Published online Jun 14, 2021. doi: 10.3748/wjg.v27.i22.3085
Peer-review started: October 30, 2020
First decision: January 17, 2021
Revised: January 28, 2021
Accepted: April 25, 2021
Article in press: April 25, 2021
Published online: June 14, 2021
Processing time: 220 Days and 21.1 Hours
Quinine oxidoreductase 1 (NQO1) plays a vital role in protecting normal cells against oxidative damage and electrophilic attack. It is highly expressed in many solid tumors, suggesting a role in cancer development and progression. However, the role of NQO1 in gastric cancer and its effect on cancer development and prognosis have not been fully investigated.
To investigate the clinical relevance of NQO1 protein expression in gastric cancer and to explore the potential of NQO1 to serve as a prognostic biomarker and therapeutic target.
In this retrospective study, gastric cancer specimens of 175 patients who were treated between 1995 and 2011 were subjected to immunohistochemistry analyses for NQO1. The correlation of NQO1 expression with gastric cancer prognosis and clinical and pathological parameters was investigated.
NQO1 protein was overexpressed in 59.43% (104/175) of the analyzed samples. Overexpression of NQO1 was associated with a significantly inferior prognosis. In addition, multivariate analysis suggested that NQO1 overexpression, along with tumor stage and patient age, are prominent prognostic biomarkers for gastric cancer. Moreover, NQO1 overexpression was correlated to a better response to 5-fluorouracil (5-FU)-based adjuvant chemotherapy.
NQO1 overexpression is associated with a significantly poor prognosis and better response to 5-FU in patients with gastric cancer. These findings are relevant for improving therapeutic approaches for gastric cancer patients.
Core Tip: Quinone oxidoreductase 1 may be used as a useful prognostic biomarker and therapeutic target for the efficient management of patients with gastric cancer.
- Citation: Jiang ZN, Ahmed SMU, Wang QC, Shi HF, Tang XW. Quinone oxidoreductase 1 is overexpressed in gastric cancer and associated with outcome of adjuvant chemotherapy and survival. World J Gastroenterol 2021; 27(22): 3085-3096
- URL: https://www.wjgnet.com/1007-9327/full/v27/i22/3085.htm
- DOI: https://dx.doi.org/10.3748/wjg.v27.i22.3085
Gastric cancer (GC) is now the fourth most common cancer and the third most common cause of cancer-related deaths worldwide. It is estimated that 951600 new GC cases and 723100 deaths occurred in 2012. East Asia, including China, is among the areas with the highest incidence of GC, accounting for > 40% of all new cases[1,2]. Presently, the prevalence rates of GC are declining in most parts of the world owing to the establishment of mass screening and advanced treatment techniques. Despite this decline, the clinical outcomes of GC patients remain disappointing. Moreover, the prognosis of GC strongly depends on the TNM stage, biological factors, and clinical pathological factors[3,4]. Therefore, finding a new biomarker for the diagnosis and treatment of GC is of great value.
Quinine oxidoreductase 1 (NQO1) is a cytoplasmic flavoenzyme located on chromosome 16q22. It is also known as DT-diaphorase, menadione reductase, or quinone reductase 1. NQO1 directly reduces quinines to hyroquinones using reduced form of nicotinamide-adenine dinucleotide (NADH) or nicotinamide adenine dinucleotide phosphate (NADPH) as an electron donor[5,6]. Numerous functions of NQO1 have been reported, such as xenobiotic detoxification, superoxide scavenging, and maintenance of endogenous antioxidant vitamins[7]. It is likely that NQO1 plays an important role in protecting normal cells against oxidative damage and electrophilic attack. Paradoxically, despite the suggestion that it exerts a protective action and plays an antioxidant role, disruption of an NQO1 genetic polymorphism increases the risk of xenobiotic-induced toxicity and cancer[8,9]. High levels of NQO1 expression have been found in many human tumors, including breast cancer, melanoma, lung cancer, cholangiocarcinoma, and pancreatic cancer[10-13]. Garate et al[10] reported that NQO1 protein expression induces cell cycle progression and proliferation in melanoma cells. In a study by Lin et al[14], NQO1 expression in GC patients, non-cancer patients, and normal controls was quantified by immunohistochemistry (IHC). They reported that NQO1 played an important role in the progression of GC, and might be a potential prognostic biomarker and therapeutic target for GC. However, the role of NQO1 in cancer progression remains controversial, and data on its expression in GC are scarce. In this study, we analyzed the expression of NQO1 and its potential association with survival and pathological parameters in primary GC. We aimed to investigate the associations between NQO1 protein expression status and responses to 5-fluorouracil (5-FU)-based adjuvant chemotherapy in patients with GC and thereby determine the potential of NQO1 to serve as a prognostic biomarker and therapeutic target.
Unless otherwise stated, all chemicals were procured from Sigma-Aldrich Co. (Shanghai, China). Antibodies against rabbit NQO1 were used as described previously[15,16]. The human gastric cancer cell line AGS was obtained from the American Type Culture Collection (Manassas, VA, United States). Cells were maintained in RPMI-1640 growth medium (Shenggong, Shanghai, China) supplemented with 10% fetal bovine serum and antibiotics in a humidified atmosphere of 5% CO2 and 95% air at 37 °C[17]. Cell extracts were prepared for sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), and NQO1 immunoblotting was performed using a standard protocol with anti-NQO1 antibodies (dilution at 1:2000)[17,18].
A total of 175 samples of GC were used in this study. All patients were enrolled and treated between 1995 and 2011 at the Department of Surgical Oncology, Affiliated Sir Run Run Shaw Hospital, Zhejiang University School of Medicine, Hangzhou, China. All patients received surgical treatment and were periodically followed for the determination of their survival status.
Tumor samples were assembled as multiple tissue arrays, as described in our previous study[19]. IHC staining for NQO1 in paraffin-embedded tumor sections was performed using standard procedures. In brief, to eliminate endogenous peroxidase activity, the slides were deparaffinized in xylene, rehydrated through a graded series of ethanol solutions, and incubated with 3% H2O2 in methanol for 15 min at room temperature. Antigen retrieval was performed by placing the slides in 0.01 M sodium citrate buffer (pH 6.0) at 121 °C for 15 min. Next, the slides were incubated with the NQO1 antibodies (1:5000) overnight at 4 °C. The immunoreactions were visualized with an ImmPRESS detection kit (Vector Laboratories), which uses a second antibody conjugated with horseradish peroxidase and a diaminobenzidine-based stain. Finally, the sections were counterstained with Mayer's hematoxylin, dehydrated, and mounted.
Cytoplasmic NQO1 staining was considered as positive for NQO1 expression. NQO1 expression was semiquantitatively assessed by two experienced pathologists who were blinded to the clinical parameters. Analyses of NQO1 expression were based on both the staining intensity and proportion of positive cells. The staining intensity was scored from 0 to 3+ as follows: 0 for no staining, 1+ for weak staining, 2+ for moderate staining, and 3+ for strong staining[20]. The proportion of NQO1-positive cells was expressed as a percentage and divided into four grades: grade 0, 0% positive; grade 1, 1%-30% positive; grade 2, 31%-60% positive; and grade 3, > 60% positive cells. The total score was obtained by multiplying these two parameters (range 0-9); for statistical purposes, the samples were divided into two groups: Low expression level (0-4) and high expression level or overexpression (> 4) groups. As the median score of NQO1 expression in the GC samples was 4.5, a cut-off value of 4 was selected[20].
The correlation between the expression of NQO1 and clinical and pathological parameters was determined by statistical analysis using SSPS 16.0 for Windows (SPSS, incorporated, Chicago, IL, United States). The χ2 or Fisher’s exact test, Kaplan-Meier survival analysis, and Cox regression analysis were used as appropriate. P values < 0.05 were considered significant.
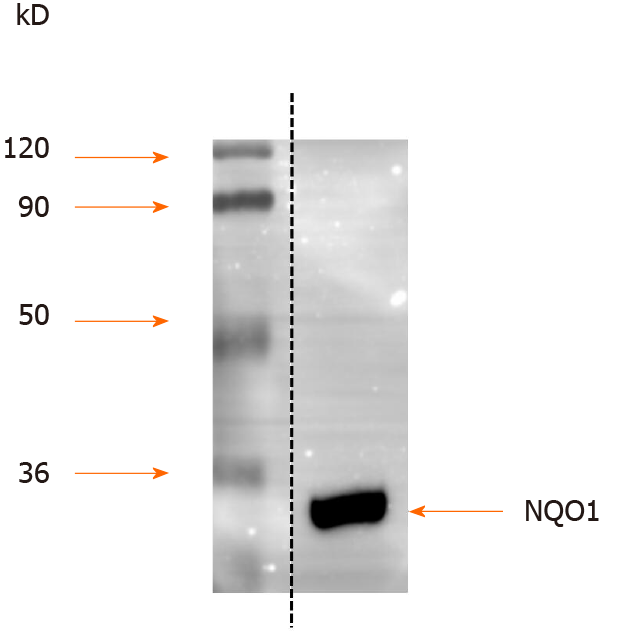
Protein extracts from AGS human GC cells were separated by SDS-PAGE, and the proteins were detected by Western blot analysis. The results showed that NQO1 can be readily detected, and no other protein cross-reacted with the NQO1 antibodies (Figure 1). Therefore, these antibodies are sensitive and specific for IHC staining to detect NQO1 expression in paraffin-embedded tumor sections.
A total of 175 patients were enrolled in this study. The baseline characteristics are described in detail and summarized in Table 1. The patients ranged from 26 to 84 years of age. The study population consisted of 124 men and 51 women. The clinical and pathological parameters consisted of age, gender, tumor size, tumor location, tumor stage, tumor depth, lymph node metastasis, histological differentiation, and survival data. The number of patients with a tumor size < 5 cm and ≥ 5 cm was 63 and 99, respectively. The stage of GC was determined at the time of analysis, according to the American Joint Commission on Cancer system AJCC TNM 8th edition. Of the 175 samples, 69 cases were stage I-II and 102 were stage III-IV GC. Among all patients, 67 underwent total gastrectomy, 83 received distal subtotal gastrectomy, 1 received partial resection, and 14 received proximal subtotal gastrectomy. The information of the remaining 10 patients was missing. None of the patients received chemotherapy prior to surgery. A total of 84 patients were treated with 5-FU + platinum-based adjuvant chemotherapy. The patients were followed until the last follow-up date or death. Among the 175 patients, there were 75 death events and 65 recurrence events in total. The follow-up period ranged from 0.5-182 mo, and the median follow-up time was 22.4 mo.
| Clinical parameter | Number of cases (n) | (%) |
| Age group | Range (26-84, mean = 58.9) | |
| < 60 yr | 83 | 47.43 |
| ≥ 60 yr | 92 | 52.57 |
| Sex | ||
| Male | 124 | 70.86 |
| Female | 51 | 29.14 |
| Tumor size (cm) | ||
| < 5 | 63 | 36.00 |
| ≥ 5 | 99 | 56.57 |
| Unknown | 13 | 7.43 |
| Tumor location | ||
| Fundus | 40 | 22.86 |
| Body | 44 | 25.14 |
| Antral | 82 | 46.68 |
| Whole | 4 | 2.29 |
| Unknown | 5 | 2.85 |
| TNM stage | ||
| I II | 69 | 39.43 |
| III IV | 102 | 58.29 |
| Unknown | 4 | 2.28 |
| Tumor depth | ||
| T1/T2 | 33 | 18.86 |
| T3/T4 | 139 | 79.43 |
| Unknown | 3 | 1.71 |
| Lymph node metastasis | ||
| Yes | 43 | 24.57 |
| No | 123 | 70.29 |
| Unknown | 9 | 5.14 |
| Vascular invasion | ||
| Yes | 19 | 10.86 |
| No | 156 | 89.14 |
| Histological Differentiation | ||
| Poor | 20 | 11.43 |
| Moderate | 44 | 25.14 |
| Well | 111 | 63.43 |
| Surgical type | ||
| Complete resection | 67 | 38.29 |
| Distal resection | 83 | 47.43 |
| Proximal resection | 14 | 8.00 |
| Partial resection | 1 | 0.57 |
| Unknown | 10 | 5.71 |
| Chemotherapy | ||
| Adjuvant chemotherapy | 84 | 48.00 |
| Without chemotherapy | 80 | 45.71 |
| Unknown | 11 | 6.29 |
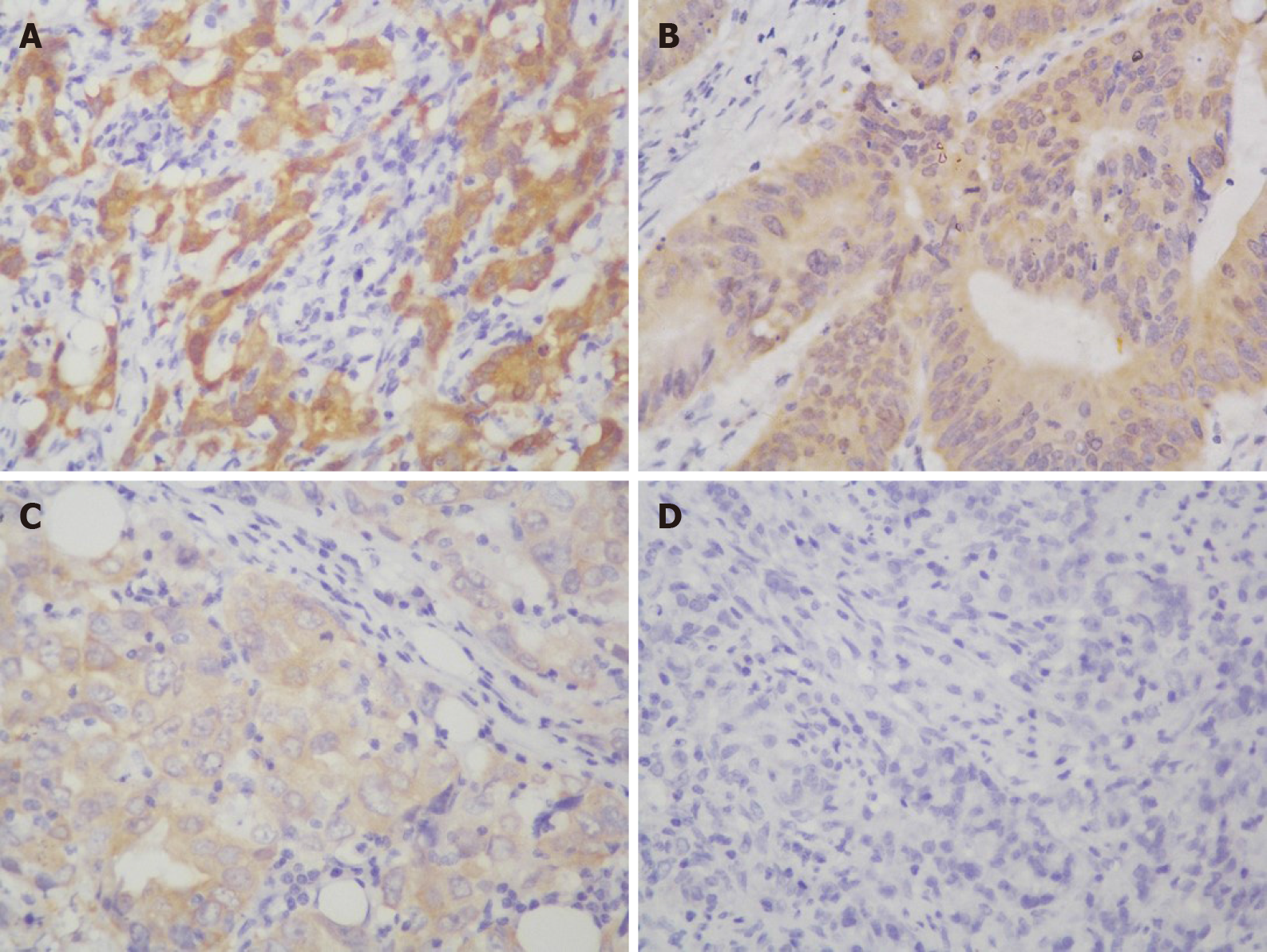
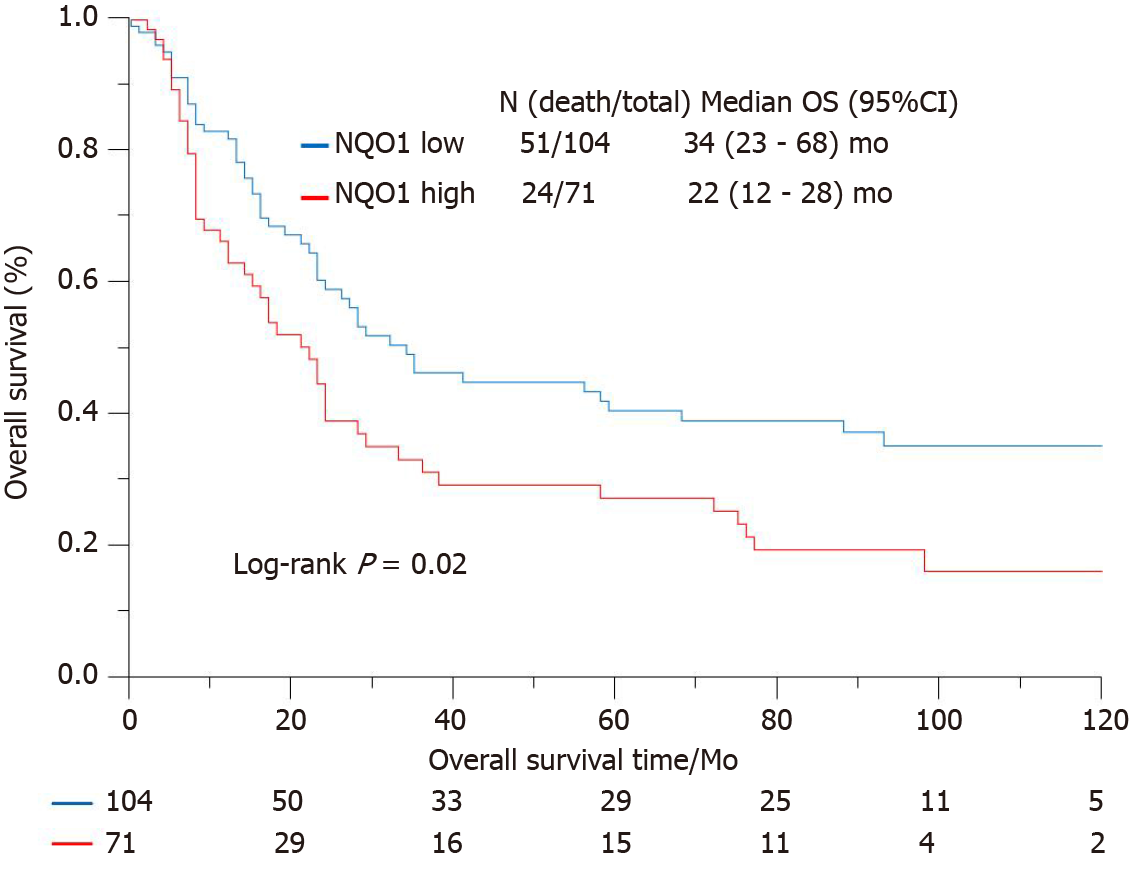
IHC staining showed that the NQO1 protein was mainly localized in the cytoplasm of GC cells (Figure 2). The level of NQO1 protein expression was high in 49.43% of the samples, with an overall score of > 4, while 71 (40.57%) samples showed low NQO1 expression levels, with an overall score of ≤ 4. To evaluate the role of the NQO1 protein in GC progression, we analyzed the correlation between its expression and the clinical and pathological parameters (Table 2). The number of patients with high levels of NQO1 expression and lymph node metastasis was higher than that of patients with low NQO1 expression, but this difference was not significant (P = 0.06; Table 2). In addition, there were no significant correlations between NQO1 and other clinical parameters (Table 2). However, the follow-up results showed that the mean survival time for patients with low levels of NQO1 expression was 34 mo, while for those with NQO1 overexpression, it was 22 mo. Kaplan-Meier survival analysis revealed that patients with NQO1 overexpression had significantly poorer survival rates than those with low levels of NQO1 expression (P = 0.02; Figure 3).
| Clinical parameter | NQO1 expression | ||
| High (%) | Low (%) | P value | |
| Age (yr) | 0.92 | ||
| < 60 | 34 (40.96) | 49 (59.04) | |
| ≥ 60 | 37 (40.22) | 55 (59.78) | |
| Gender | |||
| Male | 48 (38.71) | 76 (61.29) | |
| Female | 23 (45.10) | 28 (54.90) | |
| Tumor size (cm) | 0.81 | ||
| < 5 | 23 (36.51) | 40 (63.49) | |
| ≥ 5 | 38 (38.38) | 61 (61.62) | |
| Tumor location | 0.19 | ||
| Fundus | 18 (40.91) | 26 (59.09) | |
| Body | 11 (27.50) | 29 (72.50) | |
| Antral | 34 (41.46) | 48 (58.54) | |
| Whole | 3 (75.00) | 1(25.00) | |
| Tumor staging | 0.06 | ||
| I/II | 22 (31.88) | 47 (68.12) | |
| III/IV | 47 (46.08) | 55 (53.92) | |
| Tumor depth | 0.37 | ||
| T1 T2 | 11 (33.33) | 22 (66.67) | |
| T3 T4 | 58 (41.73) | 81 (58.27) | |
| Lymph node metastasis | |||
| Yes | 12 (27.91) | 31 (72.09) | |
| No | 54 (43.90) | 69 (56.10) | |
| Vascular invasion | 0.72 | ||
| Yes | 7 (36.84) | 12 (63.16) | |
| No | 64 (41.03) | 92 (58.97) | |
| Histological differentiation | 0.57 | ||
| Poor | 8 (40.00) | 12 (60.00) | |
| Moderate | 15 (34.10) | 29 (65.90) | |
| Well | 48 (43.24) | 63 (56.76) | |
We further used the Cox proportional hazard regression method to examine the correlations between various clinical indexes and survival (Table 3). Univariate analysis revealed significant associations of tumor size (P < 0.001), tumor stage (P < 0.001), and NQO1 expression (P = 0.01) with overall survival, whereas the other factors showed no significant association. However, multivariate analysis of these factors, including NQO1 expression, indicated that age, tumor stage, and NQO1 were independent prognostic factors for the overall survival of GC patients [hazard ratio (HR) = 1.90, P = 0.01; HR = 2.64, P < 0.001; and HR = 1.56, P = 0.05, respectively].
| Clinical factor | 1Univariate | Multivariate | ||||
| P value | HR | 95%CI | P value | HR | 95%CI | |
| Interval | ||||||
| Age (< 60/≥ 60) | ||||||
| - | - | - | 0.01 | 1.90 | 1.20-3.02 | |
| Gender (male/female) | ||||||
| - | - | - | 0.96 | 1.03 | 0.62-1.66 | |
| Tumor size (< 5/≥ 5 cm) | ||||||
| < 0.001 | 1.75 | 1.10-2.87 | 0.40 | 1.00 | 0.76-2.25 | |
| Tumor location | ||||||
| Proximal | 1 (Reference) | 1 (Reference) | ||||
| Body | 0.67 | 1.13 | 0.65-1.94 | 0.82 | 1.06 | 0.59-1.95 |
| Distal | 0.09 | 0.7 | 0.43-1.15 | 0.11 | 0.69 | 0.41-1.19 |
| Whole2 | - | - | - | - | - | - |
| Tumor stage (III, IV/I, II) | ||||||
| < 0.001 | 3.11 | 1.96-5.09 | < 0.001 | 2.64 | 1.56-4.61 | |
| Histological differentiation | ||||||
| Well | 1 (Reference) | 1 (Reference) | ||||
| Moderate | 0.3 | 1.58 | 0.68-4.30 | 0.62 | 1.26 | 0.53-3.48 |
| Poor | 0.07 | 2.03 | 0.96-5.23 | 0.15 | 1.79 | 0.83-4.67 |
| NQO1 (high/low) | ||||||
| 0.01 | 1.73 | 1.16-2.58 | 0.05 | 1.56 | 1.01-2.40 |
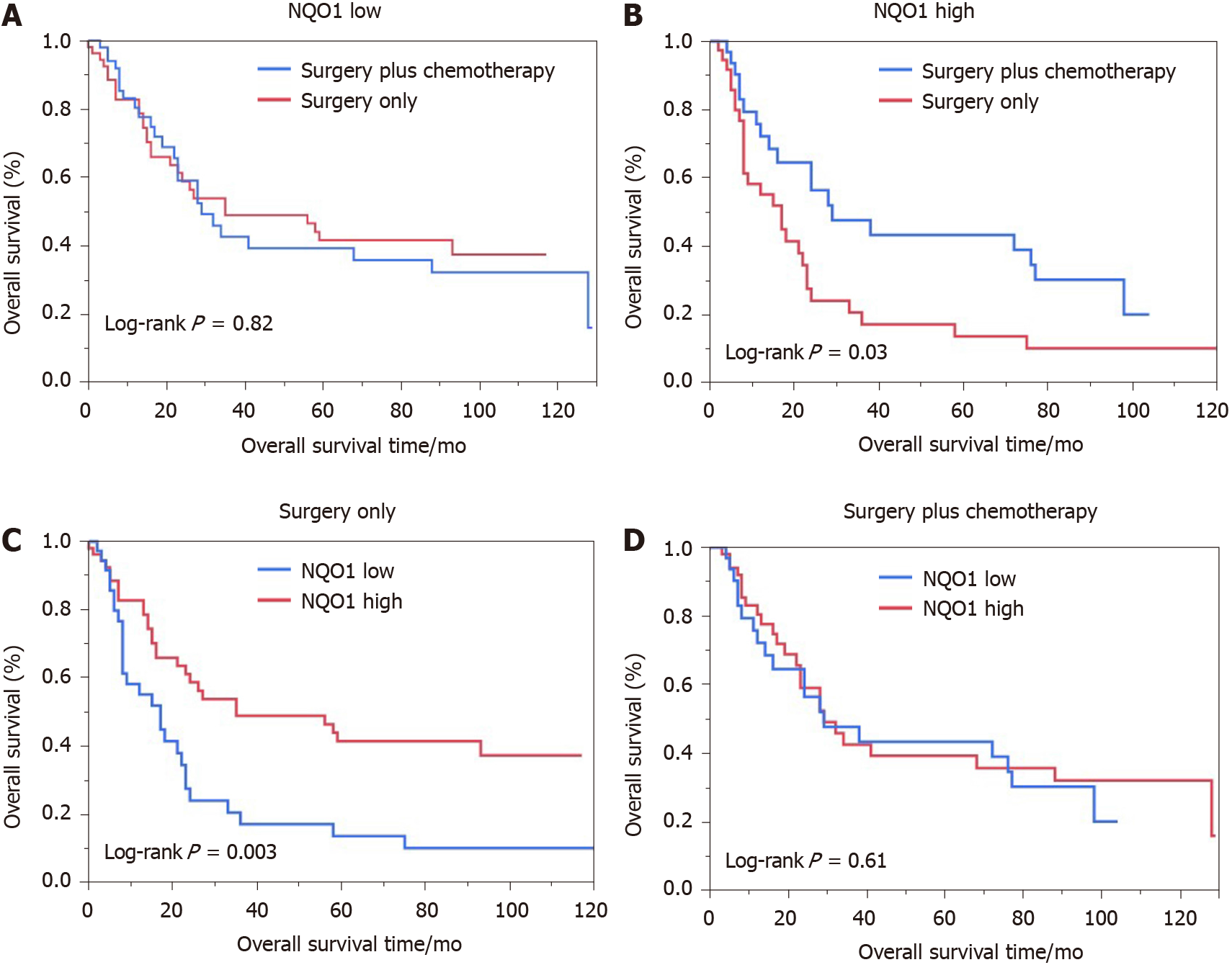
We also performed the survival analysis of patients from the various treatment subgroups based on the NQO1 expression. Among 84 patients treated with 5-FU-based chemotherapy, 52 (61.09%) had high levels of NQO1 expression and 32 (38.10%) had low levels of NQO1 expression. Similarly, high and low levels of NQO1 expression were found in 60% and 40% of patients who did not receive treatment, respectively. Patients with low levels of NQO1 expression showed poor response to adjuvant chemotherapy and similar survival, compared to those that underwent surgery alone (log rank P = 0.82) (Figure 4A). However, high levels of NQO1 expression indicated good response to adjuvant chemotherapy and longer overall survival compared with those in patients that underwent surgery alone (log rank P = 0.03) (Figure 4B). In addition, in the Kaplan-Meier analysis, we stratified NQO1 expression based on chemotherapy. Interestingly, the results showed that in patients who underwent surgery only, significant difference in overall survival was observed (log rank P = 0.003), whereas in patients who underwent surgery plus chemotherapy, no difference was found (log rank P = 0.61) (Figure 4C and D). These findings indicated that GC patients with NQO1 overexpression may be suitable for adjuvant chemotherapy.
The catalytic properties of NQO1 were first reported by Ernster and Navazio[21] in 1958. NQO1 is a homodimeric flavoprotein that reduces quinines to hydroquinones in an obligatory two-electron reduction step[6]. After years of research, cumulative data have shown that NQO1 protects cells from exogenous substances, oxidants, and ultraviolet and ionizing radiation by inducing a series of protective reactions[10]. Consistent with this finding, recent research by Nagata et al[22] and Malik et al[23] showed that the C609T polymorphism in the NQO1 gene might be linked to the risk of cancer-associated death. In addition, an NQO1 polymorphism that causes inactivation of the enzyme predicts a poor outcome in breast cancer[24]. Moreover, NQO1 plays a prominent role in regulating the stability of p53 by inhibiting its degradation and that of other labile proteins, such as ornithine decarboxylase[25,26]. Based on this evidence, NQO1 plays an important role in cancer progression.
Recent studies have reported that NQO1 protein expression is significantly elevated in the cytoplasm and nucleus in many human solid tumors[27]. For instance, Yang et al[28] showed that NQO1 is highly expressed in breast cancer tissues and may be associated with breast cancer progression. Cheng et al[29] demonstrated strong NQO1 expression in primary melanomas compared with that in dysplastic nevi, and that NQO1 may be involved in the initiation of melanoma development. Similarly, the expression of NQO1 in the cervical epithelium indicated that it might be responsible for cervical carcinogenesis[30]. Malkinson et al[31] reported elevated NQO1 expression in human lung cancer tissues. Li et al[32] showed that NQO1 is upregulated in non-small cell lung cancer and identified it as a biomarker of poor prognosis. In addition, the gene encoding NQO1 is thought to participate in the pathogenesis of lung cancer[33,34]. Consistently, Ji et al[35], Awadallah et al[13], and Lyn-Cook et al[36] demonstrated that elevated NQO1 expression is associated with pancreatic ductal adenocarcinoma and may be a potential prognostic biomarker for pancreatic cancer.
In the present study, elevated NQO1 expression was observed in cancer tissues. In addition, a higher rate of NQO1 protein overexpression in patients with lymph node metastasis than in those without metastasis was observed. This is partly consistent with the findings of Lin et al[14], which suggested that NQO1 upregulation contributes to tumorigenesis and progression of GC.
With regard to survival, we found that GC patients with overexpression of NQO1 protein had a significantly lower overall survival than those with low NQO1 expression. Buranrat et al[12] showed a significant correlation between overexpression of NQO1 protein and short overall survival in cholangiocarcinoma patients, enhancing the possibility of using NQO1 as a tumor biomarker. Univariate survival analysis revealed that tumor size, tumor location, tumor stage, histological differentiation, and NQO1 expression status were significantly associated with the overall survival of GC patients. Likewise, multivariate survival analysis revealed that NQO1 was an independent prognostic biomarker, along with the tumor stage and patient age. Interestingly, it was also notable that patients with elevated NQO1 expression may be more sensitive to 5-FU-based chemotherapy than those with low NQO1 expression. Although the mechanism underlying this phenomenon remains unclear, the results indicated that NQO1 may serve as a biomarker for the early diagnosis and prognosis of GC, and as a potential molecular target for GC therapy. However, the role of NQO1 in GC has not been fully elucidated. Further manipulation of NQO1 gene expression in different gastric cancer cell lines and investigation of the characteristics and mechanism of its effects on gastric cancer are needed in additional studies.
NQO1 is involved in the initiation and progression of GC. High NQO1 expression levels indicates a worse overall survival and better response to 5-FU therapy. NQO1 could be a useful prognostic biomarker and therapeutic target for the appropriate management of patients with GC.
Quinone oxidoreductase 1 (NQO1) plays a cytoprotective role in normal tissues. It is overexpressed in many solid tumors, suggesting a role in cancer development and progression. However, the expression of NQO1 in gastric cancer and its influence on cancer development and prognosis have not been fully addressed.
Identifying molecular markers that can be used as an independent prognostic factor for gastric cancer is of great significance for the early diagnosis and targeted therapy of gastric cancer. The expression of NQO1 in gastric cancer and its influence on clinicopathological characteristics and prognosis have not previously been extensively determined.
The objective of this study was to discover novel effective biomarkers to classify patients with low or high survival. This would provide a guide to clinicians to design personalized therapeutic approaches for gastric cancer patients. In this study, we investigated NQO1 protein, which may determine patient management strategies.
We used IHC to detect the expression of NQO1 in gastric cancer and adjacent tissues. Western blot analysis was performed to detect the expression of NQO1 in gastric cancer cell line. According to IHC scores, the patients were divided into NQO1 high expression group and NQO1 low expression group, and the relationship between the expression level and clinicalpathological data was analyzed.
We found no significant correlations between NQO1 and clinical parameters. However, the follow-up results showed that patients with NQO1 overexpression had a significantly poorer survival rates than those with low levels of NQO1.
Patients with low levels of NQO1 expression showed a poor response to adjuvant chemotherapy and similar survival, compared to those who underwent surgery alone. However, high levels of NQO1 expression indicated a good response to adjuvant chemotherapy and longer overall survival compared with those in patients who underwent surgery alone. In addition, in the Kaplan-Meier analysis, we stratified NQO1 expression based on chemotherapy. The results showed that in patients who underwent surgery only, a significant difference in overall survival was observed, whereas in patients who underwent surgery plus chemotherapy, no difference was found.
This study found that high expression of NQO1 is closely related to a poor prognosis and better response to 5-fluorouracil in patients with gastric cancer.
Our study found prognostic value of NQO1 protein expression in patients with gastric cancer. However, our results need to be validated by independent groups or cohorts in a prospective study.
We thank Ms Shiping Yan and Mr. Ahmed Hammad for their assistance with the preparation of the manuscript.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Kato S, Nath J S-Editor: Zhang L L-Editor: Wang TQ P-Editor: Liu JH
| 1. | Torre LA, Siegel RL, Ward EM, Jemal A. Global Cancer Incidence and Mortality Rates and Trends--An Update. Cancer Epidemiol Biomarkers Prev. 2016;25:16-27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2004] [Cited by in RCA: 2488] [Article Influence: 248.8] [Reference Citation Analysis (0)] |
| 2. | Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87-108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18694] [Cited by in RCA: 21366] [Article Influence: 2136.6] [Reference Citation Analysis (3)] |
| 3. | Warneke VS, Behrens HM, Hartmann JT, Held H, Becker T, Schwarz NT, Röcken C. Cohort study based on the seventh edition of the TNM classification for gastric cancer: proposal of a new staging system. J Clin Oncol. 2011;29:2364-2371. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 84] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 4. | Lagarde SM, ten Kate FJ, Reitsma JB, Busch OR, van Lanschot JJ. Prognostic factors in adenocarcinoma of the esophagus or gastroesophageal junction. J Clin Oncol. 2006;24:4347-4355. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 106] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 5. | Zhu CL, Huang Q, Liu CH, Lin XS, Xie F, Shao F. NAD(P)H: quinone oxidoreductase 1 (NQO1) C609T gene polymorphism association with digestive tract cancer: a meta-analysis. Asian Pac J Cancer Prev. 2013;14:2349-2354. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 6. | Ross D, Kepa JK, Winski SL, Beall HD, Anwar A, Siegel D. NAD(P)H:quinone oxidoreductase 1 (NQO1): chemoprotection, bioactivation, gene regulation and genetic polymorphisms. Chem Biol Interact. 2000;129:77-97. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 457] [Cited by in RCA: 468] [Article Influence: 18.7] [Reference Citation Analysis (0)] |
| 7. | Siegel D, Gustafson DL, Dehn DL, Han JY, Boonchoong P, Berliner LJ, Ross D. NAD(P)H:quinone oxidoreductase 1: role as a superoxide scavenger. Mol Pharmacol. 2004;65:1238-1247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 371] [Cited by in RCA: 361] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 8. | Su XL, Yan MR, Yang L; Qimuge-Suyila. NQO1 C609T polymorphism correlated to colon cancer risk in farmers from western region of Inner Mongolia. Chin J Cancer Res. 2012;24:317-322. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 9. | Radjendirane V, Joseph P, Lee YH, Kimura S, Klein-Szanto AJ, Gonzalez FJ, Jaiswal AK. Disruption of the DT diaphorase (NQO1) gene in mice leads to increased menadione toxicity. J Biol Chem. 1998;273:7382-7389. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 204] [Cited by in RCA: 200] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 10. | Garate M, Wani AA, Li G. The NAD(P)H:Quinone Oxidoreductase 1 induces cell cycle progression and proliferation of melanoma cells. Free Radic Biol Med. 2010;48:1601-1609. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 40] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 11. | Siegel D, Franklin WA, Ross D. Immunohistochemical detection of NAD(P)H:quinone oxidoreductase in human lung and lung tumors. Clin Cancer Res. 1998;4:2065-2070. [PubMed] |
| 12. | Buranrat B, Chau-in S, Prawan A, Puapairoj A, Zeekpudsa P, Kukongviriyapan V. NQO1 expression correlates with cholangiocarcinoma prognosis. Asian Pac J Cancer Prev. 2012;13 Suppl:131-136. [PubMed] |
| 13. | Awadallah NS, Dehn D, Shah RJ, Russell Nash S, Chen YK, Ross D, Bentz JS, Shroyer KR. NQO1 expression in pancreatic cancer and its potential use as a biomarker. Appl Immunohistochem Mol Morphol. 2008;16:24-31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 61] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 14. | Lin L, Qin Y, Jin T, Liu S, Zhang S, Shen X, Lin Z. Significance of NQO1 overexpression for prognostic evaluation of gastric adenocarcinoma. Exp Mol Pathol. 2014;96:200-205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 32] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 15. | Luo L, Chen Y, Wu D, Shou J, Wang S, Ye J, Tang X, Jun Wang X. Differential expression patterns of Nqo1, AKR1B8 and Ho-1 in the liver and small intestine of C57BL/6 mice treated with sulforaphane. Data Brief. 2015;5:416-423. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 16. | Luo L, Chen Y, Wu D, Shou J, Wang S, Ye J, Tang X, Wang XJ. Butylated hydroxyanisole induces distinct expression patterns of Nrf2 and detoxification enzymes in the liver and small intestine of C57BL/6 mice. Toxicol Appl Pharmacol. 2015;288:339-348. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 31] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 17. | Ahmed SM, Wu X, Jin X, Zhang X, Togo Y, Suzuki T, Li Y, Kanematsu A, Nojima M, Yamamoto S, Sugimoto M, Kakehi Y. Synergistic induction of apoptosis by mapatumumab and anthracyclines in human bladder cancer cells. Oncol Rep. 2015;33:566-572. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 18. | Tang X, Wang H, Fan L, Wu X, Xin A, Ren H, Wang XJ. Luteolin inhibits Nrf2 Leading to negative regulation of the Nrf2/ARE pathway and sensitization of human lung carcinoma A549 cells to therapeutic drugs. Free Radic Biol Med. 2011;50:1599-1609. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 227] [Cited by in RCA: 268] [Article Influence: 19.1] [Reference Citation Analysis (0)] |
| 19. | Wang Q, Shu X, Dong Y, Zhou J, Teng R, Shen J, Chen Y, Dong M, Zhang W, Huang Y, Xie S, Wei Q, Zhao W, Chen W, Yuan X, Qi X, Wang L. Tumor and serum gamma-glutamyl transpeptidase, new prognostic and molecular interpretation of an old biomarker in gastric cancer. Oncotarget. 2017;8:36171-36184. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 27] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 20. | Kawasaki Y, Ishigami S, Arigami T, Uenosono Y, Yanagita S, Uchikado Y, Kita Y, Nishizono Y, Okumura H, Nakajo A, Kijima Y, Maemura K, Natsugoe S. Clinicopathological significance of nuclear factor (erythroid-2)-related factor 2 (Nrf2) expression in gastric cancer. BMC Cancer. 2015;15:5. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 51] [Cited by in RCA: 51] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 21. | ERNSTER L, LINDBERG O. Animal mitochondria. Annu Rev Physiol. 1958;20:13-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 62] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 22. | Nagata M, Kimura T, Suzumura T, Kira Y, Nakai T, Umekawa K, Tanaka H, Matsuura K, Mitsuoka S, Yoshimura N, Oka T, Kudoh S, Hirata K. C609T Polymorphism of NADPH Quinone Oxidoreductase 1 Correlates Clinical Hematological Toxicities in Lung Cancer Patients Treated with Amrubicin. Clin Med Insights Oncol. 2013;7:31-39. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 23. | Malik MA, Zargar SA, Mittal B. Role of NQO1 609C>T and NQO2 -3423G>A gene polymorphisms in esophageal cancer risk in Kashmir valley and meta analysis. Mol Biol Rep. 2012;39:9095-9104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 23] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 24. | Fagerholm R, Hofstetter B, Tommiska J, Aaltonen K, Vrtel R, Syrjäkoski K, Kallioniemi A, Kilpivaara O, Mannermaa A, Kosma VM, Uusitupa M, Eskelinen M, Kataja V, Aittomäki K, von Smitten K, Heikkilä P, Lukas J, Holli K, Bartkova J, Blomqvist C, Bartek J, Nevanlinna H. NAD(P)H:quinone oxidoreductase 1 NQO1*2 genotype (P187S) is a strong prognostic and predictive factor in breast cancer. Nat Genet. 2008;40:844-853. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 155] [Cited by in RCA: 171] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 25. | Asher G, Lotem J, Cohen B, Sachs L, Shaul Y. Regulation of p53 stability and p53-dependent apoptosis by NADH quinone oxidoreductase 1. Proc Natl Acad Sci USA. 2001;98:1188-1193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 126] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 26. | Asher G, Bercovich Z, Tsvetkov P, Shaul Y, Kahana C. 20S proteasomal degradation of ornithine decarboxylase is regulated by NQO1. Mol Cell. 2005;17:645-655. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 123] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 27. | Siegel D, Kepa JK, Ross D. NAD(P)H:quinone oxidoreductase 1 (NQO1) localizes to the mitotic spindle in human cells. PLoS One. 2012;7:e44861. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 38] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 28. | Yang Y, Zhang Y, Wu Q, Cui X, Lin Z, Liu S, Chen L. Clinical implications of high NQO1 expression in breast cancers. J Exp Clin Cancer Res. 2014;33:14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 93] [Cited by in RCA: 137] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 29. | Cheng Y, Li J, Martinka M, Li G. The expression of NAD(P)H:quinone oxidoreductase 1 is increased along with NF-kappaB p105/p50 in human cutaneous melanomas. Oncol Rep. 2010;23:973-979. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 15] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 30. | Ma Y, Kong J, Yan G, Ren X, Jin D, Jin T, Lin L, Lin Z. NQO1 overexpression is associated with poor prognosis in squamous cell carcinoma of the uterine cervix. BMC Cancer. 2014;14:414. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 51] [Cited by in RCA: 71] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 31. | Malkinson AM, Siegel D, Forrest GL, Gazdar AF, Oie HK, Chan DC, Bunn PA, Mabry M, Dykes DJ, Harrison SD. Elevated DT-diaphorase activity and messenger RNA content in human non-small cell lung carcinoma: relationship to the response of lung tumor xenografts to mitomycin Cł. Cancer Res. 1992;52:4752-4757. [PubMed] |
| 32. | Li Z, Zhang Y, Jin T, Men J, Lin Z, Qi P, Piao Y, Yan G. NQO1 protein expression predicts poor prognosis of non-small cell lung cancers. BMC Cancer. 2015;15:207. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 81] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 33. | Rosvold EA, McGlynn KA, Lustbader ED, Buetow KH. Identification of an NAD(P)H:quinone oxidoreductase polymorphism and its association with lung cancer and smoking. Pharmacogenetics. 1995;5:199-206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 79] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 34. | Heller G, Zielinski CC, Zöchbauer-Müller S. Lung cancer: from single-gene methylation to methylome profiling. Cancer Metastasis Rev. 2010;29:95-107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 81] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 35. | Ji M, Jin A, Sun J, Cui X, Yang Y, Chen L, Lin Z. Clinicopathological implications of NQO1 overexpression in the prognosis of pancreatic adenocarcinoma. Oncol Lett. 2017;13:2996-3002. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 16] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 36. | Lyn-Cook BD, Yan-Sanders Y, Moore S, Taylor S, Word B, Hammons GJ. Increased levels of NAD(P)H: quinone oxidoreductase 1 (NQO1) in pancreatic tissues from smokers and pancreatic adenocarcinomas: A potential biomarker of early damage in the pancreas. Cell Biol Toxicol. 2006;22:73-80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 28] [Article Influence: 1.5] [Reference Citation Analysis (0)] |