Published online Apr 28, 2022. doi: 10.3748/wjg.v28.i16.1625
Peer-review started: March 18, 2021
First decision: July 3, 2021
Revised: July 17, 2021
Accepted: March 27, 2022
Article in press: March 27, 2022
Published online: April 28, 2022
Processing time: 402 Days and 6.4 Hours
Hepatic dysfunction represents a wide spectrum of pathological changes, which can be frequently found in hepatitis, cholestasis, metabolic diseases, and focal liver lesions. As hepatic dysfunction is often clinically silent until advanced stages, there remains an unmet need to identify affected patients at early stages to enable individualized intervention which can improve prognosis. Passive liver function tests include biochemical parameters and clinical grading systems (e.g., the Child-Pugh score and Model for End-Stage Liver Disease score). Despite widely used and readily available, these approaches provide indirect and limited information regarding hepatic function. Dynamic quantitative tests of liver function are based on clearance capacity tests such as the indocyanine green (ICG) clearance test. However, controversial results have been reported for the ICG clearance test in relation with clinical outcome and the accuracy is easily affected by various factors. Imaging techniques, including ultrasound, computed tomography, and magnetic resonance imaging, allow morphological and functional assessment of the entire hepatobiliary system, hence demonstrating great potential in evaluating hepatic dysfunction noninvasively. In this article, we provide a state-of-the-art summary of noninvasive imaging modalities for hepatic dysfunction assessment along the pathophysiological track, with special emphasis on the imaging modality comparison and selection for each clinical scenario.
Core Tip: Hepatic dysfunction can be frequently found in hepatitis, cholestasis, metabolic diseases, and focal liver lesions. It remains clinically silent until advanced stages, so there remains an unmet need to identify affected individuals at early stages. Imaging techniques, including ultrasound, computed tomography, and magnetic resonance imaging, allow morphological and functional assessment of the entire hepatobiliary system. In this article, we provide a state-of-the-art summary of noninvasive imaging modalities for assessing hepatic dysfunction in various clinical situations.
- Citation: Duan T, Jiang HY, Ling WW, Song B. Noninvasive imaging of hepatic dysfunction: A state-of-the-art review. World J Gastroenterol 2022; 28(16): 1625-1640
- URL: https://www.wjgnet.com/1007-9327/full/v28/i16/1625.htm
- DOI: https://dx.doi.org/10.3748/wjg.v28.i16.1625
Hepatic dysfunction is a common result of a wide variety of diseases, including hepatobiliary disorders and systemic diseases. The clinical symptoms of hepatic dysfunction (e.g., jaundice, anorexia, and abdominal pain) are varied and nonspecific[1]. Liver biopsy is the gold standard for hepatic dysfunction currently. Accurate as it is, liver biopsy is invasive, and susceptible to sampling errors and inter-observer variation. Besides, liver biopsy is limited by various complications and operator expertise. Therefore, the introduction of noninvasive diagnostic approaches is pivotal to addressing the above limitations of liver biopsy. Hepatic dysfunction usually manifests as biochemical abnormalities of serum markers, typically involving hepatocyte damage, cholestasis, bilirubin, synthesis function, and liver fibrosis[2,3]. Nevertheless, it is worth noting that not all patients with abnormalities in the above markers have primary liver disease, highlighting the wide differential diagnosis spectrum of abnormal liver chemistry and metabolic functions[2]. Considering the limited value of single serum markers in hepatic dysfunction evaluation, clinical grading systems integrating biochemical parameters and clinical symptoms have been developed to reveal impaired liver function. Among them, the Child-Pugh score is a widely adopted clinical scoring system that is particularly useful in selecting surgical candidates with hepatocellular carcinoma (HCC) and cirrhosis[4]. The Model for End-Stage Liver Disease score was initially developed to predict short-term survival in patients undergoing transcutaneous intrahepatic portosystemic shunt procedures and has been later expanded to stratify patients with end-stage liver disease awaiting transplantation[5]. Nevertheless, the performances of these clinical grading systems are suboptimal in mild liver injuries. Furthermore, despite widely used and readily available, biochemical parameters and clinical grading systems only provide indirect information about the hepatic function[6]. In contrast, dynamic quantitative tests, such as the indocyanine green (ICG) clearance test[7], allows direct measurements of liver clearance capacity and hence has become a routine test in preoperative liver function evaluation. However, discrepancies have been reported on the performances of ICG clearance test in clinical outcome prediction[8]. In addition, the accuracy of ICG clearance is affected by operator’s proficiency and the concentration of blood oxygen and other competitive agents[9].
Noninvasive imaging techniques, including ultrasound (US), computed tomography (CT), and magnetic resonance imaging (MRI), allow morphological and functional assessment of the entire hepatobiliary system (Table 1). These techniques permit qualitative and quantitative evaluation of hepatocyte quantity and function, fibrosis degree, type and severity of metabolic disorders, and excretory function of the biliary system. Therefore, through accurate hepatic dysfunction measurement and identification of affected individuals at early diseases stages, noninvasive imaging modalities offer appeal in individualized clinical decision-making and improving patient prognosis. Therefore, this review provides a state-of-the-art summary of noninvasive imaging modalities for assessing hepatic dysfunction along the pathophysiological track in various clinical situations.
| Imaging modality | Target changes | |
| US | B-mode ultrasonography | Echo intensity |
| Morphological changes | ||
| Color Doppler US | Phase and velocity of blood flow | |
| Contrast-enhanced US | Hemodynamic changes with better contrast than Doppler US | |
| Transient elastography | Liver stiffness | |
| Steatosis | ||
| Point shear wave elastography | Liver stiffness | |
| 2D-shear wave elastography | Liver stiffness | |
| CT | Conventional CT | CT value |
| Morphological changes | ||
| Steatosis | ||
| Dynamic enhanced CT | Portal hypertension | |
| Hemodynamic changes | ||
| CT perfusion | Quantitative measurement hemodynamic changes | |
| Liver extracellular volume on CT | Fibrosis | |
| MR | Conventional MRI | Morphological changes |
| MR elastography | Liver stiffness | |
| Diffusion-weighted MRI | Brownian motion of water molecules | |
| Gadoxetate-enhanced MRI | Number and function of hepatocytes | |
| MR perfusion | Quantitative measurement hemodynamic changes | |
| Chemical-shift-encoded MRI | Steatosis | |
| Iron overload | ||
| MR cholangiopancreatography | Biliary system | |
| Quantitative susceptibility mapping | Iron overload | |
| Liver extracellular volume on MRI | Fibrosis | |
Hepatitis is a major global public health problem affecting hundreds of millions of people. The common causes are the virus, bacteria, amoeba, and other infections. Other relatively rare causes include drug and food poisoning. Most deaths from viral hepatitis are due to hepatitis B and hepatitis C. An estimated 257 million people were living with hepatitis B and 71 million people were living with hepatitis C[10].
In mild hepatitis, edema of hepatocytes and inflammatory cells gather in the portal area at pathology. At this stage, the imaging findings are generally nonspecific, such as enhanced echo on US, slightly decreased density on CT, or increased signals on T2-weighted imaging.
With the aggravation of inflammation, histologic changes become more pronounced, including lobular disarray, acidophilic degeneration of hepatocytes, focal lobular necrosis, disruption of bile canaliculi with cholestasis, and portal and parenchymal infiltration of inflammatory cells (predominantly lymphocytes and macrophages)[11], as well as hypertrophy and hyperplasia of Kupffer cells and macrophages. These changes can lead to heterogeneous appearances of the liver parenchyma on pre-contrast imaging. Meanwhile, the microcirculation in the liver deteriorates, causing patchy enhancement or wedge-shaped enhancement pattern of the liver parenchyma on contrast-enhanced imaging. In addition, the “halo-ring sign” or “track sign” appears around the portal vein as a result of increased lymph inflow and blocked lymph backflow[12]. The transient portal hypertension (PH) leads to increased pressure in the gallbladder vein, causing subsequent subserosal edema of the gallbladder wall. With the gallbladder wall thickening and protruding into the cavity, a typical sign of “centripetal edema” appears[13]. Enlarged lymph node can be detected on US, CT, or MRI[14].
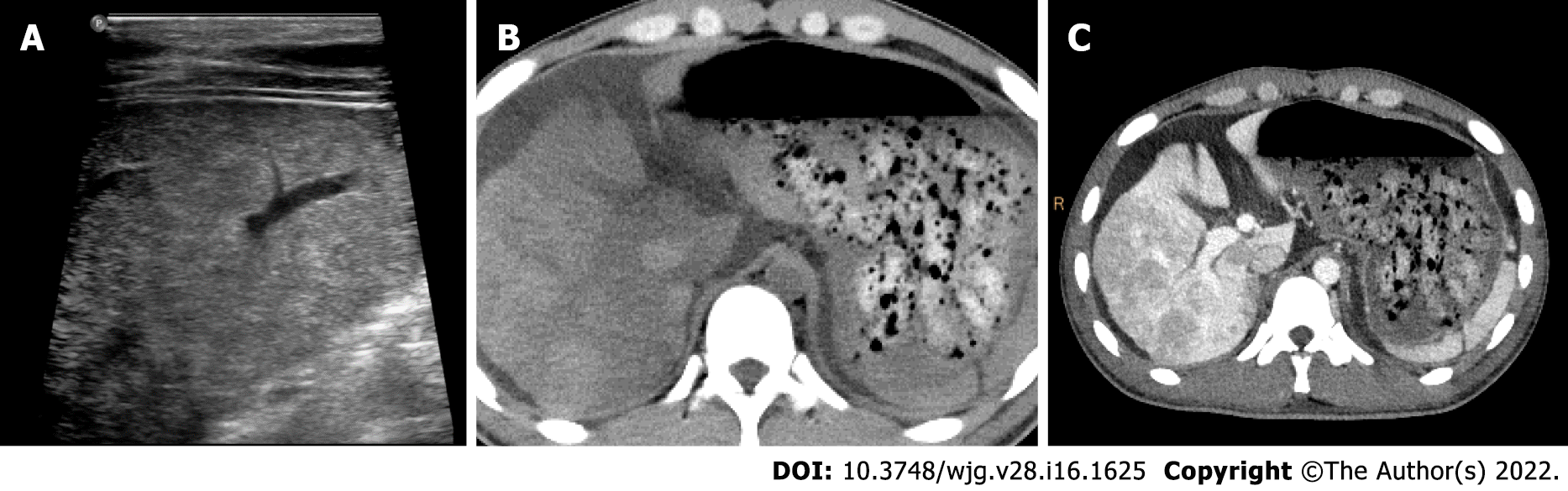
A high proportion of severe acute hepatitis cases can result in significant liver failure[15,16]. In these cases, extensive hepatocyte necrosis can lead to substantial bridging. Irregular necrosis is depicted as map-like low density on CT images. On contrast-enhanced images in the portal venous phase, the necrotic areas usually become hyper-attenuating compared with adjacent liver parenchyma due to infiltrates of inflammatory cells, increased arterial blood supply, and widened intercellular space. This sign is called “reverse enhancement”, which is a characteristic manifestation of severe hepatitis. In addition, ascites can be detected frequently[15] (Figure 1). Grillet et al[15] reported that heterogeneous liver parenchyma on CT would be particularly beneficial for patients with acute severe autoimmune hepatitis as histological examinations could be technically challenging due to complications. They also reported heterogeneous CT features of severe alcoholic hepatitis, indicating that these imaging features were mainly associated with transient heterogeneous steatosis and liver perfusion disorders[15]. Furthermore, Tana et al[17] used texture analysis to quantify the heterogeneity of the liver parenchyma, and showed that texture features of the liver could provide important quantitative information in predicting the severity and outcomes of patients with acute alcohol-associated hepatitis.
In summary, ultrasound is recommended as the first-line imaging modality for morphologic evaluation in patients with acute hepatitis. Contrast-enhanced CT or MRI should be considered when intrahepatic necrosis is suspected.
Chronic hepatitis refers to a morphologic pattern that is usually observed in patients with chronic viral hepatitis, autoimmune hepatitis, drug-induced hepatitis, and alcoholic hepatitis. Chronic hepatitis is characterized by several pathologic changes. These include inflammations of the portal veins and sometimes of the bile ducts; periportal injury and inflammation; several degeneration and apoptosis of intra-acinar hepatocytes secondary to inflammatory response; and different forms of fibrosis[18]. The end-stage progression is cirrhosis. The image findings of liver fibrosis and cirrhosis are described in later sections.
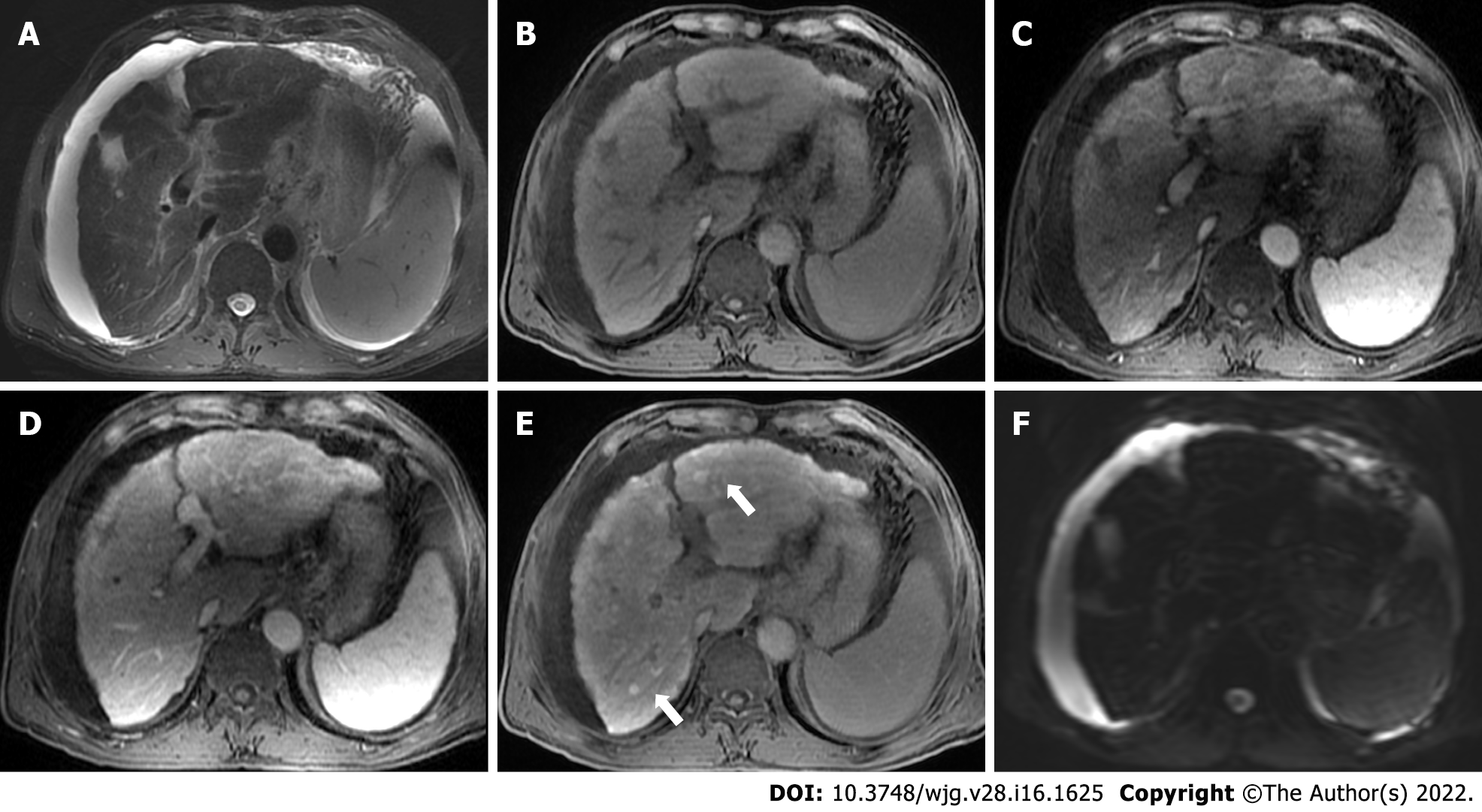
Typical imaging characteristics of chronic hepatitis include unsmooth liver margin, blunt edge, widened portal vein, enlarged spleen, and thickened gallbladder wall[19] (Figure 2). Unfortunately, when the above signs appear, liver injury has usually occurred for a long time and become irreversible.
Many efforts have been devoted to capturing the early hepatic microcirculation and perfusion changes of chronic hepatitis using imaging techniques. The deposition of collagen in the space of Disse and sinusoidal capillarization result in increased resistance to incoming sinusoidal blood flow, leading to a decrease in portal venous flow to the liver and an increase in hepatic arterial flow, and subsequently the formation of intrahepatic and portosystemic shunts. Cao et al[20] reported a significant correlation between the ICG clearance rate and MR-based portal venous perfusion, suggesting that MR-based portal venous perfusion could be used as a surrogate for liver function assessment.
Another important cause for hepatic dysfunction in chronic hepatitis is the impaired hepatocytes. Active transport of MR hepatobiliary contrast agents (e.g., gadoxetate and gadobenate dimeglumine) into the hepatocytes can reflect hepatocyte functions. Hepatobiliary phase (HBP) images can be acquired at about 20 min after contrast administration for gadoxetate and 1-2 h for gadobenate dimeglumine, with signal intensity on HBP images providing important information regarding liver function[21,22]. On this basis, studies further showed that T1 mapping could eliminate signal deviation and allow accurate liver function quantification[23-25].
Without proper and timely intervention, chronic hepatitis may progress to liver fibrosis (LF) and PH, which would be discussed in later sections.
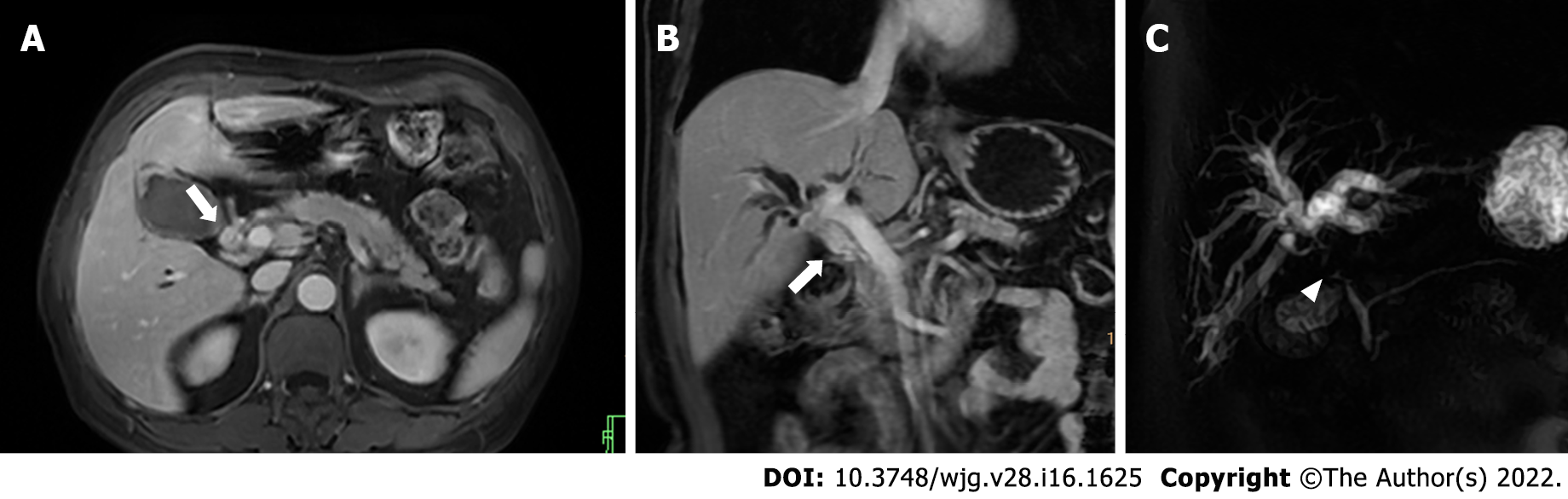
Acute cholestasis is characterized with mechanical biliary obstruction of any cause, such as choledocholithiasis, strictures (e.g., neoplastic, inflammatory, or postoperative), pancreatitis, choledochal cysts, parasitic diseases (e.g., ascariasis and fascioliasis), or even extrinsic pressure from enlarged lymph nodes[26]. US is promising for diagnosing early-stage acute cholestasis. However, magnetic resonance cholangiopancreatography (MRCP) is more sensitive in assessing the location, severity, cause, and extent of biliary obstruction[27]. MRCP images of a patient with suspected acute cholestasis can help: (1) Confirm the obstruction; (2) exclude other causes of jaundice; (3) determine the location of obstruction (intra- or extrahepatic ducts); (4) measure the approximate length of the biliary stricture; and (5) reveal the status of proximal bile ducts[28] (Figure 3).
Apart from MRCP, gadoxetate-enhanced MRI can also aid in evaluating acute cholestasis. Although less widely available than MRCP, it has a unique role in detecting bile leaks after biliary surgery or liver trauma[29].
Recently, elastography has also been applied in acute cholestasis. Kim et al[30] reported that liver stiffness measured by MRI elastography (MRE) is elevated with the increase of cholestasis, and can be predictive for the sufficiency of biliary decompression after biliary drainage.
Most chronic cholestatic disorders are insidious in onset, and chronic cholestasis progresses slowly over the course of years before it becomes clinically apparent. The most frequent causes of chronic cholestasis are primary sclerosing cholangitis (PSC) and primary biliary cirrhosis (PBC). Furthermore, allograft rejection can lead to bile duct damage and subsequent chronic cholestasis in patients who have undergone liver transplantation.
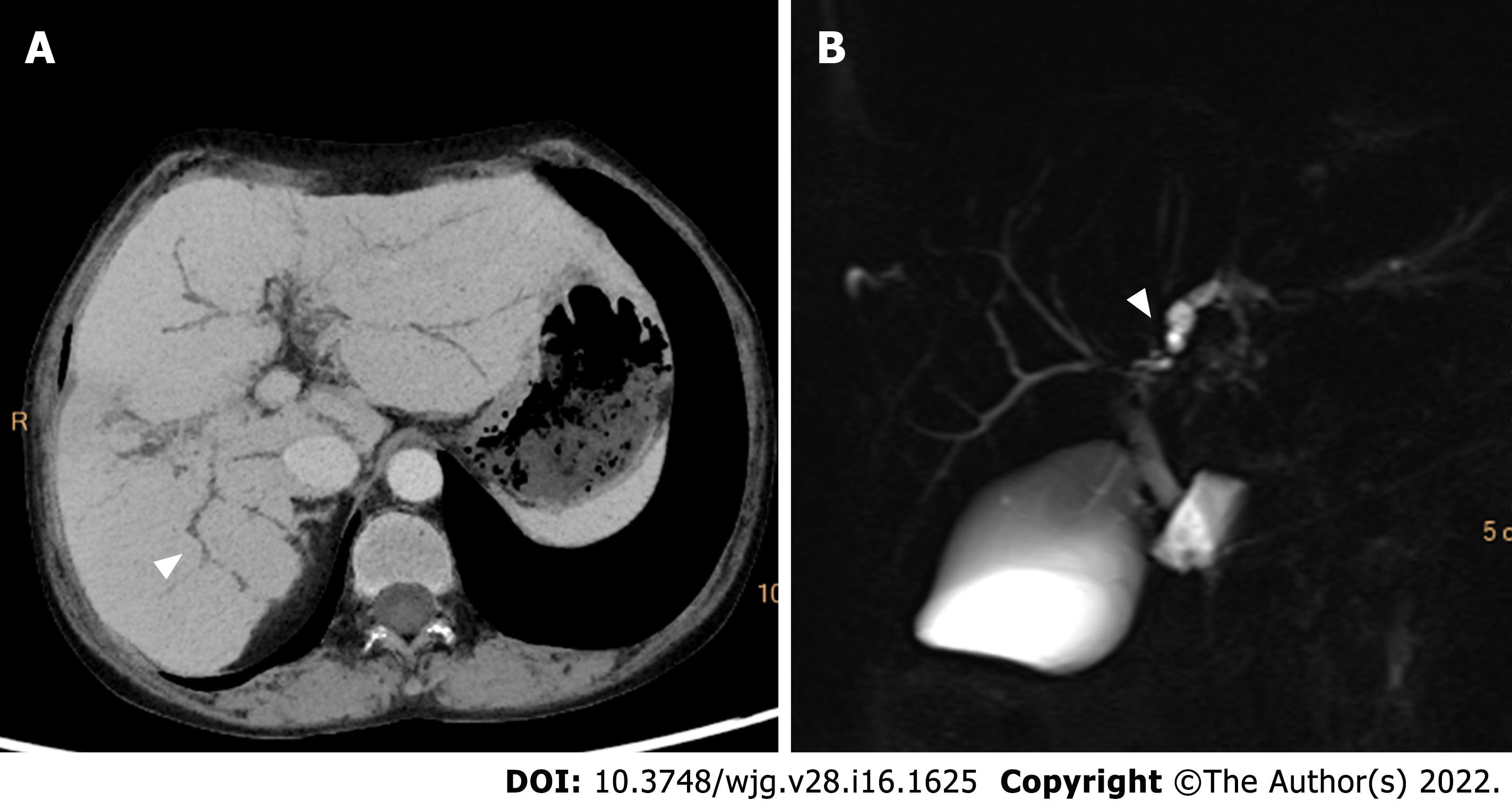
Characteristic imaging features of PSC include thickened concentric mural wall involving the extrahepatic biliary duct, with segmental intrahepatic biliary duct dilatation, preferentially affecting the left hepatic lobe. Gallbladder luminal sludge or stones and inflammatory polyps can also be depicted[31]. On MRCP, PSC can have typical features of biliary ductal changes, such as intrahepatic and extrahepatic short segmental bile duct strictures alternating with normal or mildly dilated bile ducts, giving rise to a beading appearance. At times, mild diffuse dilatation of the entire intrahepatic biliary system with a branching-tree appearance can be observed[32] (Figure 4).
On the other hand, PBC is characterized by chronic, non-suppurative lymphocytic cholangitis that predominantly affects small and interlobular bile ducts in the portal triads, leading to vanishing bile duct syndrome[33]. Diffuse hepatomegaly is the most pronounced morphological change. Patients usually develop micronodular or liver fibrosis. Most early PBCs had normal appearances on MRCP. As disease progresses, intrahepatic bile ducts become irregular. Thereafter, most peripheral branches of the intrahepatic bile ducts gradually become invisible, while medium-sized bile ducts present with reduced caliber and irregularity. These findings could be explained pathologically by destruction and disappearance of small intrahepatic bile ducts in PBC[34]. The assessments of liver function in PSC is similar to those in cirrhosis caused by chronic hepatitis[35].
In summary, when cholestasis is suspected, ultrasound is recommended for screening. When biliary obstruction or stricture is confirmed, MRI (MRCP in particular) is the preferred modality for further examinations.
Nonalcoholic fatty liver disease (NAFLD) is defined as liver fat exceeding 5%-10% by weight and exists as a spectrum from steatosis (usually stable) to nonalcoholic steatohepatitis (NASH) (characterized by cellular ballooning, necroapoptosis, inflammation, and fibrosis)[36]. Early detection and treatment of NAFLD can help prevent its progression to NASH and cirrhosis[37].
Among the imaging methods which enable liver fat quantification, transient elastography (TE) is the most widely studied US approach. A recent meta-analysis revealed that in NAFLD patients, the areas under the curve (AUC) of TE were 0.819 for S0 vs S1-S3 and 0·754 for S0-S1 vs S2-S3[38]. Another meta-analysis reported superior result of TE in the diagnosis of mild steatosis (AUC, 0.96) compared with severe steatosis (AUC, 0.70)[39]. Thus, an insufficient performance for TE in the diagnosis of moderate to severe steatosis should be noted.
The sensitivity and specificity of CT in detecting hepatic steatosis were reported ranging from 46% to 72% and from 88 to 95%, respectively[40]. However, given the potential additive radiation exposure, CT is not typically utilized as a screening test for NAFLD.
In addition, chemical-shift-encoded MRI-based proton density fat-fraction (PDFF) is increasingly accepted as an effective imaging modality in evaluating liver steatosis. A recent meta-analysis which included 2979 patients showed that MRI-PDFF offered pooled sensitivities of 0.71-0.91 and specificities of 0.88-0.93 for staging liver steatosis[41], with the optimal diagnostic performance achieved for detecting ≥ S1 (sensitivity, 0.92; specificity, 0.93) steatosis. Choi et al[42] compared the performance of MRI-PDFF and TE-based controlled attenuation parameter (CAP) in staging liver steatosis, and they found that MRI-PDFF correlated far better with hepatic fat measured (r = 0.978) than with CAP (r = 0.727). Besides, several clinical randomized controlled trials have shown that PDFF can be used to monitor and predict the therapeutic effect of NAFLD[43-45].
NASH is also characterized with a distinctive increase in liver extracellular fluid, which can be measured by an increase in T1 relaxation time. However, the accumulation of excess iron in liver tissue can be a confounding factor for T1 relaxation time. In this context, iron-corrected T1 can be generated to correct for this potential bias[46,47]. In a study of 50 patients undergoing standard-of-care liver biopsy for NAFLD, iron-corrected T1 has been demonstrated to correlate with ballooning and could accurately distinguish between steatosis and NASH patients[48].
Collectively, given the costs, availability, and diagnostic performances, US may be an appropriate modality to detect NAFLD. If accurate quantification of liver fat or monitoring of efficacy is needed, MRI PDFF should be a better choice.
Iron storage disorders are characterized by unregulated iron increase or decrease in the liver[49]. An increase in systemic iron can be a consequence of: (1) Hereditary hemochromatosis; (2) ineffective erythropoiesis or chronic liver disease; and (3) parenteral iron administration. Excessive intracellular deposition of iron ultimately results in tissue and organ damage. The diagnosis of iron overload relies on serum iron studies (elevated transferrin saturation and elevated serum ferritin levels), genetic testing, and sometimes liver biopsy to assess the hepatic iron concentration and degree of liver injury[50].
The paramagnetic effect of liver iron on the neighborhood protons affects T2 and T2* relaxation times by accelerating the signal decay. Therefore, the presence of iron results in tissue signal loss on T2 and T2* weighted images that is proportional to iron content, which is the basic principle of MRI in evaluating liver iron overload[51]. The MRI methods for liver iron quantification can be divided into signal intensity ratio methods and relaxometry methods.
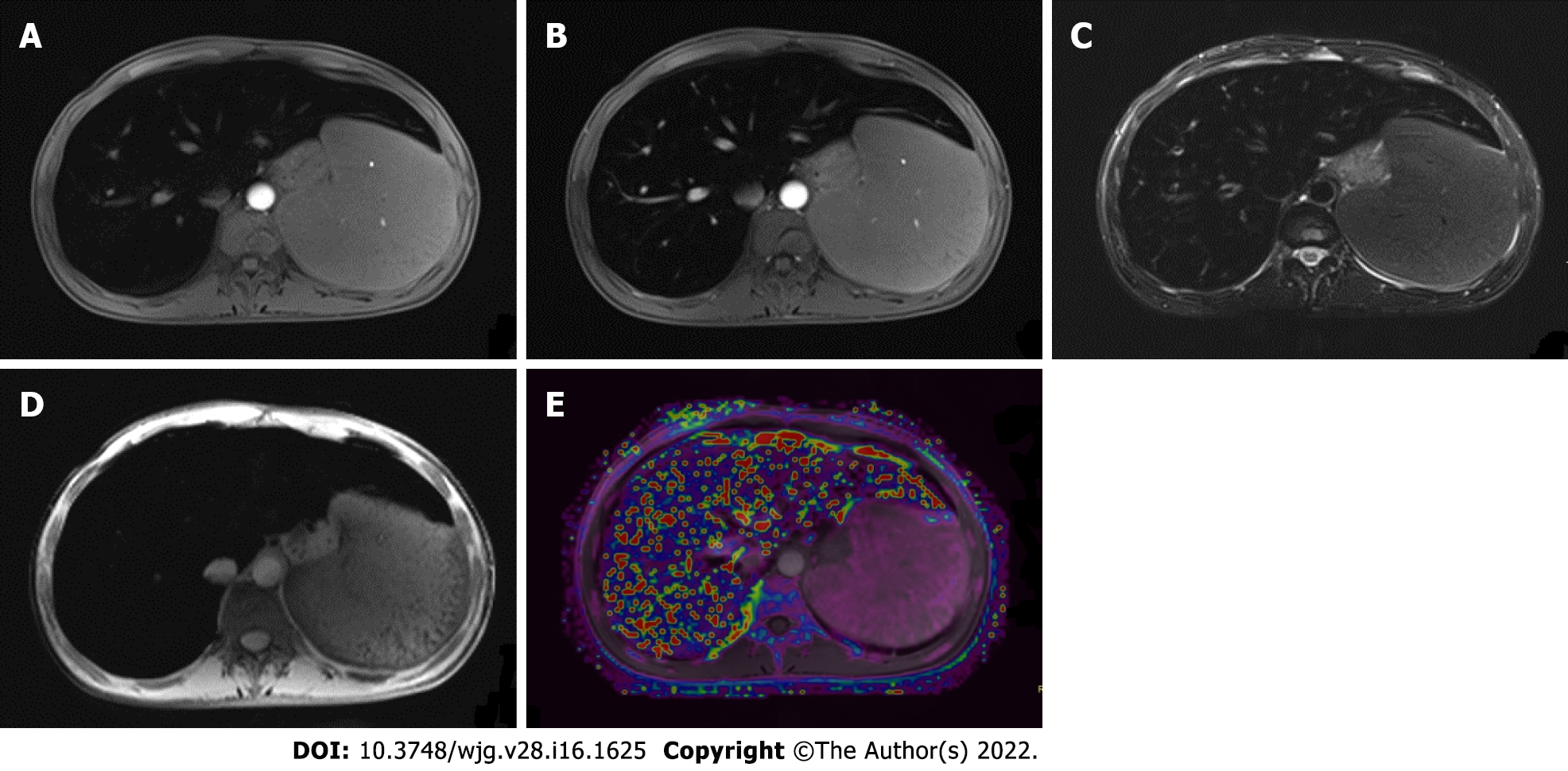
With signal intensity ratios, studies showed that although these methods tended to overestimate mild to moderate hepatic iron overload, it might be more precise in severe iron overload, particularly on 3T MRI[52,53]. On the other hand, relaxometry techniques measure the MR signal decay resulting from the shortening of T2 or T2* relaxation times. For practical purposes, the inverse of T2 or T2* (the relaxation rates, R2 or R2*) is generally used instead, because the elevation in liver iron concentration directly increases the R2 and R2*[54]. The most known R2 relaxometry method is commercially available as FerriScan and is FDA-approved for 1.5T machines[55]. Well validated across different sites and platforms, liver R2 has an excellent correlation with liver iron concentration, with low inter-exam variability and good inter-machine reproducibility[56]. However, major limitations of this technique include long acquisition times and high cost. In contrast, R2* relaxometry is performed with fast, single breath-hold spoiled GRE multi-echo sequences in most MR scanners. Several studies demonstrated an excellent linear relationship between R2* and liver iron concentration[57,58] (Figure 5). However, R2* measurements may be affected by liver fibrosis and the coexistence of fat[59].
Quantitative susceptibility mapping (QSM) was first used in the nervous system. It is based on the concept of transforming hypointense blooming artifacts into precise quantitative measurements of spatial biodistributions. Therefore, it is not affected by liver fibrosis and the coexistence of fat[59]. Tipirneni-Sajja et al[60] applied a multispectral autoregressive moving average model in QSM to liver iron concentration. They found that autoregressive moving average-QSM showed a good association with an iron concentration in both phantom study and in vivo cohort, indicating that autoregressive moving average-QSM could provide a potentially confounder-free assessment of hepatic iron overload[60].
Therefore, the influence of iron on MRI signal makes MRI the most appropriate imaging modality for quantifying liver iron concentration, and QSM may be the most potential sequence to serve this purpose.
Liver fibrosis is a scarring response that occurs in almost all chronic liver injuries mentioned above. Ultimately, liver fibrosis can lead to cirrhosis, in which PH is a common and lethal complication. Early diagnosis and accurate staging of these conditions can facilitate timely patient care and optimize prognoses.
With the deposition of collagen in the extracellular space, liver parenchyma stiffness increases as the disease progresses. These alterations can be measured by elastography techniques.
Among all elastography techniques, TE is the most widely used method to determine liver stiffness and may serve as a potential surrogate to assess liver fibrosis. The pooled AUC of TE for diagnosing liver fibrosis was 0.859 for NAFLD, 0.860 for chronic hepatitis B, and 0.830 for alcohol-related liver disease in previous meta-analyses[61-63]. In addition, shear wave elastography (SWE) was also reported with a high diagnostic accuracy for detecting early-stage liver fibrosis[64-66]. Petzold et al[67] found that a cutoff value of 8.05 kPa could differentiate patients with advanced fibrosis (F ≥ 3) from those with no or mild fibrosis (F0-F2) with AUCs ranging between 0.995 and 1.000. A meta-analysis revealed no significant difference between TE and SWE in the diagnosis of significant fibrosis, advanced fibrosis, and cirrhosis, but the proportion of failed measurements was over ten-fold greater with TE than SWE[68].
In addition to ultrasound-based elastography techniques, the MR-based elastography technique MRE is another promising noninvasive modality to assess liver fibrosis[69,70]. A prospective study of 67 PSC patients revealed a high sensitivity (87.5%) and specificity (96%) of MRE in detecting cirrhosis[71]. In another study, a significant discriminatory ability of MRE was confirmed when distinguishing between early to moderate and advanced liver fibrosis, shedding light on the incremental values of liver stiffness measurements on MRE in prognosis stratification[72]. Fu et al[73] found that the efficacy of MRE was superior compared with TE in detecting significant fibrosis (AUC: 0.965 vs 0.906) and advanced fibrosis (AUC: 0.957 vs 0.913). These results were confirmed by a meta-analysis in which the pooled AUC of MRE (0.97) was significantly higher than that of SWE (0.88) in detecting significant fibrosis[74].
As fibrosis progresses, the deposition of fibroglia can lead to enlarged extracellular space. Therefore, liver extracellular volume (LECV) measured by CT or MR T1 mapping can also be used to assess liver fibrosis[75-77]. In a cynomolgus monkey model of NASH, Lyu et al[78] found that LECV was significantly correlated with the fibrosis score (r = 0.949), and demonstrated an AUC of 0.945 in diagnosing liver fibrosis.
Diffusion-weighted imaging is a noninvasive technique based on the Brownian motion of water molecules in biological tissue and has shown potential in assessing liver fibrosis[79]. Studies showed that in chronic liver diseases, apparent diffusion coefficients in diffusion-weighted imaging decreased as the degree of fibrosis increased, but this relationship was not statistically significant due to confounding factor of blood microcirculation in the capillaries[80,81]. Recent studies have explored various diffusion models to avoid this influence. Lefebvre et al[82] reported that intravoxel incoherent motion parameter with 10 b-values was reproducible for liver tissue characterization and that perfusion fraction (f) provided good diagnostic performance for distinguishing dichotomized grades of inflammation. Park et al[83] showed that the distributed diffusion coefficient from the stretched exponential model was the most accurate diffusion-weighted imaging parameter for staging liver fibrosis as it could avoid the confounding effect by steatosis.
Besides, liver fibrosis can result in changes in hepatic microcirculation and perfusion. Fan et al[84] found that MR perfusion parameters, time to peak, and mean transit time in particular could reflect the degree of liver fibrosis. Similarly, Yoon et al[85] also found that portal blood flow was significantly lower in clinically significant hepatic fibrosis and that mean transit time and extracellular volume increased in cirrhosis.
In general, TE is the modality preferred for LF. SWE can be considered in patients who fail in TE examination. As a modality which is gaining increasingly popularity, MRE is preferred over sonographic elastography in patients with ascites and obesity, or requiring more comprehensive liver workup.
PH is defined by values of hepatic venous pressure gradient (HVPG) > 5 mmHg, whereas clinically significant PH could be diagnosed if HVPG ≥ 10 mmHg. HVPG has been widely-validated as associated with variceal bleeding, hepatic decompensation, and mortality. However, its measurement is invasive and requires extensive expertise[86].
Characteristic imaging features of PH include portosystemic shunts, splenomegaly, ascites, and widening of the portal vein. However, these findings are often detectable at end stages of the disease, thus demonstrating limited sensitivities for diagnosing PH.
For quantitative methods, similar to liver fibrosis, elastography techniques have gained increasing attention in the assessment of PH[87]. Among ultrasound-based elastography techniques, TE was the most validated method for PH assessment. A meta-analysis involving 12 studies showed that liver stiffness measured on TE was well correlated with HVPG and demonstrated a sensitivity of 91.2% and specificity of 81.3% in diagnosing clinically significant PH (cut-off values 13.6-18.6 kPa)[88]. In contrast, despite much less applied than TE, SWE also exhibited encouraging profiles in predicting PH and esophageal varices (AUC: 0.86-0.89)[89-93].
Liver and spleen stiffness measured by MRE also showed promising performances in predicting PH and esophageal varices. A recent meta-analysis found that liver and spleen stiffness on MRE could serve as supplemental noninvasive assessment tools for detecting clinically significant PH and that spleen stiffness might be more specific and accurate than liver stiffness (AUC: 0.88 vs 0.92)[94].
Hemodynamic alteration is another distinct feature in PH. In patients with cirrhosis, decreased portal and total hepatic perfusion were observed[95,96]. Studies showed that mean portal vein velocity in cirrhosis was lower than that in normal subjects and decreased with the severity of liver cirrhosis and gastroesophageal varices[97,98]. The portal vein velocity measured by doppler US could be used as noninvasive triage tests before referral to endoscopy (sensitivity, 84%-97%), but the cutoff value varied from 16-19 cm/s[99,100]. Several MR techniques have also been proposed for liver hemodynamic assessment in PH. Chouhan et al[101] used phase-contrast MR to assess the portal vein and the infrahepatic and suprahepatic inferior vena cava. The hepatic artery flow was estimated by subtracting infrahepatic from suprahepatic inferior vena cava flow and portal vein flow, which showed significant positive correlations with HVPG[101]. Additionally, 4D flow MRI also demonstrated promising capacity in quantifying blood flow in the hepatic and splanchnic vasculature[102,103]. Motosugi et al[104] found that azygos flow > 0.1 L/min and portal venous flow less than the sum of splenic and superior mesenteric vein flow were useful markers to stratify the risk of gastroesophageal varices bleeding in patients with cirrhosis. Another study revealed that the combination of liver stiffness measured by MRE and perfusion metrics measured by contrast-enhanced-MRI had an AUC of 0.903 for diagnosing PH, and an AUC of 0.785 for detecting clinically significant PH[105].
In summary, TE and SWE are promising noninvasive approaches for preliminary PH screening. Nevertheless, for patients with increased risk for esophageal and gastric varices, multiparametric MRI may be a more accurate and comprehensive modality.
Focal liver lesions include benign tumors, malignant tumors, and hepatic echinococcosis. The impact of focal liver lesions on liver function includes the decrease of normal liver volume and the reduced hepatocyte function, especially in surgical candidates with malignant liver tumors. Previous studies have shown that a high residual to total liver volume ratio (≥ 40%) was required for patients with an impaired liver function to tolerate resection[106-108]. Gadoxetate-enhanced MRI is also used to evaluate the hepatic function of patients with focal liver lesions. Yoon et al[109] reported that T1 mapping on gadoxetate-enhanced MRI provided information on global liver function and demonstrated functional heterogeneity in patients with HCC. Other studies have combined liver volume with hepatocyte function, and their results showed that combined T1 mapping and residual liver volume on gadoxetate-enhanced MRI could assess liver function with good diagnostic accuracy in patients with liver tumors[110-112]. Kim et al[113] and Wang et al[114] reported that the combination could predict post hepatectomy liver failure better than the ICG clearance test in patients with HCC who underwent hepatectomy.
To sum up, CT can be used to calculate the residual liver volume for surgical candidates. Gadoxetate-enhanced MRI can not only reflect residual liver volume, but also reveal the functional information of hepatocytes.
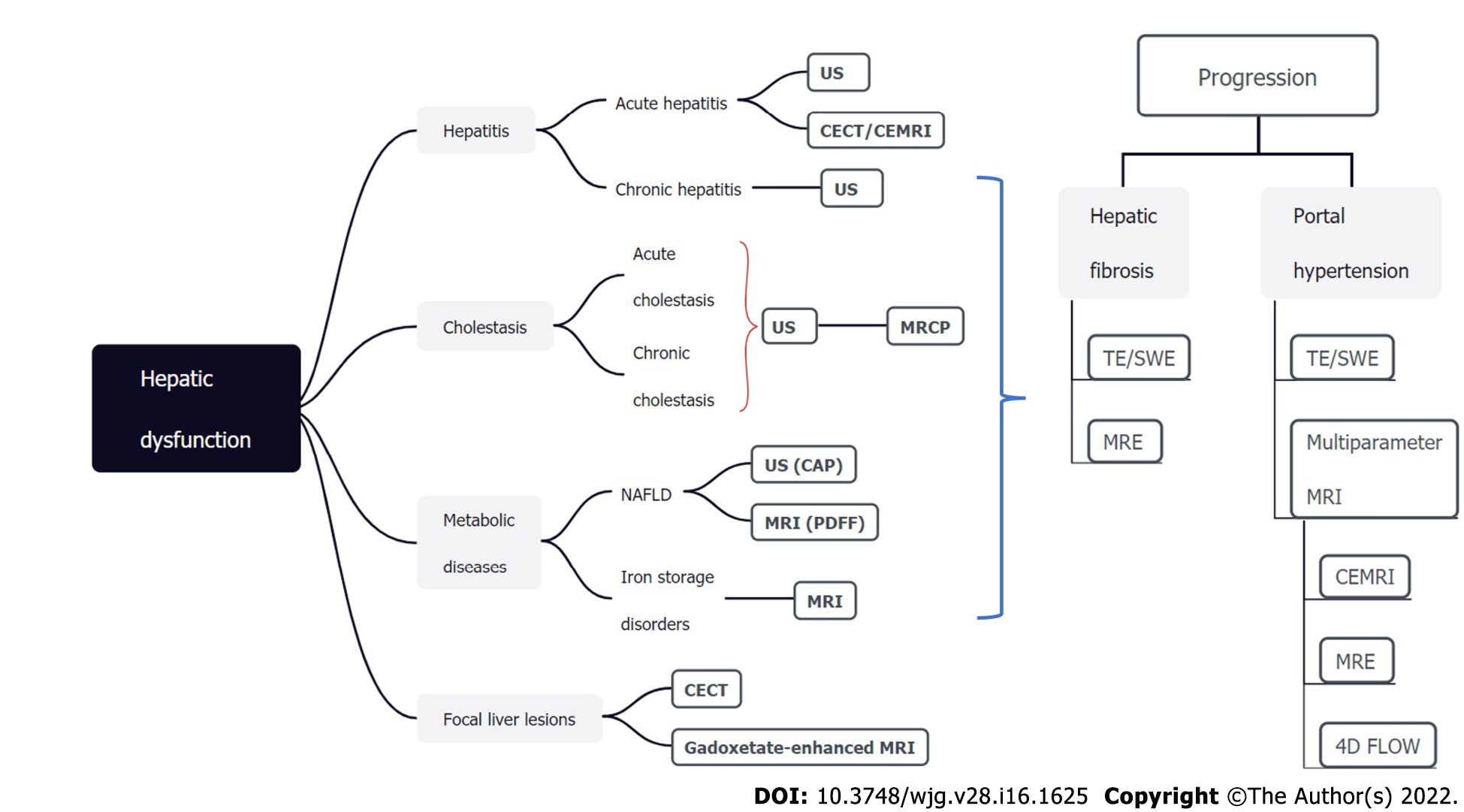
In this article, we provide a summary of noninvasive imaging modalities for assessing hepatic dysfunction in various clinical situations and case scenarios (Figure 6). Several challenges still exist in noninvasive imaging of hepatic dysfunction. First, many imaging parameters have inconsistencies on the device. Therefore, a unified threshold cannot be adopted. Second, quantification of sensitivity and specificity usually requires an effective reference standard (e.g., liver biopsy) which may not be readily available. Furthermore, most of the current studies focus on the role of a single method or sequence, with limited multiparametric, multimodal, and multidisciplinary approaches to evaluate liver dysfunction.
The long-term goal in hepatic dysfunction imaging is to develop reliable, noninvasive, and comprehensive methods which could reveal not only the disease severities but also etiologies using safe and clinically available techniques. However, to accomplish this goal will require advances in imaging sciences (improved image modalities standardization and quantitation, further exploration of US, CT, and MR imaging methods, and combination of multiparametric and multimodal imaging techniques). On this basis, radiomics and artificial intelligence may provide further assistance in quantifying high-level imaging features beyond human eyes and help in constructing effective predictive models. A better understanding of the human genetic variation underlying differences in the liver will further contribute to this field. Furthermore, the potential value of combining imaging and serum biomarkers should also be explored.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Radiology, nuclear medicine and medical imaging
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Duan S, China; Mogahed EA, Egypt S-Editor: Chang KL L-Editor: Wang TQ P-Editor: Chang KL
| 1. | Helmke S, Colmenero J, Everson GT. Noninvasive assessment of liver function. Curr Opin Gastroenterol. 2015;31:199-208. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 31] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 2. | Agrawal S, Dhiman RK, Limdi JK. Evaluation of abnormal liver function tests. Postgrad Med J. 2016;92:223-234. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 91] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 3. | Dillon JF, Miller MH, Robinson EM, Hapca A, Rezaeihemami M, Weatherburn C, McIntyre PG, Bartlett B, Donnan PT, Boyd KA, Dow E. Intelligent liver function testing (iLFT): A trial of automated diagnosis and staging of liver disease in primary care. J Hepatol. 2019;71:699-706. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 54] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 4. | Dhiman RK, Agrawal S, Gupta T, Duseja A, Chawla Y. Chronic Liver Failure-Sequential Organ Failure Assessment is better than the Asia-Pacific Association for the Study of Liver criteria for defining acute-on-chronic liver failure and predicting outcome. World J Gastroenterol. 2014;20:14934-14941. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 65] [Cited by in RCA: 65] [Article Influence: 5.9] [Reference Citation Analysis (1)] |
| 5. | Bajaj JS, O’Leary JG, Reddy KR, Wong F, Biggins SW, Patton H, Fallon MB, Garcia-Tsao G, Maliakkal B, Malik R, Subramanian RM, Thacker LR, Kamath PS; North American Consortium For The Study Of End-Stage Liver Disease (NACSELD). Survival in infection-related acute-on-chronic liver failure is defined by extrahepatic organ failures. Hepatology. 2014;60:250-256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 358] [Cited by in RCA: 433] [Article Influence: 39.4] [Reference Citation Analysis (1)] |
| 6. | Rassam F, Olthof PB, Bennink RJ, van Gulik TM. Current Modalities for the Assessment of Future Remnant Liver Function. Visc Med. 2017;33:442-448. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 53] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 7. | Lisotti A, Azzaroli F, Buonfiglioli F, Montagnani M, Cecinato P, Turco L, Calvanese C, Simoni P, Guardigli M, Arena R, Cucchetti A, Colecchia A, Festi D, Golfieri R, Mazzella G. Indocyanine green retention test as a noninvasive marker of portal hypertension and esophageal varices in compensated liver cirrhosis. Hepatology. 2014;59:643-650. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 86] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 8. | Bolondi G, Mocchegiani F, Montalti R, Nicolini D, Vivarelli M, De Pietri L. Predictive factors of short term outcome after liver transplantation: A review. World J Gastroenterol. 2016;22:5936-5949. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 64] [Cited by in RCA: 59] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 9. | Kokudo T, Hasegawa K, Shirata C, Tanimoto M, Ishizawa T, Kaneko J, Akamatsu N, Arita J, Demartines N, Uldry E, Kokudo N, Halkic N. Assessment of Preoperative Liver Function for Surgical Decision Making in Patients with Hepatocellular Carcinoma. Liver Cancer. 2019;8:447-456. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 43] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 10. | Lanini S, Ustianowski A, Pisapia R, Zumla A, Ippolito G. Viral Hepatitis: Etiology, Epidemiology, Transmission, Diagnostics, Treatment, and Prevention. Infect Dis Clin North Am. 2019;33:1045-1062. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 92] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 11. | Kwong S, Meyerson C, Zheng W, Kassardjian A, Stanzione N, Zhang K, Wang HL. Acute hepatitis and acute liver failure: Pathologic diagnosis and differential diagnosis. Semin Diagn Pathol. 2019;36:404-414. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 15] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 12. | Kim SW, Shin HC, Kim IY. Diffuse pattern of transient hepatic attenuation differences in viral hepatitis: a sign of acute hepatic injury in patients without cirrhosis. J Comput Assist Tomogr. 2010;34:699-705. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 13. | Park SJ, Kim JD, Seo YS, Park BJ, Kim MJ, Um SH, Kim CH, Yim HJ, Baik SK, Jung JY, Keum B, Jeen YT, Lee HS, Chun HJ, Kim CD, Ryu HS. Computed tomography findings for predicting severe acute hepatitis with prolonged cholestasis. World J Gastroenterol. 2013;19:2543-2549. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 12] [Cited by in RCA: 14] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 14. | Feng IC, Wang SJ, Sheu MJ, Koay LB, Lin CY, Ho CH, Sun CS, Kuo HT. Perihepatic nodes detected by point-of-care ultrasound in acute hepatitis and acute-on-chronic liver disease. World J Gastroenterol. 2015;21:12620-12627. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 15. | Grillet F, Calame P, Cervoni JP, Weil D, Thevenot T, Ronot M, Delabrousse E. Non-invasive diagnosis of severe alcoholic hepatitis: Usefulness of cross-sectional imaging. Diagn Interv Imaging. 2021;102:247-254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 16. | Sonthalia N, Rathi PM, Jain SS, Surude RG, Mohite AR, Pawar SV, Contractor Q. Natural History and Treatment Outcomes of Severe Autoimmune Hepatitis. J Clin Gastroenterol. 2017;51:548-556. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 31] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 17. | Tana MM, McCoy D, Lee B, Patel R, Lin J, Ohliger MA. Texture features from computed tomography correlate with markers of severity in acute alcohol-associated hepatitis. Sci Rep. 2020;10:17980. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 18. | Seto WK, Lo YR, Pawlotsky JM, Yuen MF. Chronic hepatitis B virus infection. Lancet. 2018;392:2313-2324. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 271] [Cited by in RCA: 367] [Article Influence: 52.4] [Reference Citation Analysis (2)] |
| 19. | Shin SW, Kim TY, Jeong WK, Kim Y, Kim J, Kim YH, Park HC, Sohn JH. Usefulness of B-mode and doppler sonography for the diagnosis of severe acute viral hepatitis A. J Clin Ultrasound. 2015;43:384-392. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 20. | Cao Y, Wang H, Johnson TD, Pan C, Hussain H, Balter JM, Normolle D, Ben-Josef E, Ten Haken RK, Lawrence TS, Feng M. Prediction of liver function by using magnetic resonance-based portal venous perfusion imaging. Int J Radiat Oncol Biol Phys. 2013;85:258-263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 57] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 21. | Van Beers BE, Pastor CM, Hussain HK. Primovist, Eovist: what to expect? J Hepatol. 2012;57:421-429. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 292] [Cited by in RCA: 321] [Article Influence: 24.7] [Reference Citation Analysis (0)] |
| 22. | Choi Y, Huh J, Woo DC, Kim KW. Use of gadoxetate disodium for functional MRI based on its unique molecular mechanism. Br J Radiol. 2016;89:20150666. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 21] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 23. | Nakagawa M, Namimoto T, Shimizu K, Morita K, Sakamoto F, Oda S, Nakaura T, Utsunomiya D, Shiraishi S, Yamashita Y. Measuring hepatic functional reserve using T1 mapping of Gd-EOB-DTPA enhanced 3T MR imaging: A preliminary study comparing with 99mTc GSA scintigraphy and signal intensity based parameters. Eur J Radiol. 2017;92:116-123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 26] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 24. | Pan S, Wang XQ, Guo QY. Quantitative assessment of hepatic fibrosis in chronic hepatitis B and C: T1 mapping on Gd-EOB-DTPA-enhanced liver magnetic resonance imaging. World J Gastroenterol. 2018;24:2024-2035. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 26] [Cited by in RCA: 29] [Article Influence: 4.1] [Reference Citation Analysis (1)] |
| 25. | Liu MT, Zhang XQ, Lu J, Zhang T, Chen Q, Jiang JF, Ding D, Du S, Chen WB. Evaluation of liver function using the hepatocyte enhancement fraction based on gadoxetic acid-enhanced MRI in patients with chronic hepatitis B. Abdom Radiol (NY). 2020;45:3129-3135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 26. | Di Serafino M, Gioioso M, Severino R, Esposito F, Vezzali N, Ferro F, Pelliccia P, Caprio MG, Iorio R, Vallone G. Ultrasound findings in paediatric cholestasis: how to image the patient and what to look for. J Ultrasound. 2020;23:1-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 27. | Alsaigh S, Aldhubayb MA, Alobaid AS, Alhajjaj AH, Alharbi BA, Alsudais DM, Alhothail HA, AlSaykhan MA. Diagnostic Reliability of Ultrasound Compared to Magnetic Resonance Cholangiopancreatography and Endoscopic Retrograde Cholangiopancreatography in the Detection of Obstructive Jaundice: A Retrospective Medical Records Review. Cureus. 2020;12:e10987. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 28. | Katabathina VS, Dasyam AK, Dasyam N, Hosseinzadeh K. Adult bile duct strictures: role of MR imaging and MR cholangiopancreatography in characterization. Radiographics. 2014;34:565-586. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 68] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 29. | Hyodo T, Kumano S, Kushihata F, Okada M, Hirata M, Tsuda T, Takada Y, Mochizuki T, Murakami T. CT and MR cholangiography: advantages and pitfalls in perioperative evaluation of biliary tree. Br J Radiol. 2012;85:887-896. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 89] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 30. | Kim DK, Choi JY, Park MS, Kim MJ, Chung YE. Clinical Feasibility of MR Elastography in Patients With Biliary Obstruction. AJR Am J Roentgenol. 2018;210:1273-1278. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 12] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 31. | Seo N, Kim SY, Lee SS, Byun JH, Kim JH, Kim HJ, Lee MG. Sclerosing Cholangitis: Clinicopathologic Features, Imaging Spectrum, and Systemic Approach to Differential Diagnosis. Korean J Radiol. 2016;17:25-38. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 38] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 32. | Khoshpouri P, Habibabadi RR, Hazhirkarzar B, Ameli S, Ghadimi M, Ghasabeh MA, Menias CO, Kim A, Li Z, Kamel IR. Imaging Features of Primary Sclerosing Cholangitis: From Diagnosis to Liver Transplant Follow-up. Radiographics. 2019;39:1938-1964. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 28] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 33. | Crosignani A, Battezzati PM, Invernizzi P, Selmi C, Prina E, Podda M. Clinical features and management of primary biliary cirrhosis. World J Gastroenterol. 2008;14:3313-3327. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 33] [Cited by in RCA: 28] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 34. | Kovač JD, Weber MA. Primary Biliary Cirrhosis and Primary Sclerosing Cholangitis: an Update on MR Imaging Findings with Recent Developments. J Gastrointestin Liver Dis. 2016;25:517-524. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 17] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 35. | Cebada Chaparro E, Lloret Del Hoyo J, Méndez Fernández R. Chronic cholangitides: Differential diagnosis and role of MRI. Radiologia (Engl Ed). 2020;62:452-463. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 36. | Byrne CD, Targher G. NAFLD: a multisystem disease. J Hepatol. 2015;62:S47-S64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1516] [Cited by in RCA: 2131] [Article Influence: 213.1] [Reference Citation Analysis (0)] |
| 37. | Castera L, Friedrich-Rust M, Loomba R. Noninvasive Assessment of Liver Disease in Patients With Nonalcoholic Fatty Liver Disease. Gastroenterology. 2019;156:1264-1281.e4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 619] [Cited by in RCA: 1027] [Article Influence: 171.2] [Reference Citation Analysis (0)] |
| 38. | Petroff D, Blank V, Newsome PN, Shalimar, Voican CS, Thiele M, de Lédinghen V, Baumeler S, Chan WK, Perlemuter G, Cardoso AC, Aggarwal S, Sasso M, Eddowes PJ, Allison M, Tsochatzis E, Anstee QM, Sheridan D, Cobbold JF, Naveau S, Lupsor-Platon M, Mueller S, Krag A, Irles-Depe M, Semela D, Wong GL, Wong VW, Villela-Nogueira CA, Garg H, Chazouillères O, Wiegand J, Karlas T. Assessment of hepatic steatosis by controlled attenuation parameter using the M and XL probes: an individual patient data meta-analysis. Lancet Gastroenterol Hepatol. 2021;6:185-198. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 175] [Cited by in RCA: 168] [Article Influence: 42.0] [Reference Citation Analysis (0)] |
| 39. | Pu K, Wang Y, Bai S, Wei H, Zhou Y, Fan J, Qiao L. Diagnostic accuracy of controlled attenuation parameter (CAP) as a non-invasive test for steatosis in suspected non-alcoholic fatty liver disease: a systematic review and meta-analysis. BMC Gastroenterol. 2019;19:51. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 140] [Cited by in RCA: 138] [Article Influence: 23.0] [Reference Citation Analysis (0)] |
| 40. | Bohte AE, van Werven JR, Bipat S, Stoker J. The diagnostic accuracy of US, CT, MRI and 1H-MRS for the evaluation of hepatic steatosis compared with liver biopsy: a meta-analysis. Eur Radiol. 2011;21:87-97. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 326] [Cited by in RCA: 395] [Article Influence: 26.3] [Reference Citation Analysis (0)] |
| 41. | Gu Q, Cen L, Lai J, Zhang Z, Pan J, Zhao F, Yu C, Li Y, Chen C, Chen W, Shen Z. A meta-analysis on the diagnostic performance of magnetic resonance imaging and transient elastography in nonalcoholic fatty liver disease. Eur J Clin Invest. 2021;51:e13446. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 33] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 42. | Choi SJ, Kim SM, Kim YS, Kwon OS, Shin SK, Kim KK, Lee K, Park IB, Choi CS, Chung DH, Jung J, Paek M, Lee DH. Magnetic Resonance-Based Assessments Better Capture Pathophysiologic Profiles and Progression in Nonalcoholic Fatty Liver Disease. Diabetes Metab J. 2021;45:739-752. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 20] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 43. | Chalasani N, Vuppalanchi R, Rinella M, Middleton MS, Siddiqui MS, Barritt AS 4th, Kolterman O, Flores O, Alonso C, Iruarrizaga-Lejarreta M, Gil-Redondo R, Sirlin CB, Zemel MB. Randomised clinical trial: a leucine-metformin-sildenafil combination (NS-0200) vs placebo in patients with non-alcoholic fatty liver disease. Aliment Pharmacol Ther. 2018;47:1639-1651. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 32] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 44. | Yan J, Yao B, Kuang H, Yang X, Huang Q, Hong T, Li Y, Dou J, Yang W, Qin G, Yuan H, Xiao X, Luo S, Shan Z, Deng H, Tan Y, Xu F, Xu W, Zeng L, Kang Z, Weng J. Liraglutide, Sitagliptin, and Insulin Glargine Added to Metformin: The Effect on Body Weight and Intrahepatic Lipid in Patients With Type 2 Diabetes Mellitus and Nonalcoholic Fatty Liver Disease. Hepatology. 2019;69:2414-2426. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 115] [Cited by in RCA: 182] [Article Influence: 30.3] [Reference Citation Analysis (0)] |
| 45. | Jiang H, Chen HC, Lafata KJ, Bashir MR. Week 4 Liver Fat Reduction on MRI as an Early Predictor of Treatment Response in Participants with Nonalcoholic Steatohepatitis. Radiology. 2021;300:361-368. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 13] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 46. | Mojtahed A, Kelly CJ, Herlihy AH, Kin S, Wilman HR, McKay A, Kelly M, Milanesi M, Neubauer S, Thomas EL, Bell JD, Banerjee R, Harisinghani M. Reference range of liver corrected T1 values in a population at low risk for fatty liver disease-a UK Biobank sub-study, with an appendix of interesting cases. Abdom Radiol (NY). 2019;44:72-84. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 60] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 47. | Imajo K, Tetlow L, Dennis A, Shumbayawonda E, Mouchti S, Kendall TJ, Fryer E, Yamanaka S, Honda Y, Kessoku T, Ogawa Y, Yoneda M, Saito S, Kelly C, Kelly MD, Banerjee R, Nakajima A. Quantitative multiparametric magnetic resonance imaging can aid non-alcoholic steatohepatitis diagnosis in a Japanese cohort. World J Gastroenterol. 2021;27:609-623. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 18] [Cited by in RCA: 33] [Article Influence: 8.3] [Reference Citation Analysis (2)] |
| 48. | Eddowes PJ, McDonald N, Davies N, Semple SIK, Kendall TJ, Hodson J, Newsome PN, Flintham RB, Wesolowski R, Blake L, Duarte RV, Kelly CJ, Herlihy AH, Kelly MD, Olliff SP, Hübscher SG, Fallowfield JA, Hirschfield GM. Utility and cost evaluation of multiparametric magnetic resonance imaging for the assessment of non-alcoholic fatty liver disease. Aliment Pharmacol Ther. 2018;47:631-644. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 80] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 49. | Bassett ML, Hickman PE, Dahlstrom JE. The changing role of liver biopsy in diagnosis and management of haemochromatosis. Pathology. 2011;43:433-439. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 50. | Yan F, He N, Lin H, Li R. Iron deposition quantification: Applications in the brain and liver. J Magn Reson Imaging. 2018;48:301-317. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 36] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 51. | Wells SA. Quantification of hepatic fat and iron with magnetic resonance imaging. Magn Reson Imaging Clin N Am. 2014;22:397-416. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 52. | Castiella A, Alústiza JM, Emparanza JI, Zapata EM, Costero B, Díez MI. Liver iron concentration quantification by MRI: are recommended protocols accurate enough for clinical practice? Eur Radiol. 2011;21:137-141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 37] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 53. | d’Assignies G, Paisant A, Bardou-Jacquet E, Boulic A, Bannier E, Lainé F, Ropert M, Morcet J, Saint-Jalmes H, Gandon Y. Non-invasive measurement of liver iron concentration using 3-Tesla magnetic resonance imaging: validation against biopsy. Eur Radiol. 2018;28:2022-2030. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 54] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 54. | Sirlin CB, Reeder SB. Magnetic resonance imaging quantification of liver iron. Magn Reson Imaging Clin N Am. 2010;18:359-381, ix. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 177] [Cited by in RCA: 154] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 55. | St Pierre TG, Clark PR, Chua-anusorn W, Fleming AJ, Jeffrey GP, Olynyk JK, Pootrakul P, Robins E, Lindeman R. Noninvasive measurement and imaging of liver iron concentrations using proton magnetic resonance. Blood. 2005;105:855-861. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 640] [Cited by in RCA: 663] [Article Influence: 31.6] [Reference Citation Analysis (0)] |
| 56. | St Pierre TG, El-Beshlawy A, Elalfy M, Al Jefri A, Al Zir K, Daar S, Habr D, Kriemler-Krahn U, Taher A. Multicenter validation of spin-density projection-assisted R2-MRI for the noninvasive measurement of liver iron concentration. Magn Reson Med. 2014;71:2215-2223. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 72] [Cited by in RCA: 101] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 57. | Sussman MS, Ward R, Kuo KHM, Tomlinson G, Jhaveri KS. Impact of MRI technique on clinical decision-making in patients with liver iron overload: comparison of FerriScan- vs R2*-derived liver iron concentration. Eur Radiol. 2020;30:1959-1968. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 58. | Henninger B, Plaikner M, Zoller H, Viveiros A, Kannengiesser S, Jaschke W, Kremser C. Performance of different Dixon-based methods for MR liver iron assessment in comparison to a biopsy-validated R2* relaxometry method. Eur Radiol. 2021;31:2252-2262. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 29] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 59. | Li J, Lin H, Liu T, Zhang Z, Prince MR, Gillen K, Yan X, Song Q, Hua T, Zhao X, Zhang M, Zhao Y, Li G, Tang G, Yang G, Brittenham GM, Wang Y. Quantitative susceptibility mapping (QSM) minimizes interference from cellular pathology in R2* estimation of liver iron concentration. J Magn Reson Imaging. 2018;48:1069-1079. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 54] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 60. | Tipirneni-Sajja A, Loeffler RB, Hankins JS, Morin C, Hillenbrand CM. Quantitative Susceptibility Mapping Using a Multispectral Autoregressive Moving Average Model to Assess Hepatic Iron Overload. J Magn Reson Imaging. 2021;54:721-727. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 61. | Nguyen-Khac E, Thiele M, Voican C, Nahon P, Moreno C, Boursier J, Mueller S, de Ledinghen V, Stärkel P, Gyune Kim S, Fernandez M, Madsen B, Naveau S, Krag A, Perlemuter G, Ziol M, Chatelain D, Diouf M. Non-invasive diagnosis of liver fibrosis in patients with alcohol-related liver disease by transient elastography: an individual patient data meta-analysis. Lancet Gastroenterol Hepatol. 2018;3:614-625. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 92] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 62. | Qi X, An M, Wu T, Jiang D, Peng M, Wang W, Wang J, Zhang C; Chess Study Group OBOT. Transient Elastography for Significant Liver Fibrosis and Cirrhosis in Chronic Hepatitis B: A Meta-Analysis. Can J Gastroenterol Hepatol. 2018;2018:3406789. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 13] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 63. | Ooi GJ, Mgaieth S, Eslick GD, Burton PR, Kemp WW, Roberts SK, Brown WA. Systematic review and meta-analysis: non-invasive detection of non-alcoholic fatty liver disease related fibrosis in the obese. Obes Rev. 2018;19:281-294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 24] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 64. | Dietrich CF, Bamber J, Berzigotti A, Bota S, Cantisani V, Castera L, Cosgrove D, Ferraioli G, Friedrich-Rust M, Gilja OH, Goertz RS, Karlas T, de Knegt R, de Ledinghen V, Piscaglia F, Procopet B, Saftoiu A, Sidhu PS, Sporea I, Thiele M. EFSUMB Guidelines and Recommendations on the Clinical Use of Liver Ultrasound Elastography, Update 2017 (Long Version). Ultraschall Med. 2017;38:e16-e47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 601] [Cited by in RCA: 575] [Article Influence: 71.9] [Reference Citation Analysis (0)] |
| 65. | Xiao G, Zhu S, Xiao X, Yan L, Yang J, Wu G. Comparison of laboratory tests, ultrasound, or magnetic resonance elastography to detect fibrosis in patients with nonalcoholic fatty liver disease: A meta-analysis. Hepatology. 2017;66:1486-1501. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 492] [Cited by in RCA: 640] [Article Influence: 80.0] [Reference Citation Analysis (0)] |
| 66. | Sande JA, Verjee S, Vinayak S, Amersi F, Ghesani M. Ultrasound shear wave elastography and liver fibrosis: A Prospective Multicenter Study. World J Hepatol. 2017;9:38-47. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 49] [Article Influence: 6.1] [Reference Citation Analysis (1)] |
| 67. | Petzold G, Bremer SCB, Knoop RF, Amanzada A, Raddatz D, Ellenrieder V, Ströbel P, Kunsch S, Neesse A. Noninvasive assessment of liver fibrosis in a real-world cohort of patients with known or suspected chronic liver disease using 2D-shear wave elastography. Eur J Gastroenterol Hepatol. 2020;32:1559-1565. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 68. | Jiang W, Huang S, Teng H, Wang P, Wu M, Zhou X, Ran H. Diagnostic accuracy of point shear wave elastography and transient elastography for staging hepatic fibrosis in patients with non-alcoholic fatty liver disease: a meta-analysis. BMJ Open. 2018;8:e021787. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 87] [Cited by in RCA: 104] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 69. | Venkatesh SK, Yin M, Takahashi N, Glockner JF, Talwalkar JA, Ehman RL. Non-invasive detection of liver fibrosis: MR imaging features vs. MR elastography. Abdom Imaging. 2015;40:766-775. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 81] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 70. | Wang XP, Wang Y, Ma H, Wang H, Yang DW, Zhao XY, Jin EH, Yang ZH. Assessment of liver fibrosis with liver and spleen magnetic resonance elastography, serum markers in chronic liver disease. Quant Imaging Med Surg. 2020;10:1208-1222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 71. | Jhaveri KS, Hosseini-Nik H, Sadoughi N, Janssen H, Feld JJ, Fischer S, Menezes R, Cheung AC. The development and validation of magnetic resonance elastography for fibrosis staging in primary sclerosing cholangitis. Eur Radiol. 2019;29:1039-1047. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 17] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 72. | Tafur M, Cheung A, Menezes RJ, Feld J, Janssen H, Hirschfield GM, Jhaveri KS. Risk stratification in primary sclerosing cholangitis: comparison of biliary stricture severity on MRCP vs liver stiffness by MR elastography and vibration-controlled transient elastography. Eur Radiol. 2020;30:3735-3747. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 73. | Fu F, Li X, Chen C, Bai Y, Liu Q, Shi D, Sang J, Wang K, Wang M. Non-invasive assessment of hepatic fibrosis: comparison of MR elastography to transient elastography and intravoxel incoherent motion diffusion-weighted MRI. Abdom Radiol (NY). 2020;45:73-82. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 74. | Dong BT, Chen YP, Lyu GR, Wang HM, Lin GF, Gu JH. Diagnostic accuracy of two-dimensional shear wave elastography and magnetic resonance elastography for staging liver fibrosis in patients with chronic hepatitis B: A systematic review and meta-analysis. J Gastroenterol Hepatol. 2021;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 3] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 75. | Morita K, Nishie A, Ushijima Y, Takayama Y, Fujita N, Kubo Y, Ishimatsu K, Yoshizumi T, Maehara J, Ishigami K. Noninvasive assessment of liver fibrosis by dual-layer spectral detector CT. Eur J Radiol. 2021;136:109575. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 19] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 76. | Evrimler S, Swensson JK, Are VS, Tirkes T, Vuppalanchi R, Akisik F. Quantitative assessment of disease severity of primary sclerosing cholangitis with T1 mapping and extracellular volume imaging. Abdom Radiol (NY). 2021;46:2433-2443. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 77. | Bak S, Kim JE, Bae K, Cho JM, Choi HC, Park MJ, Choi HY, Shin HS, Lee SM, Kim HO. Quantification of liver extracellular volume using dual-energy CT: utility for prediction of liver-related events in cirrhosis. Eur Radiol. 2020;30:5317-5326. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 78. | Lyu L, Liu XL, Rui MP, Yang LC, Wang GZ, Fan D, Wang T, Zheng J. Liver extracellular volume fraction values obtained with magnetic resonance imaging can quantitatively stage liver fibrosis: a validation study in monkeys with nonalcoholic steatohepatitis. Eur Radiol. 2020;30:5748-5757. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 79. | Taouli B, Koh DM. Diffusion-weighted MR imaging of the liver. Radiology. 2010;254:47-66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 623] [Cited by in RCA: 609] [Article Influence: 40.6] [Reference Citation Analysis (2)] |
| 80. | Tokgöz Ö, Unal I, Turgut GG, Yildiz S. The value of liver and spleen ADC measurements in the diagnosis and follow up of hepatic fibrosis in chronic liver disease. Acta Clin Belg. 2014;69:426-432. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 81. | Ding Y, Rao SX, Chen C, Li R, Zeng MS. Assessing liver function in patients with HBV-related HCC: a comparison of T₁ mapping on Gd-EOB-DTPA-enhanced MR imaging with DWI. Eur Radiol. 2015;25:1392-1398. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 42] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 82. | Lefebvre T, Hébert M, Bilodeau L, Sebastiani G, Cerny M, Olivié D, Gao ZH, Sylvestre MP, Cloutier G, Nguyen BN, Gilbert G, Tang A. Intravoxel incoherent motion diffusion-weighted MRI for the characterization of inflammation in chronic liver disease. Eur Radiol. 2021;31:1347-1358. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 83. | Park JH, Seo N, Chung YE, Kim SU, Park YN, Choi JY, Park MS, Kim MJ. Noninvasive evaluation of liver fibrosis: comparison of the stretched exponential diffusion-weighted model to other diffusion-weighted MRI models and transient elastography. Eur Radiol. 2021;31:4813-4823. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 84. | Fan G, Ya Y, Ni X, Hou J, Yu R. Application Value of Magnetic Resonance Perfusion Imaging in the Early Diagnosis of Rat Hepatic Fibrosis. Biomed Res Int. 2019;2019:5095934. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 85. | Yoon JH, Lee JM, Yu MH, Hur BY, Grimm R, Sourbron S, Chandarana H, Son Y, Basak S, Lee KB, Yi NJ, Lee KW, Suh KS. Simultaneous evaluation of perfusion and morphology using GRASP MRI in hepatic fibrosis. Eur Radiol. 2022;32:34-45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 86. | Engelmann C, Clària J, Szabo G, Bosch J, Bernardi M. Pathophysiology of decompensated cirrhosis: Portal hypertension, circulatory dysfunction, inflammation, metabolism and mitochondrial dysfunction. J Hepatol. 2021;75 Suppl 1:S49-S66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 217] [Article Influence: 54.3] [Reference Citation Analysis (1)] |
| 87. | de Franchis R; Baveno VI Faculty. Expanding consensus in portal hypertension: Report of the Baveno VI Consensus Workshop: Stratifying risk and individualizing care for portal hypertension. J Hepatol. 2015;63:743-752. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2011] [Cited by in RCA: 2282] [Article Influence: 228.2] [Reference Citation Analysis (3)] |
| 88. | You MW, Kim KW, Pyo J, Huh J, Kim HJ, Lee SJ, Park SH. A Meta-analysis for the Diagnostic Performance of Transient Elastography for Clinically Significant Portal Hypertension. Ultrasound Med Biol. 2017;43:59-68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 78] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 89. | Fofiu R, Bende F, Popescu A, Șirli R, Miuţescu B, Sporea I. Assessing Baveno VI Criteria Using Liver Stiffness Measured with a 2D-Shear Wave Elastography Technique. Diagnostics (Basel). 2021;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 90. | Fofiu R, Bende F, Popescu A, Şirli R, Lupușoru R, Ghiuchici AM, Sporea I. Spleen and Liver Stiffness for Predicting High-Risk Varices in Patients with Compensated Liver Cirrhosis. Ultrasound Med Biol. 2021;47:76-83. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 15] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 91. | Kang SH, Baik SK, Kim MY. Application of Baveno Criteria and Modified Baveno Criteria with Shear-wave Elastography in Compensated Advanced Chronic Liver Disease. J Korean Med Sci. 2020;35:e249. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 92. | Yu JB, Xiong H, Yuan XC, Zhou AY. Liver Stiffness Detected by Shear Wave Elastography Predicts Esophageal Varices in Cirrhotic Patients. Ultrasound Q. 2019;37:118-122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 93. | Yoo HW, Kim YS, Kim SG, Yoo JJ, Jeong SW, Jang JY, Lee SH, Kim HS, Kim YD, Cheon GJ, Jun B, Kim BS. Usefulness of noninvasive methods including assessment of liver stiffness by 2-dimensional shear wave elastography for predicting esophageal varices. Dig Liver Dis. 2019;51:1706-1712. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 94. | Singh R, Wilson MP, Katlariwala P, Murad MH, McInnes MDF, Low G. Accuracy of liver and spleen stiffness on magnetic resonance elastography for detecting portal hypertension: a systematic review and meta-analysis. Eur J Gastroenterol Hepatol. 2021;32:237-245. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 95. | Ma R, Hunter P, Cousins W, Ho H, Bartlett A, Safaei S. Anatomically based simulation of hepatic perfusion in the human liver. Int J Numer Method Biomed Eng. 2019;35:e3229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 11] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 96. | Donato H, França M, Candelária I, Caseiro-Alves F. Liver MRI: From basic protocol to advanced techniques. Eur J Radiol. 2017;93:30-39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 87] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 97. | Zironi G, Gaiani S, Fenyves D, Rigamonti A, Bolondi L, Barbara L. Value of measurement of mean portal flow velocity by Doppler flowmetry in the diagnosis of portal hypertension. J Hepatol. 1992;16:298-303. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 121] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 98. | Kayacetin E, Efe D, Doğan C. Portal and splenic hemodynamics in cirrhotic patients: relationship between esophageal variceal bleeding and the severity of hepatic failure. J Gastroenterol. 2004;39:661-667. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 30] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 99. | Shastri M, Kulkarni S, Patell R, Jasdanwala S. Portal vein Doppler: a tool for non-invasive prediction of esophageal varices in cirrhosis. J Clin Diagn Res. 2014;8:MC12-MC15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 100. | Elkenawy YN, Elarabawy RA, Ahmed LM, Elsawy AA. Portal vein flow velocity as a possible fast noninvasive screening tool for esophageal varices in cirrhotic patients. JGH Open. 2020;4:589-594. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 101. | Chouhan MD, Mookerjee RP, Bainbridge A, Punwani S, Jones H, Davies N, Walker-Samuel S, Patch D, Jalan R, Halligan S, Lythgoe MF, Taylor SA. Caval Subtraction 2D Phase-Contrast MRI to Measure Total Liver and Hepatic Arterial Blood Flow: Proof-of-Principle, Correlation With Portal Hypertension Severity and Validation in Patients With Chronic Liver Disease. Invest Radiol. 2017;52:170-176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 102. | Roldán-Alzate A, Frydrychowicz A, Niespodzany E, Landgraf BR, Johnson KM, Wieben O, Reeder SB. In vivo validation of 4D flow MRI for assessing the hemodynamics of portal hypertension. J Magn Reson Imaging. 2013;37:1100-1108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 92] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 103. | Frydrychowicz A, Roldan-Alzate A, Winslow E, Consigny D, Campo CA, Motosugi U, Johnson KM, Wieben O, Reeder SB. Comparison of radial 4D Flow-MRI with perivascular ultrasound to quantify blood flow in the abdomen and introduction of a porcine model of pre-hepatic portal hypertension. Eur Radiol. 2017;27:5316-5324. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 23] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 104. | Motosugi U, Roldán-Alzate A, Bannas P, Said A, Kelly S, Zea R, Wieben O, Reeder SB. Four-dimensional Flow MRI as a Marker for Risk Stratification of Gastroesophageal Varices in Patients with Liver Cirrhosis. Radiology. 2019;290:101-107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 45] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 105. | Wagner M, Hectors S, Bane O, Gordic S, Kennedy P, Besa C, Schiano TD, Thung S, Fischman A, Taouli B. Noninvasive prediction of portal pressure with MR elastography and DCE-MRI of the liver and spleen: Preliminary results. J Magn Reson Imaging. 2018;48:1091-1103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 32] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 106. | Wagener G. Assessment of hepatic function, operative candidacy, and medical management after liver resection in the patient with underlying liver disease. Semin Liver Dis. 2013;33:204-212. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 27] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 107. | Blüthner E, Jara M, Shrestha R, Faber W, Pratschke J, Stockmann M, Malinowski M. The predictive value of future liver remnant function after liver resection for HCC in noncirrhotic and cirrhotic patients. HPB (Oxford). 2019;21:912-922. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 28] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 108. | Rahbari NN, Mehrabi A, Mollberg NM, Müller SA, Koch M, Büchler MW, Weitz J. Hepatocellular carcinoma: current management and perspectives for the future. Ann Surg. 2011;253:453-469. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 310] [Cited by in RCA: 365] [Article Influence: 26.1] [Reference Citation Analysis (0)] |
| 109. | Yoon JH, Lee JM, Kim E, Okuaki T, Han JK. Quantitative Liver Function Analysis: Volumetric T1 Mapping with Fast Multisection B1 Inhomogeneity Correction in Hepatocyte-specific Contrast-enhanced Liver MR Imaging. Radiology. 2017;282:408-417. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 47] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 110. | Yoon JH, Choi JI, Jeong YY, Schenk A, Chen L, Laue H, Kim SY, Lee JM. Pre-treatment estimation of future remnant liver function using gadoxetic acid MRI in patients with HCC. J Hepatol. 2016;65:1155-1162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 38] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 111. | Haimerl M, Schlabeck M, Verloh N, Zeman F, Fellner C, Nickel D, Barreiros AP, Loss M, Stroszczynski C, Wiggermann P. Volume-assisted estimation of liver function based on Gd-EOB-DTPA-enhanced MR relaxometry. Eur Radiol. 2016;26:1125-1133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 55] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 112. | Duan T, Jiang H, Xia C, Chen J, Cao L, Ye Z, Wei Y, Song B, Lee JM. Assessing Liver Function in Liver Tumors Patients: The Performance of T1 Mapping and Residual Liver Volume on Gd-EOBDTPA-Enhanced MRI. Front Med (Lausanne). 2020;7:215. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 113. | Kim DK, Choi JI, Choi MH, Park MY, Lee YJ, Rha SE, Jung SE. Prediction of Posthepatectomy Liver Failure: MRI With Hepatocyte-Specific Contrast Agent Versus Indocyanine Green Clearance Test. AJR Am J Roentgenol. 2018;211:580-587. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 40] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 114. | Wang Y, Zhang L, Ning J, Zhang X, Li X, Chen G, Zhao X, Wang X, Yang S, Yuan C, Dong J, Chen H. Preoperative Remnant Liver Function Evaluation Using a Routine Clinical Dynamic Gd-EOB-DTPA-Enhanced MRI Protocol in Patients with Hepatocellular Carcinoma. Ann Surg Oncol. 2021;28:3672-3682. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |