Published online Feb 28, 2021. doi: 10.3748/wjg.v27.i8.751
Peer-review started: November 23, 2020
First decision: December 21, 2020
Revised: January 18, 2021
Accepted: February 11, 2021
Article in press: February 11, 2021
Published online: February 28, 2021
Processing time: 94 Days and 2.9 Hours
Endoscopic ultrasound-guided fine needle aspiration or biopsy (EUS-FNA or FNB) has become a popular method for diagnosing various lesions of the gastrointestinal tract and surrounding tissue due to the accuracy and safety. To the best of our knowledge, no case report of severe infection after EUS-FNB of a solid lesion in the spleen has been described. Herein, we report a rare case of septic shock after EUS-FNB of a splenic mass.
A 45-year-old male patient presented to the outpatient clinic due to an incidentally detected splenic mass. A definitive diagnosis could not be established based on the abdominal magnetic resonance imaging. EUS of the spleen showed a 6 cm-sized, relatively well-demarcated, heterogeneous mass, and EUS-FNB with a 22G needle was performed. Ten days after the procedure patient developed septic shock and a splenic abscess was identified. Blood culture revealed growth of Granulicatella adiacens. After the treatment with antibiotics the patient underwent surgical resection, and the pathological examination showed diffuse large B-cell lymphoma. The patient received chemotherapy and he is in complete remission.
Infection of a splenic mass after EUS-FNB is a rare complication and prophylactic antibiotics might be considered.
Core Tip: As the risk of infection after endoscopic ultrasound (EUS)-guided sampling of a solid organ is very low, prophylactic antibiotics are generally not recommended. However, our patient developed an abscess after EUS-guided fine needle biopsy of a splenic tumor. To the best of our knowledge, this is the first case of septic shock after EUS-guided fine needle biopsy of a splenic large B cell lymphoma and of an infection caused by Granulicatella adiacens during this procedure. Therefore, we suggest considering prophylactic antibiotic usage for EUS-guided sampling of splenic tumors.
- Citation: Cho SY, Cho E, Park CH, Kim HJ, Koo JY. Septic shock due to Granulicatella adiacens after endoscopic ultrasound-guided biopsy of a splenic mass: A case report. World J Gastroenterol 2021; 27(8): 751-759
- URL: https://www.wjgnet.com/1007-9327/full/v27/i8/751.htm
- DOI: https://dx.doi.org/10.3748/wjg.v27.i8.751
Endoscopic ultrasound (EUS)-guided fine needle aspiration or biopsy (FNA or FNB) has emerged as a popular and safe method for accurate diagnosis of various lesions of the gastrointestinal tract and adjacent organs. Splenic tumors are relatively rare and encompass a variety of benign and malignant diseases. Traditionally, percutaneous biopsies under ultrasound guidance have been performed for the diagnosis of these lesions. However, when the lesion is invisible by external ultrasonography, the procedure is impossible, and the complication rate of 5.3% has been reported[1]. EUS has an advantage of providing a clear image of the spleen through the gastric wall, and tissue sampling can be done with no intervening organs under real-time guidance with color Doppler, reducing major complications such as hemorrhage[2].
The incidence of infectious adverse events after EUS-FNA is less than 1%[3], and most of the severe infectious adverse events occur after EUS-FNA of cystic lesions. Therefore, current guidelines suggest antibiotic prophylaxis for the EUS-guided sampling of cystic lesions but for the sampling of solid lesions due to the very low risk of infection after the procedure[4].
Granulicatella adiacens (G. adiacens) is a catalase-negative, gram-positive coccus. It is a part of the normal flora of oral cavity, genitourinary and gastrointestinal tract rarely causing any disease. Reported infectious cases by G. adiacens include endocarditis and sepsis in immunocompromised patients and peritonitis, abscess, and severe infections in patients with prosthetic devices[5].
To the best of our knowledge, no case of severe infection after EUS-FNB of a splenic tumor and no case of sepsis by G. adiacens after EUS has been reported. We herein report the first case of septic shock due to G. adiacens after EUS-FNB of a splenic mass.
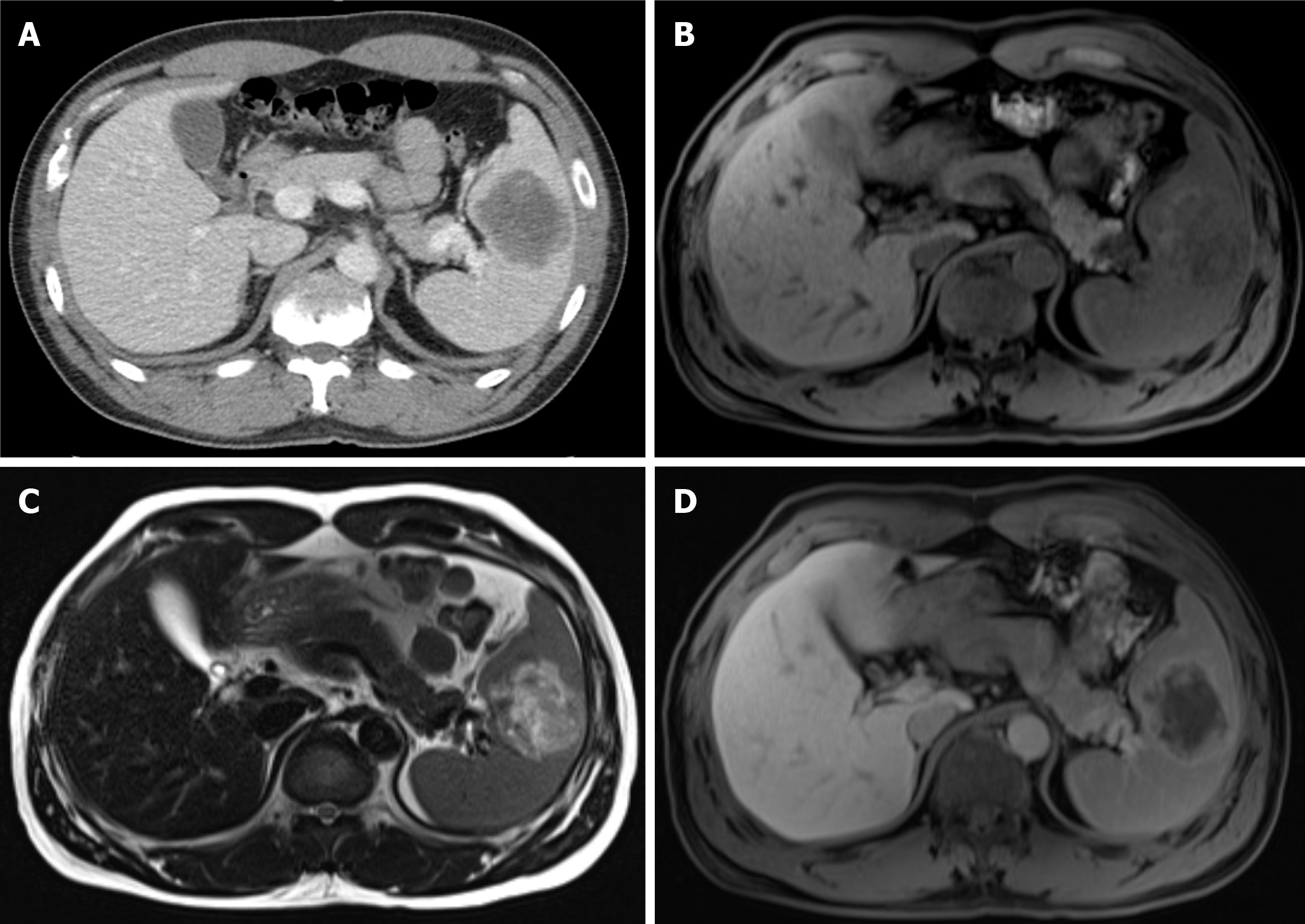
A 45-year-old male patient presented with an incidentally detected, 6 cm-sized splenic mass in the abdominal computed tomography (CT) after a traffic accident (Figure 1A).
He denied fever, night sweat, weight loss and abdominal pain but complained of mild low back pain due to the accident.
The patient had no previous medical history.
The patient had no family history.
Physical examination revealed an enlarged spleen, two finger breadths below the left costal margin and no palpable lymph node enlargement.
Laboratory findings showed slightly elevated aspartate aminotransferase (AST) of 54 U/L (normal 10-37 U/L) but normal lactate dehydrogenase (LDH), alanine aminotransferase (ALT), alkaline phosphatase (ALP), and bilirubin levels. Other examinations including complete blood count, C-reactive protein (CRP), coagulation profiles, and renal function tests were within normal limits.
Abdominal CT showed a 6 cm-sized hypodense mass with peripheral enhancing rim in the spleen (Figure 1A). Abdominal magnetic resonance imaging (MRI) revealed a 6 cm-sized splenic mass, which was heterogeneously hypointense on T1-weighted image (Figure 1B), hyperintense T-2 weighted image (Figure 1C) and peripheral enhancement with a central hypo-enhancing lesion on gadolinium-enhanced T1-weighted image (Figure 1D), which was considered as a rare splenic tumor such as sclerosing angiomatoid nodular transformation (SANT) of the spleen.
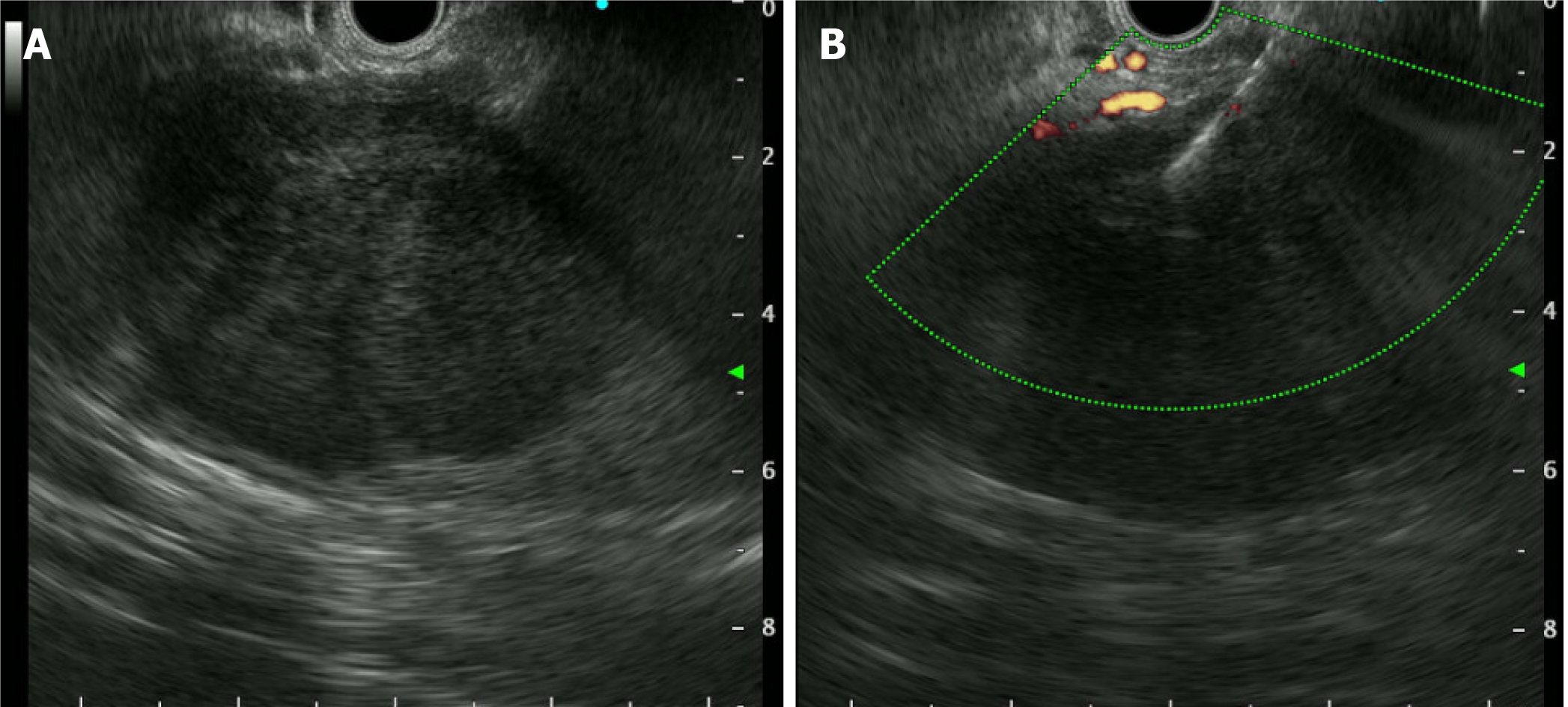
EUS-FNB using a 22G needle (EchoTip Procore®, Cook medical, Limerick, Ireland) was performed for pathologic diagnosis. EUS without contrast medium of the lesion demonstrated a 6 cm-sized, relatively well-demarcated, heterogeneously hypoechoic lesion in the spleen without any necrotic changes (Figure 2). Three needle passes were performed and three biopsy specimens and 8 cytology slides were made. No adverse events occurred during the one-day hospitalization for the procedure. The patient did not receive prophylactic antibiotics, due to the solid nature of the lesion. Histopathology revealed mostly blood clots and a few inflammatory cells and cytopathology was suspicious for poorly differentiated malignancy. Further laboratory exams were performed and splenectomy was planned. Laboratory findings showed elevated soluble interleukin-2 receptor of 3891 U/mL (normal 158-623 U/mL), decreased complement 3 of 69.1 mg/dL (normal 90-180 mg/dL), but normal immunoglobulin E of 15.6 IU/mL (normal 1.5-158 IU/mL) and complement 4 of 27.5 mg/dL (normal 10-40 mg/dL).
Ten days after the procedure the patient was readmitted due to pain in the left upper quadrant. He complained of nausea, vomiting and fever. He had suffered from chills for three days before readmission. On admission, his blood pressure was 70/40 mmHg, heart rate was 86/min, respiratory rate was 20/min, and body temperature was 38.4 °C. Laboratory findings showed leukocytosis of 19200/µL, hemoglobin 12.0 g/dL, platelet count 34000/µL, AST 102 U/L, ALT 55 U/L, ALP 426 U/L, total bilirubin 14.73 mg/dL, LDH 1300 U/L, and CRP 31.08 mg/dL (normal < 0.3 mg/dL).
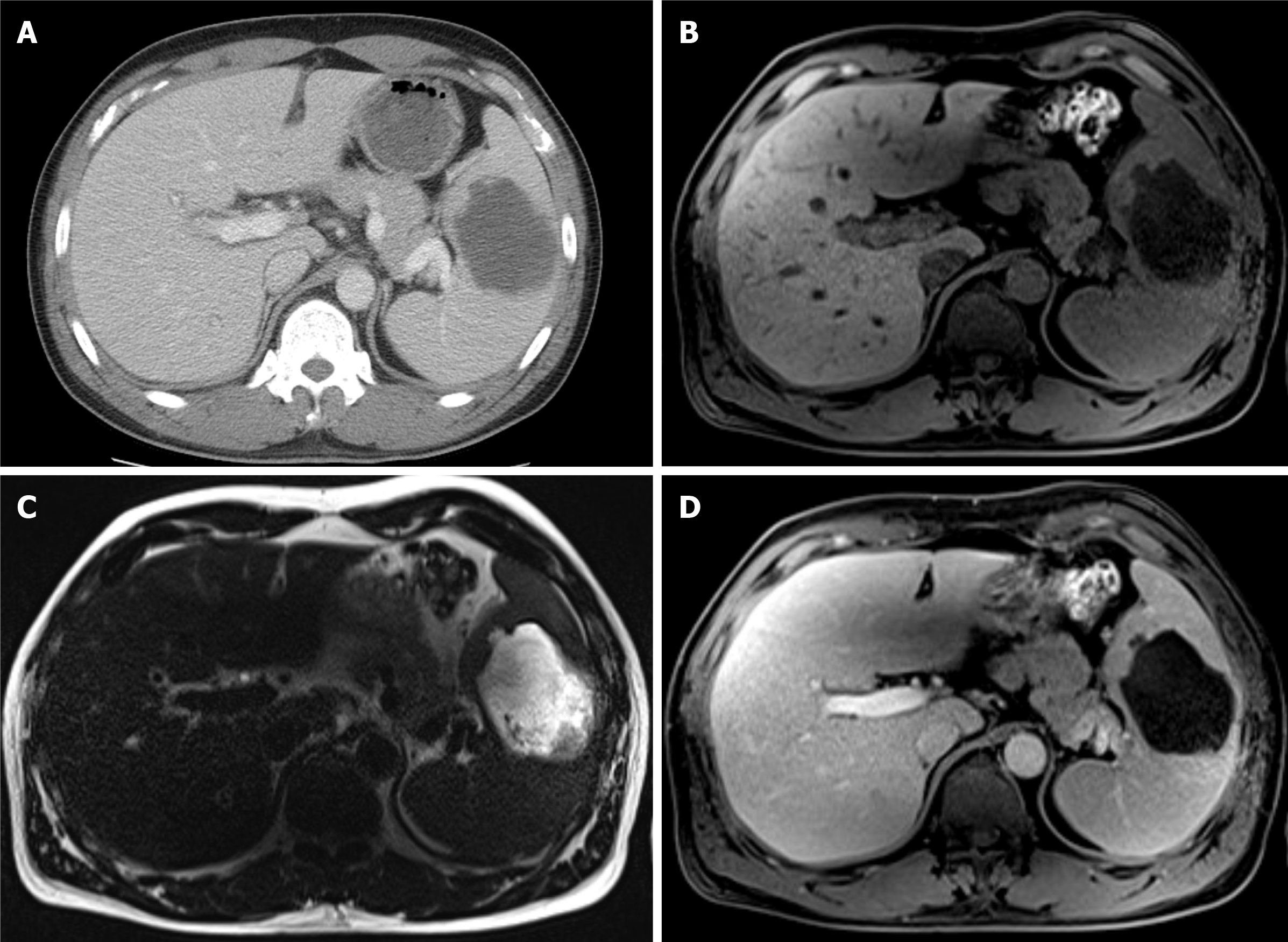
Abdominal CT and MRI, compared to previous results, revealed slightly increased splenic lesion. Abdominal CT revealed a 7 cm-sized low-density lesion (Figure 3A), MRI revealed a low signal lesion on T1-weighted image (Figure 3B) compared to initial MRI, much higher signal lesion on T2-weighted image with non-enhancing debris or necrotic portions inside (Figure 3C) and much lower signal with minimal peripheral enhancement showing a capsule development on gadolinium-enhanced T1-weighted image (Figure 3D), suggestive for abscess formation.
The blood culture revealed G. adiacens three days after readmission. The blood sample was cultivated in a liquid medium and it was identified by matrix-assisted laser desorption/ionization time-of-flight mass spectrometry.
Diagnosis of septic shock due to a splenic abscess caused by G. adiacens after EUS-FNB of a splenic tumor was made.
Intravenous piperacillin/tazobactam was initially started to cover broad-spectrum antimicrobials due to his septic condition. After identifying the pathogen, G. adiacens, and piperacillin/tazobactam was changed to ampicillin/sulbactam. Patient’s condition, vital signs and laboratory findings improved. Intravenous antibiotics were administered for 12 d and the patient was discharged. After recovery from the infection, he got vaccination to reduce the risk of infections with encapsulated organisms after splenectomy. Splenectomy was performed 4 wk after vaccination, 40 d after the septic shock event.
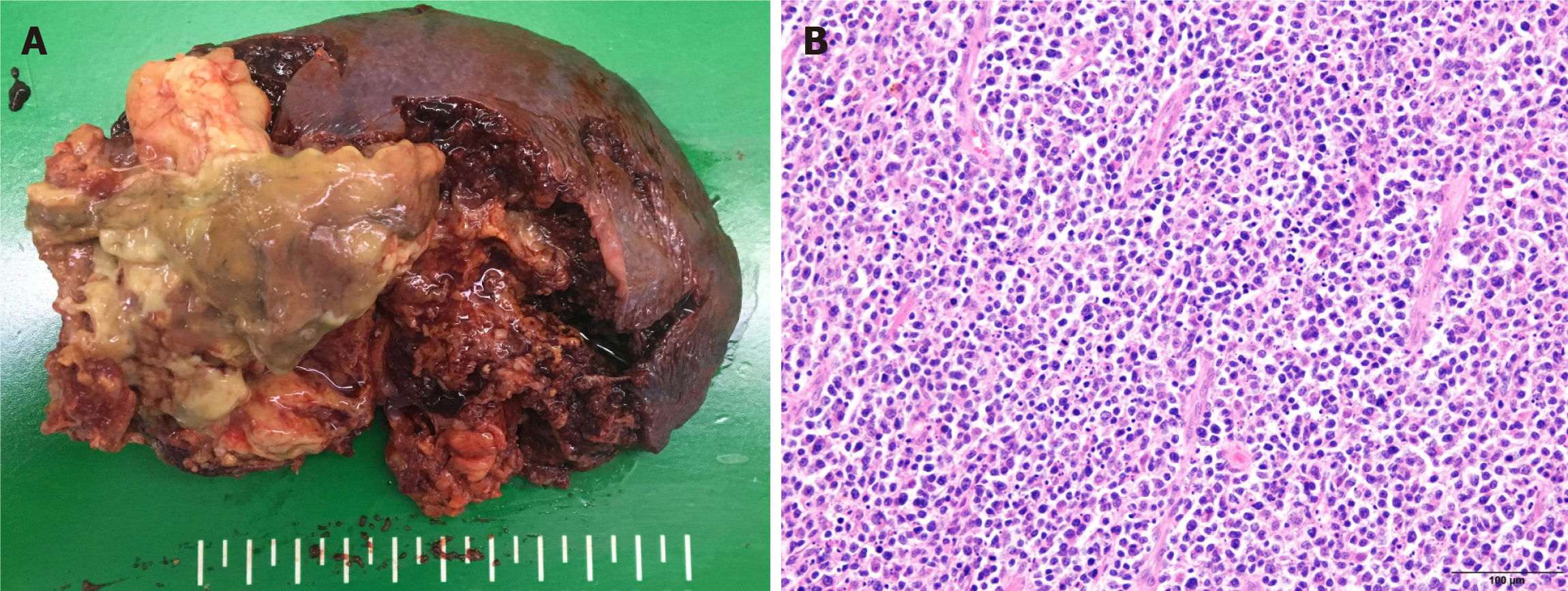
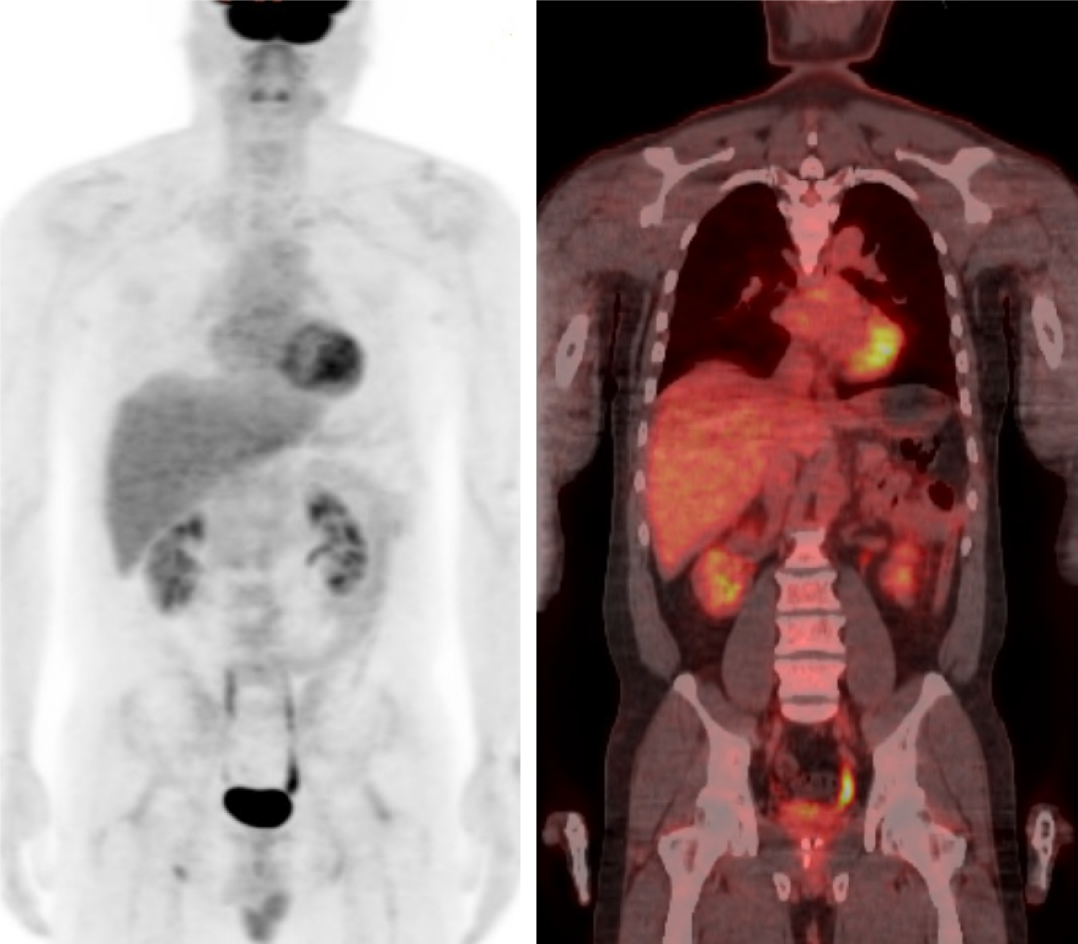
Pathological finding of the resected splenic mass was consistent with diffuse large B-cell lymphoma (DLBCL) (Figure 4). 18F-Fluorodeoxyglucose (FDG)-positron emission tomography (PET) (Figure 5) and bone marrow biopsy were examined for staging, and there was no other organ involvement of lymphoma. Our patient was in Ann Arbor stage I with non-bulky (< 7.5 cm) mass and international prognostic index was 0 (age < 60 years, normal serum LDH, performance status 0, Ann Arbor stage I, and no extranodal disease)[6]. He underwent 4 cycles of chemotherapy with rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisolone (R-CHOP) after splenectomy[6].
He has followed up blood exams and CT every 6 mo and he is in complete remission until now, 20 mo after chemotherapy.
Our patient underwent EUS-FNB of a splenic mass and developed septic shock due to a splenic abscess. To the best of our knowledge, this is the first case of septic shock after EUS-FNB of a splenic lymphoma. Also, EUS-related infections caused by G. adiacens, rarely causing any disease, have never been reported before.
Conclusive diagnosis of a splenic mass by radiology is difficult because splenic tumors are relatively uncommon and different splenic tumors often share similar radiologic findings[7]. The most common primary malignant tumor of the spleen is lymphoma[8]. More often, lymphomatous involvement of the spleen is secondary and primary splenic lymphoma (PSL), defined as lymphomatous involvement of the spleen without peripheral lymph node involvement, is rare (< 1%)[9]. Imaging findings of splenic lymphomas (in the order of most to least frequent) are homogeneous splenomegaly, diffuse infiltration with miliary lesions, multiple tumoral foci, and a large solitary mass[10]. Splenic lymphomas present with hypodense in plain CT and hypo-enhancement in post-contrast CT[11]. Significant necrosis is observed often, especially in large tumors and large cell lymphomas[12]. In MRI, primary lymphomas show hypointense or nearly isointense on T1-weighted images, hyperintense on T2-weighted images, and mild or absent enhancement on gadolinium enhanced images[9].
SANT of the spleen is a benign vascular lesion consisting of multiple angiomatoid nodules surrounded by a dense fibrous tissue[13]. It commonly presents as a solitary and well-circumscribed splenic mass with smooth borders. SANT rarely cause symptoms and therefore, most SANT lesions are found incidentally[14]. MRI of the SANT shows heterogeneously hypointense on T1 and T2-weighted images and progressive enhancement with peripheral and septal enhancement in “spoke wheel” pattern in gadolinum-enhanced T1-weighted images[13]. We suspected SANT of the spleen in our patient because there was a solitary mass in the spleen without any other organ involvement and the splenic lesion showed peripheral enhancement in contrast enhanced T1 weighted image. Also, the splenic mass was found incidentally after a traffic accident and he had no subjective symptoms such as fever, night sweat, or bodyweight loss, which are known as the B symptoms of lymphoma[11]. However, conclusive diagnosis of the splenic lesion based on the radiologic studies was impossible and pathological confirmation was needed.
EUS-guided splenic biopsy has become popular due to the advantage of providing a clear image of the spleen through the gastric wall and safety and effectiveness for diagnosis of splenic tumors[2]. Until now, there has been no report of serious infection after EUS-FNB of splenic lesion. Therefore, we decided to perform EUS-FNB of the splenic mass in our patient.
The risk of infection associated with EUS-guided sampling of solid organs is low, compared with cystic lesions[3]. In a retrospective analysis of 16 cases of EUS-guided FNA of a focal splenic lesion, no infectious events were found[15]. Recent guidelines do not recommend prophylactic antibiotics for the FNA or FNB of solid masses and lymph nodes[3]. Based on the recommendations, we did not prescribe prophylactic antibiotics at the time of EUS-FNB of the splenic mass; however, our patient developed septic shock due to the splenic abscess 10 days after the procedure.
The risk factors of infection after EUS-guided sampling are cystic lesions regardless of the location, ascites, and pleural fluid around the lesions[16]. CT or MRI findings of the splenic mass in our patient had none of these risk factors. Necrosis is also a well-known risk factor for infection[17]. However, initial EUS of the spleen in our patient showed a solid mass without necrotic changes. Also, he had no other risk factors for infection such as diabetes mellitus. Laboratory findings before EUS-FNB showed no elevation of inflammatory markers. However, septic shock developed and a new splenic abscess was found in MRI obtained at readmission. Therefore, the splenic abscess and sepsis in our patient was likely due to EUS-FNB and his immunocom-promised status, later proven as lymphoma.
Patients with hematologic malignancies usually have weak immunity and are vulnerable to bacterial infections, due to immunoglobulin abnormalities and complement system dysfunction[18]. Our patient also had decreased complement 3 level. Patients with advanced hematologic malignancies have high risk of bacteremia and sepsis after the gastrointestinal endoscopy[19]. As our patient was diagnosed with DLBCL, he had the susceptibility for a bacterial infection and sepsis after the EUS-FNB, and, consequently, a splenic abscess and septic shock developed.
Limited data are available regarding the incubation period for infection after EUS-guided sampling due to the rarity of this complication. In a systematic review and meta-analysis, infectious events after EUS-FNA of the pancreatic cystic lesions were reported to be 0.44% (19/5124 patients), and symptoms to suspect infection developed about 2 d to 7 d after EUS-FNA of pancreatic cystic lesions[20-23]. In several case series, mediastinal infection including abscess developed 2 d to 15 d after EUS-FNA of mediastinal masses or lymph nodes[24-27]. Our patient felt chillness 7 d after EUS-FNB and septic shock developed after 10 d of the procedure. Initial evaluation of the splenic lesion including CT, MRI, and EUS showed no abscess formation, but MRI obtained on readmission showed a new abscess in the splenic mass. Therefore, it is likely that the splenic abscess was the infectious complication of EUS-FNB in our patient.
G. adiacens, formerly known as nutritionally variant streptococci (NVS), is present in the normal flora of the oral cavity, gastrointestinal and genitourinary tract and rarely causes any infection. When the infection occurs, it is most commonly presented as endocarditis or bacteremia, although meningitis, osteomyelitis, peritonitis, and infections of foreign bodies such as prosthetic devices have been reported[5]. So far, no case of splenic abscess or bacteremia due to G. adiacens after the EUS-FNB has been reported.
G. adiacens is associated with bacteremia in immunocompromised patients, especially, with hematologic malignancies. In a retrospective study that included 13 patients with NVS bacteremia, 77% of the them had hematologic malignancy[28]. Our patient had DLBCL of the spleen, and he might have been contaminated with the G. adiacens during the EUS-guided sampling of the splenic mass, leading to the splenic abscess, bacteremia, and septic shock.
The protective effect of prophylactic antibiotics in patients with hematologic malignancy has not been studied. No large scale, prospective studies on the effects of prophylactic antibiotics for the EUS-FNB of splenic tumors have been conducted, and further research is necessary. However, our case showed that prophylactic antibiotic usage might be considered in splenic tumor patients, taking into account lymphoma as the most common primary tumor of the spleen, to avoid severe infection after the EUS-guided sampling. In our patient, there was no other organ involvement of lymphoma and biopsy had to be performed at the spleen. However, because most splenic lymphomas are secondary and PSL is rare, looking for other organ involvement using various imaging studies including CT, FDG-PET and laboratory tests is important before EUS-FNB. If other organ involvement such as peripheral lymph nodes are found, biopsy can be performed at these sites to decrease the risk of infection.
Studies regarding bacteremia and infectious complication following EUS revealed that blood cultures were positive for various pathogens including viridans group streptococci, coagulase negative staphylococcus, and Gram negative bacilli[16,29]. There is no prospective randomized study in which antibiotics should be administered before the EUS-guided sampling. In the studies for antibiotic prophylaxis of EUS-guided sampling of pancreatic cystic lesions, fluoroquinolones or beta-lactams were used most often, because these antibiotics can cover both gram positive and gram negative organisms[4]. The optimal duration or dosage of antibiotic therapy has not been studied adequately as well. Most studies used prophylactic antibiotics intravenously at first, followed by orally for 3-5 d[4]. Therefore, although the effectiveness of antibiotics differs as local patterns of resistance, obtaining the susceptibility and source of infection, fluoroquinolones or beta-lactam antibiotics as prophylaxis, intravenously at first followed by oral administration could be used before EUS-guided sampling of a splenic mass. Further large scale, prospective studies are needed regarding the antibiotic prophylaxis of EUS-guided sampling.
Although EUS-FNB is considered a safe method and the risk of infection in the solid lesions is low, severe infection can occur after EUS-FNB of a splenic lymphoma. Therefore, prophylactic antibiotics for EUS-guided sampling of splenic tumors should be considered.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: South Korea
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C, C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Carrara S, Kawashima H, Yumoto T S-Editor: Gao CC L-Editor: A P-Editor: Li JH
| 1. | Civardi G, Vallisa D, Bertè R, Giorgio A, Filice C, Caremani M, Caturelli E, Pompili M, De Sio I, Buscarini E, Cavanna L. Ultrasound-guided fine needle biopsy of the spleen: high clinical efficacy and low risk in a multicenter Italian study. Am J Hematol. 2001;67:93-99. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 93] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 2. | Iwashita T, Yasuda I, Tsurumi H, Goto N, Nakashima M, Doi S, Hirose Y, Takami T, Moriwaki H. Endoscopic ultrasound-guided fine needle aspiration biopsy for splenic tumor: a case series. Endoscopy. 2009;41:179-182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 24] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 3. | ASGE Standards of Practice Committee, Early DS, Acosta RD, Chandrasekhara V, Chathadi KV, Decker GA, Evans JA, Fanelli RD, Fisher DA, Fonkalsrud L, Hwang JH, Jue TL, Khashab MA, Lightdale JR, Muthusamy VR, Pasha SF, Saltzman JR, Sharaf RN, Shergill AK, Cash BD. Adverse events associated with EUS and EUS with FNA. Gastrointest Endosc. 2013;77:839-843. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 149] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 4. | Polkowski M, Jenssen C, Kaye P, Carrara S, Deprez P, Gines A, Fernández-Esparrach G, Eisendrath P, Aithal GP, Arcidiacono P, Barthet M, Bastos P, Fornelli A, Napoleon B, Iglesias-Garcia J, Seicean A, Larghi A, Hassan C, van Hooft JE, Dumonceau JM. Technical aspects of endoscopic ultrasound (EUS)-guided sampling in gastroenterology: European Society of Gastrointestinal Endoscopy (ESGE) Technical Guideline - March 2017. Endoscopy. 2017;49:989-1006. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 268] [Cited by in RCA: 254] [Article Influence: 31.8] [Reference Citation Analysis (0)] |
| 5. | Gardenier JC, Hranjec T, Sawyer RG, Bonatti H. Granulicatella adiacens bacteremia in an elderly trauma patient. Surg Infect (Larchmt). 2011;12:251-253. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 6. | Zelenetz AD, Gordon LI, Abramson JS, Advani RH, Bartlett NL, Caimi PF, Chang JE, Chavez JC, Christian B, Fayad LE, Glenn MJ, Habermann TM, Lee Harris N, Hernandez-Ilizaliturri F, Kaminski MS, Kelsey CR, Khan N, Krivacic S, LaCasce AS, Mehta A, Nademanee A, Rabinovitch R, Reddy N, Reid E, Roberts KB, Smith SD, Snyder ED, Swinnen LJ, Vose JM, Dwyer MA, Sundar H. NCCN Guidelines Insights: B-Cell Lymphomas, Version 3.2019. J Natl Compr Canc Netw. 2019;17:650-661. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 116] [Article Influence: 23.2] [Reference Citation Analysis (0)] |
| 7. | Lee HJ, Kim JW, Hong JH, Kim GS, Shin SS, Heo SH, Lim HS, Hur YH, Seon HJ, Jeong YY. Cross-sectional Imaging of Splenic Lesions: RadioGraphics Fundamentals | Online Presentation. Radiographics. 2018;38:435-436. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 24] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 8. | Siewert B, Millo NZ, Sahi K, Sheiman RG, Brook OR, Sun MRM, Kane RA. The Incidental Splenic Mass at CT: Does It Need Further Work-up? Radiology. 2018;287:156-166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 17] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 9. | Giovagnoni A, Giorgi C, Goteri G. Tumours of the spleen. Cancer Imaging. 2005;5:73-77. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 74] [Cited by in RCA: 72] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 10. | Saboo SS, Krajewski KM, O'Regan KN, Giardino A, Brown JR, Ramaiya N, Jagannathan JP. Spleen in haematological malignancies: spectrum of imaging findings. Br J Radiol. 2012;85:81-92. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 96] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 11. | Li M, Zhang L, Wu N, Huang W, Lv N. Imaging findings of primary splenic lymphoma: a review of 17 cases in which diagnosis was made at splenectomy. PLoS One. 2013;8:e80264. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 35] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 12. | Nakashima A, Nakashima K, Seto H, Kamei T, Kakishita M, Kitagawa M. Primary splenic lymphoma presenting as a large cyst. Radiat Med. 1994;12:42-45. [PubMed] |
| 13. | Lewis RB, Lattin GE Jr, Nandedkar M, Aguilera NS. Sclerosing angiomatoid nodular transformation of the spleen: CT and MRI features with pathologic correlation. AJR Am J Roentgenol. 2013;200:W353-W360. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 45] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 14. | Martel M, Cheuk W, Lombardi L, Lifschitz-Mercer B, Chan JK, Rosai J. Sclerosing angiomatoid nodular transformation (SANT): report of 25 cases of a distinctive benign splenic lesion. Am J Surg Pathol. 2004;28:1268-1279. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 182] [Cited by in RCA: 173] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 15. | Rana SS, Sharma V, Sharma R, Srinivasan R, Gupta R. Safety and utility of endoscopic ultrasound-guided fine-needle aspiration of focal splenic lesions: a retrospective analysis. Ann Gastroenterol. 2017;30:559-563. [PubMed] |
| 16. | Jenssen C, Alvarez-Sánchez MV, Napoléon B, Faiss S. Diagnostic endoscopic ultrasonography: assessment of safety and prevention of complications. World J Gastroenterol. 2012;18:4659-4676. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 80] [Cited by in RCA: 98] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 17. | Beger HG, Bittner R, Block S, Büchler M. Bacterial contamination of pancreatic necrosis. A prospective clinical study. Gastroenterology. 1986;91:433-438. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 626] [Cited by in RCA: 527] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 18. | Safdar A, Armstrong D. Infections in patients with hematologic neoplasms and hematopoietic stem cell transplantation: neutropenia, humoral, and splenic defects. Clin Infect Dis. 2011;53:798-806. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 97] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 19. | ASGE Standards of Practice Committee. , Khashab MA, Chithadi KV, Acosta RD, Bruining DH, Chandrasekhara V, Eloubeidi MA, Fanelli RD, Faulx AL, Fonkalsrud L, Lightdale JR, Muthusamy VR, Pasha SF, Saltzman JR, Shaukat A, Wang A, Cash BD. Antibiotic prophylaxis for GI endoscopy. Gastrointest Endosc. 2015;81:81-89. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 225] [Cited by in RCA: 242] [Article Influence: 24.2] [Reference Citation Analysis (2)] |
| 20. | de Jong K, Poley JW, van Hooft JE, Visser M, Bruno MJ, Fockens P. Endoscopic ultrasound-guided fine-needle aspiration of pancreatic cystic lesions provides inadequate material for cytology and laboratory analysis: initial results from a prospective study. Endoscopy. 2011;43:585-590. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 99] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 21. | Guarner-Argente C, Shah P, Buchner A, Ahmad NA, Kochman ML, Ginsberg GG. Use of antimicrobials for EUS-guided FNA of pancreatic cysts: a retrospective, comparative analysis. Gastrointest Endosc. 2011;74:81-86. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 64] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 22. | Williams DB, Sahai AV, Aabakken L, Penman ID, van Velse A, Webb J, Wilson M, Hoffman BJ, Hawes RH. Endoscopic ultrasound guided fine needle aspiration biopsy: a large single centre experience. Gut. 1999;44:720-726. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 476] [Cited by in RCA: 436] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 23. | Zhu H, Jiang F, Zhu J, Du Y, Jin Z, Li Z. Assessment of morbidity and mortality associated with endoscopic ultrasound-guided fine-needle aspiration for pancreatic cystic lesions: A systematic review and meta-analysis. Dig Endosc. 2017;29:667-675. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 72] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 24. | Valli PV, Gubler C, Bauerfeind P. Severe Infectious Complications after Endoscopic Ultrasound-Guided Fine Needle Aspiration of Suspected Mediastinal Duplication Cysts: A Case Series. Inflamm Intest Dis. 2017;1:165-171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 25. | von Bartheld M, van der Heijden E, Annema J. Mediastinal abscess formation after EUS-guided FNA: are patients with sarcoidosis at increased risk? Gastrointest Endosc. 2012;75:1104-1107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 17] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 26. | Aerts JG, Kloover J, Los J, van der Heijden O, Janssens A, Tournoy KG. EUS-FNA of enlarged necrotic lymph nodes may cause infectious mediastinitis. J Thorac Oncol. 2008;3:1191-1193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 40] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 27. | Savides TJ, Margolis D, Richman KM, Singh V. Gemella morbillorum mediastinitis and osteomyelitis following transesophageal endoscopic ultrasound-guided fine-needle aspiration of a posterior mediastinal lymph node. Endoscopy. 2007;39 Suppl 1:E123-E124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 28. | Yacoub AT, Krishnan J, Acevedo IM, Halliday J, Greene JN. Nutritionally variant streptococci bacteremia in cancer patients: a retrospective study, 1999-2014. Mediterr J Hematol Infect Dis. 2015;7:e2015030. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 29. | Janssen J, König K, Knop-Hammad V, Johanns W, Greiner L. Frequency of bacteremia after linear EUS of the upper GI tract with and without FNA. Gastrointest Endosc. 2004;59:339-344. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 66] [Article Influence: 3.1] [Reference Citation Analysis (0)] |