Published online Apr 28, 2021. doi: 10.3748/wjg.v27.i16.1816
Peer-review started: January 31, 2021
First decision: February 22, 2021
Revised: March 5, 2021
Accepted: April 13, 2021
Article in press: April 13, 2021
Published online: April 28, 2021
Processing time: 79 Days and 16.8 Hours
With improved survival in gastric cancer patients, health-related quality of life has become an important clinical endpoint alongside primary oncological outcomes.
To investigate health-related quality of life after various surgical procedures for gastric cancer treatment.
The validated Slovenian version of the European Organization for Research and Treatment of Cancer Quality of Life Core Questionnaire (QLQ-C30) and its gastric cancer-specific module (QLQ STO-22) was sent for self-completion to patients that underwent curative resection for gastric adenocarcinoma between January 2014 and December 2018 at our centre. In total, 116 patients responded. Scores were compared between patients after subtotal distal vs total gastrectomy and patients after subtotal distal gastrectomy with Billroth II vs Roux-en-Y reconstruction.
Interestingly, the extent of resection did not influence daily functioning; however, more dysphagia and eating restrictions were reported in patients after total gastrectomy when compared to patients after subtotal distal gastrectomy. Moreover, patients with Billroth II reconstruction after subtotal distal resection experienced worse physical and role functioning and reported more pain, fatigue and reflux compared to Roux-en-Y reconstruction.
Based on our results, Roux-en-Y reconstruction after subtotal distal gastrectomy should be preferred over Billroth II reconstruction. The data obtained from this study will help surgeons when preoperatively informing their patients about expected functional outcomes after gastrectomy and enable them to ensure proper supportive care of their patients in the postoperative period.
Core Tip: Quality of life assessment is an important tool to guide and evaluate treatment interventions, especially after a major surgery like gastrectomy. We conducted a cross-sectional survey among patients with gastric adenocarcinoma treated at our centre to provide insight into overall wellbeing after curative resection. The information provided will guide surgeons in selecting an optimal treatment approach and informing patients about expected treatment outcomes.
- Citation: Grosek J, Zavrtanik H, Tomažič A. Health-related quality of life after curative resection for gastric adenocarcinoma. World J Gastroenterol 2021; 27(16): 1816-1827
- URL: https://www.wjgnet.com/1007-9327/full/v27/i16/1816.htm
- DOI: https://dx.doi.org/10.3748/wjg.v27.i16.1816
Gastrectomy is a major operation that alters the physiological functions of the digestive tract. Consequently, patients that undergo this treatment commonly experience a broad range of metabolic disorders, including malnutrition, weight loss and several postgastrectomy symptoms that negatively impact patients’ wellbeing[1]. With modern gastrectomy techniques and other treatment modalities, survival in gastric cancer patients has improved and health-related quality of life (HRQoL) has become an important aspect when evaluating treatment outcomes[2].
Various surgical procedures have been described for achieving oncological radicality in gastric cancer. In previous comparative studies, subtotal distal gastrectomy was associated with shorter operative duration, reduced postoperative complications and better recovery when compared to total gastrectomy[3-6]. On the other hand, total gastrectomy could still be performed safely with low morbidity while reducing the risk of inadequate safety margins or remnant carcinoma[7,8]. Following subtotal distal gastrectomy, there is no consensus regarding the best type of reconstruction[9,10]. Billroth II reconstruction is often performed due to its simplicity, but associated reflux gastritis and esophagitis have been the limiting concerns. Roux-en-Y reconstruction is a recommended alternative, but it can result in delayed gastric emptying, nausea, vomiting and abdominal pain[11]. Selection of the procedure is usually made based on tumour location, preoperative staging, the patient’s general physical status and the surgeon’s preference. In addition, quality of life assessment has become an increasingly important index for evaluating and selecting treatment interventions. Patient-reported measures regarding their physical and emotional state after treatment as opposed to objectively defined short-term perioperative outcomes should be taken into consideration to achieve optimal care.
We conducted a cross-sectional survey among patients with gastric adenocarcinoma treated at a tertiary referral centre to provide insight into overall wellbeing after curative resection. The European Organization for Research and Treatment of Cancer (EORTC) Quality of Life Core Questionnaire (QLQ-C30) and its gastric cancer-specific module (QLQ STO-22) were used to assess HRQoL[12]. Its various aspects were compared among different surgical procedures.
Patients with gastric cancer that underwent curative resection at the Ljubljana University Medical Centre between January 2014 and December 2018 were recruited for the study. Patients were eligible for inclusion if they were older than 18, had undergone curative subtotal distal or total gastrectomy for histologically confirmed adenocarcinoma, and had not undergone previous gastric surgery. We excluded patients that were deemed unable to answer the questionnaires, provided questionnaires with missing items that prevented final scoring, suffered from other gastrointestinal diseases (e.g., chronic inflammatory bowel disease, exocrine pancreatic insufficiency or cholestasis) or other malignant diseases, had undergone emergency or palliative surgery, or had experienced disease recurrence. The study was approved by the National Medical Ethics Committee of Republic of Slovenia (No. 0120-315/2019/3; July 9, 2019). Patients that met the inclusion criteria and provided written informed consent were included for further analysis.
Data on the patients included demographics, comorbidities, American Society of Anesthesiologists score, tumour stage, type of resection, type of reconstruction, postoperative complications, hospital stay, histopathological characteristics of the tumour, cancer recurrence and possible (neo)adjuvant therapy and were collected from electronic patient records.
All patients underwent open gastrectomy, either subtotal distal or total, depending on the tumour location and preoperatively determined tumor-node-metastasis stage. Gastric adenocarcinoma was confirmed upon histopathological examination of the specimen.
After total gastrectomy, reconstruction was performed with Roux-en-Y esophagojejunostomy in all cases. After subtotal distal gastrectomy, either a Billroth II or Roux-en-Y anastomosis was constructed. Braun enteroenterostomy was routinely performed in all cases of Billroth II gastrojejunostomy. Billroth I reconstruction was not performed in our patient cohort due to the surgeon’s preference and lack of early-stage gastric cancer.
To assess postoperative HRQoL of our patient cohort, the validated Slovenian versions of the EORTC QLQ-C30 (version 0.3) and QLQ STO-22 were used[12].
The EORTC QLQ-C30 questionnaire consists of 30 questions divided into five functional scales (physical, role, cognitive, emotional and social), three symptom scales (fatigue, pain, and nausea and vomiting), the global health status/quality of life scale and six single items to report other complaints (dyspnoea, loss of appetite, insomnia, constipation, diarrhoea and financial difficulties).
The EORTC QLQ-STO22 is a gastric cancer–specific module to assess HRQoL of patients with adenocarcinoma of the stomach. It comprises 22 questions divided into five multi-item scales (dysphagia, chest and abdominal pain, reflux, eating restrictions and anxieties) and four single items (dry mouth, taste problems, body image and hair loss).
A study description with an informed consent form plus the EORTC QLQ-C30 and EORCT QLQ-STO-22 questionnaires were sent to the patients for self-completion.
All completed questionnaires were scored and linearly transformed to a 0-100 scale according to the EORTC QLQ-C30 Scoring Manual[13]. On the functional scale higher scores represent better functioning, whereas on the symptom scale higher scores indicate higher symptom burden. Missing values were processed as follows: if at least half of the items from the scale were answered, the missing items were assumed to have values equal to the average of the completed items on the scale.
HRQoL was compared between patients after subtotal distal vs total gastrectomy and between patients after subtotal distal gastrectomy with Billroth II vs Roux-en-Y reconstruction.
Means with standard deviation as well as medians with interquartile ranges of EORTC QLQ-C30 and EORTC QLQ-STO-22 scores were obtained. The Shapiro–Wilk test was used to test the normal distribution of the data.
The time that elapsed from surgery to completion of the questionnaires was compared among different surgical procedures using the Kruskal-Wallis test and Mann-Whitney U test.
Scores of EORTC QLQ-C30 and EORTC QLQ-STO-22 of patients after subtotal distal vs total gastrectomy and Billroth II vs Roux-en-Y reconstruction were compared with the Mann-Whitney U test.
To assess the correlation between the type of operation and general health status adjusted for some demographic and clinical characteristics of patients, multiple linear regression was used. The assumptions of absence of multicollinearity (assessed by variance inflation factor), normal distribution of the residuals and homoscedasticity were met.
A double-sided P value of < 0.05 was considered statistically significant. All statistical analyses were performed using SPSS 27.0 (IBM Corporation, Armonk, NY, United States).
Invitations for study participation were sent to 234 patients. A total of 116 (49.6%) patients that provided informed consent with completed questionnaires were further analyzed. There were 63 men and 53 women 44 to 88 years old. Ten questionnaires had missing items. In six questionnaires, only one item was missing. In four questionnaires, there were two missing items, which were not part of the same scale.
The time that elapsed from surgery to completion of the questionnaires is shown in Table 1 and was not statistically different among different surgical procedures (P = 0.161).
| Median (IQR), yr | |
| Total gastrectomy | 3 (2-4) |
| Subtotal distal gastrectomy | 3 (2-4) |
| Billroth II reconstruction | 3 (1-4) |
| Roux-en-Y reconstruction | 2.5 (1-4) |
Respondents and non-respondents did not differ significantly according to sex, type of gastric resection, type of reconstructive procedure and postoperative complications. Respondents were younger (P = 0.016) and had a significantly less advanced disease stage (P = 0.001). The baseline characteristics of patients eligible for inclusion are presented in Table 2.
| Respondents (n = 116) | Non-respondents (n = 118) | P value | |
| Gender, n (%) | 0.192 | ||
| Male | 63 (54.3) | 74 (62.7) | |
| Female | 53 (45.7) | 44 (37.3) | |
| Age at surgery (yr, median, IQR) | 66 (58-74) | 72 (63-78) | 0.016 |
| Performed procedure, n (%) | 0.299 | ||
| Total gastrectomy | 43 (37.1) | 55 (46.6) | |
| Distal-Billroth II | 26 (22.4) | 20 (16.9) | |
| Distal-Roux-Y | 47 (40.5) | 43 (36.4) | |
| Postoperative complications, n (%) | |||
| Yes | 28 (24.1) | 38 (32.2) | 0.170 |
| TNM stage, n (%) | 0.001 | ||
| 0 | 3 (2.6) | 4 (3.4) | |
| I | 52 (44.8) | 25 (21.2) | |
| II | 32 (27.6) | 36 (30.5) | |
| III | 29 (25.0) | 53 (44.9) |
Patients after total vs subtotal distal gastrectomy: No statistically significant differences were observed in HRQoL of patients after total gastrectomy when compared to patients after subtotal distal gastrectomy. The details are shown in Table 3.
| Subtotal gastrectomy (n = 73) | Total gastrectomy (n = 43) | P value | |||
| mean (SD) | Median (IQR) | mean (SD) | Median (IQR) | ||
| Functional scales | |||||
| Physical functioning | 79.9 (21.4) | 87 (67-100) | 80.3 (19.5) | 87 (73-100) | 0.954 |
| Role functioning | 80.2 (26.7) | 100 (67-100) | 77.9 (26.1) | 83 (67-100) | 0.509 |
| Cognitive functioning | 81.9 (21.6) | 83 (67-100) | 82.9 (19.7) | 83 (67-100) | 0.842 |
| Emotional functioning | 82.1 (20.5) | 92 (75-100) | 76.1 (21.5) | 79 (58-96) | 0.116 |
| Social functioning | 81.5 (22.5) | 83 (67-100) | 73.7 (26) | 83 (50-100) | 0.115 |
| Symptoms | |||||
| Dyspnoea | 10.5 (22.1) | 0 (0-0) | 10.8 (21.5) | 0 (0-0) | 0.869 |
| Insomnia | 28.7 (29.1) | 33 (0-33) | 27.8 (28.1) | 33 (0-33) | 0.917 |
| Appetite loss | 10 (19.7) | 0 (0-0) | 12.3 (20.6) | 0 (0-33) | 0.489 |
| Nausea/vomiting | 10.7 (17.4) | 0 (0-17) | 4.7 (9.8) | 0 (0-0) | 0.079 |
| Constipation | 10.5 (22.1) | 0 (0-0) | 6.9 (13.6) | 0 (0-0) | 0.665 |
| Diarrhoea | 13.6 (22.7) | 0 (0-33) | 19.3 (24.4) | 0 (0-33) | 0.141 |
| Fatigue | 30.5 (23.3) | 22 (17-33) | 34.2 (23.4) | 33 (22-50) | 0.263 |
| Pain | 18.3 (22.4) | 17 (0-33) | 17.9 (21.6) | 17 (0-33) | 0.906 |
| Financial problems | 16.4 (26.7) | 0 (0-33) | 27.1 (32.8) | 0 (0-67) | 0.069 |
| Global health status | 66.6 (22.9) | 67 (50-83) | 67.4 (19.9) | 67 (58-83) | 0.846 |
Patients after subtotal distal gastrectomy with Billroth II vs Roux-en-Y reconstruction: HRQoL of patients after subtotal distal gastrectomy with Billroth II reconstruction was significantly lower on the physical (P = 0.038) and role functioning (P = 0.034) scale when compared to patients with Roux-en-Y reconstruction. Patients with Billroth II reconstruction also reported more pain (P = 0.01) and fatigue (P = 0.028). No differences were observed between the two groups in global health status/quality of life scores (P = 0.635). The details are summarized in Table 4.
| Billroth II (n = 26) | Roux-Y (n = 47) | P value | |||
| mean (SD) | Median (IQR) | mean (SD) | Median (IQR) | ||
| Functional scales | |||||
| Physical functioning | 73.1 (23) | 73.5 (53-93) | 83.7 (19.6) | 92 (73-100) | 0.038 |
| Role functioning | 70.5 (31.4) | 67 (50-100) | 85.5 (22.4) | 100 (67-100) | 0.034 |
| Cognitive functioning | 76.9 (26.3) | 83 (67-100) | 84.7 (18.3) | 83 (83-100) | 0.301 |
| Emotional functioning | 74.9 (27.7) | 83 (58-100) | 86 (14) | 92 (75-100) | 0.220 |
| Social functioning | 79.5 (25.9) | 83 (67-100) | 82.6 (20.5) | 83 (67-100) | 0.792 |
| Symptoms | |||||
| Dyspnoea | 16.7 (30.3) | 0 (0-33) | 7 (15.4) | 0 (0-0) | 0.285 |
| Insomnia | 37.1 (33.2) | 33 (0-67) | 24 (25.8) | 33 (0-33) | 0.102 |
| Appetite loss | 12.8 (25.1) | 0 (0-33) | 8.4 (16.2) | 0 (0-0) | 0.651 |
| Nausea/vomiting | 15.4 (22.1) | 0 (0-33) | 8.1 (13.8) | 0 (0-17) | 0.194 |
| Constipation | 19.2 (31.5) | 0 (0-33) | 5.6 (12.5) | 0 (0-0) | 0.054 |
| Diarrhoea | 14.1 (31.6) | 0 (0-0) | 13.3 (16.4) | 0 (0-33) | 0.200 |
| Fatigue | 41 (28.6) | 33 (22-67) | 24.6 (17.5) | 22 (17-33) | 0.028 |
| Pain | 28.9 (28.1) | 17 (0-50) | 12.4 (16.1) | 0 (0-17) | 0.010 |
| Financial problems | 19.2 (28.6) | 0 (0-33) | 14.8 (25.8) | 0 (0-33) | 0.510 |
| Global health status | 68.2 (24.7) | 67 (50-83) | 65.7 (22) | 67 (50-83) | 0.635 |
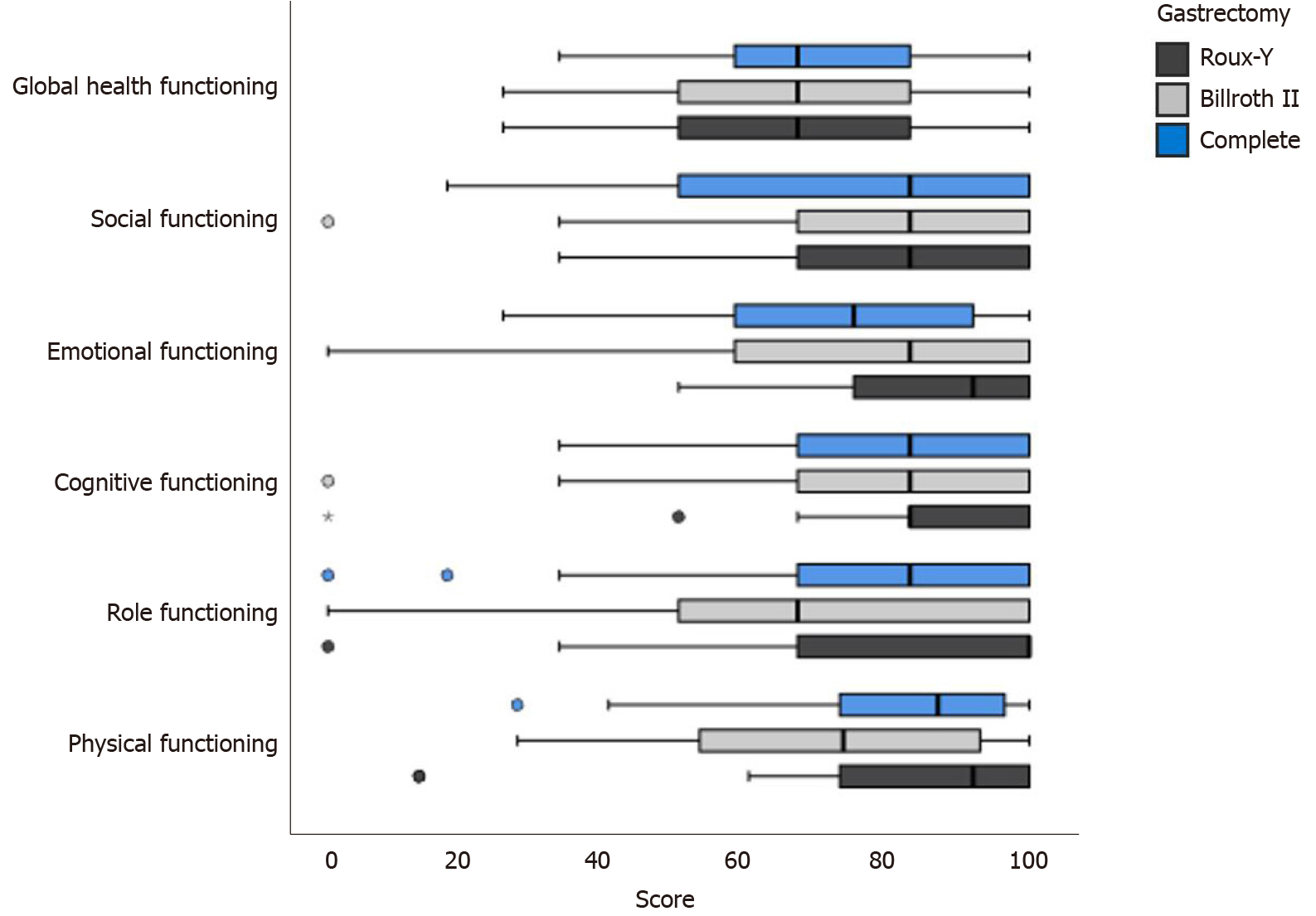
Reported scores on different functional scales and the global health status/quality of life scale among various surgical procedures are presented in Figure 1. Patients after subtotal distal gastrectomy with Roux-en-Y reconstruction scored highest on cognitive, role functioning and physical scales when compared to patients after total gastrectomy or subtotal distal gastrectomy with Billroth II reconstruction.
Quality of life and type of surgery: The type of surgery was significantly associated with the stage of the disease (P = 0.002) (Table 5). Patients with stage III gastric adenocarcinoma underwent either total gastrectomy (43.9%) or subtotal distal gastrectomy with Billroth II reconstruction (41.3%) rather than Roux-en-Y reconstruction (22.2%).
| Total gastrectomy, n (%) | Subtotal-Billroth II, n (%) | Subtotal-Roux-Y, n (%) | P value | |
| Stage | 0.002 | |||
| 0 | 4 (4.1) | 3 (6.5) | 0 (0) | |
| I | 25 (25.5) | 14 (30.4) | 38 (42.2) | |
| II | 26 (26.5) | 10 (21.7) | 32 (35.6) | |
| III | 43 (43.9) | 19 (41.3) | 20 (22.2) |
When adjusted for demographic data, disease stage and postoperative complications in a multiple linear regression model, no statistically significant differences in the global health status/quality of life scale were observed among different surgical procedures. Global health status/quality of life scores were significantly negatively associated with disease stage (β = -0.21, P = 0.029; Table 6).
| β (P value) | |
| Male | -0.11 (0.229) |
| Age (yr) | -0.05 (0.625) |
| Total vs Billroth II | -0.001 (0.990) |
| Roux-Y vs Billroth II | -0.08 (0.492) |
| Postoperative complications-yes | -0.15 (0.116) |
| Stage II/III vs 0/I | -0.21 (0.029) |
Patients with Roux-en-Y reconstruction had significantly higher scores on the emotional (β = 0.24, P = 0.041), role functioning (β = 0.24, P = 0.034) and physical scale (β = 0.23, P = 0.048) when compared to patients after Billroth II reconstruction, even when adjusted for other variables in a regression model (Table 7).
| Emotional | Social | Role | Physical | Cognitive | ||||||
| β | P value | β | P value | β | P value | β | P value | β | P value | |
| Male | 0.01 | 0.901 | 0.10 | 0.254 | 0.09 | 0.334 | 0.16 | 0.089 | 0.04 | 0.666 |
| Age (yr) | 0.09 | 0.343 | 0.05 | 0.557 | 0.08 | 0.379 | -0.09 | 0.343 | 0.18 | 0.054 |
| Total vs Billroth II | 0.04 | 0.729 | -0.11 | 0.343 | 0.15 | 0.199 | 0.15 | 0.201 | 0.16 | 0.194 |
| Roux-Y vs Billroth II | 0.24 | 0.041 | 0.04 | 0.763 | 0.24 | 0.034 | 0.23 | 0.048 | 0.17 | 0.154 |
| Postoperative complications yes | -0.03 | 0.730 | -0.13 | 0.150 | -0.15 | 0.101 | -0.10 | 0.292 | 0.01 | 0.915 |
| Saage II/III vs 0/I | -0.17 | 0.062 | -0.20 | 0.031 | -0.27 | 0.003 | -0.16 | 0.079 | -0.09 | 0.324 |
Patients after total vs subtotal distal gastrectomy: Patients after total gastrectomy reported more dysphagia (P = 0.020) and eating restrictions (P = 0.017) when compared to patients after subtotal distal gastrectomy. No differences were found in other scales of the EORTC QLQ STO-22 questionnaire (Table 8).
| Subtotal gastrectomy (n = 60) | Total gastrectomy (n = 51) | P value | |||
| mean (SD) | Median (IQR) | mean (SD) | Median (IQR) | ||
| Dysphagia | 11.1 (15.8) | 11 (0-11) | 17.2 (17.1) | 11 (0-33) | 0.020 |
| Pain | 19.7 (17.9) | 17 (8-33) | 23.3 (20.9) | 17 (8-33) | 0.477 |
| Reflux | 16 (20.9) | 11 (0-22) | 22.7 (23.6) | 11 (0-44) | 0.089 |
| Eating restrictions | 14.7 (17.4) | 8 (0-17) | 21.6 (18.2) | 17 (8-33) | 0.017 |
| Anxiety | 33.1 (23.4) | 33 (17-44) | 31.4 (25.8) | 22 (11-44) | 0.591 |
| Dry mouth | 24.1 (28.5) | 33 (0-33) | 24.7 (28.3) | 33 (0-33) | 0.871 |
| Taste | 6.4 (18.1) | 0 (0-0) | 11.6 (21.7) | 0 (0-33) | 0.109 |
| Body image | 18.7 (29.9) | 0 (0-33) | 20.1 (27.4) | 0 (0-33) | 0.567 |
| Hair loss | 16.4 (27.9) | 0 (0-33) | 8.7 (20.9) | 0 (0-0) | 0.124 |
Patients after subtotal distal gastrectomy with Billroth II vs Roux-en-Y reconstruction: Patients after subtotal distal gastrectomy with Billroth II reconstruction reported more problems with reflux when compared to patients with Roux-en-Y reconstruction. No differences were found in other scales of the EORTC QLQ STO-22 questionnaire (Table 9).
| Billroth II (n = 18) | Roux-Y (n = 42) | P value | |||
| mean (SD) | Median (IQR) | mean (SD) | Median (IQR) | ||
| Dysphagia | 15.8 (22.3) | 5.5 (0-22) | 8.5 (10.2) | 11 (0-11) | 0.526 |
| Pain | 24.3 (23.9) | 17 (8-33) | 17.2 (13.3) | 17 (8-25) | 0.471 |
| Reflux | 28.5 (26.3) | 22 (0-44) | 9.1 (13) | 0 (0-11) | 0.001 |
| Eating restrictions | 22.3 (22.8) | 17 (0-42) | 10.4 (11.7) | 8 (0-17) | 0.069 |
| Anxiety | 36.6 (26.9) | 33 (22-55) | 31.2 (21.3) | 33 (11-44) | 0.541 |
| Dry mouth | 23 (26.3) | 33 (0-33) | 24.7 (29.9) | 0 (0-33) | 0.965 |
| Taste | 11.5 (26.6) | 0 (0-0) | 3.5 (10.3) | 0 (0-0) | 0.247 |
| Body image | 26.8 (36.5) | 0 (0-33) | 14.1 (24.8) | 0 (0-33) | 0.127 |
| Hair loss | 14.1 (27) | 0 (0-33) | 17.7 (28.6) | 0 (0-33) | 0.541 |
The results of this cross-sectional survey show that the type of gastric resection influences different aspects of HRQoL. Patients after total vs subtotal gastrectomy had similar functional scores, but the former experienced more dysphagia and eating restrictions. At the same time, patients after subtotal gastrectomy with Billroth II (compared to Roux-en-Y) reconstruction reported worse physical and role functioning scores and complained of symptoms such as pain, fatigue and reflux. However, these differences appear to be clinically less relevant because similar global health scores were reported by patients after different surgical procedures. The information provided should guide the surgeon on the optimal treatment approach after considering oncological feasibility of the technique. Moreover, it should be used to inform patients about expected functional sequelae.
Previous studies evaluated longitudinal changes of HRQoL after gastrectomy for gastric cancer and used preoperative scores as a reference[14-21]. However, these scores are highly influenced by circumstances surrounding the diagnosis as well as symptoms associated with the disease itself, such as nausea and vomiting, dysphagia, postprandial fullness, loss of appetite, fatigue due to anaemia and so on, resulting in worse HRQoL[16,17]. In their multicentre study, Brenkman et al[22] compared EORTC QLQ-C30 scores of patients after gastrectomy to a Dutch reference population and concluded that global HRQoL is more or less comparable between the two cohorts despite patients’ worse scores on several functional and symptom scales. Similarly, Lee et al[23,24] found no significant difference in global HRQoL between patients more than 5 years after surgery for gastric cancer and healthy volunteers awaiting a routine screening exam.
A limited number of studies focused on HRQoL after various surgical procedures[5,11,15,16,18,20]. Hence, we opted to conduct a cross-sectional analysis to compare HRQoL after various types of gastric resection and to evaluate the actual life quality deviation caused by surgical treatment. Based on our results, the type of reconstruction appeared to have a greater effect on HRQoL than the extent of gastric resection, which is somehow unexpected. Our data show that proximal gastric preservation has marginal advantages for improving patients’ quality of life by reducing dysphagia and eating restrictions postoperatively whereas no differences in daily functioning were found. In line with our finding, subtotal distal gastrectomy was generally better tolerated in several previous studies, especially due to a higher symptom burden reported with total gastrectomy such as nausea and vomiting, dysphagia, eating restrictions and reflux[16,21]. In subtotal gastrectomy, gastric physiology is at least partly preserved, possibly leading to superior HRQoL. However, several studies found no difference in global HRQoL between the two groups[5,15,18,25]. A possible explanation for this finding is the time interval from the surgical procedure. HRQoL changes over time are well documented. In longitudinal analyses, significantly worse scores on almost all HRQoL scales were observed 1 mo to 6 mo postoperatively compared to the preoperative scores[14-17,20]. Several functional scales recovered to the baseline by 1 year after surgery, however, symptoms such as nausea and vomiting, reflux and eating restrictions persisted even 5 years after surgery[19,23,24]. In a long-term analysis by Lee et al[25], HRQoL inferiority of patients after total gastrectomy when compared to subtotal distal gastrectomy generally disappeared beyond 5 years postoperatively, remaining inferior only in eating restrictions. In our study, the median time interval from the surgery to the completion of the questionnaires was 3 years, possibly diminishing some differences between the two groups.
Regarding the type of digestive tract reconstruction after subtotal distal gastrectomy, the choice of the technique is often driven by the surgeon’s preferences, and no clear recommendations exist in the current literature[9-11]. Several studies suggested Roux-en-Y reconstruction to be superior to Billroth II reconstruction in terms of preventing bile reflux and remnant gastritis, thus allowing better quality of life[10,26-28]. However, in a proportion of patients, Roux-en-Y may be associated with a Roux stasis syndrome causing delayed gastric emptying with postprandial pain, nausea and vomiting[29,30]. Our results are partly in line with previous studies reporting reduced HRQoL following Billroth II vs Roux-en-Y reconstruction[10,26-28]. Patients after Billroth II reconstruction scored lower on some of the functional scales and reported more pain, fatigue and reflux symptoms. This occurred despite routine construction of Braun anastomosis, which supposedly diverts bile from the remnant stomach, relieving reflux symptoms, dumping syndrome or other disturbances in food intake[31]. Although patients after Billroth II reconstruction were more likely to have a higher disease stage than those after Roux-en-Y reconstruction, statistically significant superiority of the Roux-en-Y procedure on the emotional, role and physical functioning scale remained after adjustment for demographic data, disease stage and postoperative complications.
This study has some limitations. First, the cross-sectional nature does not allow longitudinal assessment of HRQoL. Nonetheless, we believe this kind of design allows us to gain important insight into the overall HRQoL of patients following gastrectomy, which represents the basis for clinical decision making. Second, the number of participating patients is relatively low (116), representing 49.6% of patients that were eligible for study inclusion. Third, mail surveys lack data related to the actual health status of the patient. We did not see the patients to obtain their health status objectively; therefore, their subjective measures could not be compared to their actual physical findings. Nonetheless, HRQoL is a multimodal construct of physical, psychological and social wellbeing in relation to disease treatment. Therefore, objective and subjective measures are not necessarily related. Even if a patient is objectively well, he or she may at the same time be subjectively unwell, which should be addressed separately from objective measures.
Our study shows that patients after gastrectomy for gastric cancer experience several functional and symptom complaints affecting quality of life. Based on our results, with regard to HRQoL, subtotal distal gastrectomy with Roux-en-Y reconstruction should be preferred over subtotal distal gastrectomy with Billroth II reconstruction. Patients should be informed preoperatively about expected functional sequelae after surgery and should be regularly monitored postoperatively to ensure proper symptomatic and supportive care.
Gastrectomy is a major operation that alters the physiological functions of the digestive tract. Consequently, these patients experience malnutrition, weight loss and several postgastrectomy symptoms that negatively impact patients’ wellbeing.
With improved survival in gastric cancer patients, health-related quality of life has become an important clinical endpoint alongside oncological outcomes.
The aim of this study was to investigate health-related quality of life after various surgical procedures for gastric cancer treatment.
Patients that underwent curative resection for gastric adenocarcinoma at a tertiary centre between January 2014 and December 2018 were recruited for inclusion in this cross-sectional survey. The validated Slovenian version of the European Organization for Research and Treatment of Cancer Quality of Life Core Questionnaire (QLQ-C30) and its gastric cancer specific module (QLQ STO-22) were sent to all eligible patients for self-completion. The scores of both questionnaires were compared between patients after subtotal distal vs total gastrectomy and patients after subtotal distal gastrectomy with Billroth II vs Roux-en-Y reconstruction using the Mann-Whitney U test. The association between the type of operation and general health status adjusted for some demographic and clinical characteristics was assessed with multiple linear regression.
Out of 234 patients that were eligible for study inclusion, 116 (49.6%) patients completed the questionnaires. No statistically significant differences were observed in scores on global or functional scales among patients after total or subtotal distal gastrectomy. However, patients after total vs subtotal gastrectomy did experience more dysphagia (P = 0.020) and eating restrictions (P = 0.017). Patients after subtotal distal gastrectomy with Billroth II reconstruction reported significantly worse scores on the physical (P = 0.038) and role functioning (P = 0.034) scales and had more problems with pain (P = 0.010), fatigue (P = 0.028) and reflux (P = 0.001). When adjusted for demographic data, disease stage and postoperative complications, no differences were observed in reported global health status/quality of life scores among different surgical procedures. However, Roux-en-Y was superior over Billroth II reconstruction in emotional (β = 0.24, P = 0.041), role (β = 0.24, P = 0.034) and physical (β = 0.23, P = 0.048) functioning when adjusted for other variables in a regression model.
Patients after gastrectomy for gastric cancer experience several functional and symptom complaints. Based on our results, subtotal distal gastrectomy with Roux-en-Y reconstruction should be preferred over subtotal distal gastrectomy with Billroth II reconstruction.
The data obtained from this study will help surgeons when preoperatively informing their patients about expected functional outcomes after gastrectomy and enable them to ensure proper supportive care for their patients in the postoperative period.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Slovenia
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B, B, B
Grade C (Good): C, C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Endo S, Mishra TS, Panduro-Correa V, Toyoshima O S-Editor: Gao CC L-Editor: A P-Editor: Liu JH
| 1. | Davis JL, Ripley RT. Postgastrectomy Syndromes and Nutritional Considerations Following Gastric Surgery. Surg Clin North Am. 2017;97:277-293. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 77] [Article Influence: 9.6] [Reference Citation Analysis (2)] |
| 2. | van den Boorn HG, Stroes CI, Zwinderman AH, Eshuis WJ, Hulshof MCCM, van Etten-Jamaludin FS, Sprangers MAG, van Laarhoven HWM. Health-related quality of life in curatively-treated patients with esophageal or gastric cancer: A systematic review and meta-analysis. Crit Rev Oncol Hematol. 2020;154:103069. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 36] [Article Influence: 7.2] [Reference Citation Analysis (1)] |
| 3. | Ji X, Yan Y, Bu ZD, Li ZY, Wu AW, Zhang LH, Wu XJ, Zong XL, Li SX, Shan F, Jia ZY, Ji JF. The optimal extent of gastrectomy for middle-third gastric cancer: distal subtotal gastrectomy is superior to total gastrectomy in short-term effect without sacrificing long-term survival. BMC Cancer. 2017;17:345. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 33] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 4. | Kong L, Yang N, Shi L, Zhao G, Wang M, Zhang Y. Total vs subtotal gastrectomy for distal gastric cancer: meta-analysis of randomized clinical trials. Onco Targets Ther. 2016;9:6795-6800. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 22] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 5. | Goh YM, Gillespie C, Couper G, Paterson-Brown S. Quality of life after total and subtotal gastrectomy for gastric carcinoma. Surgeon. 2015;13:267-270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 18] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 6. | Ahmad R, Schmidt BH, Rattner DW, Mullen JT. Factors influencing readmission after curative gastrectomy for gastric cancer. J Am Coll Surg. 2014;218:1215-1222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 28] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 7. | Tran TB, Hatzaras I, Worhunsky DJ, Vitiello GA, Squires MH 3rd, Jin LX, Spolverato G, Votanopoulos KI, Schmidt C, Weber S, Bloomston M, Cho CS, Levine EA, Fields RC, Pawlik TM, Maithel SK, Norton JA, Poultsides GA. Gastric remnant cancer: A distinct entity or simply another proximal gastric cancer? J Surg Oncol. 2015;112:877-882. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 8. | Hanyu T, Wakai A, Ishikawa T, Ichikawa H, Kameyama H, Wakai T. Carcinoma in the Remnant Stomach During Long-Term Follow-up After Distal Gastrectomy for Gastric Cancer: Analysis of Cumulative Incidence and Associated Risk Factors. World J Surg. 2018;42:782-787. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 30] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 9. | Tran TB, Worhunsky DJ, Squires MH, Jin LX, Spolverato G, Votanopoulos KI, Cho CS, Weber SM, Schmidt C, Levine EA, Bloomston M, Fields RC, Pawlik TM, Maithel SK, Norton JA, Poultsides GA. To Roux or not to Roux: a comparison between Roux-en-Y and Billroth II reconstruction following partial gastrectomy for gastric cancer. Gastric Cancer. 2016;19:994-1001. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 29] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 10. | He L, Zhao Y. Is Roux-en-Y or Billroth-II reconstruction the preferred choice for gastric cancer patients undergoing distal gastrectomy when Billroth I reconstruction is not applicable? Medicine (Baltimore). 2019;98:e17093. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 35] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 11. | So JB, Rao J, Wong AS, Chan YH, Pang NQ, Tay AYL, Yung MY, Su Z, Phua JNS, Shabbir A, Ng EKW. Roux-en-Y or Billroth II Reconstruction After Radical Distal Gastrectomy for Gastric Cancer: A Multicenter Randomized Controlled Trial. Ann Surg. 2018;267:236-242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 46] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 12. | Aaronson NK, Ahmedzai S, Bergman B, Bullinger M, Cull A, Duez NJ, Filiberti A, Flechtner H, Fleishman SB, de Haes JC. The European Organization for Research and Treatment of Cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst. 1993;85:365-376. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9802] [Cited by in RCA: 11461] [Article Influence: 358.2] [Reference Citation Analysis (0)] |
| 13. | Fayers P, Aaronson NK, Bjordal K, Groenvold M, Curran D, Bottomley A. EORTC QLQ-C30 Scoring Manual. 3rd ed. Brussels: European Organization for Research and Treatment of Cancer, 2001. |
| 14. | Avery K, Hughes R, McNair A, Alderson D, Barham P, Blazeby J. Health-related quality of life and survival in the 2 years after surgery for gastric cancer. Eur J Surg Oncol. 2010;36:148-154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 55] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 15. | Karanicolas PJ, Graham D, Gönen M, Strong VE, Brennan MF, Coit DG. Quality of life after gastrectomy for adenocarcinoma: a prospective cohort study. Ann Surg. 2013;257:1039-1046. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 122] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 16. | Kim AR, Cho J, Hsu YJ, Choi MG, Noh JH, Sohn TS, Bae JM, Yun YH, Kim S. Changes of quality of life in gastric cancer patients after curative resection: a longitudinal cohort study in Korea. Ann Surg. 2012;256:1008-1013. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 108] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 17. | Kong H, Kwon OK, Yu W. Changes of quality of life after gastric cancer surgery. J Gastric Cancer. 2012;12:194-200. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 51] [Cited by in RCA: 62] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 18. | Park S, Chung HY, Lee SS, Kwon O, Yu W. Serial comparisons of quality of life after distal subtotal or total gastrectomy: what are the rational approaches for quality of life management? J Gastric Cancer. 2014;14:32-38. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 43] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 19. | Yu W, Park KB, Chung HY, Kwon OK, Lee SS. Chronological Changes of Quality of Life in Long-Term Survivors after Gastrectomy for Gastric Cancer. Cancer Res Treat. 2016;48:1030-1036. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 62] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 20. | Kobayashi D, Kodera Y, Fujiwara M, Koike M, Nakayama G, Nakao A. Assessment of quality of life after gastrectomy using EORTC QLQ-C30 and STO22. World J Surg. 2011;35:357-364. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 111] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 21. | Kwon OK, Yu B, Park KB, Park JY, Lee SS, Chung HY. Advantages of Distal Subtotal Gastrectomy over Total Gastrectomy in the Quality of Life of Long-Term Gastric Cancer Survivors. J Gastric Cancer. 2020;20:176-189. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 22. | Brenkman HJF, Tegels JJW, Ruurda JP, Luyer MDP, Kouwenhoven EA, Draaisma WA, van der Peet DL, Wijnhoven BPL, Stoot JHMB, van Hillegersberg R; LOGICA Study Group. Factors influencing health-related quality of life after gastrectomy for cancer. Gastric Cancer. 2018;21:524-532. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 46] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 23. | Lee SS, Chung HY, Yu W. Quality of life of long-term survivors after a distal subtotal gastrectomy. Cancer Res Treat. 2010;42:130-134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 32] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 24. | Lee SS, Chung HY, Kwon OK, Yu W. Quality of life in cancer survivors 5 years or more after total gastrectomy: a case-control study. Int J Surg. 2014;12:700-705. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 35] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 25. | Lee SS, Chung HY, Kwon OK, Yu W. Long-term Quality of Life After Distal Subtotal and Total Gastrectomy: Symptom- and Behavior-oriented Consequences. Ann Surg. 2016;263:738-744. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 75] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 26. | Rausei S, Mangano A, Galli F, Rovera F, Boni L, Dionigi G, Dionigi R. Quality of life after gastrectomy for cancer evaluated via the EORTC QLQ-C30 and QLQ-STO22 questionnaires: surgical considerations from the analysis of 103 patients. Int J Surg. 2013;11 Suppl 1:S104-S109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 38] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 27. | Cai Z, Zhou Y, Wang C, Yin Y, Shen C, Yin X, Chen Z, Zhang B. Optimal reconstruction methods after distal gastrectomy for gastric cancer: A systematic review and network meta-analysis. Medicine (Baltimore). 2018;97:e10823. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 37] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 28. | Lee MS, Ahn SH, Lee JH, Park DJ, Lee HJ, Kim HH, Yang HK, Kim N, Lee WW. What is the best reconstruction method after distal gastrectomy for gastric cancer? Surg Endosc. 2012;26:1539-1547. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 113] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 29. | Hoya Y, Mitsumori N, Yanaga K. The advantages and disadvantages of a Roux-en-Y reconstruction after a distal gastrectomy for gastric cancer. Surg Today. 2009;39:647-651. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 76] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 30. | Otsuka R, Natsume T, Maruyama T, Tanaka H, Matsuzaki H. Antecolic reconstruction is a predictor of the occurrence of roux stasis syndrome after distal gastrectomy. J Gastrointest Surg. 2015;19:821-824. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 31. | Hwang HK, Lee SH, Han DH, Choi SH, Kang CM, Lee WJ. Impact of Braun anastomosis on reducing delayed gastric emptying following pancreaticoduodenectomy: a prospective, randomized controlled trial. J Hepatobiliary Pancreat Sci. 2016;23:364-372. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 18] [Article Influence: 2.0] [Reference Citation Analysis (0)] |