Published online Aug 7, 2022. doi: 10.3748/wjg.v28.i29.3854
Peer-review started: December 16, 2021
First decision: April 16, 2022
Revised: April 27, 2022
Accepted: July 6, 2022
Article in press: July 6, 2022
Published online: August 7, 2022
Processing time: 229 Days and 18.6 Hours
The mechanisms underlying gastrointestinal (GI) dysmotility with ulcerative colitis (UC) have not been fully elucidated. The enteric nervous system (ENS) plays an essential role in the GI motility. As a vital neurotransmitter in the ENS, the gas neurotransmitter nitric oxide (NO) may impact the colonic motility. In this study, dextran sulfate sodium (DSS)-induced UC rat model was used for investigating the effects of NO by examining the effects of rate-limiting enzyme nitric oxide synthase (NOS) changes on the colonic motility as well as the role of the ENS in the colonic motility during UC.
To reveal the relationship between the effects of NOS expression changes in NOS-containing nitrergic neurons and the colonic motility in a rat UC model.
Male rats (n = 8/each group) were randomly divided into a control (CG), a UC group (EG1), a UC + thrombin derived polypeptide 508 trifluoroacetic acid (TP508TFA; an NOS agonist) group (EG2), and a UC + NG-monomethyl-L-arginine monoacetate (L-NMMA; an NOS inhibitor) group (EG3). UC was induced by administering 5.5% DSS in drinking water without any other treatment (EG1), while the EG2 and EG3 were gavaged with TP508 TFA and L-NMMA, respectively. The disease activity index (DAI) and histological assessment were recorded for each group, whereas the changes in the proportion of colonic nitrergic neurons were counted using immunofluorescence histochemical staining, Western blot, and enzyme linked immunosorbent assay, respectively. In addition, the contractile tension changes in the circular and longitudinal muscles of the rat colon were investigated in vitro using an organ bath system.
The proportion of NOS-positive neurons within the colonic myenteric plexus (MP), the relative expression of NOS, and the NOS concentration in serum and colonic tissues were significantly elevated in EG1, EG2, and EG3 compared with CG rats. In UC rats, stimulation with agonists and inhibitors led to variable degrees of increase or decrease for each indicator in the EG2 and EG3. When the rats in EGs developed UC, the mean contraction tension of the colonic smooth muscle detected in vitro was higher in the EG1, EG2, and EG3 than in the CG group. Compared with the EG1, the contraction amplitude and mean contraction tension of the circular and longitudinal muscles of the colon in the EG2 and EG3 were enhanced and attenuated, respectively. Thus, during UC, regulation of the expression of NOS within the MP improved the intestinal motility, thereby favoring the recovery of intestinal functions.
In UC rats, an increased number of nitrergic neurons in the colonic MP leads to the attenuation of colonic motor function. To intervene NOS activity might modulate the function of nitrergic neurons in the colonic MP and prevent colonic motor dysfunction. These results might provide clues for a novel approach to alleviate diarrhea symptoms of UC patients.
Core Tip: This study focused on the effects of nitrergic neurons in the myenteric plexus (MP) on colonic motor function in rats with ulcerative colitis (UC). The results suggest that an increased number of nitrergic neurons in the colonic MP of the UC rats leads to reduced colon contractile function. Therefore, the regulation of the activity of nitrergic neurons in the colonic MP through interference with the activities of nitric oxide synthase might be a novel potential and prospective way to reduce the diarrhea symptoms in UC patients.
- Citation: Li YR, Li Y, Jin Y, Xu M, Fan HW, Zhang Q, Tan GH, Chen J, Li YQ. Involvement of nitrergic neurons in colonic motility in a rat model of ulcerative colitis. World J Gastroenterol 2022; 28(29): 3854-3868
- URL: https://www.wjgnet.com/1007-9327/full/v28/i29/3854.htm
- DOI: https://dx.doi.org/10.3748/wjg.v28.i29.3854
Ulcerative colitis (UC) is a nonspecific inflammatory disorder of the intestine that primarily involves the rectum, sigmoid colon, and/or, the whole colon, in severe cases. Clinical features include recurrent episodes of abdominal pain, diarrhea, and mucopurulent stools[1]. Unfortunately, the disease course is permanent and causes great suffering to the patients. UC can occur at any age (mainly in young adults), and in recent years, its incidence has increased worldwide[2]. The etiology of UC is complex, and its pathogenesis might be related to genetics[3], immunological factors, psychiatric depression and anxiety[4], environmental factors[5], dietary allergy, intestinal flora[6], and other factors. Therefore, it leads to treatment difficulty and a prolonged treatment course, and recurs easily. Although current pharmacological treatments in the clinic might improve patient’s symptoms, they still cannot achieve satisfactory results. In recent years, the research on the aspects of gastrointestinal (GI) motility disorders in UC patients has advanced, and the abnormal intestinal dynamics has become a focus of research on the pathogenesis of UC [7-9].
The enteric nervous system (ENS), including the submucous plexus (SP) and myenteric plexus (MP)[10,11], as an essential component of the peripheral nervous system (PNS), is independent of the central nervous system (CNS) and is involved in the regulation of intestinal secretion, absorption, and motility[12]. Neurons in the ENS, depending on their neurotransmission function, are divided into sensory neurons, motor neurons, and interneurons. Further, motor neurons are divided into excitatory and inhibitory ones, regulating the systolic and diastolic function of the intestine, respectively. Typically, the ENS plays a vital role in maintaining gut homeostasis; however, gut motility gets impaired once the relaxation and contraction functions of the gut become imbalanced.
As the primary inhibitory gas neurotransmitter within the ENS, nitric oxide (NO) regulates several GI functions, such as vascular permeability, mucosal defense, immune regulation, and GI motility[13]. Nitric oxide synthase (NOS), the rate-limiting enzyme of NO synthesis, is widely distributed in en
Dextran sulfate sodium (DSS; PC-99017), NG-monomethyl-L-arginine monoacetate (L-NMMA; PC-45273), and thrombin derived polypeptide 508 trifluoroacetic acid (TP508TFA; PC-50991) were purchased from PlantChemMed Co., Ltd (Shanghai, China). Rabbit Anti-HuD + HuC (ab184267) and Goat Anti-nNOS (ab1376) were purchased from Abcam (Cambridge, United Kingdom). Donkey Anti-Goat Alexa Fluor 488 (a11055) and Donkey Anti-Rabbit Alexa Fluor 594 (A21207) were purchased from Invitrogen Co., Ltd (Carlsbad, United States). Mouse Anti-β-Actin monoclonal antibody (A1978) and Immobilon Forte western HRP substrate (Cat. No. WBLUF0020) were purchased from Merck KGaA (Darmstadt, Germany). HRP-Labeled Goat Anti-Rabbit IgG (H + L) (ZB-2306) and HRP-Labeled Goat Anti-Mouse IgG (H + L) (ZB-2305) were purchased from ZhongShan GoldBridge Biotechnology Co., Ltd (Beijing, China). SDS-PAGE Gel Preparation Kit (P0012A) was purchased from Beyotime Biotechnology Co., Ltd (Shanghai, China). BCA Protein Concentration Assay Kit (AR0146) was purchased from BOSTER Biological Technology Co., Ltd (Wuhan, China). NOS1/nNOS ELISA Kit (E-EL-R1438C) were purchased from Elabscience Biotechnology Co., Ltd (Wuhan, China). All other reagents and chemicals used in this study are commercially available.
Thirty-two 8-wk-old male Sprague-Dawley rats weighing 200 ± 20 g were housed in a specific pathogen-free animal house. The animals were kept at a standard room temperature of 24 °C, with 40%-60% relative humidity, 12 h light-dark alternation, and a standard laboratory diet containing 23% protein and water. All animals were provided by the Animal Center of the Fourth Military Medical University and divided into four major groups. All experimental procedures were conducted in accordance with the Principles of Laboratory Animal Care and approved by the University Ethics Committee and performed as per institutional guidelines. Efforts were made to minimize the number of animals used.
The rats were randomly divided into four groups (n = 8/each group), including the control (CG), UC (EG1), UC + NOS agonist TP508TFA (EG2), and UC + NOS inhibitor L-NMMA (EG3) groups. The CG group was housed as described above, whereas the animals in the three experimental groups (EG1-3) were given tap water containing 5.5% DSS. The CG group was fed in the same way as the experimental groups except that DSS was not added in the tap water. All animals were given free access to water for 7 d, and the water was changed to tap water at day 15. Further, EG2 rats were treated with 3 mL of 0.01 mmol/L TP508 TFA i.g. daily for 15 d and EG3 rats were treated with 3 mL of 0.01 mmol/L L-NMMA i.g. daily for 15 d. Animals were regularly monitored for the general conditions, body weight, stool characteristics, occult blood, and hematochezia for the evaluation of the disease condition. The scoring criteria of the disease activity index (DAI) were as follows[14]: Body weight: No loss, 0 points; loss by 1%-5%, 1 point; loss by 6%-10%, 2 points; loss by 11%-15%, 3 points; loss by more than 15%, 4 points; stool characteristics: Normal (well-shaped), 0 points; bondless (mushy and semi-formed stool that does not adhere to the anus), 2 points; loose (watery stool that can adhere to the anus), 4 points; fecal occult blood or macroscopic hematochezia: Normal, 0 points; occult blood (+), 2 points; macroscopic hematochezia, 4 points. DAI score was calculated as equal to the average value of the sum of the above scores.
Rats in each group were individually subjected to the open field test on day 14 of the experiment. The rats were randomly placed into boxes with a height of 30-40 cm and a length of 100 cm on the bottom side. The box's inner walls were darkened, and the bottom surface was divided on the average 25 squares (4 cm × 4 cm) with a digital camera set 2 m above each side. The data was acquired automatically and recorded for 15 min. This technique was used to test the spontaneous activity of the animals and their anxious behavior in an open environment.
On the 15th day of the experiment, rats in each group were anesthetized with an intraperitoneal injection of 7% chloral hydrate (0.4 mL/100 g). The whole colon was removed to compare the colon length for rats in each group.
Eight rats in each group were used for histological evaluation of the colon. Following the whole colon removal, the intestinal lumen was flushed using 0.01 mmol/L PBS buffer, and transected 6-9 cm from the anus. Colons were fixed in 4% paraformaldehyde, dehydrated in graded alcohols, and then embedded in the paraffin. The block was cut into 5 μm thick sections and hematoxylin-eosin stained. Afterwards, the slides were mounted with neutral gum and dried at 37 °C overnight. A whole slide was observed under a scanning biomicroscope (SLIDEVIEW VS200, Olympus, Tokyo, Japan). Histological changes were recorded based on the staining results, and the histological index (HI) scoring was performed, with the following criteria[15]: 0 points for no damage; 1 point for disappearance of basal 1/3 crypts; 2 points for disappearance of basal 2/3 crypts; 3 points disappearance of crypts with intact epithelial cells; and 4 points for crypt and epithelial cell disappearance.
The distal colons of eight rats in each group were dissected separately. The intestinal lumens were flushed in 0.01 mol/L PBS buffer, then a colonic strip with a width of approximately 0.5-1.0 cm was cut along the travel direction of the circular muscle (CM). Afterwards, the dissected colon was fixed in 4% paraformaldehyde. The mucosal layer was fixed upward and horizontally in PBS buffer (pH = 7.4). The mucosal, submucosal, and CM layers were removed with the help of filament forceps to preserve the longitudinal muscle (LM) layer. The tissues were blocked in 10% donkey serum for 30 min and then incubated with Rabbit-Anti-HuD + HuC (1: 500) and Goat-Anti-nNOS (1: 300) in a shaker overnight at 4 °C. Slides were incubated with Donkey Anti-Goat Alexa Fluor 488 (1: 500) and Donkey Anti-Rabbit Alexa Fluor 594 (1: 500) for 4 h. Eight different fields (approximately 1.0 cm × 1.0 cm) of the specimens were observed using confocal microscopy (FV-1000, Olympus, Tokyo, Japan) with the appropriate laser beams and filter settings for Alexa 488 (excitation, 488 nm; emission, 510-530 nm) and Alexa 594 (excitation, 543 nm; emission, 590-615 nm). Digital images were captured with an FV10-ASW 4.2 from Olympus, and these images eventually saved as TIFF files to calculate changes in the proportion of colon nitrergic neurons.
Four rats in each group were anesthetized and perfused with pre-cooled PBS buffer, and the terminal colon was transected. The mucosa and submucosa were separated with silk tweezers. The muscle layer was put into a pre-cooled EP tube. The tissue was homogenized using an ultrasonic grinder, in the lysis buffer (RIPA: protease inhibitor: phosphatase inhibitors = 100:1:1). After standing for 10 min on ice, the supernatant was centrifuged at 12000 rpm (10008 × g) for 10 min. The protein concentration was measured using a BCA protein concentration assay kit and FC microplate reader (1410101, Thermo Fisher Scientific, Shanghai, China). The protein samples were kept at -80 °C for further use.
Gels were made using the SDS-PAGE gel preparation kit, and the samples were electrophoresed at a constant voltage of 80 and 120 V. Membrane transfer was achieved at a constant current of 300 mA. First, the PVDF membranes were blocked with Western blocking solution (P0252, Beyotime Biotechnology Co., Ltd., Shanghai, China) for 15 min and then incubated with Rabbit Anti-nNOS (1:1000) and Anti-β-Actin antibody (mouse monoclonal; 1:5000) on a shaker overnight at 4 °C. The PVDF membranes were then incubated with HRP-labeled Rabbit Anti-Goat IgG (H + L) (1:10000) and HRP-labeled Goat Anti-Mouse IgG (H + L) (1:10000) for 2 h. After three rinses (10 min each) in TBST, the membranes were probed by ImmobilonTM Western chemiluminescent HRP substrate (WBKLS0050, Merck KGaA, Darmstadt, Germany) and placed into ECL for the detection. The proteins were analyzed using Image-Pro Plus software (Image-Pro Plus Version 6.0, Media Cybernetics, Maryland, United States).
Whole blood from four rats in each group were kept for 2 h at room temperature and then centrifuged at 3800 rpm (1000 × g) for 20 min, followed by the removal of supernatant. Next, the terminal colon tissues were grounded and disrupted with an appropriate volume of PBS (usually at a weight to volume ratio of 1:9), homogenized using a glass homogenizer, and then centrifuged at 8460 rpm (5000 × g) for 5 to 10 min. Afterwards, the supernatant was collected. The NOS1/nNOS ELISA kit was used to determine the concentration of NOS in the colon as well as in the serum.
Four rats in each group were used to explore the changes of in vitro gut colonic tension, including eight circular and eight longitudinal colon muscle strips. Rats were anesthetized, and the abdominal cavity was exposed. Then, the intestinal tube was carefully separated with the forceps and quickly freed. The colon was excised and placed in Krebs' fluid at 37 °C with a continuous supply of 95% O2 and 5% CO2 mixture. Then, 3 mm × 10 mm circular and LM strips were cut, where both ends were anchored to tension receptors and platinum rings at the lower end, respectively. The signals acquired by the tension receptors were recorded and processed with a multi-channel physiological signal acquisition and processing system (RM6240E, INSTRUMENT FACTORY, Chengdu, China). The mean amplitude of spontaneous contractions was recorded in circular and LM strips obtained from control and UC rats at rest, when the muscle strips were allowed to rest in the incubation solution for 30 min. After 10 min of recording, TP508TFA (1 × 10-4 mol/L) was added to the bath of the UC group, and the mean amplitude changes of circular and LM strips were recorded, respectively. The liquid in the bath was replaced after 10 min, and the bath was washed. After resting for 30 min, L-NMMA (1 × 10-4 mol/L) was added, and the corresponding mean amplitude changes were re-recorded.
SPSS version 23.0 statistical software (SPSS Inc, Chicago, United States) was used for statistical analyses. Data are expressed as the mean ± SD, and comparisons between groups were performed by one-way ANOVA. A P value of < 0.05 was considered statistically significant.
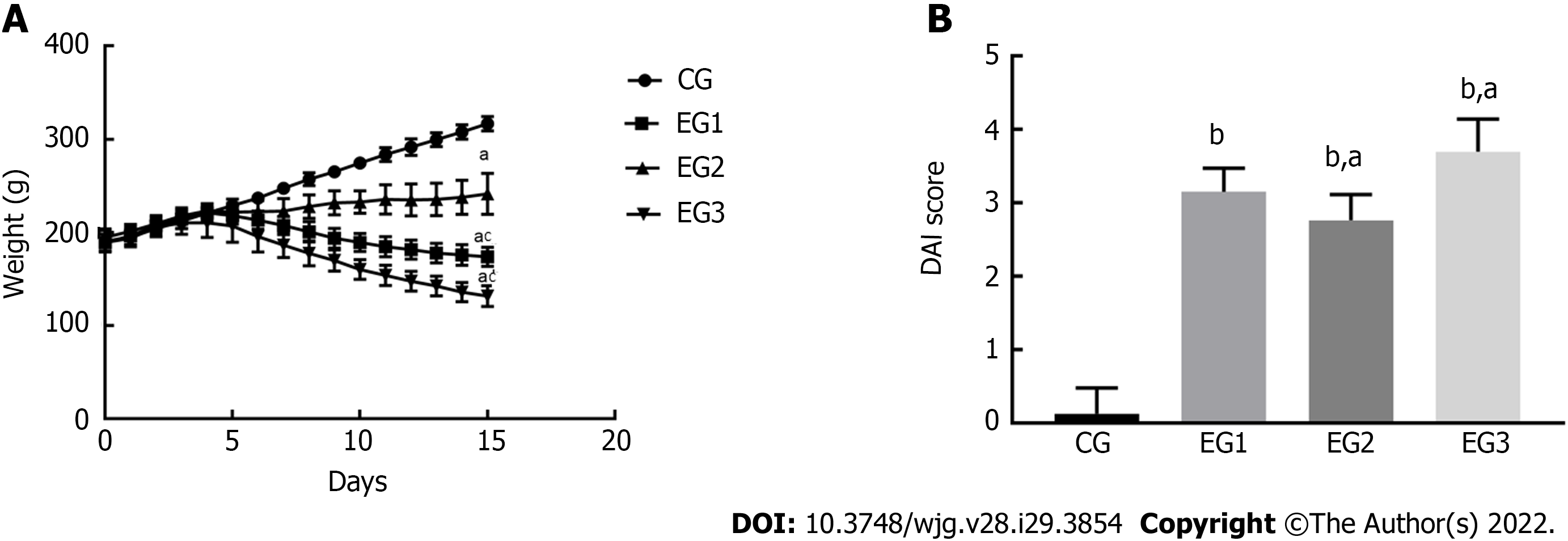
Sloth, anorexia, emaciation, decreased fur gloss, and higher stool frequency were found in all EG rats, with some developing mucopurulent bloody stools. From days 0 to 5, animals in all groups gained weight (CG group: 28.90 ± 2.43 g; EG1 group: 18.10 ± 3.23 g; EG2 group: 17.00 ± 6.17 g; EG3 group: 21.80 ± 3.63 g). The body weight of rats in each group was not significantly different (P > 0.05); however, the body weight of rats in the EG1-3 groups began to decrease from day 6. Until day 15, the CG group (116.60 ± 2.76 g) and EG2 group (58.60 ± 7.79 g) gained weight, while the EG1 group (26.00 ± 3.69 g) and EG3 group (60.40 ± 3.99 g) lost weight. The decrease in body weight was statistically significantly different between the EG groups and CG group (P < 0.05); the differences in weight change between the EG2-3 groups and EG1 group were also statistically significant (P < 0.05) (Figure 1A). As for the stool profiles of all the groups, two rats in the CG group exhibited bondless and loose stools without hematochezia. For the EG1 group, it took 3.25 ± 0.31 d to the occurrence of loose stools, 3.37 ± 0.37 d to fecal occult blood, and 4.1 2 ± 0.39 d to macroscopic hematochezia; the corresponding values in the EG2 and EG3 groups were 3.85 ± 0.50 d, 4.00 ± 0.37 d, and 4.62 ± 0.41 d, and 2.87 ± 0.29 d, 2.62 ± 0.26 d, and 3.37 ± 0.32 d, respectively. Regarding the DAI scores, no statistically significant differences was found from day 0 to day 5 for any group (P > 0.05). After day 5, the intergroup differences in DAI scores were significantly increased. The DAI scores were (0.12 ± 0.12), (3.15 ± 0.11), (2.67 ± 0.12), and (3.69 ± 0.15) for the CG, EG1, EG2 and EG3 groups, respectively, at day 15. Moreover, the scores were significantly different between the EG groups and CG group (P < 0.0001), and between the EG2-3 groups and EG1 group (P < 0.05) (Figure 1B).
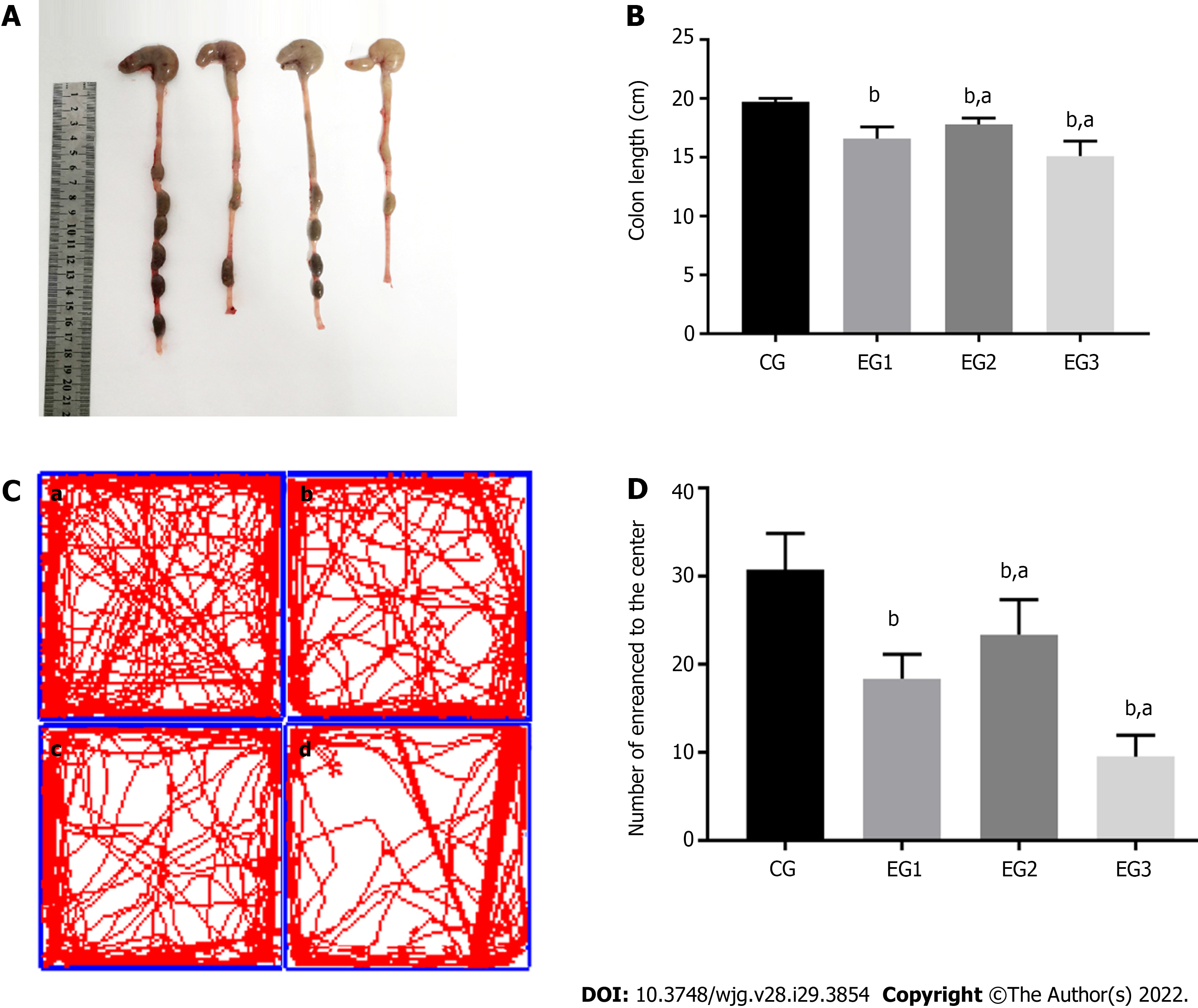
The colonic morphology of rats from each group was compared during the dissection (Figure 2A). Compared with the CG group, the colon length in the EG groups showed various degrees of shortening, and the differences were statistically significant (P < 0.0001). The difference was also significant between the EG2-3 groups and EG1 group (P < 0.05) (Figure 2B).
On the 14th day of the experiment, the behavior of rats was examined using an open field test. The number of times that the rats passed through the center of an open box within 15 min period is shown as follows: 30.75 ± 1.46 for the CG, 18.38 ± 0.98 for EG1, 23.38 ± 1.40 for EG2, and 9.50 ± 0.86 for EG3 group. Differences were statistically significant between the EG groups and CG group (P < 0.0001). Additionally, the EG2-3 groups showed statistically significant differences compared with the EG1 group (P < 0.05) (Figure 2C and D).
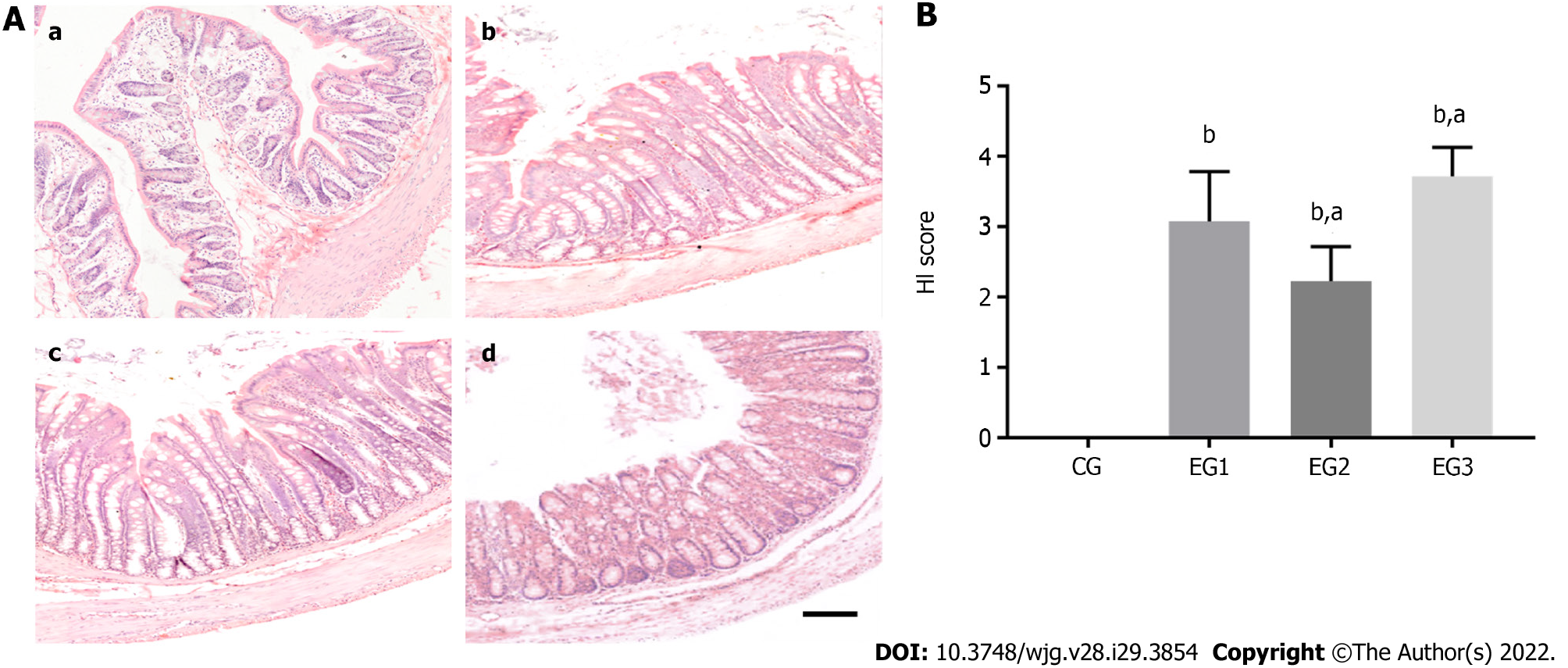
The results of HE staining indicated that the colonic tissue structure of CG rats was normal, with the mucosal layer showing a well-arranged monolayer of columnar epithelial cells, clear intestinal glands, morphologically normal goblet cells, and no inflammatory cell infiltration. In the colon of EG1 rats, most crypts disappeared, with some broken or disappearing epithelia, accompanied by inflammatory cell infiltration. In the colon of EG2 rats, 1/3-2/3 of basal crypts disappeared, with the occasional destruction of epithelial cells. In the colon of EG3 rats, crypts completely disappeared, with some broken or disappearing epithelia, accompanied by massive inflammatory cell infiltration (Figure 3A). The HI scores for the colons of the rats in the CG, EG1, EG2, and EG3 groups were 0.00 ± 0.00, 3.07 ± 0.25, 2.22 ± 0.17, and 3.71 ± 0.14, respectively. The differences were statistically significant between the EG groups and CG group (P < 0.0001), and between the EG2-3 groups and EG1 group (P < 0.05) (Figure 3B).
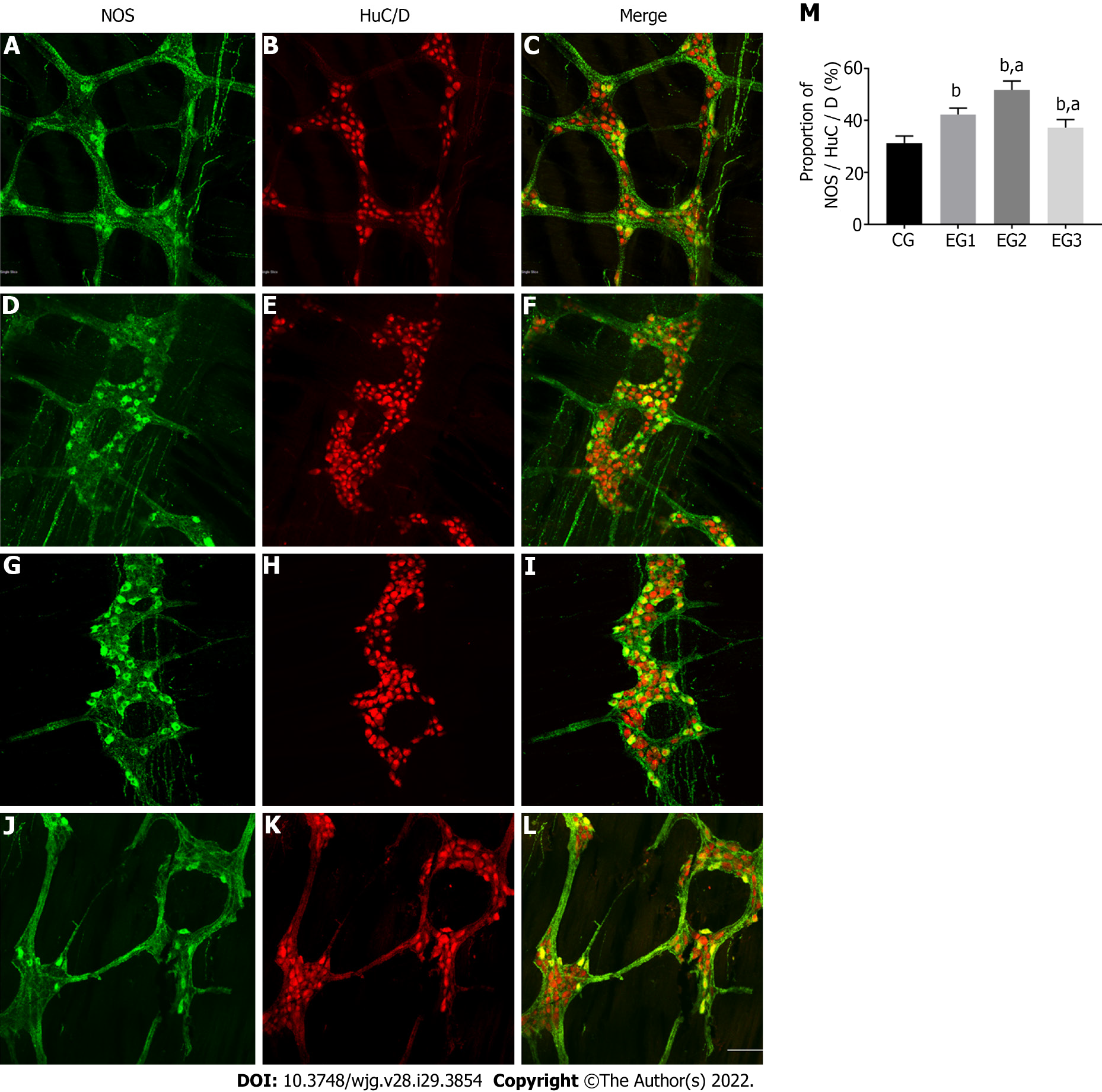
The distal rat colons were double-stained by immunofluorescence histochemistry to observe the distribution and expression of NOS-positive neurons within the colonic MP. Within the colonic MP of rats, the proportion of these neurons in each group was counted. Enteric neurons exhibited a reticular distribution in the colonic MP (Figure 4B). NOS-positive neurons accounted for 31.38 ± 0.94% in the CG group (Figure 4C) but were more distributed within the marginal side of the ganglia (Figure 4A). They were fusiform or star-shaped, and their nuclei were round with several elongated protrusions. The protrusions of these neurons were interconnected with each other to form a dense and complex neural network. In the EG1 group, the percentage of NOS-positive neurons in colonic neurons (Figure 4E) increased to 42.25% ± 0.88% under disease conditions (Figure 4F). The distribution of these neurons was no longer confined to the edges of the ganglia and began to appear elsewhere within the ganglia (Figure 4D). Moreover, the proportion of NOS-positive neurons in the EG2 group increased to 51.75% ± 1.22% (Figure 4I and H). These neurons in the ganglia were disorganized and widely distributed within the ganglia (Figure 4G). Compared with the EG1 group, the proportion of NOS-positive neurons in the EG3 group decreased to 37.25% ± 1.09% (Figure 4L and K), with their distribution within the ganglia being predominantly marginal (Figure 4J). The changes in the proportion of NOS-positive neurons to colonic neurons in the EG groups were statistically significant in comparison to the CG group (P < 0.0001); however, the EG2 and EG3 groups were also significantly different from the EG1 group (P < 0.05) (Figure 4M).
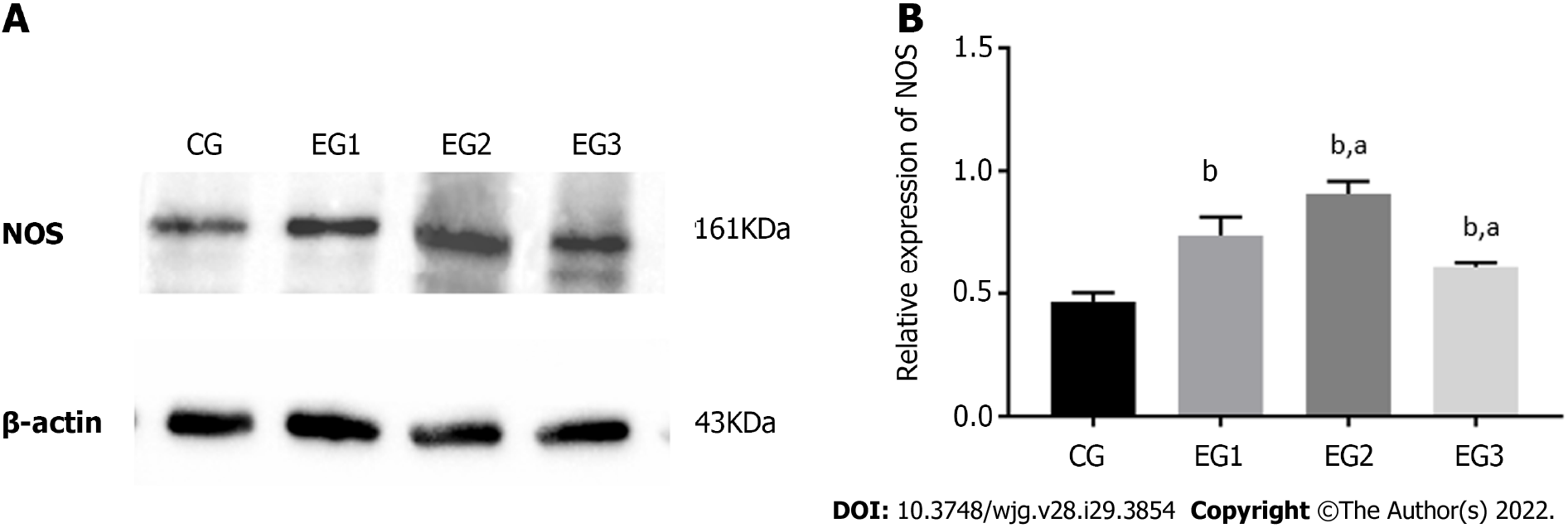
The expression level of NOS protein in the colonic MP of EG1 rats was higher than that in the CG group. The NOS expression in the EG2 rat colon was further increased, while the expression in the EG3 rat colon was lower than that in the EG1 group. The differences were statistically significant between the EG groups and CG group (P < 0.0001) and between the EG2-3 groups and EG1 group (P < 0.05) (Figure 5A and B).
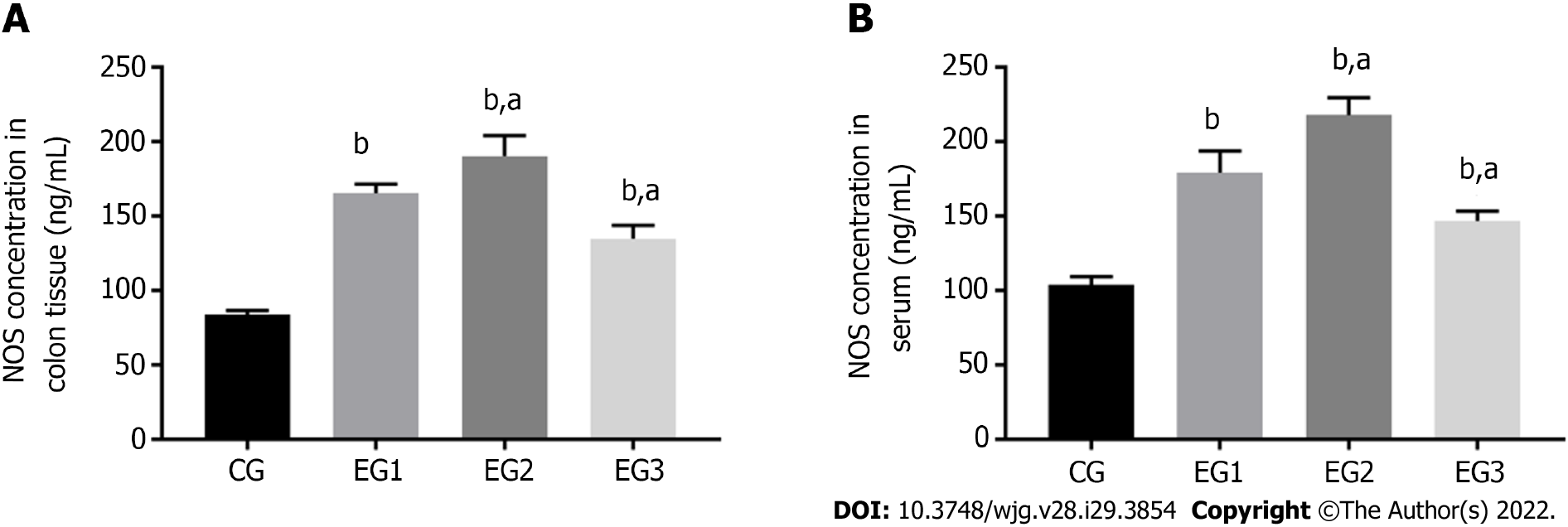
The concentration of NOS was significantly increased in the colonic myenteric tissue in the EG groups compared with the CG group (P < 0.0001); the EG2 group had a higher NOS concentration, whereas the EG3 group had a lower concentration than the EG1 group, and the difference between the EG2-3 groups and EG1 group was also statistically significant (P < 0.05) (Figure 6A). In addition, serum concentrations of NOS were significantly increased in the EG group compared with the CG group (P < 0.0001); however, the EG2 group had a significantly higher NOS concentration, whereas the EG3 group had a significantly lower concentration than the EG1 group (P < 0.05) (Figure 6B).
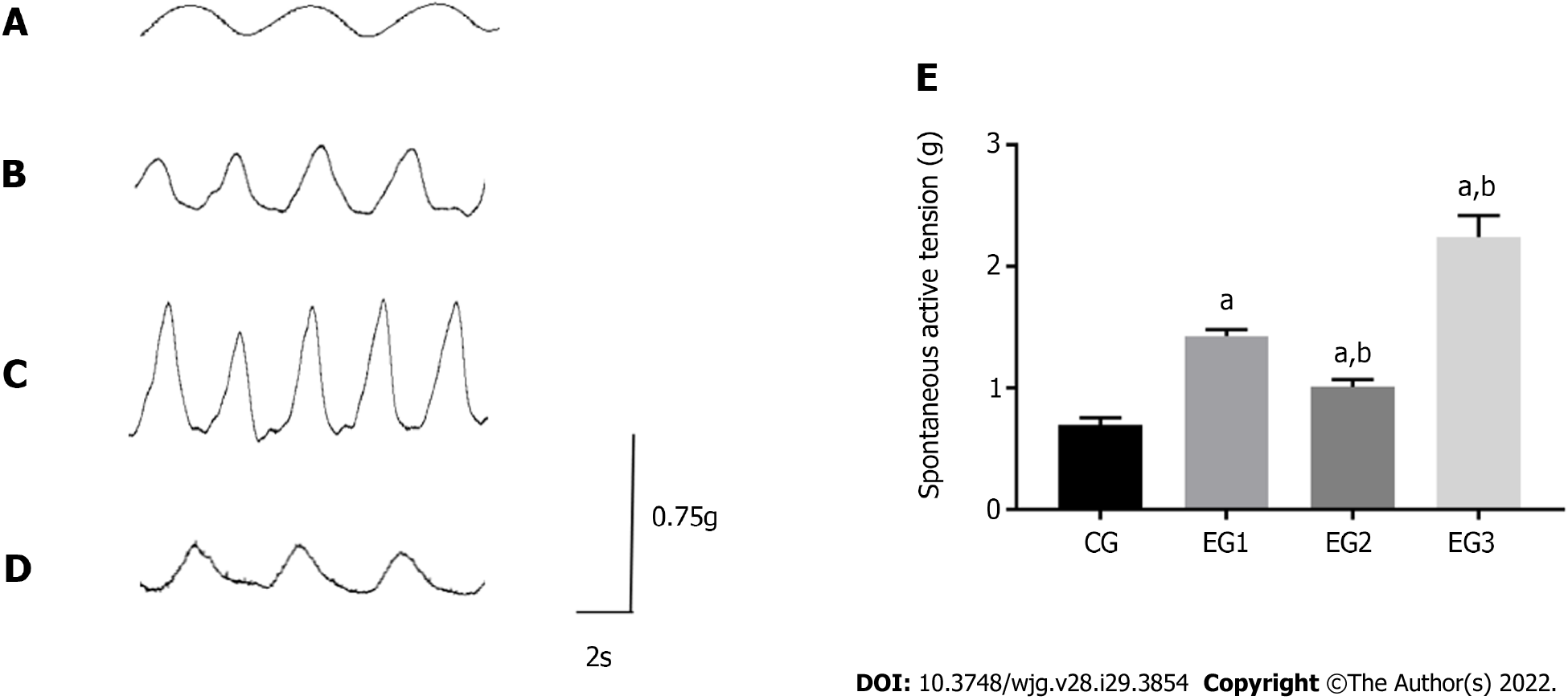
At rest, the contraction of the LM in the colon of CG rats appeared as a regular and sine wave-like curve with relatively neat amplitude (Figure 7A). In EGs rats, the contraction was significantly more frequent, with the increased amplitude (Figure 7B-D). The contraction tension of the LM of the colon was considerably more significant in EG rats than in CG rats (P < 0.0001). However, the contraction tensions of the colonic LM were significantly weakened and increased in EG2 and EG3 rats, respectively, when compared with EG1 rats (P < 0.001) (Figure 7E).
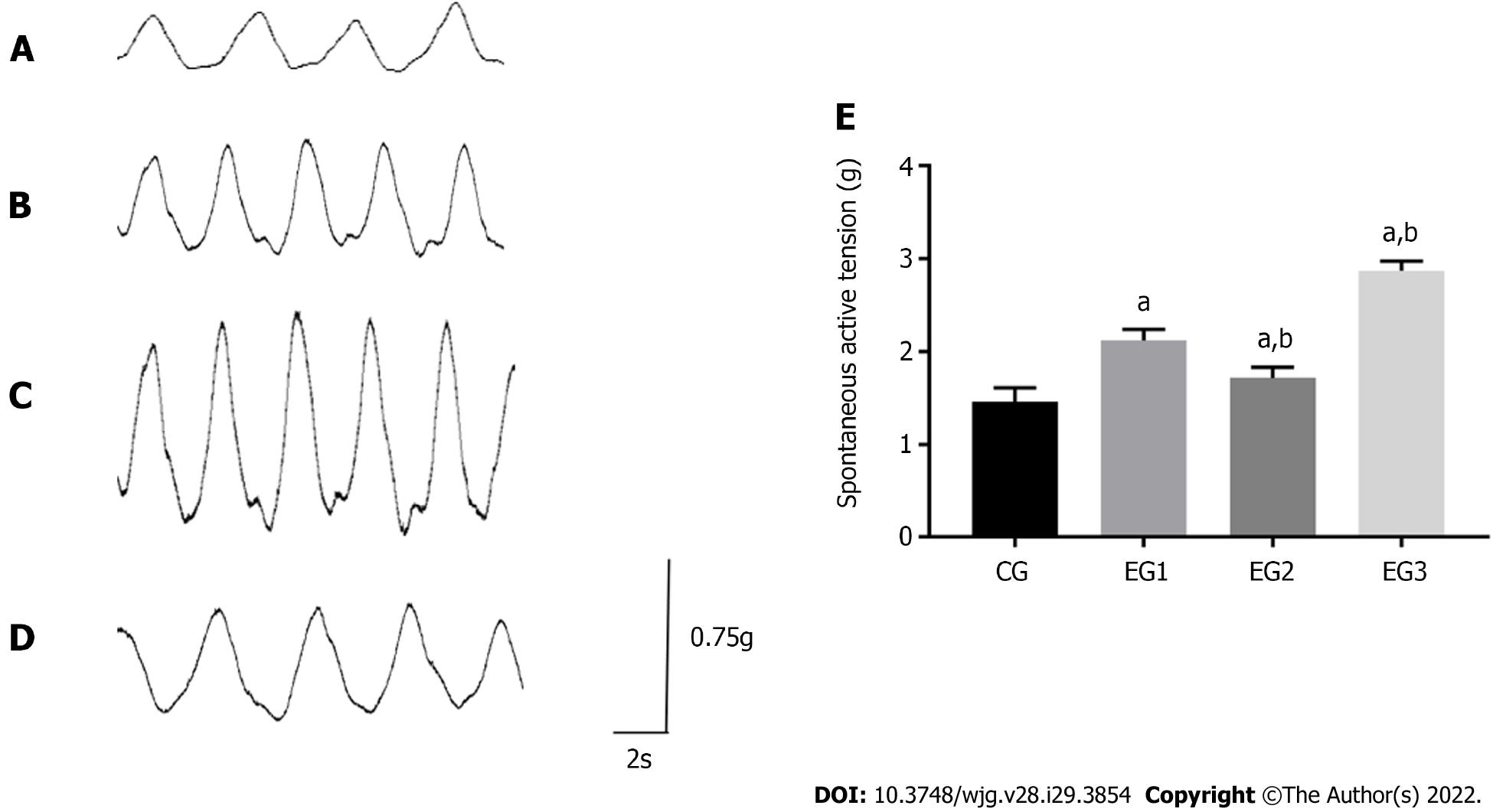
At rest, the contraction of the colonic CM in CG rats appeared as a regular and triangular wave-like curve. The amplitude was neat, with a contraction interval between the adjacent two waves (Figure 8A). In EGs rats, contraction of the CM of the colon was significantly accelerated, and its amplitude was increased (Figure 8B-D). The contraction tension of the colonic CM was significantly larger in EG rats than in CG rats (P < 0.0001). The contraction tension of the colonic CM appeared significantly weakened and increased in EG1 rats compared with EG2 and EG3 rats, respectively (P < 0.0001) (Figure 8E).
An increase or decrease in the number of neurons and/or neuronal degeneration in the ENS can lead to various diseases. For instance, congenital dysplasia of the ENS leads to congenital megacolon (Hirschsprung disease) and primary achalasia, whereas neurodegenerative ENS alterations can lead to the disorders such as Alzheimer, Huntington, and Parkinson diseases[16,17]. The secondary alterations in the ENS also result in inflammatory infiltrates or immune system pathologies such as irritable bowel syndrome[18], idiopathic enteric gangliosidosis[19], paraneoplastic syndrome[20], slow transit constipation[21], severe acute pancreatitis[22], diabetes mellitus[23,24], and UC[25].
The different types of ENS neurons have significantly different morphology and structure associated with the synthesis and secretion of neurotransmitters. However, by forming complex synaptic connections, these neurons participate in the structural basis that mainly underlies the relatively independent reflex activity of the gut and can also regulate the diverse motor and sensory activities of the digestive tract.
Despite the unclear etiology of UC, numerous studies have shown that its pathogenesis might be associated with the interactions between genetic susceptibility, environmental provoking factors, and immune-mediated tissue damage. Additionally, the relationship between abnormal intestinal motility and UC has also gained attention. Abnormal ENS is an important mechanism contributing to the abnormal colonic motility of UC, which is an important factor causing diarrhea in UC patients.
The onset of UC has a complex genetic background. Based on the gene polymorphism and heterogeneity, the impaired local barrier function of the intestinal mucosa can be the result of a combination of factors, such as altered epithelial permeability, neuroendocrine regulation, and intestinal flora translocation[26]. This, in turn, provokes the inflammatory response with symptoms such as abdominal pain, diarrhea, and colonic motor dysfunction. As the rate-limiting enzyme of NO synthesis in the body, NOS has three sub-types: iNOS, eNOS, and nNOS. Histological studies have identified intense focal iNOS expression by the inflamed bowel epithelium and in the mononuclear cell infiltrate in the intestinal tissues of both Crohn’s disease and UC patients[27]. A great number of studies suggest that iNOS in the ENS may play a part in preventing activation of mast cells, reducing leukocyte adhesion to the endothelium and protecting the host from being invaded by colonic bacteria[28-30]. In normal and UC states, eNOS expression is limited to colonic vascular endothelium[31]. Baker et al[32] confirmed that during DSS-induced UC, eNOS KO mice suffered less tissue damage and inflammation than wild-type mice, suggesting that eNOS is essential for maintaining the integrity of the GI mucosa. nNOS is one of the specific markers for nitrergic neurons within the ENS, the primary inhibitory neurons of the colonic MP[33]. By releasing inhibitory neurotransmitter NO, nitrergic neurons can regulate GI motility. The changes in expression of nNOS in the colonic MP of UC rats indicate that nitrergic neurons may be involved in NO-based neurotransmission and regulate GI motility in UC state.
Research has shown that NO is the second messenger in the smooth muscle cells (SMCs) or interstitial cells of Cajal (ICCs)[34]. NO is highly lipid-soluble and reaches target ICCs in a freely diffusible manner after synthesis. It binds to the soluble guanylyl cyclase in the cells to increase the enzymatic activity by altering its spatial configuration, which further leads to an increase in cyclic guanosine monophosphate (cGMP) within the cells, activating the cGMP protein kinase-dependent calcium pumps, and therefore finally participates in intercellular information transmission[35]. Therefore, it could be concluded that as a messenger of information transmission between the NOS-positive neurons and GI SMCs, increased NO can reduce the Ca2+ influx and directly promotes smooth muscle relaxation. In addition, studies have confirmed that NO can inhibit muscle contraction by inhibiting the release of excitatory transmitters[23,36]. Therefore, NO-mediated reduced contractility of the intestinal smooth muscle might be one of the important mechanisms contributing to colonic dysmotility[9,37].
In the present study, DSS was applied to induce the UC rat model successfully. For the first time, the finding of a secondary increase in NOS expression in the colonic MP of UC rats, combined with altered in vitro colonic contraction tension, suggests that the increased NOS expression is associated with the altered colonic motility in UC rats. Increasing (or decreasing) the number of NOS-positive neurons might enhance (or attenuate) the diastolic function of the colonic smooth muscle regulated by these neurons. However, the change in the number of NOS-positive neurons is often due to changes in the concentration and release of neurotransmitters caused by changes in the amount of NOS contained in neurons under pathological conditions, rather than caused by neuronal degeneration and regeneration. The results of in vitro studies of the colon using both agonists and inhibitors of NOS further confirmed that the altered NOS expression regulate the colonic motility in UC. A previous similar study demonstrated that the NOS expression and NO concentration within the muscular layer of the stomach and small intestine were increased in an animal model of chronic pancreatitis[38]. These results suggest that the reduced contractility of the gastric CM because of NO inhibition might be an important mechanism underlying gastric motor dysfunction in chronic pancreatitis[36,38]. Moreover, these findings can provide an interesting insight into the role of the ENS during GI dysmotility.
The initiation of colonic dysmotility in UC may be related to the structural alterations and abnormal number of ICCs, disturbed intestinal electrophysiology, changes in colonic pressure, and abnormal expression of gut-related neurotransmitters. Our present results have demonstrated that the increased NOS expression inhibits the contraction motility of the colonic smooth muscle. Therefore, appropriate adjustment of NOS levels can alter the expression of nitrergic neurons, control the motor movement of the intestinal smooth muscle, and improve the UC colonic motor function. Of note, this could improve the symptoms of UC patients, providing a basis for the screening of novel agents against UC.
The increased number of nitrergic neurons in the colonic MP of UC rats, both in vitro and in vivo, diminishes the colonic motor function. In contrast, activation and inhibition of NOS activity could induce and diminish the colon motor function, respectively. Further, an increased number of nitrergic neurons in the colonic MP of UC rats leads to reduced colon contractile function. Therefore, the regulation of nitrergic neurons in the colonic MP through interference with the activity of NOS might be a novel potential and prospective way to reduce diarrhea symptoms in UC patients.
Ulcerative colitis (UC) is a nonspecific inflammatory intestinal disorder with a complex etiology and poorly understood pathogenesis. The association between abnormal intestinal motility and UC has gained increasing attention over the past years. The enteric nervous system (ENS) regulates gut motility and based on their functions, has been divided into inhibitory and excitatory neurons, which mainly regulate the gut motility in terms of relaxation and contraction via different neurotransmitters. Nitrergic neurons are typical inhibitory neurons in the ENS and act through the neurotransmitter nitric oxide, which is synthetized by the rate-limiting enzyme nitric oxide synthase (NOS), whose expression changes might affect the motor function of the gut.
UC is an intestinal disease with abdominal pain and diarrhea as the main symptoms, which are associated with abnormal gastrointestinal motility. Alterations in the amount of enteric neurotransmitters may change the number of enteric neurons. Nitrergic neurons are well-established enteric inhibitory neurons, and modification of its expression may interfere with its regulatory effect on intestinal motility and improve the symptoms of abdominal pain and diarrhea in UC.
This study aimed to investigate the relationship between colonic NOS expression changes and colonic motility in dextran sulfate sodium (DSS)-induced UC rats, and to explore the effects of nitrergic neurons on colonic motility in UC rats to discover the potential mechanisms for the treatment of UC.
UC was induced in adult male rats with 5.5% DSS, and part of them were administered with NOS agonists and inhibitors. The rats were divided into control (CG), UC (EG1), UC + agonist (EG2), and UC + inhibitor (EG3) groups. The changes in tissue expression, relative protein expression, and concentration of NOS in rats were detected by immunofluorescence histochemical double staining, Western blot, and ELISA techniques, respectively. The effect of nitrergic neurons on colonic motility was examined by the changes in colonic circular muscle (CM) and longitudinal muscle (LM) contraction tension in vitro.
Compared with CG rats, the proportion of NOS positive neurons within the colonic myenteric plexus (MP), the relative expression of NOS, and the concentration of NOS in both serum and colonic tissue were significantly higher in EG rats. After administration of NOS agonists and inhibitors, various degrees of increase and decrease were observed in EG2 and EG3 rats, respectively. The contraction amplitude and mean contraction tension of the CM and LM in rat colon after administration of agonists and inhibitors were attenuated and enhanced in vitro, respectively. For UC, regulating the expression of NOS within the MP may improve intestinal motility, thereby favoring the recovery of intestinal function.
Nitrergic neurons within the rat colonic MP are involved in the regulation of colonic motility. Increased NOS in the colonic MP of UC rats causes nitrergic neurons amplification, leading to decreased colonic contraction function. Modulation of NOS levels within colonic MP can alter nitrergic neuron expression and adjust the motor activity of the intestinal smooth muscle, which can further improve colonic motor function, moderate UC symptoms, and provide evidence for the development of new drugs against UC.
This study demonstrated increased NOS expression in the colonic MP of UC rats, with a possible corresponding increase in nitrergic neuron expression and a decrease in colonic contraction function in UC rats. Thus, by regulating the expression of NOS in the colonic MP, colonic motor function and interruption in the pathogenesis of UC can be achieved, thus providing a novel insight into the treatment of UC.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Bernstein HG, Germany; Sahin Y, Turkey S-Editor: Ma YJ L-Editor: Wang TQ P-Editor: Cai YX
| 1. | Kuehn F, Hodin RA. Impact of Modern Drug Therapy on Surgery: Ulcerative Colitis. Visc Med. 2018;34:426-431. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 20] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 2. | Hoffmann P, Krisam J, Stremmel W, Gauss A. Real-World Outcomes of Vedolizumab Therapy in Ulcerative Colitis and Crohn's Disease at a Tertiary Referral Center. Dig Dis. 2019;37:33-44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 3. | Ponder A, Long MD. A clinical review of recent findings in the epidemiology of inflammatory bowel disease. Clin Epidemiol. 2013;5:237-247. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52] [Cited by in RCA: 119] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 4. | Higuchi LM, Khalili H, Chan AT, Richter JM, Bousvaros A, Fuchs CS. A prospective study of cigarette smoking and the risk of inflammatory bowel disease in women. Am J Gastroenterol. 2012;107:1399-1406. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 179] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 5. | Santana PT, Rosas SLB, Ribeiro BE, Marinho Y, de Souza HSP. Dysbiosis in Inflammatory Bowel Disease: Pathogenic Role and Potential Therapeutic Targets. Int J Mol Sci. 2022;23. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 180] [Cited by in RCA: 176] [Article Influence: 58.7] [Reference Citation Analysis (0)] |
| 6. | Sundin J, Aziz I, Nordlander S, Polster A, Hu YOO, Hugerth LW, Pennhag AAL, Engstrand L, Törnblom H, Simrén M, Öhman L. Evidence of altered mucosa-associated and fecal microbiota composition in patients with Irritable Bowel Syndrome. Sci Rep. 2020;10:593. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 39] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 7. | Bassotti G, Antonelli E, Villanacci V, Nascimbeni R, Dore MP, Pes GM, Maconi G. Abnormal gut motility in inflammatory bowel disease: an update. Tech Coloproctol. 2020;24:275-282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 29] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 8. | Li H, Fan C, Lu H, Feng C, He P, Yang X, Xiang C, Zuo J, Tang W. Protective role of berberine on ulcerative colitis through modulating enteric glial cells-intestinal epithelial cells-immune cells interactions. Acta Pharm Sin B. 2020;10:447-461. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 153] [Article Influence: 30.6] [Reference Citation Analysis (0)] |
| 9. | Głąbska D, Guzek D, Grudzińska D, Lech G. Influence of dietary isoflavone intake on gastrointestinal symptoms in ulcerative colitis individuals in remission. World J Gastroenterol. 2017;23:5356-5363. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 17] [Cited by in RCA: 16] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 10. | Costa M, Spencer NJ, Brookes SJH. The role of enteric inhibitory neurons in intestinal motility. Auton Neurosci. 2021;235:102854. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 24] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 11. | Schemann M. Control of gastrointestinal motility by the "gut brain"--the enteric nervous system. J Pediatr Gastroenterol Nutr. 2005;41 Suppl 1:S4-S6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 96] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 12. | Saldana-Morales FB, Kim DV, Tsai MT, Diehl GE. Healthy Intestinal Function Relies on Coordinated Enteric Nervous System, Immune System, and Epithelium Responses. Gut Microbes. 2021;13:1-14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 18] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 13. | Tse JKY. Gut Microbiota, Nitric Oxide, and Microglia as Prerequisites for Neurodegenerative Disorders. ACS Chem Neurosci. 2017;8:1438-1447. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 137] [Article Influence: 17.1] [Reference Citation Analysis (0)] |
| 14. | Sánchez-Fidalgo S, Cárdeno A, Sánchez-Hidalgo M, Aparicio-Soto M, de la Lastra CA. Dietary extra virgin olive oil polyphenols supplementation modulates DSS-induced chronic colitis in mice. J Nutr Biochem. 2013;24:1401-1413. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 110] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 15. | Murano M, Maemura K, Hirata I, Toshina K, Nishikawa T, Hamamoto N, Sasaki S, Saitoh O, Katsu K. Therapeutic effect of intracolonically administered nuclear factor kappa B (p65) antisense oligonucleotide on mouse dextran sulphate sodium (DSS)-induced colitis. Clin Exp Immunol. 2000;120:51-58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 196] [Cited by in RCA: 251] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 16. | Drossman DA, Tack J, Ford AC, Szigethy E, Törnblom H, Van Oudenhove L. Neuromodulators for Functional Gastrointestinal Disorders (Disorders of Gut-Brain Interaction): A Rome Foundation Working Team Report. Gastroenterology. 2018;154:1140-1171.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 195] [Cited by in RCA: 274] [Article Influence: 39.1] [Reference Citation Analysis (0)] |
| 17. | Filpa V, Moro E, Protasoni M, Crema F, Frigo G, Giaroni C. Role of glutamatergic neurotransmission in the enteric nervous system and brain-gut axis in health and disease. Neuropharmacology. 2016;111:14-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 81] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 18. | Niesler B, Kuerten S, Demir IE, Schäfer KH. Disorders of the enteric nervous system - a holistic view. Nat Rev Gastroenterol Hepatol. 2021;18:393-410. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 106] [Article Influence: 26.5] [Reference Citation Analysis (0)] |
| 19. | Fornai M, Pellegrini C, Antonioli L, Segnani C, Ippolito C, Barocelli E, Ballabeni V, Vegezzi G, Al Harraq Z, Blandini F, Levandis G, Cerri S, Blandizzi C, Bernardini N, Colucci R. Enteric Dysfunctions in Experimental Parkinson's Disease: Alterations of Excitatory Cholinergic Neurotransmission Regulating Colonic Motility in Rats. J Pharmacol Exp Ther. 2016;356:434-444. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 52] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 20. | Li S, Fei G, Fang X, Yang X, Sun X, Qian J, Wood JD, Ke M. Changes in Enteric Neurons of Small Intestine in a Rat Model of Irritable Bowel Syndrome with Diarrhea. J Neurogastroenterol Motil. 2016;22:310-320. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 43] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 21. | De Giorgio R, Barbara G, Stanghellini V, De Ponti F, Salvioli B, Tonini M, Velio P, Bassotti G, Corinaldesi R. Clinical and morphofunctional features of idiopathic myenteric ganglionitis underlying severe intestinal motor dysfunction: a study of three cases. Am J Gastroenterol. 2002;97:2454-2459. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 20] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 22. | Lin Z, Liu Y, Zheng Q, Hu Q. Increased proportion of nitric oxide synthase immunoreactive neurons in rat ileal myenteric ganglia after severe acute pancreatitis. BMC Gastroenterol. 2011;11:127. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 13] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 23. | Dai Y, Haque MM, Stuehr DJ. Restricting the conformational freedom of the neuronal nitric-oxide synthase flavoprotein domain reveals impact on electron transfer and catalysis. J Biol Chem. 2017;292:6753-6764. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 10] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 24. | Anitha M, Gondha C, Sutliff R, Parsadanian A, Mwangi S, Sitaraman SV, Srinivasan S. GDNF rescues hyperglycemia-induced diabetic enteric neuropathy through activation of the PI3K/Akt pathway. J Clin Invest. 2006;116:344-356. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 191] [Cited by in RCA: 198] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 25. | Gracie DJ, Guthrie EA, Hamlin PJ, Ford AC. Bi-directionality of Brain-Gut Interactions in Patients With Inflammatory Bowel Disease. Gastroenterology. 2018;154:1635-1646.e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 201] [Cited by in RCA: 291] [Article Influence: 41.6] [Reference Citation Analysis (0)] |
| 26. | Leonardi I, Paramsothy S, Doron I, Semon A, Kaakoush NO, Clemente JC, Faith JJ, Borody TJ, Mitchell HM, Colombel JF, Kamm MA, Iliev ID. Fungal Trans-kingdom Dynamics Linked to Responsiveness to Fecal Microbiota Transplantation (FMT) Therapy in Ulcerative Colitis. Cell Host Microbe. 2020;27:823-829.e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 136] [Article Influence: 27.2] [Reference Citation Analysis (0)] |
| 27. | Singer II, Kawka DW, Scott S, Weidner JR, Mumford RA, Riehl TE, Stenson WF. Expression of inducible nitric oxide synthase and nitrotyrosine in colonic epithelium in inflammatory bowel disease. Gastroenterology. 1996;111:871-885. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 422] [Cited by in RCA: 413] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 28. | Kolios G, Rooney N, Murphy CT, Robertson DA, Westwick J. Expression of inducible nitric oxide synthase activity in human colon epithelial cells: modulation by T lymphocyte derived cytokines. Gut. 1998;43:56-63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 99] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 29. | Kubes P. Inducible nitric oxide synthase: a little bit of good in all of us. Gut. 2000;47:6-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 85] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 30. | Vallance BA, Deng W, De Grado M, Chan C, Jacobson K, Finlay BB. Modulation of inducible nitric oxide synthase expression by the attaching and effacing bacterial pathogen citrobacter rodentium in infected mice. Infect Immun. 2002;70:6424-6435. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 77] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 31. | Vallance BA, Dijkstra G, Qiu B, van der Waaij LA, van Goor H, Jansen PL, Mashimo H, Collins SM. Relative contributions of NOS isoforms during experimental colitis: endothelial-derived NOS maintains mucosal integrity. Am J Physiol Gastrointest Liver Physiol. 2004;287:G865-G874. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 60] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 32. | Beck PL, Xavier R, Wong J, Ezedi I, Mashimo H, Mizoguchi A, Mizoguchi E, Bhan AK, Podolsky DK. Paradoxical roles of different nitric oxide synthase isoforms in colonic injury. Am J Physiol Gastrointest Liver Physiol. 2004;286:G137-G147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 81] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 33. | Luo CX, Zhu DY. Research progress on neurobiology of neuronal nitric oxide synthase. Neurosci Bull. 2011;27:23-35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 42] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 34. | Bulc M, Palus K, Dąbrowski M, Całka J. Hyperglycaemia-Induced Downregulation in Expression of nNOS Intramural Neurons of the Small Intestine in the Pig. Int J Mol Sci. 2019;20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 18] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 35. | Lalatta-Costerbosa G, Clavenzani P, Petrosino G, Mazzoni M. An immunohistochemical study of the distribution of nitric oxide synthase-immunoreactive neurons and fibers in the reticular groove of suckling lambs. J Anat. 2011;218:439-448. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 36. | Tomuschat C, O'Donnell AM, Coyle D, Dreher N, Kelly D, Puri P. NOS-interacting protein (NOSIP) is increased in the colon of patients with Hirschsprungs's disease. J Pediatr Surg. 2017;52:772-777. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 8] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 37. | Huber A, Saur D, Kurjak M, Schusdziarra V, Allescher HD. Characterization and splice variants of neuronal nitric oxide synthase in rat small intestine. Am J Physiol. 1998;275:G1146-G1156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 26] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 38. | Chen L, Yu B, Luo D, Lin M. Enteric motor dysfunctions in experimental chronic pancreatitis: Alterations of myenteric neurons regulating colonic motility in rats. Neurogastroenterol Motil. 2018;30:e13301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.9] [Reference Citation Analysis (0)] |