Published online Nov 21, 2021. doi: 10.3748/wjg.v27.i43.7572
Peer-review started: March 4, 2021
First decision: June 3, 2021
Revised: June 30, 2021
Accepted: October 31, 2021
Article in press: October 31, 2021
Published online: November 21, 2021
Processing time: 259 Days and 22 Hours
Of 25% of randomised controlled trials (RCTs) on interventions for inflammatory bowel disease (IBD) have no power calculation.
To systematically review RCTs reporting interventions for the management of IBD and to produce data for minimum sample sizes that would achieve appro
We included RCTs retrieved from Cochrane IBD specialised Trial register and CENTRAL investigating any form of therapy for either induction or maintenance of remission against control, placebo, or no intervention of IBD in patients of any age. The relevant data was extracted, and the studies were grouped according to the intervention used. We recalculated sample size and the achieved difference, as well as minimum sample sizes needed in the future.
A total of 105 trials were included. There was a large discrepancy between the estimated figure for the minimal clinically important difference used for the calculations (15% group differences observed vs 30% used for calculation) explaining substantial actual sample size deficits. The minimum sample sizes indicated for future trials based on the 25 years of trial data were calculated and grouped by the intervention.
A third of intervention studies in IBD within the last 25 years are underpowered, with large variations in the calculation of sample sizes. The authors present a sample size estimate resource constructed on the published evidence base for future researchers and key stakeholders within the IBD trial field.
Core Tip: This work has identified a large variation in the estimated minimal clinically important difference (MCID) between study groups in inflammatory bowel disease trials in the literature, with no standard to support study designers or reviewers. We have provided a resource to support sample size estimation based on observed MICD in the literature over the last 25 years.
- Citation: Gordon M, Lakunina S, Sinopoulou V, Akobeng A. Minimum sample size estimates for trials in inflammatory bowel disease: A systematic review of a support resource. World J Gastroenterol 2021; 27(43): 7572-7581
- URL: https://www.wjgnet.com/1007-9327/full/v27/i43/7572.htm
- DOI: https://dx.doi.org/10.3748/wjg.v27.i43.7572
Sample size estimation (SSE) is an extremely important calculation for designing a clinical trial. Failure to produce an appropriate calculation may lead to imprecise results[1]. If a sample size is too large, statistically significant outcomes may be theoretically detected that may not be clinically relevant (type 1 error). This, however, is rarely a concern as studies are rarely overpowered to balance the study power with the cost. On the other hand, if a sample size is too small then a clinically significant outcome may not be detected statistically (type 2 error)[2,3]. The reporting of SSE in randomised controlled trials (RCTs) is a standard requirement according to the consolidated standards of reporting trials (CONSORT) statement which was introduced as a guide to conducting RCTs in 1996[4].
In a previous systematic review[5], we showed that 25% of RCTs on interventions for inflammatory bowel disease (IBD) have no power calculation (PC). A third of those who report PC do not achieve their target sample size. Based on those results, we decided to conduct a further systematic review.
We set out to systematically review RCTs on interventions for the IBD management, extract the vital parameters needed for sample size calculations, and synthesise the data to demonstrate whether trials across the field are adequately powered. We also set out to use the actual clinical data across these comparisons to synthesise data for minimum sample sizes that would achieve appropriate power to support future researchers designing trials and performing SSEs.
This review was performed in alignment with Cochrane guidelines[6] in April 2020 and reported in line with the Preferred Reporting Items for Systematic Reviews and Meta-analyses statement[7].
We followed the sampling methodology described within our systematic review protocol (uploaded within our institutional repository)[8] used for our previous review of the reporting of sample size calculations[5].
In brief, we included RCTs investigating either induction or maintenance therapy with biologics, immunomodulators, and microbiome against control, placebo, or no intervention. We conducted a comprehensive search of the Cochrane IBD Specialized Trials Register, CENTRAL, Cochrane library of IBD reviews for primary RCTs. The search terms are presented in Supplementary material.
We included RCTs published since 1996 (after the publication of the CONSORT statement). We excluded reports lacking clear information on the number of participants; cluster RCTs; pilot or feasibility studies; studies with mixed population of people with and without IBD; studies on secondary analyses of follow-up data collection after discontinuation of treatment. We excluded abstracts as these rarely allow space for such information to be presented. As we wanted to assess the established evidence for a PC of treatment for the IBD, we excluded RCTs describing all interventions where work may be at phase 3 (pharmacological: e.g. ustekinumab, golimumab, tofacitinib) or not under the three core headings (biologic, immunomodulators or anti-inflammatories).
Complying to the above search strategy, two authors (SL and MG) identified RCTs titles that appeared to be applicable. These were independently screened and in cases of disagreement, a third review author (VS) was involved to reach consensus. Two review authors independently extracted and recorded data on a predefined checklist. When disagreements occurred, a third review author was involved, and the consensus was reached.
We created an excel document to extract data regarding the trials. Firstly, we separated the studies into 8 categories [Crohn’s disease (CD)–clinical relapse, clinical remission, endoscopic relapse, endoscopic remission; ulcerative colitis (UC)–clinical relapse, clinical remission, endoscopic relapse, endoscopic remission]. Secondly, we grouped the studies according to the intervention used. One author extracted the data, and in case of any problems, the data was checked by the second author.
The extracted data although is not available publicly can be obtained via direct contact with authors. The references of the included stuidies can be found in Supplementary material.
(1) Number of events and participants originally assigned to each group; (2) Characteristics of participants; (3) The proportion that we calculated according to the number of events and participants (x = n/N), in which n is a number of events and N is a number of participants); (4) The difference achieved that we calculated according to the proportions of two groups (proportion 1–proportion 2); (5) Intervention and control details; (6) Presence of SSE and calculation details [minimal clinically important difference (MCID) used for PC, power, significance level, target sample size]; and (7) Outcomes (the number of patients recruited and completing study; the number of treatment success/failures; and the difference achieved).
We used the studies in which intervention was compared to the control or placebo. We grouped those studies according to the interventions, type of treatment (induction, maintenance), and outcomes (relapse, remission) and calculated mean difference and mean MCID where it was possible.
After resolving all the inconsistencies with data extraction regarding the use of sample size calculations for the studies with achieved difference of less than 10%, we produced two tables (Tables 1 and 2). We recalculated sample size for those groups using the power of 80%, probability of type 1 error 0.05, and the achieved difference. We used those parameters as they were the most commonly used amongst the studies. The parameters we used were two independent groups, dichotomous outcomes. In group 1 we have put the rate reported by the study of the intervention drug, and in group 2 we have put the rate of the placebo.
| Total studies | Studies with power calculation | Studies with difference of 10% and less1 | How many studies didn’t achieve target sample size | Mean sample size underpowered (range) | Mean sample size needed | How many studies are underpowered2 | |
| CD induction | 39 | 26 | 12 | 6 | 28 (2-70) | 231 | 11 |
| CD maintenance | 25 | 19 | 9 | 3 | 52 (7-79) | 300 | 10 |
| UC induction | 27 | 19 | 8 | 3 | 22 (1-55) | 219 | 4 |
| UC maintenance | 16 | 10 | 0 | 1 + 1 didn’t report | 21 | 196 | 7 |
| Ulcerative colitis-comparison | Ulcerative colitis-difference achieved (Group 1–Placebo) | Ulcerative colitis-Minimum sample size needed based on data | Crohn’s disease-comparison | Crohn’s disease-difference achieved (Group 1–Placebo) | Crohn’s disease-Minimum sample size needed based on data |
| Induction studies | |||||
| Outcome–clinical remission | Outcome–clinical remission | ||||
| Vedolizumab vs Placebo | 14.8% | 190 | Glutamine-enriched diet vs Placebo | -11.1 | 634 |
| Azathioprine vs Placebo | -3.6% | NA | |||
| 6-MP vs Placebo | 5% | NA | |||
| Fecal Transplant vs Control | 20.3% | 150 | 6-MP vs Placebo | 5% | NA |
| Budesonide vs Placebo | 6.5% | NA | Interventional diet vs Control diet | 20.9% | 160 |
| Type 1 IFNs vs Placebo | 5.9% | NA | Elemental diet vs Non elemental diet | 1.6% | NA |
| Etrolizumab vs Placebo | 13.4% | 140 | N6/N9 rich feeds vs non N6/N9 rich food | -1.1% | NA |
| Low dose naltrexone vs Placebo | 9% | NA | |||
| 5-ASA vs Placebo | 11.8% | 422 | GM-CSF vs Placebo | 7.8% | NA |
| Outcome–endoscopic remission | Brakinumab vs Placebo | 8.5% | NA | ||
| Vedolizumab vs Placebo | 37.7% | 182 | Ustekinumab vs Placebo | 8.6% | NA |
| Natalizumab vs Placebo | 14.8% | 310 | |||
| Fecal Transplant vs Control | 26.4% | 160 | Methotrexate vs Placebo | -14.8% | 350 |
| Budesonide vs Placebo | 13.9% | NA | Antibiotics vs Placebo | 10% | 780 |
| Methotrexate vs Placebo | 46.7% | NA | Outcome–endoscopic remission | ||
| Etrolizumab vs Placebo | 7.7% | NA | Low dose naltrexone vs Placebo | 22.2% | 60 |
| 5-ASA vs Placebo | 53.7% | 306 | |||
| Maintenance studies | |||||
| Outcome–clinical relapse | Outcome–clinical relapse | ||||
| 5-ASA vs Placebo | -16.4% | 290 | 5-ASA vs Placebo, medically induced | 3.1% | NA |
| Vedolizumab vs Placebo | -27.4 | 84 | 5-ASA vs Placebo, surgically induced | -5.4% | NA |
| Interventional diet vs Control diet | -3.6% | NA | Anti-TB vs Placebo | -23% | 130 |
| Probiotics vs Control | -16.7 | 154 | Azathioprine vs Placebo, medically induced | -9.9% | NA |
| Azathioprine vs Placebo | -22.4 | 154 | Azathioprine vs Placebo, surgically induced | -17.3% | 254 |
| Methotrexate vs Placebo | 19.9% | 194 | 6-MP vs Placebo, surgically induced | -10.9% | 646 |
| Rectal 5-ASA vs Placebo | -29% | 90 | Omega -3 fatty acids diet vs Control diet | -8.5% | NA |
| Curcumin vs Placebo | -9.6% | NA | Elemental diet vs No supplemets | -29.4% | 88 |
| Outcome–endoscopic relapse | Interventional diet vs Control diet | -2.5% | NA | ||
| Vedolizumab vs Placebo | -34 | 60 | Antibiotics vs Placebo | -14.6% | 360 |
| Methotrexate vs Placebo | -24.2% | 128 | |||
| 5-ASA vs Placebo | -16.4% | 290 | Methotrexate vs Placebo | -24.2% | 128 |
| Outcome–endoscopic relapse | |||||
| 5-ASA vs Placebo | 2.7% | NA | |||
| Azathioprine vs Placebo | -23% | 130 | |||
| 6-MP vs Placebo | -3.8% | NA | |||
| Antibiotics vs Placebo | 6.6% | NA | |||
| Induction studies | |||||
| Outcome–clinical remission | Outcome–clinical remission | ||||
| Vedolizumab vs Placebo | 14.8% | 190 | Glutamine-enriched diet vs Placebo | -11.1 | 634 |
| Azathioprine vs Placebo | -3.6% | NA | |||
| 6-MP vs Placebo | 5% | NA | |||
| Fecal Transplant vs Control | 20.3% | 150 | 6-MP vs Placebo | 5% | NA |
| Budesonide vs Placebo | 6.5% | NA | Interventional diet vs Control diet | 20.9% | 160 |
| Type 1 IFNs vs Placebo | 5.9% | NA | Elemental diet vs Non elemental diet | 1.6% | NA |
| Etrolizumab vs Placebo | 13.4% | 140 | N6/N9 rich feeds vs non N6/N9 rich food | -1.1% | NA |
| Low dose naltrexone vs Placebo | 9% | NA | |||
| 5-ASA vs Placebo | 11.8% | 422 | GM-CSF vs Placebo | 7.8% | NA |
| Outcome–endoscopic remission | Brakinumab vs Placebo | 8.5% | NA | ||
| Vedolizumab vs Placebo | 37.7% | 182 | Ustekinumab vs Placebo | 8.6% | NA |
| Natalizumab vs Placebo | 14.8% | 310 | |||
| Fecal Transplant vs Control | 26.4% | 160 | Methotrexate vs Placebo | -14.8% | 350 |
| Budesonide vs Placebo | 13.9% | NA | Antibiotics vs Placebo | 10% | 780 |
| Methotrexate vs Placebo | 46.7% | NA | Outcome–endoscopic remission | ||
| Etrolizumab vs Placebo | 7.7% | NA | Low dose naltrexone vs Placebo | 22.2% | 60 |
| 5-ASA vs Placebo | 53.7% | 306 | |||
| Maintenance studies | |||||
| Outcome–clinical relapse | Outcome–clinical relapse | ||||
| 5-ASA vs Placebo | -16.4% | 290 | 5-ASA vs Placebo, medically induced | 3.1% | NA |
| Vedolizumab vs Placebo | -27.4 | 84 | 5-ASA vs Placebo, surgically induced | -5.4% | NA |
| Interventional diet vs Control diet | -3.6% | NA | Anti-TB vs Placebo | -23% | 130 |
| Probiotics vs Control | -16.7 | 154 | Azathioprine vs Placebo, medically induced | -9.9% | NA |
| Azathioprine vs Placebo | -22.4 | 154 | Azathioprine vs Placebo, surgically induced | -17.3% | 254 |
| Methotrexate vs Placebo | 19.9% | 194 | 6-MP vs Placebo, surgically induced | -10.9% | 646 |
| Rectal 5-ASA vs Placebo | -29% | 90 | Omega -3 fatty acids diet vs Control diet | -8.5% | NA |
| Curcumin vs Placebo | -9.6% | NA | Elemental diet vs No supplemets | -29.4% | 88 |
| Outcome–endoscopic relapse | Interventional diet vs Control diet | -2.5% | NA | ||
| Vedolizumab vs Placebo | -34 | 60 | Antibiotics vs Placebo | -14.6% | 360 |
| Methotrexate vs Placebo | -24.2% | 128 | |||
| 5-ASA vs Placebo | -16.4% | 290 | Methotrexate vs Placebo | -24.2% | 128 |
| Outcome–endoscopic relapse | |||||
| 5-ASA vs Placebo | 2.7% | NA | |||
| Azathioprine vs Placebo | -23% | 130 | |||
| 6-MP vs Placebo | -3.8% | NA | |||
| Antibiotics vs Placebo | 6.6% | NA | |||
The small lest MCID that was reported by the studies was 10%, thereby, we decided to not reproduce PC for those studies with the achieved difference of less than 10%. We also calculated the mean sample deficit in percentage based on the target sample size and achieved sample size reported by the studies.
After receiving the sample size of participants, we made a decision whether the study is underpowered, and if yes, then by how many people.
We produced descriptive statistics regarding the sample sizes for the studies grouped according to the interventions (Tables 1 and 2).
As all data included already existed within the published scholarly output, no ethical approval was sought.
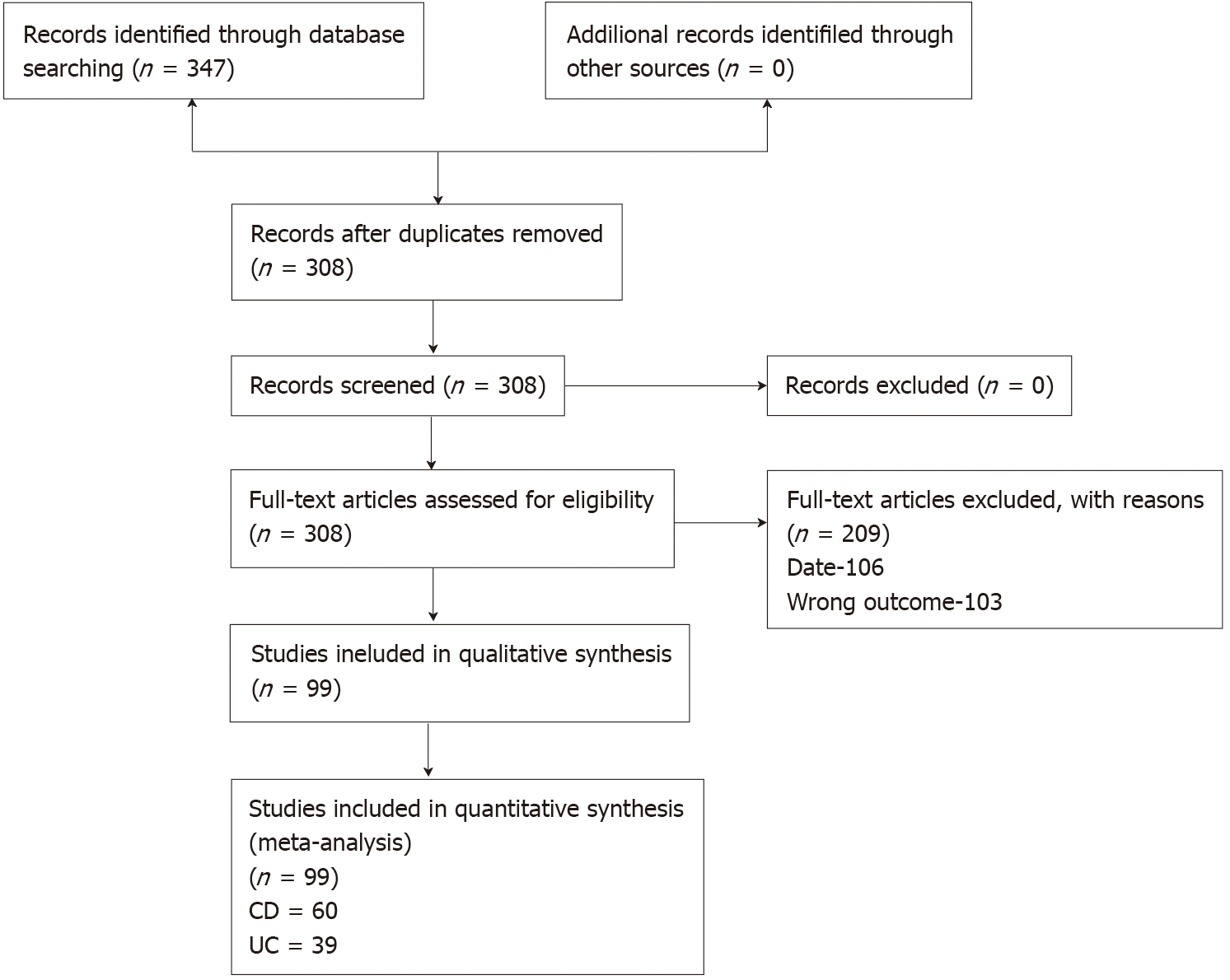
A total of 7451 potential citations were screened and 308 full texts assessed for eligibility. There were 209 texts excluded, 106 because they were published prior to the release of the CONSORT statement and 103 because they did not match our inclusion outcome. This left a total of 99 trials included, with 60 pertaining to CD and 39 to UC. The full details are shown in Figure 1.
The mean proportion of patients achieving clinical remission reported within the placebo groups of induction studies was 34.34% in CD trials and 26.79% for UC. For endoscopic remission, 0% in CD and 29.6% for UC. The mean proportion of patients achieving clinical relapse for maintenance studies were 55% for CD and 46.79% for UC. For endoscopic relapse, 78.85% in CD, and 28.7% in UC.
Within CD induction studies, 26 out of 41 (63.4%) reported a PC and 19 of 26 (73.1%) in maintenance studies. Within UC induction studies, 22 out of 31 (71%) reported a PC and 10 of 17 (58.8%) in maintenance studies.
When considering the MCID that those studies reporting a PC employed for this calculation, within CD induction studies the mean difference was 33% (range 20%-50%) and 27% difference for maintenance studies (15%-40%). Within UC induction studies the mean was 26% (range 19%-40%) and 27% for maintenance studies (18%-40%). The MCIDs these studies reported rarely matched the actual differences achieved by these studies. In fact, the discrepancy between this estimated figure for the MCID used for the PC and the actual differences seen were a mean of 22.8% higher in CD induction studies, 13.8% higher in maintenance studies, 15.7% higher in UC induction studies, and 10.2% higher in maintenance studies.
These discrepancies are proportionally large and in the context of PCs are clearly substantial and led to large numbers of studies being underpowered. These are summarised in Table 1. Study specific data with further details is available upon request.
Table 2 gives the results of our sample size calculations at the intervention specific level that employed the actual achieved clinical differences from previous studies, using the power of 80% and the probability of type 1 error 0.05. This shows the minimum sample sizes that would be indicated for RCTs compared with placebo to use. Within comparisons where the mean difference was less than 10%, no calculation has been given as this would be a very high indicative figure.
Within this review, it has been demonstrated that there is no clear basis or accepted standard for current practice for MCID estimation when producing a PC for a primary RCT within IBD. This has led to huge variations in suggested figures for recruitment. These trials present practical and logistical challenges to organisers, with potential inconvenience to patients, as well as the cost to those funding such research. Having an accurate figure for calculations is important to ensure this investment of resource is used most efficiently and effectively. It is key to note that we are not commenting at the individual study level. It is inappropriate to look at the projected MCID and PC for a project, if calculated on a reasonable basis, to then retrospectively suggest that the findings of a lesser MCID mean it is underpowered. This not just statistically inappropriate, but methodologically flawed. However, these findings propose that the basis for such MCID estimations is at worst unclear and often can be seen as flawed.
There are further ethical issues these problems raise, such as being forced to give treatments to people without having a statistically proved effect or a high certainty result within the Grading of Recommendations Assessment, Development and Evaluation analysis (due to reasons of imprecision from statistical sampling issues). The power of a study, therefore, has huge implications on the precision of estimates in the future analysis of data and in turn clinical practice guidelines. Within this review, 30% of studies appeared to be underpowered based on actual achieved clinical differences within the wider comparable evidence base, with mean sample size deficits up to 79 patients per trial. This does impact the overall certainty of the global evidence base within IBD, with precision a key limitation downgrading many outcomes within key guidelines across dozens of interventions.
Within this review, we present a resource for SSE not just for future study authors, but for study peer reviewers and most importantly professionals and the patients. This table gives an estimated PC result for a minimum sample size based on all existing studies within this period. Rather than being based on just single studies or clinical judgement, these represent estimates based on actual achieved clinical data and to our knowledge are the first time such a resource has ever been provided for researchers in the field or indeed for readers of future research. Additionally, for those wishing to calculate key statistics and measures of outcome from their primary studies, this paper provides a systematic and objective resource for baseline risk. This could be used for calculating numbers needed to treat or harm, for example.
This resource can be used by study designers to prevent PCs based on studies that offer a high MCID and as such a lower minimum sample size than is actually warranted. Conversely, it prevents unnecessary over recruitment. Funders can use this to appropriately budget and ensure viability of studies. Ethics boards and other governance groups will be able to consult this resource to support their consideration of research proposals.
There were a number of comparisons where the difference in practice was below 10% and it was deemed inappropriate to make a calculation in such cases, as no previous study has ever indicated an MCID below 10% as clinically significant to patients or practice. In these cases, consideration should be given to the overall figures presented in Table 2 or minimum sample size and MCID in practice in a similar context.
We would also recommend that in practice, patients and key stakeholders should be involved in deciding on an MCID for a given intervention prior to a new study. They may indicate that in spite of any existing MCID evidence that such a difference is not significant enough to matter to those who are most impacted by the findings and such views must be reflected in the process of SSE. It is also worth noting that there will always be settings and contexts when deviation may be warranted, thereby, a resource is not prescriptive but rather presented as evidence-based guidance. We would, however, propose that such deviations can and should be justified to support transparency for the readings these trials report.
There are weaknesses and exceptions to these approaches. The search methods used limited the parameters of the search for pragmatic reasons. However, this does not represent any systematic bias, hence we do not believe it invalidates the findings, and in the future this resource can be updated prospectively. When the achieved difference was less than 10%, rather than reporting extremely large sample size calculations, no such calculation was made. Additionally, in studies comparing active agents, accurate estimates are needed based on the contexts as the hypothesis may not be of the inferiority or superiority but of no difference, which requires a different approach to calculations.
There were some limitations to this review. There are obvious issues of heterogeneity limiting the appropriateness of pooling the data, however, the only way to obtain the previously used MCID was through looking at the past studies. These are mainly related to missing or unclear information in primary studies regarding SSE and as authors were not contacted, assumptions were made for the basis of these calculations which could confer some inaccuracy in our estimations. We also limited our studies to those from after the CONSORT statement release as we felt this was a fair time from which to expect SSE to occur, but earlier studies could potentially have offered more insight. Finally, we have focussed on studies comparing treatment with placebo or no intervention. This was a pragmatic decision as many studies of agents choose to make this comparison, although often these do not reflect current standard clinical practice. In the cases of such comparisons, SSE may not have to be based on a MCID but instead assume clinical equivalency and therefore be informed differently. In essence, this guidance may not be relevant for these scenarios, although may inform statistical considerations within similar contexts. Finally, such a resource of course is likely to become inaccurate rapidly, with the need for updates, but as often no such resource is employed, we believe this is still an improvement on current practices.
Future researcher is needed to potentially validate the calculations with clinical and patient input to ensure the SSE and MCID that the data informs has clinical, as well as statistical relevance. This could lead to a more triangulated resource that is statistically and evidentially sound, but also clinically sound and patient informed. This could conceivably lead to increases or decreases in minimally important differences to reflect complexity in specific clinical scenarios and interventional contexts.
In conclusion, a third of intervention IBD studies within the last 25 years are underpowered, with large variations in the approaches to calculating sample sizes and the minimum clinically important differences. The authors present a sample size estimate resource based on the published evidence base for future researchers and other key stakeholders within the IBD trial field.
A third of randomised controlled trials (RCTs) on interventions for inflammatory bowel disease (IBD) have no adequate power calculation (PC).
A key element of PCs is an estimation of a minimally important clinical difference. The basis of these is capricious within the literature, with many not based on any existing or prior studies and as such can lead to massive shifts in PCs for similar studies, with concerns as to the underlying power.
We systematically reviewed RCTs reporting interventions for the management of IBD and to producted a resource for minimum clinically important difference using clinical data for the future researchers to use as a starting point.
We included RCTs retrieved from Cochrane IBD trial register and CENTRAL investigating anti-inflammatory, immunomodulator and biologic therapies for either induction or maintenance of remission against control, placebo, or no intervention of IBD in patients of any age. The data was extracted and synthesized. We recalculated sample size and the achieved difference, as well as minimum sample sizes and presented in a tabular format.
Of 105 trials were included. A large discrepancy between the estimated figure for the minimal clinically important difference used for the calculations (15% differences observed vs 30% used for calculation) was observed explaining substantial actual sample size deficits. The minimum sample sizes indicated for future trials based on the 25 years of trial data were calculated and grouped by the intervention.
There are large variations in the sample size calculatins in the studies of interventions for IBD with a third of all studies being underpowered. The authors present a sample size estimate resource constructed on the published evidence base for future researchers and key stakeholders within the IBD trial field.
The use of this resource will support research staff, ethics committees and journal editors in ensuring adequate sample sizing and powering of studies across the field.
Provenance and peer review: Invited article; Externally peer reviewed.
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: United Kingdom
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Liefferinckx C, Vasudevan A, Yuksel I S-Editor: Fan JR L-Editor: A P-Editor: Fan JR
| 1. | Biau DJ, Kernéis S, Porcher R. Statistics in brief: the importance of sample size in the planning and interpretation of medical research. Clin Orthop Relat Res. 2008;466:2282-2288. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 197] [Cited by in RCA: 235] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 2. | Nayak BK. Understanding the relevance of sample size calculation. Indian J Ophthalmol. 2010;58:469-470. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 82] [Cited by in RCA: 128] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 3. | Cornish R. Statistics: An Introduction To Sample Size Calculations. Mathematics Learning Support Centre, 2006: 1-5. [cited 10 January 2021]. Available from: https://www.lboro.ac.uk/departments/mlsc/. |
| 4. | Begg C, Cho M, Eastwood S, Horton R, Moher D, Olkin I, Pitkin R, Rennie D, Schulz KF, Simel D, Stroup DF. Improving the quality of reporting of randomized controlled trials. The CONSORT statement. JAMA. 1996;276:637-639. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 409] [Cited by in RCA: 1077] [Article Influence: 37.1] [Reference Citation Analysis (0)] |
| 5. | Iheozor-Ejiofor Z, Lakunina S, Gordon M, Akintelure D, Sinopoulou V, Akobeng A. Sample-size estimation is not reported in 24% of randomised controlled trials of inflammatory bowel disease: A systematic review. United European Gastroenterol J. 2021;9:47-53. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 20] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 6. | Higgins JPT, Thomas J. Cochrane Handbook for Systematic Reviews of Interventions. In: Chandler J, Cumpston M, Li T, Page MJ, Welch VA. Chichester: John Wiley and Sons, 2019. |
| 7. | Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52948] [Cited by in RCA: 47144] [Article Influence: 2946.5] [Reference Citation Analysis (0)] |
| 8. | Gordon M, Lakunins S. [Protocol for systematic review 'The Reporting of Sample Size Estimation in Randomised Trials of Inflammatory Bowel Disease: A systematic review'.] [cited 10 January 2021]. Available from: http://clok.uclan.ac.uk/33088. |