Published online Apr 21, 2022. doi: 10.3748/wjg.v28.i15.1588
Peer-review started: November 20, 2021
First decision: January 11, 2022
Revised: February 2, 2022
Accepted: March 6, 2022
Article in press: March 6, 2022
Published online: April 21, 2022
Processing time: 145 Days and 22.5 Hours
The severity of acute pancreatitis in pregnancy (APIP) is correlated with higher risks of maternal and fetal death.
To develop a nomogram that could predict moderately severe and severe acute pancreatitis in pregnancy (MSIP).
Patients with APIP admitted to West China Hospital between January 2012 and December 2018 were included in this study. They were divided into mild acute pancreatitis in pregnancy (MAIP) and MSIP. Characteristic parameters and laboratory results were collected. The training set and test set were randomly divided at a ratio of 7:3. Least absolute shrinkage and selection operator regression was used to select potential prognostic factors. A nomogram was developed by logistic regression. A random forest model was used to validate the stability of the prediction factors. Receiver operating characteristic curves and calibration curves were used to evaluate the model’s predictive performance.
A total of 190 patients were included in this study. A total of 134 patients (70.5%) and 56 patients (29.5%) were classified as having MAIP and MSIP, respectively. Four independent predictors (lactate dehydrogenase, triglyceride, cholesterol, and albumin levels) were identified for MSIP. A nomogram prediction model based on these factors was established. The model had areas under the curve of 0.865 and 0.853 in the training and validation sets, respectively. The calibration curves showed that the nomogram has a good consistency.
A nomogram including lactate dehydrogenase, triglyceride, cholesterol, and albumin levels as independent predictors was built with good performance for MSIP prediction.
Core Tip: The severity of acute pancreatitis in pregnancy (APIP) is correlated with higher risks of maternal and fetal death. Few studies have focused on APIP severity prediction. We identified four predictors developed and established a prediction nomogram model for pregnant patients with moderate and severe acute pancreatitis. This model achieved good concordance indexes and may help guide doctors in the managementof APIP.
- Citation: Yang DJ, Lu HM, Liu Y, Li M, Hu WM, Zhou ZG. Development and validation of a prediction model for moderately severe and severe acute pancreatitis in pregnancy. World J Gastroenterol 2022; 28(15): 1588-1600
- URL: https://www.wjgnet.com/1007-9327/full/v28/i15/1588.htm
- DOI: https://dx.doi.org/10.3748/wjg.v28.i15.1588
Acute pancreatitis (AP) is the most common gastrointestinal disease requiring acute admission to the hospital[1]. The incidence of acute pancreatitis in pregnancy (APIP) varies from 1/10000 to 11.3/10000[2,3]. Geng et al[4] showed that APIP contributes to increased maternal death and fetal loss. Previous studies have shown that the maternal and perinatal mortality rates of APIP are as high as 3.3% and 11.6%-18.7%, respectively[4,5]. According to the revised Atlanta classification, AP was classified as mild acute pancreatitis (MAP), moderately severe acute pancreatitis (MSAP), and severe acute pancreatitis (SAP)[6]. MSAP and SAP develop in 20% of AP patients. Although, management strategies such as fluid resuscitation, early enteral nutrition, and organ supportive care are usually performed in the clinical setting, the mortality rate of MSAP and SAP can be as high as 35%, which is significantly higher than that of MAP[7,8]. Furthermore, some studies have shown that APIP severity is significantly associated with a higher risk of maternal and fetal death[5,9]. The first week after AP onset is usually defined as the early phase[6]. It would be useful in clinical management if the severity of APIP could be predicted in the early phase. Currently, several prediction systems, including the Acute Physiology and Chronic Health Evaluation, Ranson score, and Bedside Index for Severity in AP, are usually used for AP patients. However, the sensitivity and specificity of these prediction systems are not high enough, and cumbersome items limit their clinical use[10]. At present, few scoring systems have been designed for patients with APIP[11]. Therefore, this study aimed to develop a simple and useful prediction model to predict moderately severe and severe acute pancreatitis in pregnancy (MSIP).
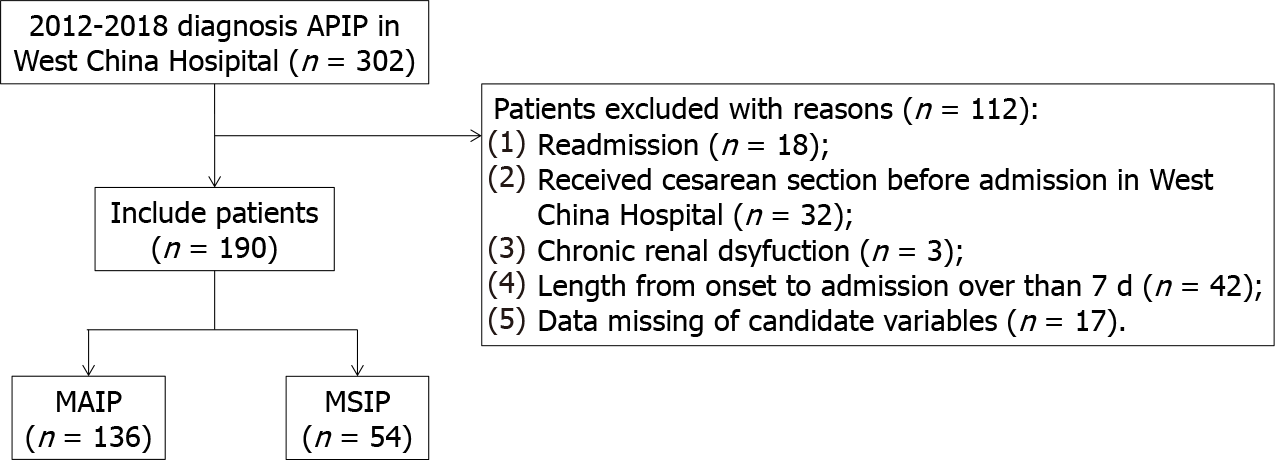
We retrospectively collected the medical records of patients who were diagnosed with AP during pregnancy at West China Hospital from January 2012 to December 2018. Patients meeting the following criteria were excluded: (1) Were readmitted (only included first-time record); (2) Received a cesarean section before admission to West China Hospital; (3) Had a length of more than 7 d from AP onset to admission; (4) Had chronic kidney dysfunction; and (5) Had any missing data of candidate variables. The Ethics Committee of West China Hospital approved the study, and it was conducted according to the Declaration of Helsinki.
The following clinical variables were collected: age, etiology (hypertriglyceridemia, gallstones, other), comorbidities (hypertension, diabetes, fatty liver), smoking, drinking, length of time from onset to admission, gestational weeks on admission, trimester of pregnancy on admission, blood infection, length of hospital stay (LOS), fetal death, and maternal hospital mortality. All laboratory variables were tested in the hospital, including hematocrit, platelet, white blood cell (WBC), and neutrophil levels. Laboratory variables were collected within 48 h of admission. The average levels of retested laboratory variables are shown.
Candidate variables were age, etiology, comorbidity, smoking, drinking, gestational weeks on admission, trimester of pregnancy on admission, length of time from onset to admission, blood infection, and hematocrit, platelet, WBC, neutrophil, lymphocyte, monocyte, alanine aminotransferase, albumin, creatinine, aspartate aminotransferase, alkaline phosphatase, creatine kinase, lactate dehydrogenase (LDH), triglyceride, cholesterol, high-density lipoprotein, low-density lipoprotein, sodium, potassium, and chlorine levels..
According to the revised Atlanta Classification of Acute Pancreatitis[6], a diagnosis of acute pancreatitis requires two of the following three features: (1) abdominal pain consistent with acute pancreatitis (acute onset of a persistent, severe, epigastric pain often radiating to the back); (2) serum lipase activity (or amylase activity) at least three times greater than the upper limit of normal; and (3) characteristic findings of acute pancreatitis on contrast-enhanced computed tomography, and less commonly on magnetic resonance imaging or transabdominal ultrasonography. The grades of severity were also based on the revised Atlanta Classification of Acute Pancreatitis[6]. Patients with persistent organ failure (> 48 h) were classified as having severe acute pancreatitis. Patients with transient organ failure (< 48 h) and/or local or systemic complications without persistent organ failure were classified as having moderately severe acute pancreatitis. Organ failure was classified according to the Modified Marshall scoring system for organ dysfunction[6]. Patients who needed mechanical ventilation or had a PaO2/FiO2 ratio less than 300 were diagnosed with respiratory failure. Patient need for vasopressor support was thought to indicate cardiovascular failure. When the serum creatinine level was over 170 μmol/L, renal failure was diagnosed. Blood infection was defined as described in a previous study[12].
Data are expressed as the mean ± SD for normally distributed continuous variables and as the median (interquartile range) for nonnormally distributed variables. Categorical data are expressed as numbers (percentages). Student’s t-test was used to compare normally distributed continuous variables, and the Wilcoxon rank-sum test was used to compare nonnormally distributed continuous variables. The χ2 -test or Fisher’s exact test was used to compare categorical variables. Statistical analysis was performed using R software. (Version 3.6.1) A 2-sided P value < 0.05 was considered statistically significant.
First, least absolute shrinkage and selection operator (LASSO) regression was used to select potential prognostic factors from the candidate variables. Logistic regression was used to develop a nomogram. The random forest model further validated the predictive performance of the selected factors. To reduce the risk of overfitting, the whole dataset was randomly divided into the training set and validation set at a ratio of 7:3. The model’s development was based on the training set, and the model’s performance assessment was based on the validation set. Finally, a new nomogram based on the selected predictors was established. Receiver operating characteristic (ROC) curves and calibration curves were used to evaluate the model’s predictive performance. ROC curves were calculated to estimate the discrimination of the prediction model. Calibration curves were plotted to evaluate the consistency between the predicted MSIP probability and actual MSIP proportion. Values of 1 and 0.5 indicated perfect discrimination and no discrimination, respectively.
Figure 1 shows the flow chart of the study. During the 7 years, 302 patients with APIP were admitted to West China Hospital. A total of 112 patients were excluded for various reasons, such as readmission, having a cesarean section before admission, and missing data. Finally, a total of 190 patients with APIP were included in this study. Among them, 134 patients (70.5%) were classified as having MAIP, and 56 patients (29.5%) were classified as having MSIP. The overall characteristics of the patients are presented in Table 1.
| Parameters | MAIP (n = 136) | MSIP (n = 54) | P value |
| Age | 27.61 ± 5.25 | 29.46 ± 5.57 | 0.032 |
| Etiology | 0.514 | ||
| Hypertriglyceridemia | 50 (36.8) | 24 (44.4) | |
| Gallstone | 45 (33.1) | 19 (35.2) | |
| Other | 41 (30.1) | 11 (20.4) | |
| Comorbidity | |||
| Hypertension | 0 (0.0) | 2 (3.7) | 0.080 |
| Diabetes | 8 (5.9) | 13 (24.1) | 0.001 |
| Fatty liver disease | 32 (23.5) | 16 (29.6) | 0.492 |
| Smoking | 3 (2.2) | 2 (3.7) | 0.937 |
| Drinking | 4 (2.9) | 1 (1.9) | 1.000 |
| Trimester of pregnancy on admission | |||
| Early (1–12 wk) | 9 (6.6) | 3(5.6) | |
| Mid (12–24 wk) | 31 (22.8) | 10(18.5) | |
| Late (24–40 wk) | 96 (70.6) | 41(75.9) | |
| Gestational weeks on admission | 28.04 ± 7.72 | 28.80 ± 6.64 | 0.520 |
| Onset to admission (days) | 1.59 ± 1.37 | 1.88 ± 1.65 | 0.220 |
| Blood infection | 0 (0.0) | 8 (14.8) | < 0.001 |
| LOS | 7.25 ± 4.27 | 11.88 ± 7.42 | < 0.001 |
| Fetal death | 3 (2.2) | 13(24.1) | < 0.001 |
| Maternal hospital mortality | 0 (0.0) | 1(2.9) | 0.284 |
| Hematocrit | 0.33 ± 0.05 | 0.32 ± 0.06 | 0.155 |
| Platelet | 164.42 ± 55.01 | 147.55 ± 65.17 | 0.072 |
| WBC | 12.59 ± 4.71 | 14.15 ± 4.15 | 0.035 |
| Neutrophils | 10.86 ± 4.40 | 12.49 ± 3.97 | 0.019 |
| Lymphocytes | 1.01 ± 0.40 | 0.88 ± 0.47 | 0.068 |
| Monocytes | 0.55 ± 0.23 | 0.48 ± 0.27 | 0.064 |
| Alanine aminotransferase | 50.94 ± 78.74 | 19.57 ± 37.40 | 0.006 |
| Albumin | 34.22 ± 3.70 | 29.36 ± 5.17 | < 0.001 |
| Creatinine | 42.57 ± 9.30 | 75.87 ± 100.15 | < 0.001 |
| Aspartate aminotransferase | 51.17 ± 67.61 | 35.07 ± 50.13 | 0.115 |
| Alkaline phosphatase | 113.56 ± 52.19 | 95.38 ± 36.87 | 0.020 |
| Creatine kinase | 36.20 ± 25.87 | 126.36 ± 213.49 | < 0.001 |
| LDH | 185.32 ± 66.39 | 346.93 ± 208.95 | < 0.001 |
| Triglyceride | 5.87 ± 6.72 | 12.57 ± 7.34 | < 0.001 |
| Cholesterol | 7.34 ± 5.63 | 12.80 ± 6.64 | < 0.001 |
| High density lipoprotein | 1.40 ± 0.48 | 1.16 ± 0.39 | 0.001 |
| Low density lipoprotein | 2.24 ± 1.23 | 1.94 ± 1.59 | 0.158 |
| Sodium | 135.62 ± 3.82 | 133.57 ± 5.43 | 0.004 |
| Potassium | 3.76 ± 0.34 | 3.83 ± 0.46 | 0.294 |
| Chlorine | 102.17 ± 4.46 | 102.44 ± 6.40 | 0.735 |
The mean ages of the MAIP and MSIP groups were 27.61 ± 5.25 years and 29.46 ± 5.57 years, respectively. Patients in the MSIP group were significantly older than those in the MAIP group (P = 0.032). The most common cause of APIP in both groups was hypertriglyceridemia. Biliary disease was the second most common cause of APIP, which was found in 45 (33.1%) and 19 (35.2%) patients in the MAIP and MSIP groups, respectively. The number of patients with diabetes in the MSIP group was significantly higher than that in the MAIP group (P = 0.001). The rate of blood infections (P < 0.001) in the MSIP group was significantly higher than that in the MAIP group. The LOS (P < 0.001) in the MSIP group was significantly longer than that in the MAIP group, and the rate of fetal deaths (P < 0.001) in the MSIP group was significantly higher than that in the MAIP group. Other clinical indicators were not different between the two groups.
Laboratory indices such as WBC (P = 0.035), neutrophil (P = 0.019), alanine aminotransferase (P = 0.006), albumin (P < 0.001), creatinine (P < 0.001), alkaline phosphatase (P = 0.020), creatine kinase (P < 0.001), LDH (P < 0.001), triglyceride (P < 0.001), cholesterol (P < 0.001), high density lipoprotein (P = 0.001), and sodium (P = 0.004) levels were significantly different between the two groups (P < 0.05).
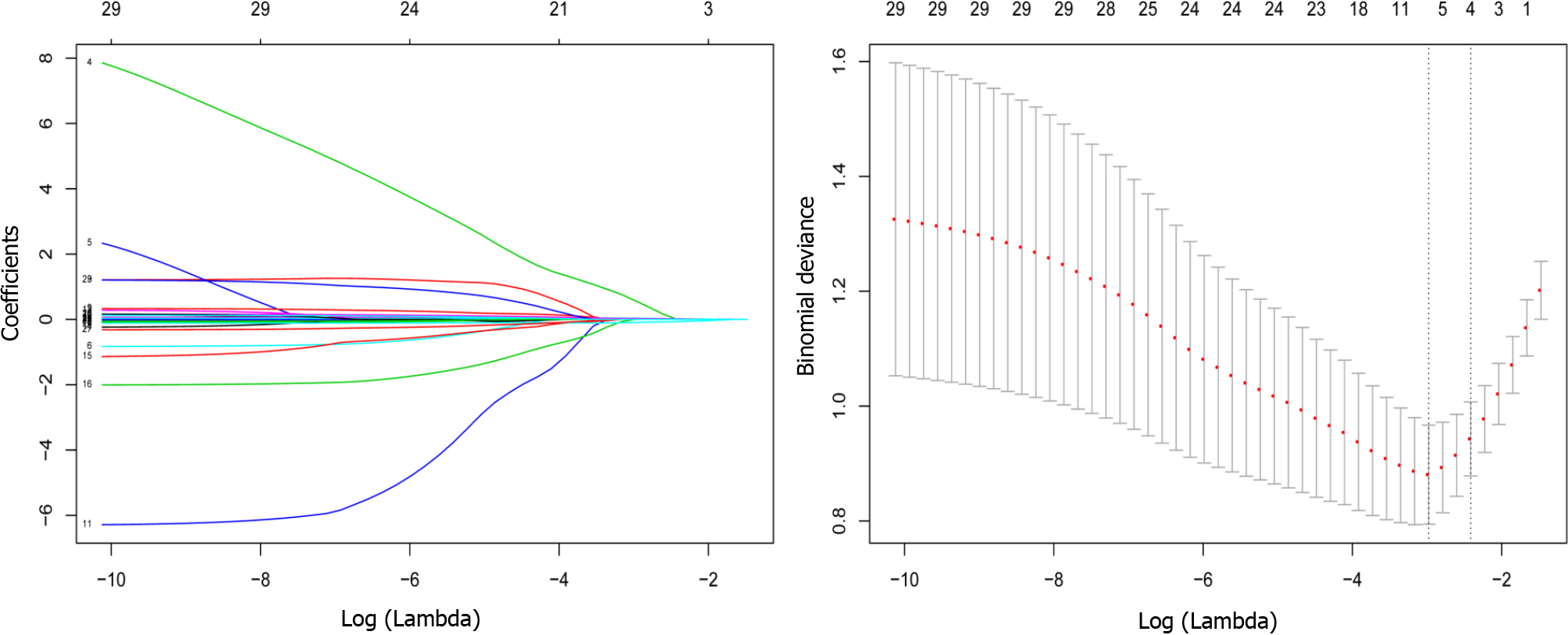
Variable selection using the LASSO regression model: The data were randomly divided into the training set and test set at a ratio of 7:3. The characteristics of the patients in the training and test sets are displayed in Table 2. Most of the included variables were well balanced between the two groups. Four variables (albumin, lactate dehydrogenase, triglyceride, and cholesterol levels) had nonzero coefficients in the LASSO regression model based on the analysis of the whole dataset (Figure 2).
| Training set | Test set | |||||
| Parameters | MAIP (96) | MSIP (38) | P value | MAIP (40) | MSIP (16) | P value |
| Age | 27.16 ± 5.46 | 30.13 ± 6.09 | 0.007 | 28.70 ± 4.60 | 27.88 ± 3.79 | 0.528 |
| Etiology | 0.620 | 0.804 | ||||
| Hypertriglyceridemia | 32 (33.3) | 18 (47.4) | 18 (45.0) | 7 (43.8) | ||
| Gallstone | 36 (37.5) | 13 (34.2) | 9 (22.5) | 5 (31.2) | ||
| Other | 28 (29.2) | 7 (18.4) | 13 (32.5) | 4 (25.0) | ||
| Comorbidity | ||||||
| Hypertension | 0 (0.0) | 2 (5.3) | 0.079 | 0 (0.0) | 0 (0.0) | - |
| Diabetes | 6 (6.3) | 9 (23.7) | 0.012 | 2 (5.0) | 4 (25.0) | 0.049 |
| Fatty liver disease | 20 (20.8) | 11 (28.9) | 0.437 | 12 (30.0) | 5 (31.2) | 1.000 |
| Smoking | 1 (1.0) | 2 (5.3) | 0.400 | 2 (5.0) | 0 (0.0) | 0.909 |
| Drinking | 3 (3.1) | 1 (2.6) | 1.000 | 1 (2.5) | 0 (0.0) | 1.000 |
| Trimester of pregnancy on admission | ||||||
| Early (1–12 wk) | 6 (6.3) | 2 (5.3) | 3 (7.5) | 1 (6.3) | ||
| Mid (12–24 wk) | 22 (22.9) | 8 (21.1) | 9 (22.5) | 2 (12.5) | ||
| Late (24–40 wk) | 68 (70.8) | 28 (73.7) | 28 (70.0) | 13 (81.3) | ||
| Gestational weeks on admission | 27.53 ± 7.52 | 29.74 ± 6.34 | 0.113 | 29.25 ± 8.15 | 26.56 ± 7.01 | 0.252 |
| Onset to admission (d) | 1.63 ± 1.35 | 2.12 ± 1.87 | 0.090 | 1.50 ± 1.44 | 1.29 ± 0.63 | 0.586 |
| Blood infection | 0 (0.0) | 6 (15.8) | < 0.001 | 0 (0.0) | 2 (12.5) | 0.139 |
| LOS | 6.99 ± 4.43 | 23.11 ± 48.52 | < 0.001 | 7.90 ± 3.63 | 18.25 ± 12.96 | 0.001 |
| Fetal death | 1 (1.0) | 9 (23.7) | < 0.001 | 2 (5.0) | 4 (25.0) | 0.049 |
| Maternal hospital mortality | 0 (0.0) | 1 (2.6) | 0.284 | 0 (0.0) | 0 (0.0) | - |
| Hematocrit | 0.33 ± 0.05 | 0.32 ± 0.06 | 0.734 | 0.33 ± 0.05 | 0.30 ± 0.05 | 0.040 |
| Platelet | 169.57 ± 56.91 | 141.97 ± 61.66 | 0.015 | 152.07 ± 48.61 | 160.80 ± 73.24 | 0.604 |
| WBC | 12.89 ± 4.82 | 13.94 ± 4.10 | 0.239 | 11.87 ± 4.42 | 14.64 ± 4.37 | 0.038 |
| Neutrophils | 11.19 ± 4.52 | 12.34 ± 3.83 | 0.166 | 10.09 ± 4.06 | 12.85 ± 4.39 | 0.029 |
| Lymphocytes | 1.00 ± 0.38 | 0.91 ± 0.49 | 0.246 | 1.01 ± 0.44 | 0.81 ± 0.41 | 0.129 |
| Monocytes | 0.55 ± 0.23 | 0.48 ± 0.29 | 0.147 | 0.56 ± 0.25 | 0.47 ± 0.22 | 0.249 |
| Alanine aminotransferase | 55.20 ± 82.18 | 22.91 ± 44.14 | 0.024 | 40.73 ± 69.69 | 11.62 ± 6.40 | 0.103 |
| Albumin | 34.44 ± 3.72 | 29.68 ± 5.49 | < 0.001 | 33.71 ± 3.62 | 28.62 ± 4.41 | < 0.001 |
| Creatinine | 42.90 ± 9.16 | 86.27 ± 117.45 | < 0.001 | 41.78 ± 9.69 | 51.17 ± 22.10 | 0.030 |
| Aspartate aminotransferase | 54.45 ± 69.25 | 40.67 ± 58.67 | 0.281 | 43.33 ± 63.66 | 21.77 ± 10.97 | 0.186 |
| Alkaline phosphatase | 114.05 ± 51.61 | 100.65 ± 37.29 | 0.148 | 112.39 ± 54.23 | 82.86 ± 33.70 | 0.048 |
| creatine kinase | 36.46 ± 26.55 | 129.89 ± 242.89 | < 0.001 | 35.58 ± 24.47 | 117.98 ± 124.10 | < 0.001 |
| LDH | 183.85 ± 63.85 | 356.97 ± 234.19 | < 0.001 | 188.86 ± 72.86 | 323.09 ± 134.59 | < 0.001 |
| Triglyceride | 5.44 ± 6.86 | 12.62 ± 8.01 | < 0.001 | 6.91 ± 6.33 | 12.46 ± 5.66 | 0.004 |
| cholesterol | 6.79 ± 4.43 | 12.19 ± 6.18 | < 0.001 | 8.68 ± 7.70 | 14.24 ± 7.63 | 0.018 |
| High density lipoprotein | 1.42 ± 0.49 | 1.18 ± 0.39 | 0.007 | 1.34 ± 0.43 | 1.12 ± 0.40 | 0.078 |
| Low density lipoprotein | 2.27 ± 1.18 | 2.00 ± 1.58 | 0.282 | 2.17 ± 1.35 | 1.78 ± 1.67 | 0.364 |
| Sodium | 135.91 ± 3.19 | 133.94 ± 5.71 | 0.012 | 134.93 ± 5.01 | 132.68 ± 4.72 | 0.129 |
| Potassium | 3.76 ± 0.30 | 3.88 ± 0.47 | 0.075 | 3.78 ± 0.44 | 3.71 ± 0.45 | 0.588 |
| Chlorine | 102.63 ± 4.22 | 103.14 ± 6.80 | 0.597 | 101.06 ± 4.85 | 100.78 ± 5.14 | 0.849 |
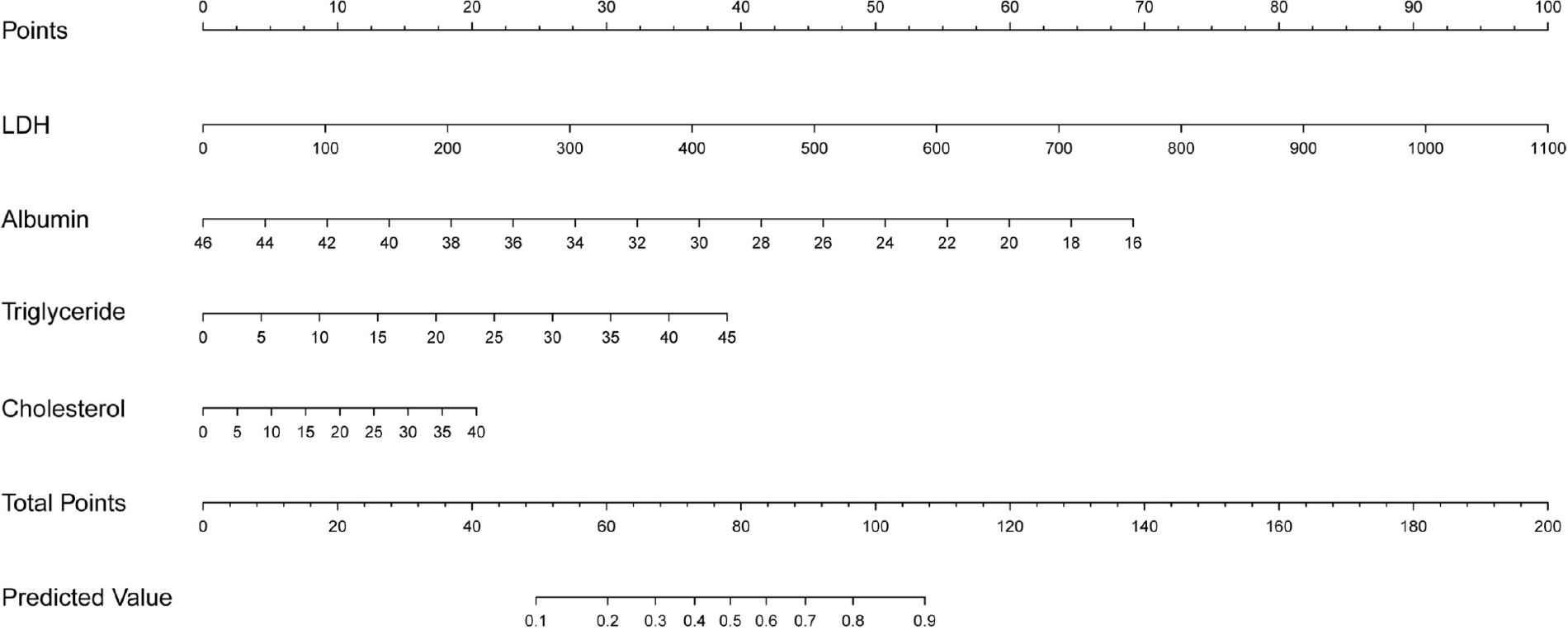
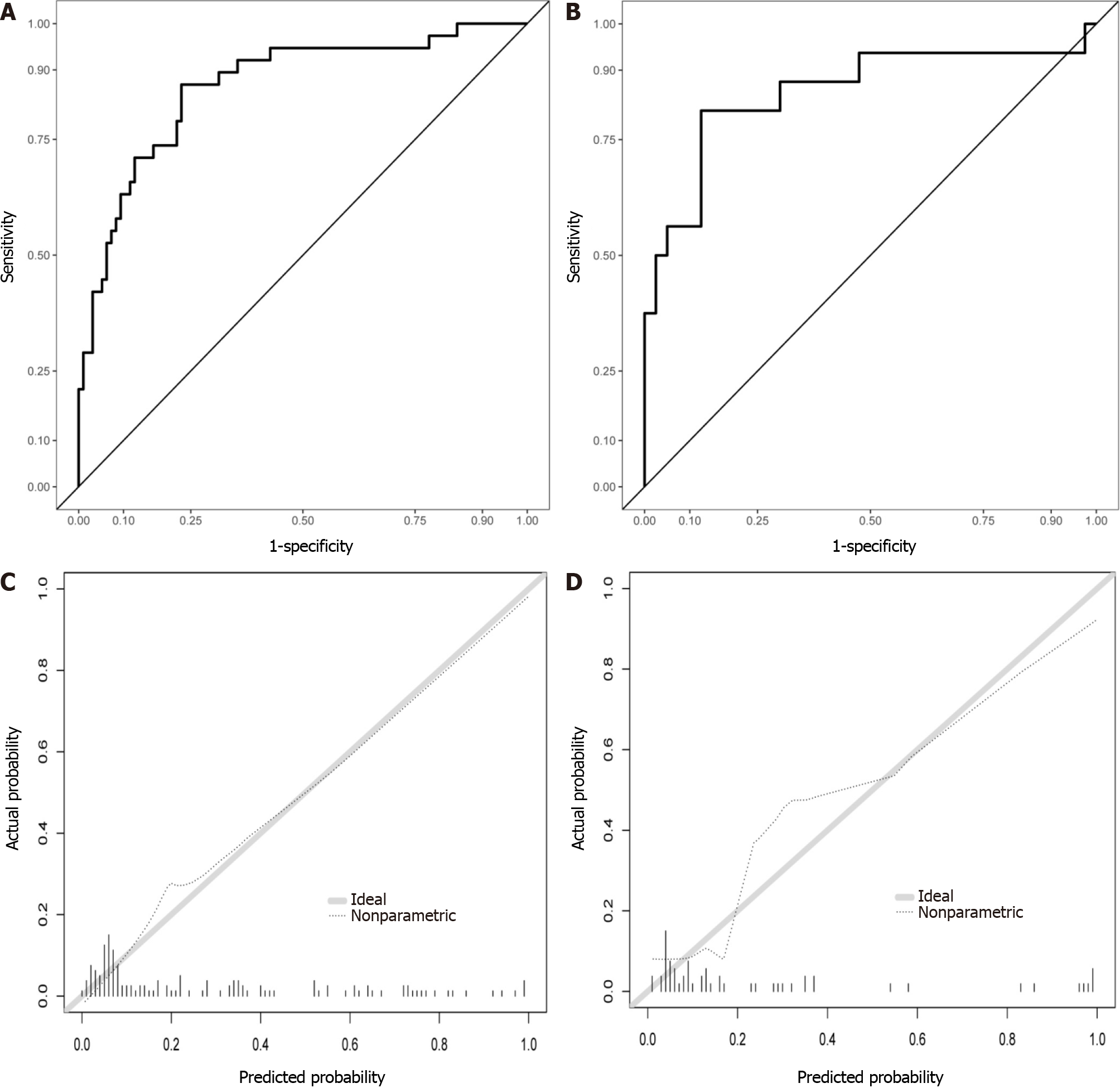
Logistic regression development and validation prediction model: Four selected variables albumin, lactate dehydrogenase, triglyceride, and cholesterol levels, were incorporated into the nomogram model (Figure 3). The ROC curves and calibration curves of the training set and test set are shown in Figure 4. The parameters of the ROC curve at the optimal cutoff point are displayed in Table 3. The areas under the curve in the training and validation sets were 0.865 and 0.853, respectively. The calibration curves showed that the nomogram has good consistency. The positive predictive value was 0.8750, and the negative predictive value was 0.8125.
| Models | AUC | Sensitivity | Specificity |
| Training set | |||
| Logistic model | 0.865 | 0.868 | 0.771 |
| Random forest model | 1.000 | 1.000 | 1.000 |
| Validation set | |||
| Logistic model | 0.853 | 0.812 | 0.875 |
| Random forest model | 0.870 | 0.812 | 0.875 |
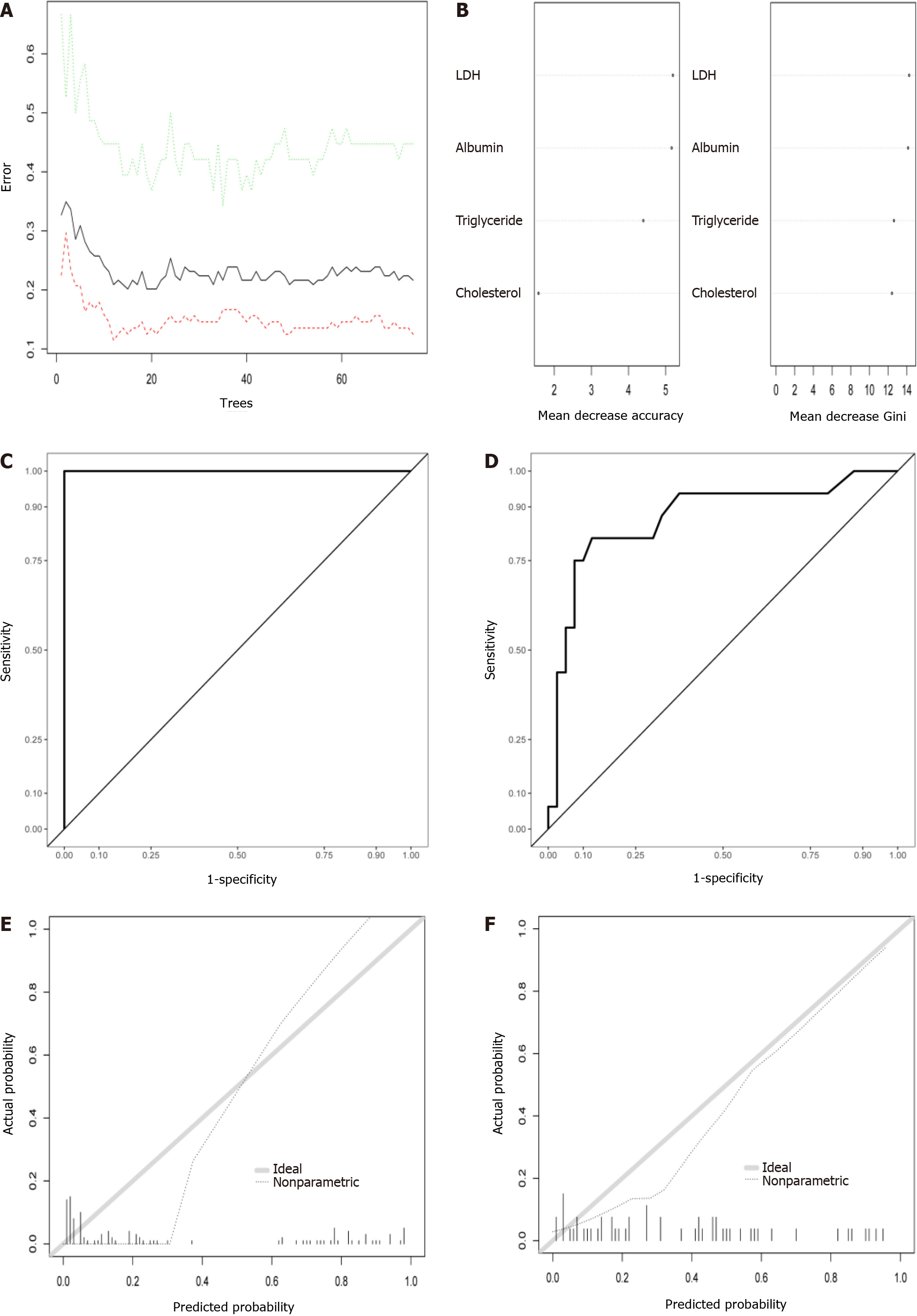
Random forest model development and validation prediction model: The relationship between out-of-bag error and the number of trees is shown in Figure 5A. In total, 100 trees were selected to establish a random forest model. Two methods were used to rank the importance of the variables (Figure 5B). The ROC curves are shown in Figure 5C and D, and the optimal cutoff point is displayed in Table 3. In addition, the calibration curves indicated good agreement between the predicted probability and observed probability for MSIP in the training and test sets (Figure 5E and F).
APIP was thought to be associated with high rates of maternal death and fetal loss. The early and accurate prediction of APIP severity is of great importance for effective therapy. Previous studies have not only focused on the treatments of APIP[13,14] but have also shown interest in the prediction factors for APIP[15]. A single prediction factor cannot achieve the expected predictive power. Therefore, it is necessary to establish a multifactor model to predict the severity of APIP to help with risk stratification and management. In the present study, a new prediction model consisting of four risk factors (albumin, lactate dehydrogenase, triglyceride, and cholesterol levels) with good predictive value was built and verified.
Hypertriglyceridemia (HTG) induced APIP has received continuous attention[16-18]. HTG-induced AP is defined as AP patients with a triglyceride level above 1000 mg/dL (11.3 mmol/L) alone, or 500 mg/dL (5.65 mmol/L) accompanied by lipemic or lactescent blood, after excluding other etiologies[19]. In a recent study by Olesen et al[20], the mean incidence rate of HTG associated pancreatitis was 1.4 (95%CI, 1.1-1.7) per 100000 person-years and it has increased year by year. In addition, AP patients with severe HTG are not rare in Asia[21]. High-fat diets are common among pregnant women in China. In some studies, HTG was the second leading cause of AP in China[22,23]. In our study, HTG (38.9%) was the leading cause of APIP. A higher level of triglycerides not only contributes to more severe pancreatitis[21,24-26] but is also associated with more severe complications[27]. Thus, the detection of HTG is very important in APIP prediction.
As a cytoplasmic enzyme, LDH is widely expressed in tissues. It converts pyruvate to lactate when oxygen is in short supply[28]. In some disease conditions, such as tissue injury, hypoxia, or necrosis, elevated LDH levels are observed[29,30]. As a systemic inflammatory disease, AP can lead to organ dysfunction and pancreatic or peripancreatic necrosis when the disease progresses. Thus, LDH was recognized as a prognostic factor for severe AP in the 1992 Atlanta criteria[31]. More studies have shown that LDH is a useful predictor of AP severity[32,33]. Furthermore, LDH is used not only for the prediction of severity but also for the prediction of organ failure in AP patients[34]. A recent study displayed the high prediction ability of LDH in SAP prediction when levels were over 273.04 U/L[35]. In a study by Cui, an LDH level over 647 U/L showed a good ability to predict persistent organ failure in patients with AP[36]. In this study, LDH was the most important factor in the accuracy and Gini rank of the random forest model. Additionally, LDH accounted for the highest score in the final nomogram model. Moreover, convenient laboratory tests for LDH could be routinely utilized in the clinical setting.
Although hypercholesterolemia is a known risk factor for cardiovascular diseases, with further investigation of AP, the relationship between AP and hypercholesterolemia has been revealed. Hypercholesterolemia may lead to inflammatory responses, lysosomal damage, and proinflammatory cytokine secretion[37,38]. In particular, it promotes the augmentation of toll-like receptor signaling, which plays a significant proinflammatory role in the progression of AP[39]. Clinical studies also found a relationship between cholesterol and AP. Cholesterol is not only associated with AP occurrence[40] but is also thought to be an early predictor of persistent organ failure and mortality in AP patients[41,42]. Some studies have produced inconsistent conclusions. Some reported that cholesterol was not identified as an independent risk factor for SAP[43,44]. However, cholesterol was thought to be a predictor of SAP development in the study by Hong et al[45]. Thus, it is unclear whether the relationship between AP severity and cholesterol is linear. A recent study suggested that cholesterol levels have a U-shaped association with AP severity[46]. This may explain the different conclusions in previous studies.
Some studies have shown that decreases in albumin levels predict the severity of AP[47,48]. An albumin level less than 30 g/L was an independent risk factor for acute respiratory distress syndrome in SAP patients[49]. In the present study, the albumin levels of patients in the MSIP group were less than 30 g/L and significantly lower than those of patients in the MAIP group. This was in accordance with previous studies.
Lactate dehydrogenase, triglyceride, albumin, and cholesterol are routine test items in clinical practice. They can be easily detected from blood samples at a low cost. Therefore, this nomogram will be easy to use and function for MSIP prediction in the clinical setting.
There are some limitations to this study. First, the sample size of 190 patients with APIP was greater than those of most previous studies, but the sample size of this study was still small. Second, this was a retrospective study, so some data were missing. Thus, some variables were not included in this study. Third, the prediction model has a good prediction ability of MSIP (consisting of MSAP and SAP), but further differentiation of MSAP and SAP cannot be achieved. The prognosis of MSAP is not as poor as that of SAP. Thus, separate predictions of MSAP and SAP should be considered in future studies. Moreover, this study only collected data from our institution. If validation can be performed in external institutions, the conclusion of this study would be more substantial.
We developed and validated a nomogram with good accordance for the prediction of MSIP. Incorporating blood indices for albumin, lactate dehydrogenase, triglyceride, and cholesterol levels into the nomogram facilitates the early individualized prediction of APIP severity.
The severity of acute pancreatitis in pregnancy is correlated with higher risks of maternal and fetal death.
There is a lack of a scoring model for predicting the moderately severe and severe acute pancreatitis in pregnancy (MSIP).
We aimed to develop a prediction model for moderately severe and severe acute pancreatitis in pregnancy.
The training set and test set were randomly divided at a ratio of 7:3. Least absolute shrinkage and selection operator regression was used to select potential prognostic factors. A nomogram was developed by logistic regression. A random forest model was used to validate the stability of the of prediction factors. Receiver operating characteristic curves and calibration curves were used to evaluate the model’s predictive performance.
A total of 190 patients were included in this study. Four predictors including lactate dehydrogenase, triglyceride, cholesterol, and albumin levels constitute the prediction model. The model had areas under the curve of 0.865 and 0.853 in the training and validation sets, respectively. The calibration curves showed that the prediction model has a good consistency.
An effective prediction model that can predict MSIP was constructed.
Our model could help to predict moderately severe and severe acute pancreatitis in pregnancy. Usability of the model needs validation by other center data.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Dambrauskas Z, Lithuania; Szakács Z, Hungary S-Editor: Zhang H L-Editor: A P-Editor: Yuan YY
| 1. | Boxhoorn L, Voermans RP, Bouwense SA, Bruno MJ, Verdonk RC, Boermeester MA, van Santvoort HC, Besselink MG. Acute pancreatitis. Lancet. 2020;396:726-734. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 276] [Cited by in RCA: 582] [Article Influence: 116.4] [Reference Citation Analysis (0)] |
| 2. | Magudapathi C, Shanthi S, Palanisamy R. Pancreatitis in Pregnancy: Case Series for 5 Years. J Obstet Gynaecol India. 2020;70:169-172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 3. | Tang SJ, Rodriguez-Frias E, Singh S, Mayo MJ, Jazrawi SF, Sreenarasimhaiah J, Lara LF, Rockey DC. Acute pancreatitis during pregnancy. Clin Gastroenterol Hepatol. 2010;8:85-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 57] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 4. | Geng Y, Li W, Sun L, Tong Z, Li N, Li J. Severe acute pancreatitis during pregnancy: eleven years experience from a surgical intensive care unit. Dig Dis Sci. 2011;56:3672-3677. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 41] [Article Influence: 2.9] [Reference Citation Analysis (1)] |
| 5. | Luo L, Zen H, Xu H, Zhu Y, Liu P, Xia L, He W, Lv N. Clinical characteristics of acute pancreatitis in pregnancy: experience based on 121 cases. Arch Gynecol Obstet. 2018;297:333-339. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 47] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 6. | Banks PA, Bollen TL, Dervenis C, Gooszen HG, Johnson CD, Sarr MG, Tsiotos GG, Vege SS; Acute Pancreatitis Classification Working Group. Classification of acute pancreatitis--2012: revision of the Atlanta classification and definitions by international consensus. Gut. 2013;62:102-111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4932] [Cited by in RCA: 4337] [Article Influence: 361.4] [Reference Citation Analysis (45)] |
| 7. | van Dijk SM, Hallensleben NDL, van Santvoort HC, Fockens P, van Goor H, Bruno MJ, Besselink MG; Dutch Pancreatitis Study Group. Acute pancreatitis: recent advances through randomised trials. Gut. 2017;66:2024-2032. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 221] [Cited by in RCA: 297] [Article Influence: 37.1] [Reference Citation Analysis (0)] |
| 8. | Lankisch PG, Apte M, Banks PA. Acute pancreatitis. Lancet. 2015;386:85-96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 880] [Cited by in RCA: 821] [Article Influence: 82.1] [Reference Citation Analysis (1)] |
| 9. | Sun L, Li W, Geng Y, Shen B, Li J. Acute pancreatitis in pregnancy. Acta Obstet Gynecol Scand. 2011;90:671-676. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 45] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 10. | Cho JH, Kim TN, Chung HH, Kim KH. Comparison of scoring systems in predicting the severity of acute pancreatitis. World J Gastroenterol. 2015;21:2387-2394. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 95] [Cited by in RCA: 112] [Article Influence: 11.2] [Reference Citation Analysis (1)] |
| 11. | Yang Z, Guo G, Li H. Predicting fetal loss in severe acute pancreatitis during pregnancy: a 5-year single-tertiary-center retrospective analysis. Postgrad Med. 2020;132:473-478. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 12. | Huerta LE, Rice TW. Pathologic Difference between Sepsis and Bloodstream Infections. J Appl Lab Med. 2019;3:654-663. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 35] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 13. | Goto S, Ookawara S, Tabei K. Effectiveness of Plasma Exchange for Acute Pancreatitis Induced by Hypertriglyceridemia During Pregnancy. Ther Apher Dial. 2016;20:98-99. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 14. | Talebi-Bakhshayesh M, Mohammadzadeh A, Zargar A. Timing of cholecystectomy after acute severe pancreatitis in pregnancy. Malays J Med Sci. 2015;22:68-70. [PubMed] |
| 15. | Zhang L, Wang Y, Han J, Shen H, Zhao M, Cai S. Neutrophil-lymphocyte ratio, gamma-glutamyl transpeptidase, lipase, high-density lipoprotein as a panel of factors to predict acute pancreatitis in pregnancy. Medicine (Baltimore). 2018;97:e11189. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 16. | Tan SYT, Teh SP, Kaushik M, Yong TT, Durai S, Tien CJ, Gardner DS. Hypertriglyceridemia-induced pancreatitis in pregnancy: case review on the role of therapeutic plasma exchange. Endocrinol Diabetes Metab Case Rep. 2021;2021. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 17. | Zeng L, Cai X, Chen J, Jin G, Zheng Y. Role of mean platelet volume in hypertriglyceridemia-induced acute pancreatitis during pregnancy. BMC Pregnancy Childbirth. 2020;20:592. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 18. | Chyzhyk V, Kozmic S, Brown AS, Hudgins LC, Starc TJ, Davila AD, Blevins TC, Diffenderfer MR, He L, Geller AS, Rush C, Hegele RA, Schaefer EJ. Extreme hypertriglyceridemia: Genetic diversity, pancreatitis, pregnancy, and prevalence. J Clin Lipidol. 2019;13:89-99. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 29] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 19. | Zafrir B, Jubran A, Hijazi R, Shapira C. Clinical features and outcomes of severe, very severe, and extreme hypertriglyceridemia in a regional health service. J Clin Lipidol. 2018;12:928-936. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 23] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 20. | Olesen SS, Harakow A, Krogh K, Drewes AM, Handberg A, Christensen PA. Hypertriglyceridemia is often under recognized as an aetiologic risk factor for acute pancreatitis: A population-based cohort study. Pancreatology. 2021;21:334-341. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 34] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 21. | Jo SI, Chang JH, Kim TH, Kim CW, Kim JK, Han SW. Subsets associated with developing acute pancreatitis in patients with severe hypertriglyceridemia and the severity of pancreatitis. Pancreatology. 2019;19:795-800. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 27] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 22. | Yin G, Cang X, Yu G, Hu G, Ni J, Xiong J, Hu Y, Xing M, Chen C, Huang Y, Tang M, Zhao Y, Cheng G, Wan R, Wang S, Wang X. Different Clinical Presentations of Hyperlipidemic Acute Pancreatitis: A Retrospective Study. Pancreas. 2015;44:1105-1110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 44] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 23. | Jin M, Bai X, Chen X, Zhang H, Lu B, Li Y, Lai Y, Qian J, Yang H. A 16-year trend of etiology in acute pancreatitis: The increasing proportion of hypertriglyceridemia-associated acute pancreatitis and its adverse effect on prognosis. J Clin Lipidol. 2019;13:947-953.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 68] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 24. | Valdivielso P, Ramírez-Bueno A, Ewald N. Current knowledge of hypertriglyceridemic pancreatitis. Eur J Intern Med. 2014;25:689-694. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 253] [Cited by in RCA: 290] [Article Influence: 26.4] [Reference Citation Analysis (0)] |
| 25. | Vipperla K, Somerville C, Furlan A, Koutroumpakis E, Saul M, Chennat J, Rabinovitz M, Whitcomb DC, Slivka A, Papachristou GI, Yadav D. Clinical Profile and Natural Course in a Large Cohort of Patients With Hypertriglyceridemia and Pancreatitis. J Clin Gastroenterol. 2017;51:77-85. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 79] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 26. | Wang SH, Chou YC, Shangkuan WC, Wei KY, Pan YH, Lin HC. Relationship between Plasma Triglyceride Level and Severity of Hypertriglyceridemic Pancreatitis. PLoS One. 2016;11:e0163984. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 35] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 27. | Deng LH, Xue P, Xia Q, Yang XN, Wan MH. Effect of admission hypertriglyceridemia on the episodes of severe acute pancreatitis. World J Gastroenterol. 2008;14:4558-4561. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 121] [Cited by in RCA: 138] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 28. | Feron O. Pyruvate into lactate and back: from the Warburg effect to symbiotic energy fuel exchange in cancer cells. Radiother Oncol. 2009;92:329-333. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 367] [Cited by in RCA: 412] [Article Influence: 25.8] [Reference Citation Analysis (0)] |
| 29. | Karlsson M, Wiberg-Itzel E, Chakkarapani E, Blennow M, Winbladh B, Thoresen M. Lactate dehydrogenase predicts hypoxic ischaemic encephalopathy in newborn infants: a preliminary study. Acta Paediatr. 2010;99:1139-1144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 43] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 30. | Kato GJ, McGowan V, Machado RF, Little JA, Taylor J 6th, Morris CR, Nichols JS, Wang X, Poljakovic M, Morris SM Jr, Gladwin MT. Lactate dehydrogenase as a biomarker of hemolysis-associated nitric oxide resistance, priapism, leg ulceration, pulmonary hypertension, and death in patients with sickle cell disease. Blood. 2006;107:2279-2285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 503] [Cited by in RCA: 480] [Article Influence: 25.3] [Reference Citation Analysis (0)] |
| 31. | Chen CC, Wang SS, Chao Y, Lu CW, Lee SD, Tsai YT, Lo KJ. C-reactive protein and lactate dehydrogenase isoenzymes in the assessment of the prognosis of acute pancreatitis. J Gastroenterol Hepatol. 1992;7:363-366. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 28] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 32. | Losurdo G, Iannone A, Principi M, Barone M, Ranaldo N, Ierardi E, Di Leo A. Acute pancreatitis in elderly patients: A retrospective evaluation at hospital admission. Eur J Intern Med. 2016;30:88-93. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 15] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 33. | Ćeranić DB, Zorman M, Skok P. Interleukins and inflammatory markers are useful in predicting the severity of acute pancreatitis. Bosn J Basic Med Sci. 2020;20:99-105. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 34. | Cui J, Xiong J, Zhang Y, Peng T, Huang M, Lin Y, Guo Y, Wu H, Wang C. Serum lactate dehydrogenase is predictive of persistent organ failure in acute pancreatitis. J Crit Care. 2017;41:161-165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 36] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 35. | Tian F, Li H, Wang L, Li B, Aibibula M, Zhao H, Feng N, Lv J, Zhang G, Ma X. The diagnostic value of serum C-reactive protein, procalcitonin, interleukin-6 and lactate dehydrogenase in patients with severe acute pancreatitis. Clin Chim Acta. 2020;510:665-670. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 35] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 36. | Plesko M, Suvada J, Makohusova M, Waczulikova I, Behulova D, Vasilenkova A, Vargova M, Stecova A, Kaiserova E, Kolenova A. The role of CRP, PCT, IL-6 and presepsin in early diagnosis of bacterial infectious complications in paediatric haemato-oncological patients. Neoplasma. 2016;63:752-760. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 22] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 37. | Triantafilou M, Miyake K, Golenbock DT, Triantafilou K. Mediators of innate immune recognition of bacteria concentrate in lipid rafts and facilitate lipopolysaccharide-induced cell activation. J Cell Sci. 2002;115:2603-2211. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 467] [Cited by in RCA: 450] [Article Influence: 19.6] [Reference Citation Analysis (0)] |
| 38. | Li HB, Jin C, Chen Y, Flavell RA. Inflammasome activation and metabolic disease progression. Cytokine Growth Factor Rev. 2014;25:699-706. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 23] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 39. | Sharif R, Dawra R, Wasiluk K, Phillips P, Dudeja V, Kurt-Jones E, Finberg R, Saluja A. Impact of toll-like receptor 4 on the severity of acute pancreatitis and pancreatitis-associated lung injury in mice. Gut. 2009;58:813-819. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 144] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 40. | Shen Z, Wang X, Zhen Z, Wang Y, Sun P. Metabolic syndrome components and acute pancreatitis: a case-control study in China. BMC Gastroenterol. 2021;21:17. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 22] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 41. | Zhou CL, Zhang CH, Zhao XY, Chen SH, Liang HJ, Hu CL, Chen NW. Early prediction of persistent organ failure by serum apolipoprotein A-I and high-density lipoprotein cholesterol in patients with acute pancreatitis. Clin Chim Acta. 2018;476:139-145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 28] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 42. | Zhang Y, Guo F, Li S, Wang F, Meng Z, Zhao J, Liu Z, Wang B, Fan P, Wang C, Wu H. Decreased high density lipoprotein cholesterol is an independent predictor for persistent organ failure, pancreatic necrosis and mortality in acute pancreatitis. Sci Rep. 2017;7:8064. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 43. | Peng YS, Chen YC, Tian YC, Yang CW, Lien JM, Fang JT, Wu CS, Hung CF, Hwang TL, Tsai YH, Lee MS, Tsai MH. Serum levels of apolipoprotein A-I and high-density lipoprotein can predict organ failure in acute pancreatitis. Crit Care. 2015;19:88. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 44] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 44. | Khan J, Nordback I, Sand J. Serum lipid levels are associated with the severity of acute pancreatitis. Digestion. 2013;87:223-228. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 27] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 45. | Hong W, Lin S, Zippi M, Geng W, Stock S, Zimmer V, Xu C, Zhou M. High-Density Lipoprotein Cholesterol, Blood Urea Nitrogen, and Serum Creatinine Can Predict Severe Acute Pancreatitis. Biomed Res Int. 2017;2017:1648385. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 34] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 46. | Hong W, Zimmer V, Basharat Z, Zippi M, Stock S, Geng W, Bao X, Dong J, Pan J, Zhou M. Association of total cholesterol with severe acute pancreatitis: A U-shaped relationship. Clin Nutr. 2020;39:250-257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 27] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 47. | Farrell PR, Hornung L, Farmer P, DesPain AW, Kim E, Pearman R, Neway B, Serrette A, Sehgal S, Heubi JE, Lin TK, Nathan JD, Vitale DS, Abu-El-Haija M. Who's at Risk? J Pediatr Gastroenterol Nutr. 2020;71:536-542. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 48. | Li S, Zhang Y, Li M, Xie C, Wu H. Serum albumin, a good indicator of persistent organ failure in acute pancreatitis. BMC Gastroentero.. 2017;17:59. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 44] [Article Influence: 5.5] [Reference Citation Analysis (1)] |
| 49. | Zhang W, Zhang M, Kuang Z, Huang Z, Gao L, Zhu J. The risk factors for acute respiratory distress syndrome in patients with severe acute pancreatitis: A retrospective analysis. Medicine (Baltimore). 2021;100:e23982. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |