Published online Jul 21, 2022. doi: 10.3748/wjg.v28.i27.3314
Peer-review started: January 17, 2022
First decision: April 11, 2022
Revised: May 3, 2022
Accepted: June 18, 2022
Article in press: June 18, 2022
Published online: July 21, 2022
Processing time: 182 Days and 1.3 Hours
The prevalence of nonalcoholic fatty liver disease (NAFLD) is rising worldwide, paralleling the epidemic of obesity. The liver is a key organ for the metabolism of proteins, fats and carbohydrates. Various types of fats and carbohydrates in isocaloric diets differently influence fat accumulation in the liver parenchyma. Therefore, nutrition can manage hepatic and cardiometabolic complications of NAFLD. Even moderately reduced caloric intake, which leads to a weight loss of 5%-10% of initial body weight, is effective in improving liver steatosis and surrogate markers of liver disease status. Among dietary patterns, the Mediterranean diet mostly prevents the onset of NAFLD. Furthermore, this diet is also the most recommended for the treatment of NAFLD patients. However, clinical trials based on the dietary interventions in NAFLD patients are sparse. Since there are only a few studies examining dietary interventions in clinically advanced stages of NAFLD, such as active and fibrotic steatohepatitis, the optimal diet for patients in these stages of the disease must still be determined. In this narrative review, we aimed to critically summarize the associations between different dietary patterns, obesity and prevention/risk for NAFLD, to describe specific dietary inter
Core Tip: In this review, we emphasize that based on the current evidence, there is no consensus on the ideal macronutrient composition of the diet for nonalcoholic fatty liver disease (NAFLD) patients. We have shown that dietary habits are the most important factor in NAFLD prevention. The Mediterranean and healthy dietary pattern, characterized by high consumption of vegetables, fruits, nuts, olive oil, low-fat dairy products and fish, were linked with a reduced NAFLD risk. The Dietary Approach to Stop Hypertension diet, intermittent fasting and ketogenic diet are other dietary regimes that have growing interest among specialists who advise patients with NAFLD. Nevertheless, new studies designed to assess the effects of these diets on liver-related outcomes and liver histology are needed. We also noted that dietary advice should be personalized in NAFLD patients.
- Citation: Ristic-Medic D, Bajerska J, Vucic V. Crosstalk between dietary patterns, obesity and nonalcoholic fatty liver disease. World J Gastroenterol 2022; 28(27): 3314-3333
- URL: https://www.wjgnet.com/1007-9327/full/v28/i27/3314.htm
- DOI: https://dx.doi.org/10.3748/wjg.v28.i27.3314
Nonalcoholic fatty liver disease (NAFLD) is the accumulation of excess fat (more than 5%) in the liver parenchyma in people with no significant alcohol consumption or secondary causes of hepatic steatosis[1]. The prevalence of NAFLD is rising in many countries, paralleling the epidemic of obesity worldwide. The highest rates of NAFLD have been observed in North Africa (31%), the Middle East (32%) and Asia (27%)[2].
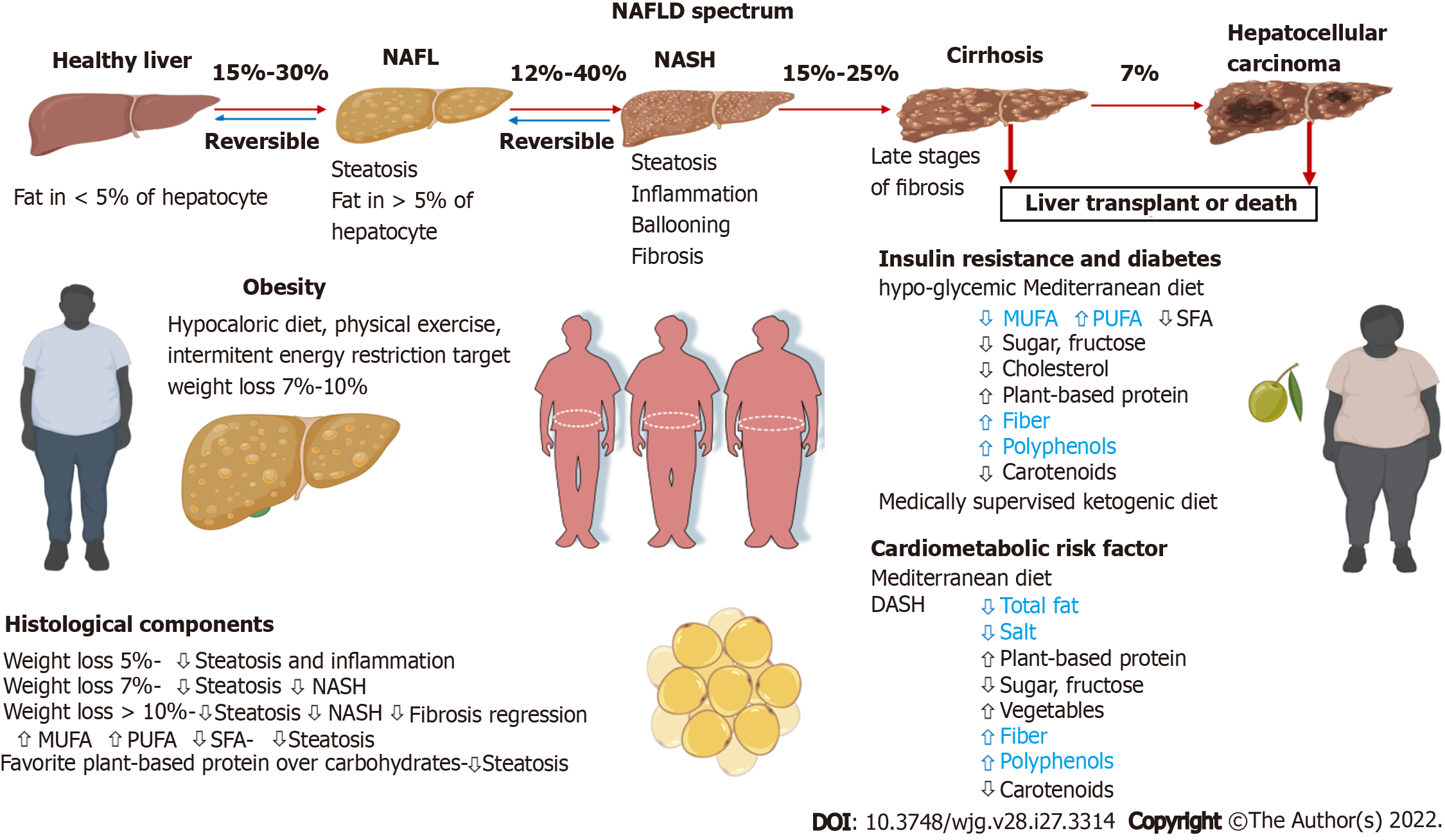
NAFLD represents a clinicopathological spectrum, ranging from benign hepatic steatosis to nonalcoholic steatohepatitis (NASH) and characterized by hepatocellular injury and inflammation, which leads to hepatic fibrosis[3,4]. Up to 20% of patients with fibrotic NASH progress to cirrhosis and associated complications[5,6]. Fibrotic NASH can lead to hepatocellular carcinoma, even at the pre-cirrhotic stage (Figure 1). Approximately 90% of the obese population, 60% of patients with diabetes type 2 and 50% of patients with dyslipidemia have NAFLD[6-8]. Moreover, NAFLD is a risk factor for severe coronavirus disease 2019, and thus nutritional prevention of coronavirus disease 2019 complications has been highlighted in a recent review[8].
Nevertheless, obesity, overnutrition, dietary components and a sedentary lifestyle are modifiable risk factors for NAFLD. Central obesity is probably the most significant modifiable risk factor for this disorder, which arises from energy imbalance[9]. The relationship between excessive caloric intake and the NAFLD development has been shown in interventional studies. Weight loss as a primary therapeutic approach produced clinically meaningful outcomes in patients with NAFLD[10,11]. However, the success of such weight loss interventions depends on the intensity of diet counseling and the frequency of visits to dietitians. Two dietary patterns that seem to promote the improvement of NAFLD with incorporated recommendations are the Mediterranean and the Dietary Approach to Stop Hypertension (DASH) diets[12].
This review critically summarizes the associations between dietary patterns, obesity and prevention/risk for NAFLD as well as the impact of specific dietary interventions on hepatic steatosis in adults with NAFLD. It also provides an updated overview of dietary recommendations that clinicians potentially need to apply in their daily practice.
This narrative review was based on PubMed electronic database search for relevant publications using the following terms (“fatty liver” OR “NAFLD” OR “non-alcoholic fatty liver disease” OR “steatosis of liver” OR “steatohepatitis” OR “steatosis”) AND “obesity” AND (“diet“ OR “dietary pattern” OR “dietary interventions“ OR “nutrition“) to identify the studies on the association between dietary patterns and NAFLD and specific clinical dietary intervention studies in adult patients with NAFLD. Also, we focused on systematic reviews with meta-analyses. Studies relevant to the topic, conducted in humans, published in English and preferably published in the last 10 years were included. All studies are checked in Reference Citation Analysis database (https://www.referencecitationanalysis.com/). The list of references was reduced because priority had been given to studies that are relevant to clinical practice. The final list of references was approved with the consent of the authors.
The pathophysiology of NAFLD involves multiple genetic and environmental factors. Genetic factors include specific polymorphisms and epigenetic modifications. As the most common genetic determinant of NAFLD, the I148M variant of patatin-like phospholipase domain-containing protein 3 gene has been recognized[13]. Environmental factors are related to diet and lifestyle, hormonal disturbances, insulin resistance (IR), obesity, oxidative stress, lipotoxicity, unfavorable gut microbiota and many others[9]. Despite well-established risk factors for NAFLD, the pathways leading to the disease are not elucidated, but the role of the diet is undeniable.
It is known that the liver utilizes fatty acids and sugars as primary metabolic substrates, but the overload of these substances results in the accumulation of toxic lipid products[14]. These products increase oxidative stress by overproduction of reactive oxygen species and inflammation in hepatocytes that leads to liver injury. Moreover, a higher intake of saturated fatty acids (SFAs) promotes hepatic liver accumulation and the development and progression of NAFLD[15]. On the contrary, intake of unsaturated fats has a protective role[16].
Recent studies revealed the underlying mechanism of this process, highlighting mitochondrial dysfunction as a key player (reviewed by Meex and Blank[17]). Hepatocytes are very rich in mitochondria, and intake of SFAs induces changes in their structure and function. The process starts with liver steatosis due to reduced oxidation and enhanced lipolysis of adipose tissues. Steatosis affects the efficacy of the respiratory transport chain[18]. Consequently, overproduction of reactive oxygen species and lipid peroxidation arise, eventually resulting in inflammation, apoptosis and damage of the liver. In addition, SFAs from food enter the mitochondrial membrane and alter its permeability and fluidity, contributing further to NAFLD progression[19].
Besides the diet itself, obesity is also associated with NAFLD pathophysiology. In obesity, the capacity of an expanded adipose tissue to store lipids is limited, and the excess of lipids is stored in hepatocytes. The main form of lipids stored in the liver are triglycerides (TGs). Namely, high levels of free fatty acids in circulation, derived from enhanced lipolysis or diminished absorption by subcutaneous adipose tissue, bring ectopic fat accumulation, mostly in the liver. The sources of free fatty acids that form in the liver TG are not only from the diet (around 15%) but from increased lipolysis of TGs in adipose tissue (approximately 60%) and de novo lipogenesis (DNL) in the liver (25%) from dietary sugars, glucose and fructose[20]. This is supported by a study using stable isotopes, which has shown that accumulated lipids in the liver of NAFLD patients are mainly attributable to DNL. This stage of fat accumulation in the liver is the beginning of NAFLD, and managing obesity at this stage is of crucial importance. The lack of successful obesity treatment leads to intrahepatic inflammation and infiltration of immune cells, such as lymphocytes, monocytes and neutrophils, which release cytokines in the liver[21]. This process not only intensifies inflammation but also promotes intrahepatic fibrogenesis, leading to progression of NAFLD to NASH.
Another relationship between obesity and NAFLD has been established through adipokines[22]. Adipokines are hormones derived from adipose tissue, and they are commonly represented by leptin and adiponectin. While their synthesis is balanced in people with normal weight, in obesity the dysregulation of pro- and anti-inflammatory adipokines is present. The enlarged, hypertrophic adipocytes produce proinflammatory adipokines and cytokines and promote IR. Adiponectin suppresses the secretion of proinflammatory cytokines (interleukin 6, tumor necrosis factor α), promotes the release of anti-inflammatory interleukin 10 and negatively correlates with visceral adipose tissue mass[23]. On the contrary, leptin is a product of white adipose tissue, and its level in circulation depends on the fat tissue mass and adipocyte size[24]. This is a satiety hormone with pleiotropic effects, and its concentration is a marker of obesity-related complications: Neuropathy and atherosclerosis[25,26]. Hyperleptinemia is considered crucial for NAFLD progression, although the exact mechanisms are still unclear. However, new findings pinpointed that leptin mediates pyroptotic-like cell death of macrophages and hepatocytes through infiltrated CD8+ T lymphocytes[27]. These results can provide a new strategy for future treatment of NAFLD.
Among the other risk factors, metabolic syndrome (MetS) has demonstrated the strongest association with NAFLD and its advanced stage, NASH. Since MetS is characterized by several features, including waist circumference, hypertension, hyperglycemia and dyslipidemia (low high-density lipoprotein cholesterol and/or high TG level), the clearest biological link with NAFLD development and progression was found for glucose level[28]. In line with this, 75% of patients with diabetes mellitus have NAFLD as well. This relation is bidirectional: Patients with NAFLD have a higher risk of developing diabetes[29]. Although IR is involved in NAFLD pathogenesis, improving IR is often insufficient to prevent further progression of NAFLD[30].
Furthermore, increased central adiposity, an important component of MetS, is considered a more significant marker of NAFLD than total body fat. This is expected, considering the role of visceral fats in the biosynthesis of adipokines. According to a recent study, there is a cross-talk between IR, adipose tissue inflammation and NAFLD, with dipeptidyl peptidase 4 as the key factor. This enzyme, secreted by the hepatocytes, has been shown to promote IR and inflammation of visceral adipose tissue[31]. In support of that, Barchetta et al[32] reported that levels and activity of dipeptidyl peptidase 4 in circulation are independently associated with NAFLD presence and severity in patients with or without other metabolic diseases and with various grades of obesity. The authors proposed dipeptidyl peptidase 4 as a novel marker for NAFLD/NASH risk stratification and follow-up of NAFLD patients.
Since people do not consume nutrients in isolation, the best option to describe the relationship between nutrition and health outcomes is the analysis of dietary patterns. Dietary patterns are a combination of a variety of foods habitually consumed by an individual, which together create synergistic effects on our health[33]. Two main dietary patterns, such as a “Western dietary pattern” and “Mediterranean dietary pattern” have been significantly associated (although in the opposite direction) with NAFLD, independently of potential confounders[34]. However, there are more dietary patterns (e.g., healthy, traditional) identified for these associations.
Mediterranean diet (MD) is a plant-based diet containing significant amounts of fiber, antioxidants, vegetable proteins, monounsaturated fat and polyunsaturated fatty acids (PUFAs), and with an appropriate n-6/n-3 PUFA ratio. This diet is known as a high-fat diet, with a fat intake of up to 45% of total daily calories[35]. The basic source of dietary fat in this diet is olive oil[33,36], where oleic acid, a monounsaturated fatty acid (MUFA), is a major component[37]. The MD is also characterized by high amounts of PUFAs. Dietary sources of the PUFAs, especially long-chain n-3 fatty acids, which include eicosapentaenoic acid and docosahexaenoic acid, in the MD are fish and nuts[38]. The MD is therefore rich in macronutrients that have been shown to have a beneficial effect on glucose and lipidic metabolism, and consequently on NAFLD[39]. The observational studies on the association between MD and NAFLD are summarized in Table 1. A reverse association between high adherence to MD and NAFLD odds, even after adjusting for some confounders such as age, sex, diabetes, physical activity, energy intake, smoking status and supplements use was seen in two case-control studies[40,41] and one cross-sectional study[42]. It should be highlighted that higher consumption of nuts, fruits and vegetables, legumes and fish as well as lower intake of meat were reported to be protective against NAFLD[43].
| Ref. | Country/Region | Assess adherence to the MD | Food groups associated with lower risk NAFLD | Study design | Number of patients and age range | Main results | Associations |
| Entezari et al[40] | Iran | MDS | ↑ Intake nuts and fruits, vegetables, legumes, high MUFA/PUFA ratio, cereals and fish. ↓ EI, low-fat dairy and meats | C-C | 247 (43.7% male); 18–55 yr | ↑ Adherence to MD was associated with ↓ risk of NAFLD after controlling for age (OR: 0.40, 95%CI: 0.17–0.95) and sex, diabetes, PA and supplement intake (OR: 0.36, 95%CI: 0.15–0.89). This association disappeared after adjusting for BMI, WHR (OR: 0.70, 95%CI: 0.25–1.97) | ↓, After controlling for anthropometrical variables ↔ |
| Giraldi et al[41] | Italy | MDS | ↑ Legumes consumption ↓ risk of NAFLD (OR: 0.62; 95%CI: 0.38-0.99) and ↑ fish intake ↓ risk of NAFLD (OR: 0.38; 95%CI: 0.17-0.85) | C-C | 815 (371 with NAFLD); 59 ± 16 yr; 444 controls; 45 ± 14 yr | ↑ Adherence to the MD was associated with ↓ risk of NAFLD (OR: 0.83; 95%CI: 0.71-0.98) after controlling for age, sex, EI, diabetes status, smoking status, BMI and PA | ↓ |
| Baratta et al[42] | Italy | MD questionnaire | ↓ Meat intake | C-S | 584 patients (61.8% males) with cardiometabolic risk factors screened for the presence of liver steatosis; 56.2 ± 12.4 yr | ↑ Adherence to MD was associated with ↓ risk of NAFLD (intermediate vs low tertile OR: 0.12; P < 0.05; high vs low tertile OR: 0.09; P < 0.05) | ↓ |
| Aller[45] | Spain | 14-item MD assessment tool | - | C-S | 82 NAFLD patients (42.7% low and 57.3% high steatosis grade, 68.3% steatohepatitis and 51.2% liver fibrosis mean age 44 + 11 yr | ↑ Adherence to MD was associated with ↓ likelihood of having steatohepatitis OR: 0.43; 95%CI: 0.29-0.64 and steatosis OR: 0.42; 95%CI: 0.26- 0.70 | ↓ |
| Park et al[46] | United States; 5 targeted racial/ethnic groups: African American, Native Hawaiian, Japanese American, Latino and White | Alternate MDS | - | Nested C-C | 2959 with NAFLD (509 with cirrhosis; 2450 without cirrhosis) and 29292 controls; mean age 44.2 + 11.3 yr | ↑ Adherence to MD was not associated with lower NAFLD risk | ↔ |
| Chan et al[47] | Hong Kong of China | MDS | ↑ Vegetables and legumes, fruits and dried fruits, vitamin C | C-S | 797 (41.7% males) 27.6% had a fatty liver aged ≥ 18 yr | MDS was not associated with the prevalence of NAFLD | ↔ |
| Kontogianni et al[44] | Greece | MDS | - | C-S | 73 overweight/obese patients with NAFLD (69% males) vs 58 age-sex- and BMI matched controls; mean age 45 yr | No difference in the MDS was observed between patients and controls. One unit increase in the MDS was associated with ↓ likelihood of having NASH (OR: 0.64; 95%CI: 0.45-0.92), after controlling for sex and abdominal fat | ↓ |
However, Entezari et al[40] observed that the reverse relationship between adherence to MD and odds of NAFLD disappeared after controlling for the anthropometric variables (body mass index and waist-to-hip ratio), which means that the MD may improve fatty liver by body weight modification, modulation of lipid profile and inflammatory markers. Although Kontogianni et al[44] did not find a significant difference between NAFLD patients and controls in terms of adherence to the MD, higher adherence to this diet was inversely associated with alcoholic steatohepatitis. Similar results were seen in the study by Aller et al[45].
On the other hand, in a nested and matched case-control study[46] as well as a cross-sectional study[47] it was found that adherence to the MD in any models (crude or adjusted to some confounders) was not associated with the risk of NAFLD. It should be highlighted that the dietary indices that measure adherence to the MD vary among the included studies. Hence, the specific dietary components and/or food items included within each of these indices and the methods used to evaluate compliance should be taken into consideration when interpreting obtained results. Nevertheless, a recent meta-analysis has proven that MD reduced the risk of NAFLD by 23%[43]. Also, the European Association for the Study of the Liver, and the European Association for the Study of Diabetes-European Association for the Study of Obesity Clinical Practice Guidelines have encouraged the MD as a lifestyle choice for treating the disease[48].
Various mechanisms may be associated with the beneficial effects of the MD on metabolic health and NAFLD, but the most important for this association is an appropriate fatty acid composition due to high MUFA content and an appropriate n-6/n-3 PUFA ratio[49]. It has been proven that MUFA may prevent the development of NAFLD by improving blood lipid concentrations, lowering body fat contents and decreasing postprandial adiponectin expression[50]. MUFAs (oleic acid) from olive oil have numerous beneficial effects on NAFLD, including decreased oxidized low-density lipoprotein, low-density lipoprotein cholesterol (LDL-C) and TG concentration, without the concomitant decrease in high-density lipoprotein cholesterol (HDL-C)[51], as well as lowering blood pressure and improving insulin sensitivity[37]. Additional effects of the MD relate to its polyphenol content. For example, polyphenols present in olive oil, such as oleuropein, hydroxytyrosol and tyrosol, have important antioxidant and anti-inflammatory effects[51]. The high content of dietary fiber both in soluble and insoluble forms in the MD is associated with a decrease in serum TGs and blood glucose[40]. The beneficial effect of the MD on NAFLD progression is also linked with an absence of added sugars and fructose in this diet.
A healthy dietary pattern is defined as an appropriate intake of fruits & vegetables, nuts, olive oil, low-fat dairy products and fish. MD is one example of a healthy dietary pattern, but there are also other specific healthy diets. In Table 2, associations between healthy dietary patterns and the risk/prevalence of NAFLD are summarized.
| Ref. | Country/Region | Dietary pattern | Food items in dietary patterns | Type of study | N, age | Main results | Associations |
| Oddy et al[52] | Australia | Western | Takeaway foods, confectionery, red meat, refined grains, processed meats, chips, sauces, full-fat dairy products and soft drinks | P | 995. FFQ completed at 14 yr and liver ultrasound at 17 yr | Higher western pattern score at 14 yr was associated with ↑ risk of NAFLD at 17 yr (OR: 1.59; 95%CI: 1.17–2.14; P < 0.05) before adjustment to BMI | ↑ |
| Healthy | Whole grains, fruit, vegetables, legumes, fish | A healthy diet at 14 yr appeared protective against NAFLD at 17 yr in centrally obese adolescents (OR: 0.63; 95%CI 0.41-0.96; P < 0.05) | ↓ | ||||
| Salehi-Sahlabadi et al[34] | Iran | Western | Fast foods, soft drinks, processed meat, high-fat dairy products, hydrogenated fats, mayonnaise, salty snacks, sugar sweetened desserts, organ meats and refined grains | C-C | 675 (450 with NAFLD) NAFLD: 38.6 ± 8.7 yr; Controls: 37.9 ± 8.9 yr | The western pattern was associated with ↑ risk for NAFLD after adjustment for age, sex, BMI, PA, SES and EI | ↑ |
| Healthy | Fish, skinless poultry, low-fat dairy, fresh fruits, natural juices, canned fruits, dried fruits, vegetables, nuts, olive and garlic | A healthy pattern was associated with ↓ risk for NAFLD, after controlling for mentioned variables | ↓ | ||||
| Traditional | Red meat, organ meats, skinless poultry, eggs, yogurt drink, tea, legumes, tomato sauce, sugar sweetened-desserts, potato, condiments, salt, pickles and broth | Lack of association between traditional pattern and risk of NAFLD adjusted for mentioned variables | ↔ | ||||
| Chung et al[60] | Korea | Western and high-carbohydrate | Processed meats, bread, soft drinks, pork, noodles, beef, cakes, snacks, beef soup, sugar, coffee, chicken, processed fish and refined grains | C-S | 1190 (331 with NAFLD) NAFLD: 53 ± 9 yr; Controls: 51 ± 10 yr | Lack of association between Western/high-carbohydrate pattern and risk of NAFLD after adjustment for age, sex, WC, smoking status, EI, diabetes and hypertension | ↔ |
| Traditional | Vegetables; fermented vegetables such as kimchi and jjangajji; fish and seafood; mush-rooms; fermented, processed, natural soybeans | ↑ Adherence to the traditional pattern was associated with ↑ risk of NAFLD (OR: 1.85; 95%CI: 1.11-3.08; P < 0.05) after controlling for mentioned variables | ↑ | ||||
| Simple meal | Fruits, root and yellow vegetables, eggs, dairy products and nuts | ↑ Adherence to the simple meal pattern was associated with ↓ risk of NAFLD (OR: 0.59; 95%CI: 0.34-1.00; P < 0.05), after controlling for mentioned variables | ↓ | ||||
| Dehghanseresht et al[54] | Iran | Ordinary | Sweets, oils, fruits, white meats, refined grains, tea and coffee, salt, biscuits, snacks as well as red and organ meats | C-C | 244 (122 with NAFLD) aged 19–70 yr | ↑ Adherence to the ordinary pattern was associated with ↑ risk of NAFLD; P < 0.001 | ↑ |
| Traditional | Red and organ meats, dairy products, condiments, salt, tea and coffee and low intake of fruits | ↑ Adherence to the traditional pattern was associated with ↑ risk of NAFLD P < 0.001 | ↑ | ||||
| Vegetables and dairy (healthy pattern) | Vegetables, whole grains, legumes and nuts and dairy products | ↑ Adherence to the vegetables and dairy pattern was ↓ association with NAFLD risk (OR: 0.23; 95%CI: 0.09–0.58; P < 0.05) | ↓ | ||||
| Fast food | Sauces, pickles, fast foods, soft drinks, snacks and biscuits | No association between Fast food patterns and the risk of NAFLD | ↔ | ||||
| Yang et al[57] | China | Traditional Chinese | Staple food, coarse grains, fruits, eggs, fish and shrimp, milk and tea | C-S | 999 (345 with NAFLD) aged 45–60 yr | No association between traditional pattern and the risk of NAFLD | ↔ |
| Animal food | Kelp/seaweed and mushroom, pork, beef, mutton, poultry, cooked meat, eggs, fish and shrimp, beans and grease | After controlling for potential confounders, animal food patterns had ↑ prevalence rate for NAFLD (PR: 1.35; 95%CI: 1.06–1.72; P < 0.05 | ↑ | ||||
| Grains-vegetables (healthy pattern) | Coarse grains, tubers, vegetables, mushroom and kelp/seaweed, cooked meat and beans | After adjustment for BMI, a vegetable pattern had ↓ prevalence rate for NAFLD (PR: 0.78; 95%CI: 0.62–0.98, P < 0.05). | ↓ | ||||
| High-salt | Rice, pickled vegetables, processed meat, bacon, salted duck egg, salted fish and tea | No association between high salt and the risk of NAFLD | ↔ | ||||
| Jia et al[65] | China | High-carbohydrate/sweet | Fruits, cakes and candied fruits | C-S | 4365 (1339 with NAFLD: adults | ↑ Adherence to a high-carbohydrate/sweet pattern was associated with ↑ the prevalence of NAFLD in females but not in males | ↑ only in females not in males |
| Kalafati et al[55] | Greece | Fast food | Energy-dense foods rich in saturated fat and sugar and included fast foods, sweetened soft drinks, fried potatoes and savory and puff pastry snacks | C-C | 351 (134 with NAFLD) Case: 50.0 ± 10.5 yr; Control 44.0 ± 11.0 yr | ↑ Adherence to a fast-food pattern was associated with ↑ odds for NAFLD after adjustment for age, sex, EI, PA, pack-yr smoked, education, MS (P < 0.01) | ↑ |
| Prudent (healthy pattern) | Oil-based cooked vegetables, legumes, potatoes, fruits, vegetables and fatty fish | ↑ Adherence to the prudent pattern was associated with ↓ TG and uric acid levels (β: -5.96; P < 0.05; β: -0.15; P < 0.05, respectively) | ↓ | ||||
| High-protein | Red meat, poultry, eggs | The high protein pattern was not associated with any NAFLD-related biomarker | ↔ | ||||
| The unsaturated FA | Nuts, chocolate and other foods rich in unsaturated FA | Individuals in the second quartile of the unsaturated FA pattern had ↓ odds of developing NAFLD vs the first quartile after being adjusted for mentioned confounders (P < 0.05) | ↓ | ||||
| Tutunchi et al[56] | Iran | Healthy | Vegetables, legumes, fruits and low-fat dairy products | C-C | 210 (105 with NAFLD) Cases 46 ± 9 yr; Controls 45 ± 9 yr | A healthy pattern was associated with ↓ odds of NAFLD (OR: 0.34; 95%CI: 0.16–0.81) after controlling for sex, education, PA, BMI, WC | ↓ |
| Western | Sweet, hydrogenated fat, red and processed meat and soft drink dietary patterns | ↑ Adherence to the western pattern was related to ↑ risk of NAFLD (OR: 2.68; 95%CI: 1.31–4.16), after controlling to mentioned confounders | ↑ | ||||
| Zhang et al[61] | China | Sugar-rich | Strawberry, kiwi fruit, persimmon, sweets, candied fruits, Chinese cakes | P | 17360 free from NAFLD at baseline; During a median follow-up of 4.2 yr, 4034 with NAFLD, aged > 18 yr | After adjusting for age, sex, BMI, smoking, alcohol, education, occupation, income, PA, EI, personal and family history of the disease, depressive symptoms, dietary supplement use, inflammation markers, WHR and each other dietary pattern score, the sugar-rich pattern was associated with ↑ risk of NAFLD (HR: 1.11; 95%CI 1.01, 1.23) | ↑ |
| Vegetable (healthy pattern) | Cucumber, green leafy vegetables, Chinese cabbage, celery, pumpkin | After adjusting for mentioned confounders, vegetable diet was associated with ↓ risk of NAFLD (HR 0.96; 95%CI: 0.86, 1.07) | ↓ | ||||
| Animal food | Animal organs, animal blood, preserved eggs, instant noodles, pork skin, sausage | After adjusting for mentioned confounders, animal food diet was associated with ↑ risk of NAFLD (HR: 1.22; 95%CI: 1.10, 1.36) | ↑ | ||||
| Alferink et al[62] | The Netherlands | Vegetable and fish (healthy pattern) | Vegetables, poultry, fish and fruit | P | 963 (343 with NAFLD) Baseline: 71.0 yr; Follow-up: 75 yr | No associations between vegetable and fish diet and NAFLD | ↔ |
| Red meat and alcohol | Red, refined or organ meat, salty snacks and beer or spirits and low intake of fruit and tea | No associations between red meat and alcohol pattern and NAFLD | ↔ | ||||
| Traditional | Vegetable oils and stanols and margarine or butter, potatoes, whole grains and sweet snacks or desserts | ↑ Adherence to the Traditional pattern was associated with ↓ risk of NAFLD (OR: 0.40; 95%CI 0.15–1.00) adjustment for sex, age, baseline education level, PA, EI, alcohol intake and follow-up time, BMI, baseline type 2 diabetes mellitus and baseline hypertension | ↓ | ||||
| Salty snacks and sauces | Savory food groups such as nuts, legumes, salty snacks and sauces | No associations between salty snacks and sauces pattern and NAFLD | ↔ | ||||
| High-fat dairy and refined grain | Fruit juice, refined grains, high-fat dairy products and sweet snacks or desserts | No associations between high-fat dairy and refined grain pattern and NAFLD | ↔ | ||||
| Fakhoury-Sayegh et al[64] | Lebanon | Traditional | Vegetables, chickpeas, red beans, lentils, peas, vegetable oil/olives | C-C | 222 (112 with NAFLD) Cases: 40 ± 6 yr; Controls: 39 ± 13 yr | ↑ Adherence to traditional pattern ↓ the odds of NAFLD (OR: 0.30; CI 95%: 0.11–0.86; P < 0.05) adjusted for MS, EI, education, PA, family history, smoking, place of residence and profession | ↓ |
| High fruits | Fruits and fruit juices | ↑ Adherence to high fruits pattern ↑ the odds of NAFLD (OR: 4.061; 95%CI: 1.320–12.100, P < 0.05, adjusted for mentioned confounders | ↑ | ||||
| The high meat and fast food diet: (Western-like dietary pattern) | Meat such as pork, chicken, beef meat and hotdog | ↑ Adherence to Western pattern ↑ the odds of NAFLD (OR: 4.081; 95%CI: 1.36–12.28, P < 0.05) adjusted for mentioned confounders | ↑ | ||||
| Nakashita et al[58] | Japan | Healthy | Seaweeds, vegetables, mushrooms, pulses, potatoes and starches | C-S | 281 men (89 with NAFLD) NAFLD: 62 (57–67) yr; Controls: 61 (56–67) yr | A healthy pattern was correlated with the ↓ risk of NAFLD | ↓ |
| Western | Fats and oils, meat, seasonings, spices | No correlation between western pattern and NAFLD | ↔ | ||||
| Snacks | Sugars and starches, beverages (tea, coffee, fruit juice, soft drinks), fruits | No correlation between snacks pattern and NAFLD | ↔ | ||||
| Adriano et al[59] | Brazil | Healthy | Fruits, vegetables/legumes, white meat, olive oil, margarine, bread/toast (with significant negative loading for beef) | C-S | 229 older adults (74.7% women) NAFLD: 67.0 ± 5.0 yr; Controls: 70.1 ± 7.0 yr | ↑ Adherence to the healthy pattern was associated with ↓ prevalence of NAFLD (PR: 0.70; 95%CI: 0.50, 0.98, P < 0.05) after adjustment for sex, age, EI, BMI, smoking status, PA, family income and use of hypoglycemic drugs | ↓ |
| Regional snacks (Northeast of Brazil) | Tea/coffee, dairy products, cassava flour/tapioca/cuscus, butter and olive oil | ↑ Adherence to the regional snacks pattern was associated with the ↑ prevalence of NAFLD (PR: 1.42; 95%CI: 1.02, 1.92, P < 0.05) after adjustment for mentioned confounders | ↑ | ||||
| Energy-dense | Processed cold meats, beef, viscera, sweet products/desserts/sugar, soft drinks, tubers/spaghetti/pastries | No association between energy density pattern and NAFLD | ↔ | ||||
| Traditional | Rice, beans, bread/toast, tea/coffee, sweet products/desserts/sugar | No association between traditional pattern and NAFLD | ↔ |
In nine out of ten collected studies–in two prospective studies[52,53], four case-control studies[33,54-56], and three cross-sectional studies[57-59], a healthy dietary pattern was associated with a decreased risk of NAFLD independent of several confounders added to the models. Moreover, in a study by Chung et al[60] “simple meal pattern” characterized by a high intake of root and yellow vegetables, fruits, dairy products, eggs and nuts also exhibited an inverse correlation with NAFLD. Kalafati et al[55] found that individuals in the second quartile of the unsaturated fatty acids pattern, a dietary pattern with strong antioxidant properties, had 55.7% reduced odds of developing NAFLD than those in the first quartile, after adjusting for several confounders. However, higher consumption of unsaturated fatty acids was not associated with further protection from NAFLD, which may be explained by the fact that a greater intake of this diet leads to higher energy intake. Moreover, the mentioned authors found that the score for the prudent pattern (recognized also as a healthy dietary pattern) based on oil-based cooked vegetables, legumes, potatoes, fruits, vegetables and fatty fish was negatively associated with TG and uric acid levels, mediators of the associations between obesity and the incidence of NAFLD[61]. Only one study, presented by Alferink et al[62], found that adherence to vegetable and fish patterns (a kind of healthy pattern) was not associated with the risk of NAFLD.
The protective effect of healthy diets on the risk of the NAFLD could be a consequence of high consumption of vegetables and moderate intake of fruits, which are sources of antioxidant vitamins, such as vitamins A, E and C (protective against oxidative stress)[43]. Moreover, fruits and vegetables are good sources of dietary fiber, which has an inverse association with IR and the risk of NAFLD progression. Fish are sources of long-chain n-3 PUFAs, which are capable of reducing TGs and have a protective role against NAFLD[38].
Although definitions of Western dietary patterns vary, this diet is often characterized by high consumption of soft drinks, red and processed meat and refined cereals, with concurrently low intake of fish, fruit and vegetables as well as whole grains[63]. Therefore, this diet is characterized by a high intake of animal and trans fats, sugar and fructose and a low intake of fiber and phytochemicals[52]. It was observed that when a western diet is provided in excess, even for a short period of 1 wk, it leads to increased hepatic steatosis[33]. In Table 2, associations between Western and traditional and healthy dietary patterns and the risk/prevalence of NAFLD are summarized.
Oddy et al[52], in their prospective cohort study, found that a higher score of the Western dietary pattern at 14 years of age was associated with a greater risk of NAFLD at 17 years. Similar results were obtained in other observational studies[34,56]. On the other hand, some studies report significant associations of this diet with the risk of NAFLD[58,60]. In the literature, the following dietary patterns familiar to the western patterns are also present: Fast food[54,55]; animal food/high protein[53,54]; red meat and alcohol[62]; high-salt[57]; high-fat dairy and refined grains[62]; high-carbohydrate/sweet/sugar/high fruits[53,64,65]; as well as, snacks and energy-dense dietary pattern[58,59,62]. The majority of these dietary patterns increased the risk of NAFLD. Although high-carbohydrate/sweet/sugar/high fruits dietary pattern was associated with a significantly higher risk of NAFLD, Jia et al[65] found that this diet was positively associated with the prevalence of NAFLD only in females but not in males. Overall, Hassani Zadeh et al[43], in their meta-analysis, found that Western dietary patterns increased the risk of NAFLD by 56%.
The Western dietary pattern rich in saturated and trans-fatty acids may affect the hepatic cell steatosis via chylomicron uptake[34]. This dietary pattern, due to high amounts of refined grains, white bread and sugar-sweetened beverages has been also strongly associated with IR, diabetes and obesity. Soft drinks, the main constituents of the Western diet, contain substantial amounts of added sugars and fructose[66]. It was indicated that a higher intake of fructose induces hepatic IR and inflammation, thereby fueling the development of NAFLD. In addition, fructose metabolism could promote hepatic lipogenesis by inhibiting the DNL pathway and regulating lipogenic gene expression in the liver[67]. It should be noted that moderate consumption of fruits due to the presence of other dietary components such as dietary fiber and antioxidant vitamins can have a protective effect against NAFLD. On the other hand, excessive fruit consumption, as was seen in a study by Fakhoury-Sayegh et al[64], may increase the risk for NAFLD, due to the high content of simple sugars (especially fructose).
The traditional diet may differ depending on the region or country and encompasses the common foods eaten there. Since this pattern comprises both healthy and unhealthy food items in different proportions, in collected studies we can observe the different influences of this pattern on the risk of NAFLD. For example, in a Korean study[60] the traditional diet was characterized by high intake of vegetables, fermented vegetables, such as kimchi, fish and seafood, mushrooms and fermented, processed and natural soybeans and was associated with a higher risk of NAFLD independent of several confounders added to this model. A traditional Iranian dietary pattern characterized by intake of red and organ meats, dairy products, condiments, salt, tea and coffee and low fruits consumption was related to an increased risk of NAFLD[54]. However, in another Iranian study[34], a traditional diet, represented by a high intake of red meat organ meats, skinless poultry, eggs, yogurt drink, tea, legumes, tomato sauce, sugars sweets-desserts, potato, condiments, salt, pickles and broth, was not associated with risk of NAFLD. Similar observations were reported by Yang et al[57] and Adriano et al[59] where traditional Chinese food items (staple food, coarse grains, fruits, eggs, fish and shrimp, milk and tea) and traditional Brazilian foods (rice, beans, bread/toast, tea/coffee, and sweet products/ desserts/sugar) were not associated with a risk of NAFLD. In turn, Alferink et al[62] found that traditional Dutch dietary patterns consisting of vegetable oils, stanols and margarine or butter, potatoes, whole grains, and sweet snacks or desserts were associated with regression of NAFLD. Similar observations revealed that the traditional Lebanon diet (characterized by high intake of vegetables, chickpeas, red beans, lentils, peas, and vegetable oil/olives) was also related to a lower risk of NAFLD[64].
Lifestyle modification, including a change in diet, weight loss target and structured exercise/physical intervention is the first-line and a cornerstone therapy for the NAFLD condition. It is implemented to reduce the cardiometabolic risk factors and cardiovascular disease events and to resolve NAFLD. Table 3 displays the NAFLD diet treatment recommendations/guidelines of The European Association for the Study of the Liver[48] and the European Society for Clinical Nutrition and Metabolism[68], in addition to the American Association for the Study of Liver Diseases[69,70], the Asian Pacific Association for the Study of the Liver[71], the American Gastroenterological Association[7] and the World Gastroenterology Organization[72].
| EASL/EASD/EASO clinical practice guidelines for the management of NAFLD[48] | ESPEN guideline on clinical nutrition in liver disease[68] | AASLD practice guidance: The diagnosis and management of NAFLD[69,70] | APASL clinical practice guidelines for the management of MAFLD[71] | AGA clinical practice guidelines for diagnosis and management of NAFLD[7] | WGO guidance for NAFLD/NASH[72] | |
| Target for weight loss | 7%-10% | 7%-10% (in obese patient); > 10% to improve fibrosis | 3%-5% (to improve steatosis); 7%-10% for histological improvement | 7%-10% | ≥ 5% if steatosis; ≥ 7% if NASH; ≥ 10% if fibrosis | 5%-10% |
| Macronutrient composition | Low to moderate fat and moderate to higher carbohydrate; low carbohydrate, ketogenic diets or high protein | Irrespective of macronutrient composition, MD to improve steatosis and IR | Less relevant | Low-carbohydrate, low-fat and Mediterranean-type diets | Minimize SFA, ↓ red and processed meat | Avoid trans-fats; ↑ omega 3/omega 6 PUFA |
| Energy restriction | Hypocaloric: Reduction of 500-1000 kcal/d target weight loss of 0.5-1.0 kg/wk | Hypocaloric diet according to obesity guidance | Hypocaloric diet reduction of 500-1000 kcal/d | Hypocaloric diet reduction of 500-1000 kcal/d | Hypocaloric: 1200-1500 kcal/d or ↓ from baseline 500-1000 kcal/d | Hypocaloric: ↓ calories intake 25% |
| Fructose intake | Avoid fructose-containing beverages and foods | Avoid fructose commercially produced | Avoid fructose and soft drinks | |||
| Coffee intake | No liver-related limitations | “More likely to benefit health than harm” | “More likely to benefit health than harm” | |||
| Alcohol intake | Risk below (< 30 g men, < 20 g women) | Abstain | Not consume heavy amounts | Restrict |
The primary dietary goal for patients with NAFLD is to implement a hypocaloric diet due to a caloric deficit. Most often, low-calorie diets lead to an energy deficit of 500-1000 calories. Ordinarily, overweight NAFLD patients are advised to have a deficit of at least 500 calories/d for weight loss[10,48,73,74]. A weight loss of 3%-5% of body weight is necessary to improve liver steatosis[10]. To improve most of the histopathological characteristics in NAFLD, hepatocyte ballooning, lobular inflammation and fibrosis, a greater loss of body weight of 7%-10% is required[75]. Meta-analysis of 8 randomized controlled trials confirm that a 7% reduction in body weight was associated with improvement of the NAFLD Activity Score[76]. But, it should be noted that 94% of patients who lost 5% of initial body weight stabilized/or improved liver fibrosis[77]. Meta-analyses of 22 randomized controlled trials with 2588 participants reported that weight-loss interventions were significantly associated with improvements in alanine aminotransferase (ALT), ultrasonography pronounced liver steatosis, NAFLD Activity Score and presence of steatohepatitis[11]. Caloric restriction alone or in combination with physical activity encourages the loss of body weight and reduces hepatic steatosis and subsequently promotes fat mobilization from the liver[70]. In adults with NAFLD, exercise alone may prevent, reduce and cured liver steatosis. However, the ability of physical activity to improve other NAFLD spectrum histological parameters remains unknown.
Based on the current evidence, there is no consensus on the ideal macronutrient composition of the diet for NAFLD patients. The best nutrition recommendation is a traceable diet, based on individual preferences, eating habits and behaviors[74]. Also, there is no solid evidence to support a particular macronutrient composition of a hypocaloric diet unique for use in NAFLD patients. Independent of weight loss, a diet low in carbohydrates and higher in protein intake is associated with improvements of metabolic parameters in NAFLD patients[73,78]. A recent meta-analysis 32 controlled isocaloric feeding studies with a constant proportion of protein in the diet and varying ratios of carbohydrate and fat indicates that diet differences are too small, which implies the importance of caloric intake in NAFLD patients[79]. Overall, more future studies on macronutrient composition in diet are needed.
As previously stated, Mediterranean dietary patterns prevent the onset of NAFLD. The MD is also the most recommended diet for the treatment of NAFLD patients[12]. It improves liver steatosis, as indicated by the results of several studies, regardless of whether there is a calorie restriction in the diet. Independent of weight loss, patients have greater reductions in intrahepatic lipid content and insulin sensitivity after following the MD compared to a low-fat/high-carbohydrate diet. Consumption of a MD with calories less than the required daily energy allowed male NAFLD patients to reduce body weight, lipid accumulation, visceral adiposity index, fatty liver index, hepatic steatosis index and IR, as well as a reduced share of SFA in the serum fatty acid profile decreased serum levels of SFAs and increased serum levels of MUFAs and n-3 PUFA[80]. The MD has well-documented metabolic benefits to reduce cardiovascular risk and thus is well valued in the medical community[81]. This observation is important because NAFLD patients have an increased risk of cardiovascular disease.
A systematic review and meta-analysis of randomized controlled trials presented that Mediterranean and hypocaloric dietary interventions favoring unsaturated fatty acids led to improved intrahepatic lipid content and transaminases levels (ALT, aspartate aminotransferase) in NAFLD patients[82]. The gamma-glutamyl transferase level does not change significantly during the Mediterranean dietary interventions[82]. Diet compositions in randomized controlled trials used in these meta-analyses can be considered comparable. Based on the calculated NAFLD fibrosis score, the composite score of age, glucose levels, platelet count, albumin and aspartate aminotransferase/ALT ratio, indicated that risk for advanced hepatic fibrosis was 11% among NAFLD patients with incidentally discovered hepatic steatosis[76]. In patients with NAFLD, gamma-glutamyl transferase levels decreased only after low glycemic index-MD intervention[83]. Hence, it is confirmed that MD without caloric restriction reduced the liver fat. Since there are only a few studies examining dietary interventions in clinically advanced stages of NAFLD (active and fibrotic NASH), the optimal dietary recommendation for nutrition intervention in NAFLD remains to be defined.
Well-discussed risk factors for hepatic steatosis are high SFA intake and overconsumption of carbohydrates, such as fructose. This type of diet leads to obesity. Intervention studies provide clear and strong evidence of a link between excessive calorie intake and NAFLD development as well as being linked to excess energy intake with increased lipolysis, induced IR and increased harmful ceramides in plasma[15,16]. Excessive intake of SFA (1000 extra kcal/d) conducted in obese patients for 3 wk increased intrahepatic TG content more than the intake of unsaturated fats (+ 55% vs + 15%, respectively)[16]. Also, overconsumption of simple sugars increased the intrahepatic TG content (+ 33%) by stimulating DNL (+ 98%). In a review by Stokes et al[84], short-term hypocaloric diets (up to 16 wk) have shown beneficial effects in reducing intrahepatic lipid content. Also, research supports that carbohydrate restriction and consumption of unsaturated fatty acids have efficacious metabolic effects in NAFLD[12,81,84]. Obesity is closely related to low levels of n-3 PUFA in plasma phospholipids[85]. Dietary modifications including n-3 PUFA supplementation are considered to be suitable therapeutic strategies for obese NAFLD patients, though further clinical trials are required.
However, among NAFLD patients, weight loss is largely unsuccessful in the real world in the ambulatory and clinical settings[86]. However, more frequent clinical encounters and controls are associated with an increased likelihood of weight loss (enhanced probability of weight reduction). Therefore, national strategies are needed for targeted success in weight loss success in high-risk populations.
The newest popular dietary intervention in the past few years is time-restricted feeding as a form of daily intermittent fasting (IF). This dietary approach restricts the time between the first and last food intake, without emphasizing calorie restriction. IF implies a > 60% energy restriction on > 2 d/wk. In time-restricted eating, daily food intake is limited to 8-10 h. These diets with a limited eating window appear to be safe in the NAFLD population. Patients tolerate this diet well. The key feature of this dietetic approach is the so-called ”metabolic switch” that occurs 12 h after the cessation of food intake, where glycogen stores in the liver are depleted, and adipose tissue lipolysis increases[87]. This type of diet seems to be effective for weight loss, whereas many authors denied that the effect is still the result of a real calorie restriction. Patients with NAFLD follow the IF diet based on metabolic changes that are presented among overweight/obese individuals. A recent meta-analysis, involving patients with NAFLD, has shown that IF is beneficial in weight loss and liver enzyme levels[88]. However, no additional metabolic benefit has been shown compared to calorie-restricted diets[89]. In patients with NAFLD improvement in fatty liver index correlates with the number of fasting days and with the degree reduction in body mass index[90].
In a study performed by Cai et al[91], 271 NAFLD patients were randomized to time-restricted feeding, alternate-day fasting and control groups and were followed for 12 wk[91]. Findings from this study indicated that alternate-day fasting could be an effective diet method for weight loss and amelioration of lipid metabolism, with no direct effect in steatosis regression. In one Malaysian randomized controlled trial, 8 wk of IF with alternate-day calorie restriction resulted in the reduction of body weight and liver enzymes as well as hepatic steatosis compared to a habitual diet[92]. 8 wk of IF with limited caloric intake on alternating days led to a decrease in body weight and liver enzymes, as well as hepatic steatosis compared to the usual diet. Additional evidence for the benefit of IF to diminish hepatic steatosis and body weight compared to common lifestyle modification has been reported by 5:2 diet (intermittent calorie restriction: 600 kcal/d for men and 500 kcal/d for women for 2 non-consecutive days per week). But, the same effect was obtained in another group of participants on a low-carbohydrate high-fat diet (daily caloric intake: 1900 kcal/d for men and 1600 kcal/d for women)[89]. Data regarding IF efficacy in the steatosis/fibrosis regression are lacking. For now, it is important for medical practitioners not to advise this diet to patients with cirrhosis caused by NAFLD due to the well-known effect of starvation on the development of sarcopenia.
Evidence from two observational studies revealed that high adherence to the DASH-style diet is inversely associated with the risk of developing NAFLD[93,94]. It is indicated that subjects who fully adhered to the DASH diet were 30% less likely to have NAFLD. DASH is a low-glycemic index and low energy-dense diet, emphasizing low sodium intake and minimal consumption of processed foods. It is well known that the DASH diet is associated with a reduction in cardiovascular risk, as originally intended for hypertension patients. A randomized controlled trial including 60 overweight/obese adults, with ultrasonography proven NAFLD showed that the DASH diet over 8 wk led to more effective weight loss, improvement of aminotransferases and markers of IR, TG and total-C/HDL-C ratio compared to a contemporary control diet[95]. The DASH diet may be a promising dietary option for NAFLD patients, as weight loss, improved cardiometabolic factors and regression of steatosis are surrogate markers of liver disease status and the main goals of NAFLD treatment. This diet has aroused interest among specialists who care for patients with NAFLD. Further studies are essential to assess the effects of the DASH diet on liver histology and the clinical outcome of patients with NAFLD.
Ketogenic diet (KD) is the most popular low-carbohydrate eating plan based on a strict restriction in carbohydrates (less than 20-50 g/d) consumption. The KD became a popular weight loss intervention among obese patients due to its effectiveness despite safety concerns of this diet plan if dyslipidemia is present[96]. Therefore, KD could have a positive impact on NAFLD, due to very low content of carbohydrates in the diet. However, it is not known if ketosis plays an additional role. Several mechanisms may be proposed links between ketosis and improvement of NAFLD. First, a ketogenic diet decreases insulin levels that lead to increased rate of fatty acid oxidation and decreased lipogenesis[97]. Then, restriction of carbohydrates encourages the formation of ketone bodies, which cause satiety by a still-unknown mechanism[98]. In turn, reduced calorie intake leads to weight loss.
Few studies have tested KD as a treatment strategy for NAFLD patients. Based on fat content, KD can be a normocaloric, hypocaloric or non-restricted caloric diet. Pérez-Guisado et al[99] conducted a pilot study on 14 overweight male patients with MetS and with ultrasonography-proven NAFLD. Patients fed unrestricted Mediterranean high-fat KD, high in unsaturated fats (i.e. olive oil and fish oil rich with omega-3 fatty acids). Adherence to Mediterranean high-fat KD showed a significant improvement in body weight, aminotransferases and LDL-C levels, and steatosis degree (21% of the patients had complete fatty liver regression)[99].
Mardinoglu et al[100] reported a 2-wk KD intervention (carbohydrate 20–30 g/d, fat 241 g/d, 3115 kcal/d) in 17 obese patients with NAFLD. Despite a slight weight loss, liver fat content (assessed by magnetic resonance spectroscopy) was reduced by 43.8% in obese patients in this study. At the same time, a concomitant decrease in de novo gene for liver lipogenesis was obtained[100]. Moreover, literature data indicated that normocaloric high-fat KD inhibits DNL and induces fatty acid oxidation, caused sustained weight loss and reduced hepatic fat accumulation[16,101]. Based on the above findings KD could be a potential therapeutic dietary intervention for addressing steatosis regression and weight loss. Future studies are needed on the KD effect on fibrosis regression and resolution of inflammation. Because ketosis may have beneficial effects independently of the diet composition, studies aiming to identify the specific role that ketone bodies play in the pathophysiology of NAFLD are warranted.
Study evidence from cross-sectional trials pronounced a directly proportional association between the intake of refined sugar (especially high fructose corn syrup) due to the consumption of sweet sugar beverages with the risk of developing NAFLD[81,102]. Patients with NAFLD consume 2-3 times more fructose. Higher fructose consumption is also related to an increased risk of having steatohepatitis and advanced fibrosis in NAFLD patients. Based on current evidence, fructose supplementation was linked with higher adiposity and enhanced visceral fat, hypertriglyceridemia and IR, occurs due to increase DNL in liver, in spite of similar weight gain when compared to glucose[103]. Increasing the frequency of the snacks with added sugar consumption led to a prominent increase in the hepatic fat content. The augmented hepatic steatosis was proportional to visceral fat accumulation and to the rise in DNL.
Fructose-rich diets, based on sugar-sweetened beverages increase hepatic synthesis of TG and are recognized as a major mediator of NAFLD[73]. It was observed that carbohydrate overfeeding in overweight persons consumed 1000 kcal/d from simple carbohydrates (sugar-sweetened soft drinks, candy, pineapple juice) for 3 wk caused a 10-times greater relative increase in fat content in the liver than in body weight (27% vs 2%, respectively)[104]. The recommendation to avoid sweet sugar beverages reduced the intake of extra empty calories and supported a caloric deficit for weight loss. Notably, high fructose consumption in NAFLD patients was compiled, with an increase in hepatic fructokinase and fatty acid synthase mRNA when compared to healthy persons[105]. Fructose can advance hepatic steatosis both directly via DNL and indirectly via DNL feedback inhibition of fatty acids. Overconsumption of fructose may increase the risk of developing NASH and advanced fibrosis, although the relationship may be confounded by excess energy intake or by unhealthy dietary patterns and sedentary lifestyle, which are common in NAFLD patients[106].
Current literature evidence suggests that higher fructose intake (> 20E% or 100–220 g/d) may adversely affect disease onset and progression[81]. Meta-analyses reported that moderate fructose consumption lower than 10% of energy (< 50 g/d for a 2000 kcal diet) does not induce weight gain or dyslipidemia. Sugar-sweetened beverage intake of ≥ 1 serving/d rises the risk of having NAFLD by 50%[33] and liver fibrosis by 250%[107]. It seems that artificially sweetened beverages and defined 100% fruit juices have similar effects as sugar-sweetened beverages. The results of a systematic review indicated that fructose in the diet isocalorically replaced with other carbohydrate sources for 1-10 wk did not affect NAFLD biomarkers[108]. Fructose overconsumption increases intrahepatic lipids and ALT levels. This effect results from excess energy intake rather than fructose consumption[108]. In the future, long-term prospective clinical trials are essential to understand and confirm a link between NAFLD progression and fructose consumption.
NAFLD patients who drink three to four cups of coffee per day will have more health benefits than harm, with the reduction in risk for various health outcome[109]. Nevertheless, a recent meta-analysis of 11 epidemiological studies confirmed association with regular coffee consumption and decreased risk of NAFLD[110]. Moreover in patients already diagnosed with NAFLD, coffee consumption reduced risk for the development of liver fibrosis[97,110]. A case-control study showed involvement of coffee in the fatty liver score, pronounced by ultrasound in all coffee consumers[111]. A systematic review deter
In conclusion, the Western dietary pattern characterized by high intake of soft drinks, red and processed meat and refined cereals with coincidentally low intake of fish, fruit and vegetables as well as whole grains tended to increase the risk of NAFLD. The healthy and Mediterranean dietary patterns characterized by high consumption of vegetables, fruits, nuts, olive oil, low-fat dairy products and fish were linked with a reduced NAFLD risk. More prospective cohort studies are needed to confirm the association between dietary patterns and NAFLD risk. Macronutrient composition and excessive caloric intake are critical determinates of obesity and liver health. DASH, IF and KD have aroused interest among specialists who care for patients with NAFLD. Further well-designed studies are needed to assess the effects of these diets on liver-related outcomes and liver histology. Dietary advice should be provided by a multidisciplinary team with a specialized dietitian as an individual approach, as we already know that our genetics and gut microbiota cause differences in the effects of the diet to our metabolism. Future research in field interaction overfeeding and genomics are warranted, as are of the inter-individual difference of liver steatoses.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Serbia
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Tziomalos K, Greece; Xing HC, China S-Editor: Fan JR L-Editor: A P-Editor: Fan JR
| 1. | Benedict M, Zhang X. Non-alcoholic fatty liver disease: An expanded review. World J Hepatol. 2017;9:715-732. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 547] [Cited by in RCA: 506] [Article Influence: 63.3] [Reference Citation Analysis (17)] |
| 2. | Wong MCS, Huang JLW, George J, Huang J, Leung C, Eslam M, Chan HLY, Ng SC. The changing epidemiology of liver diseases in the Asia-Pacific region. Nat Rev Gastroenterol Hepatol. 2019;16:57-73. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 248] [Cited by in RCA: 237] [Article Influence: 39.5] [Reference Citation Analysis (7)] |
| 3. | Cotter TG, Rinella M. Nonalcoholic Fatty Liver Disease 2020: The State of the Disease. Gastroenterology. 2020;158:1851-1864. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 411] [Cited by in RCA: 835] [Article Influence: 167.0] [Reference Citation Analysis (2)] |
| 4. | Adibi A, Maleki S, Adibi P, Etminani R, Hovsepian S. Prevalence of Nonalcoholic Fatty Liver Disease and its Related Metabolic Risk Factors in Isfahan, Iran. Adv Biomed Res. 2017;6:47. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 23] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 5. | Wong MCS, Huang J. The growing burden of liver cirrhosis: implications for preventive measures. Hepatol Int. 2018;12:201-203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 27] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 6. | Godoy-Matos AF, Silva Júnior WS, Valerio CM. NAFLD as a continuum: from obesity to metabolic syndrome and diabetes. Diabetol Metab Syndr. 2020;12:60. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 184] [Cited by in RCA: 394] [Article Influence: 78.8] [Reference Citation Analysis (0)] |
| 7. | Younossi ZM, Corey KE, Lim JK. AGA Clinical Practice Update on Lifestyle Modification Using Diet and Exercise to Achieve Weight Loss in the Management of Nonalcoholic Fatty Liver Disease: Expert Review. Gastroenterology. 2021;160:912-918. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 343] [Cited by in RCA: 327] [Article Influence: 81.8] [Reference Citation Analysis (2)] |
| 8. | Ristic-Medic D, Petrovic S, Arsic A, Vucic V. Liver disease and COVID-19: The link with oxidative stress, antioxidants and nutrition. World J Gastroenterol. 2021;27:5682-5699. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 21] [Cited by in RCA: 14] [Article Influence: 3.5] [Reference Citation Analysis (2)] |
| 9. | Polyzos SA, Kountouras J, Mantzoros CS. Obesity and nonalcoholic fatty liver disease: From pathophysiology to therapeutics. Metabolism. 2019;92:82-97. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 453] [Cited by in RCA: 800] [Article Influence: 133.3] [Reference Citation Analysis (0)] |
| 10. | Sanyal AJ. Putting non-alcoholic fatty liver disease on the radar for primary care physicians: how well are we doing? BMC Med. 2018;16:148. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 24] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 11. | Koutoukidis DA, Astbury NM, Tudor KE, Morris E, Henry JA, Noreik M, Jebb SA, Aveyard P. Association of Weight Loss Interventions With Changes in Biomarkers of Nonalcoholic Fatty Liver Disease: A Systematic Review and Meta-analysis. JAMA Intern Med. 2019;179:1262-1271. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 121] [Cited by in RCA: 176] [Article Influence: 29.3] [Reference Citation Analysis (0)] |
| 12. | Pugliese N, Plaz Torres MC, Petta S, Valenti L, Giannini EG, Aghemo A. Is there an 'ideal' diet for patients with NAFLD? Eur J Clin Invest. 2022;52:e13659. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 40] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 13. | Eslam M, Valenti L, Romeo S. Genetics and epigenetics of NAFLD and NASH: Clinical impact. J Hepatol. 2018;68:268-279. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 732] [Cited by in RCA: 689] [Article Influence: 98.4] [Reference Citation Analysis (0)] |
| 14. | Mota M, Banini BA, Cazanave SC, Sanyal AJ. Molecular mechanisms of lipotoxicity and glucotoxicity in nonalcoholic fatty liver disease. Metabolism. 2016;65:1049-1061. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 294] [Cited by in RCA: 415] [Article Influence: 46.1] [Reference Citation Analysis (0)] |
| 15. | Rosqvist F, Kullberg J, Ståhlman M, Cedernaes J, Heurling K, Johansson HE, Iggman D, Wilking H, Larsson A, Eriksson O, Johansson L, Straniero S, Rudling M, Antoni G, Lubberink M, Orho-Melander M, Borén J, Ahlström H, Risérus U. Overeating Saturated Fat Promotes Fatty Liver and Ceramides Compared With Polyunsaturated Fat: A Randomized Trial. J Clin Endocrinol Metab. 2019;104:6207-6219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 161] [Article Influence: 26.8] [Reference Citation Analysis (0)] |
| 16. | Luukkonen PK, Sädevirta S, Zhou Y, Kayser B, Ali A, Ahonen L, Lallukka S, Pelloux V, Gaggini M, Jian C, Hakkarainen A, Lundbom N, Gylling H, Salonen A, Orešič M, Hyötyläinen T, Orho-Melander M, Rissanen A, Gastaldelli A, Clément K, Hodson L, Yki-Järvinen H. Saturated Fat Is More Metabolically Harmful for the Human Liver Than Unsaturated Fat or Simple Sugars. Diabetes Care. 2018;41:1732-1739. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 220] [Cited by in RCA: 304] [Article Influence: 43.4] [Reference Citation Analysis (1)] |
| 17. | Meex RCR, Blaak EE. Mitochondrial Dysfunction is a Key Pathway that Links Saturated Fat Intake to the Development and Progression of NAFLD. Mol Nutr Food Res. 2021;65:e1900942. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 65] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 18. | Satapati S, Kucejova B, Duarte JA, Fletcher JA, Reynolds L, Sunny NE, He T, Nair LA, Livingston KA, Fu X, Merritt ME, Sherry AD, Malloy CR, Shelton JM, Lambert J, Parks EJ, Corbin I, Magnuson MA, Browning JD, Burgess SC. Mitochondrial metabolism mediates oxidative stress and inflammation in fatty liver. J Clin Invest. 2016;126:1605. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 36] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 19. | Sullivan EM, Fix A, Crouch MJ, Sparagna GC, Zeczycki TN, Brown DA, Shaikh SR. Murine diet-induced obesity remodels cardiac and liver mitochondrial phospholipid acyl chains with differential effects on respiratory enzyme activity. J Nutr Biochem. 2017;45:94-103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 30] [Article Influence: 3.8] [Reference Citation Analysis (1)] |
| 20. | Donnelly KL, Smith CI, Schwarzenberg SJ, Jessurun J, Boldt MD, Parks EJ. Sources of fatty acids stored in liver and secreted via lipoproteins in patients with nonalcoholic fatty liver disease. J Clin Invest. 2005;115:1343-1351. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2112] [Cited by in RCA: 2589] [Article Influence: 129.5] [Reference Citation Analysis (0)] |
| 21. | Nati M, Haddad D, Birkenfeld AL, Koch CA, Chavakis T, Chatzigeorgiou A. The role of immune cells in metabolism-related liver inflammation and development of non-alcoholic steatohepatitis (NASH). Rev Endocr Metab Disord. 2016;17:29-39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 106] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 22. | Zorena K, Jachimowicz-Duda O, Ślęzak D, Robakowska M, Mrugacz M. Adipokines and Obesity. Potential Link to Metabolic Disorders and Chronic Complications. Int J Mol Sci. 2020;21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 155] [Cited by in RCA: 247] [Article Influence: 49.4] [Reference Citation Analysis (0)] |
| 23. | Monda V, Polito R, Lovino A, Finaldi A, Valenzano A, Nigro E, Corso G, Sessa F, Asmundo A, Nunno ND, Cibelli G, Messina G. Short-Term Physiological Effects of a Very Low-Calorie Ketogenic Diet: Effects on Adiponectin Levels and Inflammatory States. Int J Mol Sci. 2020;21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 66] [Cited by in RCA: 57] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 24. | Flehmig G, Scholz M, Klöting N, Fasshauer M, Tönjes A, Stumvoll M, Youn BS, Blüher M. Identification of adipokine clusters related to parameters of fat mass, insulin sensitivity and inflammation. PLoS One. 2014;9:e99785. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 92] [Cited by in RCA: 109] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 25. | Morioka T, Emoto M, Yamazaki Y, Kawano N, Imamura S, Numaguchi R, Urata H, Motoyama K, Mori K, Fukumoto S, Koyama H, Shoji T, Inaba M. Leptin is associated with vascular endothelial function in overweight patients with type 2 diabetes. Cardiovasc Diabetol. 2014;13:10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 33] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 26. | Jung CH, Kim BY, Mok JO, Kang SK, Kim CH. Association between serum adipocytokine levels and microangiopathies in patients with type 2 diabetes mellitus. J Diabetes Investig. 2014;5:333-339. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 45] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 27. | Zhang Q, Wang J, Huang F, Yao Y, Xu L. Leptin induces NAFLD progression through infiltrated CD8+ T lymphocytes mediating pyroptotic-like cell death of hepatocytes and macrophages. Dig Liver Dis. 2021;53:598-605. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 27] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 28. | Käräjämäki AJ, Bloigu R, Kauma H, Kesäniemi YA, Koivurova OP, Perkiömäki J, Huikuri H, Ukkola O. Non-alcoholic fatty liver disease with and without metabolic syndrome: Different long-term outcomes. Metabolism. 2017;66:55-63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 48] [Article Influence: 6.0] [Reference Citation Analysis (1)] |
| 29. | Ballestri S, Zona S, Targher G, Romagnoli D, Baldelli E, Nascimbeni F, Roverato A, Guaraldi G, Lonardo A. Nonalcoholic fatty liver disease is associated with an almost twofold increased risk of incident type 2 diabetes and metabolic syndrome. Evidence from a systematic review and meta-analysis. J Gastroenterol Hepatol. 2016;31:936-944. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 571] [Cited by in RCA: 540] [Article Influence: 60.0] [Reference Citation Analysis (0)] |
| 30. | Loomba R, Abraham M, Unalp A, Wilson L, Lavine J, Doo E, Bass NM; Nonalcoholic Steatohepatitis Clinical Research Network. Association between diabetes, family history of diabetes, and risk of nonalcoholic steatohepatitis and fibrosis. Hepatology. 2012;56:943-951. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 363] [Cited by in RCA: 367] [Article Influence: 28.2] [Reference Citation Analysis (0)] |
| 31. | Ghorpade DS, Ozcan L, Zheng Z, Nicoloro SM, Shen Y, Chen E, Blüher M, Czech MP, Tabas I. Hepatocyte-secreted DPP4 in obesity promotes adipose inflammation and insulin resistance. Nature. 2018;555:673-677. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 215] [Article Influence: 30.7] [Reference Citation Analysis (0)] |
| 32. | Barchetta I, Ceccarelli V, Cimini FA, Barone E, Sentinelli F, Coluzzi M, Chiappetta C, Bertoccini L, Tramutola A, Labbadia G, Di Cristofano C, Silecchia G, Leonetti F, Cavallo MG. Circulating dipeptidyl peptidase-4 is independently associated with the presence and severity of NAFLD/NASH in individuals with and without obesity and metabolic disease. J Endocrinol Invest. 2021;44:979-988. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 24] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 33. | Zelber-Sagi S, Salomone F, Mlynarsky L. The Mediterranean dietary pattern as the diet of choice for non-alcoholic fatty liver disease: Evidence and plausible mechanisms. Liver Int. 2017;37:936-949. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 184] [Article Influence: 23.0] [Reference Citation Analysis (0)] |
| 34. | Salehi-Sahlabadi A, Sadat S, Beigrezaei S, Pourmasomi M, Feizi A, Ghiasvand R, Hadi A, Clark CCT, Miraghajani M. Dietary patterns and risk of non-alcoholic fatty liver disease. BMC Gastroenterol. 2021;21:41. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 59] [Article Influence: 14.8] [Reference Citation Analysis (2)] |
| 35. | Pérez-Martínez P, Mikhailidis DP, Athyros VG, Bullo M, Couture P, Covas MI, de Koning L, Delgado-Lista J, Díaz-López A, Drevon CA, Estruch R, Esposito K, Fitó M, Garaulet M, Giugliano D, García-Ríos A, Katsiki N, Kolovou G, Lamarche B, Maiorino MI, Mena-Sánchez G, Muñoz-Garach A, Nikolic D, Ordovás JM, Pérez-Jiménez F, Rizzo M, Salas-Salvadó J, Schröder H, Tinahones FJ, de la Torre R, van Ommen B, Wopereis S, Ros E, López-Miranda J. Lifestyle recommendations for the prevention and management of metabolic syndrome: an international panel recommendation. Nutr Rev. 2017;75:307-326. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 322] [Cited by in RCA: 281] [Article Influence: 35.1] [Reference Citation Analysis (0)] |
| 36. | Bajerska J, Chmurzynska A, Muzsik A, Krzyżanowska P, Mądry E, Malinowska AM, Walkowiak J. Weight loss and metabolic health effects from energy-restricted Mediterranean and Central-European diets in postmenopausal women: A randomized controlled trial. Sci Rep. 2018;8:11170. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 40] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 37. | Gosal H, Kaur H, Chakwop Ngassa H, Elmenawi KA, Anil V, Mohammed L. The Significance of the Mediterranean Diet in the Management of Non-Alcoholic Fatty Liver Disease: A Systematic Review. Cureus. 2021;13:e15618. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 38. | Gil A, Gil F. Fish, a Mediterranean source of n-3 PUFA: benefits do not justify limiting consumption. Br J Nutr. 2015;113 Suppl 2:S58-S67. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 92] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 39. | Kastorini CM, Milionis HJ, Esposito K, Giugliano D, Goudevenos JA, Panagiotakos DB. The effect of Mediterranean diet on metabolic syndrome and its components: a meta-analysis of 50 studies and 534,906 individuals. J Am Coll Cardiol. 2011;57:1299-1313. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 722] [Cited by in RCA: 734] [Article Influence: 52.4] [Reference Citation Analysis (0)] |
| 40. | Entezari MR, Talenezhad N, Mirzavandi F, Rahimpour S, Mozaffari-Khosravi H, Fallahzadeh H, Hosseinzadeh M. Mediterranean dietary pattern and non-alcoholic fatty liver diseases: a case-control study. J Nutr Sci. 2021;10:e55. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 41. | Giraldi L, Miele L, Aleksovska K, Manca F, Leoncini E, Biolato M, Arzani D, Pirro MA, Marrone G, Cefalo C, Racco S, Liguori A, Rapaccini G, Miggiano GA, Gasbarrini A, Boccia S, Grieco A. Mediterranean diet and the prevention of non-alcoholic fatty liver disease: results from a case-control study. Eur Rev Med Pharmacol Sci. 2020;24:7391-7398. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 42. | Baratta F, Pastori D, Polimeni L, Bucci T, Ceci F, Calabrese C, Ernesti I, Pannitteri G, Violi F, Angelico F, Del Ben M. Adherence to Mediterranean Diet and Non-Alcoholic Fatty Liver Disease: Effect on Insulin Resistance. Am J Gastroenterol. 2017;112:1832-1839. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 108] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 43. | Hassani Zadeh S, Mansoori A, Hosseinzadeh M. Relationship between dietary patterns and non-alcoholic fatty liver disease: A systematic review and meta-analysis. J Gastroenterol Hepatol. 2021;36:1470-1478. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 106] [Article Influence: 26.5] [Reference Citation Analysis (0)] |
| 44. | Kontogianni MD, Tileli N, Margariti A, Georgoulis M, Deutsch M, Tiniakos D, Fragopoulou E, Zafiropoulou R, Manios Y, Papatheodoridis G. Adherence to the Mediterranean diet is associated with the severity of non-alcoholic fatty liver disease. Clin Nutr. 2014;33:678-683. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 164] [Article Influence: 13.7] [Reference Citation Analysis (3)] |
| 45. | Aller R, Izaola O, de la Fuente B, De Luis Román DA. Mediterranean diet is associated with liver histology in patients with non alcoholic fatty liver disease. Nutr Hosp. 2015;32:2518-2524. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 19] [Reference Citation Analysis (0)] |
| 46. | Park SY, Noureddin M, Boushey C, Wilkens LR, Setiawan VW. Diet Quality Association with Nonalcoholic Fatty Liver Disease by Cirrhosis Status: The Multiethnic Cohort. Curr Dev Nutr. 2020;4:nzaa024. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 41] [Article Influence: 8.2] [Reference Citation Analysis (3)] |
| 47. | Chan R, Wong VW, Chu WC, Wong GL, Li LS, Leung J, Chim AM, Yeung DK, Sea MM, Woo J, Chan FK, Chan HL. Diet-Quality Scores and Prevalence of Nonalcoholic Fatty Liver Disease: A Population Study Using Proton-Magnetic Resonance Spectroscopy. PLoS One. 2015;10:e0139310. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 72] [Cited by in RCA: 79] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 48. | European Association for the Study of the Liver (EASL); European Association for the Study of Diabetes (EASD); European Association for the Study of Obesity (EASO). EASL-EASD-EASO Clinical Practice Guidelines for the management of non-alcoholic fatty liver disease. J Hepatol. 2016;64:1388-1402. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2290] [Cited by in RCA: 3173] [Article Influence: 352.6] [Reference Citation Analysis (4)] |
| 49. | Anania C, Perla FM, Olivero F, Pacifico L, Chiesa C. Mediterranean diet and nonalcoholic fatty liver disease. World J Gastroenterol. 2018;24:2083-2094. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 156] [Cited by in RCA: 174] [Article Influence: 24.9] [Reference Citation Analysis (0)] |
| 50. | Paniagua JA, Gallego de la Sacristana A, Romero I, Vidal-Puig A, Latre JM, Sanchez E, Perez-Martinez P, Lopez-Miranda J, Perez-Jimenez F. Monounsaturated fat-rich diet prevents central body fat distribution and decreases postprandial adiponectin expression induced by a carbohydrate-rich diet in insulin-resistant subjects. Diabetes Care. 2007;30:1717-1723. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 181] [Cited by in RCA: 162] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 51. | Assy N, Nassar F, Nasser G, Grosovski M. Olive oil consumption and non-alcoholic fatty liver disease. World J Gastroenterol. 2009;15:1809-1815. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 109] [Cited by in RCA: 112] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 52. | Oddy WH, Herbison CE, Jacoby P, Ambrosini GL, O'Sullivan TA, Ayonrinde OT, Olynyk JK, Black LJ, Beilin LJ, Mori TA, Hands BP, Adams LA. The Western dietary pattern is prospectively associated with nonalcoholic fatty liver disease in adolescence. Am J Gastroenterol. 2013;108:778-785. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 179] [Cited by in RCA: 207] [Article Influence: 17.3] [Reference Citation Analysis (3)] |
| 53. | Zhang S, Gu Y, Bian S, Górska MJ, Zhang Q, Liu L, Meng G, Yao Z, Wu H, Wang Y, Zhang T, Wang X, Sun S, Zhou M, Jia Q, Song K, Qi L, Niu K. Dietary patterns and risk of non-alcoholic fatty liver disease in adults: A prospective cohort study. Clin Nutr. 2021;40:5373-5382. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 31] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 54. | Dehghanseresht N, Jafarirad S, Alavinejad SP, Mansoori A. Association of the dietary patterns with the risk of non-alcoholic fatty liver disease among Iranian population: a case-control study. Nutr J. 2020;19:63. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 55. | Kalafati IP, Borsa D, Dimitriou M, Revenas K, Kokkinos A, Dedoussis GV. Dietary patterns and non-alcoholic fatty liver disease in a Greek case-control study. Nutrition. 2019;61:105-110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 44] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 56. | Tutunchi H, Saghafi-Asl M, Asghari-Jafarabadi M, Ostadrahimi A. Association between Dietary Patterns and Non-alcoholic Fatty Liver Disease: Results from a Case-Control Study. Arch Iran Med. 2021;24:35-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 15] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 57. | Yang CQ, Shu L, Wang S, Wang JJ, Zhou Y, Xuan YJ, Wang SF. Dietary Patterns Modulate the Risk of Non-Alcoholic Fatty Liver Disease in Chinese Adults. Nutrients. 2015;7:4778-4791. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 57] [Cited by in RCA: 76] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 58. | Nakashita C, Xi L, Inoue Y, Kabura R, Masuda S, Yamano Y, Katoh T. Impact of dietary compositions and patterns on the prevalence of nonalcoholic fatty liver disease in Japanese men: a cross-sectional study. BMC Gastroenterol. 2021;21:342. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 59. | Adriano LS, Sampaio HA, Arruda SP, Portela CL, de Melo MLP, Carioca AA, Soares NT. Healthy dietary pattern is inversely associated with non-alcoholic fatty liver disease in elderly. Br J Nutr. 2016;115:2189-2195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 44] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 60. | Chung GE, Youn J, Kim YS, Lee JE, Yang SY, Lim JH, Song JH, Doo EY, Kim JS. Dietary patterns are associated with the prevalence of nonalcoholic fatty liver disease in Korean adults. Nutrition. 2019;62:32-38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 38] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 61. | Zhang Q, Ma X, Xing J, Shi H, Yang R, Jiao Y, Chen S, Wu S, Zhang S, Sun X. Serum Uric Acid Is a Mediator of the Association Between Obesity and Incident Nonalcoholic Fatty Liver Disease: A Prospective Cohort Study. Front Endocrinol (Lausanne). 2021;12:657856. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 14] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 62. | Alferink LJM, Erler NS, de Knegt RJ, Janssen HLA, Metselaar HJ, Darwish Murad S, Kiefte-de Jong JC. Adherence to a plant-based, high-fibre dietary pattern is related to regression of non-alcoholic fatty liver disease in an elderly population. Eur J Epidemiol. 2020;35:1069-1085. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 41] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 63. | Drake I, Sonestedt E, Ericson U, Wallström P, Orho-Melander M. A Western dietary pattern is prospectively associated with cardio-metabolic traits and incidence of the metabolic syndrome. Br J Nutr. 2018;119:1168-1176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 88] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 64. | Fakhoury-Sayegh N, Younes H, Heraoui GNHA, Sayegh R. Nutritional Profile and Dietary Patterns of Lebanese Non-Alcoholic Fatty Liver Disease Patients: A Case-Control Study. Nutrients. 2017;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 34] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 65. | Jia Q, Xia Y, Zhang Q, Wu H, Du H, Liu L, Wang C, Shi H, Guo X, Liu X, Li C, Sun S, Wang X, Zhao H, Song K, Huang G, Wu Y, Cui N, Niu K. Dietary patterns are associated with prevalence of fatty liver disease in adults. Eur J Clin Nutr. 2015;69:914-921. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 89] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 66. | Zhang S, Gu Y, Bian S, Lu Z, Zhang Q, Liu L, Meng G, Yao Z, Wu H, Wang Y, Zhang T, Wang X, Sun S, Zhou M, Jia Q, Song K, Qi L, Niu K. Soft drink consumption and risk of nonalcoholic fatty liver disease: results from the Tianjin Chronic Low-Grade Systemic Inflammation and Health (TCLSIH) cohort study. Am J Clin Nutr. 2021;113:1265-1274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 43] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 67. | Softic S, Cohen DE, Kahn CR. Role of Dietary Fructose and Hepatic De Novo Lipogenesis in Fatty Liver Disease. Dig Dis Sci. 2016;61:1282-1293. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 444] [Cited by in RCA: 481] [Article Influence: 53.4] [Reference Citation Analysis (0)] |
| 68. | Bischoff SC, Austin P, Boeykens K, Chourdakis M, Cuerda C, Jonkers-Schuitema C, Lichota M, Nyulasi I, Schneider SM, Stanga Z, Pironi L. ESPEN guideline on home enteral nutrition. Clin Nutr. 2020;39:5-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 162] [Article Influence: 32.4] [Reference Citation Analysis (0)] |
| 69. | The diagnosis and management of nonalcoholic fatty liver disease: Practice guidance from the American Association for the Study of Liver Diseases. Clin Liver Dis (Hoboken). 2018;11:81. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 42] [Article Influence: 6.0] [Reference Citation Analysis (1)] |
| 70. | Chalasani N, Younossi Z, Lavine JE, Charlton M, Cusi K, Rinella M, Harrison SA, Brunt EM, Sanyal AJ. The diagnosis and management of nonalcoholic fatty liver disease: Practice guidance from the American Association for the Study of Liver Diseases. Hepatology. 2018;67:328-357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3544] [Cited by in RCA: 4928] [Article Influence: 704.0] [Reference Citation Analysis (9)] |
| 71. | Eslam M, Sarin SK, Wong VW, Fan JG, Kawaguchi T, Ahn SH, Zheng MH, Shiha G, Yilmaz Y, Gani R, Alam S, Dan YY, Kao JH, Hamid S, Cua IH, Chan WK, Payawal D, Tan SS, Tanwandee T, Adams LA, Kumar M, Omata M, George J. The Asian Pacific Association for the Study of the Liver clinical practice guidelines for the diagnosis and management of metabolic associated fatty liver disease. Hepatol Int. 2020;14:889-919. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 563] [Cited by in RCA: 551] [Article Influence: 110.2] [Reference Citation Analysis (0)] |
| 72. | Review Team. , LaBrecque DR, Abbas Z, Anania F, Ferenci P, Khan AG, Goh KL, Hamid SS, Isakov V, Lizarzabal M, Peñaranda MM, Ramos JF, Sarin S, Stimac D, Thomson AB, Umar M, Krabshuis J, LeMair A; World Gastroenterology Organisation. World Gastroenterology Organisation global guidelines: Nonalcoholic fatty liver disease and nonalcoholic steatohepatitis. J Clin Gastroenterol. 2014;48:467-473. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 278] [Cited by in RCA: 275] [Article Influence: 25.0] [Reference Citation Analysis (0)] |
| 73. | Barrera F, George J. The role of diet and nutritional intervention for the management of patients with NAFLD. Clin Liver Dis. 2014;18:91-112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 97] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 74. | Raynor HA, Champagne CM. Position of the Academy of Nutrition and Dietetics: Interventions for the Treatment of Overweight and Obesity in Adults. J Acad Nutr Diet. 2016;116:129-147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 185] [Cited by in RCA: 225] [Article Influence: 25.0] [Reference Citation Analysis (0)] |
| 75. | Hohenester S, Christiansen S, Nagel J, Wimmer R, Artmann R, Denk G, Bischoff M, Bischoff G, Rust C. Lifestyle intervention for morbid obesity: effects on liver steatosis, inflammation, and fibrosis. Am J Physiol Gastrointest Liver Physiol. 2018;315:G329-G338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 34] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 76. | Musso G, Cassader M, Rosina F, Gambino R. Impact of current treatments on liver disease, glucose metabolism and cardiovascular risk in non-alcoholic fatty liver disease (NAFLD): a systematic review and meta-analysis of randomised trials. Diabetologia. 2012;55:885-904. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 541] [Cited by in RCA: 492] [Article Influence: 37.8] [Reference Citation Analysis (0)] |
| 77. | Hsu CC, Ness E, Kowdley KV. Nutritional Approaches to Achieve Weight Loss in Nonalcoholic Fatty Liver Disease. Adv Nutr. 2017;8:253-265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 39] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 78. | Miller EF. Nutrition Management Strategies for Nonalcoholic Fatty Liver Disease: Treatment and Prevention. Clin Liver Dis (Hoboken). 2020;15:144-148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 21] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 79. | Hall KD, Guo J. Obesity Energetics: Body Weight Regulation and the Effects of Diet Composition. Gastroenterology. 2017;152:1718-1727.e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 189] [Cited by in RCA: 228] [Article Influence: 28.5] [Reference Citation Analysis (0)] |
| 80. | Ristic-Medic D, Kovacic M, Takic M, Arsic A, Petrovic S, Paunovic M, Jovicic M, Vucic V. Calorie-Restricted Mediterranean and Low-Fat Diets Affect Fatty Acid Status in Individuals with Nonalcoholic Fatty Liver Disease. Nutrients. 2020;13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 60] [Cited by in RCA: 51] [Article Influence: 10.2] [Reference Citation Analysis (1)] |
| 81. | George ES, Forsyth A, Itsiopoulos C, Nicoll AJ, Ryan M, Sood S, Roberts SK, Tierney AC. Practical Dietary Recommendations for the Prevention and Management of Nonalcoholic Fatty Liver Disease in Adults. Adv Nutr. 2018;9:30-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 67] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 82. | Houttu V, Csader S, Nieuwdorp M, Holleboom AG, Schwab U. Dietary Interventions in Patients With Non-alcoholic Fatty Liver Disease: A Systematic Review and Meta-Analysis. Front Nutr. 2021;8:716783. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 59] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 83. | Misciagna G, Del Pilar Díaz M, Caramia DV, Bonfiglio C, Franco I, Noviello MR, Chiloiro M, Abbrescia DI, Mirizzi A, Tanzi M, Caruso MG, Correale M, Reddavide R, Inguaggiato R, Cisternino AM, Osella AR. Effect of a Low Glycemic Index Mediterranean Diet on Non-Alcoholic Fatty Liver Disease. A Randomized Controlled Clinici Trial. J Nutr Health Aging. 2017;21:404-412. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 84] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 84. | Stokes CS, Lammert F, Krawczyk M. Short-term Dietary Interventions for the Management of Nonalcoholic Fatty Liver. Curr Med Chem. 2019;26:3483-3496. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 85. | Arsic A, Takic M, Kojadinovic M, Petrovic S, Paunovic M, Vucic V, Ristic Medic D. Metabolically healthy obesity: is there a link with polyunsaturated fatty acid intake and status? Can J Physiol Pharmacol. 2021;99:64-71. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 86. | Dudekula A, Rachakonda V, Shaik B, Behari J. Weight loss in nonalcoholic Fatty liver disease patients in an ambulatory care setting is largely unsuccessful but correlates with frequency of clinic visits. PLoS One. 2014;9:e111808. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 51] [Cited by in RCA: 58] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 87. | Kang J, Ratamess NA, Faigenbaum AD, Bush JA, Beller N, Vargas A, Fardman B, Andriopoulos T. Effect of Time-Restricted Feeding on Anthropometric, Metabolic, and Fitness Parameters: A Systematic Review. J Am Coll Nutr. 2021;1-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 17] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 88. | Yin C, Li Z, Xiang Y, Peng H, Yang P, Yuan S, Zhang X, Wu Y, Huang M, Li J. Effect of Intermittent Fasting on Non-Alcoholic Fatty Liver Disease: Systematic Review and Meta-Analysis. Front Nutr. 2021;8:709683. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 46] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 89. | Holmer M, Lindqvist C, Petersson S, Moshtaghi-Svensson J, Tillander V, Brismar TB, Hagström H, Stål P. Treatment of NAFLD with intermittent calorie restriction or low-carb high-fat diet - a randomised controlled trial. JHEP Rep. 2021;3:100256. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 107] [Article Influence: 26.8] [Reference Citation Analysis (0)] |
| 90. | Wilhelmi de Toledo F, Grundler F, Sirtori CR, Ruscica M. Unravelling the health effects of fasting: a long road from obesity treatment to healthy life span increase and improved cognition. Ann Med. 2020;52:147-161. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 71] [Article Influence: 14.2] [Reference Citation Analysis (1)] |
| 91. | Cai H, Qin YL, Shi ZY, Chen JH, Zeng MJ, Zhou W, Chen RQ, Chen ZY. Effects of alternate-day fasting on body weight and dyslipidaemia in patients with non-alcoholic fatty liver disease: a randomised controlled trial. BMC Gastroenterol. 2019;19:219. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 57] [Cited by in RCA: 133] [Article Influence: 22.2] [Reference Citation Analysis (0)] |
| 92. | Johari MI, Yusoff K, Haron J, Nadarajan C, Ibrahim KN, Wong MS, Hafidz MIA, Chua BE, Hamid N, Arifin WN, Ma ZF, Lee YY. A Randomised Controlled Trial on the Effectiveness and Adherence of Modified Alternate-day Calorie Restriction in Improving Activity of Non-Alcoholic Fatty Liver Disease. Sci Rep. 2019;9:11232. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 86] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 93. | Hekmatdoost A, Shamsipour A, Meibodi M, Gheibizadeh N, Eslamparast T, Poustchi H. Adherence to the Dietary Approaches to Stop Hypertension (DASH) and risk of Nonalcoholic Fatty Liver Disease. Int J Food Sci Nutr. 2016;67:1024-1029. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 71] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 94. | Xiao ML, Lin JS, Li YH, Liu M, Deng YY, Wang CY, Chen YM. Adherence to the Dietary Approaches to Stop Hypertension (DASH) diet is associated with lower presence of non-alcoholic fatty liver disease in middle-aged and elderly adults. Public Health Nutr. 2020;23:674-682. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 42] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 95. | Razavi Zade M, Telkabadi MH, Bahmani F, Salehi B, Farshbaf S, Asemi Z. The effects of DASH diet on weight loss and metabolic status in adults with non-alcoholic fatty liver disease: a randomized clinical trial. Liver Int. 2016;36:563-571. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 158] [Cited by in RCA: 160] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 96. | Moreno B, Bellido D, Sajoux I, Goday A, Saavedra D, Crujeiras AB, Casanueva FF. Comparison of a very low-calorie-ketogenic diet with a standard low-calorie diet in the treatment of obesity. Endocrine. 2014;47:793-805. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 113] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 97. | Watanabe M, Tozzi R, Risi R, Tuccinardi D, Mariani S, Basciani S, Spera G, Lubrano C, Gnessi L. Beneficial effects of the ketogenic diet on nonalcoholic fatty liver disease: A comprehensive review of the literature. Obes Rev. 2020;21:e13024. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 127] [Cited by in RCA: 151] [Article Influence: 30.2] [Reference Citation Analysis (0)] |
| 98. | Westerbacka J, Lammi K, Häkkinen AM, Rissanen A, Salminen I, Aro A, Yki-Järvinen H. Dietary fat content modifies liver fat in overweight nondiabetic subjects. J Clin Endocrinol Metab. 2005;90:2804-2809. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 264] [Cited by in RCA: 270] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 99. | Pérez-Guisado J, Muñoz-Serrano A. A pilot study of the Spanish Ketogenic Mediterranean Diet: an effective therapy for the metabolic syndrome. J Med Food. 2011;14:681-687. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 100. | Mardinoglu A, Wu H, Bjornson E, Zhang C, Hakkarainen A, Räsänen SM, Lee S, Mancina RM, Bergentall M, Pietiläinen KH, Söderlund S, Matikainen N, Ståhlman M, Bergh PO, Adiels M, Piening BD, Granér M, Lundbom N, Williams KJ, Romeo S, Nielsen J, Snyder M, Uhlén M, Bergström G, Perkins R, Marschall HU, Bäckhed F, Taskinen MR, Borén J. An Integrated Understanding of the Rapid Metabolic Benefits of a Carbohydrate-Restricted Diet on Hepatic Steatosis in Humans. Cell Metab. 2018;27:559-571.e5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 256] [Cited by in RCA: 334] [Article Influence: 47.7] [Reference Citation Analysis (0)] |
| 101. | Lundsgaard AM, Holm JB, Sjøberg KA, Bojsen-Møller KN, Myrmel LS, Fjære E, Jensen BAH, Nicolaisen TS, Hingst JR, Hansen SL, Doll S, Geyer PE, Deshmukh AS, Holst JJ, Madsen L, Kristiansen K, Wojtaszewski JFP, Richter EA, Kiens B. Mechanisms Preserving Insulin Action during High Dietary Fat Intake. Cell Metab. 2019;29:50-63.e4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 59] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 102. | Ouyang X, Cirillo P, Sautin Y, McCall S, Bruchette JL, Diehl AM, Johnson RJ, Abdelmalek MF. Fructose consumption as a risk factor for non-alcoholic fatty liver disease. J Hepatol. 2008;48:993-999. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 678] [Cited by in RCA: 605] [Article Influence: 35.6] [Reference Citation Analysis (0)] |
| 103. | Stanhope KL, Schwarz JM, Keim NL, Griffen SC, Bremer AA, Graham JL, Hatcher B, Cox CL, Dyachenko A, Zhang W, McGahan JP, Seibert A, Krauss RM, Chiu S, Schaefer EJ, Ai M, Otokozawa S, Nakajima K, Nakano T, Beysen C, Hellerstein MK, Berglund L, Havel PJ. Consuming fructose-sweetened, not glucose-sweetened, beverages increases visceral adiposity and lipids and decreases insulin sensitivity in overweight/obese humans. J Clin Invest. 2009;119:1322-1334. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1178] [Cited by in RCA: 1203] [Article Influence: 75.2] [Reference Citation Analysis (0)] |
| 104. | Sevastianova K, Santos A, Kotronen A, Hakkarainen A, Makkonen J, Silander K, Peltonen M, Romeo S, Lundbom J, Lundbom N, Olkkonen VM, Gylling H, Fielding BA, Rissanen A, Yki-Järvinen H. Effect of short-term carbohydrate overfeeding and long-term weight loss on liver fat in overweight humans. Am J Clin Nutr. 2012;96:727-734. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 156] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 105. | Roeb E, Weiskirchen R. Fructose and Non-Alcoholic Steatohepatitis. Front Pharmacol. 2021;12:634344. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 34] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 106. | Meng G, Zhang B, Yu F, Li C, Zhang Q, Liu L, Wu H, Xia Y, Bao X, Shi H, Su Q, Gu Y, Fang L, Yang H, Yu B, Sun S, Wang X, Zhou M, Jia Q, Jiao H, Wang B, Guo Q, Carvalhoa LA, Sun Z, Song K, Yu M, Niu K. Soft drinks consumption is associated with nonalcoholic fatty liver disease independent of metabolic syndrome in Chinese population. Eur J Nutr. 2018;57:2113-2121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 16] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 107. | Abdelmalek MF, Suzuki A, Guy C, Unalp-Arida A, Colvin R, Johnson RJ, Diehl AM; Nonalcoholic Steatohepatitis Clinical Research Network. Increased fructose consumption is associated with fibrosis severity in patients with nonalcoholic fatty liver disease. Hepatology. 2010;51:1961-1971. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 608] [Cited by in RCA: 573] [Article Influence: 38.2] [Reference Citation Analysis (0)] |
| 108. | Chiu S, Sievenpiper JL, de Souza RJ, Cozma AI, Mirrahimi A, Carleton AJ, Ha V, Di Buono M, Jenkins AL, Leiter LA, Wolever TM, Don-Wauchope AC, Beyene J, Kendall CW, Jenkins DJ. Effect of fructose on markers of non-alcoholic fatty liver disease (NAFLD): a systematic review and meta-analysis of controlled feeding trials. Eur J Clin Nutr. 2014;68:416-423. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 244] [Cited by in RCA: 216] [Article Influence: 19.6] [Reference Citation Analysis (0)] |
| 109. | Kositamongkol C, Kanchanasurakit S, Auttamalang C, Inchai N, Kabkaew T, Kitpark S, Chaiyakunapruk N, Duangjai A, Saokaew S, Phisalprapa P. Coffee Consumption and Non-alcoholic Fatty Liver Disease: An Umbrella Review and a Systematic Review and Meta-analysis. Front Pharmacol. 2021;12:786596. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 32] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 110. | Hayat U, Siddiqui AA, Okut H, Afroz S, Tasleem S, Haris A. The effect of coffee consumption on the non-alcoholic fatty liver disease and liver fibrosis: A meta-analysis of 11 epidemiological studies. Ann Hepatol. 2021;20:100254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 58] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 111. | Catalano D, Martines GF, Tonzuso A, Pirri C, Trovato FM, Trovato GM. Protective role of coffee in non-alcoholic fatty liver disease (NAFLD). Dig Dis Sci. 2010;55:3200-3206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 72] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 112. | Saab S, Mallam D, Cox GA 2nd, Tong MJ. Impact of coffee on liver diseases: a systematic review. Liver Int. 2014;34:495-504. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 131] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 113. | Zelber-Sagi S, Salomone F, Webb M, Lotan R, Yeshua H, Halpern Z, Santo E, Oren R, Shibolet O. Coffee consumption and nonalcoholic fatty liver onset: a prospective study in the general population. Transl Res. 2015;165:428-436. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 53] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 114. | Kennedy OJ, Fallowfield JA, Poole R, Hayes PC, Parkes J, Roderick PJ. All coffee types decrease the risk of adverse clinical outcomes in chronic liver disease: a UK Biobank study. BMC Public Health. 2021;21:970. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 34] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 115. | Bravi F, Tavani A, Bosetti C, Boffetta P, La Vecchia C. Coffee and the risk of hepatocellular carcinoma and chronic liver disease: a systematic review and meta-analysis of prospective studies. Eur J Cancer Prev. 2017;26:368-377. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 103] [Article Influence: 12.9] [Reference Citation Analysis (1)] |