Published online Jul 7, 2022. doi: 10.3748/wjg.v28.i25.2994
Peer-review started: December 18, 2021
First decision: March 10, 2022
Revised: March 12, 2022
Accepted: May 27, 2022
Article in press: May 27, 2022
Published online: July 7, 2022
Processing time: 198 Days and 7.5 Hours
Submucosal tumor (SMT)-like gastric cancer is rare, and almost all cases undergo curative surgical treatment because the submucosal layer is usually deeply invaded by tumor cells or because histopathologic types of SMT-like gastric cancer are undifferentiated or poorly differentiated. No report has been issued on an SMT-like gastric cancer cured by endoscopic resection alone or on changes in the endoscopic features of this type of tumor over several years.
We describe an exceptional case of a 53-year-old male with a 1.5 cm-sized SMT-like lesion covered by normal-appearing mucosa discovered by esophagogastroduodenoscopy (EGD) at the gastric antrum. Endoscopic ultrasound (EUS) visualized a homogeneous, well-circumscribed hypoechogenic lesion arising from the second sonographic layer with associated subtle obliteration of the third sonographic layer. Initial endoscopic biopsy was negative for neoplasm. The patient refused to undergo an invasive procedure and was subsequently lost to follow-up. Three years after initial detection, EGD revealed the lesion had become markedly erythematous, and at 4 years after initial EGD it had increased in size to 1.8 cm and developed a central ulcer and a heterogeneous EUS echo. Finally, endoscopic submucosal dissection (ESD) was performed, and histopathologic examination revealed a moderately differentiated adenocarcinoma had minutely invaded the submucosal layer (invasion depth 169 μm) but without lympho
This report describes an extremely rare case of early gastric cancer presenting as SMT that was cured by ESD after a treatment delay of 4 years and the endoscopic changes that occurred during this period. The report highlights the importance of considering the possibility of gastric cancer when SMT is encountered in clinical practice.
Core Tip: We experienced an exceptionally rare case of early gastric cancer presenting as submucosal tumor (SMT) that was successfully treated by endoscopic submucosal dissection (ESD) alone, although the procedure was performed four years after first detection due to patient refusal and follow-up loss. The present case cautions that SMT-like gastric cancer should be included in the differential diagnosis when a hypoechogenic mass is detected in the 2nd or 3rd layer by endoscopic ultrasound, regardless of size and the absence of findings suggesting malignancy. When diagnosis is uncertain, invasive techniques such as diagnostic endoscopic mucosal resection or ESD, which can potentially be used for therapeutic purposes, should be considered and close follow-up is recommended.
- Citation: Cho JH, Lee SH. Early gastric cancer presenting as a typical submucosal tumor cured by endoscopic submucosal dissection: A case report. World J Gastroenterol 2022; 28(25): 2994-3000
- URL: https://www.wjgnet.com/1007-9327/full/v28/i25/2994.htm
- DOI: https://dx.doi.org/10.3748/wjg.v28.i25.2994
Gastric carcinoma is an epithelial tumor exposed to the mucosal surface that occurs in lamina propria with variable gross findings. However, cases of gastric carcinoma with features of submucosal tumor (SMT) are rarely encountered in routine clinical settings and reportedly account for only 0.2% to 0.62% of all resected gastric cancers[1]. The majority of patients with gastric adenocarcinoma resembling SMT are not indicated for endoscopic resection or offered non-curative resection after endoscopic resection. Submucosal layer is deeply invaded by tumor cells, even in cases of early gastric cancer (EGC)[2], and histopathologically, most adenocarcinomas presenting as SMTs are undifferentiated or poorly differentiated[3,4], and as a result, almost all undergo curative surgical treatment. Accordingly, no case has been reported on SMT-like gastric cancer cured by endoscopic resection alone. In addition, no report has been issued on changes in the endoscopic features of this type of tumor over several years. Here, we report an exceptionally rare case of EGC presenting as SMT that was cured by endoscopic submucosal dissection (ESD) and describe changes in the endoscopic features of this tumor over a period of 4 years.
A 53-year-old male Korean patient was referred to our institution for further evaluation and treatment of a gastric SMT discovered by esophagogastroduodenoscopy (EGD) during a routine medical check-up.
The patient had no abdominal pain or related discomfort.
He had a history of abdominal surgery due to duodenal ulcer perforation 30 years previously.
The patient had diabetes that was being treated with oral hypoglycemic agents. He was a smoker (30 pack-years) and social-alcohol drinker and had no significant family history.
Physical examination was unremarkable, and his abdomen was soft, nontender, and nondistended with no palpable mass.
Laboratory tests, which included tests for common serum tumor markers such as CEA and CA 19-9, revealed no abnormalities.
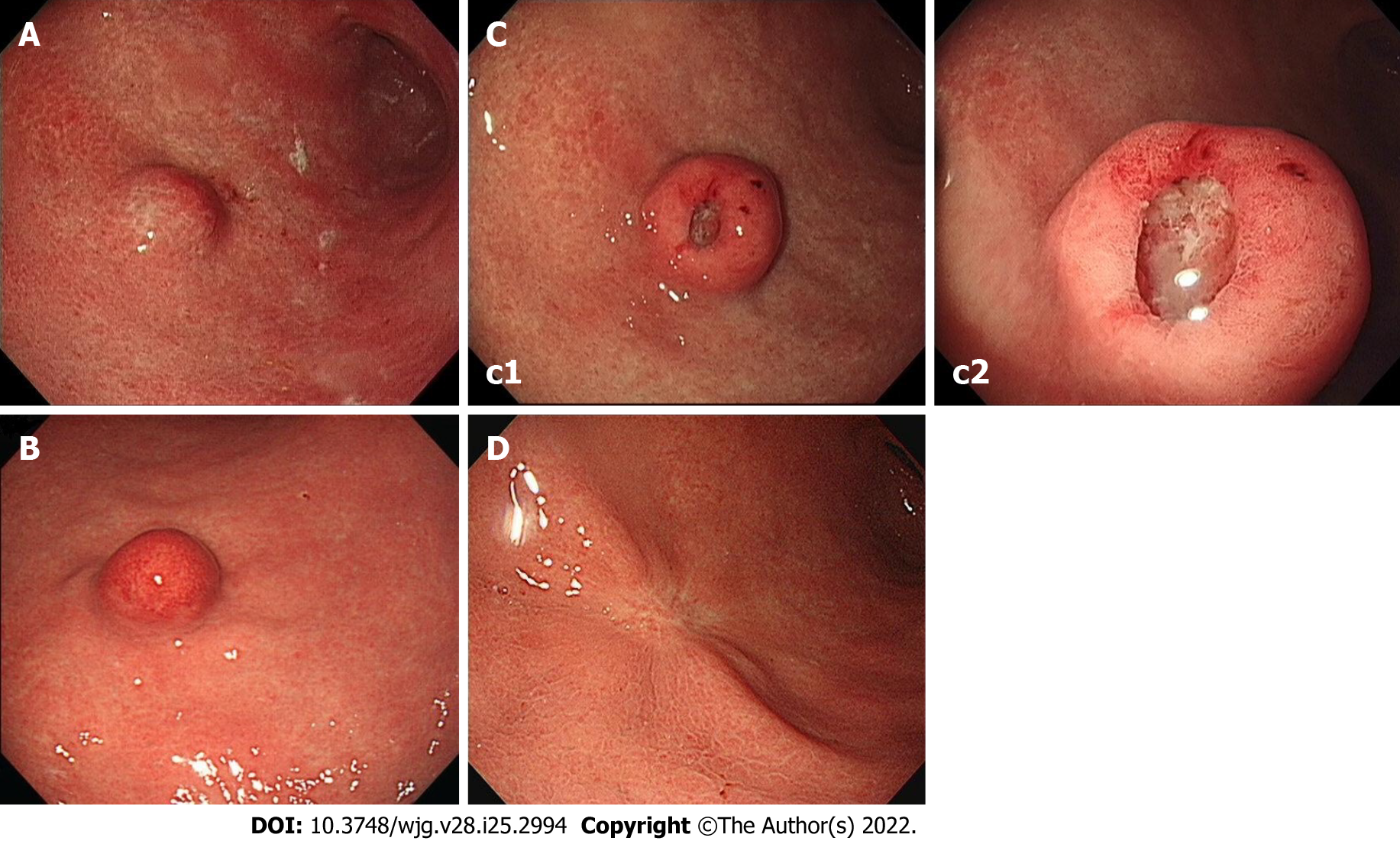
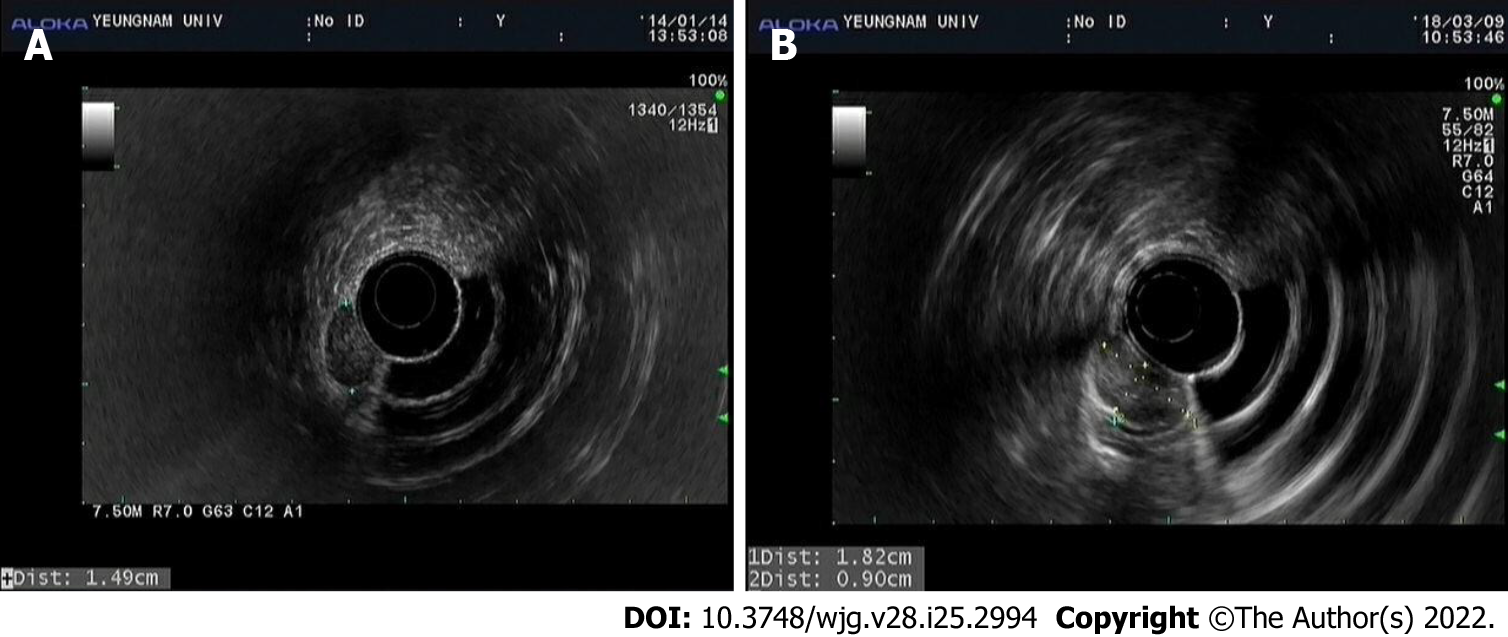
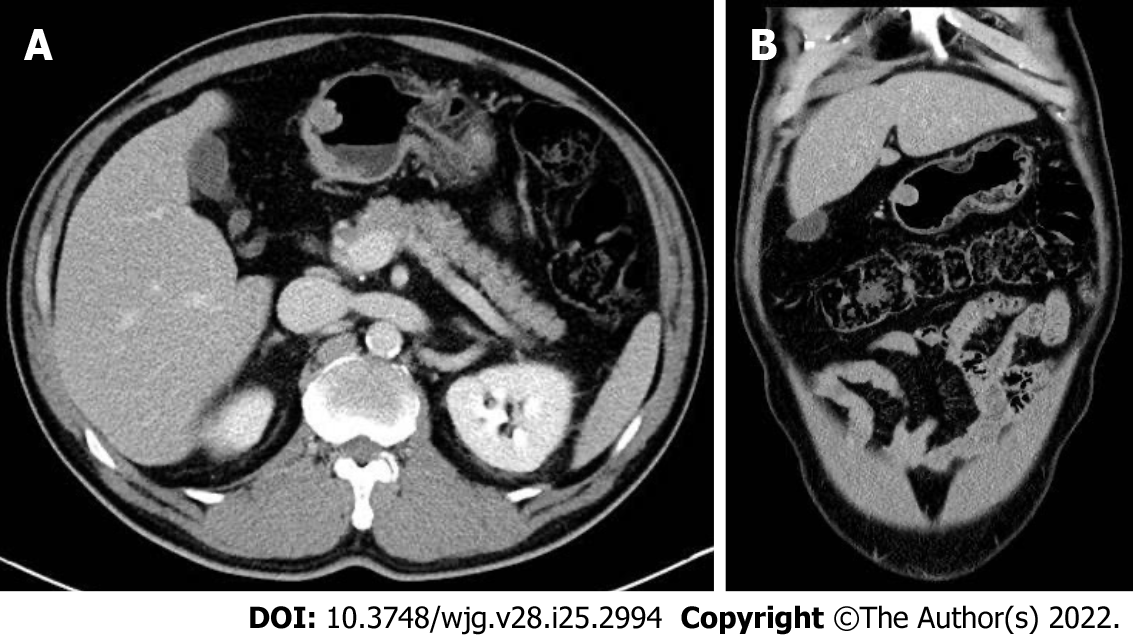
At initial EGD, an SMT-like elevated lesion of diameter 15-mm was observed at the great curvature side of the proximal part of the gastric antrum (Figure 1A). The lesion was covered with normal-appearing mucosa without any erosion, ulcer, or mucosal erythema. Mild-atrophic gastritis, confined to antrum, was observed in background mucosa. Endoscopic ultrasound (EUS; GF-UM2000, Olympus, Tokyo, Japan) demonstrated a 15 mm × 7 mm homogeneous, well-circumscribed hypoechogenic lesion arising from the second sonographic layer with associated subtle obliteration of the third sonographic layer (Figure 2A). The EUS appearance of the lesion was suggestive of a gastric neuroendocrine tumor (NET) or ectopic pancreas. Initial biopsy specimens were negative for neoplasm. Computed tomography (CT) of the abdomen showed a 15 mm protruding intraluminal mass at the gastric antrum and no evidence of lymph node enlargement or distant metastasis (Figure 3). After this initial work-up, endoscopic resection of the lesion was planned for definitive histopathologic examination and treatment. However, the patient declined any invasive procedure and was subsequently lost to follow-up.
About 3 years after initial EGD, he underwent a national health screening examination at our hospital, and the previously noted gastric SMT was detected again by EGD. The lesion showed no change in size as compared with its size 3 years previously, but marked erythema of overlying mucosa was observed (Figure 1B). Endoscopic biopsy was performed, but specimens were negative for neoplasm. During consultation regarding his health examination results, he was recommended for further evaluation and treatment at the gastroenterology department but again refused and was lost to follow-up.
About a year later, he revisited our hospital with mild indigestion. Follow-up EGD then revealed the SMT-like lesion had enlarged (to a greatest diameter of approximately 18 mm) and that a 5 mm central ulcer had developed on the top of the lesion (Figure 1C). EUS also demonstrated an 18 mm × 9 mm homogeneous, well-circumscribed hypoechogenic mass arising from the second sonographic layer with associated subtle obliteration of the third sonographic layer (Figure 2B). Echogenicity at this time was slightly more heterogeneous than that observed by initial EUS. CT of the abdomen showed no evidence of lymph node enlargement or distant metastasis, and endoscopic biopsy specimens taken from the lesion revealed tubular adenoma with high-grade dysplasia.
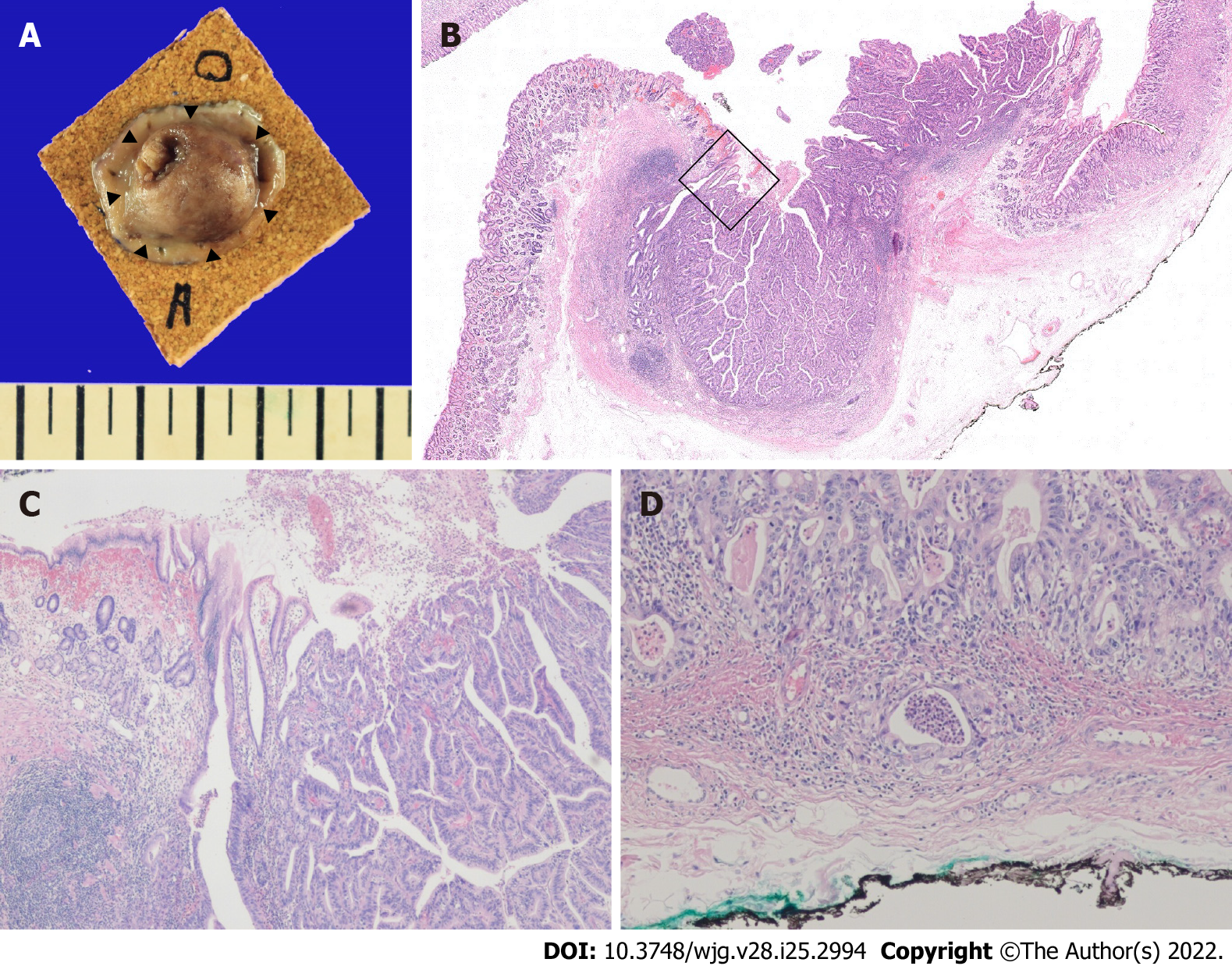
The patient was then admitted for further endoscopic treatment. A serum anti-Helicobacter pylori (H. pylori) IgG assay was negative on the day of endoscopic examination. ESD of the lesion was performed, and histopathologic examination of the resected specimen revealed moderately differentiated adenocarcinoma that invaded the submucosal layer (depth of invasion 169 μm) with no lymphovascular invasion (Figure 4).
Furthermore, resection margins were all negative for cancer. Also, rapid urease test and Giemsa staining were negative for H. pylori infection.
His post-ESD course was uneventful, and he has since been followed up at our outpatient department for 4 years without any evidence of local or distant recurrence. The latest follow-up EGD performed (approximately 3.5 years after ESD) showed only post-ESD scarring (Figure 1D).
This case report is meaningful for the following reasons. First, to the best of our knowledge, this is the first case report of SMT-like gastric cancer cured by ESD alone, and this result was obtained even though ESD was performed four years after initial detection. Second, this case report describes the natural course of SMT-like gastric cancer and the endoscopic changes that occurred after a treatment delay of 4 years. Furthermore, unlike previous reports, mucosa overlying the SMT appeared completely normal when the tumor was first detected.
Gastric cancer usually derives from the lamina propria layer and has macroscopic appearances ranging from well-defined protuberant to diffuse infiltrating. Approximately 95% of gastric cancers are adenocarcinomas[5] but only rarely appear in the form of SMT. According to the literature[2], fewer than 0.5% of gastric cancer cases present as SMT. However, when an SMT-like lesion is encountered in clinical practice, the possibility of gastric cancer should be carefully considered and the lesion differentiated from other submucosal lesions, such as gastric NET[6], leiomyoma, lymphoma, gastrointestinal stromal tumor (GIST), lipoma, ectopic pancreas, and other unusual manifestations, such as metastatic carcinoma[7] and gastric glomus tumor[8], which require completely different treatment strategies.
Pathologic diagnoses of reported cases of gastric cancer presenting as SMT include gastric adenocarcinoma[9], gastric mucinous adenocarcinoma[10,11], and gastric lymphoepithelioma-like carcinoma[12]. The most common histopathologic type is undifferentiated to poorly differentiated adenocarcinoma, which constitutes 68.8% to 71.4% of these cancers[3,4]. The mechanism of SMT-like gastric cancer development is obscure, but several pathologic mechanisms have been suggested. These mechanisms include the biologic tendencies of poorly differentiated adenocarcinomas to exhibit invasion of a deeper layer at an early stage, marked lymphocyte infiltration around tumors[1], the production of large amounts of mucin by mucinous adenocarcinoma[10,11], substantial amounts of surrounding fibrosis due to repetitive inflammation, abundant edematous fibrosis[13], and adenocarcinomas arising from a submucosal heterotopic gastric gland[9,14], which have been recognized as aberrant lamina propria components associated with repeated erosion and regeneration. These factors may facilitate a predominance of submucosal growth and penetration of muscularis mucosa during the early stage of carcinogenesis and contribute to a macroscopic appearance indistinguishable from SMT. However, the tumor in our case had a moderately differentiated histology, which was not in line with any of these mechanisms.
Although the diagnosis of SMT-like gastric cancer is usually difficult due to a deep tumor location and non-specific and overlapping imaging features, some EGD characteristics of SMT-like gastric cancer have been reported. In particular, SMT with central ulceration or depression has been described as common for SMT-like gastric cancer[10,14-16]. Erythematous surface change is another reported characteristic[14,15]. Fujiyoshi et al[16] concluded that a small SMT (3-5 cm) with a central ulcer or irregular erythematous change should raise suspicion of malignancy. However, since our case originally appeared as SMT with completely normal overlying mucosa, we suspected SMT originating from the second or third layer rather than cancer. However, EGD at 3 and 4 years after initial detection of the SMT-like lesion, showed erythematous change and central ulceration. Interestingly, definite changes were observed in the endoscopic features of the tumor 4 years after initial detection, but it was not possible to determine when these morphologic changes occurred precisely due to follow-up loss.
Histological diagnosis of gastric SMT by endoscopic biopsy is often difficult and detailed imaging usually fails to provide sufficient evidence to differentiate benign and malignant tumors. Furthermore, endoscopic biopsy specimens, even those taken from a central ulcer, may be unhelpful[17], and when a tumor is completely covered with normal mucosa, it is extremely difficult to obtain an adequate sample of the underlying lesion. In the present case, negative results for neoplasm were reported for biopsies performed during EGD on overlying normal mucosa at initial presentation and on overlying erythematous mucosa 3 years later.
EUS is useful for evaluating gastric SMT. Findings that suggest malignant SMT include[18] a size > 3 cm or > 5 cm, rapid growth, heterogeneous echogenicity, and irregular margins, whereas GIST or leiomyoma may present as a homogeneous, well-demarcated, submucosal mass with smooth margins. However, these imaging characteristics are non-specific, and EUS images alone are insufficient for accurate diagnosis. More invasive techniques such as EUS-guided fine-needle aspiration or biopsy and endoscopic mucosal resection may aid differential diagnoses[18]. In our patient, initial EUS depicted a 15 mm-sized well-circumscribed homogeneous mass, which did not suggest malignant SMT. However, EUS performed 4 years later showed the mass had increased in size to 18 mm and had slightly more heterogeneous echogenicity.
Gastric cancers resembling SMT are characterized by a predominance of submucosal or sometimes deeper invasion into the gastric wall[2], which suggests they are likely to be more advanced and pose a greater risk of metastasis than ordinary gastric cancers of similar size[13]. Furthermore, most adenocarcinomas presenting as SMTs are of the undifferentiated or poorly differentiated histopathologic types[3,4], and thus, in almost all case reports, SMT-like gastric cancer, even when small, has been treated in the same way as advanced gastric cancer. Although ESD can potentially be used for therapeutic purposes, the pathologic results of most specimens resected by ESD indicate non-curative resection, and thus, additional gastrectomy with lymphadenectomy is required[19]. To date, no case report of cure by endoscopic resection has been published in the English literature. However, in the present case, ESD was performed to provide a definitive histopathologic diagnosis and a treatment strategy, and additional surgery was not needed, based on a final pathologic report that the tumor was not poorly differentiated, did not invade deep submucosa, and had negative lateral and deep margins.
The present case emphasizes that although SMT-like gastric cancer is rare, it should be included in the differential diagnosis when a hypoechogenic mass is visualized in the 2nd or 3rd layer by EUS, regardless of lesion size and the absence of findings suggesting malignancy. In addition, if diagnosis is uncertain, the use of techniques more aggressive than EUS alone, such as diagnostic endoscopic mucosal resection or ESD, which can potentially be used for therapeutic purposes, should be considered and close follow-up is recommended.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: South Korea
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Jin X, China; Kawabata H, Japan; Zhang H, China A-Editor: Yao QG, China S-Editor: Yan JP L-Editor: A P-Editor: Yan JP
| 1. | Umehara Y, Kimura T, Okubo T, Sano Y, Nakai K, Oi S, Higashi Y, Funai K. Gastric carcinoma resembling submucosal tumor. Gastric Cancer. 1999;2:191-193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 2. | Ishiguro S, Tsukamoto Y, Kasugai T, Yasuhara Y, Tsuji N, Fujii Y, Nishizawa Y, Yamato N. Characteristic feature of the gastric carcinoma with submucosal tumor like appearance. Stomach Intest. 2003;38:1519-1526. |
| 3. | Takemoto N, Baba T. Kaku Y, Takekoshi T, Maruyama M, Takahashi T, Kato H, Yanagisawa A. Radiologic diagnosis of gastric cancer morphologically mimicking submucosal tumor. Stomach Intest. 1995;30:759-768. [DOI] [Full Text] |
| 4. | Mitsunaga A, Futami S, Murata Y, Suzuki A. Uchida KJ, Nemoto Y, Ikeda I, Nakamura S, Murata Y, Suzuki H. Endoscopic diagnosis of the gastric cancer showing the features of a submucosal tumor. tomach Intest. 1995;30:769-776. [RCA] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 5. | Nagini S. Carcinoma of the stomach: A review of epidemiology, pathogenesis, molecular genetics and chemoprevention. World J Gastrointest Oncol. 2012;4:156-169. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 324] [Cited by in RCA: 331] [Article Influence: 25.5] [Reference Citation Analysis (0)] |
| 6. | Gheorghe AV, Rimbas M, Ginghina O, Spanu A, Voiosu TA. An atypical type I gastric neuroendocrine tumor. Rom J Intern Med. 2017;55:253-256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 7. | Inagaki C, Suzuki T, Kitagawa Y, Hara T, Yamaguchi T. A case report of prostate cancer metastasis to the stomach resembling undifferentiated-type early gastric cancer. BMC Gastroenterol. 2017;17:93. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 8] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 8. | Handa Y, Kano M, Kaneko M, Hirabayashi N. Gastric Glomus Tumor: A Rare Cause of Upper Gastrointestinal Bleeding. Case Rep Surg. 2015;2015:193684. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 9. | Manabe S, Mukaisho KI, Yasuoka T, Usui F, Matsuyama T, Hirata I, Boku Y, Takahashi S. Gastric adenocarcinoma of fundic gland type spreading to heterotopic gastric glands. World J Gastroenterol. 2017;23:7047-7053. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 13] [Cited by in RCA: 16] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 10. | Yu BC, Lee WK. Two cases of mucinous adenocarcinoma of the stomach mistaken as submucosal tumor. J Korean Surg Soc. 2013;84:118-122. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 11. | Yoo CH, Park SJ, Park MI, Moon W, Kim HH, Lee JS, Song JY, Jang HK. Submucosal tumor-like early-stage mucinous gastric carcinoma: a case study. Korean J Gastroenterol. 2013;62:122-125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (1)] |
| 12. | Takahashi T, Otani Y, Yoshida M, Furukawa T, Kameyama K, Akiba Y, Saikawa Y, Kubota T, Kumai K, Kuramochi S, Mukai M, Ishii H, Kitajima M. Gastric cancer mimicking a submucosal tumor diagnosed by laparoscopic excision biopsy. J Laparoendosc Adv Surg Tech A. 2005;15:51-56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 13. | Ohara N, Tominaga O, Uchiyama M, Nakano H. A case of advanced gastric cancer resembling submucosal tumor of the stomach. Jpn J Clin Oncol. 1997;27:423-426. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 10] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 14. | Hashimoto R, Hamamoto H, Omori Y, Tanuma T. Early gastric cancer on submucosal heterotopic gastric glands. Gastrointest Endosc. 2017;85:851-852. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 15. | Teraishi F, Uno F, Kagawa S, Fujiwara T, Gouchi A, Tanaka N. Advanced gastric adenocarcinoma mimicking a submucosal tumor. Endoscopy. 2007;39 Suppl 1:E191-E192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 16. | Fujiyoshi A, Kawamura M, Ishitsuka S. Gastric adenocarcinoma mimicking a submucosal tumor: case report. Gastrointest Endosc. 2003;58:633-635. [PubMed] |
| 17. | Tio TL, Tytgat GN, den Hartog Jager FC. Endoscopic ultrasonography for the evaluation of smooth muscle tumors in the upper gastrointestinal tract: an experience with 42 cases. Gastrointest Endosc. 1990;36:342-350. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 86] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 18. | Papanikolaou IS, Triantafyllou K, Kourikou A, Rösch T. Endoscopic ultrasonography for gastric submucosal lesions. World J Gastrointest Endosc. 2011;3:86-94. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 51] [Cited by in RCA: 77] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 19. | Kim HI, Shim KN, Yoon SY, Song EM, Cho WY, Kim SE, Jung HK, Jung SA. A case of early gastric adenocarcinoma resembling subepithelial tumor. Korean J Helicobacter Up Gastrointest Res. 2013;13:60-63. [RCA] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |