Published online Jul 21, 2021. doi: 10.3748/wjg.v27.i27.4441
Peer-review started: April 27, 2021
First decision: May 27, 2021
Revised: June 6, 2021
Accepted: July 9, 2021
Article in press: July 9, 2021
Published online: July 21, 2021
Processing time: 83 Days and 7 Hours
Computed tomography colonography (CTC) may be superior to colonoscopy and barium enema for detecting diverticula. However, few studies have used CTC to diagnose diverticula.
To evaluate the current prevalence and distribution of colonic diverticula in Japan using CTC.
This study was conducted as part of the Japanese National Computed Tomogra
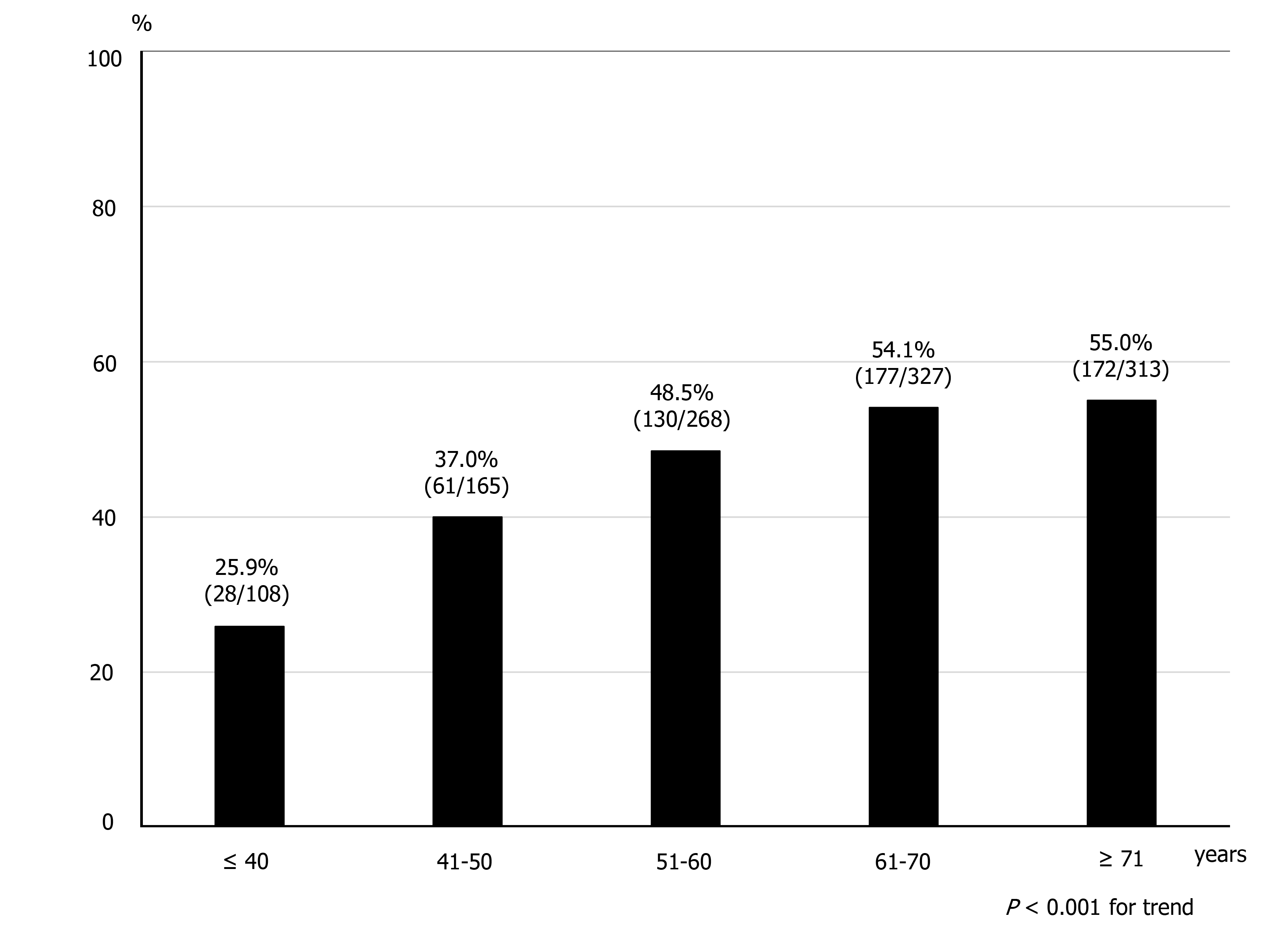
Diverticulosis was present in 48.1% of the participants. The prevalence of diverticulosis was higher in the older participants (P < 0.001 for trend). The diverticula seen in younger participants were predominantly located in the right-sided colon. Older participants had a higher frequency of bilateral type (located in the right- and left-sided colon) diverticulosis (P < 0.001 for trend). The length of the large intestine with multiple diverticula in the sigmoid colon was significantly shorter in those without diverticula (P < 0.001).
The prevalence of colonic diverticulosis in Japan is higher than that previously reported. The prevalence was higher, and the distribution tended to be bilateral in older participants.
Core Tip: In this retrospective study, we evaluated the current prevalence and distribution of colonic diverticula in Japan using computed tomography colonography. Diverticulosis was present in 48.1% of the 1181 participants. The prevalence of diverticulosis was higher among the older participants. The diverticula seen in younger participants were predominantly located in the right-sided colon. Older participants had a higher frequency of the bilateral type. The length of the large intestine with multiple diverticula in the sigmoid colon was significantly shorter in participants without diverticula.
- Citation: Isohata N, Nagata K, Utano K, Nozaki R, Nozu S, Kato T, Kijima S, Matsumoto H, Majima K, Ryu Y, Hirayama M, Endo S. Recent trends in the prevalence and distribution of colonic diverticula in Japan evaluated using computed tomography colonography. World J Gastroenterol 2021; 27(27): 4441-4452
- URL: https://www.wjgnet.com/1007-9327/full/v27/i27/4441.htm
- DOI: https://dx.doi.org/10.3748/wjg.v27.i27.4441
Colonic diverticulosis is a common abnormality of the colon. Almost all colonic diverticula are pseudodiverticula, which are small outpouchings of the colonic mucosa and submucosa that herniate through the muscle layers at sites of vascular perforation[1]. Diverticulosis refers to asymptomatic diverticula, whereas diverticular disease refers to symptomatic diverticula. Diverticular disease is classified into uncomplicated and complicated diseases, including perforation, fistulae, obstructions, and bleeding[1]. The cumulative incidences of bleeding and diverticulitis in patients with colonic diverticulosis are 10% and 10%-25%, respectively[2,3]. Additionally, the prevalence of colonic diverticulosis increases with age[1,4,5]. Due to Japan's aging population, the incidence of diverticular diseases in older patients who need treatment will also likely increase[6].
Findings from studies involving colonoscopy or barium enema have shown that the prevalence and distribution of diverticulosis differ according to country, region, and race[7-11]. These reports are from the early 2000s until the mid-2010s, and also reported that the incidence of diverticula increased with time[5,8]. Colonic diverticula occur mainly in the left colon in Western populations and in the right colon in Japanese and other Asian populations[7-9,11,12]. Recent studies have reported that the overall prevalence of diverticulosis in Japan is 20%-26.0%[5,7,8].
Colonoscopy is a standard technique that is widely performed for the screening and diagnosis of colonic and rectal diseases. However, there are reports suggesting that compared to colonoscopy, assessments based on barium enema are superior for diagnosing colonic diverticula, especially in the sigmoid colon[13,14]. In recent years, computed tomography colonography (CTC) has been widely used as an alternative colon cancer screening method. CTC can detect diverticula as outpouchings of the colonic wall from a more advantageous perspective, and it may be superior to colonoscopy and barium enema for detecting diverticula. Only one large study has evaluated the prevalence and distribution of colonic diverticula using CTC[15]. Hence, this study is the largest study in the world and is the first to accurately evaluate the prevalence and distribution of colonic diverticula in Japanese patients using CTC.
This study was conducted as a part of another study on the application of CTC for detecting polypoid lesions [Japanese National Computed Tomographic Colonography Trial (JANCT)][16]. The trial was registered with Clinical Trials. gov (No. NCT00997802) and the UMIN Clinical Trials Registry (No. UMIN000002097). The medical ethics committee of Fukushima Medical University reviewed and approved the study design. The JANCT data were analyzed using the Digital Imaging and Communications in Medicine protocol. Patients from 14 hospitals in Japan were enrolled between September 2009 and August 2011. This study was conducted in accordance with the principles of the Declaration of Helsinki. The institutional review board of each hospital approved the study. All patients provided written informed consent prior to enrollment. The patients included in this study underwent CTC for screening purposes or because they had abdominal symptoms, positive fecal immunochemical test (FIT) results, a personal history of polyps, or a familial history of colorectal cancer (CRC) or polyps. Moreover, 847 participants were screened, 96 for abdominal symptoms or change in bowel habits, 165 for positive FIT, 42 for melena or hematochezia, 11 for surveillance after polypectomy, and 19 for abnormal blood test results (anemia or tumor marker elevation). The exclusion criteria have been described in detail previously[16]. Briefly, participants with serious medical conditions for bowel preparation and colonoscopy or CTC, who underwent colonoscopy or barium enema during the preceding 3 years, with known colorectal polyps or cancers, a history of inflammatory bowel disease, and familial polyposis, and who underwent colorectal surgery were excluded from the study.
Details of the CTC procedure have been previously published[16]. A polyethylene glycol electrolyte lavage solution (Niflec; Ajinomoto Pharmaceuticals, Tokyo, Japan) was used for bowel preparation, and contrast medium containing sodium diatrizoate (Gastrografin; Bayer Yakuhin, Osaka, Japan) was added to tag the residual fluid. A tube was inserted while the patients were in the left decubitus position before CO2 insufflation and CTC scanning. CO2 insufflation was performed using an automated CO2 insufflator (HP-2; Horii Pharmaceutical Industries, Ltd., Osaka, Japan). All CTC examinations were performed using 64- or 16-channel multidetector row computed tomography (CT) scanners. CT scanning was performed under single breath-holds while the patients were in the supine and prone positions.
Four radiologists and six gastroenterologists participated in this study as readers of the CTC images. The readers analyzed CTC images from a median of 1750 patients (range: 400–8000 patients). Identification of the colonic diverticula and measuring the length of the large intestine were performed at workstations at each hospital, namely: AZE Virtual Place (AZE, Tokyo, Japan), Synapse Vincent (Fujifilm Medical Co., Tokyo, Japan), and Ziostation (Ziosoft, Tokyo, Japan). Axial 2-dimensional (2D) images, captured while the patients were in the supine position, were primarily used to determine the number of diverticula. A diverticulum was identified as an outpouching from the colonic wall. If necessary, other 2D and 3-dimensional (3D) images, comprising luminal fly-through or air-contrast barium enema images, were analyzed (Figure 1). The readers recorded the number of diverticula in each colonic segment, including the cecum and as well as the ascending, transverse, descending, sigmoid, and rectosigmoid colons. The rectosigmoid colon was defined as the segment between the promontrium and the inferior margin of the second sacral vertebra. Diverticula in the terminal ileum and appendix were also evaluated. The terminal ileum was defined as the portion of the ileum 10 cm from the ileocecal valve. If there were < 20 diverticula, the readers recorded the numbers as is; if there were > 20 diverticula, the readers recorded their findings as severe. When diverticula were found in multiple colonic segments, we evaluated the number of diverticula in each segment in duplicate. In analysis, the grading of diverticula was in accordance with a previous study to allow for comparison with the previous study[15]. The number of diverticula was graded in each segment: 1-5 diverticula were classified as rare, 6-20 diverticula were classified as multiple, and more than 20 diverticula were classified as severe. The length of the large intestine was defined as the distance from the anus to the appendiceal orifice and was measured automatically using 3D workstations.
The primary endpoint of this study was to clarify the prevalence of colonic diverticulosis by CTC and to evaluate it by sex, age group, and segment. There were two secondary endpoints; the first of which was to investigate the prevalence of diverticulosis in the terminal ileum and appendix (which have not been evaluated accurately so far), and the second was to investigate the relationship between diverticulosis and the length of the large intestine.
Continuous variables are reported as medians and ranges. To evaluate differences in the prevalence of diverticula and the length of the large intestine, the Mann-Whitney U test for unpaired samples was performed. We used the chi-squared test to assess the relationship between the prevalence of diverticulosis and sex. Trends in age-related difference and increases in the prevalence of diverticula were evaluated using the Cochran-Armitage test. Statistical significance was set at P < 0.05. Test of normal distribution was evaluated using Shapiro-Wilk test. All statistical analyses were performed using IBM® SPSS® software, version 26 (IBM Corp., Armonk, NY, United States), except for the Cochran-Armitage test and Shapiro-Wilk test, which was performed using Bell Curve for Excel version 2.20 (Social Survey Research Information Co., Ltd., Tokyo, Japan).
Our study population comprised 1181 participants, including 669 men (56.6%) and 512 women (43.4%) with a median age of 62 years (range: 23-91 years). Of the 1181 participants, 568 (48.1%) had ≥ 1 diverticula. The median age of the participants with diverticula was 64 years (range: 27-91 years); that of participants with no diverticulum was 60 years (range: 23-84 years). Participants with diverticula were significantly older than participants with no diverticulum (P < 0.001) (Table 1). The participants were stratified into five groups according to age. There were 108 participants in the 40 years or younger group, 165 in the 41-50 years group, 268 in the 51-60 years group, 327 in the 61-70 years group, and 313 in the 71 years and over group. A normal distribution of age dates was performed.
| Diverticula positive (n = 568) | Diverticulum negative (n = 613) | P value | |
| Age: Median (range) | 64 (27-91) | 60 (23-84) | < 0.001 |
| Sex, n (%): Male:female | 346 (29.3):222 (18.8) | 323 (27.3):290 (24.6) | 0.004 |
| Age group, n (%): Male:female | |||
| ≤ 40 (n = 108) | 27 (25.0):1 (0.9) | 56 (51.9):24 (22.2) | 0.004 |
| 41-50 (n = 165) | 42 (25.5):19 (11.5) | 56 (33.9):48 (29.1) | 0.058 |
| 51-60 (n = 268) | 80 (29.9):50 (18.7) | 67 (25.0):71 (26.5) | 0.033 |
| 61-70 (n = 327) | 97 (29.7):80 (24.5) | 88 (26.9):62 (19.0) | 0.485 |
| ≥ 71 (n = 313) | 100 (31.9):72 (23.0) | 56 (17.9):85 (27.2) | 0.001 |
Diverticulosis was present in 346 men (29.3%) and 222 women (18.8%). The frequency of diverticulosis in men was significantly higher than that in women (P = 0.004). The frequency of diverticulosis was significantly higher or tended to occur in men in other age groups, but did not differ between sexes in the 60s. The distribution of diverticula and the relationship between sex and location of diverticula are also shown in Table 2. Diverticula were most often found in the ascending colon. The frequency of diverticulosis in men was higher in all segments except the rectosigmoid colon. Regarding the relationship between age and frequency of diverticulosis, the prevalence of diverticulosis significantly increased with age (P < 0.001 for trend) (Figure 2). Over half of the participants aged > 60 years had diverticula.
| Segment | n (%) | Male:Female |
| Ileum | 13 (1.1) | 9:4 |
| Appendix | 6 (0.5) | 5:1 |
| Cecum | 258 (21.8) | 153:105 |
| Ascending colon | 431 (36.5) | 266:165 |
| Transverse colon | 124 (10.5) | 78:46 |
| Descending colon | 149 (12.6) | 102:47 |
| Sigmoid colon | 200 (16.9) | 129:71 |
| Rectosigmoid colon | 4 (0.3) | 1:3 |
The prevalence and the number of diverticula in each colonic segment increased with age, and diverticula were also found in the terminal ileum and appendix (Table 3). Thirteen participants (1.1%) had diverticula in the terminal ileum, and all had less than six diverticula. The appendix was visualized in 680 participants (57.6%), six (0.5%) of whom had appendiceal diverticula. Five participants had one diverticulum, with one participant in his 60s having three diverticula in the appendix.
| Age group (yr) | |||||
| ≤ 40 (n = 108) | 41-50 (n = 165) | 51-60 (n = 268) | 61-70 (n = 327) | ≥ 71 (n = 313) | |
| Sex | |||||
| Male | 83(76.9) | 98(59.4) | 147 (54.9) | 185 (56.6) | 156 (49.8) |
| Female | 25 (23.1) | 67 (40.6) | 121 (45.1) | 142 (43.4) | 157 (50.2) |
| Grade of diverticula in segment | |||||
| Ileum | |||||
| Rare | 0 | 2 (1.2) | 2 (0.7) | 5 (1.5) | 4 (1.3) |
| Multiple | 0 | 0 | 0 | 0 | 0 |
| Severe | 0 | 0 | 0 | 0 | 0 |
| Appendix | |||||
| Rare | 0 | 0 | 1 (0.4) | 4 (1.2) | 1 (0.3) |
| Multiple | 0 | 0 | 0 | 0 | 0 |
| Severe | 0 | 0 | 0 | 0 | 0 |
| Cecum | |||||
| Rare | 10 (9.3) | 21 (12.7) | 48 (17.9) | 77 (23.5) | 75 (24.0) |
| Multiple | 0 | 3 (1.8) | 9 (3.4) | 7 (2.1) | 4 (1.3) |
| Severe | 0 | 0 | 1 (0.4) | 3 (0.9) | 0 |
| Ascending colon | |||||
| Rare | 12 (11.1) | 35 (21.2) | 60 (22.3) | 81 (24.8) | 77 (24.6) |
| Multiple | 4 (3.7) | 8 (4.8) | 30 (11.2) | 34 (10.4) | 49 (15.7) |
| Severe | 1 (0.9) | 1 (0.6) | 11 (4.1) | 15 (4.6) | 13 (4.2) |
| Transverse colon | |||||
| Rare | 5 (4.6) | 8 (4.8) | 25 (9.3) | 29 (8.9) | 30 (9.6) |
| Multiple | 0 | 0 | 4 (1.5) | 12 (3.7) | 9 (2.9) |
| Severe | 0 | 1 (0.6) | 0 | 1 (0.3) | 0 |
| Descending colon | |||||
| Rare | 4 (3.7) | 6 (3.6) | 25 (9.3) | 30 (9.2) | 32 (10.2) |
| Multiple | 0 | 2 (1.2) | 8 (3.0) | 14 (4.3) | 14 (4.5) |
| Severe | 0 | 1 (0.6) | 0 | 5 (1.5) | 8 (2.6) |
| Sigmoid colon | |||||
| Rare | 7 (6.5) | 7 (4.2) | 29 (10.8) | 31 (9.5) | 34 (10.9) |
| Multiple | 0 | 0 | 10 (3.7) | 20 (6.1) | 27 (8.6) |
| Severe | 0 | 2 (1.2) | 3 (1.1) | 11 (3.4) | 19 (6.1) |
| Rectosigmoid colon | |||||
| Rare | 0 | 0 | 1 (0.4) | 0 | 2 (0.6) |
| Multiple | 0 | 0 | 0 | 0 | 1 (0.3) |
| Severe | 0 | 0 | 0 | 0 | 0 |
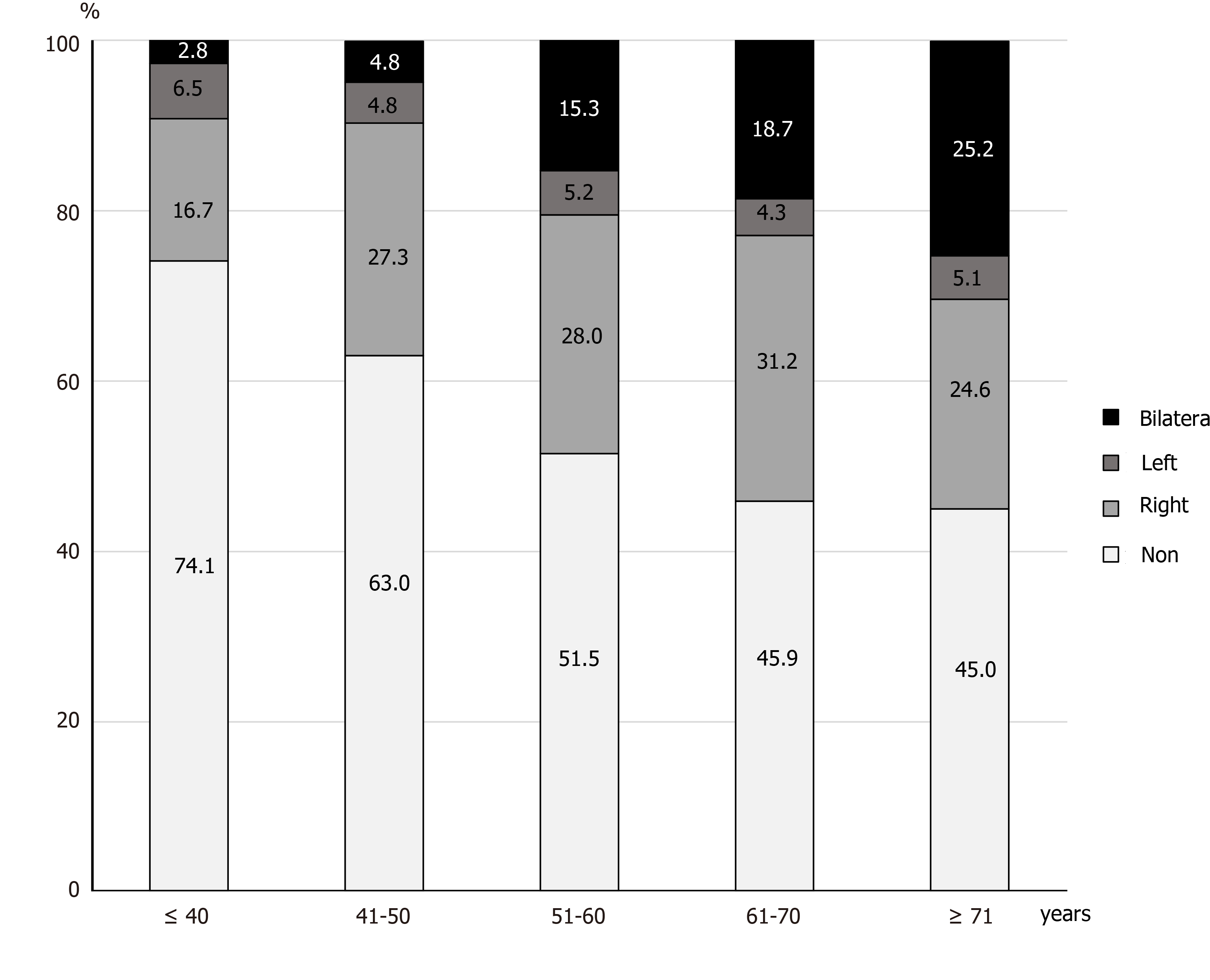
Participants with diverticula were divided into three groups according to the location of the diverticula: The right-sided type was defined as diverticula located from the ileum to the transverse colon only; the left-sided type was defined only from the descending to rectosigmoid colon; and the bilateral type was defined as located on both sides of the colon. We investigated the proportion of diverticula in each type in all patients (Figure 3). As mentioned above, the prevalence of diverticula increased with age, while the prevalence of right-sided and left-sided diverticula did not differ
There was no significant difference between the length of the large intestine without diverticula and that with diverticula (P = 0.104): median length was 157.2 cm (range: 104.9-259.3 cm) and 154.7 cm (range: 103.9-246.6 cm), respectively. The median length of the large intestine with ≥ 6 diverticula in cecum and/or ascending colon was 154.5 cm (range: 114.6-246.6 cm). This result also did not differ when compared to the length of the large intestine with no diverticula (P = 0.260). The median length of the large intestine with ≥ 6 and ≥ 21 diverticula in the sigmoid colon was 149.9 cm (range: 113.5-195.6 cm) and 145.1 cm (range: 115.0-192.0 cm), respectively, which were both significantly shorter than those with no diverticula (P < 0.001) (Table 4).
| Median (cm) | Range (cm) | |
| Diverticula negative in any segment | 157.2 | 104.9-259.3 |
| Diverticula positive in any segment | 154.7 | 103.9-246.6 |
| Multiple in cecum to ascending colon | 154.5 | 114.9-246.6 |
| Severe in cecum to ascending colon | 151.6 | 119.6-198.4 |
| Multiple in sigmoid colon | 149.9 | 113.5-195.6 |
| Severe in sigmoid colon | 145.1 | 115.0-192.0 |
Geographical and racial variations have been reported in relation to the prevalence and distribution of colonic diverticula[1,4,17]. In developed Western countries, the prevalence of diverticulosis is high, affecting 5% of people in their 40s and approximately half of those in their 80s[18], with an estimated mortality rate of 2.5% per 100000 per year. This disease is the fifth most important gastrointestinal disease in terms of healthcare costs[19]. The incidence of diverticula has also been reported to increase over time[5,8]. The most recent large studies using colonoscopy showed that the prevalence of diverticulosis was 42% in the United States[4] and 23.9% in Japan[8]. Diverticula are mainly left-sided in patients in the United States and in other western countries. On the other hand, patients in Japan often manifest right-sided diverticula, with an associated age-related increase in disease prevalence[1,4,5,8,12]. In our study, the prevalence of colonic diverticulosis detected using CTC was 48.1%, which is much higher than the rates reported in previous studies using colonoscopy in Japan[5,8]. A report from Europe on the prevalence of diverticula diagnosed by CTC reported a prevalence of 51.6%, similar to ours, with a higher prevalence in the right-sided colon than expected[15]; however, compared to our results, the prevalence of diverticula in the right-sided colon in Caucasians was lower than that in Japanese. Most of the recent epidemiologic studies of diverticulosis have been evaluated through colonoscopy, which is a standard technique that is performed worldwide for the screening and diagnosis of colonic diseases[4,5,8]. However, investigators have reported that compared to colonoscopy, assessments evaluated using barium enema are superior for diagnosing colonic diverticulosis[13,14]. Among diverticula that had been detected using barium enema, Niikura et al[14] reported colonoscopy detection ratios of 41% and 32% for diverticula in the entire colon and in the left colon, respectively. Therefore, the actual prevalence of colonoscopy-detected diverticulosis in Japan may be over 25%. Conducting clinical studies on the prevalence of diverticulosis determined that the use of barium enema is difficult, given the widespread use of colonoscopy. According to the European Society of Gastrointestinal Endoscopy and the European Society of Gastrointestinal and Abdominal Radiology Guideline - Update 2020[20], barium enema for the diagnosis of colorectal neoplasia was not recommended (strong recommendation); in this regard, barium enema cannot be used in this setting.
In recent years, CTC has become more popular for screening and diagnosing colorectal cancer[21,22]; therefore, CTC could be used as an alternative to colonoscopy due to its diagnostic ability and safety[20,23]. It might be superior for detecting diverticula because it can evaluate the colon from more advantageous perspectives and avoid impediments caused by overlapping adjacent sections of the colon. In fact, De Cecco et al[15] reported that the prevalence of diverticulosis identified using CTC was 51.6%, which was much higher than that previously reported using colonoscopes in Western countries[4]. This result is similar to our results.
Our results showed that the prevalence of diverticulosis is increasing in Japan, mimicking the trend seen in Western countries. Several studies have shown that age, low intake of dietary fiber, fat intake, red meat intake, alcohol consumption, smoking, and increasing body weight are pathogenic risk factors associated with colonic diverticulosis[1,4,7,8,24]. Increasing age and changing to a westernized diet might be the most closely associated risk factor for the increasing prevalence of diverticulosis in Japan.
In our study, the prevalence and grades of colonic diverticula increased with age, which concurs with the findings of previous studies[1,4,8]. Moreover, right-sided colonic diverticula were more predominant in patients aged < 70 years. While the prevalence of diverticula in the right- and left-sided colon did not differ, even in older patients, the prevalence of bilateral diverticula was higher in older patients. Patients with right-sided diverticula only may eventually develop left-sided diverticula with aging. Consequently, they may ultimately develop bilateral diverticula[25]. Although reports from Western countries suggest that the rate of diverticulosis does not differ between the sexes, our results showed that diverticulosis was more common in men, which is consistent with the findings of previous studies in Japan[1,4,5,8].
In this study, diverticula were also identified in the terminal ileum and appendix. Although there are few case reports and research on resected specimens[26-28], this is the first epidemiological report of asymptomatic diverticulosis of the appendix and terminal ileum. This should be considered in the differential diagnosis of acute abdomen and lower gastrointestinal bleeding. Diverticula in the appendix and terminal ileum, and as well as low numbers of right-sided diverticula, might be congenital. Similar to colonic diverticula, inflammation and hemorrhage are occasional consequences.
This is the first study to investigate the relationship between the length of the large intestine and the presence of diverticula. The large intestine with multiple (≥ 6) diverticula in the sigmoid colon was significantly shorter than that with no diverticula. There have been no reports of studies on the relationship between colonic diverticulosis and the length of the large intestine. The ability to accurately measure the length of the large intestine using a workstation is an advantage of CTC, a study made possible because of CTC. However, the length of the large intestine did not differ between the participants with multiple diverticula in the cecum and/or ascending colon in those with no diverticula. When multiple diverticula are present, the colonic wall can thicken and become rigid[18], and the sigmoid colon may shorten because it is free in the peritoneal cavity. In contrast, the colon is unlikely to shorten in patients with multiple right-sided diverticula because it is attached to the retroperitoneum.
This study had several limitations. First, about 70% of asymptomatic participants participated for screening purpose. The study also included participants who underwent CTC for the purpose of examination for any abdominal symptoms, or positive FIT, or abnormal blood test, or a familial history of CRC and polyps, or for the surveillance of polyps. Hence, the findings of this study may not necessarily reflect those of an asymptomatic general population. Second, participants aged > 50 years accounted for > 75% of the study population, and only a small number of patients aged < 40 years were included in this study. This differs from the age structure of the general population in Japan, which may explain why the prevalence of colonic diverticula in this study was much higher than what was previously reported. However, asymptomatic younger people have not been evaluated in previous studies. There might be the same bias in previous studies diagnosed by colonoscopy or barium enema on the prevalence of diverticula. Third, the patients’ diet, lifestyle, and physical factors could underlie the increase in the prevalence of diverticulosis; however, data describing these factors were absent from this study. Fourth, the number of participants was smaller than the number of studies conducted based on colonoscopy[5,7,8]. This is because CTC is not yet widely used as a screening modality for colorectal neoplasms, and the facilities that can be used are limited. However, the number of participants in this study was slightly larger than that in a previous study conducted by CTC[15]. Moreover, the data used in this study are approximately 10 years old and are a bit out of date. We believe that a large-scale prospective study should be conducted after standardization of bowel preparation and imaging protocols in the future.
This is the first investigation to use CTC to evaluate the prevalence and distribution of colonic diverticula in Japan. The prevalence of colonic diverticulosis was higher than that reported previously. In addition to the superiority of CTC in detecting colonic diverticula, the aging of the Japanese population and the change to a Western diet may be behind the high prevalence of colonic diverticulosis observed in this study. The diverticula were predominantly right-sided in the younger generation, while right- and left-sided diverticula were more common in older participants. The prevalence of asymptomatic diverticulosis in the terminal ileum and appendix can be clarified for the first time. These results may be helpful in the management of diverticular diseases.
Colonic diverticulosis is a common abnormality of the colon. The prevalence of diverticulosis and diverticular disease in Japan is increasing due to aging and a westernized diet.
Recent trends in the prevalence and distribution of colonic diverticula in Japan are not clear. Most recent studies on diverticulosis in Japan involved the use of colonoscopy. In recent years, computed tomography colonography (CTC) has been widely used as an alternative colon cancer screening method. We considered CTC to detect diverticula as outpouchings of the colonic wall from a more advantageous perspective, and it may be superior to colonoscopy and barium enema for detecting diverticula.
The main objective of this study was to evaluate the prevalence and distribution of colonic diverticula in Japan.
This study included 1181 participants from 14 hospitals in Japan. We analyzed the prevalence and distribution of colonic diverticula and their relationships with age and sex using CTC and analyzed the relationship between the diverticula and the length of the large intestine.
Diverticulosis was present in 48.1% of the participants. The prevalence of diverticulosis was higher in the older participants. The diverticula seen in younger participants were predominantly located in the right-sided colon and on the bilateral side for older participants. The length of the large intestine with multiple diverticula in the sigmoid colon was significantly shorter in those without diverticula.
The prevalence of colonic diverticulosis in Japan is 48.1%. This was much higher than that previously reported. In older participants, the prevalence was higher, and the distribution tended to be bilateral.
The data used in this study were slightly limited and a bit out of date. We hope to perform a large-scale prospective study after standardization of bowel preparation and imaging protocols, and CTC use will become widespread in the future. We also hope to determine the relationship between the trends in diverticulosis and its accompanying symptoms.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Japan
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Du P, Perez AR S-Editor: Wu YXJ L-Editor: A P-Editor: Xing YX
| 1. | Stollman N, Raskin JB. Diverticular disease of the colon. Lancet. 2004;363:631-639. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 443] [Cited by in RCA: 413] [Article Influence: 19.7] [Reference Citation Analysis (0)] |
| 2. | Niikura R, Nagata N, Shimbo T, Aoki T, Yamada A, Hirata Y, Sekine K, Okubo H, Watanabe K, Sakurai T, Yokoi C, Mizokami M, Yanase M, Akiyama J, Koike K, Uemura N. Natural history of bleeding risk in colonic diverticulosis patients: a long-term colonoscopy-based cohort study. Aliment Pharmacol Ther. 2015;41:888-894. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 39] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 3. | Shahedi K, Fuller G, Bolus R, Cohen E, Vu M, Shah R, Agarwal N, Kaneshiro M, Atia M, Sheen V, Kurzbard N, van Oijen MG, Yen L, Hodgkins P, Erder MH, Spiegel B. Long-term risk of acute diverticulitis among patients with incidental diverticulosis found during colonoscopy. Clin Gastroenterol Hepatol. 2013;11:1609-1613. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 253] [Cited by in RCA: 309] [Article Influence: 25.8] [Reference Citation Analysis (0)] |
| 4. | Peery AF, Keku TO, Martin CF, Eluri S, Runge T, Galanko JA, Sandler RS. Distribution and Characteristics of Colonic Diverticula in a United States Screening Population. Clin Gastroenterol Hepatol 2016; 14: 980-985. e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 130] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 5. | Nagata N, Niikura R, Aoki T, Shimbo T, Itoh T, Goda Y, Suda R, Yano H, Akiyama J, Yanase M, Mizokami M, Uemura N. Increase in colonic diverticulosis and diverticular hemorrhage in an aging society: lessons from a 9-year colonoscopic study of 28,192 patients in Japan. Int J Colorectal Dis. 2014;29:379-385. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 86] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 6. | Wheat CL, Strate LL. Trends in Hospitalization for Diverticulitis and Diverticular Bleeding in the United States From 2000 to 2010. Clin Gastroenterol Hepatol 2016; 14: 96-103. e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 131] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 7. | Nagata N, Niikura R, Shimbo T, Kishida Y, Sekine K, Tanaka S, Aoki T, Watanabe K, Akiyama J, Yanase M, Itoh T, Mizokami M, Uemura N. Alcohol and smoking affect risk of uncomplicated colonic diverticulosis in Japan. PLoS One. 2013;8:e81137. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 69] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 8. | Yamamichi N, Shimamoto T, Takahashi Y, Sakaguchi Y, Kakimoto H, Matsuda R, Kataoka Y, Saito I, Tsuji Y, Yakabi S, Takeuchi C, Minatsuki C, Niimi K, Asada-Hirayama I, Nakayama C, Ono S, Kodashima S, Yamaguchi D, Fujishiro M, Yamaji Y, Wada R, Mitsushima T, Koike K. Trend and risk factors of diverticulosis in Japan: age, gender, and lifestyle/metabolic-related factors may cooperatively affect on the colorectal diverticula formation. PLoS One. 2015;10:e0123688. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 77] [Cited by in RCA: 74] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 9. | Hong W, Geng W, Wang C, Dong L, Pan S, Yang X, Zippi M, Xu C, Zhou M, Pan J. Prevalence of colonic diverticulosis in mainland China from 2004 to 2014. Sci Rep. 2016;6:26237. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 10. | Golder M, Ster IC, Babu P, Sharma A, Bayat M, Farah A. Demographic determinants of risk, colon distribution and density scores of diverticular disease. World J Gastroenterol. 2011;17:1009-1017. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 24] [Reference Citation Analysis (0)] |
| 11. | Miura S, Kodaira S, Shatari T, Nishioka M, Hosoda Y, Hisa TK. Recent trends in diverticulosis of the right colon in Japan: retrospective review in a regional hospital. Dis Colon Rectum. 2000;43:1383-1389. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 86] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 12. | Nakaji S, Danjo K, Munakata A, Sugawara K, MacAuley D, Kernohan G, Baxter D. Comparison of etiology of right-sided diverticula in Japan with that of left-sided diverticula in the West. Int J Colorectal Dis. 2002;17:365-373. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 66] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 13. | Rockey DC, Koch J, Yee J, McQuaid KR, Halvorsen RA. Prospective comparison of air-contrast barium enema and colonoscopy in patients with fecal occult blood: a pilot study. Gastrointest Endosc. 2004;60:953-958. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 12] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 14. | Niikura R, Nagata N, Shimbo T, Akiyama J, Uemura N. Colonoscopy can miss diverticula of the left colon identified by barium enema. World J Gastroenterol. 2013;19:2362-2367. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 24] [Cited by in RCA: 30] [Article Influence: 2.5] [Reference Citation Analysis (2)] |
| 15. | De Cecco CN, Ciolina M, Annibale B, Rengo M, Bellini D, Muscogiuri G, Maruotti A, Saba L, Iafrate F, Laghi A. Prevalence and distribution of colonic diverticula assessed with CT colonography (CTC). Eur Radiol. 2016;26:639-645. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 30] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 16. | Nagata K, Endo S, Honda T, Yasuda T, Hirayama M, Takahashi S, Kato T, Horita S, Furuya K, Kasai K, Matsumoto H, Kimura Y, Utano K, Sugimoto H, Kato H, Yamada R, Yamamichi J, Shimamoto T, Ryu Y, Matsui O, Kondo H, Doi A, Abe T, Yamano HO, Takeuchi K, Hanai H, Saida Y, Fukuda K, Näppi J, Yoshida H. Accuracy of CT Colonography for Detection of Polypoid and Nonpolypoid Neoplasia by Gastroenterologists and Radiologists: A Nationwide Multicenter Study in Japan. Am J Gastroenterol. 2017;112:163-171. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 32] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 17. | Martel J, Raskin JB; NDSG. History, incidence, and epidemiology of diverticulosis. J Clin Gastroenterol. 2008;42:1125-1127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 57] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 18. | Petruzziello L, Iacopini F, Bulajic M, Shah S, Costamagna G. Review article: uncomplicated diverticular disease of the colon. Aliment Pharmacol Ther. 2006;23:1379-1391. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 81] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 19. | Sandler RS, Everhart JE, Donowitz M, Adams E, Cronin K, Goodman C, Gemmen E, Shah S, Avdic A, Rubin R. The burden of selected digestive diseases in the United States. Gastroenterology. 2002;122:1500-1511. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1062] [Cited by in RCA: 1038] [Article Influence: 45.1] [Reference Citation Analysis (0)] |
| 20. | Spada C, Hassan C, Bellini D, Burling D, Cappello G, Carretero C, Dekker E, Eliakim R, de Haan M, Kaminski MF, Koulaouzidis A, Laghi A, Lefere P, Mang T, Milluzzo SM, Morrin M, McNamara D, Neri E, Pecere S, Pioche M, Plumb A, Rondonotti E, Spaander MC, Taylor S, Fernandez-Urien I, van Hooft JE, Stoker J, Regge D. Imaging alternatives to colonoscopy: CT colonography and colon capsule. European Society of Gastrointestinal Endoscopy (ESGE) and European Society of Gastrointestinal and Abdominal Radiology (ESGAR) Guideline - Update 2020. Eur Radiol. 2021;31:2967-2982. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 45] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 21. | Levin B, Lieberman DA, McFarland B, Smith RA, Brooks D, Andrews KS, Dash C, Giardiello FM, Glick S, Levin TR, Pickhardt P, Rex DK, Thorson A, Winawer SJ; American Cancer Society Colorectal Cancer Advisory Group; US Multi-Society Task Force; American College of Radiology Colon Cancer Committee. Screening and surveillance for the early detection of colorectal cancer and adenomatous polyps, 2008: a joint guideline from the American Cancer Society, the US Multi-Society Task Force on Colorectal Cancer, and the American College of Radiology. CA Cancer J Clin. 2008;58:130-160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1169] [Cited by in RCA: 1203] [Article Influence: 70.8] [Reference Citation Analysis (0)] |
| 22. | US Preventive Services Task Force, Bibbins-Domingo K, Grossman DC, Curry SJ, Davidson KW, Epling JW Jr, García FAR, Gillman MW, Harper DM, Kemper AR, Krist AH, Kurth AE, Landefeld CS, Mangione CM, Owens DK, Phillips WR, Phipps MG, Pignone MP, Siu AL. Screening for colorectal cancer. US Preventive Services Task Force Recommendation Statement. JAMA. 2016;315:2564-2575. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1249] [Cited by in RCA: 1387] [Article Influence: 154.1] [Reference Citation Analysis (1)] |
| 23. | Nagata K, Takabayashi K, Yasuda T, Hirayama M, Endo S, Nozaki R, Shimada T, Kanazawa H, Fujiwara M, Shimizu N, Iwatsuki T, Iwano T, Saito H. Adverse events during CT colonography for screening, diagnosis and preoperative staging of colorectal cancer: a Japanese national survey. Eur Radiol. 2017;27:4970-4978. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 20] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 24. | Strate LL. Lifestyle factors and the course of diverticular disease. Dig Dis. 2012;30:35-45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 60] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 25. | Miura S, Kodaira S, Aoki H, Hosoda Y. Bilateral type diverticular disease of the colon. Int J Colorectal Dis. 1996;11:71-75. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 30] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 26. | Käser SA, Willi N, Maurer CA. Prevalence and clinical implications of diverticulosis of the vermiform appendix. J Int Med Res. 2013;41:1350-1356. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 27. | Iwano T, Nagata K, Egawa T, Yamashita H. Appendiceal diverticulosis incidentally detected by computed tomographic colonography. Dig Liver Dis. 2016;48:565. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 28. | Nakazawa N, Suzuki H, Ebara G, Watanabe Y, Tsukagoshi R, Ieta K, Morohara K, Osawa H, Katayama K, Yasuda Y, Tanaka S, Kuwano H. Adult intussusceptions induced by a terminal ileum diverticulum: a case report. Clin Case Rep. 2018;6:674-677. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |