Published online Aug 7, 2022. doi: 10.3748/wjg.v28.i29.3780
Peer-review started: January 13, 2022
First decision: May 29, 2022
Revised: May 30, 2022
Accepted: June 30, 2022
Article in press: June 30, 2022
Published online: August 7, 2022
Processing time: 201 Days and 21.3 Hours
The liver is the site of synthesis of the majority of circulating proteins. Besides initial polypeptide synthesis, sophisticated machinery is involved in the further processing of proteins by removing parts of them and/or adding functional groups and small molecules tailoring the final molecule to suit its physiological purpose. Posttranslational modifications (PTMs) design a network of molecules with the common protein ancestor but with slightly or considerably varying activity/localization/purpose. PTMs can change under pathological conditions, giving rise to aberrant or overmodified proteins. Undesired changes in the structure of proteins most often accompany undesired changes in their function, such as reduced activity or the appearance of new effects. Proper protein processing is essential for the reactions in living beings and crucial for the overall quality control. Modifications that occur on proteins synthesized in the liver whose PTMs are cirrhosis-related are oxidation, nitration, glycosylation, acetylation, and ubiquitination. Some of them predominantly affect proteins that remain in liver cells, whereas others predominantly occur on proteins that leave the liver or originate from other tissues and perform their function in the circulation. Altered PTMs of certain proteins are potential candidates as biomarkers of liver-related diseases, including cirrhosis. This review will focus on PTMs on proteins whose structural changes in cirrhosis exert or are suspected to exert the most serious functional consequences.
Core Tip: Chronic liver diseases and cirrhosis are accompanied by various metabolic disorders, some of which affect proteins. Besides changes in the concentration, structural alterations of proteins occur, mostly at the level of posttranslational modifications (PTMs). Five frequent cirrhosis-related PTMs are oxidation, nitration, glycosylation, acetylation, and ubiquitination. Some are more specific for the circulating proteins, whereas others are more specific for liver tissue-residing proteins. PTMs influence folding, stability, half-life, aggregation, and function of proteins. Modified proteins with altered function contribute to further progression of liver pathology. An overview of cirrhosis-related alterations of PTMs of specific proteins is the topic of this article.
- Citation: Gligorijević N, Minić S, Nedić O. Structural changes of proteins in liver cirrhosis and consequential changes in their function. World J Gastroenterol 2022; 28(29): 3780-3792
- URL: https://www.wjgnet.com/1007-9327/full/v28/i29/3780.htm
- DOI: https://dx.doi.org/10.3748/wjg.v28.i29.3780
Liver cirrhosis is an end-stage condition of chronic liver disease[1]. The speed of progression from chronic disease to cirrhosis depends on multiple factors. Cirrhosis may develop due to toxic, infectious, immunopathological, or vascular processes. Alcoholic fatty liver disease (AFLD) and non-AFLD (NAFLD), and hepatitis B- and hepatitis C-induced diseases are the most common causes of cirrhosis, with AFLD being the most frequent.
The liver is the site of synthesis of the majority of circulating proteins. Besides initial polypeptide synthesis, sophisticated machinery is involved in the further processing of proteins by removing parts of them and/or adding functional groups and small molecules tailoring the final molecule to suit its physiological purpose. Changes that occur after formation of the polypeptide chain are known as cotranslations and posttranslational modifications (PTMs). PTMs enlarge the genome coding capacity by several orders of magnitude, designing a network of molecules with the common protein ancestor but with slightly or considerably varying activity/Localization/purpose. According to review articles of Ramazi and Zahiri[2] and Khoury et al[3], more than 400 different PTMs have been discovered to date.
When talking about PTMs, one should bear in mind the dynamic nature of the protein structure, which can be seen, among other ways, in different modifications at different moments of the protein lifespan. Each modification is expected to suit or respond to certain (patho)physiological needs of an organism. Furthermore, one protein can have more than one type of PTM simultaneously and at several molecular sites. Multiple PTMs are highly dependent on conformational and steric factors. A vast number of PTMs on proteins have been experimentally detected, but in the era of computational omics and bioinformatics, the prediction of PTMs based on the structure of proteins is an additional tool in the investigation of PTMs and their consequences on protein function.
PTMs that occur under physiological conditions regulate the proper functioning of the organism. PTMs can, however, change under pathological conditions giving rise to aberrant or overmodified proteins. Undesired changes in protein structure most often accompany undesired changes in their function. Such modifications can reduce the initial activity of proteins or their lifespan with an overall outcome experienced as a decreased effect. Other modifications can, however, contribute to the appearance of new effects; these include misfolded proteins, previously unseen interactions, unusual molecular trafficking and localization, altered gene expression, creation of neoantigens, and stimulation of the immune system (which may lead to autoimmunity), protein aggregation followed by tissue deposition, prolonged half-life, impaired clearance and initiation of additional pathological processes. Thus, PTMs are essential for the reactions in living beings and crucial for the overall quality control.
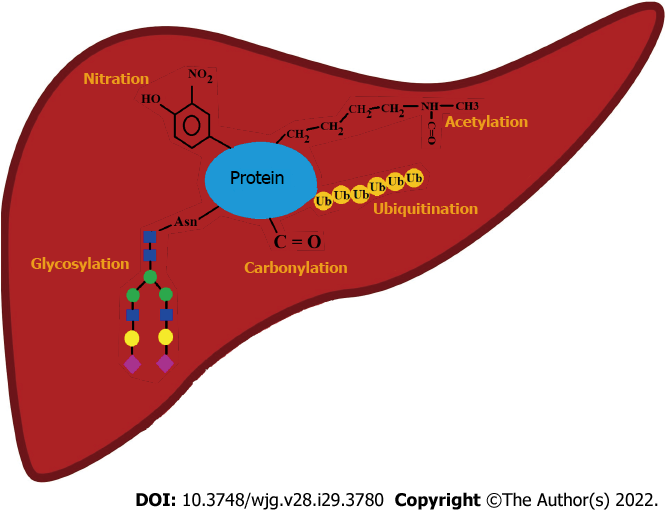
Huang et al[4] reported more than 80 modification sites in proteins that were experimentally confirmed and named 24 major PTMs. Phosphorylation of the amino acid serine (Ser) is the most frequent modification, followed by phosphorylation of other amino acids, and then acetylation and ubiquitination. Lysine (Lys) is the amino acid that can be modified in the greatest number of different ways, resulting in as many as 15 types of altered species. Modifications that occur on proteins synthesized in the liver whose PTMs were found to be cirrhosis-related (Figure 1) are oxidation, nitration, glycosylation, acetylation, and ubiquitination[5-8].
Oxidative stress plays a major role in the development of liver pathology, regardless of etiology. The liver, being a central organ of homeostasis, regulates metabolism, biosynthesis, and storage of carbohydrates, proteins, lipids, and vitamins. Due to such performances, it has intensive metabolic activity and is a site of a considerable free radical generation[9]. Any molecule possessing an unpaired electron is a free radical and is highly reactive. The most important free radicals produced by living organisms are reactive oxygen species (ROS) and reactive nitrogen species (RNS). Kupffer cells, neutrophils, and hepatocytes (mitochondria and cytochrome P450 enzymes) are involved in ROS generation during processes such as signal transduction, apoptosis, proliferation, growth, and defense against microorganisms[10]. Excessive quantities of ROS and RNS are toxic. They can induce tissue damage and are generally recognized as oxidative/nitrosative stress initiators. Cell type, intensity, and duration of the stress govern the outcome, which may be positive (beneficial) or negative (damaging). Some products of protein oxidation are chemically stable and present in large quantities, thus enabling their consideration as potential biomarkers of oxidative damage to be applied in clinical practice[11].
As mentioned before, cirrhosis usually occurs due to AFLD, NAFLD, and viral infection. The intersection point of all these conditions is oxidative stress. Alcohol in the case of AFLD, fatty acids in the case of NAFLD, and viral proteins in the case of hepatitis are the initiators of oxidative stress in these diseases. Different cellular compartments including mitochondria, cytoplasm, and endoplasmic reticulum are the sites of ROS production. Alterations in signaling pathways also play an important role in free radical induction. Detailed mechanisms underlining or assisting in oxidative stress generation in AFLD, NAFLD, and hepatitis B and C are reviewed in several papers[12-14]. Oxidation damages liver tissue by modifying proteins, DNA, and lipids. Once modified, these biomolecules gain altered structure, their function may be reduced to a different extent, their clearance rate may be increased or decreased, modified molecules may be involved in the activation of the immune system, or they alter signaling pathways contributing to further liver damage. Oxidative stress plays a role in both the initiation and progression of liver diseases[15].
Nitric oxide (NO) is a signaling molecule that can be synthesized in either enzymatically or non-enzymatically driven reactions in many cells. NO itself is not very reactive and has a short half-life. However, highly reactive peroxynitrite ion (NO3-) may form in the reaction of NO with ROS. This ion can further react with tyrosine (Tyr) residues in a process called nitration, creating 3-nitroTyr[16], and with cysteine (Cys) residues in a reaction called S-nitrosylation, creating nitrosothiols[17]. Interaction with tryptophan (Trp) also occurs[18]. These nitration modifications affect not only proteins but also DNA and lipids. If not controlled, RNS may cause progressive damage to cells, tissues, and organs. Both ROS and RNS levels increase in liver diseases, as does the activity of inducible nitric oxide synthase. Mitochondria are particularly sensitive to nitro-oxidative damage since they have lower anti-oxidative capacity than the cytoplasm[19], rendering mitochondrial proteins and DNA vulnerable to nitration. Prolonged exposure of mitochondria to nitro-oxidation deteriorates their function, affecting both mitochondrial proteins and DNA[20-22].
When immunohistochemical detection of nitroproteins was performed in liver sections from patients with cirrhosis, a significant increase in nitrated proteins was observed[23]. Changes were more significant in patients with grade C (according to Child-Pugh classification) cirrhosis than in patients with grade A or B. A positive correlation was found between the level of nitrite and the level of nitroproteins with the progression of cirrhosis.
Glycosylation is one of the major posttranslational modifications that affects most secretory proteins. This modification introduces a higher level of diversity in the protein population due to the covalent addition of specific sugar moieties. Glycosylation influences both structural and functional properties of proteins. The overall glycome is affected by genetic and environmental factors, and changes may suggest the presence of inflammatory or other pathological events in the organism[24]. Glycosylation is an enzymatically regulated process that depends on several glycosyltransferases and glycosidases. Many factors including the activity of enzymes involved, substrate availability, and localization of enzymes within organelles affect the final glycosylation pattern of the protein. Unlike the genome and proteome, glycome is produced without a template. In the O-glycosylation of proteins, glycan attachment occurs at amino acid residues Ser and threonine (Thr). N-glycosylation involves asparagine (Asn) residues but only in a specific sequence, Asn-X-Ser/Thr, where X represents any amino acid except proline[25].
Several liver diseases are accompanied by alterations in protein glycosylation: NAFLD, liver fibrosis, cirrhosis, and hepatocellular carcinoma (HCC)[7,26-28]. A number of common glycosylation changes were noted as a general pattern in liver diseases, regardless of the disease type. They include increased fucosylation (Fuc), increased branching, and an appearance of a bisecting N-acetylglucosamine (GlcNAc). Additional analyses of the responsible glycosyltransferases documented that their levels were altered, underlining the mechanism of detected changes[7]. When the overall N-glycan structure was examined in immunoglobulin (Ig)G-deprived sera from patients with cirrhosis, two general trends emerged: an increase in bisecting GlcNAc structures and a decrease in fully galactosylated (Gal) bi- and triantennary N-glycans. The ratios of certain glycans were suggested to serve as potential biomarkers of a specific stage of liver fibrosis or cirrhosis[27]. Data reported in that study confirmed the correlation between the detected glycan changes and the levels of the corresponding glycosyltransferases.
Acetylation is one of the key PTMs involved in the regulation of many proteins. It influences their stability, activity, localization, and interactions with other proteins. Metabolic pathways, including fatty acid metabolism and the Krebs cycle, are regulated by protein acetylation. This modification assumes the addition of an acetyl group from acetyl-coenzyme A on Lys residues[29]. Acetylation seems to play a significant role in liver diseases, as the pattern of protein acetylation in fatty liver is significantly different from the pattern in the healthy liver[30].
Ubiquitin and its related pathways are closely connected to chronic liver disease. Dysfunctional ubiquitination was detected in liver tissue in different stages of chronic liver disease. Ubiquitin is a protein that consists of 76 amino acids and has seven Lys and N-terminal methionine (Met) residues that can form iso-peptide-linked ubiquitin chains. It binds to substrates by a three-step enzymatic mechanism involving activating enzyme (E1), conjugating enzyme (E2) and ligase (E3)[31]. Receptors with ubiquitin-binding domains can recognize ubiquitinated substrates[32]. On the other hand, deubiquitinases remove ubiquitin from modified molecules, making this PTM a reversible, dynamic process with a plethora of diverse cellular effects[33]. Ubiquitinated proteins may be taken by proteasome which destroys them, thus controlling their lifespan and activity.
Some of the aforementioned PTMs predominantly affect proteins that remain in liver cells (ubiquitination, acetylation, and nitration), whereas others predominantly occur on proteins that leave the liver and exert their functions in the circulation (oxidation and glycosylation). Additionally, there are proteins not originating from the liver whose structure changes in cirrhosis. Some of these modifications have the potential to serve as biomarkers of liver-related diseases.
This review mostly focuses on PTMs on the circulating proteins originating from the liver whose structural changes in cirrhosis exert the most serious functional consequences. Modifications of Igs and liver tissue proteins will be also briefly mentioned.
Fibrinogen is a large, fibrillar glycoprotein with a molecular mass of 340 kDa. This protein is involved both in primary (interaction with platelets) and secondary hemostasis (fibrin formation). N-glycosylation analysis using lectin microarray with 15 Lectins demonstrated that cirrhosis induces changes in its glycans[34]. Based on the interactions with lectins Pholiota squarrosa (PhoSL), Maackia amurensis lectin I (MAL-I), Maackia amurensis lectin II (MAL-II), Galanthus nivalis lectin (GNL), Phaseolus vulgaris leucoagglutinin (PHA-L), and Phaseolus vulgaris lectin E (PHA-E), an increased content of the following carbohydrate moieties was observed: terminal α-2,3 sialic acid (Sia) and α-1,3 mannose (Man), a disaccharide composed of Gal and N-acetyl galactosamine (Galβ-1,4GlcNAc) and tri/tetra-antennary N-glucan structures. Core α-1,6 (Fuc) and bi-antennary galactosylated N-glycans with the bisecting GlcNAc were, on the other hand, reduced. An increase in the number of Sia residues on fibrinogen as a consequence of cirrhosis was confirmed by several researchers[35,36]. Sialic acid is negatively charged and is a weak binding site for calcium ions[37]. Taking into consideration that advanced chronic liver disease is characterized by hypercalcemia[38], interactions between calcium ions and an increased number of Sia residues may be seen as a modulator of fibrinogen action in cirrhosis[37]. “Proper” glycosylation of fibrinogen is essential for its function. Alterations in this PTM may even lead to complete dysfunction of the protein[39-42].
Increased oxidation of fibrinogen from patients with cirrhosis was detected as well[34,43]. Dinitrophenyl-hydrazide (DNP)-reactive sites on fibrinogen were investigated using anti-DNP antibodies to analyze protein carbonyls and it was discovered that the Aα-chain was dominantly carbonylated, followed by the Bβ-chain. It seems that the γ-chain of fibrinogen is not carbonylated in patients with cirrhosis. Carbonylation affects the function of fibrinogen[44-46], as both Aα- and Bβ-chains contain cleavage sites for thrombin action. Oxidation of αC domains on Aα-chains also affects their mutual interaction and lateral association of fibrin monomers[47]. Generally, residues that may be affected by oxidation/carbonylation are located in all structural elements of fibrinogen. Thus, alterations in the secondary and tertiary structures of fibrinogen also accompany cirrhosis[34]. Reduced content of its α-helical subunits was observed, which coincided with the formation of denser clots[46]. Although the structure of fibrinogen is altered in cirrhosis, Hugenholtz et al[43] did not detect significant structural changes in fibrin clots, even though clots formed from samples originating from patients with cirrhosis had reduced porosity. Subtle alterations, not visible by scanning electron microscopy, are most likely sufficient to influence porosity. Furthermore, carbonylated sites on fibrinogen are hydrophobic regions which prevent efficient liquid flow through the fibrin clot, increasing its resistance towards lysis by plasmin[36]. Overall, changes in fibrinogen structure due to cirrhosis are complex and lead to the formation of a molecule acquiring thrombogenic characteristics. Other factors that participate in coagulation or fibrinolysis are altered as well, defining a general pro-coagulant state in liver cirrhosis[48].
Albumin is the major plasma protein, constituting approximately 50% of the total protein content. It is a globular, single-chain, α-helicoidal protein, organized into three domains. This protein exhibits many physiological roles, such as maintenance of osmotic pressure and transport of various metal ions and biomolecules (fatty acids, metabolites, and drugs), providing anti-oxidative, anti-inflammatory, and hemostatic activities[49].
Cirrhosis is accompanied by reduced albumin concentration and its significant structural changes[49]. Oxidation of free Cys-34 residue is one of the most notable changes in albumin structure due to cirrhosis. This residue strongly contributes to the anti-oxidative capacity of albumin. Oxidized albumin differs from the native molecule pharmacokinetically and conformationally, negatively influencing its function[50]. Quantities of albumin oxidative forms, assessed by measuring the level of carbonyl groups and oxidation of free Cys-34 residue, correlate with the severity of liver failure[51]. Oxidative stress triggers the dimerization of albumin molecules (through free Cys residues) in patients with cirrhosis, with significant reduction in native albumin required for the physiological functions[52]. Furthermore, matrix-assisted laser desorption/ionization time of flight (MALDI-TOF) mass spectrometry (MS) detects cysteinylation of Cys-34 in cirrhosis, followed by other molecular changes, such as truncation of N-terminal portion and glycosylation[53]. Oxidative stress also induces structural changes of the N-terminus and the reduced capacity of albumin to bind cobalt ions. Albumin modified in that way is called ischemia-modified albumin (IMA)[50]. The cobalt-binding assay revealed increased IMA levels in patients with advanced cirrhosis[54]. Additionally, the electron paramagnetic resonance study, using 16-doxyl stearic acid as a spin probe, confirmed that the ligand-binding capacity of albumin is significantly reduced in cirrhosis[55]. A small-angle X-ray scattering study demonstrated that albumin in patients with liver disease has an altered, more open conformation compared to control samples[56]. To summarize, posttranslational alternations of albumin in cirrhosis influence its conformation, which further decreases its ligand binding.
Therefore, reduced level of serum albumin in patients with cirrhosis, as well as its substantial structural change, have important and clinically relevant consequences, such as alterations in the redox balance, hemostatic disorders, modifications in the transport of endogenous and exogenous ligands, acid-base imbalance, reduced detoxification capacity, and antioxidant activity[49].
Human transferrin (Tf), an iron-binding protein, is a 76-kDa glycoprotein produced in the liver[57,58]. The Tf molecule contains two lobes at the N- and C-termini, and each binds one Fe3+ ion[58]. Tf is stabilized by 19 intrachain disulfide bonds and is modified by three carbohydrate side chains; two of them are N-linked (Asn-413 and Asn-611) and the third one is O-linked (Ser-32)[57]. The glycosylation profile of Tf changes significantly in liver diseases. A carbohydrate-deficient Tf (CDT) is found in AFLD, and it is characterized by a loss of terminal sialic acids[59]. Lectin affinity electrophoresis of CDT also confirmed the absence of Asn-linked oligosaccharides in AFLD and cirrhosis[60]. Both Tf and iron uptake capacity of deglycosylated Tf are significantly reduced by the human hepatoma cell line PLC/PRF/5[61], suggesting impaired function of Tf in liver cirrhosis. A less advanced liver disease can be discriminated from cirrhosis by studying disialotransferrin isoforms. Poor chromatographic resolution of disialotransferrin from trisialotransferrin (the so-called “di-tri bridging”) was seen for samples originating from patients with cirrhosis[62,63]. This abnormal pattern could be ascribed to the presence of higher mass disialotransferrin isoforms due to an increased branching and fucosylation of the carbohydrate moiety[64]. This phenomenon was not seen in less-advanced liver diseases[63].
Besides changes in the glycosylation profile, the lower concentration of Tf is also related to cirrhosis, and it represents a good indicator of the survival rate of patients[65]. Lower serum Tf concentration is accompanied by higher hepatic iron concentration and lipid peroxidation levels compared to healthy subjects. In this context, Tf exerts a protective role in maintaining liver function[66].
Hemopexin (HPX) is secreted mainly by hepatocytes and binds free heme in the circulation. The formed complex is cleared from the circulation by a hepatocyte-specific membrane receptor. The serum concentration of HPX does not significantly vary in liver diseases[67]. However, significant alterations in the glycosylation profile of HPX occur in patients with cirrhosis. Lectin chemiluminescence-linked immunosorbent assay demonstrated a higher level of N-glycosylation (fucosylation) in samples from patients with cirrhosis and HCC than in samples from patients with hepatitis and healthy individuals[68]. A study using liquid chromatography-tandem MS with multiple reaction monitoring (LC-MS/MS MRM) assay revealed a nearly five-fold increase in the sialylation of site-specific Oglycoforms of HPX in cirrhosis[67]. Since HPX is an important anti-oxidative protein, its altered N-glycosylation in cirrhosis possibly interferes with the redox balance in organisms.
Haptoglobin (Hp) is a glycoprotein secreted by the liver into the plasma. Its major biological role is to capture released hemoglobin during intravascular hemolysis and prevent kidney damage by the released iron[69]. Hp is composed of two α and two β chains linked by disulfide bonds in a quaternary structure[70,71]. All four N-glycosylation sites are located in the β subunit and glycoforms are known to create additional phenotypic variants[69,72]. Many studies have reported glycan changes of Hp in diseases. MALDI-TOF MS analyses revealed N-linked glycan alterations (increased fucosylation) of serum Hp β chain in patients with cirrhosis[69,71]. Zhu et al[73] applied a similar approach to distinguish the N-glycan profile of Hp in patients with cirrhosis from those with hypophosphatasia. A degree of bifucosylation was higher in samples from patients with an early-stage HCC than in samples from patients with cirrhosis, regardless of the etiology. Thus, monitoring alterations in the glycosylation profile of the Hp β chain may become a valuable approach for detection and distinction between HCC and cirrhosis. The observed changes of Hp at the N-glycosylation level most likely affect its functional properties, including interactions with binding partners, as was suggested in the case of progressive liver diseases[69].
Ferritin plays an important role in storing intracellular iron and its segregation in a non-toxic form[74,75]. It is a 24-mer globular protein that is made up of heavy (H) and light (L) subunits, with molecular masses of 21 kDa and 19 kDa, respectively[74]. Subunits surround the central hollow core, capable of binding up to 4500 iron ions[76]. Ferritin concentration in the circulation is relatively low (< 1 μg/mL)[74]. However, it is increased in conditions such as iron overload, infection, inflammation, malignancy, diabetes and liver diseases, including NAFLD and cirrhosis[77]. Besides changes in ferritin concentration, liver cirrhosis is also accompanied by the derangement of the ordered secondary structure of the protein[78].
Approximately 50%-80% of serum ferritin is glycosylated[79]. There is no strong evidence of the connection between ferritin glycosylation and cirrhosis. Some studies have reported a decrease in glycosylated ferritin in liver necrosis[79]. On the other hand, Chapman et al[80] demonstrated that the measurement of the fraction of glycosylated serum ferritin does not provide any advantage over the estimation of the total serum ferritin concentration in the assessment of iron stores in patients with liver cirrhosis.
Insulin-like growth factors (IGFs), namely IGF-I and IGF-II, are peptides that exert growth-promoting, endocrine and cytokine effects[81,82]. They are synthesized in many tissues locally, but the liver is the origin of IGFs that enter the circulation. IGF-I is the mediator of the growth hormone (GH) action. The activity of IGFs is regulated by a network of insulin-like growth factor binding proteins (IGFBPs) and is most often inhibited when IGFs are in complexes with IGFBPs. To perform their physiological roles, IGFs need to be released from complexes and interact with IGF receptors, which are predominantly found on cell membranes. IGFs are liberated from complexes by the proteolysis of IGFBPs. The synthesis of several IGFBPs occurs in the liver, under the control of GH. IGFBP-3 is the major binding protein in the circulation and is synthesized in Kupffer cells. It forms ternary complexes with IGFs, which also contain an acid-labile subunit. These complexes are large (150 kDa), remain within blood vessels, and bind 75%-90% of the circulating IGFs, serving both as a reservoir and a guardian of the IGF activity.
IGFBP-3 has three N-glycosylation and two phosphorylation sites[83,84]. It is present in the circulation as two major glycoforms of 40 kDa and 44 kDa, although a non-glycosylated form (29 kDa) can also be detected. Diethylaminoethyl ion-exchange chromatography was shown to fractionate at least 12 IGFBP-3 species that are isoforms with different charges due to PTMs[85]. Three of these isoforms exhibited significant reactivity with lectin concanavalin A (Con A), specific for Man residues and to a lesser extent for glucose (Glc) and GlcNAc residues. In patients with alcoholic liver cirrhosis, two of these glycoforms had an increased reactivity with Con A, whereas the third one had decreased reactivity, compared to molecules originating from healthy persons. Furthermore, some differences were also detected between different stages of liver cirrhosis. IGFBP-3 from patients with Child score A stage exhibited similar isoform distribution and reactivity as in healthy persons. Child score B seems to be the turning point in the progression of cirrhosis, after which considerable changes in the concentration of IGFBP-3 and its structure occur. A reduced reactivity of IGFBP-3 due to cirrhosis was also noted with wheat germ agglutinin, specific for GlcNAc, and breadfruit lectin, specific for Gal and GalNAc residues[86]. These alterations affect the conformation of IGFBP-3 and its susceptibility to proteolytic cleavage, thus influencing its half-life and the entire mechanism that controls IGFs' activity. Since IGFBP-3 can also perform IGF-independent roles after binding to its cell surface or nuclear receptors, changes in IGFBP-3 glycosylation can contribute to the pathophysiology of several diseases such as diabetes, obesity, NAFLD, and cancer[87,88].
Sex hormone-binding globulin (SHBG) is a 90-kDa to 100-kDa homodimeric glycoprotein, mainly produced by the liver[89]. It is a transporter of sex hormones, capable of binding to them with high affinity[90]. SHBG is both N- and mucin-type O-glycosylated[90,91]. Comprehensive LC-MS/MS analysis revealed that fucosylation of N-glycoforms increases in liver cirrhosis. Additionally, the same pathology was related to an increase of the α-2−6 sialylated glycoform of the O-glycopeptide of SHBG[67]. Glycosylation of SHBG does not seem to influence binding of steroid hormones[92]. However, it is suspected that higher content of sialic acid increases the half-life of SHBG[93], elevating the total concentration of this protein in patients with cirrhosis[94]. An increased concentration of SHBG may influence the equilibrium between protein-bound and free, physiologically active steroid hormones, particularly testosterone. Consequently, SHBG seems to play an important role in the occurrence of feminization in male non-alcoholic liver cirrhosis by reducing free testosterone level[95]. On the other hand, higher concentrations of this protein were reported to possibly exert protection against NAFLD[96]. The exact effect of altered glycosylation of SHBG on is function is still not known.
Igs are principle components of the defense system known as immunity. Although Ig are not synthetized in the liver, their aberrant glycosylation has been linked to various liver diseases[97]. A study combining LC-MS/MS analysis with lectin fluorophore-linked immunosorbent assay identified changes in the glycosylation of anti-Gal IgG molecules in the sera of hepatitis C virus-infected individuals with fibrosis and cirrhosis[98]. The most prominent change was agalactosylation of heavy chains of anti-Gal IgG. The same study also revealed that truncation of Gal residues induced alterations in the tertiary structure of IgG molecules originating from patients with cirrhosis[98]. Yuan et al[97] confirmed agalactosylation of IgG molecules in cirrhosis and reported an increased degree of fucosylation in IgG1 and IgG3 glycoforms. Cirrhosis is also accompanied by Gal deficiency and decreased sialylation of IgA molecules, as well as by an increased amounts of abnormally glycosylated polymeric IgA molecules[99]. Furthermore, concentrations of IgG and IgA are increased in the circulation of patients with cirrhosis[97]. Altered glycosylation could influence the ability of IgG to bind and activate complement system. It was discovered that alpha-Gal IgG antibodies from patients with cirrhosis have reduced complement-mediated killing ability[100]. This issue is important since bacterial infection in one of the major complications in patients with cirrhosis and alpha-Gal epitope is abundantly synthetized by bacteria.
Proteins remaining in liver cells are mostly modified by ubiquitination, nitration and acetylation. Ubiquitination and associated processes play important roles in the development of cirrhosis affecting many proteins. E3 ubiquitin ligase promotes, for example, NF-E2-related factor 2 ubiquitination and degradation, disrupting the anti-oxidative pathway. The same ligase promotes the accumulation of extracellular matrix by inducing ubiquitination of procollagen1 to mature collagen1[101,102]. Sumoylation and neddylation, ubiquitin-like modifications, also play roles in liver cirrhosis. It was discovered that in vivo reduction of neddylation ameliorates liver fibrosis[103]. Sumoylation affects, for example, promyelocytic leukemia protein and nuclear factor-kappa B (NF-kB)[104]. Modification of these proteins leads to cell proliferation and fibrosis in the liver. A detailed overview of ubiquitination and its implications in chronic liver disease is given in the 2021 review paper of Park et al[33].
Transcription factors, sterol regulatory element-binding transcription factor (SREBP), and carbohydrate-response element-binding protein (ChREBP) regulate fatty acid metabolism in the liver, promoting lipogenesis. These factors are active when acetylated. Sirtuins 1 and 3 (SIRT1 and SIRT3) are deacetylases that regulate their activity. High-fat diet and obesity reduce the expression of SIRT 1 and SIRT 3, thus promoting acetylation and activation of ChREBP and SREBP, followed by an increase in the uptake of fatty acids by the liver. Liver lipid load induces inflammation and the NF-kappa B pathway, and reduces mitophagy, altogether leading to mitochondrial and liver damage and advancement towards NAFLD. Calorie restriction and exercise upregulate SIRT1 and SIRT3, thus, preventing and ameliorating NAFLD[29]. ATP-citrate lyase, microtubules, heat shock protein 90 and CCAAT/enhancer binding protein α are differently modified by acetylation in different liver diseases preceding cirrhosis. Aberrant acetylation of the mentioned proteins leads to their functional alterations and subsequently different metabolic responses, further contributing to the liver pathology[105-108].
There are many hepatic proteins with confirmed nitration status in liver diseases, whose functions are either augmented or reduced[109]. Decreased function of glutamine synthetase, 3-ketoacyl-CoA thiolase, aldehyde dehydrogenase 2, complexes I and V of oxidative phosphorylation, cytochrome p450 2E1 and B6, superoxide dismutase 1 and 2, and cluster of differentiation 95 contribute to decreased energy production, ROS leakage, steatosis, decreased anti-oxidant defense capacity, ethanol- and drug-induced toxicity, apoptosis and necrosis. Nitration of glutathione-S-transferase, however, potentiates the function of this enzyme, leading to an increased hepatic anti-oxidative defense capacity[110].
Liver disease can progress from mild damage, over moderate with a certain degree of compensation, to severe, which cannot be compensated and may lead to organ failure. While still able to compensate for tissue damage, the liver produces scars of fibrotic tissue, which reduce its function. The persistent presence of agents and/or events that cause liver damage leads to decompensated cirrhosis, resulting in several pathological outcomes, including liver cancer, and finally, functional arrest.
Early diagnosis of chronic liver disease is critical since the etiology of the disease can be discovered in this stage. When cirrhosis progresses, the etiology of a disease is very hard to determine. Appropriate treatment administered on time can prevent and reverse the progression of cirrhosis which leads to irreversible changes. Avoiding or minimizing contributing harmful factors is recommended[1]. For example, persons with chronic liver disease should avoid alcohol intake and smoking[111]. On the other hand, consumption of coffee is associated with a slower progression of liver fibrosis[112]. There are no specific curative strategies targeting alterations in PTMs in cirrhosis.
Since oxidative stress is one of the causes involved in the etiology and development of liver diseases, supplementation of vitamins and minerals, which act as anti-oxidants and/or cofactors of enzymes and other molecules that participate in anti-oxidant defense, may be recommended. However, one should bear in mind that some substances are stored in the liver and may act as pro-disease agents[113]. For example, lipophilic vitamin A is stored in liver stellate cells. If overloaded with vitamin A, these cells start to produce collagen, leading to liver fibrosis. The beta-carotene form, however, causes no such effects and is safe to consume. Iron ions can induce oxidative stress by participation in the generation of free radicals. As already said, the liver is the site of iron storage, in association with the protein ferritin. Patients with alcoholic liver cirrhosis often have an increased intrahepatic iron concentration, which is highly correlated with mortality rates[114]. Thus, when considering management strategies to treat cirrhosis, one should have in mind the complexity and limitations of interconnected metabolic pathways.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Biochemistry and molecular biology
Country/Territory of origin: Serbia
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Costache RS, Romania; El-Shabrawi MH, Egypt; Pan Y, China S-Editor: Wu YXJ L-Editor: A P-Editor: Wu YXJ
| 1. | Wiegand J, Berg T. The etiology, diagnosis and prevention of liver cirrhosis: part 1 of a series on liver cirrhosis. Dtsch Arztebl Int. 2013;110:85-91. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 80] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 2. | Ramazi S, Zahiri J. Posttranslational modifications in proteins: resources, tools and prediction methods. Database (Oxford). 2021;2021. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 411] [Article Influence: 102.8] [Reference Citation Analysis (0)] |
| 3. | Khoury GA, Baliban RC, Floudas CA. Proteome-wide post-translational modification statistics: frequency analysis and curation of the swiss-prot database. Sci Rep. 2011;1. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 556] [Cited by in RCA: 651] [Article Influence: 46.5] [Reference Citation Analysis (0)] |
| 4. | Huang KY, Lee TY, Kao HJ, Ma CT, Lee CC, Lin TH, Chang WC, Huang HD. dbPTM in 2019: exploring disease association and cross-talk of post-translational modifications. Nucleic Acids Res. 2019;47:D298-D308. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 124] [Cited by in RCA: 159] [Article Influence: 31.8] [Reference Citation Analysis (0)] |
| 5. | Osna NA, Carter WG, Ganesan M, Kirpich IA, McClain CJ, Petersen DR, Shearn CT, Tomasi ML, Kharbanda KK. Aberrant post-translational protein modifications in the pathogenesis of alcohol-induced liver injury. World J Gastroenterol. 2016;22:6192-6200. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 26] [Cited by in RCA: 18] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 6. | Karve TM, Cheema AK. Small changes huge impact: the role of protein posttranslational modifications in cellular homeostasis and disease. J Amino Acids. 2011;2011:207691. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 197] [Cited by in RCA: 246] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 7. | Ramachandran P, Xu G, Huang HH, Rice R, Zhou B, Lindpaintner K, Serie D. Serum Glycoprotein Markers in Nonalcoholic Steatohepatitis and Hepatocellular Carcinoma. J Proteome Res. 2022;21:1083-1094. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 20] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 8. | Lachiondo-Ortega S, Mercado-Gómez M, Serrano-Maciá M, Lopitz-Otsoa F, Salas-Villalobos TB, Varela-Rey M, Delgado TC, Martínez-Chantar ML. Ubiquitin-Like Post-Translational Modifications (Ubl-PTMs): Small Peptides with Huge Impact in Liver Fibrosis. Cells. 2019;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 9. | Arauz J, Ramos-Tovar E, Muriel P. Redox state and methods to evaluate oxidative stress in liver damage: From bench to bedside. Ann Hepatol. 2016;15:160-173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 49] [Reference Citation Analysis (0)] |
| 10. | Taub R. Liver regeneration: from myth to mechanism. Nat Rev Mol Cell Biol. 2004;5:836-847. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1091] [Cited by in RCA: 1175] [Article Influence: 56.0] [Reference Citation Analysis (0)] |
| 11. | Kehm R, Baldensperger T, Raupbach J, Höhn A. Protein oxidation - Formation mechanisms, detection and relevance as biomarkers in human diseases. Redox Biol. 2021;42:101901. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 77] [Cited by in RCA: 146] [Article Influence: 36.5] [Reference Citation Analysis (0)] |
| 12. | Galicia-Moreno M, Gutiérrez-Reyes G. The role of oxidative stress in the development of alcoholic liver disease. Rev Gastroenterol Mex. 2014;79:135-144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 58] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 13. | Ivanov AV, Valuev-Elliston VT, Tyurina DA, Ivanova ON, Kochetkov SN, Bartosch B, Isaguliants MG. Oxidative stress, a trigger of hepatitis C and B virus-induced liver carcinogenesis. Oncotarget. 2017;8:3895-3932. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 136] [Cited by in RCA: 120] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 14. | Masarone M, Rosato V, Dallio M, Gravina AG, Aglitti A, Loguercio C, Federico A, Persico M. Role of Oxidative Stress in Pathophysiology of Nonalcoholic Fatty Liver Disease. Oxid Med Cell Longev. 2018;2018:9547613. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 496] [Cited by in RCA: 480] [Article Influence: 68.6] [Reference Citation Analysis (0)] |
| 15. | Li S, Tan HY, Wang N, Zhang ZJ, Lao L, Wong CW, Feng Y. The Role of Oxidative Stress and Antioxidants in Liver Diseases. Int J Mol Sci. 2015;16:26087-26124. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1267] [Cited by in RCA: 1088] [Article Influence: 108.8] [Reference Citation Analysis (1)] |
| 16. | Radi R. Protein tyrosine nitration: biochemical mechanisms and structural basis of functional effects. Acc Chem Res. 2013;46:550-559. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 341] [Cited by in RCA: 379] [Article Influence: 31.6] [Reference Citation Analysis (0)] |
| 17. | Lamotte O, Bertoldo JB, Besson-Bard A, Rosnoblet C, Aimé S, Hichami S, Terenzi H, Wendehenne D. Protein S-nitrosylation: specificity and identification strategies in plants. Front Chem. 2014;2:114. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 59] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 18. | Nuriel T, Hansler A, Gross SS. Protein nitrotryptophan: formation, significance and identification. J Proteomics. 2011;74:2300-2312. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 59] [Cited by in RCA: 49] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 19. | Fernández-Checa JC, Kaplowitz N, García-Ruiz C, Colell A. Mitochondrial glutathione: importance and transport. Semin Liver Dis. 1998;18:389-401. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 163] [Cited by in RCA: 158] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 20. | Campello S, Scorrano L. Mitochondrial shape changes: orchestrating cell pathophysiology. EMBO Rep. 2010;11:678-684. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 243] [Cited by in RCA: 223] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 21. | Mansouri A, Fromenty B, Berson A, Robin MA, Grimbert S, Beaugrand M, Erlinger S, Pessayre D. Multiple hepatic mitochondrial DNA deletions suggest premature oxidative aging in alcoholic patients. J Hepatol. 1997;27:96-102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 110] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 22. | Mansouri A, Gaou I, De Kerguenec C, Amsellem S, Haouzi D, Berson A, Moreau A, Feldmann G, Lettéron P, Pessayre D, Fromenty B. An alcoholic binge causes massive degradation of hepatic mitochondrial DNA in mice. Gastroenterology. 1999;117:181-190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 139] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 23. | Annie-Jeyachristy S, Geetha A, Surendran R, Sundaram A, Lavanya K, Kumar SJ, Prakash SA. Level of nitrated proteins in the plasma, platelets and liver of patients with liver cirrhosis. Redox Rep. 2009;14:259-266. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 24. | Verhelst X, Dias AM, Colombel JF, Vermeire S, Van Vlierberghe H, Callewaert N, Pinho SS. Protein Glycosylation as a Diagnostic and Prognostic Marker of Chronic Inflammatory Gastrointestinal and Liver Diseases. Gastroenterology. 2020;158:95-110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 105] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 25. | Reily C, Stewart TJ, Renfrow MB, Novak J. Glycosylation in health and disease. Nat Rev Nephrol. 2019;15:346-366. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1352] [Cited by in RCA: 1353] [Article Influence: 225.5] [Reference Citation Analysis (0)] |
| 26. | Vanderschaeghe D, Laroy W, Sablon E, Halfon P, Van Hecke A, Delanghe J, Callewaert N. GlycoFibroTest is a highly performant liver fibrosis biomarker derived from DNA sequencer-based serum protein glycomics. Mol Cell Proteomics. 2009;8:986-994. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 100] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 27. | Callewaert N, Van Vlierberghe H, Van Hecke A, Laroy W, Delanghe J, Contreras R. Noninvasive diagnosis of liver cirrhosis using DNA sequencer-based total serum protein glycomics. Nat Med. 2004;10:429-434. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 336] [Cited by in RCA: 322] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 28. | Malaguarnera G, Bertino G, Vacante M, Malaguarnera M. Hepatocellular carcinoma markers in the omics era: the glycomic analysis. Hepatobiliary Surg Nutr. 2014;3:407-409. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 29. | Nassir F. Role of acetylation in nonalcoholic fatty liver disease: a focus on SIRT1 and SIRT3. Explor Med 2020; 1: 248-258. . [RCA] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 30. | Le-Tian Z, Cheng-Zhang H, Xuan Z, Zhang Q, Zhen-Gui Y, Qing-Qing W, Sheng-Xuan W, Zhong-Jin X, Ran-Ran L, Ting-Jun L, Zhong-Qu S, Zhong-Hua W, Ke-Rong S. Protein acetylation in mitochondria plays critical functions in the pathogenesis of fatty liver disease. BMC Genomics. 2020;21:435. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 24] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 31. | Ciechanover A. The unravelling of the ubiquitin system. Nat Rev Mol Cell Biol. 2015;16:322-324. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 221] [Article Influence: 22.1] [Reference Citation Analysis (0)] |
| 32. | Dikic I, Wakatsuki S, Walters KJ. Ubiquitin-binding domains - from structures to functions. Nat Rev Mol Cell Biol. 2009;10:659-671. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 608] [Cited by in RCA: 678] [Article Influence: 42.4] [Reference Citation Analysis (0)] |
| 33. | Park JS, Ma H, Roh YS. Ubiquitin pathways regulate the pathogenesis of chronic liver disease. Biochem Pharmacol. 2021;193:114764. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 25] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 34. | Gligorijević N, Minić S, Križáková M, Katrlík J, Nedić O. Structural changes of fibrinogen as a consequence of cirrhosis. Thromb Res. 2018;166:43-49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 12] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 35. | Martinez J, Palascak JE, Kwasniak D. Abnormal sialic acid content of the dysfibrinogenemia associated with liver disease. J Clin Invest. 1978;61:535-538. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 81] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 36. | Lisman T, Ariëns RA. Alterations in Fibrin Structure in Patients with Liver Diseases. Semin Thromb Hemost. 2016;42:389-396. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 52] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 37. | Dang CV, Shin CK, Bell WR, Nagaswami C, Weisel JW. Fibrinogen sialic acid residues are low affinity calcium-binding sites that influence fibrin assembly. J Biol Chem. 1989;264:15104-15108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 51] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 38. | Kuchay MS, Mishra SK, Farooqui KJ, Bansal B, Wasir JS, Mithal A. Hypercalcemia of advanced chronic liver disease: a forgotten clinical entity! Clin Cases Miner Bone Metab. 2016;13:15-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 6] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 39. | Marchi R, Arocha-Piñango CL, Nagy H, Matsuda M, Weisel JW. The effects of additional carbohydrate in the coiled-coil region of fibrinogen on polymerization and clot structure and properties: characterization of the homozygous and heterozygous forms of fibrinogen Lima (Aalpha Arg141-->Ser with extra glycosylation). J Thromb Haemost. 2004;2:940-948. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 40. | Maekawa H, Yamazumi K, Muramatsu S, Kaneko M, Hirata H, Takahashi N, Arocha-Piñango CL, Rodriguez S, Nagy H, Perez-Requejo JL. Fibrinogen Lima: a homozygous dysfibrinogen with an A alpha-arginine-141 to serine substitution associated with extra N-glycosylation at A alpha-asparagine-139. Impaired fibrin gel formation but normal fibrin-facilitated plasminogen activation catalyzed by tissue-type plasminogen activator. J Clin Invest. 1992;90:67-76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 32] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 41. | Yamazumi K, Shimura K, Terukina S, Takahashi N, Matsuda M. A gamma methionine-310 to threonine substitution and consequent N-glycosylation at gamma asparagine-308 identified in a congenital dysfibrinogenemia associated with posttraumatic bleeding, fibrinogen Asahi. J Clin Invest. 1989;83:1590-1597. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 39] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 42. | Sugo T, Nakamikawa C, Takano H, Mimuro J, Yamaguchi S, Mosesson MW, Meh DA, DiOrio JP, Takahashi N, Takahashi H, Nagai K, Matsuda M. Fibrinogen Niigata with impaired fibrin assembly: an inherited dysfibrinogen with a Bbeta Asn-160 to Ser substitution associated with extra glycosylation at Bbeta Asn-158. Blood. 1999;94:3806-3813. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 32] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 43. | Hugenholtz GC, Macrae F, Adelmeijer J, Dulfer S, Porte RJ, Lisman T, Ariëns RA. Procoagulant changes in fibrin clot structure in patients with cirrhosis are associated with oxidative modifications of fibrinogen. J Thromb Haemost. 2016;14:1054-1066. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 97] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 44. | Martinez M, Weisel JW, Ischiropoulos H. Functional impact of oxidative posttranslational modifications on fibrinogen and fibrin clots. Free Radic Biol Med. 2013;65:411-418. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 76] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 45. | Xu YJ, Qiang M, Zhang JL, Liu Y, He RQ. Reactive carbonyl compounds (RCCs) cause aggregation and dysfunction of fibrinogen. Protein Cell. 2012;3:627-640. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 46. | Becatti M, Marcucci R, Bruschi G, Taddei N, Bani D, Gori AM, Giusti B, Gensini GF, Abbate R, Fiorillo C. Oxidative modification of fibrinogen is associated with altered function and structure in the subacute phase of myocardial infarction. Arterioscler Thromb Vasc Biol. 2014;34:1355-1361. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 83] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 47. | Rosenfeld MA, Vasilyeva AD, Yurina LV, Bychkova AV. Oxidation of proteins: is it a programmed process? Free Radic Res. 2018;52:14-38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 31] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 48. | Zermatten MG, Fraga M, Moradpour D, Bertaggia Calderara D, Aliotta A, Stirnimann G, De Gottardi A, Alberio L. Hemostatic Alterations in Patients With Cirrhosis: From Primary Hemostasis to Fibrinolysis. Hepatology. 2020;71:2135-2148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 57] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 49. | Bernardi M, Ricci CS, Zaccherini G. Role of human albumin in the management of complications of liver cirrhosis. J Clin Exp Hepatol. 2014;4:302-311. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 77] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 50. | Carvalho JR, Verdelho Machado M. New Insights About Albumin and Liver Disease. Ann Hepatol. 2018;17:547-560. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 131] [Article Influence: 18.7] [Reference Citation Analysis (0)] |
| 51. | Oettl K, Stadlbauer V, Petter F, Greilberger J, Putz-Bankuti C, Hallström S, Lackner C, Stauber RE. Oxidative damage of albumin in advanced liver disease. Biochim Biophys Acta. 2008;1782:469-473. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 113] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 52. | Baldassarre M, Domenicali M, Naldi M, Laggetta M, Giannone FA, Biselli M, Patrono D, Bertucci C, Bernardi M, Caraceni P. Albumin Homodimers in Patients with Cirrhosis: Clinical and Prognostic Relevance of a Novel Identified Structural Alteration of the Molecule. Sci Rep. 2016;6:35987. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 31] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 53. | Domenicali M, Baldassarre M, Giannone FA, Naldi M, Mastroroberto M, Biselli M, Laggetta M, Patrono D, Bertucci C, Bernardi M, Caraceni P. Posttranscriptional changes of serum albumin: clinical and prognostic significance in hospitalized patients with cirrhosis. Hepatology. 2014;60:1851-1860. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 131] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 54. | Giannone FA, Domenicali M, Baldassarre M, Bartoletti M, Naldi M, Laggetta M, Bertucci C, Colecchia A, Viale P, Bernardi M, Caraceni P. Ischaemia-modified albumin: a marker of bacterial infection in hospitalized patients with cirrhosis. Liver Int. 2015;35:2425-2432. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 20] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 55. | Baldassarre M, Naldi M, Zaccherini G, Bartoletti M, Antognoli A, Laggetta M, Gagliardi M, Tufoni M, Domenicali M, Waterstradt K, Paterini P, Baldan A, Leoni S, Bartolini M, Viale P, Trevisani F, Bernardi M, Caraceni P. Determination of Effective Albumin in Patients With Decompensated Cirrhosis: Clinical and Prognostic Implications. Hepatology. 2021;74:2058-2073. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 56] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 56. | Paar M, Fengler VH, Rosenberg DJ, Krebs A, Stauber RE, Oettl K, Hammel M. Albumin in patients with liver disease shows an altered conformation. Commun Biol. 2021;4:731. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 18] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 57. | Gomme PT, McCann KB, Bertolini J. Transferrin: structure, function and potential therapeutic actions. Drug Discov Today. 2005;10:267-273. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 292] [Cited by in RCA: 337] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 58. | Kawabata H. Transferrin and transferrin receptors update. Free Radic Biol Med. 2019;133:46-54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 232] [Cited by in RCA: 424] [Article Influence: 70.7] [Reference Citation Analysis (0)] |
| 59. | Stibler H. Carbohydrate-deficient transferrin in serum: a new marker of potentially harmful alcohol consumption reviewed. Clin Chem. 1991;37:2029-2037. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 463] [Cited by in RCA: 459] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 60. | Inoue T, Yamauchi M, Ohkawa K. Structural studies on sugar chains of carbohydrate-deficient transferrin from patients with alcoholic liver disease using lectin affinity electrophoresis. Electrophoresis. 1999;20:452-457. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 61. | Hoefkens P, Huijskes-Heins MI, de Jeu-Jaspars CM, van Noort WL, van Eijk HG. Influence of transferrin glycans on receptor binding and iron-donation. Glycoconj J. 1997;14:289-295. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 62. | Arndt T, van der Meijden BB, Wielders JP. Atypical serum transferrin isoform distribution in liver cirrhosis studied by HPLC, capillary electrophoresis and transferrin genotyping. Clin Chim Acta. 2008;394:42-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 37] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 63. | Stewart SH, Reuben A, Anton RF. Relationship of Abnormal Chromatographic Pattern for Carbohydrate-Deficient Transferrin with Severe Liver Disease. Alcohol Alcohol. 2017;52:24-28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 64. | Landberg E, Åström E, Kågedal B, Påhlsson P. Disialo-trisialo bridging of transferrin is due to increased branching and fucosylation of the carbohydrate moiety. Clin Chim Acta. 2012;414:58-64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 22] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 65. | Viveiros A, Finkenstedt A, Schaefer B, Mandorfer M, Scheiner B, Lehner K, Tobiasch M, Reiberger T, Tilg H, Edlinger M, Zoller H. Transferrin as a predictor of survival in cirrhosis. Liver Transpl. 2018;24:343-351. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 31] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 66. | Yu Y, Jiang L, Wang H, Shen Z, Cheng Q, Zhang P, Wang J, Wu Q, Fang X, Duan L, Wang S, Wang K, An P, Shao T, Chung RT, Zheng S, Min J, Wang F. Hepatic transferrin plays a role in systemic iron homeostasis and liver ferroptosis. Blood. 2020;136:726-739. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 418] [Cited by in RCA: 405] [Article Influence: 81.0] [Reference Citation Analysis (0)] |
| 67. | Sanda M, Benicky J, Wu J, Wang Y, Makambi K, Ahn J, Smith CI, Zhao P, Zhang L, Goldman R. Increased sialylation of site specific O-glycoforms of hemopexin in liver disease. Clin Proteomics. 2016;13:24. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 68. | Morota K, Nakagawa M, Sekiya R, Hemken PM, Sokoll LJ, Elliott D, Chan DW, Dowell BL. A comparative evaluation of Golgi protein-73, fucosylated hemopexin, α-fetoprotein, and PIVKA-II in the serum of patients with chronic hepatitis, cirrhosis, and hepatocellular carcinoma. Clin Chem Lab Med. 2011;49:711-718. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 53] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 69. | Pompach P, Brnakova Z, Sanda M, Wu J, Edwards N, Goldman R. Site-specific glycoforms of haptoglobin in liver cirrhosis and hepatocellular carcinoma. Mol Cell Proteomics. 2013;12:1281-1293. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 102] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 70. | Bensi G, Raugei G, Klefenz H, Cortese R. Structure and expression of the human haptoglobin locus. EMBO J. 1985;4:119-126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 102] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 71. | Zhang S, Shu H, Luo K, Kang X, Zhang Y, Lu H, Liu Y. N-linked glycan changes of serum haptoglobin β chain in liver disease patients. Mol Biosyst. 2011;7:1621-1628. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 49] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 72. | Polticelli F, Bocedi A, Minervini G, Ascenzi P. Human haptoglobin structure and function--a molecular modelling study. FEBS J. 2008;275:5648-5656. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 69] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 73. | Zhu J, Lin Z, Wu J, Yin H, Dai J, Feng Z, Marrero J, Lubman DM. Analysis of serum haptoglobin fucosylation in hepatocellular carcinoma and liver cirrhosis of different etiologies. J Proteome Res. 2014;13:2986-2997. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 79] [Cited by in RCA: 100] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 74. | Orino K, Watanabe K. Molecular, physiological and clinical aspects of the iron storage protein ferritin. Vet J. 2008;178:191-201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 86] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 75. | Wang W, Knovich MA, Coffman LG, Torti FM, Torti SV. Serum ferritin: Past, present and future. Biochim Biophys Acta. 2010;1800:760-769. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 613] [Cited by in RCA: 564] [Article Influence: 37.6] [Reference Citation Analysis (0)] |
| 76. | Fletcher LM, Halliday JW, Powell LW. Interrelationships of alcohol and iron in liver disease with particular reference to the iron-binding proteins, ferritin and transferrin. J Gastroenterol Hepatol. 1999;14:202-214. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 67] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 77. | Czaja AJ. Review article: iron disturbances in chronic liver diseases other than haemochromatosis - pathogenic, prognostic, and therapeutic implications. Aliment Pharmacol Ther. 2019;49:681-701. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 33] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 78. | Domschke W, Meyer-Bertenrath JG. -Helical structures and iron contents of the ribosomal ferritin from normal, cirrhotic and cancerous human livers. Digestion. 1971;4:321-325. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.0] [Reference Citation Analysis (0)] |
| 79. | Cullis JO, Fitzsimons EJ, Griffiths WJ, Tsochatzis E, Thomas DW; British Society for Haematology. Investigation and management of a raised serum ferritin. Br J Haematol. 2018;181:331-340. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 126] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 80. | Chapman RW, Gorman A, Laulicht M, Hussain MA, Sherlock S, Hoffbrand AV. Binding of serum ferritin to concanavalin A in patients with iron overload and with chronic liver disease. J Clin Pathol. 1982;35:481-486. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 16] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 81. | Annunziata M, Granata R, Ghigo E. The IGF system. Acta Diabetol. 2011;48:1-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 137] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 82. | Jogie-Brahim S, Feldman D, Oh Y. Unraveling insulin-like growth factor binding protein-3 actions in human disease. Endocr Rev. 2009;30:417-437. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 219] [Cited by in RCA: 239] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 83. | Firth SM, Baxter RC. Characterisation of recombinant glycosylation variants of insulin-like growth factor binding protein-3. J Endocrinol. 1999;160:379-387. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 77] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 84. | Coverley JA, Martin JL, Baxter RC. The effect of phosphorylation by casein kinase 2 on the activity of insulin-like growth factor-binding protein-3. Endocrinology. 2000;141:564-570. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 33] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 85. | Nedić O, Nikolić JA, Prisić S, Acimovic J, hajdukovic-Dragojlovic L. Reactivity of IGF binding protein-3 isoforms towards concanavalin A in healthy adults and subjects with cirrhosis. Addict Biol. 2003;8:81-88. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 86. | Nedić O, Nikolić JA, Hajduković-Dragojlović L, Todorović V, Masnikosa R. Alterations of IGF-binding proteins in patients with alcoholic liver cirrhosis. Alcohol. 2000;21:223-229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 87. | Cai Q, Dozmorov M, Oh Y. IGFBP-3/IGFBP-3 Receptor System as an Anti-Tumor and Anti-Metastatic Signaling in Cancer. Cells. 2020;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 84] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 88. | Arab JP, Cabrera D, Sehrawat TS, Jalan-Sakrikar N, Verma VK, Simonetto D, Cao S, Yaqoob U, Leon J, Freire M, Vargas JI, De Assuncao TM, Kwon JH, Guo Y, Kostallari E, Cai Q, Kisseleva T, Oh Y, Arrese M, Huebert RC, Shah VH. Hepatic stellate cell activation promotes alcohol-induced steatohepatitis through Igfbp3 and SerpinA12. J Hepatol. 2020;73:149-160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 47] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 89. | Luo J, Chen Q, Shen T, Wang X, Fang W, Wu X, Yuan Z, Chen G, Ling W, Chen Y. Association of sex hormone-binding globulin with nonalcoholic fatty liver disease in Chinese adults. Nutr Metab (Lond). 2018;15:79. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 26] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 90. | Yuan W, Benicky J, Wei R, Goldman R, Sanda M. Quantitative Analysis of Sex-Hormone-Binding Globulin Glycosylation in Liver Diseases by Liquid Chromatography-Mass Spectrometry Parallel Reaction Monitoring. J Proteome Res. 2018;17:2755-2766. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 91. | Thaler MA, Seifert-Klauss V, Luppa PB. The biomarker sex hormone-binding globulin - from established applications to emerging trends in clinical medicine. Best Pract Res Clin Endocrinol Metab. 2015;29:749-760. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 71] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 92. | Luppa PB, Thaler M, Schulte-Frohlinde E, Schreiegg A, Huber U, Metzger J. Unchanged androgen-binding properties of sex hormone-binding globulin in male patients with liver cirrhosis. Clin Chem Lab Med. 2006;44:967-973. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 19] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 93. | Brenta G, Bedecarras P, Schnitman M, Gurfinkiel M, Damilano S, Campo S, Pisarev MA. Characterization of sex hormone-binding globulin isoforms in hypothyroid women. Thyroid. 2002;12:101-105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 94. | Kaymakoğlu S, Okten A, Cakaloğlu Y, Boztaş G, Beşişik F, Taşçioğlu C, Yalçin S. Hypogonadism is not related to the etiology of liver cirrhosis. J Gastroenterol. 1995;30:745-750. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 29] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 95. | Maruyama Y, Adachi Y, Aoki N, Suzuki Y, Shinohara H, Yamamoto T. Mechanism of feminization in male patients with non-alcoholic liver cirrhosis: role of sex hormone-binding globulin. Gastroenterol Jpn. 1991;26:435-439. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 31] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 96. | Price JC, Wang R, Seaberg EC, Brown TT, Budoff MJ, Kingsley LA, Palella FJ Jr, Witt MD, Post WS, Lake JE, Thio CL. Sex Hormone-Binding Globulin Levels Are Inversely Associated With Nonalcoholic Fatty Liver Disease in HIV-Infected and -Uninfected Men. Open Forum Infect Dis. 2019;6:ofz468. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 97. | Yuan W, Sanda M, Wu J, Koomen J, Goldman R. Quantitative analysis of immunoglobulin subclasses and subclass specific glycosylation by LC-MS-MRM in liver disease. J Proteomics. 2015;116:24-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 61] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 98. | Mehta AS, Long RE, Comunale MA, Wang M, Rodemich L, Krakover J, Philip R, Marrero JA, Dwek RA, Block TM. Increased levels of galactose-deficient anti-Gal immunoglobulin G in the sera of hepatitis C virus-infected individuals with fibrosis and cirrhosis. J Virol. 2008;82:1259-1270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 106] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 99. | Tissandié E, Morelle W, Berthelot L, Vrtovsnik F, Daugas E, Walker F, Lebrec D, Trawalé JM, Francoz C, Durand F, Moura IC, Paradis V, Moreau R, Monteiro RC. Both IgA nephropathy and alcoholic cirrhosis feature abnormally glycosylated IgA1 and soluble CD89-IgA and IgG-IgA complexes: common mechanisms for distinct diseases. Kidney Int. 2011;80:1352-1363. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 57] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 100. | Lamontagne A, Long RE, Comunale MA, Hafner J, Rodemich-Betesh L, Wang M, Marrero J, Di Bisceglie AM, Block T, Mehta A. Altered functionality of anti-bacterial antibodies in patients with chronic hepatitis C virus infection. PLoS One. 2013;8:e64992. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 21] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 101. | Wu T, Zhao F, Gao B, Tan C, Yagishita N, Nakajima T, Wong PK, Chapman E, Fang D, Zhang DD. Hrd1 suppresses Nrf2-mediated cellular protection during liver cirrhosis. Genes Dev. 2014;28:708-722. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 202] [Cited by in RCA: 301] [Article Influence: 27.4] [Reference Citation Analysis (0)] |
| 102. | Hasegawa D, Fujii R, Yagishita N, Matsumoto N, Aratani S, Izumi T, Azakami K, Nakazawa M, Fujita H, Sato T, Araya N, Koike J, Tadokoro M, Suzuki N, Nagata K, Senoo H, Friedman SL, Nishioka K, Yamano Y, Itoh F, Nakajima T. E3 ubiquitin ligase synoviolin is involved in liver fibrogenesis. PLoS One. 2010;5:e13590. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 34] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 103. | Zou T, Zhang J. Diverse and pivotal roles of neddylation in metabolism and immunity. FEBS J. 2021;288:3884-3912. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 39] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 104. | Dai J, Hu Y, Niu Q, Song G, Wang H, Li S. Role of PML SUMOylation in arsenic trioxide-induced fibrosis in HSCs. Life Sci. 2020;251:117607. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 105. | Guo L, Guo YY, Li BY, Peng WQ, Chang XX, Gao X, Tang QQ. Enhanced acetylation of ATP-citrate lyase promotes the progression of nonalcoholic fatty liver disease. J Biol Chem. 2019;294:11805-11816. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 73] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 106. | Groebner JL, Girón-Bravo MT, Rothberg ML, Adhikari R, Tuma DJ, Tuma PL. Alcohol-induced microtubule acetylation leads to the accumulation of large, immobile lipid droplets. Am J Physiol Gastrointest Liver Physiol. 2019;317:G373-G386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 21] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 107. | Yang Y, Sangwung P, Kondo R, Jung Y, McConnell MJ, Jeong J, Utsumi T, Sessa WC, Iwakiri Y. Alcohol-induced Hsp90 acetylation is a novel driver of liver sinusoidal endothelial dysfunction and alcohol-related liver disease. J Hepatol. 2021;75:377-386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 47] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 108. | Ding D, Chen LL, Zhai YZ, Hou CJ, Tao LL, Lu SH, Wu J, Liu XP. Trichostatin A inhibits the activation of Hepatic stellate cells by Increasing C/EBP-α Acetylation in vivo and in vitro. Sci Rep. 2018;8:4395. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 22] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 109. | Abdelmegeed MA, Song BJ. Functional roles of protein nitration in acute and chronic liver diseases. Oxid Med Cell Longev. 2014;2014:149627. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 44] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 110. | Ji Y, Neverova I, Van Eyk JE, Bennett BM. Nitration of tyrosine 92 mediates the activation of rat microsomal glutathione s-transferase by peroxynitrite. J Biol Chem. 2006;281:1986-1991. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 52] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 111. | Charatcharoenwitthaya P, Karaketklang K, Aekplakorn W. Cigarette Smoking Increased Risk of Overall Mortality in Patients With Non-alcoholic Fatty Liver Disease: A Nationwide Population-Based Cohort Study. Front Med (Lausanne). 2020;7:604919. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 28] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 112. | Corrao G, Zambon A, Bagnardi V, D'Amicis A, Klatsky A; Collaborative SIDECIR Group. Coffee, caffeine, and the risk of liver cirrhosis. Ann Epidemiol. 2001;11:458-465. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 102] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 113. | Herrera JL, Rodríguez R. Medical Care of the Patient With Compensated Cirrhosis. Gastroenterol Hepatol (N Y). 2006;2:124-133. [PubMed] |
| 114. | Harrison-Findik DD. Role of alcohol in the regulation of iron metabolism. World J Gastroenterol. 2007;13:4925-4930. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 105] [Cited by in RCA: 100] [Article Influence: 5.6] [Reference Citation Analysis (0)] |