Published online Jun 7, 2022. doi: 10.3748/wjg.v28.i21.2251
Peer-review started: September 13, 2021
First decision: November 16, 2021
Revised: December 8, 2021
Accepted: April 26, 2022
Article in press: April 26, 2022
Published online: June 7, 2022
Processing time: 261 Days and 13.8 Hours
Hepatocellular carcinoma (HCC) is a global health challenge. Due to the high prevalence in low-income countries, hepatitis B virus (HBV) and hepatitis C virus infections remain the main risk factors for HCC occurrence, despite the increasing frequencies of non-viral etiologies. In addition, hepatitis D virus coinfection increases the oncogenic risk in patients with HBV infection. The molecular processes underlying HCC development are complex and various, either independent from liver disease etiology or etiology-related. The reciprocal interlinkage among non-viral and viral risk factors, the damaged cellular microenvironment, the dysregulation of the immune system and the alteration of gut-liver-axis are known to participate in liver cancer induction and progression. Oncogenic mechanisms and pathways change throughout the natural history of viral hepatitis with the worsening of liver fibrosis. The high risk of cancer incidence in chronic viral hepatitis infected patients compared to other liver disease etiologies makes it necessary to implement a proper surveillance, both through clinical-biochemical scores and periodic ultrasound assessment. This review aims to outline viral and microenvironmental factors contributing to HCC occurrence in patients with chronic viral hepatitis and to point out the importance of surveillance programs recommended by international guidelines to promote early diagnosis of HCC.
Core Tip: Chronic hepatitis B virus (HBV), hepatitis C virus (HCV) and hepatitis D virus (HDV) infection represents a global health burden leading to liver fibrosis, cirrhosis and hepatocellular carcinoma (HCC). Among these complications, HCC accounts for 3.5% of all deaths worldwide. Thus, the understanding of the role of chronic hepatitis virus infection in HCC development is necessary in order to clarify the mechanisms underlying oncogenesis and design future treatments for this cancer. This review outlines pathophysiological and molecular pathways that contribute to carcinogenesis in HBV, HCV and HDV chronic infection focusing on the impact of clinical surveillance and antiviral treatment on the risk of HCC development.
- Citation: Stella L, Santopaolo F, Gasbarrini A, Pompili M, Ponziani FR. Viral hepatitis and hepatocellular carcinoma: From molecular pathways to the role of clinical surveillance and antiviral treatment. World J Gastroenterol 2022; 28(21): 2251-2281
- URL: https://www.wjgnet.com/1007-9327/full/v28/i21/2251.htm
- DOI: https://dx.doi.org/10.3748/wjg.v28.i21.2251
Primary liver cancer is the sixth most common cancer worldwide accounting for 800000 new cases per year and one of the major causes of cancer-related death in men with 745000 deaths per year. It has been estimated that there will be an increased incidence after the year 2025 with more than 1 million cases per year[1]. Hepatocellular carcinoma (HCC) represents the most common histologic type among liver cancers (70%-90%)[2]. According to recent epidemiologic studies, the mortality rate from HCC is increasing in some of the European and North American countries. Indeed, HCC has been recognized as the cause of death in 54%-70% of patients with compensated cirrhosis of different etiologies[3]. Nevertheless, HCC is one of the most prevalent cancers in less developed world regions. About 60%-70% of HCCs have been linked with chronic hepatitis B virus (HBV) or hepatitis C virus (HCV) infection, with a total incidence of 16/100000 cases globally (Table 1)[4], while the linkage with hepatitis D virus (HDV) is less clear[5].
| Location | Chronic HBV infection | Chronic HCV infection | Alcoholic liver disease | Non-alcoholic steatohepatitis | ||||
| NC | CC | NC | CC | NC | CC | NC | CC | |
| Europe | 0.12 | 2.2 | 0-1.8 | 3.7 | 0.1 | 1.8 | 0.5 | 1.1 |
| East Asia | 0.8 | 4.3 | F0/1 0.4; F2 1.5; F3 5.1 | 7.1 | 0.1 | 1.7 | a | a |
HCC is associated with many risk factors (Table 2) with the highest odds ratio (OR) for viral ones. Cirrhosis precedes most cases of HCC and may exert a promoting effect via hepatocyte regeneration and chronic inflammation[4]. There are other environmental and genetic risk factors involved in the pathogenesis of HCC such as excessive alcohol consumption, aflatoxin intake, diabetes, obesity and hereditary hemochromatosis[6]. This review outlines the pathophysiology and molecular pathways that contribute to carcinogenesis in HBV, HCV and HBV/HDV chronic infection and investigates the impact of surveillance programs and antiviral treatments on the risk of HCC occurrence.
| Risk factors in hepatocellular carcinoma (other than liver cirrhosis) | OR (95%CI) | |
| Strong risk factors (OR > 10) | Europe | |
| Untreated chronic HBV/HCV hepatitis | 191.0 | |
| Untreated chronic HCV hepatitis | 31.2 | |
| Untreated chronic HBV hepatitis | 18.8 | |
| East Asia and Africa | ||
| Untreated chronic HBV/HCV hepatitis | 75.6 | |
| Untreated chronic HBV hepatitis | 20.8 | |
| Untreated chronic HCV hepatitis | 11.5 | |
| Moderate risk factors (OR = 2-10) | Aflatoxin B1 exposure | 5.9 |
| Untreated chronic HDV infection | 3.9 | |
| Diabetes | 3.2 | |
| Asian race | 3.2 | |
| Male gender | 2.8 | |
| Alcohol intake | 2.3 | |
| Severe iron overload | 2.1 | |
| Weak risk factors (OR < 2) | Obesity (BMI > 30 kg/m2) | 1.9 |
| Mild iron overload | 1.6 | |
| Current smoking | 1.6 | |
| HCV genotype 1b | 1.6 | |
| PNPLA3 rs738409 single nucleotide polymorphism | 1.4 | |
HBV infection is the most widespread chronic viral infection in the world. Currently, 2 billion people have been infected and more than 350 million are chronic carriers of the virus[7]. Worldwide HBV surface antigen (HBsAg) seroprevalence is 3.6% with the highest prevalence in Africa (8.83%) and in Western Pacific Asia (5.26%)[8]. In contrast, the prevalence of chronic HBV infection is low in most of European countries and it is estimated to be around 0.5%-0.7%[9]. Underdiagnosis and undertreatment are still unresolved issues and public awareness is largely suboptimal with less than 25% of infected Europeans aware of HBV transmission risk at the moment of their diagnosis[9]. Thus, chronic HBV infection is the tenth leading cause of global deaths accounting for 3.5% of all deaths worldwide (786000 deaths per year). Half of the total liver cancer mortality in 2010 has been attributed to HBV infection[10].
Chronic HBV infection is the most important cause of HCC worldwide. Prospective cohort studies show a 5-100-fold increase in the risk of developing HCC among patients with untreated chronic HBV infection[2]. A systematic review estimates a yearly HCC incidence rate of 0.2% in HBV inactive carriers, of 0.6% in those with chronic infection without cirrhosis and of 3.7% in those with compensated cirrhosis[11]. Meta-analyses show a 15-20 times greater relative risk for HCC occurrence in HBsAg positive individuals than in HBsAg negative individuals[2].
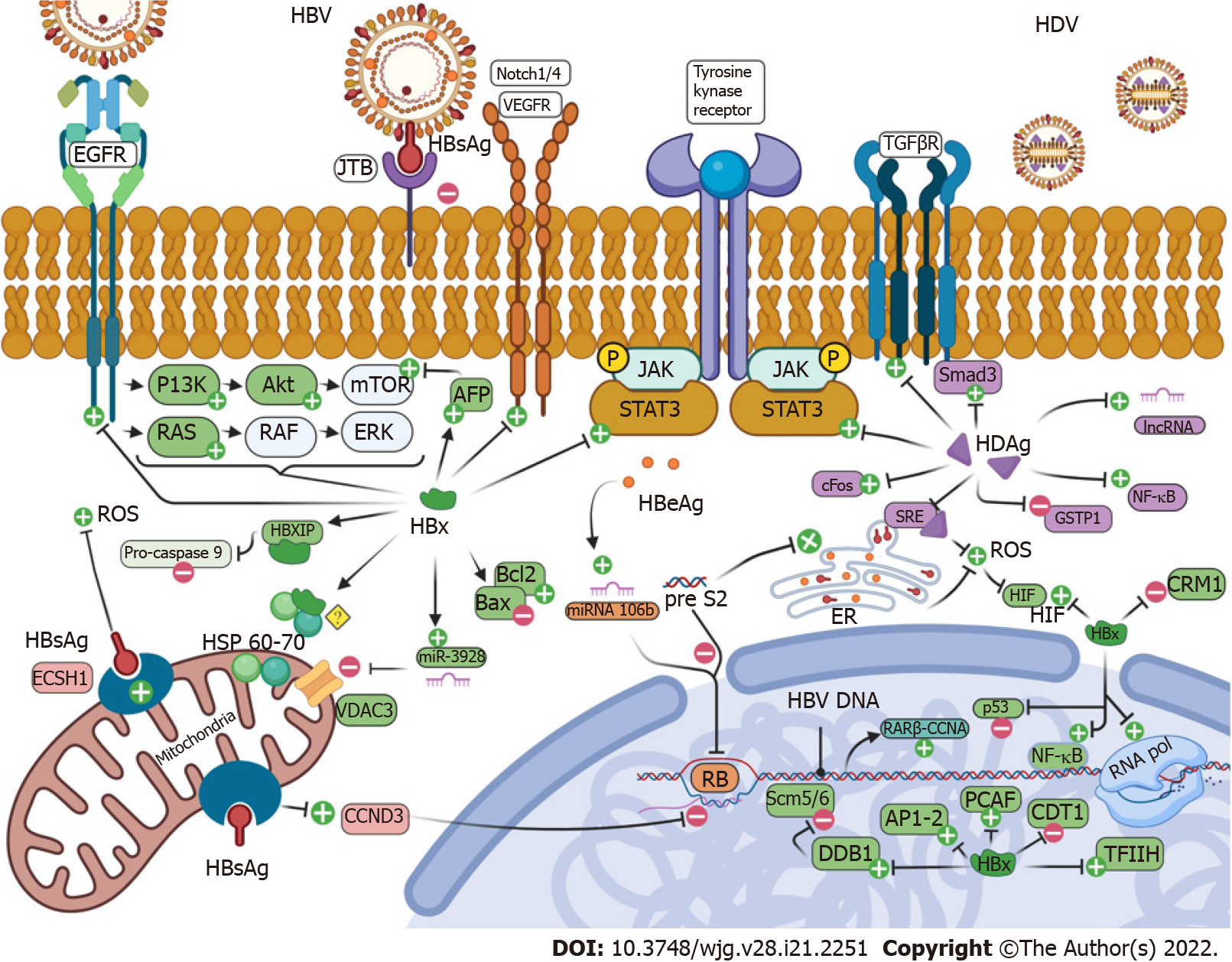
Most of HBV-infected individuals (70%-90%) develop HCC related to the onset of cirrhosis and secondary to chronic inflammation[2]. However, in up to one-third of patients, HCC occurs in the absence of cirrhosis[12]. Oncogenesis in HBV infected patients is a complex phenomenon involving most of the cell cycle regulation molecular pathways (Table 3 and Figure 1). As a noncytopathic virus, the pathogenesis of HBV tissue damage is mainly mediated by cell-mediated immune response to HBV epitopes surface proteins. Persistent HBV replication triggers immune response against the virus leading to genetic damage and perpetrating oxidative stress. In addition to chronic inflammation, other viral mechanisms contribute to HBV carcinogenesis involving the HBs and HBx proteins, the insertional mutagenesis caused by HBV DNA integration into the host genome, which induces chromosomal instability and alters the expression of endogenous genes, the epigenetic modifications through DNA methylation and the regulation of microRNA (miRNA) expression[13].
| HBx related pathways |
| DNA repair impairment and DNA instability |
| HBx - binds to DDB1 - instability of Scm5/6 - impairment in DNA replication and repair |
| HBx - interacts with TFIIH - impairment in DNA replication and repair |
| HBx - blocks BER pathway - impairment in DNA repair |
| HBx - binds to CRM1 and sequestering it in cytoplasm - aberrant centrosome duplication and chromatin’s segregation - chromosome instability |
| DNA replication increase |
| HBx - upregulates RLF and CDT1 and downregulates geminin - DNA replication |
| HBx - binds to cccDNA - recruiting PCAF - histone H3 acetylation - inhibition of chromatin’s methylation - DNA replication |
| Cell cycle deregulation via signal pathways |
| HBx - binds to p53 - impaired function of p53 - cell cycle dysregulation |
| HBx - induces AFP expression - activation of PTEN and PI3K/mTOR pathway - cell cycle deregulation |
| HBx - activates Notch1 and Notch4 receptor - cell cycle progression |
| HBx - upregulates NF-kB, AP-1, AP-2, c-EBP, RNA-polymerase, ATF - altered oncogenes expression and cell cycle deregulation |
| HBx - upregulates NF-kB - upregulation of EGR1 - upregulation of miR-3928v - downregulation of VDAC3 - tumor suppressor inhibition |
| HBx - downregulates SFRP1 and SFRP5 - DNMT1 recruitment - inhibition of WNT/β-catenin pathway - epithelial mesenchymal transition |
| Epigenetic modification impairment |
| HBx - interacts with MBD2 and CBP - P3 and P4 promoters’ activation through hypomethylation - recruitment of IGF2 - oncogenesis |
| HBx - stimulates deacetylation of IGFBP3 gene - upregulation of IGF1 - mitogenic and anti-apoptotic effects |
| HBx - upregulates DNMT1 - hypermethylation of RASSF1A - tumor suppressor inhibition |
| HBx - downregulates DNMT3a/DNMT3L and recruits HDAC1 - hypomethylation of oncogenes promoters including JAK/STAT3 - impairment in cell differentiation |
| HBx - downregulates CD82, MTA1, PCDH10 through hypermethylation - tumor progression |
| HBx - inhibits CDH1 through deacetylation - E-cadherin upregulation - metastasis promotion |
| Apoptosis impairment |
| HBx - upregulates Bcl2 and Mcl1, inhibits Bax - apoptosis inhibition |
| HBx - upregulates Foxo4 - increased resistance to ROS damage, avoiding cell death and apoptosis |
| HBx - upregulates NF-kB - increase of DR5 - TRAIL induced apoptosis |
| HBx - inhibits caspase-8 inhibitor A 20 - TRAIL induced apoptosis |
| mi/lnc RNA related pathways |
| HBx - impairs miRNA regulation and synthesis - cell cycle deregulation |
| HBx - impairs lncRNA regulation and synthesis - cell cycle deregulation |
| Oxidative stress |
| HBx - downregulates NQO1 - mitochondrial injury - ROS production |
| C-terminal truncated HBx - mitochondrial DNA damage - ROS production |
| Neoangiogenesis |
| HBx - upregulates VEGF, HIF1 and ANG2 - neoangiogenesis |
| Unknown mechanism |
| HBx - binds to HSP60 and HSP70 - unknown function but involved in HCC carcinogenesis |
| Pre-S/S related pathways |
| pre-S2 - retention of HBV proteins in ER - ROS increase - cell DNA damages |
| pre-S2 - retention of HBV proteins in ER - upregulation of CCNA - chromosome instability and centrosome overduplication |
| pre-S2 - interacts with JAB1 - RB tumor suppressor inhibition |
| HBsAg related pathways |
| HBsAg - binds to ECHS1 - ROS increase - cell DNA damages |
| HBsAg - binds to JTB - decreased apoptosis and increased cell mobility |
| HBeAg related pathways |
| HBeAg - stimulates upregulation of miR-106b - RB tumor suppressor inhibition |
| HBV DNA related pathways |
| cccDNA - triggers DNA repair pathways - histone degradation and cell cycle checkpoints activation - enhanced DNA recombination rate |
| HBV DNA - genome integration - oncogene activation or tumor suppressor inhibition with evidence of fusion proteins |
| HBV DNA - genome integration - genetic instability - clonal proliferation |
| Inflammatory pathways |
| Increased cytokines production (TGF-β, IL-4, IL-10, IL-12, IL-13) - JAK/STAT3 activation - cell proliferation |
| CD4+ T follicular helper decrease - loss of growth inhibition and death control of cancer cells |
| CD8+ cell dysfunction - impaired growth inhibition and death control of cancer cells |
| Functional exhausted CD8+TIM-3+ T cells - increased viral replication - increased viral factors in HCC development |
| NK cells - increase in IL-4 and IL-13 - activation of HSCs - increased cytokines production - cell cycle deregulation |
| NK cells - miR-146a increase - reduced cytotoxicity and decreased IFN-γ production - reduction in immunosurveillance |
| Tregs - PD1 and CTLA4 overexpression - C |
| Gut microbiota-related pathways |
| HBV related dysbiosis - circulating LPS - TLR4 activation - cytokines production - JAK/STAT3 activation - cell proliferation |
There are other factors increasing HCC risk among HBV carriers that can be divided into: Viral factors (high rates of HBV replication, long term infection, coinfection with HCV/human immunodeficiency virus/HDV, high risk genotype) and host-related factors, such as genetic or demographic (older ages, male sex, Asian or African origin, family history of HCC), clinical (presence of cirrhosis) and environmental (diabetes, overweight, alcohol abuse, aflatoxin exposure)[14].
The risk of developing HCC strictly correlates with HBV viraemia. Multiple integrations have been detected in liver tissues and integrated HBV sequences have been observed in almost 90% of HBV-related HCCs[13]. The reveal-HBV study shows increased HCC-related mortality in subjects with baseline HBV DNA higher than 106 copies/mL compared to those with baseline DNA lower than 300 copies/mL. A multivariate cyclooxygenase (COX) regression analysis identifies an increase in HBV DNA levels as the strongest independent predictor of death from HCC, after adjusting for age, sex, smoking, alcohol consumption and hepatitis B e antigen (HBeAg) serostatus[15]. Nevertheless, patients previously defined as “inactive carriers” (HBeAg negative, HBV DNA < 10000 copies/mL and normal liver function tests) have a 5-fold higher risk of developing HCC compared to HBsAg negative controls[16].
The integration of HBV DNA into the host genome represents a major pathogenetic pathway. The plasmid-like covalently closed circular DNA (cccDNA), derived from the conversion of HBV DNA soon after the entry into the hepatocyte, is recognized in the nucleus as a damaged product triggering DNA repair pathways such as the activation of cell cycle checkpoints and histone degradation, which enhances DNA recombination rates[17-19]. The integration of HBV DNA can also contribute to hepatocarcinogenesis by altering several cancer-relevant genes[20]. Viral promoter-driven human transcripts have also been identified adjacent to repetitive non-coding sequences, in particular long interspersed nuclear elements or short interspersed nuclear elements[20]. Evidence for fusion proteins have been collected for retinoic acid receptor-β and human cyclin A gene resulting in tumor-specific chimeric proteins endowed with pro-carcinogenic functions[21].
Despite this evidence, HBV integration is random and hardly ever leads to direct oncogene activation or tumor suppressor genes inactivation. However, it is widely accepted that integration contributes to hepatocytes genetic instability leading to clonal proliferation[22]; this can occur in HBV infected patients even before liver tissue damage is clinically evident[23].
A study shows that the presence of HBV-DNA integration increases more than 100 times the risk for HCC occurrence among HBsAg carriers compared with HBsAg negative individuals[13]. HBsAg seems to have effects on the mitochondrial function, as it reduces the lipid b-oxidative activity of enoyl-coenzyme A hydratase short chain 1. On one hand, this interaction induces cell apoptosis by decreasing the mitochondrial membrane potential and reduces the phosphorylation of a serine/threonine protein kinase (Akt). On the other hand, it leads to an increased reactive oxygen species (ROS) production and promotes UDP-glucose ceramide glycosyltransferase which contributes to the alteration of ceramide metabolism[24]. Furthermore, HBsAg can inhibit jumping translocation breakpoints leading to increased cell motility and decreased apoptosis[13].
The association between HBeAg and HCC has been well established by epidemiological studies although the pathophysiological mechanisms of HBeAg-mediated oncogenesis remain unknown. A large study investigates the effect of HBV replication on HCC development risk among 11893 Taiwanese men followed up for a mean of 8.5 years: The incidence rate of HCC is 1169 per 100000 person-years among HBsAg/HBeAg positive men, 324 per 100000 person-years for HBsAg positive HBeAg negative men and 39 per 100000 person-years among HBsAg negative men[25]. HBeAg enhances cell proliferation by means of promotion by a G1/S transition. Data suggests that miR-106b upregulation mediated by HBeAg is involved in the pathogenesis of HBV-related HCC by downregulating retinoblastoma (RB) gene[26].
The preS/S open reading frame encodes three envelope proteins (S or HBsAg, M, and L proteins)[27]. Various specific mutations in the preS/S gene may imbalance surface proteins’ synthesis and their consequent retention within hepatocytes’ endoplasmic reticulum (ER)[13,28]. This process occurs mainly in the presence of HBsAg or pre-S2 mutants: The viral proteins amassed in the ER can lead to oxidative stress, stimulate cell growth and survival signaling pathways and cause mutation through the generation of free radicals. The induced ER stress upregulates the cytoplasmic cyclin A pathway promoting chromosome instability through centrosome over-duplication[29]. Moreover, pre-S2 mutant protein in hepatocytes can directly interact with the c-Jun activation domain-binding protein 1 inducing hyperphosphorylation of the RB tumor-suppressor and its inactivation[13,30].
HBx is a 154 aminoacids long protein which is essential for the HBV viral life cycle. HBx is usually expressed at low levels during the first stages of infection but with the rise of HBV DNA integration frequency during infection, relative HBx expression can increase[31]. HBx is involved in multiple and complex molecular pathways linked to mitogen and apoptotic signaling cascades, resulting in one of the most relevant oncogenic proteins in HBV proteome. It does not interact directly with DNA, but rather it acts on cellular promoters, impairs DNA repair pathways, enhances DNA replication, alters epigenetic processing and inhibits apoptosis mechanisms. HBx effects are proven to contribute to HCC progression as well, by means of angiogenesis upregulation and stimulation of cell motility.
Some preclinical studies show that HBx interacts with cell cycle regulatory pathways activating a plethora of genes involved in mitogen signaling cascades and their downstream transcription factors, including, nuclear factor-κB (NF-κB), phosphatidylinositol 3-kinase (PI3K) and phosphatase and tensin homolog through upregulation of alpha-fetoprotein (AFP), Notch1 and Notch4, wingless-related integration site (WNT)/β-catenin signaling, activator protein 1 (AP-1), AP-2, CCAAT-enhancer-binding protein, RNA polymerase and nuclear factor of activated T cells. The activation of these pathways leads to altered expression of growth-control genes[32]. Other significant targets of HBx are the p53 family genes; The viral protein directly binds to p53 and impairs its function (p53-mediated apoptosis, p53-mediated transactivation properties and cell cycle regulation)[33].
In vitro studies have provided mechanistic insights towards the understanding of the role of HBx in HBV-mediated carcinogenesis showing an interesting effect on DNA replication. Indeed, HBx expressing cells exhibit increased DNA replication licensing factor, chromatin licensing and DNA replication factor 1, as well as reduced expression of geminin. The impairment in these molecules homeostatic balance leads to an increase in DNA replication[34]. Furthermore, HBx impairs DNA repair enzymes, interacting with transcription factor IIH, and deregulating all other proteins involved in the base excision repair pathway[32]. HBx is also involved in HBV transcription from cccDNA in the beginning and maintaining phase of viral replication. This leads to chromatin modulation and chromosomal instability because the HBx bond with cccDNA induces the recruitment of acetyltransferases P300/CREB-binding protein (CBP)-associated factor and acetylation of histone H3, inhibiting chromatin’s methylation[35]. HBx seems to induce multipolar spindle formation, chromosome segregation defects and aberrant centrosome duplication, probably by sequestrating the nuclear transport receptor Chromosomal Maintenance 1 in the cytoplasm[36]. HBx binds to the HBx interacting protein regulating the centrosome duplication or to the UV-damaged DNA binding protein 1 that influences the stability of proteins such as the structural maintenance of chromosome proteins 5 and 6 complex which plays a role in nuclear DNA replication and repair[37,38].
In mitochondria, HBx binds heat shock proteins 60 and 70 (HSP60 and HSP70) and increased levels of the resulting complex have been found in HCC cells, even if the underlying carcinogenetic mechanism has not been understood yet[39]. HBx, through miR-3928v upregulation, downregulates voltage dependent anion channel 3 (VDAC3) expression in mitochondria leading to the loss of its tumor suppression activity[40].
The role of HBx on apoptosis has been controversial. On one hand, HBx upregulates B-cell lymphoma 2 (Bcl-2) and myeloid cell leukemia-1 that exert an anti-apoptotic role and downregulates Bcl-2-associated X protein, a cytosolic protein inducing apoptosis by its interaction with VDAC3 channels on the mitochondrial membrane. On the other hand, HBx stimulates apoptosis by increasing the cell surface concentration of death receptor 5 and inhibits caspase-8 inhibitor A20 that have central roles in tumor necrosis factor (TNF) α-related apoptosis-inducing ligand mediated apoptosis. Moreover, HBx increases resistance to apoptosis through the forkhead box protein 4 avoiding cell death primed by oxidative stress damage[32]. In addition, HBx controls hepatic angiogenesis, upregulating vascular endothelial growth factor (VEGF), hypoxia inducible factor 1 (HIF-1) and proangiogenic growth factor angiopoietin 2 (ANG2)[41].
DNA methylation and acetylation are two of the most studied epigenetic modifications and usually occur in the early stage of HCC development. The genomic hypomethylation/hypoacetylation increases chromosome instability while localized hypermethylation/hyperacetylation decreases the expression of tumor suppressor genes[13]. DNA hypermethylation in the promoter region of specific oncosuppressor genes has been found in HBV-related HCC mainly due to HBx effect.
HBx upregulates DNA methyltransferases 1 (DNMT1) causing a hypermethylation of rat sarcoma (RAS) association domain family member 1 (RASSF1A), involved in cell cycle maintenance, altering its expression from the very early stages of HCC development[42]. On the contrary, the downregulation of DNA methyltransferases (DNMT) 3A and DNMT 3L and the recruitment of histone deacetylase 1 causes the hypomethylation of intragenic CpG islands resulting in dedifferentiation of liver cells[43]. The upregulation of methyl-CpG binding domain protein 2 and CBP triggers a molecular pathway leading to hypomethylation of promoters 3 (P3) and 4 (P4) of insulin-like growth factor 2 gene that stimulates cell duplication pathways as well as G1/S transition[44]. Hypomethylation has been found in the promoter region of Telomerase Reverse Transcriptase (TERT), increasing the proliferative potential of the cell, and in the promoter region of COX-2, leading to a proinflammatory condition[45]. Hypermethylation has also been found in a cluster of differentiation 82, a metastasis suppressor protein involved in the p53 pathway, metastasis-associated protein 1 and protocadherin 10. Deacetylation of Cadherin-1 promoter leads to E-cadherin upregulation and a progression to metastatic disease[32].
A growing number of studies identify and investigate the role of specific miRNA, small non-coding RNA molecules (up to 25 nucleotides in length) that regulate gene expression at transcriptional and post-transcriptional levels as mediators in the hepatocarcinogenic process. Recent publications, summarized by Sartorius et al[46] in a meta-analysis, have found miRNAs that are involved in HBV-related HCC leading to more aggressive cancer phenotypes by means of both tumor suppressor genes inactivation and oncogenes activation.
Studies with highly sensitive polymerase chain reaction assays show that HBV DNA may persist in the liver of people who have serologic recovery from acute HBV infection, as an occult HBV infection. A systematic review identifies a modest association between occult HBV infection and the risk of HCC occurrence [risk ratio (RR) = 2.83][47]. Another case-control study performed in Hong Kong shows an increased prevalence of occult HBV infection in cryptogenic HCC[48]. None of these studies is population based and most of them have a small sample size. Thus, the association between HBV occult infection and HCC lacks convincing evidence.
HCC is more common in patients with HBV genotype C, D and F than in those with genotype A[49]. The majority of retrospective and case-control studies indicate that patients with genotype C HBV have a more severe course of liver disease and a higher risk of HCC occurrence has been reported. A community-based Taiwanese cohort study shows that genotype C is associated with an increased risk of HCC in HBV carriers [hazard ratio (HR) = 2.35, 95% confidence interval (CI): 1.68-3.30, P < 0.001] as well[50]. The Eradicate-B study, by analyzing HBsAg positive patients without evidence of cirrhosis and a mean follow-up of 14.7 years, points out a higher annual incidence rate of HCC in genotype C than genotype B patients[51].
HBV genotype also influences the features of HCC. In genotype B patients, solitary HCC nodules are more frequent than in genotype C (94% vs 86%), but are more often associated with satellite nodules (22% vs 12%)[52]. Indeed, several reports show that HBV genotype type B is associated with a higher risk of HCC development in youth, whereas genotype C is associated with HCC development in the elderly[53]. Genotype D has been noticed to be prevalent in HCC patients younger than 40 years of age, as compared to other genotypes (63% vs 44%; P = 0.06). Furthermore, a study carried out among Alaska native HBV infected patients shows that the HCC is more prevalent in genotype F than other genotypes (OR= 7.73, 95%CI: 3.69-16.4, P < 0.001)[54]. Data about genotype E, endemic in Africa, are lacking and further studies are needed to outline the carcinogenic role of this genotype. Differently from HBV genotypes, the clinical significance of HBV subtypes is still unclear[55].
Several mutations in the X gene of the HBV genome are frequently found in HBV mutants of individuals with HCC. Some studies show that 3’-end X gene is commonly deleted in HCC cells leading to a C-terminal truncated HBx protein that may have a key role in the carcinogenetic process[56]. In a Taiwanese cohort, genotype C shows a higher prevalence of BCP A1762T/G1764A variant than genotype B. Patients with BCP A1762T/G1764A variant have a higher risk of developing HCC and a long-term follow up study reveals BCP A1762T/G1764A variant as an independent predictor for progression to HCC[57]. Mutations in enhancer II (C1653T) and elsewhere in the basal core promoter (T1753V) have also been found to be associated with HCC development[57].
Chronic tissue inflammation plays a central part in oncogenesis through various complex mechanisms. In the liver, continuous inflammation and regeneration increases the risk of hepatocarcinogenesis. Cytokines production and inflammation-mediated alteration of key signaling pathways, such as signal transducer and activator of transcription 3 (STAT3) and NF-κB pathways, play an important role as well[13,58]. The most common immune alterations found during HBV infection are: Cytokines production [such as tumor growth factor (TGF-β), interleukin (IL)-4, IL-10, IL-12, IL-13], decreased CD4+ and CD8+ cells function, Treg cells dysfunction and innate immune response suppression[59].
CD4+ Th1 and CD8+ cells play an important role in cell cycle regulation and death control of cancer cells; thus, T cells dysfunction can lead to a higher risk of HCC development[60]. The impairment of HBV-specific CD8+ T cell functions in patients with chronic hepatitis B is mainly shown by the high expression levels of inhibitory receptors such as cytotoxic T-lymphocyte antigen 4, programmed cell death protein (PD) 1 and T cell immunoglobulin and mucin-domain containing (TIM) 3. CD8+TIM-3+ T cells are functionally exhausted because of the continuous immune activation and are unable to effectively produce cytokines and exert anti-viral activity[60]. Additionally, non-specific CD8+ T cells with memory phenotypes secrete interferon (IFN)-γ, recruiting hepatic macrophages which promote HCC through the secretion of TNF-α, IL-6 and Monocyte Chemoattractant Protein 1[61]. The frequency of circulating CD4+ T follicular helper cells decreases and their function results are impaired during disease progression in patients with HBV-related HCC; CD4+ cells infiltrate seems to be significantly reduced in HCC tumor regions compared to non-tumor regions[62].
Natural killer (NK) cells from HCC patients show significant increase in the expression of miR-146a which downregulates NK cell’s function and leads to a decreased IFN-γ and TNF-α production. Activation of non-virus-specific cells may result in widespread inflammation promoting HCC development. NK cells promote hepatic stellate cells (HSCs) activation in liver fibrogenesis through the production of the inflammatory cytokines IL-4 and IL-13[63]. HSCs enhance the recruitment of regulatory T cells (Tregs) in the liver increasing the fibrotic processes and therefore hepatocarcinogenesis. Indeed, Tregs show enhanced suppressive function by PD-1 over-expression leading to a more immunosuppressive and exhausted cellular microenvironment in HBV-related HCC compared to non-virus-related HCC[64]. Thus, HBV-induced immune imbalance is a major risk factor in the pathway of HCC development.
The link between the gut-liver axis and HCC has been studied in animal models as well as small cohorts of patients and is based on the theories that inflammation related to chronic liver disease causes dysbiosis and intestinal barrier dysfunction. Translocation of bacterial products in portal and systemic circulation can trigger molecular pathways leading to inflammation, in particular lipopolysaccharide (LPS) from Gram-negative bacteria wall activates the Toll-like receptor (TLR) 4 causing an increase in inflammatory cytokines production. In turn, cytokines are linked to the activation of signaling pathways of cell proliferation, such as Janus kinase (JAK)/STAT3[65]. A well characterized pattern of inflammatory cytokines and chemokines has been associated with cirrhosis and HCC, characterized by increased plasmatic levels of IL-8, IL-13, C-C motif chemokine ligand (CCL) 3, CCL4 and CCL5[66]. This correlates alteration in gut microbiota with replication and immortalization of HCC cells. Indeed, several studies suggest that the gut microbiota might play a key role in the process. The inflammatory role of LPS is in fact mediated by TLR4; TLR4 knock-out mice show a much lower incidence of HCC[67]. Some other studies demonstrate that diethylnitrosamine (DEN)-exposed mice have an increased abundance of Gram-negative bacteria and therefore, of LPS (Escherichia coli, Atopobium, Collinsella, Eggerthella and Coriobacterium)[68]. High-dose probiotics administration in these animals is able not only to reverse dysbiosis but can reduce the number and size of cancer nodules as well.
There are limited studies comparing the gut microbiota of HBV-related HCC and non-HBV non-HCV-related HCC. A small Asian study enrolled 90 patients, divided in three groups: HBV-related HCC (B-HCC), non-HBV non-HCV-related HCC (NBNC-HCC) and healthy controls. The gut microbiota of B-HCC patients is more heterogeneous with higher bacterial alpha diversity indices, whereas, those of healthy controls and NBNC-HCC patients exhibits similar features. However, butyrate-producing bacteria (genus Ruminococcus, Feacalibacterium, Clostridium) present heterogeneity in B-HCC and NBNC-HCC in the study[69]. In addition, a set of Bifidobacterium species is found to mark predominant dysbiosis in HBV cirrhosis patients[70]. More studies are needed to clarify the possible oncogenic role of the gut microbiota related to HBV infection in the pathogenesis of HCC.
Eastern and western hepatological societies have the difficult task of identifying patient populations at high risk for HCC occurrence, in whom clinical and radiological surveillance is mandatory (risk above threshold, usually cumulative incidence > 1.5% yearly) and other populations where there is no certain benefit in carrying out the surveillance programs (risk unknown or below threshold)[71]. Based on these considerations, surveillance must include patients affected by cirrhosis of all causes and a subset of non-cirrhotic HBV carriers (Table 4). HCC screening and surveillance is recommended by the American Association for the Study of Liver Diseases (AASLD) guidelines in patients with cirrhosis of any cause and non-cirrhotic HBV carriers at higher risk (i.e., Asian men older than 40-years-old, Asian women older than 50-years-old, patients with a family history of HCC and African or North American Blacks of any age)[72,73]. The European Association for the Study of the Liver (EASL) guidelines also include non-cirrhotic patients with high or medium risk calculated with the PAGE-B score[74,75]. The Japan Society of Hepatology (JSH) differentiates ‘‘extremely-high-risk patients’’ (hepatitis B cirrhosis) in whom surveillance is recommended every 3-4 mo and ‘‘high-risk patients’’, in whom surveillance is recommended every 6 mo and ‘‘risk factors other than hepatitis virus infection or liver cirrhosis’’ in whom the benefits of surveillance are uncertain[76,77].
| HCC surveillance in HBV-infected patients | ||
| Western medical societies | ||
| EASL, 2017 | High-risk-patients: (1) HBV cirrhotic patients; (2) HBV and F3 fibrosis; and (3) HBsAg-positive patient on NA treatment with a PAGE-B of ≥ 18 at the onset of therapy. Medium risk-patients: HBsAg-positive patient on NA treatment with a PAGE-B of 10 - 17 at the onset of therapy | Screening with US examination with or without AFP every 6 mo for medium and high-risk patients. No specific HCC screening needed for low-risk patients |
| AASLD, 2018 | High-risk patients: (1) HBV cirrhotic patients; (2) Special population of HBsAg-positive adults: Asian or African men (> 40 yr) and Asian women (> 50 yr), first-degree family member with a history of HCC, HDV coinfected; and (3) HBsAg-positive children/adolescents with advanced F3 or cirrhosis and first-degree family member with HCC | Screening with US examination with or without AFP every 6 mo; if in areas where US is not readily available, screening with AFP every 6 mo |
| Eastern medical societies | ||
| JSH, 2014-2021 | Extremely-high-risk patients: HBV cirrhotic patients. High-risk patients: Special population of HBsAg positive patients: age ≥ 40, male, alcohol consumption, high HBV load, family history of HCC, HCV/HDV/HIV coinfection, F3 fibrosis, low platelet count associated with advanced fibrosis, genotype C, and core promoter mutation | Screening with US and tumor marker measurements (AFP, protein induced by vitamin K absence or antagonist-II and AFP-lectin fraction 3) every 3-4 mo in the super-high-risk population. A 6-12 mo dynamic CT scan or dynamic MRI should be performed. Screening every 6 mo in high-risk populations |
| APASL, 2016 | High-risk patients: All patients with HBV-related cirrhosis. HBsAg-positive without cirrhosis, based on the economic situation of each country and on the available risk scores | Surveillance by US and AFP should be performed every 6 mo and preferably every 3-4 mo in cirrhotic patients and those at high risk of HCC |
| KLCSG, 2014-18 | High-risk patients: HBV cirrhotic patients; chronic hepatitis B patients | Screening with US examination with or without AFP every 6 mo. If liver US cannot be performed properly, liver dynamic CT or dynamic contrast-enhanced MRI can be performed |
As individual HCC risk factors cannot adequately classify patients with chronic hepatitis B according to their HCC risk, several risk scores have been developed for those undergoing viral treatment with nucleotide analogues: CU-HCC, CAG-HCC, REACH-B, PAGE-B, modified PAGE-B (mPAGE-B)[78].
CU-HCC, GAG-HCC and REACH-B have been validated in a cohort of Asian patients with chronic hepatitis B, 22% of whom with cirrhosis, treated with entecavir (ETV): The 5-year HCC cumulative incidence rates were 12.9% in cirrhotic patients and 2.1% in non-cirrhotic ones while the accuracy of CU-HCC, GAG-HCC and REACH-B scores for HCC prediction at baseline was 0.8, 0.76 and 0.71, respectively[79]. Other studies confirmed an analogue predicting value of the REACH-B score in patients treated with tenofovir[80-82].
However, CU-HCC, GAG-HCC and REACH-B predictability is proven to be poor in Caucasian subjects with chronic hepatitis B. A large study including patients treated with ETV or tenofovir showed an accuracy of 0.66, 0.74 and 0.54, respectively[83]. Subsequently, Papatheodoridis et al[84] validated the PAGE-B score on 1815 Caucasians treated with ETV or tenofovir for at least 12 mo. The PAGE-B score had the advantage of including simple variables (platelets, age and gender) and was able to stratify the risk of HCC development within 5 years in: Low (0%), for PAGE-B score < 10; medium (4%), for PAGE-B score 10-17; or high (about 16%), for PAGE-B score > 17. Kim et al[85] developed the mPAGE-B score, adding serum albumin to the PAGE-B parameters, and validating the score on Asian populations. The mPAGE-B differentiated the 5-years HCC risk in low (< 8) or high (> 13). Therefore, the PAGE B score seems to perform with an adequate accuracy both in Caucasian and Asian chronic hepatitis B patients under the current oral antiviral drugs.
Recently, the usefulness of two novel biomarkers, hepatitis B core-related antigen (HBcrAg) and Wisteria floribunda agglutinin-positive Mac-2 binding protein (M2BPGi), has been evaluated for the prediction of HCC occurrence. HBcrAg is a novel biomarker of HCC occurrence and consists of three products of the precore/core gene: HBeAg, hepatitis B core antigen and p22cr, a 22kDa protein present in DNA-negative Dane-like particles. It is used in Japan to support HCC screening in patients with chronic hepatitis B. Serum HBcr levels correlate with HBV replication, serum HBV DNA, intrahepatic cccDNA levels and transcriptional activity. In a large cohort study, HBcrAg is proven to be superior to HBV DNA in predicting HCC development in treatment-naïve patients with chronic hepatitis B. Tseng et al[51] showed that HBcrAg level is an independent risk factor of HCC in individuals with chronic hepatitis B and intermediate viral load. Another cohort study suggests a correlation between M2BPGi, a serum glycoprotein-based biomarker (glycobiomarker), fibrosis (FIB) stage and HCC. In patients with cirrhosis M2BPGi serum levels > 1.8 cut-off index are associated with a higher risk of HCC development (P < 0.001). Although the area under the receiver operating characteristic curves for M2BPGi and AFP are similar in cirrhotic patients of all etiologies (0.77 vs 0.72; P = 0.15), M2BPGi outperforms AFP in chronic hepatitis B patients (0.84 vs 0.75; P = 0.02)[86]. Furthermore, M2BPGi serum level before receiving NA and 48 wk after the beginning of treatment is predictive of HCC occurrence.
Nowadays, only a few classes of drugs have been licensed and therefore approved for hepatitis B treatment: Nucleos(t)ide reverse transcriptase inhibitors (NRTIs) and IFN-α.
Lamivudine (LAM) has been the first nucleoside analogue used for the treatment of chronic HBV infection. In an Asian study, the cumulative incidence of HCC is 3.9% in the LAM arm vs 7.4% in the placebo arm (P = 0.047), yet the clinical benefits on disease progression and HCC occurrence are lost when patients developed resistance (11% vs 5% placebo)[87]. A meta-analysis of 5 studies including 1267 patients treated with LAM compared with 1022 untreated patients demonstrates that HCC incidence is reduced by 78% (2.5% vs 11.7%, RR = 0.22; P < 0.001)[87,88]. Therefore, LAM treatment seems to reduce but not to eliminate the risk of HCC.
Second-generation NRTIs, ETV and tenofovir [tenofovir disoproxil fumarate (TDF) or tenofovir alafenamide), have a potent suppressive effect on the DNA synthesis mechanism during HBV replication, although they have poor effect on the intracellular concentration and activity of cccDNA, which persist for years in the infected liver due to the long half-life and the resistant molecular structure. In an Asian study, ETV treated patients have a 63% reduction in the incidence of HCC at 5 years compared to those not treated (3.7% vs 13.7%; P < 0.001)[89]. A Greek study followed 321 patients with chronic hepatitis B under ETV for 30 mo comparing them with 818 patients on LAM treatment; the cumulative HCC incidence in the ETV group is 4.8% vs 5.6% (P = 0.096)[49]. In another European multicenter cohort study after a median follow-up of 39 mo, HCC developed in 71 (4.3%) out of 1666 patients with chronic hepatitis B treated with ETV or TDF; the cumulative probability of HCC results 1.3% at the first year, 3.4% at the third year and 8.7% at the fifth year after the initiation of treatment[90]. ETV and tenofovir seem to improve liver FIB to prevent cirrhosis complications and to reduce, though not eliminate, the risk of HCC.
Patients with a clinical response to conventional IFN-α therapy schemes show a lower incidence of cirrhosis decompensation and a decrease in HCC occurrence with a better overall survival compared with non-responders. A recent meta-analysis of 14 trials suggests that pegylated-IFN facilitates HBsAg clearance or seroconversion in chronic hepatitis B patients[15,91]. In another study evaluating long-term outcomes of IFN-α therapy in HBeAg seropositive patients by comparing 233 patients undergoing IFN-based treatment with 233 matched controls, the cumulative HCC incidence at the end of 15 years of follow-up is 2.7% vs 12.5% (P = 0.01)[92]. Finally, a meta-analysis on 2742 subjects pooled from 12 studies also shows that IFN-α treatment reduces the risk of HCC occurrence by 34% (RR = 0.66, 95%CI: 0.48-0.89)[93].
HBV vaccination prevents children from becoming HBV carriers and reduces the incidence of HCC in vaccinated populations. In Taiwan, after a vaccination campaign, the average incidence of HCC in children (6 years to 14 years) fell from 0.70 per 100000 children in 1981-1986 to 0.57 in 1986-1990 and further to 0.36 in 1990-1994 (P < 0.01). Moreover, after the universal HBV immunization program in the 1990s, HCC incidence reduced from 0.293-0.117 per 100000 person-years[94]. Similar findings result from South Eastern Asian and Alaskan studies[95-97]. More than 70%-80% of HCC in all developing countries are linked to chronic hepatitis B infection so widespread vaccination is the most important step to prevent more than 500000 HCC cases per year worldwide[98].
Recent studies estimate that between 20 and 72 million people are infected with HDV[99]. Indeed, the precise global prevalence of HDV infection has remained unestablished due to the non-standardized screening programs in different nations and the inaccessibility to testing in most highly endemic areas[100,101]. In Western Europe, the prevalence of the infection has decreased in the last 20 years as a consequence of ameliorated socioeconomic conditions and national vaccination campaigns against HBV[102,103].
Chronic hepatitis D is considered the most severe form of chronic viral hepatitis leading to a rapid progression towards liver cirrhosis and a higher mortality rate compared to other types of viral hepatitis. More than 10% of chronically HDV infected patients develop liver cirrhosis within 5 years from infection and in 30 years, more than 80% of patients suffer from cirrhosis decompensation[104].
Recent cohort studies have found a risk as much as nine times higher in HDV-infected patients compared to HBV monoinfected ones despite the common belief that HDV doesn’t represent a certain major risk factor for HCC development[105,106]. Furthermore, persistent HDV replication has been proven to be a risk factor for liver disease progression to liver cirrhosis and HCC[107]. A study by the European Concerted Action on Viral Hepatitis demonstrates an increase of HCC occurrence by a factor of 3.2 in anti-HDV positive cirrhotic patients compared to negative ones[105]. Other recent studies show an increased risk of HCC for HDV/HBV coinfected patients than in HBV monoinfected ones (adjusted HR = 9.30)[108]. One large study estimates a risk of HCC occurrence significantly higher among patients with chronic HDV infection (RR = 3.9) than among those with HBV monoinfection[109]. On the contrary, an English retrospective study reports that despite the increasing prevalence of HDV infected patients in South London, liver cancer occurrence is only slightly increased between HBV monoinfected and HBV/HDV coinfected patients (OR = 1.34)[110].
In conclusion, recent findings identify HDV infection as a risk factor for HCC even if a direct oncogenic mechanism is unlikely. So, it is uncertain whether HDV adds oncogenic effects beyond those carried by chronic inflammation, FIB and cirrhosis. The genomic signature of HDV-related HCC has been poorly understood but several studies have pointed out some potential oncogenic mechanisms involving: Interference of HDV antigen (HDAg) in the cell cycle through STAT3 and cyclophilin, alteration of protein synthesis, oxidative stress as a result of severe inflammation, aberrant silencing of tumor suppressor genes by DNA methyltransferases and a possible epigenetic control on HBV transcription[5]. These mechanisms are summarized in Table 5 and Figure 1.
| Cell cycle deregulation via signal pathways |
| L-HDAg - Smad 3 activation - TGFβ upregulation - cells growth and dedifferentiation |
| L-HDAg - antagonizes c-Jun inhibitory effect over TGFβ - TGFβ upregulation - cells growth and epithelial-mesenchymal transition |
| L-HDAg - TNF-α stimulation - NF-κB activation - inflammation and proliferation |
| L-HDAg - activates STAT3 downstream protein - JAK/STAT pathway activation - cell growth |
| L-HDAg - stimulates c-Fos activation - cells growth and dedifferentiation |
| L-HDAg - downregulates GSTP1 - tumor oncosuppressor inhibition |
| Oxidative stress |
| L-HDAg - NF-κB and STAT3 activation - ROS production - DNA damage |
| L-HDAg - activates promoters of GRP78 and GRP94 - ROS production - DNA damage |
| L-HDAg - activates TGFβ1 - Nox4 activity - ROS production - DNA damage |
| S-HDAg and L-HDAg - increase in TRAF2 - inflammation and ROS production |
| S-HDAg and L-HDAg - bind to SRE - targeting proinflammatory genes - inflammation and ROS production |
| Epigenetic mechanisms |
| S-HDAg and L-HDAg - increased activity of histone acetyltransferases and CBP - histone H3 acetylation of clusterin promoter - increased clusterin expression - prolonged cell survival |
| S-HDAg - stimulates Histone H1e acetylation - clusterin promoter activation - prolonged cell survival |
| HDV - DNMT1 and 3b increased activity - tumor suppressor inhibition |
| S-HDAg and L-HDAg - hypermethylation of E2F1 promoter - cell cycle dysregulation |
Even if HDV replicative activity has been related to a poor prognosis, it is still uncertain whether levels of HDV DNA increase the risk of HCC development. Some studies find no correlation between viral load and HCC occurrence in HDV infected patients while some others show an increased risk of cirrhosis complications in patients with a higher HDV viraemia[104]. Further investigations are needed to point out the role of HDV replication as a risk factor in liver oncogenesis.
HDAg expression alone does not appear to have oncogenic potential. Moreover, since high levels of antigen and viral RNA can lead to a cell cycle arrest in G1 phase, this mechanism can promote DNA damage and oncogenes mutation. L-HDAg plays a key role in HDV-related oncogenesis. It can stimulate inflammatory and cell growth pathways by enhancing TNF-α production and NF-kB upregulation triggering STAT3 downstream proteins in the JAK/STAT molecular signaling and recruiting small mothers against decapentaplegic (Smad) 3 protein, involved in TGF-β activation[104]. In a similar way, L-HDAg downregulates glutathione S-transferase pi 1 (GSTP1), a tumor suppressor, and antagonizes c-Jun, by neutralizing its inhibitory effect on TGF-β cascade[111]. HBx and L-HDAg can act synergistically on NF-kB and JAK/STAT regulation increasing each other’s effect.
The severe inflammation occurring in HDV infection can by itself produce free radicals through NF-kB and STAT3 pathways. In addition, L-HDAg-induced TGF-β1 can activate NADPH oxidase (Nox) 4, resulting in a further ROS levels increase[111]. Either L-HDAg and S-HDAg bind to ER stress response element and stimulate an increase in TNF receptor-associated factor 2 targeting proinflammatory genes in the generation of oxygen-derived reactive products[112]. Furthermore, HDAg can stimulate the promoters of immunoglobulin protein gastrin releasing peptide (GRP)78 and GRP94 targeting inflammatory genes and upregulating ROS production[111]. Even if the damage carried to the host genome by HDV through oxidative stress is indirect, it represents one of the most important oncogenic effects of the virus. Novel therapies such as bulevirtide can stop this process by inhibiting viral replication and consequently virus-related inflammation and oxidative stress.
Both HBV and HDV-related HCC enhance oncogenes and inhibit tumor suppressor genes by epigenetic regulation mostly through DNMT1 and DNMT3b proteins involved in the maintenance of methylation patterns in the human genome. The molecular mechanism of DNMT1 deregulation in HDV infected patients has not been established yet, even if some studies identify STAT3 regulation over DNMTs protein as the culprit of DNA methylation impairment in HDV-related HCC[111].
Moreover, S and L-HDAg can increase E2F transcription factor 1 promoter leading to cell cycle deregulation (G2/M switch) and Nox4 activation[111]. HDAgs impair the DNA acetylation as well, mainly by the recruitment of histone acetyltransferases and P300/CBP[104]. Histone acetyltransferases increase clusterin expression and prolongs cellular survival through its antiapoptotic effect[43].
The histological examination of liver tissue of chronically HDV-infected patients shows an increase in HDAg specific T CD4+ lymphocytes that rises gradually along the course of the infection. Recent findings suggest that hepatitis D is an immune-mediated disease and chronic immune activation may promote hepatocarcinogenesis[5].
There is increasing interest in the study of non-coding RNAs including small non-coding RNA and long non-coding RNAs (lncRNAs). According to a recent study Y3 lncRNA results significantly downregulated in HDV-related HCC suggesting a possible role in the pathogenesis of HDV-related HCC[114].
Recent long-term studies point out that pegylated (PEG)-IFN treatment is independently associated with a better clinical outcome and a longer survival. A retrospective study shows a reduced rate of liver decompensation in PEG-IFNα treated patients without any difference in terms of HCC development. In addition, complete loss of HDV RNA reached with IFN-α therapy does not seem to reduce the HCC occurrence rate[5,115]. Whether PEG-IFN treatment can reduce the risk of HCC development in HDV infected patients is unknown and more studies are needed to clarify this point. Given the recent approval of bulevirtide, data on its effect on HCC occurrence is still lacking.
Global prevalence of HCV, based on detectability of anti-HCV antibodies, has been estimated to be 1.6% which corresponds to almost 115 million individuals[116]. The prevalence of viremic individuals is estimated at 1% or 71 million[117]. HCV seroprevalence varies across geographical regions and is highest in Central Asia, East Asia, North-Africa and the Middle East where more than 3.5% of the total population is affected[118]. Russia, Egypt, Nigeria, India, Pakistan and China account for more than 50% of the total viremic HCV infection[116,118] whereas Western countries contribute only a small percentage (seroprevalence < 1%)[119]. The number of HCV-related deaths due to liver cirrhosis decompensation or HCC has been increased from almost 900000 deaths in the 1990s to almost 1500000 deaths in the 2010s as the result of the high prevalence and the lack of therapies[120].
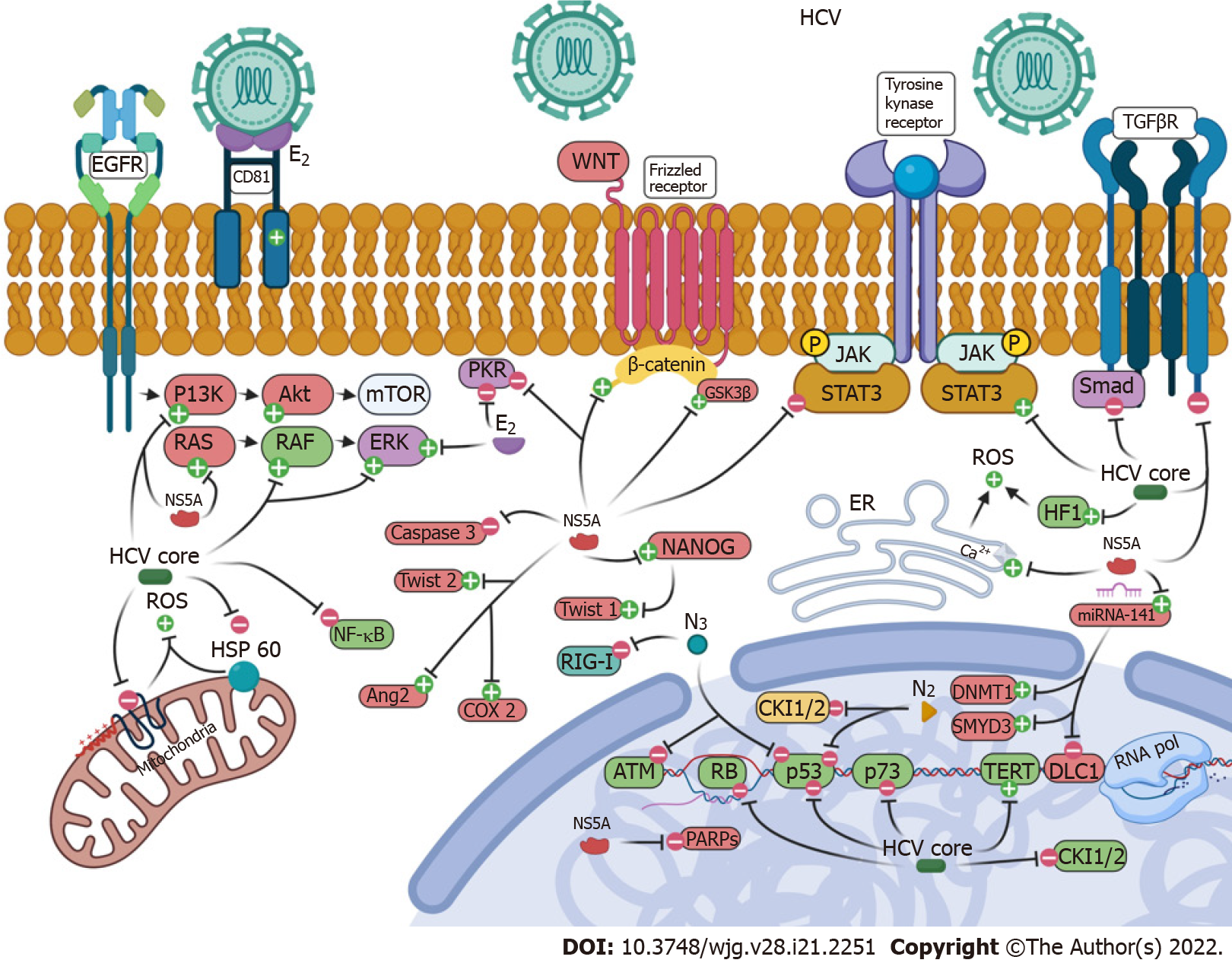
HCV infection has been identified as one of the major risk factors for HCC development worldwide. Prospective studies show a 15-30-fold increased risk of HCC occurrence among chronically HCV infected patients compared with HCV negative ones. HCV genetic material doesn’t integrate into the host genome; therefore, HCV needs continuous replication to maintain chronic infection. Despite this, HCV exerts indirect and direct effects on hepatocarcinogenesis. On one hand, the indirect effect is related to FIB and cirrhosis. The probability of HCC development rises with the stage of FIB and most of HCV-related HCCs occur in patients with advanced FIB or cirrhosis (annual rate up to 8%, average 1%-4%)[121]. On the other hand, viral factors such as HCV core protein, non-structural proteins, structural proteins and HCV genotypes and subtypes have a direct effect on the risk of HCC development through the modulation of hepatocytes gene expression. The multifaceted HCV infection effects on cells molecular pathways and microenvironment are summarized in Table 6 and Figure 2.
| HCV core protein-related pathways |
| Signaling pathways |
| HCV core protein - binds p53, p73 and RB - tumor suppressors inactivation |
| HCV core protein - increased TERT gene activity – oncogenesis |
| HCV core protein - induces expression of cyclin E/CDK2 - G1/S transition |
| HCV core protein - inhibits CKI1 - cell cycle deregulation |
| HCV core protein - induces RAF/MAPK pathway – oncogenesis |
| HCV core protein - inhibits E-cadherin expression and SFRP1 via histone modification - activation of WNT/β-catenin signaling - epithelial mesenchymal transition |
| HCV core protein - interacts with TBR1 - inhibit TGFβ signaling and prevent translocation of Smad - cell spreading, cell growth regulation |
| Oxidative stress and mitochondrial impairment |
| HCV core protein - impairs lipid β-oxidation - reduces mitochondrial electron transport chain - ROS production |
| HCV core protein - impairs mitophagy - mitochondrial damage - ROS production |
| HCV core protein -interacts with HSP60 - ROS production and inhibition of TNFα induced apoptosis |
| Angiogenesis |
| HCV core protein - stimulate an increasing in HIF1α and AP-1 - upregulation of VEGF expression - angiogenesis |
| HCV core protein - activates PI3K/Akt and JAK/STAT - AR activation - angiogenesis |
| HCV core protein - activates COX2, MMP-2 and MMP-9 – angiogenesis |
| Inflammation |
| HCV core protein - suppresses of NF-kB pathways - impaired immune response |
| HCV core protein - upregulates cytokines and deregulates HSCs activity - impaired immune response |
| E2 protein-related pathways |
| E2 protein - interacts with CD81 - impaired host immune system |
| E2 protein - activates MAPK/ERK pathway - promoting cell proliferation |
| E2 protein - inhibits PKR - inhibition of protein synthesis |
| NS2 protein-related pathways |
| NS2 - activates cyclinD/CDK4 - induces expression of cyclin E/CDK2 - G1/S transition |
| NS2 - binds p53 - tumor suppressors inactivation |
| NS3-related pathways |
| NS3 - inhibits p53 - tumor suppressor inactivation |
| NS3 - inhibits ATM - tumor suppressor inactivation |
| NS3 - suppresses of NF-kB pathways - impaired immune response |
| NS3 - blocks TLR3 and RIG-I - impaired immune response |
| NS5A-related pathways: Signaling pathways |
| NS5A - inhibits p53 - tumor suppression inactivation |
| NS5A - interacts with TGFBR1 - inhibit TGFβ signaling and prevent translocation of Smad 3/4 - cell spreading, cell growth regulation |
| NS5A - increases phosphorylation of GSK3β - activates β-catenin - upregulates c-Myc - cell growth |
| NS5A - activates Akt pathway – oncogenesis |
| NS5A - interacts with PI3K p85 subunit - upregulates cell survival cascade |
| NS5A - activates Twist 2 - epithelial mesenchymal transition |
| NS5A - activates RAS - enhance tumor cell invasiveness |
| NS5A - inhibits JAK/STAT pathway - blockage of IFN signaling |
| NS5A - inhibits PKR - inhibition of protein synthesis |
| NS5A - activates TLR4 - amplified NANOG - Twist 1 induction - oncogenesis and epithelial mesenchymal transition |
| NS5A-related pathways: Apoptosis |
| NS5A - inhibits TNFα mediated apoptosis - cell immortalization |
| NS5A - inactivates caspase 3 - inhibition of apoptosis |
| NS5A - inhibits proteolytic cleavage of death substrates (PARPs pathway) - impaired DNA repair and apoptosis |
| NS5A-related pathways: Oxidative stress |
| NS5A - induces of WNT/β-catenin signaling - upregulation of c-Myc - ROS production |
| NS5A - increases calcium release from ER - mitochondrial calcium uptake - ROS production |
| Epigenetic modifications |
| HCV - alters histone mark H3K27ac - TNFα and IL2 pathways - cell growth deregulation and epithelial mesenchymal transition |
| HCV - upregulates DNMT1 and SMYD3 - increased methylation of CDKN2A, GSTP1, APC, SOCS1, RASSF1A - tumor suppressors inhibition |
| HCV - increases miR-141 - inhibition of DLC1 - tumor suppressor inhibition |
| Inflammatory pathways |
| HCV - activates CCL20-CCR6 - endothelial cell invasion and angiogenesis |
| Switch from Th1 to Th2 - increasing in IL4-5-8-10 - loss of death control on cancer cells |
| Switch from Th1 to Th2 - decreasing in IL1-2-12-15 - loss of death control on cancer cells |
| Gut microbiota-related pathways |
| HCV-related dysbiosis - circulating LPS - TLR4 activation - cytokines production - JAK/STAT3 activation - cell proliferation |
HCV core protein presents a broad intracellular distribution in host cells suggesting a role in the modulation of multiple metabolic and replicative processes. Host proto-oncogenes and tumor-suppressors are direct targets of the HCV core protein which interact with tumor-suppressor proteins such as p53, p73 and RB, downregulating their functions. Interaction between p73 and the HCV core protein leads to inhibition of cell growth arrest mechanisms[122].
Moreover, increased TERT gene activity, typical of cells undergoing malignant transformation, is observed in primary human hepatocytes transfected with HCV core protein. TERT expression seems to be one of the earliest neoplastic events in HCC conferring an immortalized phenotype to transformed hepatocytes[123]. The HCV core protein inhibits the expression of the cyclin-dependent kinase inhibitor p21/WAF that has a role in the regulation of the cell cycle and in the induction of apoptosis. In a similar way, HCV core protein activates the rapidly accelerated fibrosarcoma 1/mitogen-activated protein kinase (MAPK) proto-oncogene pathway and nonspecific hepatitis-induced cell proliferation[124].
Furthermore, overexpression of the HCV core protein seems to be important for the regulation of epithelial mesenchymal transition (EMT) driving the activation of HSCs. The induction of EMT is mediated by the inhibition of E-cadherin expression and the induction of secreted frizzled-related protein 1 via DNA methylation and histone modifications. This pathway leads to activation of WNT/β-catenin signaling[123]. Finally, the HCV core protein can suppress NF-κB, impairing downstream immune response.
Indirect HCV core protein mediated damage is carried by oxidative stress through an impairment of lipid β-oxidation and alteration of intracellular lipid metabolism which is associated with a reduction of mitochondrial electron transport chain’s function. This process induces further ROS production and regulates apoptosis through inhibition of the HSP60 leading to a TNF-α-mediated apoptosis. Recently, the HCV core protein has also been proven to impair mitophagy contributing to mitochondrial damage; the resulting oxidative stress has been identified as a key trigger of the hepatocarcinogenetic process[122]. Dysregulation in angiogenetic processes and upregulation of inflammatory pathways represent other targets of indirect damage mediated by the HCV core protein.
HCV-encoded proteins have been directly involved in the oncogenic process through interaction with several signaling pathways mainly in experimental animal models. The HCV E2 glycoprotein interacts with CD81, a cell surface marker of NK cells. This process leads to the alteration of cytokines production and dysregulation of cytotoxic granules release which impairs the host immune system[14]. In addition, E2 protein leads to MAPK/extracellular signal-regulated kinases pathway activation promoting cell proliferation and maintaining cell survival[125]. The NS2 protein can activate cyclin D/cyclin-dependent kinase 4 (CDK4) inducing cyclin E expression and stimulating cell cycle progression from G1 phase to S phase. NS2 protein is involved in HCC carcinogenesis through tumor suppressor inactivation of p53 and consequent transcriptional activation[126]. The NS3 protein is involved in tumor cell transformation, by loss of contact inhibition and growth anchorage, evasion of innate immunity, interaction with mitochondrial signaling protein, tumor suppressors inactivation (p53 and ataxia telangiectasia mutated kinase or ATM) and alteration of immunoregulatory pathways such as TLR3, retinoic acid-inducible gene I (RIG-I) and NF-κB pathways[127].
Similarly, the NS5A protein plays a role in cell cycle dysregulation through signaling pathways, immune escape, impairment of cell apoptosis and ROS production. NS5A is involved in proliferative pathways by the inactivation of p53, blockade of IFN signaling through JAK-STAT cascade and inhibition of host protein synthesis through protein kinase R (PKR)[126]. In addition, NS5A protein, like HCV core protein, can inhibit TGF-β signaling through interaction with T-box brain transcription factor 1 or direct binding to TGF-β receptor 1 and through prevention of translocation of Smad proteins[128]. On the other hand, NS5A interacts with the PI3K p85 regulatory subunit that regulates apoptotic activity of PI3K and upregulates cell survival cascade, activating Akt and Twist family BHLH transcription factor 2 pathways favoring EMT, and interacts with RAS oncogene to enhance tumor cell invasiveness[129]. NS5A stimulates TLR4 activation and its signaling through homeobox protein NANOG and consequent Twist 1 activation leading to hepatocytes dedifferentiation[126].
NS5A affects apoptosis trough downregulatory effect on TNFα-mediated apoptosis. It deactivates caspase-3 and inhibits proteolytic cleavage of death substrates known as polyADP-ribose polymerases, impairing PD cascade[126]. Moreover, HCV seems to enhance ROS formation and aberrant cell-cycle arrest due to DNA damage through dysregulation of ER-mediated calcium uptake and induction of glycogen synthase kinase 3β phosphorylation with consequent beta-catenin-dependent upregulation of c-Myc via NS5A[122]. Non-structural viral proteins, together with HCV core protein, play a key role in HCV mediated oncogenesis. Their multifaceted interaction with basic hepatocytes signaling pathways favors deep dysregulation of cells homeostasis and accumulation of DNA damage until malignant transformation.
A higher vascular density has been reported in nodules of HCV-related HCC in relation to HCC nodules in patients affected by other liver diseases. Studies show upregulation of VEGF expression in patients with HCV-related HCC compared to the control group. HCV core protein can increase cellular VEGF expression through HIF-1α transcription factors and AP-1. Similarly, core protein can induce overexpression and stabilization of HIF-1α and ANG2 which upregulate VEGF expression[130]. Moreover, HCV core protein binds androgen receptor (AR), impairing transcriptional activity by activating several signaling pathways such as PI3K/Akt and JAK/STAT3. Since AR targets the VEGF gene in the liver tissue, HCV core protein upregulates VEGF expression through enhanced activity of the AR pathway[123].
Other mechanisms of HCV core-protein promoted angiogenesis and rely on upregulation of COX-2 and metalloproteinases 2 and 9 (MMP-2 and MMP-9)[126]. CCL20/C-C chemokine receptor type (CCR) 6 axis is directly involved in stimulating angiogenesis in the microenvironment of HCV-related HCC. In vitro experiments pointed out CCL20 as a direct promotor of angiogenesis inducing endothelial cells invasion and sprouting and migration of CCR6-positive leukocytes. A study demonstrates that HCV-induced CCL20 protein expression and secretion in hepatoma cells can be diminished and even abolished by means of antiviral treatment proving CCL20 expression to be dependent on HCV replication[131].
Epigenetic modifications in patients’ liver tissue can derive both from infected hepatocytes and from virus-induced inflammatory or fibrotic responses in the cells’ microenvironment. HCV-induced histone modifications and fibrogenesis are interdependent during the course of liver disease; epigenetic changes orchestrate fibrogenesis through the activation of HSCs. Specific epigenetic modifications have been identified in patients’ liver tissue and humanized mouse’s liver tissue where no necrotic nor inflammatory response are present, therefore, it is probable that part of the changes observed can be a direct consequence of HCV-hepatocyte interaction.
HCV upregulates methyltransferase DNMT1 that hypomethylates tumor suppressor genes such as Cyclin Dependent Kinase Inhibitor 2A, GSTP1, RUNX family transcription factor 3, suppressor of cytokine signaling 1 and RASSF1A[126]. In a similar way, an upregulation of “suppressor of variegation, enhancer of Zeste, trithorax” and myeloid nervy deaf 1 domain-containing protein 3, also named SMYD3, leads to a specific hypermethylation of histone marks H3K4 and H4K5 in tumor suppressor genes, such as RASSF1A[126].
A recent study shows that chronic HCV infection induces changes in histone mark H3K27ac which are associated with HCC risk and seem to persist after HCV cure[132]. H3K27ac is involved in pathways related to TNF-α, inflammatory response, IL-2 and activator of transcription 5 signaling. Furthermore, lower levels of H3K27ac can alter pathways associated with metabolism and coagulative cascade, such as oxidative phosphorylation, impaired fatty acid regulation or adipogenesis. Several alterations in these pathways (e.g., epigenetic alteration in TNF-α signaling, G2M-checkpoint, epithelial-mesenchymal transition, PI3K, Akt and mTOR) persist even after direct-acting antiviral agents (DAAs) therapy[132].
Recently, several studies outlined the role of miRNAs-mediated epigenetic alterations in HCV-related carcinogenetic processes. One study examines the expression profiles of miRNA in more than 50 HCV-related HCCs by quantitative real-time polymerase chain reaction. Five miRNAs are examined: MiR-122, miR-100, and miR-10a seem to be upregulated, while miR-198 and miR-145 are downregulated up to 5-fold in tumors than in normal liver tissue[133]. MiR-122 is highly expressed in the liver of HCV-infected individuals due to its essential role in the stability and replication of HCV RNA[134]. Another study develops an in vitro model of HCV infection in primary human hepatocytes to evaluate the role of miRNAs; miR-141, which targets the tumor-suppressor gene Deleted in Liver Cancer (DLC)-1, is upregulated in HCV genotypes 1a-, 1b-, and 2a-infected cells. The rise in miR-141 levels in HCV-infected cells correlates with an inhibitory effect on DLC-1 protein and a subsequent increase in HCC development risk[122].
HCV genotype 1b is associated with at least a doubled risk of HCC compared to other HCV genotypes[135,136]. HCV genotype 3 is considered a major risk factor for hepatic carcinogenesis. A multicenter Asian cohort study shows a 4.3-fold in the risk of HCC development in patients with chronic HCV genotype 3 infection compared to other genotypes[137]. Another Asian study shows a 5-year occurrence rate of HCC of 34% in genotype 3 and of 17% in non-genotype 3 groups, respectively (P = 0.002); in a multivariate analysis, HCV genotype 3 infection is independently associated with a rise of HCC occurrence rates (HR = 3.54), even after adjustment for confounding factors[138]. A further study finds an approximately doubled risk of all-cause mortality and HCC in patients with HCV genotype 3 infection, related to individuals infected with other genotypes[139]. However, the exact role of HCV genotypes in hepatocarcinogenesis processes and in the worsening of liver disease still needs to be established.
Most of HCV-related HCCs occur in cirrhotic livers following decades of chronic inflammation underscoring the key role of the virus-induced inflammatory response in the hepatic carcinogenetic processes. Generally, the inflammatory response is beneficial to the host, but in HCV patients innate and adaptive immunity result ineffective. This cycle can be primed by HCV proteins but host immune response tends to self-maintenance.
HCV interferes with various defense mechanisms; TLR3 and RIG-I gene downstream pathogen-recognition signaling pathways are blocked by the HCV protease NS3/4A; HCV proteins NS5A and core proteins can block IFN signaling interfering with JAK–STAT pathway; HCV proteins E2 and NS5A inhibit PKR, an antiviral effector that impairs protein synthesis of infected cells; HCV core protein stimulates the release of cytokines that can lead to a direct decrease in plasmacytoid dendritic cells levels among HCV-infected patients[140,141].
The persistence of chronic inflammatory response leads to the release of free radicals such as ROS and nitric oxide (NO)[142]. NO contributes to viral persistence leading to antiapoptotic effects in hepatocytes and may induce viral mutations and promote suppression of Th1 cells[143]. Also, it directly influences liver cell survival by preventing apoptosis through the activation of the NF-κB pathway. ROS induce modifications in structure and function of cancer-related proteins and genes including those fundamental for cell-cycle control, apoptosis, and DNA repair[144]. There is evidence that HCV-mediated inflammation is responsible for the promotion of hepatic carcinogenesis through oxidative DNA damage[145].
In patients affected by HCC, Th1 dominance is progressively lost due to an increase of Th2 cells[146]. Some studies typify the microenvironment of HCC metastatic phenotype as composed by a Th2-like cytokine profile, consisting in higher levels of IL-4, IL-8, IL-10 and IL-5, and lower levels of Th1-like cytokines as IL-1α, IL-1β, IL-2, IL-12p35, IL-12p40, IL-15, TNF-α and IFN-γ[147]. Moreover, the peripheral blood and the tumor tissue of HCC patients show an increased number of Tregs (with a positive direct correlation with HCV RNA levels). IL-10 seems to be highly expressed in HCC patients and its presence correlates with disease progression[148].
In conclusion, inadequate immune response is a necessary factor for hepatic cells transformation but not a sufficient one. It builds a favorable microenvironment in which all factors (etiology, genetic, epigenetic, humoral and cellular inflammation, chronic liver injury) contribute to the development of FIB and liver cells proliferation. These oncogenic factors promote DNA abnormalities that ultimately transform the hepatocytes into malignant cells.
HCV-related cirrhosis is characterized by a disruption of the gut microbiota composition leading to an increased intestinal permeability and bacterial translocation. Compared to healthy individuals, bacterial alpha diversity seems to be lower in patients affected by chronic HCV infection with a reduction in bacteria of the order Clostridiales and an expansion in Streptococcus species. Gut dysbiosis begins to occur in the very early stages of the infection with the transient increase in Bacteroidaceae and Enterobacteriaceae. A decrease in the abundance of Bifidobacteriaceae and Lactobacillaceae with an increase of Bacteroidaceae and Enterobacteriaceae has been reported in the gut microbiota of patients with HCV-related HCC[149,150]. Predicted metagenomics of microbial communities show an upregulation of urease gene, mainly encoded by Streptococcus viridans during chronic hepatic disease course, consistent with a significantly higher faecal pH than in healthy individuals[151]. A small study shows an increase in Streptococcus salivarius (S. salivarius) in oral and gut microbiota of patients with HCV-related HCC suggesting a possible role in oncogenesis. It has been noticed that S. salivarius impairs and deregulates the innate immune response of epithelial cells with a possible contribution in HCC progression[152].
DAAs treatment has proven to modulate the composition of the gut microbiota contributing to a decrease in inflammatory markers and liver stiffness but without effect on the gut barrier and its permeability. The eradication of the infection cannot resolve the intestinal dysfunction caused by liver cirrhosis but can improve the pre-existing dysbiosis[153]. Therefore, alterations of the gut microbiota may be focused as a predisposing factor for liver disease progression but further studies are necessary to establish its possible pathogenetic role in HCC development.
The genetic background of the host has a growing role in hepatic carcinogenesis. A systematic review found 16 genes associated with HCV-related HCC[154]. The polymorphisms identified in patients with HCV-related HCC involve genes encoding for: TGF-β1, TNF-α, mitochondrial aldehyde dehydrogenase enzyme 2, catalase, EGF, glutathione S-transferase mu 1 protein, glutathione S-transferase theta 1, HLA-Bw4, HLA-B1814 and HLA-DR11, HSPA1B, leptin receptor, IL-1b, a regulator of the p53 tumor suppressor named mouse double minute 2 homolog, UDP glucuronosyltransferase 1 family-polypeptide A7, manganese-dependent superoxide dismutase mitochondrial protein, IFN lambda 3 and lambda 4 cytokine, patatin-like phospholipase domain–containing protein 3 (PNPLA3) and MHC class I polypeptide-related sequence A (MICA/HCP5)[154-156]. Nowadays, the genetic risk score, designed using the combination of four high risk variants in the PNPLA3, TM6SF2, MBOAT7, and Glucokinase Regulator GCKR genes, estimates the inherited predisposition to accumulate liver fat (Genetic Risk Score) and is reported as an independent risk factor for de novo occurrence of HCC after DAAs treatment[157].
As already discussed, HCC surveillance in HCV-infected patients must include patients affected by cirrhosis of all causes and patients with chronic HCV infection with advanced liver FIB (Table 7). The AASLD and EASL practice guidelines recommend HCC screening and surveillance in patients with cirrhosis or with stage 3 FIB[73,75,158]. JSH still differentiates ‘‘super-high-risk patients’’ (hepatitis C cirrhosis) in whom surveillance is recommended every 3-4 mo and ‘‘high-risk patients’’, in whom surveillance is recommended every 6 mo[124].
| HCC surveillance in HCV infected patients | ||
| Western medical societies | ||
| EASL, 2018 | High-risk patients: HCV-related cirrhosis. Chronic hepatitis C and stage | Screening with US examination with or without AFP every 6 mo for high-risk patients (incidence > 1.5%/yr) |
| AASLD, 2018 | High-risk patients: HCV-related cirrhosis. Chronic hepatitis C and stage 3 fibrosis | Screening with US examination with or without AFP every 6 mo for high-risk group (incidence > 1.5%/yr) |
| Eastern medical societies | ||
| JSH, 2017-2021 | Extremely-high-risk patients: All patients with HCV-related cirrhosis. High-risk patients: Patients with chronic hepatitis C | Screening with US and tumor marker measurements (AFP, PIVKA-II and AFP-L3) every 3-4 mo in the super-high-risk population. A 6-12 mo dynamic CT scan, dynamic MRI should be performed or Sonazoid CEUS. Screening every 6 mo in high-risk populations |
| APASL, 2017 | High-risk patients: All patients with HCV-related cirrhosis. SVR patients with chronic hepatitis C with advanced liver fibrosis, independently of the histologic response to therapy. SVR patients with chronic hepatitis C with any histologic stage of HCV with comorbidities, such as alcohol abuse and DM | Surveillance by US and AFP should be performed every 6 mo and preferably every 3-4 mo in cirrhotic patients and those at high risk of HCC |
| KLCSG, 2014-2018 | High-risk patients: All patients with HCV-related cirrhosis. Patients with chronic hepatitis C and advanced fibrosis | Screening with US examination with or without AFP every 6 mo. If liver US cannot be performed properly, liver dynamic CT or dynamic contrast-enhanced MRI can be performed as an alternative |
The utmost importance of viral clearance in the reduction of HCC occurrence among chronically HCV-infected patients has been largely established. Since the first studies on IFN-based therapy, HCC incidence shows a significant difference between patients with sustained virological response (SVR) and non-SVR patients (5.1% and 21.8%)[159]. A large meta-analysis including 31528 HCV-infected patients shows that SVR is a key factor in reducing the incidence of HCC (1.5% in SVR patients vs 6.2% in non-SVR patients at 3-8 years of follow up)[139,160].
IFN-α was approved to treat HCV infection in the 1980s but its efficacy was limited (SVR rates 54%-56%). A meta-analysis of 8 long-term studies on 2649 patients with chronic hepatitis C demonstrates that HCC occurrence is significantly reduced in patients with SVR after IFN-based therapy compared to non-responder (4.2% vs 17.8%, HR = 0.23)[161]. The majority of the studies report that older ages, male gender, advanced liver FIB, fatty liver disease and a high post-treatment serum AFP level are the main risk factors for HCC development even after SVR[162].
The perspective of HCV therapy has changed over the years after the introduction of DAAs therapy, which has increased the SVR rate up to 90%, with excellent tolerance compared to IFN-based regimens. Even if the therapies demonstrated high efficacy, two retrospective studies, published in 2016 and conducted respectively in Spain and Italy, supposed the existence of a higher risk of HCC occurrence and recurrence following DAAs treatment course, raising the debate about the safety profile of DAAs and, in particular, the relation between SVR with and HCC occurrence and recurrence[163]. The Spanish study registered a high recurrence rate of HCC (27.6%) among 58 patients with previous history of HCC who received DAAs[164]. In the Italian study, HCC was detected in 7.6% of patients following DAAs treatment, 28.81% of patients with and 3.16% of patients without previous HCC[165]. Furthermore, an unpredictable increase in the amount of advanced stage HCC was noticed by the same authors.
Later debates arose owing some possible biases in these studies (the small size of the cohorts, lack of untreated controls and short follow-up periods). Subsequently, further data from large real-world retrospective cohort studies with extended follow-up periods after DAAs treatment have been released. On the heels of the studies listed so far, in 2017 another meta-analysis conducted by Waziry et al[166] reported lack of a statistically significant increase in HCC recurrence or occurrences in either patients having undergone DAAs or IFN treatment after achieving SVR. Subsequently in 2017, a systematic review from Ioannou et al[167] including 62354 patients treated with DAAs, IFN or a combination of both showed no significant difference in HCC occurrence or recurrence between the three treatment regimens. In fact, SVR was associated with a 71% decrease in HCC occurrence risk with no particular differences between DAAs and IFNs. Nahon et al[168] enrolled 1270 HCV-infected patients with compensated cirrhosis in France, demonstrating that there wasn’t any statistically significant increase in the risk of HCC occurrence following DAAs treatment (HR = 0.89), after adjustment for age, diabetes and reduced liver function. In 2018, another American study from Singer et al[169] showed that DAAs therapy was associated with a lower risk of HCC as compared to untreated patients (HR = 0.84) and to IFN based treatment (HR = 0.69). During the same year, an Italian study found that SVR after DAAs treatment led to a lower incidence rate of HCC in patients with HCV-related cirrhosis over a follow up of 14 mo[170]. At the end of 2018, another Italian review from Guarino et al[171] outlined that the alarmist data on HCC recurrence after DAAs treatment and more aggressive pattern of disease have not been confirmed by subsequent studies.
Recently, a French prospective cohort study on 9895 HCV-infected patients with a long term follow-up (mean 33.4 mo) reported that after adjusting for several variables (including non-modifiable risk factor as age, sex, geographical origin, HCV genotype and modifiable ones as FIB score, alcohol consumption, diabetes, arterial hypertension, and model for end-stage liver disease score in patients with cirrhosis), DAAs exposure decreased all-cause mortality (adjusted HR = 0.48) and the risk of HCC development (adjusted HR = 0.66)[172]. Another large American retrospective cohort study concluded that DAAs treatment and SVR resulted in a significant benefit in overall mortality[173].
Watanabe et al[174] recognized FIB-4 index ≥ 4.0 and albumin ≤ 3.8 g/dL before DAAs therapy and a FIB-4 index ≥ 4.0 and AFP ≥ 6.0 after the end of DAAs therapy as independent predictors for HCC occurrence. Another study confirmed a linkage between the risk of HCC and persistently high FIB-4/[aminotransferase/platelet ratio index (APRI)], either with or without cirrhosis. HCC development risk shows a significative reduction in cirrhotic patients who showed a decrease of FIB-4/APRI scores after DAAs therapy[175].
In 2020, an Egyptian study designed with three large independent cohorts, enrolled 4400 patients with chronic hepatitis C and advanced FIB, and identified predictors of HCC development after DAAs treatment developing and validating a simple risk score (GES risk score). GES identifies three groups of patients with a different risk of HCC occurrence along the follow-up by means of simple and readily available predictors: Age, male gender, low albumin, high AFP levels and presence of cirrhosis at baseline. The cumulative incidence of HCC for GES low-risk group at 2 years was 1.2% in the derivation cohort and 0.22% in validation cohorts, while GES high-risk group showed a 6 to 30 folds higher risk, with a cumulative incidence of 7.1% and 6.1% in the derivation and validation cohorts, respectively. The study enrolled mostly genotype 4-infected patients thus further studies are needed to assess the utility of this score in clinical practice[176].
These data underline that the rise in HCC incidence suggested by the first studies in patients with HCV-related cirrhosis treated with DAAs who achieve SVR compared to those treated with IFN-based therapy may be due to confounders as patients features (age, diabetes, reduced liver function) and/or lower screening frequency. Therefore, DAAs treatment should not be withheld for the potential risk of HCC occurrence or recurrence. According to long-term post-SVR observational studies, HCC development risk remains high in patients with cirrhosis who eliminate HCV, although it is significantly reduced compared to untreated patients or patients not achieving SVR (10-year cumulative incidence rate 21.8% vs 5.1%)[139].
Several studies assessed the difference in clinical manifestation in HCC patients between different etiologies. Different HCC surveillance strategies need to be set according to the viral etiologies in relation to HCC age of presentation and clinical features. Two Italian studies noticed that HBV-infected patients are younger at HCC diagnosis, with a higher staging, increased probability of vascular invasion and a worse prognosis[177]. An Asian study showed a peak incidence of HBV-related HCC in the 50-59 years old group whereas the peak annual incidence in HCV patients is over 70 years. In an HBV high endemic area, the outcome analysis of the study showed a reduced survival in HBV-related HCC compared to HCV-related HCC (1.34 years vs 2.17 years, adjusted for confounders). There was also a slight increase in frequency of large tumors (> 5 cm) and portal vein invasion at diagnosis in HBV-related HCC group, while multiple tumors occurred more frequently in HCV patients[178]. A cross sectional observational study from Pakistan, an HCV high endemic country, confirmed that HBV-related HCC patients were younger at presentation, but HCV-related HCC was larger, with higher AFP serum levels and more frequent vascular invasion at diagnosis[179]. Similar results were obtained in a large cohort including Asian and European patients[180-183]. These differences can be explained by the high prevalence of perinatal infections in high endemic areas and to the specific oncogenic potential of HBV. However, there was no difference in post-operative or post-treatment outcomes in the studies listed above between HBV and HCV-related HCC groups.
Chronic HBV- and HCV-related hepatitis are still pandemic, with an increased spread in low and middle-income continents, such as Asia and Africa. HBV- and HCV-infected patients have a higher oncogenic risk compared to other liver disease aetiology, mostly because of the interactions between viral proteome and transcriptome and host cells molecular pathways involving inflammation and cells replication. New data about gut-liver axis and systemic inflammation are pointing out their key role as a cofactor in hepatocarcinogenesis. Therefore, viral hepatitis B and C still represent a major public health problem worldwide although health organizations have made substantial efforts to contain their spread in recent years through the global HBV vaccination campaign and the widespread accessibility of DAAs for HCV. The broad availability of generic antivirals would allow low- and middle-income countries to strengthen their contribution to the HCV eradication program. In the meantime, one of the most important challenges is to adapt the timing of HCC surveillance in these patients with the aim of ensuring early diagnosis and rapid management to improve prognosis. In the era of coronavirus disease 2019, well-organized and accessible HCC surveillance becomes even more important due to the limited healthcare resources allocated to secondary prevention and the reluctance of patients to access healthcare facilities. In the future, new biomarkers and risk stratification systems may help clinicians to identify patients for closer follow-up leading to a more personalized approach to HCC surveillance.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Italy
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: El-Shabrawi MH, Egypt; Liu LJ, China; Tanwandee T, Thailand; Wang X, China; Zhang J, China S-Editor: Wang JJ L-Editor: Filipodia P-Editor: Wang JJ
| 1. | Llovet JM, Kelley RK, Villanueva A, Singal AG, Pikarsky E, Roayaie S, Lencioni R, Koike K, Zucman-Rossi J, Finn RS. Hepatocellular carcinoma. Nat Rev Dis Primers. 2021;7:6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4432] [Cited by in RCA: 3843] [Article Influence: 960.8] [Reference Citation Analysis (3)] |
| 2. | El-Serag HB. Epidemiology of viral hepatitis and hepatocellular carcinoma. Gastroenterology. 2012;142:1264-1273.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2183] [Cited by in RCA: 2504] [Article Influence: 192.6] [Reference Citation Analysis (2)] |
| 3. | Anthony PP. Hepatocellular carcinoma: an overview. Histopathology. 2001;39:109-118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 88] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 4. | Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359-E386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20108] [Cited by in RCA: 20499] [Article Influence: 2049.9] [Reference Citation Analysis (20)] |
| 5. | Romeo R, Petruzziello A, Pecheur EI, Facchetti F, Perbellini R, Galmozzi E, Khan NU, Di Capua L, Sabatino R, Botti G, Loquercio G. Hepatitis delta virus and hepatocellular carcinoma: an update. Epidemiol Infect. 2018;146:1612-1618. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 35] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 6. | Gomaa AI, Khan SA, Toledano MB, Waked I, Taylor-Robinson SD. Hepatocellular carcinoma: epidemiology, risk factors and pathogenesis. World J Gastroenterol. 2008;14:4300-4308. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 456] [Cited by in RCA: 499] [Article Influence: 29.4] [Reference Citation Analysis (1)] |
| 7. | Trépo C, Chan HL, Lok A. Hepatitis B virus infection. Lancet. 2014;384:2053-2063. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1004] [Cited by in RCA: 1156] [Article Influence: 105.1] [Reference Citation Analysis (0)] |
| 8. | Chang MS, Nguyen MH. Epidemiology of hepatitis B and the role of vaccination. Best Pract Res Clin Gastroenterol. 2017;31:239-247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 47] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 9. | Blachier M, Leleu H, Peck-Radosavljevic M, Valla DC, Roudot-Thoraval F. The burden of liver disease in Europe: a review of available epidemiological data. J Hepatol. 2013;58:593-608. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 879] [Cited by in RCA: 908] [Article Influence: 75.7] [Reference Citation Analysis (0)] |
| 10. | Lozano R, Naghavi M, Foreman K, Lim S, Shibuya K, Aboyans V, Abraham J, Adair T, Aggarwal R, Ahn SY, Alvarado M, Anderson HR, Anderson LM, Andrews KG, Atkinson C, Baddour LM, Barker-Collo S, Bartels DH, Bell ML, Benjamin EJ, Bennett D, Bhalla K, Bikbov B, Bin Abdulhak A, Birbeck G, Blyth F, Bolliger I, Boufous S, Bucello C, Burch M, Burney P, Carapetis J, Chen H, Chou D, Chugh SS, Coffeng LE, Colan SD, Colquhoun S, Colson KE, Condon J, Connor MD, Cooper LT, Corriere M, Cortinovis M, de Vaccaro KC, Couser W, Cowie BC, Criqui MH, Cross M, Dabhadkar KC, Dahodwala N, De Leo D, Degenhardt L, Delossantos A, Denenberg J, Des Jarlais DC, Dharmaratne SD, Dorsey ER, Driscoll T, Duber H, Ebel B, Erwin PJ, Espindola P, Ezzati M, Feigin V, Flaxman AD, Forouzanfar MH, Fowkes FG, Franklin R, Fransen M, Freeman MK, Gabriel SE, Gakidou E, Gaspari F, Gillum RF, Gonzalez-Medina D, Halasa YA, Haring D, Harrison JE, Havmoeller R, Hay RJ, Hoen B, Hotez PJ, Hoy D, Jacobsen KH, James SL, Jasrasaria R, Jayaraman S, Johns N, Karthikeyan G, Kassebaum N, Keren A, Khoo JP, Knowlton LM, Kobusingye O, Koranteng A, Krishnamurthi R, Lipnick M, Lipshultz SE, Ohno SL, Mabweijano J, MacIntyre MF, Mallinger L, March L, Marks GB, Marks R, Matsumori A, Matzopoulos R, Mayosi BM, McAnulty JH, McDermott MM, McGrath J, Mensah GA, Merriman TR, Michaud C, Miller M, Miller TR, Mock C, Mocumbi AO, Mokdad AA, Moran A, Mulholland K, Nair MN, Naldi L, Narayan KM, Nasseri K, Norman P, O'Donnell M, Omer SB, Ortblad K, Osborne R, Ozgediz D, Pahari B, Pandian JD, Rivero AP, Padilla RP, Perez-Ruiz F, Perico N, Phillips D, Pierce K, Pope CA 3rd, Porrini E, Pourmalek F, Raju M, Ranganathan D, Rehm JT, Rein DB, Remuzzi G, Rivara FP, Roberts T, De León FR, Rosenfeld LC, Rushton L, Sacco RL, Salomon JA, Sampson U, Sanman E, Schwebel DC, Segui-Gomez M, Shepard DS, Singh D, Singleton J, Sliwa K, Smith E, Steer A, Taylor JA, Thomas B, Tleyjeh IM, Towbin JA, Truelsen T, Undurraga EA, Venketasubramanian N, Vijayakumar L, Vos T, Wagner GR, Wang M, Wang W, Watt K, Weinstock MA, Weintraub R, Wilkinson JD, Woolf AD, Wulf S, Yeh PH, Yip P, Zabetian A, Zheng ZJ, Lopez AD, Murray CJ, AlMazroa MA, Memish ZA. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2095-2128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9500] [Cited by in RCA: 9569] [Article Influence: 736.1] [Reference Citation Analysis (0)] |
| 11. | Fattovich G, Bortolotti F, Donato F. Natural history of chronic hepatitis B: special emphasis on disease progression and prognostic factors. J Hepatol. 2008;48:335-352. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 909] [Cited by in RCA: 966] [Article Influence: 56.8] [Reference Citation Analysis (0)] |
| 12. | Zamor PJ, deLemos AS, Russo MW. Viral hepatitis and hepatocellular carcinoma: etiology and management. J Gastrointest Oncol. 2017;8:229-242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 109] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 13. | Tarocchi M, Polvani S, Marroncini G, Galli A. Molecular mechanism of hepatitis B virus-induced hepatocarcinogenesis. World J Gastroenterol. 2014;20:11630-11640. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 132] [Cited by in RCA: 142] [Article Influence: 12.9] [Reference Citation Analysis (2)] |
| 14. | Lin CL, Kao JH. Hepatitis B virus genotypes and variants. Cold Spring Harb Perspect Med. 2015;5:a021436. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 180] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 15. | Chen CJ, Iloeje UH, Yang HI. Long-term outcomes in hepatitis B: the REVEAL-HBV study. Clin Liver Dis. 2007;11:797-816, viii. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 153] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 16. | Chen JD, Yang HI, Iloeje UH, You SL, Lu SN, Wang LY, Su J, Sun CA, Liaw YF, Chen CJ; Risk Evaluation of Viral Load Elevation and Associated Liver Disease/Cancer in HBV (REVEAL-HBV) Study Group. Carriers of inactive hepatitis B virus are still at risk for hepatocellular carcinoma and liver-related death. Gastroenterology. 2010;138:1747-1754. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 236] [Cited by in RCA: 254] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 17. | Nassal M. HBV cccDNA: viral persistence reservoir and key obstacle for a cure of chronic hepatitis B. Gut. 2015;64:1972-1984. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 548] [Cited by in RCA: 691] [Article Influence: 69.1] [Reference Citation Analysis (0)] |
| 18. | Galli A, Svegliati-Baroni G, Ceni E, Milani S, Ridolfi F, Salzano R, Tarocchi M, Grappone C, Pellegrini G, Benedetti A, Surrenti C, Casini A. Oxidative stress stimulates proliferation and invasiveness of hepatic stellate cells via a MMP2-mediated mechanism. Hepatology. 2005;41:1074-1084. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 175] [Cited by in RCA: 181] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 19. | Wang Y, Jiang L, Ji X, Yang B, Zhang Y, Fu XD. Hepatitis B viral RNA directly mediates down-regulation of the tumor suppressor microRNA miR-15a/miR-16-1 in hepatocytes. J Biol Chem. 2013;288:18484-18493. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 88] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 20. | Lau CC, Sun T, Ching AK, He M, Li JW, Wong AM, Co NN, Chan AW, Li PS, Lung RW, Tong JH, Lai PB, Chan HL, To KF, Chan TF, Wong N. Viral-human chimeric transcript predisposes risk to liver cancer development and progression. Cancer Cell. 2014;25:335-349. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 210] [Cited by in RCA: 227] [Article Influence: 20.6] [Reference Citation Analysis (0)] |
| 21. | Guerrieri F, Belloni L, Pediconi N, Levrero M. Molecular mechanisms of HBV-associated hepatocarcinogenesis. Semin Liver Dis. 2013;33:147-156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 87] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 22. | Ringelhan M, O'Connor T, Protzer U, Heikenwalder M. The direct and indirect roles of HBV in liver cancer: prospective markers for HCC screening and potential therapeutic targets. J Pathol. 2015;235:355-367. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 101] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 23. | Mason WS, Gill US, Litwin S, Zhou Y, Peri S, Pop O, Hong ML, Naik S, Quaglia A, Bertoletti A, Kennedy PT. HBV DNA Integration and Clonal Hepatocyte Expansion in Chronic Hepatitis B Patients Considered Immune Tolerant. Gastroenterology. 2016;151:986-998.e4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 262] [Cited by in RCA: 317] [Article Influence: 35.2] [Reference Citation Analysis (0)] |
| 24. | Li R, Hao Y, Wang Q, Meng Y, Wu K, Liu C, Xu L, Liu Z, Zhao L. ECHS1, an interacting protein of LASP1, induces sphingolipid-metabolism imbalance to promote colorectal cancer progression by regulating ceramide glycosylation. Cell Death Dis. 2021;12:911. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 25. | Yang HI, Lu SN, Liaw YF, You SL, Sun CA, Wang LY, Hsiao CK, Chen PJ, Chen DS, Chen CJ; Taiwan Community-Based Cancer Screening Project Group. Hepatitis B e antigen and the risk of hepatocellular carcinoma. N Engl J Med. 2002;347:168-174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 924] [Cited by in RCA: 914] [Article Influence: 39.7] [Reference Citation Analysis (0)] |
| 26. | Samal J, Kandpal M, Vivekanandan P. HBeAg-induced miR-106b promotes cell growth by targeting the retinoblastoma gene. Sci Rep. 2017;7:14371. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 25] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 27. | Pollicino T, Cacciola I, Saffioti F, Raimondo G. Hepatitis B virus PreS/S gene variants: pathobiology and clinical implications. J Hepatol. 2014;61:408-417. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 175] [Cited by in RCA: 211] [Article Influence: 19.2] [Reference Citation Analysis (0)] |
| 28. | Mani SKK, Andrisani O. Hepatitis B Virus-Associated Hepatocellular Carcinoma and Hepatic Cancer Stem Cells. Genes (Basel). 2018;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 33] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 29. | Wang LH, Huang W, Lai MD, Su IJ. Aberrant cyclin A expression and centrosome overduplication induced by hepatitis B virus pre-S2 mutants and its implication in hepatocarcinogenesis. Carcinogenesis. 2012;33:466-472. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 61] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 30. | Hsieh YH, Su IJ, Yen CJ, Tsai TF, Tsai HW, Tsai HN, Huang YJ, Chen YY, Ai YL, Kao LY, Hsieh WC, Wu HC, Huang W. Histone deacetylase inhibitor suberoylanilide hydroxamic acid suppresses the pro-oncogenic effects induced by hepatitis B virus pre-S2 mutant oncoprotein and represents a potential chemopreventive agent in high-risk chronic HBV patients. Carcinogenesis. 2013;34:475-485. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 31] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 31. | Tropberger P, Mercier A, Robinson M, Zhong W, Ganem DE, Holdorf M. Mapping of histone modifications in episomal HBV cccDNA uncovers an unusual chromatin organization amenable to epigenetic manipulation. Proc Natl Acad Sci U S A. 2015;112:E5715-E5724. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 187] [Article Influence: 18.7] [Reference Citation Analysis (0)] |
| 32. | Liu S, Koh SS, Lee CG. Hepatitis B Virus X Protein and Hepatocarcinogenesis. Int J Mol Sci. 2016;17. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 51] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 33. | Knoll S, Fürst K, Thomas S, Villanueva Baselga S, Stoll A, Schaefer S, Pützer BM. Dissection of cell context-dependent interactions between HBx and p53 family members in regulation of apoptosis: a role for HBV-induced HCC. Cell Cycle. 2011;10:3554-3565. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 42] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 34. | Rakotomalala L, Studach L, Wang WH, Gregori G, Hullinger RL, Andrisani O. Hepatitis B virus X protein increases the Cdt1-to-geminin ratio inducing DNA re-replication and polyploidy. J Biol Chem. 2008;283:28729-28740. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 47] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 35. | Belloni L, Pollicino T, De Nicola F, Guerrieri F, Raffa G, Fanciulli M, Raimondo G, Levrero M. Nuclear HBx binds the HBV minichromosome and modifies the epigenetic regulation of cccDNA function. Proc Natl Acad Sci U S A. 2009;106:19975-19979. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 324] [Cited by in RCA: 399] [Article Influence: 24.9] [Reference Citation Analysis (0)] |
| 36. | Forgues M, Difilippantonio MJ, Linke SP, Ried T, Nagashima K, Feden J, Valerie K, Fukasawa K, Wang XW. Involvement of Crm1 in hepatitis B virus X protein-induced aberrant centriole replication and abnormal mitotic spindles. Mol Cell Biol. 2003;23:5282-5292. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 110] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 37. | Decorsière A, Mueller H, van Breugel PC, Abdul F, Gerossier L, Beran RK, Livingston CM, Niu C, Fletcher SP, Hantz O, Strubin M. Hepatitis B virus X protein identifies the Smc5/6 complex as a host restriction factor. Nature. 2016;531:386-389. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 309] [Cited by in RCA: 414] [Article Influence: 46.0] [Reference Citation Analysis (0)] |
| 38. | Menolfi D, Delamarre A, Lengronne A, Pasero P, Branzei D. Essential Roles of the Smc5/6 Complex in Replication through Natural Pausing Sites and Endogenous DNA Damage Tolerance. Mol Cell. 2015;60:835-846. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 96] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 39. | Hoter A, Rizk S, Naim HY. Heat Shock Protein 60 in Hepatocellular Carcinoma: Insights and Perspectives. Front Mol Biosci. 2020;7:60. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 22] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 40. | Zhang Q, Song G, Yao L, Liu Y, Liu M, Li S, Tang H. miR-3928v is induced by HBx via NF-κB/EGR1 and contributes to hepatocellular carcinoma malignancy by down-regulating VDAC3. J Exp Clin Cancer Res. 2018;37:14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 30] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 41. | Lee JO, Kwun HJ, Jung JK, Choi KH, Min DS, Jang KL. Hepatitis B virus X protein represses E-cadherin expression via activation of DNA methyltransferase 1. Oncogene. 2005;24:6617-6625. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 152] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 42. | Qiu X, Zhang L, Lu S, Song Y, Lao Y, Hu J, Fan H. Upregulation of DNMT1 mediated by HBx suppresses RASSF1A expression independent of DNA methylation. Oncol Rep. 2014;31:202-208. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 30] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 43. | Freese K, Seitz T, Dietrich P, Lee SML, Thasler WE, Bosserhoff A, Hellerbrand C. Histone Deacetylase Expressions in Hepatocellular Carcinoma and Functional Effects of Histone Deacetylase Inhibitors on Liver Cancer Cells In Vitro. Cancers (Basel). 2019;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 77] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 44. | Martinez-Quetglas I, Pinyol R, Dauch D, Torrecilla S, Tovar V, Moeini A, Alsinet C, Portela A, Rodriguez-Carunchio L, Solé M, Lujambio A, Villanueva A, Thung S, Esteller M, Zender L, Llovet JM. IGF2 Is Up-regulated by Epigenetic Mechanisms in Hepatocellular Carcinomas and Is an Actionable Oncogene Product in Experimental Models. Gastroenterology. 2016;151:1192-1205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 103] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 45. | Calvisi DF, Ladu S, Gorden A, Farina M, Lee JS, Conner EA, Schroeder I, Factor VM, Thorgeirsson SS. Mechanistic and prognostic significance of aberrant methylation in the molecular pathogenesis of human hepatocellular carcinoma. J Clin Invest. 2007;117:2713-2722. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 293] [Cited by in RCA: 320] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 46. | Sartorius K, Makarova J, Sartorius B, An P, Winkler C, Chuturgoon A, Kramvis A. The Regulatory Role of MicroRNA in Hepatitis-B Virus-Associated Hepatocellular Carcinoma (HBV-HCC) Pathogenesis. Cells. 2019;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 63] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 47. | Shi Y, Wu YH, Wu W, Zhang WJ, Yang J, Chen Z. Association between occult hepatitis B infection and the risk of hepatocellular carcinoma: a meta-analysis. Liver Int. 2012;32:231-240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 110] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 48. | Wong DK, Huang FY, Lai CL, Poon RT, Seto WK, Fung J, Hung IF, Yuen MF. Occult hepatitis B infection and HBV replicative activity in patients with cryptogenic cause of hepatocellular carcinoma. Hepatology. 2011;54:829-836. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 113] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 49. | Papatheodoridis GV, Manolakopoulos S, Touloumi G, Nikolopoulou G, Raptopoulou-Gigi M, Gogos C, Vafiadis-Zouboulis I, Karamanolis D, Chouta A, Ilias A, Drakoulis C, Mimidis K, Ketikoglou I, Manesis E, Mela M, Hatzis G, Dalekos GN; HepNet. Greece Study Group. Hepatocellular carcinoma risk in HBeAg-negative chronic hepatitis B patients with or without cirrhosis treated with entecavir: HepNet.Greece cohort. J Viral Hepat. 2015;22:120-127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 38] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 50. | Yang HI, Yeh SH, Chen PJ, Iloeje UH, Jen CL, Su J, Wang LY, Lu SN, You SL, Chen DS, Liaw YF, Chen CJ; REVEAL-HBV Study Group. Associations between hepatitis B virus genotype and mutants and the risk of hepatocellular carcinoma. J Natl Cancer Inst. 2008;100:1134-1143. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 447] [Cited by in RCA: 483] [Article Influence: 28.4] [Reference Citation Analysis (0)] |
| 51. | Tseng TC, Liu CJ, Yang HC, Su TH, Wang CC, Chen CL, Kuo SF, Liu CH, Chen PJ, Chen DS, Kao JH. High levels of hepatitis B surface antigen increase risk of hepatocellular carcinoma in patients with low HBV load. Gastroenterology. 2012;142:1140-1149.e3; quiz e13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 445] [Cited by in RCA: 436] [Article Influence: 33.5] [Reference Citation Analysis (0)] |
| 52. | Chen BF, Chen PJ, Jow GM, Sablon E, Liu CJ, Chen DS, Kao JH. High prevalence of mixed genotype infections in hepatitis B virus infected intravenous drug users. J Med Virol. 2004;74:536-542. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 34] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 53. | Ni YH, Chang MH, Wang KJ, Hsu HY, Chen HL, Kao JH, Yeh SH, Jeng YM, Tsai KS, Chen DS. Clinical relevance of hepatitis B virus genotype in children with chronic infection and hepatocellular carcinoma. Gastroenterology. 2004;127:1733-1738. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 158] [Cited by in RCA: 162] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 54. | Livingston SE, Simonetti JP, McMahon BJ, Bulkow LR, Hurlburt KJ, Homan CE, Snowball MM, Cagle HH, Williams JL, Chulanov VP. Hepatitis B virus genotypes in Alaska Native people with hepatocellular carcinoma: preponderance of genotype F. J Infect Dis. 2007;195:5-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 186] [Cited by in RCA: 193] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 55. | Orito E, Sugauchi F, Tanaka Y, Ichida T, Sata M, Tanaka E, Okanoue T, Sakugawa H, Watanabe H, Miyakawa H, Nishiguchi S, Kumada H, Ueda R, Mizokami M. Differences of hepatocellular carcinoma patients with hepatitis B virus genotypes of Ba, Bj or C in Japan. Intervirology. 2005;48:239-245. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 26] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 56. | Song C, Lv J, Liu Y, Chen JG, Ge Z, Zhu J, Dai J, Du LB, Yu C, Guo Y, Bian Z, Yang L, Chen Y, Chen Z, Liu J, Jiang J, Zhu L, Zhai X, Jiang Y, Ma H, Jin G, Shen H, Li L, Hu Z; China Kadoorie Biobank Collaborative Group. Associations Between Hepatitis B Virus Infection and Risk of All Cancer Types. JAMA Netw Open. 2019;2:e195718. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 78] [Cited by in RCA: 127] [Article Influence: 21.2] [Reference Citation Analysis (0)] |
| 57. | Yuen MF, Wong DK, Fung J, Ip P, But D, Hung I, Lau K, Yuen JC, Lai CL. HBsAg Seroclearance in chronic hepatitis B in Asian patients: replicative level and risk of hepatocellular carcinoma. Gastroenterology. 2008;135:1192-1199. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 306] [Cited by in RCA: 321] [Article Influence: 18.9] [Reference Citation Analysis (0)] |
| 58. | Fan Y, Mao R, Yang J. NF-κB and STAT3 signaling pathways collaboratively link inflammation to cancer. Protein Cell. 2013;4:176-185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 338] [Cited by in RCA: 556] [Article Influence: 46.3] [Reference Citation Analysis (0)] |
| 59. | Chen Y, Tian Z. HBV-Induced Immune Imbalance in the Development of HCC. Front Immunol. 2019;10:2048. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 90] [Cited by in RCA: 188] [Article Influence: 31.3] [Reference Citation Analysis (0)] |
| 60. | Fisicaro P, Barili V, Rossi M, Montali I, Vecchi A, Acerbi G, Laccabue D, Zecca A, Penna A, Missale G, Ferrari C, Boni C. Pathogenetic Mechanisms of T Cell Dysfunction in Chronic HBV Infection and Related Therapeutic Approaches. Front Immunol. 2020;11:849. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 104] [Article Influence: 20.8] [Reference Citation Analysis (0)] |
| 61. | Park JJ, Wong DK, Wahed AS, Lee WM, Feld JJ, Terrault N, Khalili M, Sterling RK, Kowdley KV, Bzowej N, Lau DT, Kim WR, Smith C, Carithers RL, Torrey KW, Keith JW, Levine DL, Traum D, Ho S, Valiga ME, Johnson GS, Doo E, Lok AS, Chang KM; Hepatitis B Research Network. Hepatitis B Virus--Specific and Global T-Cell Dysfunction in Chronic Hepatitis B. Gastroenterology. 2016;150:684-695.e5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 170] [Cited by in RCA: 180] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 62. | Jia Y, Zeng Z, Li Y, Li Z, Jin L, Zhang Z, Wang L, Wang FS. Impaired function of CD4+ T follicular helper (Tfh) cells associated with hepatocellular carcinoma progression. PLoS One. 2015;10:e0117458. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 65] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 63. | Xu D, Han Q, Hou Z, Zhang C, Zhang J. miR-146a negatively regulates NK cell functions via STAT1 signaling. Cell Mol Immunol. 2017;14:712-720. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 89] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 64. | Li X, Su Y, Hua X, Xie C, Liu J, Huang Y, Zhou L, Zhang M, Li X, Gao Z. Levels of hepatic Th17 cells and regulatory T cells upregulated by hepatic stellate cells in advanced HBV-related liver fibrosis. J Transl Med. 2017;15:75. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 43] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 65. | Ponziani FR, Nicoletti A, Gasbarrini A, Pompili M. Diagnostic and therapeutic potential of the gut microbiota in patients with early hepatocellular carcinoma. Ther Adv Med Oncol. 2019;11:1758835919848184. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 50] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 66. | Ponziani FR, Bhoori S, Castelli C, Putignani L, Rivoltini L, Del Chierico F, Sanguinetti M, Morelli D, Paroni Sterbini F, Petito V, Reddel S, Calvani R, Camisaschi C, Picca A, Tuccitto A, Gasbarrini A, Pompili M, Mazzaferro V. Hepatocellular Carcinoma Is Associated With Gut Microbiota Profile and Inflammation in Nonalcoholic Fatty Liver Disease. Hepatology. 2019;69:107-120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 300] [Cited by in RCA: 472] [Article Influence: 78.7] [Reference Citation Analysis (1)] |
| 67. | Yu LX, Yan HX, Liu Q, Yang W, Wu HP, Dong W, Tang L, Lin Y, He YQ, Zou SS, Wang C, Zhang HL, Cao GW, Wu MC, Wang HY. Endotoxin accumulation prevents carcinogen-induced apoptosis and promotes liver tumorigenesis in rodents. Hepatology. 2010;52:1322-1333. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 218] [Cited by in RCA: 254] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 68. | Rattan P, Minacapelli CD, Rustgi V. The Microbiome and Hepatocellular Carcinoma. Liver Transpl. 2020;26:1316-1327. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 24] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 69. | Liu Q, Li F, Zhuang Y, Xu J, Wang J, Mao X, Zhang Y, Liu X. Alteration in gut microbiota associated with hepatitis B and non-hepatitis virus related hepatocellular carcinoma. Gut Pathog. 2019;11:1. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 118] [Cited by in RCA: 145] [Article Influence: 24.2] [Reference Citation Analysis (0)] |
| 70. | Gupta H, Youn GS, Shin MJ, Suk KT. Role of Gut Microbiota in Hepatocarcinogenesis. Microorganisms. 2019;7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 59] [Cited by in RCA: 87] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 71. | Tang A, Hallouch O, Chernyak V, Kamaya A, Sirlin CB. Epidemiology of hepatocellular carcinoma: target population for surveillance and diagnosis. Abdom Radiol (NY). 2018;43:13-25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 224] [Cited by in RCA: 302] [Article Influence: 43.1] [Reference Citation Analysis (0)] |
| 72. | Terrault NA, Bzowej NH, Chang KM, Hwang JP, Jonas MM, Murad MH; American Association for the Study of Liver Diseases. AASLD guidelines for treatment of chronic hepatitis B. Hepatology. 2016;63:261-283. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1532] [Cited by in RCA: 1582] [Article Influence: 175.8] [Reference Citation Analysis (2)] |
| 73. | AASLD-IDSA HCV Guidance Panel. Hepatitis C Guidance 2018 Update: AASLD-IDSA Recommendations for Testing, Managing, and Treating Hepatitis C Virus Infection. Clin Infect Dis. 2018;67:1477-1492. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 477] [Cited by in RCA: 477] [Article Influence: 68.1] [Reference Citation Analysis (0)] |
| 74. | European Association for the Study of the Liver. EASL 2017 Clinical Practice Guidelines on the management of hepatitis B virus infection. J Hepatol. 2017;67:370-398. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3745] [Cited by in RCA: 3787] [Article Influence: 473.4] [Reference Citation Analysis (1)] |
| 75. | European Association for the Study of the Liver. EASL Recommendations on Treatment of Hepatitis C 2018. J Hepatol. 2018;69:461-511. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1281] [Cited by in RCA: 1209] [Article Influence: 172.7] [Reference Citation Analysis (0)] |
| 76. | Kudo M, Kawamura Y, Hasegawa K, Tateishi R, Kariyama K, Shiina S, Toyoda H, Imai Y, Hiraoka A, Ikeda M, Izumi N, Moriguchi M, Ogasawara S, Minami Y, Ueshima K, Murakami T, Miyayama S, Nakashima O, Yano H, Sakamoto M, Hatano E, Shimada M, Kokudo N, Mochida S, Takehara T. Management of Hepatocellular Carcinoma in Japan: JSH Consensus Statements and Recommendations 2021 Update. Liver Cancer. 2021;10:181-223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 448] [Article Influence: 112.0] [Reference Citation Analysis (0)] |
| 77. | Drafting Committee for Hepatitis Management Guidelines and the Japan Society of Hepatology. JSH Guidelines for the Management of Hepatitis B Virus Infection. Hepatol Res. 2014;44 Suppl S1:1-58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 137] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 78. | Varbobitis I, Papatheodoridis GV. The assessment of hepatocellular carcinoma risk in patients with chronic hepatitis B under antiviral therapy. Clin Mol Hepatol. 2016;22:319-326. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 77] [Cited by in RCA: 77] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 79. | Wong GL, Chan HL, Chan HY, Tse PC, Tse YK, Mak CW, Lee SK, Ip ZM, Lam AT, Iu HW, Leung JM, Wong VW. Accuracy of risk scores for patients with chronic hepatitis B receiving entecavir treatment. Gastroenterology. 2013;144:933-944. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 146] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 80. | Coffin CS, Rezaeeaval M, Pang JX, Alcantara L, Klein P, Burak KW, Myers RP. The incidence of hepatocellular carcinoma is reduced in patients with chronic hepatitis B on long-term nucleos(t)ide analogue therapy. Aliment Pharmacol Ther. 2014;40:1262-1269. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 57] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 81. | Ahn J, Lim JK, Lee HM, Lok AS, Nguyen M, Pan CQ, Mannalithara A, Te H, Reddy KR, Trinh H, Chu D, Tran T, Lau D, Leduc TS, Min A, Trong Le L, Bae H, Van Tran S, Do S, Hann HW, Wong C, Han S, Pillai A, Park JS, Tong M, Scaglione S, Woog J, Kim WR. Lower Observed Hepatocellular Carcinoma Incidence in Chronic Hepatitis B Patients Treated With Entecavir: Results of the ENUMERATE Study. Am J Gastroenterol. 2016;111:1297-1304. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 34] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 82. | Kim WR, Loomba R, Berg T, Aguilar Schall RE, Yee LJ, Dinh PV, Flaherty JF, Martins EB, Therneau TM, Jacobson I, Fung S, Gurel S, Buti M, Marcellin P. Impact of long-term tenofovir disoproxil fumarate on incidence of hepatocellular carcinoma in patients with chronic hepatitis B. Cancer. 2015;121:3631-3638. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 162] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 83. | Arends P, Sonneveld MJ, Zoutendijk R, Carey I, Brown A, Fasano M, Mutimer D, Deterding K, Reijnders JG, Oo Y, Petersen J, van Bömmel F, de Knegt RJ, Santantonio T, Berg T, Welzel TM, Wedemeyer H, Buti M, Pradat P, Zoulim F, Hansen B, Janssen HL; VIRGIL Surveillance Study Group. Entecavir treatment does not eliminate the risk of hepatocellular carcinoma in chronic hepatitis B: limited role for risk scores in Caucasians. Gut. 2015;64:1289-1295. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 167] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 84. | Papatheodoridis G, Dalekos G, Sypsa V, Yurdaydin C, Buti M, Goulis J, Calleja JL, Chi H, Manolakopoulos S, Mangia G, Gatselis N, Keskin O, Savvidou S, de la Revilla J, Hansen BE, Vlachogiannakos I, Galanis K, Idilman R, Colombo M, Esteban R, Janssen HL, Lampertico P. PAGE-B predicts the risk of developing hepatocellular carcinoma in Caucasians with chronic hepatitis B on 5-year antiviral therapy. J Hepatol. 2016;64:800-806. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 267] [Cited by in RCA: 389] [Article Influence: 43.2] [Reference Citation Analysis (0)] |
| 85. | Kim JH, Kim YD, Lee M, Jun BG, Kim TS, Suk KT, Kang SH, Kim MY, Cheon GJ, Kim DJ, Baik SK, Choi DH. Modified PAGE-B score predicts the risk of hepatocellular carcinoma in Asians with chronic hepatitis B on antiviral therapy. J Hepatol. 2018;69:1066-1073. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 189] [Cited by in RCA: 183] [Article Influence: 26.1] [Reference Citation Analysis (0)] |
| 86. | Baudi I, Inoue T, Tanaka Y. Novel Biomarkers of Hepatitis B and Hepatocellular Carcinoma: Clinical Significance of HBcrAg and M2BPGi. Int J Mol Sci. 2020;21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 24] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 87. | Rapti I, Hadziyannis S. Risk for hepatocellular carcinoma in the course of chronic hepatitis B virus infection and the protective effect of therapy with nucleos(t)ide analogues. World J Hepatol. 2015;7:1064-1073. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 43] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 88. | Sung JJ, Tsoi KK, Wong VW, Li KC, Chan HL. Meta-analysis: Treatment of hepatitis B infection reduces risk of hepatocellular carcinoma. Aliment Pharmacol Ther. 2008;28:1067-1077. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 283] [Cited by in RCA: 278] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 89. | Hosaka T, Suzuki F, Kobayashi M, Seko Y, Kawamura Y, Sezaki H, Akuta N, Suzuki Y, Saitoh S, Arase Y, Ikeda K, Kumada H. Long-term entecavir treatment reduces hepatocellular carcinoma incidence in patients with hepatitis B virus infection. Hepatology. 2013;58:98-107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 519] [Cited by in RCA: 540] [Article Influence: 45.0] [Reference Citation Analysis (0)] |
| 90. | Papatheodoridis GV, Dalekos GN, Yurdaydin C, Buti M, Goulis J, Arends P, Sypsa V, Manolakopoulos S, Mangia G, Gatselis N, Keskın O, Savvidou S, Hansen BE, Papaioannou C, Galanis K, Idilman R, Colombo M, Esteban R, Janssen HL, Lampertico P. Incidence and predictors of hepatocellular carcinoma in Caucasian chronic hepatitis B patients receiving entecavir or tenofovir. J Hepatol. 2015;62:363-370. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 130] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 91. | Chen LP, Zhao J, Du Y, Han YF, Su T, Zhang HW, Cao GW. Antiviral treatment to prevent chronic hepatitis B or C-related hepatocellular carcinoma. World J Virol. 2012;1:174-183. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 24] [Cited by in RCA: 25] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 92. | Lin SM, Yu ML, Lee CM, Chien RN, Sheen IS, Chu CM, Liaw YF. Interferon therapy in HBeAg positive chronic hepatitis reduces progression to cirrhosis and hepatocellular carcinoma. J Hepatol. 2007;46:45-52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 246] [Cited by in RCA: 242] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 93. | Fontana RJ, Hann HW, Perrillo RP, Vierling JM, Wright T, Rakela J, Anschuetz G, Davis R, Gardner SD, Brown NA. Determinants of early mortality in patients with decompensated chronic hepatitis B treated with antiviral therapy. Gastroenterology. 2002;123:719-727. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 205] [Cited by in RCA: 180] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 94. | Chang MH, Chen CJ, Lai MS, Hsu HM, Wu TC, Kong MS, Liang DC, Shau WY, Chen DS. Universal hepatitis B vaccination in Taiwan and the incidence of hepatocellular carcinoma in children. Taiwan Childhood Hepatoma Study Group. N Engl J Med. 1997;336:1855-1859. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1328] [Cited by in RCA: 1196] [Article Influence: 42.7] [Reference Citation Analysis (0)] |
| 95. | Qu C, Chen T, Fan C, Zhan Q, Wang Y, Lu J, Lu LL, Ni Z, Huang F, Yao H, Zhu J, Fan J, Zhu Y, Wu Z, Liu G, Gao W, Zang M, Wang D, Dai M, Hsia CC, Zhang Y, Sun Z. Efficacy of neonatal HBV vaccination on liver cancer and other liver diseases over 30-year follow-up of the Qidong hepatitis B intervention study: a cluster randomized controlled trial. PLoS Med. 2014;11:e1001774. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 106] [Cited by in RCA: 101] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 96. | Wiangnon S, Kamsa-ard S, Suwanrungruang K, Promthet S, Mahaweerawat S, Khuntikeo N. Trends in incidence of hepatocellular carcinoma, 1990-2009, Khon Kaen, Thailand. Asian Pac J Cancer Prev. 2012;13:1065-1068. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 16] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 97. | McMahon BJ, Bulkow LR, Singleton RJ, Williams J, Snowball M, Homan C, Parkinson AJ. Elimination of hepatocellular carcinoma and acute hepatitis B in children 25 years after a hepatitis B newborn and catch-up immunization program. Hepatology. 2011;54:801-807. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 145] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 98. | Kao JH. Hepatitis B vaccination and prevention of hepatocellular carcinoma. Best Pract Res Clin Gastroenterol. 2015;29:907-917. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 67] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 99. | Mentha N, Clément S, Negro F, Alfaiate D. A review on hepatitis D: From virology to new therapies. J Adv Res. 2019;17:3-15. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 77] [Cited by in RCA: 72] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 100. | Stockdale AJ, Kreuels B, Henrion MRY, Giorgi E, Kyomuhangi I, Geretti AM. Hepatitis D prevalence: problems with extrapolation to global population estimates. Gut. 2020;69:396-397. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 30] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 101. | Chen HY, Shen DT, Ji DZ, Han PC, Zhang WM, Ma JF, Chen WS, Goyal H, Pan S, Xu HG. Prevalence and burden of hepatitis D virus infection in the global population: a systematic review and meta-analysis. Gut. 2019;68:512-521. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 277] [Cited by in RCA: 260] [Article Influence: 43.3] [Reference Citation Analysis (0)] |
| 102. | Sagnelli E, Sagnelli C, Pisaturo M, Macera M, Coppola N. Epidemiology of acute and chronic hepatitis B and delta over the last 5 decades in Italy. World J Gastroenterol. 2014;20:7635-7643. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 91] [Cited by in RCA: 84] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 103. | Stroffolini T, Sagnelli E, Sagnelli C, Russello M, De Luca M, Rosina F, Cacopardo B, Brancaccio G, Furlan C, Gaeta GB, Licata A, Almasio PL; behalf of EPACRON study group. Hepatitis delta infection in Italian patients: towards the end of the story? Infection. 2017;45:277-281. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 33] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 104. | Farci P, Niro GA, Zamboni F, Diaz G. Hepatitis D Virus and Hepatocellular Carcinoma. Viruses. 2021;13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 35] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 105. | Fattovich G, Giustina G, Christensen E, Pantalena M, Zagni I, Realdi G, Schalm SW. Influence of hepatitis delta virus infection on morbidity and mortality in compensated cirrhosis type B. The European Concerted Action on Viral Hepatitis (Eurohep). Gut. 2000;46:420-426. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 312] [Cited by in RCA: 362] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 106. | Ji J, Sundquist K, Sundquist J. A population-based study of hepatitis D virus as potential risk factor for hepatocellular carcinoma. J Natl Cancer Inst. 2012;104:790-792. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 77] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 107. | Romeo R, Foglieni B, Casazza G, Spreafico M, Colombo M, Prati D. High serum levels of HDV RNA are predictors of cirrhosis and liver cancer in patients with chronic hepatitis delta. PLoS One. 2014;9:e92062. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 78] [Article Influence: 7.1] [Reference Citation Analysis (1)] |
| 108. | Amougou MA, Noah DN, Moundipa PF, Pineau P, Njouom R. A prominent role of Hepatitis D Virus in liver cancers documented in Central Africa. BMC Infect Dis. 2016;16:647. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 35] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 109. | Ioannou GN, Beste LA, Green PK, Singal AG, Tapper EB, Waljee AK, Sterling RK, Feld JJ, Kaplan DE, Taddei TH, Berry K. Increased Risk for Hepatocellular Carcinoma Persists Up to 10 Years After HCV Eradication in Patients With Baseline Cirrhosis or High FIB-4 Scores. Gastroenterology. 2019;157:1264-1278.e4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 181] [Cited by in RCA: 293] [Article Influence: 48.8] [Reference Citation Analysis (0)] |
| 110. | Cross TJ, Rizzi P, Horner M, Jolly A, Hussain MJ, Smith HM, Vergani D, Harrison PM. The increasing prevalence of hepatitis delta virus (HDV) infection in South London. J Med Virol. 2008;80:277-282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 124] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 111. | Abbas Z, Abbas M, Abbas S, Shazi L. Hepatitis D and hepatocellular carcinoma. World J Hepatol. 2015;7:777-786. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 36] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 112. | Puigvehí M, Moctezuma-Velázquez C, Villanueva A, Llovet JM. The oncogenic role of hepatitis delta virus in hepatocellular carcinoma. JHEP Rep. 2019;1:120-130. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 58] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 113. | Benegiamo G, Vinciguerra M, Guarnieri V, Niro GA, Andriulli A, Pazienza V. Hepatitis delta virus induces specific DNA methylation processes in Huh-7 liver cancer cells. FEBS Lett. 2013;587:1424-1428. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 31] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 114. | Zhang Q, Matsuura K, Kleiner DE, Zamboni F, Alter HJ, Farci P. Analysis of long noncoding RNA expression in hepatocellular carcinoma of different viral etiology. J Transl Med. 2016;14:328. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 56] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 115. | Wedemeyer H, Yurdaydin C, Hardtke S, Caruntu FA, Curescu MG, Yalcin K, Akarca US, Gürel S, Zeuzem S, Erhardt A, Lüth S, Papatheodoridis GV, Keskin O, Port K, Radu M, Celen MK, Idilman R, Weber K, Stift J, Wittkop U, Heidrich B, Mederacke I, von der Leyen H, Dienes HP, Cornberg M, Koch A, Manns MP; HIDIT-II study team. Peginterferon alfa-2a plus tenofovir disoproxil fumarate for hepatitis D (HIDIT-II): a randomised, placebo controlled, phase 2 trial. Lancet Infect Dis. 2019;19:275-286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 144] [Article Influence: 28.8] [Reference Citation Analysis (0)] |
| 116. | Gower E, Estes C, Blach S, Razavi-Shearer K, Razavi H. Global epidemiology and genotype distribution of the hepatitis C virus infection. J Hepatol. 2014;61:S45-S57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1325] [Cited by in RCA: 1362] [Article Influence: 123.8] [Reference Citation Analysis (0)] |
| 117. | Polaris Observatory HCV Collaborators. Global prevalence and genotype distribution of hepatitis C virus infection in 2015: a modelling study. Lancet Gastroenterol Hepatol. 2017;2:161-176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1493] [Cited by in RCA: 1471] [Article Influence: 183.9] [Reference Citation Analysis (0)] |
| 118. | Davis GL, Albright JE, Cook SF, Rosenberg DM. Projecting future complications of chronic hepatitis C in the United States. Liver Transpl. 2003;9:331-338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 442] [Cited by in RCA: 404] [Article Influence: 18.4] [Reference Citation Analysis (0)] |
| 119. | Lok AS, McMahon BJ. Chronic hepatitis B: update 2009. Hepatology. 2009;50:661-662. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2125] [Cited by in RCA: 2170] [Article Influence: 135.6] [Reference Citation Analysis (0)] |
| 120. | Alfaleh FZ, Nugrahini N, Matičič M, Tolmane I, Alzaabi M, Hajarizadeh B, Valantinas J, Kim DY, Hunyady B, Abaalkhail F, Abbas Z, Abdou A, Abourached A, Al Braiki F, Al Hosani F, Al Jaberi K, Al Khatry M, Al Mulla MA, Al Quraishi H, Al Rifai A, Al Serkal Y, Alam A, Alashgar HI, Alavian SM, Alawadhi S, Al-Dabal L, Aldins P, Alghamdi AS, Al-Hakeem R, Aljumah AA, Almessabi A, Alqutub AN, Alswat KA, Altraif I, Andrea N, Assiri AM, Babatin MA, Baqir A, Barakat MT, Bergmann OM, Bizri AR, Chaudhry A, Choi MS, Diab T, Djauzi S, El Hassan ES, El Khoury S, Estes C, Fakhry S, Farooqi JI, Fridjonsdottir H, Gani RA, Ghafoor Khan A, Gheorghe L, Goldis A, Gottfredsson M, Gregorcic S, Gunter J, Hamid S, Han KH, Hasan I, Hashim A, Horvath G, Husni R, Jafri W, Jeruma A, Jonasson JG, Karlsdottir B, Kim YS, Koutoubi Z, Lesmana LA, Liakina V, Lim YS, Löve A, Maimets M, Makara M, Malekzadeh R, Memon MS, Merat S, Mokhbat JE, Mourad FH, Muljono DH, Nawaz A, Olafsson S, Priohutomo S, Qureshi H, Rassam P, Razavi H, Razavi-Shearer D, Razavi-Shearer K, Rozentale B, Sadik M, Saeed K, Salamat A, Salupere R, Sanai FM, Sanityoso Sulaiman A, Sayegh RA, Schmelzer JD, Sharara AI, Sibley A, Siddiq M, Siddiqui AM, Sigmundsdottir G, Sigurdardottir B, Speiciene D, Sulaiman A, Sultan MA, Taha M, Tanaka J, Tarifi H, Tayyab G, Ud Din M, Umar M, Videčnik-Zorman J, Yaghi C, Yunihastuti E, Yusuf MA, Zuberi BF, Blach S. Strategies to manage hepatitis C virus infection disease burden - volume 3. J Viral Hepat. 2015;22 Suppl 4:42-65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 32] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 121. | Freeman AJ, Dore GJ, Law MG, Thorpe M, Von Overbeck J, Lloyd AR, Marinos G, Kaldor JM. Estimating progression to cirrhosis in chronic hepatitis C virus infection. Hepatology. 2001;34:809-816. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 440] [Cited by in RCA: 424] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 122. | Lin MV, King LY, Chung RT. Hepatitis C virus-associated cancer. Annu Rev Pathol. 2015;10:345-370. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 74] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 123. | Mahmoudvand S, Shokri S, Taherkhani R, Farshadpour F. Hepatitis C virus core protein modulates several signaling pathways involved in hepatocellular carcinoma. World J Gastroenterol. 2019;25:42-58. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 60] [Cited by in RCA: 54] [Article Influence: 9.0] [Reference Citation Analysis (1)] |
| 124. | Alisi A, Giambartolomei S, Cupelli F, Merlo P, Fontemaggi G, Spaziani A, Balsano C. Physical and functional interaction between HCV core protein and the different p73 isoforms. Oncogene. 2003;22:2573-2580. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 53] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 125. | Zhao LJ, Wang L, Ren H, Cao J, Li L, Ke JS, Qi ZT. Hepatitis C virus E2 protein promotes human hepatoma cell proliferation through the MAPK/ERK signaling pathway via cellular receptors. Exp Cell Res. 2005;305:23-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 87] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 126. | Vescovo T, Refolo G, Vitagliano G, Fimia GM, Piacentini M. Molecular mechanisms of hepatitis C virus-induced hepatocellular carcinoma. Clin Microbiol Infect. 2016;22:853-861. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 107] [Article Influence: 11.9] [Reference Citation Analysis (1)] |
| 127. | Deng L, Nagano-Fujii M, Tanaka M, Nomura-Takigawa Y, Ikeda M, Kato N, Sada K, Hotta H. NS3 protein of Hepatitis C virus associates with the tumour suppressor p53 and inhibits its function in an NS3 sequence-dependent manner. J Gen Virol. 2006;87:1703-1713. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 75] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 128. | Yoshida K, Murata M, Yamaguchi T, Matsuzaki K. TGF-β/Smad signaling during hepatic fibro-carcinogenesis (review). Int J Oncol. 2014;45:1363-1371. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 86] [Cited by in RCA: 100] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 129. | Street A, Macdonald A, Crowder K, Harris M. The Hepatitis C virus NS5A protein activates a phosphoinositide 3-kinase-dependent survival signaling cascade. J Biol Chem. 2004;279:12232-12241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 176] [Cited by in RCA: 171] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 130. | Zhu C, Liu X, Wang S, Yan X, Tang Z, Wu K, Li Y, Liu F. Hepatitis C virus core protein induces hypoxia-inducible factor 1α-mediated vascular endothelial growth factor expression in Huh7.5.1 cells. Mol Med Rep. 2014;9:2010-2014. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 24] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 131. | Benkheil M, Van Haele M, Roskams T, Laporte M, Noppen S, Abbasi K, Delang L, Neyts J, Liekens S. CCL20, a direct-acting pro-angiogenic chemokine induced by hepatitis C virus (HCV): Potential role in HCV-related liver cancer. Exp Cell Res. 2018;372:168-177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 42] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 132. | Hamdane N, Jühling F, Crouchet E, El Saghire H, Thumann C, Oudot MA, Bandiera S, Saviano A, Ponsolles C, Roca Suarez AA, Li S, Fujiwara N, Ono A, Davidson I, Bardeesy N, Schmidl C, Bock C, Schuster C, Lupberger J, Habersetzer F, Doffoël M, Piardi T, Sommacale D, Imamura M, Uchida T, Ohdan H, Aikata H, Chayama K, Boldanova T, Pessaux P, Fuchs BC, Hoshida Y, Zeisel MB, Duong FHT, Baumert TF. HCV-Induced Epigenetic Changes Associated With Liver Cancer Risk Persist After Sustained Virologic Response. Gastroenterology. 2019;156:2313-2329.e7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 140] [Cited by in RCA: 210] [Article Influence: 35.0] [Reference Citation Analysis (0)] |
| 133. | Varnholt H, Drebber U, Schulze F, Wedemeyer I, Schirmacher P, Dienes HP, Odenthal M. MicroRNA gene expression profile of hepatitis C virus-associated hepatocellular carcinoma. Hepatology. 2008;47:1223-1232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 332] [Cited by in RCA: 347] [Article Influence: 20.4] [Reference Citation Analysis (0)] |
| 134. | Jopling CL, Yi M, Lancaster AM, Lemon SM, Sarnow P. Modulation of hepatitis C virus RNA abundance by a liver-specific MicroRNA. Science. 2005;309:1577-1581. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1993] [Cited by in RCA: 1986] [Article Influence: 99.3] [Reference Citation Analysis (0)] |
| 135. | Bruno S, Crosignani A, Maisonneuve P, Rossi S, Silini E, Mondelli MU. Hepatitis C virus genotype 1b as a major risk factor associated with hepatocellular carcinoma in patients with cirrhosis: a seventeen-year prospective cohort study. Hepatology. 2007;46:1350-1356. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 150] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 136. | Raimondi S, Bruno S, Mondelli MU, Maisonneuve P. Hepatitis C virus genotype 1b as a risk factor for hepatocellular carcinoma development: a meta-analysis. J Hepatol. 2009;50:1142-1154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 168] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 137. | Lee SS, Kim CY, Kim BR, Cha RR, Kim WS, Kim JJ, Lee JM, Kim HJ, Ha CY, Kim TH, Jung WT, Lee OJ. Hepatitis C virus genotype 3 was associated with the development of hepatocellular carcinoma in Korea. J Viral Hepat. 2019;26:459-465. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 10] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 138. | Nkontchou G, Ziol M, Aout M, Lhabadie M, Baazia Y, Mahmoudi A, Roulot D, Ganne-Carrie N, Grando-Lemaire V, Trinchet JC, Gordien E, Vicaut E, Baghad I, Beaugrand M. HCV genotype 3 is associated with a higher hepatocellular carcinoma incidence in patients with ongoing viral C cirrhosis. J Viral Hepat. 2011;18:e516-e522. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 128] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 139. | van der Meer AJ, Veldt BJ, Feld JJ, Wedemeyer H, Dufour JF, Lammert F, Duarte-Rojo A, Heathcote EJ, Manns MP, Kuske L, Zeuzem S, Hofmann WP, de Knegt RJ, Hansen BE, Janssen HL. Association between sustained virological response and all-cause mortality among patients with chronic hepatitis C and advanced hepatic fibrosis. JAMA. 2012;308:2584-2593. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1165] [Cited by in RCA: 1167] [Article Influence: 89.8] [Reference Citation Analysis (0)] |
| 140. | Castello G, Scala S, Palmieri G, Curley SA, Izzo F. HCV-related hepatocellular carcinoma: From chronic inflammation to cancer. Clin Immunol. 2010;134:237-250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 112] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 141. | Waggoner SN, Hall CH, Hahn YS. HCV core protein interaction with gC1q receptor inhibits Th1 differentiation of CD4+ T cells via suppression of dendritic cell IL-12 production. J Leukoc Biol. 2007;82:1407-1419. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 84] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 142. | Hussain SP, Hofseth LJ, Harris CC. Radical causes of cancer. Nat Rev Cancer. 2003;3:276-285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1242] [Cited by in RCA: 1197] [Article Influence: 54.4] [Reference Citation Analysis (0)] |
| 143. | Majano PL, Garcia-Monzon C. Does nitric oxide play a pathogenic role in hepatitis C virus infection? Cell Death Differ. 2003;10 Suppl 1:S13-S15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 144. | Kato N, Yoshida H, Ono-Nita SK, Kato J, Goto T, Otsuka M, Lan K, Matsushima K, Shiratori Y, Omata M. Activation of intracellular signaling by hepatitis B and C viruses: C-viral core is the most potent signal inducer. Hepatology. 2000;32:405-412. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 173] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 145. | Maki A, Kono H, Gupta M, Asakawa M, Suzuki T, Matsuda M, Fujii H, Rusyn I. Predictive power of biomarkers of oxidative stress and inflammation in patients with hepatitis C virus-associated hepatocellular carcinoma. Ann Surg Oncol. 2007;14:1182-1190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 102] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 146. | Matsui T, Nagai H, Sumino Y, Miki K. Relationship of peripheral blood CD4-positive T cells to carcinogenesis in patients with HCV-related chronic hepatitis and liver cirrhosis. Cancer Chemother Pharmacol. 2008;62:401-406. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 147. | Budhu A, Wang XW. The role of cytokines in hepatocellular carcinoma. J Leukoc Biol. 2006;80:1197-1213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 200] [Cited by in RCA: 238] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 148. | Cabrera R, Tu Z, Xu Y, Firpi RJ, Rosen HR, Liu C, Nelson DR. An immunomodulatory role for CD4(+)CD25(+) regulatory T lymphocytes in hepatitis C virus infection. Hepatology. 2004;40:1062-1071. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 426] [Cited by in RCA: 430] [Article Influence: 20.5] [Reference Citation Analysis (0)] |
| 149. | Li L, Ye J. Characterization of gut microbiota in patients with primary hepatocellular carcinoma received immune checkpoint inhibitors: A Chinese population-based study. Medicine (Baltimore). 2020;99:e21788. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 46] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 150. | Wang L, Wan YY. The role of gut microbiota in liver disease development and treatment. Liver Res. 2019;3:3-18. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 34] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 151. | Inoue T, Nakayama J, Moriya K, Kawaratani H, Momoda R, Ito K, Iio E, Nojiri S, Fujiwara K, Yoneda M, Yoshiji H, Tanaka Y. Gut Dysbiosis Associated With Hepatitis C Virus Infection. Clin Infect Dis. 2018;67:869-877. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 130] [Article Influence: 18.6] [Reference Citation Analysis (0)] |
| 152. | Cosseau C, Devine DA, Dullaghan E, Gardy JL, Chikatamarla A, Gellatly S, Yu LL, Pistolic J, Falsafi R, Tagg J, Hancock RE. The commensal Streptococcus salivarius K12 downregulates the innate immune responses of human epithelial cells and promotes host-microbe homeostasis. Infect Immun. 2008;76:4163-4175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 169] [Cited by in RCA: 213] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 153. | Ponziani FR, Putignani L, Paroni Sterbini F, Petito V, Picca A, Del Chierico F, Reddel S, Calvani R, Marzetti E, Sanguinetti M, Gasbarrini A, Pompili M. Influence of hepatitis C virus eradication with direct-acting antivirals on the gut microbiota in patients with cirrhosis. Aliment Pharmacol Ther. 2018;48:1301-1311. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 62] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 154. | Walker AJ, Peacock CJ, Pedergnana V; STOP-HCV Consortium, Irving WL. Host genetic factors associated with hepatocellular carcinoma in patients with hepatitis C virus infection: A systematic review. J Viral Hepat. 2018;25:442-456. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 21] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 155. | Thrift AP, El-Serag HB, Kanwal F. Global epidemiology and burden of HCV infection and HCV-related disease. Nat Rev Gastroenterol Hepatol. 2017;14:122-132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 249] [Cited by in RCA: 285] [Article Influence: 35.6] [Reference Citation Analysis (0)] |
| 156. | Bandiera S, Billie Bian C, Hoshida Y, Baumert TF, Zeisel MB. Chronic hepatitis C virus infection and pathogenesis of hepatocellular carcinoma. Curr Opin Virol. 2016;20:99-105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 55] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 157. | Degasperi E, Galmozzi E, Pelusi S, D'Ambrosio R, Soffredini R, Borghi M, Perbellini R, Facchetti F, Iavarone M, Sangiovanni A, Valenti L, Lampertico P. Hepatic Fat-Genetic Risk Score Predicts Hepatocellular Carcinoma in Patients With Cirrhotic HCV Treated With DAAs. Hepatology. 2020;72:1912-1923. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 52] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 158. | Marrero JA, Kulik LM, Sirlin CB, Zhu AX, Finn RS, Abecassis MM, Roberts LR, Heimbach JK. Diagnosis, Staging, and Management of Hepatocellular Carcinoma: 2018 Practice Guidance by the American Association for the Study of Liver Diseases. Hepatology. 2018;68:723-750. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2121] [Cited by in RCA: 3228] [Article Influence: 461.1] [Reference Citation Analysis (1)] |
| 159. | Kudo M, Matsui O, Izumi N, Iijima H, Kadoya M, Imai Y, Okusaka T, Miyayama S, Tsuchiya K, Ueshima K, Hiraoka A, Ikeda M, Ogasawara S, Yamashita T, Minami T, Yamakado K; Liver Cancer Study Group of Japan. JSH Consensus-Based Clinical Practice Guidelines for the Management of Hepatocellular Carcinoma: 2014 Update by the Liver Cancer Study Group of Japan. Liver Cancer. 2014;3:458-468. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 401] [Cited by in RCA: 488] [Article Influence: 44.4] [Reference Citation Analysis (0)] |
| 160. | Ponziani FR, Mangiola F, Binda C, Zocco MA, Siciliano M, Grieco A, Rapaccini GL, Pompili M, Gasbarrini A. Future of liver disease in the era of direct acting antivirals for the treatment of hepatitis C. World J Hepatol. 2017;9:352-367. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 42] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 161. | Morgan RL, Baack B, Smith BD, Yartel A, Pitasi M, Falck-Ytter Y. Eradication of hepatitis C virus infection and the development of hepatocellular carcinoma: a meta-analysis of observational studies. Ann Intern Med. 2013;158:329-337. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 631] [Cited by in RCA: 651] [Article Influence: 54.3] [Reference Citation Analysis (0)] |
| 162. | Quartuccio L, Benucci M, De Vita S. Answer to Vieira et al. "Cytokine profile as a prognostic tool in coronavirus disease 2019". Joint Bone Spine. 2021;88:105076. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 163. | Pascut D, Pratama MY, Tiribelli C. HCC occurrence after DAA treatments: molecular tools to assess the post-treatment risk and surveillance. Hepat Oncol. 2020;7:HEP21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 164. | Reig M, Mariño Z, Perelló C, Iñarrairaegui M, Ribeiro A, Lens S, Díaz A, Vilana R, Darnell A, Varela M, Sangro B, Calleja JL, Forns X, Bruix J. Unexpected high rate of early tumor recurrence in patients with HCV-related HCC undergoing interferon-free therapy. J Hepatol. 2016;65:719-726. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 725] [Cited by in RCA: 803] [Article Influence: 89.2] [Reference Citation Analysis (0)] |
| 165. | Conti F, Buonfiglioli F, Scuteri A, Crespi C, Bolondi L, Caraceni P, Foschi FG, Lenzi M, Mazzella G, Verucchi G, Andreone P, Brillanti S. Early occurrence and recurrence of hepatocellular carcinoma in HCV-related cirrhosis treated with direct-acting antivirals. J Hepatol. 2016;65:727-733. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 708] [Cited by in RCA: 698] [Article Influence: 77.6] [Reference Citation Analysis (0)] |
| 166. | Waziry R, Hajarizadeh B, Grebely J, Amin J, Law M, Danta M, George J, Dore GJ. Hepatocellular carcinoma risk following direct-acting antiviral HCV therapy: A systematic review, meta-analyses, and meta-regression. J Hepatol. 2017;67:1204-1212. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 352] [Cited by in RCA: 380] [Article Influence: 47.5] [Reference Citation Analysis (0)] |
| 167. | Ioannou GN, Green PK, Berry K. HCV eradication induced by direct-acting antiviral agents reduces the risk of hepatocellular carcinoma. J Hepatol. 2017;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 311] [Cited by in RCA: 375] [Article Influence: 46.9] [Reference Citation Analysis (1)] |
| 168. | Nahon P, Layese R, Bourcier V, Cagnot C, Marcellin P, Guyader D, Pol S, Larrey D, De Lédinghen V, Ouzan D, Zoulim F, Roulot D, Tran A, Bronowicki JP, Zarski JP, Riachi G, Calès P, Péron JM, Alric L, Bourlière M, Mathurin P, Blanc JF, Abergel A, Serfaty L, Mallat A, Grangé JD, Attali P, Bacq Y, Wartelle C, Dao T, Thabut D, Pilette C, Silvain C, Christidis C, Nguyen-Khac E, Bernard-Chabert B, Zucman D, Di Martino V, Sutton A, Roudot-Thoraval F, Audureau E; ANRS CO12 CirVir Group. Incidence of Hepatocellular Carcinoma After Direct Antiviral Therapy for HCV in Patients With Cirrhosis Included in Surveillance Programs. Gastroenterology. 2018;155:1436-1450.e6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 185] [Cited by in RCA: 181] [Article Influence: 25.9] [Reference Citation Analysis (0)] |
| 169. | Singer AW, Reddy KR, Telep LE, Osinusi AO, Brainard DM, Buti M, Chokkalingam AP. Direct-acting antiviral treatment for hepatitis C virus infection and risk of incident liver cancer: a retrospective cohort study. Aliment Pharmacol Ther. 2018;47:1278-1287. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 63] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 170. | Calvaruso V, Cabibbo G, Cacciola I, Petta S, Madonia S, Bellia A, Tinè F, Distefano M, Licata A, Giannitrapani L, Prestileo T, Mazzola G, Di Rosolini MA, Larocca L, Bertino G, Digiacomo A, Benanti F, Guarneri L, Averna A, Iacobello C, Magro A, Scalisi I, Cartabellotta F, Savalli F, Barbara M, Davì A, Russello M, Scifo G, Squadrito G, Cammà C, Raimondo G, Craxì A, Di Marco V; Rete Sicilia Selezione Terapia–HCV (RESIST-HCV). Incidence of Hepatocellular Carcinoma in Patients With HCV-Associated Cirrhosis Treated With Direct-Acting Antiviral Agents. Gastroenterology. 2018;155:411-421.e4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 281] [Cited by in RCA: 268] [Article Influence: 38.3] [Reference Citation Analysis (0)] |
| 171. | Guarino M, Viganò L, Ponziani FR, Giannini EG, Lai Q, Morisco F; Special Interest Group on Hepatocellular carcinoma and new anti-HCV therapies” of the Italian Association for the Study of the Liver. Recurrence of hepatocellular carcinoma after direct acting antiviral treatment for hepatitis C virus infection: Literature review and risk analysis. Dig Liver Dis. 2018;50:1105-1114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 35] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 172. | Carrat F, Fontaine H, Dorival C, Simony M, Diallo A, Hezode C, De Ledinghen V, Larrey D, Haour G, Bronowicki JP, Zoulim F, Asselah T, Marcellin P, Thabut D, Leroy V, Tran A, Habersetzer F, Samuel D, Guyader D, Chazouilleres O, Mathurin P, Metivier S, Alric L, Riachi G, Gournay J, Abergel A, Cales P, Ganne N, Loustaud-Ratti V, D'Alteroche L, Causse X, Geist C, Minello A, Rosa I, Gelu-Simeon M, Portal I, Raffi F, Bourliere M, Pol S; French ANRS CO22 Hepather cohort. Clinical outcomes in patients with chronic hepatitis C after direct-acting antiviral treatment: a prospective cohort study. Lancet. 2019;393:1453-1464. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 362] [Cited by in RCA: 462] [Article Influence: 77.0] [Reference Citation Analysis (0)] |
| 173. | Butt AA, Yan P, Simon TG, Abou-Samra AB. Effect of Paritaprevir/Ritonavir/Ombitasvir/Dasabuvir and Ledipasvir/Sofosbuvir Regimens on Survival Compared With Untreated Hepatitis C Virus-Infected Persons: Results From ERCHIVES. Clin Infect Dis. 2017;65:1006-1011. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 28] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 174. | Watanabe T, Tokumoto Y, Joko K, Michitaka K, Horiike N, Tanaka Y, Tada F, Kisaka Y, Nakanishi S, Yamauchi K, Yukimoto A, Hirooka M, Abe M, Hiasa Y. Predictors of hepatocellular carcinoma occurrence after direct-acting antiviral therapy in patients with hepatitis C virus infection. Hepatol Res. 2019;49:136-146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 51] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 175. | Kanwal F, Kramer JR, Asch SM, Cao Y, Li L, El-Serag HB. Long-Term Risk of Hepatocellular Carcinoma in HCV Patients Treated With Direct Acting Antiviral Agents. Hepatology. 2020;71:44-55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 193] [Article Influence: 38.6] [Reference Citation Analysis (0)] |
| 176. | Shiha G, Waked I, Soliman R, Elbasiony M, Gomaa A, Mikhail NNH, Eslam M. GES: A validated simple score to predict the risk of HCC in patients with HCV-GT4-associated advanced liver fibrosis after oral antivirals. Liver Int. 2020;40:2828-2833. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 37] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 177. | Xue X, Liao W, Xing Y. Comparison of clinical features and outcomes between HBV-related and non-B non-C hepatocellular carcinoma. Infect Agent Cancer. 2020;15:11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 26] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 178. | Sinn DH, Gwak GY, Cho J, Paik SW, Yoo BC. Comparison of clinical manifestations and outcomes between hepatitis B virus- and hepatitis C virus-related hepatocellular carcinoma: analysis of a nationwide cohort. PLoS One. 2014;9:e112184. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 48] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 179. | Munaf A, Memon MS, Kumar P, Ahmed S, Kumar MB. Comparison of viral hepatitis-associated hepatocellular carcinoma due to HBV and HCV - cohort from liver clinics in Pakistan. Asian Pac J Cancer Prev. 2014;15:7563-7567. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 18] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 180. | Carr BI, Guerra V, Steel JL, Lu SN. A comparison of patients with hepatitis B- or hepatitis C-based advanced-stage hepatocellular carcinoma. Semin Oncol. 2015;42:309-315. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 181. | Chuang SC, La Vecchia C, Boffetta P. Liver cancer: descriptive epidemiology and risk factors other than HBV and HCV infection. Cancer Lett. 2009;286:9-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 209] [Cited by in RCA: 233] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 182. | Polesel J, Zucchetto A, Montella M, Dal Maso L, Crispo A, La Vecchia C, Serraino D, Franceschi S, Talamini R. The impact of obesity and diabetes mellitus on the risk of hepatocellular carcinoma. Ann Oncol. 2009;20:353-357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 151] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 183. | Liu Y, Chang CC, Marsh GM, Wu F. Population attributable risk of aflatoxin-related liver cancer: systematic review and meta-analysis. Eur J Cancer. 2012;48:2125-2136. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 262] [Cited by in RCA: 217] [Article Influence: 16.7] [Reference Citation Analysis (0)] |