Published online Apr 7, 2022. doi: 10.3748/wjg.v28.i13.1347
Peer-review started: October 25, 2021
First decision: January 9, 2022
Revised: January 21, 2022
Accepted: February 27, 2022
Article in press: February 27, 2022
Published online: April 7, 2022
Processing time: 156 Days and 12.7 Hours
In China, it has been well recognized that some female patients with esophageal squamous cell carcinoma (ESCC) have different overall survival (OS) time, even with the same tumor-node-metastasis (TNM) stage, challenging the prognostic value of the TNM system alone. An effective predictive model is needed to accurately evaluate the prognosis of female ESCC patients.
To construct a novel prognostic model with clinical and reproductive data for Chinese female patients with ESCC, and to assess the incremental prognostic value of the full model compared with the clinical model and TNM stage.
A new prognostic nomogram incorporating clinical and reproductive features was constructed based on univariatie and Cox proportional hazards survival analysis from a training cohort (n = 175). The results were recognized using the internal (n = 111) and independent external (n = 85) validation cohorts. The capability of the clinical–reproductive model was evaluated by Harrell’s concordance index (C-index), Kaplan–Meier curve, time-dependent receiver operating characteristic (ROC), calibration curve and decision curve analysis. The correlations between estrogen response and immune-related pathways and some gene markers of immune cells were analyzed using the TIMER 2.0 database.
A clinical–reproductive model including incidence area, age, tumor differentiation, lymph node metastasis (N) stage, estrogen receptor alpha (ESR1) and beta (ESR2) expression, menopausal age, and pregnancy number was constructed to predict OS in female ESCC patients. Compared to the clinical model and TNM stage, the time-dependent ROC and C-index of the clinical–reproductive model showed a good discriminative ability for predicting 1-, 3-, and 5-years OS in the primary training, internal and external validation sets. Based on the optimal cut-off value of total prognostic scores, patients were classified into high- and low-risk groups with significantly different OS. The estrogen response was significantly associated with p53 and apoptosis pathways in esophageal cancer.
The clinical–reproductive prognostic nomogram has an incremental prognostic value compared with the clinical model and TNM stage in predicting OS in Chinese female ESCC patients.
Core Tip: In China, some female patients with esophageal squamous cell carcinoma (ESCC), even with the same tumor-node-metastasis (TNM) stage, have significantly different overall survival (OS) time. The prognostic value of the TNM system has been challenged due to its unsatisfactory discriminative ability. A new prognostic nomogram that combines clinical and reproductive features was developed and validated in this study. Compared with the clinical model and TNM stage, clinical–reproductive model has incremental prognostic value in predicting OS in Chinese female patients with ESCC, which can help clinicians to make individual treatment and medical decisions.
- Citation: Zhang DY, Ku JW, Zhao XK, Zhang HY, Song X, Wu HF, Fan ZM, Xu RH, You D, Wang R, Zhou RX, Wang LD. Increased prognostic value of clinical–reproductive model in Chinese female patients with esophageal squamous cell carcinoma. World J Gastroenterol 2022; 28(13): 1347-1361
- URL: https://www.wjgnet.com/1007-9327/full/v28/i13/1347.htm
- DOI: https://dx.doi.org/10.3748/wjg.v28.i13.1347
Esophageal squamous cell carcinoma (ESCC) is highly invasive malignancy in China with a 5-year overall survival (OS) rate of only 10%-20% in patients with advanced disease[1]. In addition to pathological features, reproductive factors such as menopausal status, estrogen receptors, and pregnancy number are also correlated with clinical outcome in female ESCC patients[2,3]. The increased risk of esophageal cancer is associated with a decrease in estrogen level[4]. An epidemiological study of menopausal hormone therapy (MHT) confirmed that patients who have been using MHT have a reduced risk of ESCC[5]. Previous research also supports the protective effects of female hormones on the risk of ESCC[6].
The most common prognostic evaluation system for ESCC mainly depends on the tumor node metastasis (TNM) staging system of the American Joint Committee on Cancer, which has been adopted in the United States since 1959[7,8]. Recently, the prognostic value of the TNM system has been challenged due to its unsatisfactory discriminative ability[9]. Relevant studies have shown significant differences in OS in female ESCC patients even with the same TNM stage[3]. Combined with other clinical prognostic factors such as age and tumor differentiation, individual prognosis can be more accurately predicted[10,11]. An effective predictive model is needed for female ESCC to precisely assess the clinical outcome.
A nomogram is a graphical representation tool based on statistical predictive modeling[12]. So far, no nomogram including reproductive factors has been constructed to predict OS in female ESCC. The current study was designed to identify independent prognostic factors based on univariatie and Cox proportional hazards survival analysis, and then develop and validate a new prognostic nomogram incorporating clinical and reproductive characteristics to predict 1-, 3-, and 5-years OS in female ESCC. We also aimed to establish whether the nomogram model could provide more accurate prognostic prediction than the clinical model and TNM stage.
This retrospective study was approved by the Ethics Committee of the First Affiliated Hospital of Zhengzhou University and Institutional Review Board of the First Affiliated Hospital of Nanyang Medical College. The documentation of informed consent was waived due to anonymity of the participants. In the multicenter study, the analysis was performed on the two independent cohorts of female patients with ESCC (Cohort 1, 500 000 esophageal and gastric cardiac carcinoma database of First Affiliated Hospital of Zhengzhou University[3]; Cohort 2, First Affiliated Hospital of Nanyang Medical College). Patients who were pathologically diagnosed with ESCC, underwent surgery, and had detailed follow-up information where eligible. Patients who received preoperative therapy (radiotherapy and/or chemotherapy) and lost clinical and reproductive information were excluded.
Clinicopathological factors, including age at diagnosis, incidence area, tumor location, tumor differentiation, tumor invasion depth (T), lymph node metastasis (N), metastasis, and therapy methods were collected from the medical records. Reproductive factors such as menarchal age, menopausal status, menopausal age, pregnancy number, and biomarker levels [estrogen receptor alpha (ESR1) and estrogen receptor beta (ESR2)] were also recorded. All eligible patients were followed-up every 3 mo via telephone interview or outpatient review. Patients were followed to the latest lost-to-follow-up time (December 31, 2020) or time of death. In our study, the primary clinical outcome was OS which was defined with reference to our previous study[3].
Factors affecting OS in univariate analysis (P < 0.20) were included in a Cox proportional hazards regression for multivariable analysis[13]. Hazard ratio and 95% confidence interval were assessed. After testing for collinearity, a prognostic nomogram was constructed to predict 1-, 3-, and 5-years OS for female ESCC. The discrimination was evaluated using the time-dependent receiver operating characteristic (ROC) and concordance index (C-index) calculated by bootstrapping with 1000 resamples[14]. Calibration was examined via calibration plots. Decision curve analysis (DCA) was conducted to evaluate the clinical application and net benefit at different threshold probabilities[15]. Patients in the study were separated into high- or low-risk groups based on the best cut-off point of total prognostic score (TPS) that was decided by X-Tile software[16]. Survival curves were plotted using the Kaplan–Meier analysis. Sub-analysis was conducted to confirm the potential correlations between risk score and OS among different subgroups in the primary training cohort.
We analyzed the correlations between estrogen response and immune-related pathways such as p53 pathway and apoptosis pathway in esophageal cancer from The Cancer Genome Atlas (TCGA) by R packages. We also assessed the associations of ESR1 and ESR2 levels with gene markers of tumor-infiltrating immune cells, including B cells, tumor-associated macrophages (TAMs), M1 macrophages, M2 macrophages, and neutrophils by TIMER 2.0 (http://timer.comp-genomics.org/).
All statistical analyses were conducted using R language (version 4.1.0). Baseline characteristics of female ESCC patients were summarized. Student’s t test was used to analyze the continuous variables, and categorical variables were compared with the χ2 test and Fisher’s exact bilateral test. Survival curves were conducted by using the Kaplan–Meier method, and OS was analyzed with a log-rank test. The rms, Hmisc, survival, survcomp, and ggplot2 packages were used for analysis. P < 0.05 (two-sided) was considered statistically significant.
In Cohort 1, all 286 eligible patients were randomly divided into two sets according by computer-generated random numbers: 175 (60%) (mean age, 61.35 years; range, 41–78 years) were randomly assigned to a primary training set; 111 (40%) (mean age, 60.25 years; range, 42–81 years) to an internal validation set. An independent dataset (n = 85) from Cohort 2 was established to be an external validation cohort. The mean age was 60.15 years with a range of 42–80 years. The median survival time in the primary training cohort was 47.59 mo and the 1-, 3-, and 5-years OS rates were 84.0%, 58.9% and 31.4%, respectively. In the internal validation cohort, the median survival time was 45.63 mo, and the 1-, 3-, and 5-years OS rates were 85.6%, 64.0% and 31.5%, respectively. The median survival time of the external validation cohort was 39.94 mo, and the 1-, 3-, and 5-years OS rates were 84.7%, 56.5% and 23.5%, respectively. The detailed distribution of baseline characteristics of the primary training, internal validation, and external validation datasets are shown in Table 1.
| Characteristics | Primary training cohort (%) | Internal validation cohort (%) | External validation cohort (%) |
| Total | 175 (61.2) | 111 (38.8) | 85 (100) |
| Diagnose age | |||
| < 50 | 21 (12.0) | 16 (14.4) | 10 (11.8) |
| 50-60 | 59 (33.7) | 47 (42.3) | 35 (41.2) |
| > 60 | 95 (54.3) | 48 (43.2) | 40 (47.1) |
| Age | 61.35 ± 7.99 | 60.25 ± 7.97 | 60.15 ± 8.50 |
| Incidence area | |||
| High | 138 (78.9) | 89 (80.2) | 64 (75.3) |
| Low | 37 (21.1) | 22 (19.8) | 21 (24.7) |
| Tumor location | |||
| Upper | 34 (19.4) | 19 (17.1) | 11 (12.9) |
| Middle | 113 (64.6) | 79 (71.2) | 66 (77.6) |
| Lower | 28 (16.0) | 13 (11.7) | 8 (9.4) |
| Differentiation | |||
| Higher | 11 (6.3) | 8 (7.2) | 3 (3.5) |
| Moderate | 101 (57.7) | 67 (60.4) | 51 (60.0) |
| Lower | 63 (36.0) | 36 (32.4) | 31 (36.5) |
| T stage | |||
| T1/T2 | 64 (36.6) | 32 (28.8) | 26 (30.6) |
| T3/T4 | 111 (63.4) | 79 (71.2) | 59 (69.4) |
| N stage | |||
| N0 | 86 (49.1) | 60 (54.1) | 46 (54.1) |
| N1-N3 | 89 (50.9) | 51 (45.9) | 39 (45.9) |
| Therapy methods | |||
| Operation | 164 (93.7) | 106 (95.5) | 82 (96.5) |
| Others | 11 (6.3) | 5 (4.5) | 3 (3.5) |
| Family history | |||
| No | 116 (66.3) | 66 (59.5) | 56 (65.9) |
| Yes | 59 (33.7) | 45 (40.5) | 29 (34.1) |
| ESR1 | |||
| Negative | 49 (28.0) | 41 (36.9) | 29 (34.1) |
| Positive | 126 (72.0) | 70 (63.1) | 56 (65.9) |
| ESR2 | |||
| Negative | 63 (36.0) | 39 (35.1) | 24 (28.2) |
| Positive | 112 (64.0) | 72 (64.9) | 61 (71.8) |
| Menarche age | |||
| < 14 | 28 (16.0) | 23 (20.7) | 12 (14.1) |
| 14-16 | 85 (48.6) | 45 (40.5) | 41 (48.2) |
| > 16 | 62 (35.4) | 43 (38.7) | 32 (37.6) |
| Menopausal age | |||
| < 46 | 48 (27.4) | 19 (17.1) | 23 (27.1) |
| 46-55 | 47 (26.9) | 36 (32.4) | 20 (23.5) |
| > 55 | 80 (45.7) | 56 (50.5) | 42 (49.4) |
| Pregnancy number | |||
| < 4 | 61 (34.9) | 40 (36.0) | 30 (35.3) |
| 4-6 | 86 (49.1) | 52 (46.8) | 43 (50.6) |
| > 6 | 28 (16.0) | 19 (17.1) | 12 (14.1) |
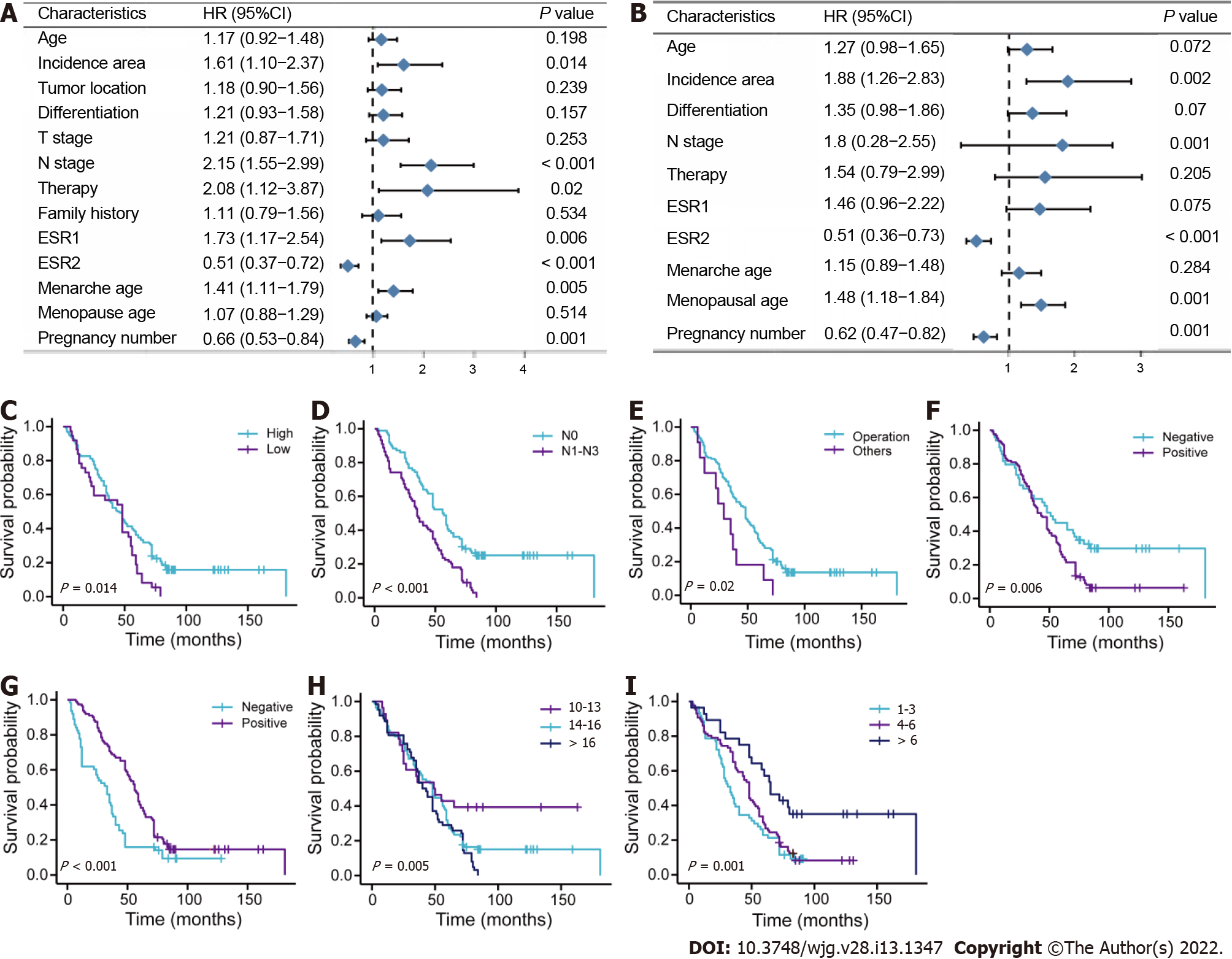
The prognostic role of each variable in OS was tested in the training cohort (Figure 1). Incidence area (P = 0.014; high vs low area), N stage (P < 0.001; N0 vs N1–N3), therapy methods (P = 0.02; operation vs other methods), ESR1 expression level (P = 0.006; negative vs positive), ESR2 level (P < 0.001; negative vs positive), menarche age (P = 0.005; > 16 years vs < 13 years), and pregnancy number (P = 0.001; > 6 vs < 4–6) were significantly associated with OS in the univariate analysis (Figure 1A). Data were also represented using Kaplan–Meier curves (Figure 1C-I). Parameters associated with P < 0.20 based on univariate analysis and relevant clinical factors were entered into a Cox proportional hazards regression model, which included age, incidence area, tumor differentiation, N stage, therapy, ESR1, ESR2, menarche age, menopausal age, and pregnancy number (Figure 1B). No evidence of problematic multicollinearity was found.
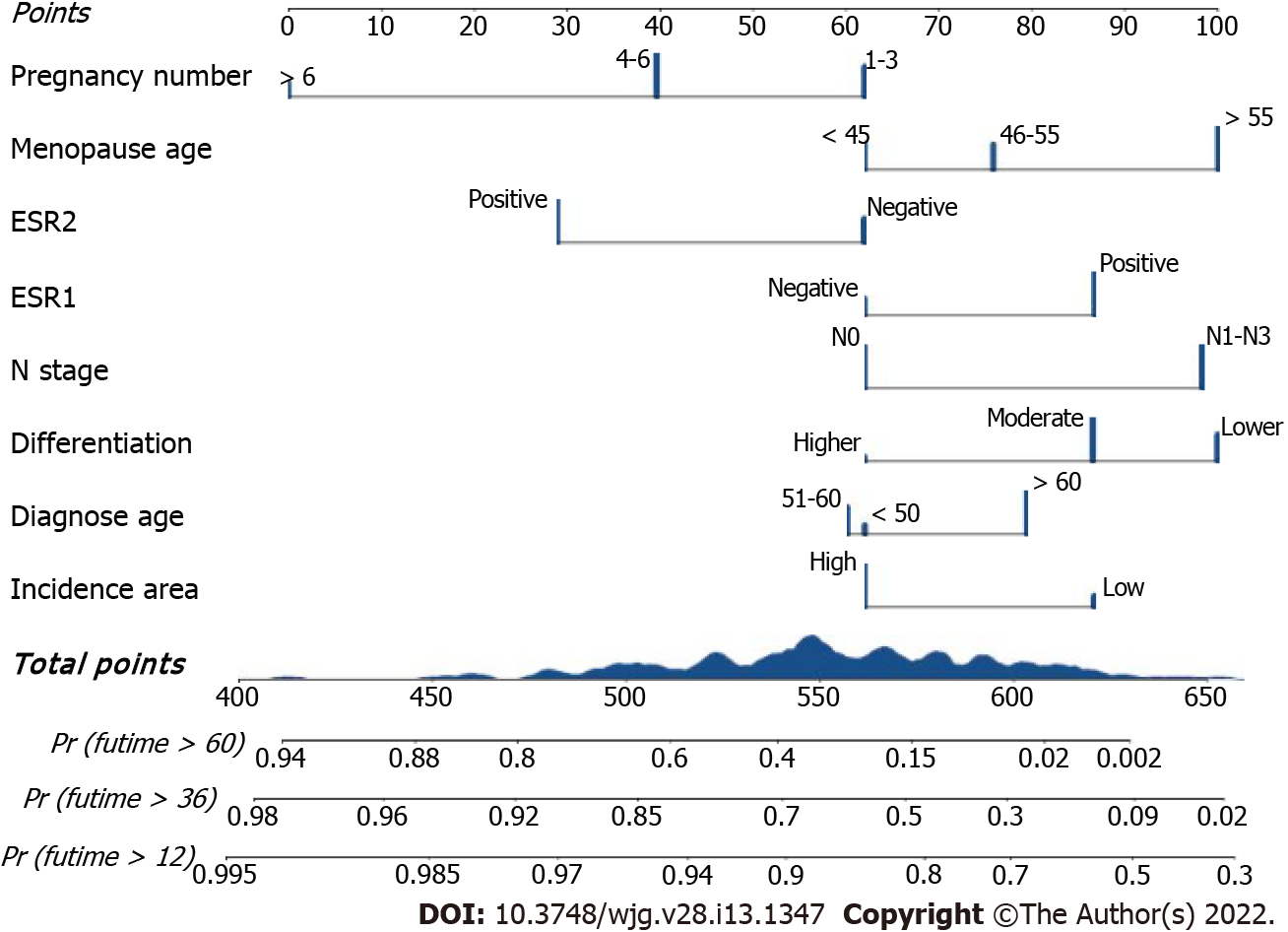
To address patient prognosis, we identified a nomogram model for the prognosis prediction of female ESCC patients at 1-, 3-, and 5-years OS in the primary training cohort. Incidence area, diagnose age, differentiation, N stage, ESR1 expression, ESR2 expression, menopausal age, and pregnancy number were finally included in the full model (Figure 2). Pregnancy number contributed to the highest points in the model, followed by menopausal age, N stage, differentiation, and ESR2 status. Incidence area, age and ESR1 Level had a minor impact on OS. To investigate whether the clinical–reproductive model had incremental prognostic value for individualized OS prediction, a clinical model was also constructed by only including incidence area, age, differentiation, and N stage.
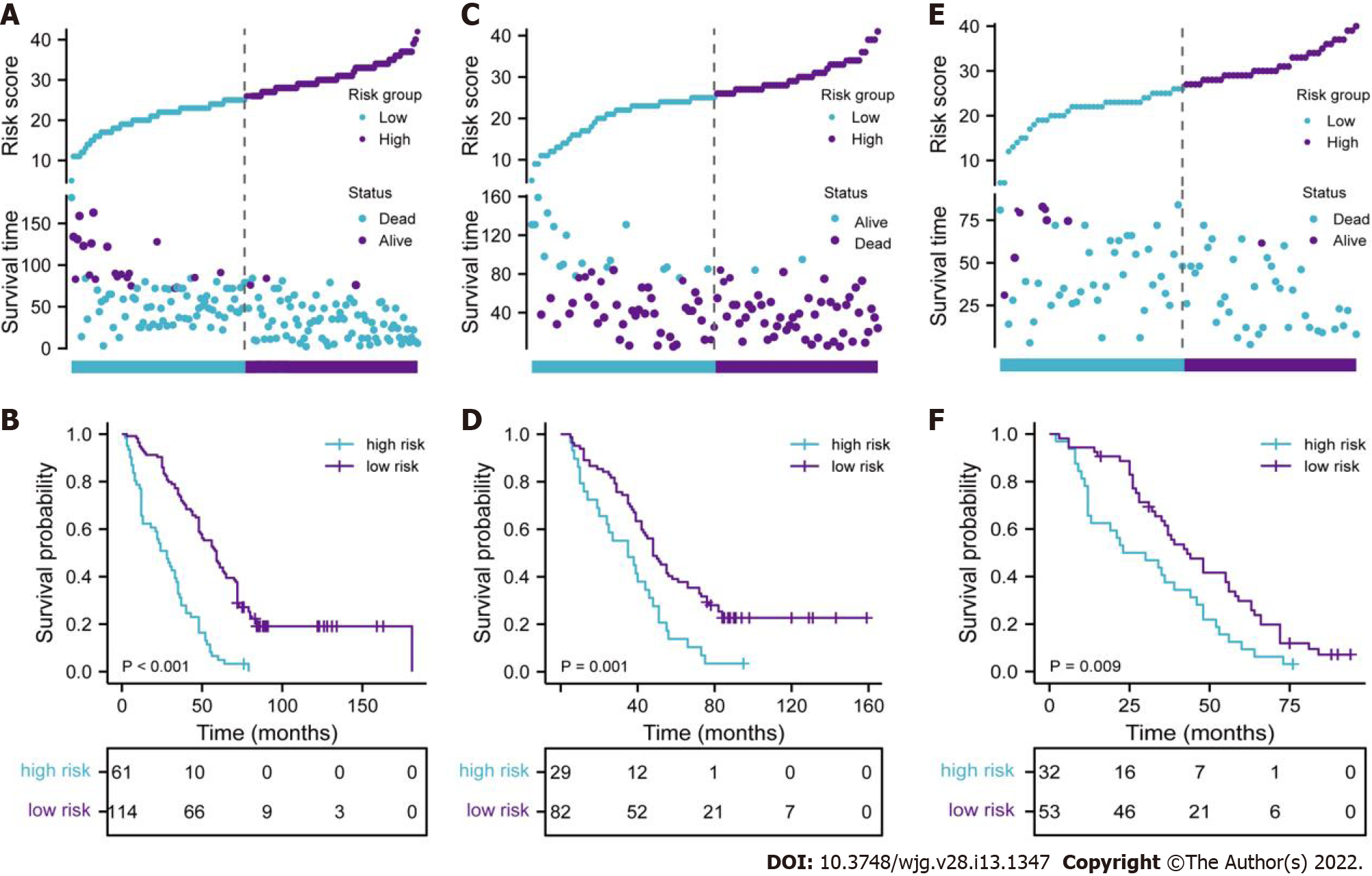
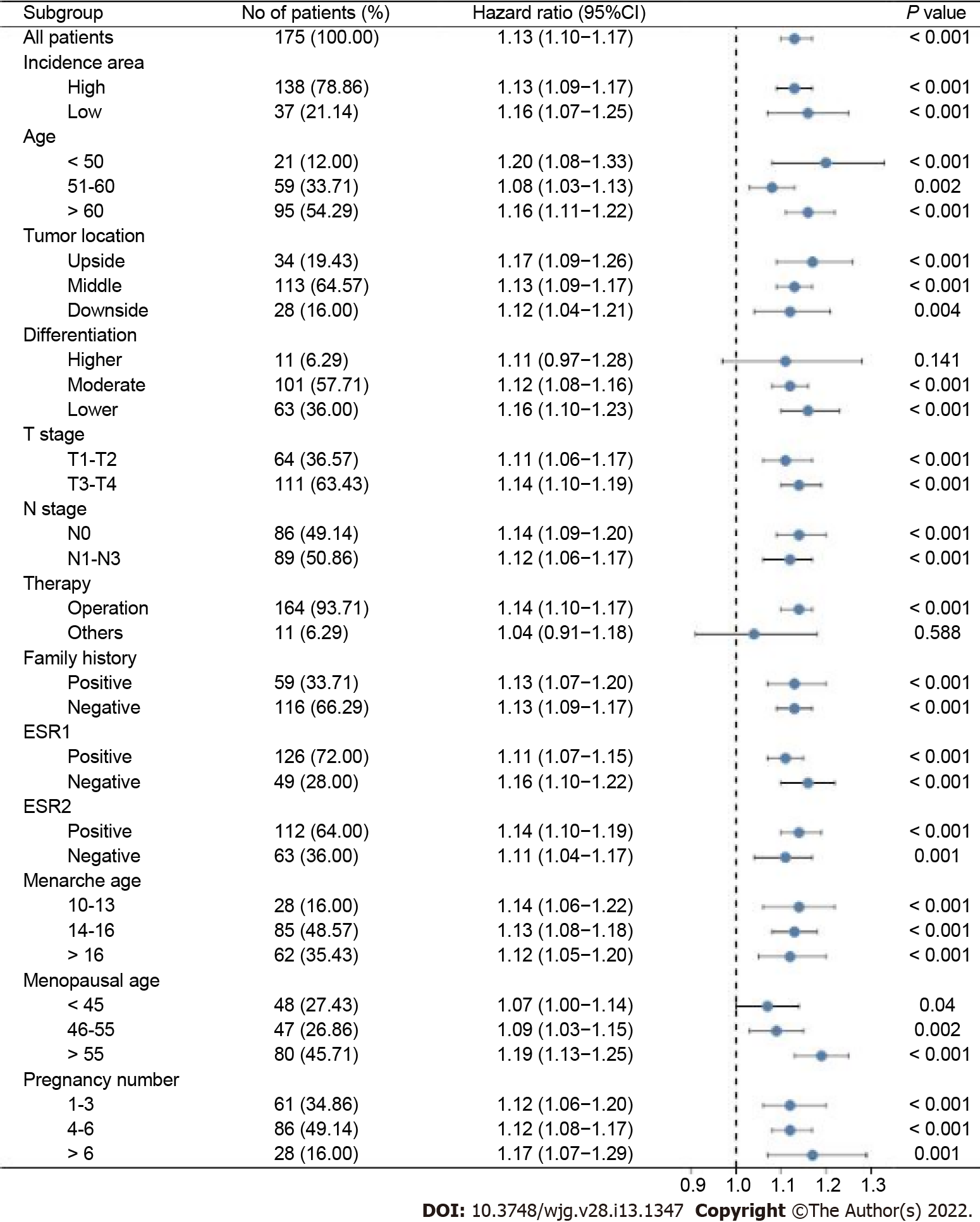
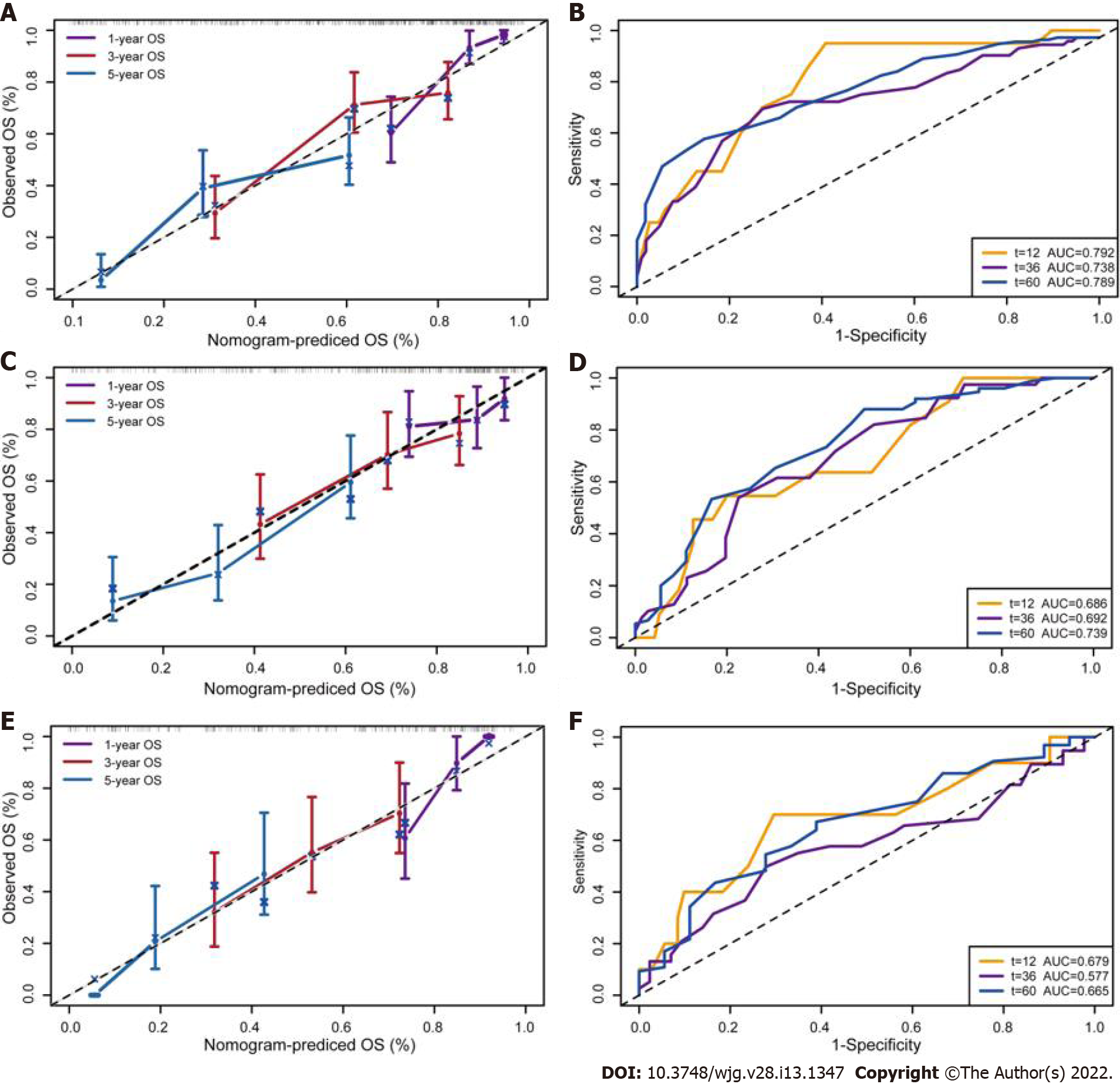
To further reveal the prognostic prediction of the full model, patients were categorized into high- and low-risk groups based on the optimal cut-off value of TPS in the primary training set. The relationships among risk scores and survival status of patients are shown in Figure 3A. Kaplan–Meier survival curves revealed that patients with higher risk scores had significantly poorer OS (0.28, 95%CI: 0.20–0.40, P < 0.001, Figure 3B). Using the same cut-off values, we identified the same risk groups in both internal and external validation sets with OS represented by Kaplan–Meier curves (0.47, 95%CI: 0.30–0.73, P = 0.001; 0.54, 95%CI: 0.34–0.85, P = 0.009, respectively, Figure 3C–F). In addition to highly differentiated patients and patients who received other treatments (surgery plus postoperative radiotherapy, surgery plus postoperative chemotherapy), the clinical–reproductive model also maintained a good and stable prediction performance among other subgroups (Figure 4).
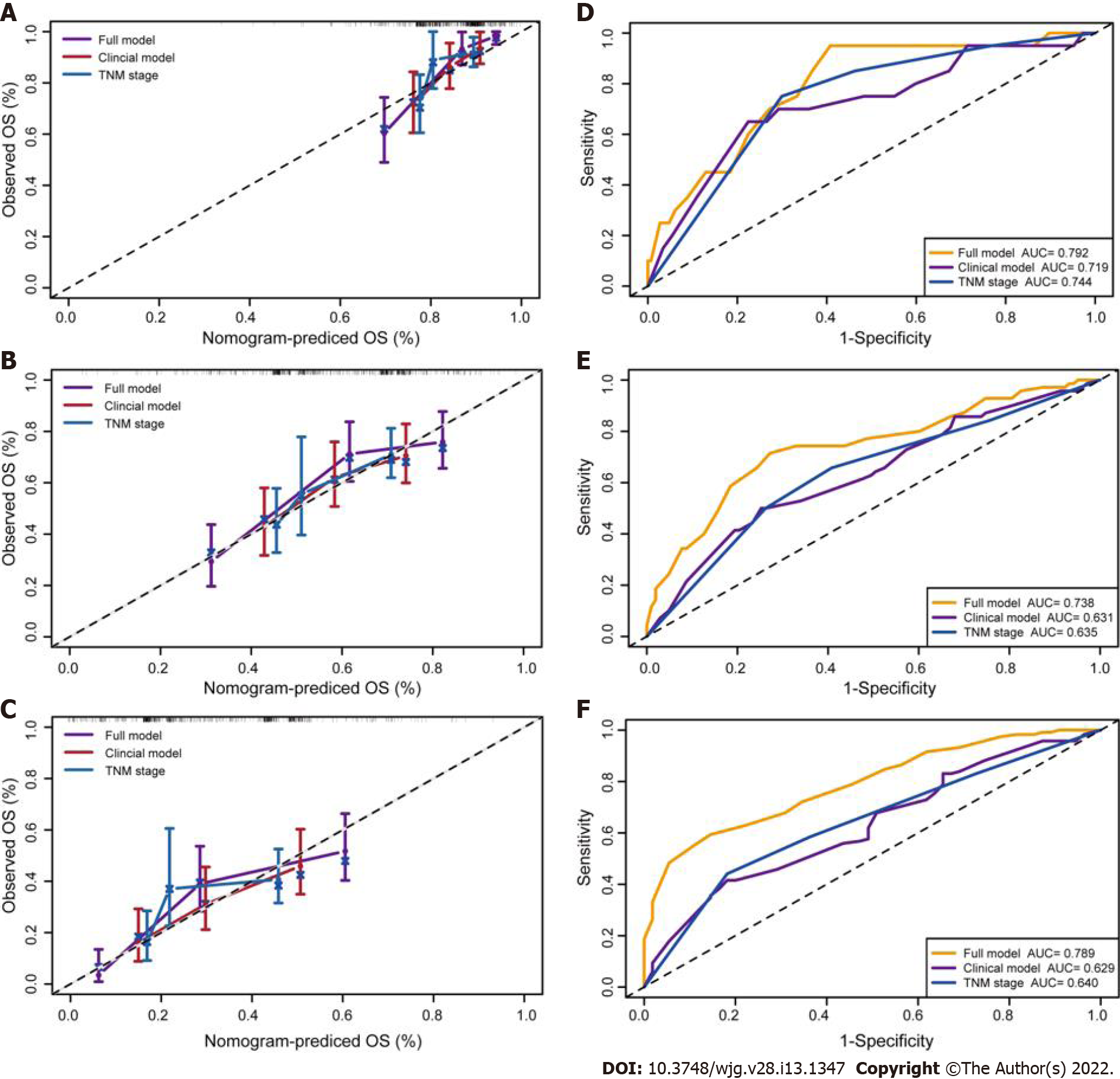
The Harrell C-indexes are summarized in Table 2 for the full model, clinical model, and TNM stage in the training, internal and external validation sets. For OS prediction, the clinical model achieved a C-index of 0.629 (95%CI: 0.578–0.681) in the training set, with internal and external validation C-index of (0.593, 95%CI: 0.531–0.655, and 0.589, 95%CI: 0.514–0.633, respectively). After integrating the clinical with reproductive risk factors, the C-index of the full model increased to 0.701 (95%CI: 0.655–0.746, P = 0.000) in the training set, 0.684 (95%CI: 0.619–0.748, P = 0.009) in the internal validation, and 0.672 (95%CI: 0.609–0.734, P = 0.014) in the external validation set. The C-indexes for OS were also higher than those for TNM stage in all of the three cohorts (P = 0.013, 0.011 and 0.033, respectively) (Table 2).
| Primary training set | Internal validation set | External validation set | ||||
| C-index (95%CI) | P value | C-index (95%CI) | P value | C-index (95%CI) | P value | |
| Full model | 0.701 (0.655-0.746) | Ref. | 0.684 (0.619-0.748) | Ref. | 0.672 (0.609-0.734) | Ref. |
| Clinical-model | 0.629 (0.578-0.681) | 0.000 | 0.593 (0.531-0.655) | 0.009 | 0.589 (0.514-0.663) | 0.014 |
| TNM stage | 0.638 (0.576-0.699) | 0.013 | 0.552 (0.462-0.641) | 0.011 | 0.560 (0.462-0.657) | 0.033 |
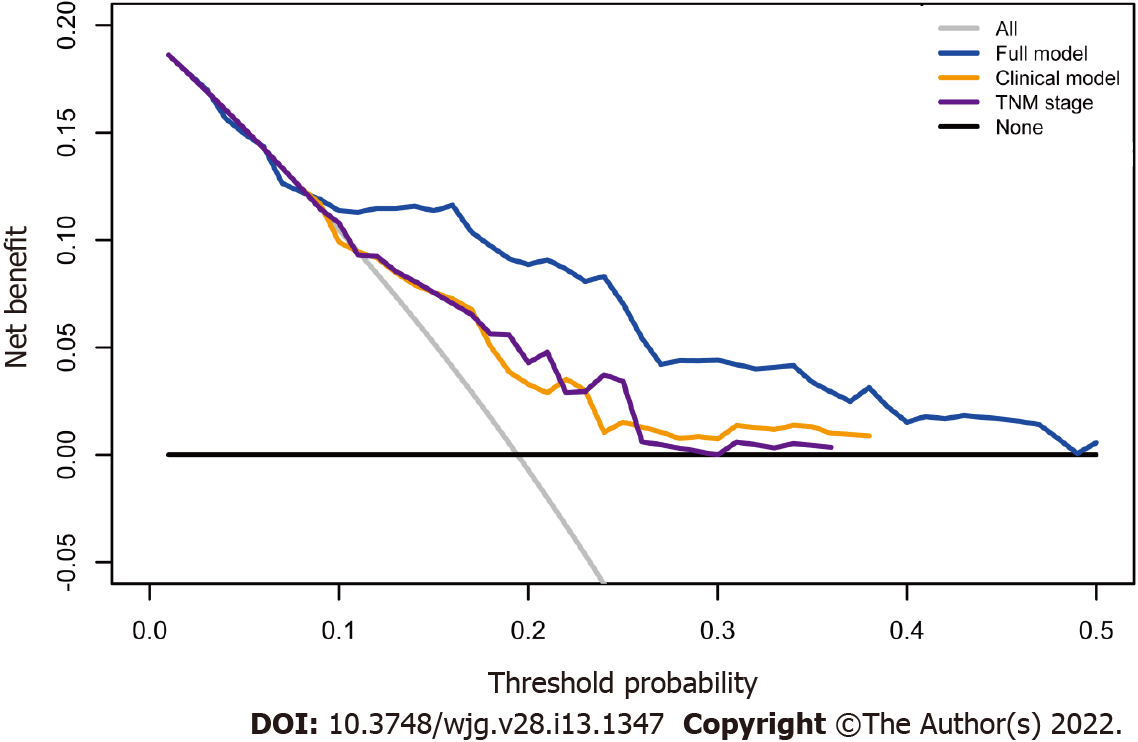
The calibration suggested that the OS prediction at 1-, 3-, and 5-years was well matched to the actual outcomes in the primary training cohort (Figure 5A). The time-dependent ROC curve also demonstrated that the full model showed a good performance in predicting OS in the training set (Figure 5B). The AUC at 1-, 3-, and 5-years was 0.792, 0.738 and 0.789, respectively. These discoveries were verified in two validation sets (Figure 5C–F). Compared with the clinical model and TNM stage, the predicted value of the full model was in good agreement with the OS at 1 year (Figure 6A), 3 years (Figure 6B), and 5 years (Figure 6C). The full model also confirmed the better discrimination for OS at 1 year (AUC: 0.792 vs 0.719 vs 0.744; Figure 6D), 3 years (AUC: 0.738 vs 0.631 vs 0.635; Figure 6E), and 5 years (AUC: 0.789 vs 0.629 vs 0.640; Figure 6F) OS in the primary training cohort. DCA for 18 mo OS prediction showed that the clinical-reproductive model yielded a larger net benefit than either clinical model or TNM stage when the threshold probability was > 0.10 (Figure 7).
The estrogen response was positively correlated with p53 pathway (r = 0.436, P < 0.001), and apoptosis pathway (r = 0.245, P < 0.001) (Supplementary Figure 1). ESR1 and ESR2 Levels were positively correlated with some biomarkers of immune cells or their subsets (Supplementary Table 1).
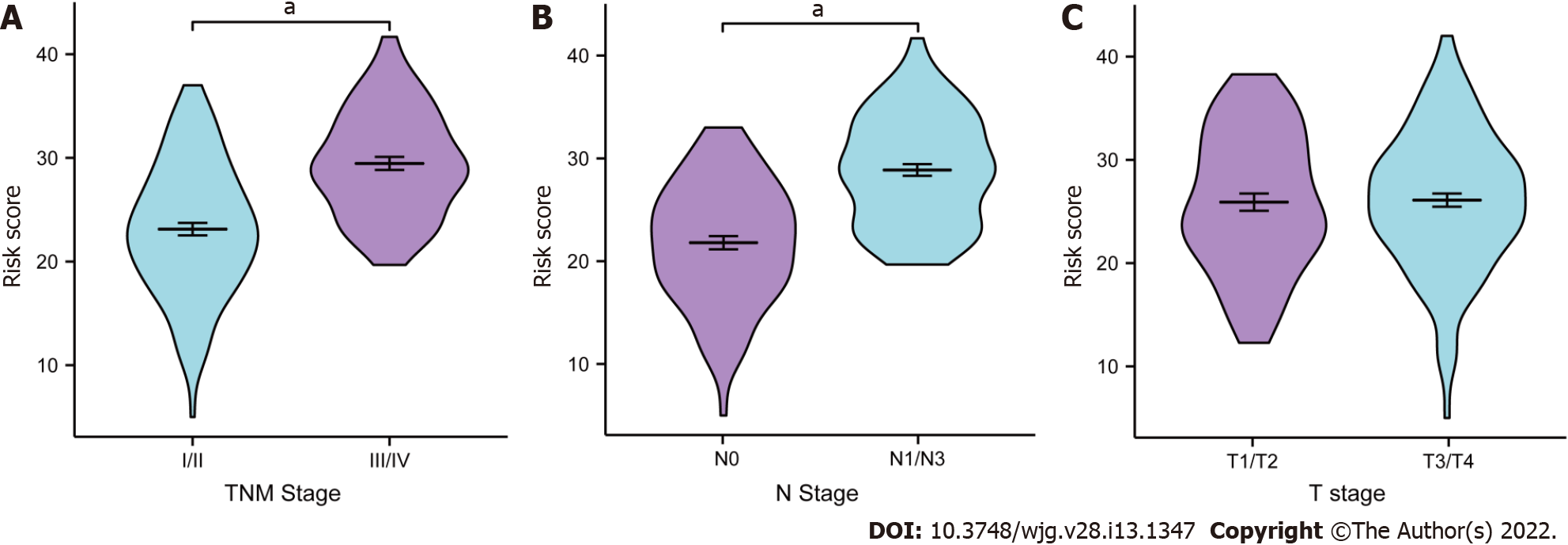
The relationships between the full model and clinical parameters are shown in Figure 8. In terms of TNM stage, female patients with III/IV stage ESCC had significantly higher risk scores than patients with I/II stage disease had (P < 0.001, Figure 8A). The risk scores were also higher for patients with than those without lymph node metastasis (P < 0.001, Figure 8B). No significant difference was found in patients with different T stages (P = 0.152, Figure 8C).
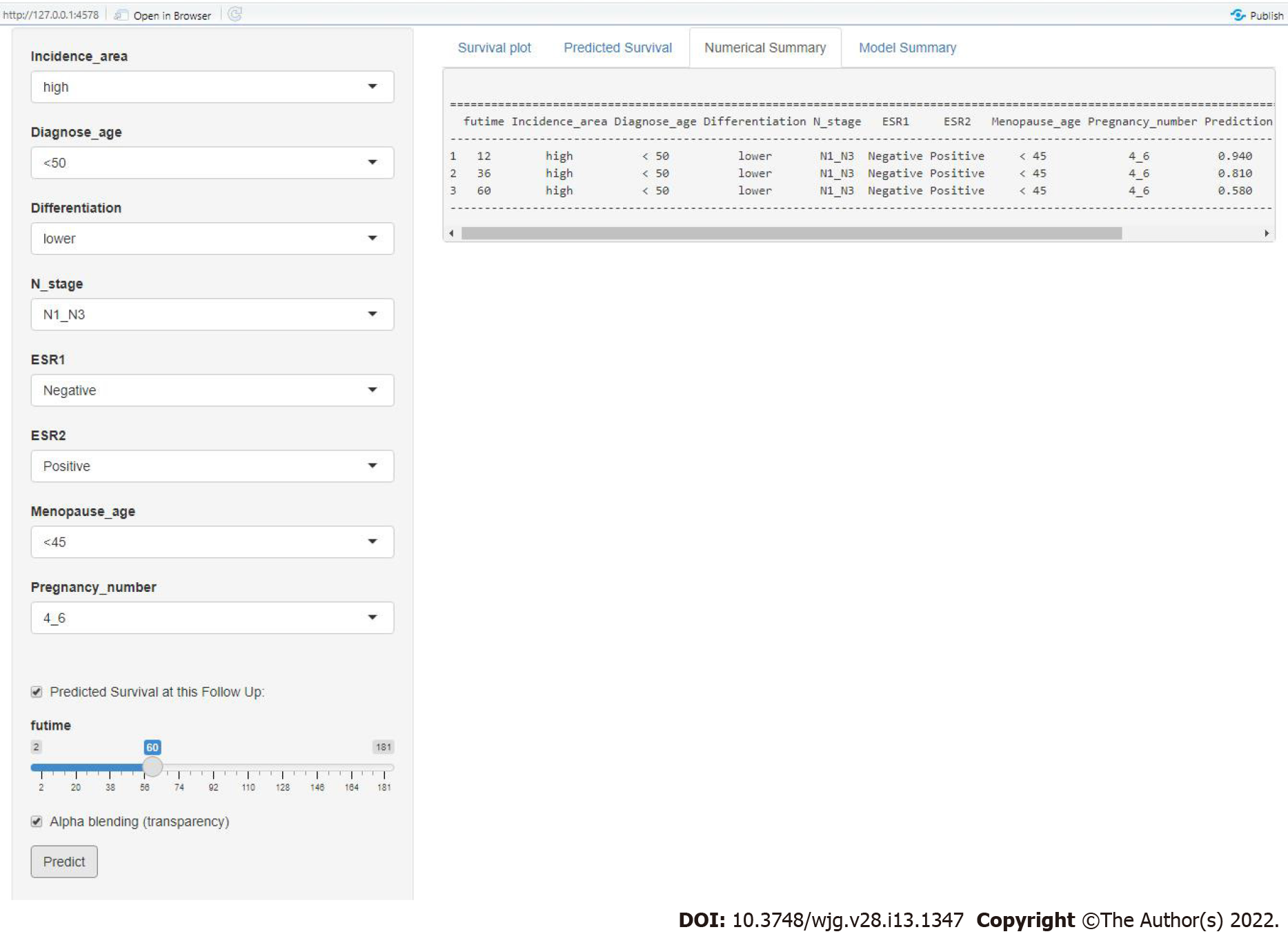
To improve the predictive performance and clinical utility of the full model, we transferred the data and formulae to a user-friendly website. Figure 9 shows a snapshot of web-based nomogram that is available on predictbcos.shaws.cn: https://female-escc-predictor.shinyapps.io/DynNomapp/. Visitors can select values from the drop-down list according to the circumstance of clinical and reproductive factors, and then click the “predict” button to predict the OS rates in Chinese female ESCC.
Although advances have been achieved in the prevention and treatment of ESCC in the past few decades, the prognosis of ESCC is still poor with high mortality rates[17,18]. Traditionally, the TNM staging system has been used to predict the prognosis of many cancers. However, accumulated studies have reported that the prognostic predictive probability of clinical nomograms is more accurate than that of TNM stage due to the incorporation of all known significant prognostic factors of individual patients[19-21]. To date, nomograms have been widely applied in clinical prognostic evaluation for several cancers[22-24].
We constructed and validated a clinical–reproductive prognostic model for assessing the added value of reproductive factors over existing risk factors in female ESCC. Our results revealed that reproductive factors have independent prognostic value with respect to clinical parameters for individualized OS prediction in female ESCC. Subgroup analysis showed that the prognosis of female patients in the low-risk group was significantly better than that in the high-risk group. Our results of discrimination, calibration and DCA curves also showed that the clinical–reproductive model had enhanced prognostic value compared with clinical model and TNM stage.
To further explore the potential molecular mechanism of increased prognostic value of our clinical–reproductive model in female ESCC, we analyzed the correlations of estrogen response with immune-related pathways and biomarker genes of immune cells in esophageal cancer. An in vitro study has found that estrogen mediates the apoptosis of esophageal cancer cells by interacting with ERs[25]. The p53 signaling pathway serves as a major barrier to prevent the occurrence and progression of cancer[26,27]. Our results revealed that estrogen response could prolong clinical outcome by promoting the p53 pathway and inducing apoptosis. ESCC expresses ERs and estrogen plays a protective role through ERs[28]. Overexpression of ERs is associated with prognosis and female ESCC patients with higher ESR2 expression have a better prognosis and those with higher ESR1 seem to have shorter survival[3]. In the present study, the expression levels of some biomarker genes in immune cells were positively correlated with ESR1 and ESR2 levels. Significant correlations were found between ESR2 and PTGS2 (Prostaglandin-Endoperoxide Synthase 2, biomarker of M1 macrophages), ESR1 and CD163, VSIG4 (V-Set And Immunoglobulin Domain Containing 4), MS4A4A (Membrane Spanning 4-Domains A4A, biomarker of M2 macrophages). It is well known that different cell subtypes may have distinct roles in the immune microenvironment. M1 macrophages showed an antitumor effect after being activated by Th1 cytokines, while M2 macrophages showed protumor activity[29]. This may partly explain the survival difference in female ESCC patients with different expression levels of ESR1 and ESR2.
Several studies have developed some prognostic nomograms to predict prognosis in ESCC. These models provide useful tools to stratify the risk and predict survival probability in ESCC. However, the patients in these studies were mainly extracted from the Surveillance, Epidemiology, and Final Results (SEER) database in the United States, and these models were not entirely suitable for predicting OS for Chinese female ESCC due to the ethnic difference. In our study, the internal C-index of our model was 0.701, which is higher than two previously released models (0.67 and 0.66 for both nomograms)[30,31]. Although the C-index of the Yu et al[32] model was higher (0.749), the nomogram can only be used to predict the clinical outcome of T1 esophageal cancer patients with positive lymph nodes. A recent study on breast cancer showed that user-friendly online prognostic tools could greatly improve patient care[33]. There is no such online tool available for female ESCC. We implemented our nomogram in an online webserver, which means that clinicians can easily use it to predict prognosis. For example, a 46-year-old menopausal ESCC patient with five pregnancies showed a poorly differentiated tumor with positive lymph node metastasis, and the tumor was ESR1 negative and ESR2 positive. The predicted results by the website show that the OS rates at 1-, 3-, and 5-years were 0.94, 0.81 and 0.58, respectively.
There were some limitations to our research. First, our analysis may have been affected by bias and loss of follow-up due to the retrospective nature of the study. Second, we must be careful to extrapolate our findings to patients of other races because most of our patients were of Han nationality. Finally, the in-depth molecular mechanisms of reproductive factors in progression and prognosis of female ESCC depend on further experimental studies to elucidate.
We developed and verified a prognostic nomogram with clinical and reproductive factors to improve the accuracy of prognostic prediction in Chinese female patients with ESCC. Compared with the clinical model and TNM stage, the full model has increased prognostic value and can help clinicians to make individual treatment and medical decisions.
Nomogram has been widely used and proved to be more accurate than the tumor-node-metastasis (TNM) staging system for predicting prognosis in different cancers. In China, female patients with esophageal squamous cell carcinoma (ESCC), even with the same TNM stage, had distinct overall survival (OS) difference, which requires an effective prediction model to accurately evaluate the prognosis.
Several studies have developed some prognosis nomogram models, which provide useful tools for predicting OS probability in patients with esophageal cancer. The included patients were mainly extracted from the Surveillance, Epidemiology, and Final Results (SEER) database in United States. Due to the ethnic difference, the models are not entirely applicable to perform the OS prediction for Chinese female ESCC.
The purpose of the study was to develop and validate a clinical-reproductive model for predicting OS in Chinese female patients with ESCC, and to further explore whether the model had higher prognostic value than the clinical model and TNM stage.
A new prognostic nomogram incorporating clinical and reproductive characteristics was constructed in the primary training cohort and verified in the internal and external validation cohorts. The performance of the full model was evaluated by Kaplan-Meier curve, time-dependent receiver operating characteristic (ROC) curve, Harrell’s concordance index (C-index), calibration curve and decision curve analysis.
The clinical-reproductive model incorporated incidence area, age, differentiation, N stage, estrogen receptor alpha expression, estrogen receptor beta expression, menopausal age, and pregnancy number. Compared to the clinical model and TNM stage, the ROC curve and C-index indicated good discriminative ability of the full model for predicting 1-, 3-, and 5-years OS in the primary, internal, and external validation sets.
A clinical-reproductive nomogram for OS prediction in Chinese female ESCC was developed and validated in the present study, which showed superior survival prediction than the clinical model and TNM stage.
The clinical–reproductive model has incremental prognostic predictive value in Chinese female ESCC, which may be beneficial to individualized treatment and medical decision-making.
We thank Professor Xue-Zhong Shi (Department of Epidemiology and Biostatistics, College of Public Health in Zhengzhou University) for help in statistical analysis.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Chen T, China; Farid K, Egypt; Yakar M, Turkey S-Editor: Fan JR L-Editor: A P-Editor: Yuan YY
| 1. | Guo YL, Shan BE, Guo W, Dong ZM, Zhou Z, Shen SP, Guo X, Liang J, Kuang G. Aberrant methylation of DACT1 and DACT2 are associated with tumor progression and poor prognosis in esophageal squamous cell carcinoma. J Biomed Sci. 2017;24:6. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 14] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 2. | Nozoe T, Oyama T, Takenoyama M, Hanagiri T, Sugio K, Yasumoto K. Significance of immunohistochemical expression of estrogen receptors alpha and beta in squamous cell carcinoma of the esophagus. Clin Cancer Res. 2007;13:4046-4050. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 54] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 3. | Zhang D, Ku J, Liu R, Wu H, Liang G, Wei Y, Chen P, Zhao X, Liu S, Li Y, Yao J, Song X, Wang L. Characterization of serum estradiol level and tissue estrogen receptor immunostaining with clinical response and reproductive factor changes in Chinese female patients with esophageal squamous cell carcinoma. Biomed Pharmacother. 2017;93:879-884. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 11] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 4. | Wang C, Wang P, Liu JC, Zhao ZA, Guo R, Li Y, Liu YS, Li SG, Zhao ZG. Interaction of Estradiol and Endoplasmic Reticulum Stress in the Development of Esophageal Carcinoma. Front Endocrinol (Lausanne). 2020;11:410. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 5. | Brusselaers N, Maret-Ouda J, Konings P, El-Serag HB, Lagergren J. Menopausal hormone therapy and the risk of esophageal and gastric cancer. Int J Cancer. 2017;140:1693-1699. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 69] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 6. | Islami F, Cao Y, Kamangar F, Nasrollahzadeh D, Marjani HA, Shakeri R, Fahimi S, Sotoudeh M, Dawsey SM, Abnet CC, Boffetta P, Malekzadeh R. Reproductive factors and risk of esophageal squamous cell carcinoma in northern Iran: a case-control study in a high-risk area and literature review. Eur J Cancer Prev. 2013;22:461-466. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 18] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 7. | Rice TW, Rusch VW, Ishwaran H, Blackstone EH; Worldwide Esophageal Cancer Collaboration. Cancer of the esophagus and esophagogastric junction: data-driven staging for the seventh edition of the American Joint Committee on Cancer/International Union Against Cancer Cancer Staging Manuals. Cancer. 2010;116:3763-3773. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 315] [Cited by in RCA: 357] [Article Influence: 23.8] [Reference Citation Analysis (0)] |
| 8. | Mo R, Chen C, Pan L, Yu A, Wang D, Wang T. Is the new distribution of early esophageal adenocarcinoma stages improving the prognostic prediction of the 8th edition of the TNM staging system for esophageal cancer? J Thorac Dis. 2018;10:5192-5198. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 6] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 9. | Wang S, Yang L, Ci B, Maclean M, Gerber DE, Xiao G, Xie Y. Development and Validation of a Nomogram Prognostic Model for SCLC Patients. J Thorac Oncol. 2018;13:1338-1348. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 145] [Article Influence: 20.7] [Reference Citation Analysis (0)] |
| 10. | Adam MA, Thomas S, Hyslop T, Scheri RP, Roman SA, Sosa JA. Exploring the Relationship Between Patient Age and Cancer-Specific Survival in Papillary Thyroid Cancer: Rethinking Current Staging Systems. J Clin Oncol. 2016;34:4415-4420. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 112] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 11. | Pan JJ, Ng WT, Zong JF, Lee SW, Choi HC, Chan LL, Lin SJ, Guo QJ, Sze HC, Chen YB, Xiao YP, Kan WK, O'Sullivan B, Xu W, Le QT, Glastonbury CM, Colevas AD, Weber RS, Lydiatt W, Shah JP, Lee AW. Prognostic nomogram for refining the prognostication of the proposed 8th edition of the AJCC/UICC staging system for nasopharyngeal cancer in the era of intensity-modulated radiotherapy. Cancer. 2016;122:3307-3315. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 128] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 12. | Shariat SF, Capitanio U, Jeldres C, Karakiewicz PI. Can nomograms be superior to other prediction tools? BJU Int. 2009;103:492-5; discussion 495. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 119] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 13. | Kang SJ, Cho YR, Park GM, Ahn JM, Han SB, Lee JY, Kim WJ, Park DW, Lee SW, Kim YH, Lee CW, Park SW, Mintz GS, Park SJ. Predictors for functionally significant in-stent restenosis: an integrated analysis using coronary angiography, IVUS, and myocardial perfusion imaging. JACC Cardiovasc Imaging. 2013;6:1183-1190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 61] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 14. | Li L, Greene T, Hu B. A simple method to estimate the time-dependent receiver operating characteristic curve and the area under the curve with right censored data. Stat Methods Med Res. 2018;27:2264-2278. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 37] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 15. | Talluri R, Shete S. Using the weighted area under the net benefit curve for decision curve analysis. BMC Med Inform Decis Mak. 2016;16:94. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 44] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 16. | Camp RL, Dolled-Filhart M, Rimm DL. X-tile: a new bio-informatics tool for biomarker assessment and outcome-based cut-point optimization. Clin Cancer Res. 2004;10:7252-7259. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1947] [Cited by in RCA: 2936] [Article Influence: 146.8] [Reference Citation Analysis (0)] |
| 17. | Abnet CC, Arnold M, Wei WQ. Epidemiology of Esophageal Squamous Cell Carcinoma. Gastroenterology. 2018;154:360-373. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 935] [Cited by in RCA: 1155] [Article Influence: 165.0] [Reference Citation Analysis (1)] |
| 18. | Thrumurthy SG, Chaudry MA, Thrumurthy SSD, Mughal M. Oesophageal cancer: risks, prevention, and diagnosis. BMJ. 2019;366:l4373. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 62] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 19. | Wang Y, Li J, Xia Y, Gong R, Wang K, Yan Z, Wan X, Liu G, Wu D, Shi L, Lau W, Wu M, Shen F. Prognostic nomogram for intrahepatic cholangiocarcinoma after partial hepatectomy. J Clin Oncol. 2013;31:1188-1195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 786] [Cited by in RCA: 833] [Article Influence: 69.4] [Reference Citation Analysis (0)] |
| 20. | Balachandran VP, Gonen M, Smith JJ, DeMatteo RP. Nomograms in oncology: more than meets the eye. Lancet Oncol. 2015;16:e173-e180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1119] [Cited by in RCA: 2394] [Article Influence: 239.4] [Reference Citation Analysis (0)] |
| 21. | Ma M, Wang J, Hu Y, Weng M, Liu X, Wang Y. Prognostic Value of Inflammatory Biomarkers in Gastric Cancer Patients and the Construction of a Predictive Model. Dig Surg. 2019;36:433-442. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 10] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 22. | Yan L, Deng W, Guan L, Xu H. Nomogram forecasting 3-, 5-, and 8-year overall survival and cancer-specific survival of gingival squamous cell carcinoma. Cancer Med. 2020;9:8266-8274. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 23. | Feng Y, Wang Y, Xie Y, Wu S, Li Y, Li M. Nomograms predicting the overall survival and cancer-specific survival of patients with stage IIIC1 cervical cancer. BMC Cancer. 2021;21:450. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 24. | Wang F, Zhang H, Wen J, Zhou J, Liu Y, Cheng B, Chen X, Wei J. Nomograms forecasting long-term overall and cancer-specific survival of patients with oral squamous cell carcinoma. Cancer Med. 2018;7:943-952. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 31] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 25. | Al-Khyatt W, Tufarelli C, Khan R, Iftikhar SY. Selective oestrogen receptor antagonists inhibit oesophageal cancer cell proliferation in vitro. BMC Cancer. 2018;18:121. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 23] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 26. | Holzer K, Ori A, Cooke A, Dauch D, Drucker E, Riemenschneider P, Andres-Pons A, DiGuilio AL, Mackmull MT, Baßler J, Roessler S, Breuhahn K, Zender L, Glavy JS, Dombrowski F, Hurt E, Schirmacher P, Beck M, Singer S. Nucleoporin Nup155 is part of the p53 network in liver cancer. Nat Commun. 2019;10:2147. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 34] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 27. | Sargolzaei J, Etemadi T, Alyasin A. The P53/microRNA network: A potential tumor suppressor with a role in anticancer therapy. Pharmacol Res. 2020;160:105179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 21] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 28. | Dong J, Jiang SW, Niu Y, Chen L, Liu S, Ma T, Chen X, Xu L, Su Z, Chen H. Expression of estrogen receptor α and β in esophageal squamous cell carcinoma. Oncol Rep. 2013;30:2771-2776. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 17] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 29. | den Breems NY, Eftimie R. The re-polarisation of M2 and M1 macrophages and its role on cancer outcomes. J Theor Biol. 2016;390:23-39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 45] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 30. | Shao CY, Liu XL, Yao S, Li ZJ, Cong ZZ, Luo J, Dong GH, Yi J. Development and validation of a new clinical staging system to predict survival for esophageal squamous cell carcinoma patients: Application of the nomogram. Eur J Surg Oncol. 2021;47:1473-1480. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 9] [Article Influence: 2.3] [Reference Citation Analysis (1)] |
| 31. | Du F, Sun Z, Jia J, Yang Y, Yu J, Shi Y, Jia B, Zhao J, Zhang X. Development and Validation of an Individualized Nomogram for Predicting Survival in Patients with Esophageal Carcinoma after Resection. J Cancer. 2020;11:4023-4029. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 28] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 32. | Yu J, Hu W, Yao N, Sun M, Li X, Wang L, Yang Y, Li B. Development and validation of a nomogram to predict overall survival of T1 esophageal squamous cell carcinoma patients with lymph node metastasis. Transl Oncol. 2021;14:101127. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 33. | Shachar SS, Muss HB. Internet tools to enhance breast cancer care. NPJ Breast Cancer. 2016;2:16011. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 36] [Article Influence: 4.0] [Reference Citation Analysis (0)] |