Published online Feb 7, 2021. doi: 10.3748/wjg.v27.i5.428
Peer-review started: November 5, 2020
First decision: December 13, 2020
Revised: December 24, 2020
Accepted: January 6, 2021
Article in press: January 6, 2021
Published online: February 7, 2021
Processing time: 85 Days and 4.6 Hours
Efficient bowel cleansing is essential for a successful colonoscopy, but the ideal cleansing agent, volume, and pharmaceutical dosage form have yet to be determined. Small-volume cleansers enhance patient compliance.
To compare the bowel cleansing efficacy of 32-tablet sodium phosphate (Quiklean®) with 2-L polyethylene glycol (PEG)/bisacodyl (Klean-Prep/ Dulcolax®) under identical dietary recommendations.
This multicenter, randomized, parallel-group, noninferiority clinical trial enrolled 472 outpatients, randomized 456 subjects, and scheduled 442 subjects to undergo colonoscopy (Quiklean® = 222 and Klean-Prep/Dulcolax® = 220). After bowel preparation, a colonoscopist performed the colonoscopy with video recorded for rating. The primary efficacy endpoint was the bowel cleansing quality using the Aronchick Scale. The secondary endpoints were the bowel cleansing efficacy of three colon segments, tolerability and acceptability, safety using the Ottawa bowel preparation scale, questionnaires by subjects, and monitoring of adverse events.
Success rates (Excellent + Good) of the bowel cleansing quality by Aronchick Scale were 98.6% (n = 205) and 97.6% (n = 204) in the Quiklean® and Klean-Prep/Dulcolax® groups, respectively. Quiklean® demonstrated noninferiority over Klean-Prep/Dulcolax® in colon cleansing efficacy. Quicken showed better tolerability and acceptability in the overall experience (was rated as excellent; 24.0% vs 17.2%; P = 0.0016) and the taste of the study preparation (was rated as excellent, 23.1% vs 13.4%; P < 0.0001) than Klean-Prep/Dulcolax®. Safety profiles did not differ between the two groups. Our data indicate that Quiklean® is an adequate, well-tolerated bowel cleansing preparation compared with the standard comparator Klean-Prep/Dulcolax®.
Quiklean® is sodium phosphate tablets available on Taiwan’s market for bowel preparation; it potentially offers patients an alternative to standard large-volume bowel preparation regimens and may, therefore, increase positive attitudes toward colonoscopies and participation rates.
Core Tip: The bowel cleanser 32-tablet sodium phosphate (Quiklean®) is an adequate bowel cleansing preparation with better tolerability and acceptability than the standard comparator 2-L polyethylene glycol/bisacodyl (Klean-Prep/Dulcolax®).
- Citation: Hung SY, Chen HC, Ke TW, Chen JH, Hsiao KH, Wang HM, Chiang HC, Chang SC, Chen YC, Hsieh MH, Tsai YY, Hsieh YW, Chen WTL. Noninferiority clinical trial comparing the bowel cleansing efficacy of sodium phosphate tablets (Quiklean®) with a polyethylene glycol/bisacodyl kit. World J Gastroenterol 2021; 27(5): 428-441
- URL: https://www.wjgnet.com/1007-9327/full/v27/i5/428.htm
- DOI: https://dx.doi.org/10.3748/wjg.v27.i5.428
Optimal bowel cleansing is essential before a colonoscopy, which is the most commonly used and cost-effective method in screening for various diseases, such as colorectal cancer[1]. Bowel preparation is a complex undertaking, involving dietary modifications and laxatives tailored to the individual patient[2]. Several agents are currently available to clean the colon, including sodium picosulfate/magnesium citrate (PSMC), polyethylene glycol (PEG), magnesium citrate, and sodium phosphate products[3]. An adequate bowel preparation that uses effective cleanser results in a high-quality colonoscopy, but the ideal clearing agent, the volume, and the pharmaceutical dosage form have yet to be determined.
The ideal bowel-cleansing agent should be well-tolerated, easily administered, inexpensive, and produce adequate clearance without allowing explosive gases to form[4]. Bowel preparation quality by different agents is assessed based on their efficacy, safety, and tolerability[5]. Most bowel preparations are either PEG-based or hyperosmotic; many of these regimens are perceived as unpalatable or unpleasant by patients[6]. PEG-containing preparations (e.g., Klean-Prep, GoLYTELY) are large-volume (2-4 L), osmotically-balanced nonabsorbable solutions that act as purgatives to evacuate the intestine; 2-L split-dose PEG preparations are better tolerated than 4-L PEG preparations[3]. Similarly, 2-L PEG/bisacodyl preparations are as effective as the standard 4-L PEG regimens but are better tolerated[3]. Sodium phosphate tablets were developed to improve patient acceptability of the bowel preparation regimen[7]. Sodium phosphate acts as an osmotic purgative, drawing water into the bowel lumen and stimulating peristalsis and evacuation[8]. Sodium phosphate has two oral dosage forms, solution and tablet[9]. Visicol® and OsmoPrep® are sodium phosphate tablets that are used for bowel cleansing before a colonoscopy in the United States[10,11]. However, although sodium phosphate provides excellent cleansing results and is well tolerated by most patients, concerns have been expressed about its safety regarding the osmotic action[8,12]. Moreover, sodium phosphate is not recommended for patients with renal or cardiac disorders or those on diuretic medications[9].
Colonoscopy is an important screening and therapeutic procedure for colon-related diseases[13]. The quality of bowel preparation impacts the success of colonoscopy[13]. Of the various agents available to clean the colon, small-volume cleansers are associated with improved patient compliance (tolerance), which affects bowel-clearing efficacy[14]. However, the efficacy of different small-volume bowel preparation agents has not been clearly defined. Our previous clinical trial found that a small-volume preparation (PSMC 300 mL) had better tolerability, higher acceptability, and compliance than a 2-L PEG/bisacodyl (5 mg)-Klean-Prep/Dulcolax preparation[14]. Tjandra et al[9] have shown that a 45-mL sodium phosphate solution was more effective in bowel cleansing than a PSMC preparation. Although these two agents were both accepted well by subjects, the PSMC preparation tasted significantly better than the 45 mL sodium phosphate preparation[9]. In comparison with the 4-L PEG solution, Jung et al[7] have shown that sodium phosphate tablets produced equivalent colon cleansing, did not cause more side effects, and had better patient acceptability and satisfaction in relatively young (aged < 60 years), healthy individuals without comorbidities. In 2011, the United States Food and Drug Administration (FDA) withdrew the 2-L PEG bowel cleansing kit HalfLytely containing bisacodyl 10 mg tablets due to safety concerns of ischemic colitis and abdominal cramping compared with the same kit using only bisacodyl 5 mg[15,16].
In Taiwan, 2-L Klean-Prep/bisacodyl (5 mg) is more commonly used than sodium phosphate tablets. The introduction of the 32-tablet sodium phosphate preparation Quiklean® (Universal Integrated Corporation, Taiwan) prompted us to conduct a multicenter, randomized, parallel-group, pre-specified noninferiority study to compare the bowel cleansing efficacy, acceptability, tolerability, and safety of Quiklean® to a 2-L PEG solution Klean-Prep (Helsinn-Birex Pharmaceuticals Limited, Ireland) combined with bisacodyl 5 mg (Dulcolax®, Boehringer Ingelheim, Germany) before the colonoscopy in 422 outpatients of Taiwanese populations. The objective was to compare the bowel cleansing efficacy, acceptability and tolerability, and safety of Quiklean® to Klean-Prep/Dulcolax® for bowel preparation.
This randomized, active-controlled, evaluator-blinded, noninferiority, parallel, multicenter phase III clinical trial (ClinicalTrials.gov Identifier: NCT03992365; first posted date: June 20, 2019) was conducted in China Medical University Hospital (Taiwan) and Taipei Tzu Chi Hospital (Taiwan).
Before trial initiation and subject enrollment, the Institutional Review Boards of China Medical University Hospital (CMUH107-REC2-151) and Taipei Tzu Chi Hospital (08-FS-030) approved the study, and Taiwan’s FDA approved the study protocol on January 17, 2019 (version: v2.0).
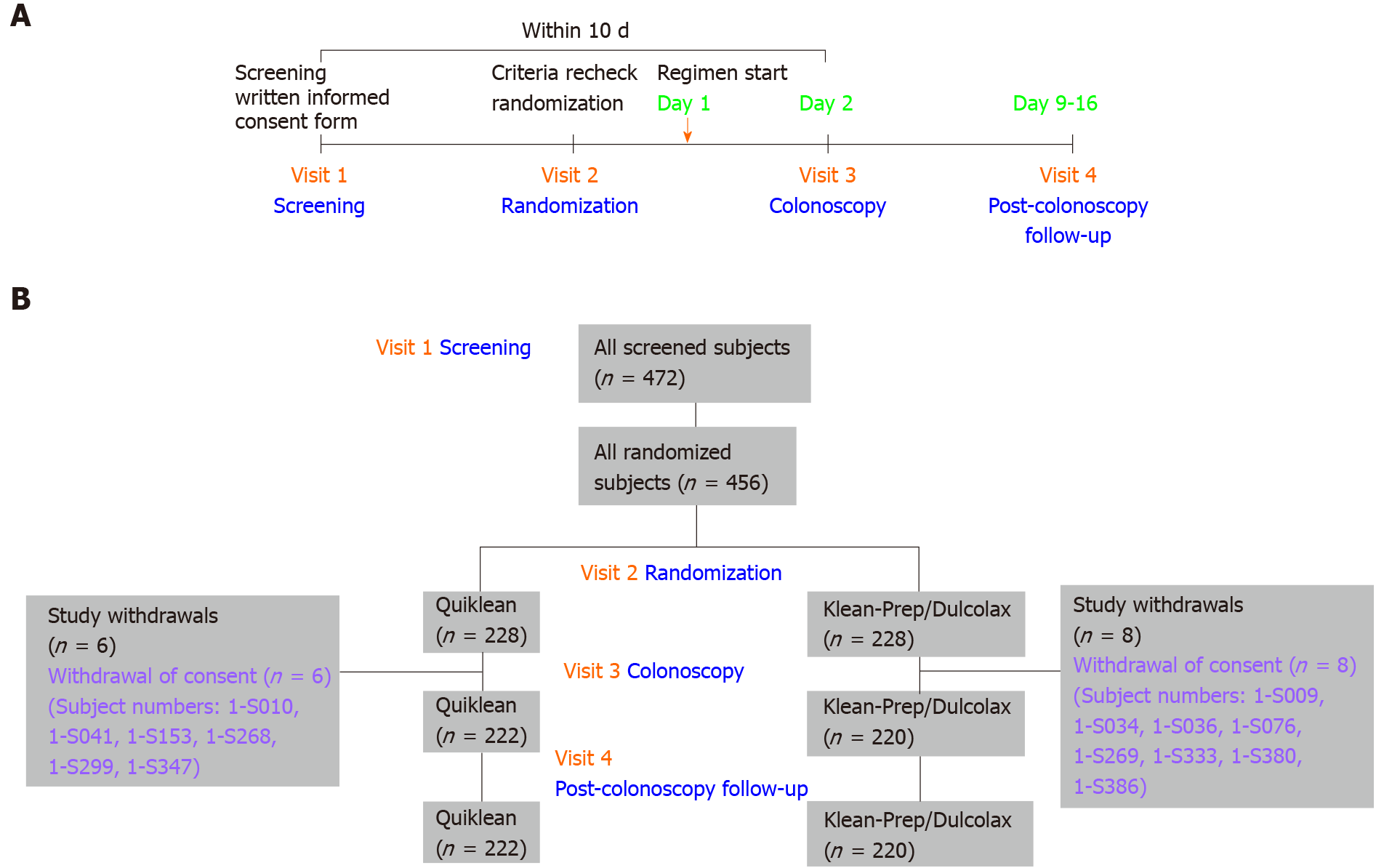
For sample size estimation, we assumed a conservative, successful cleaning response rate of 85% with Quiklean® and that the true difference in response rates between the Quiklean® and Klean-Prep/Dulcolax® groups would be zero. The sample size calculation was determined by assuming a 10% noninferiority margin, at least 80% power, and a one-sided significance level of 0.025. Based on these assumptions, this trial required 205 subjects per group to verify the noninferiority of Quiklean® over Klean-Prep/Dulcolax®. It was anticipated that up to 10% of randomized subjects were ineligible for the per-protocol population. Approximately 456 subjects were therefore recruited to provide 410 evaluable subjects. After the trial was started, a total number of 472 subjects were enrolled between June 10, 2019 and October 15, 2019; 456 subjects were subsequently randomized to receive either the Quiklean® (n = 228) or Klean-Prep/Dulcolax® (n = 228) regimen. A flow chart detailing the study design and timetable of four visits (screening, randomization, colonoscopy, and post-colonoscopy follow-up) is depicted in Figure 1A. The study event schedule performed during each visit is detailed in Table 1. After undergoing screening for inclusion and exclusion criteria in Visit 1, patients completed informed written consent forms and underwent physical examinations that assessed vital signs, signs of pregnancy, liver and renal functions, and serum electrolyte levels. The inclusion and exclusion criteria of this study are presented in Supplementary Table 1. An overview of subject disposition (enrollment, randomization, study withdrawals, colonoscopy, and post-colonoscopy follow-up) is provided in Figure 1B.
| Screening visit | Randomization visit1 | Regimen start | Colonoscopy visit | Follow-up visit | |
| Visit | 1 | 2 | 3 | 4 | |
| Day | -8 to -1 | -8 to -1 | 1 | 2 | 9-16 |
| Informed consent | X | ||||
| Inclusion/exclusion | X | X | |||
| Medical history | X | ||||
| Demographic data | X | ||||
| Physical examination | X | X | |||
| Vital signs | X | X | X | ||
| Urine pregnancy test | X | ||||
| 12-Lead electrocardiogram | X | X | |||
| Renal function | X2 | X3 | X | ||
| Electrolytes | X2 | X3 | X | ||
| Randomization | X | ||||
| Dietary control | X | ||||
| Dispensing of study drug | X | ||||
| Dosing day | X4 | X | |||
| Dietary card | X | X | |||
| Subject questionnaire | X5 | ||||
| Colonoscopy | X | ||||
| Aronchick scale | X6 | ||||
| Ottawa bowel preparation scale (OBPS) | X6 | ||||
| Bowel preparation compliance | X | ||||
| Concomitant medication | X | X | X | X | X |
| Reported events7 | X | X | |||
| Unsolicited adverse events and serious adverse events | X | X | X |
At the time of randomization, each study subject received standardized dietary advice with a dietary card containing detailed instructions about dietary measures to be taken and Quiklean® or Klean-Prep/Dulcolax® consumption. Subjects were instructed to give the completed dietary card and the empty bag or any remaining package of study regimen on the day of colonoscopy to the unblinded study coordinator, who recorded the following information: (1) Standard dietary advice; (2) The start time, the end time, and the number of bowel movements after the first regimen of the study product before colonoscopy; (3) The number of cups of clear water consumed; and (4) Adverse events experienced during consumption of the bowel preparation.
The Quiklean® regimen contained 32 tablets, each tablet containing 1.102 g of sodium phosphate monobasic monohydrate (United States Pharmacopoeia grade) and 0.398 g of sodium phosphate dibasic anhydrous (United States Pharmacopoeia grade), for a total of 1.5 g of sodium phosphate per tablet. Subjects were instructed to undertake the following procedures: (1) The evening before the colonoscopy: Ingest four tablets with 250 mL of clear liquids every 15 min, for a total of 20 tablets; and (2) On the day of the colonoscopy: Starting 3-5 h before the procedure, ingest four tablets with 250 mL of clear liquids every 15 min, for a total of 12 tablets. The Klean-Prep/Dulcolax® procedure (two sachets of Klean-Prep with one tablet of Dulcolax®) required subjects to prepare Klean-Prep (ingredients per sachet: 59 g PEG 3350, 5.685 g anhydrous sodium sulfate, 1.685 g sodium bicarbonate, 1.465 g sodium chloride, 0.7425 g potassium chloride, and 0.0494 g aspartame) immediately before each administration, by mixing one sachet of Klean-Prep with 1000 mL of cold water at room temperature, stirring thoroughly until the solution became clear as previously described[14]. The Klean-Prep/Dulcolax® administration was as follows: (1) In the afternoon before the day of colonoscopy: Ingest one 5 mg tablet of Dulcolax® (without chewing or crushing the tablet); (2) About 4 h after taking Dulcolax®, subjects were instructed to drink 1000 mL of Klean-Prep solution over a 2-h period or approximately 250 mL every 15 min; and (3) On the day of the colonoscopy: 3-5 h before the procedure, subjects were instructed to drink the other 1000 mL Klean-Prep solution at a rate of 250 mL every 15 min. The subject’s self-completed dietary card supplied details on compliance with Quiklean® or Klean-Prep/Dulcolax®, as to whether the subject drank the required amount of water or solution, as follows: (1) Excellent compliance, claiming ingestion of at least 8 cups of clear water/solution (one cup is equivalent to 250 mL); (2) Good compliance, claiming ingestion of at least 6 cups and fewer than 8 cups of clear water/solution; (3) Medium compliance, claiming ingestion of at least 4 cups and fewer than 6 cups of clear water/solution; (4) Poor compliance, claiming ingestion of fewer than 4 cups of clear water/solution; and (5) Non-compliance, claiming ingestion of 0 cups of clear water/solution.
The study consisted of four clinical visits, according to a predefined schedule. Subjects were screened on Visit 1 and randomized into the study on Visit 2. Colonoscopy was performed on Visit 3, and post-colonoscopy follow-up was conducted on Visit 4 (Figure 1A). After obtaining informed consent from the subject, the designated assessment was performed. If the eligibility criteria were satisfied, the subjects were randomly assigned (1:1) to either the Quiklean® or Klean-Prep/Dulcolax® regimen and scheduled to undergo colonoscopy. The colonoscopy visit was arranged within 10 d of screening (Figure 1A). Subjects were instructed on how to take the study medication, and each study group was issued with identical standard dietary instructions. After undergoing bowel-clearing preparation, the colonoscopy was performed in the morning by the experienced colonoscopist, with the entire colonoscopy recorded by video. After the completion of colonoscopy, the quality of bowel cleansing in the video recording was rated by independent colonoscopists blinded to treatment allocation. The colonoscopists jointly evaluated the scoring system before the study commenced in order to minimize interobserver variability.
Aronchick Scale and Ottawa bowel preparation scale (OBPS) scores graded colon cleanliness[17,18]. The primary endpoint of this study was the efficiency of colon cleansing according to the modified Aronchick Scale, as follows: Excellent = only a small volume of clear liquid or > 95% of the surface was observed; Good = a large volume of clear liquid covered 5%-25% of the surface, but > 90% of the surface was observed; Fair = some semi-solid stools were found, but these could be suctioned or washed away and > 90% of the surface was observed; Poor = semi-solid stools were found that could not be suctioned or washed away and < 90% of the surface was observed; and Inadequate = the subject was required to undergo a repeat preparation process[17]. Successful and unsuccessful bowel preparations were defined as “Excellent + Good” and “Fair + Poor + Inadequate”, respectively. For the secondary endpoint, the OBPS evaluated subjects’ acceptance and tolerance of the preparation, as well as cleansing efficacy in the ascending, mid- (transverse and descending), and rectosigmoid segments of the colon[18]. OBPS scores for each colon segment were graded on a 5-point scale, as follows: Excellent = 0; Good = 1; Fair = 2; Poor = 3; and Inadequate = 4[18]. OBPS scores ranged from 0 to 14 (fluid scores, 0-2; scores of ascending + mid + rectosigmoid segments, 0-12)[18]. The colonoscopist rated the overall fluid amount on a 3-point scale (where 0 = mild; 1 = moderate; 2 = large)[18]. Safety was assessed by monitoring adverse events and laboratory examinations. All adverse events were coded using MedDRA Preferred Terms. All study procedures, including efficacy and safety measurements, were performed according to the schedule described in Table 1.
Our analysis used the International Council for Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use recommendations in guidelines E3 and E9 regarding intent-to-treat, per-protocol, and safety analysis sets. Among 456 randomized subjects, 442 (Quiklean® = 222 and Klean-Prep/Dulcolax® = 220) who received study medications and underwent colonoscopy were included in the intent-to-treat analysis set and safety analysis set. After excluding 25 subjects from the intent-to-treat analysis set, 417 subjects (Quiklean® = 208 and Klean-Prep/ Dulcolax® = 209) were included in the per-protocol analysis set. Supplementary Table 2 (lower) lists the reasons concerning exclusions from the per-protocol analysis set; Supplementary Table 3 lists subjects in the intent-to-treat set who were excluded from the per-protocol analysis set. Supplementary Table 2 also details patient disposition data for each group, each analysis set, and reasons for study withdrawals. No significant between-group differences were identified for baseline characteristics, including sex, age, body weight, height, and body mass index in the per-protocol analysis set (Supplementary Table 4).
The statistical analysis in this study was implemented by a contract research organization, StatPlus Inc., which used SAS® Version 9.4 (Cary, NC, United States); the Taiwan FDA approved the results. The primary efficacy variable was the percentage of subjects who achieved “success rate” (Excellent + Good) in overall colon cleansing, based on the Aronchick Scale in both the intent-to-treat and per-protocol analysis sets. The differences in success rates (Excellent + Good) were calculated using Fisher’s exact test with associated exact 95% confidence intervals (CIs)[19]. Noninferiority was satisfied if the lower bound of the two-sided 95%CI for the difference in the success rate (Quiklean® minus Klean-Prep/Dulcolax®) was at least −10%; P < 0.05 was regarded as statistically significant[14]. The secondary endpoint included bowel cleaning efficacy by OBPS and the subject’s responses to acceptability and tolerability. The overall score and each colon segment score of OBPS were used to analyze the secondary endpoint by Fisher’s exact test and independent t-tests. The total OBPS score was categorized as excellent cleansing (score 0-1), good (score 2-4), sufficient (score 5-7), poor (score 8-10), or inappropriate (score 11-14). The subject’s responses in the questionnaire regarding acceptability and tolerability, numbers, and percentages were categorized by the responses on each questionnaire for each group and analyzed by the Fisher’s exact test for binary variables and the Cochran-Mantel-Haenszel test with relative to an identified distribution integral transformation scores for ordered categorical variables. All safety parameters, including adverse events, clinical laboratory evaluations, and vital signs, were summarized with descriptive statistics for the two groups, and each scheduled a return visit.
The study design and visit timetable (screening, randomization, colonoscopy, and post-colonoscopy follow-up) are depicted in the flow chart illustrated in Figure 1A. In the beginning, 472 subjects were screened in Visit 1, and 16 subjects within that did not pass criteria recheck in Visit 2; then 456 subjects were randomized in the trial. Study withdrawal rates were 2.6% and 3.5% for Quiklean® and Klean-Prep/Dulcolax®, respectively (Supplementary Table 2). In both groups, the reason cited for withdrawal was the subjects’ withdrawal of consent (Figure 1B, Supplementary Table 2).
The primary endpoint of our trial was the “success rate” (Excellent + Good) of bowel cleansing, evaluated by the Aronchick Scale, which graded 24.0%, 74.5%, 1.4%, 0%, and 0% of the Quiklean® per-protocol analysis set and 33%, 64.6%, 1.9%, 0%, and 0% of the Klean-Prep/Dulcolax® per-protocol analysis set as Excellent, Good, Fair, Poor, and Inadequate, respectively (Table 2). Success rates (Excellent + Good) were 98.6% (n = 205) and 97.6% (n = 204) in the Quiklean® and Klean-Prep/Dulcolax® groups, respectively; the between-group difference was 0.95% (95%CI: −8.620%, 10.525%) (Table 2). For the primary endpoint assessed by the Aronchick Scale, Quiklean® demonstrated noninferiority over Klean-Prep/Dulcolax® since the lower confidence bound of the two-sided 95%CI of the treatment difference (Quiklean® minus Klean-Prep/Dulcolax®) was greater than the pre-specified margin of −10%.
| Quiklean®, n = 208 | Klean-Prep/Dulcolax®, n = 209 | P value | |
| Responses | |||
| Successful (Excellent + Good) | 205 | 204 | |
| Unsuccessful (Fair + Poor + Inadequate) | 3 | 5 | |
| Success rate | 98.6% | 97.6% | |
| Between-group difference | 0.95% | ||
| 95% exact CI | (-8.620%, 10.525%) | ||
| Rating, n (%) | |||
| Excellent | 50 (24.0) | 69 (33.0) | 0.1355 |
| Good | 155 (74.5) | 135 (64.6) | |
| Fair | 3 (1.4) | 4 (1.9) | |
| Poor | 0 | 1 (0.5) | |
| Inadequate | 0 | 0 | |
One of our four secondary endpoints was bowel cleansing efficacy in three colonic segments, evaluated by the OBPS. Mean overall OBPS scores were 2.5 ± 1.48 in the Quiklean® cohort (n = 208) and 2.5 ± 1.68 in the Klean-Prep/Dulcolax® cohort (n = 208), without statistical between-group significance (P = 0.733; Table 3). Success rates (Excellent + Good + Fair) for overall bowel cleansing quality were 100% with Quiklean® and 99.0% with Klean-Prep/Dulcolax® (P = 0.4988; Table 3). The success rates for the ascending colon were 100% for both groups (P = 1.0000); 100% for the transverse colon with Quiklean® and 99.5% with Klean-Prep/Dulcolax® (P = 1.0000); 100% for the descending colon with Quiklean® and 98.6% with KleanPrep/Dulcolax® (P = 0.2482).
| Quiklean®, n = 208 | Klean-Prep/Dulcolax®, n = 209 | P value | |
| 1 How easy or difficult was it to consume the study drug? | |||
| Very easy | 68 (32.7) | 48 (23.0) | 0.12011 |
| Easy | 96 (46.2) | 110 (52.6) | |
| Tolerable | 23 (11.1) | 25 (12.0) | |
| Difficult | 18 (8.7) | 25 (12.0) | |
| Very difficult | 3 (1.4) | 1 (0.5) | |
| 2 Were you able to consume the study preparation as instructed? | |||
| Yes | 208 (100) | 207 (99.0) | 0.49882 |
| No | 0 | 2 (1.0) | |
| 3 Please describe your overall experience with the study preparation. | |||
| Excellent | 50 (24.0) | 36 (17.2) | 0.00161 |
| Good | 112 (53.8) | 109 (52.2) | |
| Fair | 21 (10.1) | 12 (5.7) | |
| Poor | 24 (11.5) | 43 (20.6) | |
| Bad | 1 (0.5) | 9 (4.3) | |
| 4 The taste of this study preparation was | |||
| Excellent | 48 (23.1) | 28 (13.4) | < 0.00011 |
| Good | 82 (39.4) | 82 (39.2) | |
| Fair | 41 (19.7) | 17 (8.1) | |
| Poor | 35 (16.8) | 65 (31.1) | |
| Bad | 2 (1.0) | 17 (8.1) | |
| 5 Would you ask your doctor for this preparation again if you need another colonoscopy in the future? | |||
| Yes | 167 (80.3) | 152 (72.7) | 0.08302 |
| No | 41 (19.7) | 27.3) | |
| 6 Would you refuse the same preparation again if it were to be prescribed to you in the future? | |||
| Yes | 42 (20.2) | 54 (25.8) | 0.20072 |
| No | 166 (79.8) | 155 (74.2) | |
The other three secondary endpoints of our trial were acceptability, tolerability, and safety, as rated by each subject. Subject ratings did not differ between the groups regarding how easy or difficult the preparations were to consume (Table 4). All 208 Quiklean® recipients (100%) and 207 Klean-Prep/Dulcolax® recipients (99%) could consume the study preparation as instructed (P = 0.4988; Table 4). A significantly higher proportion of Quiklean®vs Klean-Prep/Dulcolax® recipients rated their overall experience as “Excellent” (24.0% vs 17.2%; P = 0.0016; Table 4). The taste of the study preparation was rated as excellent by more Quiklean® than Klean-Prep/Dulcolax® recipients (23.1% vs 13.4%; P < 0.0001; Table 4). A total of 80.3% of Quiklean® recipients and 72.7% of Klean-Prep/Dulcolax® recipients stated they would choose the same regimen again in the future (P = 0.0830; Table 4). The proportions of subjects claiming they would refuse the same preparation in the future did not differ significantly between the groups (P = 0.2007; Table 5).
| Treatment-emergent adverse events | Quiklean®, n = 222 | Klean-Prep/Dulcolax®, n = 220 | P value |
| Any treatment-emergent adverse events | 218 (98.2) | 70 (31.8) | < 0.0001 |
| Mild | 217 (97.7) | 70 (31.8) | |
| Moderate | 1 (0.5) | 0 | |
| Severe | 0 | 0 | |
| Life threatening | 0 | 0 | |
| Death | 0 | 0 | |
| MedDRA preferred term | |||
| Blood phosphorus increased | 216 (97.3) | 8 (3.6) | < 0.0001 |
| Blood potassium decreased | 109 (49.1) | 9 (4.1) | < 0.0001 |
| Blood phosphorus decreased | 6 (2.7) | 42 (19.1) | < 0.0001 |
| Blood urea decreased | 16 (7.2) | 7 (3.2) | 0.0847 |
| Blood chloride decreased | 14 (6.3) | 8 (3.6) | 0.2740 |
| Blood chloride increased | 6 (2.7) | 7 (3.2) | 0.7867 |
| Blood calcium decreased | 6 (2.7) | 1 (0.5) | 0.1221 |
| Blood sodium decreased | 7 (3.2) | 0 | 0.0149 |
| Blood creatinine decreased | 2 (0.9) | 1 (0.5) | 1.0000 |
| Blood urea increased | 1 (0.5) | 2 (0.9) | 0.6224 |
| Blood magnesium increased | 2 (0.9) | 0 | 0.4989 |
| Blood sodium increased | 1 (0.5) | 1 (0.5) | 1.0000 |
| Blood creatinine increased | 1 (0.5) | 0 | 1.0000 |
| Blood potassium increased | 1 (0.5) | 0 | 1.0000 |
| Duodenal ulcer | 0 | 1 (0.5) | 0.4977 |
| Nonalcoholic fatty liver disease | 0 | 1 (0.5) | 0.4977 |
| Herpes zoster | 0 | 1 (0.5) | 0.4977 |
| Pyoderma | 0 | 1 (0.5) | 0.4977 |
| Muscle disorder | 0 | 1 (0.5) | 0.4977 |
| Calculus urinary | 1 (0.5) | 0 | 1.0000 |
| Asthma | 1 (0.5) | 0 | 1.0000 |
| Rhinitis allergic | 1 (0.5) | 0 | 1.0000 |
Reported adverse events were reported by similar proportions of Quiklean® and Klean-Prep/Dulcolax® recipients (P = 0.4944) including nausea (P = 0.0299), vomiting (P = 0.0126), abdominal pain/cramping (P = 0.0333), abdominal bloating (P = 0.0080), urticaria (P = 1.0000), anal irritation (P = 0.1865), and edema (P = 0.4483). Although Quiklean® recipients had significantly higher rates of nausea, vomiting, and abdominal bloating than Klean-Prep/Dulcolax® recipients, the Klean-Prep/Dulcolax® preparation was associated with higher rates of abdominal pain/cramping than Quiklean®; all reported adverse events were deemed to be mild in intensity. During the study period, 98.2% of Quiklean® recipients and 31.8% of Klean-Prep/Dulcolax® recipients reported at least one treatment-emergent adverse event (P < 0.0001; Table 5); corresponding proportions reporting at least one study drug-related treatment-emergent adverse event were 97.7% and 20.5%, respectively. Although Quiklean® was associated with a significantly higher incidence of any study drug-related treatment-emergent adverse event than Klean-Prep/Dulcolax®, no severe treatment-emergent adverse events resulted in discontinuation or death. The top two reported treatment-emergent adverse events associated with Quiklean® were an increase in blood phosphorus and a decrease in blood potassium (97.3% and 49.1%; P < 0.0001); the most commonly reported treatment-emergent event with Klean-Prep/Dulcolax® was a decrease in blood phosphorus (19.1%; P < 0.0001) (Table 5). Blood electrolytes that differed significantly between the study groups included phosphorous, potassium, and sodium (Table 5). None of these electrolytes differed significantly between the groups at Visit 1/screening (baseline) (Supplementary Tables 5, 6, and 7, respectively). Compared with baseline values at Visit 1 (screening), phosphorus levels were found to be significantly increased with Quiklean® and significantly decreased with Klean-Prep/Dulcolax® at Visit 3 (colonoscopy) (P < 0.0001 for both intra-group comparisons); both values were restored to baseline values by Visit 4 (post-colonoscopy follow-up) (Supplementary Table 5). The normal range of serum potassium is 3.5-5.0 mmol/L; the level in patients with moderate hypokalemia is 2.5-3.0 mmol/L[20]. Supplementary Table 6 shows that the means of blood potassium levels were significantly decreased from baseline (Quiklean® = 3.87 mmol/L and Klean-Prep/Dulcolax® = 3.89 mmoL) in both study groups at Visit 3 (Quiklean® = 3.08 mmol/L and Klean-Prep/Dulcolax® = 3.60 mmoL) and had returned to baseline in both groups at Visit 4 (Quiklean® = 3.89 mmol/L and Klean-Prep/Dulcolax® = 3.88 mmoL). Conversely, blood sodium levels were significantly increased in both study groups at Visit 3 and had returned to baseline in both groups at Visit 4 (Supplementary Table 7). On Visits 1, 3, and 4, no statistically significant between-group differences were identified for renal function (serum creatinine and blood urea nitrogen) or vital signs (body temperature, blood pressure, pulse rate, respiration rate, and body weight); all of these values remained within the normal range during the study period.
Adequate bowel preparation is essential for high-quality colonoscopies capable of detecting diseases of the colon and rectum. Ideal preparation would rapidly and reliably eliminate all fecal material from the colon without causing any gross or histological alternations of the colonic mucosa[21,22]. Moreover, the preparation would not cause any discomfort and would be safe for the patient[23]. In this study, 32 tablets of Quiklean® (1.5 g sodium phosphate per tablet) demonstrated noninferiority over two sachets of Klean-Prep combined with one tablet of 5 mg Dulcolax® (Klean-Prep/Dulcolax®) for colon cleansing before colonoscopy, according to Aronchick Scale scores. Secondary endpoint evaluations revealed similar success rates for bowel cleansing quality in the overall and individual colon segments, according to OBPS scores. Acceptability and tolerability ratings demonstrated that Quiklean® was easier to consume, tasted better, and was more often rated as an overall excellent experience during bowel preparation than Klean-Prep/Dulcolax®. Patient satisfaction scores indicated that more subjects would prefer Quiklean® over Klean-Prep/Dulcolax® in the future. Visit 3 (colonoscopy) safety data revealed that Quiklean® was associated with increases from baseline in blood phosphorus and sodium, as well as a decrease in blood potassium, while Klean-Prep/Dulcolax® was associated with reductions from baseline in blood phosphorus and potassium, and an increase in blood sodium. In both study groups, all values were restored to baseline by Visit 4 (post-colonoscopy follow-up). Our data suggest that the new bowel-clearing preparation Quiklean® may increase patients’ positive attitudes and participation in bowel preparation for colonoscopy.
The quality of bowel preparation can be rated during colonoscopy, and thus the superiority of one bowel preparation method can be effectively compared with others[24]. The Aronchick Scale is one such rating scale that is universally accepted and has been used in pivotal trials that have resulted in new drug application approvals, including that of HalfLytely[17]. The OBPS is associated with high interobserver agreement and reliability, whether used as a total score or for individual colon segments[18]. In the present study, our independent blinded colonoscopists used both the Aronchick Scale and OBPS to rate bowel cleansing quality. Their ratings demonstrate noninferiority for Quiklean® over Klean-Prep/Dulcolax®.
Many bowel-clearing preparations are available on the market. It is advised that the choice of any such regimen should be based on cleansing efficacy first and patient tolerability second, although these factors are undoubtedly closely interrelated[5]. For example, poor tolerability that prevents full compliance with a bowel cleansing regimen may mean inadequate cleansing[5]. Comparing sodium phosphate and other bowel preparation agents has shown that sodium phosphate is better tolerated by patients, making this a preferred method of preparing colonoscopy for specific patient subgroups[25,26]. Sodium phosphate tablets were developed to improve patient acceptability of the bowel preparation and have been reported to be similar or better than PEG solution for patient compliance in Western countries and Japan[11,27,28]. In the present study, Quiklean® had higher acceptability and tolerability than Klean-Prep/Dulcolax®. Quiklean® may offer Taiwanese patients a suitable agent that is very effective and well-tolerated for colon cleansing before colonoscopy.
Significant dehydration and electrolyte abnormalities have been described with the use of oral sodium phosphate[8]. Two major safety issues are related to its use: First, the osmotic action of this agent can draw fluid from the intravascular space and potentially lead to hypovolemia; second, most patients develop transient mild hyperphosphatemia that could potentially result in symptoms caused by hypocalcemia[8]. However, evidence from 26 clinical trials involving 2496 subjects administered oral sodium phosphate solution and 526 subjects who received sodium phosphate tablets show no major adverse events attributed to sodium phosphate[8]. Most of those studies excluded patients with heart failure, renal failure, ascites, and/or myocardial infarction within the previous 6 mo[8]. In our study, the exclusion criteria also included renal insufficiency, cardiovascular diseases, and myocardial infarction to avoid significant adverse events. Serum potassium is closely regulated physiologically with normal values ranging from 3.5 mmol/L to 5.0 mmol/L, and moderate hypokalemia (2.5-3.0 mmol/L) can be highly arrhythmogenic in normal hearts[20]. In subjects of Quiklean® group, the mean blood potassium levels of Visit 1, Visit 3, and Visit 4 were 3.87 mmol/L, 3.08 mmol/L, and 3.89 mmol/L, respectively. The data in Klean-Prep/Dulcolax® group were 3.89 mmol/L, 3.60 mmol/L, and 3.88 mmol/L, respectively. Indicating the changes in serum potassium levels in subjects of Quiklean® group did not cause severe and life-threatening hypokalemia. In the present study, we identified significantly higher rates of nausea, vomiting, and abdominal bloating with Quiklean® compared with Klean-Prep/Dulcolax®, while abdominal pain/cramping was significantly more likely with Klean-Prep/Dulcolax® than with Quiklean®; all reported adverse events reported in this study were judged to be mild in intensity. Moreover, treatment-emergent adverse events were similar for both study groups. No severe treatment-emergent adverse events occurred.
Our limitations in this study include the fact that during the enrollment process, we included subjects aged between 20 and 74 years and excluded subjects with significant cardiovascular or renal impairment, significant gastrointestinal disease, acute exacerbation of inflammatory bowel disease, or pregnancy. Thus, our safety data for Quiklean® should not be applied to the older elderly (> 75 years) with significant comorbidities. Significant dehydration and electrolyte abnormalities have been described with sodium phosphate. Our study detected changes from baseline (Visit 1/screening) in both study groups for renal function measures and electrolytes at Visit 3 (colonoscopy). Quiklean® induced increases in blood phosphorous and sodium and a decrease in potassium, while Klean-Prep/Dulcolax® induced decreases in blood phosphorous and potassium and an increase in sodium; all values were restored to normal levels in both groups by Visit 4 (post-colonoscopy follow-up). The 7-d to the 14-d interval between colonoscopy and follow-up prevents us from knowing the details about the electrolyte changes over this timeframe.
Adequate bowel preparations are critical for high-quality colonoscopy examinations and successful colonoscopy screening or surveillance programs. In conclusion, 32 tablets of Quiklean® (1.5 g per tablet) demonstrated noninferiority over two Klean-Prep sachets combined with one tablet of Dulcolax® for the outcome of effective colon cleansing. Patient satisfaction ratings indicated a higher preference for Quiklean® compared with Klean-Prep/Dulcolax®. Quiklean® may, therefore, increase patients’ positive attitudes and participation in bowel preparation for colonoscopy.
Efficient bowel cleansing is essential for a successful colonoscopy.
The ideal cleansing agent, volume, and pharmaceutical dosage form have yet to be determined. Small-volume cleansers enhance patient compliance.
To compare the bowel cleansing efficacy of 32-tablet sodium phosphate (Quiklean®) with 2-L polyethylene glycol (PEG)/bisacodyl (Klean-Prep/Dulcolax®) under identical dietary recommendations.
This multicenter, randomized, parallel-group, noninferiority clinical trial enrolled 472 outpatients, randomized 456 subjects, and scheduled 442 subjects to undergo colonoscopy (Quiklean® = 222 and Klean-Prep/Dulcolax® = 220). After bowel preparation, a colonoscopist performed the colonoscopy with video recorded for rating. The primary efficacy endpoint was the bowel cleansing quality using the Aronchick Scale. The secondary endpoints were the bowel cleansing efficacy of three colon segments, tolerability and acceptability, and safety using the Ottawa bowel preparation scale, questionnaires by subjects, and monitoring of adverse events.
Success rates (Excellent + Good) of the bowel cleansing quality by Aronchick Scale were 98.6% (n = 205) and 97.6% (n = 204) in the Quiklean® and Klean-Prep/Dulcolax® groups, respectively. Quiklean® demonstrated noninferiority over Klean-Prep/Dulcolax® in colon cleansing efficacy. Quicken showed better tolerability and acceptability in the overall experience (was rated as excellent; 24.0% vs 17.2%; P = 0.0016) and taste of the study preparation (was rated as excellent, 23.1% vs 13.4%; P < 0.0001) than Klean-Prep/Dulcolax®. Safety profiles did not differ between the two groups. Our data indicate that Quiklean® is an adequate, well-tolerated bowel cleansing preparation compared with the standard comparator Klean-Prep/Dulcolax®.
Quiklean® is sodium phosphate tablets available on Taiwan’s market for bowel preparation; it potentially offers patients an alternative to standard large-volume bowel preparation regimens and may, therefore, increase positive attitudes toward colonoscopies and participation rates.
The bowel cleanser 32-tablet sodium phosphate (Quiklean®) is an adequate bowel cleansing preparation with better tolerability and acceptability as compared with the standard comparator 2-L polyethylene glycol (PEG)/bisacodyl (Klean-Prep/Dulcolax®).
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Taiwan
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Ho KY S-Editor: Chen XF L-Editor: Filipodia P-Editor: Ma YJ
| 1. | Sonnenberg A, Delcò F, Inadomi JM. Cost-effectiveness of colonoscopy in screening for colorectal cancer. Ann Intern Med. 2000;133:573-584. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 338] [Cited by in RCA: 324] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 2. | Hassan C, Bretthauer M, Kaminski MF, Polkowski M, Rembacken B, Saunders B, Benamouzig R, Holme O, Green S, Kuiper T, Marmo R, Omar M, Petruzziello L, Spada C, Zullo A, Dumonceau JM; European Society of Gastrointestinal Endoscopy. Bowel preparation for colonoscopy: European Society of Gastrointestinal Endoscopy (ESGE) guideline. Endoscopy. 2013;45:142-150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 290] [Cited by in RCA: 311] [Article Influence: 25.9] [Reference Citation Analysis (1)] |
| 3. | Barkun A, Chiba N, Enns R, Marcon M, Natsheh S, Pham C, Sadowski D, Vanner S. Commonly used preparations for colonoscopy: efficacy, tolerability, and safety--a Canadian Association of Gastroenterology position paper. Can J Gastroenterol. 2006;20:699-710. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 91] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 4. | Atkin WS, Hart A, Edwards R, Cook CF, Wardle J, McIntyre P, Aubrey R, Baron C, Sutton S, Cuzick J, Senapati A, Northover JM. Single blind, randomised trial of efficacy and acceptability of oral picolax versus self administered phosphate enema in bowel preparation for flexible sigmoidoscopy screening. BMJ. 2000;320:1504-8; discussion 1509. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 35] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 5. | Johnson DA, Barkun AN, Cohen LB, Dominitz JA, Kaltenbach T, Martel M, Robertson DJ, Boland CR, Giardello FM, Lieberman DA, Levin TR, Rex DK. Optimizing adequacy of bowel cleansing for colonoscopy: recommendations from the U.S. multi-society task force on colorectal cancer. Gastrointest Endosc. 2014;80:543-562. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 103] [Article Influence: 9.4] [Reference Citation Analysis (1)] |
| 6. | Rutherford CC, Calderwood AH. Update on Bowel Preparation for Colonoscopy. Curr Treat Options Gastroenterol. 2018;16:165-181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 22] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 7. | Jung YS, Lee CK, Kim HJ, Eun CS, Han DS, Park DI. Randomized controlled trial of sodium phosphate tablets vs polyethylene glycol solution for colonoscopy bowel cleansing. World J Gastroenterol. 2014;20:15845-15851. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 13] [Cited by in RCA: 12] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 8. | Hookey LC, Depew WT, Vanner S. The safety profile of oral sodium phosphate for colonic cleansing before colonoscopy in adults. Gastrointest Endosc. 2002;56:895-902. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 34] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 9. | Tjandra JJ, Chan M, Tagkalidis PP. Oral sodium phosphate (Fleet) is a superior colonoscopy preparation to Picopre (sodium picosulfate-based preparation). Dis Colon Rectum. 2006;49:616-620. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 29] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 10. | US Food and Drug Administration. Drug Approval Package. Visicol® NDA Medical Review. 2000. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2000/21097_Visicol.cfm. |
| 11. | US Food and Drug Administration. Drug Approval Package. OsmoPrep® NDA Medical Review. 2006. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2006/021892_osmoprep_toc.cfm. |
| 12. | Vanner SJ, MacDonald PH, Paterson WG, Prentice RS, Da Costa LR, Beck IT. A randomized prospective trial comparing oral sodium phosphate with standard polyethylene glycol-based lavage solution (Golytely) in the preparation of patients for colonoscopy. Am J Gastroenterol. 1990;85:422-427. [PubMed] |
| 13. | Bechtold ML, Mir F, Puli SR, Nguyen DL. Optimizing bowel preparation for colonoscopy: a guide to enhance quality of visualization. Ann Gastroenterol. 2016;29:137-146. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 48] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 14. | Hung SY, Chen HC, Chen WT. A Randomized Trial Comparing the Bowel Cleansing Efficacy of Sodium Picosulfate/Magnesium Citrate and Polyethylene Glycol/Bisacodyl (The Bowklean Study). Sci Rep. 2020;10:5604. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 15. | Shibata M, Lu T, Furuya T, Degterev A, Mizushima N, Yoshimori T, MacDonald M, Yankner B, Yuan J. Regulation of intracellular accumulation of mutant Huntingtin by Beclin 1. J Biol Chem. 2006;281:14474-14485. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 346] [Cited by in RCA: 362] [Article Influence: 19.1] [Reference Citation Analysis (0)] |
| 16. | Clark RE, Godfrey JD, Choudhary A, Ashraf I, Matteson ML, Bechtold ML. Low-volume polyethylene glycol and bisacodyl for bowel preparation prior to colonoscopy: a meta-analysis. Ann Gastroenterol. 2013;26:319-324. [PubMed] |
| 17. | Aronchick CA, Lipshutz WH, Wright SH, DuFrayne F, Bergman G. Validation of an instrument to assess colon cleansing. Am J Gastroenterol. 1999;94:2667-2667. |
| 18. | Rostom A, Jolicoeur E. Validation of a new scale for the assessment of bowel preparation quality. Gastrointest Endosc. 2004;59:482-486. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 308] [Cited by in RCA: 343] [Article Influence: 16.3] [Reference Citation Analysis (2)] |
| 19. | Mathus-Vliegen EMH, van der Vliet K, Wignand-van der Storm IJ, Stadwijk JS. Efficacy and Safety of Sodium Picosulfate/Magnesium Citrate for Bowel Preparation in a Physically Disabled Outpatient Population: A Randomized, Endoscopist-Blinded Comparison With Ascorbic Acid-Enriched Polyethylene Glycol Solution Plus Bisacodyl (The PICO-MOVI Study). Dis Colon Rectum. 2018;61:239-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 20. | Weiss JN, Qu Z, Shivkumar K. Electrophysiology of Hypokalemia and Hyperkalemia. Circ Arrhythm Electrophysiol. 2017;10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 220] [Article Influence: 36.7] [Reference Citation Analysis (0)] |
| 21. | ASGE Technology Committee, Mamula P, Adler DG, Conway JD, Diehl DL, Farraye FA, Kantsevoy SV, Kaul V, Kethu SR, Kwon RS, Rodriguez SA, Tierney WM. Colonoscopy preparation. Gastrointest Endosc. 2009;69:1201-1209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 46] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 22. | Wexner SD, Beck DE, Baron TH, Fanelli RD, Hyman N, Shen B, Wasco KE; American Society of Colon and Rectal Surgeons; American Society for Gastrointestinal Endoscopy; Society of American Gastrointestinal and Endoscopic Surgeons. A consensus document on bowel preparation before colonoscopy: prepared by a task force from the American Society of Colon and Rectal Surgeons (ASCRS), the American Society for Gastrointestinal Endoscopy (ASGE), and the Society of American Gastrointestinal and Endoscopic Surgeons (SAGES). Gastrointest Endosc. 2006;63:894-909. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 211] [Cited by in RCA: 211] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 23. | Koshitani T, Kawada M, Yoshikawa T. Bowel preparation for colonoscopy using standard vs reduced doses of sodium phosphate: A single-blind randomized controlled study. World J Gastrointest Endosc. 2014;6:379-384. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 2] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 24. | Lee SH, Lee DJ, Kim KM, Seo SW, Kang JK, Lee EH, Lee DR. Comparison of the efficacy and safety of sodium phosphate tablets and polyethylene glycol solution for bowel cleansing in healthy Korean adults. Yonsei Med J. 2014;55:1542-1555. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 25. | Aronchick CA, Lipshutz WH, Wright SH, Dufrayne F, Bergman G. A novel tableted purgative for colonoscopic preparation: efficacy and safety comparisons with Colyte and Fleet Phospho-Soda. Gastrointest Endosc. 2000;52:346-352. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 283] [Cited by in RCA: 318] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 26. | Hsu CW, Imperiale TF. Meta-analysis and cost comparison of polyethylene glycol lavage versus sodium phosphate for colonoscopy preparation. Gastrointest Endosc. 1998;48:276-282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 143] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 27. | Hosoe N, Nakashita M, Imaeda H, Sujino T, Bessho R, Ichikawa R, Inoue N, Kanai T, Hibi T, Ogata H. Comparison of patient acceptance of sodium phosphate versus polyethylene glycol plus sodium picosulfate for colon cleansing in Japanese. J Gastroenterol Hepatol. 2012;27:1617-1622. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 28. | Kastenberg D, Chasen R, Choudhary C, Riff D, Steinberg S, Weiss E, Wruble L. Efficacy and safety of sodium phosphate tablets compared with PEG solution in colon cleansing: two identically designed, randomized, controlled, parallel group, multicenter phase III trials. Gastrointest Endosc. 2001;54:705-713. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 106] [Article Influence: 4.4] [Reference Citation Analysis (0)] |