Published online Jan 14, 2021. doi: 10.3748/wjg.v27.i2.152
Peer-review started: October 14, 2020
First decision: December 3, 2020
Revised: December 15, 2020
Accepted: December 22, 2020
Article in press: December 22, 2020
Published online: January 14, 2021
The infusion of triolein emulsion (TE) induced increased vascular permeability and a negligible and temporary decrease in liver function without specific histopathological damage.
To assess changes in doxorubicin concentration according to the percentage of TE infused via a hepatic artery to study the vascular permeability in the rabbit liver.
Thirty-nine healthy rabbits were divided into five groups according to the concentration of emulsified triolein infused into the hepatic arteries: Group 0, saline infusion (control group, n = 5); group 1, 0.3% TE (n = 13); group 2, 0.6% TE (n = 6); group 3, 0.9% TE (n = 8); and group 4, 1.5% TE (n = 6). Doxorubicin (2.4 mg/kg) was infused immediately after TE injection via the hepatic arteries. After 2 h, the livers were harvested, and doxorubicin concentrations were calculated fluorometrically. The doxorubicin concentrations were compared between TE groups and the control group, and the optimal concentrations within the TE groups were calculated. Statistical analysis was performed using the nonparametric Mann-Whitney U test and Kruskal–Wallis test. P < 0.05 were considered statistically significant.
In the liver, doxorubicin concentrations were 2.06, 2.07, 2.16 and 1.66 times higher in groups 1 through 4, respectively, and significantly higher in the TE groups than in the control group (all P < 0.05). However, there were no significant differences in the mean doxorubicin concentrations between the four TE groups (P = 0.642). In the lungs, the mean doxorubicin concentrations were not significantly different between the control and TE groups (P > 0.05).
TE infusion into the hepatic arteries significantly increased the doxorubicin concentration approximately twofold but was not different between the TE groups. These findings suggest that TE infusion might be a useful adjuvant treatment of liver cancers.
Core Tip: Triolein emulsion infused intra-arterially increases vascular permeability transiently and reversibly. This could be applicable for adjuvant treatment to enhance chemotherapy using an angiographic technique. This study assessed changes in doxorubicin concentration per the percentage of TE infused via hepatic arteries to study vascular permeability in rabbit liver.
- Citation: Kim YW, Kim HJ, Cho BM, Choi SH. Triolein emulsion infusion into the hepatic artery increases vascular permeability to doxorubicin in rabbit liver. World J Gastroenterol 2021; 27(2): 152-161
- URL: https://www.wjgnet.com/1007-9327/full/v27/i2/152.htm
- DOI: https://dx.doi.org/10.3748/wjg.v27.i2.152
Sinusoidal capillaries in the liver have a lumen of almost 30 μm or more in diameter, which is much greater than that of normal capillaries. They also have irregular, tortuous walls, not formed by a continuous layer of endothelial cells, and have wide gaps of approximately 100 nm in diameter between cells. There are an incomplete basal lamina and no morphologic barrier between the perisinusoidal space and sinusoids. Therefore, plasma has direct access to the cell surface of the liver, which is a functionally very important structural characteristic for active metabolic exchange between the liver and blood[1].
In our previous study[2], the infusion of triolein emulsion (TE) in rabbit livers induced increased vascular permeability and a negligible and temporary decrease in liver function without specific histopathological damages. The gross findings revealed evans blue staining in 33% of the TE infusion group. In that study[2], we used only a single triolein volume (0.2 mL triolein in 20 mL of normal saline), whereas other previous studies[3,4] related to TE used 0.05 or 1 mL triolein in 20 mL of normal saline. Vascular permeability is possibly related to the volume of infused triolein. However, there have been no researches on this reciprocal relationship between the infused triolein volume and vascular permeability in the liver. Therefore, it is required to determine the appropriate volume of infused triolein in order to maximize the increase in vascular permeability as required. A direct quantitative demonstration is required to determine the degree of vascular permeability increase after infusion of TE into the hepatic artery.
Doxorubicin, a widely used anticancer agent, was one of the first and most commonly used chemotherapeutic drugs for the treatment of hepatocellular carcinoma (HCC)[5,6]. Intermediate HCC is treated with angiography-guided transcatheter therapy by selectively infusing a chemotherapeutic drug, a technique called transarterial chemoembolization[7-10]. This treatment method has two purposes: To deliver the drug close to the tumor and to cause an arterial embolization close to the cancer location[5,11]. The tumor is exposed to a high concentration of the drugs when infused close to it, leading to an increase in antitumor effect, while also reducing systemic exposure and side effects[12]. Furthermore, doxorubicin and its metabolites have specific fluorescence features and can be used for follow-up on the outcome of doxorubicin[13,14]. However, if TE infusion into the liver could be used to open the sinusoids or sinusoidal capillaries in the liver, this might increase the doxorubicin concentration and lead to an increased antitumor effect, which would subsequently reduce the total dosage and side effects of chemotherapeutic drugs. Moreover, doxorubicin can be extracted from the samples of liver tissue and quantified with a fluorometer at excitation and emission wavelengths of 470 and 585 nm, respectively[15-17].
The purpose of the present study was to evaluate changes in doxorubicin concentrations in rabbit liver tissues according to the percentage of TE infused via the hepatic arteries in a rabbit liver model.
Animal experiments were performed according to our institutional laboratory animal protocol guidelines (Approval No. PNUH-2019-139). The animal house was maintained at 22 ± 2 °C with a room humidity of 50% ± 15% under a 12 h dark/light cycle. Animals were kept in an environmentally controlled breeding room and supplied with standard laboratory food and water for 3 d before the experiments.
Before drug administration, animals were subjected to a 12-hour fast with free access to water. Thirty-nine New Zealand White rabbits (Samtaco, Osan, South Korea), weighing 2.6-3 kg each, were used in this doxorubicin study. The rabbits were anesthetized intramuscularly, in the groin muscles, with ketamine HCl (2.5 mg/kg; Huons, Jecheon, South Korea) and xylazine (0.125 mg/kg; Bayer Korea, Seoul, South Korea). During the procedure, the rabbits were ventilated using room air. Body temperature was monitored using a rectal probe (MGA-III 219; Shibaura Electronics, Tokyo, Japan) and was maintained at 35.5 °C to 36.5 °C with a heating pad. After anesthesia, each rabbit was placed in a supine position on a laboratory-fabricated wooden holding table that allowed each of the four extremities to be fixed with straps.
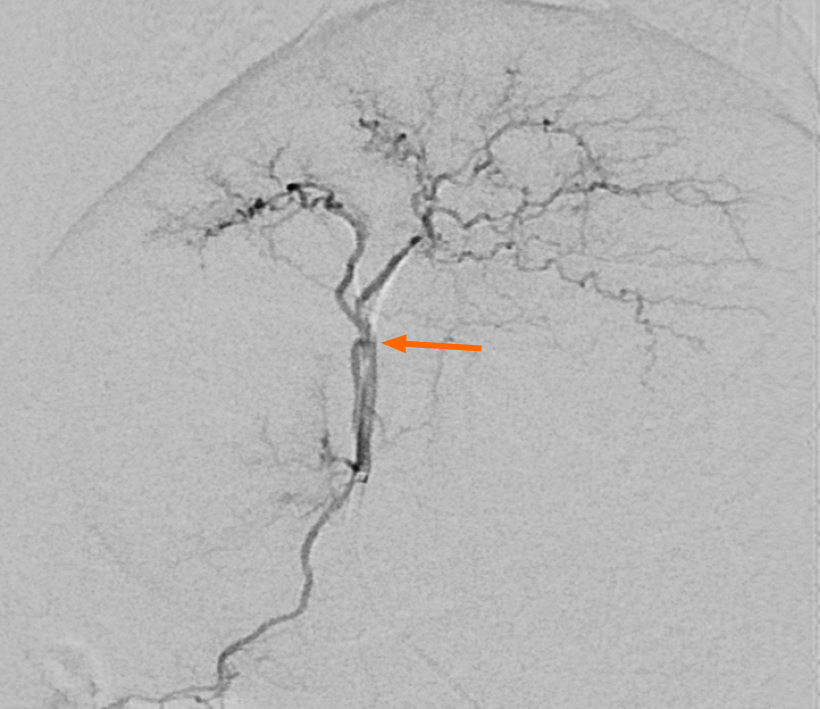
Short hairs on the dorsal ear surfaces were shaved and a dressing placed on the puncture sites. After tapping the central auricular artery repeatedly to dilate its lumen, the artery was punctured randomly in the right or left ears using a 20-gauge intravenous catheter (Insyte, Becton Dickinson, Sandy, UT, United States). After advancing the plastic sheath and simultaneously removing the inner stylet needle of the IV catheter, a 2.0-F microcatheter (Progreat α®; Terumo, Tokyo, Japan) with a 0.016-inch guidewire (GT Wire; Terumo, Tokyo, Japan) was introduced through the IV catheter and advanced to the aortic arch. Microcatheter and guidewire manipulations were performed under fluoroscopic guidance to permit selection of the celiac trunk, and angiography was performed by manual injection of contrast media (Xenetix®; donated by Guerbet Korea, Seoul, South Korea). The microcatheter tip was positioned in the proper hepatic artery following fluoroscopic guidance (Figure 1).
TE was performed as described by Kim et al[3]. The rabbits were classified according to the TE concentration infused into the hepatic artery as follows: Group 1 (0.3% TE; n = 13), group 2 (0.6% TE; n = 6), group 3 (0.9% TE; n = 8), and group 4 (1.5% TE; n = 6). TE (20 mL) was infused into each rabbit via the microcatheter at a speed of 4 mL/min. Normal saline (20 mL) was infused instead of TE in the control group (group 0, n = 5). Immediately after the infusion of TE (groups 1-4) or saline (group 0), doxorubicin hydrochloride (2.4 mg/kg; Boryung Pharmacy, Kyungkido, South Korea) was injected via the same catheter at the same speed, in all rabbits of all experimental groups. Subsequently, 3 mL of evans blue (Sigma-Aldrich, St. Louis, MO, United States) was infused through the same catheter just after the doxorubicin injection to mark targeted areas for liver tissue extraction.
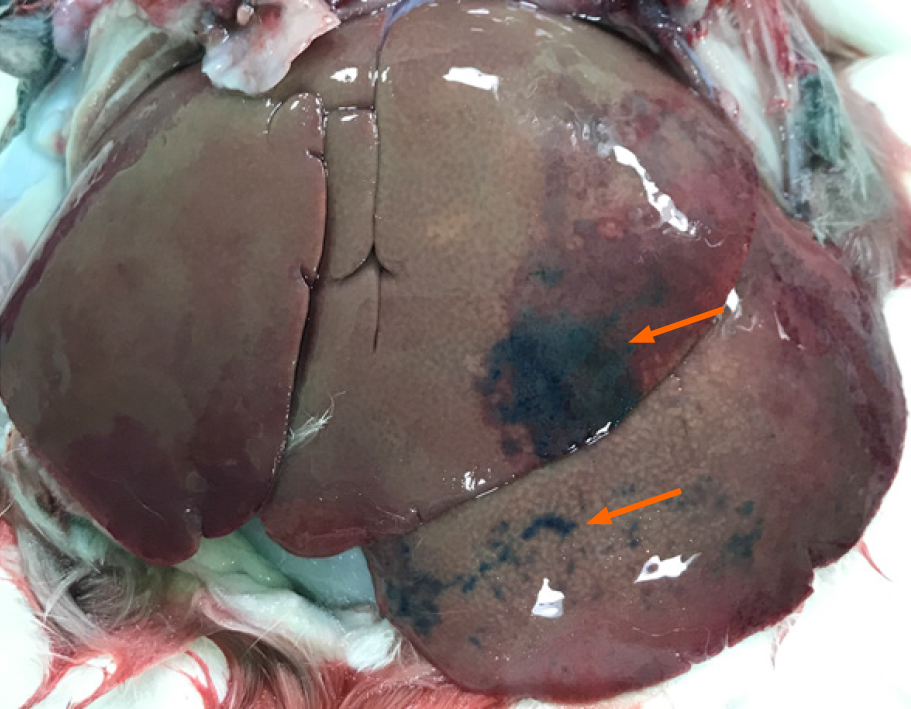
Doxorubicin concentrations in the liver and lungs were determined by fluorometric examination. Two hours after the infusion of TE, the rabbits were perfused transcardially with saline to flush unabsorbed doxorubicin from the vasculature. Liver (100 mg), identified by evans blue staining, and basal segments of both lungs were harvested in all groups (Figure 2). In the absence of a stained area, the anteroinferior portions of both lobes of the liver were selected. Tissues were soaked in four volumes of HCl (0.3 N) in 50% ethanol. The tissues were homogenized in a tissue blender and refrigerated for 24 h at 4 °C and then centrifuged (14000 × g for 25 min). For fluorescence intensities, the supernatants were measured with a fluorometer (Synergy H1, BioTek, Winooski, VT, United States) with excitation/emission of 470/585 nm. For quantitative measurement of doxorubicin concentrations, a standard curve was used, and three readings were performed per tissue sample and averaged. To prove the effect of increased vascular permeability caused by TE in the hepatic and pulmonary arteries, the concentrations in the liver and lungs were calculated.
Nonparametric tests are statistical methods used for studies with small sample sizes or non-normal distributions. Owing to the small sample size in our study, statistical analysis was conducted using the nonparametric Mann–Whitney U test and Kruskal–Wallis test (SPSS version 26.0; IBM Corp, Armonk, United States). P values of < 0.05 were considered statistically significant.
The hepatic surfaces showed blue staining after injecting evans blue in 12/34 rabbits in the TE groups, and the blue staining was patchy (Figure 2). However, the hepatic surface did not show blue staining in the control group. Lungs were not grossly stained in any of the rabbits in all groups (TE and control).
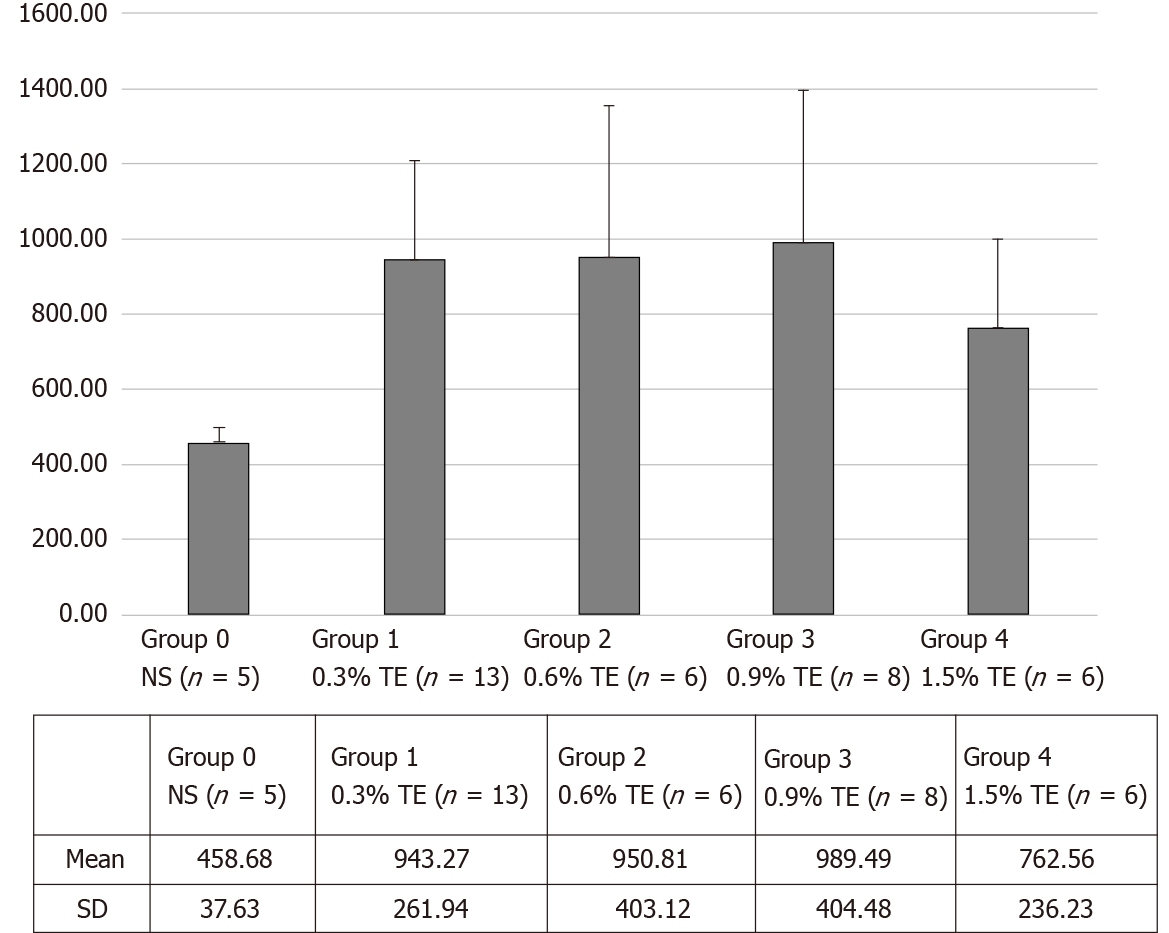
In the liver, doxorubicin concentrations were 943.27 ± 261.94 (group 1), 950.82 ± 403.11 (group 2), 989.49 ± 404.48 (group 3), and 762.55 ± 236.24 (group 4) in the TE groups and 458.68 ± 37.63 (group 0) in control group. The mean doxorubicin concentrations were 2.06, 2.07, 2.16 and 1.66 times higher in group 1 through group 4, respectively, than in the control group (Table 1 and Figure 3). The Kruskal–Wallis test showed a significant difference in doxorubicin concentration between the control and TE groups (P < 0.05). Also, the Mann–Whitney U test with Bonferroni correction compared the control group with each TE group, and all results were significantly different (all P < 0.05). However, there were no significant differences in the mean doxorubicin concentrations between the four TE groups (P = 0.642).
| Group 0 NS (n = 5) | Group 1 0.3% TE (n = 13) | Group 2 0.6% TE (n = 6) | Group 3 0.9% TE (n = 8) | Group 4 1.5% TE (n = 6) | |
| 1 | 429.5 | 1177.0 | 1553.4 | 642.7 | 1096.1 |
| 2 | 417.4 | 720.3 | 1306.8 | 910.5 | 526.2 |
| 3 | 487.9 | 629.7 | 632.0 | 1466.2 | 526.3 |
| 4 | 505.8 | 1402.3 | 788.6 | 822.0 | 924.0 |
| 5 | 452.8 | 1017.9 | 912.9 | 1088.1 | 873.4 |
| 6 | 768.2 | 511.2 | 751.4 | 629.3 | |
| 7 | 918.9 | 1693.8 | |||
| 8 | 1017.7 | 541.2 | |||
| 9 | 898.0 | ||||
| 10 | 724.9 | ||||
| 11 | 1449.4 | ||||
| 12 | 746.1 | ||||
| 13 | 792.1 | ||||
| Mean | 458.68 | 943.27 | 950.81 | 989.49 | 762.56 |
| SD | 37.63 | 261.94 | 403.12 | 404.48 | 236.24 |
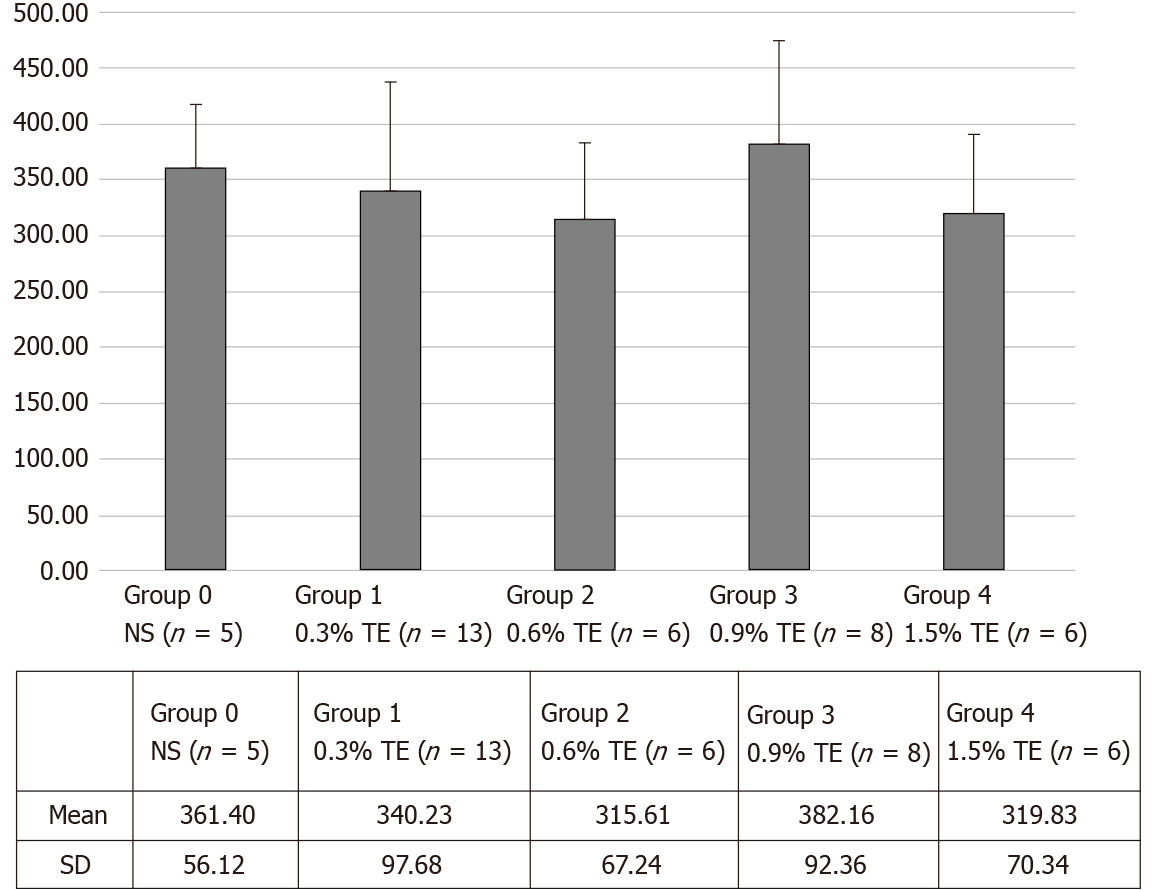
In the lungs, the doxorubicin concentrations in the TE groups were 340.23 ± 97.68 (group 1), 315.63 ± 67.24 (group 2), 382.18 ± 92.36 (group 3), and 319.85 ± 70.34 (group 4); while it was 361.40 ± 62.74 (group 0) in the control group. The mean doxorubicin concentrations were not significantly different (P > 0.05) between the control and TE groups (Figure 4).
In the present study, doxorubicin concentrations were increased after TE infusion into the hepatic arteries, compared with normal saline infusion, in a rabbit liver model. This indicates that TE increases vascular permeability in the sinusoidal capillaries in the liver, which is similar to skeletal muscle with capillaries without a blood–organ barrier, and increases vascular permeability without significant histological changes[18]. Doxorubicin, the most popular single chemotherapeutic drug in transarterial catheter treatment for hepatic malignancy, can be easily extracted from the obtained tissues and quantified by fluorometric analysis (excitation/emission wavelength, 470/585 nm)[14,16]. HCl (0.3N) in ethanol (50%) enables highly efficient recovery of doxorubicin from tissues[14], and doxorubicin concentrations in liver tissues can then be easily measured using a fluorometer. Because the high molecular weight of doxorubicin (579.98 Da) prevents its crossing the blood–organ barrier, it is not effective to treat primary or metastatic brain tumors[19]. However, a delivery study in animal models using TE revealed that doxorubicin concentrations increased in a dose-dependent manner in the brain 2 h after TE injection[13]. Unlike the brain, testis, and orbit with blood–organ barriers, the liver has no barriers between the sinusoids and adjacent hepatic tissues. In our previous study, no significant histological and biochemical changes were found in the liver tissue after TE infusion. The results of the study suggested that the infusion of TE into the hepatic artery may lead to no specific histopathological changes in the liver if the volume of injected triolein is limited[2]. Thus, although TE is used as an adjunctive agent in the chemotherapeutic treatment of liver malignancy, TE may not damage the liver and lung histopathologically but may increase doxorubicin concentration in the liver, which does not have a blood–organ barrier, rather than in the brain, which has a blood–organ barrier.
In the present study, the mean doxorubicin concentration in the liver samples of the TE groups was increased approximately twofold and was significantly higher than that in the normal saline control group (P < 0.05). However, there were no significant differences in mean doxorubicin concentrations between the four TE groups (P = 0.642). Many experimental studies on TE performed in organs with[3,4,13,15,20-25] or without[2,18] a blood–organ barrier have reported transient and reversible changes with no significant lesions, which could have been due to the particle sizes of triolein in the emulsion. Increased vascular permeability of the blood-brain barrier could be applicable to studies on drug reactions in the brain or in research on the treatment of central nervous system diseases. However, severe cerebral edema has been reported in many TE studies, and this could be an unpredictable side effect. Thus, the dosage of TE required to increase vascular permeability should be optimized to reduce unpredictable side effects. In previous studies, the concentration of TE (0.25%-1.0%) administered varied widely[4,24,25], and the optimal dosage of TE required to increase vascular permeability with minimal edema was 0.5% in a cat brain model, and this should be increased in a dose-dependent manner[26]. The present study was unable to elucidate why there were no differences in the mean doxorubicin concentrations between the TE groups. A possible explanation for this is that the pattern of increased vascular permeability may differ between the liver and brain because of the size of each organ, the vessel size for TE injection, and the difference in the type of capillary. The volume of the liver is 66.7 ± 13.7 mL, whereas that of the brain is 9.55 ± 0.35 mL. The vessel size is also larger in the liver than in the brain (1.68 ± 0.55 mm in the proper hepatic artery vs 1.13 ± 0.25 mm in the internal carotid artery). The liver has fenestrated-type capillaries without a barrier, whereas the brain has continuous-type capillaries with a barrier[18,27,28]. In the liver, sinusoids or sinusoidal capillaries are found, and the luminal diameters of these capillaries are greater than those of normal capillaries. In fact, sinusoids may be larger than 30 μm in diameter and have irregular, tortuous walls without a continuous layer of endothelial cells and with wide gaps (approximately 100 nm in diameter) between cells. The sinusoids have incomplete basal lamina, and there is no morphologic barrier between the sinusoids and the perisinusoidal space[1,2]. In the brain, continuous capillaries are found. The endothelial cells have a large amount of cytoplasm in connection with the nucleus, fine filaments, and numerous pinocytotic vesicles. The pinocytotic vesicles are 50-70 nm in diameter and are most likely related to the transport of fluid across the capillary wall, respecting the sites of the so-called large-pore system of capillary permeability. The endothelial cells are connected with either simple or interdigitated junctions and have a narrow gap between the adjacent cell membranes. However, in certain areas, opposing cell membranes are fused to form tight junctions[1,2]. Capillaries without barriers in the liver are permeable to plasma proteins. Thus, they are different from the capillaries in the brain. The increased vascular permeability in the liver did not increase in a dose-dependent manner after the infusion of TE.
In the lungs, the mean doxorubicin concentration was not significantly different between the control and TE groups. This result is associated with the high molecular weight of doxorubicin and the characteristics of the sinusoidal capillary that lacks a blood-organ barrier, causing doxorubicin concentration to increase mainly in the liver but preventing its passage into the lungs.
A limitation of the current study was that TE was infused into the proper hepatic artery. A 2.0-F microcatheter is not suitable for use in a lobar hepatic artery, and the deep insertion of the guidewire for the selection caused a vasospasm and a flow disturbance of the lobar hepatic artery. For further evaluation of the therapeutic effects of anticancer agents in liver cancer, catheters that can be used in one lobar branch of the hepatic artery are required. The use of a microcatheter with a smaller diameter would possibly overcome this limitation. Another limitation was that the concentration and amount of TE used in this study were 0.3%-1.5% and 20 mL, respectively. These values are similar to those reported in previous studies conducted on the brain[4,24-26]. In the present study, vascular permeability in the liver was increased by TE injection; however, it did not differ by TE concentration. It is possible that vascular permeability in the liver would change if different volumes of the same TE percentage were infused. Therefore, further studies using an increased amount of TE in a rabbit liver model are required.
In the present study, TE infusion into the hepatic arteries increased the doxorubicin concentration approximately twofold, a statistically significant difference in the TE groups compared with the control group. However, the differences in doxorubicin concentration between the TE groups were not statistically significant. That is, in the TE groups, doxorubicin concentrations increased in a non-dose-dependent manner. We believe our study results will be useful in the research on vascular permeability in the liver and could be useful in future studies assessing the effect and dosage of TE as adjuvant chemotherapy for the treatment of liver cancers.
The infusion of triolein emulsion (TE) induced increased vascular permeability and a negligible and temporary decrease in liver function without specific histopathological damage.
A technique of increased vascular permeability could be used for chemotherapy in hepatic malignancy.
The present study aimed to assess changes in doxorubicin concentration with TE infused via the hepatic artery in rabbit liver.
The TE was infused into each rabbit through a microcatheter via the hepatic artery. Immediately after the infusion of emulsified triolein (group 1, 0.3% TE, n = 13; group 2, 0.6% TE, n = 6; group 3, 0.9% TE, n = 8; group 4, 1.5% TE, n = 6) or saline (group 0), doxorubicin was infused through the same catheter. The concentrations of doxorubicin in the liver and lungs were measured by fluorometry. Two hours after the injection of TE, 100 mg of liver tissue, identified by evans blue staining, was harvested from all rabbits. Doxorubicin concentrations were quantified using a fluorometer. Statistical analysis was performed using the nonparametric Mann–Whitney U test and Kruskal–Wallis test (SPSS version 26.0; IBM Corp., Armonk, United States). P-values of < 0.05 were considered statistically significant.
The mean doxorubicin concentrations were 1.66 to 2.16 times higher in the TE groups than in the control group and significantly different in the TE groups (P < 0.05).
TE infusion into the hepatic arteries significantly increased the doxorubicin concentration.
TE infusion might be a useful adjuvant treatment for liver tumors.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: South Korea
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Fu T S-Editor: Fan JR L-Editor: A P-Editor: Liu JH
| 1. | Leeson THLC. The Liver. In: Leeson THLC, editor. Histology. Philadelphia, London, Toronto: WB Saunders; 1981: 383-397. [Cited in This Article: ] |
| 2. | Kim YW, Park YM, Yoon S, Kim HJ, Park DY, Cho BM, Choi SH. Effect of intra-arterial infusion with triolein emulsion on rabbit liver. World J Gastroenterol. 2014;20:14442-14449. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 4] [Cited by in F6Publishing: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 3. | Kim HJ, Lee CH, Kim HG, Lee SD, Son SM, Kim YW, Eun CK, Kim SM. Reversible MR changes in the cat brain after cerebral fat embolism induced by triolein emulsion. AJNR Am J Neuroradiol. 2004;25:958-963. [PubMed] [Cited in This Article: ] |
| 4. | Kim YW, Kim HJ, Cho BM, Moon TY, Eun CK. The study of cerebral hemodynamics in the hyperacute stage of fat embolism induced by triolein emulsion. AJNR Am J Neuroradiol. 2006;27:398-401. [PubMed] [Cited in This Article: ] |
| 5. | Dubbelboer IR, Pavlovic N, Heindryckx F, Sjögren E, Lennernäs H. Liver Cancer Cell Lines Treated with Doxorubicin under Normoxia and Hypoxia: Cell Viability and Oncologic Protein Profile. Cancers (Basel). 2019;11. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 34] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 6. | Lencioni R, de Baere T, Soulen MC, Rilling WS, Geschwind JF. Lipiodol transarterial chemoembolization for hepatocellular carcinoma: A systematic review of efficacy and safety data. Hepatology. 2016;64:106-116. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 362] [Cited by in F6Publishing: 433] [Article Influence: 54.1] [Reference Citation Analysis (0)] |
| 7. | Pecorelli A, Lenzi B, Gramenzi A, Garuti F, Farinati F, Giannini EG, Ciccarese F, Piscaglia F, Rapaccini GL, Di Marco M, Caturelli E, Zoli M, Borzio F, Sacco R, Cabibbo G, Felder M, Morisco F, Gasbarrini A, Baroni GS, Foschi FG, Biasini E, Masotto A, Virdone R, Bernardi M, Trevisani F; Italian LiverCancer (ITA. LI.CA) group. Curative therapies are superior to standard of care (transarterial chemoembolization) for intermediate stage hepatocellular carcinoma. Liver Int. 2017;37:423-433. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 35] [Cited by in F6Publishing: 46] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 8. | Galle PR, Tovoli F, Foerster F, Wörns MA, Cucchetti A, Bolondi L. The treatment of intermediate stage tumours beyond TACE: From surgery to systemic therapy. J Hepatol. 2017;67:173-183. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 117] [Cited by in F6Publishing: 155] [Article Influence: 22.1] [Reference Citation Analysis (0)] |
| 9. | Gaba RC, Lewandowski RJ, Hickey R, Baerlocher MO, Cohen EI, Dariushnia SR, Janne d'Othée B, Padia SA, Salem R, Wang DS, Nikolic B, Brown DB; Society of Interventional Radiology Technology Assessment Committee. Transcatheter Therapy for Hepatic Malignancy: Standardization of Terminology and Reporting Criteria. J Vasc Interv Radiol. 2016;27:457-473. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 78] [Cited by in F6Publishing: 83] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 10. | European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J Hepatol. 2018;69:182-236. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3934] [Cited by in F6Publishing: 4906] [Article Influence: 817.7] [Reference Citation Analysis (0)] |
| 11. | Hepatocellular Carcinoma: Diagnosis and Treatment, 3rd ed. Springer International Publishing: Cham, Switzerland, 2016. [Cited in This Article: ] |
| 12. | El Fouly A, Ertle J, El Dorry A, Shaker MK, Dechêne A, Abdella H, Mueller S, Barakat E, Lauenstein T, Bockisch A, Gerken G, Schlaak JF. In intermediate stage hepatocellular carcinoma: radioembolization with yttrium 90 or chemoembolization? Liver Int. 2015;35:627-635. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 66] [Cited by in F6Publishing: 73] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 13. | Kim HJ, Kim YW, Choi SH, Cho BM, Bandu R, Ahn HS, Kim KP. Triolein Emulsion Infusion Into the Carotid Artery Increases Brain Permeability to Anticancer Agents. Neurosurgery. 2016;78:726-733. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 10] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 14. | Bachur NR, Moore AL, Bernstein JG, Liu A. Tissue distribution and disposition of daunomycin (NCS-82151) in mice: fluorometric and isotopic methods. Cancer Chemother Rep. 1970;54:89-94. [PubMed] [Cited in This Article: ] |
| 15. | Lee IS, Kim HJ, Choi SH, Kim YW, Choi KJ. Doxorubicin concentration in brain remains high for one day after triolein emulsion infusion induced BBB opening. Int J Neurosci. 2020;130:770-776. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 16. | Treat LH, McDannold N, Vykhodtseva N, Zhang Y, Tam K, Hynynen K. Targeted delivery of doxorubicin to the rat brain at therapeutic levels using MRI-guided focused ultrasound. Int J Cancer. 2007;121:901-907. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 398] [Cited by in F6Publishing: 405] [Article Influence: 23.8] [Reference Citation Analysis (0)] |
| 17. | Park J, Zhang Y, Vykhodtseva N, Jolesz FA, McDannold NJ. The kinetics of blood brain barrier permeability and targeted doxorubicin delivery into brain induced by focused ultrasound. J Control Release. 2012;162:134-142. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 144] [Cited by in F6Publishing: 158] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 18. | Kim HJ, Kim YW, Lee IS, Song JW, Jeong YJ, Choi SH, Choi KU, Suh KT, Cho BM. Intra-arterial delivery of triolein emulsion increases vascular permeability in skeletal muscles of rabbits. Acta Vet Scand. 2009;51:30. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 19. | von Holst H, Knochenhauer E, Blomgren H, Collins VP, Ehn L, Lindquist M, Norén G, Peterson C. Uptake of adriamycin in tumour and surrounding brain tissue in patients with malignant gliomas. Acta Neurochir (Wien). 1990;104:13-16. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 41] [Cited by in F6Publishing: 48] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 20. | Kim HJ, Lee CH, Lee SH, Cho BM, Kim HK, Park BR, Ye SY, Jeon GR, Chang KH. Early development of vasogenic edema in experimental cerebral fat embolism in cats: correlation with MRI and electron microscopic findings. Invest Radiol. 2001;36:460-469. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 32] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 21. | Kim HJ, Lee JH, Lee CH, Lee SH, Moon TY, Cho BM, Kim HK, Park BR, Chang KH. Experimental cerebral fat embolism: embolic effects of triolein and oleic acid depicted by MR imaging and electron microscopy. AJNR Am J Neuroradiol. 2002;23:1516-1523. [PubMed] [Cited in This Article: ] |
| 22. | Kim HJ, Lee CH, Lee SH, Moon TY. Magnetic resonance imaging and histologic findings of experimental cerebral fat embolism. Invest Radiol. 2003;38:625-634. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 21] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 23. | Kim YW, Kim HJ, Choi SH, Cho B, Hwangbo L, Kim DC. Hemorrhage in cerebral fat embolisms in a cat model using triolein dependent on the physical properties of triolein. Jpn J Radiol. 2014;32:30-37. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 24. | Baik SK, Kim YW, Kim HJ, Lee JW, Cho BM, Kim DH, Choi SH, Lee SH, Chang KH. Proton magnetic resonance spectroscopic findings of cerebral fat embolism induced by triolein emulsion in cats. Acta Radiol. 2008;49:1174-1181. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 7] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 25. | Kim KN, Kim HJ, Lee SD, Moon TY, Lee SH, Lee JW, Lee TH. Effect of triolein emulsion on the blood-testis barrier in cats. Invest Radiol. 2004;39:445-449. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 14] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 26. | Choi SH, Kim HJ, Hwangbo L, Kim YW. The minimum percentage of triolein emulsion for studying cerebral vascular permeability with least brain edema. Iran J Radiol. 2014;11:e14887. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 27. | Tam AL, Melancon MP, Ensor J, Liu Y, Dixon K, McWatters A, Gupta S. Rabbit hepatic arterial anatomy variations: implications on experimental design. Acta Radiol. 2014;55:1226-1233. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 8] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 28. | Lee JS, Hamilton MG, Zabramski JM. Variations in the anatomy of the rabbit cervical carotid artery. Stroke. 1994;25:501-503. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 22] [Article Influence: 0.7] [Reference Citation Analysis (0)] |