Published online Jun 21, 2022. doi: 10.3748/wjg.v28.i23.2569
Peer-review started: January 5, 2022
First decision: March 9, 2022
Revised: March 23, 2022
Accepted: April 29, 2022
Article in press: April 29, 2022
Published online: June 21, 2022
Processing time: 162 Days and 7.6 Hours
Endoplasmic reticulum (ER) stress-related hepatocyte apoptosis is responsible for multiple hepatic diseases. Previous studies have revealed that endoplasmic reticulophagy (ER-phagy) promotes the selective clearance of damaged ER fragments during ER stress, playing a crucial role in maintaining ER homeostasis and inhibi
To elucidate the effect of FAM134B-mediated ER-phagy on ER stress-induced apoptosis in buffalo rat liver 3A (BRL-3A) rat hepatocytes and the potential regulatory mechanisms.
ER stress-related hepatocyte apoptosis was induced using dithiothreitol (DTT). Proteins related to ER stress and autophagy were measured with western blotting. Protein complex interactions with FAM134B were isolated by co-immunoprecipitation. ER-phagy was evaluated in immunofluorescence experiments. Cell cycle distribution and apoptosis were measured by flow cytometry. Mitochondrial Ca2+ levels were evaluated by the co-localization of intracellular Ca2+-tracker and Mito-tracker. The small interfering RNA against FAM134B was used to knockdown FAM134B in BRL-3A cells.
ER stress-related and autophagy-related proteins in BRL-3A cells were elevated by both short and long-term DTT treatment. Furthermore, co-immunoprecipitation confirmed an interaction between FAM134B, CNX, FAM134B, and LC3 in BRL-3A cells. Immunofluorescence assays revealed that autolysosomes significantly decreased following short-term DTT treatment, but increased after long-term treatment. Mitochondrial Ca2+ levels and apoptotic rates were dramatically elevated, and more cells were arrested in the G1 stage after short-term DTT treatment; however, these decreased 48 h later. Moreover, FAM134B downregulation accelerated mitochondrial apoptotic pathway activation and aggravated hepatocyte apoptosis under ER stress.
FAM134B-mediated ER-phagy attenuates hepatocyte apoptosis by suppressing the mitochondrial apoptotic pathway. Our findings provide new evidence highlighting the importance of FAM134B-mediated ER-phagy in attenuating hepatocyte apoptosis.
Core Tip: We show that family with sequence similarity 134 member B (FAM134B)-mediated reticulophagy maintains the endoplasmic reticulum (ER) homeostasis in ER-stressed hepatocytes via the clearance of damaged ER fragments. Thereby FAM134B-mediated reticulophagy ameliorates dithiothreitol-induced hepatocyte apoptosis. Our findings provide emerging evidence of the prominence of ER-phagy in ER stress-related hepatocyte apoptosis. FAM134B may represent a potential therapeutic target for liver disease treatment.
- Citation: Guo YX, Han B, Yang T, Chen YS, Yang Y, Li JY, Yang Q, Xie RJ. Family with sequence similarity 134 member B-mediated reticulophagy ameliorates hepatocyte apoptosis induced by dithiothreitol. World J Gastroenterol 2022; 28(23): 2569-2581
- URL: https://www.wjgnet.com/1007-9327/full/v28/i23/2569.htm
- DOI: https://dx.doi.org/10.3748/wjg.v28.i23.2569
Endoplasmic reticulum (ER) stress-related hepatocyte apoptosis participates in multiple hepatic diseases, including viral hepatitis[1], hepatic fibrosis[2], fatty liver[3,4] and cirrhosis[5]. Therefore, the alleviation of ER stress-mediated hepatocyte apoptosis is crucial in the treatment of hepatic diseases. Recent findings have indicated that endoplasmic reticulophagy (ER-phagy) promotes degradation of damaged ER fragments during ER stress. Although ER-phagy has a vital role in maintaining ER homeostasis and inhibiting cell apoptosis[6-8], the exact regulatory mechanisms behind this are largely unknown.
Glucose-regulated protein 78 (GRP78) is a prominent ER molecular chaperone, while calnexin (CNX) is a membrane-bound lectin protein in the ER that can increase the protein folding capacity[9,10]. Even though the excessive build-up of misfolded or unfolded proteins can be alleviated via ER stress, previous studies reported that a selective autophagic mechanism, defined as ER-phagy, can also be activated by ER stress to restore ER homeostasis[11,12]. Family with sequence similarity 134 member B (FAM134B), an ER-resident protein, may interact with CNX in the cytosol or the ER membrane[13]. Since FAM134B is not predicted to have an ER lumenal domain, there is an indirect interaction between FAM134B and lumenal proteins through the lumen-resident segment, which has a chaperone activity attributed to CNX. CNX forms transient but relatively stable complexes with unfolded ER proteins until they either become folded or are degraded. Moreover, it has been reported that as with other cargo receptor molecules, FAM134B can interact directly with microtubule-associated protein 1 light chain 3 (LC3) when its LIR motif is exposed. The CNX-FAM134B-LC3 complex can mediate the selective isolation of ER fragments containing misfolded proteins, which are subsequently transported to lysosomes for degradation[14-16]. Thus, FAM134B-mediated ER-phagy may play an essential role in maintaining ER homeostasis and promoting cell survival. However, it is unclear whether FAM134B-mediated ER-phagy is involved in the regulation of hepatocyte apoptosis induced by ER stress. In this study, dithiothreitol (DTT) was used to induce ER stress in buffalo rat liver 3A (BRL-3A) hepatocytes, and the expression of ER stress-related and autophagy-related proteins was assessed. In addition, small interfering RNA (siRNA) was used to knockdown the expression of FAM134B in hepatocytes and an apoptosis analysis followed. Our study reveals an emerging role of FAM134B-mediated ER-phagy in ER stress-mediated hepatocyte apoptosis, which may provide a novel target for the treatment of hepatic diseases.
Dulbecco's modified Eagle medium (DMEM) and fetal bovine serum (FBS) were purchased from Gibco (Grand Island, NY, United States). Trypsin-EDTA solution, trypsin solution without EDTA, and penicillin-streptomycin were purchased from Biological Industries (BioInd, Israel). Bicinchoninic acid (BCA) protein assay kit, DTT, RIPA lysis buffer, and protease inhibitor were obtained from Solarbio (Beijing, China). Annexin V-FITC/PI Apoptosis Detection Kit and Cell Cycle Detection Kit were purchased from KeyGEN BioTECH (Nanjing, China). PVDF membranes were obtained from Merck Millipore. Rabbit polyclonal antibody against FAM134B was purchased from Proteintech (Wuhan, China). Rabbit polyclonal antibodies against ATG12, cytochrome c (cyt c), and cleaved caspase-3 were obtained from Cell Signaling Technology (Danvers, MA, United States). Rabbit polyclonal antibodies against β-actin, LC3, CNX, CHOP and GRP78, and the Ca2+ indicator (Rhod-2 AM) were purchased from Abcam (Cambridge, United Kingdom). Dynabeads protein G immunoprecipitation kit and lipofecta
BRL-3A cells, bought from Cell Bank of the Chinese Academy of Sciences (Shanghai, China), were cultivated and maintained in DMEM culture media supplemented with 1% penicillin-streptomycin and 10% FBS. BRL-3A cells were seeded at 37 °C and 5% CO2 in a constant temperature and humid atmosphere, pre-cultured every 3 d, and further passaged until the density reached approximately 80%. To induce the ER stress, BRL-3A cells were treated with DTT (2.0 mmol/L based on previous studies[17]) for 0, 3, 6, 12, 24, or 48 h.
Cells were cultured to 80% confluency and treated with 2.0 mmol/L DTT for the specified point-in-time intervals. To determine the efficacy of the different DTT treatments, a cell apoptosis analysis was evaluated with flow cytometry. Each group of cells was trypsinized without EDTA and rinsed thrice with PBS. After centrifugation at 2000 rpm for 5 min, cells were loaded with 500 μL binding buffer and labeled with 5 μL of Annexin V-FITC/PI, according to the manufacturer’s instructions. Labeled cells were detected and analyzed with flow cytometry and NovoExpress® software 1.4.1. The experiments were performed in triplicate.
To determine the effect of DTT’s 0, 3, 6, 12, 24, and 48 h incubation on the cell cycle progression of BRL-3A, the harvested cells were trypsinized without EDTA and rinsed three times with cold PBS, followed by fixation with 70% ethanol in cold storage. After 24 h incubation at 4 °C, 500 μL PI/RNase was added to each group and maintained at 37 °C for 60 min in a dark place. Stained cells were processed using flow cytometry and further measured via the NovoExpress® software 1.4.1. The experiments were performed in triplicate.
BRL-3A cells were grown on 10 cm diameter dishes and treated with 2.0 mmol/L DTT for different times. Cells were rinsed three times with pre-cooled PBS after experimentation and collected with cell scrapers in 100 μL RIPA buffer containing 1 mmol/L PMSF. After centrifugation at 12000 rpm for 25 min at 4 °C, the concentrations of total cellular protein extracts were determined using the BCA kit (Solarbio Science, Beijing, China), and known concentrations of BSA were used as standard. The total cellular protein extracts were denatured by boiling at 100 °C using dry bath incubator (Hangzhou Miu Instruments Co., Ltd, Zhejiang, China). Protein samples (30–40 mg) were loaded onto SDS-PAGE and transferred onto PVDF membranes for immunostaining. After blocking with 5% defatted milk for 90 min, membranes were stained overnight with primary antibodies, including β-actin (1:1000), GRP78 (1:1000), CNX (1:3000), ATG12 (1:1000), LC3 (1:1000), FAM134B (1:1000), CHOP (1:1000), cleaved caspase-3 (1:1000), cyt c (1:1000) in cold storage, followed by incubation with secondary antibodies (1:4000). The density of protein bands on membranes was exposed and quantified via fluorography using Image J software. The images shown are representative of experiments carried out at least three times.
BRL-3A cells, treated with DTT (2.0 mmol/L for 0 h and 24 h), were lysed in RIPA lysis buffer and the lysates were centrifuged at 12000 rpm for 15 min at 4 °C. The supernatant was resuspended in ice-cold PBS to a total volume of 500 μL, and 5 μL of the designated antibody was added overnight at 4 °C. The next day, the Ab-Ag complexes were bound to Dynabeads magnetic beads on a rotary shaker for 10 min. The magnetic bead-Ab-Ag complex was washed and eluted by adding a washing buffer and elution buffer, respectively, according to the manufacturer's protocol. Immunocomplexes were heated for 5 min at 100 °C and prepared for analysis by western blot. The images shown are representative of experiments carried out at least three times.
To observe the effects of DTT treatment at 2.0 mmol/L for specified time points, mitochondrial Ca2+ levels were determined using Rhod-2 AM, a specific detection dye for calcium. The treated cells were rinsed with HBSS three times and stained with a mixture of 5 μM Rhod-2 AM and 20 nM Mito-Tracker Green at 37 °C for 30 min in the dark. Finally, live cells were extensively rinsed thrice by adding HBSS without calcium, and images were visualized with Zeiss LSM Image Browser using a Zeiss LSM 900 confocal microscope. The images shown are representative of experiments carried out at least three times.
To observe the intracellular localization of the ER and lysosomes, after treatment with 2.0 mmol/L DTT for 0, 3, 6, 12, 24, and 48 h, ER and lysosomes were stained with ER-tracker and Lyso-tracker. Prior to staining, trackers were diluted appropriately in DMEM, on the basis of the manufacturer's instructions. Following dilution, cells were simultaneously incubated with the two trackers listed above, maintained for 30 min at 37 °C, and finally rinsed thrice with HBSS. Stained cells were visualized under the Zeiss LSM 900 confocal microscope. Images shown are representative of experiments carried out at least three times.
Specific siRNA against buffalo rat FAM134B was designed and synthesized by OriGene. Product number and targeting sequence: SR510501A-rGrGrArArGrUrGrGrUrUrUrArUrCrArArArUrUrCrUrGrATA; SR510501B-rArArArUrUrUrGrArCrUrUrArCrArGrUrGrGrArArArCrCAA; SR510501C-rArArGrUrGrGrUrUrUrArUrCrArArArUrUrCrUrGrArUrAGA. Cells were cultured in six-well dishes until the density of cell fusion reached 60%. Briefly, 75 pmol of FAM134B siRNA were added to Lipofectamine 3000 Transfection Reagent and gently mixed for 15 min, then administered to BRL-3A cells, which were resuspended in DMEM. After transfection for 6 h, cells were washed, and then supplemented with fresh medium. Finally, cells were treated with DTT (2.0 mmol/L) for a further 24 h and subjected to western blot assay and apoptosis assessment.
GraphPad Prism 7 software was used to perform all the statistical analyses and prepare experimental graphs. Data are expressed as the mean ± SD. Shapiro-Wilk normality test was used to test the normal distribution of the data and all the data were fit to a normal followed by Tukey's post hoc test was performed, and a significant difference was considered as P < 0.05.
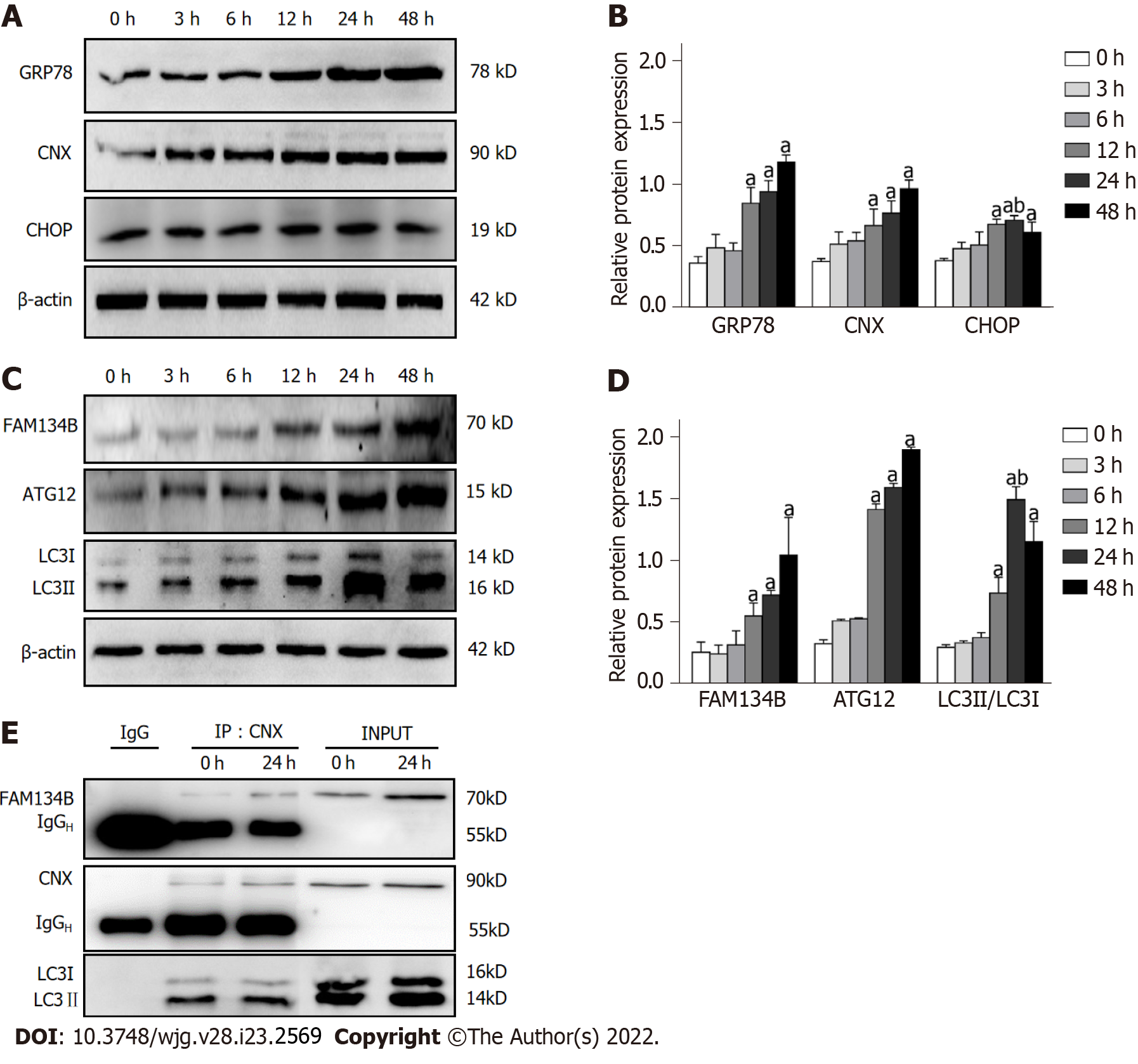
To assess whether the drug treatments could alter the protein expression of CNX and GRP78, BRL-3A cells were subjected to short-term (3, 6, 12, 24 h) or long-term (48 h) treatment with DTT, and the protein extracts from BRL-3A cells were analyzed by western blot. We found that treatment of BRL-3A cells with 2.0 mmol/L DTT resulted in a prominent increase in CNX and GRP78 levels, both in a time-dependent manner (Figure 1A and B). Moreover, CHOP is a specific and stress-responsive transcription factor during ER stress and its protein expression was significantly increased in the 12, 24, and 48 h groups (Figure 1A and B). However, the expression of CHOP in BRL-3A cells treated with DTT for 48 h was lower than that after DTT treatment for 24 h. These alterations in CNX, GRP78, and CHOP confirm that ER stress in BRL-3A was activated.
To determine the effects of ER stress on FAM134B-mediated ER-phagy, alterations in FAM134B, ATG12, and LC3 expression were detected by western blot. As expected, DTT treatment for 3, 6, 12, 24, and 48 h increased the conversion ratio of LC3-I to LC3-II and the FAM134B and ATG12 expression levels compared to those in the 0 h group (Figure 1C and D). Thus, our results revealed that the expression of FAM134B is induced in response to ER stress.
Furthermore, we used an anti-CNX antibody to immunoprecipitate the CNX-FAM134B-LC3 complex, confirming the hypothesis that FAM134B forms a complex with CNX and LC3, exerting a positive influence on ER-phagy (Figure 1E).
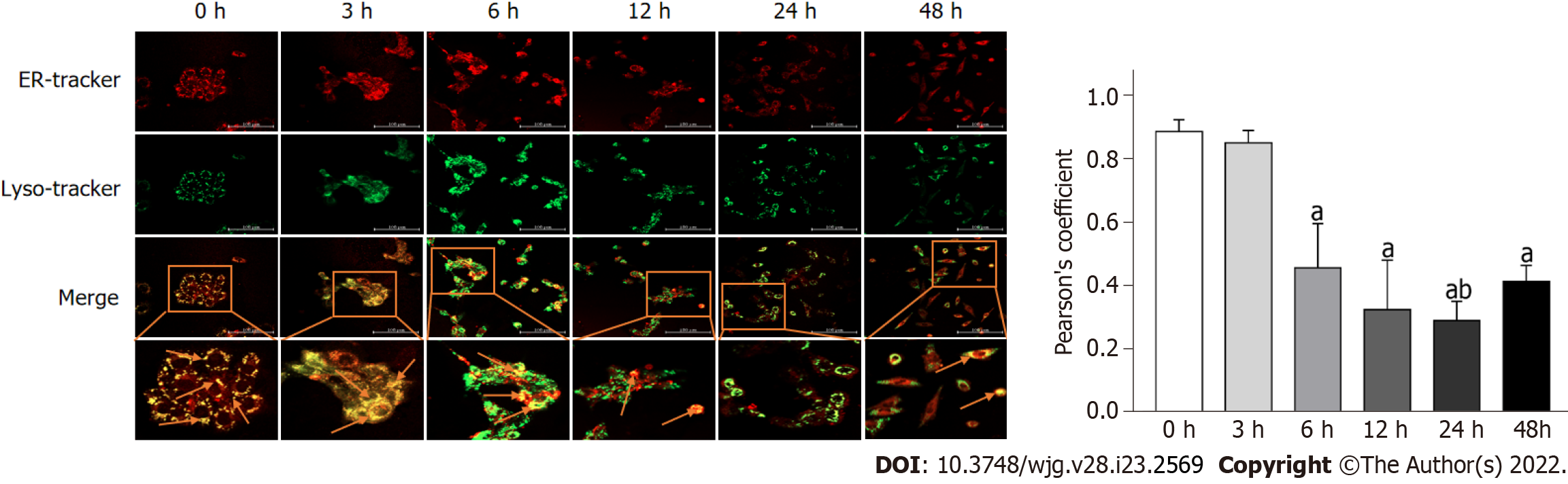
Typically, ER is delivered to lysosomes and finally degraded. To analyze whether ER autolysosomes are formed, we examined the subcellular location of the ER and lysosomes using cell organelle markers. As shown in Figure 2, the treatment groups of 3, 6, 12, 24, and 48 h DTT incubation significantly alleviated the co-localization of the ER with lysosomes, compared to that in the 0 h group. Notably, the colocalization of ER and lysosomes in BRL-3A cells treated with DTT for 48 h was increased compared to those treated for 24 h (Figure 2).
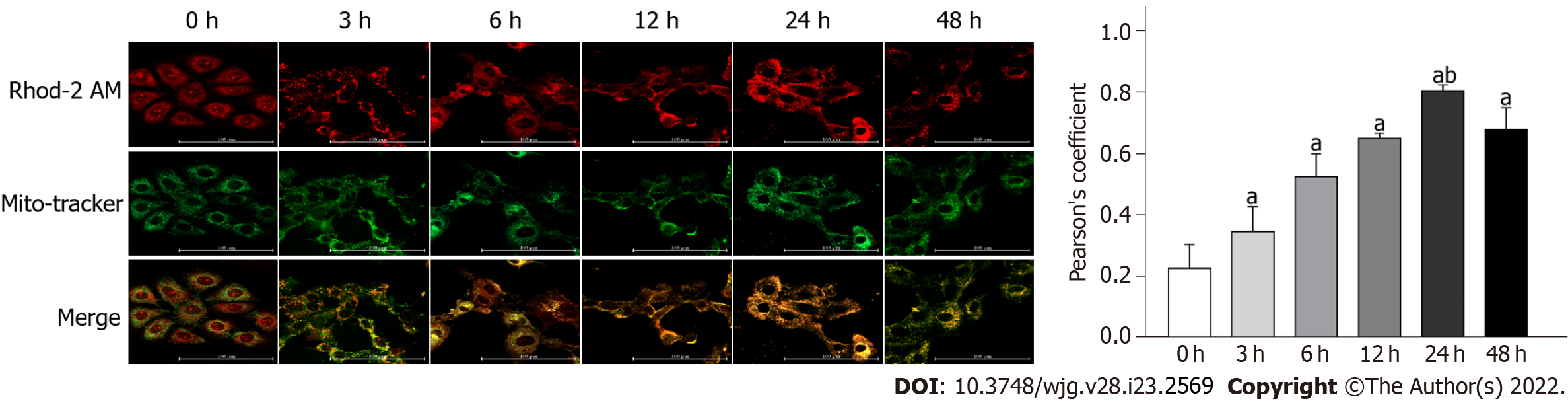
Calcium in the ER can be released and transferred to the mitochondria owing to an imbalance of ER homeostasis. To explore the altered localization of calcium, collected cells were co-loaded with Rhod-2 AM and Mito-Tracker Green. In response to DTT treatment for 3, 6, 12, 24, and 48 h, the co-localized fluorescence increased considerably (Figure 3). However, the distribution of the co-localized signal was weaker in the 48 h group, compared to that in the 24 h group (Figure 3). These results strongly suggest that mitochondrial calcium accumulation is related to DTT treatment.
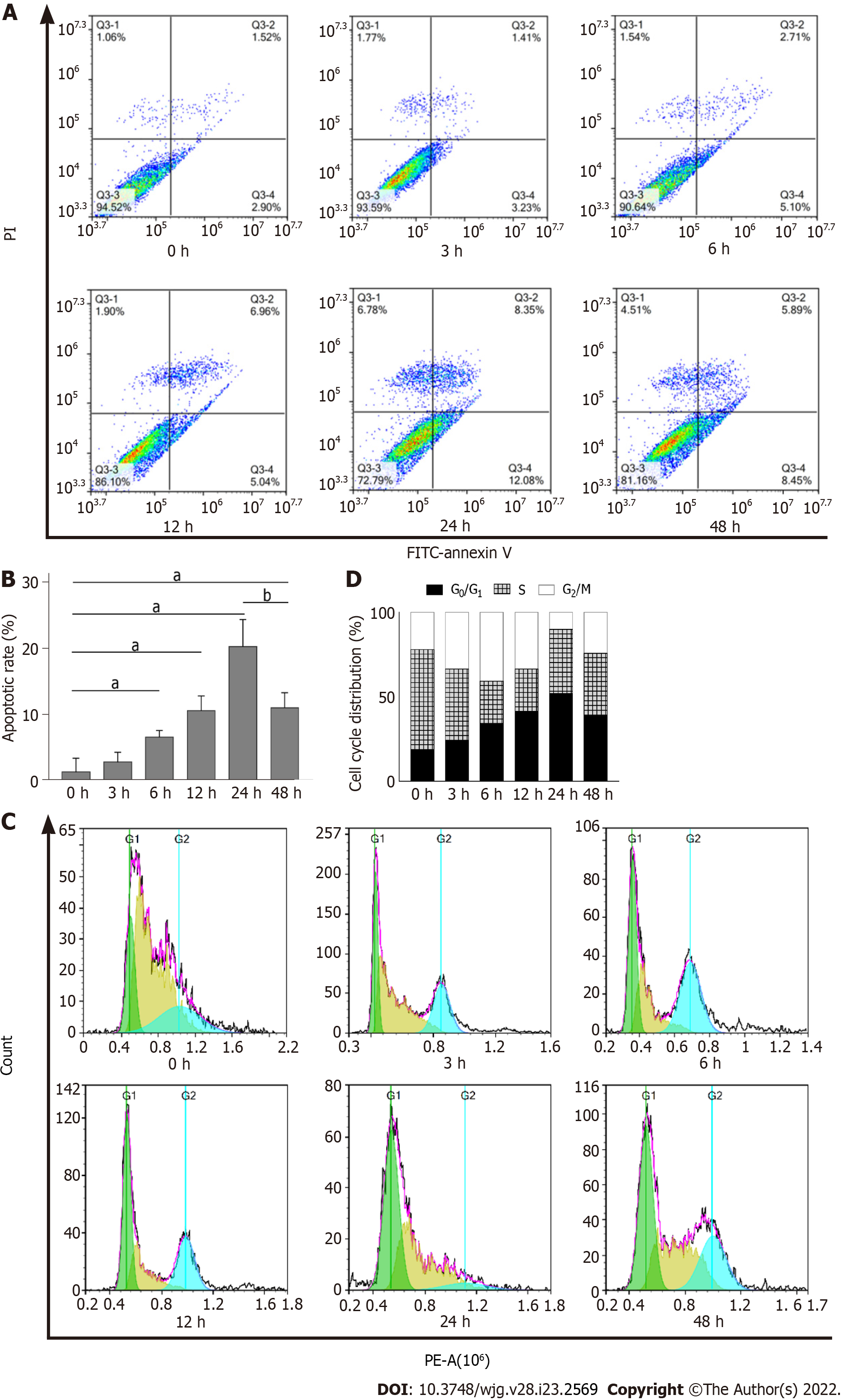
To further validate that DTT treatment leads to apoptosis in BRL-3A cells, we quantitatively measured the number of apoptotic cells using the Annexin V-FITC/PI double staining assay. As shown in Figure 4A and B, the ratio of apoptotic cells treated with DTT for 0, 3, 6, 12, and 24 h exhibited a time-dependent increase. Interestingly, the apoptotic percentage in the 48 h group was significantly lower than that in the 24 h group (Figure 4A and B). Subsequently, we sought to use flow cytometry to determine the impact of DTT treatment on the cell cycle progression, and the data suggests that the proportion of BRL-3A cells in G1 phase after DTT treatment was noticeably higher than that of the 0 h group (Figure 4C and D and Table 1). Moreover, the number of cells in G1 phase in the 48 h group was smaller than that of the 24 h group.
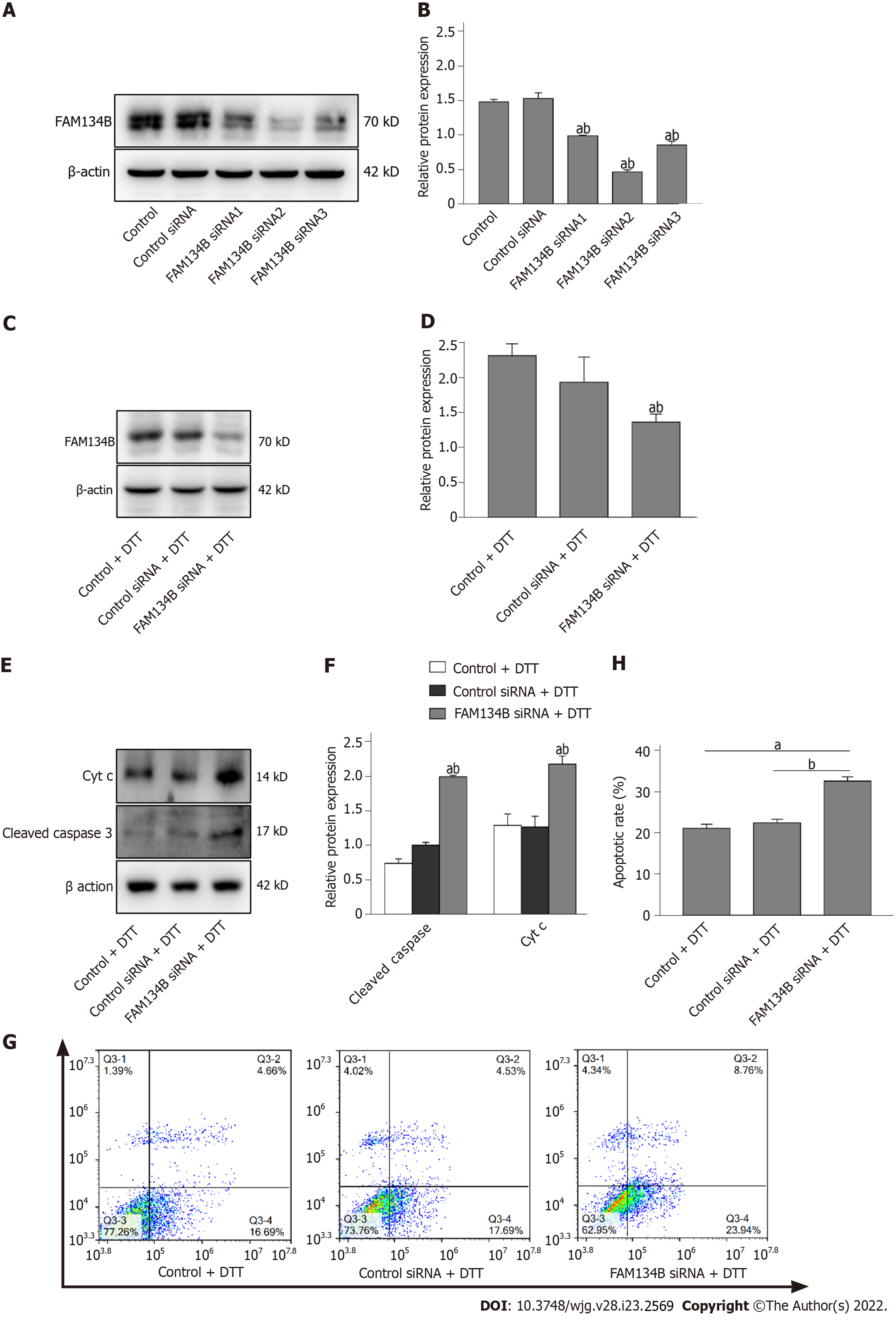
We further verified whether FAM134B knockdown could alter DTT-induced apoptosis. We first investigated the transfection efficiency of siRNA with three different siRNAs targeting FAM134B (siRNA 1, 2, and 3) and found that the FAM134B siRNA2 was the most effective (Figure 5A and B). Next, we investigated FAM134B protein levels by performing a western blot on already transfected samples, which were treated with DTT for 24 h. As shown in Figure 5C and D, FAM134B and β-actin expression levels were determined, and it was found that FAM134B protein levels were down-regulated compared with the control and control siRNA groups.
It has been reported that cyt c and cleaved caspase-3 are apoptosis-related proteins and important hallmarks of apoptosis activation involved in mitochondrial dysfunction. Consequently, siRNA-mediated silencing of FAM134B caused a high level of cleaved caspase-3 and cyt c in BRL-3A cells treated with DTT for 24 h (Figure 5E and F). We examined the rates of apoptotic cells using Annexin-V-FITC/PI staining assays, which revealed that the apoptotic rates also increased in the FAM134B siRNA group, compared with those in the control and control siRNA groups (Figure 5G and H). These results suggest that ER-phagy mediated by FAM134B is likely to serve a cytoprotective function in response to DTT treatment in BRL-3A cells.
Hepatic injury caused by multiple harmful factors is closely associated with ER stress-induced hepatocyte apoptosis[18-20]. The ER is responsible for proper protein folding, intracellular calcium storage, and lipid biosynthesis[21,22]. Various stressors, including unfolded protein aggregation in the ER, intracellular Ca2+ disturbance, and pharmacological inducers, such as DTT, can disrupt ER homeostasis and lead to ER stress in hepatocytes. If the ER stress cannot be alleviated, aberrant ER stress can trigger cell apoptosis[23]. In the present study, we found that the protein levels of GRP78 and CNX, which are ER stress biomarkers, were upregulated in BRL-3A cells during ER stress. GRP78 and CNX are ER chaperone proteins and accelerate the proper folding of the accumulated unfolded proteins in the ER, which engages effector mechanisms to rebalance ER homeostasis[24,25]. A series of studies have revealed that ER-phagy is an ER selective autophagy mechanism that can promote the clearance of damaged ER lumens containing the unfolded proteins, and helps restore ER homeostasis[26-28]. ER-phagy is a critical quality control mechanism for the ER in multiple cell types. Defects in ER-phagy pathways are associated with multiple human pathologies, including infectious and neurodegenerative diseases, aging and cancer. However, whether ER-phagy is involved in the regulation of ER homeostasis in hepatocytes under ER stress remains elusive. In this study, we assessed the levels of reticulophagy-related proteins in BRL-3A cells treated with DTT. We found that the levels of FAM134B and ATG12 were markedly elevated, and the ratio of LC3II/LC3I also increased. These data indicate that DTT-induced ER stress increases the level of reticulophagy-associated proteins.
Recent findings have indicated that receptor proteins of ER-phagy play crucial roles in driving the sequestration of isolated ER fragments into autophagosomes[29]. FAM134B, an ER-anchored protein, was recently proposed as a major mammalian receptor for reticulophagy[30,31]. FAM134B contains an LC3-interacting region that can interact with LC3 protein to form autophagosomal membranes, leading to efficient ER sequestration into an autophagosomal lumen[32-34]. In a previous report, the authors found that CNX serves as a co-receptor that recognizes misfolded proteins within the ER lumen and interacts with FAM134B[35,36]. In turn, the CNX-FAM134B complex binds with LC3, the autophagosome membrane-related protein, which delivers ER lumens containing misfolded proteins to the lysosome for degradation. To investigate how FAM134B modulates ER-phagy in BRL-3A cells, immunoprecipitation was performed to detect the interaction between CNX, FAM134B, and LC3. The results confirmed that CNX interacted with FAM134B, and FAM134B interacted with LC3 after DTT treatment. Thus, the formation of the CNX-FAM134B-LC3 complex allows for the selective delivery of ER lumens containing misfolded proteins to the lysosome for eventual degradation. Complete ER-phagy indicates that autophagosomes fuse to form autolysosomes[37,38], hence, we detected the number of autoly
The ER is the main pool for Ca2+ storage, and ER dysfunction leads to Ca2+ efflux from the ER[40,41]. In the early stages of ER stress, the suppression of the autophagosomes’ fusion with lysosomes may lead to calcium release and subsequent Ca2+ overload in mitochondria[42-44]. As expected, we found that DTT treatment dramatically elevated the levels of mitochondrial Ca2+, the apoptotic rate, and G1 arrest in BRL-3A cells. Nevertheless, these trends were relieved after treatment with DTT for 48 h. Our results reveal that hepatocytes initiate adaptive mechanisms in response to DTT-induced ER stress; consequently, apoptosis in BRL-3A cells treated with DTT for 48 h was lower than that in cells treated with DTT for 24 h.
To clarify whether FAM134B is involved in the regulation of cellular homeostasis during ER stress, we used a small interference RNA technique to knockdown FAM134B expression in hepatocytes. We found that FAM134B silencing not only significantly attenuated the DTT-upregulated FAM134B expression, but also accelerated the activation of the mitochondrial apoptotic pathway and aggravated DTT-triggered hepatocyte apoptosis.
In conclusion, DTT treatment significantly upregulated the protein levels of GRP78, CNX, FAM134B, and ATG12, and also increased the ratio of LC3II/LC3I in BRL-3A cells. Moreover, FAM134B-mediated reticulophagy ameliorates DTT-induced hepatocyte apoptosis via selective clearance of damaged ER lumens. Accordingly, knockdown of FAM134B enhanced ER stress-mediated apoptosis in BRL-3A cells. Our data show that FAM134B-mediated reticulophagy plays a key role in rebalancing ER homeostasis in hepatocytes undergoing ER stress. Therefore, FAM134B-mediated reticulophagy may be a novel therapeutic target, and our findings may provide emerging evidence to demonstrate the prominence of ER-phagy in ER stress-related hepatocyte apoptosis. Alleviation of ER stress-mediated hepatocyte apoptosis via restoring ER homeostasis is critical in the treatment of liver diseases.
Hepatocyte apoptosis induced by endoplasmic reticulum (ER) stress has a strong association with the development of fibrosis, cirrhosis, and hepatocellular carcinoma. Previous studies have revealed that endoplasmic reticulophagy (ER-phagy) promotes the selective clearance of damaged ER fragments during ER stress, playing a crucial role in maintaining ER homeostasis and inhibiting apoptosis. However, the precise regulatory mechanisms remain unclear.
Defects in ER-phagy pathways are associated with multiple human pathologies, including infectious and neurodegenerative diseases, aging and cancer. However, whether ER-phagy is involved in the regulation of ER homeostasis in hepatocytes under ER stress remains elusive.
To elucidate the effect of family with sequence similarity 134 member B (FAM134B)-mediated ER-phagy on normal buffalo rat hepatocytes apoptosis induced by dithiothreitol (DTT) and explore the potential regulatory mechanism.
A model of ER stress was established by DTT. The levels of proteins related to ER stress and ER-phagy were determined by western blot. An interaction between FAM134B, calnexin (CNX), and microtubule-associated protein 1 light chain 3 (LC3) was investigated by co-immunoprecipitation. ER-Tracker Red probe and Lyso-Tracker Green probe were used to detect the colocalization of ER with lysosome in cells. Mito-Tracker Green and Rhod-2 AM probes were used to detect the level of mitochondrial Ca2+ under the confocal microscopy. Flow cytometry was conducted to analyze the effect of DTT treatment on cell cycle distribution and apoptosis. The small interfering RNA against FAM134B was used to knockdown FAM134B in buffalo rat liver 3A (BRL-3A) cells.
DTT treatment upregulated glucose-regulated protein 78 (GRP78), CNX, FAM134B, and autophagy related gene 12 (ATG12) protein levels and increased the ratio of LC3II/LC3I in BRL-3A cells. FAM134B-mediated reticulophagy maintains ER homeostasis in ER-stressed hepatocytes via the clearance of damaged ER fragments. FAM134B-mediated reticulophagy ameliorates DTT-induced hepatocyte apoptosis. Knockdown of FAM134B enhanced ER stress-mediated apoptosis in BRL-3A cells.
FAM134B-mediated ER-phagy attenuates hepatocyte apoptosis by suppressing the mitochondrial apoptotic pathway.
FAM134B-mediated reticulophagy may be a novel therapeutic target, and our findings provide emerging evidence demonstrating the prominence of ER-phagy in ER stress-related hepatocyte apoptosis. Alleviation of the ER stress-mediated hepatocyte apoptosis via restoring ER homeostasis is critical in the treatment of liver diseases.
We thank the Basic Medical Science Research Center of Guizhou Medical University for their technical advice in using the microscope.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Elchaninov AV, Russia; Gassler N, Germany; Kulkeaw K, Thailand S-Editor: Fan JR L-Editor: A P-Editor: Qi WW
| 1. | Huang TJ, Liu SH, Kuo YC, Chen CW, Chou SC. Antiviral activity of chemical compound isolated from Artemisia morrisonensis against hepatitis B virus in vitro. Antiviral Res. 2014;101:97-104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 24] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 2. | Tsai CC, Chen YJ, Yu HR, Huang LT, Tain YL, Lin IC, Sheen JM, Wang PW, Tiao MM. Long term N-acetylcysteine administration rescues liver steatosis via endoplasmic reticulum stress with unfolded protein response in mice. Lipids Health Dis. 2020;19:105. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 25] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 3. | Zhang Y, Zhang H, Zhao Z, Lv M, Jia J, Zhang L, Tian X, Chen Y, Li B, Liu M, Han D, Ji C. Enhanced expression of glucose-regulated protein 78 correlates with malondialdehyde levels during the formation of liver cirrhosis in rats. Exp Ther Med. 2015;10:2119-2125. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 4. | Schwabe RF, Tabas I, Pajvani UB. Mechanisms of Fibrosis Development in Nonalcoholic Steatohepatitis. Gastroenterology. 2020;158:1913-1928. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 390] [Cited by in RCA: 442] [Article Influence: 88.4] [Reference Citation Analysis (1)] |
| 5. | Zuo L, Zhu Y, Hu L, Liu Y, Wang Y, Hu Y, Wang H, Pan X, Li K, Du N, Huang Y. PI3-kinase/Akt pathway-regulated membrane transportation of acid-sensing ion channel 1a/Calcium ion influx/endoplasmic reticulum stress activation on PDGF-induced HSC Activation. J Cell Mol Med. 2019;23:3940-3950. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 35] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 6. | Khaminets A, Heinrich T, Mari M, Grumati P, Huebner AK, Akutsu M, Liebmann L, Stolz A, Nietzsche S, Koch N, Mauthe M, Katona I, Qualmann B, Weis J, Reggiori F, Kurth I, Hübner CA, Dikic I. Regulation of endoplasmic reticulum turnover by selective autophagy. Nature. 2015;522:354-358. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 548] [Cited by in RCA: 718] [Article Influence: 71.8] [Reference Citation Analysis (0)] |
| 7. | Smith MD, Harley ME, Kemp AJ, Wills J, Lee M, Arends M, von Kriegsheim A, Behrends C, Wilkinson S. CCPG1 Is a Non-canonical Autophagy Cargo Receptor Essential for ER-Phagy and Pancreatic ER Proteostasis. Dev Cell. 2018;44:217-232.e11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 363] [Cited by in RCA: 341] [Article Influence: 48.7] [Reference Citation Analysis (0)] |
| 8. | Fumagalli F, Noack J, Bergmann TJ, Cebollero E, Pisoni GB, Fasana E, Fregno I, Galli C, Loi M, Soldà T, D'Antuono R, Raimondi A, Jung M, Melnyk A, Schorr S, Schreiber A, Simonelli L, Varani L, Wilson-Zbinden C, Zerbe O, Hofmann K, Peter M, Quadroni M, Zimmermann R, Molinari M. Translocon component Sec62 acts in endoplasmic reticulum turnover during stress recovery. Nat Cell Biol. 2016;18:1173-1184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 257] [Cited by in RCA: 348] [Article Influence: 38.7] [Reference Citation Analysis (0)] |
| 9. | Kopp MC, Larburu N, Durairaj V, Adams CJ, Ali MMU. UPR proteins IRE1 and PERK switch BiP from chaperone to ER stress sensor. Nat Struct Mol Biol. 2019;26:1053-1062. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 220] [Cited by in RCA: 266] [Article Influence: 44.3] [Reference Citation Analysis (0)] |
| 10. | Kozlov G, Gehring K. Calnexin cycle - structural features of the ER chaperone system. FEBS J. 2020;287:4322-4340. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 93] [Cited by in RCA: 128] [Article Influence: 25.6] [Reference Citation Analysis (0)] |
| 11. | Bernales S, McDonald KL, Walter P. Autophagy counterbalances endoplasmic reticulum expansion during the unfolded protein response. PLoS Biol. 2006;4:e423. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 735] [Cited by in RCA: 799] [Article Influence: 44.4] [Reference Citation Analysis (0)] |
| 12. | Bernales S, Schuck S, Walter P. ER-phagy: selective autophagy of the endoplasmic reticulum. Autophagy. 2007;3:285-287. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 260] [Cited by in RCA: 294] [Article Influence: 26.7] [Reference Citation Analysis (0)] |
| 13. | Forrester A, De Leonibus C, Grumati P, Fasana E, Piemontese M, Staiano L, Fregno I, Raimondi A, Marazza A, Bruno G, Iavazzo M, Intartaglia D, Seczynska M, van Anken E, Conte I, De Matteis MA, Dikic I, Molinari M, Settembre C. A selective ER-phagy exerts procollagen quality control via a Calnexin-FAM134B complex. EMBO J. 2019;38. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 116] [Cited by in RCA: 194] [Article Influence: 27.7] [Reference Citation Analysis (0)] |
| 14. | Chino H, Mizushima N. ER-Phagy: Quality Control and Turnover of Endoplasmic Reticulum. Trends Cell Biol. 2020;30:384-398. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 202] [Article Influence: 40.4] [Reference Citation Analysis (0)] |
| 15. | Wilkinson S. ER-phagy: shaping up and destressing the endoplasmic reticulum. FEBS J. 2019;286:2645-2663. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 103] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 16. | Molinari M. ER-phagy: Eating the Factory. Mol Cell. 2020;78:811-813. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 17. | Chen YS, Han B, Zheng L, Cai S, Tang L, Yang Y, Guo YX, Liang C, Zhao JY, Yang T, Yang Q. Effects of calpain-2 and autophagy-related protein 5 on hepatocyte apoptosis induced by endoplasmic reticulum stress. Zhongguo Bingli Shengli Zazhi. 2020;36:847-853. |
| 18. | Dash S, Chava S, Aydin Y, Chandra PK, Ferraris P, Chen W, Balart LA, Wu T, Garry RF. Hepatitis C Virus Infection Induces Autophagy as a Prosurvival Mechanism to Alleviate Hepatic ER-Stress Response. Viruses. 2016;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 59] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 19. | Wang K. Autophagy and apoptosis in liver injury. Cell Cycle. 2015;14:1631-1642. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 204] [Cited by in RCA: 299] [Article Influence: 33.2] [Reference Citation Analysis (0)] |
| 20. | Liu H, Lai W, Liu X, Yang H, Fang Y, Tian L, Li K, Nie H, Zhang W, Shi Y, Bian L, Ding S, Yan J, Lin B, Xi Z. Exposure to copper oxide nanoparticles triggers oxidative stress and endoplasmic reticulum (ER)-stress induced toxicology and apoptosis in male rat liver and BRL-3A cell. J Hazard Mater. 2021;401:123349. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 128] [Article Influence: 32.0] [Reference Citation Analysis (0)] |
| 21. | Phillips MJ, Voeltz GK. Structure and function of ER membrane contact sites with other organelles. Nat Rev Mol Cell Biol. 2016;17:69-82. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 559] [Cited by in RCA: 725] [Article Influence: 72.5] [Reference Citation Analysis (0)] |
| 22. | Kaneko M, Imaizumi K, Saito A, Kanemoto S, Asada R, Matsuhisa K, Ohtake Y. ER Stress and Disease: Toward Prevention and Treatment. Biol Pharm Bull. 2017;40:1337-1343. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 69] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 23. | Xie RJ, Hu XX, Zheng L, Cai S, Chen YS, Yang Y, Yang T, Han B, Yang Q. Calpain-2 activity promotes aberrant endoplasmic reticulum stress-related apoptosis in hepatocytes. World J Gastroenterol. 2020;26:1450-1462. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 8] [Cited by in RCA: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 24. | Tian G, Zhao M, Zhou J, Quan Y, Wu W, Liu X. [The potential role of calnexin in the activation of cardiac fibroblasts]. Sheng Wu Yi Xue Gong Cheng Xue Za Zhi. 2020;37:450-459. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 25. | Zheng Z, Shang Y, Tao J, Zhang J, Sha B. Endoplasmic Reticulum Stress Signaling Pathways: Activation and Diseases. Curr Protein Pept Sci. 2019;20:935-943. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 29] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 26. | Fregno I, Molinari M. Endoplasmic reticulum turnover: ER-phagy and other flavors in selective and non-selective ER clearance. F1000Res. 2018;7:454. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 55] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 27. | Birgisdottir ÅB, Lamark T, Johansen T. The LIR motif - crucial for selective autophagy. J Cell Sci. 2013;126:3237-3247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 518] [Cited by in RCA: 686] [Article Influence: 62.4] [Reference Citation Analysis (0)] |
| 28. | Liang JR, Lingeman E, Ahmed S, Corn JE. Atlastins remodel the endoplasmic reticulum for selective autophagy. J Cell Biol. 2018;217:3354-3367. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 81] [Cited by in RCA: 114] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 29. | Chen Q, Xiao Y, Chai P, Zheng P, Teng J, Chen J. ATL3 Is a Tubular ER-Phagy Receptor for GABARAP-Mediated Selective Autophagy. Curr Biol. 2019;29:846-855. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 234] [Article Influence: 39.0] [Reference Citation Analysis (0)] |
| 30. | Schultz ML, Krus KL, Kaushik S, Dang D, Chopra R, Qi L, Shakkottai VG, Cuervo AM, Lieberman AP. Coordinate regulation of mutant NPC1 degradation by selective ER autophagy and MARCH6-dependent ERAD. Nat Commun. 2018;9:3671. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 63] [Cited by in RCA: 87] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 31. | Morishita H, Mizushima N. Diverse Cellular Roles of Autophagy. Annu Rev Cell Dev Biol. 2019;35:453-475. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 278] [Article Influence: 46.3] [Reference Citation Analysis (0)] |
| 32. | Johansen T, Lamark T. Selective Autophagy: ATG8 Family Proteins, LIR Motifs and Cargo Receptors. J Mol Biol. 2020;432:80-103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 259] [Cited by in RCA: 466] [Article Influence: 93.2] [Reference Citation Analysis (0)] |
| 33. | Jiang X, Wang X, Ding X, Du M, Li B, Weng X, Zhang J, Li L, Tian R, Zhu Q, Chen S, Wang L, Liu W, Fang L, Neculai D, Sun Q. FAM134B oligomerization drives endoplasmic reticulum membrane scission for ER-phagy. EMBO J. 2020;39:e102608. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 105] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 34. | Bhaskara RM, Grumati P, Garcia-Pardo J, Kalayil S, Covarrubias-Pinto A, Chen W, Kudryashev M, Dikic I, Hummer G. Curvature induction and membrane remodeling by FAM134B reticulon homology domain assist selective ER-phagy. Nat Commun. 2019;10:2370. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 93] [Cited by in RCA: 155] [Article Influence: 25.8] [Reference Citation Analysis (0)] |
| 35. | Fregno I, Fasana E, Bergmann TJ, Raimondi A, Loi M, Soldà T, Galli C, D'Antuono R, Morone D, Danieli A, Paganetti P, van Anken E, Molinari M. ER-to-lysosome-associated degradation of proteasome-resistant ATZ polymers occurs via receptor-mediated vesicular transport. EMBO J. 2018;37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 159] [Article Influence: 22.7] [Reference Citation Analysis (0)] |
| 36. | Wilkinson S. Emerging Principles of Selective ER Autophagy. J Mol Biol. 2020;432:185-205. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 80] [Cited by in RCA: 91] [Article Influence: 18.2] [Reference Citation Analysis (0)] |
| 37. | Grumati P, Morozzi G, Hölper S, Mari M, Harwardt MI, Yan R, Müller S, Reggiori F, Heilemann M, Dikic I. Full length RTN3 regulates turnover of tubular endoplasmic reticulum via selective autophagy. Elife. 2017;6. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 358] [Cited by in RCA: 334] [Article Influence: 41.8] [Reference Citation Analysis (0)] |
| 38. | Ko SH, Jeon JI, Myung HS, Kim YJ, Kim JM. Bacteroides fragilis Enterotoxin Induces Formation of Autophagosomes in Endothelial Cells but Interferes with Fusion with Lysosomes for Complete Autophagic Flux through a Mitogen-Activated Protein Kinase-, AP-1-, and C/EBP Homologous Protein-Dependent Pathway. Infect Immun. 2017;85. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 39. | Arruda AP, Pers BM, Parlakgül G, Güney E, Inouye K, Hotamisligil GS. Chronic enrichment of hepatic endoplasmic reticulum-mitochondria contact leads to mitochondrial dysfunction in obesity. Nat Med. 2014;20:1427-1435. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 415] [Cited by in RCA: 543] [Article Influence: 49.4] [Reference Citation Analysis (0)] |
| 40. | Wiel C, Lallet-Daher H, Gitenay D, Gras B, Le Calvé B, Augert A, Ferrand M, Prevarskaya N, Simonnet H, Vindrieux D, Bernard D. Endoplasmic reticulum calcium release through ITPR2 channels leads to mitochondrial calcium accumulation and senescence. Nat Commun. 2014;5:3792. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 165] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 41. | Datta D, Khatri P, Singh A, Saha DR, Verma G, Raman R, Mazumder S. Mycobacterium fortuitum-induced ER-Mitochondrial calcium dynamics promotes calpain/caspase-12/caspase-9 mediated apoptosis in fish macrophages. Cell Death Discov. 2018;4:30. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 27] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 42. | Fan Y, Simmen T. Mechanistic Connections between Endoplasmic Reticulum (ER) Redox Control and Mitochondrial Metabolism. Cells. 2019;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 57] [Cited by in RCA: 101] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 43. | Krebs J, Agellon LB, Michalak M. Ca(2+) homeostasis and endoplasmic reticulum (ER) stress: An integrated view of calcium signaling. Biochem Biophys Res Commun. 2015;460:114-121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 308] [Cited by in RCA: 421] [Article Influence: 42.1] [Reference Citation Analysis (0)] |
| 44. | Luciani DS, Gwiazda KS, Yang TL, Kalynyak TB, Bychkivska Y, Frey MH, Jeffrey KD, Sampaio AV, Underhill TM, Johnson JD. Roles of IP3R and RyR Ca2+ channels in endoplasmic reticulum stress and beta-cell death. Diabetes. 2009;58:422-432. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 161] [Cited by in RCA: 175] [Article Influence: 10.9] [Reference Citation Analysis (0)] |