Published online Jul 28, 2021. doi: 10.3748/wjg.v27.i28.4738
Peer-review started: May 7, 2021
First decision: June 6, 2021
Revised: June 15, 2021
Accepted: July 9, 2021
Article in press: July 9, 2021
Published online: July 28, 2021
Processing time: 80 Days and 4.2 Hours
Adenocarcinoma originating from heterotopic pancreas tissue is a rare disease. Furthermore, to our knowledge, no HER2-positive cases in the duodenum have been reported in the scientific literature nor has the efficacy of trastuzumab treatment for the disease been reported.
A 65-year-old woman whose clinical diagnosis was unresectable advanced duodenal cancer with HER2 overexpression responded well to trastuzumab chemotherapy. The main tumor in the duodenum reduced drastically. The patient underwent pancreaticoduodenectomy and lymph node dissection. A small number of cancer cells remained in the submucosal layer of the duodenum and pancreas head. After histological and immunohistochemical examination, the patient was diagnosed with duodenal adenocarcinoma originating from heterotopic pancreas tissue.
Trastuzumab treatment is effective in HER2-positive adenocarcinoma originating from heterotopic pancreas tissue in the duodenum.
Core Tip: Adenocarcinoma that develops from heterotopic pancreas tissue in the duodenum is a very rare disease. Therefore, standard chemotherapy regimens to treat these patients have not been established. A 65-year-old woman presented with unresectable duodenal cancer with HER2 overexpression. Because the patient responded well to trastuzumab chemotherapy, pancreatoduodenectomy was performed. Histology and immunohistochemistry assessments revealed that the cancer developed from heterotopic pancreas tissue in the duodenum. The findings of this case suggest that HER2 status should be examined in duodenal adenocarcinoma to determine the indication for targeted therapy. Trastuzumab chemotherapy is effective for heterotopic pancreatic adenocarcinoma but not orthotopic pancreatic cancer.
- Citation: Hirokawa YS, Iwata T, Okugawa Y, Tanaka K, Sakurai H, Watanabe M. HER2-positive adenocarcinoma arising from heterotopic pancreas tissue in the duodenum: A case report. World J Gastroenterol 2021; 27(28): 4738-4745
- URL: https://www.wjgnet.com/1007-9327/full/v27/i28/4738.htm
- DOI: https://dx.doi.org/10.3748/wjg.v27.i28.4738
Heterotopic pancreas is a relatively rare event detected in 0.25% of gastrointestinal tract surgeries and in 0.6 to 13.7% of autopsy cases[1,2]. Malignant transformation was observed only in 0.7% to 1.8% of heterotopic pancreas cases[3,4]. In the literature, 16 reports of malignant tumors arising from duodenal heterotopic pancreas tissue were confirmed[5-7]. Some patients received chemotherapy, but information about the efficacy of the chemotherapy applied is limited. We describe the first case of HER2-positive adenocarcinoma originating from heterotopic pancreas tissue in the duodenum and the therapeutic effect of trastuzumab treatment in this patient.
The patient presented with epigastric discomfort that had persisted for 3 mo.
A 65-year-old woman complained of abdominal discomfort and had visited the hospital previously. Esophagogastroduodenoscopy revealed an advanced type 4 lesion in the pylorus of the stomach. The patient received a detailed medical examination and treatment at the current hospital.
The patient had an appendectomy due to acute appendicitis at the age of 15.
The patient had no smoking or alcohol intake history. There was no family history of cancer.
The abdomen was soft and without tenderness.
Initial laboratory data revealed a hemoglobin level of 7.2 g/dL, a white blood cell count of 5700 cell/µL, and a platelet count of 3.95 × 105/µl. The creatinine level was 0.64 mg/dL, total bilirubin level was 0.44 mg/dL, aspartate aminotransferase level was 11 U/L, alanine aminotransferase level was 9 U/L, albumin level was 3.6 g/dL, and amylase level was 75 U/L (normal range 37 to 125 U/L). The level of the carcinoembryonic antigen tumor marker was 445.4 ng/mL, the cancer antigen 125 level was 317.3 U/mL, the squamous cell carcinoma antigen level was 4.1 ng/mL, and the carbohydrate antigen 19-9 level was less than 2 U/mL.
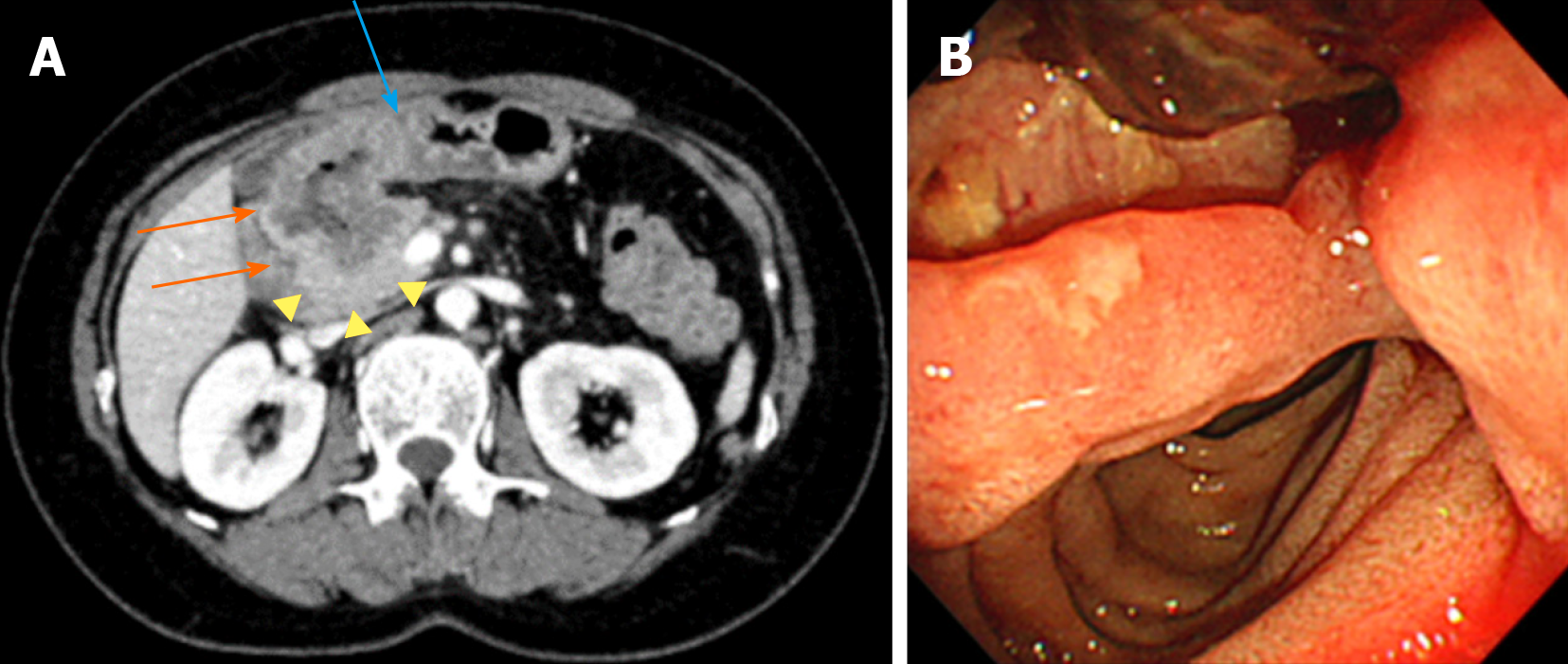
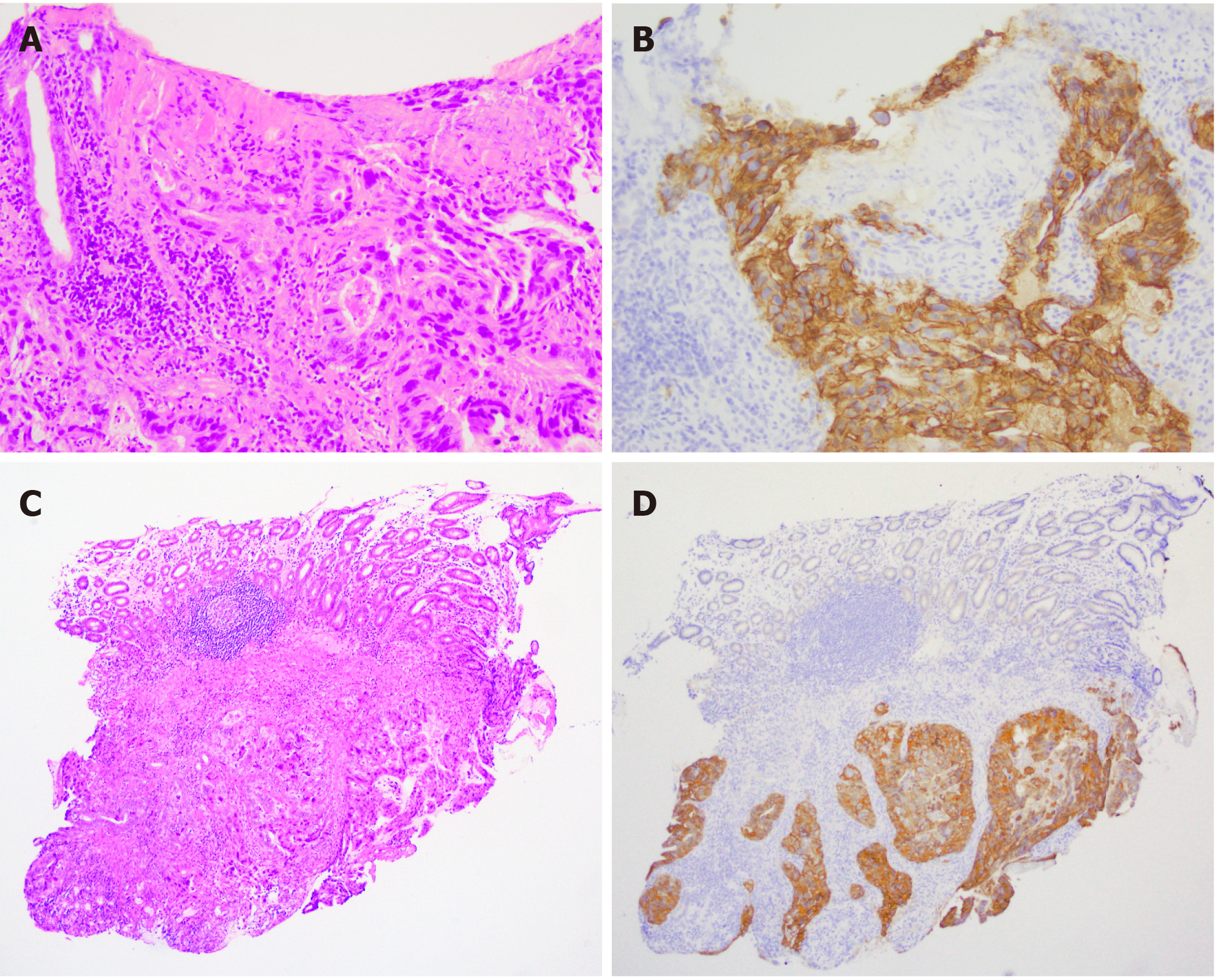
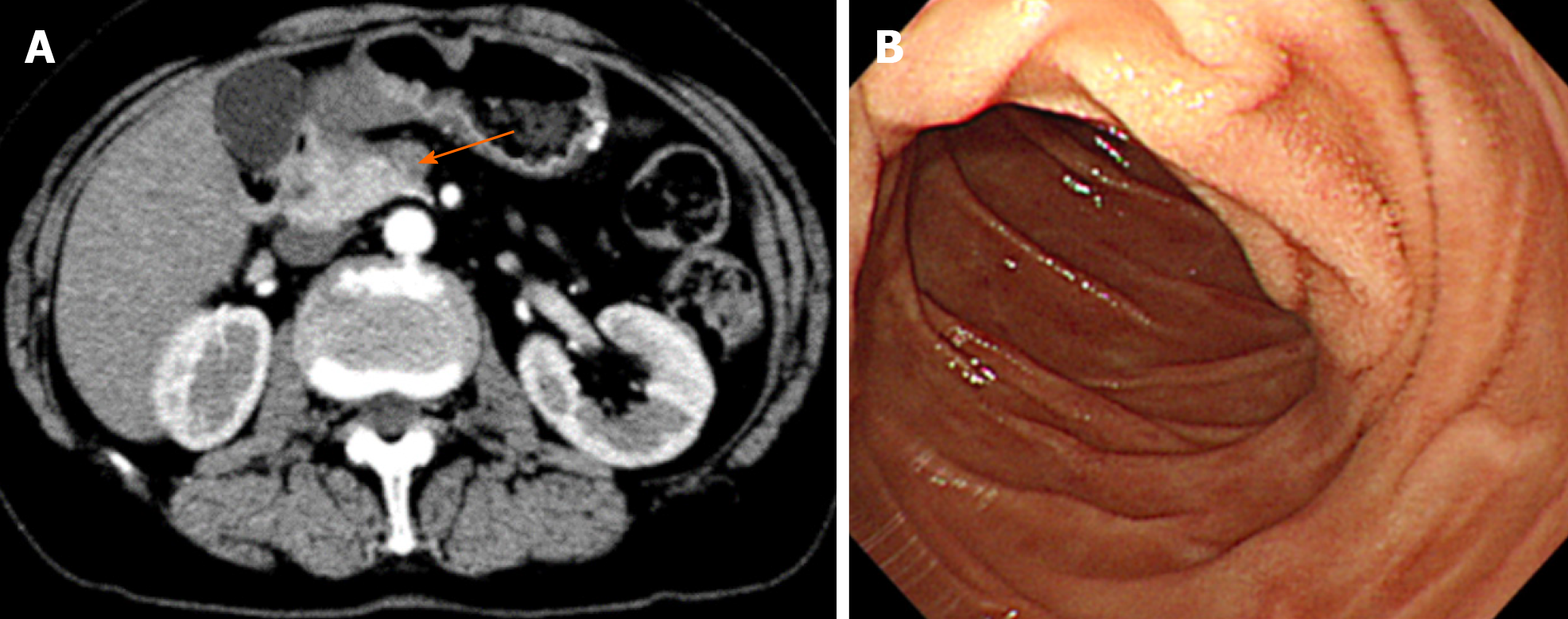
An abdominal computed tomography (CT) scan demonstrated wall thickening in the duodenal first portion extending toward the pylorus and pancreatic head (Figure 1A). Lymph node swelling in the common hepatic artery and in the portal vein and venous tumor thrombosis in the pancreatic head were also observed. There was no gross tumor mass in the orthotopic pancreas. The patient was referred for gastroduodenoscopy. The tumor was located in the first portion of the duodenum with ulcer formation (Figure 1B). The stomach pylorus was stenotic due to the extrinsic compression of the tumor. Histologic evaluation of the duodenum biopsy specimens revealed tubular adenocarcinoma, and HER2 overexpression was detected by immunohistochemistry (Figure 2). The clinical diagnosis of unresectable duodenal cancer was made based on cancer invasion of the pancreas and portal vein tumor thrombosis; thus, stomach bypass surgery and gastrojejunostomy were performed. Capecitabine and cisplatin plus trastuzumab chemotherapy was applied due to the potential efficacy of this treatment for HER2-overexpressing gastric cancer cells reported in the Trastuzumab for Gastric Cancer (ToGA) study. After 1 cycle of treatment, the patient developed renal impairment due to the side effects of cisplatin. The drug combination was changed to capecitabine and oxaliplatin plus trastuzumab. To assess the effects of treatment, a CT scan was performed after 2 cycles of capecitabine and oxaliplatin plus trastuzumab administration, and tumor size and lymph node swelling were dramatically reduced (Figure 3). The same chemotherapy was continued for two more cycles, and then pancreatoduodenectomy was conducted. No obvious tumor was visible in the duodenum or liver and there was no peritoneal metastasis.
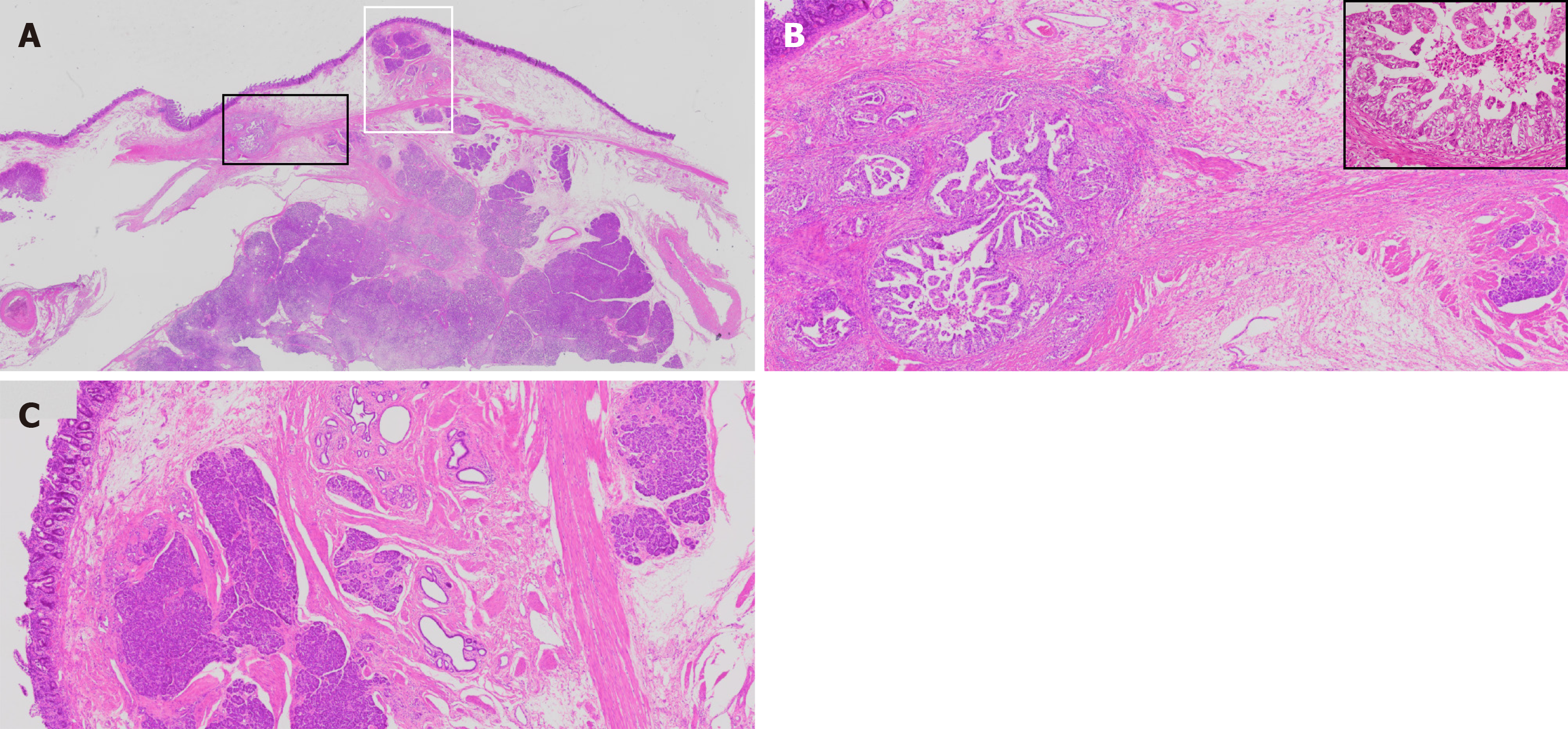
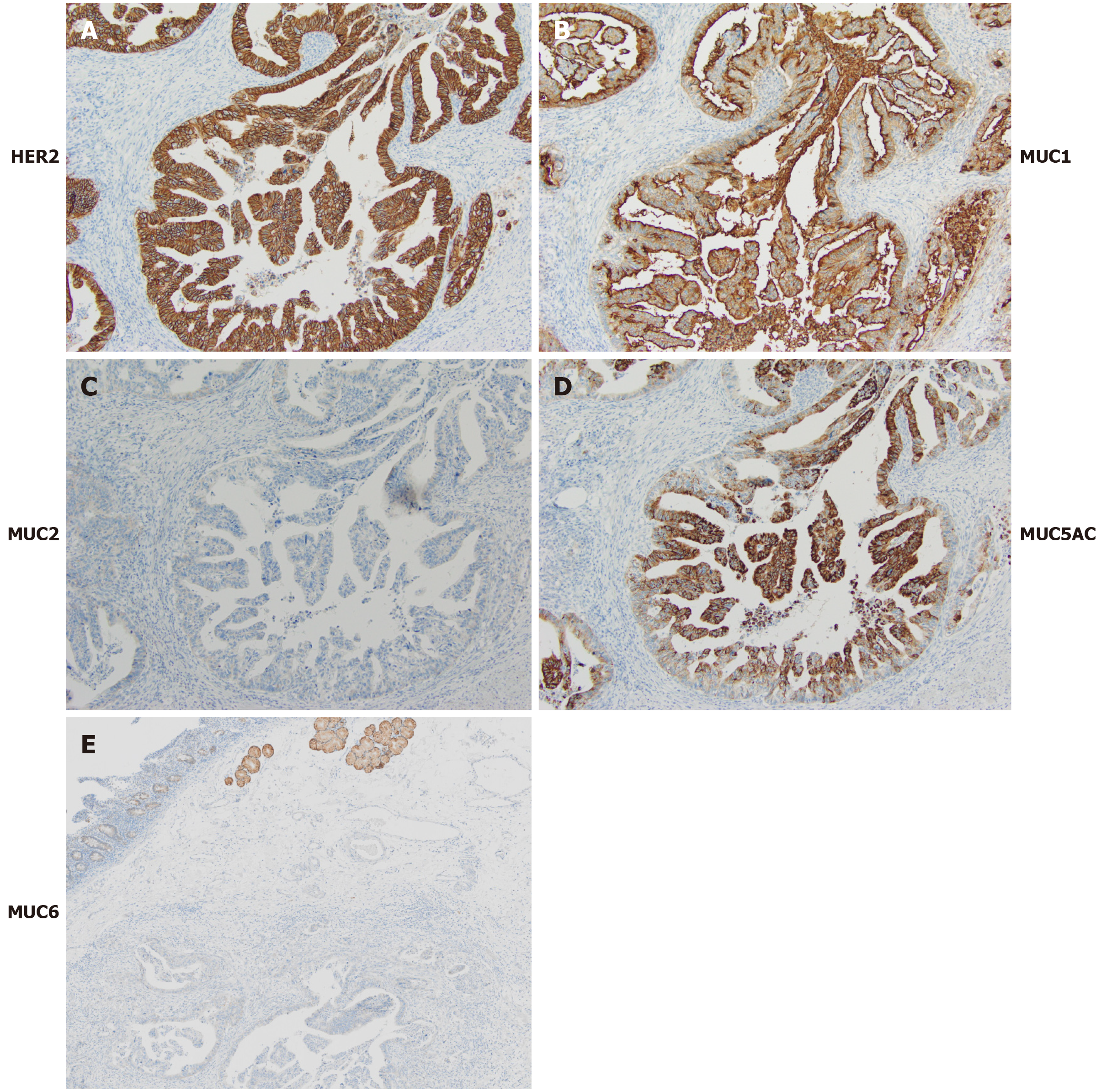
The histological evaluation of the biopsy specimens from the gastroduodenoscopy revealed an adenocarcinoma mass predominantly located in the duodenal submucosa (Figure 2). The cancer cells were strongly positive for HER2 membranous expression on immunohistochemical analysis (+3). On gross examination of the resected duodenum, no discrete mass lesion was visible, but a flat, slightly elevated mucosal fold was present in the first portion of the duodenum. On histological assessment, carcinoma cells were localized in the duodenal submucosal layer, forming small nodules, and in the pancreas head and pancreaticoduodenal lymph nodes (Figure 4A and B, data not shown). Heterotopic pancreas tissue was located close to the carcinoma cells within the submucosal layer and laminar muscularis mucosa of the duodenum, composed of acinar and duct structures (Figure 4C). In the immunohistochemical analysis, the cancer cells showed positive staining for cytokeratin (CK)7, CK20, MUC1 and MUC5AC and negative staining for MUC2 and CDX2 (Figure 5A-D and data not shown). Positive staining for MUC6, a specific marker for Brunner gland adenocarcinoma, was positive in the Brunner glands but negative in the cancer cells (Figure 5E)[8].
These findings indicated that the carcinoma cells were of pancreatic duct origin[9-11] and are consistent with the diagnosis of adenocarcinoma arising from heterotopic pancreas tissue within the duodenum. The residual cancer cells were positive for HER2 on immunohistochemical analysis (Figure 5A).
Various chemotherapies were given after surgery as follows: 1 cycle of capecitabine and oxaliplatin and 6 cycles of capecitabine. In the CT examination for evaluating chemotherapy, retroperitoneal lymph node enlargement, implying cancer metastasis, was revealed. Progressively, 3 cycles of paclitaxel, 4 cycles of S-1 and oxaliplatin plus trastuzumab, and 2 cycles of S-1 plus trastuzumab were administered. Thereafter, trastuzumab monoadministration was conducted. Chemotherapy-induced side effects guided the changes in drug selection.
Twenty-four months after the operation, the patient presented with enlarged retroperitoneal lymph nodes and was diagnosed with progressive disease according to the Evaluation Criteria in Solid Tumors. Due to the slow progression of the retroperitoneal lymph nodes, trastuzumab monoadministration was continued. When the retroperitoneal lymph nodes kept increase in size, nivolumab administration would be considered according to the gastric cancer chemotherapy regimens.
We describe a case of HER2-positive adenocarcinoma that developed in heterotopic pancreas tissue in the duodenum and outline the efficacy of HER2-targeting therapy for this disease for the first time in the English literature. Guillou et al[3] proposed the following three conditions for the definition of carcinoma arising from heterotopic pancreas tissue: (1) The tumor must be found within or close to the ectopic pancreatic tissue; (2) Direct transition between pancreatic structures and carcinoma must be observed; and (3) The nonneoplastic pancreatic tissue must comprise fully developed acini and ductal structures. In our case, the second condition, the transition between pancreatic structures and carcinoma, was not fulfilled completely; however, the desmoplasia of pancreatic cancer destroys the tissue architecture, making it impossible to identify the histological transition. Guillou et al[3]’s report included inconsistent statements; the term "close to" was used in the first condition and "direct transition" was used in the second. Furthermore, given the expanding knowledge within the field of pancreatic cancer immunohistochemistry since Gulliou et al[3]’s study was published, we propose a replacement for the second Gulliou et al[3] criterion as follows: Direct transition between pancreatic structures and carcinoma is observed; alternatively, cancer cells express proteins indicating pancreatic origin.
Since chemotherapy against HER2-positive cancer improved the outcome of breast cancer patients, an extensive search was conducted to elucidate the incidence of HER2-expressing pancreatic cancer (ductal adenocarcinoma). The incidence of pancreatic cancer with +3 membranous HER2 protein expression ranges from 6% to 12% in the literature[12-14]. Using the same approach to investigate the use of Herceptin combined chemotherapy for metastatic pancreas cancer, Safran et al[13] reported modest a benefit to overall survival, but Harder et al[14] found no such beneficial results. Harder et al[14] conducted a phase II trial of trastuzumab and capecitabine, the same drug combination administered to our patient, for metastatic pancreatic cancer. The therapeutic efficacy of HER2 chemotherapy in the current case supports the theory that HER2 overexpression drives cancer progression. The mechanism of cancer cell proliferation might differ between heterotopic and orthotopic pancreatic cancer. Given that HER2 is one of the EGFR family receptors, HER2 shares the same two main downstream signaling pathways as other EGFR receptors, the RAS-MAPK and PI3K-AKT-mTOR pathways[15]. The most reliable explanation of the failure of HER2-targeted therapy is that many pancreatic cancers possess KRAS mutations, which lead to cancer cell proliferation regardless of HER2 inactivation. The cancer in the current case had no KRAS mutation, which supports the rationale for HER2-targeted chemotherapy (data not shown). However, the current case also revealed the limitation of the therapy, since residual cancer was observed in the submucosal layer of the duodenum, lymph nodes and desmoplastic stroma in the pancreatic head. Similar to orthotopic pancreatic ductal adenocarcinoma, in the current case, the cancer cells penetrated the pancreatic parenchyma and developed a tumor microenvironment that supported cancer cell growth, invasion, and metastasis, which contribute to therapeutic resistance[16].
Trastuzumab therapy is the first-line treatment for patients with HER2-overexpressing cancer, but the mechanism of therapy resistance has not been completely established. Biological heterogeneity or clonal evolution could be the underlying mechanism. By analyzing the mechanism involved in trastuzumab resistance, targeted capture sequencing of the circulating tumor DNA (ctDNA) of advanced gastric cancer patients and a clone structure reconstruction approach were conducted[17]. Most of the gene mutation clones were concentrated in the MAPK and RAS pathways. This approach to identifying cancer gene mutations with ctDNA analysis could help to identify new targeted therapies for trastuzumab-resistant cancer patients.
HER2-positive unresectable adenocarcinoma that developed from heterotopic pancreas tissue in the duodenum was treated with trastuzumab. HER2-targeted treatment was effective for reducing the tumor volume sufficiently for surgical resection.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Japan
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Rim CH S-Editor: Gong ZM L-Editor: A P-Editor: Wang LL
| 1. | Tanaka K, Tsunoda T, Eto T, Yamada M, Tajima Y, Shimogama H, Yamaguchi T, Matsuo S, Izawa K. Diagnosis and management of heterotopic pancreas. Int Surg. 1993;78:32-35. [PubMed] |
| 2. | Dolan RV, ReMine WH, Dockerty MB. The fate of heterotopic pancreatic tissue. A study of 212 cases. Arch Surg. 1974;109:762-765. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 224] [Cited by in RCA: 213] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 3. | Guillou L, Nordback P, Gerber C, Schneider RP. Ductal adenocarcinoma arising in a heterotopic pancreas situated in a hiatal hernia. Arch Pathol Lab Med. 1994;118:568-571. [PubMed] |
| 4. | Makhlouf HR, Almeida JL, Sobin LH. Carcinoma in jejunal pancreatic heterotopia. Arch Pathol Lab Med. 1999;123:707-711. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 91] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 5. | Fukino N, Oida T, Mimatsu K, Kuboi Y, Kida K. Adenocarcinoma arising from heterotopic pancreas at the third portion of the duodenum. World J Gastroenterol. 2015;21:4082-4088. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 26] [Cited by in RCA: 30] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 6. | Minami T, Terada T, Mitsui T, Nakanuma Y. Adenocarcinoma arising from a heterotopic pancreas in the first portion of the duodenum: a case report. Surg Case Rep. 2020;6:141. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 7. | Yeo DXW, Goh N, Chuah KL, Amitbhai SK, Oo AM, Ahmed AKS, Nath KA. Malignant Transformation of Heterotopic Pancreatic Tissue in a Patient with BRCA2 Mutation. Gastroenterol Insights. 2021;12:10-16. [RCA] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 8. | Moon JH, Lee K, Yang HK, Kim WH. Duodenal Adenocarcinoma of Brunner Gland Origin: A Case Report. J Pathol Transl Med. 2018;52:179-182. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 9. | Chu P, Wu E, Weiss LM. Cytokeratin 7 and cytokeratin 20 expression in epithelial neoplasms: a survey of 435 cases. Mod Pathol. 2000;13:962-972. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 736] [Cited by in RCA: 641] [Article Influence: 25.6] [Reference Citation Analysis (0)] |
| 10. | Moskaluk CA, Zhang H, Powell SM, Cerilli LA, Hampton GM, Frierson HF Jr. Cdx2 protein expression in normal and malignant human tissues: an immunohistochemical survey using tissue microarrays. Mod Pathol. 2003;16:913-919. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 214] [Cited by in RCA: 217] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 11. | Suh H, Pillai K, Morris DL. Mucins in pancreatic cancer: biological role, implications in carcinogenesis and applications in diagnosis and therapy. Am J Cancer Res. 2017;7:1372-1383. [PubMed] |
| 12. | Safran H, Steinhoff M, Mangray S, Rathore R, King TC, Chai L, Berzein K, Moore T, Iannitti D, Reiss P, Pasquariello T, Akerman P, Quirk D, Mass R, Goldstein L, Tantravahi U. Overexpression of the HER-2/neu oncogene in pancreatic adenocarcinoma. Am J Clin Oncol. 2001;24:496-499. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 116] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 13. | Safran H, Iannitti D, Ramanathan R, Schwartz JD, Steinhoff M, Nauman C, Hesketh P, Rathore R, Wolff R, Tantravahi U, Hughes TM, Maia C, Pasquariello T, Goldstein L, King T, Tsai JY, Kennedy T. Herceptin and gemcitabine for metastatic pancreatic cancers that overexpress HER-2/neu. Cancer Invest. 2004;22:706-712. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 125] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 14. | Harder J, Ihorst G, Heinemann V, Hofheinz R, Moehler M, Buechler P, Kloeppel G, Röcken C, Bitzer M, Boeck S, Endlicher E, Reinacher-Schick A, Schmoor C, Geissler M. Multicentre phase II trial of trastuzumab and capecitabine in patients with HER2 overexpressing metastatic pancreatic cancer. Br J Cancer. 2012;106:1033-1038. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 117] [Cited by in RCA: 141] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 15. | Grapa CM, Mocan T, Gonciar D, Zdrehus C, Mosteanu O, Pop T, Mocan L. Epidermal Growth Factor Receptor and Its Role in Pancreatic Cancer Treatment Mediated by Nanoparticles. Int J Nanomedicine. 2019;14:9693-9706. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 47] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 16. | Neesse A, Bauer CA, Öhlund D, Lauth M, Buchholz M, Michl P, Tuveson DA, Gress TM. Stromal biology and therapy in pancreatic cancer: ready for clinical translation? Gut. 2019;68:159-171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 190] [Cited by in RCA: 250] [Article Influence: 41.7] [Reference Citation Analysis (0)] |
| 17. | Wang Y, Zhao C, Chang L, Jia R, Liu R, Zhang Y, Gao X, Li J, Chen R, Xia X, Bulbul A, Husain H, Guan Y, Yi X, Xu J. Circulating tumor DNA analyses predict progressive disease and indicate trastuzumab-resistant mechanism in advanced gastric cancer. EBioMedicine. 2019;43:261-269. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 67] [Article Influence: 11.2] [Reference Citation Analysis (0)] |