Published online Jul 21, 2021. doi: 10.3748/wjg.v27.i27.4429
Peer-review started: January 28, 2021
First decision: February 24, 2021
Revised: February 28, 2021
Accepted: April 25, 2021
Article in press: April 25, 2021
Published online: July 21, 2021
Processing time: 172 Days and 13.6 Hours
Multifocal-type autoimmune pancreatitis (AIP), sometimes forming multiple pancreatic masses, is frequently misdiagnosed as pancreatic malignancy in routine clinical practice. It is critical to know the imaging features of multifocal-type AIP to prevent misdiagnosis and unnecessary surgery. To the best of our knowledge, there have been no studies evaluating the value of diffusionweighted imaging (DWI), axial fat-suppressed T1 weighted image (T1WI), and dynamic contrast enhanced-computed tomography (DCE-CT) in detecting the lesions of multifocal-type AIP.
To clarify the exact prevalence and radiological findings of multifocal AIP in our cohorts and compare the sensitivity of DWI, axial fat-suppressed T1WI, and DCE-CT for detecting AIP lesions. We also compared radiological features between multifocal AIP and pancreatic ductal adenocarcinoma with several key imaging landmarks.
Twenty-six patients with proven multifocal AIP were retrospectively included. Two blinded independent radiologists rated their confidence level in detecting the lesions on a 5-point scale and assessed the diagnostic performance of DWI, axial fat-suppressed T1WI, and DCE-CT. CT and magnetic resonance imaging of multifocal AIP were systematically reviewed for typical imaging findings and compared with the key imaging features of pancreatic ductal adenocarcinoma.
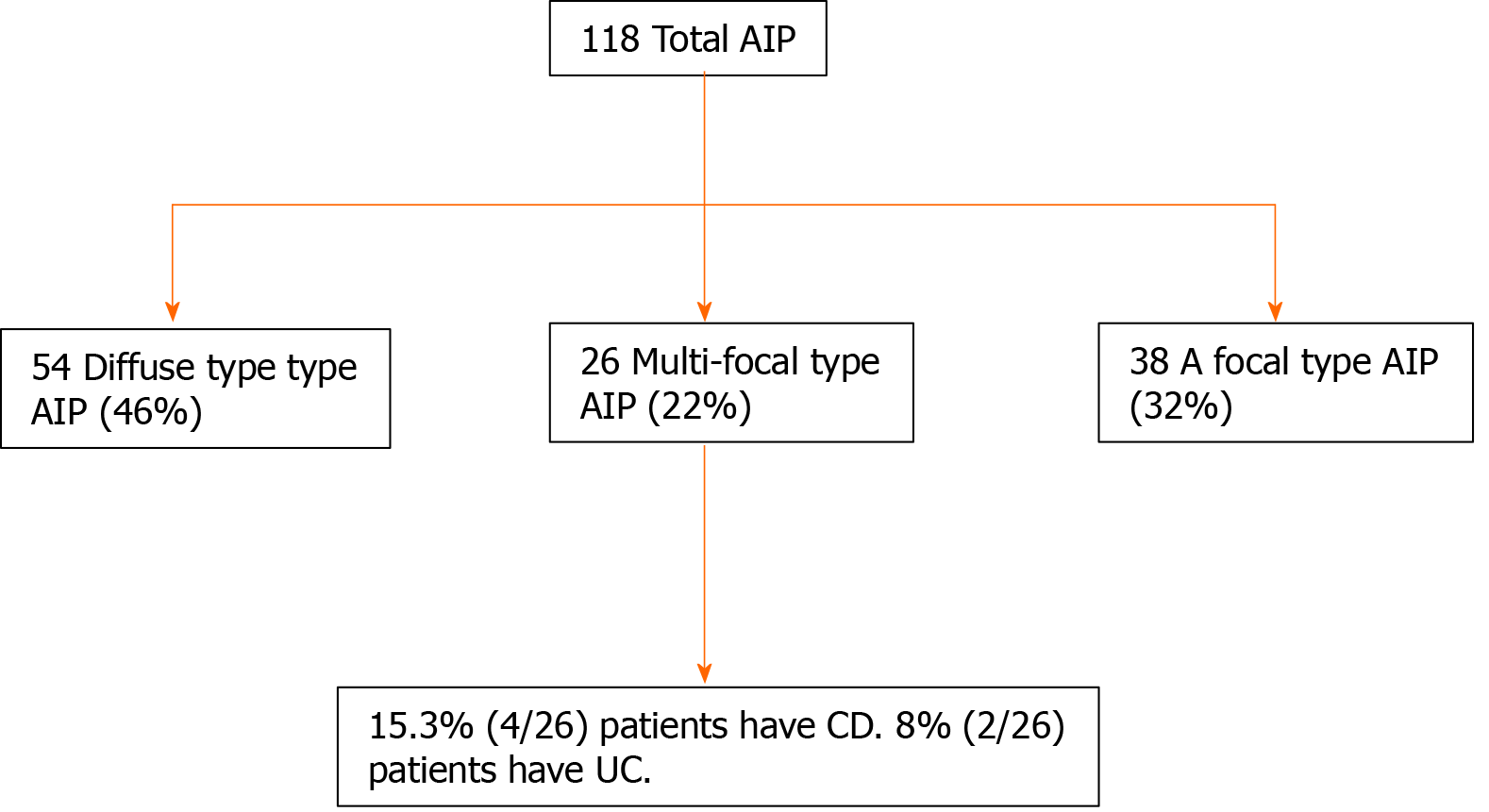
Among 118 patients with AIP, 26 (22.0%) had multiple lesions (56 lesions). Ulcerative colitis was associated with multifocal AIP in 7.7% (2/26) of patients, and Crohn’s disease was present in 15.3% (4/26) of patients. In multifocal AIP, multiple lesions, delayed homogeneous enhancement, multifocal strictures of the main pancreatic duct, capsule-like rim, lower apparent diffusion coefficient values, and elevated serum Ig4 level were observed significantly more frequently than pancreatic ductal adenocarcinoma, whereas the presence of capsule-like rim in multifocal-type AIP was lower in frequency than total AIP. Of these lesions of multifocal AIP, DWI detected 89.3% (50/56) and 82.1% (46/56) by the senior and junior radiologist, respectively.
Multifocal AIP is not as rare as previously thought and was seen in 22.0% of our patients. The diagnostic performance of DWI for detecting multifocal AIP was best followed by axial fat-suppressed T1WI and DCE-CT.
Core Tip: Multifocal autoimmune pancreatitis is not as rare as previously thought and was seen in 22.0% of our patients. The diagnostic performance of diffusionweighted imaging for detecting multifocal autoimmune pancreatitis was best followed by axial fat-suppressed T1 weighted image and dynamic contrast enhanced-computed tomography.
- Citation: Huang XM, Shi ZS, Ma CL. Multifocal autoimmune pancreatitis: A retrospective study in a single tertiary center of 26 patients with a 20-year literature review. World J Gastroenterol 2021; 27(27): 4429-4440
- URL: https://www.wjgnet.com/1007-9327/full/v27/i27/4429.htm
- DOI: https://dx.doi.org/10.3748/wjg.v27.i27.4429
Autoimmune pancreatitis (AIP) is a distinctive type of chronic pancreatitis generally characterized by an autoimmune inflammatory condition in which prominent lymphoplasmacytic infiltration with associated pancreatic fibrosis causes organ dysfunction[1]. Previous studies have shown that AIP is recognized as a heterogeneous disease classified into at least two subtypes, including lymphoplasmacytic sclerosing pancreatitis (type 1) and idiopathic duct-centric pancreatitis (type 2) with granulocytic epithelial lesions[2]. Meanwhile, AIP has also been classified radiologically as diffuse, unifocal, or multifocal[3-5]. Diffuse-type AIP is easily distinguished from pancreatic ductal adenocarcinoma (PDA) by typical imaging features, i.e. diffuse sausage-like swelling of pancreatic parenchyma with capsule-like peripancreatic rim[6,7]. Moreover, some studies[4,8] recently attempted to identify differential imaging findings between unifocal AIP and PDA by using computed tomography (CT), magnetic resonance imaging (MRI), or magnetic resonance cholangiopancreatography. Several key imaging landmarks, with reference to radiological morphology of the pancreatic mass lesion and stricture pattern of the main pancreatic duct (MPD), were clearly identified to be crucial to distinguish unifocal AIP from PDA. Hence, the differential diagnosis between unifocal AIP and PDA may not be as challenging as before.
However, multifocal-type AIP, sometimes forming multiple pancreatic masses, is frequently misdiagnosed as pancreatic malignancy in routine clinical practice[9,10]. It is critical to know the imaging features of multifocal-type AIP to prevent misdiagnosis and unnecessary surgery. Consequently, a clearer diagnostic consensus on the imaging characteristics of multifocal AIP is needed to differentiate from pancreatic malignancy. For this purpose, we herein report 26 cases of multifocal AIP and a review of the previous literature with an emphasis on CT and MRI imaging features that may help definitively distinguish between multifocal AIP and PDA.
CT is the most commonly performed, noninvasive modality to evaluate both pancreatic and extrapancreatic manifestations in patients with suspected AIP[4,11]. In our experience and according to the previous literature, CT has certain limitations in showing some specific findings of focal AIP, i.e., focal masses and focal MPD strictures, while these imaging findings seem to be more clearly demonstrated on MRI, especially on diffusionweighted imaging (DWI)[12]. In our previous study, we confirmed that axial T1 weighted image (T1WI) is the optimal sequence of identifying homogenously isoattenuating insulinoma on dynamic contrast enhanced-computed tomography (DCE-CT)[13]. Zhu et al[14] found that the appearance of multiple solid lesions can provide a diagnostic clue for identifying pancreatic nonmalignancies. Therefore, it is essential to find an optimal modality to identify pancreatic lesions as correctly as possible. To the best of our knowledge, there have been no studies evaluating the value of DWI, axial fat-suppressed T1WI, and DCE-CT in detecting the lesions of multifocal-type AIP.
We aimed to clarify the exact prevalence and radiological findings of multifocal AIP in our cohorts and compare the sensitivity of DWI, axial fat-suppressed T1WI, and DCE-CT for detecting AIP lesions. We also compared radiological features between multifocal AIP and PDA with several key imaging landmarks.
This retrospective study was approved by the Institutional Review Board of the First Affiliated Hospital of Fujian Medical University (Fuzhou, China), and written informed consent from patients was waived. The clinical, radiological, and pathological databases at our hospital between January 2005 and July 2020 were reviewed. A total of 234 consecutive patients who had undergone DCE-CT on multidetector computerized tomography and conventional MRI imaging protocols on a 3T scanner within 5 wk of each other for suspected AIP were included. Of these initially suspected cases, we finally identified 118 patients who were diagnosed with AIP according to international consensus diagnostic criteria[15].
Among these confirmed patients, 26 patients with 56 lesions were retrospectively included in our study, based on the following inclusion criteria: (1) the use of DCE-CT with the acquisition on the pancreatic arterial, pancreatic parenchyma, and portal venous phases; (2) conventional MRI sequences, including both axial fat-suppressed T1WI and DWI before steroid treatment; and (3) multiple focal-type AIP. In criterion (3), the multiple focal-type was defined by the presence of two or more focal lesions observed on DCE-CT or MRI, and lesions of AIP were separate (i.e. continuous lesion that involves at least one segment of the pancreas was previously considered to be a single lesion[14] and were thus excluded from this study).
From January 2001 to July 2020, 908 patients underwent pancreatic curative-intent surgery for PDA at our institution. To enable a 1:2 matching with the multifocal AIP group, we randomly chose 112 [78 men and 34 women; mean age ± SD, 61 ± 8.7 years (range: 38-86 years)] among the 613 patients with PDA who also underwent both DCE-CT and conventional MRI before surgery, using Statistic Package for Social Science software to generate the random numbers.
CT protocols: DCE-CT examinations were performed using 16-, 80-, or 320-detector CT scanner (Aquilion; Toshiba Medical Systems, Tokyo, Japan), using the following pancreatic scanning parameters: (1) detector collimation, 320.0 mm × 0.5 mm, 80.0 mm × 0.5 mm or 16.0 mm × 0.5 mm; (2) pitch, 1.0-1.4; (3) gantry rotation time, 0.35-0.50 s; (4) tube voltage, 120 kV; and (5) pancreatic reference tube current, 80-120 mA. All images were routinely reconstructed from the contrast-enhanced CT scans with thin-section images (slice thickness = 0.75-1.00 mm, reconstruction increment = 0.5 mm). Unenhanced and DCE-CT including the arterial, pancreatic parenchymal, and portal venous phases were acquired before and after intravenous administration of contrast agent. After unenhanced CT scanning, 300 mg/mL iodine (Ultravist 300; Schering; Bayer Healthcare Pharmaceuticals, Leverkusen, Germany) was injected intravenously using a power injector via an 18-gauge plastic antecubital catheter. An iodine dose of 1.5 mL/kg body weight was given at a flow rate of 4 mL/s. The administration of contrast agent was immediately followed by a flush of 30 mL saline. To accurately determine the timing for the standard triple-phase pancreatic imaging, a scanning delay was performed based on an automated bolus-tracking technique offered by the supplier of the Toshiba CT system. Three phases enhanced scanning were obtained at a fixed delay of 20- to 25-s, 40- to 50- s, and 80- to 90-s after the contrast agent injection.
MR protocols: The MRI examinations were conducted using a 3.0 T imaging system (Magnetom Trio; Siemens Healthineers, Erlangen, Germany) with an 8-channel phased-array coil. The routine MR sequences for imaging the pancreas, including axial fat-suppressed T1-weighted turbo spin echo sequence, coronal T2-weighted (half-Fourier acquisition single-shot turbo spin echo) sequence, axial fat-suppressed T2-weighted turbo spin echo sequence, axial T1-weighted in-phase and out-of-phase sequences with fixed echo times of 1.4 and 2.8 ms, respectively, and DWI were performed at our institution. Axial single-shot echo-planar DWI sequence was completed using the following scanning parameters: repetition time, 6900 ms; echo time, 73 ms; intersection gap, 6 mm; slice thickness, 3 mm; field of view, 350 mm × 350 mm; matrix size, 128 mm × 128 mm; flip angle, 90°; partial Fourier factor, 6/8; parallel imaging reduction factor, 2; bandwidth, 2441 Hz per pixel; and the number of excitations, 8 (water excitation technique with b-value of 50 s/mm2 and 800 s/mm2). Axial fat-suppressed T1WI was performed with the following parameters: echo time, 1.4 ms; repetition time, 3.9 ms; flip angle 9°; slice thickness, 3 mm; matrix, 320 mm × 182 mm; field of view, 250 mm × 280 mm; and the number of excitations, 1. DWI and axial fat-suppressed T1WI sequences were performed with breath hold and free breathing, respectively. The main scan parameters of the MR sequences were presented in our earlier paper[13]. Apparent diffusion coefficient values (ADC) maps were automatically generated from DW images, and ADC values were calculated on a Syngo workstation (Syngo Multimodality Workplace; Siemens Healthineers). One radiologist, who had precise knowledge of the lesion’s location based on the overall findings from the radiological images and pathological and surgical datasets, retrospectively manually drew and placed the largest possible regions of interest within the pancreatic lesion and surrounding parenchyma. ADC values of pancreatic lesions were measured and compared with the surrounding parenchyma.
Image analysis: The detection of each multifocal AIP lesion was divided into three image sets: axial fat-suppressed T1WI, DWI, and DCE-CT. Each image set was independently analyzed on a picture archiving and communication workstation (Shida picture archiving and communication system, Fuzhou, China) with the free adjustment of window width and level by two abdominal radiologists (21 and 7 years of experience in interpreting abdominal CT/MRI). The two radiologists knew that this study was being conducted to detect the lesions of multifocal AIP but were unaware of any clinical information, biological data, other conventional imaging findings, histopathological, and surgical results. The radiologists identified the absence or presence of the lesion with a 5point diagnostic confidence scales as follows: Score 1, definitely absent; Score 2, probably absent; score 3, indeterminate; Score 4, probably present; and Score 5, definitely present. In cases with diagnostic confidence score of 4 or 5, a lesion was present, and the location of the lesion was recorded on a report sheet. The signal intensity of the lesion was assessed on axial fat-suppressed T1WI and DWI image sets compared with the surrounding parenchyma and was categorized as low, intermediate, or high.
One month after the completion of the detection rating for all patients of multifocal AIP, two radiologists subsequently analyzed the DCE-CT and MRI images of all 168 patients (56 AIP and 112 PDA) in a random sequence and determined, in consensus, the absence or presence of the following characteristic imaging features as previous reports[4,16-18] for differentiating between AIP and PDA: multiple pancreatic masses; ill-defined margin; delayed homogeneous enhancement; capsule-like rim; marked upstream MPD dilation; upstream pancreatic atrophy; multiple MPD strictures; and extrapancreatic manifestations, including vascular invasion, peripancreatic fat infiltration, and peripancreatic and retroperitoneal lymphadenopathy.
Laboratory data: The serum immunoglobulin G fraction 4 (IgG4) concentrations, if available, were carefully recorded.
Statistical analysis was performed using Statistic Package for Social Science (version 19.0; IBM Corp., Armonk, NY, United States) software. Quantitative variables were summarized as the mean ± SD. Descriptive variables were determined using percentages and medians. The Wilcoxon signed rank test was performed to analyze the mean ADC values in multifocal AIP lesions and the surrounding parenchyma and to compare the ADC values in multifocal AIP and PDA. Prior to determining the agreement between the two radiologists with respect to confidence ratings for a detection task of multifocal AIP lesions, κ-values were used to assess interobserver agreement interpreted as follows: 0.81-1.00, excellent agreement; 0.61-0.80, good agreement; 0.41-0.60, moderate agreement; and 0.21-0.40, fair agreement[19,20]. Friedman’s nonparametric test was performed to evaluate the differences of diagnostic confidence level among axial fat-suppressed T1WI, DWI, and DCE-CT image sets for each radiologist. The frequency of the characteristic imaging findings for differentiating multifocal AIP from PDA was compared via the chi-squared or McNemar’s test, as appropriate. A two-tailed P value of < 0.05 was deemed to be a statistically significant difference.
Clinical findings: From 26 patients, 18 were female, and 8 were male. The median age was 60.7 ± 17.0 years (range: 26-89 years). Among 118 patients with AIP, 54 (45.8%) showed diffuse-type AIP, 38 (32.2%) showed unifocal-type AIP, and twenty-six cases (22.0%) showed multifocal-type AIP. Of the 26 patients with multifocal-type AIP, 4 (15.3%) were associated with Crohn’s disease, and 2 (7.7%) were associated with ulcerative colitis (Figure 1). A total 56 lesions were found in these 26 patients with multifocal AIP. Twenty-two patients had two lesions, and four patients had three lesions. The lesions of 16 patients were in the pancreatic head/uncinate process and tail. The lesions of 6 patients were in the pancreatic head and body; 4 patients with 3 lesions each were in the pancreatic head, body, and tail. Pancreatic tissue samples were obtained in 18 patients (69.2%) including three from surgical resection, five from endoscopic ultrasound-guided fine-needle aspiration biopsy, and ten from CT guided percutaneous biopsy. All patients were affected by type 1 AIP.
The serum concentrations of IgG4 (0.03-2.01 g/L) were elevated above the normal limit in 94.9% (112/118) of the patients with AIP, 96.2% (25/26) of the patients with multifocal-type AIP, 96.3% (52/54) of the patients with diffuse-type AIP, and 92.1% (35/38) of the patients with unifocal-type AIP. The mean serum concentration of IgG4 of the patients with multifocal-type AIP was 14.77 ± 8.05 g/L. IgG4 levels were not elevated in 53 patients with PDA, and the remaining 59 patients with PDA had no measurement of serum IgG4 before surgery.
Treatment and relapse of patients with multifocal-type AIP: Initial treatment of all patients with multifocal AIP consisted of steroid therapy in 23 patients (88.5%) or surgical resection in three patients (11.5%). After the diagnosis was rendered, 22 patients received treatment with high-dose steroid. They received an intravenous injection of daily methylprednisolone 40-80 mg for 3 to 7 d, after which 10-20 mg of oral prednisone was given per day. During the median 8.6 mo (interquartile range: 5.1 to 23.0 mo) of follow-up, all patients who underwent steroid treatment with/without surgical resection showed complete radiological and clinical remission. The remaining 4 patients refused steroid treatment and were lost to follow-up.
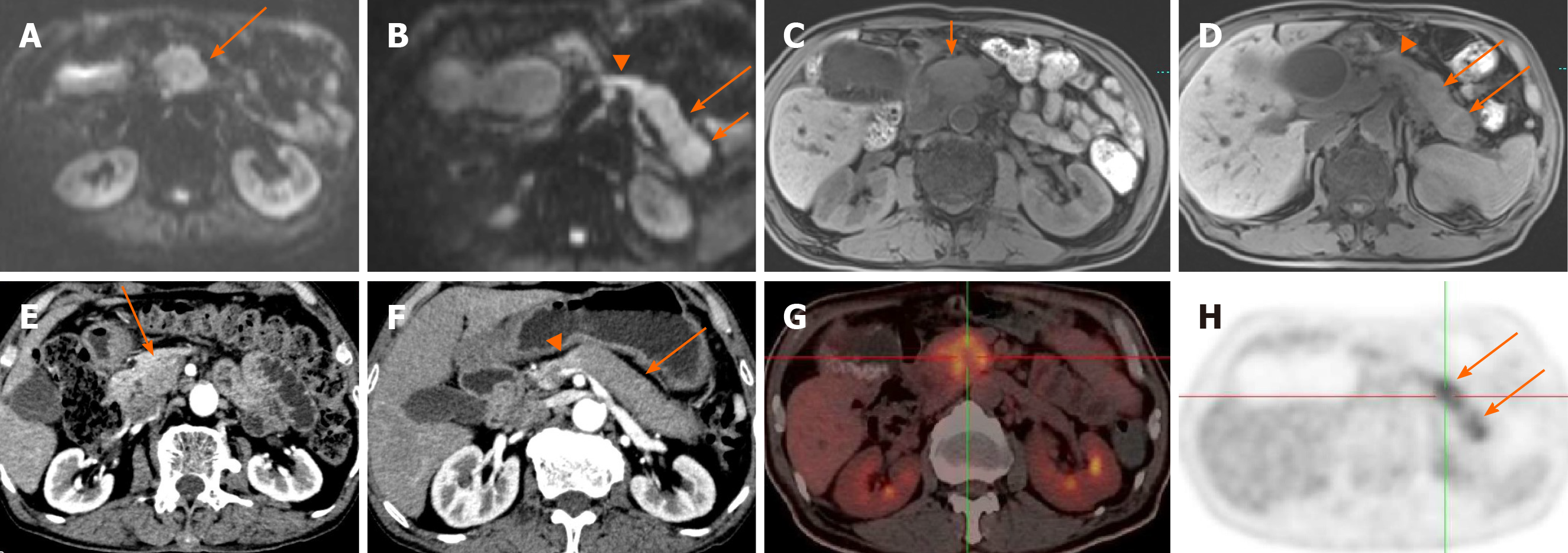
The senior and junior radiologist detected 50/56 and 46/56 lesions on DWI, 34/56 and 38/56 on axial fat-suppressed T1WI, and 22/56 and 24/56 on DCE-CT, respectively. DWI MRI had the highest relative sensitivity [4650/56 (82.1%89.3%) lesions] in the detection of lesions in multifocal AIP (Figures 2 and 3).
Statistically significant differences of the confidence level score were observed among these three image sets for the two radiologists. Diagnostic confidence level score of DWI alone was the highest, while that of DCE-CT was the lowest for each radiologist (Table 1). Weighted κvalues that rated the confidence levels of the readers in terms of image interpretation are summarized in Table 2. Good interobserver agreement was observed between the two radiologists for determining the presence or absence of lesions on DWI datasets (weighted κ = 0.878). Moderate interobserver agreement was observed between two readers on axial fat-suppressed T1WI and DCE-CT image datasets (weighted κ = 0.742 and weighted κ = 0.683, respectively).
| Groups | Junior radiologist | Senior radiologist |
| DWI | 2.32 | 2.32 |
| Axial-suppressed T1WI | 1.95 | 2.07 |
| Dynamic contrast enhanced CT | 1.73 | 1.61 |
| P value | 0.033 | 0.004 |
| Groups | κ-value |
| DWI | 0.878 |
| Axial fat-suppressed T1WI | 0.742 |
| Dynamic contrast enhanced CT | 0.683 |
The mean ADC values (× 10-3 mm2/s) of multifocal AIP lesions and the surrounding pancreatic parenchyma were 0.96 ± 0.14 (range: 0.681.26) and 1.15 ± 0.15 (range: 0.891.40), respectively. ADC values of the AIP lesions were significantly lower compared with those of the surrounding pancreatic parenchyma according to the Wilcoxon signedrank test (P = 0.001; Table 3).
| AIP lesions ADC | Surrounding parenchyma ADC | Wilcoxon signed-rank test | ||
| Values (n = 56) (× 10-3 mm2/s) | Values (n = 56) (× 10-3 mm2/s) | Z value | P value | |
| Mean | 0.96 | 1.15 | -5.11 | 0.001 |
| SD | 0.14 | 0.15 | ||
| Median | 0.96 | 1.16 | ||
| Minimum | 0.68 | 0.89 | ||
| Maximum | 1.26 | 1.40 | ||
Fifty-six patients with multifocal-type AIP and 112 patients with PDA were compared. The differences in the imaging features between multifocal-type AIP and PDA are summarized in Table 4. Multifocality (100% vs 1.7%, P < 0.01), capsule-like rim (21.4% vs 3.6%, P < 0.01), multiple MPD strictures (47.3% vs 1.8%, P < 0.01), and delayed homogeneous enhancement (92.8% vs 13.4%, P < 0.01) were significantly more frequent in multifocal AIP. Peripancreatic fat infiltration (46.4% vs 91.0%, P < 0.01), vascular invasion (14.3% vs 71.4%, P < 0.01), upstream pancreatic atrophy (19.6% vs 58.9%, P < 0.01), peripancreatic and retroperitoneal lymphadenopathy (3.6% vs 61.6%, P < 0.01), and marked upstream MPD dilatation (3.6% vs 52.7%, P < 0.01) were significantly more frequent in PDA than in multifocal AIP. ADC values of the multifocal AIP lesions were significantly lower than that of PDA. However, the indistinct margin between multifocal AIP and PDA was not statistically different. Furthermore, the capsule-like rim was present with a lower frequency (21.4%) in multifocal AIP in our study. Additionally, three multifocal AIP patients underwent 18F-fluorodeoxyglucose (FDG) positron emission tomography/CT, and increased FDG uptake was noted in seven lesions in the whole pancreas (Figures 2 and 3).
| Multifocal AIP | PDA | P value | |
| No. of radiological findings of the pancreas | 56 | 112 | |
| Multifocal lesion | 56 (100) | 2 (1.7) | < 0.01 |
| Capsule-like rim | 12 (21.4) | 4 (3.6) | < 0.01 |
| Delayed homogeneous enhancement on MDCT | 52 (92.8) | 15 (13.4) | < 0.01 |
| Margin (indistinct) | 48 (85.7) | 89 (79.5) | 0.325 |
| Peripancreatic fat infiltration | 26 (46.4) | 102 (91.0) | < 0.01 |
| Vascular invasion | 8 (14.3) | 80 (71.4) | < 0.01 |
| Multiple MPD strictures | 53 (47.3) | 2 (1.8) | < 0.01 |
| Marked upstream MPD dilatation | 2 (3.6) | 59 (52.7) | < 0.01 |
| Upstream pancreatic atrophy | 11 (19.6) | 66 (58.9) | < 0.01 |
| Lymphadenopathy | 2 (3.6) | 69 (61.6) | < 0.01 |
| ADC values (× 10-3 mm2/s) | 0.96 ± 0.16 | 1.15 ± 0.14 | < 0.01 |
AIP is an uncommon form of chronic pancreatitis that typically affects men in their fifth to sixth decades and usually presents with pancreatic enlargement and obstructive jaundice, with a reported incidence rate of up to 6% in chronic pancreatitis patients[1,21,22]. The most common radiological manifestation of AIP is a grossly enlarged sausage-shaped hypoattenuating pancreas with homogeneous decreased enhancement at the arterial phase and delayed rim-like enhancement at the portal venous phase. The unifocal form of AIP at the pancreatic head is by far the second-most common type[6]. As in our cases, the most uncommon type is the multifocal form that has only been described in a few reports[3,10,23]. All patients with multifocal AIP in our study were type 1 and responded well to steroid therapy, consistent with previous study results.
In this cohort of 118 patients of AIP, 22.0% showed multifocal-type AIP, suggesting it is not as uncommon as previously thought in actual clinical practice. One possible reason for overlooking is the radiologists have been extremely unfamiliar with its characteristic imaging features. Another possible reason may be attributed to some CT and MR imaging findings of unifocal-type or diffuse-type AIP may overlap with multifocal-type AIP. It has also been hypothesized that different diagnostic approaches in China may greatly contribute to the relatively lower incidence rate of multifocal AIP.
Current diagnostic methods available for the differentiation of pancreatic lesions are various, among which contrast-enhanced ultrasonography, endoscopic ultrasonography (EUS), contrast enhanced-CT, contrast enhanced-MRI, contrast-enhanced elastography, EUS-fine-needle aspiration, and contrast enhanced-EUS have been widely used in recent years. As one of the current gold standards for diagnosis of pancreatic neoplasms, EUS- fine-needle aspiration with 100% specificity can correctly access the pathological diagnosis directly. In addition, an accurate tissue diagnosis based on a EUS- fine-needle aspiration biopsy specimen may render surgery for those benign neoplasms or indolent cancer unnecessary. However, the best method for pancreatic mass diagnosis remains unclear[24]. In our country, DCE-CT, diagnostic endoscopic retrograde pancreatography, endoscopic biopsy, and the following therapeutic trial of steroids have been more actively conducted in the management of patients with suspected AIP than before[9,14,25]. Multifocal AIP can be mistakenly classified as diffuse or unifocal AIP when only using CT, as our study results showed that DWI and axial fat-suppressed T1WI have higher sensitivity than DCE-CT to find AIP lesions.
A literature search with the medical subject headings term ‘autoimmune pancreatitis’ in PubMed, Wanfang Database, and the China National Knowledge Infrastructure was performed by authors (Zhen-Shan Shi) on August 8, 2020. The reference lists of relevant studies were searched to identify other additional eligible articles. A PubMed search of the past 20 years revealed 783 articles describing focal-type AIP in the English and Chinese literature. However, most reported studies of focal AIP have focused on the imaging features of unifocal and not multifocal; only 18 articles involved the imaging features of multifocal-type AIP. Most of these studies were small case series or single case reports. A total of 132 cases of multifocal AIP, including our current cases, were retrospectively analyzed and summarized in the present study. We found that multifocal AIP accounted for 22% of total AIP, and most patients presented above the age of 60 years with more females than males[1,21]. The gender difference of multifocal AIP was not consistent with that of total AIP with more males than females.
In our study, 23.3% of multifocal AIP patients had associated IBDs (15.3% had Crohn’s disease, and 7.7% had ulcerative colitis) and were type 1 AIP. Most cases with coexisting AIP and IBD were type 2 AIP in the previous studies[2,26]. The possible reason is type 1 AIP is significantly more frequent in East Asia, and type 2 AIP generally prevails in Western countries[26]. Our data suggest that elevated serum IgG4 concentrations are also helpful in identifying multifocal AIP, like in previous studies[4,27].
This study confirmed the high sensitivity (82%89%) of DWI for the detection of AIP lesions[7,28], which also provided an important contribution to the highest radiologist diagnostic confidence score and the highest level of interobserver agreement (weighted κ = 0.878) between the two radiologists and also demonstrated that DWI was the optimal image sequence among the three image sets (DWI, axial fat-suppressed T1WI, and DCE-CT). We speculate that the lowest confidence score and interobserver agreement of DCE-CT between the two radiologists with different experience may be due to the inferior resolution in showing the slight difference of CT value between AIP lesions and the surrounding pancreas, which may make images more difficult to interpret than with DWI and axial fat-suppressed T1WI. Conversely, MRI has the excellent soft tissue contrast resolution, and DWI has outstanding imaging capability to detect pancreatic focal lesions, even minor lesions[29,30].
A significant difference in ADC value was also found between multifocal AIP lesions and the surrounding pancreas, similar to the results of unifocal-type AIP[4]. We hypothesize that DWI, which can be conducted without contrast media and in a relatively short time, will become a crucial imaging technique in the detection of AIP lesions.
Our present study showed that seven key distinguishing features of PDA and multifocal AIP were as follows among various CT and MR findings, particularly for multifocal AIP: (1) multiple pancreatic lesions; (2) hypoattenuating capsule-like rim; (3) delayed homogeneous good enhancement; (4) absence of distal pancreatic atrophy; (5) mild upstream MPD dilatation; (6) multiple MPD strictures; and (7) a lower ADC value are more frequently observed in multifocal AIP like other prior reports of unifocal-type AIP, which could be deemed reliable findings to prevent unnecessary invasive procedures and surgical treatment in the management of the suspected patients.
On the contrary, peripancreatic fat infiltration, vascular invasion, and peripancreatic and retroperitoneal lymphadenopathy are more often observed in PDA. Additionally, even with 18F-FDG-positron emission tomography/CT examination, when the pancreatic lesion is accurately localized, it can be often confused with PDA because it has been recently reported to show increased FDG uptake at the locally affected lesions of AIP[31-33], which is also an observation from our study. We consider that the use of these seven key differentiating features can be helpful in supporting the diagnosis of AIP when the clinical, laboratory, and other imaging features are equivocal for distinguishing between focal-type AIP and PDA.
We found that there was no statistically significant difference between multifocal AIP and PDA with respect to ill-defined margin, as in previous studies[4,16]. The capsule-like rim or halo sign has already been reported as a specific feature of AIP, with a varying frequency up to 63%[5,14,34]. This sign was observed with lower frequency (21%) in multifocal AIP in our study. We presume that multifocal-type AIP is the early manifestation of AIP, and the multiple lesions will be merged into diffuse-type along with the progression of this entity. Then, typical or uncommon imaging features will be typical, and the halo sign may be more frequently seen. However, further work is needed to confirm this hypothesis.
Our study has a few limitations. First, this study was performed by a retrospective research design, and the number of cases is relatively small. However, AIP itself is a rare entity, and multifocal AIP is even rarer. Second, the incidence rate of multifocal AIP in this study was at a single tertiary center with a specialized care clinic that has been widely recognized for its expertise and experience with pancreatic diseases in our country and may confer a considerable potential referral bias with an overestimated frequency of multifocal AIP. Third, the blinded radiologists had initially determined patient diagnosis in daily clinical practice; recall bias might have occurred. Last, we used a variety of CT scanners with different detectors (e.g., 320-, 80- and 16- detectors) from the same manufacturer with scan parameters that slightly varied from each other, which may affect the diagnostic performance among these modalities.
In conclusion, multifocal AIP is not as rare as previously thought. DWI as part of pancreatic conventional MRI protocol has the potential benefit in the detection of AIP lesions. The combinations of CT and MRI imaging features may be helpful for differentiating multifocal AIP from PDA by the key distinguishing findings, including multiple pancreatic lesions, capsule-like rim, delayed homogeneous enhancement, multiple MPD strictures, and lower ADC values.
Multifocal-type autoimmune pancreatitis (AIP), sometimes forming multiple pancreatic masses, is frequently misdiagnosed as pancreatic malignancy in routine clinical practice. It is critical to know the imaging features of multifocal-type AIP to prevent misdiagnosis and unnecessary surgery. To the best of our knowledge, there have been no studies evaluating the value of diffusionweighted imaging (DWI), axial fat-suppressed T1 weighted image (T1WI), and dynamic contrast enhanced-computed tomography (DCE-CT) in detecting the lesions of multifocal-type AIP.
The key issue is whether CT and magnetic resonance imaging features of multifocal-type AIP can help definitively distinguish from pancreatic ductal adenocarcinoma (PDA) and whether there is an optimal modality to identify pancreatic lesions as correctly as possible. The results will provide important information on the diagnostic performances of DWI, axial fat-suppressed T1WI, and DCE-CT and the key imaging features for differentiating multifocal-type AIP from PDA.
We aimed to clarify the exact prevalence and radiological findings of multifocal AIP in our cohorts and compare the sensitivity of DWI, axial fat-suppressed T1WI, and DCE-CT for detecting AIP lesions. We also compared radiological features between multifocal AIP and PDA with several key imaging landmarks.
Twenty-six patients with proven multifocal AIP were retrospectively included. Two blinded independent radiologists rated their confidence level in detecting the lesions on a 5-point scale and assessed the diagnostic performance of DWI, axial fat-suppressed T1WI, and DCE-CT. CT and magnetic resonance images of multifocal AIP were systematically reviewed for typical imaging findings and compared with the key imaging features of PDA.
Among 118 patients with AIP, 26 (22.0%) had multiple lesions (56 lesions). Ulcerative colitis was associated with multifocal AIP in 7.7% (2/26) of patients, and Crohn’s disease was associated in 15.3% (4/26) of patients. In multifocal AIP, multiple lesions, delayed homogeneous enhancement, multifocal strictures of main pancreatic duct, capsule-like rim, lower apparent diffusion coefficient values, and elevated serum Ig4 Level were observed significantly more frequently than in PDA, whereas the presence of capsule-like rim in multifocal-type AIP was lower in frequency than total AIP. Of these lesions of multifocal AIP, DWI detected 89.3% (50/56) and 82.1% (46/56) by the senior and junior radiologist, respectively.
Multifocal AIP is not as rare as previously thought and was seen in 22% of our patients. The diagnostic performance of DWI for detecting multifocal AIP was best followed by axial fat-suppressed T1WI and DCE-CT.
Larger and longer term prospective studies to investigate the important radiological findings for differential diagnosis between multifocal AIP and PDA should be performed in future studies.
Manuscript source: Unsolicited manuscript
Specialty type: Radiology, nuclear medicine and medical imaging
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Cengiz F, Triantafillidis J S-Editor: Zhang L L-Editor: Filipodia P-Editor: Wang LL
| 1. | Nagpal SJS, Sharma A, Chari ST. Autoimmune Pancreatitis. Am J Gastroenterol. 2018;113:1301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 70] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 2. | Negrelli R, Boninsegna E, Avesani G, Zamboni GA, Brozzi L, Frulloni L, Manfredi R, Pozzi Mucelli R. Type 1 and Type 2 Autoimmune Pancreatitis: Distinctive Clinical and Pathological Features, But Are There Any Differences at Magnetic Resonance? Pancreas. 2018;47:1115-1122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 14] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 3. | Shiokawa M, Kodama Y, Hiramatsu Y, Kurita A, Sawai Y, Uza N, Watanabe T, Chiba T. Autoimmune pancreatitis exhibiting multiple mass lesions. Case Rep Gastroenterol. 2011;5:528-533. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 4. | Lee S, Kim JH, Kim SY, Byun JH, Kim HJ, Kim MH, Lee MG, Lee SS. Comparison of diagnostic performance between CT and MRI in differentiating non-diffuse-type autoimmune pancreatitis from pancreatic ductal adenocarcinoma. Eur Radiol. 2018;28:5267-5274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 36] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 5. | Rehnitz C, Klauss M, Singer R, Ehehalt R, Werner J, Büchler MW, Kauczor HU, Grenacher L. Morphologic patterns of autoimmune pancreatitis in CT and MRI. Pancreatology. 2011;11:240-251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 35] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 6. | Tabata T, Kamisawa T, Takuma K, Hara S, Kuruma S, Inaba Y. Differences between diffuse and focal autoimmune pancreatitis. World J Gastroenterol. 2012;18:2099-2104. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 11] [Cited by in RCA: 15] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 7. | Kamisawa T, Takuma K, Anjiki H, Egawa N, Hata T, Kurata M, Honda G, Tsuruta K, Suzuki M, Kamata N, Sasaki T. Differentiation of autoimmune pancreatitis from pancreatic cancer by diffusion-weighted MRI. Am J Gastroenterol. 2010;105:1870-1875. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 127] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 8. | Kim M, Jang KM, Kim JH, Jeong WK, Kim SH, Kang TW, Kim YK, Cha DI, Kim K. Differentiation of mass-forming focal pancreatitis from pancreatic ductal adenocarcinoma: value of characterizing dynamic enhancement patterns on contrast-enhanced MR images by adding signal intensity color mapping. Eur Radiol. 2017;27:1722-1732. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 14] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 9. | Deng WL, Li J, Zhu L, He M, Xue HD, Jin ZY. [Multifocal IgG4-related Autoimmune Pancreatitis:Report of One Case]. Zhongguo Yi Xue Ke Xue Yuan Xue Bao. 2019;41:575-578. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 10. | Suzumura K, Hatano E, Uyama N, Okada T, Asano Y, Hai S, Nakasho K, Fujimoto J. Multifocal Mass Lesions in Autoimmune Pancreatitis. Case Rep Gastroenterol. 2017;11:678-685. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 11. | Furuhashi N, Suzuki K, Sakurai Y, Ikeda M, Kawai Y, Naganawa S. Differentiation of focal-type autoimmune pancreatitis from pancreatic carcinoma: assessment by multiphase contrast-enhanced CT. Eur Radiol. 2015;25:1366-1374. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 49] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 12. | Oki H, Hayashida Y, Oki H, Kakeda S, Aoki T, Taguchi M, Harada M, Korogi Y. DWI findings of autoimmune pancreatitis: comparison between symptomatic and asymptomatic patients. J Magn Reson Imaging. 2015;41:125-131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 13. | Shi Z, Li X, You R, Li Y, Zheng X, Ramen K, Loosa VS, Cao D, Chen Q. Homogenously isoattenuating insulinoma on biphasic contrast-enhanced computed tomography: Little benefits of diffusion-weighted imaging for lesion detection. Oncol Lett. 2018;16:3117-3125. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 14. | Zhu L, Dai MH, Wang ST, Jin ZY, Wang Q, Denecke T, Hamm B, Xue HD. Multiple solid pancreatic lesions: Prevalence and features of non-malignancies on dynamic enhanced CT. Eur J Radiol. 2018;105:8-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 11] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 15. | Shimosegawa T, Chari ST, Frulloni L, Kamisawa T, Kawa S, Mino-Kenudson M, Kim MH, Klöppel G, Lerch MM, Löhr M, Notohara K, Okazaki K, Schneider A, Zhang L; International Association of Pancreatology. International consensus diagnostic criteria for autoimmune pancreatitis: guidelines of the International Association of Pancreatology. Pancreas. 2011;40:352-358. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1050] [Cited by in RCA: 1056] [Article Influence: 75.4] [Reference Citation Analysis (0)] |
| 16. | Choi SY, Kim SH, Kang TW, Song KD, Park HJ, Choi YH. Differentiating Mass-Forming Autoimmune Pancreatitis From Pancreatic Ductal Adenocarcinoma on the Basis of Contrast-Enhanced MRI and DWI Findings. AJR Am J Roentgenol. 2016;206:291-300. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 67] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 17. | Sun GF, Zuo CJ, Shao CW, Wang JH, Zhang J. Focal autoimmune pancreatitis: radiological characteristics help to distinguish from pancreatic cancer. World J Gastroenterol. 2013;19:3634-3641. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 56] [Cited by in RCA: 56] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 18. | Muhi A, Ichikawa T, Motosugi U, Sou H, Sano K, Tsukamoto T, Fatima Z, Araki T. Mass-forming autoimmune pancreatitis and pancreatic carcinoma: differential diagnosis on the basis of computed tomography and magnetic resonance cholangiopancreatography, and diffusion-weighted imaging findings. J Magn Reson Imaging. 2012;35:827-836. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 94] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 19. | Zhu L, Xue HD, Sun H, Wang X, He YL, Jin ZY, Zhao YP. Isoattenuating insulinomas at biphasic contrast-enhanced CT: frequency, clinicopathologic features and perfusion characteristics. Eur Radiol. 2016;26:3697-3705. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 14] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 20. | Lee EK, Yun TJ, Kim JH, Lee KE, Kim SJ, Won JK, Kang KM, Choi SH, Sohn CH. Effect of tumor volume on the enhancement pattern of parathyroid adenoma on parathyroid four-dimensional CT. Neuroradiology. 2016;58:495-501. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 21. | Hart PA, Krishna SG, Okazaki K. Diagnosis and Management of Autoimmune Pancreatitis. Curr Treat Options Gastroenterol. 2017;15:538-547. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 22. | Wakabayashi T, Kawaura Y, Satomura Y, Fujii T, Motoo Y, Okai T, Sawabu N. Clinical study of chronic pancreatitis with focal irregular narrowing of the main pancreatic duct and mass formation: comparison with chronic pancreatitis showing diffuse irregular narrowing of the main pancreatic duct. Pancreas. 2002;25:283-289. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 54] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 23. | Chiang AL, Hornick JL, Sahni VA, Clancy TE, Ryou M. Autoimmune Pancreatitis Presenting as Multifocal Masses, Diagnosed on Ampullary Biopsy. Pancreas. 2016;45:e25-e27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 24. | Mei S, Wang M, Sun L. Contrast-Enhanced EUS for Differential Diagnosis of Pancreatic Masses: A Meta-Analysis. Gastroenterol Res Pract. 2019;2019:1670183. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 22] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 25. | Jung JG, Lee JK, Lee KH, Lee KT, Woo YS, Paik WH, Park DH, Lee SS, Seo DW, Lee SK, Kim MH. Comparison of endoscopic retrograde cholangiopancreatography with papillary biopsy and endoscopic ultrasound-guided pancreatic biopsy in the diagnosis of autoimmune pancreatitis. Pancreatology. 2015;15:259-264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 14] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 26. | Oh D, Song TJ, Moon SH, Kim JH, Lee NJ, Hong SM, Lee JS, Jo SJ, Cho DH, Park DH, Lee SS, Seo DW, Lee SK, Kim MH. Type 2 Autoimmune Pancreatitis (Idiopathic Duct-Centric Pancreatitis) Highlighting Patients Presenting as Clinical Acute Pancreatitis: A Single-Center Experience. Gut Liver. 2019;13:461-470. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 20] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 27. | Okazaki K, Uchida K. Recent advances in IgG4-related disease, autoimmune pancreatitis and sclerosing cholangitis. Nihon Rinsho. 2017;75:450-454. [PubMed] |
| 28. | Momtahen AJ, Balci NC, Alkaade S, Akduman EI, Burton FR. Focal pancreatitis mimicking pancreatic mass: magnetic resonance imaging (MRI)/magnetic resonance cholangiopancreatography (MRCP) findings including diffusion-weighted MRI. Acta Radiol. 2008;49:490-497. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 31] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 29. | Islim F, Salik AE, Bayramoglu S, Guven K, Alis H, Turhan AN. Non-invasive detection of infection in acute pancreatic and acute necrotic collections with diffusion-weighted magnetic resonance imaging: preliminary findings. Abdom Imaging. 2014;39:472-481. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 40] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 30. | Park MJ, Kim YK, Choi SY, Rhim H, Lee WJ, Choi D. Preoperative detection of small pancreatic carcinoma: value of adding diffusion-weighted imaging to conventional MR imaging for improving confidence level. Radiology. 2014;273:433-443. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 56] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 31. | Cheng MF, Guo YL, Yen RF, Chen YC, Ko CL, Tien YW, Liao WC, Liu CJ, Wu YW, Wang HP. Clinical Utility of FDG PET/CT in Patients with Autoimmune Pancreatitis: a Case-Control Study. Sci Rep. 2018;8:3651. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 30] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 32. | Nakamoto Y, Saga T, Ishimori T, Higashi T, Mamede M, Okazaki K, Imamura M, Sakahara H, Konishi J. FDG-PET of autoimmune-related pancreatitis: preliminary results. Eur J Nucl Med. 2000;27:1835-1838. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 64] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 33. | Lauwyck J, Piette Y, Van Walleghem L, De Geeter F. IgG4-related disease: The utility of (18)F-FDG PET/CT in diagnosis and treatment. Hell J Nucl Med. 2015;18 Suppl 1:155-159. [PubMed] |
| 34. | Hafezi-Nejad N, Singh VK, Fung C, Takahashi N, Zaheer A. MR Imaging of Autoimmune Pancreatitis. Magn Reson Imaging Clin N Am. 2018;26:463-478. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 2.0] [Reference Citation Analysis (0)] |