Published online Aug 7, 2022. doi: 10.3748/wjg.v28.i29.3981
Peer-review started: February 1, 2022
First decision: February 24, 2022
Revised: March 9, 2022
Accepted: July 6, 2022
Article in press: July 6, 2022
Published online: August 7, 2022
Processing time: 182 Days and 11.6 Hours
Hepatocellular carcinoma (HCC) is a common tumour often diagnosed with a multifocal presentation. Patients with multifocal HCC represent a heterogeneous group. Although Trans-Arterial ChemoEmbolization (TACE) is the most frequently employed treatment for these patients, previous data suggested that liver resection (LR) could be a safe and effective procedure.
To compare LR and TACE in patients with multifocal HCC in terms of procedure-related morbidity and oncologic outcomes.
All patients with multifocal HCC who underwent LR or TACE as the first procedure between May 2011 and March 2021 were enrolled. The decision to perform surgery or TACE was made after a multidisciplinary team evaluation. Only patients in Child-Pugh class A or B7 and stage B (according to the Barcelona Clinic Liver Cancer staging system, without severe portal hypertension, vascular invasion, or extrahepatic spread) were included in the final analysis. Propensity score matching was used to adjust the baseline differences between patients undergoing LR and the TACE group [number and diameter of lesions, presence of cirrhosis, alpha-fetoprotein (AFP) levels, and Model for End-Stage Liver Disease score]. The Kaplan-Meier method was used to estimate overall survival (OS) and disease-free survival (DFS). The outcomes of LR and TACE were compared using the log-rank test.
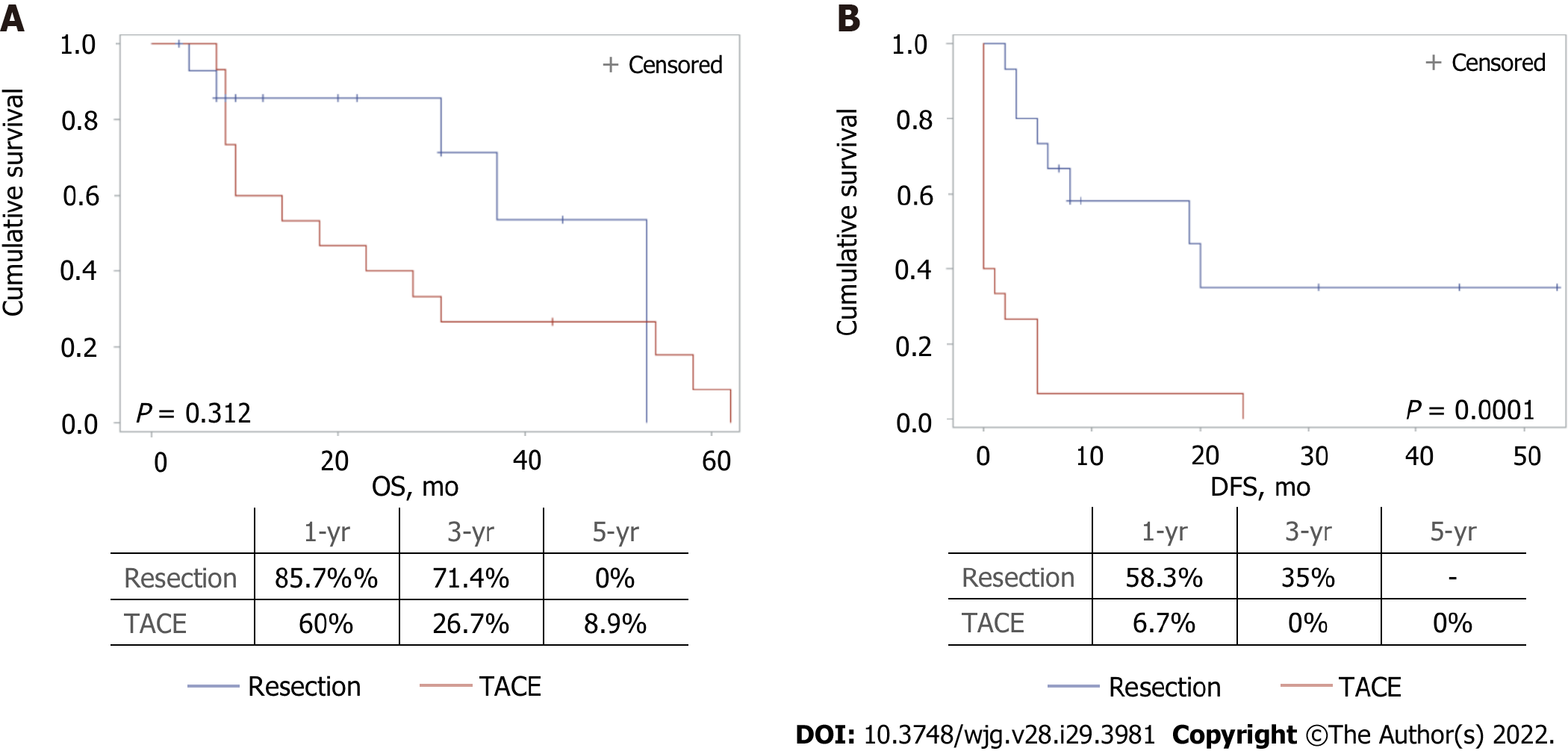
After matching, 30 patients were eligible for the final analysis, 15 in each group. Morbidity rates were 42.9% and 40% for LR and TACE, respectively (P = 0.876). Median OS was not different in the LR and TACE groups (53 mo vs 18 mo, P = 0.312), while DFS was significantly longer with LR (19 mo vs 0 mo, P = 0.0001). Subgroup analysis showed that patients in the Italian Liver Cancer (ITA.LI.CA) B2 stage, with AFP levels lower than 400 ng/mL, less than 3 lesions, and lesions bigger than 41 mm, benefited more from LR in terms of DFS. Patients classified as ITA.LI.CA B3, with AFP levels higher than 400 ng/mL and with more than 3 lesions, appeared to receive more benefit from TACE in terms of OS.
In a small cohort of patients with multifocal HCC, LR confers longer DFS compared with TACE, with similar OS and post-procedural morbidity.
Core Tip: Hepatocellular carcinoma (HCC) is a leading cause of death and often presents in a multifocal form. Trans-Arterial ChemoEmbolization (TACE) is the most frequently employed treatment for this patient category. As patients with multifocal HCC are a heterogeneous group, previous data suggested that liver resection (LR) could be a safe and effective procedure. A propensity score-matched analysis has been performed to compare LR and TACE in terms of post-procedure morbidity and survival. Despite the limited number of patients, LR conferred longer disease-free survival with similar overall survival compared to TACE. Subgroup analyses identified the patients benefiting more from a specific treatment.
- Citation: Risaliti M, Bartolini I, Campani C, Arena U, Xodo C, Adotti V, Rosi M, Taddei A, Muiesan P, Amedei A, Batignani G, Marra F. Evaluating the best treatment for multifocal hepatocellular carcinoma: A propensity score-matched analysis. World J Gastroenterol 2022; 28(29): 3981-3993
- URL: https://www.wjgnet.com/1007-9327/full/v28/i29/3981.htm
- DOI: https://dx.doi.org/10.3748/wjg.v28.i29.3981
Hepatocellular carcinoma (HCC) is the most frequent primary liver cancer worldwide, accounting for approximately 90% of cases[1]. HCC incidence has grown over the last two decades, with more than 900.000 new cases per year. Moreover, it is expected to further increase in the next few years due to the exponential growth in nonalcoholic fatty liver disease[2]. Despite the improvements in diagnosis and management, HCC is detected in a multinodular form in 35%-40% of cases[3,4] with a reported 5-year survival rate of 19.5%[5].
Over the years, various staging systems have been proposed to overcome the limitations of the tumour-node-metastasis system, which only considers tumour burden[6]. Currently, the Barcelona Clinic Liver Classification (BCLC) is one of the most widely used staging systems for HCC, and includes variables related to tumour status, liver function, and performance status (PS), and recommends one or more specific treatment modalities for each disease stage[7].
Several additional efforts have been made to deal with the heterogeneity of the BCLC-B or intermediate stage of this classification. The Italian Liver Cancer (ITA.LI.CA) group recently proposed a new staging system and prognostic score, based on the BCLC staging system, which includes tumour burden, liver function, and other patient-related variables, and provides a subclassification of this stage[8].
Surgical treatments, including liver resection (LR) and liver transplantation, are considered the best choice for survival and quality of life whenever feasible, while Trans-Arterial ChemoEmbolization (TACE) is not a curative treatment and should be used for patients with well-defined, multifocal HCC, a preserved portal flow, and adequate liver function and PS. TACE outcomes are poor, and several lines of information about the safety and effectiveness of surgery in selected patients for whom TACE is usually recommended have been reported[9-12]. Finally, liver transplantation could also have a central role for these patients, especially after successful downstaging[7], but organ shortage and increased demand for organ transplantation could lead to high drop-out rates.
The debate concerning whether selected patients with multinodular HCC may benefit from surgery rather than TACE remains unsolved.
In this study, a propensity-score matching analysis was used to compare procedure-related morbidity and oncological outcomes in multifocal HCC patients classified as BCLC-B who underwent LR or TACE to determine if one treatment should be given priority over the other.
A retrospective analysis was conducted on patients with multifocal HCC who had undergone surgical resection or TACE as first-line treatment between May 2011 and March 2021. The following exclusion criteria were considered: Age < 18 years, Child-Pugh score > 7, vascular invasion or extrahepatic spread, and hepatic venous pressure gradient > 15 mmHg. HCC was diagnosed histologically or by imaging techniques [magnetic resonance imaging and/or triphasic computed tomography (CT)] according to the available EASL guidelines version. Before LR or TACE, an evaluation of age, comorbidities, blood chemistry, tumour number and size (major radiological diameter of the largest lesion from the last available imaging test), ITA.LI.CA. stage and Eastern Cooperative Oncology Group PS.
The decision to perform surgery or TACE was taken by the local multidisciplinary team who considered patients and tumour features, including lesion location and relation with the pedicles, and the volume of the future liver remnant. During surgery, intraoperative ultrasound sonography (IOUS) was routinely performed to confirm preoperative planning. Whenever feasible, anatomic resections were preferred. Both conventional, lipiodol-based TACE and TACE with drug-eluting beads were included. The patients’ response was evaluated one month after the procedure with a CT scan. For patients who did not achieve a complete response, data and the effectiveness of each subsequent TACE were also recorded.
Post-treatment morbidity was evaluated according to the Clavien-Dindo (CD) classification[13].
All patients underwent a standardised follow-up programme that included blood tests with alpha-fetoprotein (AFP), and a triple phase-contrast enhanced CT scan every three months for the first year and then biannually for 5 years after surgery (starting 3 mo after surgery or 1 mo after TACE). Recurrence was diagnosed in the case of radiological evidence of HCC.
Quantitative data were expressed as mean ± SD or median and range, as appropriate. Qualitative data are reported as absolute and relative frequencies. Satterthwait’s test or the Mann-Whitney test was used according to the Shapiro-Wilk test and F-Test, respectively, for normality distribution and homoscedasticity to assess the difference in quantitative variables between treatment groups t-test. The chi-square test or Fisher’s test were used as appropriate to verify the association between qualitative variables and treatment groups. The propensity score matching (PSM) method was used to compare similar treatment groups for known prognostic factors (AFP levels as a dichotomous variable with a cut-off set at 400 ng/dL, presence or not of cirrhosis, Child-Pugh and Model for End-Stage Liver Disease (MELD) score, number, and diameter of lesions). An AFP level > 400 ng/mL was considered a prognostic factor of poor outcome, as suggested by recent guidelines[14]. A diagnosis of cirrhosis was established based on the presence of one or more of the following: Compatible histology, imaging showing compatible hepatic morphology, liver stiffness > 15 kPa by vibration-controlled transient elastography, clinical or endoscopic or imaging signs of portal hypertension. The propensity score was calculated by a multiple logistic regression model with a backward selection method. The nearest neighbour method was used to match the two groups with a 1:1 ratio.
Overall survival (OS) was calculated from the date of the first treatment to death of any cause or the last follow-up. As TACE patients could need more than one treatment to achieve a complete response, disease-free survival (DFS) was calculated from the date of the effective treatment to the date of first radiological recurrence. Survival was expressed as the median and 95% confidence interval.
The Kaplan-Meier curve method and the log-rank test were used to evaluate the difference in OS and DFS between the groups.
Subgroup survival analyses were performed comparing the results of the different treatment modalities and stratifying patients by the different grades of the ITA.LI.CA classification, the AFP levels (using 400 ng/mL as cut-off), and by the different number and size of HCC (dividing them into two groups using the median value as a cut-off). A P value < 0.05 was considered statistically significant.
All the analyses were conducted using SAS, version 9.2 (SAS Corporation, Cary, NC, United States), and revised by a biomedical statistician.
A total of 50 patients with multifocal HCC were included in the study, 25 underwent LR while 25 underwent TACE. The distribution of the factors used for the PSM in the general population is reported in Table 1. All patients belonging to the TACE group had cirrhosis, whereas, among those who underwent LR, cirrhosis was absent in 36% of cases (P = 0.002). The number of lesions was higher among patients who underwent TACE, whereas resected patients usually had more extensive tumours. No significant differences were found between the two groups regarding Child-Pugh score and AFP levels. In contrast, the MELD score tended to be higher in patients who underwent TACE compared with the resected patients. After the PSM, only 30 patients were eligible for the final analysis, 15 from each subgroup.
| Resection, n = 25 | TACE, n = 25 | Total, n = 50 | P value | |
| AFP, n (%) | 0.243 | |||
| < 400 ng/mL | 20 (90.9) | 17 (73.9) | 37 (82.2) | |
| > 400 ng/mL | 2 (9.1) | 6 (26.1) | 8 (17.8) | |
| Missing | 3 | 2 | 5 | |
| Cirrhosis, n (%) | 0.002 | |||
| No | 9 (36) | 0 | 9 (18) | |
| Yes | 16 (64) | 25 (100) | 41 (82) | |
| Child-Pugh score, n (%) | 0.840 | |||
| A5 | 12 (48) | 10 (40) | 22 (44) | |
| A6 | 9 (36) | 11 (44) | 20 (40) | |
| B7 | 4 (16) | 4 (16) | 8 (16) | |
| MELD | 8.4 ± 2.7 | 9.7 ± 2.2 | 9 ± 2.5 | 0.069 |
| Number of lesions | 2 (2-10) | 4 (2-7) | 2.5 (2-10) | 0.008 |
| Tumor diameter (mm) | 69.5 ± 52.2 | 41.9 ± 17 | 55.7 ± 40.9 | 0.018 |
The general baseline clinical characteristics are shown in Table 2, while data regarding liver function aspects, tumour characteristics, and patient distribution according to the Up-to-7 criteria[15] and ITA.LI.CA classification are reported in Table 3. No statistical differences were found in baseline characteristics. The median age was 69 years, and more than two/thirds of patients were male. The most common cause of liver disease was viral hepatitis, followed by NASH and alcohol use disorder. Lower platelet counts and higher bilirubin levels were present in the TACE group. There was also a trend towards a higher presence of varices in the TACE group. No significant differences across the Up-to-7 criteria in or out and ITA.LI.CA. staging distribution were found.
| Resection, n = 15 | TACE, n = 15 | Total, n = 30 | P value | |
| Age (yr) | 68.9 ± 9.2 | 70.5 ± 9.9 | 69.7 ± 9.5 | 0.651 |
| Gender, n (%) | 0.651 | |||
| Male | 13 (86.7) | 11 (73.3) | 24 (80) | |
| Female | 2 (13.3) | 4 (26.7) | 6 (20) | |
| BMI (kg/m2) | 26.1 ± 4.3 | 26.3 ± 7.7 | 26.2 ± 5.9 | 0.927 |
| Hepatitis Etiology, n (%) | 0.300 | |||
| Viral | 4 (28.6) | 9 (60) | 13 (44.8) | |
| Alcoholic | 0 | 1 (6.7) | 1 (3.5) | |
| Dysmetabolic | 3 (21.4) | 1 (6.7) | 4 (13.8) | |
| Mixed | 3 (21.4) | 1 (6.7) | 4 (13.8) | |
| Other | 4 (28.6) | 3 (20) | 7 (24.1) | |
| Missing | 1 | - | 1 | |
| Diabetes, n (%) | 0.700 | |||
| No | 9 (60) | 11 (73.3) | 20 (66.7) | |
| Yes | 6 (40) | 4 (26.7) | 10 (33.3) | |
| Heart disease, n (%) | 0.330 | |||
| No | 11 (73.3) | 14 (93.3) | 25 (83.3) | |
| Yes | 4 (26.7) | 1 (6.7) | 5 (16.7) |
| Resection, n = 15 | TACE, n = 15 | Total, n = 30 | P value | |
| INR | 1.2 ± 0.1 | 1.3 ± 0.3 | 1.2 ± 0.2 | 0.185 |
| Bilirubin (mg/dL) | 0.75 ± 0.5 | 1.2 ± 0.6 | 0.99 ± 0.6 | 0.026 |
| Platelets (103/μL) | 245 ± 118 | 95 ± 31 | 163 ± 111 | 0.003 |
| Creatinine (mg/dL) | 1.37 ± 0.8 | 0.78 ± 0.2 | 1.08 ± 1.19 | 0.188 |
| AFP, n (%) | 1.000 | |||
| < 400 ng/mL | 13 (86.7) | 12 (80) | 25 (83.3) | |
| > 400 ng/mL | 2 (13.3) | 3 (20) | 5 (16.7) | |
| Varices, n (%) | 0.081 | |||
| No | 8 (80) | 4 (36.4) | 12 (57.1) | |
| Yes | 2 (20) | 7 (63.6) | 9 (42.9) | |
| Missing | 5 | 4 | 9 | |
| Cirrhosis, n (%) | 0.100 | |||
| No | 4 (26.7) | 0 | 4 (13.3) | |
| Yes | 11 (73.3) | 15 (100) | 26 (86.7) | |
| Child-Pugh score, n (%) | 0.556 | |||
| A5 | 8 (53.3) | 5 (33.3) | 13 (43.3) | |
| A6 | 6 (40) | 7 (46.7) | 13 (43.3) | |
| B7 | 1 (6.7) | 3 (20) | 4 (13.4) | |
| MELD score | 9.1 ± 3.2 | 9.9 ± 2.4 | 9.5 ± 2.8 | 0.473 |
| Number of lesions | 2 (2-5) | 3 (2-5) | 2 (2-5) | 0.101 |
| Diameter (mm) | 46.3 ± 3 | 47 ± 5 | 46.9 ± 12.9 | 0.793 |
| Up-to-7 criteria, n (%) | 0.456 | |||
| In | 10 (66.7) | 8 (53.3) | 18 (60) | |
| Out | 5 (33.3) | 7 (46.7) | 12 (40) | |
| ITA.LI.CA classification, n (%) | 0.868 | |||
| B1 | 10 (66.7) | 8 (53.3) | 18 (60) | |
| B2 | 3 (20) | 5 (33.3) | 8 (26.7) | |
| B3 | 2 (13.3) | 2 (13.3) | 4 (13.3) |
Patients in the TACE group received a significantly higher number of treatments (Table 4, P = 0.001). Post-procedure complications were not significantly different between patients who underwent LR and TACE (Table 4). In the surgery group, complications classified as grade 1 and 2 of CD included fever, pneumonia, or the need for blood transfusion. Only one patient developed bile leakage classified as grade 3 according to CD and was treated with percutaneous drainage.
| Resection, n = 15 | TACE, n = 15 | Total, n = 30 | P value | |
| Number of treatments | 1 ± 0 | 2.4 ± 1.1 | 1.7 ± 1.1 | 0.001 |
| Complications, n (%) | 0.876 | |||
| No | 8 (57.1) | 9 (60) | 17 (58.6) | |
| Yes | 6 (42.9) | 6 (40) | 12 (41.4) | |
| Missing | 1 | - | 1 | |
| Complications, n (%) | 0.545 | |||
| CD 1-2 | 5 (83.3) | 5 (83.3) | 10 | |
| CD 3-4 | 1 (16.7) | 1 (16.7) | 2 (16.7) | |
| Hospital stay (days) | 7 ± 3 | 5 ± 5 | 6 ± 4.3 | 0.224 |
| Recurrence, n (%) | 0.006 | |||
| No | 7 (46.7) | 0 | 7 (23.3) | |
| Yes | 8 (53.3) | 15 (100) | 23 (76.7) |
In the TACE group, complications classified as grade 1 and 2 of CD included mostly transient alterations in liver biochemistry and/or fever. Only one patient developed temporary liver failure and required percutaneous drainage due to an abdominal effusion.
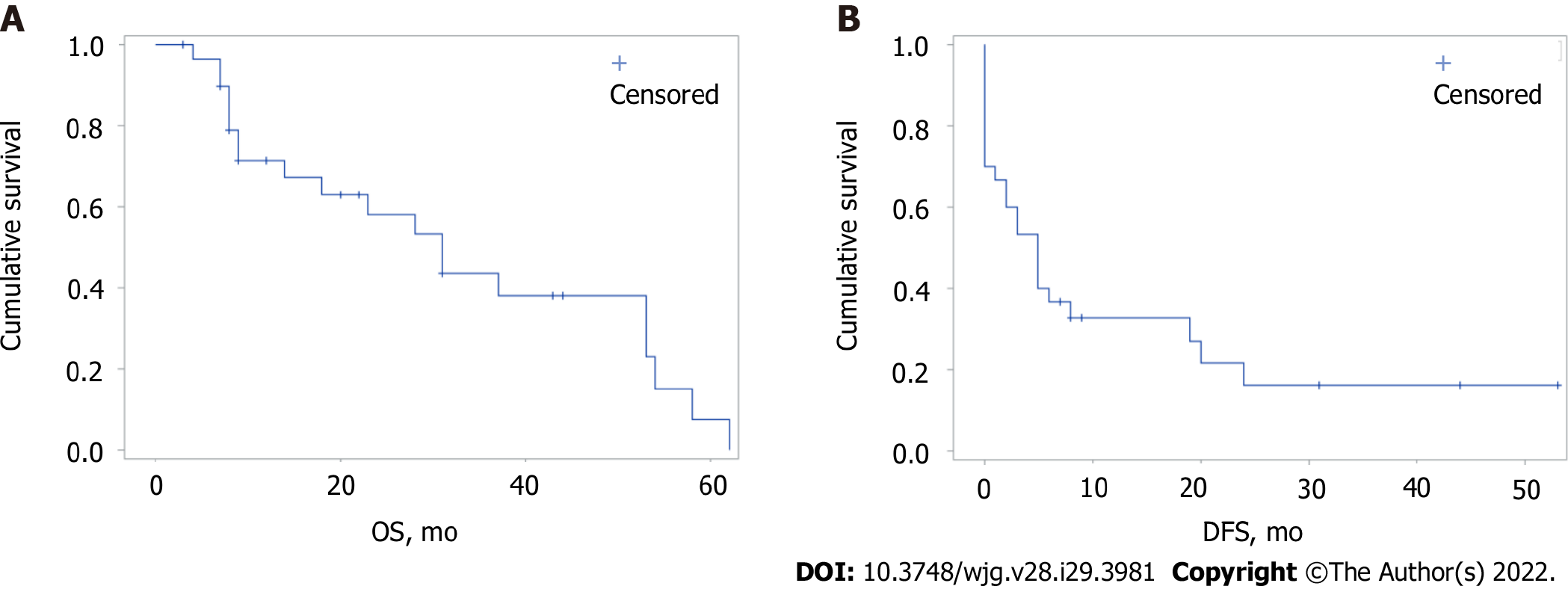
The median follow-up was 19 mo (range 3-62). The estimated global median OS and DFS were 31 and 5 mo, respectively (Figure 1). There were no significant differences in OS and DFS for the global population stratified by the ITA.LI.CA classification. Median OS for B1, B2, and B3 groups were 31, 31, and 14 mo, respectively (P = 0.803), while median DFS for B1, B2, and B3 groups were 5, 14.5, and 1.5 mo, respectively (P = 0.516).
No differences in OS were observed when the two treatment groups were compared. A significantly longer DFS was found in resected patients compared with those undergoing TACE (19 mo vs 0 mo, respectively, P = 0.0001) (Figure 2).
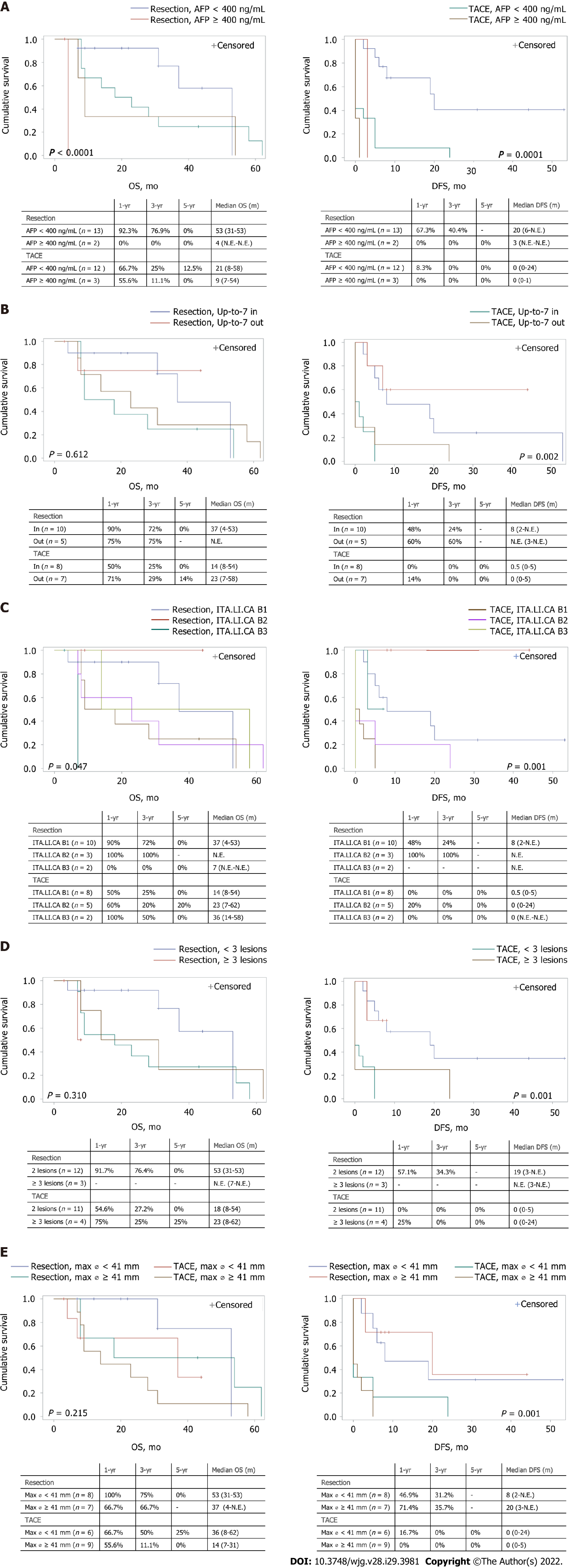
Subgroup analyses were also performed to evaluate the possible differences in OS or DFS according to AFP levels, size, lesion number, Up-to-7 criteria, and ITA.LI.CA staging between each treatment group.
A significant difference was found in both OS and DFS (P < 0.0001 and P = 0.0001, respectively, Figure 3A), with patients presenting with lower levels of AFP showing the best prognosis, in both treatment groups. Furthermore, patients with AFP levels higher than 400 ng/mL and receiving surgery showed a poor OS compared to the TACE group.
No difference in OS was found when comparing treatment modalities in patients stratified according to the Up-to-7 criteria. On the contrary, DFS was higher in the LR group (P = 0.002) (Figure 3B).
The results of different treatment modalities for patients stratified by the ITA.LI.CA classification are reported in Figure 3C. Similar to the previous analysis, a significant difference was found in both OS and DFS (P = 0.047 and P = 0.001, respectively), with patients classified as B2 and receiving resection, showing the best prognosis, while those classified as B3 and receiving resection had the worst prognosis.
Although no significant differences were found in OS, the 4 patients with more than 3 lesions receiving TACE showed a 1, 3, and 5-year OS of 75%, 25%, and 25%, respectively. Among the surgery group, patients with less than 3 lesions had a significantly higher DFS compared with those with 3 or more lesions (P = 0.001). Conversely, no differences in terms of OS and DFS were found in the TACE group stratified by the number of lesions (Figure 3D). Similar results were obtained when the treatment groups were stratified according to the size of the more extensive lesion (Figure 3E). Patients with smaller lesions in the surgery group had a better DFS.
HCC is the most common primary liver tumour. Despite recent advantages in terms of follow-up, early diagnosis, and treatment, a considerable percentage of patients are still diagnosed in multiple or advanced forms and global mortality remains high with poor long-term prognosis[16]. The term multifocal HCC comprises patients presenting with a broad disease spectrum, ranging from small, oligonodular tumours to diffuse disease. The accompanying liver cirrhosis, portal hypertension, and liver function impairment should be considered, as they are related to high post-procedure morbidity and mortality. Therefore, a tailored approach is mandatory for each patient, and the decisional role of a specialised multidisciplinary team has been highlighted and encouraged in the last version of the BCLC classification[7].
The BCLC algorithm recommends TACE as the primary treatment modality for multinodular HCC, but whether a subset of patients may benefit from a surgical approach remains unestablished. We conducted a retrospective analysis using the PSM, a method that may provide high-quality evidence in conditions when randomised clinical trials are unethical or unfeasible. For the PSM analysis, both indicators of liver function and tumour burden and aggressiveness were used. Although the Child-Pugh and MELD scores could be considered outdated after the last published version of the BCLC staging system[7], these data are still commonly used in clinical practice. Wang et al[5] showed that patients with gradual deterioration in CP score had shorter survival times and worse prognosis (5-year OS rates of 31.1%, 2.4%, and 0.9% for CP A, B, and C, respectively). AFP is a well-established independent risk factor for survival in HCC patients and levels greater than 400 ng/mL are generally considered diagnostic for HCC, in the presence of appropriate radiologic findings[5,17]. Finally, the number and dimension of the nodules are two of the major prognostic factors, and the most used guidelines underscore their importance in treatment allocation[7,8,14,18].
TACE is considered the standard treatment for BCLC-B patients, as evidence from randomised clinical trials and meta-analyses indicates that TACE provides better survival outcomes compared to the best supportive care with a 1-year mortality rate of 34.1%[19-21]. On the other hand, guidelines do not recommend LR for BCLC-B patients, due to the unfavourable prognostic impact of multinodular presentation and high postoperative morbidity[18]. Moreover, LR for multifocal HCC is a challenging surgery as wide and/or multiple resections are needed, possibly leading to bile leaks and/or postoperative liver failure.
Advances in surgical techniques, including extensive use of IOUS, better coagulation devices, and wider application of minimally invasive surgery, together with better perioperative management, have contributed to improving the results of LR in this difficult-to-treat population. Remarkably, the comparison between different studies is limited by variations in the modalities of detection and reporting of post-procedural complications.
In the present series, we did not find any differences in post-procedure morbidity when comparing resection and TACE. According to the CD classification, only three patients undergoing LR and one patient in the TACE group had a clinically relevant event. However, it should be documented that TACE patients often required more than one treatment to achieve the best response, thus partially explaining the lower morbidity rate of the procedure. Conflicting results on morbidity were previously reported. Zhong et al[22] showed a complication rate of 35% and 21% for resection and TACE, respectively[22]. On the contrary, a meta-analysis did not find any difference in post-procedure morbidity between these two treatment modalities[23].
While only a nonsignificant trend towards better OS was observed with surgery, we found that DFS was significantly longer in resected patients. These results confirm and extend previous studies conducted on patients with multinodular HCC. Favourable results with surgery were originally reported by non-controlled studies[10,24]. In a multicentric study including 736 BCLC-B patients, Torzilli et al[24] reported a 5-year survival rate of 57% and a DFS of 27%. The survival benefit of LR compared with TACE in BCLC-B patients has been previously reported in the meta-analysis by Liu et al[23], and similar conclusions were reached in a randomised controlled trial enrolling 173 patients[25].
Considering the marked population heterogeneity of patients with multinodular HCC, subgroup analyses may provide additional clues for better selection of the treatment modality. Not surprisingly, patients with lower AFP levels had the best prognosis. An exciting result was the observation that patients with high AFP levels benefit more from TACE in terms of OS, and further studies are needed to confirm these data and investigate the possible mechanisms thereof. Another aspect which deserves further investigation is to what extent some of these patients with multinodular HCC could benefit from new systemic combination treatments which include the use of immunotherapy with excellent results[26].
Interesting results were also provided by patient stratification according to the ITA.LI.CA classification. Patients in the B2 subgroup, characterised by nodules of smaller size or lower number, showed the best prognosis when receiving LR. In contrast, those classified as B3, i.e., with larger nodules and higher numbers, had the worst prognosis when resected. Patients with two HCC nodules had a more significant benefit from LR compared with TACE in terms of DFS. These data emphasise the relevance of the number of nodules, which is considered a poor prognostic factor and a predictor of early recurrence.
Nonetheless, a clear cut-off value for the number of nodules beyond which resection is contraindicated has not been determined[10,12,25,27]. Furthermore, although using the same parameters of the Up-to-7 criteria (number of lesions and a maximum diameter of the bigger lesions), the ITA.LI.CA. classification showed better ability in patient stratification.
We also evaluated the impact of nodule size on survival in our series. Interestingly, patients with lesions larger than 41 mm had an even more significant benefit from LR in terms of DFS. Previously published studies reported that patients with large solitary HCC had better survival rates when treated with resection than TACE[28,29]. Furthermore, conflicting results have been reported regarding the potential role of tumour diameter as a prognostic factor. While some studies indicated that tumour size alone was not a predictor of poor prognosis[24,30], other reports mentioned tumour dimension as a predictor of survival[31-33]. In particular, in the analysis of 2887 HCC patients, tumour size was an independent prognostic factor of poor survival at multivariate analysis[5], and Wada et al[12] concluded that while size alone was not a contraindication for resection, a diameter lower than 5 cm was a favourable factor[12].
Several limitations of this study must be acknowledged, including its retrospective nature with the inherent selection bias. Moreover, the fact that a limited number of patients from a single centre were enrolled should lead to caution in the general applicability of the results. In addition, the relatively long period of enrollment could have been associated with differences due to modifications in the HCC management. On the other hand, we performed a rigorous matching using a powerful statistical tool such as the propensity score, although some additional variables not included in the score could have influenced the outcomes.
Although the small sample analysed should lead to careful interpretation of the results, after a propensity score matching analysis, patients with multinodular HCC appear to significantly benefit from a surgical approach over TACE in terms of DFS. These results are more evident in the sub-population belonging to the less advanced B2 subgroup according to the ITA.LI.CA. classification, with AFP levels lower than 400 ng/mL, 2 lesions, and with lesions bigger than 41 mm. Patients classified as ITA.LI.CA B3, with AFP levels higher than 400 ng/mL, and more than 3 lesions had higher benefits from TACE in terms of OS. Future studies are needed to confirm these results in a larger population and to identify other HCC subgroups of patients who would benefit from personalised treatment.
Hepatocellular carcinoma (HCC) is the most frequent primary liver tumour and a leading cause of death. Despite follow-up programmes for cirrhotic patients, HCC is diagnosed in a multifocal form in up to 40% of the patients. Although being a heterogeneous group, the existing classifications consider together the patients with multifocal HCC and generally recommend Trans-Arterial ChemoEmbolization (TACE) as the main treatment. Considering the progress in perioperative care, a growing body of literature has started to propose liver resection (LR) in selected cases, which has shown the best long-term oncological results.
A consensus and detailed guidelines that also consider LR for these patients have not yet been proposed. Moreover, the characteristics of the patients that could benefit more from LR have still to be determined. Defining these aspects could help clinicians in patient management and potentially improve their prognosis.
A comparison between LR and TACE as the first main treatment in terms of post-procedural results and long-term oncological outcomes was performed in patients with multifocal HCC.
To reduce the influence of the well-known prognostic factors [i.e., Alpha-fetoprotein (AFP) levels as a dichotomous variable with a cut-off set at 400 ng/dL, presence or absence of cirrhosis, Child-Pugh and Model for End-Stage Liver Disease score, number, and diameter of lesions], a propensity score-matched analysis was performed. Two homogeneous groups (with a 1:1 ratio) were compared to assess the difference in short- and long-term post-procedural results.
After matching, 30 patients were eligible for the final analysis. Morbidity rates were 42.9% and 40% for LR and TACE, respectively (P = 0.876). Median overall survival (OS) was not different when comparing LR and TACE (53 mo vs 18 mo, P = 0.312), while disease-free survival (DFS) was significantly longer with LR (19 mo vs 0 mo, P = 0.0001). Subgroup analysis showed that patients in the Italian Liver Cancer (ITA.LI.CA) B2 stage, with AFP levels lower than 400 ng/mL, 2 lesions, and lesions bigger than 41 mm benefited more from LR in terms of DFS. Patients classified as ITA.LI.CA B3, with AFP levels higher than 400 ng/mL and more than 3 lesions appeared to receive more benefit from TACE in terms of OS. However, not all patients with multifocal HCC are amenable to treatment with LR or TACE. Consequently, only a small sample of patients resulted in being eligible for the analysis. Therefore, these results should be considered with caution and further studies are needed.
There are subgroups of patients with multifocal HCC that seem to benefit more from LR than TACE.
Further studies are needed to include LR in the guidelines as a potential treatment to be offered to specific subgroups of patients with multifocal HCC.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Italy
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Gorrell MD, Australia; Nakano M, Japan S-Editor: Fan JR L-Editor: Webster JR P-Editor: Chen YX
| 1. | Llovet JM, Kelley RK, Villanueva A, Singal AG, Pikarsky E, Roayaie S, Lencioni R, Koike K, Zucman-Rossi J, Finn RS. Hepatocellular carcinoma. Nat Rev Dis Primers. 2021;7:6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4432] [Cited by in RCA: 3887] [Article Influence: 971.8] [Reference Citation Analysis (3)] |
| 2. | Younossi ZM, Henry L. Epidemiology of non-alcoholic fatty liver disease and hepatocellular carcinoma. JHEP Rep. 2021;3:100305. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 66] [Cited by in RCA: 106] [Article Influence: 26.5] [Reference Citation Analysis (0)] |
| 3. | Fukami Y, Kaneoka Y, Maeda A, Kumada T, Tanaka J, Akita T, Kubo S, Izumi N, Kadoya M, Sakamoto M, Nakashima O, Matsuyama Y, Kokudo T, Hasegawa K, Yamashita T, Kashiwabara K, Takayama T, Kokudo N, Kudo M; Liver Cancer Study Group of Japan. Liver Resection for Multiple Hepatocellular Carcinomas: A Japanese Nationwide Survey. Ann Surg. 2020;272:145-154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 64] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 4. | Roayaie S, Jibara G, Tabrizian P, Park JW, Yang J, Yan L, Schwartz M, Han G, Izzo F, Chen M, Blanc JF, Johnson P, Kudo M, Roberts LR, Sherman M. The role of hepatic resection in the treatment of hepatocellular cancer. Hepatology. 2015;62:440-451. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 247] [Cited by in RCA: 324] [Article Influence: 32.4] [Reference Citation Analysis (0)] |
| 5. | Wang CY, Li S. Clinical characteristics and prognosis of 2887 patients with hepatocellular carcinoma: A single center 14 years experience from China. Medicine (Baltimore). 2019;98:e14070. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 56] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 6. | Brierley JD, Gospodarowicz MK, Wittekind C. TNM Classification of Malignant Tumours, 8 th edition due December 2016. J Clin Pathol. 1998;1:84. [RCA] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 7. | Reig M, Forner A, Rimola J, Ferrer-Fàbrega J, Burrel M, Garcia-Criado Á, Kelley RK, Galle PR, Mazzaferro V, Salem R, Sangro B, Singal AG, Vogel A, Fuster J, Ayuso C, Bruix J. BCLC strategy for prognosis prediction and treatment recommendation: The 2022 update. J Hepatol. 2022;76:681-693. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1904] [Cited by in RCA: 2615] [Article Influence: 871.7] [Reference Citation Analysis (59)] |
| 8. | Farinati F, Vitale A, Spolverato G, Pawlik TM, Huo TL, Lee YH, Frigo AC, Giacomin A, Giannini EG, Ciccarese F, Piscaglia F, Rapaccini GL, Di Marco M, Caturelli E, Zoli M, Borzio F, Cabibbo G, Felder M, Sacco R, Morisco F, Biasini E, Foschi FG, Gasbarrini A, Svegliati Baroni G, Virdone R, Masotto A, Trevisani F, Cillo U; ITA. LI.CA study group. Development and Validation of a New Prognostic System for Patients with Hepatocellular Carcinoma. PLoS Med. 2016;13:e1002006. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 87] [Cited by in RCA: 113] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 9. | Ruzzenente A, Capra F, Pachera S, Iacono C, Piccirillo G, Lunardi M, Pistoso S, Valdegamberi A, D'Onofrio M, Guglielmi A. Is liver resection justified in advanced hepatocellular carcinoma? J Gastrointest Surg. 2009;13:1313-1320. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 58] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 10. | Ishizawa T, Hasegawa K, Aoki T, Takahashi M, Inoue Y, Sano K, Imamura H, Sugawara Y, Kokudo N, Makuuchi M. Neither multiple tumors nor portal hypertension are surgical contraindications for hepatocellular carcinoma. Gastroenterology. 2008;134:1908-1916. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 513] [Cited by in RCA: 583] [Article Influence: 34.3] [Reference Citation Analysis (0)] |
| 11. | Kamiyama T, Orimo T, Wakayama K, Shimada S, Nagatsu A, Yokoo H, Kamachi H, Yamashita K, Shimamura T, Taketomi A. Survival outcomes of hepatectomy for stage B Hepatocellular carcinoma in the BCLC classification. World J Surg Oncol. 2017;15:156. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 16] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 12. | Wada H, Eguchi H, Noda T, Ogawa H, Yamada D, Tomimaru Y, Tomokuni A, Asaoka T, Kawamoto K, Gotoh K, Marubashi S, Umeshita K, Nagano H, Doki Y, Mori M. Selection criteria for hepatic resection in intermediate-stage (BCLC stage B) multiple hepatocellular carcinoma. Surgery. 2016;160:1227-1235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 64] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 13. | Clavien PA, Barkun J, de Oliveira ML, Vauthey JN, Dindo D, Schulick RD, de Santibañes E, Pekolj J, Slankamenac K, Bassi C, Graf R, Vonlanthen R, Padbury R, Cameron JL, Makuuchi M. The Clavien-Dindo classification of surgical complications: five-year experience. Ann Surg. 2009;250:187-196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6210] [Cited by in RCA: 8636] [Article Influence: 539.8] [Reference Citation Analysis (0)] |
| 14. | European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J Hepatol. 2018;69:182-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5593] [Cited by in RCA: 6064] [Article Influence: 866.3] [Reference Citation Analysis (3)] |
| 15. | Mazzaferro V, Llovet JM, Miceli R, Bhoori S, Schiavo M, Mariani L, Camerini T, Roayaie S, Schwartz ME, Grazi GL, Adam R, Neuhaus P, Salizzoni M, Bruix J, Forner A, De Carlis L, Cillo U, Burroughs AK, Troisi R, Rossi M, Gerunda GE, Lerut J, Belghiti J, Boin I, Gugenheim J, Rochling F, Van Hoek B, Majno P; Metroticket Investigator Study Group. Predicting survival after liver transplantation in patients with hepatocellular carcinoma beyond the Milan criteria: a retrospective, exploratory analysis. Lancet Oncol. 2009;10:35-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1267] [Cited by in RCA: 1574] [Article Influence: 92.6] [Reference Citation Analysis (1)] |
| 16. | Pecorelli A, Lenzi B, Gramenzi A, Garuti F, Farinati F, Giannini EG, Ciccarese F, Piscaglia F, Rapaccini GL, Di Marco M, Caturelli E, Zoli M, Borzio F, Sacco R, Cabibbo G, Felder M, Morisco F, Gasbarrini A, Baroni GS, Foschi FG, Biasini E, Masotto A, Virdone R, Bernardi M, Trevisani F; Italian LiverCancer (ITA. LI.CA) group. Curative therapies are superior to standard of care (transarterial chemoembolization) for intermediate stage hepatocellular carcinoma. Liver Int. 2017;37:423-433. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 53] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 17. | Bai DS, Zhang C, Chen P, Jin SJ, Jiang GQ. The prognostic correlation of AFP level at diagnosis with pathological grade, progression, and survival of patients with hepatocellular carcinoma. Sci Rep. 2017;7:12870. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 86] [Cited by in RCA: 161] [Article Influence: 20.1] [Reference Citation Analysis (0)] |
| 18. | Llovet JM, Brú C, Bruix J. Prognosis of hepatocellular carcinoma: the BCLC staging classification. Semin Liver Dis. 1999;19:329-338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2645] [Cited by in RCA: 2876] [Article Influence: 110.6] [Reference Citation Analysis (1)] |
| 19. | Llovet JM, Bruix J. Systematic review of randomized trials for unresectable hepatocellular carcinoma: Chemoembolization improves survival. Hepatology. 2003;37:429-442. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2207] [Cited by in RCA: 2271] [Article Influence: 103.2] [Reference Citation Analysis (0)] |
| 20. | Llovet JM, Real MI, Montaña X, Planas R, Coll S, Aponte J, Ayuso C, Sala M, Muchart J, Solà R, Rodés J, Bruix J; Barcelona Liver Cancer Group. Arterial embolisation or chemoembolisation vs symptomatic treatment in patients with unresectable hepatocellular carcinoma: a randomised controlled trial. Lancet. 2002;359:1734-1739. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2502] [Cited by in RCA: 2611] [Article Influence: 113.5] [Reference Citation Analysis (0)] |
| 21. | Lo CM, Ngan H, Tso WK, Liu CL, Lam CM, Poon RT, Fan ST, Wong J. Randomized controlled trial of transarterial lipiodol chemoembolization for unresectable hepatocellular carcinoma. Hepatology. 2002;35:1164-1171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1904] [Cited by in RCA: 1987] [Article Influence: 86.4] [Reference Citation Analysis (0)] |
| 22. | Zhong JH, Peng NF, You XM, Ma L, Li LQ. Hepatic resection is superior to transarterial chemoembolization for treating intermediate-stage hepatocellular carcinoma. Liver Int. 2017;37:1083-1084. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 23. | Liu W, Zhou JG, Sun Y, Zhang L, Xing BC. Hepatic Resection Improved the Long-Term Survival of Patients with BCLC Stage B Hepatocellular Carcinoma in Asia: a Systematic Review and Meta-Analysis. J Gastrointest Surg. 2015;19:1271-1280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 34] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 24. | Torzilli G, Belghiti J, Kokudo N, Takayama T, Capussotti L, Nuzzo G, Vauthey JN, Choti MA, De Santibanes E, Donadon M, Morenghi E, Makuuchi M. A snapshot of the effective indications and results of surgery for hepatocellular carcinoma in tertiary referral centers: is it adherent to the EASL/AASLD recommendations? Ann Surg. 2013;257:929-937. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 333] [Cited by in RCA: 417] [Article Influence: 34.8] [Reference Citation Analysis (0)] |
| 25. | Yin L, Li H, Li AJ, Lau WY, Pan ZY, Lai EC, Wu MC, Zhou WP. Partial hepatectomy vs. transcatheter arterial chemoembolization for resectable multiple hepatocellular carcinoma beyond Milan Criteria: a RCT. J Hepatol. 2014;61:82-88. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 194] [Cited by in RCA: 271] [Article Influence: 24.6] [Reference Citation Analysis (0)] |
| 26. | Finn RS, Qin S, Ikeda M, Galle PR, Ducreux M, Kim TY, Kudo M, Breder V, Merle P, Kaseb AO, Li D, Verret W, Xu DZ, Hernandez S, Liu J, Huang C, Mulla S, Wang Y, Lim HY, Zhu AX, Cheng AL; IMbrave150 Investigators. Atezolizumab plus Bevacizumab in Unresectable Hepatocellular Carcinoma. N Engl J Med. 2020;382:1894-1905. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2542] [Cited by in RCA: 4704] [Article Influence: 940.8] [Reference Citation Analysis (2)] |
| 27. | Donadon M, Fontana A, Procopio F, Del Fabbro D, Cimino M, Viganò L, Palmisano A, Torzilli G. Dissecting the multinodular hepatocellular carcinoma subset: is there a survival benefit after hepatectomy? Updates Surg. 2019;71:57-66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 28. | Liu PH, Su CW, Hsu CY, Hsia CY, Lee YH, Huang YH, Lee RC, Lin HC, Huo TI. Solitary Large Hepatocellular Carcinoma: Staging and Treatment Strategy. PLoS One. 2016;11:e0155588. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 41] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 29. | Hong SK, Lee KW, Hong SY, Suh S, Hong K, Han ES, Lee JM, Choi Y, Yi NJ, Suh KS. Efficacy of Liver Resection for Single Large Hepatocellular Carcinoma in Child-Pugh A Cirrhosis: Analysis of a Nationwide Cancer Registry Database. Front Oncol. 2021;11:674603. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 13] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 30. | Chang WT, Kao WY, Chau GY, Su CW, Lei HJ, Wu JC, Hsia CY, Lui WY, King KL, Lee SD. Hepatic resection can provide long-term survival of patients with non-early-stage hepatocellular carcinoma: extending the indication for resection? Surgery. 2012;152:809-820. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 87] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 31. | Delis SG, Bakoyiannis A, Tassopoulos N, Athanassiou K, Kelekis D, Madariaga J, Dervenis C. Hepatic resection for hepatocellular carcinoma exceeding Milan criteria. Surg Oncol. 2010;19:200-207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 27] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 32. | Truant S, Boleslawski E, Duhamel A, Bouras AF, Louvet A, Febvay C, Leteurtre E, Huet G, Zerbib P, Dharancy S, Hebbar M, Pruvot FR. Tumor size of hepatocellular carcinoma in noncirrhotic liver: a controversial predictive factor for outcome after resection. Eur J Surg Oncol. 2012;38:1189-1196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 40] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 33. | Bartolini I, Nelli T, Russolillo N, Cucchetti A, Pesi B, Moraldi L, Ferrero A, Ercolani G, Grazi G, Batignani G. Multiple hepatocellular carcinoma: Long-term outcomes following resection beyond actual guidelines. An Italian multicentric retrospective study. Am J Surg. 2021;222:599-605. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |