Published online Jul 21, 2022. doi: 10.3748/wjg.v28.i27.3282
Peer-review started: January 11, 2022
First decision: March 8, 2022
Revised: March 15, 2022
Accepted: June 13, 2022
Article in press: June 13, 2022
Published online: July 21, 2022
Processing time: 187 Days and 23 Hours
The circadian rhythm in humans is determined by the central clock located in the hypothalamus’s suprachiasmatic nucleus, and it synchronizes the peripheral clocks in other tissues. Circadian clock genes and clock-controlled genes exist in almost all cell types. They have an essential role in many physiological processes, including lipid metabolism in the liver, regulation of the immune system, and the severity of infections. In addition, circadian rhythm genes can stimulate the immune response of host cells to virus infection. Hepatitis B virus (HBV) infection is the leading cause of liver disease and liver cancer globally. HBV infection depends on the host cell, and hepatocyte circadian rhythm genes are associated with HBV replication, survival, and spread. The core circadian rhythm proteins, REV-ERB and brain and muscle ARNTL-like protein 1, have a crucial role in HBV replication in hepatocytes. In addition to influencing the virus’s life cycle, the circadian rhythm also affects the pharmacokinetics and efficacy of antiviral vaccines. Therefore, it is vital to apply antiviral therapy at the appropriate time of day to reduce toxicity and improve the effectiveness of antiviral treatment. For these reasons, understanding the role of the circadian rhythm in the regulation of HBV infection and host responses to the virus provides us with a new perspective of the interplay of the circadian rhythm and anti-HBV therapy. Therefore, this review emphasizes the importance of the circadian rhythm in HBV infection and the optimization of antiviral treatment based on the circadian rhythm-dependent immune response.
Core Tip: Some studies in the literature contribute to the association of the circadian rhythm and liver pathophysiology associated with hepatitis B virus (HBV) infection. However, this is the first review to report the latest molecular mechanisms of HBV infection, including cellular circadian rhythm disorders, circadian clock-related immune response, therapeutic strategies in improving health outcomes, and the significance of personalized medicine and chronotherapy in treating chronic HBV infections.
- Citation: Skrlec I, Talapko J. Hepatitis B and circadian rhythm of the liver. World J Gastroenterol 2022; 28(27): 3282-3296
- URL: https://www.wjgnet.com/1007-9327/full/v28/i27/3282.htm
- DOI: https://dx.doi.org/10.3748/wjg.v28.i27.3282
The circadian rhythm in humans is determined by the central clock found in the hypothalamus’s suprachiasmatic nucleus (SCN) which receives light signals from the retina[1]. Endogenous functional circadian rhythms are active in peripheral tissues. For example, the circadian clock in humans, expressed in almost every cell, consists of a whole range of transcription-translation feedback loops[2]. Furthermore, all peripheral clocks in every tissue are synchronized by the central clock in the SCN[3]. Therefore, circadian clock genes and clock-controlled genes (CCG) exist in almost all cell types. Approximately 10%-40% of the genome is encoded in a circadian manner[4]. Furthermore, most physiological processes, such as lipid metabolism, immune system regulation, and infection severity, are under circadian control, and therefore circadian rhythm disorders can result in pathophysiological changes downstream[4,5].
The liver has a vital role in maintaining energy homeostasis within the body. The primary biochemical reactions within the liver are involved in the breakdown and generation of glucose, which is associated with fatty acid metabolism[6]. In addition, the metabolic functions of the liver show rhythmic fluctuations with a periodicity of 24 h[6]. Hepatitis-causing viruses depend closely on hepatocytes for replication, survival, and spread[6]. In addition, viral infection can disrupt the hepatocytes’ circadian rhythm[5]. Circadian rhythm transcription factors alter liver metabolism and are linked with various conditions, including viral infection, fatty liver disease, diabetes, and hepatocellular carcinoma (HCC)[1].
What makes the hepatitis B virus (HBV) significantly different from other hepatotropic viruses is its global impact on human health[7], namely, infections caused by this virus result in liver disease, which is a growing global problem[8]. According to the World Health Organization (WHO), it is estimated that 296 million people lived with chronic hepatitis B in 2019, and that 1.5 million new infections are detected each year[9]. Furthermore, due to HBV infection in 2019, mainly from cirrhosis and HCC, about 820000 people died[10]. Hepatitis B can develop in an acute or chronic form[11]. Most infected people do not have any symptoms, which does not mean that there is no disease. Symptoms of acute infection usually occur 2 mo to 3 mo after infection and are nonspecific[12,13].
The HBV begins to multiply within the first 3 d of infection[14]. Whether and to what extent liver damage occurs depends on the infected person’s immune system, the infectious dose, and the site of virus entry. Tissue compatibility antigens and interferon promote the viral antigens’ [hepatitis B surface antigen (HBsAg), hepatitis B envelope antigen (HbeAg), and hepatitis B core antigen (HBcAg)] exposure to cytotoxic T lymphocytes[15]. If there is no adequate response by these cells, milder signs of the disease occur, which may progress to the development of chronic hepatitis[16]. About 10% of acute HBV infections progress to a chronic form[17]. Chronic HBV can be clinically manifested as mild conditions to severe chronic hepatitis[18], which, in untreated cases, results in 8%-20% of cases with cirrhosis and HCC[19]. Persistent hepatitis is a mild disease that occurs in 8%-10% of patients, occasionally causing elevated aminotransferases, but generally does not progress toward cirrhosis and has a favorable prognosis[20]. HBV plays a significant role in the development of HCC, which can occur many years after chronic infection[21]. Still, typically the HBV is not a cytopathogen, and cell damage is thought to be mediated by a persistently unproductive immune response[17].
Therefore, this mini-review highlights the significance of the circadian rhythm in HBV infection, and the effectiveness of therapy in relation to the circadian rhythm-dependent immune response.
The epidemiological model of the spread of HBV and human immunodeficiency virus (HIV) is the same, but HBV is 50-100 times more infectious than HIV[22]. The source of infection is people with HBV infection[23]. However, the geographical prevalence of HBV infection varies[24]. In addition, it depends on several factors, such as different modes of transmission in the population and the age at which the infection originated, which relates to the likelihood of progression to chronic infection[25].
HBV is mainly transmitted by percutaneous or mucosal exposure to infected blood or body fluids[26]. Perinatal transmission is the primary route of infection in endemic regions[27], while the most direct route in the low-endemic regions is sexual transmission[28]. Globally, the epidemiology of HBV infection is changing, influenced by infant vaccination programs and migration between low- and high-prevalence populations[29]. On the basis of the available data, it is estimated that the prevalence of new chronic HBV cases is 70% in developing countries[30].
On the basis of the prevalence of the HBsAg, the epidemiology of HBV in certain areas of the world can be classified into one of three categories (Table 1)[31]: Low prevalence areas (< 2%), medium prevalence areas (2%-7%), and high prevalence areas (> 8% HBsAg prevalence)[32]. These categories are important for understanding the transmission and outcome of infection, and the consequences of chronic hepatitis B[25].
| Low prevalence area (< 2%) | Area of medium prevalence (2%-7%) | Area of high prevalence (> 8%) | |
| Geographical areas | United states, Canada, and Western European countries | Parts of Russia, border Eurasian, and Asian-African areas | Asia, sub-Saharan Africa, South, and Central America |
| % of the world population | 12% | 43% | 45% |
HBV is a DNA virus with ten known genotypes, labeled A-J. HBV belongs to the group of hepatotropic viruses from the family Hepadnaviridae, genus Orthohepadnavirus[33]. It is a small round virus of a very complex structure, 42 nm in diameter, with a double shell (Dane particle) (Table 2)[33]. Genotypes are divided into subgenotypes due to the significant variability of the nucleotide sequences[34]. They are based on a more than 4% difference along the entire genome. In addition, HBV genotypes and subgenotypes are related by geographical distribution, and also related to the pathogenesis and outcome of HBV infection (Table 3)[35].
| Genus | Orthohepadnavirus |
| Family | Hepadnaviridae |
| Species | HBV |
| Genotypes | A-J |
| Virion | 42 nm, spherical |
| Envelope | Yes (HBs) |
| Genome | Circular ds/ssDNA |
| Genome size | 3.2 kb |
| Sensitivity | Acid-sensitive |
| Virus antigens | HBsAg, HBcAg, HBeAg, polymerase |
| Genotype | Subgenotype | Prevalence |
| A | A1 -A6 | Africa, India, Northern Europe, USA |
| B | B1-B8 | Asia, USA |
| C | C1-C14 | Asia, USA |
| D | D1-D9 | India, Middle East, Southern Europe, USA |
| E | F1-F4 | West and South Africa |
| F | Central and South America | |
| G | Europe, USA | |
| H | Central and South America, California (USA) | |
| I | I1-12 | Vietnam |
| J | Japan |
The HBV genome has 3.2 kb long, relaxed circular, partially double-stranded DNA (rcDNA)[36]. The minus strand is complete, while the plus strand is regularly incomplete with a stable 5’ end, but an inconsistent 3’ end. The 5’ end of the plus strand is covalently linked to the RNA with a 5’ cap formation. In addition, the viral polymerase (reverse transcriptase) is covalently bound to the 5’ end of the minus strand[36,37].
Linear DNA is the direct precursor of viral DNA that is randomly incorporated into the hepatocytes’ DNA throughout infection. Incorporated DNA has no role in virus replication[37,38]. The HBV genome is highly compact, with four open reading frames (ORFs) overlapping multiple times and encoding seven proteins[39]. All ORFs are identical orientations and encoded by the negative strand of DNA[40]. Thanks to the ORFs, the coding capacity increases, and DNA is transcribed one and a half to two times because there is no stop codon[41]. In addition to ORF, there are six start codons, four promoters, and two transcription enhancers[42].
ORF C encodes the nuclear core antigen HBc and envelope E antigen (HBe)[39]. The precore protein in serum indicates active virus replication[43]. The presence of HBeAg in serum suggests prevented or decreased virus replication. HBeAg can repress the cellular immune response to HBsAg, thereby reducing perinatal transmission and promoting chronic infection[37,44]. The longest ORF is the P gene, encoding most of the virus genome (80%) and viral polymerase (reverse transcriptase)[45]. The ORF X encoding hepatitis B protein X (HBx) activates viral RNA transcription[37,38]. HBx causes the progression of the cell cycle and is essential for activating the transcription of covalently closed circular DNA (cccDNA) and pregenomic RNA (pgRNA) after infection[37]. ORF S codes three different sizes of the surface S antigen (S-small, M-medium, and L-large HBs)[46]. The M- and L-HBs have additional PreS2 and PreS1 domains[39]. The PreS1 region, present in L-HBsAg, possesses a binding domain for the sodium taurocholate co-transporting polypeptide (NTCP) receptors, essential for viral internalization[37,47]. HBsAg are embedded in the outer lipid envelope, and the inner nucleocapsid structure contains the DNA genome, HBcAg, and viral polymerase (reverse transcriptase)[48]. In addition, HbeAg is between the nucleus and the outer shell[49].
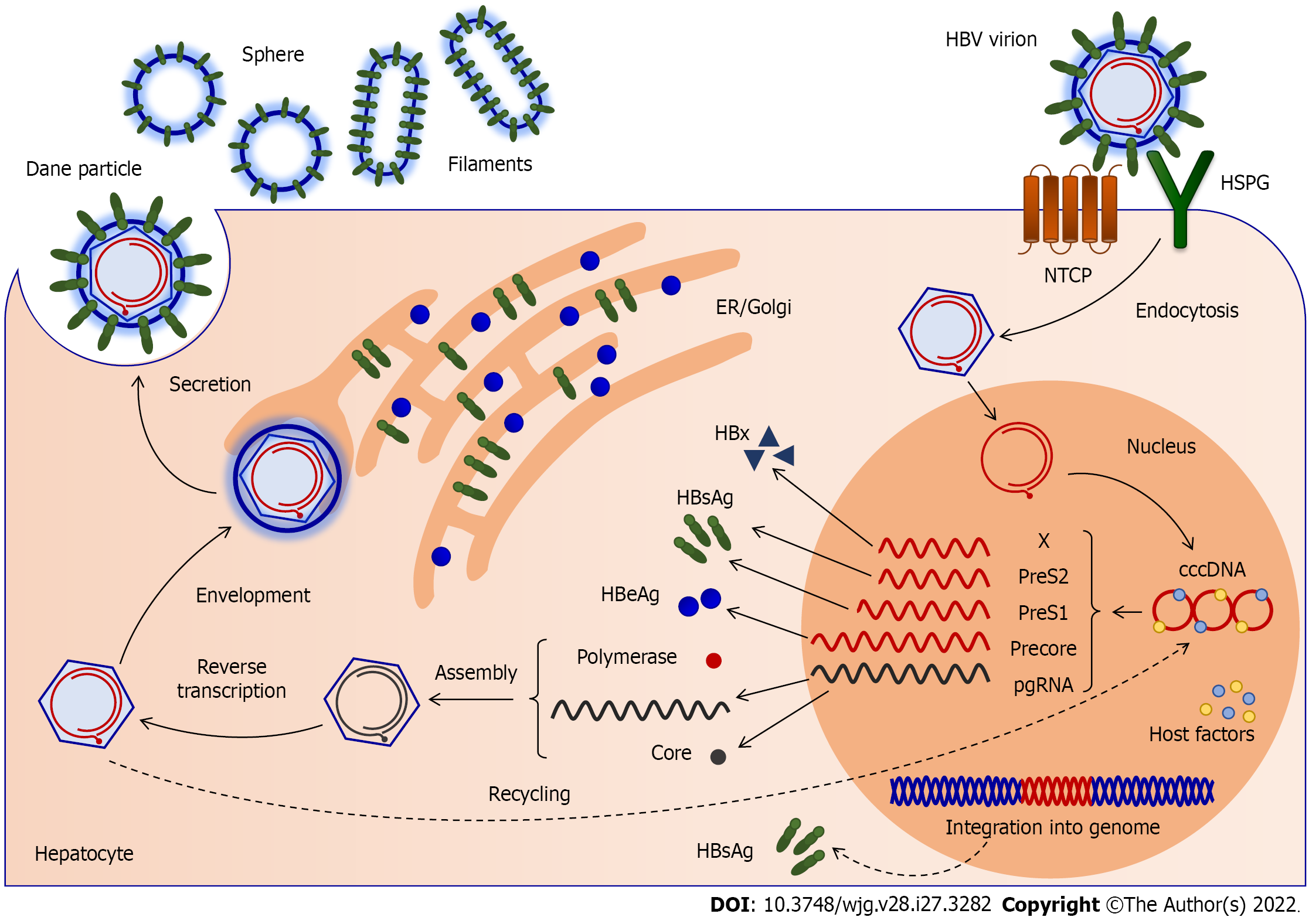
HBV replicates in hepatocytes via pgRNA (Figure 1)[35]. The entry of the virus into the hepatocyte is the first step in its life cycle[50]. After the virus enters the liver, S-HBsAg recognizes heparan sulfate proteoglycans (HSPG) on the hepatocytes, and binds them with low affinity[51]. A high-affinity interaction between the PreS1 region of the L-HBs protein and NTCP follows[52]. Later, the virus envelope and the hepatocytes’ membrane merge, releasing the nucleocapsid into the cytoplasm[53]. After the capsid shell is removed, the viral genome is transferred to the nucleus. As a result, viral DNA enters the nucleus of the hepatocytes. The relaxed circular (rc) genome is restored, resulting in cccDNA[54]. Viral DNA can be integrated into the hepatocytes’ DNA, and is a constant HBsAg source[55]. The cccDNA is a template for pgRNA production and the transcription of all viral mRNAs[37]. On this basis, cccDNA in the hepatocytes can stimulate virus replication[56]. The cccDNA is associated with cellular histones and forms a minichromosome. Each viral protein has a promoter and mRNA, except for reverse transcriptase, translated from the same pgRNA as the virus core protein[37,44]. The onset of infection is indicated by the expression of core proteins and polymerase from pgRNA. HBc is a phosphoprotein and a subunit of the viral nucleocapsid. The core proteins are constructed into nucleocapsids, icosahedral shells of 120 dimers[57]. They merge with RNA and are assembled into core particles. Viral DNA synthesis takes place in the core particles, followed by packaging of pgRNA and polymerase[37,47]. Upon completing viral DNA synthesis by reverse transcription, viral particles can exit the hepatocytes in two ways. First, while envelope protein levels are low, core particles recycle and enter the nucleus, resulting in the replenishment of cccDNA[37,39]. Later, the core particles gather with the envelope proteins in the endoplasmic reticulum, enter the secretory pathway, and are discharged from hepatocytes into the blood[58]. In addition to infectious virions (Dane particles), incomplete subviral particles are secreted by secretory pathways (such as spheres and filaments lacking nucleocapsid proteins and naked nucleocapsids)[59]. The virus emerges on the surface of the hepatocytes by budding, and can thus infect another cell[52]. Once created in the cell nucleus, the cccDNA minichromosome is very difficult to eliminate from infected cells, where it can survive until the death of the host cell[56].
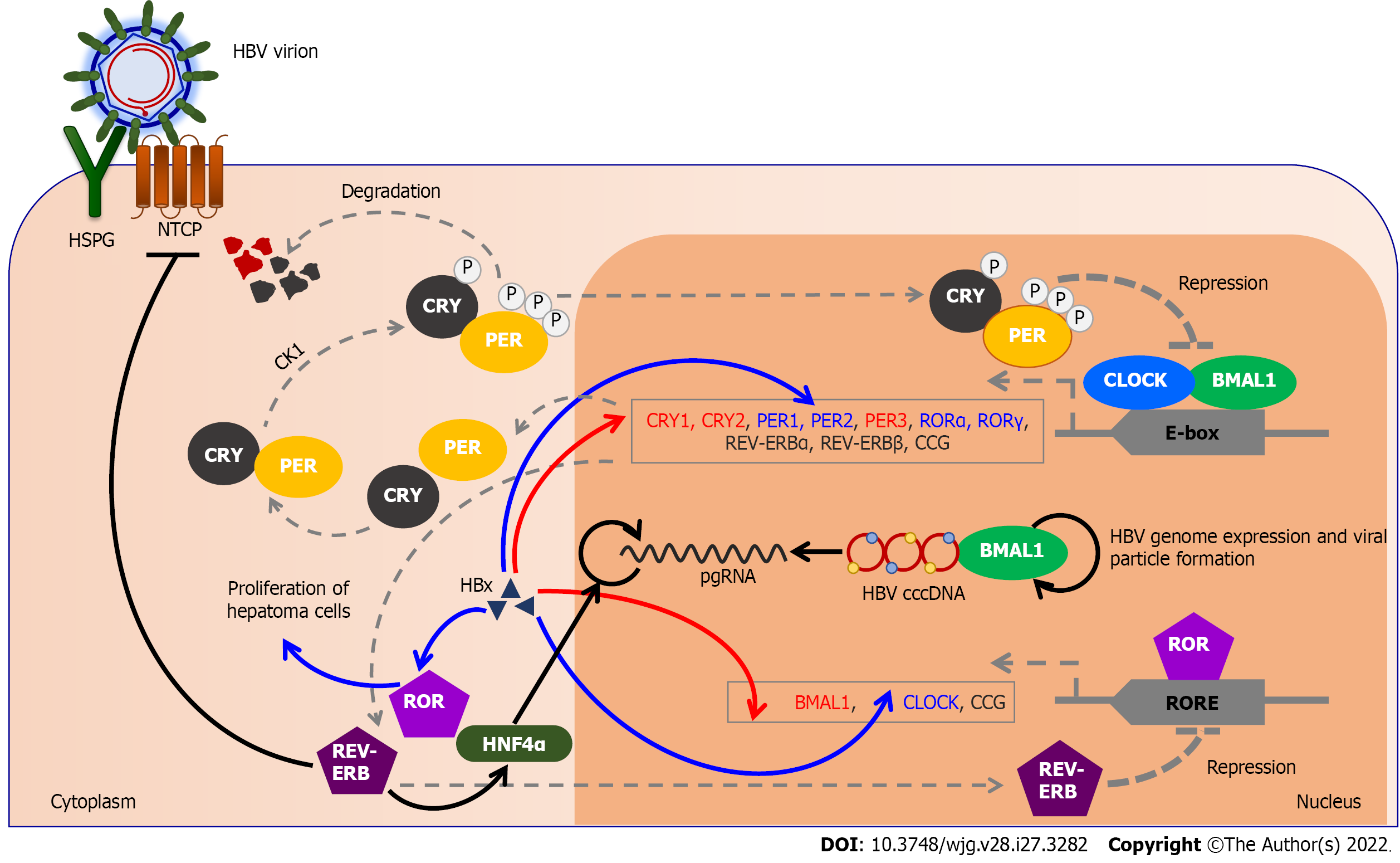
The molecular basis of the circadian rhythm includes transcriptional and translocation feedback loops. In addition, the circadian rhythm is driven by the circadian locomotor output cycles kaput (CLOCK), and brain and muscle ARNTL-like protein 1 (BMAL1 or ARNTL) transcription factors. In contrast, transcription repressors are period (PER) and cryptochrome (CRY) transcription factors. The central transcription factors that make up the activation and positive part of the molecular clock are BMAL1 and CLOCK. The heterodimer CLOCK-BMAL1 enters the nucleus, where it initiates transcription by binding to a specific sequence, the E-box, in promoters of the target genes. CLOCK’s main downstream goals include BMAL1 and its repressors, cryptochrome (CRY1, CRY2), and period (PER1, PER2, and PER3) and multiple CCG[60]. CRYs and PERs accumulate during the positive loop in the cytoplasm. They are controlled by F-box/LRR-repeat protein 3 (FBXL3) and casein kinase 1 (CK1) ε and CK1δ[4,61]. CK1ε and CK1δ phosphorylate PERs for degradation, while FBXL3 stimulates CRYs degradation. If CK1ε phosphorylates heterodimer PER-CRY, it enters the nucleus and suppresses the CLOCK-BMAL1 heterodimer. By suppressing their activator, CRYs and PERs suppress their own expression[62]. Posttranslational phosphorylation of CRYs and PERs promotes their degradation, which triggers a new circadian cycle, with increased binding of the CLOCK-BMAL1 heterodimer to the E-box of CCG[2,63].
The second regulatory loop includes the retinoic acid receptor-related orphan receptor (ROR) α and RORγ, and the REV-ERBα and REV-ERBβ genes. The CLOCK-BMAL1 heterodimer initiates their transcription by binding to the E-box elements of their promoters. RORs and REV-ERBs receptors bind to the ROR element (RORE). REV-ERBα and β inhibit transcription, while RORα and γ stimulate expression of target genes. RORs and REV-ERBs together create cyclic fluctuations in the expression of many CCG, including the regulation of BMAL1 transcription[6,64]. REV-ERBα accumulates rapidly and prevents BMAL1 transcription, while RORα accumulates more slowly and promotes BMAL1 transcription. In this way, the stability and robustness of the rhythmicity of the internal clock system are enhanced[2,63].
An additional independent feedback loop includes the helix-loop-helix e40 family (DEC1 or BHLHE40) and DEC2 (BHLHE41) that prevent CLOCK-BMAL1 activity[65]. The CLOCK-BMAL1 heterodimer stimulates the expression of DEC1 and DEC2 by binding to the E-box of their promoters. Conversely, DECs suppress transcription of genes with E-box elements in the promoter, including transcription of itself and CRYs and PERs, due to its binding to the E-box[66]. In addition, the CLOCK-BMAL1 heterodimer affects the expression of other CCG by stimulating D-box binding protein (DBP) transcription, by binding to E-box elements on the promoter[67]. DBP rhythmically triggers genes with D-box elements in the promoter[2,4].
The transcriptional and translational feedback loop creates rhythms in the expression and levels of downstream CCG[4]. All of these connected feedback loops create a circadian rhythm. For example, CCGs containing RORE elements in promoters are transcribed during the active phase. In contrast, the genes’ promoters containing E-box and D-box elements are transcribed during the resting phase[63].
The circadian rhythm is controlled at the transcriptional and posttranslational levels, including cellular pathways, transcription factors, epigenetic changes, and posttranslational modifications[68]. Many posttranslational modifications of clock elements include acetylation, phosphorylation, ubiquitylation, and sumoylation[4,63]. Epigenetic modifications, such as DNA methylation, histone modifications, and non-coding RNAs, interfere with the target genes’ transcription and post-transcriptional expression, including clock genes[69]. Different patterns of histone modifications, such as histone deacetylase sirtuin 1 (SIRT1), or microRNA, can be direct and indirect modulators in maintaining different aspects of circadian rhythm function[53,70].
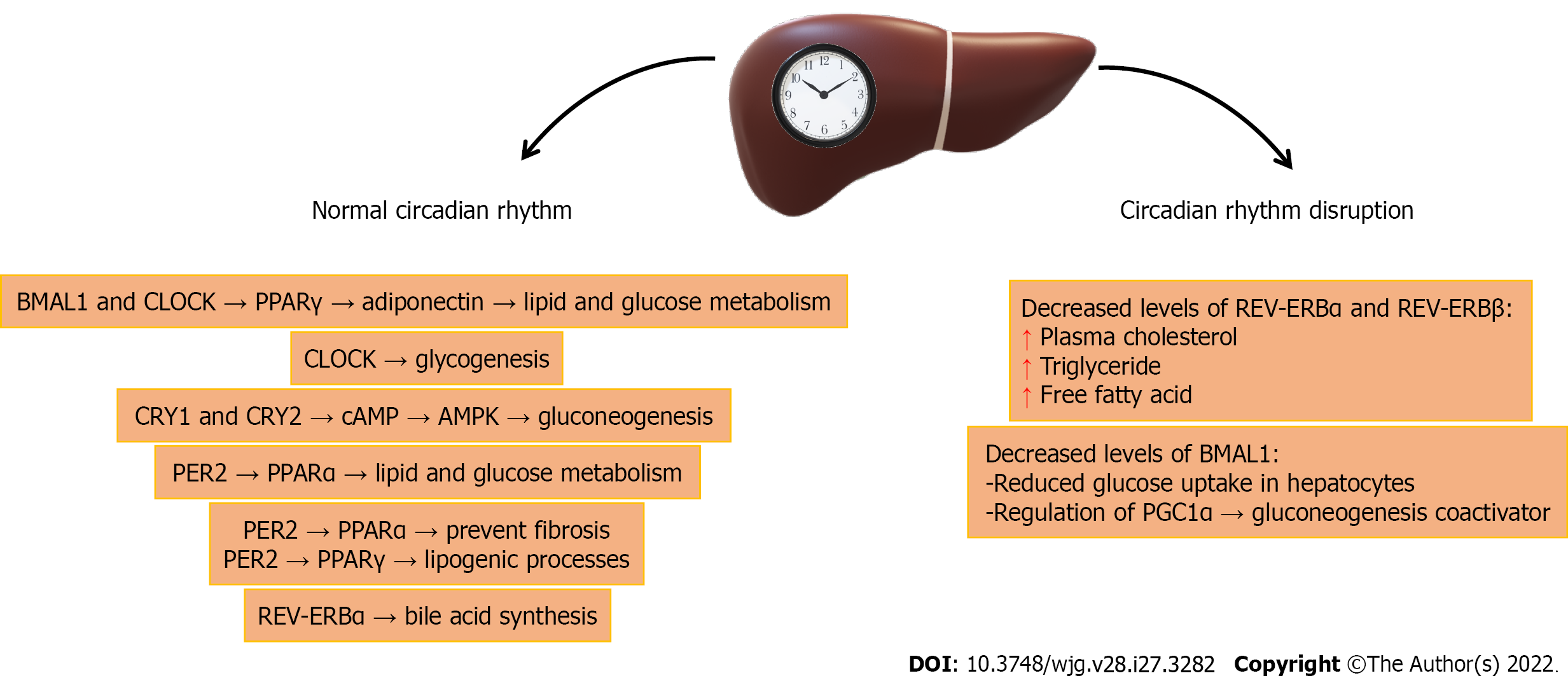
With a lack of environmental signs, such as the alternation of light and dark, food intake affects the circadian rhythm of the liver[6]. Circadian rhythms largely control different genes, levels of proteins, and the enzymes in the liver[2]. The circadian rhythm and liver metabolism are connected through the peroxisome proliferator-activated receptors (PPARs) α and γ[6,71]. PPARα controls the transcription of genes participating in lipid and glucose metabolism, while PPARγ is involved in lipogenic processes because it binds eicosanoids from omega-3 or omega-6 fatty acids[4,72]. Both genes are rhythmically expressed, and their expression is controlled by PER2, which directly regulates BMAL1 transcription (Figure 2). The binding of PPARα to PPAR response elements (PPRE) in the BMAL1 promoter leads to its transcription. Also, PPARα interacts with PER2 in the liver to impact the expression of target genes[73]. In the positive feedback loop in the liver, BMAL1 and CLOCK control the circadian oscillations of PPARα[4], and thus the expression of enzymes participating in glucose and lipid homeostasis, and the biosynthesis of bile acids and apolipoproteins[6]. REV-ERBα is implicated in the control of bile acid synthesis[74]. Adiponectin, triggered by BMAL1 and CLOCK via the transcription of PPARγ and its coactivator 1α (PGC-1α), is involved in glucose and lipid metabolism[75].
In addition, gluconeogenesis is controlled in a circadian manner in the liver via CRY1 and CRY2, which prevent signaling via the secondary messenger cyclic adenosine monophosphate (cAMP)[76]. Moreover, AMP-activated protein kinase (AMPK) can influence circadian cycle length by phosphorylating CRYs and directing it to degradation[4]. Activation of RORα in the liver may impact lipid metabolism and control hepatic steatosis by triggering AMPK[77]. Furthermore, CLOCK controls glycogenesis in the liver by acting on glycogen synthase 2 expression[78]. Disorders of the CLOCK and BMAL1 genes lead to impaired glucose homeostasis[6]. Histone deacetylase SIRT1 prevents hepatic steatosis by controlling lipid homeostasis by positive binding of PPARα and PGC1α coactivators, or by direct action on BMAL1[70].
There is a functional association between virus replication and circadian dysfunction in the pathogenesis of liver disease[79]. HBV has been revealed to impact the liver clock genes and disrupt the internal molecular clock in order to make better use of hepatocytes for self-replication[80]. HBV is randomly incorporated into the human genome, yet there are sites where it is incorporated more frequently, such as circadian rhythm-related elements, CLOCK, and BMAL1. Those elements are one way that the circadian rhythm is associated with diseases caused by HBV infection[81]. HBV replicates in the liver where around 20% of genes exhibit a rhythmic expression pattern, implying that the virus has successfully evolved to persist in the liver[5,50]. Integrated copies of HBV, which have regulatory elements similar to circadian rhythm genes, present additional circadian rhythm motifs in the infected cell, resulting in undesirable oscillations of specific genes, or disrupted circadian rhythms of the hepatocytes, and are a risk factor for cancer[50].
The circadian rhythm impacts gene expression in the liver and thus HBV replication[50]. In patients with HBV infection, a reduction in BMAL1 and an increase in REV-ERBα and REV-ERBβ transcription have been observed compared to healthy subjects, which indicates that circadian rhythm gene transcription is impaired in HBV infection[50]. In hepatocytes, decreased expression or deletion of REV-ERBα and REV-ERBβ in hepatocytes increases plasma cholesterol, triglycerides, and free fatty acid levels[63]. In addition, REV-ERB binds and controls NTCP expression, and stimulation of REV-ERB prevents HBV from entering the hepatocytes[50]. The NTCP receptor has a circadian pattern in hepatocytes[82]. Decreased expression of BMAL1 in hepatocytes leads to reduced glucose uptake. In addition, BMAL1 regulates the transcription of the PGC1α gene, a coactivator of gluconeogenesis[63]. Thus, PPARα can prevent the development of fibrosis[63], and it is known that HBV dysregulates PPARα and PPARγ[6]. Also, BMAL1 binds HBV DNA, and controls viral genome expression and new viral particle formation[50].
The impaired circadian rhythm in HCC may favor the selective survival of cancer cells and facilitate carcinogenesis[83]. An increased risk of developing HCC is linked with chronic HBV infection. The HBx protein is linked with HCC due to the disturbance of cell proliferation[19]. It is a possible cause of significantly reduced transcription levels of the BMAL1, PER3, CRY1, CRY2, and CK1ε genes in HCC[84]. At the same time, elevated HBx expression in HCC results in increased CLOCK, PER1, and PER2 expression[1,85]. In addition, abnormal expression of REV-ERBα was observed in a cell line that stably expressed HBV[86]. The circadian rhythm acts via REV-ERBα in the liver on hepatocyte nuclear factor 4 alpha (HNF4α), and thus directs the action of glucocorticoid receptors on energy metabolism (Figure 3). Consequently, the interactions between CRY and glucocorticoid receptors affect carbohydrate metabolism, hence transacting PER2[87]. HNF4α increases the transcription of pgRNA in hepatoma cells and thus affects HBV biosynthesis[88]. In addition, P2-HNF4α inhibits the expression of BMAL1, leading to the localization of P1-HNF4α from the nucleus to the cytoplasm. BMAL1 is expressed in healthy hepatocytes, but tumor growth is prevented if BMAL1 expression is induced in HNF4α-positive tumor cells[89]. The possible reason for the inhibition of tumor growth is that BMAL1 mediates the trans
HBV infection leads to overexpression of RORα and RORγ[92]. RORγ overexpression is associated with promoter methylation and HBx protein. Furthermore, HBx-induced RORγ may facilitate the proliferation and migration of hepatoma cells[93], and RORα may be a possible diagnostic and prognostic biomarker for disease severity[94]. Overexpression of RORα, CRY2, and PER1 is associated with a better survival of HCC patients, and regulating the circadian rhythm gene may help in the chronotherapy of such patients[94]. HBV infection leads to disruptions of the CLOCK gene and its downstream circadian genes and other CCGs[2]. All the above indicates that circadian rhythm disorders caused by a virus may contribute to the pathogenesis of cancer.
Clinical manifestations of chronic hepatitis B result from a cellular and humoral immune response to the recognized target antigenic viral epitopes, HBcAg and HBsAg[21]. Integration of HBV into the hepatocytes’ genome serves as a constant source of HBsAg during chronic infection. It may induce dysfunctional T-cell responses, and favor harmful immunopathology and disease progression to fibrosis and cirrhosis[95]. Hepatocytes expressing HBcAg are recognized and attacked by cytotoxic CD8+ lymphocytes (CTL)[96]. The increase in alanine aminotransferase is the result of hepatocyte lysis[97]. HBcAg has strong immunogenicity and can cause the antigen-specific T-cell responses necessary for controlling HBV infection. HBcAg stimulates the production of interleukin 6 (IL)-6, IL-10, IL-17A, IL-22, IL-23, and transforming growth factor β (TGFβ) by peripheral blood mononuclear cells[98]. In addition, the immunosuppressive cytokine IL-10 negatively controls the expansion of Th17 cells, which plays a part in the existence and promotion of HBV infection[46]. Persistent HBV infection is marked by a poor immune response due to the poor response of CD4+ lymphocytes in the early phases of infection. This results in qualitatively and quantitatively weaker CD8+ T cell responses[21].
In addition to other viral proteins, HBeAg is also important for the persistence of infection, as it suppresses the humoral and cellular immune response to HbcAg, weakens the action of CTL on HBcAg, and allows the maintenance of the infection[99]. The predominance of Th-1 over the Th-2 cytokine response, with the production of IL-2, interferon-alpha (IFN-α), and lymphotoxin-β receptor (LT-βR), is responsible for virus elimination during acute and chronic HBV infections[100]. Conversely, the predominance of Th-2 cytokine response is responsible for maintaining chronic infection, with IL-4, IL-5, and IL-10, which enhance the humoral immune response[98].
Many innate and adaptive immune system elements are under circadian regulation[1], and the adaptive immune response mediates virus removal and liver disease[95]. Cellular immune rhythms are synchronized with the central clock in the SCN[6]. Innate and adaptive immune system cells have a molecular clock that controls their rhythmic activity over 24 h[101]. Certain functions of the innate immune system rely on the hepatocytes’ circadian rhythm[5,65]. Increased amounts of pro-inflammatory hormones such as growth hormone, prolactin, and melatonin and cytokines such as IL-1 and TNF-α are present during the rest period. In addition, immune rhythms are affected by hormonal rhythms, so cortisol regulates the number of naive T cells. Epinephrine influences the number of CTL cells over 24 h[102].
Furthermore, the transcription factor CRY2 links the circadian rhythm and the innate immune system[103]. Moreover, CLOCK and BMAL1 control transcription of the pattern recognition receptor, which is involved in nucleic acid detection during viral infections[104]. RORγ regulates the differentiation of Th17 cells, which secrete IL-17, an essential regulator of pro-inflammatory signaling[93]. REV-ERBα has a protective effect in various inflammatory processes[105]. Additionally, the suppression of REV-ERB receptors elevates virus-associated mortality by promoting the inflammatory response[106]. The circadian hormone melatonin has protective and antiviral actions and a role in the inflammatory response[105]. Melatonin increased IFN-γ levels during viral infection, decreased Venezuelan equine encephalomyelitis virus (VEE) levels, and reduced mortality rates in mice infected with VEE. The protective impact of melatonin is associated with increased IL-1β production because it acts as a cytokine modulator and antioxidant[107]. This shows the positive role of the circadian rhythm in regulating antiviral immunity[91].
There are two main drugs for HBV infection therapy: Nucleoside/nucleotide analogs (NAs), which interfere with viral DNA synthesis during reverse transcription, and IFN-α[108,109]. NAs effectively inhibit virus replication and may suppress the generation of new cccDNA[109]. Existing drugs can suppress virus replication, slow fibrosis progression, and decrease infectivity, but rarely remove the cccDNA responsible for HBV persistence[108]. Therefore, the development of therapy aims to achieve a "functional healing" state, i.e., the removal of cccDNA from hepatocytes[110,111]. However, in the treatment of HBV infection, NAs rarely eliminate the virus, that is, they do not eradicate HBV from the liver[95]. This is because each HBV surface protein (S, M, and L) has an HBsAg determinant. Therefore, HBsAg circulates in the bloodstream of HBV patients, mainly in non-infectious subviral spheres and filaments[95].
Alternative therapeutic strategies are based on silencing viral genes using RNA interference (RNAi)[108] and antisense oligonucleotide (ASO) technology[110]. Both approaches are based on the effective suppression of HBV replication in patients with chronic HBV infection[108,110]. RNAi is a mechanism for silencing genes after transcription, and can be used in mammalian cells against viral infections[108]. ASO technology prevents HBV antigen production by targeting all HBV genome transcripts in infected hepatocytes[10,110]. It is based on NAs that act on viral polymerase but do not influence the cccDNA transcription[110]. ASO and other nucleic acid-based therapies may potentially decrease the tolerogenic outcome of the high HBsAg levels characteristic of chronic infection[63,110].
One of the principles of personalized medicine is to optimize the time of day for administration of drugs whose action is affected by the circadian rhythm[5,112]. Circadian oscillations can affect vaccine responses to viral pathogens[91]. The direct impact of the circadian rhythm on the cellular and humoral immune reaction is mediated by melatonin and cortisol. Research on the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has shown that melatonin can affect circadian clocks and modulate the immune response during viral infections and thus impact virus replication[113]. Therefore, vaccination in the morning or evening may impact the immune response to the vaccine[114]. Thus, it was observed that patients vaccinated against the SARS-CoV-2 in the morning had significantly lower C-reactive protein levels compared to patients vaccinated in the evening[115]. Making a simple effort to determine the appropriate time for administration of drugs or vaccine can improve the drug’s effectiveness and reduce side effects[5]. However, studying the interaction of the circadian rhythm and virus replication may lead to a better knowledge of viral infections and the associated immune response, discovery of new antiviral targets, improvement of existing treatments, and therapy for chronic infections[5]. Chronic infection is linked with a weakened immune response, and poor T-cell responses that fail to control HBV replication[50]. The effectiveness of current antiviral therapies could be enhanced by modulating the timing of vaccine administration. For example, the engineered T cell receptor activity showed a circadian pattern upon antigen activation. However, this daily effect was attenuated in CLOCK mutant mice, emphasizing the importance of timing T cell therapy to maximize antiviral immunity[1].
Many studies show that the efficacy or toxicity of treatment depends on the dosing time in many diseases. Also, inter-individual variations in the circadian rhythm resulting from lifestyle differences should be considered[116]. The efficiency of the DNA virus vaccine can be enhanced by specifying the time of day for vaccination. For example, some studies have found that patients immunized in the morning produce a more significant antibody response to hepatitis A and influenza vaccines[1,117]. In addition, morning vaccination is considered to significantly increase responses to viral-specific antibodies, compared to afternoon vaccination[1]. As viruses are intracellular pathogens that replicate within host cells, given the high association of circadian rhythm transcription factors with cell transcription, the circadian rhythm plays a vital role in determining host susceptibility to viral infections and the immune response[1].
Circadian clock-modulating small molecules may help inhibit or activate circadian rhythm proteins and enzymes in viral infections. Thus, the small molecule SRT2183 modulates the circadian clock that inhibits SARS-CoV-2 replication[118] because it modulates physiological and circadian rhythm gene expression[119]. Inhibiting BMAL1 expression and overexpression of REV-ERB via circadian rhythm-modulating small molecules may prevent dengue virus, hepatitis C virus, Zika virus, and HIV1 virus replication. In addition, clock genes have antiviral abilities that can be applied to HBV[120]. One of the effective circadian clock-modulating small molecules in treating HBV infections is GSK4112, a synthetic ligand for REV-ERB, but it is not suitable for in vivo use due to its poor pharmacokinetic properties. In contrast, ARN5187 is a REV-ERBβ agonist with dual function, an inhibitor of REV-ERB and autophagy[121]. SR9009 is a REV-ERBα agonist based on the chemical structure of GSK4112. It has better pharmacokinetic properties than GSK4112 and affects many oncogenes. In addition, REV-ERBα is known to regulate cancer development by inhibiting proliferation[121], and hence SR9009 inhibits BMAL1 and prevents the entry and replication of HBV into hepatocytes[50].
Challenges associated with antiviral drug development, including circadian clock-modulating small molecules, may be adverse effects or suboptimal pharmacokinetics. Therefore, for an antiviral agent to succeed, the target drug should be distributed locally to avoid unfavorable consequences on other tissues.
Interaction exists between viruses and the circadian rhythm of hepatocytes. Infection susceptibility depends on the infectivity of the inoculum, the mode of transmission, the length of exposure to the virus, and the time of the day infection occurs[1]. Understanding how HBV interacts with the circadian rhythm of hepatocytes may influence the treatment of infections[1]. Viruses are among the most critical human carcinogens[6], and pharmacotherapy and chronotherapy should be united to treat or prevent viral infections[1]. Research on the impact of viral infections on the circadian rhythm may help detect new antiviral targets and optimize the timing of immune-based therapies[1]. The main challenge in treating HBV infection is eradicating or silencing the cccDNA[50]. By acting on specific components of the circadian rhythm, such as REV-ERB and BMAL1, HBV can be prevented from entering hepatocytes and producing new virions. In addition to lifestyle changes, circadian rhythm-focused approaches may provide new therapeutic options in treating HBV infections[63].
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Croatia
Peer-review report’s scientific quality classification
Grade A (Excellent): A, A
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Jiang W, China; Miyoshi E, Japan; Shkodina A, Ukraine A-Editor: de Melo FF, Brazil S-Editor: Chen YL L-Editor: Wang TQ P-Editor: Chen YL
| 1. | Zhuang X, Rambhatla SB, Lai AG, McKeating JA. Interplay between circadian clock and viral infection. J Mol Med (Berl). 2017;95:1283-1289. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 47] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 2. | Zhou D, Wang Y, Chen L, Jia L, Yuan J, Sun M, Zhang W, Wang P, Zuo J, Xu Z, Luan J. Evolving roles of circadian rhythms in liver homeostasis and pathology. Oncotarget. 2016;7:8625-8639. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 29] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 3. | Škrlec I, Milic J, Heffer M, Peterlin B, Wagner J. Genetic variations in circadian rhythm genes and susceptibility for myocardial infarction. Genet Mol Biol. 2018;41:403-409. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 21] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 4. | Saran AR, Dave S, Zarrinpar A. Circadian Rhythms in the Pathogenesis and Treatment of Fatty Liver Disease. Gastroenterology. 2020;158:1948-1966.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 109] [Article Influence: 21.8] [Reference Citation Analysis (0)] |
| 5. | Borrmann H, McKeating JA, Zhuang X. The Circadian Clock and Viral Infections. J Biol Rhythms. 2021;36:9-22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 51] [Cited by in RCA: 59] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 6. | Vinciguerra M, Mazzoccoli G, Piccoli C, Tataranni T, Andriulli A, Pazienza V. Exploitation of host clock gene machinery by hepatitis viruses B and C. World J Gastroenterol. 2013;19:8902-8909. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 9] [Cited by in RCA: 11] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 7. | Cheng Z, Lin P, Cheng N. HBV/HIV Coinfection: Impact on the Development and Clinical Treatment of Liver Diseases. Front Med (Lausanne). 2021;8:713981. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 32] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 8. | Al-Sadeq DW, Taleb SA, Zaied RE, Fahad SM, Smatti MK, Rizeq BR, Al Thani AA, Yassine HM, Nasrallah GK. Hepatitis B Virus Molecular Epidemiology, Host-Virus Interaction, Coinfection, and Laboratory Diagnosis in the MENA Region: An Update. Pathogens. 2019;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 25] [Article Influence: 4.2] [Reference Citation Analysis (1)] |
| 9. | Liang Y, Bai X, Liu X, Zhang Z, Pang X, Nie L, Qiu W, Zhao W, Hu G. Hepatitis B Vaccination Coverage Rates and Associated Factors: A Community-Based, Cross-Sectional Study Conducted in Beijing, 2019-2020. Vaccines (Basel). 2021;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 10. | Yuen MF, Heo J, Jang JW, Yoon JH, Kweon YO, Park SJ, Tami Y, You S, Yates P, Tao Y, Cremer J, Campbell F, Elston R, Theodore D, Paff M, Bennett CF, Kwoh TJ. Safety, tolerability and antiviral activity of the antisense oligonucleotide bepirovirsen in patients with chronic hepatitis B: a phase 2 randomized controlled trial. Nat Med. 2021;27:1725-1734. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 111] [Cited by in RCA: 104] [Article Influence: 26.0] [Reference Citation Analysis (0)] |
| 11. | Splawn LM, Bailey CA, Medina JP, Cho JC. Heplisav-B vaccination for the prevention of hepatitis B virus infection in adults in the United States. Drugs Today (Barc). 2018;54:399-405. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 12. | Kappus MR, Sterling RK. Extrahepatic manifestations of acute hepatitis B virus infection. Gastroenterol Hepatol (N Y). 2013;9:123-126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 13. | Chen L, Bao D, Gu L, Gu Y, Zhou L, Gao Z, Huang Y. Co-infection with hepatitis B virus among tuberculosis patients is associated with poor outcomes during anti-tuberculosis treatment. BMC Infect Dis. 2018;18:295. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 39] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 14. | Yue L, Li C, Xu M, Wu M, Ding J, Liu J, Zhang X, Yuan Z. Probing the spatiotemporal patterns of HBV multiplication reveals novel features of its subcellular processes. PLoS Pathog. 2021;17:e1009838. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 15. | Le Bert N, Gill US, Hong M, Kunasegaran K, Tan DZM, Ahmad R, Cheng Y, Dutertre CA, Heinecke A, Rivino L, Tan A, Hansi NK, Zhang M, Xi S, Chong Y, Pflanz S, Newell EW, Kennedy PTF, Bertoletti A. Effects of Hepatitis B Surface Antigen on Virus-Specific and Global T Cells in Patients With Chronic Hepatitis B Virus infection. Gastroenterology. 2020;159:652-664. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 134] [Article Influence: 26.8] [Reference Citation Analysis (0)] |
| 16. | Terrault NA, Lok ASF, McMahon BJ, Chang KM, Hwang JP, Jonas MM, Brown RS Jr, Bzowej NH, Wong JB. Update on prevention, diagnosis, and treatment of chronic hepatitis B: AASLD 2018 hepatitis B guidance. Hepatology. 2018;67:1560-1599. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2290] [Cited by in RCA: 2845] [Article Influence: 406.4] [Reference Citation Analysis (0)] |
| 17. | Lim HK, Jeffrey GP, Ramm GA, Soekmadji C. Pathogenesis of Viral Hepatitis-Induced Chronic Liver Disease: Role of Extracellular Vesicles. Front Cell Infect Microbiol. 2020;10:587628. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 27] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 18. | Peng L, Gao ZL, Wang YM, He DM, Zhao JM, Bai XF, Wang XJ, Peng L. Clinical Manifestations and Laboratory Tests of AECHB and Severe Hepatitis (Liver Failure). In: Ning Q. Acute Exacerbation of Chronic Hepatitis B. Dordrecht: Springer, 2019:1-89. |
| 19. | Alqahtani SA, Colombo M. Viral hepatitis as a risk factor for the development of hepatocellular carcinoma. Hepatoma Res. 2020;6:58. [RCA] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 20. | Wu J, Han M, Li J, Yang X, Yang D. Immunopathogenesis of HBV Infection. Adv Exp Med Biol. 2020;1179:71-107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 21. | Cai Y, Yin W. The Multiple Functions of B Cells in Chronic HBV Infection. Front Immunol. 2020;11:582292. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 38] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 22. | Kafeero HM, Ndagire D, Ocama P, Kudamba A, Walusansa A, Sendagire H. Prevalence and predictors of hepatitis B virus (HBV) infection in east Africa: evidence from a systematic review and meta-analysis of epidemiological studies published from 2005 to 2020. Arch Public Health. 2021;79:167. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 28] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 23. | Nelson NP, Easterbrook PJ, McMahon BJ. Epidemiology of Hepatitis B Virus Infection and Impact of Vaccination on Disease. Clin Liver Dis. 2016;20:607-628. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 168] [Cited by in RCA: 216] [Article Influence: 24.0] [Reference Citation Analysis (0)] |
| 24. | MacLachlan JH, Cowie BC. Hepatitis B virus epidemiology. Cold Spring Harb Perspect Med. 2015;5:a021410. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 159] [Cited by in RCA: 200] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 25. | Nguyen MH, Wong G, Gane E, Kao JH, Dusheiko G. Hepatitis B Virus: Advances in Prevention, Diagnosis, and Therapy. Clin Microbiol Rev. 2020;33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 311] [Article Influence: 62.2] [Reference Citation Analysis (0)] |
| 26. | Schillie S, Vellozzi C, Reingold A, Harris A, Haber P, Ward JW, Nelson NP. Prevention of Hepatitis B Virus Infection in the United States: Recommendations of the Advisory Committee on Immunization Practices. MMWR Recomm Rep. 2018;67:1-31. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 337] [Cited by in RCA: 465] [Article Influence: 66.4] [Reference Citation Analysis (0)] |
| 27. | Drazilova S, Kristian P, Janicko M, Halanova M, Safcak D, Dorcakova PD, Marekova M, Pella D, Madarasova-Geckova A, Jarcuska P, HepaMeta Team. What is the Role of the Horizontal Transmission of Hepatitis B Virus Infection in Young Adult and Middle-Aged Roma Population Living in the Settlements in East Slovakia? Int J Environ Res Public Health. 2020;17. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 28. | Weis-Torres SMDS, Fitts SMF, Cardoso WM, Higa Junior MG, Lima LA, Bandeira LM, Castro VOL, Carneiro FA, Iglecias LMM, Cesar GA, Tanaka TSO, Puga MAM, Rezende GR, Croda J, Lago BV, Motta-Castro ARC. High level of exposure to hepatitis B virus infection in a vulnerable population of a low endemic area: A challenge for vaccination coverage. Int J Infect Dis. 2020;90:46-52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 29. | de Villiers MJ, Nayagam S, Hallett TB. The impact of the timely birth dose vaccine on the global elimination of hepatitis B. Nat Commun. 2021;12:6223. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 59] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 30. | Guimarães LCDC, Brunini S, Guimarães RA, Galdino-Júnior H, Minamisava R, da Cunha VE, Santos JRS, Silveira-Lacerda EP, Souza CM, de Oliveira VLB, Albernaz GC, de Menezes TG, Rezza G. Epidemiology of hepatitis B virus infection in people living in poverty in the central-west region of Brazil. BMC Public Health. 2019;19:443. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 31. | Makuza JD, Rwema JOT, Ntihabose CK, Dushimiyimana D, Umutesi J, Nisingizwe MP, Serumondo J, Semakula M, Riedel DJ, Nsanzimana S. Prevalence of hepatitis B surface antigen (HBsAg) positivity and its associated factors in Rwanda. BMC Infect Dis. 2019;19:381. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 29] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 32. | Fattahi MR, Mehrabani D, Mehvarz S, Jaliani NZ, Alipour A, Davarpanah MA. The Seroprevalence of Hepatitis B in Akbar Abad Village, Kavar, Southern Iran. Int J Prev Med. 2014;5:S223-S230. [PubMed] |
| 33. | McNaughton AL, D'Arienzo V, Ansari MA, Lumley SF, Littlejohn M, Revill P, McKeating JA, Matthews PC. Insights From Deep Sequencing of the HBV Genome-Unique, Tiny, and Misunderstood. Gastroenterology. 2019;156:384-399. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 90] [Cited by in RCA: 80] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 34. | Locarnini SA, Littlejohn M, Yuen LKW. Origins and Evolution of the Primate Hepatitis B Virus. Front Microbiol. 2021;12:653684. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 20] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 35. | Zhang ZH, Wu CC, Chen XW, Li X, Li J, Lu MJ. Genetic variation of hepatitis B virus and its significance for pathogenesis. World J Gastroenterol. 2016;22:126-144. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 88] [Cited by in RCA: 88] [Article Influence: 9.8] [Reference Citation Analysis (3)] |
| 36. | Tsukuda S, Watashi K. Hepatitis B virus biology and life cycle. Antiviral Res. 2020;182:104925. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 232] [Article Influence: 46.4] [Reference Citation Analysis (0)] |
| 37. | Seeger C, Mason WS, Lai MMC. Molecular Biology of Hepatitis Viruses. In: Arias IM, Alter HJ, Boyer JL, Cohen DE, Shafritz DA, Thorgeirsson SS, Wolkoff AW. The Liver: Biology and Pathobiology. Oxford, UK: John Wiley & Sons, Ltd, 2020: 793-820. |
| 38. | Mohebbi A, Lorestani N, Tahamtan A, Kargar NL, Tabarraei A. An Overview of Hepatitis B Virus Surface Antigen Secretion Inhibitors. Front Microbiol. 2018;9:662. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 20] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 39. | Seeger C, Mason WS. Molecular biology of hepatitis B virus infection. Virology. 2015;479-480:672-686. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 569] [Cited by in RCA: 625] [Article Influence: 62.5] [Reference Citation Analysis (0)] |
| 40. | Patel N, White SJ, Thompson RF, Bingham R, Weiß EU, Maskell DP, Zlotnick A, Dykeman E, Tuma R, Twarock R, Ranson NA, Stockley PG. HBV RNA pre-genome encodes specific motifs that mediate interactions with the viral core protein that promote nucleocapsid assembly. Nat Microbiol. 2017;2:17098. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 63] [Cited by in RCA: 69] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 41. | Ko C, Su J, Festag J, Bester R, Kosinska AD, Protzer U. Intramolecular recombination enables the formation of hepatitis B virus (HBV) cccDNA in mice after HBV genome transfer using recombinant AAV vectors. Antiviral Res. 2021;194:105140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 21] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 42. | Altinel K, Hashimoto K, Wei Y, Neuveut C, Gupta I, Suzuki AM, Dos Santos A, Moreau P, Xia T, Kojima S, Kato S, Takikawa Y, Hidaka I, Shimizu M, Matsuura T, Tsubota A, Ikeda H, Nagoshi S, Suzuki H, Michel ML, Samuel D, Buendia MA, Faivre J, Carninci P. Single-Nucleotide Resolution Mapping of Hepatitis B Virus Promoters in Infected Human Livers and Hepatocellular Carcinoma. J Virol. 2016;90:10811-10822. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 28] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 43. | Watanabe T, Inoue T, Tanaka Y. Hepatitis B Core-Related Antigen and New Therapies for Hepatitis B. Microorganisms. 2021;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 21] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 44. | Philips CA, Ahamed R, Abduljaleel JK, Rajesh S, Augustine P. Critical Updates on Chronic Hepatitis B Virus Infection in 2021. Cureus. 2021;13:e19152. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 45. | Pavesi A. Origin, Evolution and Stability of Overlapping Genes in Viruses: A Systematic Review. Genes (Basel). 2021;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 22] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 46. | Zhao F, Xie X, Tan X, Yu H, Tian M, Lv H, Qin C, Qi J, Zhu Q. The Functions of Hepatitis B Virus Encoding Proteins: Viral Persistence and Liver Pathogenesis. Front Immunol. 2021;12:691766. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 46] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 47. | Pazienza V, Niro GA, Fontana R, Vinciguerra M, Andriulli A. Advance in molecular diagnostic tools for hepatitis B virus detection. Clin Chem Lab Med. 2013;51:1707-1717. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 48. | Hu J, Liu K. Complete and Incomplete Hepatitis B Virus Particles: Formation, Function, and Application. Viruses. 2017;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 154] [Cited by in RCA: 215] [Article Influence: 26.9] [Reference Citation Analysis (0)] |
| 49. | Pastor F, Herrscher C, Patient R, Eymieux S, Moreau A, Burlaud-Gaillard J, Seigneuret F, de Rocquigny H, Roingeard P, Hourioux C. Direct interaction between the hepatitis B virus core and envelope proteins analyzed in a cellular context. Sci Rep. 2019;9:16178. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 23] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 50. | Zhuang X, Forde D, Tsukuda S, D'Arienzo V, Mailly L, Harris JM, Wing PAC, Borrmann H, Schilling M, Magri A, Rubio CO, Maidstone RJ, Iqbal M, Garzon M, Minisini R, Pirisi M, Butterworth S, Balfe P, Ray DW, Watashi K, Baumert TF, McKeating JA. Circadian control of hepatitis B virus replication. Nat Commun. 2021;12:1658. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 35] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 51. | Watashi K, Wakita T. Hepatitis B Virus and Hepatitis D Virus Entry, Species Specificity, and Tissue Tropism. Cold Spring Harb Perspect Med. 2015;5:a021378. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 42] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 52. | Zeyen L, Prange R. Host Cell Rab GTPases in Hepatitis B Virus Infection. Front Cell Dev Biol. 2018;6:154. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 25] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 53. | Lamontagne RJ, Bagga S, Bouchard MJ. Hepatitis B virus molecular biology and pathogenesis. Hepatoma Res. 2016;2:163-186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 122] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 54. | Marchetti AL, Guo H. New Insights on Molecular Mechanism of Hepatitis B Virus Covalently Closed Circular DNA Formation. Cells. 2020;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 35] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 55. | Tu T, Zhang H, Urban S. Hepatitis B Virus DNA Integration: In Vitro Models for Investigating Viral Pathogenesis and Persistence. Viruses. 2021;13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 53] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 56. | Wang G, Guan J, Khan NU, Li G, Shao J, Zhou Q, Xu L, Huang C, Deng J, Zhu H, Chen Z. Potential capacity of interferon-α to eliminate covalently closed circular DNA (cccDNA) in hepatocytes infected with hepatitis B virus. Gut Pathog. 2021;13:22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 19] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 57. | Karayiannis P. Hepatitis B virus: virology, molecular biology, life cycle and intrahepatic spread. Hepatol Int. 2017;11:500-508. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 85] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 58. | Jiang B, Hildt E. Intracellular Trafficking of HBV Particles. Cells. 2020;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 54] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 59. | Inan N, Tabak F. Hepatitis B Virus: Biology and Life Cycle. Viral Hepat J. 2015;21:1-7. [RCA] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 60. | Škrlec I, Milić J, Cilenšek I, Petrovič D, Wagner J, Peterlin B. Circadian clock genes and myocardial infarction in patients with type 2 diabetes mellitus. Gene. 2019;701:98-103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 61. | Zhao M, Xing H, Chen M, Dong D, Wu B. Circadian clock-controlled drug metabolism and transport. Xenobiotica. 2020;50:495-505. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 20] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 62. | Škrlec I, Milić J, Steiner R. The Impact of the Circadian Genes CLOCK and ARNTL on Myocardial Infarction. J Clin Med. 2020;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 19] [Article Influence: 3.8] [Reference Citation Analysis (1)] |
| 63. | Mukherji A, Bailey SM, Staels B, Baumert TF. The circadian clock and liver function in health and disease. J Hepatol. 2019;71:200-211. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 152] [Article Influence: 25.3] [Reference Citation Analysis (1)] |
| 64. | Škrlec I. Circadian rhythm and myocardial infarction. Med Flum. 2019;55:32-42. [RCA] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 65. | Haspel JA, Anafi R, Brown MK, Cermakian N, Depner C, Desplats P, Gelman AE, Haack M, Jelic S, Kim BS, Laposky AD, Lee YC, Mongodin E, Prather AA, Prendergast BJ, Reardon C, Shaw AC, Sengupta S, Szentirmai É, Thakkar M, Walker WE, Solt LA. Perfect timing: circadian rhythms, sleep, and immunity - an NIH workshop summary. JCI Insight. 2020;5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 136] [Article Influence: 27.2] [Reference Citation Analysis (0)] |
| 66. | Shi D, Chen J, Wang J, Yao J, Huang Y, Zhang G, Bao Z. Circadian Clock Genes in the Metabolism of Non-alcoholic Fatty Liver Disease. Front Physiol. 2019;10:423. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 63] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 67. | Mukherji A, Dachraoui M, Baumert TF. Perturbation of the circadian clock and pathogenesis of NAFLD. Metabolism. 2020;111S:154337. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 25] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 68. | Kim YH, Lazar MA. Transcriptional Control of Circadian Rhythms and Metabolism: A Matter of Time and Space. Endocr Rev. 2020;41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 86] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 69. | Dandri M. Epigenetic modulation in chronic hepatitis B virus infection. Semin Immunopathol. 2020;42:173-185. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 58] [Cited by in RCA: 70] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 70. | Kong F, Li Q, Zhang F, Li X, You H, Pan X, Zheng K, Tang R. Sirtuins as Potential Therapeutic Targets for Hepatitis B Virus Infection. Front Med (Lausanne). 2021;8:751516. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 14] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 71. | Du L, Ma Y, Liu M, Yan L, Tang H. Peroxisome Proliferators Activated Receptor (PPAR) agonists activate hepatitis B virus replication in vivo. Virol J. 2017;14:96. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 19] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 72. | Dubuquoy L, Louvet A, Hollebecque A, Mathurin P, Dharancy S. Peroxisome proliferator-activated receptors in HBV-related infection. PPAR Res. 2009;2009:145124. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 11] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 73. | Charoensuksai P, Xu W. PPARs in Rhythmic Metabolic Regulation and Implications in Health and Disease. PPAR Res. 2010;2010. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 28] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 74. | Le Martelot G, Claudel T, Gatfield D, Schaad O, Kornmann B, Lo Sasso G, Moschetta A, Schibler U. REV-ERBalpha participates in circadian SREBP signaling and bile acid homeostasis. PLoS Biol. 2009;7:e1000181. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 326] [Cited by in RCA: 366] [Article Influence: 22.9] [Reference Citation Analysis (0)] |
| 75. | Kawai M, Rosen CJ. PPARγ: a circadian transcription factor in adipogenesis and osteogenesis. Nat Rev Endocrinol. 2010;6:629-636. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 231] [Cited by in RCA: 245] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 76. | Zhang EE, Liu Y, Dentin R, Pongsawakul PY, Liu AC, Hirota T, Nusinow DA, Sun X, Landais S, Kodama Y, Brenner DA, Montminy M, Kay SA. Cryptochrome mediates circadian regulation of cAMP signaling and hepatic gluconeogenesis. Nat Med. 2010;16:1152-1156. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 471] [Cited by in RCA: 440] [Article Influence: 29.3] [Reference Citation Analysis (0)] |
| 77. | Kim EJ, Yoon YS, Hong S, Son HY, Na TY, Lee MH, Kang HJ, Park J, Cho WJ, Kim SG, Koo SH, Park HG, Lee MO. Retinoic acid receptor-related orphan receptor α-induced activation of adenosine monophosphate-activated protein kinase results in attenuation of hepatic steatosis. Hepatology. 2012;55:1379-1388. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 54] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 78. | Kalsbeek A, la Fleur S, Fliers E. Circadian control of glucose metabolism. Mol Metab. 2014;3:372-383. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 185] [Cited by in RCA: 234] [Article Influence: 21.3] [Reference Citation Analysis (0)] |
| 79. | Diallo AB, Coiffard B, Leone M, Mezouar S, Mege JL. For Whom the Clock Ticks: Clinical Chronobiology for Infectious Diseases. Front Immunol. 2020;11:1457. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 29] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 80. | Mazzoccoli G, Vinciguerra M, Carbone A, Relógio A. The Circadian Clock, the Immune System, and Viral Infections: The Intricate Relationship Between Biological Time and Host-Virus Interaction. Pathogens. 2020;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 47] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 81. | Wu C, Guo X, Li M, Shen J, Fu X, Xie Q, Hou Z, Zhai M, Qiu X, Cui Z, Xie H, Qin P, Weng X, Hu Z, Liang J. DeepHBV: a deep learning model to predict hepatitis B virus (HBV) integration sites. BMC Ecol Evol. 2021;21:138. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 82. | Zhuang X, Edgar RS, McKeating JA. The role of circadian clock pathways in viral replication. Semin Immunopathol. 2022;44:175-182. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 3] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 83. | Sulli G, Lam MTY, Panda S. Interplay between Circadian Clock and Cancer: New Frontiers for Cancer Treatment. Trends Cancer. 2019;5:475-494. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 159] [Cited by in RCA: 308] [Article Influence: 51.3] [Reference Citation Analysis (0)] |
| 84. | Jiang Y, Shen X, Fasae MB, Zhi F, Chai L, Ou Y, Feng H, Liu S, Liu Y, Yang S. The Expression and Function of Circadian Rhythm Genes in Hepatocellular Carcinoma. Oxid Med Cell Longev. 2021;2021:4044606. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 13] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 85. | Yang SL, Yu C, Jiang JX, Liu LP, Fang X, Wu C. Hepatitis B virus X protein disrupts the balance of the expression of circadian rhythm genes in hepatocellular carcinoma. Oncol Lett. 2014;8:2715-2720. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 40] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 86. | Li H, Lu YF, Chen H, Liu J. Dysregulation of metallothionein and circadian genes in human hepatocellular carcinoma. Chronobiol Int. 2017;34:192-202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 25] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 87. | Caratti G, Iqbal M, Hunter L, Kim D, Wang P, Vonslow RM, Begley N, Tetley AJ, Woodburn JL, Pariollaud M, Maidstone R, Donaldson IJ, Zhang Z, Ince LM, Kitchen G, Baxter M, Poolman TM, Daniels DA, Stirling DR, Brocker C, Gonzalez F, Loudon AS, Bechtold DA, Rattray M, Matthews LC, Ray DW. REVERBa couples the circadian clock to hepatic glucocorticoid action. J Clin Invest. 2018;128:4454-4471. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 61] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 88. | Li M, Wang Y, Xia X, Mo P, Xu J, Yu C, Li W. Steroid receptor coactivator 3 inhibits hepatitis B virus gene expression through activating Akt signaling to prevent HNF4α nuclear translocation. Cell Biosci. 2019;9:64. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 89. | Fekry B, Ribas-Latre A, Baumgartner C, Deans JR, Kwok C, Patel P, Fu L, Berdeaux R, Sun K, Kolonin MG, Wang SH, Yoo SH, Sladek FM, Eckel-Mahan K. Incompatibility of the circadian protein BMAL1 and HNF4α in hepatocellular carcinoma. Nat Commun. 2018;9:4349. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 71] [Cited by in RCA: 81] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 90. | Shkodina AD, Tan SC, Hasan MM, Abdelgawad M, Chopra H, Bilal M, Boiko DI, Tarianyk KA, Alexiou A. Roles of clock genes in the pathogenesis of Parkinson’s disease. Ageing Res Rev. 2022;74:101554. [RCA] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 36] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 91. | Pearson JA, Voisey AC, Boest-Bjerg K, Wong FS, Wen L. Circadian Rhythm Modulation of Microbes During Health and Infection. Front Microbiol. 2021;12:721004. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (1)] |
| 92. | Qi ZX, Wang LY, Fan YC, Zhang JJ, Li T, Wang K. Increased peripheral RORα and RORγt mRNA expression is associated with acute-on-chronic hepatitis B liver failure. J Viral Hepat. 2012;19:811-822. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 93. | Huang Y, Liang H, He C, Peng F. Hepatitis B Virus X Protein-Induced RORγ Expression to Promote the Migration and Proliferation of Hepatocellular Carcinoma. Biomed Res Int. 2019;2019:5407126. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 94. | Liang Y, Wang S, Huang X, Chai R, Tang Q, Yang R, Wang X, Zheng K. Dysregulation of Circadian Clock Genes as Significant Clinic Factor in the Tumorigenesis of Hepatocellular Carcinoma. Comput Math Methods Med. 2021;2021:8238833. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 15] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 95. | Iannacone M, Guidotti LG. Immunobiology and pathogenesis of hepatitis B virus infection. Nat Rev Immunol. 2022;22:19-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 291] [Article Influence: 97.0] [Reference Citation Analysis (0)] |
| 96. | Tan A, Koh S, Bertoletti A. Immune Response in Hepatitis B Virus Infection. Cold Spring Harb Perspect Med. 2015;5:a021428. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 90] [Article Influence: 9.0] [Reference Citation Analysis (1)] |
| 97. | Ghosh A, Onsager C, Mason A, Arriola L, Lee W, Mubayi A. The role of oxygen intake and liver enzyme on the dynamics of damaged hepatocytes: Implications to ischaemic liver injury via a mathematical model. PLoS One. 2021;16:e0230833. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 9] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 98. | Gu Y, Lian Y, Gu L, Chen L, Li X, Zhou L, Huang Y, Wang J. Correlations between cytokines produced by T cells and clinical-virological characteristics in untreated chronic hepatitis B patients. BMC Infect Dis. 2019;19:216. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 15] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 99. | Matsumura S, Yamamoto K, Shimada N, Okano N, Okamoto R, Suzuki T, Hakoda T, Mizuno M, Higashi T, Tsuji T. High frequency of circulating HBcAg-specific CD8 T cells in hepatitis B infection: a flow cytometric analysis. Clin Exp Immunol. 2001;124:435-444. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 12] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 100. | Meier MA, Suslov A, Ketterer S, Heim MH, Wieland SF. Hepatitis B virus covalently closed circular DNA homeostasis is independent of the lymphotoxin pathway during chronic HBV infection. J Viral Hepat. 2017;24:662-671. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 101. | Pick R, He W, Chen CS, Scheiermann C. Time-of-Day-Dependent Trafficking and Function of Leukocyte Subsets. Trends Immunol. 2019;40:524-537. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 90] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 102. | Dimitrov S, Benedict C, Heutling D, Westermann J, Born J, Lange T. Cortisol and epinephrine control opposing circadian rhythms in T cell subsets. Blood. 2009;113:5134-5143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 210] [Cited by in RCA: 258] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 103. | Narasimamurthy R, Hatori M, Nayak SK, Liu F, Panda S, Verma IM. Circadian clock protein cryptochrome regulates the expression of proinflammatory cytokines. Proc Natl Acad Sci U S A. 2012;109:12662-12667. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 261] [Cited by in RCA: 333] [Article Influence: 25.6] [Reference Citation Analysis (0)] |
| 104. | Silver AC, Arjona A, Walker WE, Fikrig E. The circadian clock controls toll-like receptor 9-mediated innate and adaptive immunity. Immunity. 2012;36:251-261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 287] [Cited by in RCA: 346] [Article Influence: 26.6] [Reference Citation Analysis (0)] |
| 105. | Crespo I, Fernández-Palanca P, San-Miguel B, Álvarez M, González-Gallego J, Tuñón MJ. Melatonin modulates mitophagy, innate immunity and circadian clocks in a model of viral-induced fulminant hepatic failure. J Cell Mol Med. 2020;24:7625-7636. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 29] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 106. | Griffett K, Bedia-Diaz G, Elgendy B, Burris TP. REV-ERB agonism improves liver pathology in a mouse model of NASH. PLoS One. 2020;15:e0236000. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 22] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 107. | Srinivasan V, Mohamed M, Kato H. Melatonin in bacterial and viral infections with focus on sepsis: a review. Recent Pat Endocr Metab Immune Drug Discov. 2012;6:30-39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 75] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 108. | van den Berg F, Limani SW, Mnyandu N, Maepa MB, Ely A, Arbuthnot P. Advances with RNAi-Based Therapy for Hepatitis B Virus Infection. Viruses. 2020;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 53] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 109. | Pierra Rouviere C, Dousson CB, Tavis JE. HBV replication inhibitors. Antiviral Res. 2020;179:104815. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 44] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 110. | Liang TJ. Hepatitis B: a new weapon against an old enemy. Nat Med. 2021;27:1672-1673. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 111. | Raimondo G, Locarnini S, Pollicino T, Levrero M, Zoulim F, Lok AS; Taormina Workshop on Occult HBV Infection Faculty Members. Update of the statements on biology and clinical impact of occult hepatitis B virus infection. J Hepatol. 2019;71:397-408. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 375] [Cited by in RCA: 351] [Article Influence: 58.5] [Reference Citation Analysis (0)] |
| 112. | Greco CM, Sassone-Corsi P. Personalized medicine and circadian rhythms: Opportunities for modern society. J Exp Med. 2020;217. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 113. | Maiese K. Circadian Clock Genes: Targeting Innate Immunity for Antiviral Strategies Against COVID-19. Curr Neurovasc Res. 2020;17:531-533. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 114. | Karabay O, Temel A, Koker AG, Tokel M, Ceyhan M, Kocoglu E. Influence of circadian rhythm on the efficacy of the hepatitis B vaccination. Vaccine. 2008;26:1143-1144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 16] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 115. | De Giorgi A, Fabbian F, Di Simone E, Greco S, De Giorgio R, Zuliani G, Passaro A, Caselli E, Manfredini R; OUTCOME-INTMED-COV19 Study Collaborators. Morning vs. evening administration of antiviral therapy in COVID-19 patients. A preliminary retrospective study in Ferrara, Italy. Eur Rev Med Pharmacol Sci. 2020;24:8219-8225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 116. | Ruben MD, Smith DF, FitzGerald GA, Hogenesch JB. Dosing time matters. Science. 2019;365:547-549. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 171] [Cited by in RCA: 165] [Article Influence: 27.5] [Reference Citation Analysis (0)] |
| 117. | Kurupati RK, Kossenkoff A, Kannan S, Haut LH, Doyle S, Yin X, Schmader KE, Liu Q, Showe L, Ertl HCJ. The effect of timing of influenza vaccination and sample collection on antibody titers and responses in the aged. Vaccine. 2017;35:3700-3708. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 28] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 118. | Sultan A, Ali R, Sultan T, Ali S, Khan NJ, Parganiha A. Circadian clock modulating small molecules repurposing as inhibitors of SARS-CoV-2 Mpro for pharmacological interventions in COVID-19 pandemic. Chronobiol Int. 2021;38:971-985. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 16] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 119. | Sultan A. Identification and development of clock-modulating small molecules-an emerging approach to fine-tune the disrupted circadian clocks. Biol Rhythm Res. 2018;50:769-786. [RCA] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 120. | Borrmann H, Davies R, Dickinson M, Pedroza-Pacheco I, Schilling M, Vaughan-Jackson A, Magri A, James W, Balfe P, Borrow P, McKeating JA, Zhuang X. Pharmacological activation of the circadian component REV-ERB inhibits HIV-1 replication. Sci Rep. 2020;10:13271. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 34] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 121. | Wang S, Li F, Lin Y, Wu B. Targeting REV-ERBα for therapeutic purposes: promises and challenges. Theranostics. 2020;10:4168-4182. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 66] [Cited by in RCA: 91] [Article Influence: 18.2] [Reference Citation Analysis (0)] |