Published online Mar 21, 2022. doi: 10.3748/wjg.v28.i11.1172
Peer-review started: February 26, 2021
First decision: June 14, 2021
Revised: June 26, 2021
Accepted: January 29, 2022
Article in press: January 29, 2022
Published online: March 21, 2022
Processing time: 383 Days and 18.7 Hours
Hepatitis C virus (HCV) genotype 6 (HCV-6) infection is prevalent predominantly in Southeast Asia, and the data on the virologic response of HCV-6 to direct-acting antivirals (DAAs) are sparse in people living with human immunodeficiency virus (HIV) (PLWH).
To assess the virologic response of HCV-6 to DAAs in PLWH.
From September 2016 to July 2019, PLWH coinfected with HCV-6 initiating DAAs were included. Laboratory investigations were performed at baseline, the end of treatment, and 12 wk off-therapy.
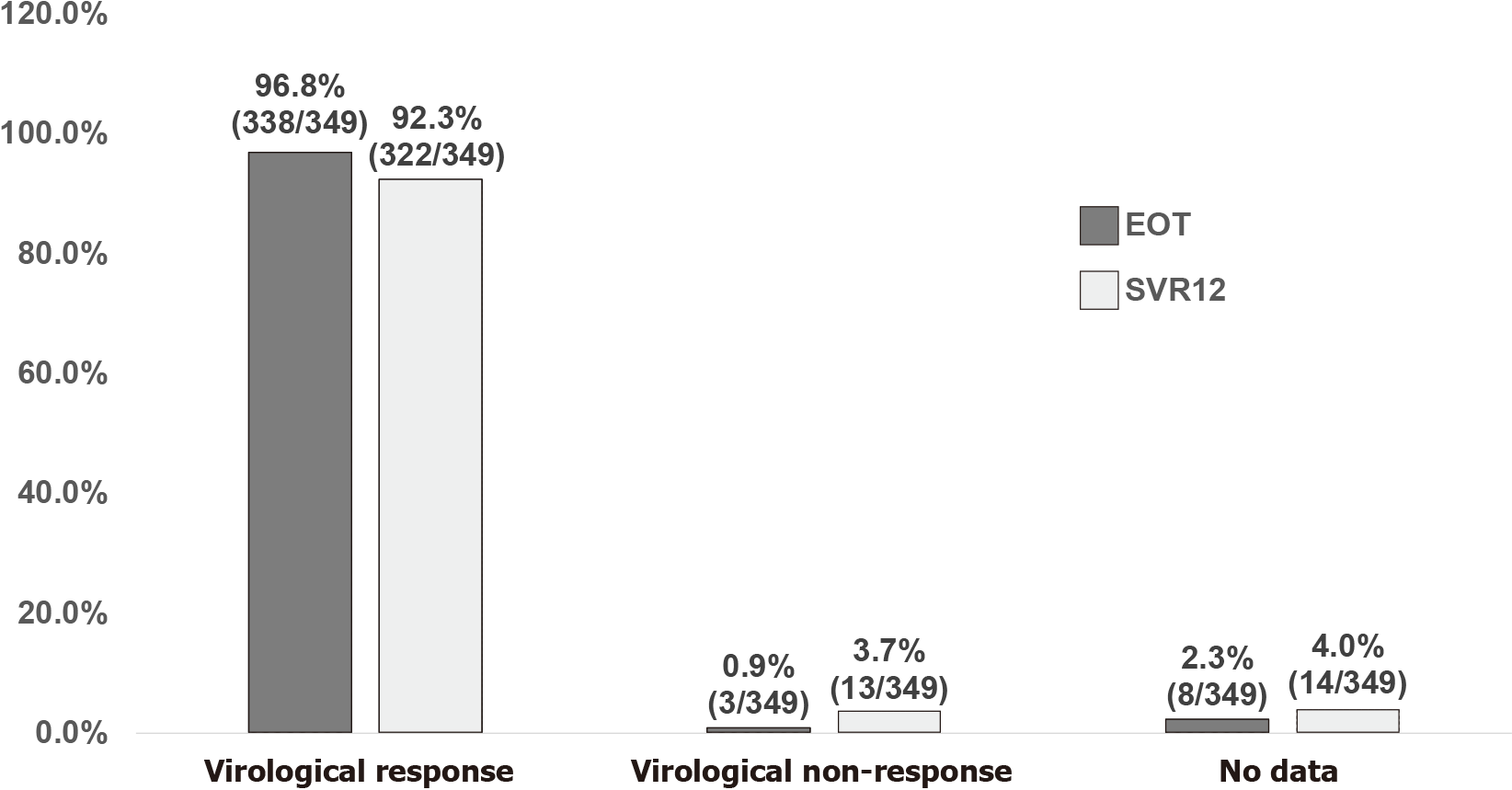
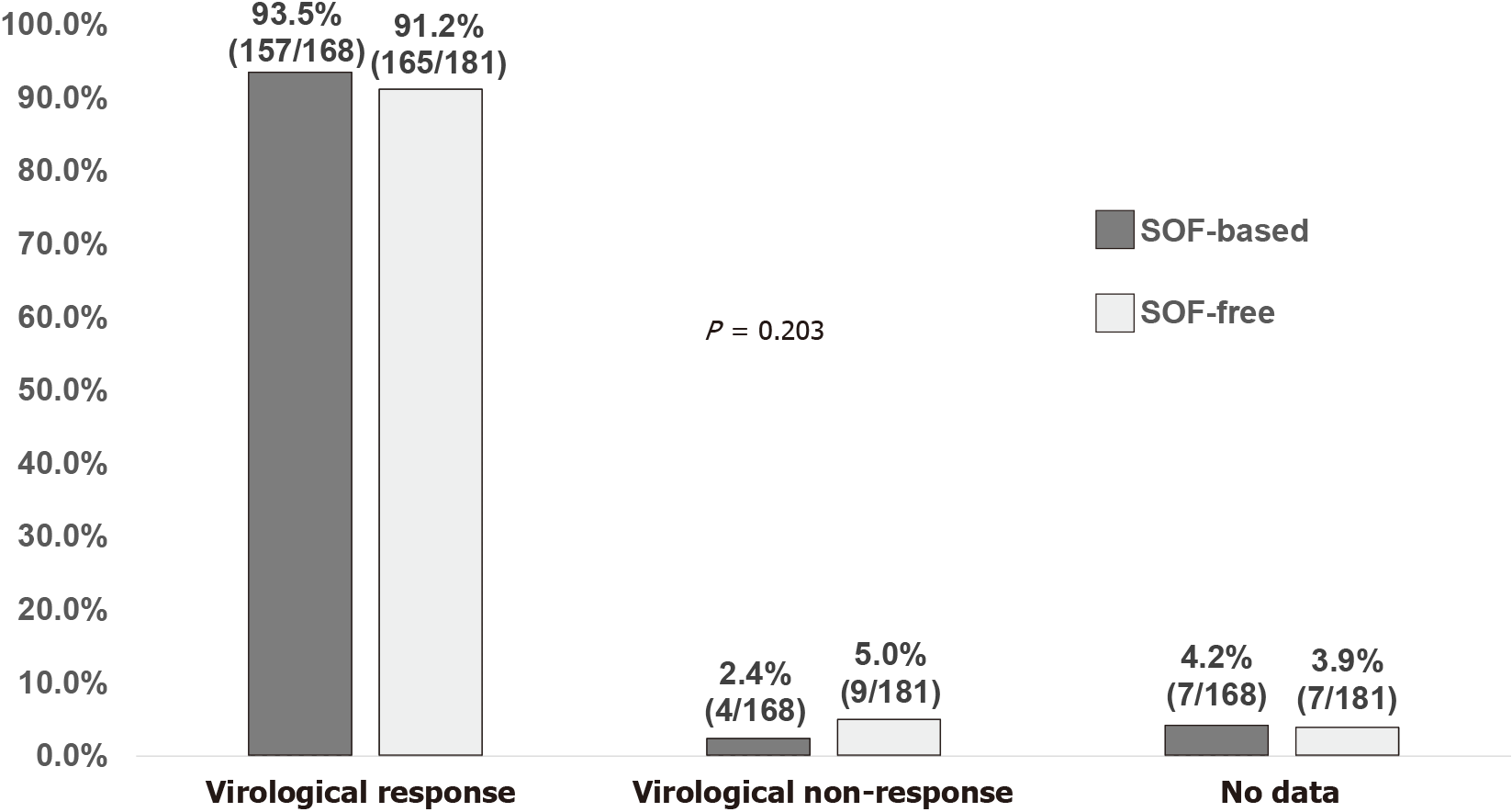
Of the 349 PLWH included (mean age 48.9 years, 82.5% men), 80.5% comprised people who inject drugs, 18.1% men who have sex with men, and 1.4% heterosexuals. Coexistent hepatitis B virus infection was present in 12.3% of the included PLWH, liver cirrhosis 10.9%, hepatocellular carcinoma 0.9%, and previous HCV treatment experience 10.9%. The mean baseline plasma HCV RNA was 6.2 log10 IU/mL. Treatment with glecaprevir/pibrentasvir was initiated in 51.9%, sofosbuvir/ledipasvir 41.5%, sofosbuvir/velpatasvir 6.3%, and sofosbuvir/daclatasvir 0.3%. At DAA initiation, antiretroviral therapy containing tenofovir alafenamide was given in 26.4%, tenofovir disoproxil fumarate 34.4%, non-tenofovir alafenamide/tenofovir disoproxil fumarate 39.3%, non-nucleoside reverse-transcriptase inhibitors 30.4%, protease inhibitors 4.0%, and integrase strand transfer inhibitors 66.8%; 94.8% of the included patients had CD4 counts ≥ 200 cells/mm3 and 96.0% had plasma HIV RNA < 50 copies/mL. Overall, 96.8% achieved undetectable plasma HCV RNA (< 30 IU/mL) at end of treatment; and 92.3% achieved sustained virologic response 12 wk off-therapy in the intention-to-treat analysis (93.5% in patients receiving sofosbuvir-based DAAs and 91.2% in those receiving glecaprevir/pibrentasvir).
Similar to the observation made in HIV-negative patients, sustained virologic response 12 wk off-therapy with DAAs is high in PLWH coinfected with HCV-6.
Core Tip: Similar to the observation made in human immunodeficiency virus-negative patients, virologic response 12 wk off-therapy achieved with direct-acting antivirals (DAAs) was high in human immunodeficiency virus-positive patients coinfected with hepatitis C virus genotype 6. Estimated glomerular filtration rate declined with DAA initiation in patients receiving sofosbuvir and tenofovir disoproxil fumarate or tenofovir alafenamide, which recovered after the completion of DAA treatment.
- Citation: Sun HY, Cheng CY, Lin CY, Yang CJ, Lee NY, Liou BH, Tang HJ, Liu YM, Lee CY, Chen TC, Huang YC, Lee YT, Tsai MJ, Lu PL, Tsai HC, Wang NC, Hung TC, Cheng SH, Hung CC. Real-world effectiveness of direct-acting antivirals in people living with human immunodeficiency virus and hepatitis C virus genotype 6 infections. World J Gastroenterol 2022; 28(11): 1172-1183
- URL: https://www.wjgnet.com/1007-9327/full/v28/i11/1172.htm
- DOI: https://dx.doi.org/10.3748/wjg.v28.i11.1172
With the introduction of highly effective direct-acting antivirals (DAAs), the treatment paradigm of acute or chronic hepatitis C virus (HCV) infections has shifted and the majority of HCV-infected patients with access to DAAs can be cured[1,2]. Nevertheless, an estimated 71 million people globally have chronic HCV infection, of which a significant number develop cirrhosis of the liver or liver cancer[3]. Furthermore, 1.34 million deaths are caused by viral hepatitis, constituting a mortality rate comparable to that of tuberculosis and higher than that of human immunodeficiency virus (HIV) infection[3].
Currently, HCV is classified into seven genotypes and 67 subtypes[4]. Each genotype has its own major geographic distribution[5,6]. The prevalence of HCV viremia due to genotype 6 (GT6) is high in Southeast (34.8%-95.6%) and East Asia (27.4%)[6]. People who inject drugs (PWID) and individuals with thalassemia major are noted to have a higher prevalence of HCV GT6 infection[7]. In Taiwan, genotypes 1b (60.1%), 2a (15.5%), and 2b (11.9%) are the main HCV genotypes in the general population, with genotype 6a being rare[8]. After the outbreak of HCV infection among HIV-positive PWID in Taiwan, genotypes 1a (29.2%), 6a (23.5%), and 3a (20.2%) have emerged as the main circulating HCV genotypes in this population[9].
Compared to patients with HCV non-GT6-related cirrhosis, those with HCV GT6-related cirrhosis have a higher risk of developing hepatocellular carcinoma[10]. In clinical trials, HCV/HIV-co-infected patients are no longer considered special populations, with a sustained virologic response (SVR) rate of 90% or higher with the use of pangenotypic DAAs[11]. In the real world, however, 90% of HCV/HIV-coinfected patients are ineligible for participation in clinical trials[12]. A systematic review concludes that the SVR rates with DAAs are high in patients with HCV GT6 infections in the modern era of DAAs except for patients with cirrhosis of the liver and prior treatment[13]. However, few patients with HIV/HCV GT6 coinfections were included in both clinical trials and real-world studies[14-16]. The present multicenter study aimed to assess the real-world SVR rates in HIV/HCV GT6-coinfected patients receiving contemporary DAAs.
In Taiwan, DAAs were conditionally included in the National Health Insurance coverage since January 2017[17,18]. In January 2019, the HCV treatment program providing free-of-charge testing and DAAs was expanded to cover all patients with HCV viremia, including those with acute HCV infections. Hepatologists and HIV-treating physicians were permitted to screen and treat HIV/HCV-coinfected patients who meet the inclusion criteria. Standardized clinical care and data collection, including serum albumin, alanine aminotransferase and aspartate aminotransferase, prothrombin time and partial thromboplastin time, hepatitis B virus (HBV) serological markers, HCV genotype, abdominal sonography, and plasma HCV RNA load at baseline, the end of treatment (EOT), and 12 wk off-therapy, are strictly required by the HCV treatment program.
People living with HIV (PLWH) in Taiwan are provided with free-of-charge combination antiretroviral therapy (ART) and monitoring of plasma HIV RNA load, CD4 lymphocyte count, renal and hepatic function, lipid profile, and serological markers of viral hepatitis and viral loads, if necessary, according to the national HIV treatment guidelines[19]. HIV care is provided by HIV-treating infectious disease specialists in collaboration with case managers at designated hospitals around Taiwan.
Eligible patients included in this multicenter retrospective observational study were PLWH aged 20 years or older who were diagnosed with HCV GT6 coinfection, either HCV treatment-naïve or -experienced, and received oral DAAs. According to Taiwan’s National Health Insurance regulation, oral DAAs for HCV GT6 infections include glecaprevir/pibrentasvir (GLE/PIB) for 8 wk (for patients without cirrhosis) or 12 wk (for those with compensated cirrhosis, Child-Pugh A), sofosbuvir/ledipasvir (SOF/LDV) +/- ribavirin for 12 wk, SOF/velpatasvir (SOF/VEL) +/- ribavirin for 12 wk, and SOF/daclatasvir (SOF/DCV) +/- ribavirin for 12 wk. The regimen of oral DAAs for HCV GT6 infections was chosen at the discretion of the treating physicians.
A standardized data collection form was used to record information on demographics (year of birth and sex), clinical characteristics (HIV and HCV transmission routes and ART regimens), and laboratory test results (CD4 count, plasma HIV RNA load, hemogram, and biochemistry). The estimated glomerular filtration rate (eGFR) was assessed using the Chronic Kidney Disease Epidemiology Collaboration equation. The Fibrosis-4 index was calculated according to a previous report[20]. Serum samples to determine HCV RNA load were obtained at baseline (i.e. at DAAs initiation), EOT, and 12 wk off-therapy. This retrospective study was approved by the Institutional Review Board or Research Ethics Committee of each participating hospital and the requirement for informed consent was waived.
The Roche cobas® HCV GT test with real-time reverse transcription-polymerase chain reaction was used in eight hospitals to identify HCV genotypes 1 to 6 and subtypes 1a and 1b via genotype- and subtype-specific primers and fluorescent dye-labeled oligonucleotide probes; the Abbott Realtime HCV Genotype II assay was used in 7 hospitals. If there were mixed types or any indeterminate results, a sequencing assay was used for final confirmation.
The primary efficacy end-point, analyzed according to the Food and Drug Administration (FDA) Snapshot algorithm, was SVR with undetectable HCV RNA 12 wk off-therapy (SVR12), defined as having HCV RNA < 30 IU/mL 12 wk after DAA treatment completion. The safety end-point was any adverse event leading to failure of completion of the DAA treatment course. The secondary end-point was HIV virologic suppression after completing DAA therapy, which was defined as plasma HIV RNA load < 50 copies/mL.
All analyses were performed using the Statistical Program for Social Sciences (SPSS Statistics Version 21, IBM Corp., Armonk, New York, United States). Categorical variables were compared using X2 or Fisher’s exact test and non-categorical variables using Student’s t test or a Mann-Whitney U test. The statistical methods of this study were reviewed by the staff of National Taiwan University Hospital -Statistical Consulting Unit.
From September 2016 to August 2019, a total of 349 PLWH with HCV GT6 infections receiving DAAs were included, with a mean age of 48.9 years (Table 1). The study population consisted mainly of PWID (80.5%), followed by men who have sex with men (MSM) (18.1%), and heterosexuals (1.4%). All had received ART at the time of DAA initiation; 94.8% had CD4 counts ≥ 200 cells/mm3, 96.0% had plasma HIV RNA loads < 50 copies/mL, and 7.7% had adjusted ART regimens for concerns about potential drug-drug interactions with DAAs (7.2%) or simplification (0.5%).
| Variables | Data |
| Age at DAA initiation, mean (SD), yr | 48.9 (11.7) |
| Male sex, n (%) | 288 (82.5) |
| Transmission route of HIV infection, n (%) | |
| Men who have sex with men | 63 (18.1) |
| Heterosexuals | 5 (1.4) |
| People who inject drugs | 281 (80.5) |
| CD4 counts ≥ 200 cells/mm3, n (%) | 331 (94.8) |
| Plasma HIV viral loads < 50 copies/mL, n (%) | 335 (96.0) |
| eGFR at baseline, mean (SD), mL/min/1.73m2 | 94.0 (20.5) |
| Receiving ART, n (%) | 349 (100.0) |
| Switch of ART before DAA, n (%) | 27 (7.7) |
| Backbone antiretroviral agent, n (%) | |
| TAF-based | 92 (26.4) |
| TDF-based | 120 (34.4) |
| Non-TDF/TAF-based | 137 (39.3) |
| The third antiretroviral agent | |
| nNRTI | 106 (30.4) |
| PI | 14 (4.0) |
| InSTI, n/N (%) | 233 (66.8) |
| RAL | 10/233 (4.3) |
| EVG | 92/233 (39.5) |
| DTG | 131/233 (56.2) |
| Cirrhosis of the liver, n (%) | 38 (10.9) |
| Hepatocellular carcinoma, n (%) | 3 (0.9) |
| HCV treatment-experienced, n (%) | 38 (10.9) |
| DAA, n | 4 |
| Interferon/ribavirin, n | 35 |
| HCV seroconversion1 within 1 yr, n (%) | 18 (5.2) |
| Transmission route of HCV infection, n (%) | |
| Sexual transmission | 43 (12.3) |
| Injection drug use | 279 (79.9) |
| Blood transfusion | 1 (0.3) |
| Unknown | 26 (7.4) |
| HCV RNA viral load, mean (SD), log10 IU/mL | 6.2 (1.1) |
| Mixed HCV infection, n (%) | 8 (2.3) |
| Positive HBsAg, n (%) | 43 (12.3) |
| Undetectable HBV DNA load (< 20 IU/mL) before DAA initiation, n/N (%) | 10/13 (76.9) |
| Undetectable HBV DNA load after completion of DAA therapies, n/N (%) | 6/7 (85.7) |
| DAA agents, n (%) | |
| GLE/PIB | 181 (51.9) |
| SOF/LDV | 145 (41.5) |
| SOF/VEL | 22 (6.3) |
| SOV/DCV | 1 (0.3) |
| Plasma HIV RNA < 50 copies/mL after DAA, n/N (%) | 289/306 (94.4) |
Cirrhosis of the liver and hepatocellular carcinoma were documented in 10.9% and 0.9% of the included PLWH, respectively. Thirty-eight PLWH (10.9%) had received HCV treatment, mainly interferon-based therapy (n = 35, 10.0%), before DAA initiation. Seroconversion of anti-HCV antibody from negativity to positivity within 1 year was documented in 5.2%. Injection drug use (IDU) (79.9%) was the predominant transmission route of HCV infection followed by sexual transmission (12.3%). Mixed infection with other genotypes was noted in 2.3% (n = 8), mainly genotype 2 (3) followed by 1a (2), 1b (1), both 1a and 1b (1), and 1 (1). Hepatitis B surface antigen testing was positive in 43 PLWH (12.3%). Of the 13 HBV-coinfected PLWH with determinations of HBV DNA load before DAA initiation, 10 (76.9%) had undetectable HBV DNA (< 20 IU/mL). After DAA completion, 85.7% (6/7) had undetectable HBV DNA.
At DAA initiation, ART included tenofovir alafenamide (TAF)-based regimens in 26.4%, tenofovir disoproxil fumarate (TDF)-based regimens in 34.4%, and non-TAF/TDF-based regimens in 39.3%. The third agent of the ART regimen varied between non-nucleoside reverse-transcriptase inhibitors in 30.4% of the included PLWH, protease inhibitors (PIs) in 4.0%, and integrase strand transfer inhibitors (InSTIs) in 66.8% (raltegravir 4.3%, elvitegravir 39.5%, and dolutegravir 56.2%).
GLE/PIB (51.9%) was the most frequently prescribed DAA, followed by SOF/LDV (41.5%), SOF/VEL (6.3%), and SOF/DCV (0.3%). Before DAA initiation, the mean plasma HCV RNA load was 6.2 log10 IU/mL, and the overall virologic response at EOT and SVR12 was 96.8% and 92.3%, respectively, in the FDA Snapshot algorithm (Figure 1). At EOT, plasma HCV RNA was detectable in 2 PWID (plasma HCV RNA 238 and 10,743,433 IU/mL after treatment with GLE/PIB and SOF/VEL, respectively) and 1 heterosexual (plasma HCV RNA 963 IU/mL after treatment with GLE/PIB). Additionally, 8 PLWH (2.3%) had no data available for assessment at EOT (Figure 1), including 4 PLWH (3 treated with GLE/PIB and 1 SOF/VEL) who were lost to follow-up, 3 (2 GLE/PIB and 1 SOF/VEL) who missed the blood testing but achieved SVR12 during follow-up, and 1 (SOF/LDV) who died of a morphine overdose.
At 12 wk off-DAA therapy, 14 PLWH (11 PWID and 3 MSM) were classified as having no data (Figure 1), including 13 PLWH (7 treated with GLE/PIB, 4 SOF/LDV, and 2 SOF/VEL) who were lost to follow-up and 1 (treated with SOF/LDV) who died of a morphine overdose. None discontinued DAA due to adverse effects. Of the 13 PLWH (3.7%, including 11 PWID, 1 MSM, and 1 heterosexual) classified as having virologic non-response (9 treated with GLE/PIB and 4 SOF/LDV), their median plasma HCV RNA load was 5.0 log10 IU/mL (interquartile range 3.7-6.7 log10 IU/mL). Reinfection was considered by the treating physicians as the cause of virologic non-response in 10 PLWH and treatment failure in 1 PLWH; and the causes in the remaining 2 PLWH were unclear. Of the 10 PLWH with HCV reinfection, 5 had HCV genotyping after SVR12, and 3 underwent a genotype switch (GT3 in 2 PLWH and 1a in 1).
The virologic response to SOF-based regimens (168 PLWH) and GLE/PIB (181 PLWH) at EOT by FDA Snapshot algorithm was 97.6% and 96.1%, respectively (P = 0.793) (Supplementary Figure 1), and the SVR12 rate was 93.5% and 91.2%, respectively (P = 0.203) (Figure 2). In the per-protocol (PP) analysis, the virologic response rates were 99.4% (164/165) and 98.9% (174/176) at EOT, and 97.5% (157/161) and 94.8% (165/174) at SVR12 for SOF-based regimens and GLE/PIB, respectively (both P > 0.05).
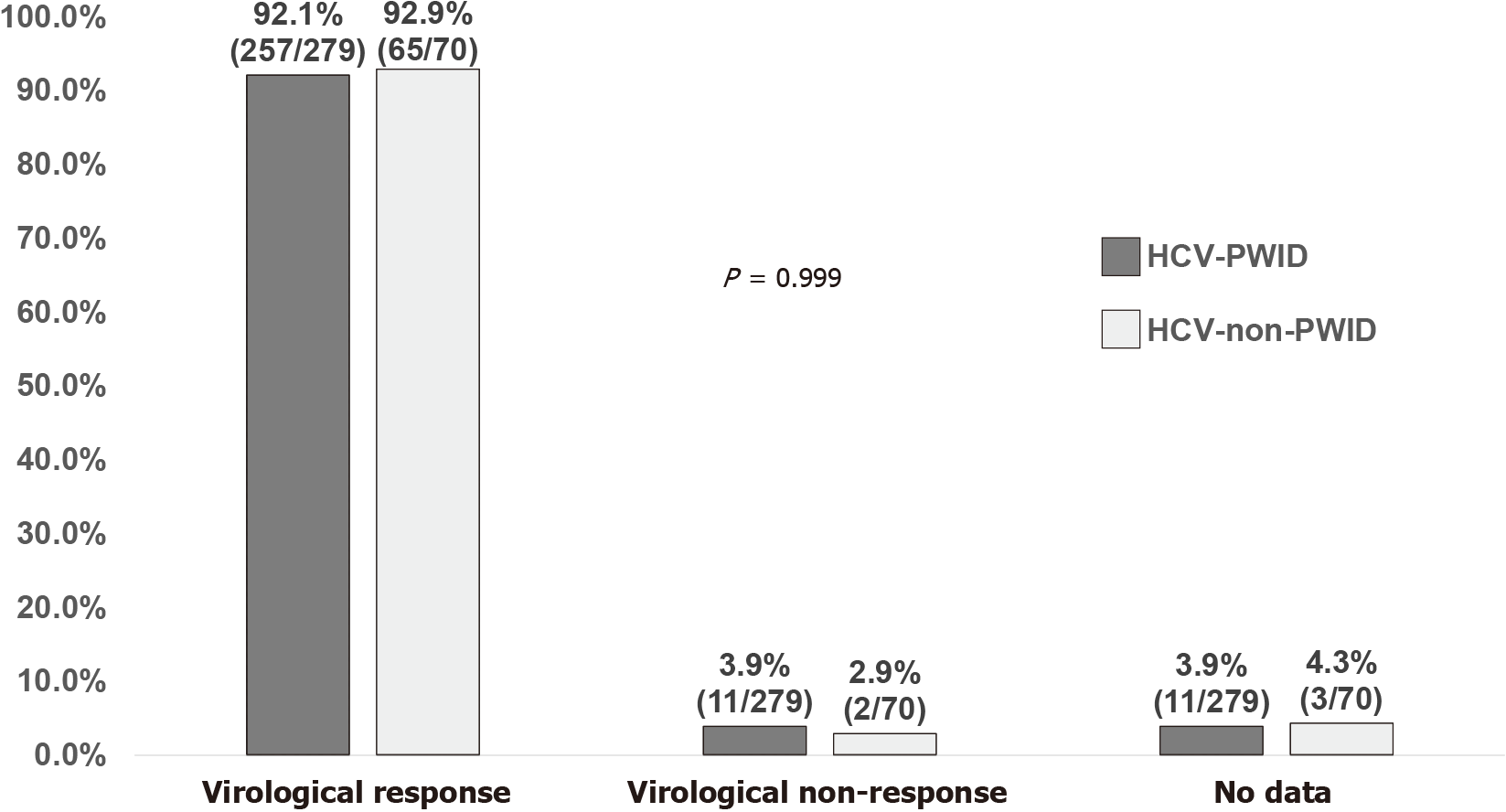
There was no statistically significant difference in virologic response at EOT (97.5% vs 94.3%; P = 0.210) and SVR12 (92.1% vs 92.9%; P = 0.999) between the 279 PLWH acquiring HCV infection via IDU and the 70 PLWH who were MSM or heterosexuals (Supplementary Figure 2, and Figure 3). In the PP analysis, no statistically significant difference was detected in virologic response between the two groups (IDU vs sexual transmission) at EOT [99.3% (272/274) vs 98.5% (66/67); P = 0.482] and SVR12 [95.9% (257/268) vs 97.0% (65/67); P = 0.999]. Of the 11 PWID who had no data available for assessment of virologic response 12 wk off-therapy, loss to follow-up was the main reason (90.9%, 10/11).
Improvement of elevated serum transaminases and Fibrosis-4 index scores was observed as soon as 4 wk after DAA initiation (Supplementary Figure 3A-C). The median absolute and percentage changes of eGFR were compared among the six groups of PLWH according to the agent used (SOF, TDF, and TAF), which included 53 PLWH receiving SOF/TDF, 44 SOF/TAF, 71 SOF/non-TDF/non-TAF, 67 non-SOF/TDF, 48 non-SOF/TAF, and 66 non-SOF/non-TDF/non-TAF during the DAA treatment course and after DAA discontinuation.
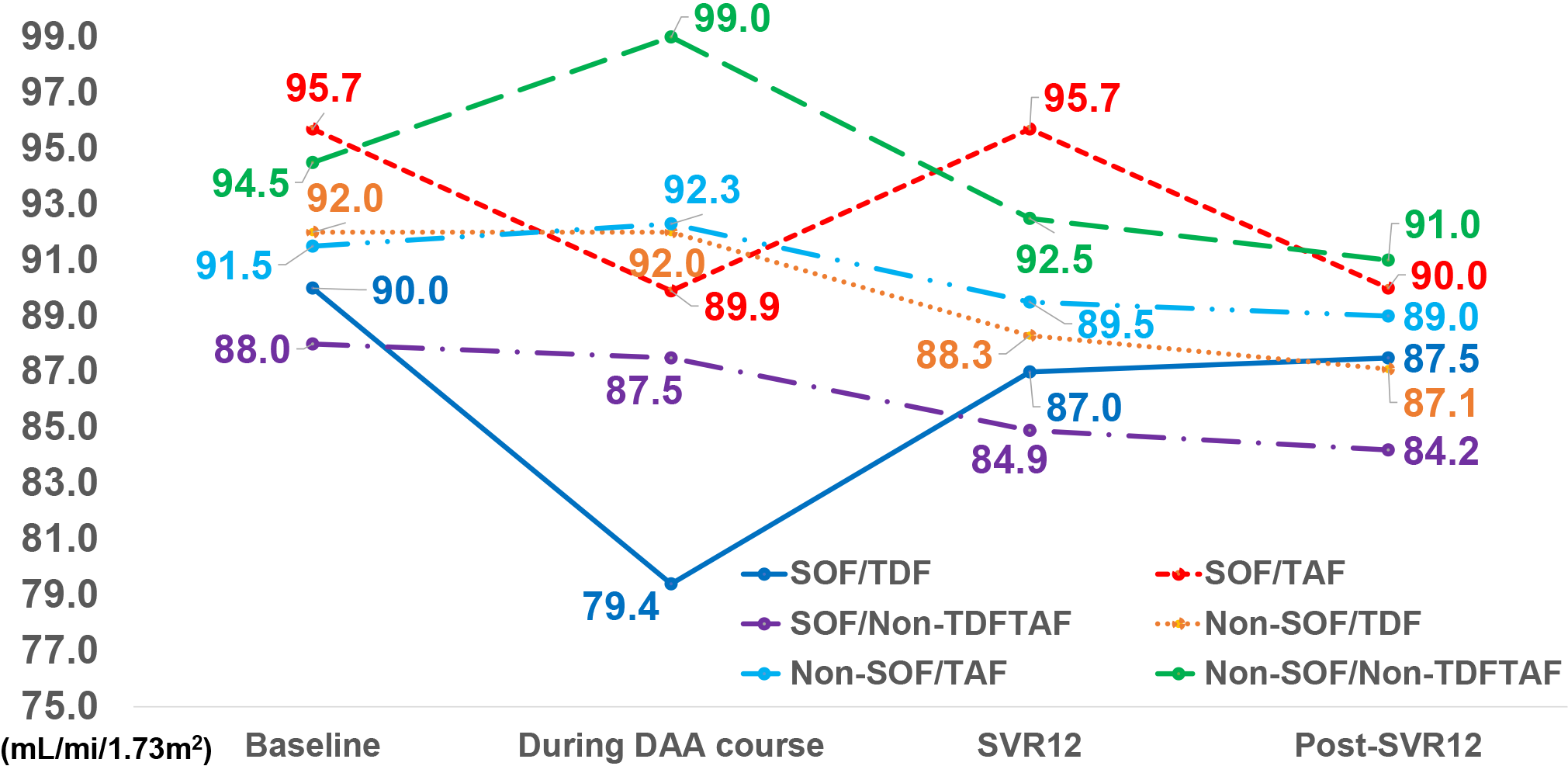
The sequential median eGFR at baseline, during the DAA course, at SVR12, and post-SVR12 of the six groups, are shown in Figure 4. The median eGFR of the PLWH receiving SOF-containing regimens declined initially after the initiation of DAA but recovered later, while that of PLWH taking non-SOF-containing regimens increased or remained the same after DAA initiation and declined after DAA discontinuation. The median absolute and percentage changes of eGFR are presented in Supplementary Figure 4A and B. The eGFR — both absolute and percentage changes — after DAA initiation decreased most significantly in PLWH receiving SOF/TDF compared with other regimens (P = 0.025 and 0.013, respectively) and improved during the DAA course and after DAA discontinuation (Supplementary Figure 3A and B). Plasma HIV RNA loads remained < 50 copies/mL in 94.4% after DAA discontinuation.
Our study with a large number (n = 349) of HIV/HCV GT6-coinfected patients receiving DAAs shows that the overall SVR12 rates in the intention-to-treat and the PP analyses were 92.3% and 96.1%, respectively. The majority of the included PLWH acquired HIV (80.5%) and HCV (79.9%) via IDU, and loss to follow-up was the main reason (92.9%, 13/14) for the lack of data required in the assessment of virologic response 12 wk off-therapy. Eleven of the 13 PLWH (84.6%) with virologic non-response were PWID, of which 10/13 (76.9%) were likely due to re-infection. Nevertheless, the SVR12 rates were similar when stratified by HCV transmission risk between those with and without IDU (Figure 3), as were the rates between SOF-based regimens and GLE/PIB (Figure 2). The median eGFR in the included PLWH treated with SOF/TDF declined most significantly after DAA initiation (Figure 4, Supplementary Figure 4A and B).
DAAs used in the current study were GLE/PIB, SOF/LDV, SOF/VEL +/- ribavirin, and SOF/DCV +/- ribavirin. Their excellent efficacy has been well-documented in a wide variety of patients, including treatment-naïve or -experienced patients and those with and without cirrhosis of the liver, HIV coinfection, chronic renal disease, and solid-organ transplantation[21-23]. For patients with HCV GT6 infections, the reported overall SVR12 was 98% in 108 patients receiving GLE/PIB, 100% in 171 receiving SOF/VEL +/- voxilaprevir, and 64.1-100% in 427 receiving SOF/LDV[13]. A meta-analysis shows that the pooled SVR of DAAs in patients with HCV GT6 infections is 95%, similar to that of patients with HCV GT1 and GT3 infections, respectively[24]. Nevertheless, only 28 HIV/HCV GT6-coinfected patients were included in previous studies[14-16]. Our study provides real-world evidence to fill the current knowledge gap regarding use of DAAs in the HIV/HCV GT6-coinfected population.
Despite excellent effectiveness of DAAs against HCV infections, there are numerous concerns (low treatment completion rates, high rates of loss to follow-up and reinfections) and barriers (psychiatric diseases, poor access to health services, ongoing drug use, inadequate HCV testing, and reluctance of physicians) to including PWID for DAA treatment[25]. In our study consisting mainly of PWID, the SVR12 rate of 95.9% in PLWH acquiring HCV infection via IDU and the rate of loss to follow-up (3.9%, 11/279) seen in the PP analysis are in line with those of previous reports in the literature. In two Spanish cohorts, SVR12 rates were lower for ongoing drug users [with or without opioid agonist therapy (OAT)] than non-drug users (79% vs 95%; P < 0.001)[26]. Furthermore, ongoing drug users had a high rate of loss to follow-up (17%) and reinfection (3.5%)[26]. Nevertheless, in the PP analysis, SVR12 rates did not differ among never-injectors (97%), PWID without OAT (95%), and those with OAT (95%) (P = 0.246). Ongoing drug use was associated with lower SVR12 rates, mainly due to loss to follow-up and not virologic failure. The German Hepatitis C-Registry also reported similar rates of SVR12 (93.7-95.9% in the PP analysis) and loss to follow-up (8.5%-10.2% in PWID with or without OAT)[27].
A meta-analysis performed on post-treatment HCV reinfection rates among people with recent drug use and IDU and those receiving OAT concluded that HCV reinfection rates were higher in the IDU than the OAT group (6.2 vs 3.8/100 person-years), and HCV reinfections developed early post-treatment[28]. Thus, the authors advocated that HCV reinfection should not be the reason to withhold DAA from people with ongoing IDU, but harm reduction services should be integrated into DAA treatment programs to avoid reinfection. Moreover, regular HCV testing to detect early reinfection should be performed to initiate retreatment[28]. To eliminate HCV among PWID, concerted efforts should be made to follow the recommendations for action in a health system framework[25].
Acute kidney injury (AKI) has been reported in 1%-15% of patients receiving SOF-based DAAs[29]. Risk factors for AKI following SOF-based DAAs include baseline stage of chronic kidney disease, presence of ascites and diabetes, and concurrent use of nephrotoxic drugs[29]. Concurrent SOF use increases intracellular tenofovir (TFV) diphosphate concentrations in HIV/HCV-coinfected patients receiving TDF-based ART via inhibition of TDF hydrolysis by SOF[30,31]. There is a positive correlation between the TFV area-under-the-curve concentration and the levels of urine retinol binding protein-4 and beta-2 microglobulin in a dose-dependent manner in HIV/HCV-coinfected patients receiving SOF/LDV[32]. A study reported significant decreases in eGFR in 273 HIV/HCV-coinfected patients receiving SOF/LDV with concomitant use of ART containing TDF-free, non-boosted TDF, or TDF plus boosted PIs, but the eGFR changes were small and reversible at 12 wk off-therapy[33]. No significant renal dysfunction was observed in HIV/HCV-coinfected patients with TDF-based ART or TDF plus boosted PIs in combination with SOF/LDV[34,35]. Our findings and the literature should provide reassurance that eGFR changes are minimal with the current ART and DAAs among PLWH.
Our study had several limitations. First, our study population consisted mainly of PWID who may have poor treatment adherence and a higher rate of loss to follow-up and reinfection, and only a small proportion of our patients had cirrhosis of the liver or were HCV treatment-experienced. Thus, our findings may not be generalizable to other populations. Second, inherently limited by the observational study design, detailed data regarding the types of adverse effects of DAAs, ongoing injection, enrollment into an OAT program, ongoing substance use during DAA, and adherence of the included PLWH were not available. Nevertheless, it has been known that DAAs are well-tolerated[21-23], and none of our included PLWH discontinued DAAs due to adverse effects. The main reason for DAA discontinuation was loss to follow-up. Third, not all (77.9%-91.1%) of the eGFR data during the DAA course, at SVR12, and post-SVR12 were available, which may have compromised our analysis and interpretation of the pre- and post-treatment eGFR changes. Failure to determine the urinary biomarkers precludes us from getting a better understanding regarding the causality of eGFR changes in PLWH who received TFV-based ART with or without concomitant use of SOF-based DAAs. Finally, HCV strains of PLWH with virologic failure were not available for analysis of emergent resistance mutations to provide more insight into retreatment options.
In conclusion, similar to the results in HIV-negative patients with HCV GT6 infections, our PLWH coinfected with HCV GT6 had an SVR12 rate of 96.1% with DAAs in the PP analysis. Small declines of eGFR were observed with DAA initiation in PLWH receiving SOF-based DAAs and TDF- or TAF-based ART, which recovered after the completion of DAA treatment.
Hepatitis C virus (HCV) genotype 6 (GT6) infection is common in Southeast Asia, and its virologic response to direct-acting antivirals (DAAs) in people living with HIV (PLWH) in a large scale is unknown.
The virologic responses of HCV GT6 to DAAs in PLWH will guide national DAA treatment policies for HCV infection in this population.
The study aimed to assess the virologic responses of HCV GT6 to DAAs in PLWH with HCV GT6 infections.
From September 2016 to August 2019, the overall virologic responses at the end of treatment and sustained virologic response 12 wk off-therapy were assessed in the 349 included PLWH with HCV GT6 infections receiving DAAs.
The overall virologic response at end of treatment and sustained virologic response 12 wk off-therapy. SVR12 was 96.8% and 92.3%, respectively, in PLWH with HCV GT6 infections receiving DAAs.
PLWH coinfected with HCV GT6 responded well to DAAs.
National DAA treatment policies of HCV infection in Southeast Asia should take PLWH coinfected with HCV GT6 into consideration.
The authors would like to express their thanks to the staff of National Taiwan University Hospital-Statistical Consulting Unit for statistical consultation and analyses.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Infectious diseases
Country/Territory of origin: Taiwan
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Kamal H S-Editor: Gong ZM L-Editor: Filipodia P-Editor: Wang LYT
| 1. | Basyte-Bacevice V, Kupcinskas J. Evolution and Revolution of Hepatitis C Management: From Non-A, Non-B Hepatitis Toward Global Elimination. Dig Dis. 2020;1-6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 24] [Article Influence: 4.8] [Reference Citation Analysis (1)] |
| 2. | Do A, Reau NS. Chronic Viral Hepatitis: Current Management and Future Directions. Hepatol Commun. 2020;4:329-341. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 26] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 3. | International Agency for Research on Cancer. World Cancer Report [Internet]. 2020 [cited 4 April 2021] Available from: https://www.iarc.who.int/cards_page/world-cancer-report/. |
| 4. | Smith DB, Bukh J, Kuiken C, Muerhoff AS, Rice CM, Stapleton JT, Simmonds P. Expanded classification of hepatitis C virus into 7 genotypes and 67 subtypes: updated criteria and genotype assignment web resource. Hepatology. 2014;59:318-327. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 965] [Cited by in RCA: 980] [Article Influence: 89.1] [Reference Citation Analysis (1)] |
| 5. | Bukh J. The history of hepatitis C virus (HCV): Basic research reveals unique features in phylogeny, evolution and the viral life cycle with new perspectives for epidemic control. J Hepatol. 2016;65:S2-S21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 158] [Cited by in RCA: 176] [Article Influence: 19.6] [Reference Citation Analysis (0)] |
| 6. | Polaris Observatory HCV Collaborators. Global prevalence and genotype distribution of hepatitis C virus infection in 2015: a modelling study. Lancet Gastroenterol Hepatol. 2017;2:161-176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1493] [Cited by in RCA: 1470] [Article Influence: 183.8] [Reference Citation Analysis (0)] |
| 7. | Bunchorntavakul C, Chavalitdhamrong D, Tanwandee T. Hepatitis C genotype 6: A concise review and response-guided therapy proposal. World J Hepatol. 2013;5:496-504. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 20] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 8. | Wu CH, Lee MF, Kuo HS. Distribution of hepatitis C virus genotypes among blood donors in Taiwan. J Gastroenterol Hepatol. 1997;12:625-628. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 9. | Liu JY, Lin HH, Liu YC, Lee SS, Chen YL, Hung CC, Ko WC, Huang CK, Lai CH, Chen YS, Shih YL, Chung HC, Liang SH, Lin JN. Extremely high prevalence and genetic diversity of hepatitis C virus infection among HIV-infected injection drug users in Taiwan. Clin Infect Dis. 2008;46:1761-1768. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 47] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 10. | Lee MH, Hsiao TI, Subramaniam SR, Le AK, Vu VD, Trinh HN, Zhang J, Jin M, Wong VW, Wong GL, Nguyen MH. HCV Genotype 6 Increased the Risk for Hepatocellular Carcinoma Among Asian Patients With Liver Cirrhosis. Am J Gastroenterol. 2017;112:1111-1119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 38] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 11. | Pol S, Parlati L. Treatment of hepatitis C: the use of the new pangenotypic direct-acting antivirals in "special populations". Liver Int. 2018;38 Suppl 1:28-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 34] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 12. | Maughan A, Sadigh K, Angulo-Diaz V, Mandimika C, Villanueva M, Lim JK, Ogbuagu O. Contemporary HCV pangenotypic DAA treatment protocols are exclusionary to real world HIV-HCV co-infected patients. BMC Infect Dis. 2019;19:378. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 13. | Mettikanont P, Bunchorntavakul C, Reddy KR. Systematic review: epidemiology and response to direct-acting antiviral therapy in genotype 6 chronic hepatitis C virus infection. Aliment Pharmacol Ther. 2019;49:492-505. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 14. | Rockstroh JK, Nelson M, Katlama C, Lalezari J, Mallolas J, Bloch M, Matthews GV, Saag MS, Zamor PJ, Orkin C, Gress J, Klopfer S, Shaughnessy M, Wahl J, Nguyen BY, Barr E, Platt HL, Robertson MN, Sulkowski M. Efficacy and safety of grazoprevir (MK-5172) and elbasvir (MK-8742) in patients with hepatitis C virus and HIV co-infection (C-EDGE CO-INFECTION): a non-randomised, open-label trial. Lancet HIV. 2015;2:e319-e327. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 245] [Cited by in RCA: 249] [Article Influence: 24.9] [Reference Citation Analysis (0)] |
| 15. | Rockstroh JK, Lacombe K, Viani RM, Orkin C, Wyles D, Luetkemeyer AF, Soto-Malave R, Flisiak R, Bhagani S, Sherman KE, Shimonova T, Ruane P, Sasadeusz J, Slim J, Zhang Z, Samanta S, Ng TI, Gulati A, Kosloski MP, Shulman NS, Trinh R, Sulkowski M. Efficacy and Safety of Glecaprevir/Pibrentasvir in Patients Coinfected With Hepatitis C Virus and Human Immunodeficiency Virus Type 1: The EXPEDITION-2 Study. Clin Infect Dis. 2018;67:1010-1017. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 105] [Cited by in RCA: 118] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 16. | Li Y, Li L, Liu J, Zhang DW, Zhao F, Wang L, Mahemure A, Xie R, Lei S, Cai W, Wang X, Shu Z, Chen X, Wang H, Wang FS. Tolerable and curable treatment in HIV/HCV co-infected patients using anti-HCV direct antiviral agents: a real-world observation in China. Hepatol Int. 2018;12:465-473. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 17. | Yu ML, Chen PJ, Dai CY, Hu TH, Huang CF, Huang YH, Hung CH, Lin CY, Liu CH, Liu CJ, Peng CY, Lin HC, Kao JH, Chuang WL. 2020 Taiwan consensus statement on the management of hepatitis C: part (I) general population. J Formos Med Assoc. 2020;119:1019-1040. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 64] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 18. | Yu ML, Chen PJ, Dai CY, Hu TH, Huang CF, Huang YH, Hung CH, Lin CY, Liu CH, Liu CJ, Peng CY, Lin HC, Kao JH, Chuang WL. 2020 Taiwan consensus statement on the management of hepatitis C: Part (II) special populations. J Formos Med Assoc. 2020;119:1135-1157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 60] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 19. | Taiwan AIDS Society. Guidelines of HIV testing and treatment, 2020 edition [Internet]. 2020 [cited 4 April 2021] Available from: http://www.aids-care.org.tw/journal/treatment.asp. |
| 20. | Sterling RK, Lissen E, Clumeck N, Sola R, Correa MC, Montaner J, S Sulkowski M, Torriani FJ, Dieterich DT, Thomas DL, Messinger D, Nelson M; APRICOT Clinical Investigators. Development of a simple noninvasive index to predict significant fibrosis in patients with HIV/HCV coinfection. Hepatology. 2006;43:1317-1325. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2633] [Cited by in RCA: 3541] [Article Influence: 186.4] [Reference Citation Analysis (0)] |
| 21. | Mensa FJ, Lovell S, Pilot-Matias T, Liu W. Glecaprevir/pibrentasvir for the treatment of chronic hepatitis C virus infection. Future Microbiol. 2019;14:89-110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 22. | Greig SL. Sofosbuvir/Velpatasvir: A Review in Chronic Hepatitis C. Drugs. 2016;76:1567-1578. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 51] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 23. | Scott LJ. Ledipasvir/Sofosbuvir: A Review in Chronic Hepatitis C. Drugs. 2018;78:245-256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 22] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 24. | Luo A, Xu P, Wang J, Li Z, Wang S, Jiang X, Ren H, Luo Q. Efficacy and safety of direct-acting antiviral therapy for chronic hepatitis C genotype 6: A meta-analysis. Medicine (Baltimore). 2019;98:e15626. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 25. | Day E, Hellard M, Treloar C, Bruneau J, Martin NK, Øvrehus A, Dalgard O, Lloyd A, Dillon J, Hickman M, Byrne J, Litwin A, Maticic M, Bruggmann P, Midgard H, Norton B, Trooskin S, Lazarus JV, Grebely J; International Network on Hepatitis in Substance Users (INHSU). Hepatitis C elimination among people who inject drugs: Challenges and recommendations for action within a health systems framework. Liver Int. 2019;39:20-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 95] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 26. | Macías J, Morano LE, Téllez F, Granados R, Rivero-Juárez A, Palacios R, Ríos M, Merino D, Pérez-Pérez M, Collado A, Figueruela B, Morano A, Freyre-Carrillo C, Martín JM, Rivero A, García F, Pineda JA; HEPAVIR group from the Sociedad Andaluza de Enfermedades Infecciosas (SAEI) and the GEHEP group from the Sociedad Española de Enfermedades Infecciosas y Microbiología (SEIMC). Response to direct-acting antiviral therapy among ongoing drug users and people receiving opioid substitution therapy. J Hepatol. 2019;71:45-51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 47] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 27. | Christensen S, Buggisch P, Mauss S, Böker KHW, Schott E, Klinker H, Zimmermann T, Weber B, Reimer J, Serfert Y, Wedemeyer H. Direct-acting antiviral treatment of chronic HCV-infected patients on opioid substitution therapy: Still a concern in clinical practice? Addiction. 2018;113:868-882. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 63] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 28. | Hajarizadeh B, Cunningham EB, Valerio H, Martinello M, Law M, Janjua NZ, Midgard H, Dalgard O, Dillon J, Hickman M, Bruneau J, Dore GJ, Grebely J. Hepatitis C reinfection after successful antiviral treatment among people who inject drugs: A meta-analysis. J Hepatol. 2020;72:643-657. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 123] [Article Influence: 24.6] [Reference Citation Analysis (0)] |
| 29. | Dashti-Khavidaki S, Khalili H, Nasiri-Toosi M. Potential nephrotoxicity of sofosbuvir-based treatment in patients infected with hepatitis C virus: a review on incidence, type and risk factors. Expert Rev Clin Pharmacol. 2018;11:525-529. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 17] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 30. | MacBrayne CE, Marks KM, Fierer DS, Naggie S, Chung RT, Hughes MD, Kim AY, Peters MG, Brainard DM, Seifert SM, Castillo-Mancilla JR, Bushman LR, Anderson PL, Kiser JJ. Effects of sofosbuvir-based hepatitis C treatment on the pharmacokinetics of tenofovir in HIV/HCV-coinfected individuals receiving tenofovir disoproxil fumarate. J Antimicrob Chemother. 2018;73:2112-2119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 31. | Brooks KM, Castillo-Mancilla JR, Blum J, Huntley R, MaWhinney S, Alexander K, Kerr BJ, Ellison L, Bushman LR, MacBrayne CE, Anderson PL, Kiser JJ. Increased tenofovir monoester concentrations in patients receiving tenofovir disoproxil fumarate with ledipasvir/sofosbuvir. J Antimicrob Chemother. 2019;74:2360-2364. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 32. | Chan A, Park L, Collins LF, Cooper C, Saag M, Dieterich D, Sulkowski M, Naggie S. Correlation Between Tenofovir Drug Levels and the Renal Biomarkers RBP-4 and ß2M in the ION-4 Study Cohort. Open Forum Infect Dis. 2019;6:ofy273. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 33. | Soeiro CASP, Gonçalves CAM, Marques MSC, Méndez MJV, Tavares APRA, Horta AMLMFCA, Sarmento-Castro RMDR. Glomerular filtration rate change during chronic hepatitis C treatment with Sofosbuvir/Ledipasvir in HCV/HIV Coinfected patients treated with Tenofovir and a boosted protease inhibitor: an observational prospective study. BMC Infect Dis. 2018;18:364. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 8] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 34. | Bhattacharya D, Belperio PS, Shahoumian TA, Loomis TP, Goetz MB, Mole LA, Backus LI. Effectiveness of All-Oral Antiviral Regimens in 996 Human Immunodeficiency Virus/Hepatitis C Virus Genotype 1-Coinfected Patients Treated in Routine Practice. Clin Infect Dis. 2017;64:1711-1720. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 72] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 35. | Taramasso L, Ricci E, Celesia BM, Bonfanti P, Quirino T, Squillace N, Nicolini LA, Maggi P, Martinelli C, De Socio GV, Di Biagio A; on behalf CISAI Study Group. Co-administration of tenofovir plus protease inhibitor based antiretroviral therapy during sofosbuvir/ledipasvir treatment for HCV infection: Much Ado About Nothing? Clin Res Hepatol Gastroenterol. 2017;41:e76-e79. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.8] [Reference Citation Analysis (0)] |