Published online Mar 21, 2022. doi: 10.3748/wjg.v28.i11.1123
Peer-review started: October 15, 2021
First decision: December 12, 2021
Revised: December 24, 2021
Accepted: February 20, 2022
Article in press: February 20, 2022
Published online: March 21, 2022
Processing time: 152 Days and 9 Hours
Rectal neuroendocrine neoplasms (r-NENs) are considered among the most frequent digestive NENs, together with small bowel NENs. Their incidence has increased over the past few years, and this is probably due to the widespread use of endoscopic screening for colorectal cancer and the advanced endoscopic procedures available nowadays. According to the current European Neuroendocrine Tumor Society (ENETS) guidelines, well-differentiated r-NENs smaller than 10 mm should be endoscopically removed in view of their low risk of local and distant invasion. R-NENs larger than 20 mm are candidates for surgical resection because of their high risk of distant spreading and the involvement of the muscularis propria. There is an area of uncertainty regarding tumors between 10 and 20 mm, in which the metastatic risk is intermediate and the endoscopic treatment can be challenging. Once removed, the indications for surveillance are scarce and poorly codified by international guidelines, therefore in this paper, a possible algorithm is proposed.
Core Tip: Rectal neuroendocrine neoplasms (r-NENs) represent 1%-2% of all rectal tumors, but an increasing incidence rate has been recently reported. Up to 90% of r-NENs are < 10 mm in size, confined to the submucosa, and well-differentiated, so that the endoscopic resection represents their gold-standard therapy. Advanced metastatic NENs are usually poorly differentiated G3 carcinomas treated with chemotherapy, except for rare G1-G2 neoplasms, which can be targeted with less aggressive systemic options. Locally advanced r-NENs invading submucosal layers, with a ≥ 10 mm diameter, but still without distant metastases, represent the real therapeutic challenge among rectal tumors.
- Citation: Gallo C, Rossi RE, Cavalcoli F, Barbaro F, Boškoski I, Invernizzi P, Massironi S. Rectal neuroendocrine tumors: Current advances in management, treatment, and surveillance. World J Gastroenterol 2022; 28(11): 1123-1138
- URL: https://www.wjgnet.com/1007-9327/full/v28/i11/1123.htm
- DOI: https://dx.doi.org/10.3748/wjg.v28.i11.1123
The gastrointestinal tract is the most frequent site for the onset of neuroendocrine neoplasms (NENs). NENs are a heterogeneous group of epithelial neoplasms, ranging from indolent well-differentiated neuroendocrine tumors (NETs) to very aggressive poorly differentiated neuroendocrine carcinomas (NECs). They can arise virtually from any organ or system in the human body. The rectum is the second localization by frequency after the small intestine[1] and rectal NENs (r-NENs) represent 12%–27% of all gastrointestinal NENs[2,3]. On the other hand, 1%–2% of all rectal tumors are neuroendocrine. An increasing incidence of r-NENs has been reported over the past few years, as illustrated in The Surveillance, Epidemiology, and End Results (SEER) database[1], and the same trend was confirmed in the German registry[4], as well as in the Asian registers[5], even if the highest incidence rate (IR) was reported in the United States, where IR was approximately 1.1 per 100000 population and increased tenfold between 1970 and 2000s[6]. In Europe, the IR was lower when compared to the SEER database, with the highest IR reported in Norway (0.25 per 100000 population) and the lowest in Austria[6]. This is probably due to an underreporting of the disease because of a lack of national registries.
The increasing incidence is related to the increased participation in screening colonoscopy programs. Even if there are limited data on the r-NENs diagnosed through colorectal cancer (CRC) screening programs, in a Polish screening CRC program cohort of 50148 participants, a prevalence of r-NENs of 0.05%-0.07% was reported[7]. Similarly in another English study, the diagnosis rates of NENs identified in the English bowel cancer screening program were 29 rectal and 18 colonic per 100000 colonoscopies, accounting for a prevalence of 0.047%[8].
Despite their increasing incidence, r-NENs are not yet sufficiently recognized by endoscopists in Western countries, since a recent study demonstrated that the neuroendocrine nature was suspected for only 18% of neuroendocrine lesions[9].
Suspicion of rNENs before their resection is clinically very important, given the fact that it significantly drives the decision on how to treat them. In fact, it has been reported that patients whose rNENs were diagnosed or suspected before resection showed a much higher complete resection rate than those whose tumors were resected as polyps and then diagnosed[10].
In the increasingly vast panorama of artificial intelligence, its application to gastrointestinal endoscopy may help in detecting and diagnosing r-NENs, thus leading to a further increase in incidence but in parallel to a more effective therapeutic approach.
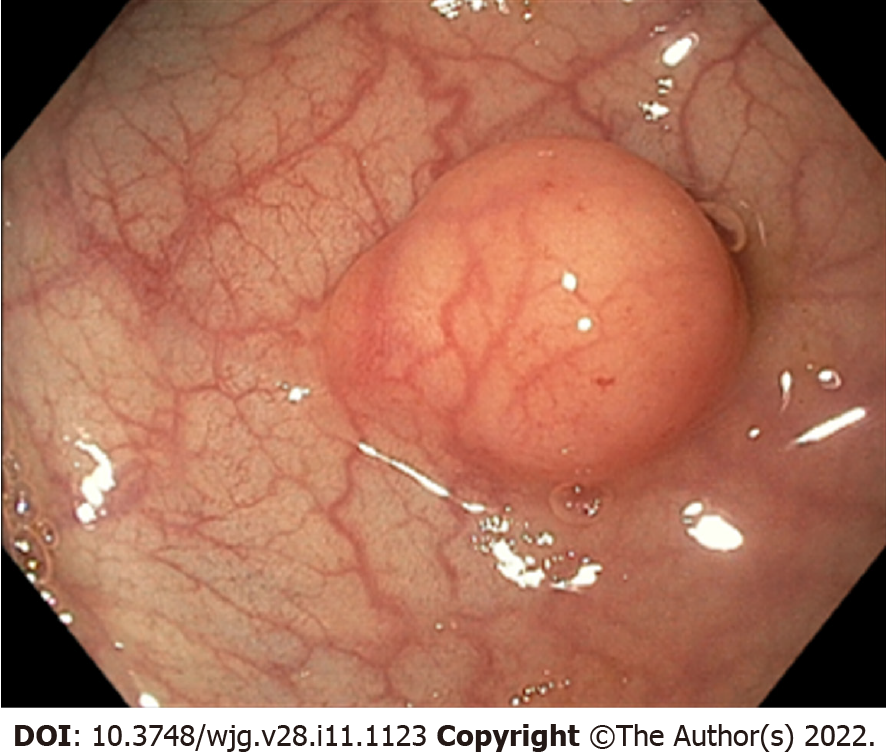
Most of r-NENs are asymptomatic and they are diagnosed incidentally during endoscopic evaluation for CRC screening or unrelated gastrointestinal symptoms. Less frequently, r-NENs may present with anal discomfort, rectal bleeding, and change in bowel habits[11]. Most r-NENs, which arise from the neuroendocrine epithelial cells, appear as small, round polypoid lesions characterized by a smooth, normal-appearing, or yellow-discolored mucosa[12], with round shape pit pattern, type I on Kudo classification, and invisible vessels, as described by Sano as type I[13] (Figure 1). R-NENs are usually located about 4 to 10 cm above the dentate line, on the frontal or lateral wall of the mid-rectum[14]. Up to 90% of r-NENs, at the time of the diagnosis, are well-differentiated epithelial lesions, less than 10 mm in size[15], usually developing towards the submucosal layer, but without invading the muscularis propria layer. However, atypical endoscopic findings (including semi-pedunculated appearance, hyperemia, central depression, erosion, and ulceration) have been reported for r-NEN exceeding 5 mm in diameter[14]. In addition, it has been recently reported that virtual chromoendoscopy with NBI, showing an absence of pit pattern with large amorphous areas (Kudo V), may be of value in detecting invasive r-NENs[13]. Unfortunately, the macroscopic appearance of r-NENs resembles that of hyperplastic or adenomatous polyps, making the differential diagnosis from other polypoid lesions challenging, and often the diagnosis is established at pathological examination after routine snare polypectomy or mucosectomy[16].
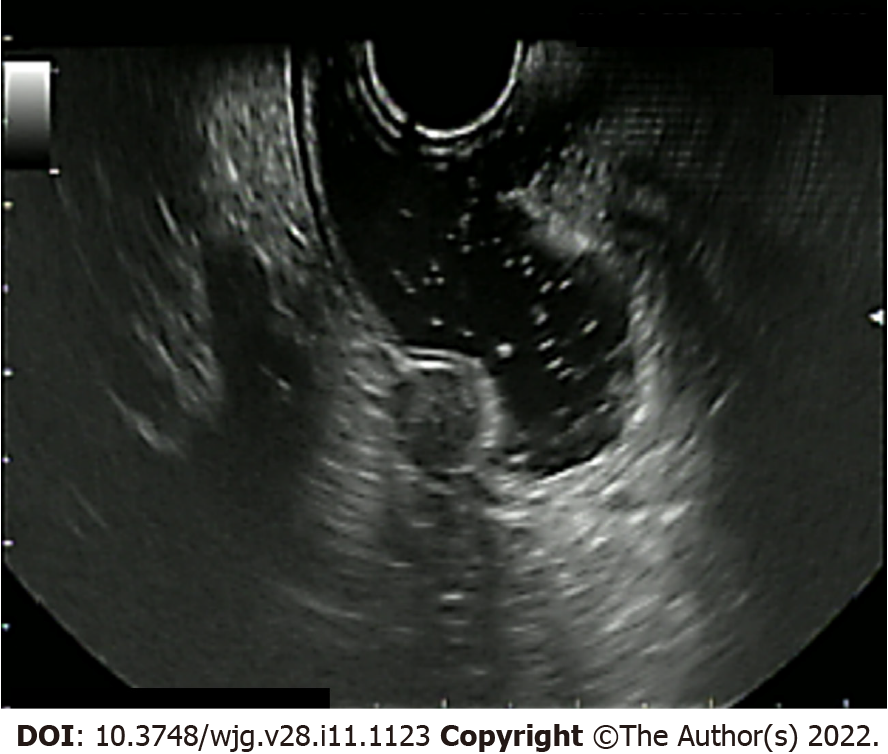
Moreover, the expanding use of artificial intelligence in endoscopy with computer-aided softwares could help the detection and characterization of polypoid lesions including r-NENs[17]. In the last years, EUS has extended the role of endoscopic evaluation of rectal NENs and it proved to help defining accurately the tumor size, the depth of invasion, and the presence of pararectal nodal metastases[18]. On EUS, rectal NETs usually appear as well-defined, hypoechoic lesions, located in the second and third wall layer[19] (Figure 2). In addition, EUS evidence of lobulated forms, irregular margins, and echogenic foci, may predict a higher grade of malignancy[20].
The risk factors associated with r-NENs are not fully clear given the overall low incidence rate of these tumors and the consequent scarcity of large epidemiological studies on rare cases. R-NENs include a heterogeneous group of neoplasms that are not completely indolent, as they used to be traditionally considered, but they are characterized by a risk of metastatic disease ranging from 3% to 60%. The identification of specific subgroups that are at increased risk of developing r-NENs is, therefore, of extreme relevance for early detection and removal of these neoplasms. According to a large Asian retrospective study[21], higher levels of cholesterol and ferritin, the presence of metabolic syndrome, and a family history of cancer were associated with an increased prevalence of r-NENs. Ko et al[22] observed that higher fasting plasma glucose and hypertriglyceridemia, younger age, and a previous history of malignancies were significantly associated with an increased risk of r-NENs. According to a retrospective cross-sectional study that compared the risk factors for 101 cases of r-NENs found during screening colonoscopies, male gender and low high-density lipoprotein cholesterol levels were significantly associated with these neoplasms[23].
As metabolic syndrome appears to be a recognized risk factor for the development of r-NENs, this might partially explain the increasing incidence of these tumors together with the improvement in screening colonoscopy. The actual mechanism underlying the association between metabolic risk factors and r-NENs is still unclear; however, it might be possible that insulin resistance represents a risk factor in r-NEN pathogenesis, also considering that insulin and insulin-like growth factors have been associated with cell proliferation, differentiation, and apoptosis[24].
Several predictors of metastases, progression, and/or recurrence of r-NENs have been proposed; however, the relevance of each of these factors has not been clearly established and clear-cut prognostic factors which might help in the management of these neoplasms have not been yet incorporated in current guidelines. Table 1 summarizes available prognostic factors.
| Prognostic factor | Cut-off | Risk of metastases | Evidence |
| Tumor size | 15 mm | 14% | Retrospective large study[27] |
| Grading | G2 | 50% | Retrospective study[30] |
| G3 | 33% | Retrospective study[30] | |
| Lymph node status | N2 (≥ 5 positive lymph nodes) | Not quantified | Retrospective study[35] |
| Depth of invasion | Invasion of the muscolaris propria | Not quantified | Retrospective study[37] |
The most important factor predicting aggressive disease is the size of the primary tumor, with lesions > 20 mm being metastatic in 60%-80% of the cases[25]. On the other hand, patients with r-NENs measuring 10-19 mm develop synchronous or metachronous metastases in 4%-20% of the cases[25]. According to some large series, the optimal size cut-off to predict the risk of metastases is 15 mm[26-28]; in detail, Concors et al[28], in their study assessing a total of 4893 r-NENs patients identified in the National Cancer Database, observed that tumors larger than 15 mm are associated with a higher risk of distant metastasis, even if one should keep in mind that local and distant metastases might occur also in lesions < 15 mm, thus suggesting that the tumor’s behavior is not influenced only by the size. As recently highlighted by Capurso et al[29], size alone has limited accuracy, as 26% of patients with stage IV and 16% with G3 neoplasm have a primary tumor ≤ 10 mm; instead, both staging and grading could accurately predict r-NEN prognosis. According to this study, the ENETS TNM staging accurately predict prognosis in patients with r-NENs, as stage IV was associated with a worse overall survival (OS) (hazard ratio [HR] = 8.16; P = 0.002) and progression-free survival (PFS) (HR = 14.26; P < 0.0001).
As concerned tumor grading, r-NENs are also classified according to the WHO 2019 classification of digestive system tumors[30], which divided NEN into NETs and NECs based on their molecular differences. NETs are then graded based on the proliferation index (mitotic count and Ki67-related proliferation index) and divided into three groups (NET G1, NET G2, and NET G3), while NECs are by definition high-grade neoplasms. There is growing evidence that, besides the diameter of the primary neoplasm, the tumor grade might play a relevant role in the development of metastases. In a recent retrospective study including 98 patients with r-NENs[31], characterized by a proportion of metastatic disease of 12%, patients with G2 or G3 tumors, regardless of size, were found to be at high risk for the development of metastases; only in G1 tumors, the size (i.e., > 20 mm) was found to be an important predictor of aggressive behavior. Therefore, the authors concluded that grade is a dominant risk factor for metastasis in small and diminutive r-NENs. Again Capurso et al[29] confirmed that, besides stage, grade could predict prognosis in r-NENs, with G3 tumors being associated with a worse OS (HR = 15.57; P = 0.0004) and PFS (HR = 6.42; P = 0.0007).
Other important prognostic factors explored in the literature are: The depth of invasion (with involvement of the muscularis propria), the presence of lymphovascular invasion and/or perineural invasion, the presence of regional nodal metastasis, atypical histology (including anaplasia, frequent mitotic cells, cellular pleomorphism, and mucin production), and mitotic rate, all of which have been suggested as possible prognostic factors for aggressive behavior[32], although evidence is elusive. To date, in fact, it is not yet clear whether these can be considered as independent risk factors[28,33].
The lymph node status is considered as a relevant prognostic factor and current guidelines have always classified r-NENs as N0 vs N1 according to the presence or absence of metastatic lymph nodes, respectively[34,35]. As reported in a recent study[36], which included 687 patients with r-NENs retrieved from the National Cancer Database, there is a significant difference in survival among patients with zero positive lymph nodes (N0), 1 to 4 positive lymph nodes (N1), and ≥ 5 positive lymph nodes (N2), which might suggest a new nodal staging system to provide a more accurate prognosis.
Furthermore, these findings might highlight the importance of an adequate lymphadenectomy in this specific setting, even if further studies are warranted to help quantify the optimal number of lymph nodes that need to be examined.
Finally, it should be underlined that the evaluation of lymphovascular invasion may frequently suffer from inter-observer variations[37].
Fahy et al[38], in a study including 70 r-NENs, reported that tumor size, depth of invasion, presence of lymphovascular invasion, and mitotic rate all correlated with poor outcome and generated a carcinoid of the rectum risk stratification (CaRRS) score based on these factors. The score was reliably correlated with recurrence-free survival and disease-specific survival, and it was based on histopathologic variables which might be assessed at biopsy and might guide the treatment strategy.
In summary, according to the ENETS 2016 Consensus Guidelines and the Union for International Cancer Control/American Joint Committee on Cancer (UICC/AJCC) indications, the size of the primary tumor, considering a potential more updated cut-off of 15 mm, and the degree of differentiation with G2 and, indeed, G3 tumors, are considered to be the main independent predictors of a dismal prognosis in r-NENs[39,40]. The number of positive nodes considering ≥ 5 positive lymph nodes as a cut-off and the depth of invasion with the invasion of the muscolaris propria are also associated with a poor prognosis[40].
Further studies are needed to determine whether more aggressive surgical approaches, as well as standardized follow-up protocols, might be beneficial in this specific subgroup of neoplasms.
The therapeutic approach of R-NENs depends on whether a localized, locally advanced, or advanced metastatic disease is present.
Due to the progressive greater awareness of these neoplasms, the improvement of endoscopic technology, and the increasingly adequate training of endoscopists, 90%-100% of r-NENs are to date detected with a diameter ≤ 10 mm. These lesions are usually intramucosal and nearly every ≤ 10 mm intramucosal r-NEN is diagnosed as a completely resected incidental tumor. Less than 10 mm NENs have a low risk of both lymphatic invasion and distant metastases, nearly 0.7% and less than 2%, respectively. Endoscopic ultrasound (EU) is highly recommended to exclude this remote occurrence, and to choose the best-suited type of endoscopic resection and its feasibility[39]. Even if, to date, not all studies unequivocally affirm that the en bloc resection of small r-NENs is associated with a statistically significant increase in OS, complete mini-invasive endoscopic en bloc resection still represents the therapeutic aim for these lesions. A wide variety of endoscopic procedures have been traditionally used to resect r-NENs.
Standard polypectomy: Standard polypectomy is performed by using a hot or cold snare. This kind of polypectomy does not guarantee a sufficient complete resection rate of the lesion margins, thus, this technique is no more recommended to treat r-NENs[39].
Endoscopic mucosal resection: Conventional endoscopic mucosal resection (EMR) consists of a snare resection of the lesion, which is usually lifted by the submucosal injection of saline solution, in order to guarantee a lower risk of wall perforation and incomplete margin resection, especially in case of sessile lesions[41]. It is a safe, cost-effective, and technically easy procedure, but it results in a remarkable rate of piece-meal resection, and, thus, a high rate of incomplete removal. According to the most reliable evidence, in fact, conventional EMR only leads to 56%-59% of R0 resections in ≤ 10 mm r-NENs[26,42]. Moreover, a submucosal fluid injection may paradoxically flatten or even depress loose connective tissue lesions, increase the tissue tension, and even displace the lesion to a hardly accessible different localization.
The underwater EMR (U-EMR) technique was inspired by the observation that the mucosa and submucosa float apart from the muscular layer when colon air is removed from the lumen and it is replaced with water. By doing so, the colon lumen gets less outstretched, it gives birth to a pseudopedicle, and a larger mucosal surface can be captured. U-EMR has been shown to be effective in removing en bloc > 20 mm lesions; a multicenter randomized controlled trial comparing conventionally injection-assisted and U-EMR in 219 15-70 mm colorectal laterally spreading tumors demonstrated a significantly higher en bloc resection rate for U-EMR (51% vs 25%, P = 0.001). To date, no comparative studies are available on the en bloc resection efficacy between conventional EMR and U-EMR for < 20 mm r-NENs[43].
According to the largest samples available so far, EMR performed with a suction cap, which represents another modified EMR technique (m-EMR), reaches an en bloc resection success rate of 100% and a histological complete resection rate of 93% for ≤ 10 mm rNENs[44].
The circumferential incision m-EMR (CI-EMR) technique, first described as an option technique for CRC, performed by marking dots by argon plasma coagulation (APC) around the lesion and lifting only the mucosal layer from the muscularis propria with a mixture of glycerin fructose and methylene blue, similarly guarantees an en bloc resection rate and a complete resection rate of 97% and 94%, respectively[45]. If an elastic band is placed around the rectal lesion in addition to its cap-assisted suction and its injection-mediated lifting, an even higher rate of complete resection can be guaranteed: In a prospective Korean study enrolling 77 patients who underwent a ligation-assisted m-EMR (L-EMR) of a ≤ 10 mm r-NEN, 100% of them achieved an en bloc histologically complete resection of the lesion[46]. The m-EMR technique performed with a double-channel gastroscope rather proved to be less effective in terms of complete resection rate and it was associated with a higher rate of adverse events[47].
Pooled data deriving from a systematic review and meta-analysis including 11 studies for a total of 811 patients who underwent endoscopic treatment by EMR or any m-EMR of ≤ 10 mm localized r-NENs limited to the mucosal layer, demonstrated a statistically significant higher rate of en bloc endoscopic removal (odds ratio [OR] = 0.13, 95% confidence interval [CI]: 0.02-0.74, P = 0.02) and complete histological resection (OR = 0.23, 95%CI: 0.10-0.51, P < 0.01) for m-EMR when compared to conventional EMR. The safety of the different procedures proved to be equal[48]. However, it is interesting to notice that, according to a multivariate analysis including 277 any size r-NENs treated by conventional EMR (243 of them), dual-channel EMR, and CI-EMR (for a total of 44 m-EMRs), the histological complete resection rate was similar among the techniques, proving that the tumor size influences the histological complete resection regardless of the endoscopic treatment modality[49].
Whatever the endoscopic mucosal resection technique used, the EMR area should be always marked after the rectal lesion resection, in order to facilitate future salvage therapy in case that the histological examination of the tumor margins does not result in complete resection.
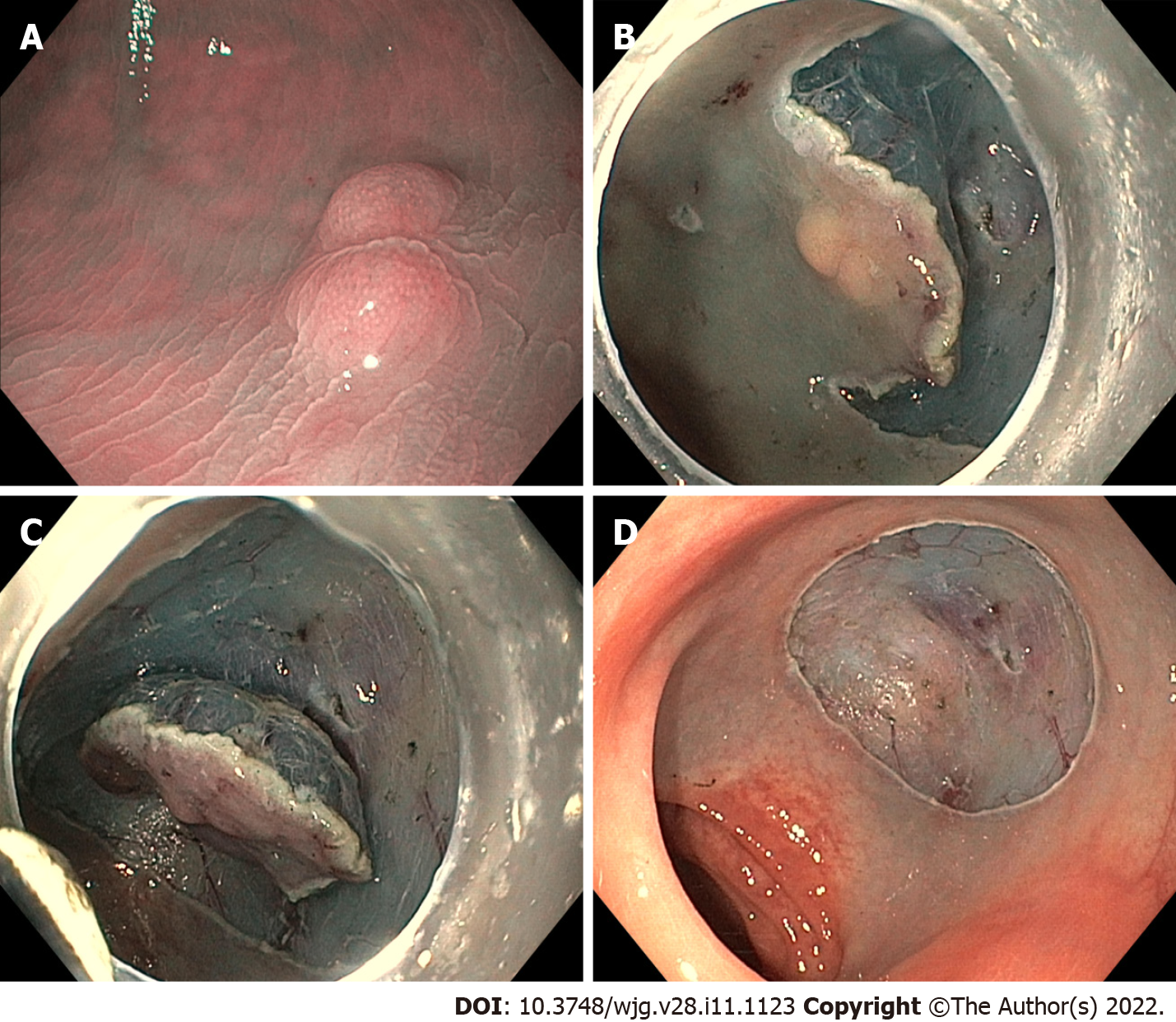
Endoscopic submucosal dissection: Endoscopic submucosal dissection (ESD) consists of a delimitation of a circumferential excision zone by using an electrocauterization knife around the lesion, followed by the creation of a cushion beneath the lesion by the injection of saline solution, or better of 0.4% sodium hyaluronate diluted with the same volume of normal saline solution[50] and, thus, by the execution of a dissection underneath the submucosal layer under direct visualization[37] (Figure 3). It represents a technically difficult procedure, requiring endoscopists’ specific training and with a long learning curve[51]. It also requires a longer execution time and is associated with a higher peri-procedural adverse event rate (especially bleeding and perforation) compared to EMR, but no significant differences were demonstrated between ESD and any m-EMR technique in terms of operating time for r-NENs[52].
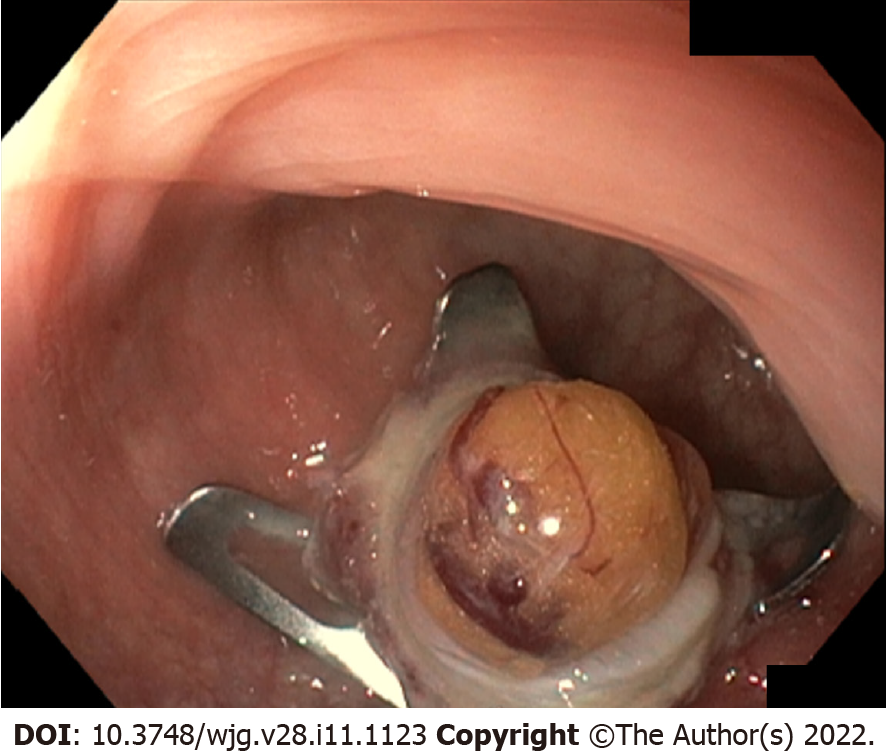
Sometimes even ESD may lead to incomplete resection and thus persistent positive vertical margin, due to deep submucosal invasion of tumor cells. In these situations, other endoscopic resection techniques may overcome the problem: The full thickness resection device (FTRD) Ovesco (Ovesco, Tubingen, Germany) is an over-the-scope clip (OTSC) consisting of a clip and a snare both preloaded on an applicator cap. The FTRD system allows endoscopic full-thickness resection, providing at the same time the closure of the wall defect; it has been shown to be a good option in case of subepithelial lesions (Figure 4).
A broad systematic review and meta-analysis including ten retrospective studies and 650 patients undergoing r-NEN resection, demonstrated a significantly higher complete resection rate in the ESD group compared with the EMR group (relative risk [RR] = 0.89, 95%CI: 0.79-0.99), but a comparable complete resection rate between the ESD group and the m-EMR group (RR = 1.03, 95%CI: 0.95-1.11)[52]. Remarkably, a comparative observational study on 115 patients did not demonstrate statistical significance between ESD and U-EMR in terms of R0 on < 10 mm G1 lesions not invading the muscularis propriae (ESD vs U-EMR 86.1%R0 vs 86.1%R0, P = 0.996)[53]. It should be emphasized, however, that EMR often presupposes a piecemeal resection, while ESD guarantees an en bloc resection, thus representing a possible bias in the definition of complete histological resection R0. As a matter of fact, U-ESD has been already proposed as a promising modified ESD technique[54]. Furthermore, another systematic review and meta-analysis including 25 studies for 1094 patients, reported L-EMR and ESD to be the most effective endoscopic techniques in guaranteeing histological complete resection of ≤ 10 mm r-NENs (94.8% and 89.6% for L-EMR and ESD R0 vs 59.1% and 72.4% for polypectomy/EMR and CI-EMR R0, respectively)[26]. Interestingly, an additional meta-analysis regrouping seven studies for a total of 386 patients, proposed L-EMR to be the most suitable technique for endoscopic resection of ≤ 10 mm r-NENs as far as it proved to achieve a higher R0 rate than ESD (OR = 4.08, 95%CI: 2.42-6.88, P < 0.00001), to take significantly less operative time (SMD: -1.59, 95%CI: -2.27 to -0.90, P < 0.00001), and to be associated with no significantly higher complications rate (OR = 0.56, 95%CI: 0.28-1.14, P = 0.11)[55]. Conversely, one study reported that ESD, in comparison to m-EMR, achieved a higher R0 resection rate (100% vs 70%) and lower recurrence (0% vs 17%) in 55 patients with 10-16 mm r-NENs. Thus, ESD appears to be the most appropriate endoscopic technique for the resection of > 10 mm r-NENs[25]. Both m-EMR and ESD were demonstrated to be feasible also for salvage treatment in incomplete primary endoscopic resection, especially for ≤ 10 mm r-NENs[56].
What has been revealed so far must always consider that comparative studies between endoscopic resection techniques are heterogeneous, and limited in samples and volume, with limited follow-up time. To date, a Chinese open-label double-arm randomized clinical trial comparing cap-assisted EMR to ESD for the treatment of rectal NENs less than 10 mm is about to start the recruiting phase (NCT03982264).
Locally advanced r-NENS, which are neoplasms invading submucosal layers, conventionally with a lateral spreading diameter ≥ 10 mm, but still without distant metastatic invasion, represent the real frontier of radical removal in the field of rectal tumors. They are associated with a moderate metastatic frequency rate of 5%−15% and with a moderate risk of lymphatic involvement[57], and thus, a long debate on the most appropriate resection technique is nowadays still ongoing. According to the ENETS and UICC/AJCC guidelines, rectal NENs measuring 10−19 mm at their first endoscopic diagnosis, should be investigated in detail to exclude an invasion of the muscularis mucosae and of the locoregional lymph nodes. Rectal magnetic resonance imaging (MRI) and/or rectal endoscopic ultrasound (r-EUS) represent the most reliable investigating techniques for staging rectal lesions: If the rectal lesion is limited to the mucosa or submucosa, which corresponds to a T1 lesion, regardless of its lateral spreading > 10 mm, the only local resection treatment (may it be endoscopic or transanal) has proved to be effective in guaranteeing a radical resection and a very limited recurrence rate over follow-up[58]. Radical surgical resection for 10-19 mm r-NENS without muscularis or lymphatic invasion does not relate to a higher rate of radical resection and radically reduces the patients’ quality of life[59].
Contrariwise, T2 or N+ stage lesions should be accurately studied by total body imaging such as 68-Ga-DOTATATE-PET and CT scanning. Once distant metastases are excluded, a surgical radical attempt by laparoscopic low anterior resection (LAR) with or without total mesorectal excision (TME), or even abdomino-perineal resection should be preferred. It has to be noted that, according to the latest European and American guidelines, rectal tumors above 20 mm are automatically considered as T2 stage lesions, as far as they are related to an up to 80% rate of distant metastases.
Transanal endoscopic microsurgery: Transanal endoscopic microsurgery (TEM) is a minimally invasive surgical technique that allows the local full-thickness excision of rectal lesions localized up to 20 cm from the anal verge, avoiding the segmental surgical resection[60]. TEM is usually performed under general anesthesia, in the lithotomy or clasp knife position; it implies the use of anal retractors to dilate the anal sphincter and to maintain exposure[61]. It requires a multi-channel transanal device, which combines the use of a rigid rectoscope with the magnified tridimensional vision. The device is able to control the endoluminal pressure, in order to precisely follow the electrocautery dots previously performed to delimitate the scheduled operating area[37]. Normal saline solution is injected into the submucosal plane with an injector syringe to create a visible submucosal cushion for the elevation of the lesion; the tumor then is excised with electrocautery or an ultrasonic knife under direct vision, then the wound can be closed with absorbable sutures[61].
R-NENs between 10 and 20 mm, limited to the submucosal layer, and without lymphatic invasion can be either approached with a complete en bloc endoscopic resection through ESD or with a complete microsurgical excision through TEM.
Both ESD and TEM demonstrated to be superior to m-EMR for > 10 mm r-NENS, but the two of them have not been appropriately compared in prospective observational studies yet[62]. According to single centers experiences, TEM implies a longer operative time than ESD and a major incidence of anesthesia-related adverse events (such as acute retention of urine); moreover, anal dilation or retraction may cause higher postoperative morbidity[63]. On the other hand, TEM provides deeper vertical resection margins in a full-thickness fashion[63]. Furthermore, in case of local recurrence, the existence of fibrosis in the submucosal layer may represent a limit for the mucosal lifting during ESD and thus may reduce the complete en bloc resection success rate[64].
Hence, according to these limited experiences, ESD might be preferred to TEM in case of a first incidental 10-19 mm r-NEN, based on its lower morbidity rate, shorter operative time and, consequently, lower costs; TEM on the contrary might be the first therapeutic choice of scar embedded recurrent 10-19 mm r-NENs located within the first 20 cm of the rectum from the anal verge. Overall, to date, the indication to perform one of the two proposed techniques still depends more on the team expertise rather than on robust superiority evidence[63]. Prospective comparative studies should be launched to better define the treatment algorithm of resectable 10-19 mm r-NENs.
Salvage TEM for incomplete endoscopic resection proved to be effective also in case of > 10 mm lesions[65].
Conventional transanal resection (TAR) represents the exact equivalent to TEM for < 20 mm r-NENs localized within 6 cm from the anal verge.
Low anterior resection or intersphincteric resection, with or without TME: R-NENs extended to the muscularis mucosae or those with lymph node involvement, as previously mentioned, are indicated for surgical resection. Low anterior resection (LAR) and intersphincteric resection, with appropriate lymph-node resection through TME, represent the most commonly performed laparoscopic techniques, which are chosen depending on the localization of the r-NEN. Either laparoscopic LAR or intersphicteric resection often implies a temporary protective ileostomy[66]. Anal preservation, if possible, is mandatory, as far as it is associated with a much higher quality of life. Fortunately, the anal localization of rectal neoplasms is rare, so that anal preservation is statistically guaranteed in most cases.
In the case of anus involvement, abdominoperineal resection with definitive colostomy represents the most appropriate surgical technique.
As previously mentioned, only around 10% of r-NENs measure > 10 mm at the diagnosis, so it can be assumed that r-NENs are diagnosed as an advanced metastatic disease in a low number of cases[15]. While most r-NETs are localized, their management should be tailored depending on the presence or absence of metastases-predicting factors, including tumor size, endoscopic aspect, T stage, grade, and lymphovascular invasion, as explained before. Endoscopic ultrasonography is the most relevant technique for locoregional assessment[37]. Moreover, it has been recommended to perform pelvic MRI (otherwise, CT scan) as part of the initial workup to evaluate loco-regional spreading and additional explorations in all cases of high-risk r-NENs (i.e., the ones that show factors predicting aggressive disease, see above): They should include contrast-enhanced thoracic-abdominal-pelvic CT scan, liver MRI – especially diffusion-weighted sequences – which has a better sensitivity than CT scan and somatostatin-receptor isotopic imaging (scintigraphy or positron-emitting tomography)[37]. In the presence of advanced disease with distant metastases, a systemic approach represents the first-line therapeutic strategy. In this context, the goal of systemic therapy is disease control and palliation of symptoms. In fact, none of the up-to-date available systemic treatments yet provided a radical cure for r-NENs, but rather a disease progression-free stabilization with variable duration, depending on the different prognostic factors. Given the fact that r-NENs are usually non-functional, systemic therapies are usually used for their antiproliferative effect rather than for their symptom palliation effect. There are several systemic therapeutic options for advanced r-NENs, not substantially different from the ones for other NENs, although studies evaluating specific response rates in r-NENs are limited. Finally, the potential advantage of palliative surgery, either as primary tumor resection or debulking surgery, is still controversial.
Somatostatin analogs: The use of somatostatin analogs (SSAs), mainly lanreotide and octreotide, is the standard first-line therapy in both functioning and non-functioning NENs, arising from both the gastrointestinal and pancreatic tract[67,68]. However, these studies collected only a few cases of r-NENs and none of these studies were focused specifically on r-NENs; in particular, only in the CLARINET study, 14 patients with hindgut NENs were included[68], demonstrating a significantly prolonged PFS (HR = 0.47; 95%CI: 0.30-0.73) in lanreotide treated patients; the treatment of r-NENs with SSAs is thus based on a very low number of patients.
Octreotide long-lasting release is used at a dose of 30 mg every 4 wk i.m. and lanreotide auto-gel is used at a dose of 120 mg every 4 wk, injected subcutaneously. They are traditionally very well-tolerated drugs[69]. A tumor gross-size reduction with SSAs was achieved in no more than 10% of the cases[69].
A superiority trial comparing octreotide and lanreotide is today active (NCT03289741).
Interferon-alpha: Interferon-alpha (INF-α) has been also approved as a symptomatic therapeutic option, especially in addition to SSAs for refractory syndromes[70].
It can be used in advanced NENs as an anti-proliferative option whenever other traditional anti-proliferative systemic drugs are not feasible[71]. An open-label single-arm interventional pharmaco-immunological study has recruited patients with histologically proved NETs to evaluate the potential immunomodulatory synergy of the association of metronomic cyclophosphamide and IFN-α (NCT02838342); no preliminary results have been posted so far.
Cases of r-NENs included in these studies are sporadic.
Targeted agents (everolimus and sunitinib): Based on the results of two randomized, double-blind, prospective, placebo-controlled studies, the mTOR inhibitor everolimus has been used in advanced pancreatic (RADIANT-3) and extra-pancreatic NENs (RADIANT-4). Specifically, in the RADIANT-4 trial, an everolimus benefit in terms of PFS compared to placebo (HR = 0.56; 95%CI 0.40-1.05) has been demonstrated in the GI or lung NENs subgroups’ analysis[72].
The multitargeted tyrosine kinase inhibitor (TKI) sunitinib has demonstrated an improved PFS from 5.5 to 11.4 mo only in metastatic pancreatic NENs (p-NENs)[73]. Therefore, it cannot be recommended in GI-NENs outside clinical trials, which are still ongoing to date (NCT00056693 and NCT02315625).
Other multikinase inhibitors such as cabozantinib and sorafenib have been proposed as alternative options in metastatic disease, but strong evidence should still be spread (NCT05048901, NCT00605566, and NCT00131911).
Furthermore, the association between TKI and chemotherapy has been studied; a single-arm, open-label trial testing the efficacy of the association between everolimus 10 mg daily and temozolomide 150 mg/m2 for 7 d every 2 wk has been recruiting patients with a histologically proven primary gastro-entero-pancreatic (GEP) neuroendocrine carcinoma with a Ki67 index of 20%-55%; the results are about to be published (NCT02248012).
Radioligand therapy: Radioligand therapy (RLT) represents a therapeutic option in progressive well-differentiated G1/2 NENs with homogenous somatostatin receptor expression[74].
NETTER-1, a recent multicenter prospective phase III trial including 229 patients with metastatic gastro-intestinal well-differentiated G1 or G2 NENs, compared patients’ prognosis between those undergoing 177Lu-DOTATATE (Lutathera) in association with octreotide long-lasting release treatment, and those only undergoing high-dose octreotide long-lasting release; 177Lu-DOTATATE proved its superiority in terms of PFS (median PFS > 19.9 mo) and promising results are on the point to be published also regarding its effect on the OS and the patients’ quality of life[75]. This study did not explicitly enroll patients affected by r-NENs, rather it focused on midgut neoplasms, with four colonic NENs (none of them were rectal).
The NETTER-2 phase III, multi-center, randomized, open-label trial is today ongoing: It aims to determine if first-line treatment Lutathera in combination with LAR prolongs PFS in G2/3 GEP-NENs patients (NCT03972488). A prospective single-arm, multicenter trial is today enrolling 195 patients with progressed NETs to evaluate the efficacy of 177Lu-DOTATATE in terms of 12-mo PFS (NCT02743741).
Chemotherapy: G3 NENs are represented preferentially by poorly differentiated r-NECs, whereas r-NET G3 are rare. R-NECs represent a distinct category separated from other r-NENs, because of a highly aggressive biological behavior with a poor prognosis and distinctive histological picture (small and large cell carcinomas)[76]. This entity is usually treated with chemotherapy, also according to the latest ESMO guidelines[77], in which the use of chemotherapy is highly recommended for metastatic low-differentiated G3 NECs.
Chemotherapy regimens containing cisplatin or carboplatin in combination with etoposide could be considered as a first-line therapy in patients with advanced GEP-NECs. Irinotecan is a valid alternative to etoposide in the same cases[78]. Regarding the rare cases of advanced G1/2 r-NENs, streptozocin and its association with 5-fluorouracil and doxorubicin are the most used schemes, even if they are associated with a < 25% clinical and radiological response rate[79]. Three-drug regimens (5-FU, dacarbazine, and epirubicin), used historically since the 1990s, are associated with a response rate of up to 30% of cases[80].
Combined radiation therapy and chemotherapy in high-grade r-NECs: Interesting data were published regarding the efficacy of chemotherapy combined with radiotherapy in poorly differentiated and aggressive high-grade G3 rNECs, which by definition have a Ki67 index ≥ 20% and a mitotic index ≥ 20/10 high power fields.
According to a preliminary retrospective univariate and multivariate analysis, radiation therapy (RT) proved to significantly improve OS in patients affected by G3 r-NECs treated by both surgery and RT compared with G3 r-NECs that underwent only surgery (HR = 0.393; 95%CI: 0.206-0.750; P = 0.009)[81]. Furthermore, according to a multicenter American multidisciplinary study, chemotherapy (based on platinum, etoposide, and fluoropyrimidine) with or without RT and surgery, obtained similar outcomes in terms of both PFS (13.0 mo vs 13.2 mo, P = 0.75) and OS (49.1 mo vs 39.2 mo, P = 0.42)[82]. These findings highlighted that not only does RT improve r-NECs prognosis, but also when combined with chemotherapy, it leads to similar outcomes compared to surgery. Table 2 shows a resume of the main systemic therapies for advanced disease.
| Pharmacological class | Therapeutic agent | Superiority vs placebo | Main ongoing clinical trials |
| Somatostatin analogs | Octreotide (30 mg/4 wk i.m.) | Yes[70] | NCT04129255 |
| Lanreotide (120 mg/4 wk s.c.) | Yes[69] | NCT03289741 (octreotide vs lanreotide) | |
| Cytokines | Interferon-α (3 MIU/three times a week s.c.) | Yes[72] | NCT02838342 (association with cyclophosfamide) |
| Targeted agents | Everolimus (10 mg/d p.o.) | Yes[73] | NCT02248012 (association with temozolomide) |
| Sunitinib (50 mg/d p.o.) | Yes[74]1 | NCT00056693NCT02315625 | |
| Cabozantinib (40-60 mg/d p.o.) | NA | NCT05048901 | |
| Sorafenib (800 mg/d p.o.) | NA | NCT00605566, NCT00131911 | |
| Radioligand therapy | 177Lu-DOTATATE (Lutathera) | Yes[76] | NCT02743741, NCT03972488 (combination with LAR) |
| Chemotherapy | Cisplatin/carboplatin ± etoposide/irinotecan | Yes[79] | NCT03963193 (etoposide + cisplatin vs irinotecan + cisplatin) |
| Streptozocin ± 5-FU ± doxorubicin | Yes[80] | NCT00602082 | |
| 5-FU ± dacarbazine ± epirubicin | Yes [81] | NA |
The indications for the follow-up of r-NENs are scarce in the literature. Furthermore, the few indications that we can find in the consensus guidelines are mostly based on expert opinions rather than evidence-based data. Moreover, the main guidelines give follow-up indications mainly based on the size of the r-NEN[83], while it is documented that grade is the most heavily prognostic factor.
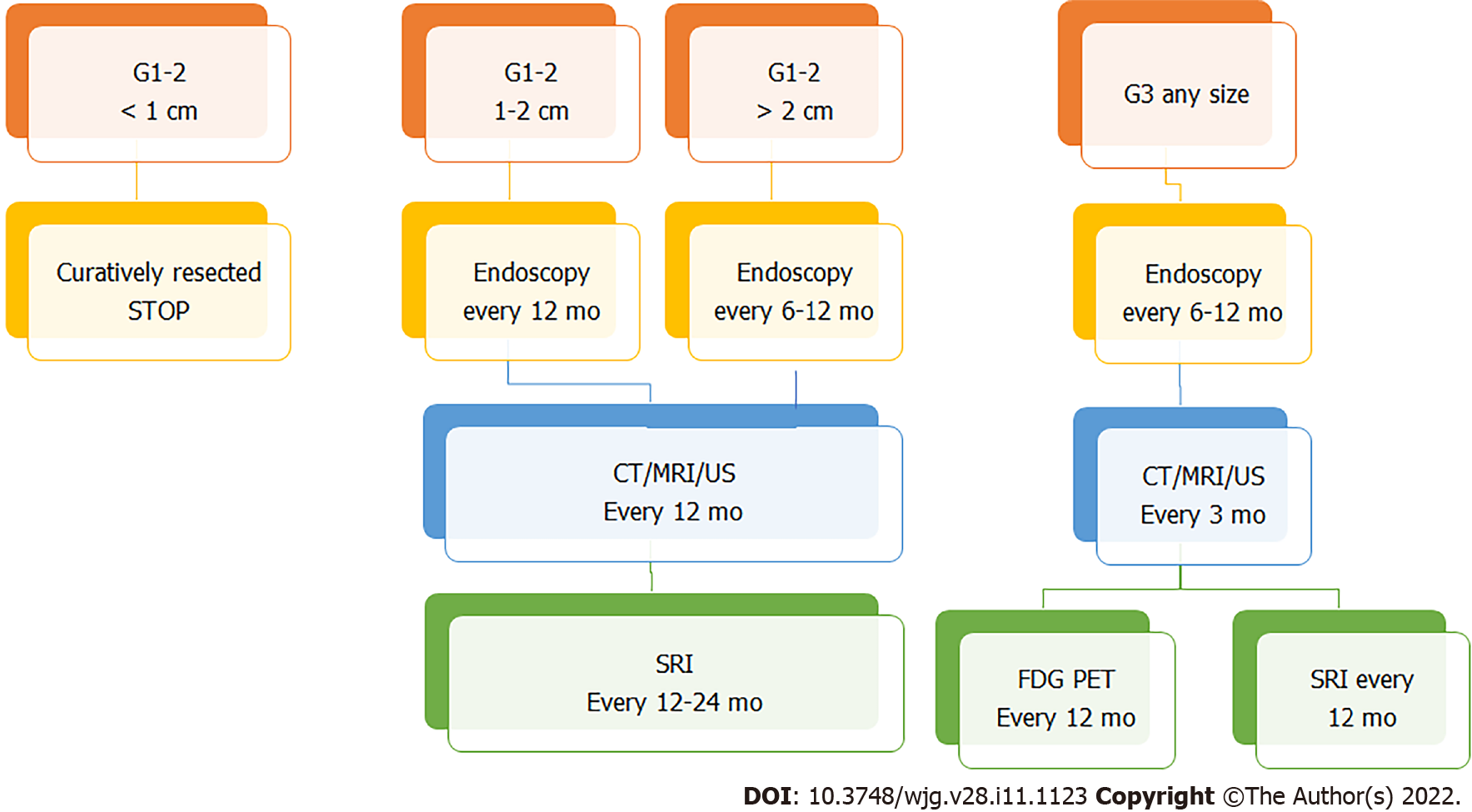
According to the ENETS 2017 guidelines, it is advocated that follow-up occurs in specialized NEN centers or at least in hospitals with close collaboration with specialized NEN centers[84]. The suggested type and timing of the follow-up are based on the tumor size, grade, and operative outcome (if curatively resected), respectively. In the case of small (< 10 mm) G1/2 r-NENs, when curatively resected, only one endoscopic check at 12 mo from the endoscopic resection is advisable. No further investigations are needed. For 10-20 mm G1/2 lesions, an annual endoscopic follow-up is recommended. Moreover, classical radiological imaging by using either CT or MRI is recommended. Also, somatostatin receptor imaging (SRI) is advisable every 12-24 mo. For G1/2 lesions > 20 mm, both curatively and non-curatively resected, a closer follow-up (every 3-12 mo) is recommended, by using endoscopy every 6-12 mo, CT or MRI every 3-12 mo, and SRI every 12-24 mo. In rare cases of G3 NEC/NETs, both curatively and non-curatively resected, a 3-mo follow-up is suggested, by performing CT or MRI; an endoscopy is recommended every 6-12 mo; nuclear medicine imaging (both SRI and FDG-PET) is advisable every 12 mo. In any case, an EUS examination may be required if recurrence or progression is suspected (Figure 5).
R-NENs are among the most frequent digestive NENs, together with small bowel NENs and their incidence has hugely increased over the last few years, likely due to the widespread use of endoscopic screening for CRC and the improvement in endoscopic techniques. As metabolic syndrome appears as a recognized risk factor for the development of r-NENs, this might have partially contributed to the increasing incidence of these neoplasms.
To date, however, there is still an underestimation of the incidence and prevalence of these neoplasms, as they are often not recognized by the endoscopist. In the increasingly vast panorama of artificial intelligence, its application to gastrointestinal endoscopy may help in detecting and thus treating r-NENs. Several predictors of metastases, progression, and/or recurrence of r-NENs have been proposed, and among them, the diameter of the primary neoplasm and the tumor grade are the most important, although the relevance of each of these factors has not been clearly established. Traditionally, well-differentiated r-NENs smaller than 10 mm are endoscopically removed given their low metastatic risk, whereas tumors larger than 20 mm are suggested to be surgically resected given their high risk of distant spreading. However, a potential more updated cut-off size of 15 mm and the degree of differentiation with G2 and, indeed, G3 tumors, are considered to be the main independent predictors of metastatic spread and dismal prognosis. Moreover, the number of positive nodes (≥ 5 as a cut-off) and the depth of invasion with the invasion of the muscolaris propria are also associated with a poor prognosis.
Locally advanced r-NENs with a lateral spreading diameter ≥ 10 mm, but still T1N0 should be addressed at first endoscopically; while surgical techniques should be adopted to address lesions invading the muscolaris propria or loco-regional lymph nodes. TEM is the treatment of choice for scar-embedded recurrent resectable lesions.
The indications for surveillance are scarce and mainly based on experts’ opinions rather than evidence-based guidelines. According to the ENETS 2017 guidelines, the follow-up is based on the tumor size, grade, and operative outcome.
Large prospective studies should be encouraged to define standardized guidelines for r-NENs and to identify clear-cut prognostic factors and scores which might help in the management of these neoplasms.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Italy
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): E
P-Reviewer: Baeg MK, Oda M, Wang G, Yamamura T S-Editor: Ma YJ L-Editor: Wang TQ P-Editor: Ma YJ
| 1. | Dasari A, Shen C, Halperin D, Zhao B, Zhou S, Xu Y, Shih T, Yao JC. Trends in the Incidence, Prevalence, and Survival Outcomes in Patients With Neuroendocrine Tumors in the United States. JAMA Oncol. 2017;3:1335-1342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1510] [Cited by in RCA: 2480] [Article Influence: 310.0] [Reference Citation Analysis (4)] |
| 2. | Wang XY, Chai NL, Linghu EQ, Li HK, Zhai YQ, Feng XX, Zhang WG, Zou JL, Li LS, Xiang JY. Efficacy and safety of hybrid endoscopic submucosal dissection compared with endoscopic submucosal dissection for rectal neuroendocrine tumors and risk factors associated with incomplete endoscopic resection. Ann Transl Med. 2020;8:368. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 3. | Osagiede O, Habermann E, Day C, Gabriel E, Merchea A, Lemini R, Jabbal IS, Colibaseanu DT. Factors associated with worse outcomes for colorectal neuroendocrine tumors in radical versus local resections. J Gastrointest Oncol. 2020;11:836-846. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 19] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 4. | Ploeckinger U, Kloeppel G, Wiedenmann B, Lohmann R; representatives of 21 German NET Centers. The German NET-registry: an audit on the diagnosis and therapy of neuroendocrine tumors. Neuroendocrinology. 2009;90:349-363. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 67] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 5. | Chang JS, Chen LT, Shan YS, Chu PY, Tsai CR, Tsai HJ. An updated analysis of the epidemiologic trends of neuroendocrine tumors in Taiwan. Sci Rep. 2021;11:7881. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 34] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 6. | Fraenkel M, Kim M, Faggiano A, de Herder WW, Valk GD; Knowledge NETwork. Incidence of gastroenteropancreatic neuroendocrine tumours: a systematic review of the literature. Endocr Relat Cancer. 2014;21:R153-R163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 232] [Cited by in RCA: 220] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 7. | Scherübl H, Cadiot G. Early Gastroenteropancreatic Neuroendocrine Tumors: Endoscopic Therapy and Surveillance. Visc Med. 2017;33:332-338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 32] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 8. | Basuroy R, O'Donnell CM, Srirajaskanthan R, Ramage JK. Ileocolonic neuroendocrine tumours identified in the English bowel cancer screening programme. Colorectal Dis. 2018;20:O85-O91. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 19] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 9. | United European Gastroenterology Journal. Volume 7, Number 8, Oct 01, 2019. Available from: https://journals.sagepub.com/toc/uega/7/8. |
| 10. | Moon CM, Huh KC, Jung SA, Park DI, Kim WH, Jung HM, Koh SJ, Kim JO, Jung Y, Kim KO, Kim JW, Yang DH, Shin JE, Shin SJ, Kim ES, Joo YE. Long-Term Clinical Outcomes of Rectal Neuroendocrine Tumors According to the Pathologic Status After Initial Endoscopic Resection: A KASID Multicenter Study. Am J Gastroenterol. 2016;111:1276-1285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 51] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 11. | Volante M, Grillo F, Massa F, Maletta F, Mastracci L, Campora M, Ferro J, Vanoli A, Papotti M. Neuroendocrine neoplasms of the appendix, colon and rectum. Pathologica. 2021;113:19-27. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 50] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 12. | Dąbkowski K, Szczepkowski M, Kos-Kudła B, Starzynska T. Endoscopic management of rectal neuroendocrine tumours. How to avoid a mistake and what to do when one is made? Endokrynol Pol. 2020;71:343-349. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 13. | Veyre F, Lambin T, Fine C, Fenouil T, Rostain F, Walter T, Pioche M. Endoscopic characterization of rectal neuroendocrine tumors with virtual chromoendoscopy: differences between benign and malignant lesions. Endoscopy. 2021;53:E215-E216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 14. | Shim KN, Yang SK, Myung SJ, Chang HS, Jung SA, Choe JW, Lee YJ, Byeon JS, Lee JH, Jung HY, Hong WS, Kim JH, Min YI, Kim JC, Kim JS. Atypical endoscopic features of rectal carcinoids. Endoscopy. 2004;36:313-316. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 48] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 15. | Ahmed M. Gastrointestinal neuroendocrine tumors in 2020. World J Gastrointest Oncol. 2020;12:791-807. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 153] [Cited by in RCA: 136] [Article Influence: 27.2] [Reference Citation Analysis (11)] |
| 16. | Maione F, Chini A, Milone M, Gennarelli N, Manigrasso M, Maione R, Cassese G, Pagano G, Tropeano FP, Luglio G, De Palma GD. Diagnosis and Management of Rectal Neuroendocrine Tumors (NETs). Diagnostics (Basel). 2021;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 41] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 17. | Current status and limitations of artificial intelligence in colonoscopy - Hann - 2021 - United European Gastroenterology Journal - Wiley Online Library. Available from: https://onlinelibrary.wiley.com/doi/full/10.1002/ueg2.12108. |
| 18. | Park SB, Kim DJ, Kim HW, Choi CW, Kang DH, Kim SJ, Nam HS. Is endoscopic ultrasonography essential for endoscopic resection of small rectal neuroendocrine tumors? World J Gastroenterol. 2017;23:2037-2043. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 14] [Cited by in RCA: 20] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 19. | Chen HT, Xu GQ, Teng XD, Chen YP, Chen LH, Li YM. Diagnostic accuracy of endoscopic ultrasonography for rectal neuroendocrine neoplasms. World J Gastroenterol. 2014;20:10470-10477. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 26] [Cited by in RCA: 29] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 20. | Moon JS. Endoscopic ultrasound-guided fine needle aspiration in submucosal lesion. Clin Endosc. 2012;45:117-123. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 41] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 21. | Pyo JH, Hong SN, Min BH, Lee JH, Chang DK, Rhee PL, Kim JJ, Choi SK, Jung SH, Son HJ, Kim YH. Evaluation of the risk factors associated with rectal neuroendocrine tumors: a big data analytic study from a health screening center. J Gastroenterol. 2016;51:1112-1121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 20] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 22. | Ko SH, Baeg MK, Ko SY, Jung HS. Clinical characteristics, risk factors and outcomes of asymptomatic rectal neuroendocrine tumors. Surg Endosc. 2017;31:3864-3871. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 23. | Jung YS, Yun KE, Chang Y, Ryu S, Park JH, Kim HJ, Cho YK, Sohn CI, Jeon WK, Kim BI, Park DI. Risk factors associated with rectal neuroendocrine tumors: a cross-sectional study. Cancer Epidemiol Biomarkers Prev. 2014;23:1406-1413. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 40] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 24. | Djiogue S, Nwabo Kamdje AH, Vecchio L, Kipanyula MJ, Farahna M, Aldebasi Y, Seke Etet PF. Insulin resistance and cancer: the role of insulin and IGFs. Endocr Relat Cancer. 2013;20:R1-R17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 206] [Cited by in RCA: 204] [Article Influence: 17.0] [Reference Citation Analysis (1)] |
| 25. | Matsuhashi N, Takahashi T, Tomita H, Araki H, Ibuka T, Tanaka K, Tanahashi T, Matsui S, Sasaki Y, Tanaka Y, Okumura N, Yamaguchi K, Osada S, Yoshida K. Evaluation of treatment for rectal neuroendocrine tumors sized under 20 mm in comparison with the WHO 2010 guidelines. Mol Clin Oncol. 2017;7:476-480. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 15] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 26. | de Mestier L, Brixi H, Gincul R, Ponchon T, Cadiot G. Updating the management of patients with rectal neuroendocrine tumors. Endoscopy. 2013;45:1039-1046. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 81] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 27. | Park CH, Cheon JH, Kim JO, Shin JE, Jang BI, Shin SJ, Jeen YT, Lee SH, Ji JS, Han DS, Jung SA, Park DI, Baek IH, Kim SH, Chang DK. Criteria for decision making after endoscopic resection of well-differentiated rectal carcinoids with regard to potential lymphatic spread. Endoscopy. 2011;43:790-795. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 99] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 28. | Concors SJ, Sinnamon AJ, Folkert IW, Mahmoud NN, Fraker DL, Paulson EC, Roses RE. Predictors of Metastases in Rectal Neuroendocrine Tumors: Results of a National Cohort Study. Dis Colon Rectum. 2018;61:1372-1379. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 29] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 29. | Capurso G, Gaujoux S, Pescatori LC, Panzuto F, Panis Y, Pilozzi E, Terris B, de Mestier L, Prat F, Rinzivillo M, Coriat R, Coulevard A, Delle Fave G, Ruszniewski P. The ENETS TNM staging and grading system accurately predict prognosis in patients with rectal NENs. Dig Liver Dis. 2019;51:1725-1730. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 5] [Article Influence: 0.8] [Reference Citation Analysis (1)] |
| 30. | Nagtegaal ID, Odze RD, Klimstra D, Paradis V, Rugge M, Schirmacher P, Washington KM, Carneiro F, Cree IA; WHO Classification of Tumours Editorial Board. The 2019 WHO classification of tumours of the digestive system. Histopathology. 2020;76:182-188. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2554] [Cited by in RCA: 2428] [Article Influence: 485.6] [Reference Citation Analysis (3)] |
| 31. | Folkert IW, Sinnamon AJ, Concors SJ, Bennett BJ, Fraker DL, Mahmoud NN, Metz DC, Stashek KM, Roses RE. Grade is a Dominant Risk Factor for Metastasis in Patients with Rectal Neuroendocrine Tumors. Ann Surg Oncol. 2020;27:855-863. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 32. | Shields CJ, Tiret E, Winter DC; International Rectal Carcinoid Study Group. Carcinoid tumors of the rectum: a multi-institutional international collaboration. Ann Surg. 2010;252:750-755. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 107] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 33. | Wang Z, Liu Z, Wen Z, Li R, An K, Mei S, Chen J, Shen H, Li J, Zhao F, Wei F, Xiao T, Liu Q. Evaluation of radical surgical treatment in the management of 58 locally advanced rectal neuroendocrine neoplasms, one multicenter retrospective study. Eur J Surg Oncol. 2021;47:3166-3174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 34. | Anthony LB, Strosberg JR, Klimstra DS, Maples WJ, O'Dorisio TM, Warner RR, Wiseman GA, Benson AB 3rd, Pommier RF; North American Neuroendocrine Tumor Society (NANETS). The NANETS consensus guidelines for the diagnosis and management of gastrointestinal neuroendocrine tumors (nets): well-differentiated nets of the distal colon and rectum. Pancreas. 2010;39:767-774. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 185] [Cited by in RCA: 211] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 35. | Rindi G, Klöppel G, Couvelard A, Komminoth P, Körner M, Lopes JM, McNicol AM, Nilsson O, Perren A, Scarpa A, Scoazec JY, Wiedenmann B. TNM staging of midgut and hindgut (neuro) endocrine tumors: a consensus proposal including a grading system. Virchows Arch. 2007;451:757-762. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 680] [Cited by in RCA: 653] [Article Influence: 36.3] [Reference Citation Analysis (1)] |
| 36. | Fields AC, McCarty JC, Ma-Pak L, Lu P, Irani J, Goldberg JE, Bleday R, Chan J, Melnitchouk N. New lymph node staging for rectal neuroendocrine tumors. J Surg Oncol. 2019;119:156-162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 15] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 37. | de Mestier L, Lorenzo D, Fine C, Cros J, Hentic O, Walter T, Panis Y, Couvelard A, Cadiot G, Ruszniewski P. Endoscopic, transanal, laparoscopic, and transabdominal management of rectal neuroendocrine tumors. Best Pract Res Clin Endocrinol Metab. 2019;33:101293. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 44] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 38. | Fahy BN, Tang LH, Klimstra D, Wong WD, Guillem JG, Paty PB, Temple LK, Shia J, Weiser MR. Carcinoid of the rectum risk stratification (CaRRs): a strategy for preoperative outcome assessment. Ann Surg Oncol. 2007;14:1735-1743. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 71] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 39. | Ramage JK, De Herder WW, Delle Fave G, Ferolla P, Ferone D, Ito T, Ruszniewski P, Sundin A, Weber W, Zheng-Pei Z, Taal B, Pascher A; Vienna Consensus Conference participants. ENETS Consensus Guidelines Update for Colorectal Neuroendocrine Neoplasms. Neuroendocrinology. 2016;103:139-143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 185] [Cited by in RCA: 231] [Article Influence: 25.7] [Reference Citation Analysis (0)] |
| 40. | Keung EZ, Gershenwald JE. The eighth edition American Joint Committee on Cancer (AJCC) melanoma staging system: implications for melanoma treatment and care. Expert Rev Anticancer Ther. 2018;18:775-784. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 368] [Article Influence: 61.3] [Reference Citation Analysis (0)] |
| 41. | Son HJ, Sohn DK, Hong CW, Han KS, Kim BC, Park JW, Choi HS, Chang HJ, Oh JH. Factors associated with complete local excision of small rectal carcinoid tumor. Int J Colorectal Dis. 2013;28:57-61. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 57] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 42. | Kim JS, Kim YJ, Chung JW, Kim JH, Kim KO, Kwon KA, Park DK, An JS. Usefulness of endoscopic resection using the band ligation method for rectal neuroendocrine tumors. Intest Res. 2016;14:164-171. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 13] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 43. | Binmoeller KF. Underwater EMR without submucosal injection: Is less more? Gastrointest Endosc. 2019;89:1117-1119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 44. | Park SB, Kim HW, Kang DH, Choi CW, Kim SJ, Nam HS. Advantage of endoscopic mucosal resection with a cap for rectal neuroendocrine tumors. World J Gastroenterol. 2015;21:9387-9393. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 33] [Cited by in RCA: 42] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 45. | Chen R, Liu X, Sun S, Wang S, Ge N, Wang G, Guo J. Comparison of Endoscopic Mucosal Resection With Circumferential Incision and Endoscopic Submucosal Dissection for Rectal Carcinoid Tumor. Surg Laparosc Endosc Percutan Tech. 2016;26:e56-e61. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 13] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 46. | Bang BW, Park JS, Kim HK, Shin YW, Kwon KS, Kim JM. Endoscopic Resection for Small Rectal Neuroendocrine Tumors: Comparison of Endoscopic Submucosal Resection with Band Ligation and Endoscopic Submucosal Dissection. Gastroenterol Res Pract. 2016;2016:6198927. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 35] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 47. | Lee HJ, Kim SB, Shin CM, Seo AY, Lee DH, Kim N, Park YS, Yoon H. A comparison of endoscopic treatments in rectal carcinoid tumors. Surg Endosc. 2016;30:3491-3498. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 48. | Zheng JC, Zheng K, Zhao S, Wang ZN, Xu HM, Jiang CG. Efficacy and safety of modified endoscopic mucosal resection for rectal neuroendocrine tumors: a meta-analysis. Z Gastroenterol. 2020;58:137-145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 49. | Kim J, Kim JH, Lee JY, Chun J, Im JP, Kim JS. Clinical outcomes of endoscopic mucosal resection for rectal neuroendocrine tumor. BMC Gastroenterol. 2018;18:77. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 46] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 50. | Boškoski I, Volkanovska A, Tringali A, Bove V, Familiari P, Perri V, Costamagna G. Endoscopic resection for gastrointestinal neuroendocrine tumors. Expert Rev Gastroenterol Hepatol. 2013;7:559-569. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 51. | Ono H, Yao K, Fujishiro M, Oda I, Nimura S, Yahagi N, Iishi H, Oka M, Ajioka Y, Ichinose M, Matsui T. Guidelines for endoscopic submucosal dissection and endoscopic mucosal resection for early gastric cancer. Dig Endosc. 2016;28:3-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 354] [Cited by in RCA: 406] [Article Influence: 45.1] [Reference Citation Analysis (0)] |
| 52. | Zhou X, Xie H, Xie L, Li J, Cao W, Fu W. Endoscopic resection therapies for rectal neuroendocrine tumors: a systematic review and meta-analysis. J Gastroenterol Hepatol. 2014;29:259-268. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 55] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 53. | Park SS, Han KS, Kim B, Chang Kim B, Hong CW, Sohn DK, Chang HJ. Comparison of underwater endoscopic mucosal resection and endoscopic submucosal dissection of rectal neuroendocrine tumors (with videos). Gastrointest Endosc. 2020;91:1164-1171.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 39] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 54. | Liu S, Chai N, Linghu E. Underwater EMR or endoscopic submucosal dissection for rectal neuroendocrine tumors: What are the advantages? Gastrointest Endosc. 2020;92:230-231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 55. | Pan J, Zhang X, Shi Y, Pei Q. Endoscopic mucosal resection with suction vs. endoscopic submucosal dissection for small rectal neuroendocrine tumors: a meta-analysis. Scand J Gastroenterol. 2018;53:1139-1145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 42] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 56. | Pagano N, Ricci C, Brighi N, Ingaldi C, Pugliese F, Santini D, Campana D, Mosconi C, Ambrosini V, Casadei R. Incidental diagnosis of very small rectal neuroendocrine neoplasms: when should endoscopic submucosal dissection be performed? Endocrine. 2019;65:207-212. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 15] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 57. | Eick J, Steinberg J, Schwertner C, Ring W, Scherübl H. [Rectal neuroendocrine tumors: endoscopic therapy]. Chirurg. 2016;87:288-291. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 58. | Gleeson FC, Levy MJ, Dozois EJ, Larson DW, Wong Kee Song LM, Boardman LA. Endoscopically identified well-differentiated rectal carcinoid tumors: impact of tumor size on the natural history and outcomes. Gastrointest Endosc. 2014;80:144-151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 56] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 59. | Shigeta K, Okabayashi K, Hasegawa H, Ishii Y, Ochiai H, Tsuruta M, Mukai M, Kameyama K, Uraoka T, Yahagi N, Kitagawa Y. Long-term outcome of patients with locally resected high- and low-risk rectal carcinoid tumors. J Gastrointest Surg. 2014;18:768-773. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 28] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 60. | Kumar AS, Sidani SM, Kolli K, Stahl TJ, Ayscue JM, Fitzgerald JF, Smith LE. Transanal endoscopic microsurgery for rectal carcinoids: the largest reported United States experience. Colorectal Dis. 2012;14:562-566. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 35] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 61. | Yan FH, Lou Z, Hu SJ, Xu XD, Wang H, Wang HT, Meng RG, Fu CG, Zhang W, He J, Yu ED. Endoscopic submucosal dissection versus transanal local excision for rectal carcinoid: a comparative study. World J Surg Oncol. 2016;14:162. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 62. | Chen T, Yao LQ, Xu MD, Zhang YQ, Chen WF, Shi Q, Cai SL, Chen YY, Xie YH, Ji Y, Chen SY, Zhou PH, Zhong YS. Efficacy and Safety of Endoscopic Submucosal Dissection for Colorectal Carcinoids. Clin Gastroenterol Hepatol. 2016;14:575-581. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 45] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 63. | Ortenzi M, Ghiselli R, Cappelletti Trombettoni MM, Cardinali L, Guerrieri M. Transanal endoscopic microsurgery as optimal option in treatment of rare rectal lesions: A single centre experience. World J Gastrointest Endosc. 2016;8:623-627. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 15] [Cited by in RCA: 13] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 64. | Mashimo Y, Matsuda T, Uraoka T, Saito Y, Sano Y, Fu K, Kozu T, Ono A, Fujii T, Saito D. Endoscopic submucosal resection with a ligation device is an effective and safe treatment for carcinoid tumors in the lower rectum. J Gastroenterol Hepatol. 2008;23:218-221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 97] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 65. | Ramage JK, Valle JW, Nieveen van Dijkum EJM, Sundin A, Pascher A, Couvelard A, Kloeppel G; the ENETS 2016 Munich Advisory Board Participants; ENETS 2016 Munich Advisory Board Participants. Colorectal Neuroendocrine Neoplasms: Areas of Unmet Need. Neuroendocrinology. 2019;108:45-53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 25] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 66. | Takatsu Y, Fukunaga Y, Nagasaki T, Akiyoshi T, Konishi T, Fujimoto Y, Nagayama S, Ueno M. Short- and Long-term Outcomes of Laparoscopic Total Mesenteric Excision for Neuroendocrine Tumors of the Rectum. Dis Colon Rectum. 2017;60:284-289. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 31] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 67. | Rinke A, Wittenberg M, Schade-Brittinger C, Aminossadati B, Ronicke E, Gress TM, Müller HH, Arnold R; PROMID Study Group. Placebo-Controlled, Double-Blind, Prospective, Randomized Study on the Effect of Octreotide LAR in the Control of Tumor Growth in Patients with Metastatic Neuroendocrine Midgut Tumors (PROMID): Results of Long-Term Survival. Neuroendocrinology. 2017;104:26-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 184] [Cited by in RCA: 259] [Article Influence: 32.4] [Reference Citation Analysis (0)] |
| 68. | Caplin ME, Pavel M, Ćwikła JB, Phan AT, Raderer M, Sedláčková E, Cadiot G, Wolin EM, Capdevila J, Wall L, Rindi G, Langley A, Martinez S, Blumberg J, Ruszniewski P; CLARINET Investigators. Lanreotide in metastatic enteropancreatic neuroendocrine tumors. N Engl J Med. 2014;371:224-233. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1142] [Cited by in RCA: 1290] [Article Influence: 117.3] [Reference Citation Analysis (0)] |
| 69. | Massironi S, Conte D, Rossi RE. Somatostatin analogues in functioning gastroenteropancreatic neuroendocrine tumours: literature review, clinical recommendations and schedules. Scand J Gastroenterol. 2016;51:513-523. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 24] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 70. | Oberg K. Interferon in the management of neuroendocrine GEP-tumors: a review. Digestion. 2000;62 Suppl 1:92-97. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 114] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 71. | Yao JC, Guthrie KA, Moran C, Strosberg JR, Kulke MH, Chan JA, LoConte N, McWilliams RR, Wolin EM, Mattar B, McDonough S, Chen H, Blanke CD, Hochster HS. Phase III Prospective Randomized Comparison Trial of Depot Octreotide Plus Interferon Alfa-2b Versus Depot Octreotide Plus Bevacizumab in Patients With Advanced Carcinoid Tumors: SWOG S0518. J Clin Oncol. 2017;35:1695-1703. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 118] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 72. | Yao JC, Fazio N, Singh S, Buzzoni R, Carnaghi C, Wolin E, Tomasek J, Raderer M, Lahner H, Voi M, Pacaud LB, Rouyrre N, Sachs C, Valle JW, Fave GD, Van Cutsem E, Tesselaar M, Shimada Y, Oh DY, Strosberg J, Kulke MH, Pavel ME; RAD001 in Advanced Neuroendocrine Tumours, Fourth Trial (RADIANT-4) Study Group. Everolimus for the treatment of advanced, non-functional neuroendocrine tumours of the lung or gastrointestinal tract (RADIANT-4): a randomised, placebo-controlled, phase 3 study. Lancet. 2016;387:968-977. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 749] [Cited by in RCA: 903] [Article Influence: 100.3] [Reference Citation Analysis (0)] |
| 73. | Raymond E, Dahan L, Raoul JL, Bang YJ, Borbath I, Lombard-Bohas C, Valle J, Metrakos P, Smith D, Vinik A, Chen JS, Hörsch D, Hammel P, Wiedenmann B, Van Cutsem E, Patyna S, Lu DR, Blanckmeister C, Chao R, Ruszniewski P. Sunitinib malate for the treatment of pancreatic neuroendocrine tumors. N Engl J Med. 2011;364:501-513. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2032] [Cited by in RCA: 1828] [Article Influence: 130.6] [Reference Citation Analysis (0)] |
| 74. | Thang SP, Lung MS, Kong G, Hofman MS, Callahan J, Michael M, Hicks RJ. Peptide receptor radionuclide therapy (PRRT) in European Neuroendocrine Tumour Society (ENETS) grade 3 (G3) neuroendocrine neoplasia (NEN) - a single-institution retrospective analysis. Eur J Nucl Med Mol Imaging. 2018;45:262-277. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 93] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 75. | Nicolini S, Severi S, Ianniello A, Sansovini M, Ambrosetti A, Bongiovanni A, Scarpi E, Di Mauro F, Rossi A, Matteucci F, Paganelli G. Investigation of receptor radionuclide therapy with 177Lu-DOTATATE in patients with GEP-NEN and a high Ki-67 proliferation index. Eur J Nucl Med Mol Imaging. 2018;45:923-930. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 61] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 76. | Suresh PK, Sahu KK, Pai RR, Sridevi HB, Ballal K, Khandelia B, Minal J, Annappa R. The Prognostic Significance of Neuroendocrine Differentiation in Colorectal Carcinomas: Our Experience. J Clin Diagn Res. 2015;9:EC01-EC04. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 8] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 77. | Pavel M, Öberg K, Falconi M, Krenning EP, Sundin A, Perren A, Berruti A; ESMO Guidelines Committee. Gastroenteropancreatic neuroendocrine neoplasms: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2020;31:844-860. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 465] [Cited by in RCA: 696] [Article Influence: 139.2] [Reference Citation Analysis (0)] |
| 78. | Bongiovanni A, Riva N, Ricci M, Liverani C, La Manna F, De Vita A, Foca F, Mercatali L, Severi S, Amadori D, Ibrahim T. First-line chemotherapy in patients with metastatic gastroenteropancreatic neuroendocrine carcinoma. Onco Targets Ther. 2015;8:3613-3619. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 30] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 79. | Turner NC, Strauss SJ, Sarker D, Gillmore R, Kirkwood A, Hackshaw A, Papadopoulou A, Bell J, Kayani I, Toumpanakis C, Grillo F, Mayer A, Hochhauser D, Begent RH, Caplin ME, Meyer T. Chemotherapy with 5-fluorouracil, cisplatin and streptozocin for neuroendocrine tumours. Br J Cancer. 2010;102:1106-1112. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 136] [Cited by in RCA: 134] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 80. | Walter T, Bruneton D, Cassier PA, Hervieu V, Pilleul F, Scoazec JY, Chayvialle JA, Lombard-Bohas C. Evaluation of the combination 5-fluorouracil, dacarbazine, and epirubicin in patients with advanced well-differentiated neuroendocrine tumors. Clin Colorectal Cancer. 2010;9:248-254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 27] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 81. | Modrek AS, Hsu HC, Leichman CG, Du KL. Radiation therapy improves survival in rectal small cell cancer - Analysis of Surveillance Epidemiology and End Results (SEER) data. Radiat Oncol. 2015;10:101. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 16] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 82. | Brieau B, Lepère C, Walter T, Lecomte T, Guimbaud R, Manfredi S, Tougeron D, Desseigne F, Lourenco N, Afchain P, El Hajbi F, Terris B, Rougier P, Coriat R. Radiochemotherapy Versus Surgery in Nonmetastatic Anorectal Neuroendocrine Carcinoma: A Multicenter Study by the Association des Gastro-Entérologues Oncologues. Medicine (Baltimore). 2015;94:e1864. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 24] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 83. | Basuroy R, Haji A, Ramage JK, Quaglia A, Srirajaskanthan R. Review article: the investigation and management of rectal neuroendocrine tumours. Aliment Pharmacol Ther. 2016;44:332-345. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 77] [Article Influence: 8.6] [Reference Citation Analysis (1)] |
| 84. | Knigge U, Capdevila J, Bartsch DK, Baudin E, Falkerby J, Kianmanesh R, Kos-Kudla B, Niederle B, Nieveen van Dijkum E, O'Toole D, Pascher A, Reed N, Sundin A, Vullierme MP; Antibes Consensus Conference Participants; Antibes Consensus Conference participants. ENETS Consensus Recommendations for the Standards of Care in Neuroendocrine Neoplasms: Follow-Up and Documentation. Neuroendocrinology. 2017;105:310-319. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 89] [Article Influence: 11.1] [Reference Citation Analysis (0)] |