Published online Aug 28, 2021. doi: 10.3748/wjg.v27.i32.5392
Peer-review started: April 7, 2021
First decision: May 27, 2021
Revised: June 3, 2021
Accepted: July 20, 2021
Article in press: July 20, 2021
Published online: August 28, 2021
Processing time: 139 Days and 10.8 Hours
Constipation is one of the chronic gastrointestinal functional diseases. It seriously affects the quality of life. Cistanche deserticola (C. deserticola) can treat constipation obviously, but its mechanism has not been clarified. We supposed that mechanism of it improved the intestinal motility by stimulating interstitial Cajal cells (ICC). Activation of the C-kit receptor on the surface of ICC is closely related to ICC function, and the stem cell factor (SCF)/C-kit signaling pathways plays an important role on it. To investigate the mechanism of how C. deserticola treats constipation, this study aimed to establish a constipation model in rats and explore the role of SCF/C-kit signaling pathway in the treatment.
To explore the SCF/C-kit signaling pathways in the role of C. deserticola for treatment of constipation by a constipation rat model.
Forty-eight 8-mo-old Sprague–Dawley rats were divided into 4 groups by random weight method: Normal group (n = 12), model group (n = 12), C. deserticola group (n = 12) and blocker group (n = 12). The normal group received normal saline by gavage; the model group received loperamide by gavage; the blocker group received loperamide and C. deserticola by gavage, and STI571 was injected by intraperitoneally. During treatment, the weight, fecal granules and fecal quality were recorded every 10 d. On day 20 after model induction, the colon tissues of each group were removed. Hematoxylin and eosin staining was used to observe pathological changes. Expression levels of SCF, C-kit and Aquaporin genes were detected by immunohistochemistry, western blotting, and real-time-quantitative polymerase chain reaction. The colonic epithelial mitochondria and goblet cells were observed by transmission electron microscopy.
Compared with the normal group, as treatment progressed, the weight of rats in the model and blocker groups decreased significantly, the stool weight decreased, and the stool quality was dry (P < 0.05). C. deserticola reversed the decrease in body weight and stool weight and improved stool quality. Histopathological analysis indicated that the colonic mucosal epithelium in the model group was incomplete, and the arrangement of the glands was irregular or damaged. Treatment with C. deserticola improved the integrity and continuity of the epithelial cells and regular arrangement of the glands. The blocking agents inhibited the effects of C. deserticola Immunohistochemistry and real-time-quantitative polymerase chain reaction showed that expression of SCF and C-kit protein or genes in the colonic tissue of the model group was decreased (P < 0.05), while treatment with C. deserticola increased protein or gene expression (P < 0.05). Western blotting showed that expression of aquaporin APQ3 was increased, while the expression of Cx43 decreased in the model group. Treatment with C. deserticola inhibited expression of APQ3 and promoted expression of Cx43. Transmission electron microscopy showed that the mitochondria of the colonic epithelium in the model group were swollen and arranged disorderly, and microvilli were sparse. The condition was better in the C. deserticola group. Mice treated with STI571 blocker confirmed that blocking the SCF/C-kit pathway inhibited the improvement of constipation by C. deserticola.
C. deserticola improved defecation in rats with constipation, and the SCF/C-kit signaling pathway, which is a key link of ICC function, played an important role of the treatment.
Core Tip: We studied a possible mechanism of Cistanche deserticola (C. deserticola) in the treatment of constipation. The mechanism might improve colon motility through stem cell factor (SCF)/C-kit signaling pathway in colon Cajal stromal cells. Therefore, the constipation rat model was replicated, and then rats were treated by direct administration of C. deserticola and specific blocking of the SCF/C-kit signaling pathway. The defecation of rats, changes of colonic pathology and ultrastructure as well as the protein expression related to the SCF/C-kit signaling pathway were observed. Our results support that the SCF/C-kit signaling pathway plays an important mechanism on the therapeutic effect of C. deserticola.
- Citation: Zhang X, Zheng FJ, Zhang Z. Therapeutic effect of Cistanche deserticola on defecation in senile constipation rat model through stem cell factor/C-kit signaling pathway. World J Gastroenterol 2021; 27(32): 5392-5403
- URL: https://www.wjgnet.com/1007-9327/full/v27/i32/5392.htm
- DOI: https://dx.doi.org/10.3748/wjg.v27.i32.5392
Senile constipation (SC) is one of the common chronic gastrointestinal functional diseases in geriatric patients, which induces or aggravates cerebrovascular events and other diseases and seriously affects the quality of life of the elderly population[1,2]. According to traditional Chinese medicine, the main etiology and pathogenesis of SC are gastrointestinal heat, deficiency of Yin and Jin and intestinal de-wetting[3,4], while Cistanche deserticola (C. deserticola) can warm the kidneys and relax the bowels. Researchers have reported that the effect of intestinal motility improvement and treatment of SC is clear, but its mechanism has not been clarified[5,6]. Interstitial Cajal cells (ICCs) are a special type of mesenchymal cells in the gastrointestinal tract. Activation of the C-kit receptor on the surface of ICCs is closely related to cell proliferation, differentiation and function. ICCs participate in the pathogenesis of many gastrointestinal motor dysfunctions[7,8]. Activation of C-kit depends on the binding of its ligand stem cell factor (SCF)[9]. To investigate the effect and mechanism of action of C. deserticola on SC, this study aimed to establish an SC model in rats induced by loperidol to explore the role of the SCF/C-kit signaling pathway in the treatment of constipation by C. deserticola.
Experimental animals: The experimental animals were 48 male 8-mo-old Sprague-Dawley rats weighing 500-550 g; all purchased from Changsha Tianqin Biotechnology Co. Ltd. Rats were raised in the animal laboratory of Chongqing Weisiteng Biomedical Technology Co. Ltd., given normal day and night light, food and drink. All procedures complied with the management guidelines issued by the Ethics Committee of Chongqing Traditional Chinese Medicine Hospital.
Primary reagents: The primary reagents included loperamide (Sigma, St. Louis, MO, United States), C. deserticola from Hubei Jurui Traditional Chinese Medicine Decoction Co. Ltd., STI571 from Chinese Selleck Co. Ltd., and SCF, C-kit, connexin 43 (Cx43), aquaporin 3 (AQP3) and β-actin antibodies (Abcam, Cambridge, MA, United States), PrimeScript II 1st Strand cDNA Synthesis Kit and Premix Taq™ (Ex Taq™ Version 2.0) (Takara Co. Ltd., Japan).
SC model preparation and grouping: To avoid the influence of confounding factors such as body weight on the experimental results, the rats were randomly grouped by the block random grouping method. Forty-eight rats were fed adaptively for 1 wk, and the rats were numbered in order of weight from light to heavy. According to the order of body weight, the rats were divided into 12 zones (the weight of rats in each zone was similar), with 4 rats in each zone. The 12 zones were divided into 4 groups of 12 (normal, model, C. deserticola and blocker). Rats in the model group were given loperamide (0.0625/100 g, 10 mL/kg) by gavage. The first gavage dose was doubled, and the drug was given at 10:00 every day, once a day for 20 consecutive days. The normal group was given the same amount of normal saline by gavage. In addition, the model group was treated with C. deserticola (0.156 g/100 g, 10 ml/kg) by gavage. The blocker group was given C. deserticola (0.156 g/100 g, 10 mL/kg) by gavage and STI571 (25 mg/mL, 1 mL/kg) by intraperitoneal injection. During the experiment, body weight, stool weight and stool condition were recorded every 10 d. No accidental death occurred in any of the rats. Twenty days later, the rats were killed, and the colonic tissue was removed for examination and liquid nitrogen preservation. The remaining parts were fixed and embedded.
Histopathological analysis: The rats fasted overnight were anesthetized by intraperitoneal injection with 7% chloral hydrate (5 mL/kg) on the next day. The abdomen was opened to separate the colonic tissue, and 8-10 mm of colon was removed. The intestinal contents were removed by gently shaking in precooled polybutylene succinate and fixed for 24 h in 4% paraformaldehyde fixative solution. Conventional paraffin embedding was performed. Tissue sections (5 μm) were removed for hematoxylin and eosin staining, and the histopathological morphology was observed under a microscope.
Detection of expression of SCF and C-kit by immunohistochemical staining: The paraffin tissues examined by histopathology above were sectioned, rehydrated with sectioned xylene dewaxed gradient ethanol, antigen remedied by heat and sealed for 1 h with goat serum at room temperature. SCF and C-kit primary antibodies were added dropwise, and tissues were kept overnight at 4 °C. After rinsing with polybutylene succinate, horseradish peroxidase was dropped to label secondary antibody, rinsed with polybutylene succinate for 5 min, drop 2,4-diaminobutyric acid chromogen solution, re-dye with hematoxylin, reverse gradient alcohol dehydration, transparentized by xylene and neutral resin seal. Microscopically, the nuclei were purplish blue, and the positive result products were brownish yellow or yellow particles.
Detection of expression of C-kit mRNA in colonic tissue by real-time-quantitative polymerase chain reaction (RT-qPCR): Frozen rat colonic tissue was ground by homogenizer, and 1 mL RNAiso Plus was added. Total RNA of colonic tissue was extracted by chloroform/isopropanol method, and the RNA quality was detected by gel electrophoresis. After identified by Bio-Rad gel imager, 1 μg RNA was reverse transcribed into cDNA by Takara PrimeScript II 1st Strand cDNA Synthesis Kit. According to NCBI database Gene ID: 64030, the CDS region of the C-kit gene was found and combined with NCBI Primer-Blast design Primer sequence, which was synthesized by Sangon Biotech Co. Ltd, C-kit primer sequence sense strand 5'-AGGT
Detection of protein expression level of Cx43 and AQP3 in colonic tissue by Western blotting: About 1 cm of frozen colonic tissue was collected and lysed with RIPA strong pyrolysis liquid (100 μL). The tissue was homogenized with a glass homogenizer 20 times and transferred to a 1.5 mL centrifuge tube. The supernatant was extracted after centrifugation at 12000 rpm at 4 °C for 5 min, and the concentration of total protein in the supernatant was determined by BCA method. We removed 25 μg denatured protein to perform sodium dodecyl sulfate-polyacrylamide gel electrophoresis and then transferred the proteins in the gel to polyvinylidene fluoride membrane at a constant current of 200 mA then sealed by 5% skim milk confining liquid for 2 h at room temperature. Corresponding primary antibodies were diluted with sealing fluid, incubated at 4 °C overnight, then washed the membrane resistance and added second antibodies. After adding ECL fluid, they were put under the exposure meter for test by optical density analysist.
Transmission electron microscopy: We took about 1 cm of proximal colonic tissue, fixed it in 2.5% glutaraldehyde for 24 h, dehydrated it with graded acetone, embedded it in Epon812 and cut 50 nm ultrathin sections on an ultramicrotome. The sections were stained with uranium dioxide acetate and lead citrate dyeing liquid at room temperature for 10 min. We observed colonic villi, epithelial cell mitochondria and goblet cells under a Philips Tecnai-10 transmission electron microscope.
SPSS 19.0 software was used for data analysis, and GraphPad Prism8.4 software was used for plotting the results. The Shapiro-Wilk’s test was used for normality testing. Data with normal distribution were represented by mean ± SD. Error items were used to represent the mean ± SD of hemoglobin after treatment. Student’s t-test was used for comparison between two groups. Analysis of variance was used for comparison between multiple groups. The Student-Newman-Keuls method was used for comparison between multiple groups in pairs, and Dunnett’s test was used to measure the differences between each experimental group and control group in sequence. Associated data were measured by multiple time point, and repeated measurement data analysis of variance was conducted. If the sphericity test showed P > 0.05, repeated measurement data analysis of variance was used. If P < 0.05, the generalized estimation equation was taken. Data that did not conform to a normal distribution were represented by M (P25, P75), and the Kruskal-Wallis H rank sum test was used for comparison between groups. The χ2 test was used to compare between groups when the data was unordered data, and Pearson’s χ2 test was used if the proportion of cells with theoretical frequency < 5 was < 20%. If the proportion of cells with theoretical frequency < 5 was ≥ 20%, Fisher’s exact test was used. P < 0.05 was used to determine that the difference was statistically significant.
One day after gavage of loperamide compared with the normal group, the body weight and stool weight in the model group were significantly decreased (P < 0.05), but there was no significant difference in stool quality score (P > 0.05). C. deserticola treatment in the model group slowed the decrease in body weight and stool weight (P < 0.05), but the body weight in the blocker group was not significantly reduced. After 10 d of treatment, compared with the normal group, the body weight and stool volume of rats in the model group were further significantly reduced, and the stools were dry and almost contained no water (P < 0.05). The body weight loss of rats was also significantly reduced by treatment with C. deserticola (P < 0.05). The body weight of rats in the blocker group was not significantly different from that in the model group (P > 0.05). On day 20 before sampling, the body weight of rats in the model group was significantly lower than that in the normal group, and the stool quality score was significantly increased (P < 0.05), while the weight loss caused by constipation was inhibited by C. deserticola treatment, and the stool quality score was decreased (P < 0.05).
Hematoxylin and eosin staining of colonic tissue of rats is shown in Figure 1. In the normal group, intestinal villi were intact, intestinal mucosa was intact and continuous, cells and glands were arranged regularly. Mucosa and submucosa were not infiltrated by inflammatory cells, and tissue structure was normal. In the model group, intestinal villi and mucosa were largely destroyed, local villi were shed and loosely arranged, and part of the glandular structure disappeared. In the C. deserticola group, histopathological staining was less than that of the model group, but there was necrosis in some goblet cells. After intraperitoneal injection in the blocker group, compared with the C. deserticola group, there was still local loss of intestinal villi, and there was fracture and loss of intestinal mucosa.
Immunohistochemical staining showed that SCF and C-kit were expressed in the colon of the control group. Compared with the control group, expression of SCF and C-kit in the model group was significantly decreased (P < 0.05). Compared with the model group, expression of SCF and C-kit in the C. deserticola group was significantly increased (P < 0.05). There was no difference in expression of SCF and C-kit between the blocker and model groups (P < 0.05, Figures 2 and 3).
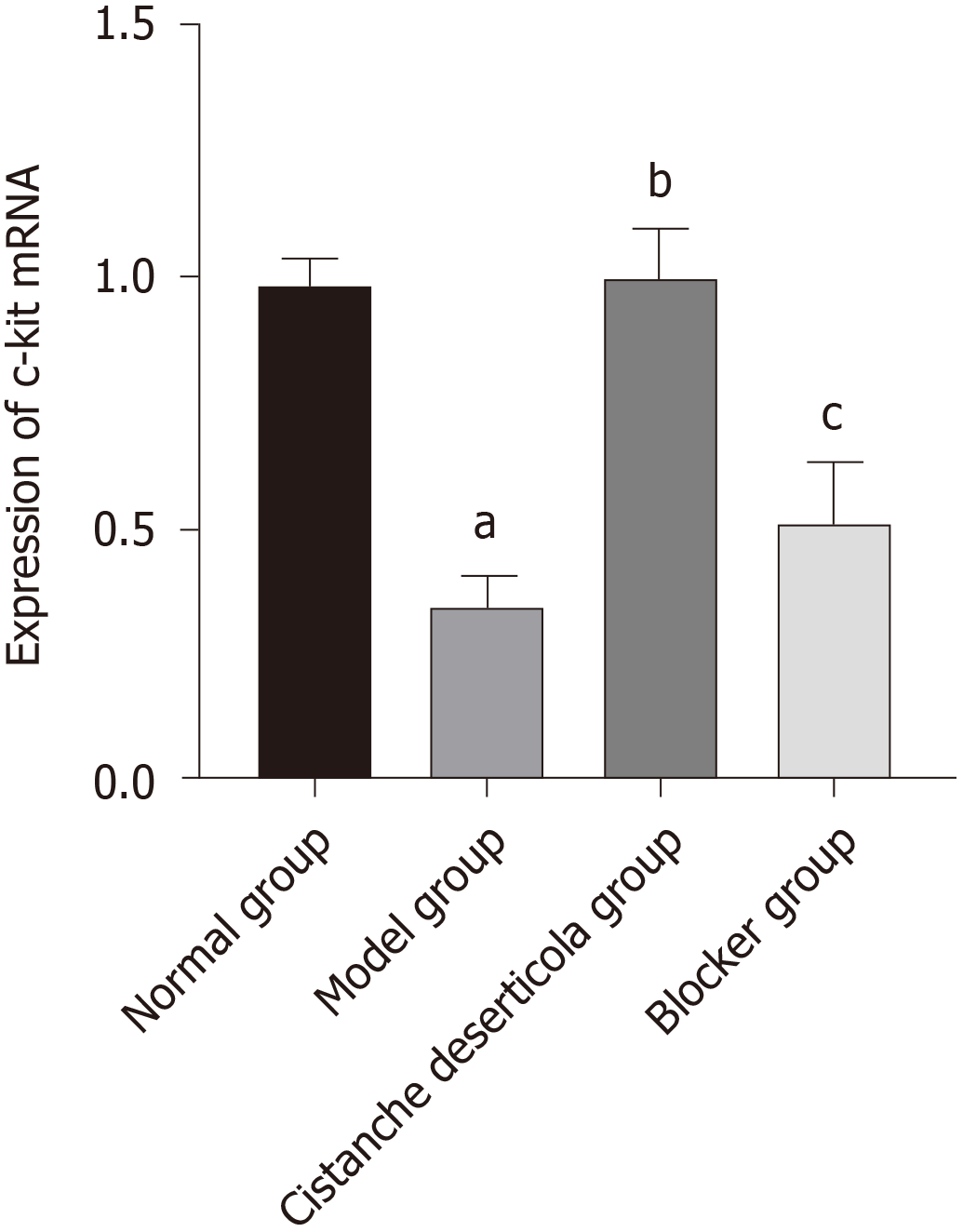
RT-qPCR showed that expression of C-kit mRNA in the model group was significantly lower than in the normal group (Figure 4, P < 0.05). Compared with the model group, C-kit in the C. deserticola group was significantly increased (P < 0.05). There was no significant difference in C-kit mRNA level between the model and blocker groups (P > 0.05). These results indicated that C-kit expression adjusted by C. deserticola participated in the improvement of constipation, and blockers inhibited the efficacy of C. deserticola.
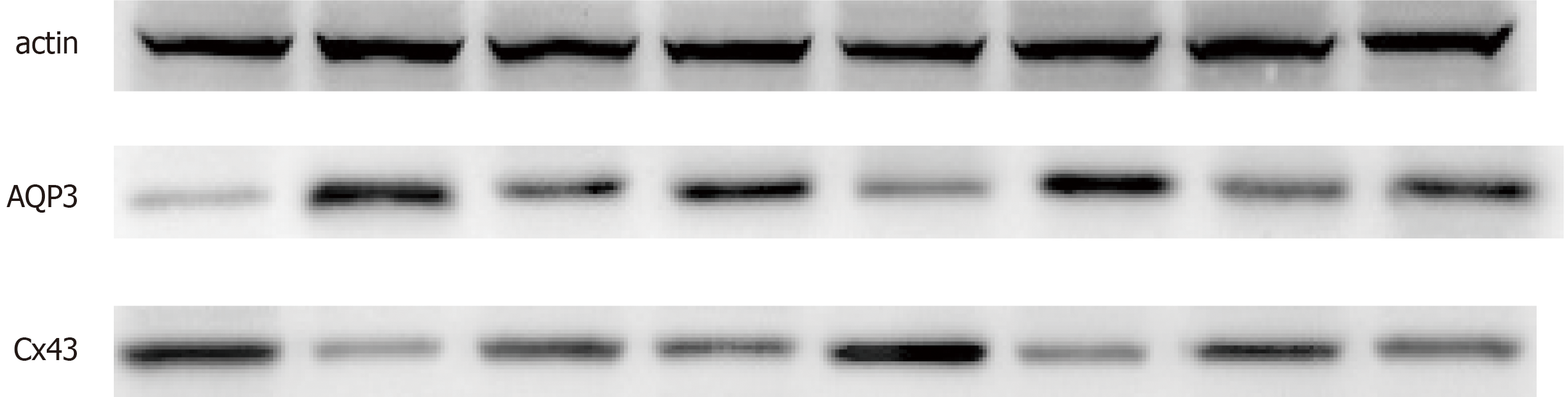
Western blotting showed that AQP3 protein was expressed at a low level in the normal control group, while it was significantly increased in the model group compared with the control group (Figure 5) (P < 0.05). Expression of AQP3 protein was decreased after treatment with C. deserticola, and the inhibitory effect of C. deserticola on the expression of AQP3 was inhibited by blocker treatment (P < 0.05). Expression of Cx43 was the opposite of that of AQP3. These results suggested that the pharmacology effect of C. deserticola on rats with SC was achieved by promoting expression of Cx43 and inhibiting expression of AQP3 (Figures 5-7).
After loperamide induction, mitochondrial swelling and autophagy occurred in some of the colonic mucosal epithelial cells in the model group. The number and length of intestinal microvilli decreased, and goblet cell pyknosis occurred (Figure 8). After treatment with C. deserticola, the number of intestinal microvilli increased. The microvilli were arranged neatly, and the structure was complete. The goblet cells regained some structural integrity but still did not return to normal length. After inhibitor treatment, the number of microvilli in colonic epithelial cells decreased significantly compared with those in the C. deserticola group, with loose arrangement, shorter length, irregular morphology of mitochondria and severe pyknosis of goblet cells.
Constipation is common in the elderly population and is one of the common symptoms that affect the health status and quality of life of elderly people[10,11]. In terms of traditional Chinese medicine, Bai et al[12] believed that the basic pathogenesis of SC was dysfunction of large intestine conduction function, senile body failure, gradual loss of liver and kidney functions, lack of qi and blood and loss of body fluid, which led to dry stools, resulting in constipation[13]. SC puts pressure on patients and their families in many aspects, and it is important to explore its pathogenesis.
C. deserticola has the functions of tonifying kidney Yang, benefiting blood and regulating body immunity[14]. C. deserticola and Chinese herbal decoction containing C. deserticola have been widely used in the treatment of constipation, and the clinical effect is remarkable. Du et al[15] found in a rat model of Yang deficiency and constipation that C. deserticola improved the contraction amplitude of isolated colon, strengthened the contractility of the intestinal tract and returned the level of gastrointestinal hormones to normal. In addition, dietary fiber of C. deserticola, a processing byproduct of C. deserticola, improves the water-holding capacity of feces and has the advantage of low dose and good effect in nourishing intestines and improving defecation[16]. Our study found that C. deserticola treatment improved fecal moisture and colonic histopathology of rats with constipation.
ICCs are found throughout the intestinal tract and play a vital role in the neural control of intestinal movement as pacemakers and intestinal nerve transmission[17]. Proto-oncogene coding receptor tyrosine kinase, C-kit, is expressed by ICCs, and SCF is a ligand of C-kit[18]. The SCF/C-kit system is crucial in the development of ICCs. The SCF/C-kit signaling pathway has been analyzed during treatment of slow transfer constipation in rats by acupuncture[19]. Electroacupuncture at Tianshu point increased the number of colon ICCs in ACK2-treated mice, upregulated expression of SCF and C-kit proteins and reversed the phenotype of ICC. Prescription for invigorating qi and spleen can increase expression of ICC in colonic tissue and upregulate expression of C-kit and SCF mRNA, thus improving constipation symptoms[20]. In our study, immunohistochemistry showed that C. deserticola significantly upregulated expression of SCF and C-kit protein in a rat model of SC, and RT-qPCR showed that C. deserticola significantly upregulated expression of C-kit mRNA. C-kit blocker inhibited the upregulation of SCF and C-kit expression induced by C. deserticola.
Fecal water content and colonic water transport are closely related to AQPs, which play an important role in intestinal water metabolism[21]. In a study of rats with slow transport constipation, Defecation decoction significantly improved constipation symptoms by regulating intestinal water absorption through downregulating AQP3 and AQP4[22]. In a rat model of functional constipation, the treatment mechanism of Oral Xiaofu Tongjie Fluid for functional constipation may be realized by regulating expression of vasoactive intestinal polypeptide and AQP3 in the colon[23]. Gap junctions play an essential role in mediating synchronous contraction of smooth muscle cells. Cx43 is the most important protein during that process[24]. Loss of Cx43 expression may be partially responsible for smooth muscle motor dysfunction[25]. Repair of the intestinal nervous system network structure in rats with slow transit constipation can improve the expression of Cx43 etc. and constipation symptoms[26]. These results indicate that the increased expression of AQP3 decreases fecal water content, leading to constipation, which may be an important mechanism of constipation. Expression of Cx43 may be related to contraction of intestinal smooth muscle and reduce the occurrence of constipation. In our study, western blotting showed that Cx43 expression was increased and AQP3 expression was inhibited by C. deserticola treatment, which resulted in increased smooth muscle contraction and decreased water absorption, and blocker treatment reversed these effects.
In conclusion, C. deserticola can inhibit expression of AQP3 and promote expression of Cx43 through the SCF/C-kit pathway, thereby improving the constipation induced by loperamine and reducing colonic tissue damage in aged rats. This study provided a good theoretical basis for clinical use of C. deserticola.
Chronic constipation is a common functional gastrointestinal disease that seriously affects the quality of life, especially for senile patients. Cistanche deserticola (C. deserticola) is one kind of herb that can improve constipation obviously, but the mechanism of it is unclear. Since it increases the frequency of defecation, we suppose that its therapeutic effect is due to increased intestinal motility by an important signaling pathway, stem cell factor (SCF)/C-kit, located on the surface of interstitial Cajal cells.
The treatment of chronic constipation is not encouraging, and the available drugs cannot meet the clinical needs. New drugs are needed to safely increase intestinal motility and improve symptoms. New drugs should be based on in-depth studies of the mechanisms of some foods that are currently widely and safely used. C. deserticola is one kind of herb that has been used for thousands of years in Traditional Chinese Medicine for constipation. Benefits would be evident from more studies about the treatment mechanisms.
To investigate the mechanism of how C. deserticola treats constipation, this study aimed to establish a constipation model in rats and explore the role of the SCF/C-kit signaling pathway in the treatment.
In the case of blank control group, a constipation rat model was first established. While these rats were treated with C. deserticola, a group of rats were specifically blocked from the target signaling pathway. The symptoms and defecation of these different groups of rats were observed, and the tissue and gene expression in which the target signaling pathway was located were observed to explain whether the SCF/C-kit signaling pathway plays a key role in the therapeutic effect of C. deserticola.
The model was successfully established, and the therapeutic effect of C. deserticola was also obvious. In the group where the target signaling pathway was blocked, the therapeutic effect of C. deserticola was significantly reduced, as reflected by histological and immunohistochemical changes as well as signal-pathway-related genes and proteins such as connexin 43, aquaporin 3 expression changes. C. deserticola can inhibit expression of aquaporin 3 and promote expression of connexin 43 through the SCF/C-kit pathway, thereby improving the constipation induced by loperamine and reducing colonic tissue damage in aged rats.
This study provided a good theoretical basis for clinical use of C. deserticola. Furthermore, the SCF/C-kit signal pathway plays an important role of constipation treatment of C. deserticola. There is likely more mechanisms related to it, for the effect of C. deserticola was not blocked completely.
The improvement of intestinal motility is the core point in the treatment of constipation. However, further research on intestinal dynamics is still needed. The research on intestinal dynamics of interstitial Cajal cells is still a focus of attention, but whether there is an unknown mechanism of its function is one of the directions of future research. In the meantime, herbs should also be more widely valued.
Manuscript source: Unsolicited manuscript
Corresponding Author’s Membership in Professional Societies: Anorectal Branch of China Association of Traditional Chinese Medicine, No. 201905365.
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Rangel-Corona R S-Editor: Wu YXJ L-Editor: Filipodia P-Editor: Li JH
| 1. | Li XF. Effect evaluation of lactulose and bifidobacterium tetragalus in the treatment of elderly functional constipation. Zhongguo Yaowu Yu Linchuang. 2019;19:1294-1295. |
| 2. | Liu X, Wang WH, Xia P, Cai FW. Clinical study of maren pill combined with prucalride in the treatment of elderly chronic constipation. Xiandai Yaowu Yu Linchuang. 2019;34:3329-3332. |
| 3. | Zhang HL, Xia Y. Observation on the curative effect of Recipes for Nourishing Yin and Moistening Intestines on senile constipation. Shiyong Zhongyiyiao Zazhi. 2019;35:405-406. |
| 4. | Ye SR. Experience in the treatment of senile constipation. Zhongguo Zhongyiyiao Xinxi Zazhi. 2003;S1. |
| 5. | Liu CY, Hu M. Clinical observation on 80 cases of elderly patients with intractable constipation treated by Cistanche Tongbian Decoction. Hebei Zhongyi. 2010;32:44-45. |
| 6. |
Tian PP, Clinical study on treatment of constipation in Parkinson's disease with Cistanche granules.
|
| 7. | Lorincz A, Redelman D, Horváth VJ, Bardsley MR, Chen H, Ordög T. Progenitors of interstitial cells of cajal in the postnatal murine stomach. Gastroenterology. 2008;134:1083-1093. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 125] [Cited by in RCA: 134] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 8. | Feng J, Gao J, Zhou S, Liu Y, Zhong Y, Shu Y, Meng MS, Yan J, Sun D, Fang Q. Role of stem cell factor in the regulation of ICC proliferation and detrusor contraction in rats with an underactive bladder. Mol Med Rep. 2017;16:1516-1522. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 18] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 9. | Yin J, Liang Y, Wang D, Yan Z, Yin H, Wu D, Su Q. Naringenin induces laxative effects by upregulating the expression levels of c-Kit and SCF, as well as those of aquaporin 3 in mice with loperamide-induced constipation. Int J Mol Med. 2018;41:649-658. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 50] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 10. | Cai YQ, Wang HJ, Zhang X, Wang YH, Gu ZW, Wang HX, Liu Y. Investigation on the prevalence of constipation and its relationship with sub-health symptoms in the elderly in Nanjing city. Zhonghua Laonian Yixue Zazhi. 2004;23:267-269. |
| 11. | Mugie SM, Benninga MA, Di Lorenzo C. Epidemiology of constipation in children and adults: a systematic review. Best Pract Res Clin Gastroenterol. 2011;25:3-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 665] [Cited by in RCA: 565] [Article Influence: 40.4] [Reference Citation Analysis (0)] |
| 12. | Bai L, Wang CJ. Professor Wang Chuijie's experience in the treatment of senile constipation. Jilin Zhongyiyiao. 2008;28:167-168. |
| 13. | Bao GQ, Huang L. Treatment analysis of 173 cases of senile constipation by syndrome differentiation. Heilongjiang Zhongyiyiao. 2000;5:22-22. |
| 14. | Ding XH, Zhang J, Wang YC. Observation on the curative effect of Cistanche defaecation oral liquid on senile constipation. Zhongguo Wuzhenxue Zazhi. 2009;31:7609-7610. |
| 15. | Du Q, Wu Z. Study on the dose-effect relationship and mechanism of the laxative effect of Cistanche deserticola based on the model of Yang deficiency and constipation. Zhongnan Yaoxue. 2016;1:23-27. |
| 16. | Wang LW, Sun J, Zhao B, Zhao MX. Study on the function of Cistanche deserticola dietary fiber to nourish intestines and defecate. Shipin Anquan Zhiliang Jiance Xuebao. 2016;7:3740-3744. |
| 17. | Iino S, Ward SM, Sanders KM. Interstitial cells of Cajal are functionally innervated by excitatory motor neurones in the murine intestine. J Physiol. 2004;556:521-530. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 87] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 18. | Torihashi S, Ward SM, Nishikawa S, Nishi K, Kobayashi S, Sanders KM. c-kit-dependent development of interstitial cells and electrical activity in the murine gastrointestinal tract. Cell Tissue Res. 1995;280:97-111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 139] [Article Influence: 4.6] [Reference Citation Analysis (2)] |
| 19. | HX. Discussions on regulation of acupuncture on SCF/c-kit signaling pathway and mechanism of treatment of slow transpose constipation. Nanjing: Nanjing University of Traditional Chinese Medicine. 2012;. |
| 20. | Wang JM, Li M, Tang R, Wang PS, Xu LJ, Zhang R, Han Y. Effects of Recipe for invigorating qi, invigorating spleen and relieving constipation on ICC and SCF/ C-KIT signal pathway in colonic tissue of rats with slow transmission constipation. Zhonghua Zhongyiyao Xuekan. 2019;37:156-160+264. |
| 21. | Ding YR, Zheng PY, Li FG, Mei L, Huang H, Bai LM, Liu SM. Effects of Lactitol and Bifidobacterium infantis on AQP3 and ICC in rats with constipation. Zhonghua Weishengwuxue He Mianyixue Zazhi. 2015;35:890-895. |
| 22. | Ji TL. Effect of Defaecation Decoction on AQP3 and AQP4 in rats with slow transit constipation. Nanjing: Nanjing University of Traditionl Chinese Medicine. 2017;. |
| 23. | Wang YJ, Zhou YX, Zhang H, Yan SG, Xie P, Man SY, Li S. Effects of N Oral Xiaofu Tongjie Decoction on the Expression of VIP and AQP3 in Colonic Tissue of Rats with Functional Constipation. Zhongguo Shiyan Fangjixue Zazhi. 2015;9:116-119. |
| 24. | Yu W, Zeidel ML, Hill WG. Cellular expression profile for interstitial cells of cajal in bladder-a cell often misidentified as myocyte or myofibroblast. PLoS One. 2012;7:e48897. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 40] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 25. | Nemeth L, Maddur S, Puri P. Immunolocalization of the gap junction protein Connexin43 in the interstitial cells of Cajal in the normal and Hirschsprung's disease bowel. J Pediatr Surg. 2000;35:823-828. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 58] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 26. | Kong JY. Experimental study on the effect of Oral Xiaofu Tongjie Fluid on the repair of intestinal nervous system network structure in rats with slow transit constipation. Chengdu Zongyiyao Daxue Xuebao. 2019;. |