INTRODUCTION
Severe acute respiratory syndrome by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) was first recognized in December 2019 in Wuhan (Hubei province, China), but its origin is still unknown and debated. Since then, the disease spread worldwide, leading the World Health Organization to declare a global pandemic on March 11, 2020[1]. More than 21 million confirmed cases of coronavirus disease 2019 (COVID-19) have been reported on all continents except Antarctica, and the incidence is steadily increasing[2]. In all territories affected by the COVID-19 pandemic, the diagnostic tests, the workload of the intensive care units and the initiation of mitigation strategies, such as the restriction of interpersonal contacts, have been established and increased over time[3]. Men seem to be disproportionately more commonly affected by a SARS-CoV-2 infection, and the hospital mortality rate among males is significantly higher than female patients[4]. Main vehicles of transmission of SARS-CoV-2 disease are respiratory droplets being released during coughing, sneezing or conversation between subjects and accumulate on surfaces causing an indirect contamination[5].
SARS-CoV-2 pathogenic agent of this pandemic disease belongs to the Coronaviridae family. Coronaviruses are nonsegmented enveloped RNA viruses with a single-strand linear positive-sense RNA[6]. They are routinely present among animals as well as humans. They are the most common cause of colds, particularly in cats and dogs[7]. Although the origin of the new mutant strain SARS-CoV-2 remains uncertain, it is likely that it originated in a wet market in Wuhan, where animals of all kinds are slaughtered in poor sanitary conditions and their meats are sometimes eaten raw[8].
Six types of coronaviruses causing human disease have been identified: Four of them cause mild respiratory symptoms, whereas the other two, Middle East respiratory syndrome coronavirus and SARS-CoV-1, have previously resulted in epidemics with high mortality rates[9]. SARS-CoV-2 has 80% genomic compatibility with SARS-CoV-1 and uses the same angiotensin-converting enzyme 2 (ACE2) receptor to enter cells. In fact, ACE2 is an integral membrane protein that seems to be the host cell receptor for SARS-CoV-2, which appears significantly increased in COVID-19 positive patients[10]. ACE2 positive endothelial cells from patients with COVID-19 show significant changes in their morphology, disruption of the intercellular junctions, cell swelling and loss of contact with the basement membrane[11]. The presence of the SARS-CoV-2 virus within endothelial cells suggests that direct viral effect as well as perivascular inflammation may contribute to endothelial damage[11]. This underlines the importance of microcirculation alterations in the pathogenesis and subsequent manifestations at the systemic level[11,12].
Pulmonary involvement in the COVID-19 pandemic is the most known and largely studied because progressive severe respiratory failure represents the leading cause of death in affected patients. Pathological samples of peripheral lung of patients who died from COVID-19 showed a histological pattern of diffuse alveolar damage with perivascular infiltration of T cells[13]. Pulmonary parenchymal tissue also showed typical vascular findings, represented by severe endothelial lesions with the presence of intracellular viruses and interrupted cell membranes[14].
Histological analysis of pulmonary vessels in affected patients showed diffuse thrombosis with microangiopathy; alveolar capillary microthrombi seem to be nine times more frequent as well as the amount of new vessel growth was reported to be 2.7 times higher in patients with COVID-19 in respect to those affected by influenza virus disease[15]. Therefore, three distinctive angiocentric characteristics of COVID-19 were found in the lung: (1) severe endothelial injury associated with the intracellular SARS-CoV-2 virus and rupture of endothelial cell membranes; (2) diffuse vascular thrombosis with microangiopathy and occlusion of the alveolar capillaries; and (3) significant growth of new vessels through an intussusceptive angiogenesis mechanism[13]. Although COVID-19 typically manifests as a respiratory illness and most of the literature described pulmonary signs and symptoms, this disease can affect other anatomical districts and structures, whose clinical manifestations are relatively poorly known. A whole series of signs and symptoms related to systemic manifestations are known to have a certain relevance. In particular, it has been reported that 57% of patients with low severity COVID-19 disease could have reported abdominal discomfort alone or in combination with pulmonary symptomatology[16]. The abdominal clinical manifestations have been related to the gastrointestinal tract and the hepato-biliary-pancreatic system, whereas urinary tract and spleen involvement have been less frequently reported[17].
Our review was aimed to report the current abdominal and gastrointestinal imaging features in COVID-19 patients as well as to define the role of the diagnostic imaging modalities in the abdominal manifestations.
GASTROINTESTINAL TRACT
Abdominal and gastrointestinal signs and symptoms in COVID-19 are being increasingly reported[18]. Indeed, on January 19, 2020, the first known patient of COVID-19 in Washington, United States reported a history of nausea and vomiting in addition to respiratory symptoms[19]. In the literature, it has been reported that series of COVID-19 patients presenting to the emergency room with abdominal pain but without the typical respiratory symptoms of SARS-CoV-2; therefore, the abdominal radiologist was the first to suggest COVID-19 infection because of the typical findings in the lung lower lobes on computed tomography (CT) scans of the abdomen, such as peripheral and subpleural ground-glass opacities[18,20,21] (Figure 1). In this context, some authors have proposed additional CT of the whole chest as part of a CT imaging pathway of acute abdominal pain during the COVID-19 pandemic; this did not get approval because in these patients it was enough just to review the pulmonary bases on abdominal CT scans[22].
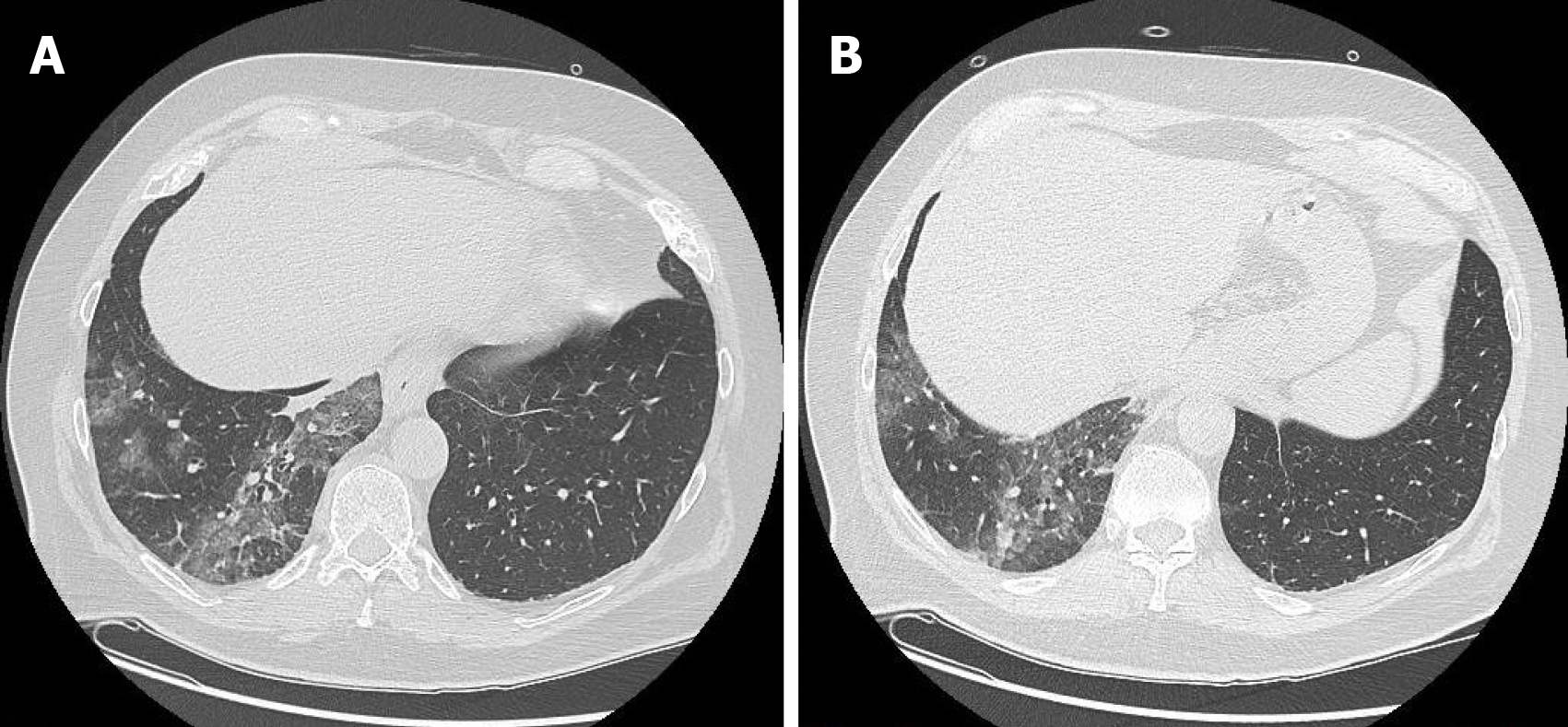
Figure 1 A 63-year-old man who presented to the emergency room for abdominal pain and no significant alterations on abdominal computed tomography.
A: Computed tomography scan with pulmonary window at the level of lung inferior lobes (included in the volume acquisition) shows the presence of ground-glass opacities and crazy-paving pattern in the right side; B: Computed tomography scan with pulmonary window at lower levels of lung inferior lobes always exhibited ground-glass opacities and crazy-paving pattern in the right side.
Patients with primarily mild gastrointestinal symptoms may not be identified as COVID-19 patients, with serious consequences for themselves and their contacts[18]; in this setting the most common gastrointestinal symptom is loss of appetite, followed by nausea and vomiting, while diarrhea and abdominal pain are the presenting symptoms in only a small percentage of cases[18].
In a retrospective study by Han et al[16], patients with gastrointestinal symptoms compared with patients with only respiratory symptoms, tended to have a longer course between symptom onset and viral clearance and took longer to report for medical care, a finding observed in other studies[23]; this suggests that in these patients the COVID-19 involvement was not initially recognized leading to delayed diagnosis[24]. Moreover, SARS-CoV-2 has been identified in stool samples of a proportion of infected patients[16]; viral replication in both small and large intestine was confirmed by the result of electron microscopy of autopsy biopsy specimen[24,25]. In particular, an autoptic study on the small intestine of two COVID-19 patients showed endothelitis of the submucosa vessels with mononuclear cell infiltrates within the intima along the lumen of many vessels, besides the evidence of direct viral infection of endothelial cells[11].
The inflammatory response in the gut due to active viral replication is also supported by the evidence of elevated fecal calprotectin concentrations in COVID-19 patients with diarrhea when compared with COVID-19 patients without diarrhea[26].
The radiological alterations of the gastrointestinal system in COVID-19 patients are represented by nonspecific thickening of various regions of the small and large bowel wall[27,28] (Figures 2 and 3), sometimes associated with hyperemia and mesenteric thickening[28] (Figure 4). Goldberg-Stein et al[29] retrospectively reported that the most common gastrointestinal symptom in 141 COVID-19 patients was the abdominal pain, present in 73.8% of patients with negative abdominal CT findings and in 53.8% of patients with positive abdominal CT findings; in this series the most commonly reported CT finding was represented by segmental wall thickening of the gastrointestinal tract[29]. In addition, 64% of patients with no positive CT abdominal findings but gastrointestinal symptoms showed suggestive features for COVID-19 pneumonia at the lung bases[29]. This suggests that abdominal symptoms may be present in COVID-19 patients without correlative CT abdominal findings; in fact, in patients with pneumonia it was hypothesized that the abdominal and back pain may be secondary to pleural irritation[30,31].
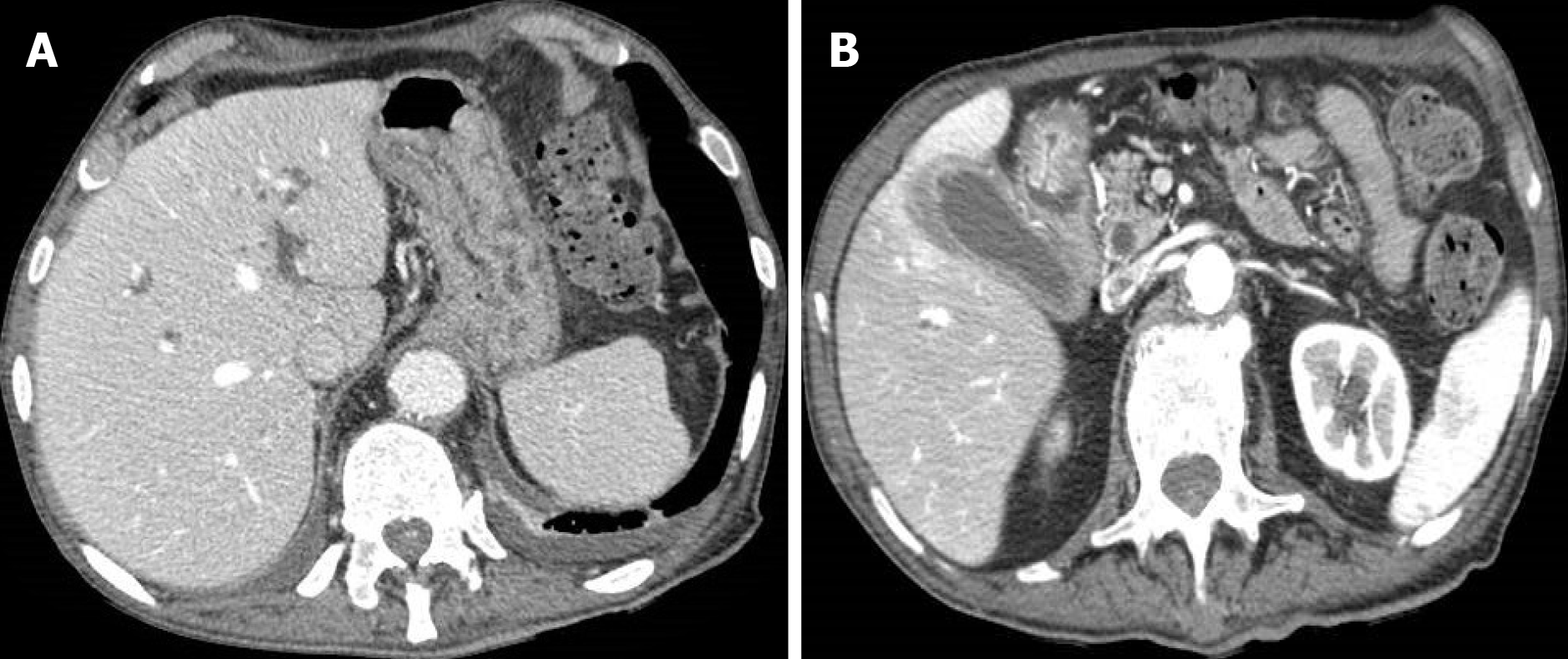
Figure 2 An 83-year-old woman with epigastric pain.
A: Abdominal contrast-enhanced portal-venous phase computed tomography showed diffuse thickening of the submucosa of the gastric walls and intrahepatic biliary dilatation; B: Abdominal contrast-enhanced portal-venous phase computed tomography also depicts thickening of the submucosa of the pyloric region and signs of cholecystitis.
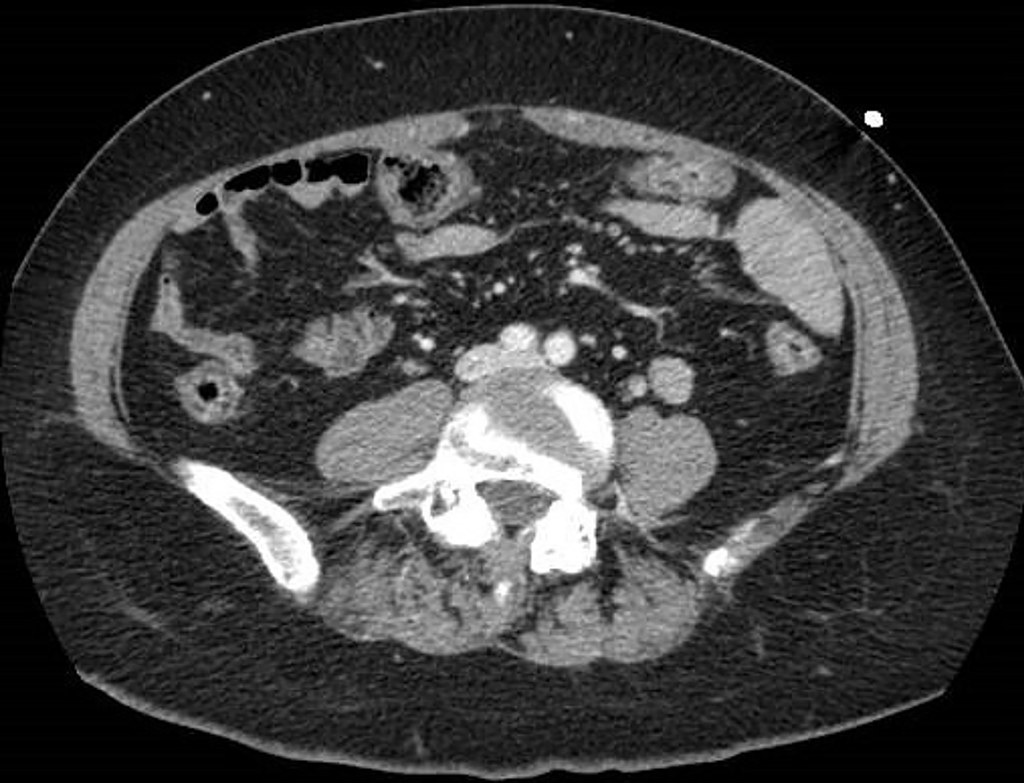
Figure 3 A 68-year-old woman with abdominal pain.
Abdominal contrast-enhanced portal-venous phase computed tomography image showed circumferential thickening of the submucosa of the right colon that appeared hypodense, in the absence of both significant contrast-enhancement and perivisceral fat stranding.
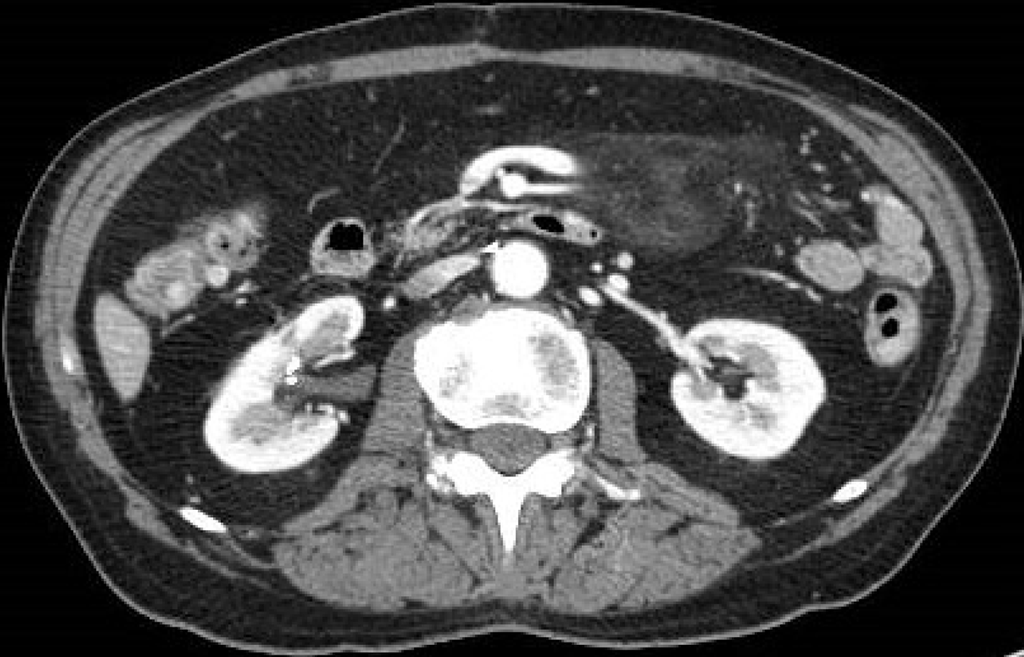
Figure 4 A 74-year-old man with abdominal pain.
Abdominal contrast-enhanced portal-venous phase computed tomography image showed well-circumscribed hyperattenuation of the fat surrounding the mesenteric vessels.
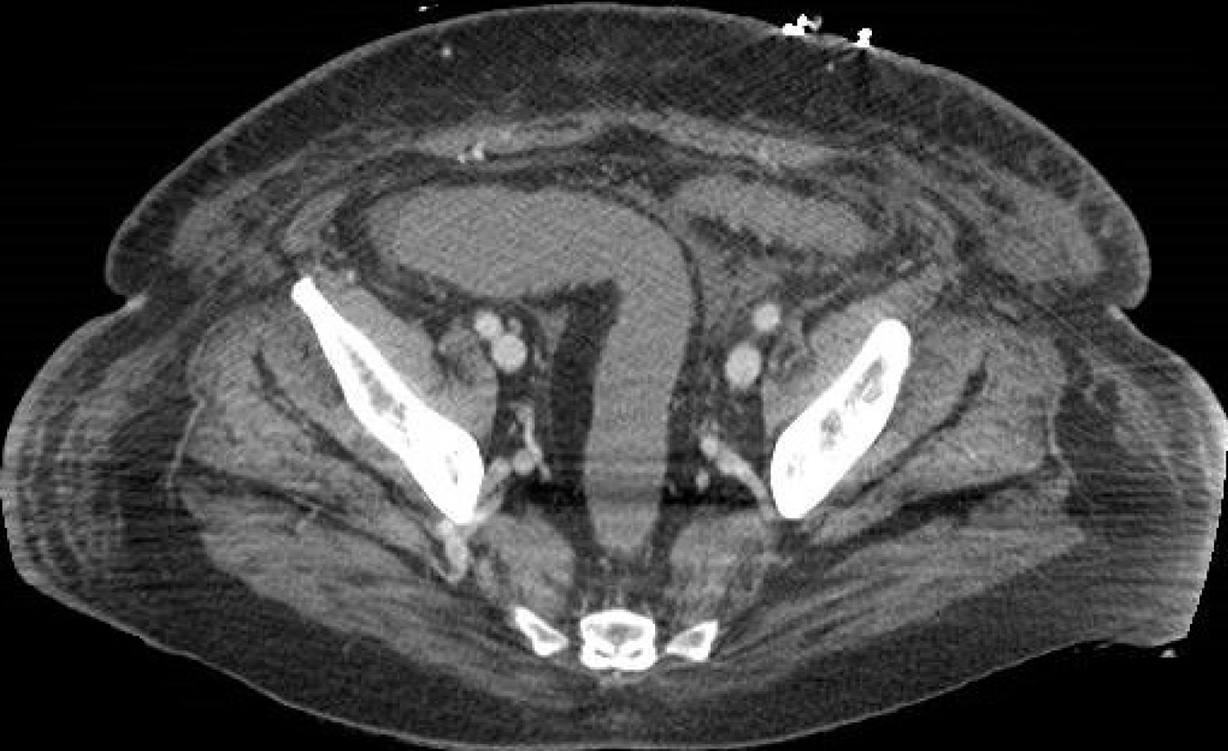
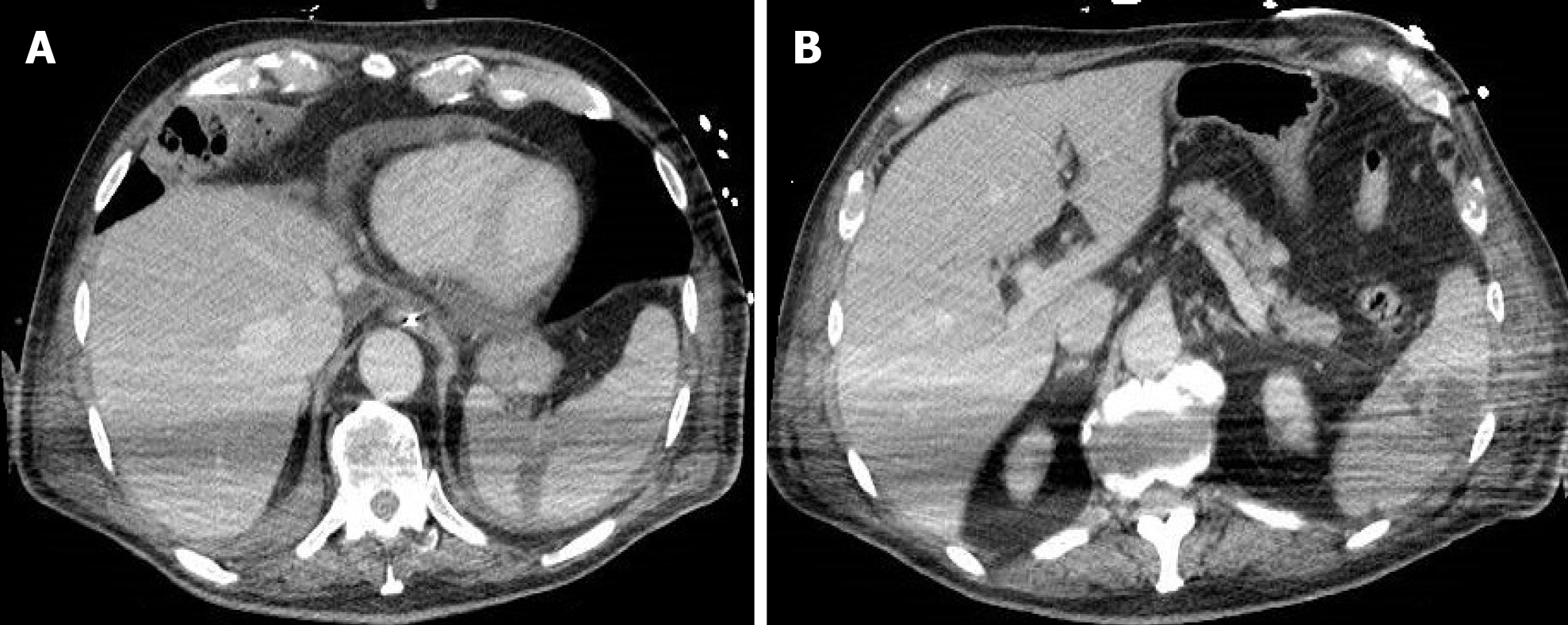
Tirumani et al[32], instead, in a retrospective study identified the incidence of abdominal findings in COVID-19 patients with and without abdominal symptoms and concluded that bowel abnormalities are the most common finding in the abdomen in patients with COVID-19, often without abdominal symptoms and especially regardless of the severity of lung involvement[32]. The most common CT abdominal findings are a fluid-filled colon with no wall thickening (Figures 5 and 6), severe colitis (characterized by a thickened and edematous wall with fat stranding), gastritis and small bowel pneumatosis with portal venous gas[32].
Figure 5 A 73-year-old woman with abdominal pain.
A: Contrast-enhanced portal-venous phase computed tomography image of the lower abdomen demonstrated gas distension of the sigma-rectum with evidence of multiple stools inside the lumen; free effusion was also appreciable around the colon; B: Contrast-enhanced portal-venous phase computed tomography image of the abdomen showed distention of the left bowel and hyperemic thickened walls (particularly affecting the mucosa) of the right colon with perivisceral fat suffusion; C: Coronal multiplanar reconstruction from contrast-enhanced computed tomography well demonstrated these findings.
Figure 6 A 69-year-old woman with diarrhea.
Contrast-enhanced portal-venous phase computed tomography image of the abdomen showed evidence of fluid-filled distension of the large bowel, particularly of the sigma and rectum, without evidence of parietal thickening. Free effusion was also present in the abdomen and between the intestinal loops with associated diffuse imbibition of the subcutaneous soft tissues.
Other minor manifestations are also reported. In a case report, Noda et al[33] described a COVID-19 healthy teenager who initially presented with abdominal discomfort. The patient underwent CT scan that demonstrated only isolated mesenteric adenopathy and adjacent fat stranding associated to ground-glass opacities and solid consolidation as well as interlobular septal thickening at lung bases[33]. This case highlights how abdominal findings in COVID-19 patients are not specific and should be suspected given the continued emergence of new manifestations of the disease.
Bhayana et al[34] retrospectively analyzed 42 abdominal CT scans performed in COVID-19 patients for abdominal pain or septic status. Colorectal and small bowel wall thickening (defined as single-wall thickness greater than 3 mm in distended intestine and greater than 5 mm in collapsed intestine) were found in 12 out of 42 abdominal CT, whereas pneumatosis associated to gas in the portal vein and fluid-filled colon (defined as homogeneous, low-attenuation colonic content) were identified in 4 and 18 out of 42 cases, respectively. The remaining 8 patients did not exhibit CT alterations of the gastrointestinal tract[34]. The 4 cases of pneumatosis and portal vein gas underwent exploratory laparotomy; 2 cases had necrotic bowel at surgery with a yellow discoloration of the small bowel in contrast with the usual black or purple color of a necrotic bowel. One patient also underwent a bowel resection demonstrating ischemic enteritis with patchy necrosis and submucosal arterioles containing fibrin thrombi[34].
Bowel ischemia has also been described in COVID-19 patients, particularly in those admitted to the intensive care unit[35], constituting a life-threatening clinical emergency[9]. It is well known that the hypercoagulable state induced by COVID-19 results in micro- and macrovascular complications[36]. The microvascular complications are detected in the early stages of the disease, while the macrovascular ones are more typically observed in severely ill patients[37]. Anyway, thromboembolic disease within the mesenteric vascular system is not frequently identified on CT imaging[35].
Revzin et al[38] divided the COVID-19 related bowel ischemia into early, intermediate and late presentations[38]. On CT images the early phase shows contracted gasless bowel that may transform into dilated gas-filled bowel with a paper-thin bowel wall in the intermediate phase. The CT findings of the late phase include intestinal wall pneumatosis, absence of mucosal enhancement and luminal dilatation. It is important to note that these phases reflect those of a classic intestinal ischemia regardless of etiology[39] and that the presence of pneumatosis intestinalis suggests bowel ischemia[36], but its presence must be interpreted with caution because it may be secondary to mechanical ventilation in patients with severe COVID-19[40] (Figure 7).
Figure 7 A 65-year-old man with abdominal pain and severe pulmonary involvement.
A: Unenhanced computed tomography scan of the upper abdomen showed signs of pneumoperitoneum secondary to mechanical ventilation and cholelithiasis; B: The presence of gas within the peritoneal cavity was also appreciable at lower levels of the abdomen on unenhanced computed tomography scan.
Actually, the exact pathological mechanism of intestinal ischemic disorders is not clearly known. In a letter to the editor, Parry et al[41] reported four possible mechanisms that work alone or in varying combination: coagulation disorder, elevated levels of von Willebrand Factor, expression of ACE2 on enterocytes of the small bowel and shock/hemodynamic compromise associated with COVID-19 pneumonia[41].
COVID-19 patients may be in a state of hypercoagulability induced by a systemic inflammatory state, endothelial activation, hypoxia and immobilization. These could lead to mesenteric microvascular thrombosis without involvement of the large mesenteric vessels, resulting in a condition of thrombosis in situ rather than an embolic event[41].
Elevated levels of von Willebrand factor have been reported in severe COVID-19 patients[42]. von Willebrand factor is released from endothelium in response to damage caused by SARS-CoV-2 and consequent endothelium dysfunction and vascular thrombosis[43].
Regarding the last two points, the enterocytes express ACE2, the target receptor for SARS-CoV-2 with intestinal tropism and direct bowel damage. Lastly, shock or hemodynamic compromise, which is commonly associated with severe COVID-19 pneumonia, may lead to a non-occlusive mesenteric ischemia[42].
LIVER, BILIARY TRACT AND PANCREAS
The liver is the second most frequently injured organ after the lung in COVID-19[44]. The mechanism of liver damage is probably due to a series of events that can occur simultaneously. Direct cytopathic effect of SARS-CoV-2, indirect damage from systemic inflammation, drug hepatotoxicity[44] and hypoxic alterations related to ventilation have been mainly described[45]. SARS-CoV-2 causes direct liver damage because the ACE2 receptor is widely expressed in the liver, more on cholangiocytes than on hepatocytes[46]. The virus alters the barrier and bile acid transport functions of cholangiocytes through the dysregulation of genes involved in tight junction formation and bile acid transport[47]. Drugs commonly used during SARS-CoV-2 infection causing liver toxicity include remdesivir, tocilizumab, chloroquine, hydroxychloroquine and azithromycin[48]. The immune-mediated cytokine storm also participates in the damage; in fact, we have a marked activation of inflammatory markers, including abnormal levels of C-reactive protein, lymphocytes, neutrophils and cytokines, in particular interleukin-6. The control of cytokine dysregulation at an early stage could be useful to slow down the progression of the disease[45]. In addition, respiratory-induced hypoxia can cause elevated serum aminotransferase concentrations, a laboratory marker of liver injury[49]. The combination of these events determines a generalized coagulopathy state that determines an alteration of the microcirculation with microthrombosis within the hepatic sinusoids[20]. As a support to this etiopathogenetic theory, liver autopsy results by Medeiros et al[50] show periportal necrosis, lymphocytic infiltration of the sinusoids, dense infiltration of the gate by abnormally small lymphocytes, central venous thrombosis and cirrhotic alterations with fibrosis in a retrospective series of 316 patients[50].
Liver injury usually manifests as an increase in enzyme levels. Current literature data show that 14.8%-53.0% of COVID-19 patients have abnormal levels of alanine aminotransferase and aspartate aminotransferase and a slight increase in serum bilirubin levels during the course of the disease[16]. Phipps et al[51] found that an alanine aminotransferase spike was significantly associated with clinical outcome[51]. In respect to mild to moderate or no liver injury, patients with severe liver injury have a higher rate of intubation and renal replacement therapy. Additionally, approximately 50% of COVID-19 patients had increased levels of γ-glutamyl transferase[52].
To the best of our knowledge, hepatic manifestations of COVID-19 were in most cases mild and transient. Despite this, there is an ever increasing number of subjects with severe hepatic manifestations that are very often associated with the pulmonary ones[53].
For the management of COVID-19 patients with liver injury, the American Association for the Study of Liver Diseases provides recommendations, highlighting that there are no contraindications to the use of drugs such as remdesivir, tocilizumab, chloroquine, hydroxychloroquine and azithromycin unless alanine aminotransferase or aspartate aminotransferase are no more than five times the upper normal limit[48].
A systematic review and meta-analysis of international data on the hepatic manifestations of COVID-19 was performed by The American Gastroenterological Association Institute[54]. Among COVID-19 patients with liver injury, more than 60% of patients had mild hepatic injury (1 time upper normal limit to 5 times upper normal limit)[51]. It should be noted that previous liver disease and underlying liver function may have influenced the results. In fact, some patients may have abnormal liver function prior to SARS-CoV-2 infection, such as nonalcoholic fatty liver disease or chronic hepatitis B[55]. Moreover, there is a higher prevalence of hepatic steatosis that is probably due to the known association between infection and obesity[50].
Hepatic steatosis is a very frequent and nonspecific finding of COVID-19 patients, which has been identified with both ultrasound (US) and CT. The findings of hepatic steatosis are the same as those found in non-COVID-19 patients. On US we can observe the typical “bright liver” that is characterized by an increase in echogenicity compared to the renal cortex or spleen (in the case of renal pathology), loss of physiological hyperechogenicity of the portal branches walls and posterior attenuation of the ultrasonic beam with failure to visualize the diaphragm (Figure 8). On the other hand, diffuse liver hypodensity is the typical sign on CT scans. In the presence of a slight steatosis a hepatic attenuation of less than 10 HU compared to the density of the spleen is observed, whereas in moderate/severe forms hepatic attenuation is less than 40 HU compared to the spleen[56] (Figure 9).
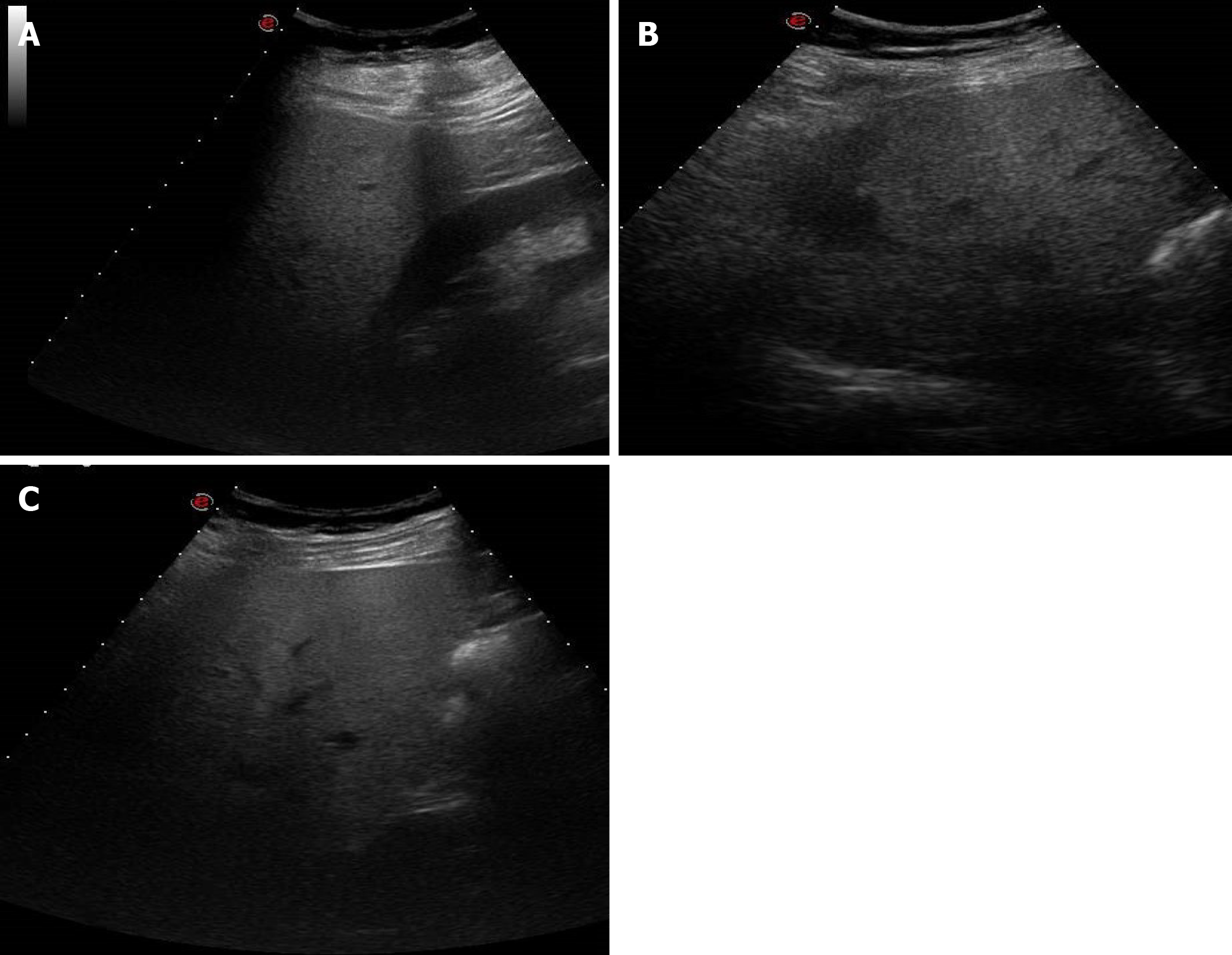
Figure 8 A 38-year-old man with abdominal discomfort.
A: Abdominal ultrasound image demonstrated hepatic steatosis as an increase in echogenicity compared to the renal cortex; B: Abdominal ultrasound image demonstrated hepatic steatosis as loss of physiological hyperechogenicity of the wall of the portal branches; C: Abdominal ultrasound image demonstrated hepatic steatosis as posterior attenuation of the ultrasonic beam with failure to visualize the diaphragm.
Figure 9 A 46-year-old woman with abdominal discomfort.
Unenhanced computed tomography image showed increased liver hypodensity compared to the spleen, with attenuation value less than 40 HU.
Ji et al[57] found that COVID-19 patients with nonalcoholic fatty liver disease were more likely to have liver damage and disease progression than patients without nonalcoholic fatty liver disease[57]. Singh et al[58] studied the impact of pre-existing liver disease on outcomes in a large cohort of COVID-19 patients and found that the risk of hospitalization and death in patients with pre-existing liver disease was significantly higher[58].
Further, in 54% of patients with COVID-19, biliary sludge and gallstones were found and were closely related to an increase in cholestasis indexes[59], values that appear to be markedly increased in comparison with the incidence in the general population (about 10%-20%)[38]. US is considered the gold standard for detecting gallstones. It allows the evaluation of macro- and microlithiasis, sludge and cholesterol deposits as well as the structural evaluation of the gallbladder[60]. The cholesterol deposition along the gallbladder walls appears as hyperechogenic spots with the typical “comet sign” whereas the biliary sludge as sediment in the declivous portion of the gallbladder lumen[60] (Figure 10). Although the role of CT in the evaluation of biliary lithiasis is marginal compared to US in the normal patient, this technique easily allows the visualization of hyperdense calcium stones and hypodense cholesterol stones in COVID-19 patients because US is not easily performed in the most compromised patients[61].
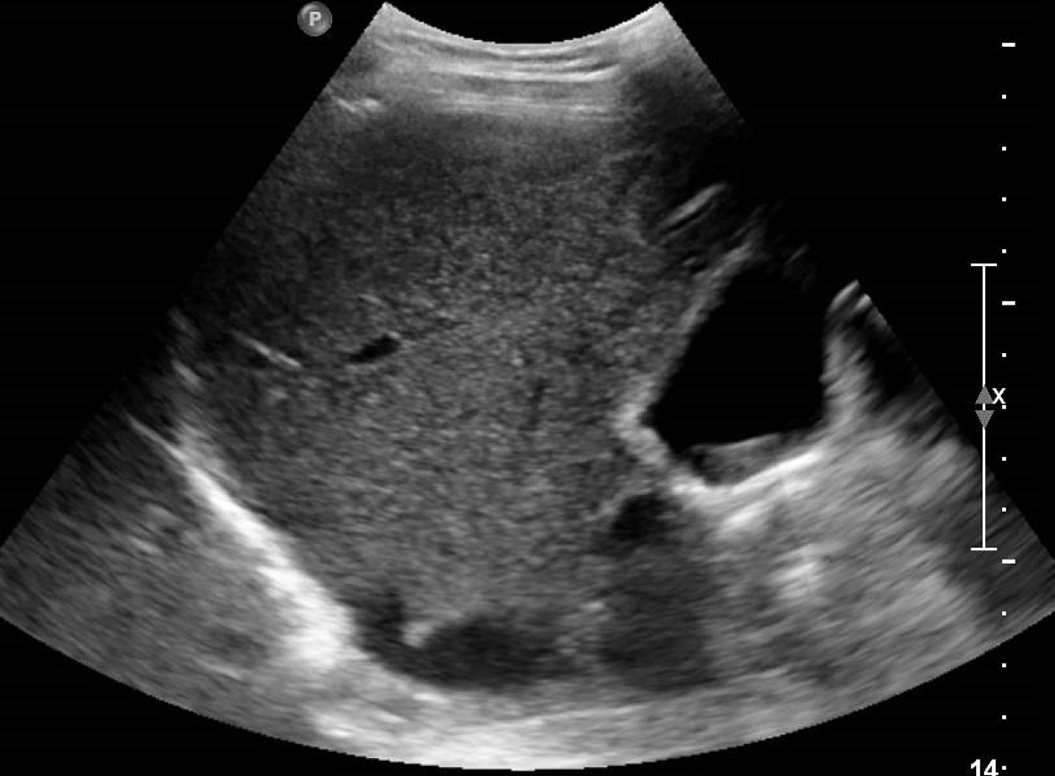
Figure 10 A 46-year-old woman with right hypochondrium pain.
Abdominal ultrasound showed an enlarged gallbladder containing deposit of biliary sludge in the infundibular region.
Pancreatic involvement has also been described in COVID-19 patients. As for other districts, the pathogenetic mechanism is not yet clear and could be the result of direct (cytopathic effect of the virus) or indirect (immune-mediated storm) mechanisms[62].
The presence of the ACE2 receptor on the cells of the pancreatic islets and exocrine glands allows the penetration of the virus thus determining the manifestations. In a series of 52 patients with COVID-19 pneumonia by Wang et al[63], 17% of them had pancreatic injury with elevated blood glucose levels. These results show potential mild pancreatic injury patterns in patients with COVID-19 pneumonia with no severe pancreatitis as a common manifestation[63]. However, some cases of pancreatitis have been reported in the literature[64]; in 64 patients with severe COVID-19 Liu et al[62] reported that 17.9% and 16.4% had increased amylase and lipase levels, respectively[62]. Thirteen out of 64 patients were examined with CT scans; of these only 5 patients showed radiological pancreatic alterations. It is worth underlining that most of these cases have been reported in moderate or severe disease. This seemingly suggests that the pathophysiology of pancreatitis could be based on the systemic inflammatory response rather than a direct histopathological effect[62].
The radiological signs of pancreatitis in COVID-19 patients were the same as we find in classic acute pancreatitis: Focal or diffuse parenchymal enlargement and density changes due to edema with indistinct pancreatic margins and stranding of retroperitoneal fat[65] (Figure 11). More rarely, necrotic-hemorrhagic forms of pancreatitis have been described[65]. The presence of gas (emphysematous pancreatitis) and the presence of calcifications as a sign of acute on chronic pancreatitis have also been reported[66].
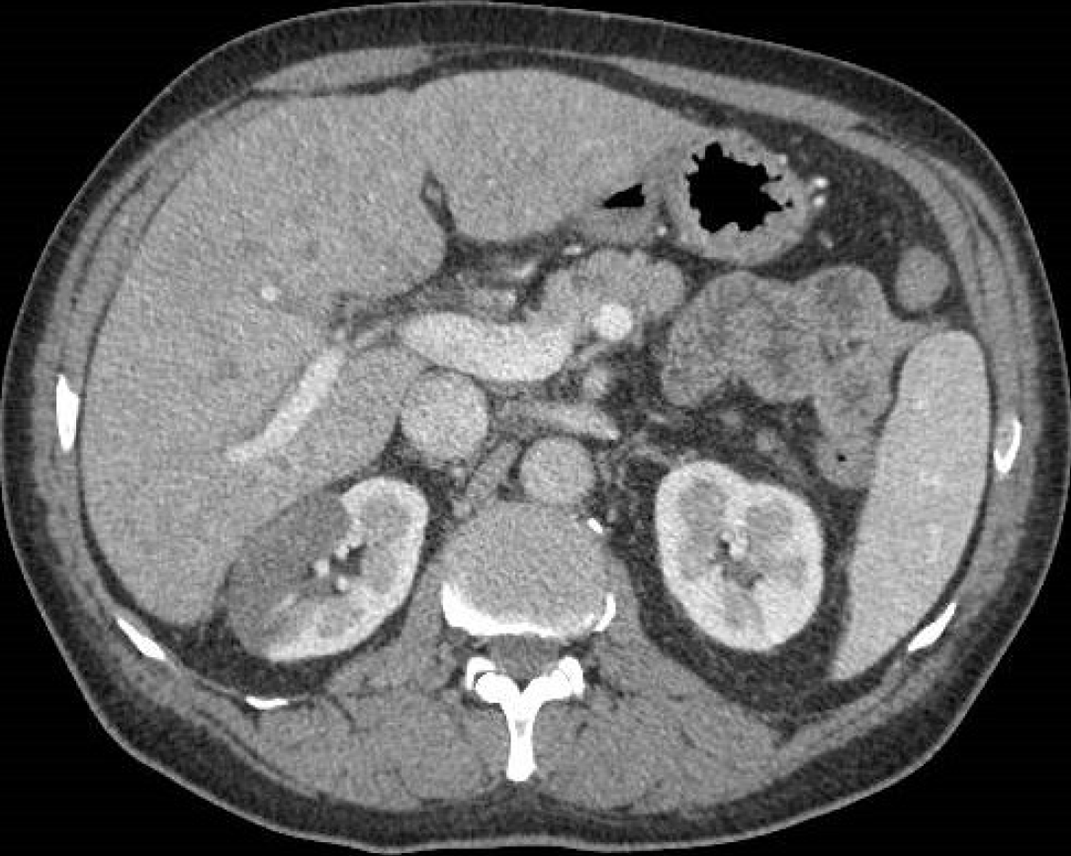
Figure 11 A 60-year-old man with abdominal pain and increased amylase and lipase levels.
Abdominal contrast-enhanced portal-venous phase computed tomography image showed fluid collections at the level of the pancreatic head and isthmic region and thickening of the left anterior pararenal fascia and perivisceral fat.
URINARY TRACT
There is increasing evidence that acute kidney injury (AKI) develops commonly in COVID-19 patients[67] because it affects approximately 20%-40% of patients admitted to the hospital and particularly to the intensive care unit in Europe and in the United States[68,69]. COVID-19 patients developing AKI, in conjunction with respiratory symptoms, have a poor prognosis, with a 35% reported mortality[70].
Possible causes of COVID-19-related AKI include dehydration, hypoperfusion from myocardial dysfunction, immune response dysregulation (cytokine storm) or direct kidney endothelial damage by SARS-CoV-2[69], which manifests clinically and pathologically by the development of acute tubular necrosis, interstitial inflammation, podocytopathy, microangiopathy and collapsing glomerulopathy[69].
In a postmortem renal histopathological analysis of 26 COVID-19 patients, Su et al[71] reported a histopathological finding of acute tubular necrosis due to endothelial damage causing microvascular lumen occlusion. The authors hypothesized that their results had been dependent on the possible kidney cells’ infections with SARS-CoV-2[71].
In this setting the imaging modality of choice is US. It is a bedside examination that can show increased cortical echogenicity or heterogeneity and loss of cortico-medullary differentiation in patients with COVID-19 and AKI[72]. In cases of renal infarction, heterogeneity and hypoperfusion of the renal parenchyma and wedge-shaped areas of decreased perfusion and/or enhancement may be visualized on US and contrast-enhanced CT and may be multifocal, involving both kidneys[72] (Figure 12). It is important to note that if renal function is impaired, the use of iodinated contrast material is not recommended, thus making US the imaging modality of choice in the evaluation of COVID-19 patients with suspected renal vascular injury.
Figure 12 A 69-year-old man with hematuria and right abdominal pain.
Abdominal contrast-enhanced portal-venous phase computed tomography image depicted wedge-shaped parenchymal defects that involved both the renal cortex and medulla with extension to the capsular surface, suggesting a renal infarct.
In a retrospective study, Hectors et al[73] found that cortex-to-aorta enhancement index (i.e. the ratio of renal cortical density to aorta density on contrast-enhanced CT) at the time of COVID-19 diagnosis was significantly reduced in patients who ultimately developed AKI. The authors suggest that reduced renal perfusion in COVID-19 precedes full-fledged AKI[73].
In another retrospective study, Huang et al[74] highlighted the usefulness of non-contrast CT on the assessment of renal impairment associated with COVID-19, featuring as perinephric fat stranding (PFS) and decreased renal parenchymal density. PFS corresponds to the thickening of perinephric bridging septa, which are fibrous lamellae that divide the perinephric space into multiple compartments, limiting the distribution of fluids, such as urine, pus and blood[75]. In this study, patients with PFS showed elevated serum creatinine levels, higher than that of the group of COVID-19 patients without PFS. Always in this study renal parenchymal attenuation in COVID-19 patients decreased. Moreover, patients with PFS showed a greater attenuation decrease, whereas patients without PFS presented a smaller decrease[75]. Therefore, the authors propose the use of PFS and renal parenchymal attenuation on non-contrast CT as a qualitative indicator for detecting renal damage associated with COVID-19.
Currently, no report has demonstrated the association between COVID-19 and urolithiasis[76], despite the nonsteroidal anti-inflammatory drugs (commonly used in stone-related colic pain) increase ACE2. This might increase the risk of developing severe and fatal COVID-19[77]. Nevertheless, the United States Food and Drug Administration recently announced that there was not enough scientific evidence connecting the use nonsteroidal anti-inflammatory drugs with worsening COVID-19 symptoms[78].
SPLEEN
Splenic injury is commonly encountered in COVID-19 patients. Red pulp cells and endothelial cells of blood vessels show an abundance of ACE2 receptors on their surfaces, so COVID-19 can directly target macrophages and dendritic cells in the spleen[79]. Autopsies in patients who died from COVID-19 exhibited splenic parenchymal congestion, hemorrhage and lack of lymphoid follicles with splenic parenchymal atrophy[79].
Splenic injuries are characterized by splenomegaly and by the development of either solitary or multifocal splenic infarcts[79]. On US examination splenic infarcts are hypoechoic compared to the remaining splenic parenchyma, although in the acute phases they may appear isoechoic and therefore difficult to identify. The morphology of the infarcts can vary as they may be classically wedge-shaped but also rounded, with irregular or smooth profiles. Over time, phenomena of contraction and scarring can develop, and in this case, they will appear as a hyperechoic region with retraction of the splenic capsule. In case of liquefaction, the area may be rounded and anechoic (splenic pseudocyst)[80]. CT is often considered the imaging method of choice, with the best visualization of infarcts during the portal-venous phase, to avoid confusing the heterogeneous improvement normally seen during the arterial phase. The CT imaging characteristics may vary with the stage of the infarct. In the hyperacute phase, there are areas of greater mottled attenuation, which represent areas of a hemorrhagic infarction typically with a wedge-shaped morphology and peripheral localization[81]. In the chronic phase, splenic infarcts may no longer be seen, or more commonly they may undergo fibrotic contraction with consequent loss of volume of the splenic parenchyma (Figure 13). As mentioned for the US, if the infarct liquefies, a cystic lesion can be left with central fluid density[81].
Figure 13 A 77-year-old man with abdominal tenderness.
A: Contrast-enhanced portal-venous phase computed tomography image of the abdomen demonstrated a wedge-shaped low-attenuation area at the level of the spleen, typical of infarction. Pericardial effusion was also present; B: A further rounded low-attenuation area with peripheral localization was present in a lower portion of the spleen on contrast-enhanced portal-venous phase computed tomography scan.
RADIOLOGICAL COMMENTS
As pointed out by the data of the most recent literature, COVID-19 is a systemic disease and not just a pulmonary disease, with a specific tropism for the vascular system. In fact, many other organs besides the lung can be involved by SARS-CoV-2 and thus the clinical manifestations can be variable. The gastrointestinal tract and hepato-biliary system are mainly affected, although the pancreas, urinary tract and spleen may also be involved.
The abdominal radiological findings are nonspecific and with poor pathological correlation reported in the literature. The correct use of imaging modalities in the management of these patients can be extremely helpful for the clinicians. US and particularly CT with multiphasic acquisition are the diagnostic methods mainly utilized in the COVID-19 patients with abdominal clinical symptoms and signs.
Although US can be quickly performed at the bedside and in intensive care units, this diagnostic modality is not always able to provide us reliable information in critically ill, unprepared and uncooperative patients. It also has the problem of exposing healthcare personnel to a consistent risk of infection and this limits its routine use in this setting. On the basis of the published data, US is mainly used for the hepatobiliary system, kidney and spleen evaluation, less for the pancreas and almost not utilized for gastrointestinal system assessment.
CT plays a pivotal role in identifying the signs of abdominal involvement, particularly for the gastrointestinal system, and is extremely useful for the evaluation of the vascular and parenchymal structures. Thanks to this method we can establish the severity and the evolution of the disease in COVID-19 patients. CT study should be performed at the baseline and after iodinated contrast medium injection in the various phases, paying attention to the arterial phase for ischemic lesions of the intestine and kidneys, in the portal-venous phase for wall thickening in colitis or for signs of acute pancreatitis or splenic infarcts and in the late phase for the evaluation of renal nephritis or ischemia.
Actually, magnetic resonance imaging has no defined role, and no significant experience is reported to our best knowledge. This is probably due to the fact that COVID-19 patients have difficulty undergoing magnetic resonance examination and the serious problems of management of these patients.
What is desirable in the future is that the abdominal and gastrointestinal manifestations identified by imaging may have a pathological correspondence in order to further clarify the etiopathogenetic mechanisms of the damage caused by COVID-19. Furthermore, larger series of COVID-19 patients studied with imaging methods could help us to identify the most characteristic signs of abdominal and gastrointestinal involvement. In this setting, magnetic resonance imaging could be particularly useful for the evaluation of the hepato- biliary system, pancreas and kidney.
CONCLUSION
Although radiological signs are not specific of abdominal and gastrointestinal involvement, the diagnostic imaging modalities and in particular CT are helpful for the clinician in the management, evaluation of the severity and evolution of COVID-19 patients.