Published online Jun 28, 2021. doi: 10.3748/wjg.v27.i24.3581
Peer-review started: January 15, 2021
First decision: March 29, 2021
Revised: April 9, 2021
Accepted: May 27, 2021
Article in press: May 27, 2021
Published online: June 28, 2021
Processing time: 161 Days and 1.2 Hours
Fasudil, as a Ras homology family member A (RhoA) kinase inhibitor, is used to improve brain microcirculation and promote nerve regeneration clinically. Increasing evidence shows that Rho-kinase inhibition could improve liver fibrosis.
To evaluate the anti-fibrotic effects of Fasudil in a mouse model of liver fibrosis induced by thioacetamide (TAA).
C57BL/6 mice were administered TAA once every 3 d for 12 times. At 1 wk after induction with TAA, Fasudil was intraperitoneally injected once a day for 3 wk, followed by hematoxylin and eosin staining, sirius red staining, western blotting, and quantitative polymerase chain reaction (qPCR), and immune cell activation was assayed by fluorescence-activated cell sorting. Furthermore, the effects of Fasudil on hepatic stellate cells and natural killer (NK) cells were assayed in vitro.
First, we found that TAA-induced liver injury was protected, and the positive area of sirius red staining and type I collagen deposition were significantly decreased by Fasudil treatment. Furthermore, western blot and qPCR assays showed that the levels of alpha smooth muscle actin (α-SMA), matrix metalloproteinase 2 (MMP-2), MMP-9, and transforming growth factor beta 1 (TGF-β1) were inhibited by Fasudil. Moreover, flow cytometry analysis revealed that NK cells were activated by Fasudil treatment in vivo and in vitro. Furthermore, Fasudil directly promoted the apoptosis and inhibited the proliferation of hepatic stellate cells by decreasing α-SMA and TGF-β1.
Fasudil inhibits liver fibrosis by activating NK cells and blocking hepatic stellate cell activation, thereby providing a feasible solution for the clinical treatment of liver fibrosis.
Core Tip: Liver fibrosis is caused by inflammation and characterized by accumulation of the extracellular matrix; there is no clinically safe and efficient drug to treat this condition. Fasudil treatment inhibited liver injury and liver fibrosis in vivo, and prevented liver fibrosis via activating natural killer cells but suppressing hepatic stellate cells in a thioacetamide-induced model. As a drug used clinically, these results provide a feasible solution for the clinical treatment of liver fibrosis.
- Citation: Han QJ, Mu YL, Zhao HJ, Zhao RR, Guo QJ, Su YH, Zhang J. Fasudil prevents liver fibrosis via activating natural killer cells and suppressing hepatic stellate cells. World J Gastroenterol 2021; 27(24): 3581-3594
- URL: https://www.wjgnet.com/1007-9327/full/v27/i24/3581.htm
- DOI: https://dx.doi.org/10.3748/wjg.v27.i24.3581
Liver fibrosis is caused by inflammation and characterized by the accumulation of extracellular matrix (ECM), which are major characteristics of chronic liver diseases such as cirrhosis and primary hepatic carcinoma[1]. The traditional view is that liver fibrosis is irreversible, but recent studies have shown that liver fibrosis can be partially reversed in experimental models of liver fibrosis[2]. Unfortunately, to date, there is no clinically safe and efficient drug to treat liver fibrosis in humans.
The mechanisms underlying hepatic fibrosis are inflammatory reaction, hepatic stellate cell (HSC) activation, and the biological function of various cytokines[3]. HSCs, as hepatic lipid-storing cells, are sensitive to hepatic injury and participate in the pathogenesis of liver fibrosis[4]. During chronic diseases, agents from damaged hepatocytes or other cells can promote the activation of HSCs. Then these activated HSCs become highly proliferative myofibroblast-like cells, exhibiting a migratory phenotype and producing large amounts of extracellular matrix proteins such as collagens and fibronectin, thereby leading to the development of hepatic fibrosis[5,6]. Therefore, HSC-specific targeted strategies are required for the effective treatment of liver fibrosis.
Immune cells in the liver exert different roles during the pathogenesis of liver fibrosis. Hepatic macrophages promote fibrosis by perpetuating an inflammatory phase, which leads to the release of pro-inflammatory cytokines and chemokines, and then the activation of HSCs[7]. T helper type 2 (Th2) cells can produce cytokines, including interleukin (IL)-13, IL-4, and IL-5, which are shown to promote fibrosis[8]. Natural killer (NK) cells suppress the pathogenesis of liver fibrosis by killing HSCs and producing interferon-γ (IFN-γ). Also, NK cells can induce apoptosis of HSCs and help clear senescent-activated HSCs, thereby facilitating the resolution of fibrosis, while the exact mechanisms remain unknown. NK cells are suppressed during the advanced stage of liver fibrosis in mice and patients[9,10]. Thus, properly regulating the activation of intrahepatic immune cells is essential for treating liver fibrosis. Practically, the restoration and promotion of NK cell activity might be an attractive strategy for the regression of liver fibrosis.
Ras homology family member A (RhoA) and its major downstream effector Rho-associated protein kinase (ROCK) play important roles in several downstream effects of the small GTP-binding protein Rho. Several cellular events including adhesion, motility, and contractility are regulated by Rho kinase[11]. Furthermore, increasing evidence has demonstrated that Rho kinase inhibition can improve liver fibrosis. The Rho kinase, Y27632, decreases fibrotic parameters in models of liver fibrosis, and inhibits the activation status of the primary HSCs[12]. Additionally, RhoA-ROCK signaling pathway also plays a key role in regulating the function of immune cells. For example, the RhoA-ROCK pathway promotes the activation of downstream chemokine receptors. Therefore, T-cell activation, polarization, and migration are promoted by chemokines[13]. Fasudil, as a first-generation selective Rho/ROCK inhibitor in the clinic, is clinically used to improve brain microcirculation and promote nerve regeneration[14]. More importantly, Fasudil can prevent lung and skin fibroses in hypochlorous acid-injected mice[15]. Recently, Fasudil was shown to exert anti-inflammatory effects and markedly reduce the accumulation of ECM in type 1 diabetic rats[16]. However, the viability of Fasudil for liver fibrosis therapy and the associated mechanisms are still unclear. The regulatory effects of Fasudil on NK cells in liver fibrosis have not been fully explained.
In this study, we determined the effects of Fasudil on the progression of liver fibrosis and clarified the related mechanisms. We found that Fasudil performed anti-proliferative effects in a mouse model of thioacetamide (TAA)-induced liver fibrosis, providing a feasible solution for the clinical treatment of liver fibrosis.
C57BL/6 mice (male, 4-6 wk old) were provided by HuaFuKang Biological Technology Co., Ltd. (Beijing, China). The animals were caged under specific pathogen-free conditions, housed under a controlled temperature 23 ± 1°C and relative humidity 45%. The animal model of hepatic fibrosis was induced by administration of TAA at 200 mg/kg (T104039; Aladdin, Shanghai, China) once every 3 d for 12 times[17]. At 1 wk after induction with TAA, Fasudil (10 mg/kg) was intraperitoneally injected once a day for 3 wk. The procedures were approved by the Research Ethics Committee of Shandong University (Jinan, China).
LX-2, an immortalized human hepatic stellate cell line preserved in our laboratory, was grown in Dulbecco’s modified Eagle medium (Thermo Fisher Scientific, Inc., Waltham, MA, United States). This cell line carries a typical phenotype and biochemical properties of activated HSCs, and was checked by referring to the International Cell Line Authentication Committee and National Center for Biotechnology Information databases[18]. Fasudil was purchased from Tianjin Chase Sun Pharmaceutical Co. Ltd. (Tianjin Shi, China).
In a TAA-induced hepatic fibrosis model, the mice were sacrificed under mild ether anesthesia after Fasudil treatment. Then mouse liver specimens were fixed overnight in 4% paraformaldehyde/phosphate-buffered saline (PBS) and embedded in paraffin. Then liver sections were examined and photographed under a light microscope after hematoxylin and eosin (Beyotime, Shanghai, China) staining. The criteria used for scoring fibrosis and inflammation were as follows: Score 0, normal (no visible fibrosis and inflammation); score 1, fibrosis and inflammation present (5%-30%); score 2, mild fibrosis and inflammation (31%-50%); and score 3, severe fibrosis and inflammation (51%-75%).
Liver specimens were stained with 0.4% sirius red in saturated picric acid for 0.5 h. The positively stained area was selected by threshold adjustment on a gray scale picture using Image J software (NIH, Bethesda, MD, United States) and the ratio of positively stained area/total area was then calculated. At least five different fields on each slide were measured. The stained area were determined using Image-pro Plus 6.0 software.
The treated cells were added to TRIzol (Invitrogen, Thermo Fisher Scientific, Inc.), and the total RNA was extracted from cells following the manufacturer’s instructions. Then cDNA was obtained and synthesized using M-MLV Reverse Transcriptase (Invitrogen; Thermo Fisher scientific, Inc.). The mRNA levels of matrix metalloproteinase 2 (MMP-2), MMP-9, transforming growth factor beta (TGF-β), B-cell lymphoma 2 (Bcl-2), Bcl-2-associated X protein (Bax), and Ki67 were detected according to the SYBR Green Master Mix kit instructions (Roche Diagnostics, Indianapolis, IN, United States). The primer sequences for quantitative polymerase chain reaction (qPCR) amplification are listed in Table 1. PCR was conducted using the iCycleriQ real-time PCR system (Bio-Rad Laboratories, Inc., Hercules, CA, United States).
| Species | Gene | Primer (5' to 3') |
| Mouse | β-actin | F: ATCATGTTTGAGACCTTCAACA |
| R: CATCTCTTGCTCGAAGTCCA | ||
| Mouse | MMP2 | F: CTCCTGGTATGAGATAGCAAA |
| R: TGCACCACCAACTGCTTAGC | ||
| Mouse | MMP9 | F: TGCATCTTGGCTTTGCAGCTCTTCCTCATGGC |
| R: TGGACCTGTGGGTTGTTGACCTCAAACTTGGC | ||
| Mouse | α-SMA | F: CTGACAGAGGCACCACTGAA |
| R: GAAGGAATAGCCACGCTCAG | ||
| Human | α-SMA | F: GATCTCAGTGCAGAGGCTCG |
| R: TTTGCTTGTCCAGGTGGTCC | ||
| Human | Ki67 | F: TCCTCCAGGGATCCAACGA |
| R: GGCAGGCGGGAGGTCTT | ||
| Human | Bcl-2 | F: CTTCCCTCATCCTCCTGCTAC |
| R: ACAAACTGGGTAAAGGTGATGG | ||
| Human | Bax | F: CTCCTGGTATGAGATAGCAAA |
| R: TGCACCACCAACTGCTTAGC | ||
| Human | Rho | F: TCCTCCAGGGATCCAACGA |
| R: GGCAGGCGGGAGGTCTT | ||
| Human | β-actin | F: CACTGTGTTGGCGTACAGGT |
| R: TCATCACCATTGGCAATGAG |
After treatment, the cell cycle distribution of HSCs seeded was assayed by flow cytometry. Briefly, HSCs (1.5 × 105) were plated in 12-well plates and treated with Fasudil at the indicated concentrations (5 and 10 mM). After 24 h, cells were harvested and washed with ice-cold PBS. Then they were fixed in cold 70% ethanol and stored at 4°C overnight, and the fixed cells were washed and re-suspended in 1 mL staining solution containing 50 mg/mL propidium iodide (PI) (Beijing Solarbio Science & Technology, Co., Ltd.) and 100 mg/mL RNase for 30 min at 37°C. Then cell cycle analysis was conducted using a flow cytometer (BD Calibur).
FITC-Annexin V/PI (eBioscience; Thermo Fisher Scientific, Inc.) was utilized to determine rates of apoptosis. LX2 cells were plated and treated with Fasudil (5 mM, 10 mM) for 24 h. Cells were harvested and stained following the manufacturer’s instructions. Finally, the flow cytometry data were analyzed using WinMDI.
Protein was extracted from LX-2 cells, NK-92 cells, or liver tissues with RIPA protein lysis buffer (1% Triton X-100, 1% deoxycholate, 0.1% sodium dodecyl sulfate [SDS]) (Beyotime, Shanghai, China). The protein concentration of each sample was determined by the BCA assay. Proteins (30 μg/lane) were separated by SDS-polyacrylamide gel electrophoresis using a 10% polyacrylamide gel, and then transferred to PVDF membranes (Millipore, Billerica, MA, United States). The following primary antibodies were used: Anti-phosphorylated extracellular signal-related kinase (p-ERK) (#14227S; Cell Signaling Technology, Danvers, MA, USA), anti-ERK (#4348S, Cell signaling Technology), alpha smooth muscle actin (α-SMA) (#4668S; Cell signaling Technology), Collagen I (WL008; Wanleibio Co., Ltd., Shenyang, China), and β-actin (#3700; Cell signaling Technology). For ECL detection, the membranes were incubated with horseradish peroxidase-conjugated goat anti-rabbit secondary antibody (#A0208; Beyotime, Shanghai, China) at a dilution of 1:10000 for 1 h at 37ºC. ECL detection was performed according to the manufacturer’s protocol (Millipore). Analysis of blots was performed using Image J.
Mice were euthanized and the liver tissues were harvested, and then intrahepatic liver mononuclear cells were isolated as indicated previously[19]. Antibodies used for fluorescence-activated cell sorting (FACS) analysis were PerCP-Cy5.5 anti-cluster of differentiation 3 (CD3) (#35-0031-82), FITC anti-NK1.1 (#11-5941-82), PE anti-CD69 (#12-0691-82), PE anti-NKG2A (#12-5897-81), PE anti-NKG2D (#12-5882-82), or isotype-matched controls (eBioscience, San Diego, CA, United States). Cells were analyzed on a FACSCalibur flow cytometer, and then analyzed using WinMDI analysis software.
The cytolytic activity of NK-92 cells against LX2 was determined using the MTT assay. Briefly, 1 × 104 target cells (LX2 cells) were seeded into 96-well plates. NK-92 cells, pretreated with Fasudil (10 mM) overnight, were added to target cells at effector/target ratios of 1:20, 1:10, or 1:5. Then the effector and target cell were co-incubated for 12 h, and then 20 µL MTT (5 mg/mL) were added. After 4 h, the absorbance (A) at 490 nm was determined using a scanning multi-well spectrophotometer (Bio-Rad). The percent specific lysis was calculated using the following formula: % specific lysis =1- (ODE+T- ODE) / ODT × 100%.
Data are shown as mean ± SD of three independent experiments. Significant differences were analyzed using the Student’s t-test or one-way analysis of variance. Statistical analyses were conducted using GraphPad Prism (version 5.0a). aP < 0.05, bP < 0.01, cP < 0.001.
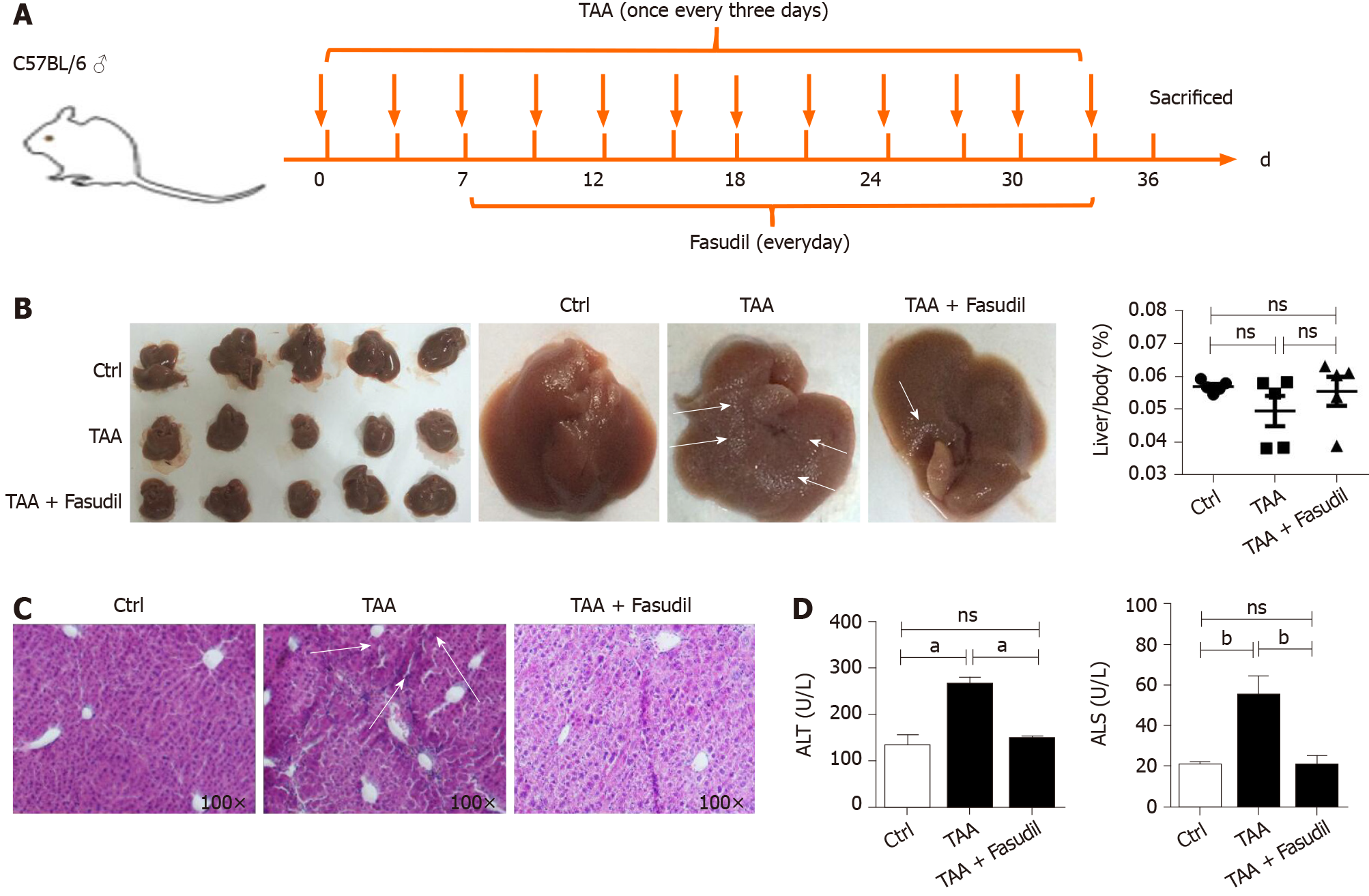
To clarify whether Fasudil influences hepatic fibrosis, a fibrotic mouse model was induced by intraperitoneal injection of TAA (200 mg/mL) once time every 3 d[11]. At 7 d after induction with TAA, Fasudil (10 mg/kg) was injected once a day for 3 wk, and then mice were sacrificed (Figure 1A). As shown in Figure 1B, compared with the normal gross features of the livers observed in the control group, the liver surface from the TAA-treated group showed several spots of nodules, while the livers from the TAA + Fasudil group had much fewer nodular spots. There was no significant difference in liver/body ratio among these three groups. Meanwhile, the liver of the TAA-treated group exhibited a lobular structure destruction and inflammatory cell infiltration. Also, livers from TAA-treated mice gained loss of structural integrity, while TAA + Fasudil-treated mice revealed structural integrity of liver with no significant inflammatory cell infiltration (Figure 1C). Fasudil significantly lowered the indices of liver (Table 2). Furthermore, we observed a significant reduction of alanine aminotransferase and aspartate aminotransferase in serum after Fasudil treatment compared with TAA-treated mice (P < 0.05; Figure 1D). These data suggest that Fasudil might blunt TAA-induced liver fibrosis by preventing liver injury.
| Group | Pathologic grading of hepatic fibrosis | |||
| 0 | I | II | III | |
| Ctrl (n = 5) | 15 | 0 | 0 | 0 |
| TAA (n = 5) | 0 | 0 | 6 | 7 |
| TAA + Fasudil | 0 | 7 | 6 | 2 |
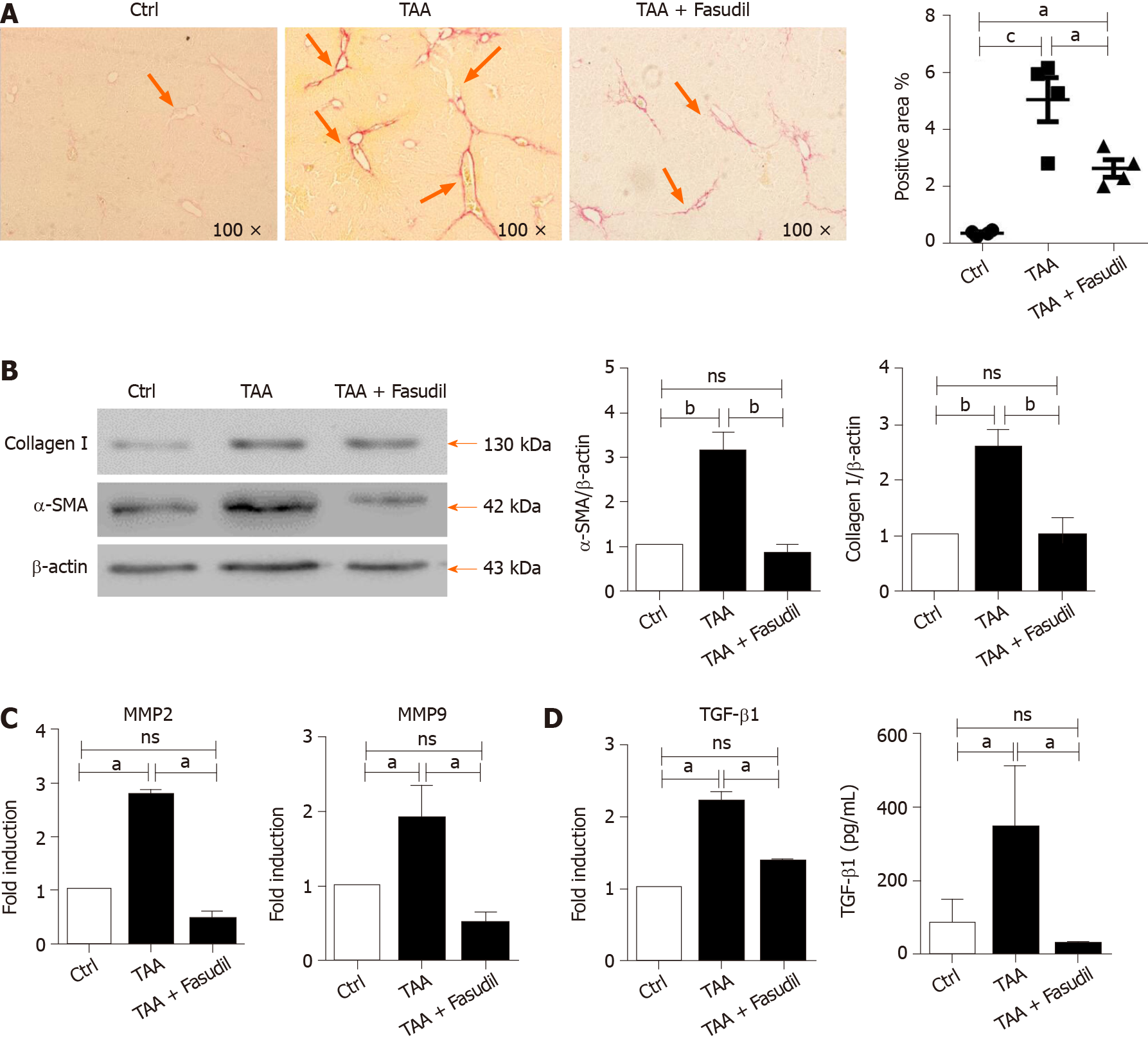
Hepatic collagen I deposition is an important characteristic of liver fibrosis level in mice[20]. As shown in Figure 2A, sirius red staining exhibited a significant increase in perisinusoidal collagen fibers in the TAA-treated group, which was significantly decreased by Fasudil treatment (P < 0.05). Furthermore, fibrosis-related genes such as α-SMA, MMP-2/9, and TGF-β1[21] were analyzed to evaluate the impact of Fasudil on hepatic fibrosis. As shown in Figure 2B, the expression of α-SMA and collagen I in TAA + Fasudil-treated mice was significantly lower than that in TAA-treated group. Meanwhile, mRNA levels of MMP-2 and MMP-9 were also downregulated by Fasudil treatment (Figure 2C). TGF-β1 induced by TAA was significantly decreased at both the mRNA and protein levels after Fasudil treatment (P < 0.05; Figure 2D). These data clearly demonstrate that TAA-induced liver fibrogenesis is markedly inhibited by Fasudil treatment.
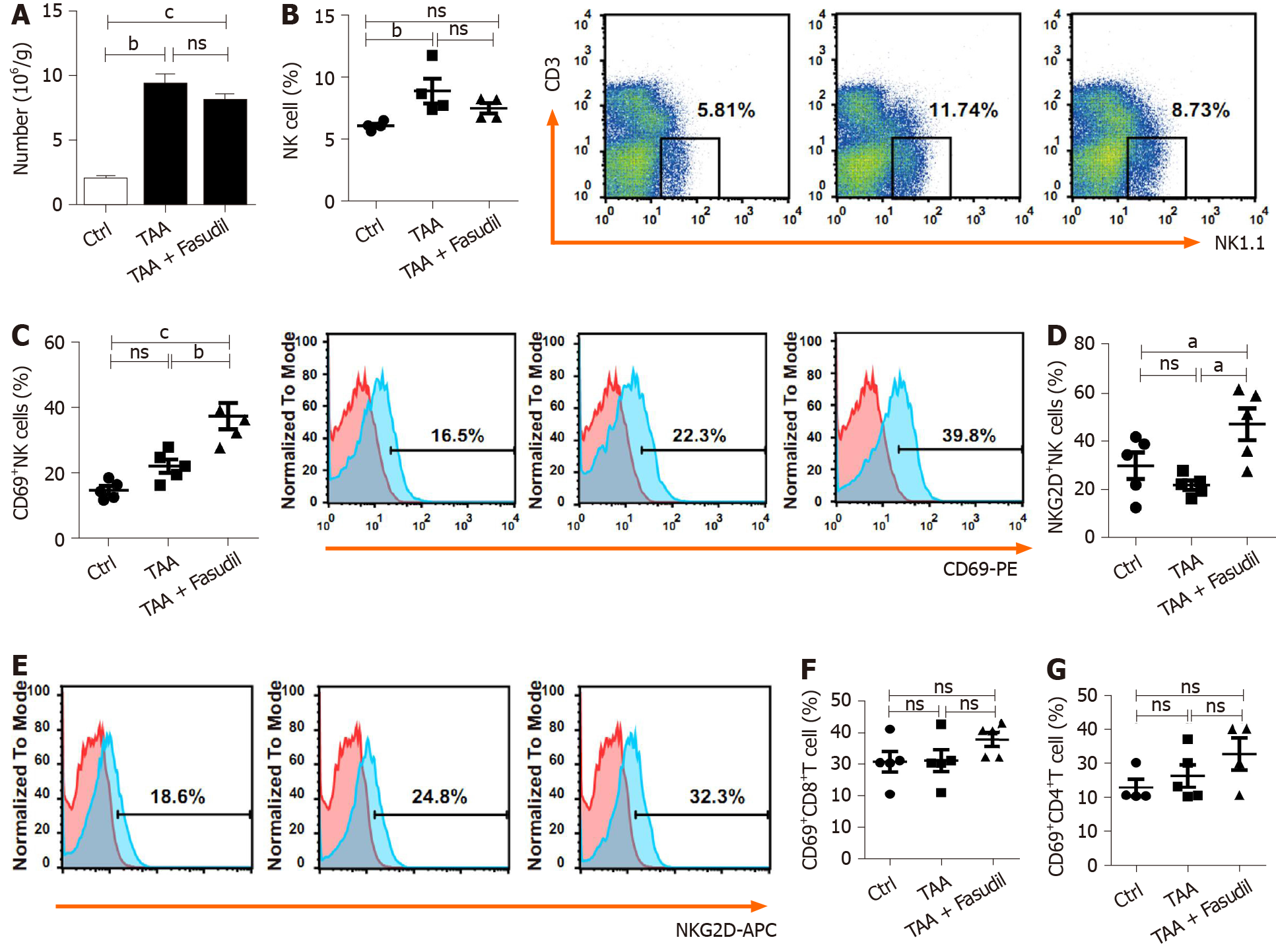
Immune cells in the liver play critical roles during the development of liver fibrosis[22]. As shown in Figure 3A, TAA treatment increased the total number of mononuclear cells in the liver compared to the normal control group, while no significant differences were observed between TAA-treated and TAA + Fasudil-treated groups. NK cells suppress liver fibrosis via strong cytolysis against HSCs[23,24], and impaired NK cell may contribute to accelerated liver fibrosis progression in a TAA-induced fibrotic mouse model. Here, the proportion of NK cells was not significantly affected after Fasudil treatment (Figure 3B), while the level of the activation marker CD69 was markedly increased in NK cells from TAA + Fasudil-treated mice than that from TAA-treated mice (P < 0.05; Figure 3C), accompanied by the elevation of activation receptor NKG2D (Figure 3D). Meanwhile, the proportion of CD69+CD8+T cells (Figure 3E) and CD69+CD4+T cells (Figure 3F) in the liver was not significantly affected by Fasudil treatment. These data suggest that NK cells might be activated by Fasudil and exhibit anti-liver fibrosis effects in a TAA-induced fibrotic mouse model.
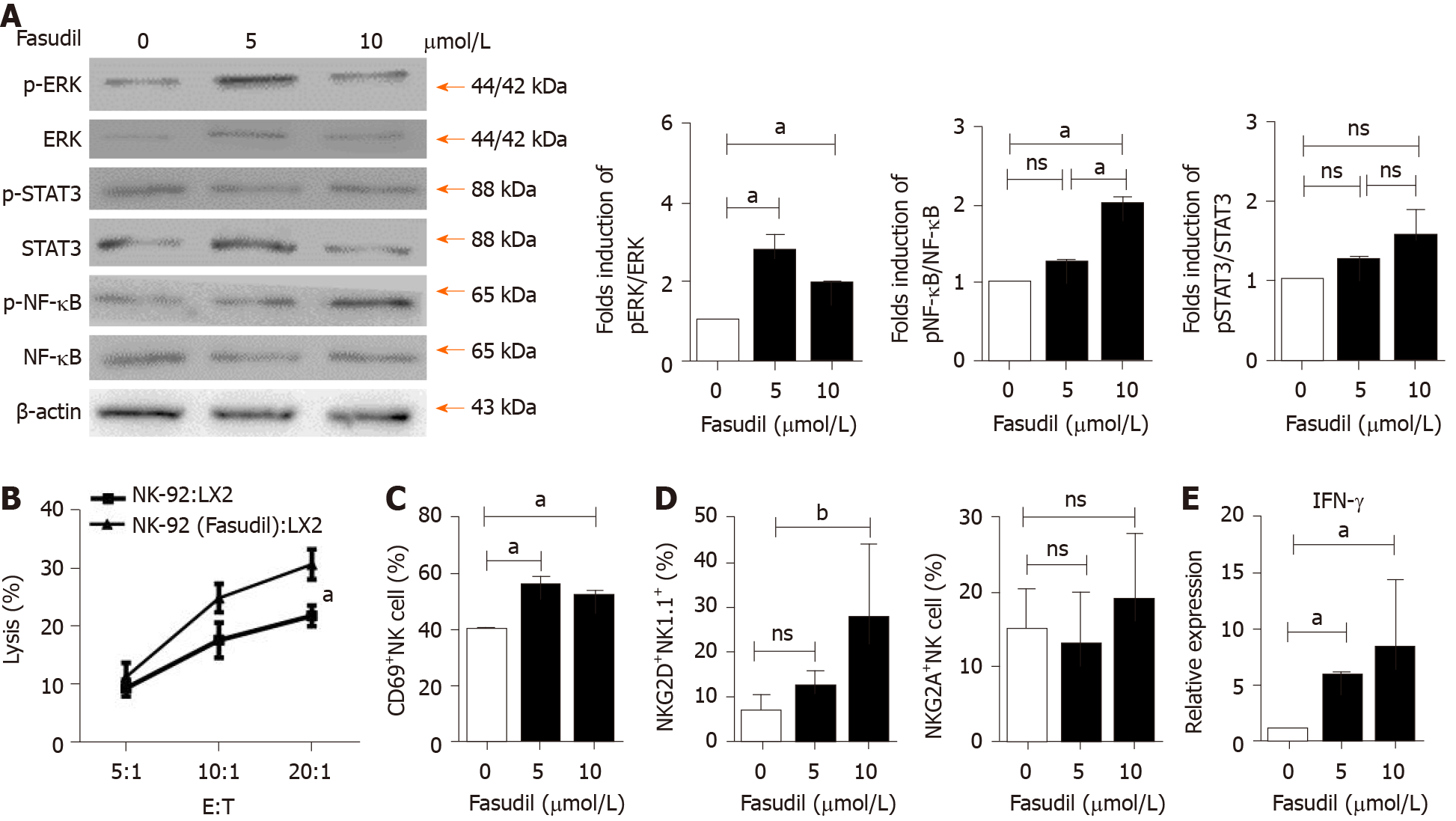
To investigate the mechanism by which Fasudil treatment suppresses the progression of liver fibrosis by activating NK cells, human NK-92 cells were treated with different concentrations of Fasudil (5 mM, 10 mM) for 24 h in vitro, and the concentration of 10 mM was equivalent to in vivo experiments. Then the levels of ERK, nuclear factor kappa B (NF-κB) and signal transducer and activator of transcription 3 (STAT3) were examined by western blotting. The levels of p-ERK and p-NF-kB were significantly increased in Fasudil-treated NK-92 cells, while activation of the STAT3 signaling pathway was not affected by Fasudil (Figure 4A). To determine the effects of NK cells activated by Fasudil on HSCs, we assayed the lysis of LX2 cells (human HSC cell line) by NK cells at different effector: target ratios. First, we treated NK-92 cells with Fasudil (10 mM) for 24 h, and assayed the cytotoxicity against cultured LX2 cells. We found that Fasudil treatment increased the lysis activity of NK cells against cultured LX2 cells (Figure 4B). Then, liver mononuclear cells from normal mice were isolated and treated with different concentrations of Fasudil for 24 h in vitro. FACS analysis showed the frequency of CD69+ NK cells increased after Fasudil treatment (Figure 4C). Furthermore, the activation receptor NKG2D was enhanced in NK cells, while the inhibitory receptor NKG2A was not significantly influenced by Fasudil treatment (Figure 4D). In addition, the proportion of IFN-γ+ NK cells was increased after Fasudil treatment (Figure 4E).
Taken together, these results demonstrate that Fasudil can promote NK cell activation and cytotoxicity by activating the ERK and NF-κB signaling pathways.
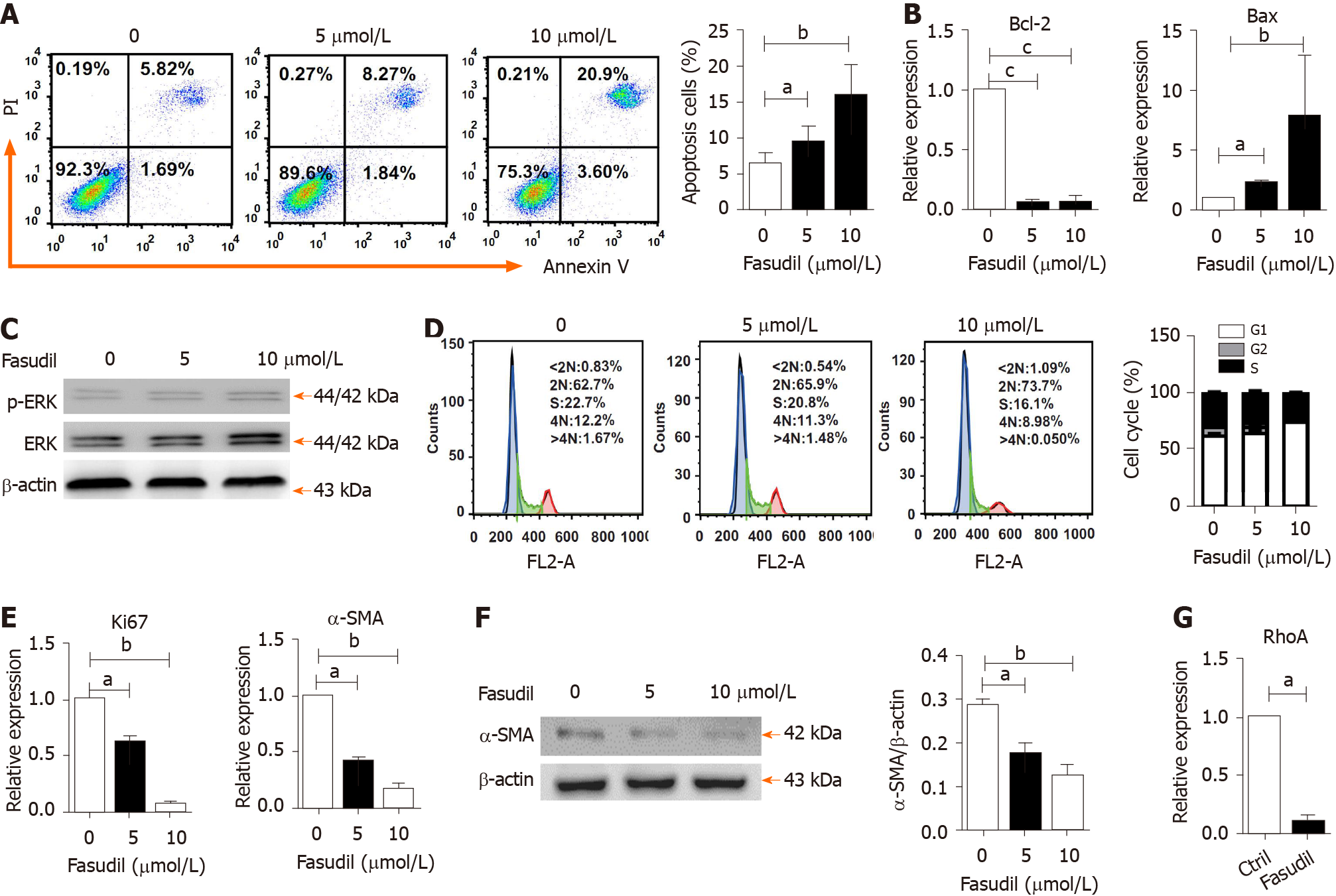
The proliferation and activation of HSCs are one of the main triggers of liver fibrosis[25]. To observe the direct effects of Fasudil on HSCs, LX2 cells were treated with different concentrations of Fasudil (5 mM, 10 mM) for 24 h in vitro, and then the survival of these HSCs was observed. As shown in Figure 5A, the percentage of apoptotic HSCs was promoted by Fasudil treatment. Meanwhile, the level of the anti-apoptotic gene Bcl-2 was decreased, while the level of the pro-apoptotic gene Bax was induced in LX2 cells treated with Fasudil (Figure 5B), accompanied by an increase in p-ERK level (Figure 5C). Furthermore, Fasudil arrested the HSC cell cycle in the G1 phase (Figure 5D). Accordingly, the proliferation-related gene Ki67 in LX2 cells was downregulated (Figure 5E). Previous studies have reported that RhoA is involved in expression of the fibrosis-associated protein α-SMA[26]. Fasudil is a RhoA kinase inhibitor; we found that Fasudil decreased the expression of α-SMA (Figure 5E and F) accompanied by the downregulation of RhoA (Figure 5G). These results indicate that Fasudil has a direct inhibitory effect on HSCs.
The current therapeutic interventions for liver fibrosis include removing the injurious stimuli, decreasing hepatic inflammation, suppressing HSC activation, and accelerating matrix degradation[27]. Repurposing old drugs for new clinical applications is a superior strategy for drug development. Recently, Rilpivirine, a widely used anti-HIV drug, was found to ameliorate liver fibrosis through selective STAT1-dependent induction of HSC apoptosis[28]. So, repositioning existing drugs might be an effective strategy for obtaining therapeutics against liver fibrosis.
Fasudil, a RhoA kinase inhibitor, is clinically applied for improving brain microcirculation and promoting nerve regeneration. Xie et al[16] found that RhoA/ROCK signaling pathway activation plays an important role in the development of diabetic hepatic fibrosis. Interestingly, the RhoA/ROCK-2 signaling pathway is necessary for activated HSC; the administration of RhoA/ROCK inhibitor can exert anti-inflammatory effects, markedly decrease collagen synthesis, and delay hepatic fibrosis[16,29]. In accordance with this, we found that liver injury and liver fibrosis induced by repeated injection of TAA were prevented by Fasudil treatment (Figures 1 and 2). In addition, several studies have proved that RhoA/ROCK pathway activation promotes TGF-β1 secretion in several models[30]. As a RhoA/ROCK inhibitor, Fasudil inhibited the induction of TGF-β1 secretion in TAA-induced liver fibrosis, consistent with previous studies.
The immune response plays important roles in the development and treatment of liver fibrosis[31]. NK cells can promote the secretion of IFN-γ through the Janus kinase-STAT signaling pathway, thereby promoting the killing of HSCs[32]. The inhibition of NK cells cytotoxicity promotes the accumulation of LX2 cells[33]. In this study, with the elevation of CD69 and NKG2D, hepatic NK cell activation was promoted by Fasudil treatment (Figure 3). Additionally, NK cells were activated in spleen from mice with Fasudil treatment (data not shown). ERK and NF-κB signals are critical for NK cell-mediated cytolysis of target cells[34]. Here, we found that Fasudil drove ERK and NF-κB activation, and augmented the cytolysis activity of NK cells against HSCs. In addition, Fasudil promoted the secretion of IFN-γ in NK cells (Figure 4). Although STAT3 activation is essential for NK cells to exert anti-tumor effects[35], it was not significantly influenced by Fasudil treatment (Figure 4). These results demonstrate the impact of Fasudil on NK cell function in fibrotic mouse model.
In terms of T cells, different T-cell subpopulations play different roles in the process of liver fibrosis. Previous studies have reported that T-cell impairment generally does not alter fibrogenesis, while Th2 and Th17 CD4+T cells are involved in the inflammatory process, promoting cytokine production and HSC activation[36]. Here, the ratio of CD4+ T cells and CD8+ T cells was upregulated in the liver and spleen (data not shown), but the activation of these T cells was not significantly affected by Fasudil treatment (Figure 3). The exact roles of T cell subtypes in Fasudil-treated liver fibrosis need to be further investigated.
HSCs play a vital role in the pathogenesis of liver fibrosis. They can secret fibrogenic factors and then promote several kinds of cell types including portal fibrocytes, fibroblasts, and bone marrow-derived myofibroblasts to produce collagen and thereby propagate fibrosis[4,37]. Interestingly, we found that Fasudil treatment significantly increased apoptosis and inhibited the proliferation of LX2 cells, indicating the direct effect of Fasudil on HSCs (Figure 5), consistent with previous studies[38]. Actually, the effect of ROCK signal pathway on apoptosis is complicated. For example, inhibition of the RhoA/ROCK signaling pathway promotes the apoptosis of human urethral scar fibroblasts[38], cardiac fibroblasts[39], and neutrophils[40]. However, the decrease of apoptosis by RhoA/ROCK inhibition is also found in several cell types, such as nitrergic neurons[41] and progenitor cells[42]. RhoA is positively correlated with the expression of α-SMA, a gene involved in liver fibrosis[43]. Indeed, Fasudil, as an inhibitor targeting RhoA kinases, significantly inhibited both the expression of RhoA and α-SMA in HSCs. In addition, ERK is an important mediator of signal transduction in HSCs, which is involved in the growth, differentiation, survival, and apoptosis of HSCs[44]. In line with previous studies, we observed an increase in the p-ERK level by Fasudil treatment, which would promote HSC apoptosis. Thus, Fasudil directly disrupts HSC activation and survival in TAA-induced liver fibrosis.
In conclusion, our findings demonstrated that Fasudil prevented TAA-induced liver fibrosis by directly suppressing the cell cycle and activation of HSCs, and activating NK cells. Because Fasudil is a drug that has been used clinically, it has high safety and reliability and can provide a feasible solution for clinical treatment of liver fibrosis.
Rho kinase inhibition reportedly improves liver fibrosis. Fasudil, as a RhoA kinase inhibitor, is used to improve brain microcirculation and promote nerve regeneration clinically. The viability of Fasudil in liver fibrosis is still unknown.
Repositioning existing drugs e.g., Fasudil might be an effective strategy for obtaining therapeutics against liver fibrosis.
To evaluate the anti-fibrotic effects of Fasudil in vitro and in a mouse model of thioacetamide (TAA)-induced liver fibrosis.
The anti-fibrotic effect of Fasudil was investigated in a TAA-induced mouse model. At 1 wk after induction with TAA, Fasudil was intraperitoneally injected once a day for 3 wk, hepatic pathological changes, liver fibrosis and immune cell activation were determined using hematoxylin and eosin staining, sirius red staining, western blotting and quantitative polymerase chain reaction and fluorescence-activated cell sorting. Furthermore, the effect of Fasudil on hepatic stellate cell (HSC) and natural killer (NK) cells was assayed in vitro.
Treatment with Fasudil alleviated hepatic pathological changes and reversed hepatic fibrosis in the TAA-chronic models with decreased deposition of collagen fibers, reduced expression of HSC activation marker (alpha smooth muscle actin), and reduced secretion of transforming growth factor beta 1 (TGF-β1), matrix metalloproteinase 2 (MMP-2), and MMP-9. Fasudil treatment increased NK cell activation and cytotoxicity by activating the extracellular signal-related kinase and nuclear factor kappa B signaling pathways. Fasudil directly promoted the apoptosis and inhibited the proliferation of HSC by decreasing α-SMA and TGF-β1.
Fasudil inhibited liver fibrosis by activating NK cells and blocking HSC activation.
Fasudil treatment prevents liver fibrosis via activating NK cells but suppressing HSCs. As a drug used clinically, these results provide a feasible solution for the clinical treatment of liver fibrosis.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Sugioka A S-Editor: Zhang H L-Editor: Filipodia P-Editor: Liu JH
| 1. | Gaba RC, Mendoza-Elias N, Regan DP, Garcia KD, Lokken RP, Schwind RM, Eichner M, Thomas FM, Rund LA, Schook LB, Schachtschneider KM. Characterization of an Inducible Alcoholic Liver Fibrosis Model for Hepatocellular Carcinoma Investigation in a Transgenic Porcine Tumorigenic Platform. J Vasc Interv Radiol 2018; 29: 1194-1202. e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 14] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 2. | Bansal MB, Chamroonkul N. Antifibrotics in liver disease: are we getting closer to clinical use? Hepatol Int. 2019;13:25-39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 27] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 3. | Trivedi P, Wang S, Friedman SL. The Power of Plasticity-Metabolic Regulation of Hepatic Stellate Cells. Cell Metab. 2021;33:242-257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 261] [Article Influence: 65.3] [Reference Citation Analysis (0)] |
| 4. | Gandhi CR. Hepatic stellate cell activation and pro-fibrogenic signals. J Hepatol. 2017;67:1104-1105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 117] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 5. | Yuan X, Gong Z, Wang B, Guo X, Yang L, Li D, Zhang Y. Astragaloside Inhibits Hepatic Fibrosis by Modulation of TGF-β1/Smad Signaling Pathway. Evid Based Complement Alternat Med. 2018;2018:3231647. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 24] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 6. | Tan Z, Liu Q, Jiang R, Lv L, Shoto SS, Maillet I, Quesniaux V, Tang J, Zhang W, Sun B, Ryffel B. Interleukin-33 drives hepatic fibrosis through activation of hepatic stellate cells. Cell Mol Immunol. 2018;15:388-398. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 95] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 7. | Sun YY, Li XF, Meng XM, Huang C, Zhang L, Li J. Macrophage Phenotype in Liver Injury and Repair. Scand J Immunol. 2017;85:166-174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 98] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 8. | Gieseck RL 3rd, Wilson MS, Wynn TA. Type 2 immunity in tissue repair and fibrosis. Nat Rev Immunol. 2018;18:62-76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 445] [Cited by in RCA: 742] [Article Influence: 92.8] [Reference Citation Analysis (0)] |
| 9. | Li X, Zhang M, Liu J, Huang Z, Zhao Q, Huang Y, Li X, Gao Z. Intrahepatic NK cells function suppressed in advanced liver fibrosis. Eur J Clin Invest. 2016;46:864-872. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 18] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 10. | Amer J, Salhab A, Doron S, Morali G, Safadi R. A novel flow cytometry tool for fibrosis scoring through hepatic stellate cell differentiation. Cytometry A. 2018;93:427-435. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 11. | Abedi F, Hayes AW, Reiter R, Karimi G. Acute lung injury: The therapeutic role of Rho kinase inhibitors. Pharmacol Res. 2020;155:104736. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 100] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 12. | Görtzen J, Trebicka J. Rho-kinase inhibition is beneficial in fibrosis. Hepatology. 2017;65:1780-1781. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 13. | Pan W, Nagpal K, Suárez-Fueyo A, Ferretti A, Yoshida N, Tsokos MG, Tsokos GC. The Regulatory Subunit PPP2R2A of PP2A Enhances Th1 and Th17 Differentiation through Activation of the GEF-H1/RhoA/ROCK Signaling Pathway. J Immunol. 2021;206:1719-1728. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 28] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 14. | Han J, Zhao Y, Zhang Y, Li C, Yi Y, Pan C, Tian J, Yang Y, Cui H, Wang L, Liu S, Liu J, Deng N, Liang A. RhoA/ROCK Signaling Pathway Mediates Shuanghuanglian Injection-Induced Pseudo-allergic Reactions. Front Pharmacol. 2018;9:87. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 25] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 15. | Bei Y, Hua-Huy T, Nicco C, Duong-Quy S, Le-Dong NN, Tiev KP, Chéreau C, Batteux F, Dinh-Xuan AT. RhoA/Rho-kinase activation promotes lung fibrosis in an animal model of systemic sclerosis. Exp Lung Res. 2016;42:44-55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 32] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 16. | Xie Y, Song T, Huo M, Zhang Y, Zhang YY, Ma ZH, Wang N, Zhang JP, Chu L. Fasudil alleviates hepatic fibrosis in type 1 diabetic rats: involvement of the inflammation and RhoA/ROCK pathway. Eur Rev Med Pharmacol Sci. 2018;22:5665-5677. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 17. | Boyer-Diaz Z, Aristu-Zabalza P, Andrés-Rozas M, Robert C, Ortega-Ribera M, Fernández-Iglesias A, Broqua P, Junien JL, Wettstein G, Bosch J, Gracia-Sancho J. Pan-PPAR agonist lanifibranor improves portal hypertension and hepatic fibrosis in experimental advanced chronic liver disease. J Hepatol. 2021;74:1188-1199. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 102] [Article Influence: 25.5] [Reference Citation Analysis (0)] |
| 18. | Chen L, Li J, Zhang J, Dai C, Liu X, Wang J, Gao Z, Guo H, Wang R, Lu S, Wang F, Zhang H, Chen H, Fan X, Wang S, Qin Z. S100A4 promotes liver fibrosis via activation of hepatic stellate cells. J Hepatol. 2015;62:156-164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 119] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 19. | Han Q, Hou Z, Yin C, Zhang C, Zhang J. 5'-triphosphate siRNA targeting HBx elicits a potent anti-HBV immune response in pAAV-HBV transfected mice. Antiviral Res. 2019;161:36-45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 24] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 20. | Saha D, Martuza RL, Rabkin SD. Macrophage Polarization Contributes to Glioblastoma Eradication by Combination Immunovirotherapy and Immune Checkpoint Blockade. Cancer Cell 2017; 32: 253-267. e5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 328] [Cited by in RCA: 438] [Article Influence: 54.8] [Reference Citation Analysis (0)] |
| 21. | Vicente A, Byström B, Lindström M, Stenevi U, Pedrosa Domellöf F. Aniridia-related keratopathy: Structural changes in naïve and transplanted corneal buttons. PLoS One. 2018;13:e0198822. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 15] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 22. | Nakano R, Ohira M, Yano T, Imaoka Y, Tanaka Y, Ohdan H. Hepatic irradiation persistently eliminates liver resident NK cells. PLoS One. 2018;13:e0198904. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 9] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 23. | Fan Y, Zhang W, Wei H, Sun R, Tian Z, Chen Y. Hepatic NK cells attenuate fibrosis progression of non-alcoholic steatohepatitis in dependent of CXCL10-mediated recruitment. Liver Int. 2020;40:598-608. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 37] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 24. | Li T, Yang Y, Song H, Li H, Cui A, Liu Y, Su L, Crispe IN, Tu Z. Activated NK cells kill hepatic stellate cells via p38/PI3K signaling in a TRAIL-involved degranulation manner. J Leukoc Biol. 2019;105:695-704. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 29] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 25. | Ma L, Yang X, Wei R, Ye T, Zhou JK, Wen M, Men R, Li P, Dong B, Liu L, Fu X, Xu H, Aqeilan RI, Wei YQ, Yang L, Peng Y. MicroRNA-214 promotes hepatic stellate cell activation and liver fibrosis by suppressing Sufu expression. Cell Death Dis. 2018;9:718. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 61] [Cited by in RCA: 75] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 26. | Kim J, Kang W, Kang SH, Park SH, Kim JY, Yang S, Ha SY, Paik YH. Proline-rich tyrosine kinase 2 mediates transforming growth factor-beta-induced hepatic stellate cell activation and liver fibrosis. Sci Rep. 2020;10:21018. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 28] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 27. | Kisseleva T, Brenner D. Molecular and cellular mechanisms of liver fibrosis and its regression. Nat Rev Gastroenterol Hepatol. 2021;18:151-166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 274] [Cited by in RCA: 1150] [Article Influence: 287.5] [Reference Citation Analysis (0)] |
| 28. | Martí-Rodrigo A, Alegre F, Moragrega ÁB, García-García F, Martí-Rodrigo P, Fernández-Iglesias A, Gracia-Sancho J, Apostolova N, Esplugues JV, Blas-García A. Rilpivirine attenuates liver fibrosis through selective STAT1-mediated apoptosis in hepatic stellate cells. Gut. 2020;69:920-932. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 90] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 29. | Lai SS, Fu X, Cheng Q, Yu ZH, Jiang EZ, Zhao DD, Yu DC, Qiu YD, Gao X, Ju HX, Wang W, Jiang Q, Zhu MS, Li CJ, Xue B. HSC-specific knockdown of GGPPS alleviated CCl4-induced chronic liver fibrosis through mediating RhoA/Rock pathway. Am J Transl Res. 2019;11:2382-2392. [PubMed] |
| 30. | Tian Y, Han YX, Guo HF, Jin HT, Sun C, Qi X, Ma LY, Bo SW. Upregulated microRNA-485 suppresses apoptosis of renal tubular epithelial cells in mice with lupus nephritis via regulating the TGF-β-MAPK signaling pathway by inhibiting RhoA expression. J Cell Biochem. 2018;119:9154-9167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 10] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 31. | Shi Y, Wang Y, Li Q, Liu K, Hou J, Shao C. Immunoregulatory mechanisms of mesenchymal stem and stromal cells in inflammatory diseases. Nat Rev Nephrol. 2018;14:493-507. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 433] [Cited by in RCA: 807] [Article Influence: 134.5] [Reference Citation Analysis (0)] |
| 32. | Dufva O, Kankainen M, Kelkka T, Sekiguchi N, Awad SA, Eldfors S, Yadav B, Kuusanmäki H, Malani D, Andersson EI, Pietarinen P, Saikko L, Kovanen PE, Ojala T, Lee DA, Loughran TP Jr, Nakazawa H, Suzumiya J, Suzuki R, Ko YH, Kim WS, Chuang SS, Aittokallio T, Chan WC, Ohshima K, Ishida F, Mustjoki S. Aggressive natural killer-cell leukemia mutational landscape and drug profiling highlight JAK-STAT signaling as therapeutic target. Nat Commun. 2018;9:1567. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 80] [Cited by in RCA: 104] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 33. | Jin H, Jia Y, Yao Z, Huang J, Hao M, Yao S, Lian N, Zhang F, Zhang C, Chen X, Bian M, Shao J, Wu L, Chen A, Zheng S. Hepatic stellate cell interferes with NK cell regulation of fibrogenesis via curcumin induced senescence of hepatic stellate cell. Cell Signal. 2017;33:79-85. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 42] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 34. | Niogret C, Miah SMS, Rota G, Fonta NP, Wang H, Held W, Birchmeier W, Sexl V, Yang W, Vivier E, Ho PC, Brossay L, Guarda G. Shp-2 is critical for ERK and metabolic engagement downstream of IL-15 receptor in NK cells. Nat Commun. 2019;10:1444. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 34] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 35. | Verdeil G, Lawrence T, Schmitt-Verhulst AM, Auphan-Anezin N. Targeting STAT3 and STAT5 in Tumor-Associated Immune Cells to Improve Immunotherapy. Cancers (Basel). 2019;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 38] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 36. | Lacoste B, Raymond VA, Lapierre P, Bilodeau M. Protection against Acute Hepatocellular Injury Afforded by Liver Fibrosis Is Independent of T Lymphocytes. PLoS One. 2016;11:e0165360. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 37. | Gao J, Wei B, de Assuncao TM, Liu Z, Hu X, Ibrahim S, Cooper SA, Cao S, Shah VH, Kostallari E. Hepatic stellate cell autophagy inhibits extracellular vesicle release to attenuate liver fibrosis. J Hepatol. 2020;73:1144-1154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 213] [Article Influence: 42.6] [Reference Citation Analysis (0)] |
| 38. | Li XD, Wu YP, Chen SH, Liang YC, Lin TT, Lin T, Wei Y, Xue XY, Zheng QS, Xu N. Fasudil inhibits actin polymerization and collagen synthesis and induces apoptosis in human urethral scar fibroblasts via the Rho/ROCK pathway. Drug Des Devel Ther. 2018;12:2707-2713. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 39. | Shi J, Xiao P, Liu X, Chen Y, Xu Y, Fan J, Yin Y. Notch3 Modulates Cardiac Fibroblast Proliferation, Apoptosis, and Fibroblast to Myofibroblast Transition via Negative Regulation of the RhoA/ROCK/Hif1α Axis. Front Physiol. 2020;11:669. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 40. | Galvão I, Athayde RM, Perez DA, Reis AC, Rezende L, de Oliveira VLS, Rezende BM, Gonçalves WA, Sousa LP, Teixeira MM, Pinho V. ROCK Inhibition Drives Resolution of Acute Inflammation by Enhancing Neutrophil Apoptosis. Cells. 2019;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 23] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 41. | Gao Y, Yan Y, Fang Q, Zhang N, Kumar G, Zhang J, Song LJ, Yu J, Zhao L, Zhang HT, Ma CG. The Rho kinase inhibitor fasudil attenuates Aβ1-42-induced apoptosis via the ASK1/JNK signal pathway in primary cultures of hippocampal neurons. Metab Brain Dis. 2019;34:1787-1801. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 33] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 42. | Wu Y, Shu J, He C, Li M, Wang Y, Ou W, He Y. ROCK inhibitor Y27632 promotes proliferation and diminishes apoptosis of marmoset induced pluripotent stem cells by suppressing expression and activity of caspase 3. Theriogenology. 2016;85:302-314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 28] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 43. | Wang Q, Cheng F, Xu Y, Zhang J, Qi J, Liu X, Wang R. Thymol alleviates lipopolysaccharide-stimulated inflammatory response via downregulation of RhoA-mediated NF-κB signalling pathway in human peritoneal mesothelial cells. Eur J Pharmacol. 2018;833:210-220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 29] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 44. | Widjaja AA, Singh BK, Adami E, Viswanathan S, Dong J, D'Agostino GA, Ng B, Lim WW, Tan J, Paleja BS, Tripathi M, Lim SY, Shekeran SG, Chothani SP, Rabes A, Sombetzki M, Bruinstroop E, Min LP, Sinha RA, Albani S, Yen PM, Schafer S, Cook SA. Inhibiting Interleukin 11 Signaling Reduces Hepatocyte Death and Liver Fibrosis, Inflammation, and Steatosis in Mouse Models of Nonalcoholic Steatohepatitis. Gastroenterology 2019; 157: 777-792. e14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 220] [Cited by in RCA: 198] [Article Influence: 33.0] [Reference Citation Analysis (0)] |