Published online Feb 14, 2021. doi: 10.3748/wjg.v27.i6.523
Peer-review started: November 7, 2020
First decision: December 3, 2020
Revised: December 12, 2020
Accepted: December 27, 2020
Article in press: December 27, 2020
Published online: February 14, 2021
Processing time: 90 Days and 10.2 Hours
Nonalcoholic fatty liver disease (NAFLD) and type-2 diabetes mellitus (T2DM) have an intricate bidirectional relationship. Individuals with T2DM, not only have a higher prevalence of non-alcoholic steatosis, but also carry a higher risk of progression to nonalcoholic steatohepatitis. Experts still differ in their recommendations of screening for NAFLD among patients with T2DM.
To study the prevalence of NAFLD and advanced fibrosis among our patient population with T2DM.
During the study period (November 2018 to January 2020), 59 adult patients with T2DM and 26 non-diabetic control group individuals were recruited prospectively. Patients with known significant liver disease and alcohol use were excluded. Demographic data and lab parameters were recorded. Liver elastography was performed in all patients.
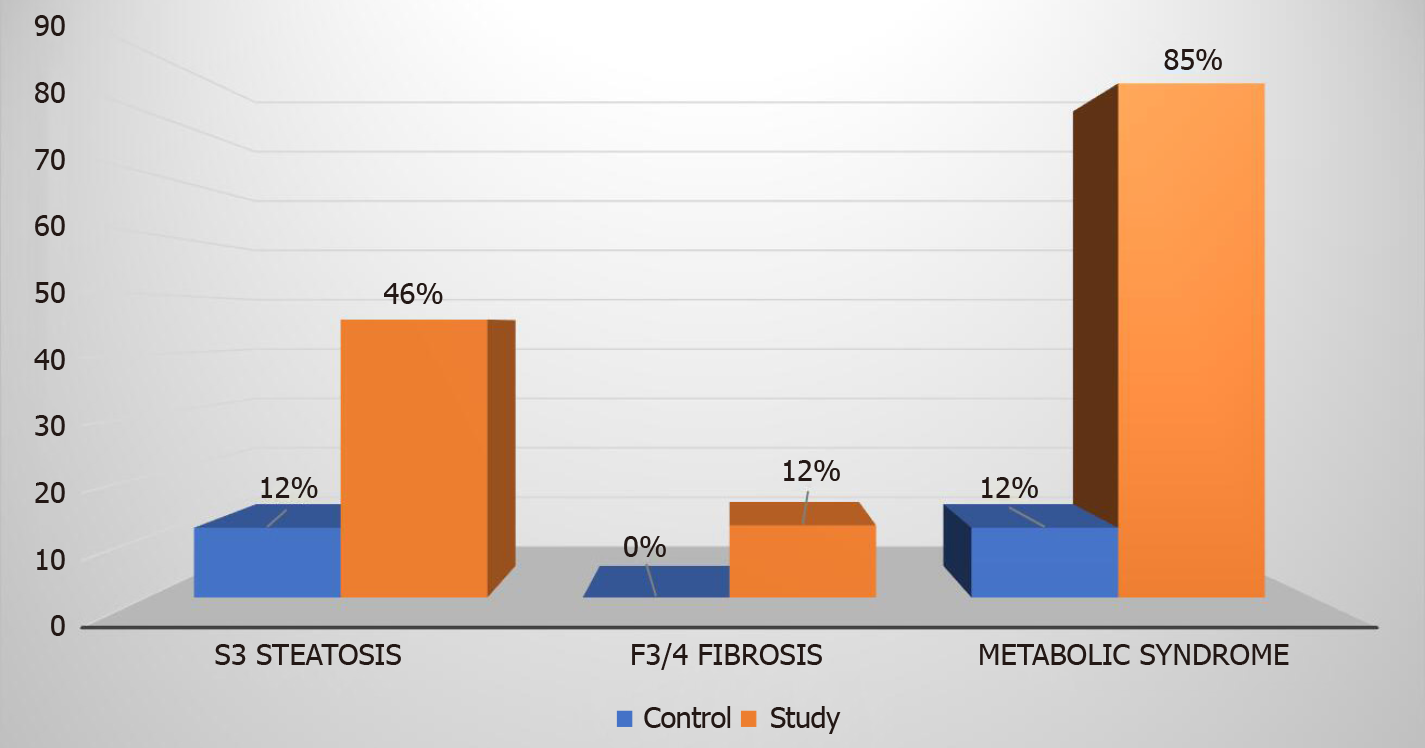
In the study group comprised of patients with T2DM and normal alanine aminotransferase levels (mean 17.8 ± 7 U/L), 81% had hepatic steatosis as diagnosed by elastography. Advanced hepatic fibrosis (stage F3 or F4) was present in 12% of patients with T2DM as compared to none in the control group. Patients with T2DM also had higher number of individuals with grade 3 steatosis [45.8% vs 11.5%, (P < 0.00001) and metabolic syndrome (84.7% vs 11.5%, P < 0.00001)].
A significant number of patients with T2DM, despite having normal transaminase levels, have NAFLD, grade 3 steatosis and advanced hepatic fibrosis as measured by liver elastography.
Core Tip: Individuals with type 2 diabetes mellitus (T2DM) have a higher prevalence of non-alcoholic steatosis and a higher risk of progression to non-alcoholic steatohepatitis and cirrhosis. Experts differ in their screening recommendations for nonalcoholic fatty liver disease among patients with T2DM. We prospectively recruited and performed liver elastography on 59 diabetics and 26 non-diabetic control patients. Patients with known liver disease and alcohol use were excluded. Our study shows advanced fibrosis is prevalent among patients with T2DM as compared to non-diabetics, even with normal liver enzymes. Screening for liver fibrosis in all patients with T2DM should be considered, regardless of liver enzyme levels.
- Citation: Makker J, Tariq H, Kumar K, Ravi M, Shaikh DH, Leung V, Hayat U, Hassan MT, Patel H, Nayudu S, Chilimuri S. Prevalence of advanced liver fibrosis and steatosis in type-2 diabetics with normal transaminases: A prospective cohort study. World J Gastroenterol 2021; 27(6): 523-533
- URL: https://www.wjgnet.com/1007-9327/full/v27/i6/523.htm
- DOI: https://dx.doi.org/10.3748/wjg.v27.i6.523
Nonalcoholic fatty liver disease (NAFLD) encompasses a spectrum of clinico-pathological conditions ranging from simple fatty liver disease to nonalcoholic steatohepatitis (NASH). The risk of progression of NASH to cirrhosis has previously been estimated at 21%-26% over a mean follow up of 8.2 years[1]. With the globalization of western diets and increasingly sedentary lifestyles, obesity rates have skyrocketed to epidemic proportions. In parallel with obesity, the prevalence of NAFLD has dramatically increased. The prevalence of NAFLD worldwide ranges from 6% to 35% depending on the method used for diagnosis and the study population[2]. The prevalence in United States has ranged between 11%-34% with the most recent study reporting a prevalence of 46%[3]. As a result of its increasing prevalence, NAFLD has now become the leading cause of liver disease in the western countries[2]. With the rising rates of NAFLD, and the availability of effective durable treatment options for other chronic liver diseases like chronic hepatitis C, experts have predicted NAFLD to become the leading cause of liver transplantation in the near future.
NAFLD and type-2 diabetes mellitus (T2DM) are intricately related to each other and this relationship is bidirectional. Development of insulin resistance is a key phenomenon underlying NAFLD as well as T2DM and as a result, these two disorders commonly occur together. Individuals with T2DM have a higher prevalence of NAFLD[4]. Individuals with T2DM who develop non-alcoholic steatosis also carry a higher risk of progression to NASH[5]. Development of NASH is characterized by progressive hepatocyte necroinflammation which is an important harbinger of cirrhosis and its associated complications such as hepatocellular cancer. In a study from the United States involving 1249 patients with T2DM and biopsy proven NAFLD, prevalence of NASH and advanced liver fibrosis was 69.2% and 41% respectively[6]. A Canadian study with a 12-year follow-up involving nearly 2.5 million participants, among which nearly one-fifth had newly diagnosed T2DM, found more than two-fold risk of incident liver cirrhosis in patients with newly diagnosed T2DM[7].
Despite the alarming rise in NAFLD prevalence among patients with T2DM, major societies differ in their recommendations about screening for NAFLD among patients with T2DM. The American Association for the Study of Liver Diseases does not recommend NAFLD screening in individuals with T2DM due to its unknown long-term benefits and cost-effectiveness[8]. On the other hand, American Diabetes Association recommends assessment of liver fibrosis in patients with T2DM and elevated transaminases[9]. Our study was conducted to explore the prevalence of NAFLD and advanced fibrosis among our minority inner city population with T2DM who had normal transaminases.
From November 2018 to January 2020, all patients aged 18 years and above with T2DM attending the endocrine clinic at our hospital were offered enrollment in this prospective cohort study. All patients who agreed, provided a written and informed consent for their participation. Patients with a known diagnosis of cirrhosis, chronic hepatitis B, chronic hepatitis C, autoimmune hepatitis, drug induced hepatitis, cholestatic liver disease, hemochromatosis and alcoholic liver disease were excluded. Patients with significant alcohol use defined as more than 20 g of daily alcohol use were excluded. Furthermore, any patient with alanine transaminase (ALT) level more than 40 (upper limit of normal at our lab) was excluded.
The study protocol conformed to the ethical guidelines of the 1975 Declaration of Helsinki, as reflected in the Bronx Care Health System Institutional Review Board’s approval (IRB #05 10 18 04).
The control group comprised of patients without T2DM and normal ALT level (< 40 U/L) as defined by our lab. Patients were recruited from the gastroenterology clinic at our hospital. These patients had no known history of fatty liver disease. Exclusion criteria similar to the study group were applied.
Demographic data including age, gender and ethnicity were collected. All comorbid medical conditions and a complete drug history were recorded via patient interviews. A physical examination including measurement of waist circumference, weight, height and body mass index (BMI) was performed at the time of inclusion. Laboratory data including liver enzymes, hemoglobin A1c, lipid profile available within one year of enrollment were recorded.
All patients were required to fast for three hours prior to elastography. Liver elastography was performed using the M or XL probe from Fibroscan 502 touch model (Echosens). The probe is automatically selected by the software based on thoracic perimeter and skin capsule distance measurements. Liver stiffness measurement and controlled attenuation parameter (CAP) which estimates the amount of liver fat was obtained. Elastography examination was considered reliable if at least 10 measurements were taken with an interquartile range interval per median LS less than 30%. Liver stiffness and CAP are calculated as a median over minimum 10 validated measurements and expressed in kilopascals (Kpa) and decibel per meter (db/m), respectively. Cut off for stage F0-1, F2, F3 and F4 fibrosis were ≤ 7 Kpa, ≥ 7.5 Kpa, ≥ 10 Kpa and ≥ 14.0 Kpa, respectively[10]. Cut off for CAP to diagnose hepatic steatosis grade 1 (5%-33% steatosis), grade 2 (34%-66% steatosis) and grade 3 (> 66% steatosis) were 238 db/m, 259 db/m and 290 db/m respectively[11].
Metabolic syndrome was defined by the National Cholesterol Education Program’s Adult Treatment Panel III criteria. It is defined as presence of at least three of the following criteria: Waist circumference > 102 cm in men or > 88 cm in women, plasma triglycerides ≥ 150 mg/dL, high density lipoprotein cholesterol < 40 mg/dL in men or < 50 mg/dL in women, blood pressure ≥ 130/85 mmHg, and fasting plasma glucose ≥ 110 mg/dL[12].
Sample size estimation was based on the assumption that prevalence of advanced fibrosis among patients with T2DM is around 20%. For a power of 80% at 5% significance level, 57 patients were required in the study group.
Statistical analysis was performed using statistical analysis system software, version 9.4 and graphpad prism software version 8.4.3. Frequencies and percentages were reported for categorical variables. Mean and standard deviations were reported for numerical continuous variables. Dichotomous variables were compared by chi-square analysis using the pearson test. A two-tailed value of < 0.05 was considered statistically significant. Bivariate analysis was done using analysis of variance to determine predictors of advanced fibrosis.
The study group comprised of 59 patients, whereas the control group had 26 patients. Demographics and baseline characteristics of both the groups were compared as shown in Table 1. The mean duration of T2DM in the study group was 15 ± 9 years. Diabetic microvascular complications including retinopathy, nephropathy, and neuropathy were prevalent in 22%, 30% and 17% of study patients, respectively. 41% of these patients were on insulin, 59% on metformin, 17% on sulfonylureas, 20% on glucagon-like peptide 1 receptor agonists, 8% on pioglitazone, 12% on sodium-glucose cotransporter-2 inhibitors, and 19% on dipeptidyl peptidase 4 inhibitors. Mean BMI of patients in the study group was significantly higher as compared to those in the control group (33.1 ± 8.4 vs 27.6 ± 4.7, P = 0.0002). A total of 7 patients in the study group were not obese and had BMI < 25, 18 patients were with BMI: 25-29.9, 11 had a BMI: 30-34.9, 14 had a BMI: 35-39.9, and 9 patients had a BMI of > 40.
| Variate | Control group, n = 26 | Study group, n = 59 | P value |
| Age (yr) | 53 ± 12.3 | 62 ± 11.7 | 0.0014 |
| Gender, n (%) | 0.8099 | ||
| Male | 9 (35) | 23 (39) | |
| Female | 17 (65) | 36 (61) | |
| Ethnicity, n (%) | 0.5005 | ||
| Hispanic | 21 (81) | 41 (69.5) | |
| African American | 3 (12) | 13 (22) | |
| Others | 2 (7) | 5 (8.5) | |
| Waist circumference (cm) | 97.5 ± 8.4 | 98. 6 ± 18.5 | 0.7595 |
| BMI (kg/m2) | 27.6 ± 4.7 | 33.1 ± 8.4 | 0.0002 |
| Hypertension, n (%) | 15 (58) | 43 (73) | 0.2083 |
| Hyperlipidemia, n (%) | 10 (38) | 35 (59) | 0.0999 |
| ALT (U/L) | 17 ± 7 | 17.8 ± 7 | 0.7189 |
| Platelet count (109/L) | 258 ± 81 | 237 ± 61 | 0.2007 |
| Albumin (g/L) | 4.4 ± 0.3 | 4.3 ± 0.3 | 0.0687 |
| HDL (mg/dL) | 57.7 ± 18.2 | 49.7 ± 15.5 | 0.0588 |
| LDL (mg/dL) | 106.3 ± 33.6 | 87.4 ± 35.5 | 0.0231 |
| TG (mg/dL) | 122.5 ± 79.3 | 154.7 ± 77.9 | 0.0883 |
| HbA1c | 5.4 ± 0.3 | 7.9 ± 1.8 | < 0.0001 |
| Liver stiffness using elastography (kPa) | 5.2 ± 1.6 | 7.5 ± 9.6 | 0.2138 |
| CAP (dB/m) | 230 ± 70 | 284 ± 64 | 0.0008 |
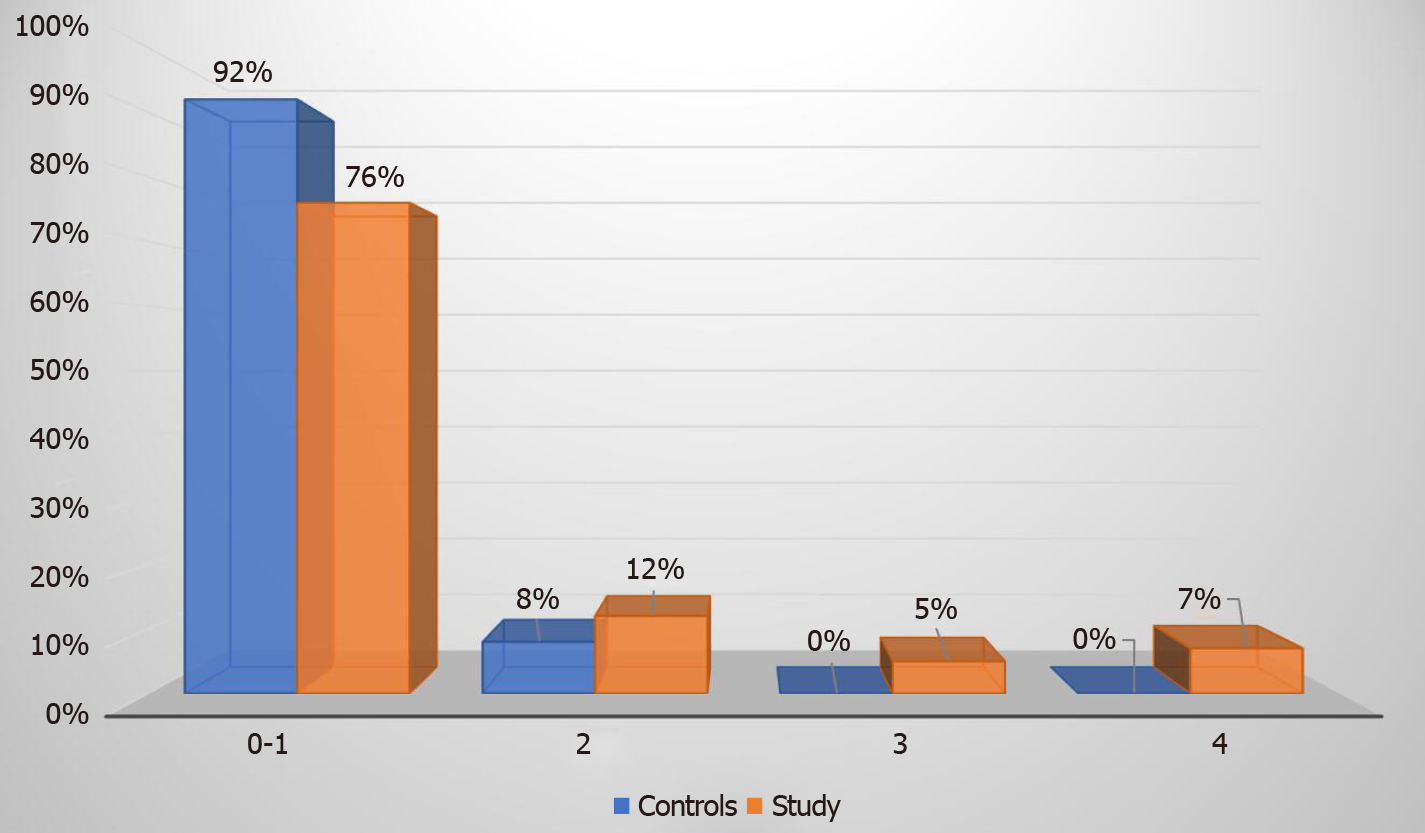
Elastography revealed that 76% of our study group patients had absent or low-grade (F0-1) fibrosis, 12% had F2 stage fibrosis, 5% had F3 stage fibrosis, and 7% had F4 stage fibrosis. Advanced fibrosis stage (F3 or F4) was diagnosed in a total of seven (12%) patients from the study group (Figure 1). In the control group none of the patients had advanced stage fibrosis, 2 (8%) patients had F2 fibrosis and 24 (92%) patients had F0-1 stage fibrosis (Figure 2).
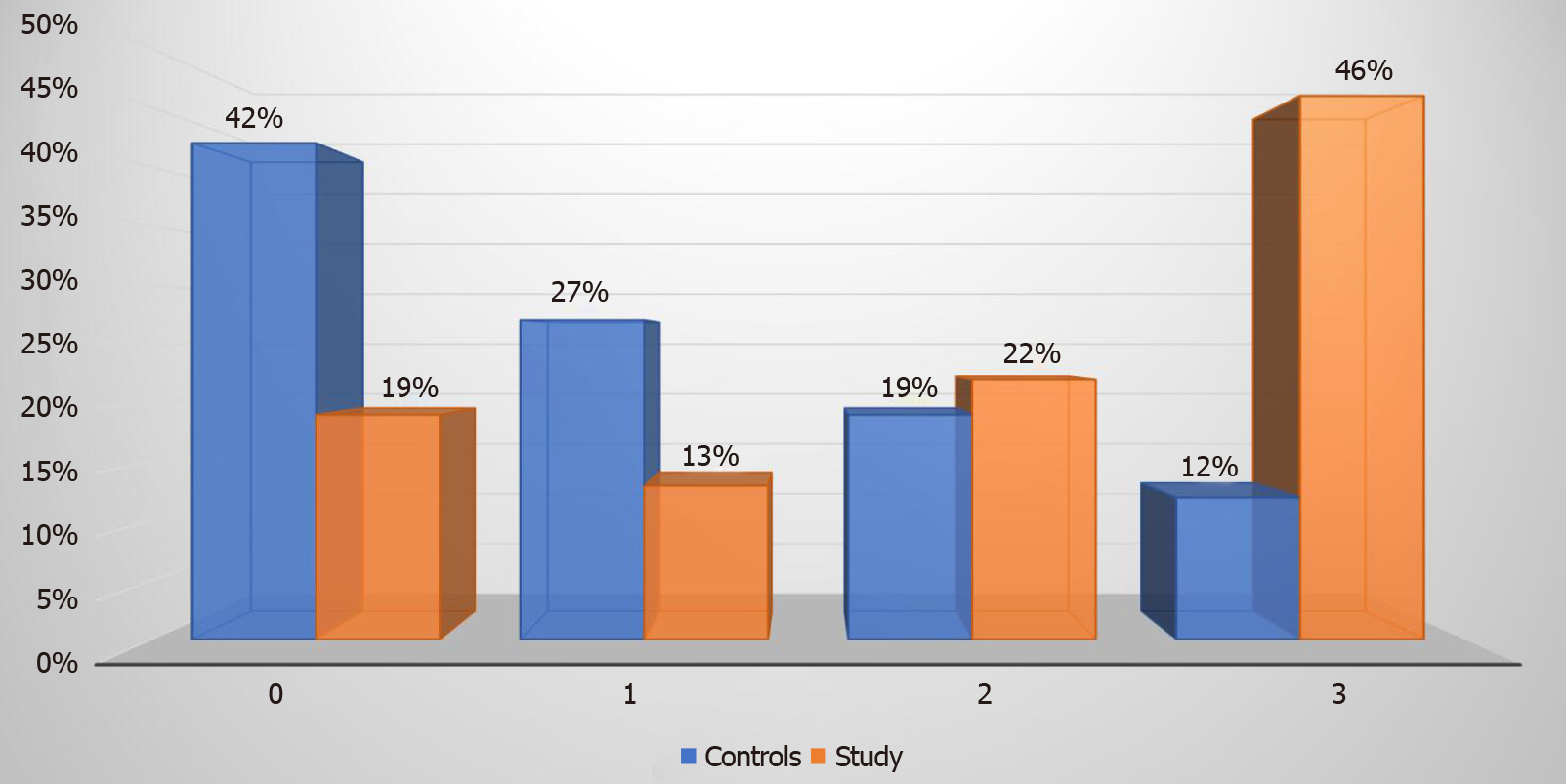
Eighty-one percent of our study group patients with T2DM had hepatic steatosis as diagnosed by liver elastography. Steatosis grade 3 was prevalent in 27 (46%) patients as compared to 3 (12%) patients in the control group (P value < 0.00001). Steatosis stage 0, 1, and 2 were diagnosed in 11 (19%), 8 (13%), and 13 (22%) patients respectively in the study group (Figure 3). In comparison, the control group had 11 (42%), 7 (27%), and 5 (19%) patients with steatosis stage 0, 1, and 2 respectively.
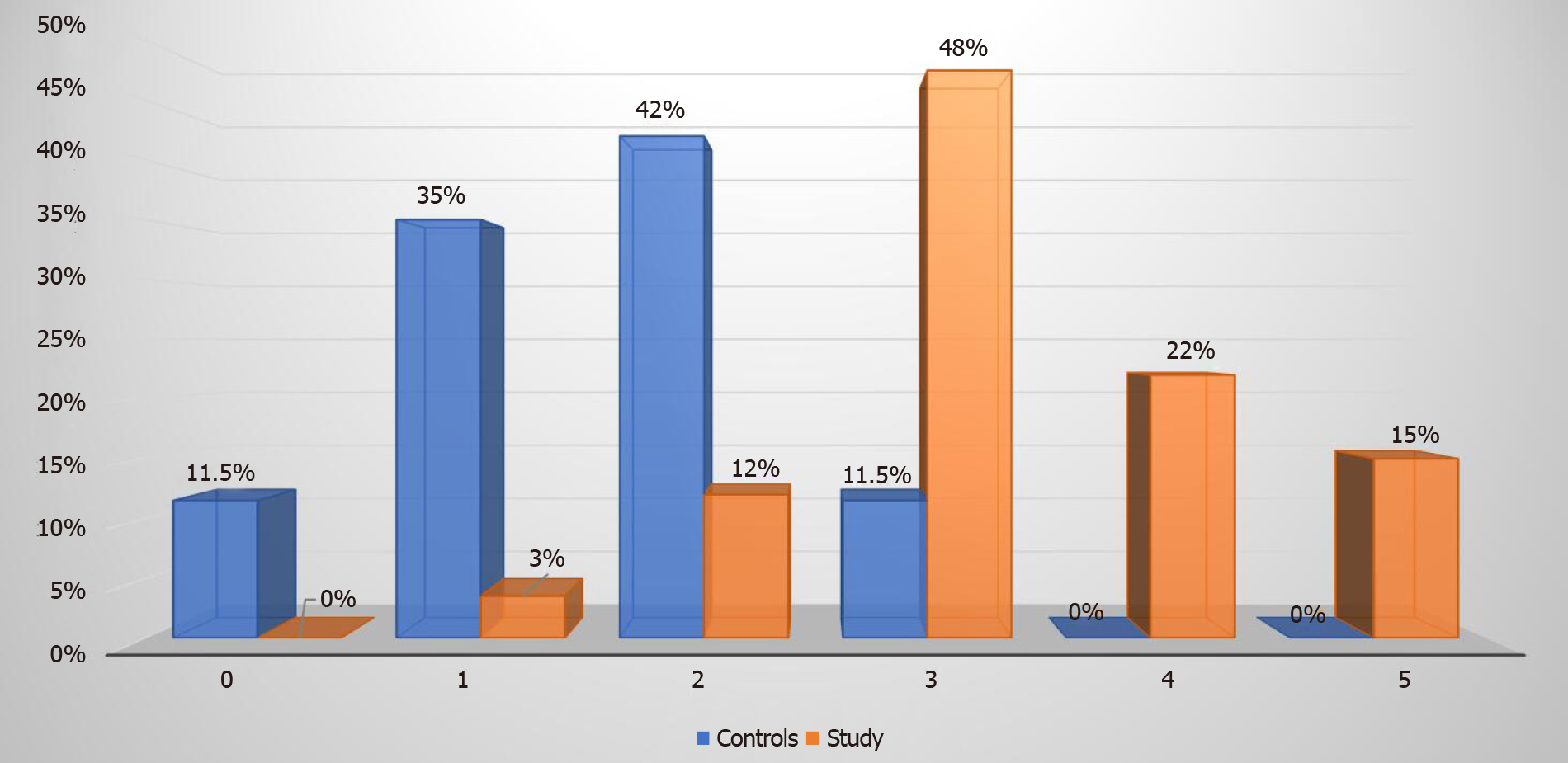
Metabolic syndrome was more prevalent among the study group patients. Fifty (85%) patients were diagnosed with metabolic syndrome in the study group and on the contrary only three (12%) patients in the control group had metabolic syndrome (P < 0.00001). Among the 27 (46%) patients in the study group who had S3 steatosis, 26 (96%) had metabolic syndrome. Twenty-eight out of the 59 (48%) patients who had metabolic syndrome in the study group met three criteria for metabolic syndrome, 13 (22%) patients met four criteria and remaining 9 (15%) patients met all the five criteria for metabolic syndrome (Figure 4).
Bivariate analysis was performed using analysis of variance to determine predictors of advanced fibrosis. Duration of T2DM was found to be a significant predictor (P < 0.05) of advanced fibrosis. Age (P = 0.29), BMI (P = 0.30), and waist circumference (P = 0.46) had no association with advanced fibrosis.
With the prevalence rate of obesity and T2DM reaching epidemic proportions, NAFLD has become a health crisis. NAFLD is seen frequently in T2DM with prevalence reported as high as 74% reported in one of the studies[13]. The pathophysiology of NAFLD and T2DM are intricately intertwined with insulin resistance serving as the common mediator. Severe hepatic necro-inflammation can progress to cirrhosis, especially more so in patients with T2DM. A study conducted by McPherson[14] has previously shown an alarmingly high rate of fibrosis progression not only in patients with NASH but also in individuals with NAFLD. The same study also showed that 22% of their patients who had NAFLD at their index liver biopsy showed progression to advanced fibrosis on their follow-up biopsy. Co-occurrence of NAFLD and T2DM poses a heightened risk of extrahepatic morbidity and mortality from cardiovascular disease, chronic kidney disease and hepatocellular cancer[15]. Despite these findings, guidelines from the expert societies differ in their opinion regarding the utility of screening for hepatic fibrosis in patients with NAFLD.
The prevalence of NAFLD in our study patients was higher than in previous reports, ranging from 40% to 70% in patients with T2DM[16]. Eighty-one percent of our patients with T2DM were found to have hepatic steatosis and nearly half of them had S3 steatosis. The prevalence of NAFLD in patients with T2DM previously reported in literature varies depending on the ethnicity and method used to diagnose NAFLD. Hispanics have been reported to have highest prevalence of non-alcoholic fatty liver disease[13] and more than 80% of our study patients were of Hispanic ethnicity.
Our study demonstrated that even patients with T2DM who had normal ALT levels were at risk of severe progressive liver disease. Twelve percent of our patients with T2DM had undiagnosed advanced fibrosis. Importantly, these patients who were noted to have advanced fibrosis had normal transaminases (mean ALT = 17.8 ± 7 U/L). Thus, based on current guidelines, these patients without clinically obvious cirrhosis would have gone undiagnosed for years. Among the seven study patients with BMI less than 25, five patients met criteria for metabolic syndrome, four patients had hepatic steatosis and one patient had advanced stage hepatic fibrosis. Thus, even non-obese patients with T2DM and normal transaminases could not be assumed to have a safer metabolic profile.
A cross sectional study done by Tuong et al[17] similarly studied the prevalence of hepatic fibrosis among patients with T2DM and found 73.3% of their diabetic patients to have NAFLD. The prevalence of F3 and F4 in their study group was 5.9% and 3.6% respectively, but they included patients with elevated transaminases as well, with a mean ALT of 60.6 U/L in the advanced fibrosis group. Another cross-sectional study by Lai et al[18] using transient elastography in patients with T2DM, showed a prevalence of cirrhosis and advanced fibrosis in 13.5% and 21% of patients, respectively. This study also included patients with elevated transaminases with a mean ALT of 38 U/L in patients with cirrhosis. A Turkish study found prevalence of advanced fibrosis of 16.9% and cirrhosis of 8% in their patients with T2DM. The mean ALT in their patients with F3 fibrosis was 39 U/L[19]. In a large prospective cohort study from Hong Kong involving 1918 patients with T2DM, increased liver stiffness suggestive of F3 or F4 fibrosis was found in 18.8% of their diabetic patients with normal ALT, defined as ALT less than 30 U/L in men and less than 19 U/L in women. This data is predominantly from an Asian population and does not reflect our study population[20]. In a study from Australia, prevalence of significant fibrosis, defined by the study as stage F2 and above, was 35% in diabetics. The mean ALT level was 38 U/L among these patients with significant fibrosis[21]. In a prospective French study, significant fibrosis defined as liver stiffness > 8.7 kPa was found in 12.9% of patients with T2DM. However, this study did not exclude patients with significant alcohol use and some of their patients found to have cirrhosis reported > 70 drinks of alcohol per week[22]. Prevalence of F4 stage fibrosis defined as liver stiffness > 9.5 kPA in another study from Romania was 18.6%. However, like most of the other studies, they too included patients with elevated ALT, with a significant number of patients having an ALT more than 40 U/L[23]. Currently, it is not the standard of care to assess liver fibrosis using liver biopsy in patients with normal ALT levels. However, it has been shown before that ALT is not a reliable criterion to exclude patients for assessment of liver fibrosis[24]. In this Italian study involving 458 liver biopsy confirmed NAFLD patients, 63 patients had normal ALT. NASH was demonstrated in 27% of those who had ALT levels less than 30 U/L in men and 19 U/L in women.
Liver biopsy is considered a gold standard diagnostic method for evaluating chronic liver diseases. In patients with NAFLD, it is particularly useful to assess for the presence of steatohepatitis which may progress to the development of cirrhosis. However, liver biopsy is an imperfect tool that is costly and invasive. It is associated with complications including pain in up to 50% of patients, serious bleeding in 0.6%, injury to other internal organs in about 0.08% and rarely death in 0.1% of patients[25]. Considering the enormous burden of NAFLD in our communities and the invasive nature of liver biopsy, it is not practically feasible to subject all patients with NAFLD to liver biopsy. Surrogate non-invasive methods of liver fibrosis assessment have been developed and are widely available. Vibration controlled liver elastography is one such tool, that is commonly available in United States. It is a quick office-based procedure with acceptable intra-observer and inter-observer variability which has been validated worldwide[26].
We noticed striking gender differences in our study population. In general, higher prevalence of NAFLD has been reported in males as compared to female patients. However, when age specific gender differences are explored further, post-menopausal women have a higher prevalence of NAFLD as compared to men[27]. Our study results are consistent with these findings, demonstrating that 86% of all female patients as compared to 78% of all male patients had NAFLD. Among the 23 male patients in the study group (mean age of 63 years) only one (4%) patient had advanced fibrosis. In comparison, 36 patients were females (mean age of 62 years) and a total of six (17%) had advanced fibrosis. Female patients not only had higher prevalence of NAFLD, but also had a higher prevalence of grade 3 steatosis which was observed in 55% of females as compared to 30% of males. Although gender has been consistently shown to be a significant modifier, gender specific personalized therapy for NAFLD has not been explored and further research is needed to investigate this area.
Considering the high prevalence of T2DM and NAFLD in our modern world, the burden of undiagnosed cirrhosis is enormous. Diagnosing these patients in a timely fashion would provide the opportunity to adopt intensive lifestyle modifications, and consequently avoid or at the least delay the progression to advanced hepatic fibrosis. Thus, it is important to recognize these patients early on, emphasize the progression of untreated NAFLD to liver cirrhosis, and potentially reduce morbidity, mortality and its related health care burden.
Our study has several limitations. Firstly, the number of overall patients in our study group is small and hence further studies with larger sample sizes are needed to validate our results. Second, we utilized liver elastography to diagnose NAFLD and liver biopsy was not performed in our patients, hence, we do not know the prevalence of NASH, which is the main driver for cirrhosis. However, it is not only unethical but risky too, to perform a liver biopsy in all T2DM patients especially with normal transaminase levels. Thirdly, the inclusion criteria in our study required a normal ALT level, defined as less than 40 U/L by our lab, which is higher than the current accepted standard. Nevertheless, the mean ALT level in the control and study group was 17 and 17.8 U/L, respectively. Fourthly, patients in our study group are older than the control group. Multiple studies have explored the influence of age on liver stiffness, and the results have been conflicting so far, reporting no difference across age groups[28,29], higher liver stiffness measurement in younger[30] or older[31] age. Lastly, our study and control group populations comprised predominantly of Hispanics and African Americans and hence these results cannot be generalized.
In conclusion, our study data shows that significant liver disease is prevalent among patients with T2DM, even with normal ALT levels. Measuring ALT levels in our study as a predictor of significant liver disease was unreliable. Timely work up with non-invasive techniques for estimation of liver fibrosis is warranted to prevent presentation at advanced stages of fibrosis. Primary care physicians and endocrinologists should be aware of this complication and should consider screening for liver fibrosis in all patients with T2DM, regardless of liver enzyme levels. Larger studies involving other ethnic groups are needed to validate our results.
With the current obesity epidemic, prevalence of non-alcoholic fatty liver disease (NAFLD) has increased. Individuals with type 2 diabetes mellitus (T2DM) have a higher prevalence of non-alcoholic steatosis, may carry a higher risk of progression to nonalcoholic steatohepatitis and eventually cirrhosis.
Experts still differ in their recommendations of screening for NAFLD among patients with T2DM.
To study the prevalence of NAFLD and advanced fibrosis among our patient population with T2DM with normal transaminases and without known liver disease.
Prospective cohort study assessing hepatic steatosis and fibrosis using transient elastography in 59 patients with T2DM, compared to 26 non-diabetic control group patients.
In our study group, 81% of patients had hepatic steatosis and 12% had advanced fibrosis on liver elastography. In the control group none of the patients had advanced stage fibrosis. Grade 3 steatosis was prevalent in 46% of patients in the study group as compared to 12% patients in the control group (P value < 0.00001).
A significant number of patients with T2DM, despite having normal transaminase levels, have advanced fibrosis or steatosis as measured by liver elastography.
Physicians should be aware of prevalence of significant liver disease among patients with T2DM, even with normal liver enzymes and should consider earlier screening to prevent presentation at advanced stages of fibrosis. Larger studies are needed to confirm and validate our results.
Manuscript source: Unsolicited manuscript
Corresponding Author's Membership in Professional Societies: American College of Gastroenterology; American Society for Gastrointestinal Endoscopy; American Gastroenterological Association.
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Malnick SD, Zhang LL S-Editor: Zhang L L-Editor: A P-Editor: Liu JH
| 1. | Matteoni CA, Younossi ZM, Gramlich T, Boparai N, Liu YC, McCullough AJ. Nonalcoholic fatty liver disease: a spectrum of clinical and pathological severity. Gastroenterology. 1999;116:1413-1419. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2357] [Cited by in RCA: 2345] [Article Influence: 90.2] [Reference Citation Analysis (0)] |
| 2. | Vernon G, Baranova A, Younossi ZM. Systematic review: the epidemiology and natural history of non-alcoholic fatty liver disease and non-alcoholic steatohepatitis in adults. Aliment Pharmacol Ther. 2011;34:274-285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2405] [Cited by in RCA: 2294] [Article Influence: 163.9] [Reference Citation Analysis (0)] |
| 3. | Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016;64:73-84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5322] [Cited by in RCA: 7542] [Article Influence: 838.0] [Reference Citation Analysis (0)] |
| 4. | Williamson RM, Price JF, Glancy S, Perry E, Nee LD, Hayes PC, Frier BM, Van Look LA, Johnston GI, Reynolds RM, Strachan MW; Edinburgh Type 2 Diabetes Study Investigators. Prevalence of and risk factors for hepatic steatosis and nonalcoholic Fatty liver disease in people with type 2 diabetes: the Edinburgh Type 2 Diabetes Study. Diabetes Care. 2011;34:1139-1144. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 312] [Cited by in RCA: 294] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 5. | Bian H, Zhu X, Xia M, Yan H, Chang X, Hu X, Pan B, Guo W, Li X, Gao X. Impact of Type 2 Diabetes on Nonalcoholic Steatohepatitis and Advanced Fibrosis in Patients with Nonalcoholic Fatty Liver Disease. Endocr Pract. 2020;26:444-453. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 29] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 6. | Bazick J, Donithan M, Neuschwander-Tetri BA, Kleiner D, Brunt EM, Wilson L, Doo E, Lavine J, Tonascia J, Loomba R. Clinical Model for NASH and Advanced Fibrosis in Adult Patients With Diabetes and NAFLD: Guidelines for Referral in NAFLD. Diabetes Care. 2015;38:1347-1355. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 131] [Cited by in RCA: 154] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 7. | Porepa L, Ray JG, Sanchez-Romeu P, Booth GL. Newly diagnosed diabetes mellitus as a risk factor for serious liver disease. CMAJ. 2010;182:E526-E531. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 87] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 8. | Chalasani N, Younossi Z, Lavine JE, Charlton M, Cusi K, Rinella M, Harrison SA, Brunt EM, Sanyal AJ. The diagnosis and management of nonalcoholic fatty liver disease: Practice guidance from the American Association for the Study of Liver Diseases. Hepatology. 2018;67:328-357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3544] [Cited by in RCA: 4954] [Article Influence: 707.7] [Reference Citation Analysis (9)] |
| 9. | American Diabetes Association. 4. Comprehensive Medical Evaluation and Assessment of Comorbidities: Standards of Medical Care in Diabetes-2020. Diabetes Care. 2020;43:S37-S47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 141] [Article Influence: 28.2] [Reference Citation Analysis (0)] |
| 10. | Bonder A, Afdhal N. Utilization of FibroScan in clinical practice. Curr Gastroenterol Rep. 2014;16:372. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 94] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 11. | Wang Y, Fan Q, Wang T, Wen J, Wang H, Zhang T. Controlled attenuation parameter for assessment of hepatic steatosis grades: a diagnostic meta-analysis. Int J Clin Exp Med. 2015;8:17654-17663. [PubMed] |
| 12. | Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, And Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III). JAMA. 2001;285:2486-2497. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20476] [Cited by in RCA: 20694] [Article Influence: 862.3] [Reference Citation Analysis (2)] |
| 13. | Williams CD, Stengel J, Asike MI, Torres DM, Shaw J, Contreras M, Landt CL, Harrison SA. Prevalence of nonalcoholic fatty liver disease and nonalcoholic steatohepatitis among a largely middle-aged population utilizing ultrasound and liver biopsy: a prospective study. Gastroenterology. 2011;140:124-131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1522] [Cited by in RCA: 1620] [Article Influence: 115.7] [Reference Citation Analysis (1)] |
| 14. | McPherson S, Hardy T, Henderson E, Burt AD, Day CP, Anstee QM. Evidence of NAFLD progression from steatosis to fibrosing-steatohepatitis using paired biopsies: implications for prognosis and clinical management. J Hepatol. 2015;62:1148-1155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 687] [Cited by in RCA: 793] [Article Influence: 79.3] [Reference Citation Analysis (0)] |
| 15. | Byrne CD, Targher G. NAFLD: a multisystem disease. J Hepatol. 2015;62:S47-S64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1516] [Cited by in RCA: 2140] [Article Influence: 214.0] [Reference Citation Analysis (0)] |
| 16. | Lazo M, Clark JM. The epidemiology of nonalcoholic fatty liver disease: a global perspective. Semin Liver Dis. 2008;28:339-350. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 521] [Cited by in RCA: 526] [Article Influence: 30.9] [Reference Citation Analysis (1)] |
| 17. | Tuong TTK, Tran DK, Phu PQT, Hong TND, Dinh TC, Chu DT. Non-Alcoholic Fatty Liver Disease in Patients with Type 2 Diabetes: Evaluation of Hepatic Fibrosis and Steatosis Using Fibroscan. Diagnostics (Basel). 2020;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 31] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 18. | Lai LL, Wan Yusoff WNI, Vethakkan SR, Nik Mustapha NR, Mahadeva S, Chan WK. Screening for non-alcoholic fatty liver disease in patients with type 2 diabetes mellitus using transient elastography. J Gastroenterol Hepatol. 2019;34:1396-1403. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 53] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 19. | Demir M, Deyneli O, Yılmaz Y. Screening for hepatic fibrosis and steatosis in Turkish patients with type 2 diabetes mellitus: A transient elastography study. Turk J Gastroenterol. 2019;30:266-270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 16] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 20. | Kwok R, Choi KC, Wong GL, Zhang Y, Chan HL, Luk AO, Shu SS, Chan AW, Yeung MW, Chan JC, Kong AP, Wong VW. Screening diabetic patients for non-alcoholic fatty liver disease with controlled attenuation parameter and liver stiffness measurements: a prospective cohort study. Gut. 2016;65:1359-1368. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 298] [Cited by in RCA: 363] [Article Influence: 40.3] [Reference Citation Analysis (0)] |
| 21. | Casey SP, Kemp WW, McLean CA, Topliss DJ, Adams LA, Roberts SK. A prospective evaluation of the role of transient elastography for the detection of hepatic fibrosis in type 2 diabetes without overt liver disease. Scand J Gastroenterol. 2012;47:836-841. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 26] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 22. | de Lédinghen V, Vergniol J, Gonzalez C, Foucher J, Maury E, Chemineau L, Villars S, Gin H, Rigalleau V. Screening for liver fibrosis by using FibroScan(®) and FibroTest in patients with diabetes. Dig Liver Dis. 2012;44:413-418. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 50] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 23. | Sima A, Sporea I, Timar R, Vlad M, Braha A, Popescu A, Nistorescu S, Mare R, Sirli R, Albai A, Albai O, Diaconu L, Sorescu T, Popescu S, Sima L. Non-Invasive Assessment of Liver Steatosis and Fibrosis using Transient Elastography and Controlled Attenuation Parameter in Type 2 Diabetes Patients. Acta Endocrinol (Buchar). 2018;14:394-400. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 24. | Fracanzani AL, Valenti L, Bugianesi E, Andreoletti M, Colli A, Vanni E, Bertelli C, Fatta E, Bignamini D, Marchesini G, Fargion S. Risk of severe liver disease in nonalcoholic fatty liver disease with normal aminotransferase levels: a role for insulin resistance and diabetes. Hepatology. 2008;48:792-798. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 462] [Cited by in RCA: 468] [Article Influence: 27.5] [Reference Citation Analysis (0)] |
| 25. | Tapper EB, Lok AS. Use of Liver Imaging and Biopsy in Clinical Practice. N Engl J Med. 2017;377:756-768. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 212] [Cited by in RCA: 282] [Article Influence: 35.3] [Reference Citation Analysis (0)] |
| 26. | Fraquelli M, Rigamonti C, Casazza G, Conte D, Donato MF, Ronchi G, Colombo M. Reproducibility of transient elastography in the evaluation of liver fibrosis in patients with chronic liver disease. Gut. 2007;56:968-973. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 676] [Cited by in RCA: 654] [Article Influence: 36.3] [Reference Citation Analysis (0)] |
| 27. | Lonardo A, Nascimbeni F, Ballestri S, Fairweather D, Win S, Than TA, Abdelmalek MF, Suzuki A. Sex Differences in Nonalcoholic Fatty Liver Disease: State of the Art and Identification of Research Gaps. Hepatology. 2019;70:1457-1469. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 689] [Cited by in RCA: 698] [Article Influence: 116.3] [Reference Citation Analysis (0)] |
| 28. | Ling W, Lu Q, Quan J, Ma L, Luo Y. Assessment of impact factors on shear wave based liver stiffness measurement. Eur J Radiol. 2013;82:335-341. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 56] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 29. | Mulabecirovic A, Mjelle AB, Gilja OH, Vesterhus M, Havre RF. Liver elasticity in healthy individuals by two novel shear-wave elastography systems-Comparison by age, gender, BMI and number of measurements. PLoS One. 2018;13:e0203486. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 34] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 30. | Colombo S, Belloli L, Zaccanelli M, Badia E, Jamoletti C, Buonocore M, Del Poggio P. Normal liver stiffness and its determinants in healthy blood donors. Dig Liver Dis. 2011;43:231-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 53] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 31. | Roulot D, Costes JL, Buyck JF, Warzocha U, Gambier N, Czernichow S, Le Clesiau H, Beaugrand M. Transient elastography as a screening tool for liver fibrosis and cirrhosis in a community-based population aged over 45 years. Gut. 2011;60:977-984. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 225] [Cited by in RCA: 247] [Article Influence: 17.6] [Reference Citation Analysis (0)] |