Published online Feb 7, 2021. doi: 10.3748/wjg.v27.i5.416
Peer-review started: November 23, 2020
First decision: December 17, 2020
Revised: December 20, 2020
Accepted: January 15, 2021
Article in press: January 15, 2021
Published online: February 7, 2021
Hepatic encephalopathy (HE) remains an enormous challenge in patients who undergo transjugular intrahepatic portosystemic shunt (TIPS) implantation. The preoperative indocyanine green retention rate at 15 min (ICG-R15), as one of the liver function assessment tools, has been developed as a prognostic indicator in patients undergoing surgery, but there are limited data on its role in TIPS.
To determine whether the ICG-R15 can be used for prediction of post-TIPS HE in decompensated cirrhosis patients with portal hypertension (PHT) and compare the clinical value of ICG-R15, Child-Pugh score (CPS), and model for end-stage liver disease (MELD) score in predicting post-TIPS HE with PHT.
This retrospective study included 195 patients with PHT who underwent elective TIPS at Beijing Shijitan Hospital from January 2018 to June 2019. All patients underwent the ICG-R15 test, CPS evaluation, and MELD scoring 1 wk before TIPS. According to whether they developed HE or not, the patients were divided into two groups: HE group and non-HE group. The prediction of one-year post-TIPS HE by ICG-R15, CPS and MELD score was evaluated by the areas under the receiver operating characteristic curves (AUCs).
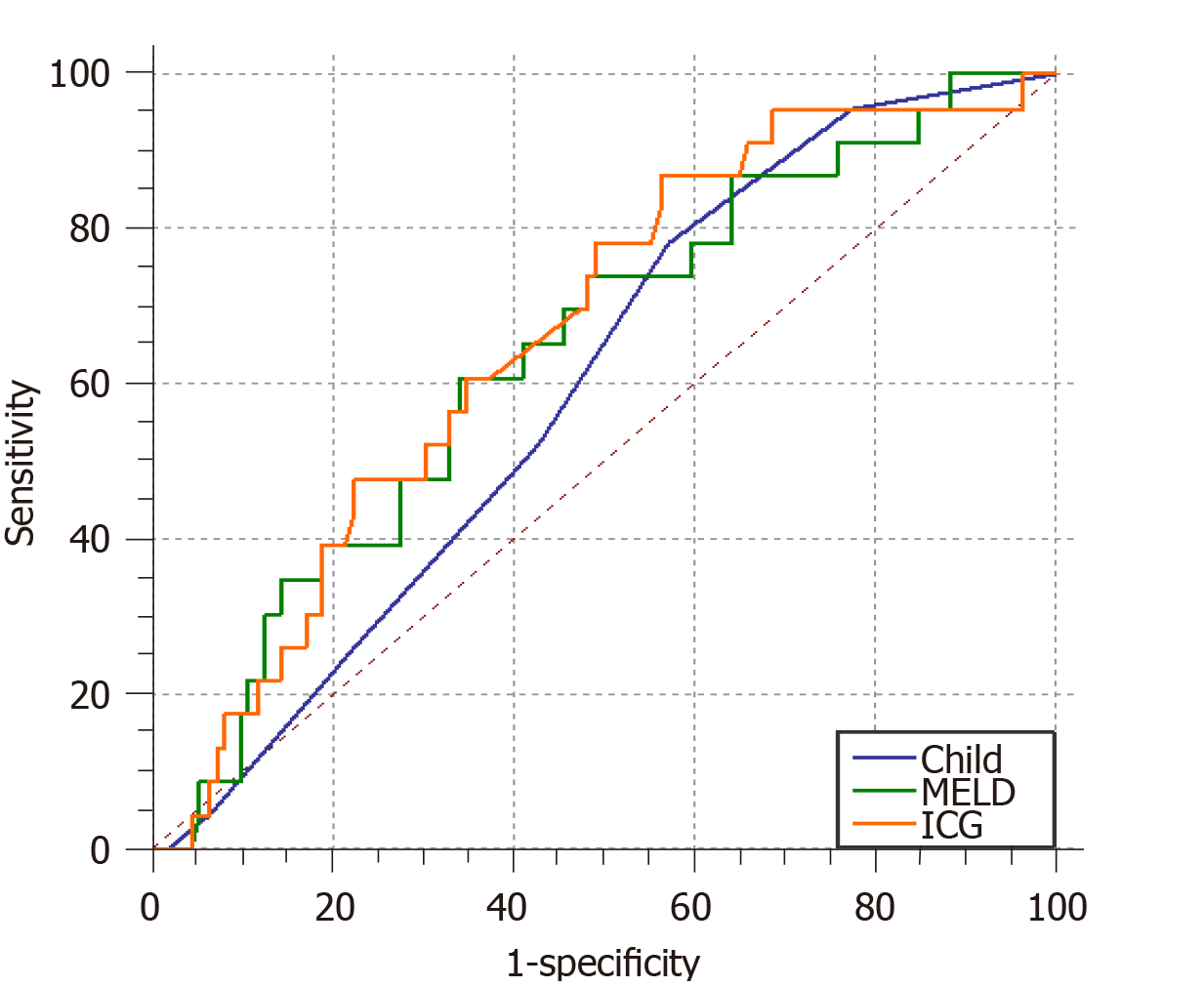
A total of 195 patients with portal hypertension were included and 23% (45/195) of the patients developed post-TIPS HE. The ICG-R15 was identified as an independent predictor of post-TIPS HE. The AUCs for the ICG-R15, CPS, and MELD score for predicting post-TIPS HE were 0.664 (95% confidence interval [CI]: 0.557-0.743, P = 0.0046), 0.596 (95%CI: 0.508-0.679, P = 0.087), and 0.641 (95%CI: 0.554-0.721, P = 0.021), respectively. The non-parametric approach (Delong-Delong & Clarke-Pearson) showed that there was statistical significance in pairwise comparison between AUCs of ICG-R15 and MELD score (P = 0.0229).
The ICG-R15 has appreciated clinical value for predicting the occurrence of post-TIPS HE and is a choice for evaluating the prognosis of patients undergoing TIPS.
Core Tip: We studied whether the indocyanine green retention rate at 15 min (ICG-R15) can be used for prediction of post-transjugular intrahepatic portosystemic shunt (TIPS) hepatic encephalopathy (HE) in decompensated cirrhosis patients with portal hypertension (PHT) and compare the clinical value of ICG-R15, Child-Pugh score, and model for end-stage liver disease score in predicting post-TIPS HE with PHT.
- Citation: Wang Z, Wu YF, Yue ZD, Zhao HW, Wang L, Fan ZH, Zhang Y, Liu FQ. Comparative study of indocyanine green-R15, Child-Pugh score, and model for end-stage liver disease score for prediction of hepatic encephalopathy after transjugular intrahepatic portosystemic shunt. World J Gastroenterol 2021; 27(5): 416-427
- URL: https://www.wjgnet.com/1007-9327/full/v27/i5/416.htm
- DOI: https://dx.doi.org/10.3748/wjg.v27.i5.416
Portal hypertension (PHT) is a very common and serious complication of chronic liver disease that often causes variceal bleeding and refractory ascites[1]. Transjugular intrahepatic portosystemic shunt (TIPS) is an important treatment option that has been shown to be efficacious in the management of PHT[2]. This procedure can alleviate portal hypertension by creating a large channel between the portal vein and hepatic vein[3]. Unfortunately, TIPS can cause severe complications such as heart failure, liver failure, and hepatic encephalopathy (HE). HE has a high incidence rate and is one of the most debilitating complications, which has a serious effect on the prognosis and survival of patients[4-6]. Although some risk factors are known, the identification of patients at risk of HE needs additional research. It is important to predict post-TIPS HE so that prevention and treatment measures can be implemented in high-risk HE patients to avoid adverse outcomes.
The indocyanine green retention rate at 15 min (ICG-R15), the Child-Pugh score (CPS), and the model for end-stage liver disease (MELD) score have been developed to assess liver function[7,8]. The ICG-R15 is a relatively non-invasive, quick, and inexpensive method that has been widely used in patients with end-stage liver disease[9]. Zipprich et al[10] reported that ICG is the most accurate predictor among quantitative liver function tests of the survival of patients with cirrhosis[10]. A recent retrospective study demonstrated that preoperative ICG clearance was predictive of the surgical prognosis in patients undergoing hepatectomy[11]. The CPS was developed to assess the severity of liver cirrhosis in the clinic. This scoring system includes the bilirubin level, the albumin level, the prothrombin time, HE, and ascites[12]. The MELD score is used to predict the survival of patients undergoing TIPS and to evaluate patients with severe liver disease prior to transplantation. It includes three objective variables: The total bilirubin level, the creatinine level, and the international normalized ratio (INR)[13]. However, there are limited data on the use of liver function tools, especially the ICG-R15, to predict post-TIPS HE. Therefore, the aim of this study was to compare the clinical value of the MELD score, CPS, and ICG-R15 for the prediction of post-TIPS HE in patients with PHT.
This retrospective study was approved by the Ethics Committee of Beijing Shijitan Hospital of Capital Medical University. The need to obtain informed consent was waived due to its retrospective nature.
All patients who underwent TIPS between January 2018 and June 2019 in the interventional department of Beijing Shijitan Hospital were included in this study.
The following inclusion criteria were set: (1) Patients between 18 and 70 years old; (2) Patients diagnosed with PHT; and (3) Patients who underwent TIPS using a polytetrafluoroethylene-covered stent. The following exclusion criteria were set: (1) Preoperative HE; (2) Liver cancer; (3) Liver transplantation; (4) TIPS retreatment; (5) Non-cirrhotic PHT; (6) Surgical splenectomy; (7) Portal vein thrombosis; and (8) Urgent TIPS.
PHT was defined as the radiological presence of significant splenomegaly, umbilical vein recanalization, and/or portosystemic shunts as well as a preoperative platelet count < 100 × 109/L. Portal pressure gradient values greater than or equal to 10 mmHg indicated clinically significant PHT[14].
HE was defined as neuropsychiatric abnormalities ranging from mild neuro-psychological dysfunction to deep coma and abnormal ammonia levels, after the exclusion of other possible causes of altered mental status by computed tomography or magnetic resonance imaging[15].
All patients included in this study underwent the ICG-R15 test with a dye-densitogram (DDG) analyser (Japan, NIHON KOHDEN, model DDG-3300K) and an ICG clearance rate test (Japan, NIHON KOHDEN, model A). Within 30 s after the injection of ICG (0.5 mg/kg) into the median cubital vein, plasma ICG concentrations were monitored via a sensor attached to the patients’ finger. The ICG-R15 was subsequently assessed by a computer. The CPS score can be divided into three grades depending on the total points: Grade A (5-6 points), B (7-9 points), and C (≥ 10 points). The MELD score was calculated based on the following formula: R = 3.8 × ln (bilirubin mg/dL) + 11.2 × ln (INR) + 9.6 ln (creatinine mg/dL) + 6.4 × aetiology (biliary and alcoholic 0, others 1)[8,16] (Figure 1).
Under fluoroscopic guidance, a standard TIPS procedure was performed by an experienced interventional radiologist. A pigtail catheter was inserted into the right internal jugular vein leading to the hepatic veins. After finding the portal vein through the superior mesenteric artery or splenic artery using indirect portal venography, a stent (7, 8, or 10 mm, Fluency, Bard, United States) was placed to create a channel between the hepatic vein and the portal vein. Afterward, the portal vein pressure was measured at least three times, and a pressure transducer system (Combitrans, Braun Melsungen, Germany) with a multichannel monitor (Sirecust, Siemens, Germany) was used to measure the haemodynamic parameters.
Clinical and laboratory characteristics were collected from the medical records. SPSS (version 20.0, SPSS Inc., United States) and MedCalc were used for the statistical analyses. Descriptive data are presented as the mean ± SD, and qualitative variables are presented as frequencies or percentages. Student's t test or the Mann-Whitney U test was used to compare quantitative variables between groups, and the chi-square test or Fisher's exact test was used for qualitative variables. Univariate and multivariable logistic regression analyses were used to determine HE-related risk factors after TIPS. The areas under the receiver operating characteristic curves (AUCs) for the ICG-R15, CPS, and MELD score were evaluated. The non-parametric approach (Delong-Delong & Clarke-Pearson)[17] was used for pairwise comparison among AUCs of ICG-R15, CPS, and MELD score. Statistical significance was established at P < 0.05.
A total of 221 patients who underwent TIPS were included in this study. After applying the inclusion and exclusion criteria of the study, data were collected from a total of 195 decompensated cirrhosis patients with PHT who underwent TIPS. The basic clinical characteristics are listed in Table 1. The study population comprised 140 men and 55 women, with a mean age of 51.2 ± 11.4 years. The indications included variceal bleeding in 118 (60.7%) patients, refractory ascites in 36 (18.5%), variceal bleeding combined with ascites in 27 (14.1%), and other (including pleural fluid and hepatorenal syndrome) in 14 (6.7%). The most common cause of cirrhosis was viral cirrhosis (60.7%), followed by alcoholic cirrhosis (15.9%), biliary cirrhosis (4.6%), and drug-induced and autoimmune hepatitis cirrhosis (4.6%). According to the CPS, 108 patients were classified as having grade A, 75 as having grade B and 12 as having grade C. The median MELD score and ICG-R15 were 7 (4.1, 10) and 38.4 (22, 50), respectively.
| Parameter | Patients enrolled (n = 195) | Patients without post-TIPS HE (n = 150) | Patients with post-TIPS HE (n = 45) | P value |
| Age (years)1 | 51.2 ± 11.4 | 49.8 ± 13.2 | 58.3 ± 9.9 | 0.004b |
| Gender (F/M), n2 | 140/55 | 0.79 | ||
| TIPS indication, n2 | 0.10 | |||
| Variceal bleeding | 118 (60.7) | 93 | 25 | |
| Ascites | 36 (18.5) | 29 | 7 | |
| Bleeding combine with ascites | 27 (14.1) | 21 | 6 | |
| Other | 14 (6.7) | 7 | 7 | |
| Cirrhotic aetiology, n2 | 0.98 | |||
| Viral | 146 (74.9) | 111 | 35 | |
| Alcoholic | 31 (15.9) | 25 | 6 | |
| Biliary | 9 (4.6) | 7 | 2 | |
| Drug-induced | 4 (2) | 3 | 1 | |
| Autoimmune | 5 (2.6) | 4 | 1 | |
| Preoperative HVPG, mmHg3 | 17.1 (11, 23) | 18 (13, 21) | 20 (12, 20) | 0.28 |
| Preoperative PPG, mmHg3 | 23 (18, 29) | 23 (16, 29) | 23 (20, 28) | 0.6 |
| Stent size, mm (%)2 | 0.057 | |||
| 7 | 59 (30.4) | 51 | 8 | |
| 8 | 117 (60) | 87 | 30 | |
| 10 | 19 (9.6) | 12 | 7 | |
| Puncture site of portal vein, n2 | 0.013a | |||
| Left branch | 113 (57.7) | 96 | 17 | |
| Bifurcation | 22 (11.1) | 15 | 7 | |
| Right branch | 60 (31.2) | 40 | 20 | |
| Child-Pugh score1 | 7.1 ± 1.5 | 7.0 ± 1.6 | 8.0 ± 2.1 | |
| Child-Pugh grade, n2 | 0.029a | |||
| A | 108 (55.6) | 89 | 19 | |
| B | 75 (38.5) | 55 | 20 | |
| C | 12 (5.9) | 6 | 6 | |
| MELD score3 | 7.0 (4.1, 10.0) | 6.5 (3.8, 10.0) | 7.9 (5.8, 13.0) | 0.07 |
| ICG-R15, %3 | 38.4 (22, 50) | 34.6 (21.8, 47.7) | 44.4 (35.4, 63.0) | 0.013a |
| Total protein, g/L1 | 64.0 ± 9.0 | 64.6 ± 9.6 | 61.8 ± 6.9 | 0.19 |
| Total bilirubin, μmoL/L3 | 24.5 (16.8, 35.4) | 24.0 (16.9, 35.2) | 29.8 (19.5, 44.5) | 0.169 |
| Creatinine, μmoL/L3 | 63 (52, 77) | 61 (52, 73) | 70 (59, 85) | 0.084 |
| ALT, U/L3 | 21 (15, 33) | 22 (16, 33) | 21 (16, 29) | 0.889 |
| AST, U/L3 | 28 (20, 42) | 28 (20, 44) | 31 (23, 50) | 0.442 |
| ALP, U/L3 | 91 (67, 122) | 90 (67, 131) | 92 (65, 121) | 0.39 |
| Albumin, g/L1 | 34.9 ± 5.4 | 33.4 ± 5.2 | 35.2 ± 6.9 | 0.12 |
| Serum potassium, mmoL/L1 | 4.0 ± 0.5 | 3.9 ± 0.3 | 4.0 ± 0.4 | 0.62 |
| Serum clozapine, mmoL/L1 | 105.2 ± 5.8 | 105.3 ± 5.4 | 105.5 ± 6.6 | 0.81 |
| Serum sodium, mmoL/L1 | 138.7 ± 5.1 | 136.2 ± 4.9 | 140.7 ± 6.1 | 0.39 |
| WBC, × 109/L3 | 3.27 (2.1, 4.6) | 3.1 (2.1, 4.4) | 3.3 (2.5, 5.7) | 0.26 |
| Platelet count, × 109/L3 | 80 (58.3, 120) | 85 (61.2, 127.7) | 83 (63, 107) | 0.49 |
| Hb, g/L1 | 97.4 ± 27.1 | 95.3 ± 25.5 | 98.1 ± 27.9 | 0.9 |
| BUN, mmoL/L3 | 5.7 (4.3, 7.7) | 5.4 (4.2, 7.0) | 7.6 (5.4, 8.8) | 0.07 |
| NH3, μg/Dl1 | 49.5 ± 19.5 | 46.6 ± 15.4 | 54.9 ± 23.8 | 0.037a |
| APTT, s3 | 33.4 ± 4.4 | 33.8 ± 4.5 | 33.5 ± 4.2 | 0.76 |
| INR1 | 1.4 ± 0.3 | 1.37 ± 0.3 | 1.32 ± 0.19 | 0.50 |
| FIB, g/L1 | 2.17 ± 0.8 | 2.2 ± 0.8 | 2.3 ± 0.7 | 0.39 |
| PT, s1 | 14.8 ± 2.5 | 14.7 ± 2.6 | 14.4 ± 2.0 | 0.69 |
Of the 195 patients who underwent TIPS, 45 (23%) developed HE at the 12 mo follow-up. The factors associated with HE in univariable analysis included age, stent size, puncture site, ICG-R15, CPS, MELD score, blood urea nitrogen (BUN) level, and NH3 level (P = 0.006, 0.019, 0.006, 0.027, 0.022, 0.020, 0.025, and 0.044, respectively). As shown in Table 2, multivariate regression analysis identified the following variables as independent risk factors for post-TIPS HE: Older age, 10 mm stent size, puncture site in the right branch of the portal vein, high ICG-R15, high BUN level, high NH3 level, high CPS, and high MELD score.
| Parameter | Univariable logistic regression | Multivariable logistic regression | ||||
| Regression coefficient | OR | P value | Regression coefficient | OR | P value | |
| Age | 0.53 | 1.055 | 0.006b | 0.057 | 1.059 | 0.021a |
| Gender (F/M) | 0.134 | 1.143 | 0.789 | |||
| TIPS indication (variceal bleeding/ascites/bleeding combined with ascites/other) | 0.147 | 1.159 | 0.522 | |||
| Cirrhotic aetiology (viral/alcoholic/biliary/drug-induced/autoimmune) | 0.053 | 0.761 | 0.437 | |||
| Preoperative HVPG | 0.019 | 1.019 | 0.502 | |||
| Preoperative PPG | 0.022 | 1.023 | 0.289 | |||
| Stent size (7/8/10) | -0.975 | 0.377 | 0.019a | |||
| Puncture site of portal vein (left branch/bifurcation/right branch) | 0.763 | 2.145 | 0.006b | 0.566 | 1.762 | 0.018a |
| Child-Pugh score | 0.027 | 1.027 | 0.022a | 0.053 | 1.055 | 0.046a |
| Child-Pugh grade (A/B/C) | 0.196 | 0.594 | 1.216 | |||
| MELD score | 0.256 | 1.291 | 0.020a | 0.516 | 1.068 | 0.043a |
| ICG-R15 | 0.026 | 1.027 | 0.027a | 0.028 | 1.029 | 0.031a |
| Total protein | -0.029 | 0.972 | 0.203 | |||
| Total bilirubin | 0.000 | 1.000 | 0.962 | |||
| Creatinine | 0.006 | 1.006 | 0.151 | |||
| ALT | 0.000 | 1.000 | 0.892 | |||
| AST | 0.003 | 1.003 | 0.232 | |||
| ALP | 0.002 | 1.002 | 0.248 | |||
| Albumin | -0.058 | 0.94 | 0.1364 | |||
| Serum potassium | 0.233 | 1.262 | 0.689 | |||
| Serum clozapine | 0.007 | 1.007 | 0.864 | |||
| Serum sodium | -0.035 | 0.965 | 0.395 | |||
| WBC | 0.047 | 1.049 | 0.544 | |||
| Platelet count | -0.003 | 0.997 | 0.359 | |||
| Hb | -0.001 | 0.999 | 0.908 | |||
| BUN | 0.084 | 1.087 | 0.025a | 0.085 | 1.008 | 0.028a |
| NH3 | 0.025 | 1.025 | 0.044a | 0.032 | 1.033 | 0.025a |
| APTT | -0.015 | 0.985 | 0.768 | |||
| INR | -0.644 | 0.525 | 0.504 | |||
| FIB | 0.248 | 1.281 | 0.389 | |||
| PT | -0.059 | 0.943 | 0.559 | |||
The area under the receiver operating characteristic (ROC) curve for the ICG-R15 (AUC = 0.664, 95% confidence interval [CI]: 0.557-0.743, P = 0.0046) for the prediction of post-TIPS HE was larger than those of the CPS (AUC = 0.596, 95%CI: 0.508-0.679, P = 0.087) and the MELD score (AUC = 0.641, 95%CI: 0.554-0.721, P = 0.021). The non-parametric approach (Delong-Delong & Clarke-Pearson)[17] showed that there was no statistical significance in pairwise comparison between AUCs of ICG-R15 and MELD score (P = 0.0229). The cut-off value for the ICG-R15, which was determined by the maximum of Youden index, was 30, with a sensitivity of 86.96% and specificity of 56.25% for the prediction of post-TIPS HE (Table 3). Of the 126 patients with an ICG-R15 > 30, 36 (28.5%) developed post-TIPS HE, while 9 (13%) of 69 patients with an ICG ≤ 30 developed post-TIPS HE.
| Parameter | CPS | MELD | ICG-R15 |
| AUC | 0.596 | 0.641 | 0.664 |
| 95%CI | 0.508-0.679 | 0.554-0.721 | 0.557-0.743 |
| Z statistic | 1.709 | 2.293 | 2.832 |
| P value | 0.087 | 0.021a | 0.0046b |
| Youden index J | 0.211 | 0.269 | 0.307 |
| Cut-off value | 6 | 7.5 | 30 |
| Sensitivity | 78.2 | 60.87 | 86.96 |
| Specificity | 42.6 | 66.07 | 43.75 |
The patients were divided into two groups according to the ICG-R15 cut-off value, 30%, determined by the maximal Youden index. Patients with an ICG-R15 > 30% had a higher incidence of HE than those with an ICG-R15 < 30% (13% vs 28.5%; P = 0.014). There were significant differences in age, CPS, preoperative portal pressure gradient, NH3, albumin, aspartate transaminase, Cl, Na, white blood cells, active partial thromboplastin, or prothrombin time in patients with different ICG-R15 levels below and above 30% (Table 4).
| Characteristic | ICG-R15 ≤ 30% (n = 69) | ICG-R15 > 30% (n = 126) | P value |
| HE/non HE1 | 9 (13%)/60 | 36 (28.5%)/90 | 0.014a |
| Age (yr)2 | 48.3 ± 11.6 | 52.9 ± 14.1 | 0.033a |
| Child-Pugh score2 | 6.3 ± 1.2 | 7.6 ± 1.6 | < 0.001c |
| Preoperative PPG, mmHg3 | 15.6 (11, 18) | 18 (16, 24) | 0.005b |
| NH3, μg/dL2 | 45.0 ± 15.0 | 52.0 ± 21.2 | 0.021a |
| Albumin, g/L2 | 38.0 ± 4.4 | 33.3 ± 5.6 | |
| AST, U/L3 | 25 (18, 36) | 28.5 (22, 45.7) | 0.018a |
| Serum clozapine, mmoL/L2 | 106.6 ± 4.8 | 104.5 ± 5.9 | 0.024a |
| Serum sodium, mmoL/L2 | 140.4 ± 3.7 | 137.7 ± 5.5 | < 0.001c |
| WBC, × 109/L3 | 2.77 (1.81, 3.86) | 3.6 (2.3, 4.9) | 0.031a |
| APTT, s3 | 32.8 ± 3.2 | 34.3 ± 5.0 | 0.024a |
| PT, s2 | 14.0 ± 1.9 | 15.3 ± 2.6 | 0.043a |
TIPS has been widely used to treat complications of PHT, including varices and ascites, by creating a large channel between the hepatic vein and portal vein. This procedure changes the liver haemodynamics by shunting a fraction of the portal venous blood directly into the systemic circulation, which can lead to decreased liver blood supply and impaired liver function reserve. In addition, HE occurs because of an increase in the amount of natural toxins such as ammonia travelling to the brain as a result of the shunting of the blood directly from the portal vein to the hepatic vein[18]. HE can produce a spectrum of neurological/psychiatric syndromes ranging from subclinical alterations to coma. It remains one of the most common and worrisome complications of end-stage liver disease after TIPS[15].
The ICG-R15 test is simple, fast, less invasive, and inexpensive, and can be performed in less than half an hour. The ICG-R15 retention trial was introduced as a relatively noninvasive tool for the classification of pediatric and adult patients with acute and chronic liver failure[19]. A particular advantage of the ICG-R15 test is that it is more suitable for pediatric patients. In addition, the trial appears to be an ideal way to assess the risks of surgical procedures such as liver resection. In addition to assessing the predictive value of varices and ascites, the results of some papers suggest that the ICG-R15 test may also predict mortality[20].
In this study, univariate and multivariate logistic analyses showed that 10 mm stent size, puncture site in the right branch of the portal vein, age, ICG-R15, BUN level, NH3 level, CPS, and MELD score were predictors of post-TIPS HE in patients with portal hypertension. Patients with puncture sites in the right portal vein had a high incidence of post-TIPS HE. This is related to the right branch of the portal vein contains more poisons mainly received from the superior mesenteric vein[21-23]. It was reported that choosing the left branch of the portal vein as the puncture site during the placement of TIPS may decrease the incidence of HE significantly[24,25]. HE occurs more often in patients with a stent diameter of 10 mm than in those with smaller-diameter stents. A larger stent can effectively reduce portal vein pressure, but at the same time, more blood that has not been detoxified by the liver directly enters the systemic circulation, which can further impair liver function and lead to HE. However, some studies showed that the incidence of post-TIPS HE was unrelated to stent diameter[26]. Li et al[27] also found that age and Child-Pugh classification were independent risk factors for early post-TIPS HE[27], which was in accord with previous studies[28,29]. Fonio et al[4] demonstrated that the MELD grade was a risk factor for post-TIPS HE; the results of this study support this finding[4]. Normally, ammonia is detoxified by conversion to urea by Krebs-Henseleit buffer or the urea cycle in the liver. In total, 40% to 60% of the urea nitrogen in the primary urine is absorbed in the renal tubules and collecting tubes. A high incidence of HE was found in patients with high BUN levels, which is related to the aggravation of liver function involving the kidneys, renal decompensation, and azotaemia[30].
Hiwatashi et al[31] found that a higher ICG-R15 was significantly correlated with surgical complications and liver dysfunction after surgical resection and chemotherapy in patients with colorectal cancer[31]. Wang et al[32] demonstrated that the ICG-R15 could more accurately predict preoperative liver reserve function than the Child-Pugh and MELD scores in patients who suffered from liver cancer[32]. Another study showed that the ALICE scoring system (including serum albumin and ICG-R15) could simply and effectively predict the prognosis of liver cancer patients undergoing surgery[33]. In this study, we analysed and compared the areas under the ROC curves for the ICG-R15, CPS, and MELD score. The results were as follows: AUCICG-R15 > AUCMELD > AUCCPS, and there was statistical significance in pairwise comparison between AUCs of ICG-R15 and MELD score. This suggests that the ICG-R15 has clinical equivalent value to predict post-TIPS HE compared to MELD score. The CPS is relatively restricted because it includes two subjective variables (HE and severity of ascites)[12], and the limited values ranging from 5 to 15 make it imprecise. The boundary values for the five parameters were chosen empirically and have not been formally validated[34]. In some situations, the predictive value of MELD may be reduced. Malabsorption of vitamin K secondary to cholestasis can cause an increase in the INR; starvation and infection can increase the level of bilirubin; and the use of diuretics can increase the level of creatinine[35]. As a quantitative assessment, the ICG-R15 is a simple and practical way to assess liver function and is widely used in patients undergoing liver surgery. The ICG-R15 may play a role in predicting post-TIPS HE; consequently, it may be useful for identifying high-risk patients.
The optimal ICG-R15 cut-off value in our study was 30%, and it divided the patients into two groups with different risks of post-TIPS HE. This difference was highly significant (P = 0.014), which implies that TIPS patients with an ICG-R15 > 30% should be given special care during perioperative management. However, we did not compare the role of the CPS, MELD score, and ICG-R15 for predicting the survival of patients after TIPS, which needs further study. In addition, future studies should analyse the incidence of complications and survival between different ICG-R15 groups. A small number of patients with Child-Pugh C cirrhosis and the low median MELD score were limitations of this study. Since this may be related to the small sample size, we will increase the amount of the sample in the future.
In summary, the ICG-R15 can be used for predicting post-TIPS HE in patients with PHT. We propose using the ICG-R15 to evaluate the risk of HE in PHT patients undergoing TIPS.
TIPS for PHT in patients with cirrhosis should be considered after careful selection based on patient characteristics and liver function. The ICG-R15 has appreciated clinical value for predicting the occurrence of post-TIPS HE and is a choice for evaluating the prognosis of patients undergoing TIPS.
Transjugular intrahepatic portosystemic shunt (TIPS) is a technique for the treatment of portal hypertension-related complications such as esophageal variceal bleeding and refractory ascites by establishing shunt channels in the hepatic parenchyma between the hepatic vein and the portal vein. It can also be used as a bridging therapy for decompensated patients with cirrhosis and other patients waiting for liver transplantation. However, the high incidence of postoperative hepatic encephalopathy (HE) seriously affects the prognosis and survival of patients, and it is particularly important to find accurate methods to predict post-TIPS HE. Some studies have shown that the clearance rate of indocyanine green before operation has good predictive value for the prognosis of patients undergoing hepatectomy. We hypothesized that indocyanine green retention rate at 15 min (ICG-R15) may can predict postoperative HE after TIPS (post-TIPS HE); therefore, prevention and treatment can be implemented in high-risk HE patients to avoid adverse outcomes.
TIPS is currently used in the management of complications of portal hypertension. However, the incidence of HE remains an issue in TIPS placement and affects patient quality of life and long-term outcomes. The preoperative ICG-R15 has been developed as a prognostic indicator in patients undergoing surgery, but there are limited data on its role in TIPS. The purpose of this study was to explore whether ICG-R15 can be used as a predictor of post-TIPS HE and compared the clinical value of the ICG-R15, Child-Pugh score (CPS), and model for end-stage liver disease (MELD) score for the prediction of post-TIPS HE in decompensated cirrhosis patients with portal hypertension (PHT).
The aim of this study was to explore whether ICG-R15 can be used as a predictor of post-TIPS HE and compared the clinical value of the ICG-R15, CPS, and MELD score for the prediction of post-TIPS HE in decompensated cirrhosis patients with PHT. According to the ICG-R15 value, appropriate and timely intervention can be implemented in patients with high-risk HE patients.
We conducted a prospective study of 195 patients with PHT who underwent elective TIPS. All patients underwent the ICG-R15 test, CPS evaluation, and MELD scoring. According to whether they developed HE or not, the patients were divided into two groups: HE group and non-HE group. Descriptive data are presented as the mean ± SD, and qualitative variables are presented as frequencies or percentages. Student's t test or the Mann-Whitney U test was utilized to compare quantitative variables between groups, and the chi-square test or Fisher's exact test was used for qualitative variables. Univariate and multivariable logistic regression analyses were used to determine HE-related risk factors after TIPS. The prediction of one-year post-TIPS HE by ICG-R15, CPS, and MELD score was evaluated by the areas under the receiver operating characteristic curves (AUCs). Pairwise comparison of AUCs in three different function tools was analysed.
A total of 195 patients with PHT were included and 23% (45/195) of the patients developed post-TIPS HE. The ICG-R15 was identified as an independent predictor of post-TIPS HE. The AUCs for the ICG-R15, CPS, and MELD score for predicting post-TIPS HE were 0.664 (95% confidence interval [CI]: 0.557-0.743, P = 0.0046), 0.596 (95%CI: 0.508-0.679, P = 0.087), and 0.641 (95%CI: 0.554-0.721, P = 0.021), respectively. Molodianovitch et al[17] showed that there was statistical significance in pairwise comparison between AUCs of ICG-R15 and MELD score (P = 0.0229).
TIPS for PHT in patients with cirrhosis should be considered after careful selection based on patient characteristics and liver function. The ICG-R15 has appreciated clinical value for predicting the occurrence of post-TIPS HE and is a choice for evaluating the prognosis of patients undergoing TIPS.
We can learn from this study that monitoring patients who underwent TIPS with an ICG-R15 value above 30% can better prevent adverse outcomes. Future studies will focus on the incidence of complications and survival in terms of the value of ICG-R15 and randomized controlled trials are needed in order to verify our results.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Joko K, Liem S, Martins VH S-Editor: Fan JR L-Editor: Wang TQ P-Editor: Wang LL
| 1. | Tsochatzis EA, Bosch J, Burroughs AK. Liver cirrhosis. Lancet. 2014;383:1749-1761. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1139] [Cited by in F6Publishing: 1188] [Article Influence: 118.8] [Reference Citation Analysis (0)] |
| 2. | Hung ML, Lee EW. Role of Transjugular Intrahepatic Portosystemic Shunt in the Management of Portal Hypertension: Review and Update of the Literature. Clin Liver Dis. 2019;23:737-754. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 3. | Sankar K, Moore CM. Transjugular Intrahepatic Portosystemic Shunts. JAMA. 2017;317:880. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 12] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 4. | Fonio P, Discalzi A, Calandri M, Doriguzzi Breatta A, Bergamasco L, Martini S, Ottobrelli A, Righi D, Gandini G. Incidence of hepatic encephalopathy after transjugular intrahepatic portosystemic shunt (TIPS) according to its severity and temporal grading classification. Radiol Med. 2017;122:713-721. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 34] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 5. | Teng D, Zuo H, Liu L, Dong J, Ding L. Long-term clinical outcomes in patients with viral hepatitis related liver cirrhosis after transjugular intrahepatic portosystemic shunt treatment. Virol J. 2018;15:151. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 6. | Zuo L, Lv Y, Wang Q, Yin Z, Wang Z, He C, Guo W, Niu J, Bai W, Li K, Yu T, Yuan X, Chen H, Liu H, Xia D, Wang E, Luo B, Li X, Yuan J, Han N, Nie Y, Fan D, Han G. Early-Recurrent Overt Hepatic Encephalopathy Is Associated with Reduced Survival in Cirrhotic Patients after Transjugular Intrahepatic Portosystemic Shunt Creation. J Vasc Interv Radiol. 2019;30:148-153.e2. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 19] [Article Influence: 3.8] [Reference Citation Analysis (1)] |
| 7. | Pind ML, Bendtsen F, Kallemose T, Møller S. Indocyanine green retention test (ICG-r15) as a noninvasive predictor of portal hypertension in patients with different severity of cirrhosis. Eur J Gastroenterol Hepatol. 2016;28:948-954. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 25] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 8. | Haj M, Rockey DC. Predictors of clinical outcomes in cirrhosis patients. Curr Opin Gastroenterol. 2018;34:266-271. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 14] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 9. | Reinhart MB, Huntington CR, Blair LJ, Heniford BT, Augenstein VA. Indocyanine Green: Historical Context, Current Applications, and Future Considerations. Surg Innov. 2016;23:166-175. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 175] [Cited by in F6Publishing: 257] [Article Influence: 28.6] [Reference Citation Analysis (0)] |
| 10. | Zipprich A, Kuss O, Rogowski S, Kleber G, Lotterer E, Seufferlein T, Fleig WE, Dollinger MM. Incorporating indocyanin green clearance into the Model for End Stage Liver Disease (MELD-ICG) improves prognostic accuracy in intermediate to advanced cirrhosis. Gut. 2010;59:963-968. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 65] [Cited by in F6Publishing: 71] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 11. | Schwarz C, Plass I, Fitschek F, Punzengruber A, Mittlböck M, Kampf S, Asenbaum U, Starlinger P, Stremitzer S, Bodingbauer M, Kaczirek K. The value of indocyanine green clearance assessment to predict postoperative liver dysfunction in patients undergoing liver resection. Sci Rep. 2019;9:8421. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 20] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 12. | Kaplan DE, Dai F, Aytaman A, Baytarian M, Fox R, Hunt K, Knott A, Pedrosa M, Pocha C, Mehta R, Duggal M, Skanderson M, Valderrama A, Taddei TH; VOCAL Study Group. Development and Performance of an Algorithm to Estimate the Child-Turcotte-Pugh Score From a National Electronic Healthcare Database. Clin Gastroenterol Hepatol. 2015;13:2333-41.e1-6. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 78] [Cited by in F6Publishing: 90] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 13. | Hoekstra LT, de Graaf W, Nibourg GA, Heger M, Bennink RJ, Stieger B, van Gulik TM. Physiological and biochemical basis of clinical liver function tests: a review. Ann Surg. 2013;257:27-36. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 204] [Cited by in F6Publishing: 220] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 14. | Turco L, Garcia-Tsao G. Portal Hypertension: Pathogenesis and Diagnosis. Clin Liver Dis. 2019;23:573-587. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 42] [Cited by in F6Publishing: 40] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 15. | Montagnese S, Russo FP, Amodio P, Burra P, Gasbarrini A, Loguercio C, Marchesini G, Merli M, Ponziani FR, Riggio O, Scarpignato C. Hepatic encephalopathy 2018: A clinical practice guideline by the Italian Association for the Study of the Liver (AISF). Dig Liver Dis. 2019;51:190-205. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 49] [Cited by in F6Publishing: 61] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 16. | Asrani SK, Kamath PS. Model for end-stage liver disease score and MELD exceptions: 15 years later. Hepatol Int. 2015;9:346-354. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 41] [Cited by in F6Publishing: 39] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 17. | Molodianovitch K, Faraggi D, Reiser B. Comparing the areas under two correlated ROC curves: parametric and non-parametric approaches. Biom J. 2006;48:745-757. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 70] [Cited by in F6Publishing: 72] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 18. | Routhu M, Safka V, Routhu SK, Fejfar T, Jirkovsky V, Krajina A, Cermakova E, Hosak L, Hulek P. Observational cohort study of hepatic encephalopathy after transjugular intrahepatic portosystemic shunt (TIPS). Ann Hepatol. 2017;16:140-148. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 22] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 19. | Lisotti A, Azzaroli F, Buonfiglioli F, Montagnani M, Cecinato P, Turco L, Calvanese C, Simoni P, Guardigli M, Arena R, Cucchetti A, Colecchia A, Festi D, Golfieri R, Mazzella G. Indocyanine green retention test as a noninvasive marker of portal hypertension and esophageal varices in compensated liver cirrhosis. Hepatology. 2014;59:643-650. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 81] [Cited by in F6Publishing: 82] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 20. | Stauber RE, Wagner D, Stadlbauer V, Palma S, Gurakuqi G, Kniepeiss D, Iberer F, Smolle KH, Haas J, Trauner M. Evaluation of indocyanine green clearance and model for end-stage liver disease for estimation of short-term prognosis in decompensated cirrhosis. Liver Int. 2009;29:1516-1520. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 30] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 21. | Mogicato G, Vautravers G, Meynaud-Collard P, Deviers A, Sautet J. Blood flows in tributaries of the portal vein: anatomical and angiographic studies in normal beagle dogs. Anat Histol Embryol. 2015;44:460-467. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 7] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 22. | Ursic M, Ravnik D, Hribernik M, Pecar J, Butinar J, Fazarinc G. Gross anatomy of the portal vein and hepatic artery ramifications in dogs: corrosion cast study. Anat Histol Embryol. 2007;36:83-87. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 30] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 23. | Maruyama H, Okugawa H, Ishibashi H, Takahashi M, Kobayashi S, Yoshizumi H, Yokosuka O. Carbon dioxide-based portography: an alternative to conventional imaging with the use of iodinated contrast medium. J Gastroenterol Hepatol. 2010;25:1111-1116. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 6] [Article Influence: 0.4] [Reference Citation Analysis (1)] |
| 24. | Chen SL, Hu P, Lin ZP, Zhao JB. The Effect of Puncture Sites of Portal Vein in TIPS with ePTFE-Covered Stents on Postoperative Long-Term Clinical Efficacy. Gastroenterol Res Pract. 2019;2019:2935498. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 25. | Luo SH, Chu JG, Huang H, Zhao GR, Yao KC. Targeted puncture of left branch of intrahepatic portal vein in transjugular intrahepatic portosystemic shunt to reduce hepatic encephalopathy. World J Gastroenterol. 2019;25:1088-1099. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 18] [Cited by in F6Publishing: 19] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 26. | Trebicka J, Bastgen D, Byrtus J, Praktiknjo M, Terstiegen S, Meyer C, Thomas D, Fimmers R, Treitl M, Euringer W, Sauerbruch T, Rössle M. Smaller-Diameter Covered Transjugular Intrahepatic Portosystemic Shunt Stents Are Associated With Increased Survival. Clin Gastroenterol Hepatol. 2019;17:2793-2799.e1. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 51] [Cited by in F6Publishing: 52] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 27. | Li Y, He X, Pang H. A model to predict early hepatic encephalopathy in patients undergoing transjugular intrahepatic portosystemic shunt. Turk J Gastroenterol. 2019;30:702-707. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 28. | Casadaban LC, Parvinian A, Minocha J, Lakhoo J, Grant CW, Ray CE, Knuttinen MG, Bui JT, Gaba RC. Clearing the Confusion over Hepatic Encephalopathy After TIPS Creation: Incidence, Prognostic Factors, and Clinical Outcomes. Dig Dis Sci. 2015;60:1059-1066. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 59] [Cited by in F6Publishing: 70] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 29. | Bai M, Qi X, Yang Z, Yin Z, Nie Y, Yuan S, Wu K, Han G, Fan D. Predictors of hepatic encephalopathy after transjugular intrahepatic portosystemic shunt in cirrhotic patients: a systematic review. J Gastroenterol Hepatol. 2011;26:943-951. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 120] [Cited by in F6Publishing: 115] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 30. | Bigot A, Tchan MC, Thoreau B, Blasco H, Maillot F. Liver involvement in urea cycle disorders: a review of the literature. J Inherit Metab Dis. 2017;40:757-769. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 38] [Cited by in F6Publishing: 42] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 31. | Hiwatashi K, Ueno S, Sakoda M, Iino S, Minami K, Mori S, Kita Y, Baba K, Kurahara H, Mataki Y, Maemura K, Shinchi H, Natsugoe S. The Evaluation of Liver Function and Surgical Influence by ICGR15 after Chemotherapy for Colorectal Liver Metastases. J Cancer. 2016;7:595-599. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 8] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 32. | Wang YY, Zhao XH, Ma L, Ye JZ, Wu FX, Tang J, You XM, Xiang BD, Li LQ. Comparison of the ability of Child-Pugh score, MELD score, and ICG-R15 to assess preoperative hepatic functional reserve in patients with hepatocellular carcinoma. J Surg Oncol. 2018;118:440-445. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 42] [Cited by in F6Publishing: 50] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 33. | Kokudo T, Hasegawa K, Amikura K, Uldry E, Shirata C, Yamaguchi T, Arita J, Kaneko J, Akamatsu N, Sakamoto Y, Takahashi A, Sakamoto H, Makuuchi M, Matsuyama Y, Demartines N, Malagó M, Kokudo N, Halkic N. Assessment of Preoperative Liver Function in Patients with Hepatocellular Carcinoma - The Albumin-Indocyanine Green Evaluation (ALICE) Grade. PLoS One. 2016;11:e0159530. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 38] [Cited by in F6Publishing: 48] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 34. | Wong M, Busuttil RW. Surgery in Patients with Portal Hypertension. Clin Liver Dis. 2019;23:755-780. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 35. | Cheng XP, Zhao J, Chen Y, Meng FK, Xu B, Yu HW, Meng QH, Liu YM, Zhang SB, Meng S, Zhang JY, Zhang JY, Duan ZP, Zheng SJ. Comparison of the ability of the PDD-ICG clearance test, CTP, MELD, and MELD-Na to predict short-term and medium-term mortality in patients with decompensated hepatitis B cirrhosis. Eur J Gastroenterol Hepatol. 2016;28:444-448. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 18] [Article Influence: 2.3] [Reference Citation Analysis (0)] |