Published online Aug 7, 2021. doi: 10.3748/wjg.v27.i29.4879
Peer-review started: February 19, 2021
First decision: May 13, 2021
Revised: May 17, 2021
Accepted: July 5, 2021
Article in press: July 5, 2021
Published online: August 7, 2021
Processing time: 165 Days and 21.5 Hours
Gut microbial dysbiosis contributes to the development and progression of colorectal cancer (CRC). Natural killer (NK) cells are involved in early defense mechanisms to kill infective pathogens and tumor cells by releasing chemokines and cytokines. To better understand the relationship between the gut microbiome and CRC, it was hypothesized here that a high abundance of Fusobacterium nucleatum (F. nucleatum) in the gastrointestinal tract could cause reduced NK cell activity.
To identify associations between gastrointestinal tract F. nucleatum levels and NK cell activity.
In vitro experiments were performed on NK cells treated with F. nucleatum, Peptostreptococcus anaerobius, and Parvimonas micra to identify the effects of gut microbiome species on NK cells. Following 24 and 48 h of treatment, NK cell counts were measured. In parallel studies, C57BL/6 mice were given broad-spectrum antibiotics in their drinking water to reduce resident gut flora. After 3 wk, the mice received the various bacterial species or phosphate-buffered saline (PBS) via oral gavage every 2 d for 6 wk. At the study end, blood samples were acquired to perform NK cell activity assessment and cytokine analysis. Intestinal tissues were collected and analyzed via immunohistochemistry (IHC).
The data show that after 3 wk of broad-spectrum antibiotic treatment, levels of total bacteria and F. nucleatum were markedly decreased in mice. Gavage of F. nucleatum significantly decreased NK cell activity relative to the activities of cells from mice treated with antibiotics only and PBS. The administration of F. nucleatum decreased the proportion of NK46+ cells based on IHC staining and increased the production of interleukin-1β and tumor necrosis factor-α.
High levels of F. nucleatum in the gastrointestinal tract reduced NK cell activity in mice, and the decrease in NK cell activity might be affected by increased pro-inflammatory cytokines after F. nucleatum treatment.
Core Tip: Gut microbial dysbiosis contributes to the development and progression of colorectal cancer (CRC). Natural killer (NK) cells are involved in early defense mechanisms to kill infective pathogens and tumor cells by releasing chemokines and cytokines. To better understand the relationship between the gut microbiome and CRC, it was hypothesized here that a high abundance of Fusobacterium nucleatum (F. nucleatum) in the gastrointestinal tract could cause reduced NK cell activity. Accor
- Citation: Kim YJ, Kim BK, Park SJ, Kim JH. Impact of Fusobacterium nucleatum in the gastrointestinal tract on natural killer cells. World J Gastroenterol 2021; 27(29): 4879-4889
- URL: https://www.wjgnet.com/1007-9327/full/v27/i29/4879.htm
- DOI: https://dx.doi.org/10.3748/wjg.v27.i29.4879
Increasing evidence indicates that gut microbial dysbiosis contributes to the deve
NK cells are large granular lymphocytes (unique type of cytotoxic lymphocyte) that play a role in innate immunity[12]. NK cells are involved in early defense mechanisms to kill infective pathogens and tumor cells by releasing chemokines and cytokines, as well as by helping other immune cells in targeted cell elimination[13]. Imai et al[14] in a follow-up study of 154 patients with cancer for 11 years found that patients with high cytotoxic activity among their peripheral blood lymphocytes had decreased cancer risk and, conversely, those with low cytotoxic activity had increased cancer risk[14]. In a study that evaluated 140 patients with Stage III CRC who underwent sur
To better understand any relationship between the gut microbiome and CRC, it was hypothesized here that a high abundance of F. nucleatum in the gastrointestinal tract could cause reduced NK cell activity. Accordingly, this association would impact upon the development of CRC in a host. To test this hypothesis, associations between the abundance of F. nucleatum in the murine gastrointestinal tract and NK cell activity were evaluated.
F. nucleatum (ATCC 25586), P. anaerobius (ATCC 27337), and P. micra (ATCC 33270) were purchased from American Type Culture Collection (ATCC, Manassas, VA, USA). All bacteria were cultured at 37°C in Anaerobe basal broth (CM0957, OXOID, Thermo Fisher Scientific, West Palm Beach, FL, USA) in an anaerobic jar (Hardy Diagnostics, Santa Maria, CA, USA). Anaerobic conditions were maintained using AnaeroGen packets (Thermo Fisher Scientific).
For in vitro studies, NK92 cells (CRL-2407, human NK line) were obtained from ATCC. The cells were cultured in a minimum essential medium (MEM, Sigma, St. Louis, MO, USA) containing 12.5% fetal bovine serum (HyClone, Logan, UT, USA), 12.5% horse serum (HyClone), 200 U recombinant IL-2/mL (Invitrogen, Carlsbad, CA, USA), 2 mmol/L L-glutamine, 0.2 mmol/L myoinositol, 0.1 mmol/L 2-mercaptoethanol, and 0.02 mmol/L folic acid (all Sigma, St. Louis, MO, USA) at 37°C in a 5% CO2 incubator. Fresh medium was replaced every 2 d; cells reached confluence at 5 d of culture.
Cell viability was determined by trypsin blue exclusion. Here, NK92 cells were plated at 105 cells/well in 6-well plates. The cells were infected with bacteria at 100:1, 250:1, and 500:1 multiplicity of infection (MOI); control cells received phosphate-buffered saline (PBS, pH 7.4). After 24 and 48 h of culture, cell viabilities were assessed.
C57BL/6 mice (male, 6-wk-of-age) were purchased from Orient Bio (Gyeonggi, Korea) and housed in pathogen-free facilities maintained at 20°C with 50% relative humidity and a 12-h light:dark cycle. All mice had ad libitum access to standard rodent chow and filtered water. The mice were acclimated for 1 wk and randomly divided into four groups (5 mice per group): Control, anti-control (antibiotics only), PBS (PBS after antibiotics), and FN (F. nucleatum after antibiotics). All mice (except control) were treated with a cocktail of broad-spectrum antibiotics (0.2 g/L ampicillin, 0.1 g/L vancomycin, 0.2 g/L neomycin, and 0.2 g/L metronidazole) in their drinking water for 3 wk to establish flora-deficient status. After 3 wk, the mice were administered 109 CFU F. nucleatum or the same dose of PBS by oral gavage every 2 d for 6 wk. At the end of the experiments, mice were anaesthetized with chloroform and euthanized by CO2 gas to permit collection of biomaterials. All experimental procedures were per
Stool samples were collected from all mice once a week to assess the flora-deficient status and to compare the levels of total bacteria and F. nucleatum. For this, total DNA was isolated using the GeneAll Exgene Stool DNA mini kit (GeneAll Biotechnology Co. Seoul, Korea) according to the manufacturer’s instructions. After quantification and confirmation of purity using the Exgene Stool DNA mini kit (GeneAll, Seoul, Korea), an aliquot of total DNA (1 mg) was used as a template to be mixed with TB Green Premix Ex Taq II (Takara Bio, Shiga, Japan). The primers used for amplification were: F. nucleatum 5’-CGAGGAACCTTACCAGCGTT-3’ (F) and 5’-CCCAACATCTCACGACACGA-3’ (R); total bacteria 5’-GTGSTGCAYGGYTGTCGTCA-3’ (F) and 5’-ACGTCRTCCMCACCTTCCTC-3’ (R); and GAPDH 5’-TGGCCTTCCGTGTTCCTAC-3’ (F) and 5’-GAG-TTGCTGTTGAAGTCGCA-3’ (R). All reactions were performed in triplicate in a 7300 Real-time polymerase chain reaction (PCR) system (Applied Biosystems, Waltham, MA, USA).
At necropsy, whole blood was collected from the heart and placed in sodium heparin-coated tubes for subsequent isolation of plasma. NK cell activity was then assessed (in triplicate) using a Murine NK activity kit (ATGen Co. Ltd., Seoul, Korea) according to the manufacturer’s instructions. The absorbance generated for each sample reflected the relative amount of NK cell activity in the sample.
Murine colon tissues were collected and placed in 10% buffered formalin overnight for fixing. Sections 4-μm thick were then prepared for immunohistochemical (IHC) staining using an automated immunostainer (Bond-MAX, Leica, Buffalo Grove, IL, USA). Antibodies against mouse CD3 (rabbit polyclonal, Dako, Agilent Technologies, city, state) and NKp46 (rabbit polyclonal, R&D Systems, Minneapolis, MN, USA) were used. Stained samples were then evaluated by light microscopy and immuno-activity was assessed. The CD3+ or NKp46+ cells were counted in five different fields (total = 500 counted cells) under 400 × magnification; cell counts were then converted to percentages for data presentation.
Plasma levels of pro-inflammatory interleukin (IL)-6, -1β, -18, and -12p70, and tumor necrosis factor (TNF)-α were measured (in duplicate) using commercial ELISA kits (Mouse IL-6 Uncoated ELISA, Mouse IL-18 Platinum ELISA, Mouse IL-1β Uncoated ELISA, Mouse IL-12p70 Platinum ELISA and Mouse TNF-α Uncoated ELISA; all Invitrogen). All experimental steps were performed at room temperature and in duplicate. Samples were quantified by spectrophotometry at 450 nm. The sensitivity levels of the kits were 4-500 pg/mL for IL-6, 8-1000 pg/mL for IL-1β, 31.2-2000 pg/mL for IL-18, 15.6-1000 pg/mL for IL-12p70, and 8-1000 pg/mL for TNF-α.
Each experiment was repeated at least three times. Data are expressed as means ± SD from each independent experiment. A Student’s t-test was performed for continuous variables; outcomes with P ≤ 0.05 were considered statistically significant. Statistical analyses were performed using SPSS Statistics software (v. 23.0, IBM, Armonk, NY, USA).
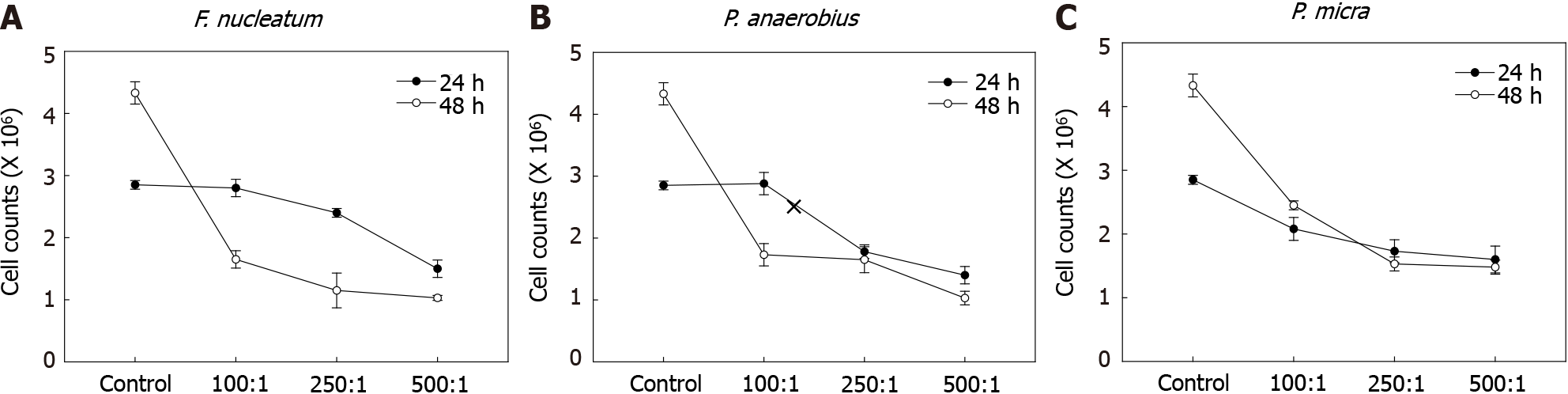
The effects of various gut microbiota species on NK cell survival were first assessed. Here, F. nucleatum, P. anaerobius, and P. micra -all known to be associated with CRC- were cultured with NK cells for 24 or 48 h. To identify optimal amounts of gut microbiome species affecting NK cells, experiments with each were carried out at 100:1, 250:1, or 500:1 MOI. As shown in Figure 1, NK cell counts were decreased in co-cultures with F. nucleatum, P. anaerobius, and P. micra. The results were remarkable in co-cultures at both 24 and 48 h; however, F. nucleatum most markedly decreased NK cell counts after 48 h. Therefore, F. nucleatum was selected for further study here as the representative microbiome species for use in subsequent in vivo studies.
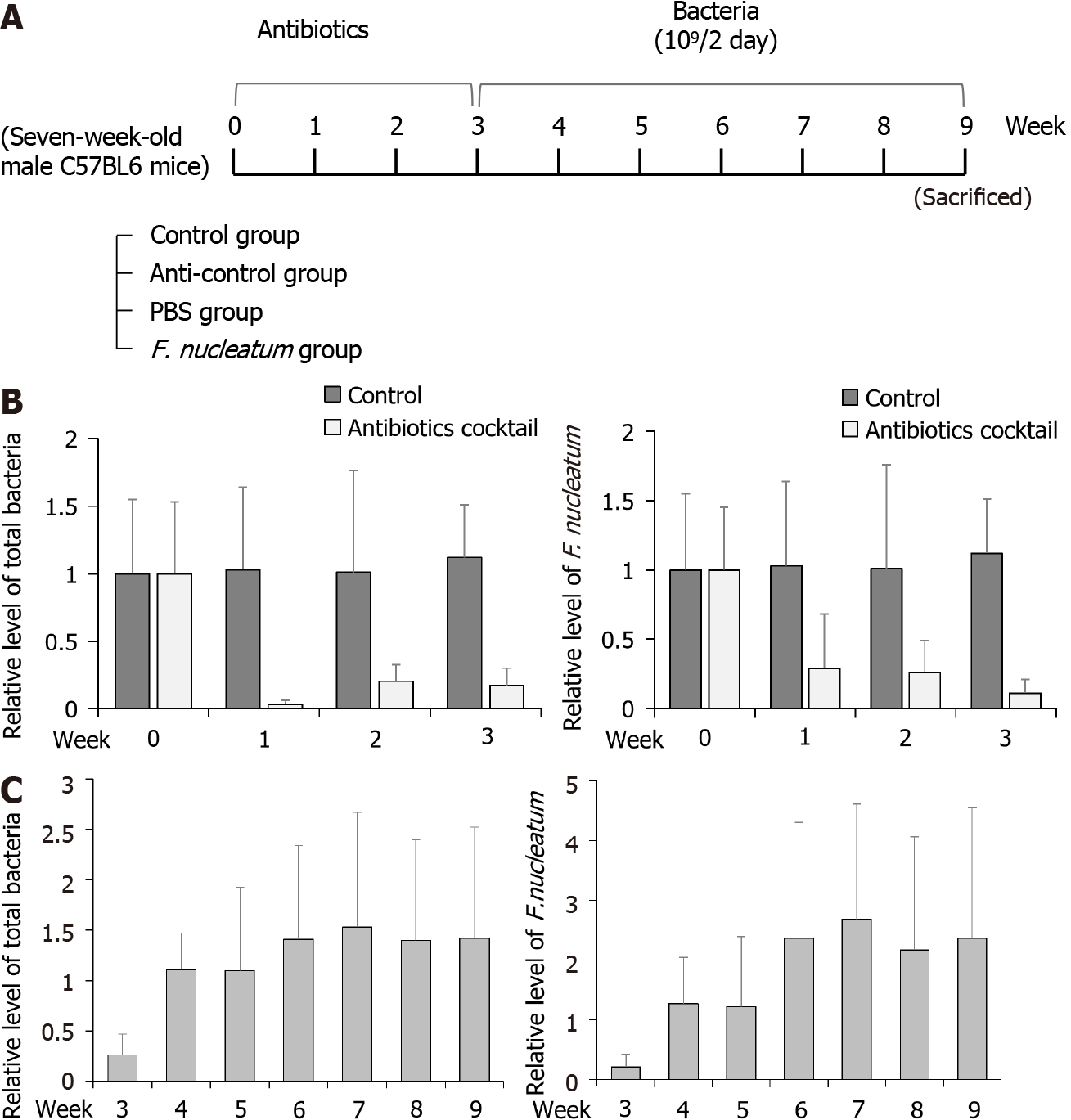
To test whether F. nucleatum could reduce the activity of NK cells in mice, the animals were divided into four groups (5 mice per group): Control, anti-control, PBS and FN (Figure 2A). To establish flora-deficient status, mice were treated with a cocktail of broad-spectrum antibiotics for 3 wk. Confirmation of flora-deficiency was then assessed by quantitative PCR analyses of stool samples; the data confirmed that the levels of total bacteria and F. nucleatum were decreased (Figure 2B). At that point, F. nucleatum was re-introduced by oral gavage to the flora-deficient mice every 2 d for 6 wk. Analyses of their stools showed that levels of total bacteria and F. nucleatum gradually increased and then attained a plateau (Figure 2C).
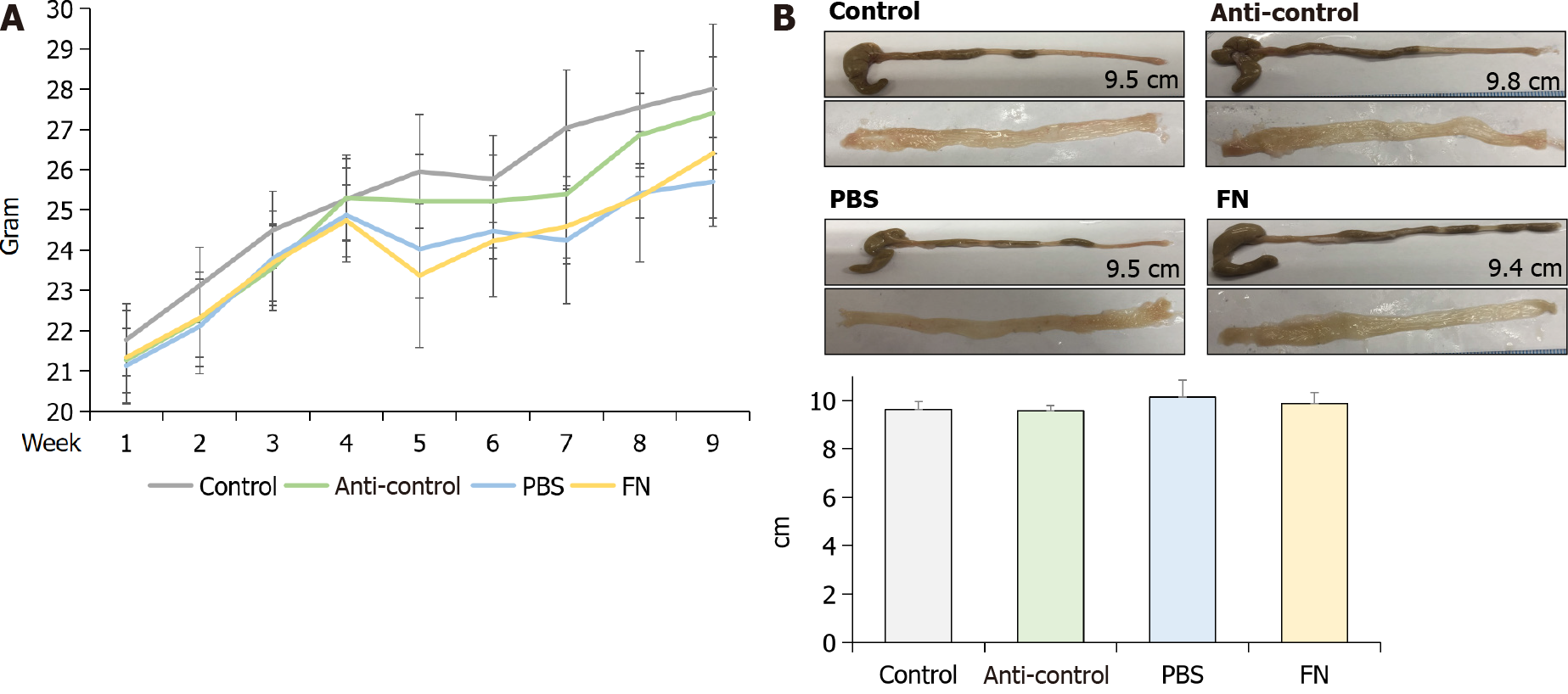
The body weights of the mice were checked every week and compared. The weights of PBS and FN mice were lower compared to those of the control and anti-control groups after oral gavage; however, the differences were not significant (Figure 3A). At sacrifice (i.e., after 6 wk of gavage treatments), the mice were euthanized and the entire colon of each was isolated, longitudinally-opened, and examined. No significant differences in colonic length or colonic mucosal surface were found between the groups (Figure 3B).
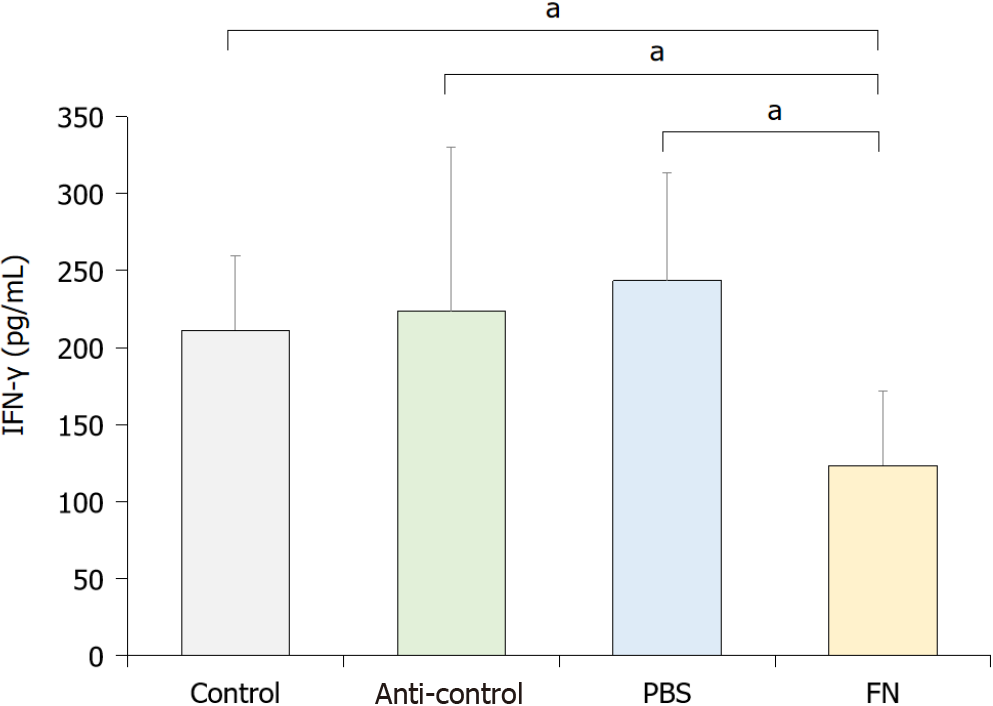
To evaluate the activity of NK cells in situ, levels of interferon-gamma (IFN-γ) in the blood of each mouse was evaluated. The data indicated that the activity of NK cells in the FN group was significantly lower than in the control, anti-control, and PBS mice (Figure 4).
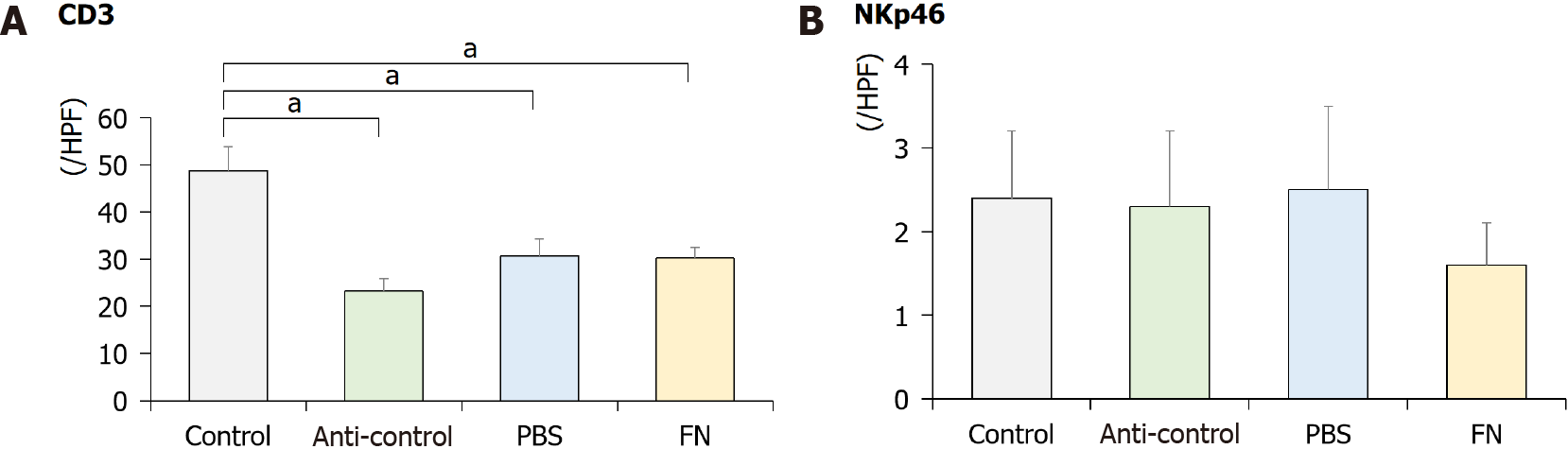
Using IHC staining, the intestinal tract of the mice was evaluated for the presence of NK cells. The data indicate that the levels of CD3+ cells were significantly lower in the anti-control, PBS, and FN mice than in the control mice (Figure 5). The levels of NKp46+ cells were lower in the FN group than in the control group, although the difference was not significant.
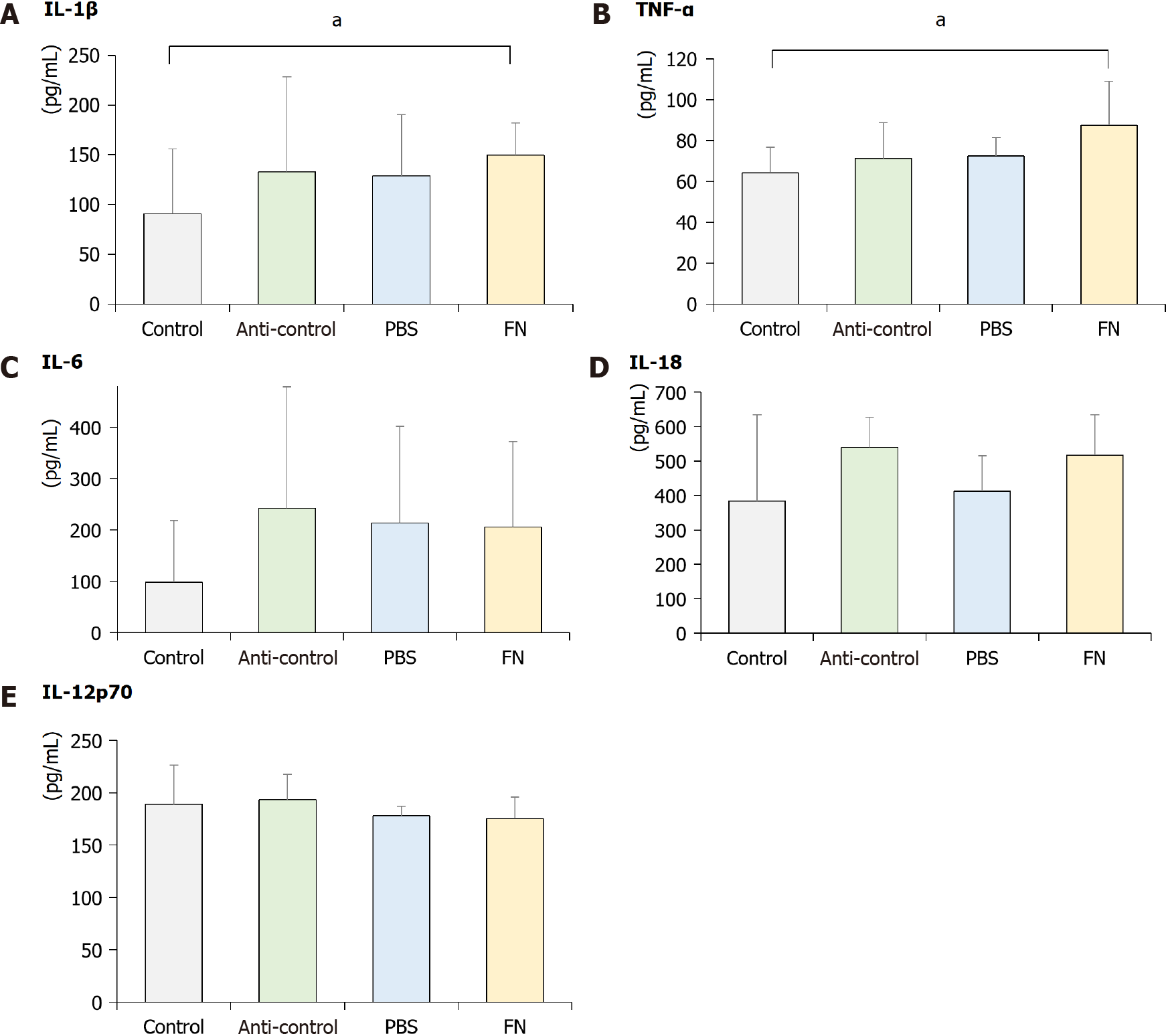
As shown in Figure 6, plasma levels of select pro-inflammatory cytokines were also evaluated at the end of the treatment regimens. The data indicated that circulating levels of IL-1β and TNF-α in the FN mice were significantly higher than in the control mice; values in the anti-control and PBS groups were not significantly higher than in the controls. Plasma levels of IL-6, IL-18, and IL-12p70 in the FN mice were not significantly higher than in the control. In addition, the values in the anti-control and PBS groups were not significantly changed compared to the control group.
Emerging evidence has shown a role for the gut microbiome, especially F. nucleatum, in cancer biology. Although F. nucleatum, a common bacterial resident in the oral cavity, has been shown to be involved in the proliferation of CRC cells and tumor development, data on how the Fusobacteria engage in the development of CRC are lacking. This study showed that a high abundance of F. nucleatum in the gastroin
The importance of the gut microbiome in immunity/inflammation has been more clearly elucidated in the past two decades. In a healthy state, the host immune system responds to intestinal microbiome strictly confined to the mucosal surface[18]. The mucus layer, epithelial cells, immunoglobulin A, dendritic cells, and T-cells comprise the mucosal barrier; these prevent the exposure and passage of gut microbiome to the gut-associated lymphoid tissue[19]. When the maintenance of tissue homeostasis is destroyed, translocating bacteria into the mucosal tissue causes a local (and systemic) effect on inflammatory cells, i.e., tissue inflammatory monocytes can respond to microbial-derived ligands by producing prostaglandin E2 which limits neutrophil activation and tissue damage[20].
F. nucleatum, whose presence has been proposed to correlate with CRC, can induce inflammation and suppress host immunity. There is increased evidence that F. nucleatum modulates immune cells in the CRC microenvironment and promotes intestinal tumorigenesis[21]. F. nucleatum can also stimulate autophagic pathways and alter chemotherapeutic responses of patients[22]. The current study focused on the impact of F. nucleatum upon gastrointestinal tract immune cells, especially NK cells. This study found that F. nucleatum could reduce local NK cell counts and decrease the activity of NK cells in the gut. Normally, NK cell activity is controlled by a balance of various signals that are delivered to the cells via inhibitory and activating NK cell receptors[23]. One study revealed that the ability of NK cells to kill tumors was inhibited by hemagglutinating F. nucleatum strains and that F. nucleatum inhibited immune cell activity by specifically targeting the inhibitory receptor TIGIT via the Fap2 protein[8]. Although the present study did not investigate the detailed mecha
NK cells usually present as CD56+CD3- cells and can be categorized into CD3-CD56dimCD16+ and CD3-CD56brightCD16- cell classes. CD56dim cells appear to have more cytotoxic activity, while CD56bright cells produce high levels of cytokines including IFN-γ and TNF-α[24]. The activation and inhibition of NK cells are induced by an interplay among the cells’ activating and inhibitory receptors[25]. NKp46 is expressed on NK cells in the tumor bed and is a major determinant of NK cell function[26]. In the current study, levels of CD3+ cells significantly decreased after antibiotic treatment and NKp46+ cells decreased after administration of F. nucleatum, although the difference was not significant. Based on these results, it is thought that the microenvironment change after antibiotic treatment could affect these alterations, and the administration of F. nucleatum might decrease NK cell number.
Several inflammatory cytokines (including TNF-α, IL-6, IL-18, and IL-1β) are known to be involved in the initiation and progression of cancers[27], and could modulate the activation of NK cells. TNF-α can induce DNA damage through reactive oxygen and nitrogen species, IL-18 can induce the production of angiogenic and tumor growth stimulating factors, IL-6 can induce oxidative stress and promote early tumorigenesis, and IL-1β can promote neo-angiogenesis and evoke antiapoptotic signaling in tumor cells[28-30]. It is plausible that any changes in the expression in one or more of these could modulate tumor growth/survival in situ by also modulating the activation of NK cells[31]. For example, IL-12 can enhance the activity of NK cells and CD8+ T-lymphocytes, and so is expected to impart anti-tumor activity[32,33]. In this study, it was seen that circulating levels of IL-1β and TNF-α (but not IL-6, IL-18, or IL-12p70) were significantly increased after administration of F. nucleatum to mice. Further studies are needed to determine if these changes directly/indirectly impact the activity of NK cells themselves in hosts.
This study has some limitations. First, whether decreased NK cell activity induces the development of CRC was not assessed. Further studies including the association between NK cell activity and CRC could strengthen the results. Second, the pro-inflammatory cytokine levels were not evaluated in NK92 cells and in colonic tissues treated with F. nucleatum. Therefore, changes in plasma pro-inflammatory cytokine levels in NK92 cells and in colonic tissues treated with F. nucleatum were not con
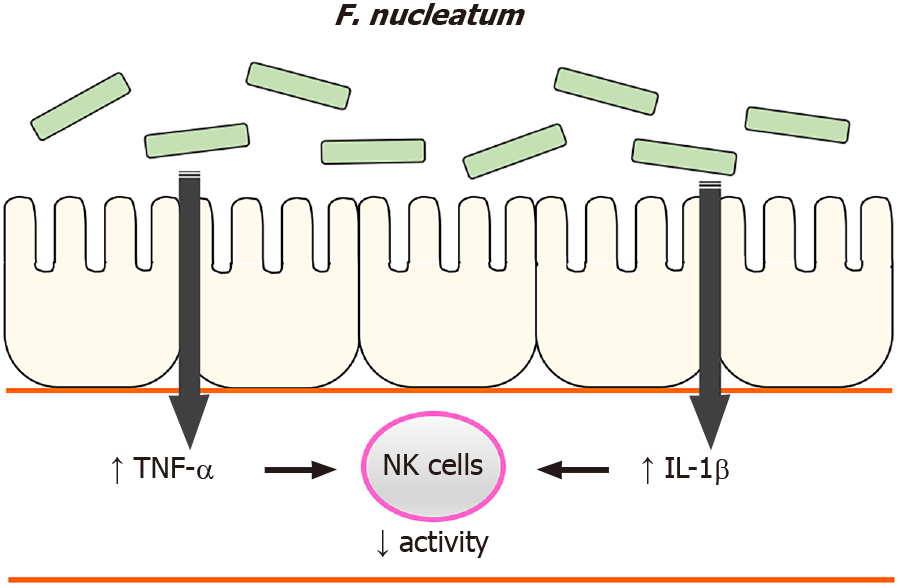
In summary, the current study identified that high levels of F. nucleatum in the gastrointestinal tract reduced NK cell activity in mice and suggests insights on how NK cell activity was affected, i.e., F. nucleatum induced systemic pro-inflammatory responses in the hosts. Based on these findings, we suggest a possible mechanism by which high levels of F. nucleatum in the gastrointestinal tract induce an increase in plasma pro-inflammatory cytokines, which reduces NK cell activity (Figure 7). Our findings illustrate another potential means by which gut microbiome can influence NK cells which, in turn could impact the development of CRC.
Gut microbial dysbiosis contributes to the development and progression of colorectal cancer (CRC). Natural killer (NK) cells are involved in early defense mechanisms to kill infective pathogens and tumor cells by releasing chemokines and cytokines.
This study was designed to better understand the relationship between the gut microbiome and CRC.
The objective of this study was to identify associations between gastrointestinal tract Fusobacterium nucleatum (F. nucleatum) levels and NK cell activity.
In vitro experiments were performed on NK cells treated with F. nucleatum, Peptostreptococcus anaerobius, and Parvimonas micra to identify the effects of gut microbiome species on NK cells. After 24 and 48 h of treatment, NK cell counts were measured. In parallel studies, C57BL/6 mice were given broad-spectrum antibiotics in their drinking water to reduce resident gut flora. After 3 wk, the mice received the various bacterial species or phosphate-buffered saline via oral gavage every 2 d for 6 wk. At the study end, blood samples were acquired to perform NK cell activity assessment and cytokine analysis. Intestinal tissues were collected and analyzed via immunohistochemistry (IHC).
After 3 wk of broad-spectrum antibiotic treatment, levels of total bacteria and F. nucleatum were markedly decreased in mice. F. nucleatum by gavage significantly decreased NK cell activity relative to the activities of cells from mice treated with antibiotics only and phosphate-buffered saline. The administration of F. nucleatum decreased the proportion of NK46+ cells based on IHC staining and increased the production of interleukin-1β and tumor necrosis factor-α.
High levels of F. nucleatum in the gastrointestinal tract reduced NK cell activity in mice, and the decrease in NK cell activity might be affected by increased pro-inflammatory cytokines after F. nucleatum treatment.
Based on these findings, we suggest that increases in F. nucleatum in the gastroin
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: South Korea
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Gao F, Perisetti A, Wu HT, Yau TO S-Editor: Fan JR L-Editor: Webster JR P-Editor: Yuan YY
| 1. | Mima K, Nishihara R, Qian ZR, Cao Y, Sukawa Y, Nowak JA, Yang J, Dou R, Masugi Y, Song M, Kostic AD, Giannakis M, Bullman S, Milner DA, Baba H, Giovannucci EL, Garraway LA, Freeman GJ, Dranoff G, Garrett WS, Huttenhower C, Meyerson M, Meyerhardt JA, Chan AT, Fuchs CS, Ogino S. Fusobacterium nucleatum in colorectal carcinoma tissue and patient prognosis. Gut. 2016;65:1973-1980. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 530] [Cited by in RCA: 756] [Article Influence: 84.0] [Reference Citation Analysis (0)] |
| 2. | Louis P, Hold GL, Flint HJ. The gut microbiota, bacterial metabolites and colorectal cancer. Nat Rev Microbiol. 2014;12:661-672. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1596] [Cited by in RCA: 1970] [Article Influence: 179.1] [Reference Citation Analysis (0)] |
| 3. | Irrazábal T, Belcheva A, Girardin SE, Martin A, Philpott DJ. The multifaceted role of the intestinal microbiota in colon cancer. Mol Cell. 2014;54:309-320. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 218] [Cited by in RCA: 258] [Article Influence: 23.5] [Reference Citation Analysis (0)] |
| 4. | Nakatsu G, Li X, Zhou H, Sheng J, Wong SH, Wu WK, Ng SC, Tsoi H, Dong Y, Zhang N, He Y, Kang Q, Cao L, Wang K, Zhang J, Liang Q, Yu J, Sung JJ. Gut mucosal microbiome across stages of colorectal carcinogenesis. Nat Commun. 2015;6:8727. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 558] [Cited by in RCA: 512] [Article Influence: 51.2] [Reference Citation Analysis (0)] |
| 5. | Yu J, Feng Q, Wong SH, Zhang D, Liang QY, Qin Y, Tang L, Zhao H, Stenvang J, Li Y, Wang X, Xu X, Chen N, Wu WK, Al-Aama J, Nielsen HJ, Kiilerich P, Jensen BA, Yau TO, Lan Z, Jia H, Li J, Xiao L, Lam TY, Ng SC, Cheng AS, Wong VW, Chan FK, Yang H, Madsen L, Datz C, Tilg H, Wang J, Brünner N, Kristiansen K, Arumugam M, Sung JJ. Metagenomic analysis of faecal microbiome as a tool towards targeted non-invasive biomarkers for colorectal cancer. Gut. 2017;66:70-78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 917] [Cited by in RCA: 783] [Article Influence: 97.9] [Reference Citation Analysis (0)] |
| 6. | Abed J, Emgård JE, Zamir G, Faroja M, Almogy G, Grenov A, Sol A, Naor R, Pikarsky E, Atlan KA, Mellul A, Chaushu S, Manson AL, Earl AM, Ou N, Brennan CA, Garrett WS, Bachrach G. Fap2 Mediates Fusobacterium nucleatum Colorectal Adenocarcinoma Enrichment by Binding to Tumor-Expressed Gal-GalNAc. Cell Host Microbe. 2016;20:215-225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 326] [Cited by in RCA: 581] [Article Influence: 72.6] [Reference Citation Analysis (0)] |
| 7. | Yang Y, Weng W, Peng J, Hong L, Yang L, Toiyama Y, Gao R, Liu M, Yin M, Pan C, Li H, Guo B, Zhu Q, Wei Q, Moyer MP, Wang P, Cai S, Goel A, Qin H, Ma Y. Fusobacterium nucleatum Increases Proliferation of Colorectal Cancer Cells and Tumor Development in Mice by Activating Toll-Like Receptor 4 Signaling to Nuclear Factor-κB, and Up-regulating Expression of MicroRNA-21. Gastroenterology. 2017;152:851-866.e24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 455] [Cited by in RCA: 716] [Article Influence: 89.5] [Reference Citation Analysis (1)] |
| 8. | Gur C, Ibrahim Y, Isaacson B, Yamin R, Abed J, Gamliel M, Enk J, Bar-On Y, Stanietsky-Kaynan N, Coppenhagen-Glazer S, Shussman N, Almogy G, Cuapio A, Hofer E, Mevorach D, Tabib A, Ortenberg R, Markel G, Miklić K, Jonjic S, Brennan CA, Garrett WS, Bachrach G, Mandelboim O. Binding of the Fap2 protein of Fusobacterium nucleatum to human inhibitory receptor TIGIT protects tumors from immune cell attack. Immunity. 2015;42:344-355. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1015] [Cited by in RCA: 977] [Article Influence: 97.7] [Reference Citation Analysis (0)] |
| 9. | Nosho K, Sukawa Y, Adachi Y, Ito M, Mitsuhashi K, Kurihara H, Kanno S, Yamamoto I, Ishigami K, Igarashi H, Maruyama R, Imai K, Yamamoto H, Shinomura Y. Association of Fusobacterium nucleatum with immunity and molecular alterations in colorectal cancer. World J Gastroenterol. 2016;22:557-566. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 292] [Cited by in RCA: 276] [Article Influence: 30.7] [Reference Citation Analysis (3)] |
| 10. | Huynh T, Kapur RV, Kaplan CW, Cacalano N, Kinder Haake S, Shi W, Sieling P, Jewett A. The role of aggregation in Fusobacterium nucleatum- induced immune cell death. J Endod. 2011;37:1531-1535. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 11. | Shenker BJ, Datar S. Fusobacterium nucleatum inhibits human T-cell activation by arresting cells in the mid-G1 phase of the cell cycle. Infect Immun. 1995;63:4830-4836. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 64] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 12. | Kiessling R, Klein E, Pross H, Wigzell H. "Natural" killer cells in the mouse. II. Cytotoxic cells with specificity for mouse Moloney leukemia cells. Characteristics of the killer cell. Eur J Immunol. 1975;5:117-121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 746] [Cited by in RCA: 757] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 13. | Bryceson YT, Chiang SC, Darmanin S, Fauriat C, Schlums H, Theorell J, Wood SM. Molecular mechanisms of natural killer cell activation. J Innate Immun. 2011;3:216-226. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 157] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 14. | Imai K, Matsuyama S, Miyake S, Suga K, Nakachi K. Natural cytotoxic activity of peripheral-blood lymphocytes and cancer incidence: an 11-year follow-up study of a general population. Lancet. 2000;356:1795-1799. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 803] [Cited by in RCA: 842] [Article Influence: 33.7] [Reference Citation Analysis (0)] |
| 15. | Kondo E, Koda K, Takiguchi N, Oda K, Seike K, Ishizuka M, Miyazaki M. Preoperative natural killer cell activity as a prognostic factor for distant metastasis following surgery for colon cancer. Dig Surg. 2003;20:445-451. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 54] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 16. | Jobin G, Rodriguez-Suarez R, Betito K. Association Between Natural Killer Cell Activity and Colorectal Cancer in High-Risk Subjects Undergoing Colonoscopy. Gastroenterology. 2017;153:980-987. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 68] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 17. | Halama N, Braun M, Kahlert C, Spille A, Quack C, Rahbari N, Koch M, Weitz J, Kloor M, Zoernig I, Schirmacher P, Brand K, Grabe N, Falk CS. Natural killer cells are scarce in colorectal carcinoma tissue despite high levels of chemokines and cytokines. Clin Cancer Res. 2011;17:678-689. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 196] [Cited by in RCA: 232] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 18. | Konrad A, Cong Y, Duck W, Borlaza R, Elson CO. Tight mucosal compartmentation of the murine immune response to antigens of the enteric microbiota. Gastroenterology. 2006;130:2050-2059. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 66] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 19. | Belkaid Y, Hand TW. Role of the microbiota in immunity and inflammation. Cell. 2014;157:121-141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2488] [Cited by in RCA: 3430] [Article Influence: 311.8] [Reference Citation Analysis (1)] |
| 20. | Schreiber S, Rosenstiel P, Albrecht M, Hampe J, Krawczak M. Genetics of Crohn disease, an archetypal inflammatory barrier disease. Nat Rev Genet. 2005;6:376-388. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 225] [Cited by in RCA: 220] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 21. | Brennan CA, Garrett WS. Gut Microbiota, Inflammation, and Colorectal Cancer. Annu Rev Microbiol. 2016;70:395-411. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 307] [Cited by in RCA: 428] [Article Influence: 53.5] [Reference Citation Analysis (0)] |
| 22. | Yu T, Guo F, Yu Y, Sun T, Ma D, Han J, Qian Y, Kryczek I, Sun D, Nagarsheth N, Chen Y, Chen H, Hong J, Zou W, Fang JY. Fusobacterium nucleatum Promotes Chemoresistance to Colorectal Cancer by Modulating Autophagy. Cell. 2017;170:548-563.e16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 814] [Cited by in RCA: 1477] [Article Influence: 184.6] [Reference Citation Analysis (0)] |
| 23. | Koch J, Steinle A, Watzl C, Mandelboim O. Activating natural cytotoxicity receptors of natural killer cells in cancer and infection. Trends Immunol. 2013;34:182-191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 213] [Cited by in RCA: 242] [Article Influence: 20.2] [Reference Citation Analysis (0)] |
| 24. | Robertson MJ, Ritz J. Biology and clinical relevance of human natural killer cells. Blood. 1990;76:2421-2438. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 25. | Cooper MA, Fehniger TA, Caligiuri MA. The biology of human natural killer-cell subsets. Trends Immunol. 2001;22:633-640. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2049] [Cited by in RCA: 2195] [Article Influence: 91.5] [Reference Citation Analysis (0)] |
| 26. | Chretien AS, Devillier R, Fauriat C, Orlanducci F, Harbi S, Le Roy A, Rey J, Bouvier Borg G, Gautherot E, Hamel JF, Ifrah N, Lacombe C, Cornillet-Lefebvre P, Delaunay J, Toubert A, Arnoulet C, Vey N, Blaise D, Olive D. NKp46 expression on NK cells as a prognostic and predictive biomarker for response to allo-SCT in patients with AML. Oncoimmunology. 2017;6:e1307491. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 40] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 27. | Landskron G, De la Fuente M, Thuwajit P, Thuwajit C, Hermoso MA. Chronic inflammation and cytokines in the tumor microenvironment. J Immunol Res. 2014;2014:149185. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1033] [Cited by in RCA: 1233] [Article Influence: 112.1] [Reference Citation Analysis (0)] |
| 28. | Palma G, Barbieri A, Bimonte S, Palla M, Zappavigna S, Caraglia M, Ascierto PA, Ciliberto G, Arra C. Interleukin 18: friend or foe in cancer. Biochim Biophys Acta. 2013;1836:296-303. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 39] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 29. | Murata M, Thanan R, Ma N, Kawanishi S. Role of nitrative and oxidative DNA damage in inflammation-related carcinogenesis. J Biomed Biotechnol. 2012;2012:623019. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 118] [Cited by in RCA: 132] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 30. | Meng F, Wehbe-Janek H, Henson R, Smith H, Patel T. Epigenetic regulation of microRNA-370 by interleukin-6 in malignant human cholangiocytes. Oncogene. 2008;27:378-386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 186] [Cited by in RCA: 183] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 31. | Zwirner NW, Domaica CI. Cytokine regulation of natural killer cell effector functions. Biofactors. 2010;36:274-288. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 134] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 32. | Tugues S, Burkhard SH, Ohs I, Vrohlings M, Nussbaum K, Vom Berg J, Kulig P, Becher B. New insights into IL-12-mediated tumor suppression. Cell Death Differ. 2015;22:237-246. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 258] [Cited by in RCA: 408] [Article Influence: 37.1] [Reference Citation Analysis (0)] |
| 33. | Lu X. Impact of IL-12 in Cancer. Curr Cancer Drug Targets. 2017;17:682-697. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 79] [Article Influence: 11.3] [Reference Citation Analysis (0)] |