Published online May 14, 2022. doi: 10.3748/wjg.v28.i18.1902
Peer-review started: February 4, 2022
First decision: February 24, 2022
Revised: February 28, 2022
Accepted: March 27, 2022
Article in press: March 27, 2022
Published online: May 14, 2022
Processing time: 96 Days and 23.5 Hours
Crohn’s disease (CD) remains a chronic, incurable disorder that presents unique challenges to the surgeon. Multiple factors must be considered to allow development of an appropriate treatment plan. Medical therapy often precedes or complements the surgical management. The indications for operative management of CD include acute and chronic disease complications and failed medical therapy. Elective surgery comes into play when patients are refractory to medical treatment if they have an obstructive phenotype. Toxic colitis, acute obstruction, perforation, acute abscess, or massive hemorrhage represent indications for emergency surgery. These patients are generally in critical conditions and present with intra-abdominal sepsis and a preoperative status of immunosuppression and malnutrition that exposes them to a higher risk of complications and mortality. A multidisciplinary team including surgeons, gastroenterologists, radiologists, nutritional support services, and enterostomal therapists are required for optimal patient care and decision making. Mana
Core Tip: Crohn’s disease remains a chronic and incurable disorder. Multiple factors must be considered to allow the development of an appropriate treatment. The indications for operative management of Crohn’s disease include acute and chronic disease complications and failed medical therapy. Progression into a complicated phenotype can be characterized by the formation of stenosis or abscesses/fistulas. Elective surgery comes into play when patients are refractory to medical treatment if they have an obstructive phenotype. Indications for emergency surgery include intestinal obstruction, abdominal and perineal sepsis, toxic colitis, or massive hemorrhage.
- Citation: Chiarello MM, Pepe G, Fico V, Bianchi V, Tropeano G, Altieri G, Brisinda G. Therapeutic strategies in Crohn’s disease in an emergency surgical setting. World J Gastroenterol 2022; 28(18): 1902-1921
- URL: https://www.wjgnet.com/1007-9327/full/v28/i18/1902.htm
- DOI: https://dx.doi.org/10.3748/wjg.v28.i18.1902
Crohn’s disease (CD) is a chronic and unremitting inflammatory disease that can involve all the gastrointestinal tract. It was first described by Crohn et al[1] in a case series presented at the American Medical Association annual meeting in 1932, at which time they noted a transmural inflammatory condition of the terminal ileum[1].
The incidence of CD has increased in recent years[2,3]; Ng et al[4] described that the higher prevalence was in Europe (322 per 100000 in Germany) and North America (319 per 100000 in Canada). In the last 30 years, a major increase has also been described in Asia, Africa, and South America[5,6].
There is no agreement between the various authors on the gender predominance[7]. Some case studies propose a female predominance in Western population and a male predominance in Eastern countries; in others there are no differences[8]. The age of presentation shows a bimodal trend with a first peak between 15-30 years (men manifest the disease earlier than women) and a second peak between 60 years and 70 years (mostly women)[9].
CD has a multifactorial etiology[10-12]. It has been described in the literature that patients who had an appendectomy for perforating appendix have a major risk to develop CD, while appendectomy for other causes is linked to a lower risk of CD[13].
The pathogenesis is unclear[5]. An excessive immune response against intraluminal bacteria occurs in the mucosa in susceptible patients and leads to a chronic inflammation that can involve all the gastrointestinal tract[7]. It is localized in the small intestine or proximal right colon with a preference of the terminal ileum in most cases[14]. The histologic findings that characterized the disease are non-caseating granulomas and discontinuous transmural lesions with inflammation penetrating to the muscolaris propria, resulting in structuring and fistulization[15].
Due to the high variability of localization and severity of inflammation, the disease can have different clinical manifestations that range from very mild to severe. Sometimes, the disease can present as an acute and severe form since from beginning[7,14,16,17].
A cure remains elusive, and efficient management of CD is essentially multidisciplinary[18,19]. Surgery is still required even if the number of operations has decreased in recent years mainly for the introduction of biological therapy[19,20].
Uncomplicated forms identified patients with a prevalent inflammatory phenotype[7,19]. On the other hand, patients affected by a form of CD with a progressive complicated phenotype present with stenosis (structuring phenotype) or abscesses/fistulas (penetrating phenotype). Elective surgery comes into play when patients are refractory to medical treatment if they have an obstructive phenotype. According to the existing literature, the risk of surgery at 1, 5, and 10 years after diagnosis of CD is 16.3%, 33.3%, and 46.6%, respectively[21]. It is still controversial whether medical treatment reduces the need for surgery[22] or not[23,24].
The indications for operative management of CD also include acute and chronic disease complications. Since CD is characterized by periods of exacerbation and remission, it is not uncommon that an acute presentation of complications that requires urgent/emergency surgery in 6%-16% of cases[24]. Patients requiring emergency surgery are generally in critical conditions and present with intra-abdominal sepsis and a preoperative status of immunosuppression and malnutrition that exposes them to a higher risk of complications and mortality. Although most acute complications of CD are managed nonoperatively, it is important to recognize those that require a surgical approach. In this review, we aimed to discuss the acute complications of CD and their treatment, focusing on the recent results of surgical, endoscopic, and percutaneous treatments, as individual treatments and as integrated therapy.
Patients with CD, who present to the emergency department with symptoms of acute complications, must be properly framed to formulate an accurate diagnosis and decide what is the best possible treatment. First, a blood chemistry panel must be obtained. It must include full blood count, electrolytes, liver enzymes, renal function, and inflammatory biomarkers, such as C-reactive protein, serum albumin, and fecal calprotectin level. It is fundamental to rule out possible blood infections by carrying out blood and stool cultures and toxin test for Clostridium difficile[7,19].
Typically, the laboratory panel of a patient with acute CD complications is characterized by anemia, increased leukocytosis, thrombocytosis, altered liver and kidney function, and increased inflammatory markers. Neutrophil/lymphocyte ratio, obtained by dividing the absolute number of neutrophils by the absolute number of lymphocytes, is described by many authors as a good predictor of sepsis and in the case of CD as an index of acute disease complicated by the presence of abscesses. In this context, Khoury et al[25] developed a clinical score to predict the presence of intra-abdominal abscess (Table 1). For patients with a low score (≤ 7) the probability of an intra-abdominal abscess is very low, and therefore an urgent computerized tomography (CT) scan can be avoided. On the other hand, a high score (> 9) would warrant urgent imaging for the elevated risk of this intra-abdominal finding. In this scenario, the disease severity indices, such as Harvey-Bradshaw Index[26] (Table 2), Crohn’s disease activity index (Table 3)[27-29], and Prognostic Nutrition Index[30], are also useful. This last index, calculated using the formula Prognostic Nutrition Index = 10 × albumin (g/dL) + 0.005 × total lymphocyte count/μL measured in peripheral blood with a reference value ≤ 40, is widely used to evaluate the nutritional status of the patient and to predict the risk of postoperative complications after gastrointestinal surgery[30,31]. A preoperative low nutritional prognostic index is a useful predictor of postoperative infectious complications in patients with CD-related bowel resection[31,32].
| Parameters | Coefficient estimate | P value | Weight appointed for the score |
| Ileo-colonic location | 0.32 ± 0.16 | 0.04 | × 1 |
| Perianal disease | 1.17 ± 0.32 | 0.0002 | × 3 |
| Absence of current corticosteroids | 0.62 ± 0.21 | 0.003 | × 2 |
| NLR > 11.75 | 1.15 ± 0.17 | < 0.0001 | × 3 |
| CRP > 0.5 mg/dL | 1.67 ± 0.21 | 0.03 | × 5 |
| Variable No. | Variable description |
| 1 | General well-being (0 = Very well; 1 = Slightly below par; 2 = Poor; 3 = Very poor; 4 = Terrible) |
| 2 | Abdominal pain (0 = None; 1 = Mild; 2 = Moderate; 3 = Severe) |
| 3 | Number of liquid stools daily |
| 4 | Abdominal mass (0 = None; 1 = Dubious; 2 = Definite; 3 = Definite and tender) |
| 5 | Complications: arthralgia, uveitis, erythema nodosum, aphthous ulcer, pyoderma gangrenosum, anal fissure, new fistula, abscess (score 1 for item) |
| Variable No. | Variable description | Multiplier |
| 1 | Number of liquid or soft stools (each day for 7 d) | × 2 |
| 2 | Abdominal pain, sum of 7 daily ratings (0 = None, 1 = Mild, 2 = Moderate, 3 = Severe) | × 5 |
| 3 | General well-being, sum of 7 daily ratings (0 = Generally well, 1 = Slightly under par, 2 = Poor, 3 = Very poor, 4 = Terrible) | × 7 |
| 4 | Number of listed complications (arthritis or arthralgia, iritis or uveitis, erythema nodosum or pyoderma gangrenosum or aphthous stomatitis, anal fissure or fistula or abscess, other fistula, fever over 37.8 °C | × 20 |
| 5 | Use of diphenoxylate or loperamide for diarrhea (0 = No, 1 = Yes) | × 30 |
| 6 | Abdominal mass (0 = No, 2 = Questionable, 5 = Definite) | × 10 |
| 7 | Hematocrit (males 47-Hct %, females 42-Hct %) | × 6 |
| 8 | Body weight (1-weight/standard weight) × 100 (add or subtract according to sign) | × 1 |
Abdominal CT scan with intravenous contrast is the first choice of examination for the diagnosis[33]. It allows the evaluation of the presence of complications such as bleeding, perforation, obstruction due to strictures, and the presence of abscesses. The procedure is essential to decide whether to proceed with a surgical treatment or opt for initial nonoperative management[34]. A frequent use of CT scan has been reported for all patients who were admitted to the emergency department for abdominal pain[35], but CD patients are irradiated more often for diagnostic purpose, with a higher exposure to X-rays[36]. Several studies evidenced that CD patients are exposed to CT scan radiation up to 2-3 times per year[36-39]. It is also described that children who are exposed early to CT scan have an increased risk of brain tumor and leukemia[40,41]. Craig et al[41] evaluated a low dose CT protocol, reaching radiation levels as low as a plain abdominal X-ray, which provided similar diagnostic efficacy even with low-quality images. The diagnostic accuracy of magnetic resonance imaging (MRI) enterography/enteroclysis is like CT scan and prevents exposure to ionizing radiations. It has a few detriments such as limited availability, high cost, and time consumption[27].
As recommended by recent guidelines for the management of CD in the emergency setting[7], point of care ultrasonography can have a role in showing free fluid, abscess, or intestinal distension, especially when CT scan is not available. Bettenworth et al[42] in their review showed that sensitivity of the ultrasonography for stricture diagnosis runs from 80% to 100% with specificity rates of 63%-75%. A systematic review by Panés et al[43] revealed that the sensitivity and specificity for ultrasound in the detection of complication are high and comparable to MRI[43].
Since urgent surgery in patients with CD is burdened by a high rate of complications due to intra-abdominal sepsis, immunosuppression, and malnutrition, it is necessary to optimize their general conditions and nutritional status, delaying surgery` if feasible.
Preoperative corticosteroid use is associated with increased risk of postoperative complications[44,45]. Reduction of corticosteroid doses may decrease postoperative complications but should be carefully monitored to avoid increasing CD burden[19]. Meta-analyses of prospective and retrospective studies reported up to a doubling of surgical site infection for patients on steroids[46,47].
The application of biologic therapy before urgent surgery is still unclear. The PUCCINI trial showed no effect of anti-tumor necrosis factor α on postoperative complications[48]. Current evidence suggests that preoperative treatment with vedolizumab or ustekinumab does not increase the risk of postoperative complications in patients with CD having abdominal surgery, and cessation of these therapies prior to surgery is not mandatory[49-51].
Malnutrition is an independent risk factor for postoperative poor outcomes[52,53]. Preoperative nutritional assessment should be performed for all patients with CD who need surgery[19]. The European Society for Clinical Nutrition and Metabolism stated that serum albumin of < 3 g/dL, body mass index < 18.5 kg/m2, and weight loss > 10%-15% within 6 mo are the best indicators of severe malnutrition in CD patients[54]. The nutrition of choice should be the enteral nutrition that can be replaced with the parenteral one only in those patients who cannot tolerate the first or have severe contraindications such as a high flow intestinal fistula, intestinal perforation, or intestinal ischemia[55,56]. Brennan et al[57] observed that patients who received preoperative nutritional support had a rate of postoperative complications of 20% compared with 61.3% in the group without nutritional support. Zhu et al[58] suggested that enteral nutrition is feasible in CD patients with non-radiologically-drainable abdominal abscess.
Acute bowel obstruction, intra-abdominal sepsis, hemorrhage, acute toxic colitis, and perianal sepsis are the indications for an emergency procedure in patients with CD. The incidence of acute complications of CD are shown in Table 4.
| Complication | Incidence (%) | Ref. |
| Small bowel obstruction | 35.0-59.0 | [63] |
| Gastroduodenal obstruction | 0.5-15.0 | [64] |
| Colonic strictures | 5.0-17.0 | [73] |
| Intra-abdominal abscess | 10.0-28.0 | [85,156] |
| Free bowel perforation | 1.0-6.5 | [104,105] |
| Acute appendicitis | 0.1-2.0 | [113] |
| Bleeding | 1.0-6.0 | [126] |
| Perianal disease with left-sided colon or rectal involvement | 17.0-43.0 | [145] |
| Isolated perianal involvement | 5.0 | [146] |
Acute surgical emergencies in patients with CD are associated with high morbidity and mortality. Postoperative mortality rates for nonelective surgeries in CD have decreased but remain significantly higher than the mortality rate following elective surgery (3.6% vs 0.6%)[59,60]. Although most acute complications of CD are managed nonoperatively, it is important to recognize those that require surgical management. Another issue is the prevention of recurrence, which is still much debated.
Acute intestinal obstruction is the most frequent complication in patients with CD, especially in patients with ileo-colic localization (35%-54%), while in jejunal (22%-36%) or colic (5%-17%) localizations the frequency is lower[19,61-63]. Gastroduodenal obstruction is rare, occurring in 0.5% to 13% of patients[62,64]. Patients with gastroduodenal strictures present with postprandial fullness, early satiety, vomiting, and upper abdominal pain[65,66]. Patients with obstruction of the small intestine present with nausea, vomiting, dehydration, crampy abdominal pain, bloating, obstipation, and constipation. Patients with colonic obstruction may present without nausea and vomiting but with bloating, distension, and abdominal pain, in addition to constipation. Initial management of bowel obstruction includes bowel rest, intravenous hydration, and nasogastric decompression in the presence of vomiting[42].
Strictures complicating CD can be classified as inflammatory or fibrostenotic[67]. Patients with inflammatory disease deserve medical therapy, such as steroids for reducing inflammation. Patients who do not improve with medical therapy and in whom an occlusion due to fibrosis can be hypothesized need receive a CT scan or an MRI. In these patients, an interventional approach, endoscopic or surgical, is usually needed[7]. Deferred surgery is the preferred option in adult patients with small bowel obstruction without bowel ischemia or peritonitis. Endoscopic balloon dilatation (EBD) is a suitable treatment option for patients with short (< 5 cm) strictures[68]. For fibrotic strictures, EBD has a technical success rate of 89% to 92%, with 70% to 81% of patients presenting free of occlusive symptoms in the short term[69]. In contrast for the long term, 73.5% of patients require repeat dilatation and 43% a surgical intervention within 2 years[70]. Fully covered self-expandable metal stents (FCSEMS) have been used for endoscopic treatment of patients for whom EBD was unsuccessful. In a multicenter, open-label, randomized trial[71], 80 patients were randomly assigned to treatment: 39 (49%) patients to the FCSEMS group and 41 (51%) patients to the EBD group. Overall, 33 (80%) of 41 patients in the EBD group and 20 (51%) of 39 patients in the FCSEMS group could avoid a new therapeutic intervention in 1 year (odds ratio: 3.9; 95% confidence interval: 1.4–10.6; P = 0.0061). Two of 80 patients had perforations (1 patient in the EBD group and 1 patient in the FCSEMS group)[71]. EBD is more effective than FCSEMS for CD strictures, with a good safety profile for both the treatments.
Surgery is mandatory for intestinal obstruction with potential impending perforation, with long or multiple strictures, when the strictures are not endoscopically accessible, when medical or endoscopic treatment does not result in an adequate reduction of clinical symptoms, or when there is a suspicion of an underlying malignancy[7,72,73]. If emergency surgery is indicated, a laparoscopic approach is recommended when appropriate expertise exists[74,75].
The surgical treatment of choice is a segmental resection of the involved segment with primary anastomosis. In fact, the most important principle in the surgical management of CD is to resect only the segment of bowel affected by the complication to prevent short bowel syndrome. Even if adjacent areas of bowel are clearly diseased, that should be ignored[76]. Furthermore, when two intestinal segments are involved, but with a healthy segment in between, the resection of the healthy segment depends on its length, too[77]. If it is 10 cm or longer, it probably should be saved. If it is 5 cm or shorter it should be included. In the other cases, the surgical attitude will depend on previous resection, configuration of blood supply, and mobility of the mesentery. The anastomosis could be either hand-sewn or stapled, end-to-end or side-to-side[78]. The literature does not describe a superiority of one technique over another, and the final decision can be left to the surgeon preference[78-80]. If a stapled anastomosis is performed, some authors advise performing it with an isoperistaltic configuration to allow an easier subsequent endoscopic surveillance. If the bowel wall is edematous due to chronically inflammation, a hand-sewn anastomosis would be better[80]. The general conclusion favors stapled side-to-side anastomosis. The diameter of the anastomosis plays a role as well, with the assumption that a wider anastomosis will have a lower rate of clinical and surgical complications[81,82].
Other surgical options include stricturoplasty, intestinal bypass, and creation of ileostomy. Short fibrous strictures, multiple strictures, short bowel syndrome, rapid recurrence of obstruction due to CD, and duodenal CD are indications for stricturoplasty. Contraindications to stricturoplasty are multiple strictures in a short intestinal segment, a long stricture (> 20 cm), or stricture close to the resection site[83]. Bypass operations have largely been abandoned because of the subsistence of disease activity[83]. An ileostomy is required when it is not safe to perform an anastomosis, such as in case of severe patient instability, malnutrition, intra-abdominal sepsis, chronic immunosuppression, or when a small bowel resection is performed together with colonic or rectal resection[76,79].
The presence of an abscess occurs in up to 20% of CD patients[84-86]. It typically results from a penetrating disease phenotype with a distal stricture[80]. An abscess is categorized as active at Harvey-Bradshaw Index[26] and severe with a Crohn’s disease activity index > 450[27-29]. Abscess is defined as an inflammatory mass consequent to bowel perforation promptly covered by fibrin that adheres to the loops surface causing adhesions among them. It can be associated with other CD complications such as fistulas (40%) and stenosis (51%)[14,85]. Usually, these patients present to the emergency department with fever associated with abdominal pain, palpable masses, and rebound tenderness. In 28% of cases they can have a generalized sepsis[84]. Therefore, it is crucial that they receive a proper clinical evaluation. If an abscess is suspected, cross-sectional imaging is recommended. CT scan is the gold standard for their detection in the emergency setting, and it also identifies concomitant fistulae or bleedings. MRI has a similar accuracy with a lower ionizing radiation exposure, but it is not always accessible in the emergency department. Point of care in the United States can have a role in the initial assessment, and it is extremely useful in rural hospitals where CT is not available[7,87,88]. Moreover, if there is an indication to the positioning of a percutaneous drainage, both ultrasonography and CT can be used as guides[86,89]. In selected cases, endoscopic ultrasound can be employed for draining the cavity by a transvaginal or transrectal approach[90,91].
When the abscess diagnosis is confirmed, the best patient tailored treatment must be defined. In stable patients with abscesses smaller than 3 cm, early empiric antimicrobial therapy is recommended with a close clinical and biochemical monitoring. If the abscess is > 3 cm, percutaneous drainage with antibiotics is endorsed[7,89]. The drainage approach depends on the abscess site. It can be transgluteal for pelvic abscesses but also transabdominal. The catheter, whose size must be chosen considering the viscosity of the fluid collected, is left in place, and the daily quantity and quality of the drain must be recorded carefully[84,92].
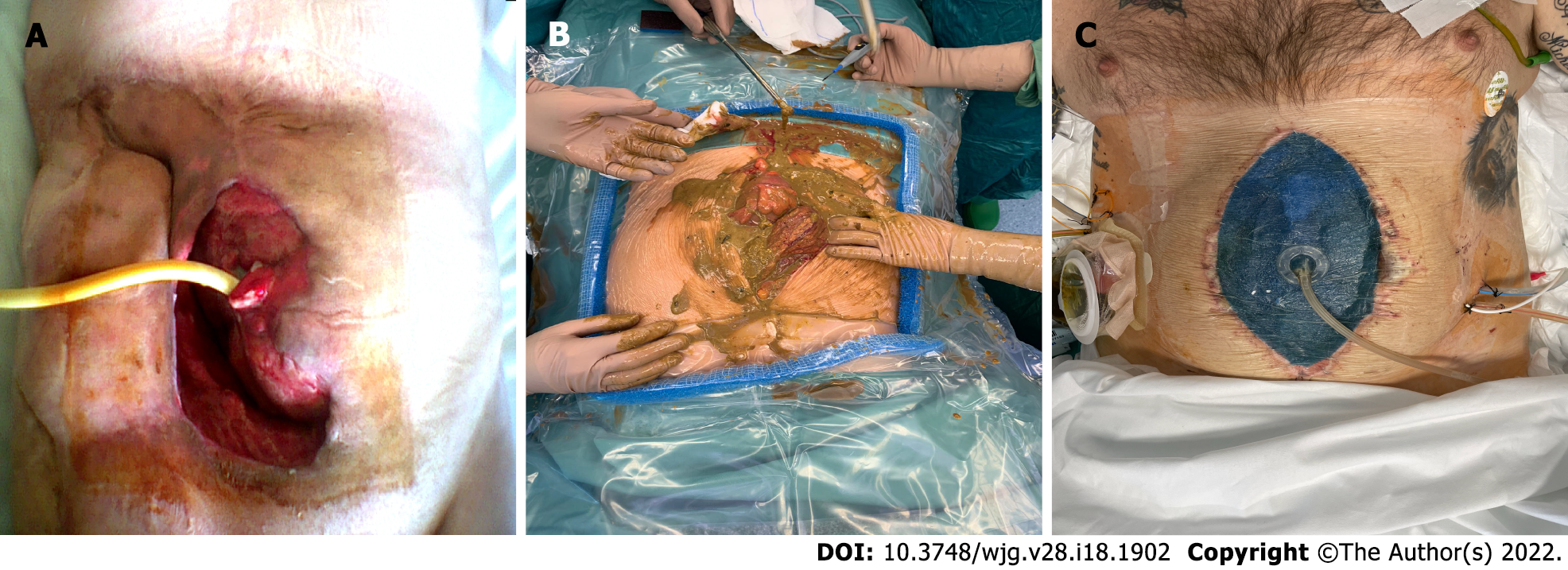
Percutaneous drainage is a validated procedure because it may avoid an immediate operation, delaying to elective surgery[93,94]. Inflammation will have enough time to resolve, making possible limited intestinal resections and reducing the need of a diverting stoma creation. In addition, the nutritional status could be optimized and steroids interrupted before surgery, reducing the risk of anastomotic leak[53,84]. According to some studies, percutaneous drainage is a bridge to definitive surgical treatment. On the other hand, more recent studies have demonstrated that most patients do well with only percutaneous drainage and do not need any other surgical treatment with success rates that vary from 50% to 95%. The risk of recurrence, more often within 3 years, must be considered[95-97]. Immediate surgery should be considered in cases of patients with septic shock or failure of percutaneous drainage due to multiple and multilocular abscesses and those associated with a fistula (Figure 1A)[84,98].
Another key factor in the conservative treatment in CD patients with abscess is antibiotic therapy. Therefore, it is important to collect a sample of drained fluid and send it for a microbiological test. The culture can reveal the type of microorganisms in the pus, allowing the optimization of the antimicrobial therapy, increasing the chances of conservative treatment success. Reuken et al[99] found that the culture was positive in about 88% of patients and usually polimicrobic. The most common pathogens were Escherichia coli, Streptococcus spp., Enterococci, Candida, and anaerobes. The most used empirical first-line antibiotics were broad spectrum penicillins, followed by cephalosporins and quinolones[99]. These treatments were inadequate in patients on immunosuppression or with a prolonged hospitalization and that could be linked to a higher risk of failure[80]. The appropriate duration of antibiotic therapy is unclear. Also, anti-tumor necrosis factor therapy can have a role in the treatment of an abscess, but only if associated to antimicrobial treatment that must be continued for 3 mo[100].
After antimicrobial treatment and percutaneous drainage clinical improvement is expected in 3–5 d, with a gradual decrease in the drain output. According to the American Society of Colon and Rectal surgeons, after a successful resolution of an abscess with percutaneous drainage and antibiotics, a drain study to exclude a concomitant fistula could be beneficial[80]. If a patient’s condition does not improve, a re-evaluation is mandatory[84], and if the abscess has not been adequately drained, repositioning of the drain or an operation must be considered.
When a surgical exploration must be performed for conservative treatment failure or for concomitant CD complications, some issues need to be considered. First, if the patient is unstable, an open approach is recommended, considering the damage control surgery principles. There is also room for open abdomen use. An operation with a bowel resection gives better results than an operative drainage alone, which determines a higher rate of reoperation and enterocutaneous fistula formation. In case of septic shock, it may be appropriate to perform bowel resection with stapled off bowel stumps, peritoneal lavage, and open abdomen with a planned second exploration in the next 24-48 h after the patient has been stabilized in the intensive care unit. At this point, surgeons must decide whether to create a stoma or anastomize the stumps[89,101]. In the case of a stable patient and technical skills available, laparoscopy is recommended to reduce hospital stay and morbidity. Both open and laparoscopic extensive resections must be avoided because they can lead to immediate or future short bowel syndrome in these patients[80].
Even after success of conservative treatment, patients with a concomitant stenosis, an enterocutaneous fistula, or refractory active disease are likely to require surgery[102]. It is believed that a window of 6 to 8 wk after the resolution of the acute clinical scenario could permit a good improvement of the clinical and nutritional status as well as a proper imaging and endoscopic work-up. This work-up aims to avoid unexpected findings during the elective operation[93]. Moreover, as already discussed, the surgical outcomes will be better and comparable to CD patients with non-penetrating disease, as it is also confirmed by recent research[103]. In their study on 592 patients, Collard et al[103] compared patients who underwent an ileocolonic resection after a conservative treatment for an abscess to CD patients without a penetrating disease who had the same operation[103]. In the first group, all the patients underwent a CT scan after the percutaneous drainage to demonstrate a complete resolution of the abscess. Nevertheless, the persistence of an abscess, defined by the presence of a pus-like or purulent collection in the site of the original abscess, was found in 25% of cases. There were no differences between the two groups for rate of primary anastomosis, need of additional intestinal resection, incidence of intraoperative and postoperative complications, and length of hospital stay.
Free peritoneal perforation in CD is a rare condition that occurs in 1%-3% of CD patients as a first manifestation or in the course of the disease[104,105]. It is one of the main indications for emergency surgery with a high rate of morbidity and mortality in the case of delayed surgery[104]. Greenstein et al[106] reported that perforation can occur in the ileum or in the colon indifferently, without a preference for location. Instead, the Connect study of Doh et al[105] found that ileal perforation had a higher incidence (86.2%), while colonic perforation occurred in only 7.4% of the patients. Some authors described that steroid treatment increases the risk of free perforation[107-110]. The role of anti-tumor necrosis factor agents in the etiopathogenesis of free perforation is not clear yet[111]. The mechanism leading to free perforation is still unclear, but there are two most likely hypotheses. The first and more conceivable is that the increase of intraluminal pressure proximal to a stenosis causes a bowel distension that can perforate the thin layer overlying a deep ulcer, while the second suggests that the perforation is consequent to intestinal ischemia due to inflammation of the small vessels[105].
The intestinal segment affected by the perforation should be resected by either directly performing a primary anastomosis or proceeding with an enterostomy. According to our practice, in the case of severe abdominal contamination (Figure 1B), we recommend not to close the laparotomy but to perform a second look as already described by Liu et al[112], using the technique of open abdomen with negative pressure wound therapy (Figure 1C). In this way, it is possible to prevent intra-abdominal sepsis and during the second look, optimize the resection. In some selected cases, a primary anastomosis could be feasible as the vacuum system allows a good intra-abdominal clearance. We also recommend this technique in the setting of damage control surgery with a hemodynamically unstable patient, when the decision to perform an enterostomy will be postponed to the second look.
Appendiceal CD is a rare condition, whose incidence varies from 0.1% to 2.0%[113]. After the first case described in 1953[114], there was an initial interest in the topic with most of the cases described in the late 1980s and early 1990s[115,116]. In 1990, Ruiz et al[117] identified 85 cases of CD limited to the appendix[117]. The disease was found most frequently in the second and third decades. The authors suggest that a prolonged preoperative history of symptoms should alert the surgeon to this possibility[117]. Lindhagen et al[118] identified 50 cases and added 12 of their own[118]. The indication for surgery in their patients were appendicitis in 8 patients, appendiceal abscess in 2 patients, suspected pyosalpinx in 1 patient, and ovarian cyst in 1 patient. All but 2 of the 8 appendicitis patients underwent appendectomy; the others underwent a more extensive resection. There were no postoperative complications. The authors concluded that when CD is confined to the appendix the prognosis is very favorable[118].
When considering acute appendicitis in CD patients, it is essential to differentiate between patients admitted to the emergency department with a possible appendicitis who already have been diagnosed with CD and patients who underwent an appendectomy. The histopathology reveals the disease. In a recent study, a group from Seoul analyzed 2179 appendectomies. Among these only 12 (0.55%) were compatible with CD at histology[113]. The 12 patients presented to the emergency department with right lower quadrant pain, abdominal pain, and diarrhea. The duration of symptoms varied from 2 to 5 mo[113]. They did not present systemic manifestations such as uveitis, arthralgia, and arthritis. The histological examination revealed transmural inflammation, lymphoid aggregates, and epithelioid granulomas as peculiar features present in all the cases, followed by appendiceal wall thickening and mucosal ulceration. When the diagnosis is made, the patient must be referred to a tertiary center for future gastroenterology follow-up.
Sometimes the appendix inflammation is consequent/reactive to terminal ileitis in patients affected by CD. Since the acute presentation is like common appendicitis, it is crucial to focus on symptoms like recurrent abdominal pain or diarrhea, lack of fever, weight loss, microcytic anemia, hypoalbuminemia, and hypoproteinemia. In fact, these symptoms are more typical of CD rather than appendicitis. If there is a suspicion of CD, the entire small and large bowel should be carefully examined to verify the presence of the disease and its extent.
In patients with known CD, medical treatment for ileocecal CD is recommended. In fact, if in the rare cases of CD localized only in the appendix, the appendectomy can be performed safely. The clinical scenario is completely different when there is active ileocecal disease. In this situation, there are some issues that need to be considered. The main goal is to avoid an ileocecal resection, when possible, for limiting the unnecessary excision of intestinal segments. An ileocecal resection is the operation of choice in the case of cecum involvement to reduce the risk of leak from the appendix stump and formation of enterocutaneous fistula. Furthermore, if the patient has experienced severe symptoms due to ileitis, an ileocecal resection is the treatment of choice, followed by biologic therapy. Otherwise, the patient will continue suffering CD symptoms with 38% requiring a resection within 1 year. Finally, it is not necessary to remove a macroscopically normal appendix during an exploration for suspected appendicitis if there are findings that raise doubts of CD[119].
Toxic colitis represents a potentially fatal condition that may in occur in patients affected by inflammatory bowel disease. Studies have demonstrated that as many as 50% of all toxic colitis cases may be secondary to Crohn’s colitis[72,120,121]. Diagnostic criteria include: Severe diarrhea (6 or more bloody bowel movements per day); temperature higher than 37.8 °C; heart rate > 90 beats/min; anemia (hemoglobin < 10.5 g/dL); an elevated erythrocyte sedimentation rate (< 30 mm/h); hypoalbuminemia (< 3.0 g/L); and leukocytosis (> 10.5 × 109 cells/L)[122]. Toxic megacolon is defined when radiographic imaging demonstrating dilation of the transverse colon to at least 6 cm is associated to the previously mentioned criteria[7,72]. Cytomegalovirus infection has been identified in 25% to 36% of patients with steroid-refractory acute colitis. Studies have demonstrated that Clostridium difficile infection in the setting of severe or toxic colitis is associated with increased morbidity and mortality.
When toxic colitis is diagnosed, intensive medical therapy should be promptly administered. Intravenous methylprednisolone, 60 mg per day, or hydrocortisone, 300 mg, are the medications of choice[123]. Treatment with higher doses of intravenous corticosteroids have not been demonstrated to reduce colectomy rate and should not be considered. In general, a response to steroid therapy includes improvement or resolution of the systemic inflammatory response syndrome and a decrease in stool frequency. In the case of overall clinical improvements over the course of the following 3 d, maintenance therapy could be considered. If there is no response to corticosteroid therapy, rescue therapy is used. Infliximab has now emerged as the most widely used rescue therapy due to its low toxicity, ease of administration, and consequently the possibility to be used for maintenance therapy. One recent literature review demonstrated immediate response rates to infliximab as a rescue therapy agent range between 50% and 83%, with long-term response rates slightly lower at 50% to 63%[124].
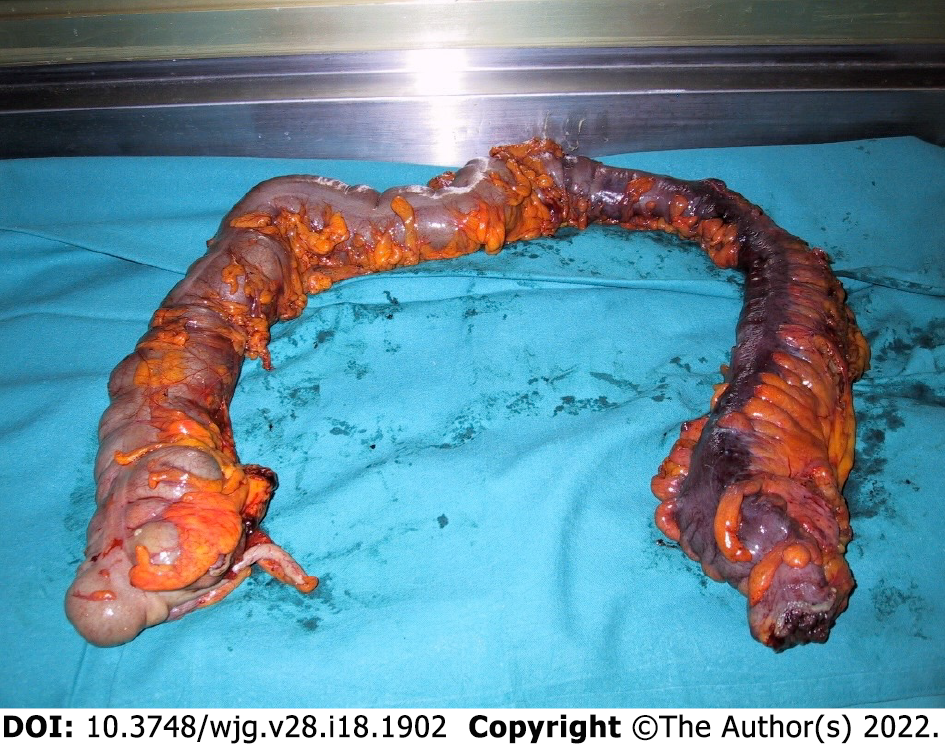
Contrarily, in the case of worsening clinical condition or failure to improve, a transition to either rescue therapy or an urgent colectomy should be taken into account. If a patient presents with peritonitis or evidence of free perforation on imaging, surgical exploration is mandatory. Perforation in patients with toxic megacolon is associated with a high mortality rate (27%–57%), regardless of whether the perforation is contained or free[7]. The transverse colon is the area of greatest concern in toxic megacolon. The surgical option of choice is subtotal colectomy with end ileostomy, which allows for future reconstructive options avoiding permanent stoma by ileorectal anastomosis (Figure 2). This could be suggested for patients without rectal or perianal involvement. A total proctocolectomy should be avoided even if total proctocolectomy with permanent stoma is the safest option to avoid further operation in high-risk patients[72] because it is associated with greater postoperative morbidity and mortality due to increased operative time, risk of blood loss, pelvic sepsis, and pelvic nerve damage. The patient typically improves over the ensuing few days. An ileoproctostomy can be recommended 6 mo later in selected patients who demonstrate minimal mucosal inflammation, adequate rectal compliance, absence of significant anoperineal disease, and sufficient sphincter strength[72].
Gastrointestinal bleeding is a common complication in patients affected by CD. Inflammation/ ulceration of the bowel are the basis of its onset. Usually, conservative treatment and endoscopy are sufficient to resolve this condition that rarely requires an emergency surgical procedure. Severe intestinal hemorrhage, on the other hand, is a rare occurrence in CD, with reported incidence between 0% and 6%[125,126]. The underlying pathophysiology of the hemorrhage most often is a result of focal erosion into an intestinal vessel. Due to the infrequency of significant bleeding in CD, as stated before, all common causes of upper and lower gastrointestinal bleeding should be assessed. Localization of the bleeding site is essential regardless of the planned therapy.
Management of gastrointestinal bleeding related to CD begins with hemodynamic assessment. In a stable patient the localization of the lesion could be identify by endoscopy or radiology. Nasogastric tube, in case of hematemesis, should be inserted to protect the airway and decompress the stomach. Gastroscopy could be required to rule out upper gastrointestinal bleeding. If a lower gastrointestinal source is suspected, a retrospective study[127] reported that the detection rate for vascular lesions was higher for colonoscopy following contrast-enhanced CT scan than for colonoscopy alone.
The role of angiography and of angioembolization must be still clarified. Most of the data suggest that angiography/angioembolization could be feasible in stable patients, but further studies are needed to better define outcomes in the emergency setting[7]. The major advantage is that it can control severe bleeding without bowel preparation. Disadvantages include the risk of bowel ischemia and the administration of intravenous contrast. In particular the rate of bowel ischemia following embolization was 1%–4% in recent studies[128].
In the case of hemodynamic instability, resuscitation by intravenous fluid/blood product administration is fundamental aiming to normalize blood pressure and heart rate prior to endoscopic evaluation/intervention. Packed red blood cells should be transfused to maintain the hemoglobin above 7 g/dL. A threshold of 9 g/dL should be considered in patients with massive bleeding, significant comorbidities (especially cardiovascular ischemia), or possible delay in receiving therapeutic interventions[7]. The decision to perform an urgent surgical procedure depends on the bleeding source itself, the hemodynamic condition of the patient, and on the availability/feasibility of less invasive therapeutic options (i.e. endoscopy or angiography). In the case of persistent hemodynamic instability, operative management is necessary. The most challenging situation is represented by patients who have a primary or recurrent hemorrhage without an identifiable source of bleeding. In these cases, the likelihood of successfully eradicating the source of hemorrhage through a “blind” intestinal resection must be weighed against the risk of recurrent bleeding and of creating short bowel syndrome in patients with extensive disease or multiple previous resections.
Postoperative recurrence is the appearance of new lesions after surgery for CD. After the first intestinal resection, 25%-45% of patients are likely to need further operations within 10 years[129]. One year after an initial resection, 60%-80% of patients possess endoscopic recurrence, 10%-20% experience clinical relapse, and 5% demonstrate operative recurrence[130]. Diagnosis of postoperative recurrence is primarily based on clinical symptoms and endoscopic findings. Rutgeerts et al[131] developed an endoscopic scoring system to assess endoscopic recurrence (Table 5).
| Grade | Endoscopic findings |
| 0 | No endoscopic recurrence |
| 1 | ≤ 5 aphthous lesions |
| 2 | > 5 aphthous lesions with normal mucosa between the lesions, or skip areas of larger lesions, or lesions confined to ileocolonic anastomosis |
| 3 | Diffuse aphthous ileitis with diffuse inflammation of the mucosa |
| 4 | Diffuse inflammation with larger ulcers, nodules, and/or narrowing |
Therefore, ileocolonoscopy is the gold standard to identify the presence and severity of CD recurrence, and it is recommended within the first year after surgery[20]. An emerging and less invasive method to identify postoperative recurrence is the small intestine contrast ultrasonography, which is comparable to ileocolonoscopy to detect lesions related to CD[132]. Fecal calprotectin is another noninvasive diagnostic and monitoring tool because a later correlation was found between endoscopic severity and calprotectin levels[133].
In the last few decades, the risk of major abdominal surgery within the first 5 years of diagnosis has declined, due to the advances in medical treatment of CD. However, there is still a great debate in the literature about risk factors for postoperative recurrence in Crohn’s disease (Figure 3). According to the European Crohn’s and Colitis Organization guidelines, smoking, prior intestinal surgery, penetrating disease, absence of prophylactic treatment, perianal location, presence of granulomas in the surgical specimen, and myenteric plexitis are considered predictors of early postoperative recurrence after ileocolonic resection[20]. Santiago et al[134] identified the presence of colic disease as an independent risk factor for postoperative recurrence and reoperation, while colectomy or proctocolectomy performed during the first surgery have a protective role against reoperation in patients with CD[134]. Liu et al[112] in 2020 published a series on the comparison between primary anastomosis and staged surgery in emergency treatment of complicated CD. The results of this study showed a lower surgical recurrence rate for staged surgery, probably linked to the reduction of inflammatory activity when a fecal diversion is performed[112]. A young age, less than 16 years, was identified as another risk factor for repeated abdominal surgery in CD patients by a multicenter study of the Korean Inflammatory Bowel Disease Study Group as well as the presence of stricturing disease behavior[135].
Other recent fields of investigation focus on the role of anastomotic technique in the pathogenesis of postoperative recurrence. In 2014 and 2018, two meta-analyses were performed to define the optimal surgical technique of anastomosis. The conclusions were that stapled isoperistaltic side-to-side anastomosis, because of its larger lumen, can significantly reduce the incidence of postoperative complications and recurrence[136,137]. In 2011, Kono et al[138] introduced a new anastomotic technique. The Kono-S is an antimesenteric functional end-to-end hand sewn anastomosis, with the two stumps sutured to create a “supporting column,” which allows preservation of the diameter and prevents stenosis and distortion of the anastomosis[138]. The SuPREMe-CD Study, a randomized clinical trial that compared Kono-S anastomosis and stapled side-to-side anastomosis, confirmed the reduction of postoperative recurrence when the Kono-S anastomosis was performed[139].
Few studies in the literature analyzed the correlation between emergency surgery for CD and postoperative recurrence. However, emergency surgery would seem to be another independent risk factor for surgical recurrence[140]. Urgent surgery may be related to more aggressive disease, and greater variation in the anastomotic technique in urgent surgery may affect the risk of recurrence[81]. Furthermore, a recent paper highlights that an end-to-end anastomosis is associated with an increased risk of early postoperative recurrence in the subset of patients undergoing an emergency operation as well as smoking and the failure to commence early medical prophylaxis[141].
Another important topic about the risk of recurrence in CD is represented by the role of the mesentery. Patients with CD are characterized by a peculiar form of adipose tissue hypertrophy, known as “creeping fat”[142], which seems to be an independent predictor of early clinical recurrence of CD when there is an advanced mesenteric involvement in the surgical specimen[143]. An international, multicenter, randomized controlled trial by Li et al[144] is ongoing to compare conservative limited resection and surgery with mesenteric excision in CD, and its results may provide important insights about the most appropriate surgical technique. One of the most common components of CD that manifests itself after proctocolectomy is the recurrence of disease in the ileostomy or remaining small bowel.
Perianal fistulas and abscesses are common in CD with an incidence up to 43% and are more frequent in patients with distant colonic or rectal disease[145,146]. They require a multidisciplinary (both medical and surgical) approach to achieve a complete fistula healing and reduce the negative effect on the patient’s lifestyle[147,148].
Abscess is a consequence of cryptoglandular inflammation/infection as described by Eisenhammer[149] in 1956 and can be the first sign of CD. It may result in a fistula that is classified based on its location as described by Parks and Gordon[150], in up to 40% of cases. Both abscesses and fistulas are manifestations that can bring the patient to emergency physicians attention (Table 6)[151].
| Categories affected by fistulas | Score |
| Discharge | |
| No discharge | 0 |
| Minimal mucous discharge | 1 |
| Moderate mucous or purulent discharge | 2 |
| Substantial discharge | 3 |
| Gross fecal soiling | 4 |
| Pain/restriction of activities | |
| No activity restriction | 0 |
| Mild discomfort, no restriction | 1 |
| Moderate discomfort, some limitation of activities | 2 |
| Marked discomfort, marked limitation | 3 |
| Severe pain, severe limitation | 4 |
| Restriction of sexual activity | |
| No restriction sexual activity | 0 |
| Slight restriction sexual activity | 1 |
| Moderate limitation sexual activity | 2 |
| Marked limitation sexual activity | 3 |
| Unable to engage in sexual activity | 4 |
| Type of perineal disease | |
| No perineal disease/skin tags | 0 |
| Anal fissure or mucosal tear | 1 |
| < 3 Perianal fistulae | 2 |
| ≥ Perianal fistulae | 3 |
| Anal sphincter ulceration or fistulae with significant undermining of skin | 4 |
| Degree of induration | |
| No induration | 0 |
| Minimal induration | 1 |
| Moderate induration | 2 |
| Substantial induration | 3 |
| Gross fluctuance/abscess | 4 |
Most patients with a perianal abscess present with pain, swelling, and redness of the interested area. High abscesses also known as supraelevator abscesses do not extend to the more superficial perianal tissues and are not associated to visible and palpable inflammatory signs but more often to systemic signs like fever and malaise[7,149]. If a supraelevator source is suspected, a CT scan or MRI is useful because it gives more information about the abscess extension but also on eventual associated intrabdominal findings[7].
When approaching a patient with a perianal abscess or fistula, the initial goal must be the sepsis control. The early drainage is the only accepted treatment for this purpose, eventually associated to gram negative and anaerobic spectrum antibiotics in patients on immunosuppression and in those with septic shock[152,153]. It is recommended to leave a Petzer or a Malecot catheter in wide ischiorectal abscesses[147,153]. It is better to perform the drainage under general anesthesia, especially if the patient is in pain, because this will allow a better exploration.
As a result of the inflammation, it could be challenging to find the underlying fistula, and unsuccessful attempts could cause tissue destruction and create an iatrogenic track. There is no evidence to support wound packing following the drainage. Nevertheless, it could have a hemostatic role in the case of bleeding. If the fistula track is evident, a loose draining seton should be inserted. There should be no attempt to lay the fistula open at the same time to preserve future anal function whose damage would negatively impact the patient’s lifestyle[153].
In the emergency setting, there is no room for treatment such as fibrin glue, fistula plug, ligation of the intersphincteric fistula tract, advancement flap, and stem cells. These will be considered when the acute process is completely solved. At this point, the patient will be referred to a tertiary center, and a multidisciplinary team will decide the best treatment plan[154]. The work-up will also include a sigmoidoscopy to define if perianal sepsis was associated to an active proctitis[147].
A recent United Kingdom survey summarized the state of the art on acute management of perianal sepsis in CD; 32% of the responding surgeons (133/179) confirmed that they would drain sepsis, 31.1% would consider placement of a seton, and only 0.6% would consider excision of the fistula track. That is in accordance with the previous evidence. When further investigations are needed, MRI is the imaging procedure of choice (96.1%). Most respondents (42.2%) would start antimicrobial therapy preoperatively, and the antibiotic of choice is metronidazole (77.9%). In the case of recurrent sepsis, most respondents created a diverting stoma. In fact, as demonstrated in other studies, it may improve quality of life in patients with severe and recurrent perianal CD[155,156].
CD is a chronic and recurrent inflammatory disease that can present with acute complications. The patient’s clinical status and assessment of the background disease location and severity must be considered to guide diagnosis and treatment of the acute complications. CD is unremitting and incurable. Therefore, treatment aims to induce a deep and prolonged remission, prevent and address complications of the disease, avoid extensive bowel resections, and improve quality of life. Acute surgical emergencies in patients with CD are associated with a high morbidity and mortality. Although most acute complications of CD are managed nonoperatively, it is important to recognize those that require surgical management.
The authors thank Dr Neill James Adams who supervised the writing of the manuscript and proofread the text for the appropriate use of the English language, grammar, punctuation and spelling.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Surgery, gastroenterology and hepatology
Country/Territory of origin: Italy
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Keese D, Germany; Matsumoto S, Japan S-Editor: Fan JR L-Editor: Filipodia P-Editor: Fan JR
| 1. | Crohn BB, Ginzburg L, Oppenheimer GD. Landmark article Oct 15, 1932. Regional ileitis. A pathological and clinical entity. By Burril B. Crohn, Leon Ginzburg, and Gordon D. Oppenheimer. JAMA. 1984;251:73-79. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 38] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 2. | Armuzzi A, Riegler G, Furfaro F, Baldoni M, Costa F, Fortuna M, Iaquinto G, Paese P, Papi C, Bossa F, Tontini GE, Di Fino S, Gualberti G, Merolla R, Rizzello F. Epidemiological features and disease-related concerns of a large cohort of Italian patients with active Crohn's disease. Dig Liver Dis. 2019;51:804-811. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 3. | Terlizzi EP, Dahlhamer JM, Xu F, Wheaton AG, Greenlund KJ. Health Care Utilization Among U.S. Adults With Inflammatory Bowel Disease, 2015-2016. Natl Health Stat Report. 2021;1-7. [PubMed] |
| 4. | Ng SC, Shi HY, Hamidi N, Underwood FE, Tang W, Benchimol EI, Panaccione R, Ghosh S, Wu JCY, Chan FKL, Sung JJY, Kaplan GG. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: a systematic review of population-based studies. Lancet. 2017;390:2769-2778. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2677] [Cited by in RCA: 4106] [Article Influence: 513.3] [Reference Citation Analysis (110)] |
| 5. | Kaplan GG, Windsor JW. The four epidemiological stages in the global evolution of inflammatory bowel disease. Nat Rev Gastroenterol Hepatol. 2021;18:56-66. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 187] [Cited by in RCA: 766] [Article Influence: 191.5] [Reference Citation Analysis (0)] |
| 6. | GBD 2017 Inflammatory Bowel Disease Collaborators. The global, regional, and national burden of inflammatory bowel disease in 195 countries and territories, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet Gastroenterol Hepatol. 2020;5:17-30. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1466] [Cited by in RCA: 1455] [Article Influence: 291.0] [Reference Citation Analysis (0)] |
| 7. | De Simone B, Davies J, Chouillard E, Di Saverio S, Hoentjen F, Tarasconi A, Sartelli M, Biffl WL, Ansaloni L, Coccolini F, Chiarugi M, De'Angelis N, Moore EE, Kluger Y, Abu-Zidan F, Sakakushev B, Coimbra R, Celentano V, Wani I, Pintar T, Sganga G, Di Carlo I, Tartaglia D, Pikoulis M, Cardi M, De Moya MA, Leppaniemi A, Kirkpatrick A, Agnoletti V, Poggioli G, Carcoforo P, Baiocchi GL, Catena F. WSES-AAST guidelines: management of inflammatory bowel disease in the emergency setting. World J Emerg Surg. 2021;16:23. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 34] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 8. | Mak WY, Zhao M, Ng SC, Burisch J. The epidemiology of inflammatory bowel disease: East meets west. J Gastroenterol Hepatol. 2020;35:380-389. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 158] [Cited by in RCA: 413] [Article Influence: 82.6] [Reference Citation Analysis (0)] |
| 9. | Molodecky NA, Soon IS, Rabi DM, Ghali WA, Ferris M, Chernoff G, Benchimol EI, Panaccione R, Ghosh S, Barkema HW, Kaplan GG. Increasing incidence and prevalence of the inflammatory bowel diseases with time, based on systematic review. Gastroenterology. 2012;142:46-54.e42; quiz e30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3789] [Cited by in RCA: 3527] [Article Influence: 271.3] [Reference Citation Analysis (5)] |
| 10. | Ananthakrishnan AN. Epidemiology and risk factors for IBD. Nat Rev Gastroenterol Hepatol. 2015;12:205-217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 930] [Cited by in RCA: 1239] [Article Influence: 123.9] [Reference Citation Analysis (0)] |
| 11. | Hashash JG, Sigal R, Wein-Levy P, Szigethy EM, Merusi JJ, Regueiro MD. Inflammatory Bowel Disease (IBD) Connect: A Novel Volunteer Program for Hospitalized Patients with IBD and Their Families. Inflamm Bowel Dis. 2016;22:2748-2753. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 12] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 12. | Lightner AL, Vogel JD, Carmichael JC, Keller DS, Shah SA, Mahadevan U, Kane SV, Paquette IM, Steele SR, Feingold DL. The American Society of Colon and Rectal Surgeons Clinical Practice Guidelines for the Surgical Management of Crohn's Disease. Dis Colon Rectum. 2020;63:1028-1052. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 69] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 13. | Andersson RE, Olaison G, Tysk C, Ekbom A. Appendectomy is followed by increased risk of Crohn's disease. Gastroenterology. 2003;124:40-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 133] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 14. | Fontana T, Falco N, Torchia M, Tutino R, Gulotta G. Bowel perforation in Crohn's Disease: correlation between CDAI and Clavien-Dindo scores. G Chir. 2017;38:303-312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 15. | Podolsky DK. Inflammatory bowel disease. N Engl J Med. 2002;347:417-429. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2693] [Cited by in RCA: 2747] [Article Influence: 119.4] [Reference Citation Analysis (2)] |
| 16. | Ballou S, Hirsch W, Singh P, Rangan V, Nee J, Iturrino J, Sommers T, Zubiago J, Sengupta N, Bollom A, Jones M, Moss AC, Flier SN, Cheifetz AS, Lembo A. Emergency department utilisation for inflammatory bowel disease in the United States from 2006 to 2014. Aliment Pharmacol Ther. 2018;47:913-921. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 22] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 17. | Goldstone RN, Steinhagen RM. Abdominal Emergencies in Inflammatory Bowel Disease. Surg Clin North Am. 2019;99:1141-1150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 24] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 18. | Torres J, Bonovas S, Doherty G, Kucharzik T, Gisbert JP, Raine T, Adamina M, Armuzzi A, Bachmann O, Bager P, Biancone L, Bokemeyer B, Bossuyt P, Burisch J, Collins P, El-Hussuna A, Ellul P, Frei-Lanter C, Furfaro F, Gingert C, Gionchetti P, Gomollon F, González-Lorenzo M, Gordon H, Hlavaty T, Juillerat P, Katsanos K, Kopylov U, Krustins E, Lytras T, Maaser C, Magro F, Marshall JK, Myrelid P, Pellino G, Rosa I, Sabino J, Savarino E, Spinelli A, Stassen L, Uzzan M, Vavricka S, Verstockt B, Warusavitarne J, Zmora O, Fiorino G. ECCO Guidelines on Therapeutics in Crohn's Disease: Medical Treatment. J Crohns Colitis. 2020;14:4-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 991] [Cited by in RCA: 905] [Article Influence: 181.0] [Reference Citation Analysis (2)] |
| 19. | Adamina M, Bonovas S, Raine T, Spinelli A, Warusavitarne J, Armuzzi A, Bachmann O, Bager P, Biancone L, Bokemeyer B, Bossuyt P, Burisch J, Collins P, Doherty G, El-Hussuna A, Ellul P, Fiorino G, Frei-Lanter C, Furfaro F, Gingert C, Gionchetti P, Gisbert JP, Gomollon F, González Lorenzo M, Gordon H, Hlavaty T, Juillerat P, Katsanos K, Kopylov U, Krustins E, Kucharzik T, Lytras T, Maaser C, Magro F, Marshall JK, Myrelid P, Pellino G, Rosa I, Sabino J, Savarino E, Stassen L, Torres J, Uzzan M, Vavricka S, Verstockt B, Zmora O. ECCO Guidelines on Therapeutics in Crohn's Disease: Surgical Treatment. J Crohns Colitis. 2020;14:155-168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 431] [Cited by in RCA: 367] [Article Influence: 73.4] [Reference Citation Analysis (0)] |
| 20. | Gionchetti P, Dignass A, Danese S, Magro Dias FJ, Rogler G, Lakatos PL, Adamina M, Ardizzone S, Buskens CJ, Sebastian S, Laureti S, Sampietro GM, Vucelic B, van der Woude CJ, Barreiro-de Acosta M, Maaser C, Portela F, Vavricka SR, Gomollón F; ECCO. 3rd European Evidence-based Consensus on the Diagnosis and Management of Crohn's Disease 2016: Part 2: Surgical Management and Special Situations. J Crohns Colitis. 2017;11:135-149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 594] [Cited by in RCA: 531] [Article Influence: 66.4] [Reference Citation Analysis (0)] |
| 21. | Frolkis AD, Dykeman J, Negrón ME, Debruyn J, Jette N, Fiest KM, Frolkis T, Barkema HW, Rioux KP, Panaccione R, Ghosh S, Wiebe S, Kaplan GG. Risk of surgery for inflammatory bowel diseases has decreased over time: a systematic review and meta-analysis of population-based studies. Gastroenterology. 2013;145:996-1006. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 536] [Cited by in RCA: 663] [Article Influence: 55.3] [Reference Citation Analysis (0)] |
| 22. | Schnitzler F, Fidder H, Ferrante M, Noman M, Arijs I, Van Assche G, Hoffman I, Van Steen K, Vermeire S, Rutgeerts P. Long-term outcome of treatment with infliximab in 614 patients with Crohn's disease: results from a single-centre cohort. Gut. 2009;58:492-500. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 397] [Cited by in RCA: 403] [Article Influence: 25.2] [Reference Citation Analysis (0)] |
| 23. | Cosnes J, Nion-Larmurier I, Beaugerie L, Afchain P, Tiret E, Gendre JP. Impact of the increasing use of immunosuppressants in Crohn's disease on the need for intestinal surgery. Gut. 2005;54:237-241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 499] [Cited by in RCA: 490] [Article Influence: 24.5] [Reference Citation Analysis (0)] |
| 24. | Lazarev M, Ullman T, Schraut WH, Kip KE, Saul M, Regueiro M. Small bowel resection rates in Crohn's disease and the indication for surgery over time: experience from a large tertiary care center. Inflamm Bowel Dis. 2010;16:830-835. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 74] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 25. | Khoury T, Daher S, Massarwa M, Hakimian D, Benson AA, Viener E, Farah R, Mari A, Hazou W, Kadah A, Sbeit W, Mahamid M, Israeli E. A Validated Score Assessing the Risk of an Intra-Abdominal Abscess in Patients with Crohn's Disease Presenting at the Emergency Department. J Crohns Colitis. 2019;13:1131-1137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 26. | Harvey RF, Bradshaw JM. A simple index of Crohn's-disease activity. Lancet. 1980;1:514. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1940] [Cited by in RCA: 2189] [Article Influence: 48.6] [Reference Citation Analysis (0)] |
| 27. | Gajendran M, Loganathan P, Catinella AP, Hashash JG. A comprehensive review and update on Crohn's disease. Dis Mon. 2018;64:20-57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 190] [Cited by in RCA: 329] [Article Influence: 41.1] [Reference Citation Analysis (1)] |
| 28. | Van Assche G, Dignass A, Reinisch W, van der Woude CJ, Sturm A, De Vos M, Guslandi M, Oldenburg B, Dotan I, Marteau P, Ardizzone A, Baumgart DC, D'Haens G, Gionchetti P, Portela F, Vucelic B, Söderholm J, Escher J, Koletzko S, Kolho KL, Lukas M, Mottet C, Tilg H, Vermeire S, Carbonnel F, Cole A, Novacek G, Reinshagen M, Tsianos E, Herrlinger K, Bouhnik Y, Kiesslich R, Stange E, Travis S, Lindsay J; European Crohn's and Colitis Organisation (ECCO). The second European evidence-based Consensus on the diagnosis and management of Crohn's disease: Special situations. J Crohns Colitis. 2010;4:63-101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 569] [Cited by in RCA: 547] [Article Influence: 36.5] [Reference Citation Analysis (0)] |
| 29. | Best WR, Becktel JM, Singleton JW. Rederived values of the eight coefficients of the Crohn's Disease Activity Index (CDAI). Gastroenterology. 1979;77:843-846. [PubMed] |
| 30. | Onodera T, Goseki N, Kosaki G. [Prognostic nutritional index in gastrointestinal surgery of malnourished cancer patients]. Nihon Geka Gakkai Zasshi. 1984;85:1001-1005. [PubMed] |
| 31. | Zhou W, Cao Q, Qi W, Xu Y, Liu W, Xiang J, Xia B. Prognostic Nutritional Index Predicts Short-Term Postoperative Outcomes After Bowel Resection for Crohn's Disease. Nutr Clin Pract. 2017;32:92-97. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 32] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 32. | Maeda K, Nagahara H, Shibutani M, Otani H, Sakurai K, Toyokawa T, Tanaka H, Kubo N, Muguruma K, Kamata N, Yamagami H, Hirakawa K. A preoperative low nutritional prognostic index correlates with the incidence of incisional surgical site infections after bowel resection in patients with Crohn's disease. Surg Today. 2015;45:1366-1372. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 20] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 33. | Guglielmo FF, Anupindi SA, Fletcher JG, Al-Hawary MM, Dillman JR, Grand DJ, Bruining DH, Chatterji M, Darge K, Fidler JL, Gandhi NS, Gee MS, Grajo JR, Huang C, Jaffe TA, Park SH, Rimola J, Soto JA, Taouli B, Taylor SA, Baker ME. Small Bowel Crohn Disease at CT and MR Enterography: Imaging Atlas and Glossary of Terms. Radiographics. 2020;40:354-375. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 36] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 34. | Verdalle-Cazes M, Charpentier C, Benard C, Joly LM, Dacher JN, Savoye G, Savoye-Collet C. Abdominopelvic CT-scan in emergency departments for patients with suspected complications of Crohn's disease: a single tertiary center experience. BMC Emerg Med. 2021;21:113. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 35. | Larson DB, Johnson LW, Schnell BM, Salisbury SR, Forman HP. National trends in CT use in the emergency department: 1995-2007. Radiology. 2011;258:164-173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 320] [Cited by in RCA: 323] [Article Influence: 23.1] [Reference Citation Analysis (0)] |
| 36. | Chatu S, Subramanian V, Pollok RC. Meta-analysis: diagnostic medical radiation exposure in inflammatory bowel disease. Aliment Pharmacol Ther. 2012;35:529-539. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 75] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 37. | Desmond AN, O'Regan K, Curran C, McWilliams S, Fitzgerald T, Maher MM, Shanahan F. Crohn's disease: factors associated with exposure to high levels of diagnostic radiation. Gut. 2008;57:1524-1529. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 279] [Cited by in RCA: 265] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 38. | Palmer L, Herfarth H, Porter CQ, Fordham LA, Sandler RS, Kappelman MD. Diagnostic ionizing radiation exposure in a population-based sample of children with inflammatory bowel diseases. Am J Gastroenterol. 2009;104:2816-2823. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 58] [Cited by in RCA: 50] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 39. | Kroeker KI, Lam S, Birchall I, Fedorak RN. Patients with IBD are exposed to high levels of ionizing radiation through CT scan diagnostic imaging: a five-year study. J Clin Gastroenterol. 2011;45:34-39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 85] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 40. | Pearce MS, Salotti JA, Little MP, McHugh K, Lee C, Kim KP, Howe NL, Ronckers CM, Rajaraman P, Sir Craft AW, Parker L, Berrington de González A. Radiation exposure from CT scans in childhood and subsequent risk of leukaemia and brain tumours: a retrospective cohort study. Lancet. 2012;380:499-505. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2528] [Cited by in RCA: 2636] [Article Influence: 202.8] [Reference Citation Analysis (1)] |
| 41. | Craig O, O'Neill S, O'Neill F, McLaughlin P, McGarrigle A, McWilliams S, O'Connor O, Desmond A, Walsh EK, Ryan M, Maher M, Shanahan F. Diagnostic accuracy of computed tomography using lower doses of radiation for patients with Crohn's disease. Clin Gastroenterol Hepatol. 2012;10:886-892. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 42] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 42. | Bettenworth D, Bokemeyer A, Baker M, Mao R, Parker CE, Nguyen T, Ma C, Panés J, Rimola J, Fletcher JG, Jairath V, Feagan BG, Rieder F; Stenosis Therapy and Anti-Fibrotic Research (STAR) Consortium. Assessment of Crohn's disease-associated small bowel strictures and fibrosis on cross-sectional imaging: a systematic review. Gut. 2019;68:1115-1126. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 230] [Cited by in RCA: 227] [Article Influence: 37.8] [Reference Citation Analysis (0)] |
| 43. | Panés J, Bouzas R, Chaparro M, García-Sánchez V, Gisbert JP, Martínez de Guereñu B, Mendoza JL, Paredes JM, Quiroga S, Ripollés T, Rimola J. Systematic review: the use of ultrasonography, computed tomography and magnetic resonance imaging for the diagnosis, assessment of activity and abdominal complications of Crohn's disease. Aliment Pharmacol Ther. 2011;34:125-145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 534] [Cited by in RCA: 475] [Article Influence: 33.9] [Reference Citation Analysis (0)] |
| 44. | Aberra FN, Lewis JD, Hass D, Rombeau JL, Osborne B, Lichtenstein GR. Corticosteroids and immunomodulators: postoperative infectious complication risk in inflammatory bowel disease patients. Gastroenterology. 2003;125:320-327. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 244] [Cited by in RCA: 238] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 45. | Nguyen GC, Elnahas A, Jackson TD. The impact of preoperative steroid use on short-term outcomes following surgery for inflammatory bowel disease. J Crohns Colitis. 2014;8:1661-1667. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 97] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 46. | Huang W, Tang Y, Nong L, Sun Y. Risk factors for postoperative intra-abdominal septic complications after surgery in Crohn's disease: A meta-analysis of observational studies. J Crohns Colitis. 2015;9:293-301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 136] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 47. | Subramanian V, Saxena S, Kang JY, Pollok RC. Preoperative steroid use and risk of postoperative complications in patients with inflammatory bowel disease undergoing abdominal surgery. Am J Gastroenterol. 2008;103:2373-2381. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 183] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 48. | Cohen BL, Fleshner P, Kane SV, Herfarth HH, Palekar N, Farraye FA, Leighton JA, Katz J, Cohen RD, Gerich ME, Cross RK, Higgins PD, Tinsley A, Glover SC, Siegel CA, Bohl JL, Iskandar H, Raymond S, Huang R, Suarez-Farinas M, Sands BE. 415a – Anti-Tumor Necrosis Factor Therapy is Not Associated with Post-Operative Infection: Results from Prospective Cohort of Ulcerative Colitis and Crohn's Disease Patients Undergoing Surgery to Identify Risk Factors for Postoperative Infection I (Puccini). Gastroenterology. 2019;156(6, Supplement 1):S80. [DOI] [Full Text] |
| 49. | Amiot A, Bouguen G, Bonnaud G, Bouhnik Y, Hagege H, Peyrin-Biroulet L; French National Consensus Clinical guidelines for the management of IBD study group. Clinical guidelines for the management of inflammatory bowel disease: Update of a French national consensus. Dig Liver Dis. 2021;53:35-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 23] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 50. | Cohen RD, Bhayat F, Blake A, Travis S. The Safety Profile of Vedolizumab in Ulcerative Colitis and Crohn's Disease: 4 Years of Global Post-marketing Data. J Crohns Colitis. 2020;14:192-204. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 54] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 51. | Armuzzi A, Ardizzone S, Biancone L, Castiglione F, Danese S, Gionchetti P, Orlando A, Rizzello F, Scribano ML, Vecchi M, Daperno M. Ustekinumab in the management of Crohn's disease: Expert opinion. Dig Liver Dis. 2018;50:653-660. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 52. | Mijac DD, Janković GL, Jorga J, Krstić MN. Nutritional status in patients with active inflammatory bowel disease: prevalence of malnutrition and methods for routine nutritional assessment. Eur J Intern Med. 2010;21:315-319. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 146] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 53. | Chiarello MM, Brisinda G. A commentary on: Preoperative hypoalbuminemia is an independent risk factor for postoperative complications in Crohn's disease patients with normal BMI: A cohort study. Int J Surg. 2020;81:100-101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 54. | Bischoff SC, Escher J, Hébuterne X, Kłęk S, Krznaric Z, Schneider S, Shamir R, Stardelova K, Wierdsma N, Wiskin AE, Forbes A. ESPEN practical guideline: Clinical Nutrition in inflammatory bowel disease. Clin Nutr. 2020;39:632-653. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 219] [Article Influence: 43.8] [Reference Citation Analysis (1)] |
| 55. | Schwartz E. Perioperative Parenteral Nutrition in Adults With Inflammatory Bowel Disease: A Review of the Literature. Nutr Clin Pract. 2016;31:159-170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 21] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 56. | Akobeng AK, Zhang D, Gordon M, MacDonald JK. Enteral nutrition for maintenance of remission in Crohn's disease. Cochrane Database Syst Rev. 2018;8:CD005984. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 14] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 57. | Brennan GT, Ha I, Hogan C, Nguyen E, Jamal MM, Bechtold ML, Nguyen DL. Does preoperative enteral or parenteral nutrition reduce postoperative complications in Crohn's disease patients: a meta-analysis. Eur J Gastroenterol Hepatol. 2018;30:997-1002. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 75] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 58. | Zhu Y, Xu L, Liu W, Qi W, Cao Q, Zhou W. Safety and Efficacy of Exclusive Enteral Nutrition for Percutaneously Undrainable Abdominal Abscesses in Crohn's Disease. Gastroenterol Res Pract. 2017;2017:6360319. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 16] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 59. | Justiniano CF, Aquina CT, Becerra AZ, Xu Z, Boodry CI, Swanger AA, Monson JRT, Fleming FJ. Postoperative Mortality After Nonelective Surgery for Inflammatory Bowel Disease Patients in the Era of Biologics. Ann Surg. 2019;269:686-691. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 30] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 60. | Singh S, Al-Darmaki A, Frolkis AD, Seow CH, Leung Y, Novak KL, Ghosh S, Eksteen B, Panaccione R, Kaplan GG. Postoperative Mortality Among Patients With Inflammatory Bowel Diseases: A Systematic Review and Meta-analysis of Population-Based Studies. Gastroenterology. 2015;149:928-937. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 108] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 61. | Hanauer SB, Sandborn W; Practice Parameters Committee of the American College of Gastroenterology. Management of Crohn's disease in adults. Am J Gastroenterol. 2001;96:635-643. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 51] [Reference Citation Analysis (0)] |
| 62. | Lowenfeld L, Michelassi F. Management of acute complications in patients with Crohn’s Disease. In: Nigri G, Tsoulfa G, editors. Gastrointestinal surgical emergencies. 2021 ed. United States of America: American College of Surgeons, 2021: 180-193. |
| 63. | Toh JW, Stewart P, Rickard MJ, Leong R, Wang N, Young CJ. Indications and surgical options for small bowel, large bowel and perianal Crohn's disease. World J Gastroenterol. 2016;22:8892-8904. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 53] [Cited by in RCA: 48] [Article Influence: 5.3] [Reference Citation Analysis (2)] |
| 64. | Annunziata ML, Caviglia R, Papparella LG, Cicala M. Upper gastrointestinal involvement of Crohn's disease: a prospective study on the role of upper endoscopy in the diagnostic work-up. Dig Dis Sci. 2012;57:1618-1623. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 102] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 65. | Balendran K, Udumalagala S, Nawaraththne NMM. Pyloric stenosis as a manifestation of isolated gastric Crohn's disease responding to intralesional steroid injection and balloon dilation: a case report. J Med Case Rep. 2019;13:331. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 66. | Guellouz S, Pariente B, Benet C, Baudry C, Lourenco N, Kraemer A, Allez M, Gornet JM. Cephalic duodenopancreatectomy for hyperalgic duodenal Crohn's disease fistulized in the pancreatic gland. Case Rep Gastroenterol. 2014;8:72-76. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 67. | Shivaji UN, Evans M, Critchlow T, Gui X, Smith SCL, Pinkney T, Iacucci M, Cooney R, Ghosh S, Skordilis K. Chronic inflammation and other changes are significant components of clinically fibrotic strictures in Crohn's disease: a histological study of resected strictures clinically characterized as noninflamed. Eur J Gastroenterol Hepatol. 2020;32:1432-1439. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 68. | Shen B, Kochhar G, Navaneethan U, Farraye FA, Schwartz DA, Iacucci M, Bernstein CN, Dryden G, Cross R, Bruining DH, Kobayashi T, Lukas M, Shergill A, Bortlik M, Lan N, Tang SJ, Kotze PG, Kiran RP, Dulai PS, El-Hachem S, Coelho-Prabhu N, Thakkar S, Mao R, Chen G, Zhang S, Suárez BG, Lama YG, Silverberg MS, Sandborn WJ. Practical guidelines on endoscopic treatment for Crohn's disease strictures: a consensus statement from the Global Interventional Inflammatory Bowel Disease Group. Lancet Gastroenterol Hepatol. 2020;5:393-405. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 104] [Article Influence: 20.8] [Reference Citation Analysis (0)] |
| 69. | Lamb CA, Kennedy NA, Raine T, Hendy PA, Smith PJ, Limdi JK, Hayee B, Lomer MCE, Parkes GC, Selinger C, Barrett KJ, Davies RJ, Bennett C, Gittens S, Dunlop MG, Faiz O, Fraser A, Garrick V, Johnston PD, Parkes M, Sanderson J, Terry H; IBD guidelines eDelphi consensus group, Gaya DR, Iqbal TH, Taylor SA, Smith M, Brookes M, Hansen R, Hawthorne AB. British Society of Gastroenterology consensus guidelines on the management of inflammatory bowel disease in adults. Gut. 2019;68:s1-s106. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1402] [Cited by in RCA: 1567] [Article Influence: 261.2] [Reference Citation Analysis (0)] |
| 70. | Lan N, Shen B. Endoscopic Stricturotomy Versus Balloon Dilation in the Treatment of Anastomotic Strictures in Crohn's Disease. Inflamm Bowel Dis. 2018;24:897-907. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 70] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 71. | Loras C, Andújar X, Gornals JB, Sanchiz V, Brullet E, Sicilia B, Martín-Arranz MD, Naranjo A, Barrio J, Dueñas C, Foruny JR, Busquets D, Monfort D, Pineda JR, González-Huix F, Pérez-Roldán F, Pons V, González B, Reyes Moreno J, Sainz E, Guardiola J, Bosca-Watts MM, Fernández-Bañares F, Mayor V, Esteve M; Grupo Español de Trabajo de la Enfermedad de Crohn y Colitis Ulcerosa (GETECCU). Self-expandable metal stents versus endoscopic balloon dilation for the treatment of strictures in Crohn's disease (ProtDilat study): an open-label, multicentre, randomised trial. Lancet Gastroenterol Hepatol. 2022;7:332-341. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 30] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 72. | Chiarello MM, Cariati M, Brisinda G. Colonic Crohn's disease - decision is more important than incision: A surgical dilemma. World J Gastrointest Surg. 2021;13:1-6. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 7] [Cited by in RCA: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 73. | Freeman HJ. Natural history and long-term clinical course of Crohn's disease. World J Gastroenterol. 2014;20:31-36. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 101] [Cited by in RCA: 118] [Article Influence: 10.7] [Reference Citation Analysis (5)] |
| 74. | Lesperance K, Martin MJ, Lehmann R, Brounts L, Steele SR. National trends and outcomes for the surgical therapy of ileocolonic Crohn's disease: a population-based analysis of laparoscopic vs. open approaches. J Gastrointest Surg. 2009;13:1251-1259. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 68] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 75. | McLeod RS, Wolff BG, Ross S, Parkes R, McKenzie M; Investigators of the CAST Trial. Recurrence of Crohn's disease after ileocolic resection is not affected by anastomotic type: results of a multicenter, randomized, controlled trial. Dis Colon Rectum. 2009;52:919-927. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 181] [Cited by in RCA: 195] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 76. | Celentano V, O'Leary DP, Caiazzo A, Flashman KG, Sagias F, Conti J, Senapati A, Khan J. Longer small bowel segments are resected in emergency surgery for ileocaecal Crohn's disease with a higher ileostomy and complication rate. Tech Coloproctol. 2019;23:1085-1091. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 23] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 77. | Kosmidis C, Anthimidis G. Emergency and elective surgery for small bowel Crohn's disease. Tech Coloproctol. 2011;15 Suppl 1:S1-S4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 78. | Guo Z, Li Y, Zhu W, Gong J, Li N, Li J. Comparing outcomes between side-to-side anastomosis and other anastomotic configurations after intestinal resection for patients with Crohn's disease: a meta-analysis. World J Surg. 2013;37:893-901. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 52] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 79. | Celentano V, Pellino G, Spinelli A, Selvaggi F; SICCR Current status of Crohn’s disease surgery collaborative, Celentano V, Pellino G, Rottoli M, Poggioli G, Sica G, Giglio MC, Campanelli M, Coco C, Rizzo G, Sionne F, Colombo F, Sampietro G, Lamperti G, Foschi D, Ficari F, Vacca L, Cricchio M, Giudici F, Selvaggi L, Sciaudone G, Peltrini R, Manfreda A, Bucci L, Galleano R, Ghazouani O, Zorcolo L, Deidda S, Restivo A, Braini A, Di Candido F, Sacchi M, Carvello M, Martorana S, Bordignon G, Angriman I, Variola A, Di Ruscio M, Barugola G, Geccherle A, Tropeano FP, Luglio G, Tanzanu M, Sasia D, Migliore M, Giuffrida MC, Marrano E, Moretto G, Impellizzeri H, Gallo G, Vescio G, Sammarco G, Terrosu G, Calini G, Bondurri A, Maffioli A, Zaffaroni G, Resegotti A, Mistrangelo M, Allaix ME, Botti F, Prati M, Boni L, Perotti S, Mineccia M, Giuliani A, Romano L, Graziano GMP, Pugliese L, Pietrabissa A, Delaini G, Spinelli A, Selvaggi F; , on behalf of the Italian Society of Colorectal Surgery SICCR. Anastomosis configuration and technique following ileocaecal resection for Crohn's disease: a multicentre study. Updates Surg. 2021;73:149-156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 80. | Strong S, Steele SR, Boutrous M, Bordineau L, Chun J, Stewart DB, Vogel J, Rafferty JF; Clinical Practice Guidelines Committee of the American Society of Colon and Rectal Surgeons. Clinical Practice Guideline for the Surgical Management of Crohn's Disease. Dis Colon Rectum. 2015;58:1021-1036. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 72] [Article Influence: 7.2] [Reference Citation Analysis (1)] |
| 81. | Aaltonen G, Keränen I, Carpelan-Holmström M, Lepistö A. Risk factors for anastomotic recurrence after primary ileocaecal resection in Crohn's disease. Eur J Gastroenterol Hepatol. 2018;30:1143-1147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 14] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 82. | Bertucci Zoccali M, Fichera A. Anastomotic Techniques for Abdominal Crohn's Disease: Tricks and Tips. J Laparoendosc Adv Surg Tech A. 2021;31:861-866. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 83. | Gardiner KR, Dasari BV. Operative management of small bowel Crohn's disease. Surg Clin North Am. 2007;87:587-610. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 53] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 84. | Richards RJ. Management of abdominal and pelvic abscess in Crohn's disease. World J Gastrointest Endosc. 2011;3:209-212. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 29] [Cited by in RCA: 29] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 85. | Yamaguchi A, Matsui T, Sakurai T, Ueki T, Nakabayashi S, Yao T, Futami K, Arima S, Ono H. The clinical characteristics and outcome of intraabdominal abscess in Crohn's disease. J Gastroenterol. 2004;39:441-448. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 67] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 86. | Gervais DA, Hahn PF, O'Neill MJ, Mueller PR. Percutaneous abscess drainage in Crohn disease: technical success and short- and long-term outcomes during 14 years. Radiology. 2002;222:645-651. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 101] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 87. | Yarur AJ, Mandalia AB, Dauer RM, Czul F, Deshpande AR, Kerman DH, Abreu MT, Sussman DA. Predictive factors for clinically actionable computed tomography findings in inflammatory bowel disease patients seen in the emergency department with acute gastrointestinal symptoms. J Crohns Colitis. 2014;8:504-512. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 26] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 88. | Lowe SC, Ream J, Hudesman D, Malter L, Bosworth B, Xia Y, Zhong H, Dane B, Megibow A, Chang S. A clinical and radiographic model to predict surgery for acute small bowel obstruction in Crohn's disease. Abdom Radiol (NY). 2020;45:2663-2668. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 89. | Clancy C, Boland T, Deasy J, McNamara D, Burke JP. A Meta-analysis of Percutaneous Drainage Versus Surgery as the Initial Treatment of Crohn's Disease-related Intra-abdominal Abscess. J Crohns Colitis. 2016;10:202-208. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 57] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 90. | Varadarajulu S, Drelichman ER. Effectiveness of EUS in drainage of pelvic abscesses in 25 consecutive patients (with video). Gastrointest Endosc. 2009;70:1121-1127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 46] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 91. | Fernandez-Urien I, Vila JJ, Jimenez FJ. Endoscopic ultrasound-guided drainage of pelvic collections and abscesses. World J Gastrointest Endosc. 2010;2:223-227. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 12] [Cited by in RCA: 13] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 92. | Golfieri R, Cappelli A, Giampalma E, Rizzello F, Gionchetti P, Laureti S, Poggioli G, Campieri M. CT-guided percutaneous pelvic abscess drainage in Crohn's disease. Tech Coloproctol. 2006;10:99-105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 37] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 93. | da Luz Moreira A, Stocchi L, Tan E, Tekkis PP, Fazio VW. Outcomes of Crohn's disease presenting with abdominopelvic abscess. Dis Colon Rectum. 2009;52:906-912. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 38] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 94. | Alves A, Panis Y, Bouhnik Y, Pocard M, Vicaut E, Valleur P. Risk factors for intra-abdominal septic complications after a first ileocecal resection for Crohn's disease: a multivariate analysis in 161 consecutive patients. Dis Colon Rectum. 2007;50:331-336. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 242] [Cited by in RCA: 244] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 95. | Lobatón T, Guardiola J, Rodriguez-Moranta F, Millán-Scheiding M, Peñalva M, De Oca J, Biondo S. Comparison of the long-term outcome of two therapeutic strategies for the management of abdominal abscess complicating Crohn's disease: percutaneous drainage or immediate surgical treatment. Colorectal Dis. 2013;15:1267-1272. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 26] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 96. | Gutierrez A, Lee H, Sands BE. Outcome of surgical versus percutaneous drainage of abdominal and pelvic abscesses in Crohn's disease. Am J Gastroenterol. 2006;101:2283-2289. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 83] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 97. | Bermejo F, Garrido E, Chaparro M, Gordillo J, Mañosa M, Algaba A, López-Sanromán A, Gisbert JP, García-Planella E, Guerra I, Domènech E. Efficacy of different therapeutic options for spontaneous abdominal abscesses in Crohn's disease: are antibiotics enough? Inflamm Bowel Dis. 2012;18:1509-1514. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 42] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 98. | Kumar RR, Kim JT, Haukoos JS, Macias LH, Dixon MR, Stamos MJ, Konyalian VR. Factors affecting the successful management of intra-abdominal abscesses with antibiotics and the need for percutaneous drainage. Dis Colon Rectum. 2006;49:183-189. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 94] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 99. | Reuken PA, Kruis W, Maaser C, Teich N, Büning J, Preiß JC, Schmelz R, Bruns T, Fichtner-Feigl S, Stallmach A; German IBD Study group [GISG]. Microbial Spectrum of Intra-Abdominal Abscesses in Perforating Crohn's Disease: Results from a Prospective German Registry. J Crohns Colitis. 2018;12:695-701. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 100. | Cullen G, Vaughn B, Ahmed A, Peppercorn MA, Smith MP, Moss AC, Cheifetz AS. Abdominal phlegmons in Crohn's disease: outcomes following antitumor necrosis factor therapy. Inflamm Bowel Dis. 2012;18:691-696. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 37] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 101. | Udholm LS, Rasmussen SL, Madsbøll TK, Omairi M, El-Hussuna A. A systemic review and metaanalysis of postoperative outcomes in urgent and elective bowel resection in patients with Crohn's disease. Int J Colorectal Dis. 2021;36:253-263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 18] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 102. | de Groof EJ, Carbonnel F, Buskens CJ, Bemelman WA. Abdominal abscess in Crohn's disease: multidisciplinary management. Dig Dis. 2014;32 Suppl 1:103-109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 48] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 103. | Collard MK, Benoist S, Maggiori L, Zerbib P, Lefevre JH, Denost Q, Germain A, Cotte E, Beyer-Berjot L, Corté H, Desfourneaux V, Rahili A, Duffas JP, Pautrat K, Denet C, Bridoux V, Meurette G, Faucheron JL, Loriau J, Souche R, Vicaut E, Panis Y, Brouquet A. A Reappraisal of Outcome of Elective Surgery After Successful Non-Operative Management of an Intra-Abdominal Abscess Complicating Ileocolonic Crohn's Disease: A Subgroup Analysis of a Nationwide Prospective Cohort. J Crohns Colitis. 2021;15:409-418. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 12] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 104. | Werbin N, Haddad R, Greenberg R, Karin E, Skornick Y. Free perforation in Crohn's disease. Isr Med Assoc J. 2003;5:175-177. [PubMed] |
| 105. | Doh YS, Kim YS, Bae SI, Im JP, Cheon JH, Ye BD, Kim JW, Park YS, Lee JH, Kim YH, Kim JS, Han DS, Kim WH. The clinical characteristics of patients with free perforation in Korean Crohn's disease: results from the CONNECT study. BMC Gastroenterol. 2015;15:31. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 17] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 106. | Greenstein AJ, Sachar DB, Mann D, Lachman P, Heimann T, Aufses AH Jr. Spontaneous free perforation and perforated abscess in 30 patients with Crohn's disease. Ann Surg. 1987;205:72-76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 57] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 107. | Steinberg DM, Cooke WT, Alexander-Williams J. Free perforation in Crohn's disease. Gut. 1973;14:187-190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 44] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 108. | Katz S, Schulman N, Levin L. Free perforation in Crohn's disease: a report of 33 cases and review of literature. Am J Gastroenterol. 1986;81:38-43. [PubMed] |
| 109. | Cooke WT, Fielding JF. Corticosteroid or corticotrophin therapy in Crohn's disease (regional enteritis). Gut. 1970;11:921-927. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 33] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 110. | Abascal J, Diaz-Rojas F, Jorge J, Sanchez-Vegazo I, Escartin P, Abreu L, Chantar C. Free perforation of the small bowel in Crohn's disease. World J Surg. 1982;6:216-220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 16] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 111. | Ye BD. Could early anti-tumor necrosis factor therapy change the prognosis of Crohn's disease? Intest Res. 2014;12:263-265. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 112. | Liu RQ, Guo D, Qiao SH, Yin Y, Guo Z, Gong JF, Li Y, Zhu WM. Comparison of primary anastomosis and staged surgery in emergency treatment of complicated Crohn's disease. J Dig Dis. 2020;21:724-734. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 113. | Han H, Kim H, Rehman A, Jang SM, Paik SS. Appendiceal Crohn's disease clinically presenting as acute appendicitis. World J Clin Cases. 2014;2:888-892. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 21] [Cited by in RCA: 21] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 114. | Meyerding EV, Bertram HF. Nonspecific granulomatous inflammation (Crohn's disease) of the appendix; a case report. Surgery. 1953;34:891-894. [PubMed] |
| 115. | Vanek VW, Spirtos G, Awad M, Badjatia N, Bernat D. Isolated Crohn's disease of the appendix. Two case reports and a review of the literature. Arch Surg. 1988;123:85-87. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 21] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 116. | Haddad M, Azim F, Koren A, Stelman E, Mor C, Zelikovski A. Crohn's disease of the appendix. Eur J Surg. 1993;159:191-192. [PubMed] |
| 117. | Ruiz V, Unger SW, Morgan J, Wallack MK. Crohn's disease of the appendix. Surgery. 1990;107:113-117. [PubMed] |
| 118. | Lindhagen T, Ekelund G, Leandoer L, Hildell J, Lindström C, Wenckert A. Crohn's disease confined to the appendix. Dis Colon Rectum. 1982;25:805-808. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 20] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 119. | Quaresma AB, Miranda EF, Kotze PG. Management of ileocecal Crohn’s disease during surgical treatment for acute appendicitis: A systematic review. Arq Gastroenterol. 2021;58:560-565. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 120. | Farmer RG, Easley KA, Rankin GB. Clinical patterns, natural history, and progression of ulcerative colitis. A long-term follow-up of 1116 patients. Dig Dis Sci. 1993;38:1137-1146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 269] [Cited by in RCA: 235] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 121. | Cima RR. Timing and indications for colectomy in chronic ulcerative colitis: Surgical consideration. Dig Dis. 2010;28:501-507. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 34] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 122. | Truelove SC, Witts LJ. Cortisone in ulcerative colitis; final report on a therapeutic trial. Br Med J. 1955;2:1041-1048. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1832] [Cited by in RCA: 1868] [Article Influence: 26.7] [Reference Citation Analysis (1)] |
| 123. | Kornbluth A, Sachar DB; Practice Parameters Committee of the American College of Gastroenterology. Ulcerative colitis practice guidelines in adults: American College Of Gastroenterology, Practice Parameters Committee. Am J Gastroenterol. 2010;105:501-23; quiz 524. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 899] [Cited by in RCA: 942] [Article Influence: 62.8] [Reference Citation Analysis (0)] |
| 124. | Gisbert JP, Chaparro M. Acute severe ulcerative colitis: State of the art treatment. Best Pract Res Clin Gastroenterol. 2018;32-33:59-69. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 26] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 125. | Berg DF, Bahadursingh AM, Kaminski DL, Longo WE. Acute surgical emergencies in inflammatory bowel disease. Am J Surg. 2002;184:45-51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 89] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 126. | Podugu A, Tandon K, Castro FJ. Crohn's disease presenting as acute gastrointestinal hemorrhage. World J Gastroenterol. 2016;22:4073-4078. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 20] [Cited by in RCA: 13] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 127. | Nagata N, Niikura R, Aoki T, Moriyasu S, Sakurai T, Shimbo T, Shinozaki M, Sekine K, Okubo H, Watanabe K, Yokoi C, Yanase M, Akiyama J, Uemura N. Role of urgent contrast-enhanced multidetector computed tomography for acute lower gastrointestinal bleeding in patients undergoing early colonoscopy. J Gastroenterol. 2015;50:1162-1172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 64] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 128. | Ali M, Ul Haq T, Salam B, Beg M, Sayani R, Azeemuddin M. Treatment of nonvariceal gastrointestinal hemorrhage by transcatheter embolization. Radiol Res Pract. 2013;2013:604328. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 15] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 129. | Heimann TM, Greenstein AJ, Lewis B, Kaufman D, Heimann DM, Aufses AH Jr. Comparison of primary and reoperative surgery in patients with Crohns disease. Ann Surg. 1998;227:492-495. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 52] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 130. | Froehlich F, Juillerat P, Felley C, Mottet C, Vader JP, Burnand B, Michetti P, Gonvers JJ. Treatment of postoperative Crohn's disease. Digestion. 2005;71:49-53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 12] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 131. | Rutgeerts P, Geboes K, Vantrappen G, Beyls J, Kerremans R, Hiele M. Predictability of the postoperative course of Crohn's disease. Gastroenterology. 1990;99:956-963. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1226] [Cited by in RCA: 1222] [Article Influence: 34.9] [Reference Citation Analysis (0)] |
| 132. | Rispo A, Imperatore N, Testa A, Nardone OM, Luglio G, Caporaso N, Castiglione F. Diagnostic Accuracy of Ultrasonography in the Detection of Postsurgical Recurrence in Crohn's Disease: A Systematic Review with Meta-analysis. Inflamm Bowel Dis. 2018;24:977-988. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 61] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 133. | Schoepfer AM, Beglinger C, Straumann A, Trummler M, Vavricka SR, Bruegger LE, Seibold F. Fecal calprotectin correlates more closely with the Simple Endoscopic Score for Crohn's disease (SES-CD) than CRP, blood leukocytes, and the CDAI. Am J Gastroenterol. 2010;105:162-169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 387] [Cited by in RCA: 421] [Article Influence: 28.1] [Reference Citation Analysis (0)] |
| 134. | Santiago M, Magro F, Correia L, Portela F, Ministro P, Lago P, Trindade E, Dias CC. Inflammatory Bowel Disease Reoperation Rate Has Decreased Over Time If Corrected by Prevalence. Clin Transl Gastroenterol. 2020;11:e00227. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 135. | Lee KY, Yu CS, Lee KY, Cho YB, Park KJ, Choi GS, Yoon SN, Yoo H; IBD Study Group, Korean Society of Coloproctology. Risk factors for repeat abdominal surgery in korean patients with Crohn's disease: a multi-center study of a korean inflammatory bowel disease study group. J Korean Soc Coloproctol. 2012;28:188-194. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 136. | He X, Chen Z, Huang J, Lian L, Rouniyar S, Wu X, Lan P. Stapled side-to-side anastomosis might be better than handsewn end-to-end anastomosis in ileocolic resection for Crohn's disease: a meta-analysis. Dig Dis Sci. 2014;59:1544-1551. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 85] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 137. | Feng JS, Li JY, Yang Z, Chen XY, Mo JJ, Li SH. Stapled side-to-side anastomosis might be benefit in intestinal resection for Crohn's disease: A systematic review and network meta-analysis. Medicine (Baltimore). 2018;97:e0315. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 60] [Cited by in RCA: 44] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 138. | Kono T, Ashida T, Ebisawa Y, Chisato N, Okamoto K, Katsuno H, Maeda K, Fujiya M, Kohgo Y, Furukawa H. A new antimesenteric functional end-to-end handsewn anastomosis: surgical prevention of anastomotic recurrence in Crohn's disease. Dis Colon Rectum. 2011;54:586-592. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 151] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 139. | Luglio G, Rispo A, Imperatore N, Giglio MC, Amendola A, Tropeano FP, Peltrini R, Castiglione F, De Palma GD, Bucci L. Surgical Prevention of Anastomotic Recurrence by Excluding Mesentery in Crohn's Disease: The SuPREMe-CD Study - A Randomized Clinical Trial. Ann Surg. 2020;272:210-217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 134] [Article Influence: 26.8] [Reference Citation Analysis (0)] |
| 140. | Yang KM, Yu CS, Lee JL, Kim CW, Yoon YS, Park IJ, Lim SB, Park SH, Ye BD, Yang SK, Kim JC. Risk factors for postoperative recurrence after primary bowel resection in patients with Crohn's disease. World J Gastroenterol. 2017;23:7016-7024. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 27] [Cited by in RCA: 23] [Article Influence: 2.9] [Reference Citation Analysis (1)] |
| 141. | Thin LWY, Picardo S, Sooben S, Murray K, Ryan J, Wallace MH. Ileocolonic End-to-End Anastomoses in Crohn's Disease Increase the Risk of Early Post-operative Endoscopic Recurrence in Those Undergoing an Emergency Resection. J Gastrointest Surg. 2021;25:241-251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 142. | Sheehan AL, Warren BF, Gear MW, Shepherd NA. Fat-wrapping in Crohn's disease: pathological basis and relevance to surgical practice. Br J Surg. 1992;79:955-958. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 189] [Cited by in RCA: 190] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 143. | Petagna L, Antonelli A, Ganini C, Bellato V, Campanelli M, Divizia A, Efrati C, Franceschilli M, Guida AM, Ingallinella S, Montagnese F, Sensi B, Siragusa L, Sica GS. Pathophysiology of Crohn's disease inflammation and recurrence. Biol Direct. 2020;15:23. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 162] [Cited by in RCA: 146] [Article Influence: 29.2] [Reference Citation Analysis (0)] |
| 144. | Li Y, Mohan H, Lan N, Wu X, Zhou W, Gong J, Shen B, Stocchi L, Coffey JC, Zhu W. Mesenteric excision surgery or conservative limited resection in Crohn's disease: study protocol for an international, multicenter, randomized controlled trial. Trials. 2020;21:210. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 33] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 145. | Safar B, Sands D. Perianal Crohn's disease. Clin Colon Rectal Surg. 2007;20:282-293. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 50] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 146. | Hurst RD, Molinari M, Chung TP, Rubin M, Michelassi F. Prospective study of the features, indications, and surgical treatment in 513 consecutive patients affected by Crohn's disease. Surgery. 1997;122:661-7; discussion 667. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 66] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 147. | Spinelli A, Armuzzi A, Ciccocioppo R, Danese S, Gionchetti P, Luglio G, Orlando A, Rispo A, Rizzello F, Sofo L, Solina G, Poggioli G. Management of patients with complex perianal fistulas in Crohn's disease: Optimal patient flow in the Italian clinical reality. Dig Liver Dis. 2020;52:506-515. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 148. | Pogacnik JS, Salgado G. Perianal Crohn's Disease. Clin Colon Rectal Surg. 2019;32:377-385. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 30] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 149. | Sneider EB, Maykel JA. Anal abscess and fistula. Gastroenterol Clin North Am. 2013;42:773-784. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 22] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 150. | Jimenez M, Mandava N. Anorectal Fistula. StatPearls. Treasure Island (FL), 2022. |
| 151. | Irvine EJ. Usual therapy improves perianal Crohn's disease as measured by a new disease activity index. McMaster IBD Study Group. J Clin Gastroenterol. 1995;20:27-32. [PubMed] |
| 152. | Steele SR, Kumar R, Feingold DL, Rafferty JL, Buie WD; Standards Practice Task Force of the American Society of Colon and Rectal Surgeons. Practice parameters for the management of perianal abscess and fistula-in-ano. Dis Colon Rectum. 2011;54:1465-1474. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 201] [Cited by in RCA: 168] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 153. | Lee MJ, Heywood N, Sagar PM, Brown SR, Fearnhead NS; pCD Collaborators. Surgical management of fistulating perianal Crohn's disease: a UK survey. Colorectal Dis. 2017;19:266-273. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 39] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 154. | Kasparek MS, Glatzle J, Temeltcheva T, Mueller MH, Koenigsrainer A, Kreis ME. Long-term quality of life in patients with Crohn's disease and perianal fistulas: influence of fecal diversion. Dis Colon Rectum. 2007;50:2067-2074. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 85] [Article Influence: 4.7] [Reference Citation Analysis (1)] |
| 155. | Archampong D, Borowski D, Wille-Jørgensen P, Iversen LH. Workload and surgeon's specialty for outcome after colorectal cancer surgery. Cochrane Database Syst Rev. 2012;CD005391. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 84] [Cited by in RCA: 122] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 156. | Nagler SM, Poticha SM. Intraabdominal abscess in regional enteritis. Am J Surg. 1979;137:350-354. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 35] [Article Influence: 0.8] [Reference Citation Analysis (0)] |