Published online Jul 21, 2021. doi: 10.3748/wjg.v27.i27.4322
Peer-review started: February 9, 2021
First decision: May 13, 2021
Revised: May 21, 2021
Accepted: June 23, 2021
Article in press: June 23, 2021
Published online: July 21, 2021
Pancreatic cancer is a challenging malignancy with limited treatment options and poor life expectancy. The only curative option is surgical resection, but only 15%-20% of patients are resectable at presentation because more than 50% of patients has distant metastasis at diagnosis and the rest of them has locally advanced pancreatic cancer (LAPC). The standard of care first line treatment for LAPC patients is chemotherapy with or without radiation therapy. Recent developments in minimally invasive ablative techniques may add to the treatment armamenta
Core Tip: During the last decade new therapeutic options arose with the advancement of minimally invasive technologies to treat pancreatic cancer patients. These new therapies have been a topic of increasing interest due to the severe prognostic implications of pancreatic cancer and the low comorbid risk of these procedures. This review summarizes new ablative options for patients with locally advanced pancreatic cancer and percutaneous liver-directed therapies for patients with liver-dominant metastatic disease.
- Citation: Bibok A, Kim DW, Malafa M, Kis B. Minimally invasive image-guided therapy of primary and metastatic pancreatic cancer. World J Gastroenterol 2021; 27(27): 4322-4341
- URL: https://www.wjgnet.com/1007-9327/full/v27/i27/4322.htm
- DOI: https://dx.doi.org/10.3748/wjg.v27.i27.4322
Pancreatic cancer carries an extremely poor prognosis with the 5-year survival is around 8% and goes down to 3% in patients with metastatic disease[1]. Although the overall cancer-survival statistics are improving, pancreatic cancer is an exception with no major therapeutic advancement in the last 30 years since the introduction of pancreaticoduodenectomy[2].
As the survival statistics of other malignancies are improving, it is expected that pancreatic cancer becomes the second leading cause of cancer-related death by 2030[3].
The only curative option of pancreatic cancer is complete surgical resection. However, only 15%-20% of patients have potentially resectable disease at presentation[4] approximately 30% patients are unresectable due to locally advanced pancreatic cancer (LAPC)[5] and approximately 50% of patients have stage IV pancreatic cancer with distant metastasis at diagnosis[1]. The most common metastatic site is the liver as 90% of pancreatic cancer patients develop liver metastasis[6].
During the last decade new therapeutic options arose with the advancement of minimally invasive technologies. This review summarizes new ablative options for patients with LAPC and percutaneous liver-directed therapies for patients with liver-dominant metastatic disease. The term pancreatic cancer includes a histologically heterogenous group. This article focuses only on pancreatic ductal adenocarcinoma, as the most common malignancy of the pancreas, accounting for 85%-95% of cases[4,5].
Non-metastatic LAPC comprises 30% of all newly diagnosed pancreatic cancer cases[7]. Patients with LAPC are not surgical candidates because of unresectable involvement of adjacent vessels like the portal vein, celiac or superior mesenteric artery or their major branches. Current standard-of-care therapy of LAPC is chemotherapy with or without radiation therapy. Adjuvant chemotherapy can downstage 20% of LAPC patients to be potentially resectable. Downstaged, surgically resected patients had a significantly improved survival (35.3 mo) compared to those who did not became surgical candidate after chemotherapy (16.2 mo)[8].
Patients with LAPC who do not became surgical candidate, may benefit from image-guided local ablation therapies, either percutaneously or during intraoperative open approach. The ablation technologies used in patients with LAPC include heat-based ablations, like radiofrequency ablation (RFA), microwave ablation (MWA) and cryoablation and the new non-thermal ablation technique, irreversible electroporation (IRE).
RFA is the most widely used heat-based ablative method which utilizes high frequency alternating electric current that causes cell death by heating the tissue through rapid electron vibration generating frictional heat[9]. This method of heat generation means that RFA is heavily dependent on conductivity which has close correlation to the water content of the tissue[9,10]. This is one of the disadvantages of RFA because as the tissue adjacent to the electrode heats up, it becomes desiccated and then acts as an “insulating sleeve” around the ablation probe hindering electron flow and further heat generation, thus limiting the ablation zone size[9]. Another major factor which limits the extent of the ablation zone is the cooling effect of flowing blood which works as a “heat sink”[11].
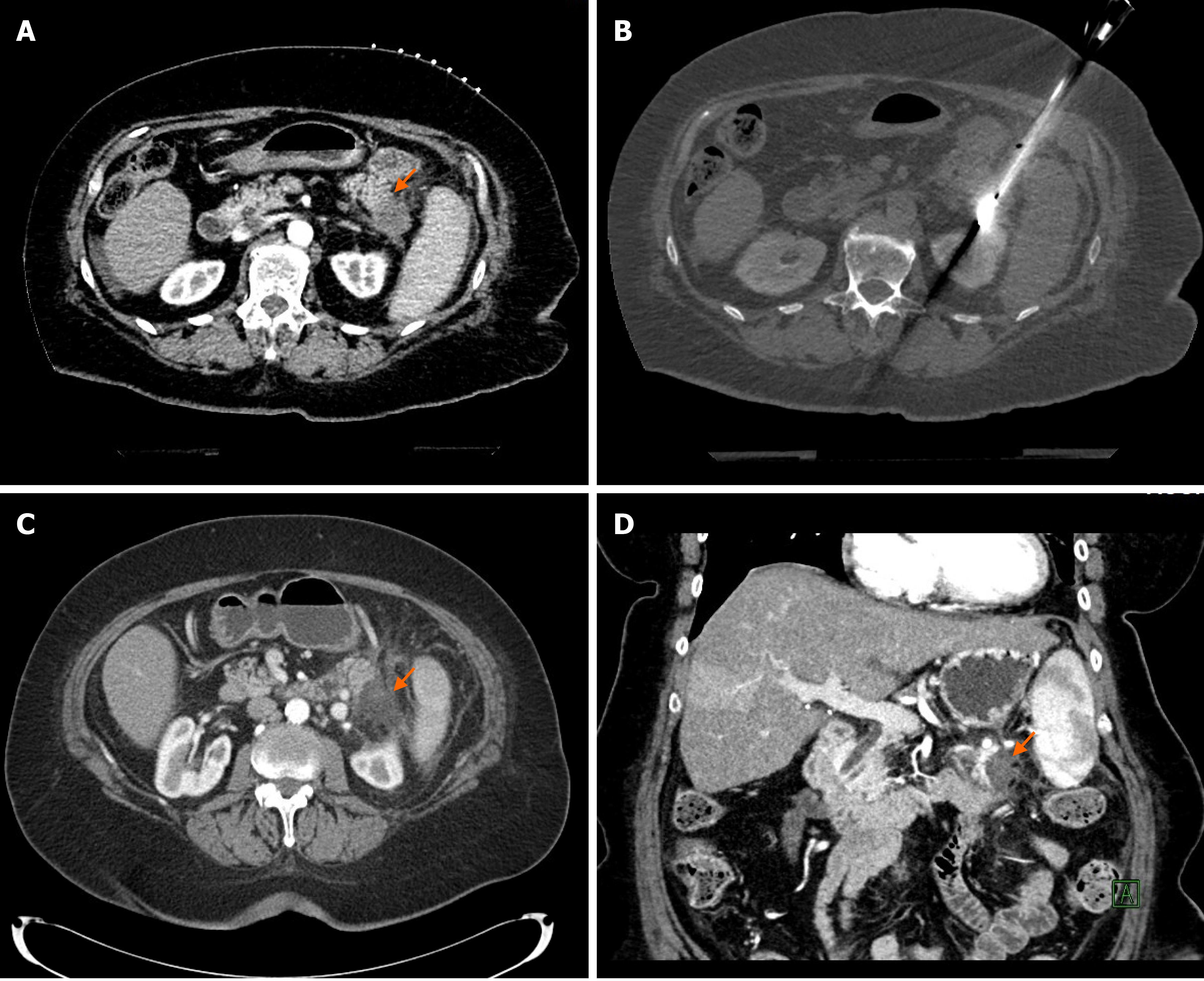
RFA treatment of the primary pancreatic cancer usually performed during open laparotomy. The studies where pancreatic RFA was used during open laparotomy are listed in Table 1. The initial publication by Matsui et al[12] included 20 patients and demonstrated that RFA is feasible in pancreatic cancer. This milestone publication was followed by multiple studies using RFA in pancreatic cancer with different endpoints. Wu et al[13] studied the pain relief effect of RFA and reported 50% pain reduction after pancreas cancer RFA. Girelli et al[14] also reported significantly reduced pain score in 69% of symptomatic patients. Several studies evaluated overall survival following RFA of pancreatic cancer and reported overall survival from 14.7 mo up to 33 mo[15,16]. The largest study[16] included 107 patients and divided patients into 2 arms: 47 patients underwent RFA as a first-line treatment and 60 patients received neoadjuvant therapy first followed by RFA. All patients received standard of care post-RFA treatment which included systemic chemotherapy and/or radiation and 29 patients also received intra-arterial chemotherapy which consisted of injection of epirubicin and cisplatin into the celiac artery in every 4 wk until disease progression. Median overall survival was 14.7 mo in the RFA group and 25.6 mo in the neoadjuvant treatment + RFA group. They also reported that 32 patients treated with the triple combination of RFA, radiation therapy, and intraarterial chemotherapy had an even longer median survival of 34 mo.
| Ref. | Study type | Patients | Outcome measure | Results | Complica-tions |
| Giardino et al[16], 2013 | Retrospective | 107 | OS | RFA 1st line: 14.7 mo RFA + adjuvant: 25.6 mo | Mortality: 1.8%; morbidity: 28% |
| Girelli et al[18], 2013 | Prospective | 100 | OS and DSS | 20 and 23 mo | Mortality 3%; morbidity 24% |
| Girelli et al[14], 2010 | Prospective | 50 | Safety and feasibility | - | Mortality 2%; morbidity 24% |
| Spiliotis et al[15], 2007 | Retrospective | 25 | OS | OS: 33 mo1 | Mortality: 0%; morbidity: 23% |
| Wu et al[13], 2006 | Prospective | 16 | Pain relief | 50% pain relief | 90-d mortality: 25%; |
| Matsui et al[12], 2000 | Prospective | 29 | OS | OS: 3 mo | Mortality: 10% |
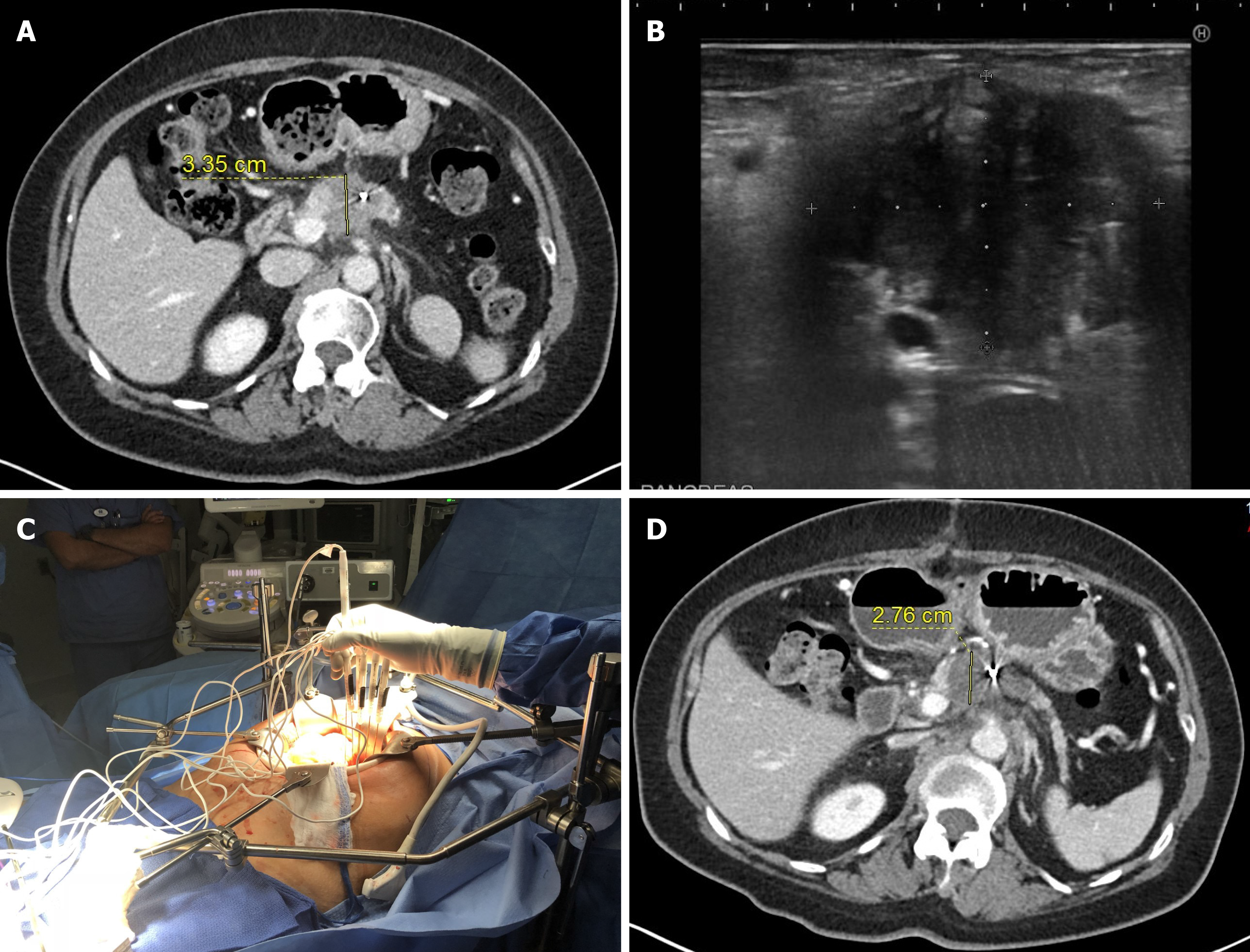
There is only one publication reporting data of percutaneous RFA of the pancreas tumor[17]. The authors analyzed data of 23 patients who underwent ultrasonography (US)-guided percutaneous RFA. There was no severe adverse event reported, which may be explained by the fact that in all patients the tumor located in the pancreatic tail or body and none in the head of the pancreas. Follow-up imaging showed good response to RFA, however, the median overall survival or pancreatic progression-free survival was not reported.
While RFA of pancreatic cancer has proven survival benefit, it also comes with a high morbidity risk of up to 40%[14] and mortality rate of up to 25%[13]. The most frequent major complications are portal vein stenosis (15%) and heat-injury of the duodenum (8%)[14]. The reported most common cause of death is massive gastrointestinal hemorrhage[13]. Girelli et al[18] reported that avoiding temperatures to exceed 90 °C during ablation and preserving surrounding tissue from overheating can reduce complications.
Despite the reported potential survival benefit of RFA, it remains unclear when RFA is best incorporated into the first line standard of care systemic chemotherapy/ chemoradiation treatment protocol; should RFA performed before or after the standard of care systemic therapy? Does its potential high complication rates worth its benefit?
MWA is another heat-based ablation technology which appears to have several advantages over RFA. Microwave generates heat using electromagnetic radiation-induced rotation of dipole molecules, such as water[19]. MWA is more powerful than RFA and generates higher temperatures in a shorter time. This leads to larger ablation zones and less heat-sink effect from the adjacent blood vessels[20]. MWA can be effective in tissues with high impedance, such as charred desiccated tissue, what is a weakness of RFA[20].
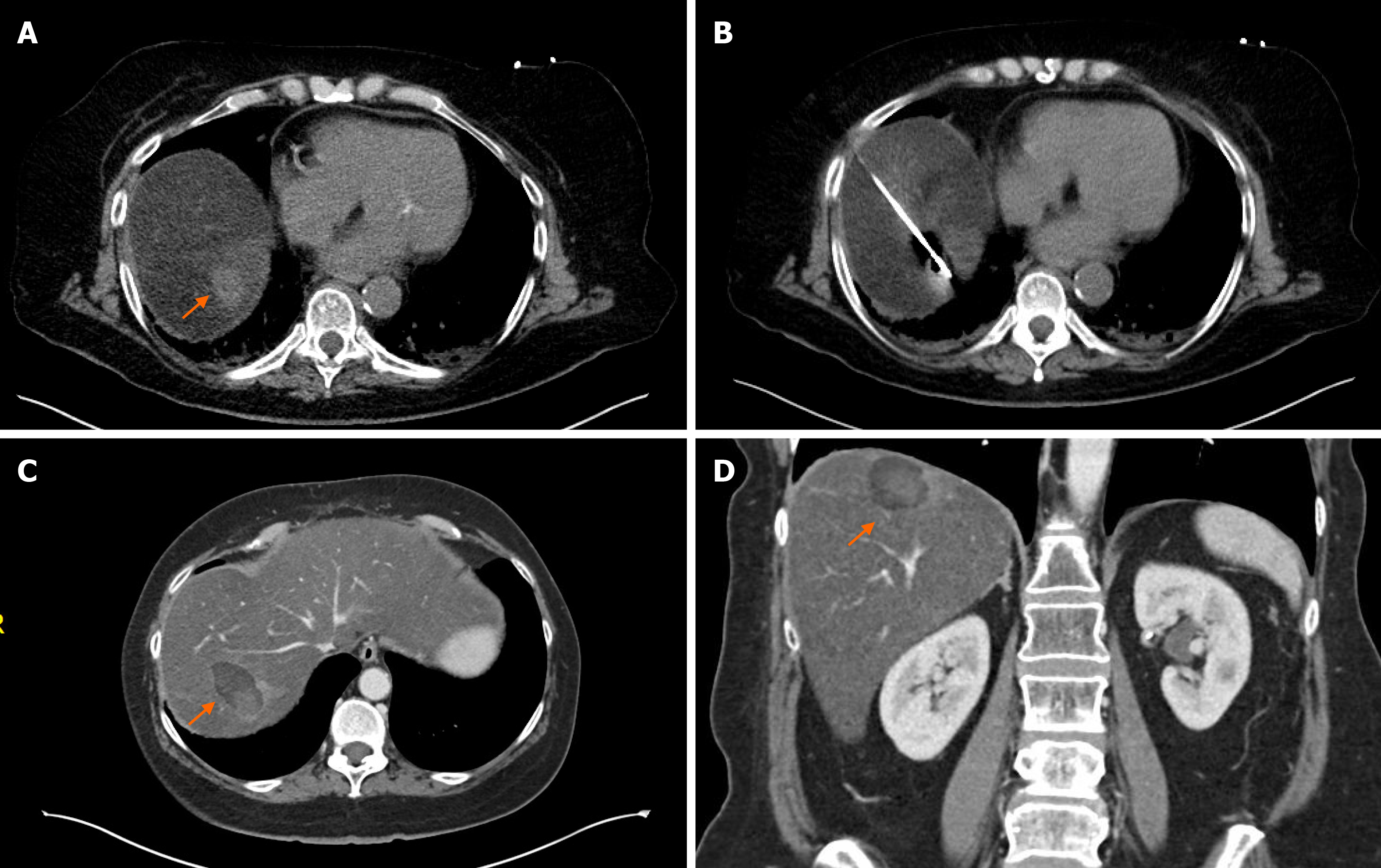
The available data on the usage of MWA in LAPC is very limited[21-24]; only 4 studies have been published with limited follow-up and survival data included (Table 2). The largest series of 20 patients reported 100% technical success without major complications and only 9.8% minor complications. However, 3-mo follow-up imaging was available in 10 patients (50%) only and no overall survival was reported[24]. In the study of Lygidakis et al[23] 15 patients were included with large pancreatic tumors (average tumor size of 6 cm) and only partial ablation was achieved in all patients. No major complications were reported. The longest follow-up was 22 mo; survival data of the group was not published. Carrafiello et al[21] reported of 10 patients with LAPC who underwent MWA; 5 during open surgery, and 5 percutane
In our experience of 2 patients with pancreatic tail cancer, MWA was very effective without major complications; in one patient the ablation was complete (Figure 1), in the other patient the tumor recurred in the pancreatic tail 17 mo after ablation. Besides the recurrence, the MWA was also complicated with a development of an asympto
During cryoablation intra- and extracellular ice crystals causing damage to the cell membrane, that will lead to cell death due to dehydration and osmotic pressure changes[25]. The cooling mechanism of the cryoablation probe based on the Joule-Thomson effect as high pressure argon gas is circulated via tiny tubes inside the probe with sudden expansion of the gas in a chamber in the ablation probe tip. The probe tip reaches temperatures as low as -160 C. The major advantage of cryoablation over other ablation technologies is that ice is visible on US, computed tomography (CT) and magnetic resonance imaging, therefore, the size of the iceball can be monitored during the ablation to prevent inadvertent damage to adjacent tissues. The other advantage is the analgesic property of cold during cryoablation which is associated with reduced intra- and postprocedural pain[26,27]. The heat sink effect of adjacent vessels can negatively influence the size of the ablation zone in cryoablation, but this effect is not as pronounced compared to RFA[28]. One of the potential major complications of cryoablation is cryoshock which occur in 0.3 to 2.0% of patients[29,30] and is characterized by multiorgan failure and disseminated intravascular coagulation[31]. Cryoshock was observed most often in large volume liver ablations[32].
Similar to MWA, the available data on cryoablation of pancreatic cancer is very limited. There is no data regarding the effect of cryoablation in patients with LAPC. There are 3 papers which reported pancreatic cryoablation, but two of them included patients with stage 4 pancreatic cancer[33,34] the third study used combination of cryoablation with radioactive iodine-125 treatment[35]. Song et al[33] compared gastrojejunal bypass surgery alone vs bypass surgery with pancreatic tumor cryoablation in 118 patients with advanced pancreatic cancer. Cryoablation was performed in 42 patients. There were no differences between the patient characteristics and postoperative mortality; however, the 1-year survival rate was superior in the cryoablation group (4.8% vs 2.6%). The reason for the poor overall survival rate is the inclusion of patients with distant metastases.
Niu et al[34] compared 4 groups of patients with metastatic pancreatic cancer who underwent cryotherapy alone (36 patients), immunotherapy alone (17 patients), chemotherapy alone (22 patients) or cryoimmunotherapy (31 patients). Cryotherapy was used to treat both the primary tumor and the metastatic sites. Median overall survival (OS) was significantly higher in the cryoimmunotherapy (13 mo) group compared to cryotherapy (7 mo), immunotherapy (5 mo) and chemotherapy (3.5 mo) groups. There was no major complication.
Xu et al[36] reported outcomes of 49 patients of whom 38 underwent a combination therapy of cryoablation and iodine-125 seed implantation for LAPC. Both laparotomic and percutaneous approach were used in this study. Complete response was seen in 20.4% of patients, partial response in 38.8%, stable disease in 30.6% and progressive disease in 10.2%. The median OS was 16.2 mo, and the 12-mo and 24-mo survival was 63.1% and 22.8%, respectively. Six patients (12.2%) developed acute pancreatitis, in one case it was considered as severe.
Given the promising results with cryoablation, further studies are warranted in patients with LAPC.
Irreversible electroporation is a novel ablation technology[36], which, unlike RFA, MWA, and cryoablation, is non-thermal. IRE delivers high-voltage electrical current (1500 to 3000 V) between the IRE ablation probes which creates nanoscale defects in the cell membranes[37-39]. The cells within the ablation zone lose the ability to maintain homeostasis which results in apoptotic cell death with narrow zone of transition[36-38]. Since this is a non-thermal ablation, its efficacy is independent of thermal conductivity of the tissues and not influenced by the “heat-sink” effect of adjacent vessels[36,39]. The ablation zone is very predictable and easy to monitor using US or CT[39]. The high voltage delivered by IRE may cause muscular contraction and cardiac arrhythmia. Therefore, IRE must be performed under general anesthesia with complete neuromuscular blockade and electrocardiogram synchronization. Patients with pacemakers may not be a candidate for IRE[40].
The main advantage of IRE is the controlled apoptosis of the cells without harming the adjacent collagen matrix and proteins, thus saving scaffolds of blood vessels and bile ducts[41-44]. As IRE leads to apoptotic cell death it enhances antigen presentation to immunocompetent cells which may improve immunological response locally and at abscopal metastatic sites. Irreversible nanopore formation at the ablation site and reversible nanopore formation in the vicinity of the ablation zone can lead to higher intracellular concentration of chemotherapeutic agents in the damaged cancer cells[45,46]. The disadvantage of IRE is that it requires placements of multiple ablation probes because the high electric currents delivered between the probes. The probes have to be parallel to each other and at the same tissue depth which adds complexity to the probe placements and increases procedure time.
IRE most commonly performed intraoperatively during open laparotomy (Table 3, Figure 2). Although, some surgeons use palpation and visual guidance for ablation probe placements, most commonly intraoperative US is used to precise identification of the tumor extent and visualization of major arteries inside the tumor to maximize ablation success and minimize bleeding complications.
| Author | Patients | Median OS(mo) | Guidancemodality | Major adverse events |
| Paiella et al[53], 2015 | 10 | 7.5 | US | 20% |
| Martin et al[48], 2015 | 200 | 24.9 | US | 22% |
| Kluger et al[52], 2015 | 50 | 7.7 | Not reported | 38% |
| Lambert et al[54], 2015 | 21 | 10.2 | Not reported | 24% |
| Yan et al[57], 2016 | 25 | Not reported | US | 16% |
| Vogel et al[50], 2017 | 15 | 16 | US | 53% |
| Huang et al[47], 2018 | 70 | 22.6 | US | 4% |
| Yang et al[105], 2020 | 74 | 53% (3 yr)1 | US | 12% |
| He et al[106], 2020 | 36 | 53.5% (2 yr)2 | US | 5% |
| He et al[49], 2020 | 167 | 16 | US | N/A |
Several studies described survival benefit for patients who underwent IRE (Table 3)[47-50]. Huang et al[47] found that median OS was significantly extended to 22.6 mo. The authors analyzed data of 70 patients who were treated with IRE for LAPC. All patients received neoadjuvant chemotherapy with either a gemcitabine-based or TS-1 (tegafur, gimeracil, and oteracil) chemotherapy based on the patient's age and performance status. Local recurrence rate was only 8.6% at 28 mo follow-up. Disease progression was noted in 25 patients (36%) at distant sites, mostly in the liver (12 patients). Both median OS (28.7 mo vs 19.1 mo) and progression-free survival (PFS, 26.4 mo vs 13.2 mo) were significantly longer in patients who received TS-1 adjuvant therapy compared to patients with gemcitabine-based adjuvant therapy. Patient selection bias could play a role in the excellent survival results because patients with larger than 4 cm pancreatic tumor were excluded.
Martin et al[48] analyzed data of 200 patients and so far this is the largest study on IRE of the pancreas. All patients received chemo- or chemoradiation therapy before the IRE (the chemotherapy was either gemcitabine- or FOLFIRINOX-based). In 50 patients IRE was used only for surgical margin extension and in 150 patients IRE was used without resection. In the resection + IRE group 75% of patients had body and neck tumors, while in the IRE group most patients (63%) had pancreatic head tumors. There was no significant difference in median OS between the two groups, however, the median OS was slightly longer in patients who received resection + IRE (28.3 mo vs 23.2 mo). The median OS and PFS for the entire group were 24.9 mo and 12.4 mo, respectively.
There were several studies which could not demonstrate survival benefit of IRE over standard of care chemotherapy with reported 9.3 mo median OS[51-54]. However, these are relatively early studies with potential biases (patient selection, different chemotherapeutic protocols, lack of experience) that may have influenced the outcomes. Although survival benefit of IRE was not proved compared to a matching control group, Lambert et al[54] recommended IRE in cases where unresectability was noted during laparotomy.
IRE can also be performed with laparoscopic approach using endoscopic or laparoscopic US guidance, but there are only 9 cases reported when laparoscopic technique was used[47,55,56]. In the study of Huang et al[47] 5 out of the 70 patients underwent laparoscopic ablation and their survival and complication results were not significantly different compared to the laparotomy group. Stillström et al[56] used an advanced stereotactic navigation system to perform laparoscopic IRE in 3 patients. The technical success rate was 100% and a prospective, randomized trial is planned. Tartaglia et al[55] reported a single case of laparoscopic IRE, where operators used laparoscopic ultrasound for guidance. The procedure was uneventful, and the 6-mo follow-up positron emission tomography-CT showed no fluorodeoxyglucose-uptake in the treated region.
In our experience probe placement-related bleeding is almost always seen due to the requirements of multiple probe placements and due to the rich blood supply of the upper gastrointestinal tract and porta hepatis. The IRE probe placements often performed via transgastric or transduodenal approach. During the open procedure the bleeding complications can be easily managed; in case of arterial bleed most of the times 5-10 min of manual compression is sufficient. Surgical vascular ligation is also readily available if needed. The other major advantage of open IRE over percutaneous IRE is the ability to sample and analyze suspicious lymph nodes with frozen section during the surgery and to detect obscure peritoneal metastases which are not seen on prior imaging. This reduces the chance to perform IRE on patients who have stage IV disease and unlikely would have benefit from the pancreatic ablation. Laparotomy is unavoidable anyway in a large number of LAPC patients since pancreatic head and neck tumors often cause gastric outlet obstruction. In these cases, IRE can be performed at the same time as the gastrojejunal bypass procedure.
Major adverse events of open IRE were reported in a wide range, from 4% to 53%. These can be laparotomy-related or IRE-related complications. Kluger et al[52] evaluated IRE-related and surgery related adverse events and found that 44% of grade 3-4 adverse events were IRE-related and 56% related to the surgical procedure. Authors concluded that IRE should not be considered as a minimally invasive treatment due to its high adverse event rate. Several authors did not describe whether they used imaging guidance during the IRE for probe placement and to monitor the ablation zone. Lack of imaging guidance may explain the high morbidity rates. Lambert et al[54] also included patients who underwent percutaneous IRE, but the percutaneous arm was closed after two patients due to high complication rate; one patient developed cholangitis, liver abscess, and biliary peritonitis resulting in surgical revision and antibiotic treatment, the other patient developed pancreatic fistula treated with stoma bag and antibiotics.
The most common IRE-related serious adverse events are pancreatic abscess formation, pancreatic fistula, gastrointestinal bleeding, and duodenal ulceration[47,48,57].
Percutaneous IRE is most commonly performed with CT guidance. CT-guided IRE requires administration of intravenous contrast to best visualize the tumor and its relationship to adjacent major vessels. US-guided percutaneous IRE has been reported[58-60] and US-guidance may be feasible in patients with low body mass index and without overlying gas-filled stomach and bowel loops, but in most patients percutaneous US is not suitable to guide pancreatic IRE ablation. The percutaneous approach is less invasive, and the hospital stay is shorter compared to open IRE, however, the management of procedural bleeding complications can be challenging and there are limitations to detect peritoneal and nodal metastases. The median OS of the percutaneous IRE studies is ranging from 10 to 19.8 mo. The longest overall survival was recently reported by Ma et al[51] The authors evaluated LAPC patients treated with gemcitabine plus IRE (33 patients) and gemcitabine alone (35 patients). The median OS of 19.8 mo in the combination therapy group indicates that including IRE into the treatment protocol provides significant survival benefit over gemcitabine alone (9.3 mo).
Narayanan et al[61] performed CT-guided percutaneous IRE in 50 LAPC patients. Nine of the 50 patients underwent a second IRE due to evidence of residual tumor. Three patients were downstaged and underwent surgical resection: pathologic examination showed negative margins in all the 3 patients with complete (1 patient) or partial (2 patients) tumor necrosis. Median OS was 14.2 mo. Most common major adverse event was abdominal pain in 7 patients. Ruarus et al[62] also published outcome of 50 patients who underwent CT-guided percutaneous IRE. This study included 16 patients who did not received neoadjuvant chemotherapy (FOLFIRINOX, gemcitabine, or capecitabine and oxaliplatin combination). For all patients, the median OS from IRE was 10 mo. Interestingly, the median OS was slightly longer in the patient group that received no chemotherapy, compared to those who did (11 mo vs 9 mo). Uni- and multivariate analysis revealed large tumor size, baseline CA19-9 Level higher than 2000 U/mL, and less than 50% reduction of CA19-9 at 3 mo follow-up as prognostic factors for poor survival.
A recent systematic review[63] compared open and percutaneous IRE. The mortality and morbidity rates were significantly higher in the open IRE group, meanwhile the median OS was found to be superior compared to the percutaneous approach. The authors concluded that the treatment plan needs to be tailored individually.
There is a big difference between major centers in the rate of reported major adverse events ranging from 0% to 42%[58,62,64,65]. Belfiore et al[64] and Zhang et al[58] treated 20 and 21 patients, respectively, without any serious adverse events. On the other hand, most studies reported 20% or higher major adverse events after percutaneous IRE (Table 4).
| Ref. | Patients | Median OS from IRE | Guidance | Major adverse events |
| Belfiore et al[64], 2015 | 20 | 13.951 mo | CT | 0% |
| Narayanan et al[61], 2017 | 50 | 14.2 mo | CT | 20% |
| Zhang et al[58], 2017 | 21 | N/A | CT/US | 0% |
| Scheffer et al[65], 2017 | 25 | 11 mo | CT | 40% |
| Månsson et al[59], 2019 | 24 | 13.3 mo | US | 25% |
| Flak et al[60], 2019 | 33 | 10.7 mo | US | 20% |
| Ruarus et al[62], 2020 | 50 | 10 mo | CT | 42% |
| Ma et al[51], 2020 | 33 | 19.8 mo | CT | 9% |
Some patients may require special attention before IRE. Patients with pacemakers may not be a candidate for IRE. Metal stents and surgical clips may also contraindicate IRE. Månsson et al[66] reported a case of a patient with implanted metallic biliary stent who underwent IRE for the tumor in the head of the pancreas and developed severe late complications (persistent pain, abscess formation, diarrhea, peritonitis) within 8 wk of the procedure, that ended in emergency laparotomy and subsequent right hemicolectomy (for severe diarrhea). Martin et al[67] strongly recommends removal of metal biliary stents before IRE procedures. The metal stent can be removed endoscopically and replaced with a plastic stent or can be removed surgically with hepatico-jejunostomy creation at the same laparotomy for open IRE. Dunki-Jacobs et al[68] demonstrated significant energy deflection during IRE in the presence of a metal stent which can lead to high current conditions, incomplete ablation, and possible thermal injury to adjacent organs. On the other hand, Melenhorst et al[69] successfully used IRE to treat a patient with hilar cholangiocarcinoma who had metallic stent in place. In our practice we haven’t experience increased rate of complications of IRE in patients with metal stent in place (Kis et al unpublished data). At this point, the relationships between metal stent and IRE complications are not fully understood.
The non-thermal nature of IRE and its ability to preserve the structural integrity of blood vessels and bile ducts made IRE the preferred ablation modality in the pancreas. There are several ongoing clinical studies investigating the role of IRE in pancreatic cancer patients and to identify potential combination treatments which could improve survival (Table 5).
| Title | Location | Hyperlink | Patients | Estimated completion |
| An open-label, multicenter, prospective study of IRE (Nano Knife) combined with radiotherapy and chemotherapy in patients with LAPC | Shanghai, China | https://clinicaltrials.gov/ct2/show/NCT04310553 | 40 | December, 2020 |
| Ablation of unresectable LAPC with IRE system | Teaneck, New Jersey, United States | https://clinicaltrials.gov/ct2/show/NCT03614910 | 30 | May, 2023 |
| Chemotherapy followed by irreversible electroporation in patients with unresectable LAPC | Aalborg, Denmark | https://clinicaltrials.gov/ct2/show/NCT04093141 | 30 | May, 2024 |
| Chemotherapy and IRE in the treatment of advanced pancreatic adenocarcinoma | Louisville, Kentucky, United States | https://clinicaltrials.gov/ct2/show/NCT03484299 | 20 | December, 2023 |
| PANFIRE-3 trial: Assessing safety and efficacy of IRE + Nivolumab + CpG for metastatic pancreatic cancer | Amsterdam, North-Holland, Netherlands | https://clinicaltrials.gov/ct2/show/NCT04612530 | 18 | October, 2022 |
| Outcomes of ablation of unresectable pancreatic cancer using the nanoknife IRE system | Baltimore, Maryland, United States | https://clinicaltrials.gov/ct2/show/NCT02041936 | 12 | December, 2021 |
| Immunotherapy and IRE in the treatment of advanced pancreatic adenocarcinoma | Louisville, Kentucky, United States | https://clinicaltrials.gov/ct2/show/NCT03080974 | 10 | April, 2022 |
| A study of the use of IRE in pancreatic ductal cancer | Toronto, Ontario, Canada | https://clinicaltrials.gov/ct2/show/NCT03257150 | 47 | September, 2021 |
| Safety and efficacy of IRE for LAPC | Seoul, Korea, Republic of | https://clinicaltrials.gov/ct2/show/NCT02898649 | 100 | August, 2019 |
| IRE (Nano Knife) for the treatment of pancreatic adenocarcinoma | Poitiers, France | https://clinicaltrials.gov/ct2/show/NCT03105921 | 20 | June, 2020 |
| IRE for inoperable hepatic and pancreatic malignancy | Hong Kong | https://clinicaltrials.gov/ct2/show/NCT02822716 | 35 | December, 2021 |
| Phase II/III of randomized controlled clinical research on IRE synchronous chemotherapy for LAPC | Guangzhou, Guangdong, China | https://clinicaltrials.gov/ct2/show/NCT03673137 | 120 | November, 2021 |
| Anti-tumor immunity induced by IRE of unresectable pancreatic cancer | Guangzhou, Guangdong, China | https://clinicaltrials.gov/ct2/show/NCT02343835 | 20 | January, 2025 |
| A pivotal study of safety and effectiveness of Nano Knife IRE for stage 3 pancreatic cancer | USA, Multicentre | https://clinicaltrials.gov/ct2/show/NCT03899636 | 528 | December, 2023 |
| Immunologic signatures following surgery for pancreatic cancer | Durham, North Carolina, United States | https://clinicaltrials.gov/ct2/show/NCT03001518 | 30 | April, 2027 |
At the time of diagnosis 53% of patients have distant metastases from pancreatic cancer and the 5-year survival of patients with metastatic disease is only 3%[1]. Liver is the most common site of metastasis with approximately 90% of patients develop hepatic metastasis[6]. In very selected cases of oligometastatic liver disease surgery can be considered[70-72], but the majority of patients are unresectable[73]. Chemotherapy is the only treatment option for most patients with metastatic pancreatic cancer[74]. The ACCORD11 trial reported 11.1 mo survival in metastatic pancreatic cancer patients treated with FOLFIRINOX, but the treatment had significant grade 3 and 4 toxicities including neutropenia (45.7%), thrombocytopenia (9.1%), diarrhea (12.7%), sensory neuropathies (9.0%), and fever (5.4%)[6]. For unresectable patients with liver-dominant metastatic disease liver-directed therapies may offer survival benefit and can provide chemotherapy holiday.
Percutaneous liver ablation is a widely used technique to treat primary and secondary malignancies in the liver[75]. The results of thermal ablations that covers the entire metastatic lesion is comparable to the results of surgical resection[76]. Studies evaluating percutaneous liver ablation in metastatic pancreatic cancer are summarized in Table 6.
Park et al[77] published a retrospective study of 34 patients who underwent liver RFA for metastatic pancreatic cancer. Median OS was 14 mo. Tumor size of less than 2 cm and well differentiated histology were predictive factors of longer survival. Second ablation due to new or recurrent metastasis was performed in 18 patients (58.1%), and in 16 of them the hepatic disease was controlled with the repeated ablation. Nine and one patient underwent a third and fourth ablation session, respectively.
Hua et al[78] performed a retrospective analysis of 102 pancreatic cancer patients who underwent RFA of liver metastases. Median OS was 11.4 mo. Univariate analysis showed that tumors in the head of the pancreas, tumor size between 3 and 5 cm and neutrophil-lymphocyte ratio > 2.5 were associated with worse prognosis. Recently Lee et al[79] published a retrospective study of 60 patients who underwent liver RFA for metachronous pancreatic cancer metastases and compared survival to a group of 66 patients who received systemic therapy. The median OS was significantly longer in the RFA group (12 mo) compared to the systemic therapy group (9.1 mo).
Although MWA and cryoablation is widely used in liver malignancies, there are no dedicated reports to date on pancreatic cancer metastases. Since MWA has several advantages over RFA it can be assumed that MWA is at least as effective as RFA ablating pancreatic cancer liver metastases. Bailey et al[80] published a study which included 20 metastatic pancreatic cancer patients who underwent any kind of liver directed treatment. In this study 10 patients had MWA of liver metastasis, but no survival or complication data was reported. During last 10 years we performed MWA of pancreatic liver metastasis in only 2 patients despite we are a high-volume ablation center and using almost exclusively MWA for liver metastases (Figure 3). Our practice reflects the recommendations of Ghidini et al[81] who reviewed the available data of surgical hepatic metastasectomy and ablative techniques for metastatic pancreatic cancer. They concluded that despite the advancements in both fields, neither surgery nor ablation improved overall survival significantly during the last decade. Therefore, metastasectomy and ablation of liver metastasis are not recommended routinely for stage IV pancreatic cancer patients.
All trans-arterial embolization therapies for liver tumors are predicated upon the fact that the liver has dual blood supply and while the normal liver receives approximately 80% of the blood from the portal vein and 20% from the hepatic artery, the blood supply of tumors are almost exclusively is from the hepatic artery. Therefore, by embolizing the tumor feeding hepatic arteries, tumor ischemia can be achieved with minimal hypoxic damage to the normal liver parenchyma.
During transarterial chemoembolization (TACE) the embolic agent is delivered simultaneously with a chemotherapeutic agent. Direct intra-arterial administration of chemotherapy increases concentration in the target area and decreases systemic toxicities of the drug. Embolization reduces intra-tumoral blood flow, therefore prolongs the dwell time of chemotherapy within the tumor and further decreases the systemic side effects of the drug.
TACE is a well-studied intraarterial therapy in the management of hepatocellular carcinoma and colon cancer, but there is limited data on TACE in metastatic pancreatic cancer (Table 7). In the largest series Vogl et al[82] analyzed the data of 112 patients with metastatic pancreatic cancer who underwent TACE using a combination of mitomycin C, cisplatin, and gemcitabine, followed by iodized oil and 50 µm embolization microspheres until stasis. Median OS was 19.2 mo with an impressive 5-year survival rate of 50%. Azizi et al[83] retrospectively analyzed the data of 32 patients who were treated with TACE, using the same technique as Vogl et al[82]. The median OS was 16 mo. Sun et al[84] reported a very promising 23 mo median OS of the 27 patients who underwent TACE for metastatic pancreatic cancer. The authors found that TACE prolongs survival and improves quality of life.
| Ref. | Patients | Median OS from TACE | Technique |
| Vogl et al[82], 2015 | 112 | 19.2 mo | cTACE after mitomycin C, cisplatin, and gemcitabine chemoperfusion |
| Azizi et al[83], 2011 | 32 | 16 mo | cTACE after mitomycin C, cisplatin, and gemcitabine chemoperfusion |
| Sun et al[84], 2017 | 27 | 23 mo | Chemoperfusion with gemcitabine, oxaplatin, and irinotecan plus embolization with Lipiodol + pirarubicin or DEB with pirarubicin |
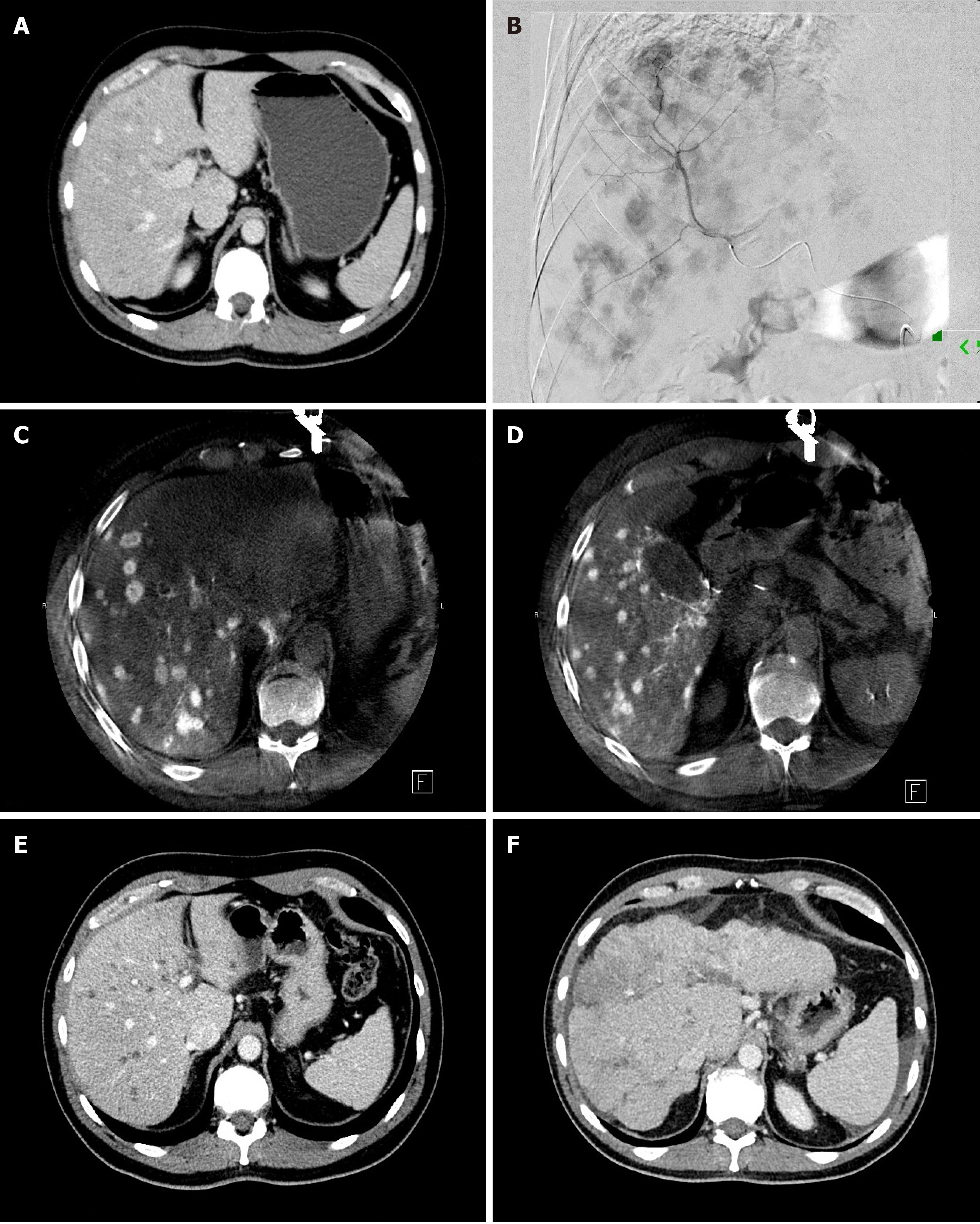
During transarterial radioembolization (TARE) treatment Yttrium-90 (Y90) containing microspheres are delivered into the arteries feeding the liver metastases (Figure 4). Y90 is a pure β-emitting isotope with a physical half-life of 64.2 h[85]. It has a high energy radiation with average β-emission is 0.9367 MeV, with a mean tissue penetration of 2.5 mm and maximum tissue penetration of 11 mm[86,87], allowing delivery of high radiation doses to hepatic tumors with a “cross-fire” mechanism between the Y90 microspheres, while limiting the radiation dose to the surrounding liver parenchyma[88]. The antitumoral effect of Y90 is thought to be secondary to irreversible damage to tumor epithelial, stromal, and endothelial cells[89]. The absorbed dose of Y90 microspheres in the liver may be heterogeneous as it depends on hemodynamics and intratumoral vessel density[90]. The injected microspheres implant mostly in the terminal arterioles of tumors[88] in a 3: 1 to 20: 1 ratio compared to normal liver, with a preferential deposition in the tumor periphery[91]. There are currently two type of Food and Drug Administration-approved microspheres on the market: a glass (TeraSphere, Boston Scientific, Marlborogh, MA) and a resin (SIR-Spheres, Sirtex Medical Pty. Ltd, St Leonards, Australia) based microspheres. The two types of Y90 microspheres differ in several physical parameters. The most important difference is the approximately 50 times higher radioactivity/beads in glass microspheres compared to resin.
TARE is generally better tolerated by patients than TACE. TARE was associated with better quality-of-life scores compared to TACE in patients with hepatocellular carcinoma[92]. Similarly, fewer side effects were reported after TARE compared to TACE in patients with intrahepatic cholangiocarcinoma and liver-dominant metastatic breast cancer patients[93-95].
Three workgroups published studies that evaluated the safety and efficacy of TARE in liver-dominant metastatic pancreatic cancer (Table 8)[96-100]. Michl et al[96] published a retrospective study of 19 patients treated with Y90-labelled resin microspheres. Six (31%) grade 3 or higher toxicity was reported. Overall survival was 9 mo. Kim et al[98] also used Y90-labelled resin microspheres in 33 patients and reported 8.1 mo median OS. Grade 3 adverse events were noted in 2 patients. Very recently we reported our experience of Y90 TARE using Y90-labelled glass microspheres in 26 patients with liver dominant metastatic pancreatic cancer[100]. Median OS was 7 mo and 3 patients (11.5%) had grade 3 clinical toxicity in this study. We found that longer hepatic progression free survival was associated with younger age (< 65 years) and decreased or stable CA19-9 tumor marker level following TARE treatment.
There was no head-to-head comparison between TACE and TARE, but it appears that TACE resulted in better OS in patients with metastatic pancreatic cancer. The reason for this difference is unknown.
The introduction of minimally invasive technologies in cancer care opened new therapeutic options for patients with malignancies beyond surgery, chemotherapy and radiation treatment. Interventional oncology is becoming the fourth pillar of cancer care besides the classic trio of medical oncology, surgery and radiation oncology. The data presented above demonstrates only modest improvement in overall survival of patients with LAPC and patients with metastatic pancreatic cancer, but still this is a significant step forward in the treatment of a disease which has dismal prognosis. In the current medical practice these new minimally invasive treatments are used mostly as salvage therapies, when a patient has no other option. This approach introduces a selection bias into the studies and may mask the real potential of these novel treatment modalities. Despite the selection bias, they have one obvious advantage, the low mortality and morbidity rates of image guided-procedures compared to surgery, radiation therapy and even to chemotherapy. Interventional oncology has many tools in its armamentarium to manage patients with primary and secondary pancreatic cancer; however, most of the new treatment options are in the experimental phase and only performed by large-volume centers.
We are in an era of a paradigm shift from conventional oncology to immuno-oncology which could promote the concurrent use of ablative technologies together with immune therapies[45,101-104]. It has been found that ablation of solid tumors triggers a tumor-specific immune response which can be beneficial in combination with immune checkpoint inhibitors or other immune modulator agents. Future studies are needed to define the specific role of interventional oncology in every stages of pancreatic cancer with special attention on the immunological effect of ablative techniques.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Chen YH, Shiryajev YN, Yang Y S-Editor: Zhang H L-Editor: A P-Editor: Xing YX
| 1. | Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69:7-34. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13300] [Cited by in F6Publishing: 14452] [Article Influence: 2890.4] [Reference Citation Analysis (2)] |
| 2. | Cohen SJ, Pinover WH, Watson JC, Meropol NJ. Pancreatic cancer. Curr Treat Options Oncol. 2000;1:375-386. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 28] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 3. | Rahib L, Smith BD, Aizenberg R, Rosenzweig AB, Fleshman JM, Matrisian LM. Projecting cancer incidence and deaths to 2030: the unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res. 2014;74:2913-2921. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3888] [Cited by in F6Publishing: 4589] [Article Influence: 458.9] [Reference Citation Analysis (0)] |
| 4. | Pietryga JA, Morgan DE. Imaging preoperatively for pancreatic adenocarcinoma. J Gastrointest Oncol. 2015;6:343-357. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 50] [Reference Citation Analysis (0)] |
| 5. | Ilic M, Ilic I. Epidemiology of pancreatic cancer. World J Gastroenterol. 2016;22:9694-9705. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 877] [Cited by in F6Publishing: 835] [Article Influence: 104.4] [Reference Citation Analysis (21)] |
| 6. | Conroy T, Desseigne F, Ychou M, Bouché O, Guimbaud R, Bécouarn Y, Adenis A, Raoul JL, Gourgou-Bourgade S, de la Fouchardière C, Bennouna J, Bachet JB, Khemissa-Akouz F, Péré-Vergé D, Delbaldo C, Assenat E, Chauffert B, Michel P, Montoto-Grillot C, Ducreux M; Groupe Tumeurs Digestives of Unicancer; PRODIGE Intergroup. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med. 2011;364:1817-1825. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4838] [Cited by in F6Publishing: 5100] [Article Influence: 392.3] [Reference Citation Analysis (1)] |
| 7. | Sahani DV, Shah ZK, Catalano OA, Boland GW, Brugge WR. Radiology of pancreatic adenocarcinoma: current status of imaging. J Gastroenterol Hepatol. 2008;23:23-33. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 103] [Cited by in F6Publishing: 102] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 8. | Gemenetzis G, Groot VP, Blair AB, Laheru DA, Zheng L, Narang AK, Fishman EK, Hruban RH, Yu J, Burkhart RA, Cameron JL, Weiss MJ, Wolfgang CL, He J. Survival in Locally Advanced Pancreatic Cancer After Neoadjuvant Therapy and Surgical Resection. Ann Surg. 2019;270:340-347. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 167] [Cited by in F6Publishing: 241] [Article Influence: 60.3] [Reference Citation Analysis (0)] |
| 9. | Hong K, Georgiades C. Radiofrequency ablation: mechanism of action and devices. J Vasc Interv Radiol. 2010;21:S179-S186. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 124] [Cited by in F6Publishing: 124] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 10. | Solazzo SA, Liu Z, Lobo SM, Ahmed M, Hines-Peralta AU, Lenkinski RE, Goldberg SN. Radiofrequency ablation: importance of background tissue electrical conductivity--an agar phantom and computer modeling study. Radiology. 2005;236:495-502. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 96] [Cited by in F6Publishing: 100] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 11. | Goldberg SN, Gazelle GS, Solbiati L, Livraghi T, Tanabe KK, Hahn PF, Mueller PR. Ablation of liver tumors using percutaneous RF therapy. AJR Am J Roentgenol. 1998;170:1023-1028. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 164] [Cited by in F6Publishing: 170] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 12. | Matsui Y, Nakagawa A, Kamiyama Y, Yamamoto K, Kubo N, Nakase Y. Selective thermocoagulation of unresectable pancreatic cancers by using radiofrequency capacitive heating. Pancreas. 2000;20:14-20. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 85] [Cited by in F6Publishing: 100] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 13. | Wu Y, Tang Z, Fang H, Gao S, Chen J, Wang Y, Yan H. High operative risk of cool-tip radiofrequency ablation for unresectable pancreatic head cancer. J Surg Oncol. 2006;94:392-395. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 88] [Cited by in F6Publishing: 108] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 14. | Girelli R, Frigerio I, Salvia R, Barbi E, Tinazzi Martini P, Bassi C. Feasibility and safety of radiofrequency ablation for locally advanced pancreatic cancer. Br J Surg. 2010;97:220-225. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 127] [Cited by in F6Publishing: 135] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 15. | Spiliotis JD, Datsis AC, Michalopoulos NV, Kekelos SP, Vaxevanidou A, Rogdakis AG, Christopoulou AN. Radiofrequency ablation combined with palliative surgery may prolong survival of patients with advanced cancer of the pancreas. Langenbecks Arch Surg. 2007;392:55-60. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 79] [Cited by in F6Publishing: 94] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 16. | Giardino A, Girelli R, Frigerio I, Regi P, Cantore M, Alessandra A, Lusenti A, Salvia R, Bassi C, Pederzoli P. Triple approach strategy for patients with locally advanced pancreatic carcinoma. HPB (Oxford). 2013;15:623-627. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 36] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 17. | D'Onofrio M, Crosara S, De Robertis R, Butturini G, Salvia R, Paiella S, Bassi C, Mucelli RP. Percutaneous Radiofrequency Ablation of Unresectable Locally Advanced Pancreatic Cancer: Preliminary Results. Technol Cancer Res Treat. 2017;16:285-294. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 30] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 18. | Girelli R, Frigerio I, Giardino A, Regi P, Gobbo S, Malleo G, Salvia R, Bassi C. Results of 100 pancreatic radiofrequency ablations in the context of a multimodal strategy for stage III ductal adenocarcinoma. Langenbecks Arch Surg. 2013;398:63-69. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 67] [Cited by in F6Publishing: 78] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 19. | van den Berg PM, De Hoop AT, Segal A, Praagman N. A computational model of the electromagnetic heating of biological tissue with application to hyperthermic cancer therapy. IEEE Trans Biomed Eng. 1983;30:797-805. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 83] [Cited by in F6Publishing: 84] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 20. | Andreano A, Brace CL. A comparison of direct heating during radiofrequency and microwave ablation in ex vivo liver. Cardiovasc Intervent Radiol. 2013;36:505-511. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 67] [Cited by in F6Publishing: 71] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 21. | Carrafiello G, Ierardi AM, Fontana F, Petrillo M, Floridi C, Lucchina N, Cuffari S, Dionigi G, Rotondo A, Fugazzola C. Microwave ablation of pancreatic head cancer: safety and efficacy. J Vasc Interv Radiol. 2013;24:1513-1520. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 54] [Cited by in F6Publishing: 55] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 22. | Ierardi AM, Biondetti P, Coppola A, Fumarola EM, Biasina AM, Alessio Angileri S, Carrafiello G. Percutaneous microwave thermosphere ablation of pancreatic tumours. Gland Surg. 2018;7:59-66. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 24] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 23. | Lygidakis NJ, Sharma SK, Papastratis P, Zivanovic V, Kefalourous H, Koshariya M, Lintzeris I, Porfiris T, Koutsiouroumba D. Microwave ablation in locally advanced pancreatic carcinoma--a new look. Hepatogastroenterology. 2007;54:1305-1310. [PubMed] [Cited in This Article: ] |
| 24. | Vogl TJ, Panahi B, Albrecht MH, Naguib NNN, Nour-Eldin NA, Gruber-Rouh T, Thompson ZM, Basten LM. Microwave ablation of pancreatic tumors. Minim Invasive Ther Allied Technol. 2018;27:33-40. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 27] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 25. | Rubinsky B, Lee CY, Bastacky J, Onik G. The process of freezing and the mechanism of damage during hepatic cryosurgery. Cryobiology. 1990;27:85-97. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 160] [Cited by in F6Publishing: 163] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 26. | Callstrom MR, Atwell TD, Charboneau JW, Farrell MA, Goetz MP, Rubin J, Sloan JA, Novotny PJ, Welch TJ, Maus TP, Wong GY, Brown KJ. Painful metastases involving bone: percutaneous image-guided cryoablation--prospective trial interim analysis. Radiology. 2006;241:572-580. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 166] [Cited by in F6Publishing: 179] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 27. | Orlacchio A, Bazzocchi G, Pastorelli D, Bolacchi F, Angelico M, Almerighi C, Masala S, Simonetti G. Percutaneous cryoablation of small hepatocellular carcinoma with US guidance and CT monitoring: initial experience. Cardiovasc Intervent Radiol. 2008;31:587-594. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 63] [Cited by in F6Publishing: 65] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 28. | Ei S, Hibi T, Tanabe M, Itano O, Shinoda M, Kitago M, Abe Y, Yagi H, Okabayashi K, Sugiyama D, Wakabayashi G, Kitagawa Y. Cryoablation provides superior local control of primary hepatocellular carcinomas of >2 cm compared with radiofrequency ablation and microwave coagulation therapy: an underestimated tool in the toolbox. Ann Surg Oncol. 2015;22:1294-1300. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 37] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 29. | Xu KC, Niu LZ, He WB, Hu YZ, Zuo JS. Percutaneous cryosurgery for the treatment of hepatic colorectal metastases. World J Gastroenterol. 2008;14:1430-1436. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 43] [Cited by in F6Publishing: 39] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 30. | Yang Y, Wang C, Lu Y, Bai W, An L, Qu J, Gao X, Chen Y, Zhou L, Wu Y, Feng Y, Zhang M, Chang X, Lv J. Outcomes of ultrasound-guided percutaneous argon-helium cryoablation of hepatocellular carcinoma. J Hepatobiliary Pancreat Sci. 2012;19:674-684. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 39] [Cited by in F6Publishing: 44] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 31. | Seifert JK, Morris DL. World survey on the complications of hepatic and prostate cryotherapy. World J Surg. 1999;23:109-13; discussion 113. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 191] [Cited by in F6Publishing: 201] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 32. | Seifert JK, France MP, Zhao J, Bolton EJ, Finlay I, Junginger T, Morris DL. Large volume hepatic freezing: association with significant release of the cytokines interleukin-6 and tumor necrosis factor a in a rat model. World J Surg. 2002;26:1333-1341. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 77] [Cited by in F6Publishing: 51] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 33. | Song ZG, Hao JH, Gao S, Gao CT, Tang Y, Liu JC. The outcome of cryoablation in treating advanced pancreatic cancer: a comparison with palliative bypass surgery alone. J Dig Dis. 2014;15:561-569. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 10] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 34. | Niu L, Chen J, He L, Liao M, Yuan Y, Zeng J, Li J, Zuo J, Xu K. Combination treatment with comprehensive cryoablation and immunotherapy in metastatic pancreatic cancer. Pancreas. 2013;42:1143-1149. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 57] [Cited by in F6Publishing: 60] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 35. | Xu KC, Niu LZ, Hu YZ, He WB, He YS, Li YF, Zuo JS. A pilot study on combination of cryosurgery and (125)iodine seed implantation for treatment of locally advanced pancreatic cancer. World J Gastroenterol. 2008;14:1603-1611. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 39] [Cited by in F6Publishing: 37] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 36. | Davalos RV, Mir IL, Rubinsky B. Tissue ablation with irreversible electroporation. Ann Biomed Eng. 2005;33:223-231. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 861] [Cited by in F6Publishing: 809] [Article Influence: 42.6] [Reference Citation Analysis (0)] |
| 37. | Narayanan G. Irreversible Electroporation. Semin Intervent Radiol. 2015;32:349-355. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 43] [Cited by in F6Publishing: 27] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 38. | Savic LJ, Chapiro J, Hamm B, Gebauer B, Collettini F. Irreversible Electroporation in Interventional Oncology: Where We Stand and Where We Go. Rofo. 2016;188:735-745. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 27] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 39. | Rubinsky B, Onik G, Mikus P. Irreversible electroporation: a new ablation modality--clinical implications. Technol Cancer Res Treat. 2007;6:37-48. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 498] [Cited by in F6Publishing: 484] [Article Influence: 28.5] [Reference Citation Analysis (0)] |
| 40. | Ball C, Thomson KR, Kavnoudias H. Irreversible electroporation: a new challenge in "out of operating theater" anesthesia. Anesth Analg. 2010;110:1305-1309. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 146] [Cited by in F6Publishing: 134] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 41. | Maor E, Ivorra A, Leor J, Rubinsky B. The effect of irreversible electroporation on blood vessels. Technol Cancer Res Treat. 2007;6:307-312. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 233] [Cited by in F6Publishing: 245] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 42. | Cannon R, Ellis S, Hayes D, Narayanan G, Martin RC 2nd. Safety and early efficacy of irreversible electroporation for hepatic tumors in proximity to vital structures. J Surg Oncol. 2013;107:544-549. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 241] [Cited by in F6Publishing: 258] [Article Influence: 23.5] [Reference Citation Analysis (0)] |
| 43. | Narayanan G, Bhatia S, Echenique A, Suthar R, Barbery K, Yrizarry J. Vessel patency post irreversible electroporation. Cardiovasc Intervent Radiol. 2014;37:1523-1529. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 85] [Cited by in F6Publishing: 74] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 44. | Silk MT, Wimmer T, Lee KS, Srimathveeravalli G, Brown KT, Kingham PT, Fong Y, Durack JC, Sofocleous CT, Solomon SB. Percutaneous ablation of peribiliary tumors with irreversible electroporation. J Vasc Interv Radiol. 2014;25:112-118. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 107] [Cited by in F6Publishing: 125] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 45. | Calvet CY, Mir LM. The promising alliance of anti-cancer electrochemotherapy with immunotherapy. Cancer Metastasis Rev. 2016;35:165-177. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 76] [Cited by in F6Publishing: 50] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 46. | Bulvik BE, Rozenblum N, Gourevich S, Ahmed M, Andriyanov AV, Galun E, Goldberg SN. Irreversible Electroporation versus Radiofrequency Ablation: A Comparison of Local and Systemic Effects in a Small-Animal Model. Radiology. 2016;280:413-424. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 70] [Cited by in F6Publishing: 81] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 47. | Huang KW, Yang PC, Pua U, Kim MD, Li SP, Qiu YD, Song TQ, Liang PC. The efficacy of combination of induction chemotherapy and irreversible electroporation ablation for patients with locally advanced pancreatic adenocarcinoma. J Surg Oncol. 2018;118:31-36. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 27] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 48. | Martin RC 2nd, Kwon D, Chalikonda S, Sellers M, Kotz E, Scoggins C, McMasters KM, Watkins K. Treatment of 200 locally advanced (stage III) pancreatic adenocarcinoma patients with irreversible electroporation: safety and efficacy. Ann Surg. 2015;262:486-94; discussion 492. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 255] [Cited by in F6Publishing: 265] [Article Influence: 29.4] [Reference Citation Analysis (0)] |
| 49. | He C, Huang X, Zhang Y, Cai Z, Lin X, Li S. Comparison of Survival Between Irreversible Electroporation Followed by Chemotherapy and Chemotherapy Alone for Locally Advanced Pancreatic Cancer. Front Oncol. 2020;10:6. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 12] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 50. | Vogel JA, Rombouts SJ, de Rooij T, van Delden OM, Dijkgraaf MG, van Gulik TM, van Hooft JE, van Laarhoven HW, Martin RC, Schoorlemmer A, Wilmink JW, van Lienden KP, Busch OR, Besselink MG. Induction Chemotherapy Followed by Resection or Irreversible Electroporation in Locally Advanced Pancreatic Cancer (IMPALA): A Prospective Cohort Study. Ann Surg Oncol. 2017;24:2734-2743. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 56] [Cited by in F6Publishing: 61] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 51. | Ma YY, Leng Y, Xing YL, Li HM, Chen JB, Niu LZ. Gemcitabine plus concurrent irreversible electroporation vs gemcitabine alone for locally advanced pancreatic cancer. World J Clin Cases. 2020;8:5564-5575. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 6] [Cited by in F6Publishing: 5] [Article Influence: 1.3] [Reference Citation Analysis (1)] |
| 52. | Kluger MD, Epelboym I, Schrope BA, Mahendraraj K, Hecht EM, Susman J, Weintraub JL, Chabot JA. Single-Institution Experience with Irreversible Electroporation for T4 Pancreatic Cancer: First 50 Patients. Ann Surg Oncol. 2016;23:1736-1743. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 70] [Cited by in F6Publishing: 73] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 53. | Paiella S, Butturini G, Frigerio I, Salvia R, Armatura G, Bacchion M, Fontana M, D'Onofrio M, Martone E, Bassi C. Safety and feasibility of Irreversible Electroporation (IRE) in patients with locally advanced pancreatic cancer: results of a prospective study. Dig Surg. 2015;32:90-97. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 88] [Cited by in F6Publishing: 89] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 54. | Lambert L, Horejs J, Krska Z, Hoskovec D, Petruzelka L, Krechler T, Kriz P, Briza J. Treatment of locally advanced pancreatic cancer by percutaneous and intraoperative irreversible electroporation: general hospital cancer center experience. Neoplasma. 2016;63:269-273. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 18] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 55. | Tartaglia E, Fabozzi M, Rizzuto A, Settembre A, Abete R, Guerriero L, Favoriti P, Cuccurullo D, Corcione F. Irreversible electroporation for locally advanced pancreatic cancer through a minimally invasive surgery supported by laparoscopic ultrasound. Int J Surg Case Rep. 2018;42:290-294. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 6] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 56. | Stillström D, Nilsson H, Jesse M, Peterhans M, Jonas E, Freedman J. A new technique for minimally invasive irreversible electroporation of tumors in the head and body of the pancreas. Surg Endosc. 2017;31:1982-1985. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 19] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 57. | Yan L, Chen YL, Su M, Liu T, Xu K, Liang F, Gu WQ, Lu SC. A Single-institution Experience with Open Irreversible Electroporation for Locally Advanced Pancreatic Carcinoma. Chin Med J (Engl). 2016;129:2920-2925. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 37] [Cited by in F6Publishing: 36] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 58. | Zhang Y, Shi J, Zeng J, Alnagger M, Zhou L, Fang G, Long X, Pan Z, Li Y, Chen J, Xu K, Qian W, Niu L. Percutaneous Irreversible Electroporation for Ablation of Locally Advanced Pancreatic Cancer: Experience From a Chinese Institution. Pancreas. 2017;46:e12-e14. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 26] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 59. | Månsson C, Brahmstaedt R, Nygren P, Nilsson A, Urdzik J, Karlson BM. Percutaneous Irreversible Electroporation as First-line Treatment of Locally Advanced Pancreatic Cancer. Anticancer Res. 2019;39:2509-2512. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 16] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 60. | Flak RV, Stender MT, Jensen TM, Andersen KL, Henriksen SD, Mortensen PB, Sall M, Thorlacius-Ussing O. Treatment of locally advanced pancreatic cancer with irreversible electroporation - a Danish single center study of safety and feasibility. Scand J Gastroenterol. 2019;54:252-258. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 61. | Narayanan G, Hosein PJ, Beulaygue IC, Froud T, Scheffer HJ, Venkat SR, Echenique AM, Hevert EC, Livingstone AS, Rocha-Lima CM, Merchan JR, Levi JU, Yrizarry JM, Lencioni R. Percutaneous Image-Guided Irreversible Electroporation for the Treatment of Unresectable, Locally Advanced Pancreatic Adenocarcinoma. J Vasc Interv Radiol. 2017;28:342-348. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 68] [Cited by in F6Publishing: 69] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 62. | Ruarus AH, Vroomen LGPH, Geboers B, van Veldhuisen E, Puijk RS, Nieuwenhuizen S, Besselink MG, Zonderhuis BM, Kazemier G, de Gruijl TD, van Lienden KP, de Vries JJJ, Scheffer HJ, Meijerink MR. Percutaneous Irreversible Electroporation in Locally Advanced and Recurrent Pancreatic Cancer (PANFIRE-2): A Multicenter, Prospective, Single-Arm, Phase II Study. Radiology. 2020;294:212-220. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 46] [Cited by in F6Publishing: 70] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 63. | Moris D, Machairas N, Tsilimigras DI, Prodromidou A, Ejaz A, Weiss M, Hasemaki N, Felekouras E, Pawlik TM. Systematic Review of Surgical and Percutaneous Irreversible Electroporation in the Treatment of Locally Advanced Pancreatic Cancer. Ann Surg Oncol. 2019;26:1657-1668. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 33] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 64. | Belfiore MP, Ronza FM, Romano F, Ianniello GP, De Lucia G, Gallo C, Marsicano C, Di Gennaro TL, Belfiore G. Percutaneous CT-guided irreversible electroporation followed by chemotherapy as a novel neoadjuvant protocol in locally advanced pancreatic cancer: Our preliminary experience. Int J Surg. 2015;21 Suppl 1:S34-S39. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 39] [Cited by in F6Publishing: 45] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 65. | Scheffer HJ, Vroomen LG, de Jong MC, Melenhorst MC, Zonderhuis BM, Daams F, Vogel JA, Besselink MG, van Kuijk C, Witvliet J, de van der Schueren MA, de Gruijl TD, Stam AG, van den Tol PM, van Delft F, Kazemier G, Meijerink MR. Ablation of Locally Advanced Pancreatic Cancer with Percutaneous Irreversible Electroporation: Results of the Phase I/II PANFIRE Study. Radiology. 2017;282:585-597. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 80] [Cited by in F6Publishing: 86] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 66. | Månsson C, Nilsson A, Karlson BM. Severe complications with irreversible electroporation of the pancreas in the presence of a metallic stent: a warning of a procedure that never should be performed. Acta Radiol Short Rep. 2014;3:2047981614556409. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 21] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 67. | Martin RC 2nd, Durham AN, Besselink MG, Iannitti D, Weiss MJ, Wolfgang CL, Huang KW. Irreversible electroporation in locally advanced pancreatic cancer: A call for standardization of energy delivery. J Surg Oncol. 2016;114:865-871. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 50] [Cited by in F6Publishing: 55] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 68. | Dunki-Jacobs EM, Philips P, Martin RC 2nd. Evaluation of thermal injury to liver, pancreas and kidney during irreversible electroporation in an in vivo experimental model. Br J Surg. 2014;101:1113-1121. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 78] [Cited by in F6Publishing: 81] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 69. | Melenhorst MC, Scheffer HJ, Vroomen LG, Kazemier G, van den Tol MP, Meijerink MR. Percutaneous Irreversible Electroporation of Unresectable Hilar Cholangiocarcinoma (Klatskin Tumor): A Case Report. Cardiovasc Intervent Radiol. 2016;39:117-121. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 11] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 70. | Michalski CW, Erkan M, Hüser N, Müller MW, Hartel M, Friess H, Kleeff J. Resection of primary pancreatic cancer and liver metastasis: a systematic review. Dig Surg. 2008;25:473-480. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 72] [Cited by in F6Publishing: 78] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 71. | Hackert T, Niesen W, Hinz U, Tjaden C, Strobel O, Ulrich A, Michalski CW, Büchler MW. Radical surgery of oligometastatic pancreatic cancer. Eur J Surg Oncol. 2017;43:358-363. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 114] [Cited by in F6Publishing: 126] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 72. | Zanini N, Lombardi R, Masetti M, Giordano M, Landolfo G, Jovine E. Surgery for isolated liver metastases from pancreatic cancer. Updates Surg. 2015;67:19-25. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 48] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 73. | Garrido-Laguna I, Hidalgo M. Pancreatic cancer: from state-of-the-art treatments to promising novel therapies. Nat Rev Clin Oncol. 2015;12:319-334. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 398] [Cited by in F6Publishing: 423] [Article Influence: 47.0] [Reference Citation Analysis (0)] |
| 74. | Kamisawa T, Wood LD, Itoi T, Takaori K. Pancreatic cancer. Lancet. 2016;388:73-85. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1397] [Cited by in F6Publishing: 1547] [Article Influence: 193.4] [Reference Citation Analysis (0)] |
| 75. | Vogl TJ, Emam A, Naguib NN, Eichler K, Zangos S. How Effective Are Percutaneous Liver-Directed Therapies in Patients with Non-Colorectal Liver Metastases? Viszeralmedizin. 2015;31:406-413. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 8] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 76. | Bale R, Widmann G, Stoffner DI. Stereotaxy: breaking the limits of current radiofrequency ablation techniques. Eur J Radiol. 2010;75:32-36. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 51] [Cited by in F6Publishing: 56] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 77. | Park JB, Kim YH, Kim J, Chang HM, Kim TW, Kim SC, Kim PN, Han DJ. Radiofrequency ablation of liver metastasis in patients with locally controlled pancreatic ductal adenocarcinoma. J Vasc Interv Radiol. 2012;23:635-641. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 36] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 78. | Hua YQ, Wang P, Zhu XY, Shen YH, Wang K, Shi WD, Lin JH, Meng ZQ, Chen Z, Chen H. Radiofrequency ablation for hepatic oligometastatic pancreatic cancer: An analysis of safety and efficacy. Pancreatology. 2017;17:967-973. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 31] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 79. | Lee SJ, Kim JH, Kim SY, Won HJ, Shin YM, Kim PN. Percutaneous Radiofrequency Ablation for Metachronous Hepatic Metastases after Curative Resection of Pancreatic Adenocarcinoma. Korean J Radiol. 2020;21:316-324. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 16] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 80. | Bailey RE, Surapaneni PK, Core J, Vidal LLC, LeGout J, Ritchie C, Frey G, McKinney JM, Sella D, Paz-Fumagalli R, Toskich B, Mody K. Safety and efficacy of locoregional therapy for metastatic pancreatic ductal adenocarcinoma to the liver: a single-center experience. J Gastrointest Oncol. 2019;10:688-694. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 81. | Ghidini M, Petrillo A, Salati M, Khakoo S, Varricchio A, Tomasello G, Grossi F, Petrelli F. Surgery or Locoregional Approaches for Hepatic Oligometastatic Pancreatic Cancer: Myth, Hope, or Reality? Cancers (Basel). 2019;11. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 16] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 82. | Vogl TJ, Mohamed SA, Albrecht MH, Gruber-Roh T, Lin H, Nour Eldin NEA, Bednarova I, Naguib NN, Panahi B. Transarterial chemoembolization in pancreatic adenocarcinoma with liver metastases: MR-based tumor response evaluation, apparent diffusion coefficient (ADC) patterns, and survival rates. Pancreatology. 2018;18:94-99. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 18] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 83. | Azizi A, Naguib NN, Mbalisike E, Farshid P, Emami AH, Vogl TJ. Liver metastases of pancreatic cancer: role of repetitive transarterial chemoembolization (TACE) on tumor response and survival. Pancreas. 2011;40:1271-1275. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 35] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 84. | Sun JH, Zhou TY, Zhang YL, Zhou GH, Nie CH, Zhu TY, Chen SQ, Wang BQ, Ye S, Shen Y, Guo H, Wang WL, Zheng SS. Efficacy of transcatheter arterial chemoembolization for liver metastases arising from pancreatic cancer. Oncotarget. 2017;8:39746-39755. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 13] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 85. | Salem R. Radioembolization with 90Y microspheres: technical considerations. J Vasc Interv Radiol. 2007;18:1460-1461. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 8] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 86. | Vesselle G, Petit I, Boucebci S, Rocher T, Velasco S, Tasu JP. Radioembolization with yttrium-90 microspheres work up: Practical approach and literature review. Diagn Interv Imaging. 2015;96:547-562. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 21] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 87. | Salem R, Lewandowski RJ, Sato KT, Atassi B, Ryu RK, Ibrahim S, Nemcek AA Jr, Omary RA, Madoff DC, Murthy R. Technical aspects of radioembolization with 90Y microspheres. Tech Vasc Interv Radiol. 2007;10:12-29. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 101] [Cited by in F6Publishing: 100] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 88. | Morgan B, Kennedy AS, Lewington V, Jones B, Sharma RA. Intra-arterial brachytherapy of hepatic malignancies: watch the flow. Nat Rev Clin Oncol. 2011;8:115-120. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 21] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 89. | Folkman J, Camphausen K. Cancer. What does radiotherapy do to endothelial cells? Science. 2001;293:227-228. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 69] [Cited by in F6Publishing: 75] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 90. | Kennedy AS, Kleinstreuer C, Basciano CA, Dezarn WA. Computer modeling of yttrium-90-microsphere transport in the hepatic arterial tree to improve clinical outcomes. Int J Radiat Oncol Biol Phys. 2010;76:631-637. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 65] [Cited by in F6Publishing: 56] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 91. | Kennedy AS, Nutting C, Coldwell D, Gaiser J, Drachenberg C. Pathologic response and microdosimetry of (90)Y microspheres in man: review of four explanted whole livers. Int J Radiat Oncol Biol Phys. 2004;60:1552-1563. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 301] [Cited by in F6Publishing: 298] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 92. | Salem R, Gilbertsen M, Butt Z, Memon K, Vouche M, Hickey R, Baker T, Abecassis MM, Atassi R, Riaz A, Cella D, Burns JL, Ganger D, Benson AB 3rd, Mulcahy MF, Kulik L, Lewandowski R. Increased quality of life among hepatocellular carcinoma patients treated with radioembolization, compared with chemoembolization. Clin Gastroenterol Hepatol 2013; 11: 1358-1365. e1. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 162] [Cited by in F6Publishing: 174] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 93. | Al-Adra DP, Gill RS, Axford SJ, Shi X, Kneteman N, Liau SS. Treatment of unresectable intrahepatic cholangiocarcinoma with yttrium-90 radioembolization: a systematic review and pooled analysis. Eur J Surg Oncol. 2015;41:120-127. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 118] [Cited by in F6Publishing: 115] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 94. | Ray CE Jr, Edwards A, Smith MT, Leong S, Kondo K, Gipson M, Rochon PJ, Gupta R, Messersmith W, Purcell T, Durham J. Metaanalysis of survival, complications, and imaging response following chemotherapy-based transarterial therapy in patients with unresectable intrahepatic cholangiocarcinoma. J Vasc Interv Radiol. 2013;24:1218-1226. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 55] [Cited by in F6Publishing: 57] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 95. | Davisson NA, Bercu ZL, Friend SC, Paplomata E, Ermentrout RM, Newsome J, Majdalany BS, Kokabi N. Predictors of Survival after Yttrium-90 Radioembolization of Chemotherapy-Refractory Hepatic Metastases from Breast Cancer. J Vasc Interv Radiol. 2020;31:925-933. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 96. | Michl M, Haug AR, Jakobs TF, Paprottka P, Hoffmann RT, Bartenstein P, Boeck S, Haas M, Laubender RP, Heinemann V. Radioembolization with Yttrium-90 microspheres (SIRT) in pancreatic cancer patients with liver metastases: efficacy, safety and prognostic factors. Oncology. 2014;86:24-32. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 37] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 97. | Kim AY, Unger K, Wang H, Pishvaian MJ. Incorporating Yttrium-90 trans-arterial radioembolization (TARE) in the treatment of metastatic pancreatic adenocarcioma: a single center experience. BMC Cancer. 2016;16:492. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 20] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 98. | Kim AY, Frantz S, Brower J, Akhter N. Radioembolization with Yttrium-90 Microspheres for the Treatment of Liver Metastases of Pancreatic Adenocarcinoma: A Multicenter Analysis. J Vasc Interv Radiol 2019; 30: 298-304. e2. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 16] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 99. | Kim AY, Frantz S, Akhter N, Zhang J. Radioembolization with yttrium-90 resin microspheres for liver metastases of pancreatic adenocarcinoma: A retrospective multicenter analysis. JCO. 2017;35:e15745-e15745. [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 100. | Kayaleh R, Krzyston H, Rishi A, Naziri J, Frakes J, Choi J, El-Haddad G, Parikh N, Sweeney J, Kis B. Transarterial Radioembolization Treatment of Pancreatic Cancer Patients with Liver-Dominant Metastatic Disease Using Yttrium-90 Glass Microspheres: A Single-Institution Retrospective Study. J Vasc Interv Radiol. 2020;31:1060-1068. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 101. | Giardino A, Innamorati G, Ugel S, Perbellini O, Girelli R, Frigerio I, Regi P, Scopelliti F, Butturini G, Paiella S, Bacchion M, Bassi C. Immunomodulation after radiofrequency ablation of locally advanced pancreatic cancer by monitoring the immune response in 10 patients. Pancreatology. 2017;17:962-966. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 42] [Cited by in F6Publishing: 47] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 102. | Haen SP, Pereira PL, Salih HR, Rammensee HG, Gouttefangeas C. More than just tumor destruction: immunomodulation by thermal ablation of cancer. Clin Dev Immunol. 2011;2011:160250. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 117] [Cited by in F6Publishing: 150] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 103. | Reccia I, Kumar J, Habib N, Sodergren M. The use of radiofrequency ablation in pancreatic cancer in the midst of the dawn of immuno-oncology. Med Oncol. 2018;35:151. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 104. | Go EJ, Yang H, Chon HJ, Yang D, Ryu W, Kim DH, Han DK, Kim C, Park W. Combination of Irreversible Electroporation and STING Agonist for Effective Cancer Immunotherapy. Cancers (Basel). 2020;12. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 17] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 105. | Yang PC, Huang KW, Pua U, Kim MD, Li SP, Li XY, Liang PC. Prognostic factor analysis of irreversible electroporation for locally advanced pancreatic cancer - A multi-institutional clinical study in Asia. Eur J Surg Oncol. 2020;46:811-817. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 106. | He C, Wang J, Zhang Y, Cai Z, Lin X, Li S. Comparison of combination therapies in the management of locally advanced pancreatic cancer: Induction chemotherapy followed by irreversible electroporation vs radiofrequency ablation. Cancer Med. 2020;9:4699-4710. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |