Published online Jun 28, 2021. doi: 10.3748/wjg.v27.i24.3429
Peer-review started: March 17, 2021
First decision: May 1, 2021
Revised: May 6, 2021
Accepted: May 20, 2021
Article in press: May 20, 2021
Published online: June 28, 2021
Processing time: 99 Days and 17.5 Hours
Although hepatocellular carcinoma is considered a highly lethal malignancy, recent therapeutic advances have been achieved during the last 10 years. This scenario resulted in an unprecedented improvement in survival for patients with advanced hepatocellular carcinoma, almost reaching 20-26 mo of overall survival after first-second line sequential treatment. The advent of the combination of atezolizumab with bevacizumab showed, for the first time, superiority over sorafenib with improvement in overall survival. However, first and second-line trials were correctly based on the premise that a strict selection of patients enhances the power to capture the positive effect of treatment by excluding competing risks for mortality such as liver failure, decompensated cirrhosis or other underlying medical conditions. As a result, the inclusion criteria used in clinical trials do not support the use of novel therapies in several real-world scenarios involving underrepresented subgroups, such as patients with unpreserved liver function, other comorbid conditions, a history of solid-organ transplantation, autoimmune disorders and those with a high risk of bleeding. The present text aims at discussing treatment strategies in these subgroups.
Core Tip: The strict criteria used in clinical trials in advanced hepatocellular carcinoma have led to a scarcity of available data in a considerable proportion of patients in the real-world practice. The daily challenge of treating these underrepresented subgroups can be overcome by future clinical trials addressing special situations, collaborative studies and real-world data.
- Citation: Piñero F, da Fonseca LG. Trial eligibility in advanced hepatocellular carcinoma: Does it support clinical practice in underrepresented subgroups? World J Gastroenterol 2021; 27(24): 3429-3439
- URL: https://www.wjgnet.com/1007-9327/full/v27/i24/3429.htm
- DOI: https://dx.doi.org/10.3748/wjg.v27.i24.3429
Although hepatocellular carcinoma (HCC) is considered a highly lethal malignancy, recent therapeutic advances have been achieved during the last 10 years. These achievements were unthinkable 20 years before. Historically, patients with HCC at advanced stages or refractory to locoregional therapies (such as surgery, ablation or intra-arterial treatments) were associated with a dismal prognosis[1]. This scenario has fortunately changed.
In 2008, the first positive phase III trial (SHARP trial) using a systemic agent for HCC was published, showing that sorafenib improved overall survival over placebo in a selected population[2]. This result was observed in the Asia-Pacific trial, repeating similar observations yet in another population[3]. Sorafenib has succeeded due to its activity against different tumor pathways, particularly angiogenesis and proliferation signaling activation even in the absence of significant tumor shrinkage. It showed a favorable safety profile, particularly in patients with a well-preserved liver function (Child-Pugh A), a performance status of 2 or less and no other organ failure.
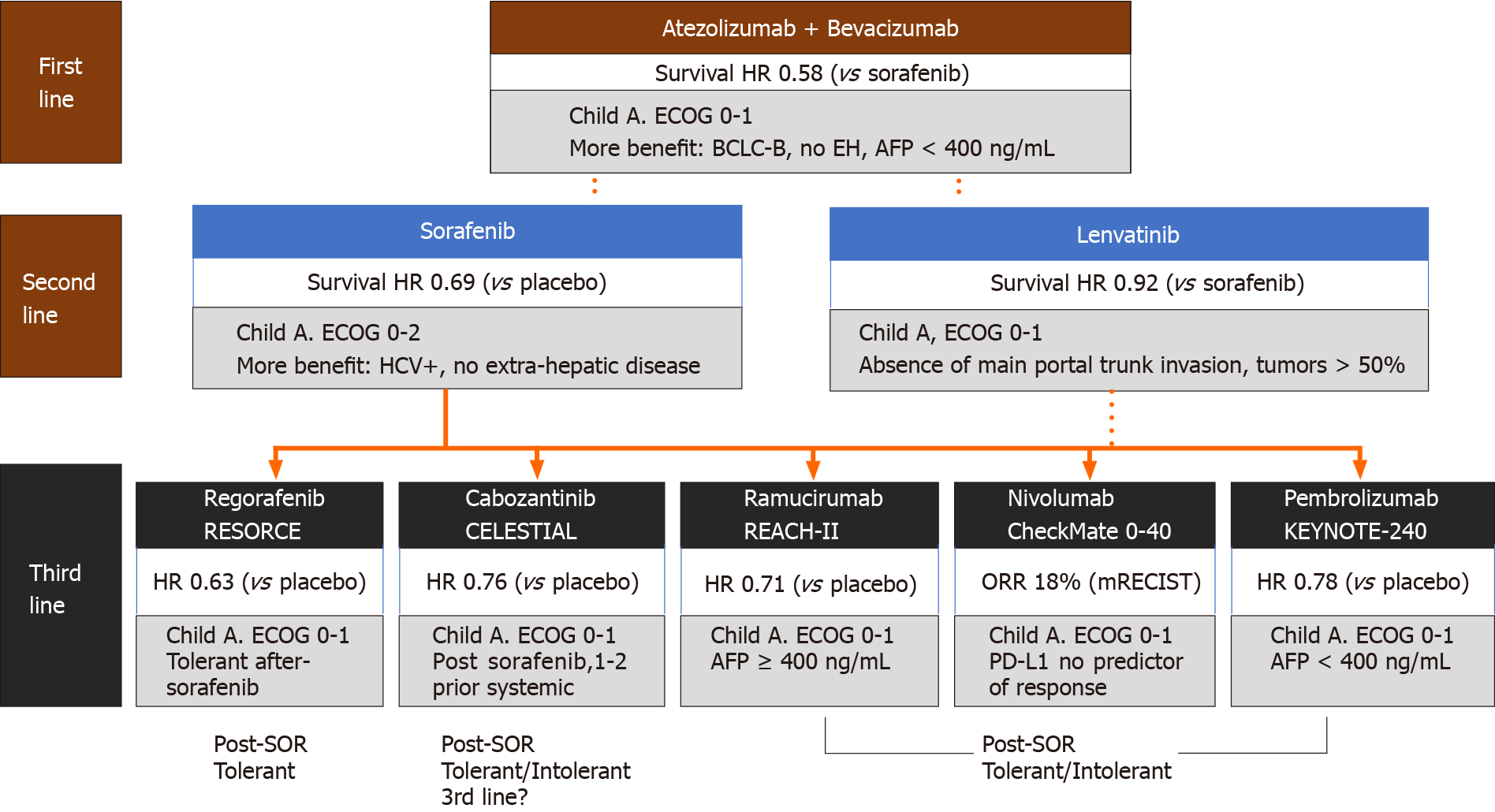
Following sorafenib, several drugs with similar or different targets were tested with disappointing results in phase III trials[4]. On the other hand, lenvatinib, shown to be non-inferior to sorafenib in the phase III REFLECT trial in patients without main portal trunk tumor invasion or without more than 50% of liver involvement[5], became an alternative in the first-line setting. Other agents such as regorafenib[6], cabozantinib[7] and ramucirumab[8] were incorporated as second-line options after sorafenib failure. This scenario resulted in an unprecedented improvement in survival for patients with advanced HCC, almost reaching 20-26 mo of overall survival after first-second line sequential treatment[9,10].
The advent of immune checkpoint inhibitors (ICI) with impressive results in solid tumors underpinned trials in advanced HCC. ICIs were rapidly incorporated after encouraging results with nivolumab and pembrolizumab in phase II trials with HCC patients, with durable objective response rates in 15%-20% of the patients[11,12]. In 2020, the combination of atezolizumab (a programmed death ligand 1 inhibitor) with bevacizumab [an anti-vascular endothelial growth factor-vascular endothelial growth factor (VEGF)-antibody] showed for the first time superiority over sorafenib in the phase III IMBRAVE150 trial[13]. This result was followed by approval of this combination as the standard first-line treatment for advanced HCC in different countries.
However, first and second-line trials were correctly based on the premise that a strict selection of patients enhances the power to capture the positive effect of treatment by excluding competing risks of mortality such as liver failure, decom
In Western countries (mainly Europe and the United States), the leading risk factor for HCC is chronic hepatitis C virus (HCV) infection. In contrast, hepatitis B virus (HBV) chronic infection is predominant in China, Asia and sub-Saharan Africa, where a higher burden of HCC is found compared to the rest of the world[14]. In Latin America, HCV represents the most prevalent risk factor for HCC[15,16], but other etiologies, such as nonalcoholic fatty liver disease, are steadily increasing[17,18].
This geographic heterogeneity directly impacts the recruitment of patients. Trials that restrict enrollment to a specific region are likely to be enriched with the predominant local etiology. Clinical trials that recruit globally tend to have HBV as the most frequent etiology when the Asian population predominates. A noticeable transition in risk factors has been observed in Western countries, with growing evidence that metabolic-associated fatty liver disease is an increasing cause of HCC, often associated with other comorbidities such as obesity, hypertension and diabetes[19].
This geographical eligibility is exemplified by the pivotal trials exploring sorafenib. In the SHARP trial, only 18.4% of the enrolled population had HBV-related HCC[2], while 73% of the patients enrolled in the Asia-Pacific trial had HBV-related HCC[3]. Although both trials showed a benefit in overall survival irrespective from underlying liver disease, a combined analysis of these two trials demonstrated a significant benefit in patients with HCV[20].
Nonviral etiologies represent only 30% to 45% of the included population in recent immunotherapy trials (Table 1). Whether immunotherapy is equally effective across all etiologies is still uncertain[13], shown through subgroup analysis in the IMBRAVE150 study suggesting that atezolizumab plus bevacizumab may have a lower benefit over sorafenib in nonviral etiologies [hazard ratio (HR): 0.91, 95% confidence interval (CI): 0.51-1.60], when compared to HBV (HR: 0.51; 95%CI: 0.32-0.81) or HCV-associated HCC (HR: 0.43; 95%CI: 0.22-0.87). This was also shown in the first-line trial comparing nivolumab vs sorafenib (Checkmate-459 trial, NCT02576509), in which nivolumab did not reach superiority over sorafenib in the overall population. Stratified subgroup analysis showed an HR of 0.91 (95%CI: 0.72-1.16) in nonviral etiologies when compared to HBV (HR: 0.79; 95%CI: 0.59-1.07) or HCV-associated HCC (HR: 0.72; 95%CI: 0.51-1.02). More recently, it has been shown that chronic inflammation in nonalcoholic fatty liver leads to liver injury and promotes liver cancer, impairing tumor surveillance. A meta-analysis of three randomized-control trials (IMbrave150, Checkmate-459 and KEYNOTE-240), showed that treatment with ICIs in these patients is associated with reduced survival compared to other etiologies[21].
| IMbrave: Phase III, open-label | REFLECT: Phase III, open-label | CheckMate-459: Phase III, open-label | ||||
| Atezo + Bev | Sorafenib | Lenvatinib | Sorafenib | Nivolumab | Sorafenib | |
| n | 336 | 165 | 478 | 476 | 371 | 372 |
| Age, median | 64 | 66 | 63 | 62 | 65 | 65 |
| ≥ 65 yr, % | 48 | 55 | 44 | 41 | NR | NR |
| Male, % | 82 | 83 | 85 | 84 | 85 | 85 |
| Asia, % | 56 | 58 | 70 | 68 | NR | NR |
| ECOG 1, % | 38 | 38 | 36 | 37 | 27 | 30 |
| AFP ≥ 200 ng/mL, % | 43 | 45 | 46 | 39 | 39 | 43 |
| HBV, % | 49 | 46 | 53 | 48 | 31 | 31 |
| HCV, % | 21 | 22 | 19 | 26 | 23 | 23 |
| Nonviral, % | 30 | 32 | 28 | 26 | 45 | 45 |
| MVI trunk | Excluded | Excluded | ||||
| MVI, % | 38 | 43 | 23 | 19 | NR | NR |
| EH, % | 63 | 56 | 61 | 62 | NR | NR |
Although not conclusive, subgroup analysis might offer partial information and generate a hypothesis for future trials. In regard with etiology, there is some molecular background that supports the existence of different molecular activated pathways according to molecular and transcriptomic-based features, which may lead to distinct activation of antitumor immunity or even to ICI resistance[22].
Approximately 70% of patients with cancer are aged 65 or older. The number of patients with cancer in this age group is projected to increase over the next decades significantly[23]. The aging process has been associated with changes in antineoplastic agents’ pharmacokinetics due to a number of age-related changes, including modifications in renal and liver function, leading to altered drug absorption, metabolism and distribution.
The mean age of patients with HCC included in clinical trials is around 65-years-old. However, a substantial proportion of HCC patients are older. In HCC and other malignancies, elderly patients are underrepresented in clinical trials. In HCC, exceptionally, the concomitance of advanced age with chronic liver disease raises concerns about toxicity and clinical benefit.
A pooled analysis of both sorafenib pivotal trials (SHARP and Asia-Pacific) did not demonstrate prognostic differences between patients < or ≥ 75 years[20], suggesting that well-selected individuals could derive benefit from systemic treatment irre
A concern that elderlies may present a poor tolerance to systemic treatment primes a trend for early discontinuation of sorafenib in field-practice studies[24]. However, in this cohort study (SOFIA Italian study), patients with half dosing sorafenib were associated with improved overall survival and discontinuation with worse outcomes. Consequently, early reduction avoiding definitive treatment discontinuation should be mandatory[24]. On the other hand, other studies did not show significant differences in overall survival and class-specific adverse events with lenvatinib in older patients[25]. A subanalysis of the IMBRAVE150 trial evaluating patients aged < or ≥ 65 years showed a similar toxicity profile, patient-reported outcomes and survival outcomes[26].
Most available data come from retrospective studies and subanalysis of prospective trials, mainly with sorafenib. Most of these data support that age alone should not restrict treatment in advanced HCC, but a multidisciplinary approach and frailty metrics, apart from Eastern Cooperative Oncology Group grades, can be helpful in managing this group.
Cirrhosis and its complications (ascites among others) are the most significant competing risk for mortality in patients with HCC. In fact, prior evaluation of liver function, liver decompensation (prior history of ascites and its complications) or clinically significant portal hypertension are mandatory before systemic therapy initiation or selection (e.g., presence of gastric or esophageal varices, other abdominal collaterals, enlarged spleen more than 120 mm, low platelet count < 150000 mm3, among others). Due to this fact, clinical trials specifically selected those populations in which HCC determines the risk of mortality so that the antitumor treatment effect is more likely to be captured without distortion by cirrhosis-imposed threats. The majority of trials strictly included patients with preserved liver function, Child-Pugh A, or without liver decompensation events. It results in the lack of robust data showing how to manage patients with advanced HCC and impaired liver function. On the other hand, liver decompensation during systemic therapy leads to a significant impact on overall survival and an exclusion of sequencing systemic options[27,28].
The GIDEON study[29], the most extensive real-world data including patients treated with sorafenib, demonstrated that the median survival of patients with unpreserved liver function or Child-Pugh B and C was 5.2 mo and 2.6 mo, res
Some authors recommend against grading ascites due to its subjective assessment, showing that the albumin-bilirubin score may be an alternative tool to evaluate prognosis in candidates for systemic treatment[32]. However, events of liver decompensation, such as ascites, jaundice or encephalopathy, have been associated with a significant worse prognosis and should always be part of eligibility criteria in trials and in the real-world setting.
Safety and efficacy in patients with liver dysfunction should not be extrapolated to all tyrosine kinase inhibitors (TKIs). The GIDEON cohort study showed an increasing incidence rate of serious adverse events from Child Pugh A to B or C, with a rising rate of sorafenib discontinuation[29]. Moreover, almost 20% of the patients may experience clinical deterioration due to liver impairment with the treatment with TKIs, particularly during the first 4 wk of therapy[6,24,33]. Nevertheless, in the second-line setting, patients allocated to cabozantinib in the CELESTIAL trial who presented worsening in liver function by week 8 had a manageable safety profile and maintained treatment benefit compared to the total cohort[7].
Whether these data could be extrapolated to ICIs is a matter of debate. Nivolumab was tested in a prospective Child-Pugh B cohort (75% of Child-Pugh B7)[11]. The median overall survival was 7.6 mo, with a disease control rate of 55.1%. Although safety profile may be more favorable, there is a paucity of data on other immune-oncology drugs in the setting of liver dysfunction.
The safety of combined therapies, including ICIs and VEGF targeted pathways (TKIs or anti-VEGF), in patients with unpreserved liver function is a matter to be clarified in prospective real-world data. Tyrosine kinase inhibitors and immunotherapy seems to be feasible in patients with a mild liver alteration. A close follow-up and multidisciplinary management are paramount to secure safety and better outcomes.
Liver transplantation has been an exclusion criterion in all clinical trials enrolling patients with advanced HCC, TKIs or ICIs. Safety concerns and overall survival in immunosuppressed patients has been one of the main explanations of this exclusion criteria.
However, the use of TKIs has been reported in retrospective cohort studies with acceptable results. Sorafenib was shown to be safe and effective, with a median overall survival of 20.1 mo[34]. The toxicity profile and the risk for liver graft deterioration have been reported to be similar and lower than patients with no history of transplantation, respectively[35]. Favorable outcomes were observed in a multicenter retrospective study exploring the sorafenib-regorafenib sequencing therapy in the post-transplant setting. The median survival was 12.9 mo (95%CI: 6.7-19.1) since regorafenib initiation and 38.4 mo (95%CI: 18.5-58.4) since sorafenib discontinuation[36]. Other studies have already reported outcomes with lenvatinib in the post-transplant setting.
The risk of allograft rejection with ICI therapy precludes these patients from being treated with ICIs, either monotherapy or in combination with TKIs or anti-VEGF[37]. Therefore, sequencing TKIs is the optimal approach for patients with tumor recurrence after liver transplantation not amenable to local treatment.
Patients with HCC and coexisting cirrhosis have an increased risk of bleeding events due to portal hypertension. However, the risk of spontaneous bleeding in other organs is rare, and these patients are paradoxically at a higher risk of thrombotic events[38,39]. The risk of bleeding goes in parallel with the presence and severity of portal hypertension. In patients without prior endoscopy, at least during the last 6 mo, the risk of variceal hemorrhage should be assessed before systemic therapy, particularly with bevacizumab. Primary or secondary prophylaxis of variceal hemorrhage should be implemented according to International Consensus guidelines (e.g., BAVENO VI), either with beta-blockers or endoscopic variceal banding or both for secondary prophylaxis. In some patients without any surrogate marker of clinically significant portal hypertension (e.g., presence of enlarged spleen more than 120 mm, low platelet count < 150000 mm3 or other abdominal collaterals), upper endoscopy may be replaced by transient elastography as a first or additional approach to rule-out gastroeso
Bleeding can occur either due to variceal cause or spontaneous tumor rupture, both dramatic events associated with dismal outcome in patients with advanced HCC. In fact, HCC leads to an increase in portal hypertension, and consequently the risk of bleeding should be reassessed in these patients. Drugs with antiangiogenic activity, TKIs or anti-VEGF are associated with an increased risk of bleeding that usually does not require significant interventions. In pivotal trials, sorafenib was associated with a low risk of severe bleeding events (7% any grade, 1% grade 3) as well as ramucirumab (1% of grade 3-4)[2,8].
On the contrary, the IMBRAVE150 trial did not include patients with untreated or incompletely treated esophageal or gastric varices (according to local clinical practice, either beta-blockers or endoscopic procedures)[13]. This concern was based on the risk of tumor-associated hemorrhage with bevacizumab (3%-5%), with reported fatal bleeding cases in earlier trials[40]. Despite the exclusion of high-risk patients and a well-balanced risk of bleeding (26% of each group had varices), there was a 25.2% rate of any grade bleeding events in the atezolizumab-bevacizumab arm, and fatal bleeding events occurred in 6 patients in the IMBRAVE150 trial (1.8%). Specifically, variceal bleeding occurred more frequently in the atezolizumab plus bevacizumab arm vs sorafenib (7% vs 4.5%)[13].
The risk of bleeding should be extensively assessed in systemic treatment candidates, and a careful follow-up should be carried out in the real-world setting. Particular attention is required for those patients considered for atezolizumab and bevacizumab, patients using anticoagulants and those with a recent history or higher risk of variceal bleeding (e.g., esophageal or gastric varices with red spots).
The classes of agents used for treating advanced HCC have particular prescribing concerns due to their mechanism of action. TKIs with antiangiogenic properties may increase the risk of cardiovascular disease and ischemic events. Consequently, patients with risk for cardiovascular events, such as diabetes or prior cardiovascular complications, are underrepresented in clinical trials, although they were not entirely excluded from enrollment. The challenge in such situation relies on the proper management of risk factors. ICIs, on the other hand, carried a low risk of cardiovascular events.
Drug interaction is a crucial topic, particularly with antiretroviral therapy for HIV. Patients with HIV are not included in clinical trials, but a real-world study showed that sorafenib does not impact viral load and CD4-T cell count[41]. Data with immunotherapy for HIV-positive patients lack as they were excluded from pivotal trials with ICIs.
Patients under supportive renal care or hemodialysis have been excluded from clinical trials, and more recent real-world data has been reported with sorafenib treatment[42]. Finally, ICIs may exacerbate autoimmune disorders. Some of these disorders are associated with an increased risk of HCC, such as autoimmune hepatitis.
Exacerbation of immune disorders and immune-related adverse events may occur in up to 75% of the cases. In this regard, ICIs should be used with caution in this population[43]. Many of these events can be managed without discontinuing therapy, but further data are required. Also, there is a deep concern with extrapolating the management of these adverse events in patients with cirrhosis. Most clinical guideline recommendations are based on non-cirrhotic patients[43]. Although immune-related events should be promptly recognized and adequately treated, the use of high steroid doses should be cautiously implemented in cirrhosis[44]. It is already known that the use of steroids may accelerate or result in liver decompensation (e.g., ascites development, among other events).
In the second-line setting, all effective options were explored after sorafenib, either intolerance or tumor progression. There is no comparative study that evaluated how second-line drugs perform after lenvatinib or atezolizumab plus bevacizumab. Regorafenib was superior to placebo in sorafenib-tolerant patients[6], ramucirumab was effective in patients with high alpha-fetoprotein (AFP) levels[8], and cabozantinib showed better survival in second or third-lines over placebo[7]. In addition, the combination of nivolumab-ipilimumab (a dual ICI combination) was granted approval after sorafenib based on an encouraging phase II trial[45].
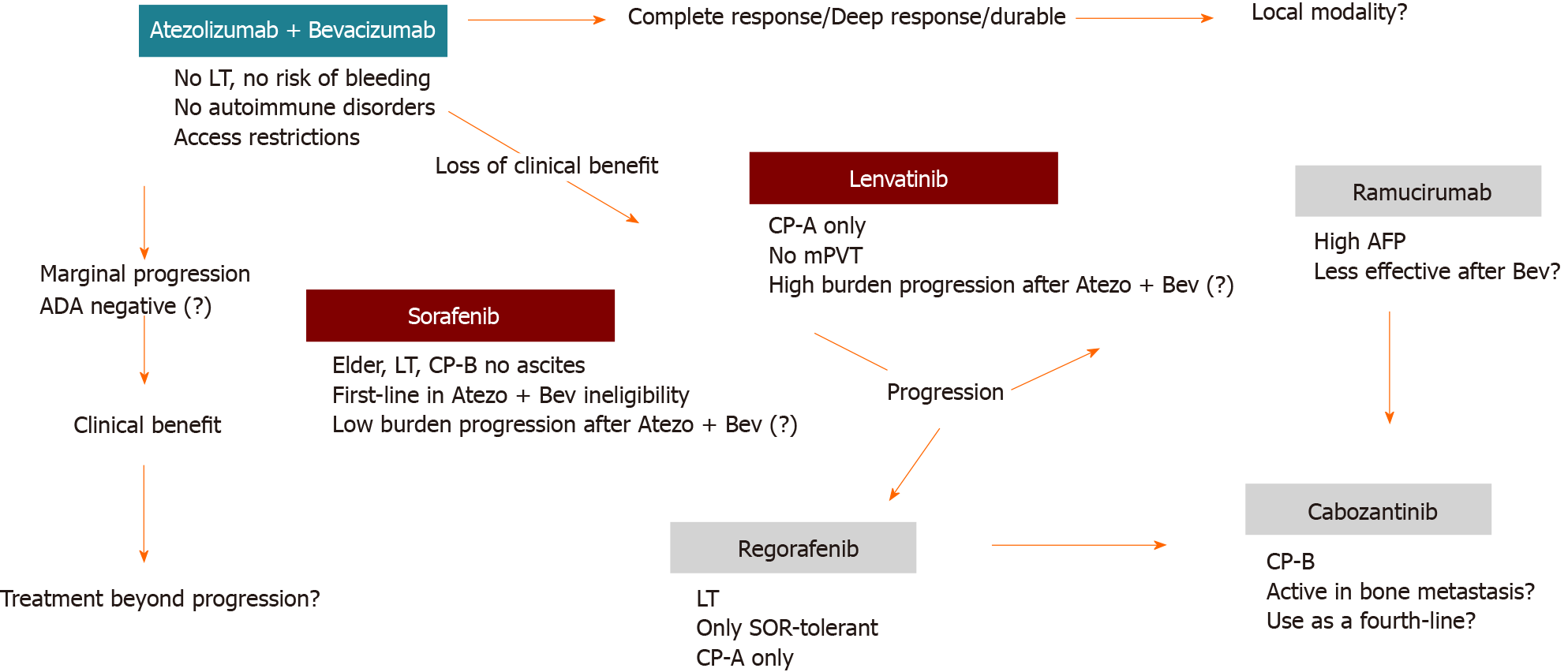
Although more recent retrospective studies have compared nivolumab vs regorafenib efficacy, all second-line competitors have not been compared face-to-face in clinical trials[46]. Head-to-head comparisons between all these options are unlikely to be addressed in future trials, so sequencing strategies will be an unmet knowledge requiring real-world data outside clinical trials. Some assumptions are reasonable to be considered when choosing the best strategy (Figure 2).
The selection based on the safety profile is crucial. For example, risk of bleeding, cardiovascular events or immune-related adverse events may impact negatively if not correctly assessed. Survival is the primary objective, but patients with tumor-related symptoms may also benefit from therapies with a higher response rate, such as lenvatinib or atezolizumab plus bevacizumab. Special subgroups not included in trials may be more safely managed based on real-world data showing favorable results. For example, this is the case of sorafenib-regorafenib therapy in transplanted patients.
Alternating treatments with different mechanisms of action instead of using sequences of drugs directed to the same target is a reasonable strategy, although not evidenced-based in clinical trials, particularly for third or even fourth-line therapies. For example, after progression on immunotherapy-based therapy, a TKI is more likely to be effective and vice versa. This issue will be a major discussion when novel therapies are incorporated following the results of ongoing clinical trials.
There is still an unmet need in HCC. The use of biomarkers for treatment selection, except high AFP levels for ramucirumab therapy, is lacking. Even the expression of programmed death ligand 1 in tumor tissue has not been associated with a predictive response. While the neutrophil-lymphocyte ratio has already been associated with better response with sorafenib[20] and lenvatinib[47], other biomarkers in other settings have been extensively explored without clinical implication[48,49].
The strict criteria used in clinical trials in advanced HCC have led to a scarcity of available data in a considerable proportion of patients in real-world practice. The daily challenge of treating these underrepresented subgroups can be overcome by future clinical trials addressing special situations, collaborative studies and real-world data[50]. A critical view of study design is essential to avoid excessive extrapolation and not limit efforts to provide better care to some subgroups that are not widely included in clinical research.
On behalf of the Latin American Liver Research, Education and Awareness Network (LALREAN).
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Argentina
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Chiu KW, Elbaz A, Poggio PD, Wang J S-Editor: Zhang L L-Editor: Filipodia P-Editor: Liu JH
| 1. | Llovet JM, Brú C, Bruix J. Prognosis of hepatocellular carcinoma: the BCLC staging classification. Semin Liver Dis. 1999;19:329-338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2645] [Cited by in RCA: 2876] [Article Influence: 110.6] [Reference Citation Analysis (1)] |
| 2. | Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, de Oliveira AC, Santoro A, Raoul JL, Forner A, Schwartz M, Porta C, Zeuzem S, Bolondi L, Greten TF, Galle PR, Seitz JF, Borbath I, Häussinger D, Giannaris T, Shan M, Moscovici M, Voliotis D, Bruix J; SHARP Investigators Study Group. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378-390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9016] [Cited by in RCA: 10271] [Article Influence: 604.2] [Reference Citation Analysis (2)] |
| 3. | Cheng AL, Kang YK, Chen Z, Tsao CJ, Qin S, Kim JS, Luo R, Feng J, Ye S, Yang TS, Xu J, Sun Y, Liang H, Liu J, Wang J, Tak WY, Pan H, Burock K, Zou J, Voliotis D, Guan Z. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2009;10:25-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3854] [Cited by in RCA: 4653] [Article Influence: 273.7] [Reference Citation Analysis (0)] |
| 4. | Bruix J, da Fonseca LG, Reig M. Insights into the success and failure of systemic therapy for hepatocellular carcinoma. Nat Rev Gastroenterol Hepatol. 2019;16:617-630. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 150] [Article Influence: 25.0] [Reference Citation Analysis (0)] |
| 5. | Kudo M, Finn RS, Qin S, Han KH, Ikeda K, Piscaglia F, Baron A, Park JW, Han G, Jassem J, Blanc JF, Vogel A, Komov D, Evans TRJ, Lopez C, Dutcus C, Guo M, Saito K, Kraljevic S, Tamai T, Ren M, Cheng AL. Lenvatinib vs sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised phase 3 non-inferiority trial. Lancet. 2018;391:1163-1173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3128] [Cited by in RCA: 3831] [Article Influence: 547.3] [Reference Citation Analysis (1)] |
| 6. | Bruix J, Qin S, Merle P, Granito A, Huang YH, Bodoky G, Pracht M, Yokosuka O, Rosmorduc O, Breder V, Gerolami R, Masi G, Ross PJ, Song T, Bronowicki JP, Ollivier-Hourmand I, Kudo M, Cheng AL, Llovet JM, Finn RS, LeBerre MA, Baumhauer A, Meinhardt G, Han G; RESORCE Investigators. Regorafenib for patients with hepatocellular carcinoma who progressed on sorafenib treatment (RESORCE): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2017;389:56-66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2160] [Cited by in RCA: 2717] [Article Influence: 339.6] [Reference Citation Analysis (0)] |
| 7. | Abou-Alfa GK, Meyer T, Cheng AL, El-Khoueiry AB, Rimassa L, Ryoo BY, Cicin I, Merle P, Chen Y, Park JW, Blanc JF, Bolondi L, Klümpen HJ, Chan SL, Zagonel V, Pressiani T, Ryu MH, Venook AP, Hessel C, Borgman-Hagey AE, Schwab G, Kelley RK. Cabozantinib in Patients with Advanced and Progressing Hepatocellular Carcinoma. N Engl J Med. 2018;379:54-63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1630] [Cited by in RCA: 1771] [Article Influence: 253.0] [Reference Citation Analysis (0)] |
| 8. | Zhu AX, Kang YK, Yen CJ, Finn RS, Galle PR, Llovet JM, Assenat E, Brandi G, Pracht M, Lim HY, Rau KM, Motomura K, Ohno I, Merle P, Daniele B, Shin DB, Gerken G, Borg C, Hiriart JB, Okusaka T, Morimoto M, Hsu Y, Abada PB, Kudo M; REACH-2 study investigators. Ramucirumab after sorafenib in patients with advanced hepatocellular carcinoma and increased α-fetoprotein concentrations (REACH-2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2019;20:282-296. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1027] [Cited by in RCA: 1251] [Article Influence: 208.5] [Reference Citation Analysis (0)] |
| 9. | Finn RS, Merle P, Granito A, Huang YH, Bodoky G, Pracht M, Yokosuka O, Rosmorduc O, Gerolami R, Caparello C, Cabrera R, Chang C, Sun W, LeBerre MA, Baumhauer A, Meinhardt G, Bruix J. Outcomes of sequential treatment with sorafenib followed by regorafenib for HCC: Additional analyses from the phase III RESORCE trial. J Hepatol. 2018;69:353-358. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 226] [Cited by in RCA: 275] [Article Influence: 39.3] [Reference Citation Analysis (0)] |
| 10. | Alsina A, Kudo M, Vogel A, Cheng AL, Tak WY, Ryoo BY, Evans TRJ, López López C, Daniele B, Misir S, Ren M, Izumi N, Qin S, Finn RS. Effects of Subsequent Systemic Anticancer Medication Following First-Line Lenvatinib: A Post Hoc Responder Analysis from the Phase 3 REFLECT Study in Unresectable Hepatocellular Carcinoma. Liver Cancer. 2020;9:93-104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 59] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 11. | El-Khoueiry AB, Sangro B, Yau T, Crocenzi TS, Kudo M, Hsu C, Kim TY, Choo SP, Trojan J, Welling TH Rd, Meyer T, Kang YK, Yeo W, Chopra A, Anderson J, Dela Cruz C, Lang L, Neely J, Tang H, Dastani HB, Melero I. Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): an open-label, non-comparative, phase 1/2 dose escalation and expansion trial. Lancet. 2017;389:2492-2502. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3278] [Cited by in RCA: 3314] [Article Influence: 414.3] [Reference Citation Analysis (1)] |
| 12. | Zhu AX, Finn RS, Edeline J, Cattan S, Ogasawara S, Palmer D, Verslype C, Zagonel V, Fartoux L, Vogel A, Sarker D, Verset G, Chan SL, Knox J, Daniele B, Webber AL, Ebbinghaus SW, Ma J, Siegel AB, Cheng AL, Kudo M; KEYNOTE-224 investigators. Pembrolizumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib (KEYNOTE-224): a non-randomised, open-label phase 2 trial. Lancet Oncol. 2018;19:940-952. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1184] [Cited by in RCA: 1901] [Article Influence: 271.6] [Reference Citation Analysis (0)] |
| 13. | Finn RS, Qin S, Ikeda M, Galle PR, Ducreux M, Kim TY, Kudo M, Breder V, Merle P, Kaseb AO, Li D, Verret W, Xu DZ, Hernandez S, Liu J, Huang C, Mulla S, Wang Y, Lim HY, Zhu AX, Cheng AL; IMbrave150 Investigators. Atezolizumab plus Bevacizumab in Unresectable Hepatocellular Carcinoma. N Engl J Med. 2020;382:1894-1905. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2542] [Cited by in RCA: 4705] [Article Influence: 941.0] [Reference Citation Analysis (2)] |
| 14. | Kim D, Li AA, Perumpail BJ, Gadiparthi C, Kim W, Cholankeril G, Glenn JS, Harrison SA, Younossi ZM, Ahmed A. Changing Trends in Etiology-Based and Ethnicity-Based Annual Mortality Rates of Cirrhosis and Hepatocellular Carcinoma in the United States. Hepatology. 2019;69:1064-1074. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 153] [Article Influence: 25.5] [Reference Citation Analysis (0)] |
| 15. | Fassio E, Díaz S, Santa C, Reig ME, Martínez Artola Y, Alves de Mattos A, Míguez C, Galizzi J, Zapata R, Ridruejo E, de Souza FC, Hernández N, Pinchuk L; Multicenter Group for Study of Hepatocarcinoma in Latin America; Asociación Latinoamericana para el Estudio del Hígado (ALEH). Etiology of hepatocellular carcinoma in Latin America: a prospective, multicenter, international study. Ann Hepatol. 2010;9:63-69. [PubMed] |
| 16. | Carrilho FJ, Paranaguá-Vezozzo DC, Chagas AL, Alencar RSSM, da Fonseca LG. Epidemiology of Liver Cancer in Latin America: Current and Future Trends. Semin Liver Dis. 2020;40:101-110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 17. | Piñero F, Costa P, Boteon YL, Duque SH, Marciano S, Anders M, Varón A, Zerega A, Poniachik J, Soza A, Padilla Machaca M, Menéndez J, Zapata R, Vilatoba M, Muñoz L, Maraschio M, Podestá LG, McCormack L, Gadano A, Boin ISFF, García P, Silva M; Latin American Liver Research; Education, Awareness Network (LALREAN). A changing etiologic scenario in liver transplantation for hepatocellular carcinoma in a multicenter cohort study from Latin America. Clin Res Hepatol Gastroenterol. 2018;42:443-452. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 21] [Article Influence: 3.0] [Reference Citation Analysis (1)] |
| 18. | Piñero F, Pages J, Marciano S, Fernández N, Silva J, Anders M, Zerega A, Ridruejo E, Ameigeiras B, D'Amico C, Gaite L, Bermúdez C, Cobos M, Rosales C, Romero G, McCormack L, Reggiardo V, Colombato L, Gadano A, Silva M. Fatty liver disease, an emerging etiology of hepatocellular carcinoma in Argentina. World J Hepatol. 2018;10:41-50. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 33] [Article Influence: 4.7] [Reference Citation Analysis (2)] |
| 19. | Eslam M, Newsome PN, Sarin SK, Anstee QM, Targher G, Romero-Gomez M, Zelber-Sagi S, Wai-Sun Wong V, Dufour JF, Schattenberg JM, Kawaguchi T, Arrese M, Valenti L, Shiha G, Tiribelli C, Yki-Järvinen H, Fan JG, Grønbæk H, Yilmaz Y, Cortez-Pinto H, Oliveira CP, Bedossa P, Adams LA, Zheng MH, Fouad Y, Chan WK, Mendez-Sanchez N, Ahn SH, Castera L, Bugianesi E, Ratziu V, George J. A new definition for metabolic dysfunction-associated fatty liver disease: An international expert consensus statement. J Hepatol. 2020;73:202-209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2883] [Cited by in RCA: 2841] [Article Influence: 568.2] [Reference Citation Analysis (1)] |
| 20. | Bruix J, Cheng AL, Meinhardt G, Nakajima K, De Sanctis Y, Llovet J. Prognostic factors and predictors of sorafenib benefit in patients with hepatocellular carcinoma: Analysis of two phase III studies. J Hepatol. 2017;67:999-1008. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 360] [Cited by in RCA: 453] [Article Influence: 56.6] [Reference Citation Analysis (0)] |
| 21. | Pfister D, Núñez NG, Pinyol R, Govaere O, Pinter M, Szydlowska M, Gupta R, Qiu M, Deczkowska A, Weiner A, Müller F, Sinha A, Friebel E, Engleitner T, Lenggenhager D, Moncsek A, Heide D, Stirm K, Kosla J, Kotsiliti E, Leone V, Dudek M, Yousuf S, Inverso D, Singh I, Teijeiro A, Castet F, Montironi C, Haber PK, Tiniakos D, Bedossa P, Cockell S, Younes R, Vacca M, Marra F, Schattenberg JM, Allison M, Bugianesi E, Ratziu V, Pressiani T, D'Alessio A, Personeni N, Rimassa L, Daly AK, Scheiner B, Pomej K, Kirstein MM, Vogel A, Peck-Radosavljevic M, Hucke F, Finkelmeier F, Waidmann O, Trojan J, Schulze K, Wege H, Koch S, Weinmann A, Bueter M, Rössler F, Siebenhüner A, De Dosso S, Mallm JP, Umansky V, Jugold M, Luedde T, Schietinger A, Schirmacher P, Emu B, Augustin HG, Billeter A, Müller-Stich B, Kikuchi H, Duda DG, Kütting F, Waldschmidt DT, Ebert MP, Rahbari N, Mei HE, Schulz AR, Ringelhan M, Malek N, Spahn S, Bitzer M, Ruiz de Galarreta M, Lujambio A, Dufour JF, Marron TU, Kaseb A, Kudo M, Huang YH, Djouder N, Wolter K, Zender L, Marche PN, Decaens T, Pinato DJ, Rad R, Mertens JC, Weber A, Unger K, Meissner F, Roth S, Jilkova ZM, Claassen M, Anstee QM, Amit I, Knolle P, Becher B, Llovet JM, Heikenwalder M. NASH limits anti-tumour surveillance in immunotherapy-treated HCC. Nature. 2021;592:450-456. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 259] [Cited by in RCA: 849] [Article Influence: 212.3] [Reference Citation Analysis (1)] |
| 22. | Kalbasi A, Ribas A. Tumour-intrinsic resistance to immune checkpoint blockade. Nat Rev Immunol. 2020;20:25-39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 443] [Cited by in RCA: 1018] [Article Influence: 169.7] [Reference Citation Analysis (0)] |
| 23. | Smith BD, Smith GL, Hurria A, Hortobagyi GN, Buchholz TA. Future of cancer incidence in the United States: burdens upon an aging, changing nation. J Clin Oncol. 2009;27:2758-2765. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1195] [Cited by in RCA: 1318] [Article Influence: 82.4] [Reference Citation Analysis (0)] |
| 24. | Iavarone M, Cabibbo G, Piscaglia F, Zavaglia C, Grieco A, Villa E, Cammà C, Colombo M; SOFIA (SOraFenib Italian Assessment) study group. Field-practice study of sorafenib therapy for hepatocellular carcinoma: a prospective multicenter study in Italy. Hepatology. 2011;54:2055-2063. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 287] [Cited by in RCA: 299] [Article Influence: 21.4] [Reference Citation Analysis (0)] |
| 25. | Tada T, Kumada T, Hiraoka A, Michitaka K, Atsukawa M, Hirooka M, Tsuji K, Ishikawa T, Takaguchi K, Kariyama K, Itobayashi E, Tajiri K, Shimada N, Shibata H, Ochi H, Toyoda H, Nouso K, Tsutsui A, Nagano T, Itokawa N, Hayama K, Imai M, Joko K, Koizumi Y, Hiasa Y. Safety and efficacy of lenvatinib in elderly patients with unresectable hepatocellular carcinoma: A multicenter analysis with propensity score matching. Hepatol Res. 2020;50:75-83. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 37] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 26. | Li D, Toh H, Merle P, Tsuchiya K, Hernandez S, Shao H, Mulla S, Ding B, Kudo M. Atezolizumab + bevacizumab vs sorafenib for unresectable hepatocellular carcinoma: Results from older adults enrolled in IMbrave150. Ann Oncol. 2020;31:234. [RCA] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 27. | Iavarone M, Cabibbo G, Biolato M, Della Corte C, Maida M, Barbara M, Basso M, Vavassori S, Craxì A, Grieco A, Cammà C, Colombo M. Predictors of survival in patients with advanced hepatocellular carcinoma who permanently discontinued sorafenib. Hepatology. 2015;62:784-791. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 117] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 28. | Piñero F, Marciano S, Fernández N, Silva J, Anders M, Zerega A, Ridruejo E, Romero G, Ameigeiras B, D'Amico C, Gaite L, Bermúdez C, Reggiardo V, Colombato L, Gadano A, Silva M. Intermediate-advanced hepatocellular carcinoma in Argentina: Treatment and survival analysis. World J Gastroenterol. 2019;25:3607-3618. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 13] [Cited by in RCA: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 29. | Marrero JA, Kudo M, Venook AP, Ye SL, Bronowicki JP, Chen XP, Dagher L, Furuse J, Geschwind JH, de Guevara LL, Papandreou C, Takayama T, Sanyal AJ, Yoon SK, Nakajima K, Lehr R, Heldner S, Lencioni R. Observational registry of sorafenib use in clinical practice across Child-Pugh subgroups: The GIDEON study. J Hepatol. 2016;65:1140-1147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 205] [Cited by in RCA: 297] [Article Influence: 33.0] [Reference Citation Analysis (0)] |
| 30. | Kim HY, Park JW, Joo J, Kim H, Woo SM, Lee WJ, Kim CM. Worse outcome of sorafenib therapy associated with ascites and Child-Pugh score in advanced hepatocellular carcinoma. J Gastroenterol Hepatol. 2013;28:1756-1761. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 43] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 31. | Hiraoka A, Kumada T, Kariyama K, Takaguchi K, Atsukawa M, Itobayashi E, Tsuji K, Tajiri K, Hirooka M, Shimada N, Shibata H, Ishikawa T, Ochi H, Tada T, Toyoda H, Nouso K, Tsutsui A, Itokawa N, Imai M, Joko K, Hiasa Y, Michitaka K; Real-life Practice Experts for HCC (RELPEC) Study Group; HCC 48 Group (hepatocellular carcinoma experts from 48 clinics in Japan). Clinical features of lenvatinib for unresectable hepatocellular carcinoma in real-world conditions: Multicenter analysis. Cancer Med. 2019;8:137-146. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 101] [Cited by in RCA: 95] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 32. | Johnson PJ, Berhane S, Kagebayashi C, Satomura S, Teng M, Reeves HL, O'Beirne J, Fox R, Skowronska A, Palmer D, Yeo W, Mo F, Lai P, Iñarrairaegui M, Chan SL, Sangro B, Miksad R, Tada T, Kumada T, Toyoda H. Assessment of liver function in patients with hepatocellular carcinoma: a new evidence-based approach-the ALBI grade. J Clin Oncol. 2015;33:550-558. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1962] [Cited by in RCA: 2014] [Article Influence: 201.4] [Reference Citation Analysis (0)] |
| 33. | Hiraoka A, Kumada T, Atsukawa M, Hirooka M, Tsuji K, Ishikawa T, Takaguchi K, Kariyama K, Itobayashi E, Tajiri K, Shimada N, Shibata H, Ochi H, Tada T, Toyoda H, Nouso K, Tsutsui A, Nagano T, Itokawa N, Hayama K, Imai M, Joko K, Koizumi Y, Hiasa Y, Michitaka K; On behalf of the Real-Life Practice Experts for HCC (RELPEC) Study Group and HCC 48 Group (hepatocellular carcinoma experts from 48 clinics in Japan). Early Relative Change in Hepatic Function with Lenvatinib for Unresectable Hepatocellular Carcinoma. Oncology. 2019;97:334-340. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 31] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 34. | Sposito C, Mariani L, Germini A, Flores Reyes M, Bongini M, Grossi G, Bhoori S, Mazzaferro V. Comparative efficacy of sorafenib vs best supportive care in recurrent hepatocellular carcinoma after liver transplantation: a case-control study. J Hepatol. 2013;59:59-66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 127] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 35. | Weinmann A, Niederle IM, Koch S, Hoppe-Lotichius M, Heise M, Düber C, Schuchmann M, Otto G, Galle PR, Wörns MA. Sorafenib for recurrence of hepatocellular carcinoma after liver transplantation. Dig Liver Dis. 2012;44:432-437. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 55] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 36. | Iavarone M, Invernizzi F, Czauderna C, Sanduzzi-Zamparelli M, Bhoori S, Amaddeo G, Manini MA, López MF, Anders M, Pinter M, Rodríguez MJB, Cristóbal MR, Soteras GA, Piñero F, Villadsen GE, Weinmann A, Crespo G, Mazzaferro V, Regnault H, Giorgio M, González-Diéguez ML, Donato MF, Varela M, Wörns MA, Bruix J, Lampertico P, Reig M. Preliminary experience on safety of regorafenib after sorafenib failure in recurrent hepatocellular carcinoma after liver transplantation. Am J Transplant. 2019;19:3176-3184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 65] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 37. | Aguirre LE, Guzman ME, Lopes G, Hurley J. Immune Checkpoint Inhibitors and the Risk of Allograft Rejection: A Comprehensive Analysis on an Emerging Issue. Oncologist. 2019;24:394-401. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 55] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 38. | Tripodi A, Chantarangkul V, Primignani M, Fabris F, Dell'Era A, Sei C, Mannucci PM. The international normalized ratio calibrated for cirrhosis (INR(liver)) normalizes prothrombin time results for model for end-stage liver disease calculation. Hepatology. 2007;46:520-527. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 130] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 39. | Tripodi A, Mannucci PM. The coagulopathy of chronic liver disease. N Engl J Med. 2011;365:147-156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 963] [Cited by in RCA: 951] [Article Influence: 67.9] [Reference Citation Analysis (1)] |
| 40. | Siegel AB, Cohen EI, Ocean A, Lehrer D, Goldenberg A, Knox JJ, Chen H, Clark-Garvey S, Weinberg A, Mandeli J, Christos P, Mazumdar M, Popa E, Brown RS Jr, Rafii S, Schwartz JD. Phase II trial evaluating the clinical and biologic effects of bevacizumab in unresectable hepatocellular carcinoma. J Clin Oncol. 2008;26:2992-2998. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 381] [Cited by in RCA: 396] [Article Influence: 23.3] [Reference Citation Analysis (0)] |
| 41. | Merchante N, Ibarra S, Revollo B, Rodríguez-Arrondo F, Merino E, Delgado-Fernández M, Montero-Alonso M, Téllez F, Galindo MJ, Rivero-Juárez A, García MA, Mínguez C, Romero-Palacios A, Garcia-Deltoro M, Pineda JA; GEHEP-002 Study Group. Real-life experience with sorafenib for the treatment of hepatocellular carcinoma in HIV-infected patients. AIDS. 2017;31:89-95. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 15] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 42. | Díaz-González Á, Sanduzzi-Zamparelli M, da Fonseca LG, Di Costanzo GG, Alves R, Iavarone M, Leal C, Sacco R, Matilla AM, Hernández-Guerra M, Aballay Soteras G, Wörns MA, Pinter M, Varela M, Ladekarl M, Chagas AL, Mínguez B, Arenas JI, Granito A, Sánchez-Torrijos Y, Rojas Á, Rodríguez de Lope C, Alvares-da-Silva MR, Pascual S, Rimassa L, Lledó JL, Huertas C, Sangro B, Giannini EG, Delgado M, Vergara M, Perelló C, Lue A, Sala M, Gallego A, Coll S, Hernáez T, Piñero F, Pereira G, França A, Marín J, Anders M, Mello V, Lozano M, Nault JC, Menéndez J, García Juárez I, Bruix J, Reig M. International and multicenter real-world study of sorafenib-treated patients with hepatocellular carcinoma under dialysis. Liver Int. 2020;40:1467-1476. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 43. | Brahmer JR, Lacchetti C, Schneider BJ, Atkins MB, Brassil KJ, Caterino JM, Chau I, Ernstoff MS, Gardner JM, Ginex P, Hallmeyer S, Holter Chakrabarty J, Leighl NB, Mammen JS, McDermott DF, Naing A, Nastoupil LJ, Phillips T, Porter LD, Puzanov I, Reichner CA, Santomasso BD, Seigel C, Spira A, Suarez-Almazor ME, Wang Y, Weber JS, Wolchok JD, Thompson JA; National Comprehensive Cancer Network. Management of Immune-Related Adverse Events in Patients Treated With Immune Checkpoint Inhibitor Therapy: American Society of Clinical Oncology Clinical Practice Guideline. J Clin Oncol. 2018;36:1714-1768. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2245] [Cited by in RCA: 2591] [Article Influence: 370.1] [Reference Citation Analysis (0)] |
| 44. | Sangro B, Chan SL, Meyer T, Reig M, El-Khoueiry A, Galle PR. Diagnosis and management of toxicities of immune checkpoint inhibitors in hepatocellular carcinoma. J Hepatol. 2020;72:320-341. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 193] [Article Influence: 38.6] [Reference Citation Analysis (0)] |
| 45. | Yau T, Kang YK, Kim TY, El-Khoueiry AB, Santoro A, Sangro B, Melero I, Kudo M, Hou MM, Matilla A, Tovoli F, Knox JJ, Ruth He A, El-Rayes BF, Acosta-Rivera M, Lim HY, Neely J, Shen Y, Wisniewski T, Anderson J, Hsu C. Efficacy and Safety of Nivolumab Plus Ipilimumab in Patients With Advanced Hepatocellular Carcinoma Previously Treated With Sorafenib: The CheckMate 040 Randomized Clinical Trial. JAMA Oncol. 2020;6:e204564. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 908] [Cited by in RCA: 960] [Article Influence: 192.0] [Reference Citation Analysis (0)] |
| 46. | Choi WM, Choi J, Lee D, Shim JH, Lim YS, Lee HC, Chung YH, Lee YS, Park SR, Ryu MH, Ryoo BY, Lee SJ, Kim KM. Regorafenib Versus Nivolumab After Sorafenib Failure: Real-World Data in Patients With Hepatocellular Carcinoma. Hepatol Commun. 2020;4:1073-1086. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 35] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 47. | Tada T, Kumada T, Hiraoka A, Michitaka K, Atsukawa M, Hirooka M, Tsuji K, Ishikawa T, Takaguchi K, Kariyama K, Itobayashi E, Tajiri K, Shimada N, Shibata H, Ochi H, Yasuda S, Toyoda H, Fukunishi S, Ohama H, Kawata K, Nakamura S, Nouso K, Tsutsui A, Nagano T, Itokawa N, Hayama K, Arai T, Imai M, Joko K, Koizumi Y, Hiasa Y. Neutrophil-to-lymphocyte ratio is associated with survival in patients with unresectable hepatocellular carcinoma treated with lenvatinib. Liver Int. 2020;40:968-976. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 43] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 48. | Teufel M, Seidel H, Köchert K, Meinhardt G, Finn RS, Llovet JM, Bruix J. Biomarkers Associated With Response to Regorafenib in Patients With Hepatocellular Carcinoma. Gastroenterology. 2019;156:1731-1741. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 167] [Article Influence: 27.8] [Reference Citation Analysis (0)] |
| 49. | Finn RS, Kudo M, Cheng A-L, Wyrwicz L, Ngan R, Blanc J-F, Baron AD, Vogel A, Ikeda M, Piscaglia F, Han K-H, Qin S, Minoshima Y, Funahashi Y, Ren M, Dairiki R, Sachdev P, Tamai T, Dutcus C, Evans TRJ. Analysis of serum biomarkers (BM) in patients from a phase 3 study of lenvatinib (LEN) vs sorafenib (SOR) as first-line treatment for unresectable hepatocellular carcinoma. Ann Oncol. 2017;28:V617. [RCA] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 30] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 50. | Mendizabal M, Silva MO. Developing multicenter consortia in liver disease in Latin America: Challenges and opportunities. Liver Transpl. 2017;23:1210-1215. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |