Published online Jun 14, 2022. doi: 10.3748/wjg.v28.i22.2429
Peer-review started: January 6, 2022
First decision: March 9, 2022
Revised: March 18, 2022
Accepted: April 22, 2022
Article in press: April 22, 2022
Published online: June 14, 2022
Processing time: 154 Days and 10.1 Hours
Many metabolic factors are associated with chronic hepatitis C virus (HCV) infection and can influence the course of the illness and impact the progression of liver and non-liver-related diseases through complex interactions. Several of these factors impact the course of chronic HCV (CHC) and result in the conceptual translation of CHC from a localized to systemic disease. Besides the traditional liver manifestations associated with CHC infection, such as cirrhosis and hepatocellular carcinoma, various extrahepatic disorders are associated with HCV infection, including atherosclerosis, glucose and lipid metabolic disturbances, alterations in the iron metabolic pathways, and lymphoproliferative diseases. The coexistence of metabolic disorders and CHC is known to influence the chronicity and virulence of HCV and accelerates the progression to liver fibrosis and hepatocellular carcinoma. Insulin resistance is one of the key factors that have a tremendous metabolic impact on CHC. Therefore, there is a great need to properly evaluate patients with CHC infection and correct the modifiable metabolic risk factors. Furthermore, patients with HCV who achieved a sustained virological response showed an overall improvement in glucose metabolism, but the exact evidence still requires further studies with long-term follow-up. This review delineates the most recent evidence on the main metabolic factors associated with CHC and the possible influence of chronic HCV infection on metabolic features.
Core Tip: Hepatitis C virus (HCV) infection has several metabolic aspects that are largely well understood; as such, HCV is nowadays considered a systemic disease rather than a local disease with different metabolic consequences. Moreover, these metabolic factors may affect the natural history of chronic liver disease and of diseases not related to the liver, which constitute a significant burden on the overall health of the human body, with an increased economic burden to patients, healthcare systems, and society if not adequately addressed and appropriately managed. More studies are needed to evaluate metabolic aspects associated with HCV infection and delineate their effects and the long-term outcome of antiviral therapies.
- Citation: El-Kassas M, Awad A. Metabolic aspects of hepatitis C virus. World J Gastroenterol 2022; 28(22): 2429-2436
- URL: https://www.wjgnet.com/1007-9327/full/v28/i22/2429.htm
- DOI: https://dx.doi.org/10.3748/wjg.v28.i22.2429
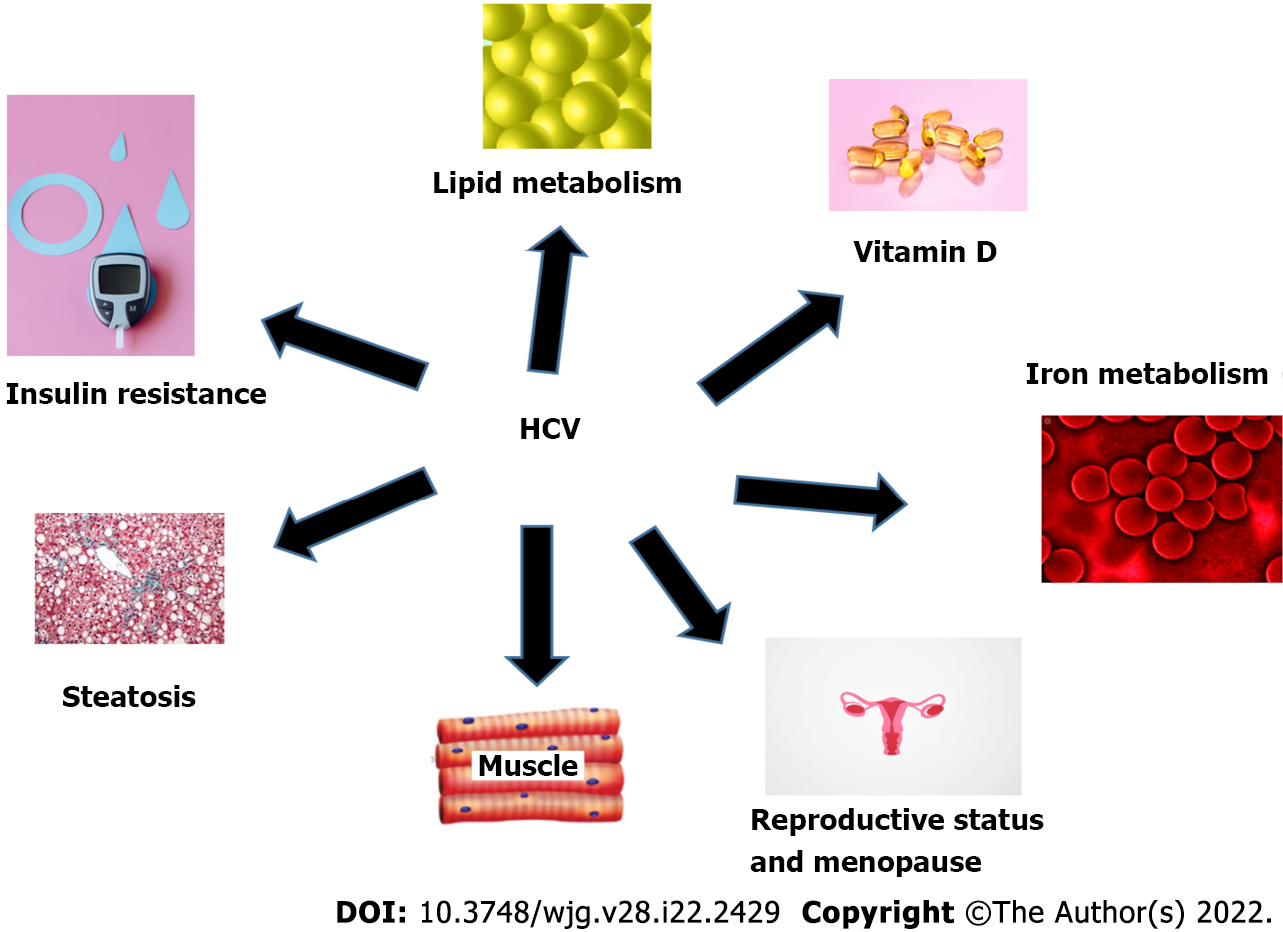
Hepatitis C virus (HCV) infection is considered one of the most notable causes of chronic liver disease worldwide[1]. Not only does HCV infection confer the risk of developing chronic hepatitis, cirrhosis, and hepatocellular carcinoma (HCC), it also has many extrahepatic manifestations, such as disorders of glucose and lipid metabolism, polyarthritis resembling rheumatoid arthritis, vascular atheromatous disease, mixed cryoglobulinemia, lymphoproliferative diseases, renal disorders, insulin resistance (IR), type 2 diabetes (T2DM), sicca syndrome, and autoimmune disorders[2-4]. Different metabolic aspects of HCV are demonstrated in Figure 1. There are several studies on the effect of metabolic factors on the natural history of patients with chronic HCV (CHC)[5]. The deleterious effects of metabolic complications resulting from HCV infection are mainly related to glucose and lipid metabolism impairments[6].
Given that it is a systemic disease, CHC infection could influence the metabolic homeostasis of the host through several complex interactions, even in light of pre-existent metabolic status and genetic background[5]. Several factors can accelerate the disease course from CHC infection to cirrhosis and may impact the likelihood of achieving a sustained virological response (SVR) after receiving antiviral therapy. Among the reported factors are metabolic factors that may change the course of illness in CHC patients and impact the results of antiviral treatments[7], despite the apparent improvement in HCV management results after the introduction of direct-acting antivirals compared with the previous standard of care interferon-based therapy[8,9]. Interestingly, this is not a “one-way street,” as CHC infection can have metabolic effects due to its influence on glucose and lipid metabolism, which impacts the host's metabolic homeostasis and may result in its extrahepatic sequelae[10]. The extrahepatic burden of HCV infection exceeds its effect on the liver as it is a significant burden on the overall health of the human body, causing increased economic burden to patients and healthcare systems[11,12].
We have discussed the most recent evidence on the main metabolic factors related to CHC, along with the proposed pathophysiological machineries essential to the correlation between HCV infection and metabolic disorders and the possible influence of CHC infection on metabolic features.
IR is considered a keystone of metabolic syndrome (MS) with increasing incidence worldwide, representing a major cause of morbidity and mortality[13]. As reported in the literature, HCV infection is associated with IR in up to 80% of cases; consequently, the risk of developing T2DM is found to be twice as high as in subjects without HCV[14,15]. There is a high chance of coexistence between MS and CHC owing to many related host factors, such as the presence of visceral obesity; moreover, HCV infection itself is reported to affect glucosidic homeostasis, leading to hepatic and extrahepatic IR[1]. Moreover, CHC is found to increase the risk of developing metabolic diseases with these complications[16]. IR in patients with CHC significantly impacts the severity and progression of chronic liver disease via direct and indirect effects by inducing steatosis[17]. Meanwhile, steatosis activates stellate cells via collagenous deposition and the generation of lipid peroxides[18], which, in turn, promotes fibrogenesis via the direct activation of hepatic stellate cells, tumor necrosis factor-α and connective growth factor production, and ductular reactions induction[19]. In this context, a high prevalence of cirrhosis and non-SVR was observed among patients with diabetes and CHC with an observed lower rate of SVR in patients with IR, not only in interferon-based treatment[7].
Moreover, there is a reported association between IR and the presence of esophageal varices in patients with HCV-related compensated cirrhosis[20]. The potential of insulin to control dynamic components of portal hypertension, such as endothelial nitric oxide and endothelin production, might explain this[21,22]. Not only are IR and DM are more prevalent in the course of HCV infection, but they also occur post-liver transplantation in patients with CHC infection[23-25]. It's not surprising, then, that T2D is linked to a three-fold increased risk of HCC, with a higher risk seen in patients who have both HCV and T2D; this could be due to the possible molecular mechanisms and intermediaries involved in hepatic carcinogenesis, such as IR and hyperinsulinemia, oxidative stress, and reported cytokine imbalances between proinflammatory and anti-inflammatory cytokines[26]. Several studies have reported the impact of IR and steatosis and both rapid virological response and SVR in patients with CHC treated with antiviral therapy; the plausible explanations that IR and steatosis may affect the response to antiviral therapy and the reasons for the disparities in results might be due to pre-existing variances in metabolic dysfunctions and genetic diversities among the tested groups[27]. other studies reported the efficiency of proper glucose control in HCV infected patients that improve early after antiviral treatment, with benefits that are not restricted to the diabetic patient only furthermore, achievement of SVR by direct-acting antivirals (DAAs) to eliminate HCV improves their glycemic control with a possible reduction on the faster progression of hepatic fibrosis[28,29].
Also, treatment of HCV with DAAs found to improve steatosis, hepatic inflammation, and the nutritional status in most of the studied patients[30-32].
This explains how profound and widespread effects of the impairment of insulin pathways exerted by HCV infection and vice versa.
Consequently, further evidence from long-term follow-up studies is still required to determine if successful eradication of HCV can help to ameliorate IR and improve glycemic control and clinical outcomes in patients with established DM2[33,34].
In individuals with CHC, hepatic steatosis is a frequent histological finding, with a frequency of up to 80%, which is higher than that in noninfected individuals; thus, it is considered as a distinct entity in the setting of HCV viral infection with specific clinical and prognostic implications[35,36]. Not only viral factors are responsible for steatosis in patients with CHC, but there are also different common risk factors for steatosis, such as obesity, T2D, alcohol, and dyslipidemia, which are common in the examined cohorts[5]. Specific genotypes of HCV, especially the HCV genotype 3, are more correlated with hepatic steatosis; moreover, HCV has the ability to promote the intracytoplasmic deposition of fat in the liver by enhancing hepatic fatty acid production and decreasing lipid release and breakdown processes, both directly and indirectly[37]. Interestingly, steatosis has also been related to HCV viral load and was found to decrease after SVR was achieved[35]. Many studies reported that steatosis could be a predictor of liver fibrosis in patients with CHC; additionally, in untreated CHC patients, worsening of steatosis may be an independent factor related with the advancement of liver fibrosis. This could be explained by "viral" and "metabolic" steatosis, in which elevated insulin levels and inflammatory mediators on liver stellate cells promote the advancement of fibrosis and liver disease[10,38]. Steatosis may improve and even vanish following effective antiviral treatment with interferon and ribavirin, according to some reports; however, evidence for a similar effect of direct-acting antivirals is currently limited[39,40].
In contrast, cross-sectional and longitudinal studies have shown that despite achieving SVR during CHC treatment, some patients have been found with clinically significant steatosis and fibrosis[41,42]. In this clinical setting, many studies reported the association between steatosis in fatty liver and HCC development in patients with CHC[43].
In the context of steatosis and IR, visceral obesity has been associated with liver fat accumulation in healthy subjects[44,45] and is also related to viral load. Several studies have discussed the association between HCV RNA status and obesity[46].
Plausible explanations include the feasibility of adipose tissue to promote fatty substrates and a proinflammatory status that accelerate HCV replication; moreover, the ability of HCV to interfere with adipocyte function through indirect methods, and increase the inflammatory status or via a direct mechanism that helps to increase the colonizing adipocytes and immune cells infiltrating adipose tissue[47]. Further studies are needed to delineate the potential role of obesity in affecting SVR rates after treatment with antiviral agents.
HCV infection is involved in disrupted lipoprotein homeostasis via impairment of the very low-density lipoprotein levels (LDLs)-releasing pathway, which is one of the main causes of hepatic fat deposition[48]. Several studies have discussed the relationship between lipoproteins and HCV cell cycle[49] and found that patients with CHC have lower serum LDL[50], which are inversely associated with the severity of liver fibrosis[51]; however, this is still a controversial issue. As reported by Nevola et al[15], the average LDL levels increased significantly after viral eradication, although there were no effects on triglycerides and high-density lipoprotein[15]. However, it is still debatable whether infections with HCV are linked to an increased risk of cardiovascular events such as carotid atherosclerosis, myocardial infarction, and heart attacks[52]. Notably, studies reported that patients with CHC had more atherosclerosis, as measured by carotid artery plaques and/or intima-media thickness (IMT), than healthy controls; likewise, the frequency of asymptomatic carotid atherosclerosis was higher in patients with CHC than in matched controls[7].
HCV infection could be an independent risk factor for increased carotid IMT[53] and cerebrovascular deaths[54], as reported in many types of study; this may be explained by the proinflammatory mechanisms that underlie liver fibrogenesis and could be systemically activated, leading to the promotion of atherosclerosis[7]. In contrast, several published studies have failed to show the association between atherosclerosis and HCV infection, even with an increased prevalence of IR in patients with HCV infection[55]. Therefore, further studies are needed to validate those data.
Of 25-Hydroxyvitamin D deficiency has been discovered in patients with CHC, even in those with minimal liver damage[56]; however, some studies reported no association between vitamin D status and fibrosis stage[57]. The role of vitamin D status in treatment regimens for HCV infection is still not well understood, although it is interesting that vitamin D3 supplement augments the response to antiviral therapy in infections with HCV genotypes 1-4, as reported in some randomized clinical trials[58-60].
It is debatable whether iron promotes or suppresses HCV viral replication, but it is considered a central component for HCV virus replication and translation[10]. In patients with CHC, elevated serum ferritin and the associated increased iron load in liver were more evident and were considered a significant predictor for hepatic fibrosis progression[61,62]. Hepcidin is a peptide hormone with an essential role in regulating iron levels under homeostatic states. Accordingly, iron metabolism alterations in CHC are related to the decreased hepcidin concentrations, although the exact underlying mechanisms remain unclear[10,63].
Still, there is an urgent need for a better understanding of how HCV impacts iron metabolism and if it could be implemented to control disease advancement[10].
It is well known that sarcopenia, increased intramyocellular lipid accumulation, myosteatosis, and reduced muscle mass are all connected with CHC infection, especially in the advanced stages[64,65]. A high incidence rate of sarcopenia was reported, up to 70%, in patients with cirrhosis. Because of anabolic resistance, current nutritional supplementation methods have not been successful in reversing sarcopenia[66,67]. The association between CHC infection of the liver and muscle loss is well documented[68,69]. High body mass index, IR, diabetes, hepatic steatosis, increased inflammation, increased oxidative stress, lipotoxicity, and multiple factors involved in muscle depletion all are considered as independent risk factors that predispose patients with CHC to skeletal muscle disorders[70-73].
Several studies performed on pregnant women with HCV infection reported reduced necro-inflammatory activity, and the rate of fibrosis advancement in CHC is nearly twice as fast in males compared to females[74,75]. Another report stated that long-term hormonal replacement therapy could prevent accelerated liver fibrosis in menopausal women with CHC[76]. Noteworthy improvement in sexual dysfunction was reported in males and females after HCV treatment with direct-acting antivirals[77].
The eradication of HCV remains an essential target for preventing the progression of liver disease and improving or preventing HCV-related metabolic extrahepatic manifestations that have an essential role in morbidity and mortality, affecting the patient’s health-related quality of life.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Corresponding Author's Membership in Professional Societies: Egyptian Association for Research and Training in Hepatogastroenterology, No. 01.
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Egypt
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Muhammad Irham L, Indonesia; Sahin TT, Turkey; Sirli RLD, Romania S-Editor: Fan JR L-Editor: A P-Editor: Fan JR
| 1. | Lavanchy D. The global burden of hepatitis C. Liver Int. 2009;29 Suppl 1:74-81. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 933] [Cited by in RCA: 939] [Article Influence: 58.7] [Reference Citation Analysis (1)] |
| 2. | Qasim SF, Jami A, Imran P, Mushtaque R, Khan RN. Frequency of Metabolic Syndrome in Chronic Hepatitis C Patients: Findings From a Lower Middle Income Country. Cureus. 2020;12:e11975. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 3. | Fabrizi F, Donato FM, Messa P. Hepatitis C and Its Metabolic Complications in Kidney Disease. Ann Hepatol. 2017;16:851-861. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 13] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 4. | Younossi Z, Park H, Henry L, Adeyemi A, Stepanova M. Extrahepatic Manifestations of Hepatitis C: A Meta-analysis of Prevalence, Quality of Life, and Economic Burden. Gastroenterology. 2016;150:1599-1608. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 326] [Cited by in RCA: 299] [Article Influence: 33.2] [Reference Citation Analysis (0)] |
| 5. | Macaluso FS, Maida M, Minissale MG, Li Vigni T, Attardo S, Orlando E, Petta S. Metabolic factors and chronic hepatitis C: a complex interplay. Biomed Res Int. 2013;2013:564645. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 24] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 6. | Chang ML. Metabolic alterations and hepatitis C: From bench to bedside. World J Gastroenterol. 2016;22:1461-1476. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 90] [Cited by in RCA: 97] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 7. | Petta S. Insulin resistance and diabetes mellitus in patients with chronic hepatitis C: spectators or actors? Dig Liver Dis. 2012;44:359-360. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 8. | Esmat G, El Kassas M, Hassany M, Gamil M, El Raziky M. Optimizing treatment for HCV genotype 4: PEG-IFN alfa 2a vs. PEG-IFN alfa 2b; the debate continues. Liver Int. 2014;34 Suppl 1:24-28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 26] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 9. | El Kassas M, Elbaz T, Hafez E, Esmat G. Safety of direct antiviral agents in the management of hepatitis C. Expert Opin Drug Saf. 2016;15:1643-1652. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 25] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 10. | Bugianesi E, Salamone F, Negro F. The interaction of metabolic factors with HCV infection: does it matter? J Hepatol. 2012;56 Suppl 1:S56-S65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 137] [Article Influence: 10.5] [Reference Citation Analysis (1)] |
| 11. | Deltenre P, Louvet A, Lemoine M, Mourad A, Fartoux L, Moreno C, Henrion J, Mathurin P, Serfaty L. Impact of insulin resistance on sustained response in HCV patients treated with pegylated interferon and ribavirin: a meta-analysis. J Hepatol. 2011;55:1187-1194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 65] [Article Influence: 4.6] [Reference Citation Analysis (1)] |
| 12. | Chaudhari R, Fouda S, Sainu A, Pappachan JM. Metabolic complications of hepatitis C virus infection. World J Gastroenterol. 2021;27:1267-1282. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 38] [Cited by in RCA: 40] [Article Influence: 10.0] [Reference Citation Analysis (3)] |
| 13. | Ziolkowska S, Binienda A, Jabłkowski M, Szemraj J, Czarny P. The Interplay between Insulin Resistance, Inflammation, Oxidative Stress, Base Excision Repair and Metabolic Syndrome in Nonalcoholic Fatty Liver Disease. Int J Mol Sci. 2021;22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 102] [Article Influence: 25.5] [Reference Citation Analysis (0)] |
| 14. | Adinolfi LE, Jacobson I, Bondin M, Cacoub P. Expert opinion on managing chronic HCV infection in patients with type 2 diabetes mellitus. Antivir Ther. 2018;23:11-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 6] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 15. | Nevola R, Rinaldi L, Zeni L, Sasso FC, Pafundi PC, Guerrera B, Marrone A, Giordano M, Adinolfi LE. Metabolic and renal changes in patients with chronic hepatitis C infection after hepatitis C virus clearance by direct-acting antivirals. JGH Open. 2020;4:713-721. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 20] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 16. | Kierepa A, Witkowska A, Kaczmarek M, Książek K, Mikuła-Pietrasik J, Żeromski J, Kowala-Piaskowska A, Mozer-Lisewska I. Impact of chronic HCV treatment on quality of life of patients with metabolic disorders in context of immunological disturbances. Sci Rep. 2020;10:10388. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 17. | Stepanova M, Rafiq N, Younossi ZM. Components of metabolic syndrome are independent predictors of mortality in patients with chronic liver disease: a population-based study. Gut. 2010;59:1410-1415. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 201] [Cited by in RCA: 239] [Article Influence: 15.9] [Reference Citation Analysis (0)] |
| 18. | Browning JD, Horton JD. Molecular mediators of hepatic steatosis and liver injury. J Clin Invest. 2004;114:147-152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 734] [Article Influence: 35.0] [Reference Citation Analysis (0)] |
| 19. | Bridle KR, Li L, O'Neill R, Britton RS, Bacon BR. Coordinate activation of intracellular signaling pathways by insulin-like growth factor-1 and platelet-derived growth factor in rat hepatic stellate cells. J Lab Clin Med. 2006;147:234-241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 20. | Wang XX, Song ZZ. Insulin resistance is a risk factor for esophageal varices in hepatitis C virus cirrhosis. Hepatology. 2009;49:1775; author reply 1776. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 21. | Vincent MA, Montagnani M, Quon MJ. Molecular and physiologic actions of insulin related to production of nitric oxide in vascular endothelium. Curr Diab Rep. 2003;3:279-288. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 166] [Cited by in RCA: 167] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 22. | Iwakiri Y, Groszmann RJ. Vascular endothelial dysfunction in cirrhosis. J Hepatol. 2007;46:927-934. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 228] [Cited by in RCA: 207] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 23. | Zimmet P. The burden of type 2 diabetes: are we doing enough? Diabetes Metab. 2003;29:6S9-618. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 136] [Cited by in RCA: 145] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 24. | Wang CS, Wang ST, Yao WJ, Chang TT, Chou P. Hepatitis C virus infection and the development of type 2 diabetes in a community-based longitudinal study. Am J Epidemiol. 2007;166:196-203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 120] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 25. | Algarem N, Sholkamy A, Alshazly M, Daoud A. New-onset diabetes and hypertension as complications of liver transplantation. Transplant Proc. 2014;46:870-872. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 26. | Petta S, Craxì A. Hepatocellular carcinoma and non-alcoholic fatty liver disease: from a clinical to a molecular association. Curr Pharm Des. 2010;16:741-752. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 51] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 27. | Petta S, Rosso C, Leung R, Abate ML, Booth D, Salomone F, Gambino R, Rizzetto M, Caviglia P, Smedile A, Grimaudo S, Cammà C, Craxì A, George J, Bugianesi E. Effects of IL28B rs12979860 CC genotype on metabolic profile and sustained virologic response in patients with genotype 1 chronic hepatitis C. Clin Gastroenterol Hepatol. 2013;11:311-7.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 27] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 28. | Adinolfi LE, Rinaldi L, Marrone A, Giordano M. The effect of sustained virological response by direct-acting antivirals on insulin resistance and diabetes mellitus in patients with chronic hepatitis C. Expert Rev Anti Infect Ther. 2018;16:595-597. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 29. | Gualerzi A, Bellan M, Smirne C, Tran Minh M, Rigamonti C, Burlone ME, Bonometti R, Bianco S, Re A, Favretto S, Bellomo G, Minisini R, Carnevale Schianca GP, Pirisi M. Improvement of insulin sensitivity in diabetic and non diabetic patients with chronic hepatitis C treated with direct antiviral agents. PLoS One. 2018;13:e0209216. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 30. | Saldarriaga OA, Dye B, Pham J, Wanninger TG, Millian D, Kueht M, Freiberg B, Utay N, Stevenson HL. Comparison of liver biopsies before and after direct-acting antiviral therapy for hepatitis C and correlation with clinical outcome. Sci Rep. 2021;11:14506. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 31. | Shimada M, Iwase H, Hirashima N, Ryuge N, Urata N. Nutritional status and liver steatosis after direct-acting antiviral treatment for chronic hepatitis C virus infection. J Transl Sci. 2017;3. [DOI] [Full Text] |
| 32. | Kawagishi N, Suda G, Nakamura A, Kimura M, Maehara O, Suzuki K, Ohara M, Izumi T, Umemura M, Nakai M, Sho T, Natsuizaka M, Morikawa K, Ogawa K, Kudo Y, Nishida M, Miyoshi H, Sakamoto N. Liver steatosis and dyslipidemia after HCV eradication by direct acting antiviral agents are synergistic risks of atherosclerosis. PLoS One. 2018;13:e0209615. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 35] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 33. | Ciancio A, Bosio R, Bo S, Pellegrini M, Sacco M, Vogliotti E, Fassio G, Bianco Mauthe Degerfeld AGF, Gallo M, Giordanino C, Terzi di Bergamo L, Ribaldone D, Bugianesi E, Smedile A, Rizzetto M, Saracco GM. Significant improvement of glycemic control in diabetic patients with HCV infection responding to direct-acting antiviral agents. J Med Virol. 2018;90:320-327. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 75] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 34. | Estefan S, Brandão-Melo CE, Dos Santos Silva CM, Gomes DCK, Cardoso P, Costa MHS. Metabolic Evaluation in Patients With Hepatitis C Treated With Direct Antiviral Agents. Front Med (Lausanne). 2021;8:631600. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 35. | Adinolfi LE, Gambardella M, Andreana A, Tripodi MF, Utili R, Ruggiero G. Steatosis accelerates the progression of liver damage of chronic hepatitis C patients and correlates with specific HCV genotype and visceral obesity. Hepatology. 2001;33:1358-1364. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 778] [Cited by in RCA: 774] [Article Influence: 32.3] [Reference Citation Analysis (0)] |
| 36. | Lonardo A, Loria P, Adinolfi LE, Carulli N, Ruggiero G. Hepatitis C and steatosis: a reappraisal. J Viral Hepat. 2006;13:73-80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 116] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 37. | Negro F. Mechanisms and significance of liver steatosis in hepatitis C virus infection. World J Gastroenterol. 2006;12:6756-6765. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 110] [Cited by in RCA: 105] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 38. | Castéra L, Hézode C, Roudot-Thoraval F, Bastie A, Zafrani ES, Pawlotsky JM, Dhumeaux D. Worsening of steatosis is an independent factor of fibrosis progression in untreated patients with chronic hepatitis C and paired liver biopsies. Gut. 2003;52:288-292. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 227] [Cited by in RCA: 214] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 39. | Mihm S. Hepatitis C virus, diabetes and steatosis: clinical evidence in favor of a linkage and role of genotypes. Dig Dis. 2010;28:280-284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 23] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 40. | Esmat G, El Akel W, Metwally M, Soliman A, Doss W, Hamid MA, Kamal M, Zalata K, Khattab H, El-Kassas M, Esmat M, Hasan A, El-Raziky M. Improvement of steatosis after interferon therapy in HCV genotype 4 is related to weight loss. Indian J Gastroenterol. 2009;28:45-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 41. | Persico M, Iolascon A. Steatosis as a co-factor in chronic liver diseases. World J Gastroenterol. 2010;16:1171-1176. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 30] [Cited by in RCA: 35] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 42. | Younossi ZM, Stepanova M, Nader F, Younossi Z, Elsheikh E. Associations of chronic hepatitis C with metabolic and cardiac outcomes. Aliment Pharmacol Ther. 2013;37:647-652. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 121] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 43. | Pekow JR, Bhan AK, Zheng H, Chung RT. Hepatic steatosis is associated with increased frequency of hepatocellular carcinoma in patients with hepatitis C-related cirrhosis. Cancer. 2007;109:2490-2496. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 143] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 44. | González-Reimers E, Castellano-Higuera A, Alemán-Valls R, Alvarez-Argüelles H, de la Vega-Prieto MJ, Abreu-González P, López-Prieto J, Santolaria-Fernández F, Valladares-Parrilla F. Relation between body fat and liver fat accumulation and cytokine pattern in non-alcoholic patients with chronic HCV infection. Ann Nutr Metab. 2009;55:351-357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 45. | Leandro G, Mangia A, Hui J, Fabris P, Rubbia-Brandt L, Colloredo G, Adinolfi LE, Asselah T, Jonsson JR, Smedile A, Terrault N, Pazienza V, Giordani MT, Giostra E, Sonzogni A, Ruggiero G, Marcellin P, Powell EE, George J, Negro F; HCV Meta-Analysis (on) Individual Patients' Data Study Group. Relationship between steatosis, inflammation, and fibrosis in chronic hepatitis C: a meta-analysis of individual patient data. Gastroenterology. 2006;130:1636-1642. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 416] [Cited by in RCA: 408] [Article Influence: 21.5] [Reference Citation Analysis (0)] |
| 46. | Chen W, Wong T, Tomlinson G, Krahn M, Heathcote EJ. Prevalence and predictors of obesity among individuals with positive hepatitis C antibody in a tertiary referral clinic. J Hepatol. 2008;49:711-717. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 21] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 47. | Everhart JE, Lok AS, Kim HY, Morgan TR, Lindsay KL, Chung RT, Bonkovsky HL, Ghany MG; HALT-C Trial Group. Weight-related effects on disease progression in the hepatitis C antiviral long-term treatment against cirrhosis trial. Gastroenterology. 2009;137:549-557. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 114] [Cited by in RCA: 103] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 48. | Yoshimura T, Oppenheim JJ. Chemerin reveals its chimeric nature. J Exp Med. 2008;205:2187-2190. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 71] [Cited by in RCA: 83] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 49. | André P, Perlemuter G, Budkowska A, Bréchot C, Lotteau V. Hepatitis C virus particles and lipoprotein metabolism. Semin Liver Dis. 2005;25:93-104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 134] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 50. | Kanda T, Moriyama M. Direct-acting antiviral agents against hepatitis C virus and lipid metabolism. World J Gastroenterol. 2017;23:5645-5649. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 7] [Cited by in RCA: 7] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 51. | Lange CM, von Wagner M, Bojunga J, Berg T, Farnik H, Hassler A, Sarrazin C, Herrmann E, Zeuzem S. Serum lipids in European chronic HCV genotype 1 patients during and after treatment with pegylated interferon-α-2a and ribavirin. Eur J Gastroenterol Hepatol. 2010;22:1303-1307. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 23] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 52. | Butt AA, Xiaoqiang W, Budoff M, Leaf D, Kuller LH, Justice AC. Hepatitis C virus infection and the risk of coronary disease. Clin Infect Dis. 2009;49:225-232. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 216] [Cited by in RCA: 226] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 53. | Marzouk D, Sass J, Bakr I, El Hosseiny M, Abdel-Hamid M, Rekacewicz C, Chaturvedi N, Mohamed MK, Fontanet A. Metabolic and cardiovascular risk profiles and hepatitis C virus infection in rural Egypt. Gut. 2007;56:1105-1110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 74] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 54. | Lee MH, Yang HI, Wang CH, Jen CL, Yeh SH, Liu CJ, You SL, Chen WJ, Chen CJ. Hepatitis C virus infection and increased risk of cerebrovascular disease. Stroke. 2010;41:2894-2900. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 121] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 55. | Miyajima I, Kawaguchi T, Fukami A, Nagao Y, Adachi H, Sasaki S, Imaizumi T, Sata M. Chronic HCV infection was associated with severe insulin resistance and mild atherosclerosis: a population-based study in an HCV hyperendemic area. J Gastroenterol. 2013;48:93-100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 31] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 56. | Petta S, Grimaudo S, Marco VD, Scazzone C, Macaluso FS, Cammà C, Cabibi D, Pipitone R, Craxì A. Association of vitamin D serum levels and its common genetic determinants, with severity of liver fibrosis in genotype 1 chronic hepatitis C patients. J Viral Hepat. 2013;20:486-493. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 39] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 57. | Esmat G, El Raziky M, Elsharkawy A, Sabry D, Hassany M, Ahmed A, Assem N, El Kassas M, Doss W. Impact of vitamin D supplementation on sustained virological response in chronic hepatitis C genotype 4 patients treated by pegylated interferon/ribavirin. J Interferon Cytokine Res. 2015;35:49-54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 30] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 58. | Kitson MT, Dore GJ, George J, Button P, McCaughan GW, Crawford DH, Sievert W, Weltman MD, Cheng WS, Roberts SK. Vitamin D status does not predict sustained virologic response or fibrosis stage in chronic hepatitis C genotype 1 infection. J Hepatol. 2013;58:467-472. [RCA] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 59] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 59. | Abu-Mouch S, Fireman Z, Jarchovsky J, Zeina AR, Assy N. Vitamin D supplementation improves sustained virologic response in chronic hepatitis C (genotype 1)-naïve patients. World J Gastroenterol. 2011;17:5184-5190. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 150] [Cited by in RCA: 160] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 60. | Nimer A, Mouch A. Vitamin D improves viral response in hepatitis C genotype 2-3 naïve patients. World J Gastroenterol. 2012;18:800-805. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 103] [Cited by in RCA: 114] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 61. | Chang ML, Hu JH, Yen CH, Chen KH, Kuo CJ, Lin MS, Lee CH, Chen SC, Chien RN. Evolution of ferritin levels in hepatitis C patients treated with antivirals. Sci Rep. 2020;10:19744. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 16] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 62. | Zou DM, Sun WL. Relationship between Hepatitis C Virus Infection and Iron Overload. Chin Med J (Engl). 2017;130:866-871. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 37] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 63. | Fujita N, Sugimoto R, Takeo M, Urawa N, Mifuji R, Tanaka H, Kobayashi Y, Iwasa M, Watanabe S, Adachi Y, Kaito M. Hepcidin expression in the liver: relatively low level in patients with chronic hepatitis C. Mol Med. 2007;13:97-104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 157] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 64. | Petta S, Ciminnisi S, Di Marco V, Cabibi D, Cammà C, Licata A, Marchesini G, Craxì A. Sarcopenia is associated with severe liver fibrosis in patients with non-alcoholic fatty liver disease. Aliment Pharmacol Ther. 2017;45:510-518. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 180] [Article Influence: 22.5] [Reference Citation Analysis (0)] |
| 65. | Gowda C, Compher C, Amorosa VK, Lo Re V 3rd. Association between chronic hepatitis C virus infection and low muscle mass in US adults. J Viral Hepat. 2014;21:938-943. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 19] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 66. | Dasarathy S. Cause and management of muscle wasting in chronic liver disease. Curr Opin Gastroenterol. 2016;32:159-165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 50] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 67. | Gumucio JP, Qasawa AH, Ferrara PJ, Malik AN, Funai K, McDonagh B, Mendias CL. Reduced mitochondrial lipid oxidation leads to fat accumulation in myosteatosis. FASEB J. 2019;33:7863-7881. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 64] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 68. | Fernández-Mincone T, Contreras-Briceño F, Espinosa-Ramírez M, García-Valdés P, López-Fuenzalida A, Riquelme A, Arab JP, Cabrera D, Arrese M, Barrera F. Nonalcoholic fatty liver disease and sarcopenia: pathophysiological connections and therapeutic implications. Expert Rev Gastroenterol Hepatol. 2020;14:1141-1157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 19] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 69. | Cruz-Jentoft AJ, Sayer AA. Sarcopenia. Lancet. 2019;393:2636-2646. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1036] [Cited by in RCA: 2298] [Article Influence: 383.0] [Reference Citation Analysis (0)] |
| 70. | Zhai Y, Xiao Q. The Common Mechanisms of Sarcopenia and NAFLD. Biomed Res Int. 2017;2017:6297651. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 43] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 71. | Hsu CS, Kao JH. Sarcopenia and chronic liver diseases. Expert Rev Gastroenterol Hepatol. 2018;12:1229-1244. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 77] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 72. | Hiraoka A, Michitaka K, Ueki H, Kaneto M, Aibiki T, Okudaira T, Kawakami T, Yamago H, Suga Y, Tomida H, Miyamoto Y, Azemoto N, Mori K, Miyata H, Tsubouchi E, Ninomiya T, Hirooka M, Abe M, Matsuura B, Hiasa Y. Sarcopenia and two types of presarcopenia in Japanese patients with chronic liver disease. Eur J Gastroenterol Hepatol. 2016;28:940-947. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 58] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 73. | Kalafateli M, Konstantakis C, Thomopoulos K, Triantos C. Impact of muscle wasting on survival in patients with liver cirrhosis. World J Gastroenterol. 2015;21:7357-7361. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 47] [Cited by in RCA: 44] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 74. | Poynard T, Bedossa P, Opolon P. Natural history of liver fibrosis progression in patients with chronic hepatitis C. The OBSVIRC, METAVIR, CLINIVIR, and DOSVIRC groups. Lancet. 1997;349:825-832. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2199] [Cited by in RCA: 2160] [Article Influence: 77.1] [Reference Citation Analysis (0)] |
| 75. | Deuffic-Burban S, Poynard T, Valleron AJ. Quantification of fibrosis progression in patients with chronic hepatitis C using a Markov model. J Viral Hepat. 2002;9:114-122. [RCA] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 52] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 76. | Di Martino V, Lebray P, Myers RP, Pannier E, Paradis V, Charlotte F, Moussalli J, Thabut D, Buffet C, Poynard T. Progression of liver fibrosis in women infected with hepatitis C: long-term benefit of estrogen exposure. Hepatology. 2004;40:1426-1433. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 216] [Cited by in RCA: 203] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 77. | El Kassas M, Salah E, Gad A, Hosny A. Improvement of sexual dysfunction in patients after treatment of hepatitis C virus using directly acting antivirals. Curr Med Res Opin. 2021;37:967-972. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |