.0] [Reference Citation Analysis (0)] |
| 28. | Okada H, Yoshida S, Hara A, Ogura S, Tomita H. Vascular endothelial injury exacerbates coronavirus disease 2019: The role of endothelial glycocalyx protection. Microcirculation. 2021;28:e12654. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 62] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 29. | Pons S, Fodil S, Azoulay E, Zafrani L. The vascular endothelium: the cornerstone of organ dysfunction in severe SARS-CoV-2 infection. Crit Care. 2020;24:353. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 360] [Cited by in RCA: 347] [Article Influence: 69.4] [Reference Citation Analysis (0)] |
| 30. | Wazny V, Siau A, Wu KX, Cheung C. Vascular underpinning of COVID-19. Open Biol. 2020;10:200208. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 31. | Roumenina LT, Rayes J, Frimat M, Fremeaux-Bacchi V. Endothelial cells: source, barrier, and target of defensive mediators. Immunol Rev. 2016;274:307-329. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 82] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 32. | Evans PC, Rainger GE, Mason JC, Guzik TJ, Osto E, Stamataki Z, Neil D, Hoefer IE, Fragiadaki M, Waltenberger J, Weber C, Bochaton-Piallat ML, Bäck M. Endothelial dysfunction in COVID-19: a position paper of the ESC Working Group for Atherosclerosis and Vascular Biology, and the ESC Council of Basic Cardiovascular Science. Cardiovasc Res. 2020;116:2177-2184. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 207] [Cited by in RCA: 317] [Article Influence: 63.4] [Reference Citation Analysis (0)] |
| 33. | Gryglewski RJ. Pharmacology of vascular endothelium. Delivered on 27 June 2004 at the 29th FEBS Congress in Warsaw. FEBS J. 2005;272:2956-2967. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 8] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 34. | Alexander Y, Osto E, Schmidt-Trucksäss A, Shechter M, Trifunovic D, Duncker DJ, Aboyans V, Bäck M, Badimon L, Cosentino F, De Carlo M, Dorobantu M, Harrison DG, Guzik TJ, Hoefer I, Morris PD, Norata GD, Suades R, Taddei S, Vilahur G, Waltenberger J, Weber C, Wilkinson F, Bochaton-Piallat ML, Evans PC. Endothelial function in cardiovascular medicine: a consensus paper of the European Society of Cardiology Working Groups on Atherosclerosis and Vascular Biology, Aorta and Peripheral Vascular Diseases, Coronary Pathophysiology and Microcirculation, and Thrombosis. Cardiovasc Res. 2021;117:29-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 159] [Cited by in RCA: 216] [Article Influence: 54.0] [Reference Citation Analysis (0)] |
| 35. | Deanfield J, Donald A, Ferri C, Giannattasio C, Halcox J, Halligan S, Lerman A, Mancia G, Oliver JJ, Pessina AC, Rizzoni D, Rossi GP, Salvetti A, Schiffrin EL, Taddei S, Webb DJ; Working Group on Endothelin and Endothelial Factors of the European Society of Hypertension. Endothelial function and dysfunction. Part I: Methodological issues for assessment in the different vascular beds: a statement by the Working Group on Endothelin and Endothelial Factors of the European Society of Hypertension. J Hypertens. 2005;23:7-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 455] [Cited by in RCA: 451] [Article Influence: 22.6] [Reference Citation Analysis (0)] |
| 36. | Michiels C. Endothelial cell functions. J Cell Physiol. 2003;196:430-443. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 481] [Cited by in RCA: 498] [Article Influence: 22.6] [Reference Citation Analysis (0)] |
| 37. | Pries AR, Kuebler WM. Normal endothelium. Handb Exp Pharmacol. 2006;1-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 131] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 38. | Sumpio BE, Riley JT, Dardik A. Cells in focus: endothelial cell. Int J Biochem Cell Biol. 2002;34:1508-1512. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 309] [Cited by in RCA: 311] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 39. | Bonfanti R, Furie BC, Furie B, Wagner DD. PADGEM (GMP140) is a component of Weibel-Palade bodies of human endothelial cells. Blood. 1989;73:1109-1112. [PubMed] |
| 40. | McEver RP, Beckstead JH, Moore KL, Marshall-Carlson L, Bainton DF. GMP-140, a platelet alpha-granule membrane protein, is also synthesized by vascular endothelial cells and is localized in Weibel-Palade bodies. J Clin Invest. 1989;84:92-99. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 680] [Cited by in RCA: 690] [Article Influence: 19.2] [Reference Citation Analysis (0)] |
| 41. | Mojzisch A, Brehm MA. The Manifold Cellular Functions of von Willebrand Factor. Cells. 2021;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 29] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 42. | van Mourik JA, Romani de Wit T, Voorberg J. Biogenesis and exocytosis of Weibel-Palade bodies. Histochem Cell Biol. 2002;117:113-122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 105] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 43. | Durante W. Carbon monoxide and bile pigments: surprising mediators of vascular function. Vasc Med. 2002;7:195-202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 59] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 44. | Hannan RL, Kourembanas S, Flanders KC, Rogelj SJ, Roberts AB, Faller DV, Klagsbrun M. Endothelial cells synthesize basic fibroblast growth factor and transforming growth factor beta. Growth Factors. 1988;1:7-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 86] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 45. | Wu S, Wu X, Zhu W, Cai WJ, Schaper J, Schaper W. Immunohistochemical study of the growth factors, aFGF, bFGF, PDGF-AB, VEGF-A and its receptor (Flk-1) during arteriogenesis. Mol Cell Biochem. 2010;343:223-229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 33] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 46. | Ferrara N. VEGF-A: a critical regulator of blood vessel growth. Eur Cytokine Netw. 2009;20:158-163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 208] [Cited by in RCA: 238] [Article Influence: 15.9] [Reference Citation Analysis (0)] |
| 47. | Tarnawski A, Stachura J, Hollander D, Sarfeh IJ, Bogdal J. Cellular aspects of alcohol-induced injury and prostaglandin protection of the human gastric mucosa. Focus on the mucosal microvessels. J Clin Gastroenterol. 1988;10 Suppl 1:S35-S45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 17] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 48. | Shasby DM, Ries DR, Shasby SS, Winter MC. Histamine stimulates phosphorylation of adherens junction proteins and alters their link to vimentin. Am J Physiol Lung Cell Mol Physiol. 2002;282:L1330-L1338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 73] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 49. | Tarnawski AS, Ahluwalia A, Jones MK. Increased susceptibility of aging gastric mucosa to injury: the mechanisms and clinical implications. World J Gastroenterol. 2014;20:4467-4482. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 46] [Cited by in RCA: 31] [Article Influence: 2.8] [Reference Citation Analysis (4)] |
| 50. | Ahluwalia A, Patel K, Hoa N, Brzozowska I, Jones MK, Tarnawski AS. Melatonin ameliorates aging-related impaired angiogenesis in gastric endothelial cells via local actions on mitochondria and VEGF-survivin signaling. Am J Physiol Gastrointest Liver Physiol. 2021;321:G682-G689. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 2.3] [Reference Citation Analysis (2)] |
| 51. | Wang Q, Zhang Y, Wu L, Niu S, Song C, Zhang Z, Lu G, Qiao C, Hu Y, Yuen KY, Wang Q, Zhou H, Yan J, Qi J. Structural and Functional Basis of SARS-CoV-2 Entry by Using Human ACE2. Cell. 2020;181:894-904.e9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2090] [Cited by in RCA: 2209] [Article Influence: 441.8] [Reference Citation Analysis (0)] |
| 52. | Zhang H, Penninger JM, Li Y, Zhong N, Slutsky AS. Angiotensin-converting enzyme 2 (ACE2) as a SARS-CoV-2 receptor: molecular mechanisms and potential therapeutic target. Intensive Care Med. 2020;46:586-590. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1522] [Cited by in RCA: 1709] [Article Influence: 341.8] [Reference Citation Analysis (0)] |
| 53. | Zhou P, Yang XL, Wang XG, Hu B, Zhang L, Zhang W, Si HR, Zhu Y, Li B, Huang CL, Chen HD, Chen J, Luo Y, Guo H, Jiang RD, Liu MQ, Chen Y, Shen XR, Wang X, Zheng XS, Zhao K, Chen QJ, Deng F, Liu LL, Yan B, Zhan FX, Wang YY, Xiao GF, Shi ZL. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579:270-273. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15248] [Cited by in RCA: 14104] [Article Influence: 2820.8] [Reference Citation Analysis (1)] |
| 54. | Donoghue M, Hsieh F, Baronas E, Godbout K, Gosselin M, Stagliano N, Donovan M, Woolf B, Robison K, Jeyaseelan R, Breitbart RE, Acton S. A novel angiotensin-converting enzyme-related carboxypeptidase (ACE2) converts angiotensin I to angiotensin 1-9. Circ Res. 2000;87:E1-E9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2045] [Cited by in RCA: 2186] [Article Influence: 87.4] [Reference Citation Analysis (0)] |
| 55. | Hikmet F, Méar L, Edvinsson Å, Micke P, Uhlén M, Lindskog C. The protein expression profile of ACE2 in human tissues. Mol Syst Biol. 2020;16:e9610. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 631] [Cited by in RCA: 709] [Article Influence: 141.8] [Reference Citation Analysis (0)] |
| 56. | Hoffmann M, Kleine-Weber H, Schroeder S, Krüger N, Herrler T, Erichsen S, Schiergens TS, Herrler G, Wu NH, Nitsche A, Müller MA, Drosten C, Pöhlmann S. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell. 2020;181:271-280.e8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11946] [Cited by in RCA: 14242] [Article Influence: 2848.4] [Reference Citation Analysis (0)] |
| 57. | Sardu C, Gambardella J, Morelli MB, Wang X, Marfella R, Santulli G. Hypertension, Thrombosis, Kidney Failure, and Diabetes: Is COVID-19 an Endothelial Disease? J Clin Med. 2020;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 318] [Cited by in RCA: 358] [Article Influence: 71.6] [Reference Citation Analysis (0)] |
| 58. | Puelles VG, Lütgehetmann M, Lindenmeyer MT, Sperhake JP, Wong MN, Allweiss L, Chilla S, Heinemann A, Wanner N, Liu S, Braun F, Lu S, Pfefferle S, Schröder AS, Edler C, Gross O, Glatzel M, Wichmann D, Wiech T, Kluge S, Pueschel K, Aepfelbacher M, Huber TB. Multiorgan and Renal Tropism of SARS-CoV-2. N Engl J Med. 2020;383:590-592. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1254] [Cited by in RCA: 1421] [Article Influence: 284.2] [Reference Citation Analysis (0)] |
| 59. | Monteil V, Kwon H, Prado P, Hagelkrüys A, Wimmer RA, Stahl M, Leopoldi A, Garreta E, Hurtado Del Pozo C, Prosper F, Romero JP, Wirnsberger G, Zhang H, Slutsky AS, Conder R, Montserrat N, Mirazimi A, Penninger JM. Inhibition of SARS-CoV-2 Infections in Engineered Human Tissues Using Clinical-Grade Soluble Human ACE2. Cell. 2020;181:905-913.e7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1779] [Cited by in RCA: 1660] [Article Influence: 332.0] [Reference Citation Analysis (0)] |
| 60. | Kim JM, Chung YS, Jo HJ, Lee NJ, Kim MS, Woo SH, Park S, Kim JW, Kim HM, Han MG. Identification of Coronavirus Isolated from a Patient in Korea with COVID-19. Osong Public Health Res Perspect. 2020;11:3-7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 322] [Cited by in RCA: 301] [Article Influence: 60.2] [Reference Citation Analysis (0)] |
| 61. | Park WB, Kwon NJ, Choi SJ, Kang CK, Choe PG, Kim JY, Yun J, Lee GW, Seong MW, Kim NJ, Seo JS, Oh MD. Virus Isolation from the First Patient with SARS-CoV-2 in Korea. J Korean Med Sci. 2020;35:e84. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 137] [Cited by in RCA: 149] [Article Influence: 29.8] [Reference Citation Analysis (0)] |
| 62. | Laue M, Kauter A, Hoffmann T, Möller L, Michel J, Nitsche A. Morphometry of SARS-CoV and SARS-CoV-2 particles in ultrathin plastic sections of infected Vero cell cultures. Sci Rep. 2021;11:3515. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 89] [Cited by in RCA: 101] [Article Influence: 25.3] [Reference Citation Analysis (0)] |
| 63. | Liu C, Mendonça L, Yang Y, Gao Y, Shen C, Liu J, Ni T, Ju B, Liu C, Tang X, Wei J, Ma X, Zhu Y, Liu W, Xu S, Liu Y, Yuan J, Wu J, Liu Z, Zhang Z, Liu L, Wang P, Zhang P. The Architecture of Inactivated SARS-CoV-2 with Postfusion Spikes Revealed by Cryo-EM and Cryo-ET. Structure. 2020;28:1218-1224.e4. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 88] [Cited by in RCA: 141] [Article Influence: 28.2] [Reference Citation Analysis (0)] |
| 64. | Bonetti PO, Lerman LO, Lerman A. Endothelial dysfunction: a marker of atherosclerotic risk. Arterioscler Thromb Vasc Biol. 2003;23:168-175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1535] [Cited by in RCA: 1618] [Article Influence: 73.5] [Reference Citation Analysis (0)] |
| 65. | Sena CM, Pereira AM, Seiça R. Endothelial dysfunction - a major mediator of diabetic vascular disease. Biochim Biophys Acta. 2013;1832:2216-2231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 603] [Cited by in RCA: 562] [Article Influence: 46.8] [Reference Citation Analysis (0)] |
| 66. | Fauci AS, Lane HC, Redfield RR. Covid-19 - Navigating the Uncharted. N Engl J Med. 2020;382:1268-1269. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1124] [Cited by in RCA: 1046] [Article Influence: 209.2] [Reference Citation Analysis (0)] |
| 67. | Froldi G, Dorigo P. Endothelial dysfunction in Coronavirus disease 2019 (COVID-19): Gender and age influences. Med Hypotheses. 2020;144:110015. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 47] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 68. | Gavriilaki E, Anyfanti P, Gavriilaki M, Lazaridis A, Douma S, Gkaliagkousi E. Endothelial Dysfunction in COVID-19: Lessons Learned from Coronaviruses. Curr Hypertens Rep. 2020;22:63. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 80] [Cited by in RCA: 105] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 69. | Hayden MR. Endothelial activation and dysfunction in metabolic syndrome, type 2 diabetes and coronavirus disease 2019. J Int Med Res. 2020;48:300060520939746. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 41] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 70. | Kaur S, Tripathi DM, Yadav A. The Enigma of Endothelium in COVID-19. Front Physiol. 2020;11:989. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 63] [Cited by in RCA: 69] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 71. | Mosleh W, Chen K, Pfau SE, Vashist A. Endotheliitis and Endothelial Dysfunction in Patients with COVID-19: Its Role in Thrombosis and Adverse Outcomes. J Clin Med. 2020;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 54] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 72. | Huertas A, Montani D, Savale L, Pichon J, Tu L, Parent F, Guignabert C, Humbert M. Endothelial cell dysfunction: a major player in SARS-CoV-2 infection (COVID-19)? Eur Respir J. 2020;56. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 204] [Cited by in RCA: 240] [Article Influence: 48.0] [Reference Citation Analysis (0)] |
| 73. | Amraei R, Rahimi N. COVID-19, Renin-Angiotensin System and Endothelial Dysfunction. Cells. 2020;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 188] [Cited by in RCA: 194] [Article Influence: 38.8] [Reference Citation Analysis (0)] |
| 74. | Jung F, Krüger-Genge A, Franke RP, Hufert F, Küpper JH. COVID-19 and the endothelium. Clin Hemorheol Microcirc. 2020;75:7-11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 61] [Cited by in RCA: 72] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 75. | Karakas M, Jarczak D, Becker M, Roedl K, Addo MM, Hein F, Bergmann A, Zimmermann J, Simon TP, Marx G, Lütgehetmann M, Nierhaus A, Kluge S. Targeting Endothelial Dysfunction in Eight Extreme-Critically Ill Patients with COVID-19 Using the Anti-Adrenomedullin Antibody Adrecizumab (HAM8101). Biomolecules. 2020;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 76. | Poor HD, Ventetuolo CE, Tolbert T, Chun G, Serrao G, Zeidman A, Dangayach NS, Olin J, Kohli-Seth R, Powell CA. COVID-19 Critical Illness Pathophysiology Driven by Diffuse Pulmonary Thrombi and Pulmonary Endothelial Dysfunction Responsive to Thrombolysis. medRxiv. 2020;. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 77. | Olbei M, Hautefort I, Modos D, Treveil A, Poletti M, Gul L, Shannon-Lowe CD, Korcsmaros T. SARS-CoV-2 Causes a Different Cytokine Response Compared to Other Cytokine Storm-Causing Respiratory Viruses in Severely Ill Patients. Front Immunol. 2021;12:629193. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 80] [Cited by in RCA: 79] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 78. | Ragab D, Salah Eldin H, Taeimah M, Khattab R, Salem R. The COVID-19 Cytokine Storm; What We Know So Far. Front Immunol. 2020;11:1446. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1044] [Cited by in RCA: 1096] [Article Influence: 219.2] [Reference Citation Analysis (0)] |
| 79. | Farinacci M, Krahn T, Dinh W, Volk HD, Düngen HD, Wagner J, Konen T, von Ahsen O. Circulating endothelial cells as biomarker for cardiovascular diseases. Res Pract Thromb Haemost. 2019;3:49-58. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 53] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 80. | Abdel Hamid M, Bakhoum SW, Sharaf Y, Sabry D, El-Gengehe AT, Abdel-Latif A. Circulating Endothelial Cells and Endothelial Function Predict Major Adverse Cardiac Events and Early Adverse Left Ventricular Remodeling in Patients With ST-Segment Elevation Myocardial Infarction. J Interv Cardiol. 2016;29:89-98. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 16] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 81. | Deanfield JE, Halcox JP, Rabelink TJ. Endothelial function and dysfunction: testing and clinical relevance. Circulation. 2007;115:1285-1295. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1478] [Cited by in RCA: 1756] [Article Influence: 97.6] [Reference Citation Analysis (0)] |
| 82. | Hill JM, Zalos G, Halcox JP, Schenke WH, Waclawiw MA, Quyyumi AA, Finkel T. Circulating endothelial progenitor cells, vascular function, and cardiovascular risk. N Engl J Med. 2003;348:593-600. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2631] [Cited by in RCA: 2583] [Article Influence: 117.4] [Reference Citation Analysis (0)] |
| 83. | Gambardella J, Khondkar W, Morelli MB, Wang X, Santulli G, Trimarco V. Arginine and Endothelial Function. Biomedicines. 2020;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 168] [Cited by in RCA: 140] [Article Influence: 28.0] [Reference Citation Analysis (0)] |
| 84. | Cotran R, Kumar V, Robbins S. Gastric ulceration. In: Cotran R, Kumar V, Robbins S, editors. Robbins Pathologic Basis of Disease. 5th Edition ed. Philadelphia: Saunder, 1999; 298-299, 773-777. |
| 85. | Ahluwalia A, Brzozowski T, Jones MK, Ichikawa Y, Tarnawski AS. Formation of new blood vessels during gastric ulcer healing. Role of bone marrow derived endothelial progenitor cells. J Physiol Pharmacol. 2017;68:585-589. [PubMed] |
| 86. | Di Franco S, Alfieri A, Petrou S, Damiani G, Passavanti MB, Pace MC, Leone S, Fiore M. Current status of COVID-19 treatment: An opinion review. World J Virol. 2020;9:27-37. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 11] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 87. | Iturricastillo G, Ávalos Pérez-Urría E, Couñago F, Landete P. Scientific evidence in the COVID-19 treatment: A comprehensive review. World J Virol. 2021;10:217-228. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 6] [Cited by in RCA: 4] [Article Influence: 1.0] [Reference Citation Analysis (3)] |
| 88. | Fischer W, Eron JJ, Holman W, Cohen MS, Fang L, Szewczyk LJ, Sheahan TP, Baric R, Mollan KR, Wolfe CR, Duke ER, Azizad MM, Borroto-Esoda K, Wohl DA, Loftis AJ, Alabanza P, Lipansky F, Painter WP. Molnupiravir, an Oral Antiviral Treatment for COVID-19. medRxiv. 2021;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 96] [Article Influence: 24.0] [Reference Citation Analysis (0)] |
| 89. | Vandyck K, Deval J. Considerations for the discovery and development of 3-chymotrypsin-like cysteine protease inhibitors targeting SARS-CoV-2 infection. Curr Opin Virol. 2021;49:36-40. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 63] [Cited by in RCA: 106] [Article Influence: 26.5] [Reference Citation Analysis (0)] |
| 90. | Lea AP, Faulds D. Ritonavir. Drugs. 1996;52:541-6; discussion 547. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 66] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 91. | Ledford H. COVID antiviral pills: what scientists still want to know. Nature. 2021;599:358-359. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 51] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 92. | Zhou S, Hill CS, Sarkar S, Tse LV, Woodburn BMD, Schinazi RF, Sheahan TP, Baric RS, Heise MT, Swanstrom R. β-d-N4-hydroxycytidine Inhibits SARS-CoV-2 Through Lethal Mutagenesis But Is Also Mutagenic To Mammalian Cells. J Infect Dis. 2021;224:415-419. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 105] [Cited by in RCA: 220] [Article Influence: 55.0] [Reference Citation Analysis (0)] |
| 93. | Boga JA, Coto-Montes A, Rosales-Corral SA, Tan DX, Reiter RJ. Beneficial actions of melatonin in the management of viral infections: a new use for this "molecular handyman"? Rev Med Virol. 2012;22:323-338. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52] [Cited by in RCA: 72] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 94. | Escames G, Acuña-Castroviejo D, López LC, Tan DX, Maldonado MD, Sánchez-Hidalgo M, León J, Reiter RJ. Pharmacological utility of melatonin in the treatment of septic shock: experimental and clinical evidence. J Pharm Pharmacol. 2006;58:1153-1165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 82] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 95. | Galley HF, Lowes DA, Allen L, Cameron G, Aucott LS, Webster NR. Melatonin as a potential therapy for sepsis: a phase I dose escalation study and an ex vivo whole blood model under conditions of sepsis. J Pineal Res. 2014;56:427-438. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 105] [Cited by in RCA: 120] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 96. | Mazini L, Rochette L, Malka G. Exosomes contribution in COVID-19 patients' treatment. J Transl Med. 2021;19:234. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 25] [Article Influence: 6.3] [Reference Citation Analysis (0)] |